HNF4α is a nuclear receptor produced as 12 isoforms from two promoters by alternative splicing. An analysis integrating protein interactions by proteomics and gene expression by transcriptomics enabled the identification of transcriptional regulatory mechanisms specific to the different HNF4α isoforms, demonstrating the importance of considering all isoforms given their seemingly diverse functions.
Keywords: Transcriptional regulation, transcription, RNA SEQ, quantification, bioinformatics, affinity proteomics, colorectal cancer, SILAC, nuclear receptors, protein isoforms
Graphical Abstract
Highlights
Analysis of the twelve isoforms of HNF4α for DNA binding and expression.
The entire transcriptome associated with each isoform were analyzed as well as their respective interacting proteome using BioID.
Isoforms α4–5–6 do not bind the known DNA response element and demonstrate little gene transactivation.
Several transcription factors and coregulators were identified as potential specific partners for different HNF4α isoforms.
Abstract
HNF4α is a nuclear receptor produced as 12 isoforms from two promoters by alternative splicing. To characterize the transcriptional capacities of all 12 HNF4α isoforms, stable lines expressing each isoform were generated. The entire transcriptome associated with each isoform was analyzed as well as their respective interacting proteome. Major differences were noted in the transcriptional function of these isoforms. The α1 and α2 isoforms were the strongest regulators of gene expression whereas the α3 isoform exhibited significantly reduced activity. The α4, α5, and α6 isoforms, which use an alternative first exon, were characterized for the first time, and showed a greatly reduced transcriptional potential with an inability to recognize the consensus response element of HNF4α. Several transcription factors and coregulators were identified as potential specific partners for certain HNF4α isoforms. An analysis integrating the vast amount of omics data enabled the identification of transcriptional regulatory mechanisms specific to certain HNF4α isoforms, hence demonstrating the importance of considering all isoforms given their seemingly diverse functions.
Nuclear receptors (NR)1 represent a class of transcription factors that encompasses 48 proteins in humans (1). The nomenclature surrounding the superfamily of NR, based on their phylogeny, consists of six subfamilies comprised of several groups (Nuclear Receptors Nomenclature Committee, 1999). NR share a structural organization of five to six distinct regions designated from A to F (2). The A/B region, located at the N-terminal end, is highly variable between the various NR. It typically contains an AF-1 transactivation region (Activation Function) which is active independently of ligand binding and allows the interaction of the receptor with various coregulators and other transcription factors (3). The C domain, or DNA-binding domain (DBD), is the most conserved among NR. It allows the recognition of specific DNA response elements via two cysteine-rich zinc finger motifs (C-X2-C-X13-C-X2-C and C-X5-C-X9-C-X2-C) (2). These response elements are composed of repeated or inverted hexameric DNA sequences and separated by linkers varying from one to five nucleotides in length (4). The D region, also called hinge region, is less conserved and its main function is to facilitate free rotation between the DBD and the ligand binding domain (LBD). A nuclear localization signal (NLS) contained in this region participates in the regulation of the subcellular distribution of NR (5). The E domain, or LBD, consists of a hydrophobic pouch for binding a multitude of small lipophilic molecules such as steroid hormones, phospholipids, fatty acids and xenobiotics (6). Some NR for which no ligand has yet been identified are considered orphan receptors (7). A second AF-2 activator region is in the LBD. In contrast to the AF-1 region, the activity of the AF-2 region is dependent on ligand binding to LBD. This induces a conformational change in the LBD, generating a pocket that can interact with the LXXLL motif present on a host of transcriptional coactivators (8). The LBD, like the DBD, contains an important interface for receptor dimerization. Finally, at the C terminus of the NR is the F domain. Because of its highly variable sequence, the exact function of the F domain remains to be established and several NR have no F domain (9). Nevertheless, the deletion of this domain in those receptors bearing the domain have revealed its importance in certain instances in connection with various functions such as dimerization, activation and interaction with different coregulators (9).
HNF4α (Hepatocyte Nuclear Factor 4 alpha) (also referred to as NR2A1) is a transcription factor of the nuclear receptor family that was initially identified as a regulator of liver-specific gene expression (10, 11). Since its initial discovery in the liver, HNF4α has also been detected in the kidneys, pancreas, stomach, small intestine and colon (12). HNF4α is crucial for the development and maintenance of hepatocyte function, including lipid homeostasis, transport and metabolism, as well as the detoxification of xenobiotics (13–15). Additional functions for HNF4α in the gut and pancreas have also emerged (16–18). In contrast to other types of NR, HNF4α is constitutively localized in the nucleus and does not require binding of a ligand to homodimerize and interact with the response elements of its target genes (19). HNF4α has long been considered an orphan nuclear receptor, although crystallography of the LBD initially revealed the presence of fatty acids of various compositions bound at the level of the ligand binding pocket of HNF4α (20). Subsequent studies have identified linoleic acid, a long chain polyunsaturated fatty acid (C18: 2ω6), as the molecule preferentially binding to its LBD (19); however, this binding is reversible and does not modulate the transcriptional activity of HNF4α. The nature of the HNF4α ligand therefore remains controversial, because linoleic acid is endogenously present and does not appear necessary for receptor activity, unlike the typical mode of action of NR requiring binding to their ligand. HNF4α recognizes DR1 sites (Direct Repeat 1), consisting of two repeated hexameric half-sites separated by a nucleotide, typically an adenosine (21). HNF4α also recognizes direct repeats separated by two nucleotides (DR2), but with lower specificity (22). The consensus sequence of its half-sites, AGGTCA, is shared by most nonsteroidal NR (21). HNF4α is an exclusive homodimer, this form being stably found in solution and necessary to bind DNA (23). However, both RXRα/β/γ and RARα nuclear receptors, known for their ability to form heterodimers with several NR, do not assemble into heterodimers with HNF4α (23, 24).
Alternative splicing is a major source of cellular protein diversity. The estimated percentage of human gene products undergoing alternative splicing has been proposed to be as high as 95% of multiexon genes, although it is still unclear how many of these splicing variants are expressed or functional (25). However, very few of these alternative protein isoforms have well-characterized cellular functions, given that studies on these proteins have either mostly concentrated on a single isoform, or do not specify which isoform was under study, hence leading to discrepancies or contradictions in protein function (26). In addition, many alternatively spliced transcripts studied for protein-protein interaction by yeast two-hybrid assay were shown to display significant differences between reference and alternative isoforms, with many alternative isoforms interacting with different protein partners (27). These differences in protein complexes underline the importance of considering each protein isoform to understand its unique role(s).
In the present study, the transcriptional functions of the 12 annotated isoforms of HNF4α (28, 29) were specifically characterized by generating stable lines expressing each HNF4α isoform in HCT 116 cells. The entire transcriptome associated with each isoform was analyzed by RNA sequencing, as well as their respective proteome by a BioID approach coupled to quantitative mass spectrometry. This analysis integrating the vast amount of transcriptomic and proteomic data enabled the identification of transcriptional regulatory mechanisms specific to each isoform, demonstrating the importance of considering all isoforms which can exhibit distinct functions.
MATERIALS AND METHODS
Experimental Model and Subject Details
All cell lines were obtained from the ATCC. HCT 116 (human colorectal cancer (hCRC)), Caco-2/15 (hCRC), Capan-2 (human pancreatic adenoma), HepG2 (human hepatocellular carcinoma) and 293T (transformed human embryonic kidney) cell lines were cultured in DMEM. AsPC-1 (human pancreatic adenocarcinoma), COLO 205 (hCRC), and DLD-1 (hCRC) cell lines were cultured in RPMI. The T84 (hCRC) cell line was cultured in DMEM/F-12. The LoVo (hCRC) cell line was cultured in F-12K. The HT-29 (hCRC) cell line was cultured in McCoy's 5A. All cultured media were supplemented with 10% FBS and cell lines were grown in a humidified incubator at 37 °C with 5% CO2. The Human Digestive System MTC Panel cDNA library was purchased from Clontech Laboratories (Mountain View). The cDNA preparations found in this library are derived from the combination of cDNAs from several healthy Caucasian individuals between 18 and 61 years of age and were provided at a concentration of 1.0 ng/μl following first-strand cDNA preparation for each tissue. A total of 12 tissues of the digestive system were included in the bank: liver, stomach, esophagus, duodenum, jejunum, ileum, ileocecum, cecum, ascending, transverse and descending colon, as well as the rectum.
Total RNA isolation and Reverse Transcription
Total RNA of HCT 116, Caco-2/15, T84, COLO 205, LoVo, DLD-1, HT-29, HepG2, AsPC-1, Capan-2, and HCT 116 HNF4α (1–12) -GFP cell lines was isolated using RNeasy RNA isolation kit (Qiagen). The stable HCT 116 HNF4α (1–12) -GFP cell lines were induced for 48 h with 2.5 μg/ml doxycycline (Clontech Laboratories, Mountain View) before extraction of total RNAs. cDNA synthesis was performed with the SuperScript IV-RT reverse transcriptase enzyme (Thermo Fisher Scientific, Waltham). Two micrograms of RNA were added in a total volume of 10 μl by supplementing with DEPC water. A mixture containing 2.4 μl of 0.5 μg/μl poly (dT) oligos (Amersham Biosciences, Little Chalfont, United Kingdom) and 0.8 μl of 25 mm dNTPs (Amersham Biosciences, Little Chalfont, United Kingdom) was added to the RNA, then heated for 5 min at 75 °C and placed on ice for an additional 5 min. A 10 μl volume of RT reaction mixture according to the manufacturer's conditions was added to the RNA. The reaction was incubated for 1 h at 50 °C, before inactivating the SuperScript IV-RT by heating for 5 min at 95 °C. The cDNA samples were subsequently stored at −20 °C.
Expression of Isoforms in Different Human Cancer Cell Lines
Oligonucleotides specific for each isoform and the reference genes were obtained from Integrated DNA Technologies (IDT, San Jose) (supplemental TableS6). The cDNA from HCT 116 HNF4α (1–12) -GFP lines was used as a positive control for each isoform. Expression levels of the HPRT and PUM1 genes were used as references, with an amplification of 26 cycles at a hybridization temperature of 60 °C and an elongation time of 20 s.
Expression of Isoforms in the Different Tissues of the Human Digestive System
The expression of the 12 HNF4α isoforms in the different human gastrointestinal tract tissues was assessed by PCR. The primers specific to each HNF4α isoform (supplemental Table S6) were used to assess the expression of the isoforms by PCR in each of the previously listed tissues. The reagents used for the amplification were the same as described previously, the template DNA being in this instance a volume of 3.7 μl of cDNA. The PCR reactions were performed according to the PCR conditions detailed in the previous section, for a first round of 30 cycles. A 10 μl aliquot of the reaction was retrieved, and the remainder of the reaction was supplemented again at 20 μl at the initial reaction concentrations for a second round of PCR of 6 additional cycles. The expression levels of the POLR2A and PSMB2 genes were used as references, with an amplification of 26 cycles. A PCR product corresponding to each isoform was sequenced to ensure the specificity of the amplification. Plasmid pUC19 was digested with the SmaI restriction enzyme (New England Biolabs, Ipswich) in CutSmart buffer (New England Biolabs, Ipswich) for 1 h at 25 °C, and subsequently purified on gel agarose. Ligation between the PCR product and the digested pUC19 plasmid was carried out in a 20:1 ratio (insert: vector) with T4 DNA ligase (New England Biolabs, Ipswich) for 3 h at room temperature. Sequencing was performed via the Genome Sequencing and Genotyping Platform (Université Laval, Quebec, Canada).
Cloning of HNF4α Isoforms
The 12 HNF4α isoforms were cloned into the donor vector pENTR11 (Thermo Fisher Scientific, Waltham) in three distinct steps consisting in initially cloning the sequence common to the 12 isoforms, inserting the different N-terminal termini (A/B domains) on each side, followed by the different C termini (F domain). The Gateway system (Invitrogen, Carlsbad) was subsequently used to obtain constructs allowing the expression of isoforms in fusion with the GFP and BioID2 protein labels.
Cloning of the Common Sequence in pENTR11
The common sequence to the 12 isoforms was amplified by PCR from the pLenti-HNF4α2-GFP plasmid. This plasmid contains the α2 isoform of HNF4α (NM_000457.3) synthesized by Feldan Inc. and cloned into pLenti6/V5 (Invitrogen) (30). The amplified common sequence measures 1014 base pairs, covering 71–86% of the complete sequence of the different HNF4α isoforms, and contains the C, D, and E domains of HNF4α. Oligonucleotides used for amplification (supplemental Table S6) were obtained from Integrated DNA Technologies (IDT, San Jose). These enabled the addition of SpeI and SfoI restriction sites within the common sequence, at the 5 'and 3′ ends, respectively, without changing the amino acid composition of the HNF4α protein. In parallel, these primers also allowed the upstream and downstream addition of the sequence of SalI and XhoI restriction sites, in consecutive order. The amplification reaction was performed with iProof High-Fidelity DNA Polymerase enzyme (Bio-Rad, Hercules) according to the manufacturer's recommendations. The reactions were performed in the T100 thermal cycler (Bio-Rad, Hercules).
The PCR product was purified on agarose gel using the EZ-10 Spin Column DNA Gel Extraction Kit (Bio Basic, Markham, Canada). The purified PCR product and the pENTR11 plasmid were digested with SalI and XhoI restriction enzymes (New England Biolabs, Ipswich) in NEBuffer 3.1 digest buffer (New England Biolabs, Ipswich) for 2 h at 37 °C, then purified on agarose gel. The digested and purified pENTR11 plasmid was then dephosphorylated by the Antarctic Phosphatase enzyme (New England Biolabs, Ipswich) for 30 min at 37 °C, before ligation between the PCR product and pENTR11 in a 5: 1 ratio (insert: vector) with T4 DNA ligase (New England Biolabs, Ipswich) for 2 h at room temperature. The pENTR11- (common sequence of HNF4α) plasmid was sequenced via the Genome Sequencing and Genotyping Platform (Laval University, Quebec, Canada).
Inserting the N-Termini into pENTR11
The four different N-terminal ends of the HNF4α isoforms, surrounded by SalI (5 ') and SpeI (3′) restriction sites, were obtained from Integrated DNA Technologies (IDT, San Jose). The sequences corresponding to the N-terminal ends of the α1/2/3 and α4/5/6 isoform groups were synthesized directly in the form of double-stranded DNA (gBlocks Gene Fragments). The sequences corresponding to the N-terminal ends of the α7/8/9 and α10/11/12 isoform groups were obtained in the form of single-stranded oligonucleotides. To obtain a double-stranded sequence, the sense and antisense oligonucleotides were mixed at a concentration of 1 μm in a 5 mm NaCl buffer, 20 mm Tris pH 7.5. Hybridization of the oligonucleotides was performed by placing the reaction mixture in a heated dry bath set at 98 °C for 2 min and subsequently allowing the reaction to return to room temperature after turning off the bath. The pENTR11- (common HNF4α sequence) plasmid as well as the four N termini were digested with SalI and SpeI restriction enzymes (New England Biolabs, Ipswich) in CutSmart buffer (New England Biolabs, Ipswich)) for 2 h at 37 °C, before being purified on agarose gel. A ligation reaction with a 10: 1 ratio (insert: vector) with T4 DNA ligase for 2 h at room temperature was then performed to insert each N terminus upstream of the common sequence of HNF4α already present in pENTR11. The four different plasmids thus obtained were sequenced via the Genome Sequencing and Genotyping Platform (Université Laval, Quebec, Canada).
Inserting the C-Termini into pENTR11
The three different C-terminal ends of the HNF4α isoforms, lined with a 3 'XhoI restriction site, were synthesized directly as double-stranded DNA (gBlocks Gene Fragments) (IDT, San Jose). These three sequences as well as the four pENTR11 plasmids containing the different N-terminal ends in front of the common HNF4α sequence were digested by the restriction enzymes SfoI (only for the plasmids) and XhoI (New England Biolabs, Ipswich) in the CutSmart buffer for 2 h at 37 °C, then gel purified. A ligation reaction as described previously was subsequently performed to insert each C terminus downstream of the common HNF4α sequence into pENTR11. The pairing of three C-terminal ends at the four N termini clones previously cloned hence generated 12 pENTR11-HNF4α plasmids (1–12), containing the complete sequence of each of the 12 HNF4α isoforms. These plasmids were sequenced via the Genome Sequencing and Genotyping Platform (Université Laval, Quebec, Canada).
Generation of pcDNA-DEST47, pgLAP5.2-GFP and pgLAP5.2-BioID2–3myc
The sequences of the 12 isoforms cloned into the pENTR11 vector were transferred into the pcDNA-DEST47 expression vectors (Thermo Fisher Scientific, Waltham), pgLAP5.2 (a gift from Peter Jackson (Addgene plasmid #19706)) and pgLAP5.2-BioID2–3Xmyc by Gateway cloning, via an LR reaction according to the manufacturer's instructions (Thermo Fisher Scientific, Waltham). The pcDNA-DEST47-HNF4α (1–12), pgLAP5.2-HNF4α (1–12) and pgLAP5.2-HNF4α (1–12) -BioID2–3Xmyc plasmids were sequenced via the Genome Sequencing and Genotyping Platform (Université Laval, Quebec, Canada). The control empty pGLAP5.2-BioID2–3Xmyc- plasmid was cloned to express the BioID2–3Xmyc protein label alone as a control for mass spectrometry experiments. To achieve the latter, the pGLAP5.2-BioID2–3Xmyc plasmid was amplified by PCR to remove an ∼1.8 kb fragment containing the chloramphenicol resistance gene and the ccdB gene.
Generation of Inducible Stable Cell Lines and Treatment with MG132
The stable cell lines HCT 116 HNF4α (1–12) -GFP, HCT 116 HNF4α (1–12) BioID2–3Xmyc and HCT 116 BioID2–3Xmyc-control were generated using the Flp-In T-REx system (Thermo Fisher Scientific, Waltham) using the pgLAP5.2-HNF4α (1–12), pgLAP5.2-HNF4α (1–12) -BioID2–3Xmyc and pGLAP5.2-BioID2–3Xmyc-empty plasmids, respectively.
Transfections for stable lines were performed with HCT 116 Flp-In T-Rex cells, upon reaching ∼70% confluence, in a 60 mm Petri dish using Lipofectamine LTX (Thermo Fisher Scientific, Waltham) as a transfection agent. A total of 500 ng of plasmid DNA was mixed with 4.5 μg of the Flp-Recombinase expression vector pOG44 (Thermo Fisher Scientific, Waltham), 5 μl of Plus Reagent (Thermo Fisher Scientific, Waltham) and Opti-MEM medium (Thermo Fisher Scientific, Waltham) to complete the reaction at a final volume of 300 μl. A second mixture was prepared simultaneously containing 8 μl of lipofectamine LTX and 292 μl of Opti MEM medium. These two reactions were incubated for 5 min at room temperature before being mixed and incubated for 30 min at room temperature. Meanwhile, HCT 116 cells were washed once with 1X PBS followed by the addition of 2 ml of DMEM culture medium (Thermo Fisher Scientific, Waltham) containing 10% FBS (Wisent, St. John the Baptist, Canada). The selection was started 24 h later, by adding the antibiotics blasticidin S (5 μg/ml) and hygromycin B (100 μg/ml). The clonal population was maintained over time by the sustained use of these antibiotics, and the induction of constructs was achieved via the addition of 2.5 μg/ml doxycycline (Clontech Laboratories, Mountain View) in the medium at the desired moment. Cells expressing HNF4α isoforms for 48 h by the addition of doxycycline were either treated or not with the proteasome inhibitor MG132 at a final concentration of 5 μm for 6 h. Cells were lysed directly in LDS sample buffer, resolved by SDS-PAGE and transferred to nitrocellulose. Immunoblotting was performed with either a myc antibody (Abcam ab9106) or a GAPDH antibody (Cell Signaling #D16H11).
EMSA
Oligonucleotides containing the HNF4α consensus DR1 response element (DR1 WT, Biotin-GGAGATTAGGTCAAAGGTCAGTAGTCG) or a mutated sequence (DR1 MUT, Biotin-GGAGATTACCTCTTAGCAGAGTAGTCG) labeled with a biotin molecule at the 5′ end, were obtained from Integrated DNA Technologies (IDT, San Jose). Hybridized probes were diluted to a concentration of 20 nm for binding reactions. Nuclear extracts were isolated from 293T cell lines transfected with the different pcDNA-DEST47-HNF4α (1–12) plasmids. The cells, cultured at an approximate 90% confluence in 60 mm Petri dishes, were trypsinized and subsequently centrifuged at 1500 × g for 5 min. The cell pellet was washed twice with 1 ml of 1× PBS, followed by centrifugation after each wash. The cells were then resuspended in 1 ml of nuclear extraction buffer A (10 mm HEPES pH 7.9, 10 mm KCl, 1.5 mm MgCl 2, 0.34 m sucrose, 10% glycerol, 1 mm DTT, and EDTA-free Protease Inhibitor Mixture (Roche Applied Science, Penzberg, Germany)). A volume of 10 μl of 10% Triton X-100 solution was added to the cell suspension to obtain a final concentration of 0.1% Triton X-100. The cells were lysed in this buffer for 8 min on ice, to only rupture the plasma membrane while maintaining the integrity of the nuclear membrane. The cells were centrifuged at 1300 × g for 5 min at 4 °C. The supernatant, corresponding to the cytoplasmic protein fraction, was collected and stored at −80 °C. The nuclei-containing pellets were next resuspended in 150 μl of nuclear extraction buffer B (1% Triton X-100, 150 mm NaCl, 20 mm Tris pH 7.4, 1 mm DTT, and cOmplete EDTA-free Protease Inhibitor Mixture). The nuclear fraction was incubated with stirring for 30 to 60 min at 4 °C, before being stored at −80 °C. The nuclear and cytoplasmic fractions were centrifuged at 20,000 × g for 15 min to eliminate cell debris. These fractions were assayed using the Pierce BCA Protein Assay kit (Thermo Fisher Scientific, Waltham) as recommended by the manufacturer. Immunoblots of the HNF4α, histone H3 and GAPDH proteins were performed to validate the quality of the nuclear extraction.
The binding reactions were prepared in several incubation steps at room temperature, in a final volume of 20 μl. A first incubation of 10 min was carried out by mixing 6 μl of nuclear extracts (6 μg), 2 μl of 10X binding buffer (100 mm Tris pH 7.5, 10 mm EDTA, 1 m KCl, 50% glycerol and 1 mm DTT) and 1 μl poly [d (IC)] (1 μg/μl) (cat. # 10108812001, Roche Applied Science, Penzberg, Germany). The biotinylated probe was then added to the mixture at a final concentration of 1 nm and incubated for 25 min. Supershift was performed by adding 1.5 μl of purified GFP monoclonal antibody (0.4 μg/μl) (cat. # 11814460001, Roche Applied Science, Penzberg, Germany) for an additional incubation period of 15 min. A 2 μl volume of 6X-EMSA loading buffer (6× TBE, 30% glycerol, 0.125% bromphenol blue) was subsequently added to the reaction mixture before loading on a non-denaturing polyacrylamide gel.
The non-denaturing polyacrylamide gels were prepared using the SureCast Gel HandCast System (Invitrogen, Carlsbad), in a “mini gel” format (8 × 8 × 0.1 cm) at a concentration of 4% acrylamide: bis, 10% glycerol and TBE 0.5X (45 μm Tris, 45 μm boric acid, 1 mm EDTA pH 8.0). A 60-min pre-migration at 100 V was performed using cold 0.5X TBE buffer as migration buffer. The samples were then loaded onto the gel and migrated at 100 V for 100 min. Binding reactions were transferred onto a positively-charged nylon membrane Amersham Biosciences Hybond-N + (cat #RPN203B, GE Healthcare Life Sciences, Marlborough). The blot was performed in 0.5× TBE buffer at 200 mA for 90 min at 4 °C. Membrane crosslinking was subsequently achieved using a UV Stratalinker 2400 (Stratagene, San Diego), equipped with UV light bulbs (254 nm, 5 × 15 W). The auto-crosslink function was used, equivalent to an emitted energy of 1200 μJ (×100) for 45 s.
The steps leading to detection of the probe on the positively-charged nylon membrane were performed using the Chemiluminescent Nucleic Acid Detection Module (Thermo Fisher Scientific, Waltham). Detection of biotinylated probes was performed using the ChemiDoc MP imaging system (Bio-Rad, Hercules) and Amersham Biosciences Hyperfilm ECL autoradiographic films (GE Healthcare Life Sciences, Marlborough).
Quantitative Real-time PCR
The transactivation of different HNF4α target genes by its isoforms was analyzed by real-time quantitative PCR (RT-qPCR), using LightCycler 96 (Roche Applied Science, Penzberg, Germany) and cDNA from 48 h-induced HCT 116 HNF4α (1–12) -GFP lines. The oligonucleotides used for the amplification of the tested genes were obtained from Integrated DNA Technologies (IDT, San Jose) (supplemental Table S7). The reactions were performed with the SYBR Green Master Fast Start reaction mixture (Roche Applied Science, Penzberg, Germany) as recommended by the manufacturer. Analysis of the amplification and melting curves was performed using LightCycler 96 software version 1.1.0.1320 (Roche Applied Science, Penzberg, Germany). The relative expression of the genes was calculated by comparison with the TBP reference gene with the formula Etarget (CpControl − CpSample) × Ereference (CpSample − CpControl).
Transcriptomics
Preparation of Total RNA
The HCT 116 HNF4α (1–12) -GFP and HCT 116 Flp-In T-Rex lines were inoculated in 60 mm Petri dishes and incubated for 48 h in the presence of 2.5 μg/ml doxycycline. Total RNA of these 13 lines was extracted into triplicates using RNeasy RNA isolation kit (Qiagen). The concentration as well as the quality of the RNAs was evaluated first by NanoDrop (Thermo Fisher Scientific, Waltham, United States) via the RNomics platform of the Université de Sherbrooke and by a 2100 Bioanalyzer (Agilent, Santa Clara). The samples were then sent to McGill University's Innovation Center and Génome Québec.
Preparation of Libraries and Sequencing
Libraries were generated from 250 ng of total RNA. Enrichment of the mRNA was performed using the NEBNext Poly (A) Magnetic Insulation Module (New England Biolabs, Ipswich). The cDNA synthesis was performed via the use of NEBNext RNA First Strand Synthesis and NEBNext Ultra Directional RNA Second Strand Synthesis modules (New England Biolabs, Ipswich). The final steps for the preparation of the libraries were carried out using the NEBNext Ultra II DNA Library Prep Kit for Illumina (New England Biolabs, Ipswich). PCR adapters and primers were obtained from New England Biolabs. The libraries were quantified using Quant-iTT PicoGreen® dsDNA Assay Kit (Thermo Fisher Scientific, Waltham) and Kapa Illumina GA with Revised Primers-SYBR Universal Fast Kit (Kapa Biosystems, Wilmington). The average size of the RNA fragments was determined using the LabChip GX instrument (PerkinElmer, Waltham). Sequencing of the libraries was performed using NovaSeq 6000 (Illumina, San Diego) using a S2 PE100 protocol.
Sequence Alignment
The quality of the sequencing results was visualized using the FastQC 0.11.5 tool (31). The sorting of the data according to their quality score was performed using the Trimmomatic 0.36 software (32). The transcriptome used for the alignment of the reads was constructed from the human genome GRCh38.p12 and annotations from RefSeq (NCBI Homo sapiens Annotation Release 109) (33). Transcriptome alignment and quantification were performed using the Kallisto 0.44.0 tool (34). Results from Kallisto counts were used to quantify transcripts, as well as genes by addition of transcripts. The DESeq2 1.14.1 software was used to calculate the differential expression of transcripts and genes for each isoform compared with the control sample (35). The repository workflow (36) of the RNA-seq analysis can be found at http://gitlabscottgroup.med.usherbrooke.ca/Joel/hnf4a-isoforms-rna-seq/tree/master.
Mass Spectrometry Experimental Design and Statistical Rationale
Cell Culture and Induction of Different Constructions
The stable HCT 116 HNF4α (1–12) -BioID2–3Xmyc and HCT 116 BioID2–3Xmyc-control cell lines were cultured in three different SILAC media designated as light (R0K0), medium (R6K4) and heavy (R10K8). SILAC media contained DMEM + 4.5 g/L glucose, l-glutamine, sodium pyruvate (Thermo Fisher Scientific, Waltham) supplemented with 10% of triple-dialyzed FBS (Thermo Fisher Scientific, Waltham), 10 mm HEPES, 2 mm GlutaMAX, 100 U/ml penicillin and 100 μg/ml streptomycin. To these media were added different l-arginine and l-lysine isotopes at final concentrations of 42 μg/ml and 63.5 μg/ml respectively to obtain light, medium and heavy media. The light medium contained l-arginine R0 (Sigma-Aldrich A6969, St. Louis) and l-lysine K0 (Sigma Aldrich A8662, St. Louis). The medium medium contained l-arginine R6 (Cambridge Isotope Laboratories, Inc. CLM-2265, Tewksbury) and l-lysine K4 (Cambridge Isotope Laboratories, Inc. DLM-2640, Tewksbury). The heavy medium contained l-arginine R10 (Cambridge Isotope Laboratories, Inc. CNLM-539, Tewksbury) and l-lysine K8 (Cambridge Isotope Laboratories, Inc. CNLM-291, Tewksbury). The different SILAC culture media were then filtered on a Stericup filtration unit (EMD Millipore, Burlington) before use. The HCT 116 BioID2–3Xmyc cells, serving as a control condition for the mass spectrometry experiments, were cultured in the light medium. The HCT 116 HNF4α (1–6) -BioID2–3Xmyc lines were cultured in the medium medium whereas the HCT 116 HNF4α (7–12) -BioID2–3Xmyc lines were cultured in the heavy medium. Culture in SILAC medium involved a minimum of 5 passages at a ratio of 1:4 followed by incubation for 2–3 days in polystyrene Petri dishes 100 or 150 mm, at 37 °C in a controlled atmosphere at 5% CO2.
A 150 mm Petri dish was used for each condition. During the last passage in SILAC medium, doxycycline (Clontech Laboratories, Mountain View) was added to the cells at a concentration of 2.5 μg/ml 48 h before the streptavidin pulldown experiment, when the cells were at a confluence of 40 to 50%. The next day, 24 h before the pulldown, biotin (Sigma-Aldrich, St. Louis) was added to the cells at a final concentration of 50 μm. The cells were then washed three times with 1× PBS, trypsinized and centrifuged at 1500 × g for 5 min at 4 °C. The cell pellets were washed again twice with 1 × PBS.
Immunoprecipitation of Biotinylated Proteins
The cell pellets were lysed in 1 ml of RIPA buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1.5 mm MgCl 2, 0.1% SDS, 1% IGEPAL CA-630 (Sigma-Aldrich, St. Louis)), 1 mm PMSF, 0.4% sodium deoxycholate, 1 mm DTT, and the EDTA-free Protease Inhibitor Mixture inhibitor per 150 mm. The cell lysates were rotated for 20 to 30 min at 4 °C and sonicated on ice with a Sonic Dismembrator Model 120 (Thermo Fisher Scientific, Waltham) at 30% amplitude three times for 10 to 15 s interspaced by 5-s pauses. A 40 μl aliquot of each sample was taken for assay using the Pierce BCA Protein Assay kit (Thermo Fisher Scientific, Waltham). The samples were then rotated for an additional hour at 4 °C, after the addition of 10 μl of 0.1 m EGTA (1 mm final). A combination of the three SILAC conditions (light, medium and heavy) was performed before the pulldown by mixing 3.5 mg of total extract of each condition and supplementing to a volume of 3 ml with RIPA buffer. A 45 μl aliquot of 20% SDS was added to each sample (0.4% final). The samples were rotated for 15 min at 4 °C and subsequently centrifuged at 20,000 × g for 20 min at 4 °C.
High performance streptavidin Sepharose beads (GE Healthcare Life Sciences, Marlborough) were used for the pulldown at 50 μl per combined sample. The beads were washed three times with 1 ml of RIPA buffer containing 0.4% SDS. Washings were performed by rotating the beads for 5 min at 4 °C and subsequently centrifuging at 6000 × g for 3 min at 4 °C before removing the supernatant. The washed beads were resuspended at 50% concentration in the same buffer and added to the samples in 5 ml tubes. The samples were rotated for 3 h at 4 °C. The beads were centrifuged at 6000 × g for 3 min at 4 °C, and a 100 μl aliquot of the supernatant was removed. The beads were transferred to Low Binding microtubes (Sarstedt, Nümbrecht, Germany) and washed according to the parameters described above. A first wash was performed with 1.5 ml of BioID wash buffer (2% SDS, 50 mm Tris-HCl, pH 7.5), followed by three washes with 1 ml of RIPA buffer containing 0.4% SDS and five washes with 1 ml of 20 mm ammonium bicarbonate buffer. An aliquot corresponding to 5% of the total amount of beads was collected. The final washed beads were stored at −80 °C, as were all the aliquots collected during the experiment.
Reduction, Alkylation and Digestion of Proteins
All buffers used in this stage were prepared with MS-grade water. The protein reduction step was carried out by incubating the beads in 100 μl of 20 mm ammonium bicarbonate buffer containing 10 mm DTT (Thermo Fisher Scientific, Waltham) with stirring (1250 rpm) for 30 min at 60 °C. The alkylation of the proteins was carried out by adding another 100 μl of 20 mm ammonium bicarbonate buffer, and then supplementing with 15 mm iodoacetamide (Sigma-Aldrich, Saint-Louis) final before stirring for 1 h at room temperature away from light. The IAA was then neutralized by completing with 15 mm DTT and stirring for 10 min at 37 °C. The proteins were digested by adding 1 μg Pierce MS-grade trypsin (Thermo Fisher Scientific, Waltham) and incubated overnight at 37 °C with shaking.
Purification and Desalting of the Peptides on C18 Columns
Digestion was stopped by adding 1% formic acid (FA) (Thermo Fisher Scientific, Waltham) to a total volume of 200 μl, followed by stirring for 5 min at room temperature. The beads were spun at 6000 × g for 3 min before harvesting the supernatant and transferring to a new low binding microtube. The beads were resuspended in 200 μl of buffer containing 60% acetonitrile (ACN) (Sigma-Aldrich, St. Louis, MO) and 0.1% FA, and subsequently stirred for 5 min at room temperature. The supernatant was harvested and combined with that obtained previously. These samples were thereafter concentrated by a centrifugal evaporator at 65 °C until complete drying (∼2 h), and resuspended in 30 μl of 0.1% trifluoroacetic acid (TFA) buffer (Sigma-Aldrich). The peptides were purified with ZipTip 10-μl micropipette tips containing a C18 column (EMD Millipore, Burlington, VT). The ZipTip was first moistened by suctioning 10 μl of 100% ACN solution three times, then equilibrated by suctioning 10 μl of 0.1% TFA buffer three times. Each peptide sample was passed on the balanced ZipTip by 10 succeeding up-and-downs of 10 μl of the sample. This step was performed three times to pass the entire sample on the column. The ZipTip was then washed with 10 μl of 0.1% TFA buffer three times. The elution of the peptides was performed in a new low-binding microtube, 10 times with a volume of 10 μl of 50% ACN and 0.1% FA buffer. This step was carried out three times to obtain a final volume of 30 μl. The peptides were then concentrated by centrifugal evaporator at 65 °C until complete drying (∼30 min) and then resuspended in 25 μl of 1% FA buffer. Peptides were assayed using a NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA) and read at an absorbance of 205 nm. The peptides were then transferred to a glass vial (Thermo Fisher Scientific) and stored at −20 °C until analysis by mass spectrometry.
LC-MS/MS Analysis
Trypsin-digested peptides were separated using a Dionex Ultimate 3000 nanoHPLC system. Ten microliters of sample (a total of 2 μg) in 1% (v/v) formic acid were loaded with a constant flow of 4 μl/min onto an Acclaim PepMap100 C18 column (0.3 mm id × 5 mm, Dionex Corporation). After trap enrichment, peptides were eluted onto an EasySpray PepMap C18 nano column (75 μm × 50 cm, Dionex Corporation) with a linear gradient of 5–35% solvent B (90% acetonitrile with 0.1% formic acid) over 240 min with a constant flow of 200 nl/min. The HPLC system was coupled to an OrbiTrap QExactive mass spectrometer (Thermo Fisher Scientific Inc) via an EasySpray source. The spray voltage was set to 2.0 kV and the temperature of the column set to 40 °C. Full scan MS survey spectra (m/z 350–1600) in profile mode were acquired in the Orbitrap with a resolution of 70,000 after accumulation of 1,000,000 ions. The ten most intense peptide ions from the preview scan in the Orbitrap were fragmented by collision-induced dissociation (normalized collision energy 35% and resolution of 17,500) after the accumulation of 50,000 ions. Maximal filling times were 250 ms for the full scans and 60 ms for the MS/MS scans. Precursor ion charge state screening was enabled and all unassigned charge states as well as singly, 7 and 8 charged species were rejected. The dynamic exclusion list was restricted to a maximum of 500 entries with a maximum retention period of 40 s and a relative mass window of 10 ppm. The lock mass option was enabled for survey scans to improve mass accuracy. Data were acquired using the Xcalibur software.
Protein Identification by MaxQuant Analysis
The raw files were analyzed using the MaxQuant version 1.6.2.2 software (37) and the Uniprot human database (16/07/2013, 88,354 entries). Isoform analyses were initially performed separately to obtain enrichment ratios for the complete sequence of each isoform. A common analysis was then performed to integrate all raw MS/MS analysis files. The settings used for the MaxQuant analysis were: 2 miscleavages were allowed; fixed modification was carbamidomethylation on cysteine; enzymes were Trypsin (K/R not before P); variable modifications included in the analysis were methionine oxidation and protein N-terminal acetylation. A mass tolerance of 7 ppm was used for precursor ions and a tolerance of 20 ppm was used for fragment ions, and the following parameters: multiplicity of 3 SILAC media (R0K0, R6K4 and R10K8), identification values “PSM FDR” “Protein FDR,” and “Site decoy fraction” 0.05, minimum ratio count of 1 and selection of the “Re-quantify” option. Following the analysis, the results were sorted according to several parameters. Proteins positive for at least one of the “Reverse” “Only.identified.by.site,” and “Potential.contaminant” categories were eliminated, as well as proteins identified from a single peptide. The ratios identified in only one of the three replicas for each experiment were eliminated. The ratios identified in two of the three replicas were eliminated when they were too divergent, i.e., when the standard deviation was greater than the average of the two enrichment ratios. Outliers for the ratios measured in the three replicas were detected using the Grubbs test at a value of α = 0.05 and then eliminated. Following this sorting, proteins for which no ratio was calculated in at least one experiment were removed. Gene ontology enrichment analyses were performed using the Panther 13.1 tool (38). Heatmap visualizations were created using the Morpheus software (https://software.broadinstitute.org/morpheus/).
Experimental Design and Statistical Rationale
For the mass spectrometry analysis, the specific protein-protein interaction networks involving each of the HNF4α isoforms was identified using a BioID approach coupled to SILAC (Stable isotope labeling with amino acids in cell culture)-based quantitative mass spectrometry. Three conditions were thus compared using SILAC, namely the control cell line in light medium (R0K0), a cell line expressing a P1 isoform in the intermediate SILAC medium (R6K4) and a line expressing a P2 isoform in heavy medium (R10K8). The biotin-labeled protein extracts from these three conditions were then mixed in a ratio of 1: 1: 1. The biotinylated proteins were digested with trypsin, and the resulting peptides analyzed by mass spectrometry. Quantification of the identified proteins for each isoform was performed by measuring the enrichment compared with the control condition. The experiments were performed in biological triplicates. Enrichment ratios obtained for each quantified protein were compared between isoforms and their triplicates via a Spearman correlation. Following this validation, the remaining analyses were carried out based on the average enrichment ratios obtained through the triplicates, with the enrichment ratios normalized relative to the median.
RESULTS
New Classification of HNF4α Isoforms
An important issue arising from the existence of the HNF4α isoforms is the lack of detail and uniformity in their descriptions in the literature and databases. Indeed, the main protein databases such as RefSeq (33), Uniprot (39) and Ensembl (40) use different numbering and identification strategies for protein isoforms, and studies on proteins in the literature often do not specify which isoform is under consideration (supplemental Table S1). Since the initial identification of HNF4α in the liver, a total of 12 HNF4α isoforms have been reported or predicted in the literature encompassing protein isoforms with distinct N- and C termini regions (28, 29, 41). A first classification of the different isoforms separates the different proteins depending on promoter usage (termed P1 and P2, (42, 43)) generating differences in the N terminus which will include either exon 3 (isoforms α1–3) or exon 4 (isoforms α4–6) for isoforms transcribed from the P1 promoter, or exon 1 (isoforms α7–9) or exon 2 (isoforms α10–12) for isoforms transcribed from the P2 promoter (Fig. 1). Interestingly, only the N termini from isoforms α1–3 include the originally defined activation function 1 domain (AF-1 domain), whereas it has not been identified in the other isoforms. In addition, alternative splicing regulation in exons 11, 12, and 13 yields another level of complexity, resulting in three different C termini combinations within the F domain, constituting the highly variable C-terminal region typical of NR.
Fig. 1.
The HNF4A gene leads to the expression of 12 isoforms, through the use of alternative promoters and alternative splicing. The proximal P1 promoter can produce two distinct N-terminal ends through either exon 3 (P1a) or exon 4 (P1b), and the same number of isoforms from the distal P2 promoter are possible from either exon 1 (P2a) or exon 2 (P2b). Furthermore, alternative splicing at the C-terminal end can produce an additional three isoforms for each of these subgroups, resulting in twelve possible isoforms. The P1 and P2 promoters of the HNF4A gene are separated by ∼46,000 bp.
Different numbering and identifiers are currently used when referring to the HNF4α isoforms (supplemental Table S1), and several studies on HNF4α either do not precisely indicate which isoform was used, do not provide the cDNA sequence, or simply refer to a previous study. This has led to contradictory functions being attributed to HNF4α as a consequence of studying different isoforms (44–46). To simplify the nomenclature of the isoforms, we now propose to follow the P1 and P2 classification of isoforms, first by sorting the isoforms from the N termini, and then by the different C termini as also suggested by (47). This leads to four main classes of isoforms (P1a, P1b, P2a, P2b) (Fig. 1), and maintains the order of the different C termini to remain consistent with the nomenclature used in most previous studies. The isoforms a (P1a and P2a) represents the canonical HNF4α isoforms, whereas the isoforms b (P1b and P2b) are isoforms that have been less studied, or that have not been detected. To be consistent with the order of the C-terminal between the different isoforms, we also propose to inverse the nomenclature of the α4 and α5 isoforms (18).
Expression of HNF4α Isoforms in Different Tissues
Although multiple HNF4α isoforms have been considered in several studies, the expression of some of these isoforms has only been limited to a some studies (α5 (18), α10, α11 and α12 (41), as well as α4 and α6 (47). The expression of the different classes of HNF4α isoforms is tightly regulated depending to the spatial context and stage of development (48). Immunohistochemical analysis of several human tissues reveals a variable distribution of P1 and P2 isoform expression (12). The intestine is the only adult organ expressing both the P1 and P2 isoforms. The expression levels of these isoform classes have been modulated in the colon, notably by RNA interference in a colorectal cancer line or by exon exchange in the mouse, to analyze the functional differences between P1 and P2. These studies revealed significant disparities, with P1 isoforms being involved in the regulation of cell differentiation and metabolism, whereas P2 isoforms were associated with cell proliferation and cancer progression (44, 46).
To validate the existence of these 12 isoforms, different tissues of the human gastrointestinal tract were selected in the present study. A human cDNA library from healthy individuals was used to study isoform expression by semi-quantitative PCR (Fig. 2), and the PCR products were sequenced to confirm the exact nature of the isoform. For a positive identification of an isoform, the PCR product sequenced had to match the predicted HFN4α isoform, otherwise it was concluded as a nonspecific PCR product. For example, the faint band for the HNF4α4 PCR product observed in the liver was not HNF4α. Seven isoforms were identified in these tissues (Fig. 2). The canonical isoforms of HNF4α (P1: α1, α2, and α3; P2: α7, α8, and α9) were detected in the liver, stomach as well as all segments of the small intestine and colon. No isoforms were found in the esophagus, a tissue previously reported not to express HNF4α (12). Although the above approach was non-quantitative, a predominance of P1 isoforms in the liver was observed, consistent with the literature (49). HNF4α5 was the only non-canonical isoform identified in this experiment and found exclusively in the liver.
Fig. 2.
HNF4α isoforms are expressed at varying levels in different tissues of the human gastrointestinal tract. Expression of isoforms was assessed by semi-quantitative PCR in the liver, esophagus, stomach, and in various segments of the small intestine and colon. The negative control is the reaction mix without a template plasmid for each of the isoform, whereas the positive control is a plasmid which includes the cDNA for the amplified isoform. The α4, α6, α10, α11 and α12 isoforms were not detected in any of these tissues (nonspecific PCR products are marked with a *). The POLR2A and PSMB2 genes were used as reference genes. (Li = Liver, Oe = Esophagus, St = Stomach, Du = Duodenum, Je = Jejunum, Ic = Ileocecum, Ce = Cecum, Ac = Ascending, Tc = Transverse colon, Dc = Descending colon and Re = Rectum).
Given that the expression of P1 and P2 HNF4α isoforms is known to be modulated in various cancer types (12), various human cancer lines were hence selected to investigate the expression of additional isoforms. Two cell lines of pancreatic origin, AsPC-1 and Capan-2, were also included into this assay given that the pancreas is a well-known organ for expressing HNF4α. Expression of the 12 isoforms was measured in these cell lines by semi-quantitative RT-PCR (supplemental Fig. S1), and confirmed by sequencing of the PCR products. In the context of the cell lines selected for this assay, HNF4α1, α2, α3, α7, α8, and α9 were detected according to a pattern similar to the expression in the corresponding healthy human tissues.
Generation of Stable Cell Lines Expressing Inducible HNF4α with GFP and BioID2
Colorectal cancer cell lines that show endogenous expression of HNF4α unequivocally express several isoforms in a concomitant manner (supplemental Fig. S1). To study the specific functions of the different HNF4α isoforms, the latter had to be expressed in a cell line lacking endogenous expression of HNF4α. The HCT 116 cell line was ultimately selected for this purpose because HNF4α mRNA (supplemental Fig. S1) and protein is not detected (45, 46) possibly because of a deletion of the HNF4A gene in this cell line (50) (supplemental Fig. S1). The Flp-In T-REx system was used to generate stable lines in HCT 116 cells for each of the HNF4α isoforms, with either a GFP or a BioID2–3myc fusion protein at their C-terminal end (Fig. 3A). This system allows integration of a gene at a single and known location within the genome, under the control of a doxycycline-inducible CMV promoter resulting in clonal populations. In addition, this strategy supports gene expression levels that are much closer to an endogenous expression level comparatively to other transfection approaches, with these levels remaining relatively similar between the different stable cell lines.
Fig. 3.
Generation of stable cell lines expressing inducible HNF4α with GFP and BioID2. A, The Flp-In T-REx system was used to generate stable lines in HCT 116 cells for each of the HNF4α isoforms, with either a GFP or a BioID2–3myc fusion protein at its C-terminal. The constructs of the 12 HNF4α isoforms fused with the GFP or BioID2–3myc protein labels were expressed following the induction of the stable lines in the HCT 116 cells. B, Immunoblots against the GFP and myc protein labels were performed to detect the expression of the different fusion proteins following induction with doxycycline of the cell lines stably expressing HNF4α1. The expression levels of the GAPDH protein in these total protein extracts were used as a reference. C, Nuclear localization of HNF4α1 was confirmed by immunofluorescence to detect the GFP tag (left) or the myc tag (right).
The inducible expression of HNF4α was confirmed by both immunoblotting (Fig. 3B) and immunofluorescence microscopy when comparing cells incubated or not with doxycycline (Fig. 3C). A similar nuclear localization was observed for all 12 isoforms, whether HNF4α was fused to a GFP (supplemental Fig. S2) or to BioID2–3myc protein tag (supplemental Fig. S3). Given the single integration site, the overall expression of HNF4α was expected to be relatively similar. However, differences in protein expression were observed when comparing immunoblot analysis of whole cell lysates (supplemental Fig. S4). This difference was most likely because of post-translational stability, because no correlative pattern of transcript expression was observed when measured by qPCR (supplemental Fig. S5). Indeed, treatment of cells with the proteasome inhibitor MG132 resulted in a re-establishment of protein expression in the isoforms with lower expression, suggesting that some of these isoforms are specifically and partially targeted for proteasome degradation (supplemental Fig. S6).
HNF4a Isoforms have Different DNA Binding Capacities
Following confirmation of the expression and localization of the 12 HNF4α isoforms, a first functional validation was performed by measuring their ability to bind the known consensus DNA binding sequence DR1 (21, 22). Electrophoretic mobility shift assays (EMSA) were carried out to determine whether the 12 isoforms could bind this consensus DNA sequence (Fig. 4A). Isoform binding to a probe containing the HNF4α DR1 response element resulted in a major complex shift for all isoforms except the α4, α5 and α6 isoforms (Fig. 4A). Inclusion of an antibody against GFP in the binding reaction was able to supershift this complex demonstrating that the latter indeed consisted of the HNF4α isoforms fused to GFP. A probe featuring a mutated DR1 sequence was used to demonstrate the specificity of interaction of the isoforms with the consensus DR1 sequence (Fig. 4A). This test demonstrates for the first time that the α4, α5 and α6 isoforms are unable to bind the DR1 response element in the same manner as other HNF4α isoforms, even though they share the same DNA binding domain. This is not because of differences in expression, as transient expression of the different isoforms of HNF4α in 293T cells results in similar expression, at least when comparing isoforms binding the DR1 element (1–3) to the isoforms that do not bind the DNA element (4–6) (Fig. 4B). These results therefore suggest that the A/B domain from exon 4 of these isoforms could negatively regulate their DNA binding capacity, because this domain is the only common sequence for α4, α5, and α6 that is absent from the other nine HNF4α isoforms.
Fig. 4.

HNF4α isoforms do not all recognize the DR1 consensus response element of the nuclear receptor. A, 293T cells were transfected for 24 h with the different HNF4α (1–12)-GFP constructs. Nuclear extracts from these cells were used to perform EMSA. A 5′-biotinylated DNA probe containing the normal or mutated DR1 consensus sequence of 5′-biotinylated HNF4α was incubated with each cell extract and the DNA-protein complexes were resolved by non-denaturing electrophoresis. Supershifts were performed using an antibody against GFP to confirm the presence of GFP-HNF4α in the observed complexes. The biotinylated probes were revealed with streptavidin-HRP. B, Nuclear extracts were isolated from 293T cell lines transfected with the different pcDNA-DEST47-HNF4α (1–12) plasmids. The different isoforms were revealed with a GFP antibody, along with an antibody recognizing a nuclear protein (RNA Polymerase I Subunit A, RPA194) as loading control. C, Known target genes of HNF4α were measured by quantitative RT-PCR reactions (qPCR) performed on samples from stable HCT 116 HNF4α (1–12)-GFP lines that were induced for 48 h in the presence of 2.5 μg/ml doxycycline.
HNF4α is known to transactivate the expression of a multitude of genes, the majority of which are involved in cell differentiation, metabolism and transport of nutrients. The expression levels of four selected genes known to be up-regulated by HNF4α (APOA1, CREB3L3, HNF1A, and VIL1) and involved in several of these functions were measured by qPCR following the induction of expression of each isoform in stable HCT 116 lines (HNF4α (1–12)-GFP) (Fig. 4C). Significant differences in the transactivation of these genes were observed between isoforms. Although the isoform transactivation profiles were similar from one gene to another, there were a few notable exceptions. The α1 and α2 isoforms (canonical P1) were the strongest transcription inducers of the four genes tested, whereas the α3 isoform positively regulated CREB3L3 and VIL1 at lower levels, but not APOA1 and HNF1A (Fig. 4C), thus demonstrating specificity in the regulated genes between the different isoforms. The α4, α5 and α6 isoforms showed no effect on the genes tested, suggesting that the absence of binding to the DR1 element by these isoforms has a major impact on their transactivation activity (Fig. 4A). The canonical (α7, α8) and non-canonical (α10, α11) P2 isoforms exhibited a much lower transactivation capacity than the α1 and α2 isoforms. Of note, for all analyzed genes, the variants of each subgroup sharing a shorter F domain (α3, α6, α9 and α12) all showed a strongly reduced or non-existent transactivation capacity for these genes. Importantly, although the isoforms exhibit differences in HNF4α protein expression (as demonstrated in supplemental Fig. S5), the results presented in Fig. 4C show that these variations in protein expression are not necessarily consistent with the transcriptional levels of target genes.
Transcriptomics Analysis of HNF4a Isoforms
To further study the transcriptional functions of the 12 HNF4α isoforms, the transcriptome of HCT 116 HNF4α (1–12) -GFP lines were analyzed by RNAseq. To achieve this, RNA from these lines were sequenced in triplicate, and compared with the parental cell line lacking HNF4α expression. The readings were aligned to the human transcriptome constructed from the annotations of the RefSeq database (supplemental Table S2). The differential expression of transcripts and genes was obtained by comparing the specific data for each isoform to the transcriptome of the control condition (HCT 116 Flp-In T-REx).
Principal component analysis (PCA) of the data sets was performed to determine the variance found between the transcripts quantified for all isoforms as well as to validate the reproducibility of the triplicates for each experiment. This analysis confirmed the propinquity of each triplicate, with the exception of the first replica for the α3 isoform (symbolized by A3N1) (Fig. 5A). The divergence of this replica is likely explained by a weaker expression of the HNF4α3 isoform in the sample (405 counts against 832 and 771 in the other two replicas). The subsequent analyses were therefore carried out by excluding this sample and using the average value of the counts between the different biological replicas. The control condition is annotated as A0, located on the left and center of the PC1 and PC2 components respectively. From these analyses, the α1 and α2 isoforms caused the most variance when compared with control, whereas α3 exhibited a reduced effect. In contrast, the α4, α5 and α6 isoforms caused very little divergence. The P2 isoforms displayed an intermediate effect with respect to these two subgroups of P1 isoforms. Of note, the isoforms generally appeared to cluster strongly according to their A/B domain, with the exception of a larger divergence observed between the α10, α11 and α12 isoforms, indicating that the majority of the functional differences between the HNF4α isoforms were associated with this domain rather than with the F domain. Notwithstanding the latter, a shorter F domain effect contained in the α3, α6, α9, α12 isoforms resulted in closer proximity to the negative control than to the other two isoforms containing the same A/B domain.
Fig. 5.
Transcriptomics analysis of HNF4a isoforms by RNAseq. A, PCA analysis of the RNAseq data from GFP cell lines expressing each of the 12 HNF4α isoforms (A1–A12) and the control condition (A0). The biological triplicates for each sample are represented by N1/N2/N3 following the isoform number. The two main components PC1 and PC2 were used for two-dimensional visualization of the analysis. B, Hierarchical analysis of the cell lines expressing the different HNF4α (1–12)-GFP were compared based on their RNA expression profiles using the log2 fold change. C, Representation of the number of transcripts showing significant up and down-regulation for each of the HNF4α isoforms. A minimum absolute modulation threshold of 2 combined with an adjusted p value threshold ≤ 0.001 was used to determine significance. The size of the circles is proportional to the total number of genes modulated for each isoform. The blue color represents the proportion of up-modulated genes relative to the control condition, whereas the black-colored portion represents the negatively modulated genes. D, Graphical representation with the number of regulated genes common to the different isoforms or unique to specific isoforms, with green indicating up-regulation and red indicating down-regulation. Only groups with more than 10 genes are shown.
Gene quantification analysis revealed that the annotation contained ∼45,000 genes, with ∼18,000 detected in at least one sample (using a minimal quantification of 1 TPM, supplemental Table S3). To further analyze the observed variance, only those genes positively or negatively modulated by at least 2-fold compared with the control, and with a maximum Benjamini-corrected p value of 0.001, were considered. The results are viewed globally in Fig. 5C as circles whose respective sizes are proportional to the number of significantly modulated genes. Positive and negative modulation levels are also presented as volcano plot representations of the sorted results to visualize the distribution of the genes found across the different data sets (supplemental Fig. S7). The most striking difference between the transcriptome profiles induced by the 12 HNF4alpha isoforms was the total number of modulated genes (Fig. 5C). The α1 and α2 isoforms were by far the isoforms with the greatest impact on the transcriptome in this setting. The α4, α5 and α6 isoforms conversely displayed an extremely weak effect on gene expression, consistent with the absence of DNA binding to the DR1 consensus sequence. This observation also suggests that they do not bind to a different response element with functional impact on the transcriptome. An intermediate effect was noted for the canonical and non-canonical P2 isoforms. In general, HNF4α was found to be more involved in the activation of gene expression than in their repression. The genes modulated by the α1 and α2 isoforms, which are the most important regulators in this setting, were mostly modulated upward (67 and 63%, respectively). Certain isoforms such as α9 were mainly responsible for repression of transcription (57%), whereas others such as α3 had an even stronger activating effect (76%), but with a much less overall number of genes regulated (Fig. 5C). Comparison of the overlap of up-regulated or downregulated genes between the different isoforms showed the largest group being regulated by α1 and α2 isoforms (293 genes), although a large number of genes were also specific to α1 (202 genes) (Fig. 5D). Of note, most of the genes regulated by the P2 isoforms were common to all isoforms in this group and were also regulated by at least one P1 isoform, whereas the few genes regulated by the α4–5–6 isoforms were completely different from the remaining isoforms (Fig. 5D). Overall, these data demonstrate major differences in transcriptional function between the 12 isoforms of HNF4α.
HNF4α Isoforms have Distinct Interaction Networks, Mostly Comprised of Transcription Factors and Transcriptional Coregulators
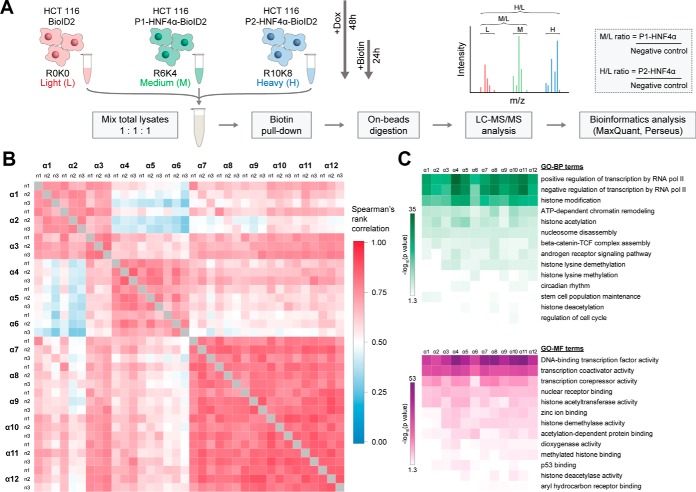
The functional activity of HNF4α is regulated by two characteristics related to its structure. Its DBD mediates the recognition of specific regulatory sequences at its target genes, whereas different transactivation domains promote the interaction with multiple transcriptional coregulators. Because the 12 isoforms have the same DBD, the differences in the number of modulated genes observed between the HNF4α isoforms likely arise from a variable interaction of the isoforms with transcriptional coregulators via the transactivating A/B or F domains, except for isoforms α4–5 that do not bind the consensus site. To determine the specific protein-protein interaction networks involving each of the HNF4α isoforms, a BioID approach coupled to SILAC (Stable isotope labeling with amino acids in cell culture)-based quantitative mass spectrometry was used (Fig. 6A) (51). Stable HCT116 cell lines were generated from constructs expressing each of the 12 HNF4α isoforms in fusion with BioID2 (52). Three conditions were thus compared using SILAC, namely the control cell line in light medium (R0K0), a cell line expressing a P1 isoform in the intermediate SILAC medium (R6K4) and a line expressing a P2 isoform in heavy medium (R10K8) (Fig. 6A and supplemental Table S4). The biotin-labeled protein extracts from these three conditions were then mixed in a ratio of 1: 1: 1. The biotinylated proteins were digested with trypsin, and the resulting peptides analyzed by mass spectrometry. Quantification of the identified proteins for each isoform was performed by measuring the enrichment compared with the control condition (Fig. 6A).
Fig. 6.
Identification of protein interactions of HNF4α isoforms using the BioID approach coupled with quantitative mass spectrometry. A, The HCT 116 HNF4α (1–12) -BioID2–3myc and the HCT 116 control line BioID2–3myc-empty cell lines were cultured in SILAC medium, induced for 48 h with doxycycline and incubated for 24 h with biotin. The total extracts from these cells were combined equally. The biotinylated proteins were precipitated with streptavidin-coupled beads and then trypsin-digested on beads. The peptides were analyzed by LC-MS/MS, before quantifying the results using MaxQuant. The experiments were performed in triplicates B, Diagram showing the enrichment ratios according to the experimental conditions used for culture in SILAC medium. Heatmap visualization comparing the association between the protein enrichment ratios identified for each isoform according to a Spearman correlation. The data are presented according to the biological triplicates carried out for each isoform. C, The identified interacting proteins were analyzed for gene ontology enrichment for biological processes (GO-BP, green) and molecular functions (GO-MF, purple) annotations for the proteins identified by each of the HNF4α isoforms. The Panther 13.1 tool was used for the above analyses whereas the Morpheus software was used to visualize the results as a heatmap. A minimum enrichment ratio threshold of 2 was used. The negative value of the logarithm in base 10 of the p value is represented according to a color scale for each annotation.
Over a thousand proteins were identified for each isoform (supplemental Table S5). Enrichment ratios obtained for each quantified protein were compared between isoforms and their triplicates via a Spearman correlation. These correlations were then plotted against a color scale for easier visualization (Fig. 6B). This initial analysis validated the reproducibility of the obtained results, yielding a very strong correlation between the triplicates (0.72 to 0.95). In addition, this representation highlighted certain differences between the proteomes associated with each isoform. The lists of potential interactants for the α4, α5 and α6 isoforms were the most dissimilar compared with the other isoforms. Overall, it appeared that most of the identified proteins were similarly enriched for the 12 isoforms. Following this validation, the remaining analyses were carried out based on the average enrichment ratios obtained through the triplicates. Given the differential isoform expressions observed (supplemental Fig. S4), the enrichment ratios were normalized relative to the median.
To determine the type of proteins enriched by the BioID approach, enrichment analyses of GO annotations were carried out using the Panther 13.1 software. A minimum enrichment threshold of 2 was used for these assays, reducing the number of proteins to ∼200 interactants for each isoform. Annotations of biological processes (GO-BP) regulated by these proteins showed an enrichment in the regulation of transcription, notably through histone modification and chromatin remodeling (Fig. 6C). As expected, the proteins associated with these enriched biological processes were annotated with molecular functions (GO-MF) for transcription factors and transcriptional coregulators (Fig. 6C). There were, however, no significant differences between the proteomes of the 12 isoforms that could be identified by this type of analysis, demonstrating that all these isoforms interact with proteins with known functions in transcription.
Identification of Isoform-specific Interaction Partners of HNF4α
To determine whether specific interactors could be identified for each isoform, the respective effects of the A/B domain and F domain were decoupled. Four different A/B domains and three F domains are found among all isoforms. By pooling the identified proteins for all of the isoforms having one of these domains in common, this analysis enabled to provide clues as to the identification of proteins that can interact specifically with certain HNF4α domains generated by alternative splicing.
The Venn diagrams presented in Fig. 7 illustrate the results of this comparative analysis, which used a minimum enrichment threshold of 2. Overall, most of the identified proteins were once again enriched equally between the 12 HNF4α isoforms, which included 69 proteins that were enriched by a 2-fold ratio for all isoforms, in all triplicates. Among these, there were several coregulators and transcription factors known to interact with HNF4α, such as CBP (53), NCOA-1 and NCOA-2 (54), NCOR2 (55) and FOSL1 (FRA-1, (45)). This analysis, however, highlighted certain proteins that appeared to interact specifically with subgroups of isoforms that had a common A/B domain or F domain. Most of these proteins have never been shown to specifically interact with HNF4α, although their functions in transcriptional regulation strongly support this possibility. These include members of ATP-dependent chromatin remodeling complexes (BRD9 - SWI/SNF, VPS72 - NuA4, SRCAP), enzymes associated with histone modification (SIRT1, SIN3B, KDM2A, KANSL1), and members of the Mediator and PIC complexes (MED26, MED27, TAF4B; Table 1). Comparison of proteins interacting with the different HNF4α F domains also demonstrated specific interactions with the three different C-terminal domains (Fig. 7B), including the transcriptional corepressors IRF-2BP1 and IRF-2BP2.
Fig. 7.

Several proteins specifically interact with isoforms that have a common A/B or F domain. Venn diagrams comparing specifically identified proteins in certain subgroups of HNF4α isoforms. A minimum enrichment ratio threshold of 2 was used. A, Comparison of isoform-specific interactants sharing the same A/B domain. B, Comparison of isoform-specific interactants sharing the same F domain.
Table I. Different subunits of labeled protein complexes that are commonly or differentially associated with the different HNF4a isoforms.
A table presented as a heat map with the values representing the average fold-enrichment of biological triplicates of different proteins over the control, highlighting in particular the BAF/PBAF, NuRD, and NuAR/Tip60 transcription complexes, as well as proteins with significant differences between the HFN4α isoforms.
To confirm some of these interactions, co-transfections between these newly identified FLAG-tagged interactants and GFP-tagged HNF4α isoforms showing the highest SILAC enrichment ratio were performed. Following immunoprecipitation against GFP, immunoblotting against FLAG led to the validation of the interactions involving five proteins, namely GATAD2B (α11), HNF4γ (α4), IRF2BP2 (α2), MTA1 (α11) and ZNF629 (α11) (supplemental Fig. S8A). The control condition, which only expressed the FLAG construct, confirmed the specificity of interaction between HNF4α and these proteins. To further validate the quantification and differential interaction between the different isoforms and the proteins identified, we performed co-immunoprecipitations experiments of these five proteins, by comparing the isoform with the highest ratio with an isoform with the lowest ratio (supplemental Fig. S8B). These experiments were performed in triplicate, quantified and are displayed as relative FLAG over GFP ratios. The differences in interactions were confirmed for three of the five proteins, including the interaction which is more prominent between HNF4α4 and HNF4γ when compared with HNF4α1. Thus, the identification of interactions by BioID coupled to SILAC quantification provided novel information regarding the transcription factor interaction networks of HNF4α.
DISCUSSION
In the present study, we characterized the 12 annotated isoforms of HNF4α by generating stable lines expressing each HNF4α isoform in HCT 116 cells. The entire transcriptome associated with each isoform was analyzed by RNA sequencing, as well as their respective interactome by a BioID approach coupled to quantitative mass spectrometry. HNF4A has been reported to produce several isoforms using two different promoters and various alternative splicing events (28). Two major groups of isoforms were classified as P1- and P2-HNF4α, according to the usage of their specific promoter. Given the presence of four different N-terminal regions, we have proposed a novel nomenclature based on these differences. Although we were successful in detecting seven of the twelve possible HNF4A isoform transcripts among specific gastrointestinal tissues and cell lines, we cannot exclude that the remaining isoforms are specifically produced in other tissues or in a timely manner during development. However, different studies have now detected ten of the twelve isoforms, which are also present in several transcriptome databases such as RefSeq, Gencode and FANTOM. Despite several of these transcripts being shown to produce proteins (12), the repetitive and combinatorial nature of A/B and F domains in certain isoforms makes it impossible to detect single protein isoforms. However, the generation herein of a cellular system with controlled expression for each recombinant single isoform led to the demonstration that each isoform produces protein with possible differing post-translational stability as previously reported for subclasses of P1 isoforms (44). It is important to note that protein expression differences between the isoforms that were transduced into the HCT116 cells may also affect the results presented here. Because functional differences in the cell transcriptome were identified among each HNF4α isoform, our study supports the importance for the design of thorough analyses to measure the biological impact of each single isoform in a specific cellular and developmental context.
Although all HNF4α isoforms share the same DBD, our data support that P1B isoforms (α4, α5 and α6) do not functionally interact with the classical HFN4α consensus DNA element. Although it is not possible to rule out binding to a different DNA sequence, the transcriptomics experiment supports this hypothesis considering the near-background number of genes whose expression is influenced when these isoforms are expressed as single isoforms producing homodimers, despite the reduced protein stability of these isoforms in HCT116 cells. Our BioID approach coupled to quantitative mass spectrometry identified several transcriptional co-regulators interacting with these isoforms similarly to other HNF4α isoforms. Because the common difference between these three isoforms resides in their A/B domain, we speculate that this region could interfere with DNA binding and down-modulate the transcriptional influence of P1B isoforms on gene transcription. Of note, similar observations were made for two RAR-related orphan alpha (RORα) nuclear receptor isoforms where RORα1 was shown to strongly activate transcription whereas RORα2 interaction with RORE elements was more restricted with the result of weaker transcriptional activity (56). Similarly to HNF4α-P1A and B isoforms, RORα2 and 1 differ in their A/B domain. Whether the HNF4α-P1B subclass of isoforms act as dominant negative regulators for other HNF4α isoforms in each cellular condition will require further investigation.
HNF4α was initially considered to be an exclusive homodimer (23). However, recent evidence demonstrate that HNF4α can also form heterodimers, with distinct gene targets from the corresponding homodimers (47), and several tissues and cell lines express more than one isoform (Fig. 2, supplemental Fig. S1 and (47)). The overexpression of different homodimers and heterodimers appears to regulate differentially a subset of previously reported genes, as well as inflammatory genes (47), underlining the importance of further studies that will tackle the complete transcriptome regulated by different combinations of HNF4α isoforms. Interestingly, we identified the nuclear receptor HNF4γ (Hepatocyte nuclear factor 4 gamma) (NR2A2) as an interaction partner of HNF4α, with which it shares strong homology. We have shown by the BioID approach a preferential interaction profile for HNF4γ for the α4–6 isoforms, and this interaction has been validated for HNF4α4 (supplemental Fig. S8B). HNF4γ has also been identified recently as a transcription factor capable of transactivating the same genes as HNF4α, and even more strongly in the case of its isoform HNF4γ2 (57). An interesting possibility would therefore be that the HNF4α4–6 isoforms act as co-regulators, which could dimerize with other isoforms of HNF4α or other transcription factors such as HNF4γ, to act as dominant negative. Throughout the literature, HNF4α is recognized as an exclusive homodimer, but this terminology refers to the formation of dimers between different transcription factors (23). The concept of “heterodimers” consisting of two distinct isoforms of the same isoform has been little studied in nuclear receptors. However, it has been proposed by EMSA that the isoforms α1 and α7 can form dimers with each other (49).
Several studies on HNF4α have recently started to focus on the specific functions of its classes of isoforms P1 and P2. However, no one had yet been interested in comparing individually the set of isoforms comprising these two classes. We have shown that isoforms regulate gene expression at varying levels by comparing the number of genes modulated following the expression of each isoforms. These variations can be associated with subgroups of isoforms sharing the same A/B or F domain. HNF4α1 and HNF4α2 are by far the most powerful transcriptional regulators in the context of HCT 116 cells, because they lead to the modulation of more than a thousand genes in this context. It may seem surprising that a transcription factor can so significantly affect the transcriptome of a cell. However, it is known that HNF4α is a particularly important transcriptional regulator for the expression of a multitude of genes. It has been shown that 42% of the genes occupied by RNA polymerase II in hepatocytes are also occupied by HNF4α. This occupancy rate was almost identical for the pancreatic islets, at 43% (58). The canonical P2 isoforms α7-α8 and non-canonical α10-α11 have a more modest effect on transcription, because there are ∼250 to 500 genes significantly modulated by these isoforms. Interestingly, we have shown that the non-canonical P1 isoforms (α4, α5 and α6) have an extremely limited transcriptional function. These results are consistent with the reduced DNA binding capacity of these isoforms at least in the context of DR1 element. In addition, we noted a functional impact associated with the F domain of certain isoforms. Indeed, it seems that the short form of the F domain, which is present in α3, α6, α9, and α12, is associated with a reduced transcriptional function for these isoforms compared with isoforms sharing the same A/B domain. For example, HNF4α3 modulates only 200 genes, a reduction of more than five times for the total number of genes affected compared with the isoforms α1 and α2. Very few studies have so far been interested in characterizing isoforms having this shorter form of the F domain, because they seem to be less expressed compared with other isoforms. The transcriptional activity of α3 has only been evaluated in the context of luciferase reporter assays and EMSA. HNF4α3 is an approximately twice as strong transactivator for the murine Hnf1α promoter and the human APOCIII promoter compared with α1 and α2, respectively (59, 60). These observations seem at first glance contradictory compared with the reduced transcriptional activity of α3 that we report in here. However, several factors may explain these differences. These past studies expressed constructs of isoforms by overexpression, whereas the Flp-In T-REx expression system we used allowed expression levels closer to endogenous levels. Thus, we noted that the α3 isoform was expressed more weakly than the α1 and α2 isoforms, whereas the previous studies did not show any differences at this level. The weaker expression of HNF4α in our system model could explain the reduction on its impact on the transcriptome. The analysis of the transcriptome obviously represents a global vision of the activity of isoforms, whereas the luciferase reporter assays were limited to the study of only a few promoters. The cellular context was also not the same in these different studies. For all these reasons, we can affirm that our transcriptomic study is the first to demonstrate that the isoform α3 has a reduced transcriptional activity compared with the isoforms α1 and α2.
Interestingly, another study compared isoforms at the transcriptional level, using RNA-seq and ChIP-seq approaches. HNFα2 and HNFα8 had very similar binding domains but high dysregulation(45). Our RNA-seq experiments confirm a high level of dysreulation specifically with HNFα2, which can differentially turn on many more genes compared with HNFα8 (1294 versus 69 uniquely up-regulated genes), with very little overlapping. When comparing these unique differentially up-regulated genes, coming from the two isoforms RNA-seq analyses (FC cutoff ≥ 1.5), with the ChIP-seq library of two isoforms coming from (45), we have comparable results (Wilcoxon rank sum test p values 0.535 and 0.62, respectively for HNFα2 and HNFα8 RNA-seq libraries). Specifically, there was still a substantial overlap of genes differently upregulated just by HNFα2 with HNFα2 peaks (358 genes, supplemental Fig. S10A), although a significant number overlap either with HNFα2 and HNFα8 (supplemental Fig. S10A), with many more being probably indirect targets not showing any overlap (707, supplemental Fig. S10). The relative proportions were pretty much similar (26, 6, and 37, respectively) when comparing our unique up-regulated genes from HNFα8 RNA-seq analysis with the two ChIP-seq libraries from (45)(supplemental Fig. S10B).
The BioID quantitative mass spectrometry performed for each HNF4α isoform identified a prominent number of common proteins that constitute ATP-dependent chromatin remodeling complexes such as the BRG1/BRM-associated factor (BAF) and the nucleosome remodeling and deacetylase (NuRD) complex (supplemental Fig. S9). These observations suggest that all HNF4α isoforms have the potential to act on chromatin structure via these specific interactions. Our results also suggest that the A/B domain is the major determinant of the differential transcriptional function of the different isoforms. However, we also observed differences in protein interactions such as IRF-2BP2. This protein was initially shown to be a corepressor of the IRF-2 transcription factor, and more recently for other transcription factors such as p53 and NFAT (61–63). The BioID approach used herein show that isoforms that have the short form of the F repressor domain do not interact with this coregulator. Considering the differential interaction profile of IRF-2BP2 with the HNF4α isoforms, we have identified transcriptome variations that can be explained by these preferential interactions. Accordingly, our results show that the proportion of down-modulated genes was similar between the α1 and α2 isoforms (33 and 37%, respectively). This proportion fell to 24% at the level of the α3 isoform, with a total of ∼8 times less negatively modulated genes. Because the IRF-2BP2 corepressor was shown to specifically interact with α1 and α2 isoforms relative to α3, it is possible that IRF-2BP2 may be responsible for part of this variation in the negative transcriptional regulatory function of these isoforms.
Although the initial goal of the study was to identify and define new specific interactions for certain isoforms of HNF4α, we have also identified several proteins enriched by the 12 isoforms equally. The majority of potential interactants identified showed a constant enrichment profile. This observation is consistent with the fact that a large part of the HNF4α sequence is common to the 12 isoforms, including the AF-2 activating region. Among the most enriched proteins which did not show specificity with respect to certain isoforms, we have identified a remarkable quantity of proteins forming part of large ATP-dependent chromatin remodeling complexes. The most overrepresented complexes of this type were BAF (BRG1/BRM-associated factor) and PBAF (Polybromo-associated BAF) of the SWI/SNF family, NuRD and NuA4 (Nucleosome acetyltransferase of H4). The NuRD complex is a nucleosome remodeling complex that also has HDAC activity and is generally associated with transcriptional repression functions (64). Ten proteins making up NuRD were enriched by the different isoforms of HNF4α, out of a total of 12 possible subunits. The NuA4 complex has HAT activity in addition to its chromatin remodeling activity (65). Of the 16 subunits forming this large complex in humans, we have identified half of them as potential interactants of HNF4α. The identification of the members of the SWI/SNF complexes BAF and PBAF is probably the most promising given the large number of subunits enriched at very high levels for all the isoforms. The BAF and PBAF complexes share most of these subunits and are made up of at least 14 proteins. We identified 10 BAF subunits and 11 PBAF subunits, most of them highly enriched by the 12 isoforms of HNF4α. Even if these examples of complexes do not exhibit an interaction profile specific to certain isoforms, the identification of these proteins by the 12 isoforms can be considered as an observation of these interactions having been overly repeated 12 times for HNF4α. Considering the biological triplicates for each isoform, this represents an identification that has been carried out a total of 36 times for most of the proteins composing these complexes.
In conclusion, the present study provides an exhaustive analysis of the transcriptional co-interacting complexes and transcriptomic impact of each single HNF4α isoforms in a specific cellular setting. Given that specificity of biological functions was previously attributed to HNF4α-P1 and -P2 subclasses (44, 46), this work provides a strong rationale to further detail the exact nature of contributing HNF4α isoforms in given biological systems and to design innovative strategies in exploring the specific biological functions that may be coincidental to the expression of these specific isoforms in the biological field. Similar analyses could be designed for the study of other human NR isoforms that result from similar alternative promoter and splicing events.
DATA AVAILABILITY
The HNF4α RNAseq data set was deposited to the NCBI Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/) under the accession number GSE125852. The mass spectrometry proteomics data was deposited to the ProteomeXchange Consortium via the PRIDE (66) partner repository with the data set identifier PXD012146.
Supplementary Material
Footnotes
* This work was supported by the Canadian Institutes of Health Research (CIHR) (grant number MOP-123469 to F.M.B. and grant number PJT-156180 to F.B.), the Natural Sciences and Engineering Research Council of Canada (NSERC) (grant number RGPIN-2017-06096 to F.B.) and by the Cancer Research Society (to F.M.B.). F.M.B. and M.S.S. are FRQS Junior II scholars (award number 32956 to F.M.B. and 34877 to M.S.S.). É.L. is recipient of NSERC and FRQS scholarships and J.S. is a recipient of FRQNT and NSERC scholarships. M.S.S., F.B., and F.M.B. are members of the FRQS-funded ≪ Centre de Recherche du CHUS. The authors declare that they have no conflicts of interest with the contents of this article.
 This article contains supplemental Figures and Tables.
This article contains supplemental Figures and Tables.
1 The abbreviations used are:
- NR
- Nuclear receptors
- DBD
- DNA-binding domain
- LBD
- ligand binding domain
- HFN 4α
- Hepatocyte Nuclear Factor 4 alpha
- DR1
- Direct Repeat 1.
REFERENCES
- 1. Zhang Z., Burch P. E., Cooney A. J., Lanz R. B., Pereira F. A., Wu J., Gibbs R. A., Weinstock G., and Wheeler D. A. (2004) Genomic analysis of the nuclear receptor family: new insights into structure, regulation, and evolution from the rat genome. Genome Res. 14, 580–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robinson-Rechavi M., Escriva Garcia H., and Laudet V. (2003) The nuclear receptor superfamily. J. Cell Sci. 116, 585–586 [DOI] [PubMed] [Google Scholar]
- 3. Lavery D. N., and McEwan I. J. (2005) Structure and function of steroid receptor AF1 transactivation domains: induction of active conformations. Biochem. J. 391, 449–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khorasanizadeh S., and Rastinejad F. (2001) Nuclear-receptor interactions on DNA-response elements. Trends Biochem. Sci. 26, 384–390 [DOI] [PubMed] [Google Scholar]
- 5. Germain P., Staels B., Dacquet C., Spedding M., and Laudet V. (2006) Overview of nomenclature of nuclear receptors. Pharmacol. Rev. 58, 685–704 [DOI] [PubMed] [Google Scholar]
- 6. Pawlak M., Lefebvre P., and Staels B. (2012) General molecular biology and architecture of nuclear receptors. Curr. Top Med. Chem. 12, 486–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giguere V. (1999) Orphan nuclear receptors: from gene to function. Endocr. Rev. 20, 689–725 [DOI] [PubMed] [Google Scholar]
- 8. Heery D. M., Kalkhoven E., Hoare S., and Parker M. G. (1997) A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature 387, 733–736 [DOI] [PubMed] [Google Scholar]
- 9. Patel S. R., and Skafar D. F. (2015) Modulation of nuclear receptor activity by the F domain. Mol. Cell. Endocrinol. 418 Pt 3:298–305, [DOI] [PubMed] [Google Scholar]
- 10. Costa R. H., Grayson D. R., and Darnell J. E. Jr. (1989) Multiple hepatocyte-enriched nuclear factors function in the regulation of transthyretin and alpha 1-antitrypsin genes. Mol. Cell. Biol. 9, 1415–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sladek F. M., Zhong W. M., Lai E., and Darnell J. E. Jr. (1990) Liver-enriched transcription factor HNF-4 is a novel member of the steroid hormone receptor superfamily. Genes Dev. 4, 2353–2365 [DOI] [PubMed] [Google Scholar]
- 12. Tanaka T., Jiang S., Hotta H., Takano K., Iwanari H., Sumi K., Daigo K., Ohashi R., Sugai M., Ikegame C., Umezu H., Hirayama Y., Midorikawa Y., Hippo Y., Watanabe A., Uchiyama Y., Hasegawa G., Reid P., Aburatani H., Hamakubo T., Sakai J., Naito M., and Kodama T. (2006) Dysregulated expression of P1 and P2 promoter-driven hepatocyte nuclear factor-4alpha in the pathogenesis of human cancer. J. Pathol. 208, 662–672 [DOI] [PubMed] [Google Scholar]
- 13. Hayhurst G. P., Lee Y. H., Lambert G., Ward J. M., and Gonzalez F. J. (2001) Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol. Cell. Biol. 21, 1393–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wortham M., Czerwinski M., He L., Parkinson A., and Wan Y. J. (2007) Expression of constitutive androstane receptor, hepatic nuclear factor 4 alpha, and P450 oxidoreductase genes determines interindividual variability in basal expression and activity of a broad scope of xenobiotic metabolism genes in the human liver. Drug Metab. Dispos. 35, 1700–1710 [DOI] [PubMed] [Google Scholar]
- 15. Yin L., Ma H., Ge X., Edwards P. A., and Zhang Y. (2011) Hepatic hepatocyte nuclear factor 4alpha is essential for maintaining triglyceride and cholesterol homeostasis. Arterioscler. Thromb. Vasc. Biol. 31, 328–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eeckhoute J., Moerman E., Bouckenooghe T., Lukoviak B., Pattou F., Formstecher P., Kerr-Conte J., Vandewalle B., and Laine B. (2003) Hepatocyte nuclear factor 4 alpha isoforms originated from the P1 promoter are expressed in human pancreatic beta-cells and exhibit stronger transcriptional potentials than P2 promoter-driven isoforms. Endocrinology 144, 1686–1694 [DOI] [PubMed] [Google Scholar]
- 17. Wang H., Maechler P., Antinozzi P. A., Hagenfeldt K. A., and Wollheim C. B. (2000) Hepatocyte nuclear factor 4alpha regulates the expression of pancreatic beta -cell genes implicated in glucose metabolism and nutrient-induced insulin secretion. J. Biol. Chem. 275, 35953–35959 [DOI] [PubMed] [Google Scholar]
- 18. Drewes T., Senkel S., Holewa B., and Ryffel G. U. (1996) Human hepatocyte nuclear factor 4 isoforms are encoded by distinct and differentially expressed genes. Mol. Cell. Biol. 16, 925–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yuan X., Ta T. C., Lin M., Evans J. R., Dong Y., Bolotin E., Sherman M. A., Forman B. M., and Sladek F. M. (2009) Identification of an endogenous ligand bound to a native orphan nuclear receptor. PLoS ONE 4, e5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dhe-Paganon S., Duda K., Iwamoto M., Chi Y. I., and Shoelson S. E. (2002) Crystal structure of the HNF4 alpha ligand binding domain in complex with endogenous fatty acid ligand. J. Biol. Chem. 277, 37973–37976 [DOI] [PubMed] [Google Scholar]
- 21. Fang B., Mane-Padros D., Bolotin E., Jiang T., and Sladek F. M. (2012) Identification of a binding motif specific to HNF4 by comparative analysis of multiple nuclear receptors. Nucleic Acids Res. 40, 5343–5356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jiang G., and Sladek F. M. (1997) The DNA binding domain of hepatocyte nuclear factor 4 mediates cooperative, specific binding to DNA and heterodimerization with the retinoid X receptor alpha. J. Biol. Chem. 272, 1218–1225 [DOI] [PubMed] [Google Scholar]
- 23. Jiang G., Nepomuceno L., Hopkins K., and Sladek F. M. (1995) Exclusive homodimerization of the orphan receptor hepatocyte nuclear factor 4 defines a new subclass of nuclear receptors. Mol. Cell. Biol. 15, 5131–5143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee S., and Privalsky M. L. (2005) Multiple mutations contribute to repression by the v-Erb A oncoprotein. Oncogene 24, 6737–6752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pan Q., Shai O., Lee L. J., Frey B. J., and Blencowe B. J. (2008) Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat. Genet. 40, 1413–1415 [DOI] [PubMed] [Google Scholar]
- 26. Kelemen O., Convertini P., Zhang Z., Wen Y., Shen M., Falaleeva M., and Stamm S. (2013) Function of alternative splicing. Gene 514, 1–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang X., Coulombe-Huntington J., Kang S., Sheynkman G. M., Hao T., Richardson A., Sun S., Yang F., Shen Y. A., Murray R. R., Spirohn K., Begg B. E., Duran-Frigola M., MacWilliams A., Pevzner S. J., Zhong Q., Trigg S. A., Tam S., Ghamsari L., Sahni N., Yi S., Rodriguez M. D., Balcha D., Tan G., Costanzo M., Andrews B., Boone C., Zhou X. J., Salehi-Ashtiani K., Charloteaux B., Chen A. A., Calderwood M. A., Aloy P., Roth F. P., Hill D. E., Iakoucheva L. M., Xia Y., and Vidal M. (2016) Widespread expansion of protein interaction capabilities by alternative splicing. Cell 164, 805–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Babeu J. P., and Boudreau F. (2014) Hepatocyte nuclear factor 4-alpha involvement in liver and intestinal inflammatory networks. World J Gastroenterol 20, 22–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sladek F. M., and Seidel S. D. (2001) Hepatocyte nuclear factor 4α. In Nuclear Receptors and Genetic Disease. Burris T.P. and McCabe E.R.B., editors. Academic Press, San Diego, CA, 309–361 [Google Scholar]
- 30. Babeu J. P., Wilson S. D., Lambert E., Levesque D., Boisvert F. M., and Boudreau F. (2019) Quantitative proteomics identifies DNA repair as a novel biological function for hepatocyte nuclear factor 4alpha in colorectal cancer cells. Cancers 11, pii: E626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wingett S. W., and Andrews S. (2018) FastQ Screen: A tool for multi-genome mapping and quality control. F1000Res 7, 1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bolger A. M., Lohse M., and Usadel B. (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O'Leary N. A., Wright M. W., Brister J. R., Ciufo S., Haddad D., McVeigh R., Rajput B., Robbertse B., Smith-White B., Ako-Adjei D., Astashyn A., Badretdin A., Bao Y., Blinkova O., Brover V., Chetvernin V., Choi J., Cox E., Ermolaeva O., Farrell C. M., Goldfarb T., Gupta T., Haft D., Hatcher E., Hlavina W., Joardar V. S., Kodali V. K., Li W., Maglott D., Masterson P., McGarvey K. M., Murphy M. R., O'Neill K., Pujar S., Rangwala S. H., Rausch D., Riddick L. D., Schoch C., Shkeda A., Storz S. S., Sun H., Thibaud-Nissen F., Tolstoy I., Tully R. E., Vatsan A. R., Wallin C., Webb D., Wu W., Landrum M. J., Kimchi A., Tatusova T., DiCuccio M., Kitts P., Murphy T. D., and Pruitt K. D. (2016) Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 44, D733–D745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bray N. L., Pimentel H., Melsted P., and Pachter L. (2016) Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34, 525–527 [DOI] [PubMed] [Google Scholar]
- 35. Love M. I., Huber W., and Anders S. (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Koster J., and Rahmann S. (2012) Snakemake–a scalable bioinformatics workflow engine. Bioinformatics 28, 2520–2522 [DOI] [PubMed] [Google Scholar]
- 37. Cox J., and Mann M. (2008) MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol 26, 1367–1372 [DOI] [PubMed] [Google Scholar]
- 38. Mi H., Dong Q., Muruganujan A., Gaudet P., Lewis S., and Thomas P. D. (2010) PANTHER version 7: improved phylogenetic trees, orthologs and collaboration with the Gene Ontology Consortium. Nucleic Acids Res. 38, D204–D210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. UniProt C. (2019) UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 47, D506–D515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zerbino D. R., Achuthan P., Akanni W., Amode M. R., Barrell D., Bhai J., Billis K., Cummins C., Gall A., Giron C. G., Gil L., Gordon L., Haggerty L., Haskell E., Hourlier T., Izuogu O. G., Janacek S. H., Juettemann T., To J. K., Laird M. R., Lavidas I., Liu Z., Loveland J. E., Maurel T., McLaren W., Moore B., Mudge J., Murphy D. N., Newman V., Nuhn M., Ogeh D., Ong C. K., Parker A., Patricio M., Riat H. S., Schuilenburg H., Sheppard D., Sparrow H., Taylor K., Thormann A., Vullo A., Walts B., Zadissa A., Frankish A., Hunt S. E., Kostadima M., Langridge N., Martin F. J., Muffato M., Perry E., Ruffier M., Staines D. M., Trevanion S. J., Aken B. L., Cunningham F., Yates A., and Flicek P. (2018) Ensembl 2018. Nucleic Acids Res. 46, D754–D761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang J., Levitsky L. L., and Rhoads D. B. (2009) Novel P2 promoter-derived HNF4alpha isoforms with different N-terminus generated by alternate exon insertion. Exp. Cell Res. 315, 1200–1211 [DOI] [PubMed] [Google Scholar]
- 42. Boj S. F., Parrizas M., Maestro M. A., and Ferrer J. (2001) A transcription factor regulatory circuit in differentiated pancreatic cells. Proc. Natl. Acad. Sci. U.S.A. 98, 14481–14486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thomas H., Jaschkowitz K., Bulman M., Frayling T. M., Mitchell S. M., Roosen S., Lingott-Frieg A., Tack C. J., Ellard S., Ryffel G. U., and Hattersley A. T. (2001) A distant upstream promoter of the HNF-4alpha gene connects the transcription factors involved in maturity-onset diabetes of the young. Hum. Mol. Genet. 10, 2089–2097 [DOI] [PubMed] [Google Scholar]
- 44. Chellappa K., Deol P., Evans J. R., Vuong L. M., Chen G., Briancon N., Bolotin E., Lytle C., Nair M. G., and Sladek F. M. (2016) Opposing roles of nuclear receptor HNF4alpha isoforms in colitis and colitis-associated colon cancer. Elife 5, pii: e10903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vuong L. M., Chellappa K., Dhahbi J. M., Deans J. R., Fang B., Bolotin E., Titova N. V., Hoverter N. P., Spindler S. R., Waterman M. L., and Sladek F. M. (2015) Differential effects of hepatocyte nuclear factor 4alpha isoforms on tumor growth and T-cell factor 4/AP-1 interactions in human colorectal cancer cells. Mol. Cell. Biol. 35, 3471–3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Babeu J. P., Jones C., Geha S., Carrier J. C., and Boudreau F. (2018) P1 promoter-driven HNF4alpha isoforms are specifically repressed by beta-catenin signaling in colorectal cancer cells. J. Cell Sci. 131, pii: jcs214734. [DOI] [PubMed] [Google Scholar]
- 47. Ko H. L., Zhuo Z., and Ren E. C. (2019) HNF4alpha combinatorial isoform heterodimers activate distinct gene targets that differ from their corresponding homodimers. Cell Rep. 26, 2549–2557 e2543 [DOI] [PubMed] [Google Scholar]
- 48. Chen W. S., Manova K., Weinstein D. C., Duncan S. A., Plump A. S., Prezioso V. R., Bachvarova R. F., and Darnell J. E. Jr. (1994) Disruption of the HNF-4 gene, expressed in visceral endoderm, leads to cell death in embryonic ectoderm and impaired gastrulation of mouse embryos. Genes Dev. 8, 2466–2477 [DOI] [PubMed] [Google Scholar]
- 49. Torres-Padilla M. E., Fougere-Deschatrette C., and Weiss M. C. (2001) Expression of HNF4alpha isoforms in mouse liver development is regulated by sequential promoter usage and constitutive 3′ end splicing. Mech. Dev. 109, 183–193 [DOI] [PubMed] [Google Scholar]
- 50. Barretina J., Caponigro G., Stransky N., Venkatesan K., Margolin A. A., Kim S., Wilson C. J., Lehar J., Kryukov G. V., Sonkin D., Reddy A., Liu M., Murray L., Berger M. F., Monahan J. E., Morais P., Meltzer J., Korejwa A., Jane-Valbuena J., Mapa F. A., Thibault J., Bric-Furlong E., Raman P., Shipway A., Engels I. H., Cheng J., Yu G. K., Yu J., Aspesi P. Jr, de Silva M., Jagtap K., Jones M. D., Wang L., Hatton C., Palescandolo E., Gupta S., Mahan S., Sougnez C., Onofrio R. C., Liefeld T., MacConaill L., Winckler W., Reich M., Li N., Mesirov J. P., Gabriel S. B., Getz G., Ardlie K., Chan V., Myer V. E., Weber B. L., Porter J., Warmuth M., Finan P., Harris J. L., Meyerson M., Golub T. R., Morrissey M. P., Sellers W. R., Schlegel R., and Garraway L. A. (2012) The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483, 603–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Varnaite R., and MacNeill S. A. (2016) Meet the neighbors: Mapping local protein interactomes by proximity-dependent labeling with BioID. Proteomics 16, 2503–2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kim D. I., Jensen S. C., Noble K. A., Kc B., Roux K. H., Motamedchaboki K., and Roux K. J. (2016) An improved smaller biotin ligase for BioID proximity labeling. Mol. Biol. Cell 27, 1188–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dell H., and Hadzopoulou-Cladaras M. (1999) CREB-binding protein is a transcriptional coactivator for hepatocyte nuclear factor-4 and enhances apolipoprotein gene expression. J. Biol. Chem. 274, 9013–9021 [DOI] [PubMed] [Google Scholar]
- 54. Martinez-Jimenez C. P., Gomez-Lechon M. J., Castell J. V., and Jover R. (2006) Underexpressed coactivators PGC1alpha and SRC1 impair hepatocyte nuclear factor 4 alpha function and promote dedifferentiation in human hepatoma cells. J. Biol. Chem. 281, 29840–29849 [DOI] [PubMed] [Google Scholar]
- 55. Ruse M. D. Jr, Privalsky M. L., and Sladek F. M. (2002) Competitive cofactor recruitment by orphan receptor hepatocyte nuclear factor 4alpha1: modulation by the F domain. Mol. Cell. Biol. 22, 1626–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Giguere V., Tini M., Flock G., Ong E., Evans R. M., and Otulakowski G. (1994) Isoform-specific amino-terminal domains dictate DNA-binding properties of ROR alpha, a novel family of orphan hormone nuclear receptors. Genes Dev. 8, 538–553 [DOI] [PubMed] [Google Scholar]
- 57. Sasaki S., Urabe M., Maeda T., Suzuki J., Irie R., Suzuki M., Tomaru Y., Sakaguchi M., Gonzalez F. J., and Inoue Y. (2018) Induction of hepatic metabolic functions by a novel variant of hepatocyte nuclear factor 4gamma. Mol. Cell. Biol. 38, pii: e00213–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Odom D. T., Zizlsperger N., Gordon D. B., Bell G. W., Rinaldi N. J., Murray H. L., Volkert T. L., Schreiber J., Rolfe P. A., Gifford D. K., Fraenkel E., Bell G. I., and Young R. A. (2004) Control of pancreas and liver gene expression by HNF transcription factors. Science 303, 1378–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Huang G., Eisenberg R., Yan M., Monti S., Lawrence E., Fu P., Walbroehl J., Lowenberg E., Golub T., Merchan J., Tenen D. G., Markowitz S. D., and Halmos B. (2008) 15-Hydroxyprostaglandin dehydrogenase is a target of hepatocyte nuclear factor 3beta and a tumor suppressor in lung cancer. Cancer Res. 68, 5040–5048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Suaud L., Formstecher P., and Laine B. (1999) The activity of the activation function 2 of the human hepatocyte nuclear factor 4 (HNF-4alpha) is differently modulated by F domains from various origins. Biochem. J. 340 (Pt 1), 161–169 [PMC free article] [PubMed] [Google Scholar]
- 61. Carneiro F. R., Ramalho-Oliveira R., Mognol G. P., and Viola J. P. (2011) Interferon regulatory factor 2 binding protein 2 is a new NFAT1 partner and represses its transcriptional activity. Mol. Cell. Biol. 31, 2889–2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Childs K. S., and Goodbourn S. (2003) Identification of novel co-repressor molecules for Interferon Regulatory Factor-2. Nucleic Acids Res. 31, 3016–3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Koeppel M., van Heeringen S. J., Smeenk L., Navis A. C., Janssen-Megens E. M., and Lohrum M. (2009) The novel p53 target gene IRF2BP2 participates in cell survival during the p53 stress response. Nucleic Acids Res. 37, 322–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Basta J., and Rauchman M. (2015) The nucleosome remodeling and deacetylase complex in development and disease. Transl. Res. 165, 36–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jacquet K., Fradet-Turcotte A., Avvakumov N., Lambert J. P., Roques C., Pandita R. K., Paquet E., Herst P., Gingras A. C., Pandita T. K., Legube G., Doyon Y., Durocher D., and Cote J. (2016) The TIP60 complex regulates bivalent chromatin recognition by 53BP1 through direct H4K20me binding and H2AK15 acetylation. Mol. Cell 62, 409–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vizcaino J. A., Csordas A., del-Toro N., Dianes J. A., Griss J., Lavidas I., Mayer G., Perez-Riverol Y., Reisinger F., Ternent T., Xu Q. W., Wang R., and Hermjakob H. (2016) 2016 update of the PRIDE database and its related tools. Nucleic Acids Res. 44, D447–D456 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The HNF4α RNAseq data set was deposited to the NCBI Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/) under the accession number GSE125852. The mass spectrometry proteomics data was deposited to the ProteomeXchange Consortium via the PRIDE (66) partner repository with the data set identifier PXD012146.