Abstract
The effect of potassium nitrate on the status of fermentative and sucrose metabolizing pathways was studied in two maize (Zea mays L.) genotypes, viz., LM 5 (relatively susceptible to flooding) and I 167 (relatively tolerant to flooding) under water logging stress. The higher increase in pyruvate decarboxylase, alcohol dehydrogenase and aldehyde dehydrogenase activities in the hypoxic roots of I 167 seedlings over LM 5 showed the former’s efficient tolerance mechanism towards anaerobic conditions. Foliar application of KNO3 reduced these enzymatic activities in the roots of both the genotypes. The shoots of I 167 seedlings also showed a parallel increase in alcohol dehydrogenase and pyruvate decarboxylase activities under water logging stress. These enzymatic activities, however, remained unaffected in shoots of water logged LM 5 seedlings. There was a higher decrease in acid and alkaline invertase activities in the hypoxic roots of I 167 seedlings. KNO3 treatment led to higher acid invertase activity in roots of I 167 seedlings than those of LM 5. Sucrose synthase (synthesis) and sucrose phosphate synthase activities decreased, but sucrose synthase (breakdown) activity increased in the roots of both the genotypes, during water logging. KNO3 increased sucrose synthesizing activities with a parallel increase in the sucrose content of the roots. Sucrose synthesis was comparatively unaffected in I 167 shoots under water logging stress while LM 5 shoots showed higher reduction in its sucrose synthase (synthesis) and sucrose phosphate synthase activities. It may thus be concluded that KNO3 induced a network of reactions for improving water logging tolerance. The nitrate ions acted as an alternate electron acceptor and thus reduced the activities of fermentative enzymes. It promoted the funneling of sugars into the glycolytic pathway by inducing the activities of acid and alkaline invertases in the roots and shoots of maize genotypes. It also directed the hexoses towards biosynthetic pathway by increasing the activities of sucrose synthesizing enzymes.
Keywords: Water logging, Fermentative pathway, Sucrose metabolism, Potassium nitrate
Introduction
Plants, being sessile, are exposed to various environmental challenges in their life cycle. Among the various factors, water logging is the major abiotic stress that affects crop growth and productivity worldwide (Sasidharan et al. 2018). It occurs when the water content of the surface layer exceeds 20% above the field capacity, leading to free standing water on the soil (Aggarwal et al. 2006). It may result due to excessive precipitation, faulty irrigation practices, poor drainage and heavy rain falls. It leads to hypoxia in soil that when prolongs, leads to anoxia. Maize (Zea mays L.) that ranks at third position among cereals, with a global production of 1099.61 million metric tons (Statista 2019), is highly sensitive towards water logging during its vegetative stage leading to heavy reductions in crop yield (Ren et al. 2014).
During water logging, the limited oxygen availability shifts the metabolism of plants towards a less efficient pathway of energy production, the alcoholic fermentation (Kato-Naguchi 2000). It, however, acts as a boon for the plants under stressful conditions. An up regulation of anaerobic proteins (ANPs) that principally consist of alcohol dehydrogenase, pyruvate decarboxylase, lactate dehydrogenase, sucrose synthase, phosphohexose isomerase and fructose-1,6-bisphosphate aldolase have been reported in plants exposed to water logging stress (Sairam et al. 2008).
Sucrose metabolism also gets significantly affected under varied environmental stresses that directly or indirectly affect the synthesis, concentration, transport and storage of sugars. Sustaining adequate levels of readily fermentable sugars under low oxygen conditions is an important adaptive mechanism towards water logging (Sairam et al. 2008). The status of sugar reserve and the activities of sucrose hydrolyzing enzymes are important determinants of water logging tolerance in crop plants (Hossain and Uddin 2011).
Water logging leads to leaching of nitrate ions from the soil that result in their lower availability for the plants (Manik et al. 2019). The nitrogen status of the plants is indirectly related to their water logging tolerance and carbon metabolism (Rocha et al. 2010). Therefore, a wide range of nitrogen fertilizers have been explored to improve the tolerance of plants towards anaerobic conditions (Habibzadeh et al. 2013). The application of nitrate nitrogen fertilizers, predominantly potassium nitrate, has been reported to improve carbon dioxide assimilation and biosynthesis of sucrose (Carvalho et al. 2018) and activation of fermentative enzymes in flooded plants (Jain et al. 2016).
We had earlier observed that potassium nitrate alleviated the affects of water logging and improved the growth of maize genotypes under stress conditions (unpublished). A comparative analysis of the effects of potassium nitrate on the fermentative and sucrose metabolism of differentially tolerant maize genotypes needs to be deciphered. Therefore, the present study was carried out to investigate the effects of potassium nitrate on the fermentative and sucrose metabolising pathways of contrasting maize genotypes with differential response towards water logging stress.
Materials and methods
Germination of seeds and seedling growth
The seeds of two maize genotypes, viz., LM 5 (water logging susceptible) and I 167 (water logging tolerant) were sown in three sets of disposable plastic cups containing farmyard manure and siphoned soil. There were five seeds per cup with fifteen seedlings per treatment. All the cups were kept at 25 ± 1 °C for four days with 90% relative humidity. On the fifth day of seedling growth (DSG), one set was exposed to water logging treatment by maintaining water level up to 5 cm above the soil surface. Another set was foliar sprayed with 1% KNO3, kept for two hours under normal conditions and then subjected to water logging stress and considered as second treatment. The third untreated set, control, was considered as the third treatment. On 6 and 8 DSG, the seedlings from each treatment were taken in triplicate. The activities of fermentative and sucrose metabolizing enzymes and the content of sugars were determined in seedlings of both genotypes at 6 and 8 DSG stages.
Determination of the activities of fermentative enzymes
Pyruvate decarboxylase (PDC) [EC 4.1.1.1] activity was extracted with 0.05 M Tris-HCl buffer (pH 7.5) containing 1 mM dithiothreitol and 2% PVP (w/v) and estimated by the method of Zanandrea et al. (2009). Alcohol dehydrogenase (ADH) [EC 1.1.1.1] and aldehyde dehydrogenase (ALDH) [EC 1.2.1.3] were extracted with 100 mM HEPES buffer containing 2 mM dithiothreitol (pH 6.5). ADH activity was determined by the method of Ke et al. (1994), while ALDH activity was determined as described by Liu et al. (2001).
Determination of sucrose metabolizing enzymes
Invertases [EC 3.2.1.26] were extracted with 20 mM sodium phosphate buffer (pH 7.0). The activities of acid and alkaline invertases (AI and AKI) were determined by the method of Dey (1986). Sucrose synthase (SS) [EC 2.4.1.13] and sucrose-6-phosphate synthase (SPS) [2.4.1.14] activities were extracted with chilled 100 mM HEPES buffer (pH 8.2) containing 5 mM β-mercaptoethanol, 10 mM EDTA, 5 mM PVP and 5 mM MgCl2. The activities of SS (synthesis) and SPS were determined as described by Kerr et al. (1987). For determining SS (breakdown) activity, the reaction mixture consisted of 250 mM HEPES buffer (pH 6.5), 125 mM sucrose and 5 mM UDP. To initiate the reaction, 100 µl of enzyme extract was added and incubated at 37 ºC for 30 min. The fructose released was determined by the procedure of Nelson (1944).
Determination of carbohydrates
The sugars were extracted from roots and shoots of seedlings with 80% and 70% ethanol (Kaur et al. 2007). The contents of total soluble sugars (TSS) and reducing sugars were determined by the method of Dubois et al. (1956) and Nelson (1944) respectively. Sucrose content was determined by the method of Gascon and Lampen (1968).
Statistical analysis
The data obtained for all the biochemical parameters was analyzed by Duncan’s multiple range test (DMRT) at 0.05% level of significance using DSAASTAT software ver. 1.101 software.
Results
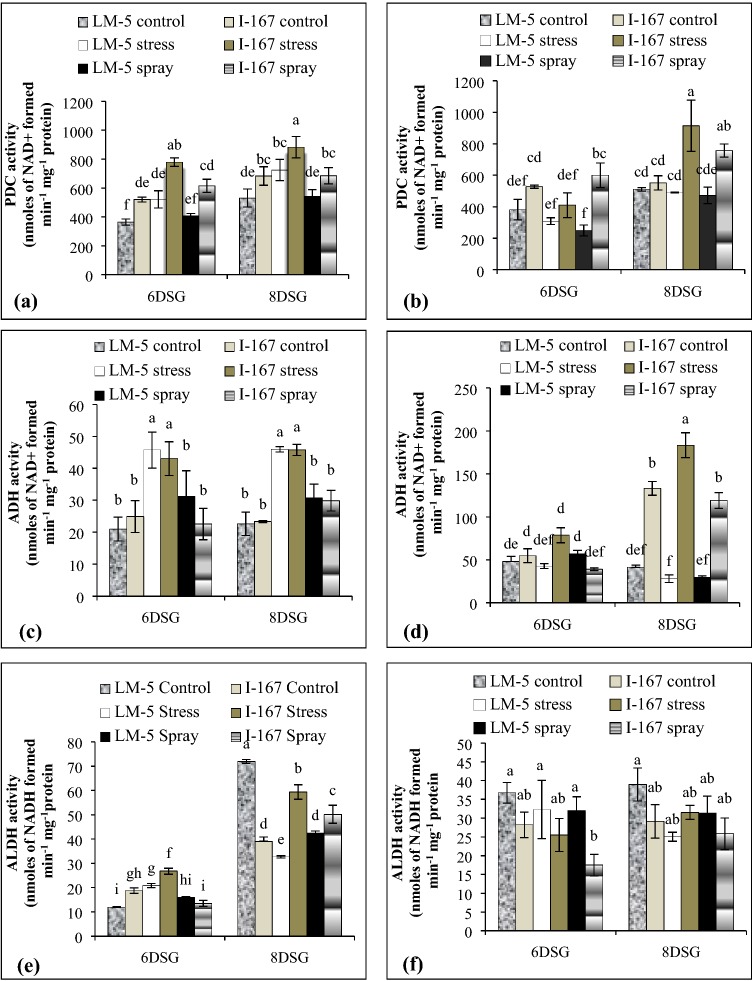
Under water logging stress, the roots of I 167 seedlings showed higher PDC activity at 6 and 8 DSG than those of LM 5 seedlings (Fig. 1a). Foliar application of KNO3 reduced PDC activity in I 167 roots at both the growth stages while only at 8 DSG in those of LM 5 seedlings though, the values remained higher in water logged I 167 roots (Fig. 1a). Water logging as well as foliar treatment of seedlings with KNO3 did not affect PDC activity in shoots of LM 5 genotype (Fig. 1b). However, both these treatments showed higher PDC activity in shoots of I 167 seedlings at 8 DSG when compared to their control counterparts (Fig. 1b).
Fig. 1.
Effect of KNO3 on ADH, PDC and ALDH activity in the roots (a, c, e) and shoots (b, d, f) of LM-5 and I-167 seedlings respectively, under water logging conditions. Error bars represent standard deviations. Bars with different alphabets represent significant differences at p < 0.05
KNO3 application reduced the up regulated ADH and ALDH activities in hypoxic roots of both the genotypes at 6 and 8 DSG stages (Fig. 1c, e). It also reduced the up regulated ADH activity in the shoots of waterlogged I 167 seedlings at 8 DSG but showed no effect on their ALDH activity (Fig. 1d). The shoots of untreated as well as KNO3 treated water logged LM 5 seedlings remained unaffected with respect to their ADH and ALDH activities (Fig. 1d, f).
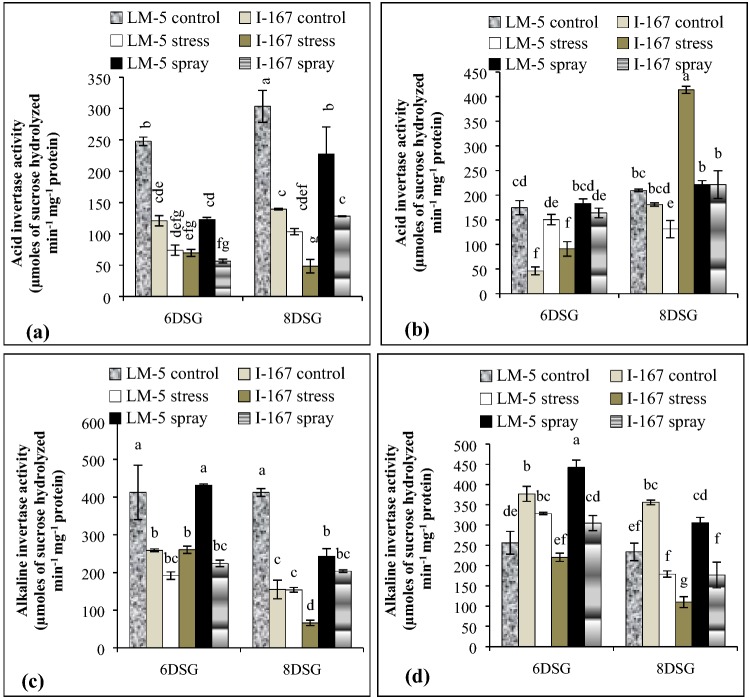
Under water logging stress, I 167 roots had lower AI and AKI activities as compared to those of LM 5 seedlings (Fig. 2a, c). KNO3 treatment increased the AI and AKI activities in the roots of both the genotypes (Fig. 2a, c). It also increased AI activity in I 167 and LM 5 shoots at 6 and 8 DSG respectively (Fig. 2b). The shoots of water logged I 167 seedlings showed reduction in AKI activity at both the growth stages while those of LM 5 showed an increase in AKI activity at 6 DSG which then gradually decreased at 8 DSG stage (Fig. 2d). Foliar application of KNO3 increased the AKI activity in shoots of both the genotypes (Fig. 2d). The increase was, nevertheless, higher in the shoots of susceptible genotype as compared to the tolerant one (Fig. 2d).
Fig. 2.
Effect of KNO3 on AI and AKI activity in the roots (a, c) and shoots (b, d) of LM-5 and I-167 seedlings respectively, under water logging conditions. Error bars represent standard deviations. Bars with different alphabets represent significant differences at p < 0.05
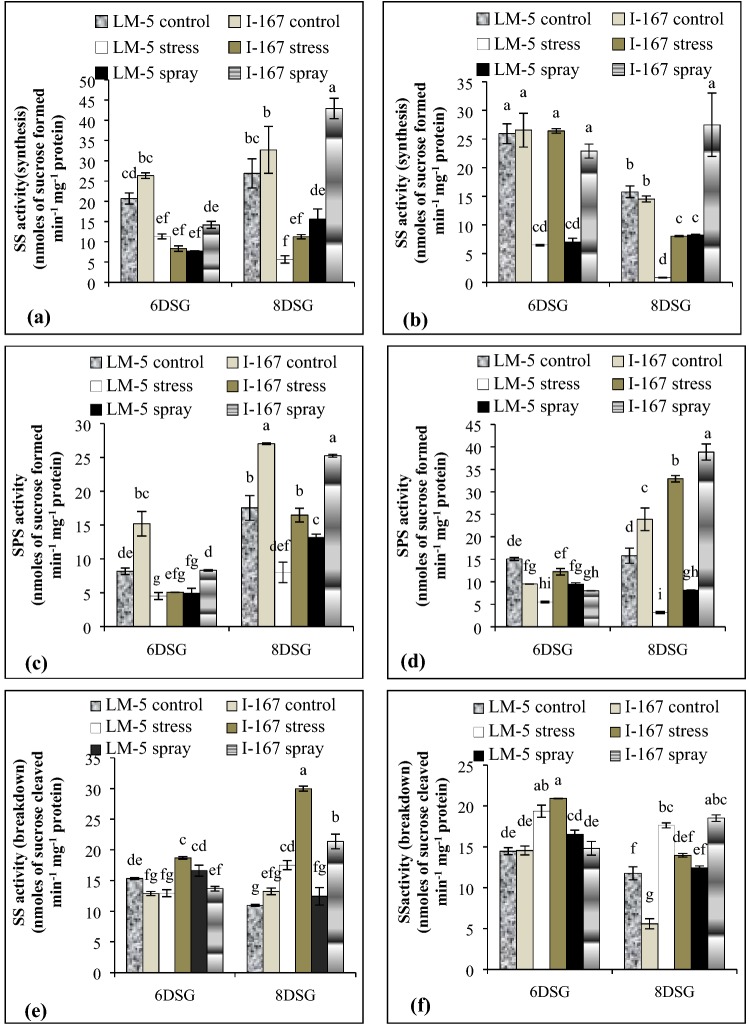
The activities of SS (synthesis) and SPS decreased in the roots of I 167 and LM 5 seedlings under water logging stress (Fig. 3a, c). SPS activity showed a twofold reduction in the hypoxic roots of LM 5 seedlings at both the growth stages. However, in I 167 roots, SPS activity was reduced by 3-folds at 6 DSG stage followed by ~ twofold reduction at 8 DSG (Fig. 3c). KNO3 spray resulted in increased SS and SPS activities in the hypoxic roots of I 167 seedlings as compared to those of LM 5 (Fig. 3a, c). The shoots of LM 5 seedlings showed reduced SS and SPS activities at 6 and 8 DSG under water logging stress. I 167 shoots, however, showed no effect of water logging on their SS and SPS activities at 6 DSG. With further seedling growth, SS activity decreased while that of SPS increased in the shoots of I 167 seedlings (Fig. 3b, d). Foliar application of KNO3 resulted in increased SS and SPS activities in shoots of both I 167 and LM 5 genotypes at 8 DSG stage. However, the values remained higher in the former as compared to the latter (Fig. 3b, d).
Fig. 3.
Effect of KNO3 on SS (synthesis), SPS and SS (breakdown) activity in the roots (a, c, e) and shoots (b, d, f) of LM-5 and I-167 seedlings respectively, under water logging conditions. Error bars represent standard deviations. Bars with different alphabets represent significant differences at p < 0.05
The activity of SS (breakdown) increased in the hypoxic roots of I 167 seedlings at both the growth stages, while, only at 8 DSG in those of LM 5 (Fig. 3e). SS activity was observed to be higher in I 167 roots over those of LM 5 seedlings (Fig. 3e). The shoots of both the genotypes also showed increased SS specific activity under water logging stress (Fig. 3f). Foliar application of KNO3, in general, reduced SS activity in the roots and shoots of waterlogged seedlings of both the genotypes (Fig. 3e, f).
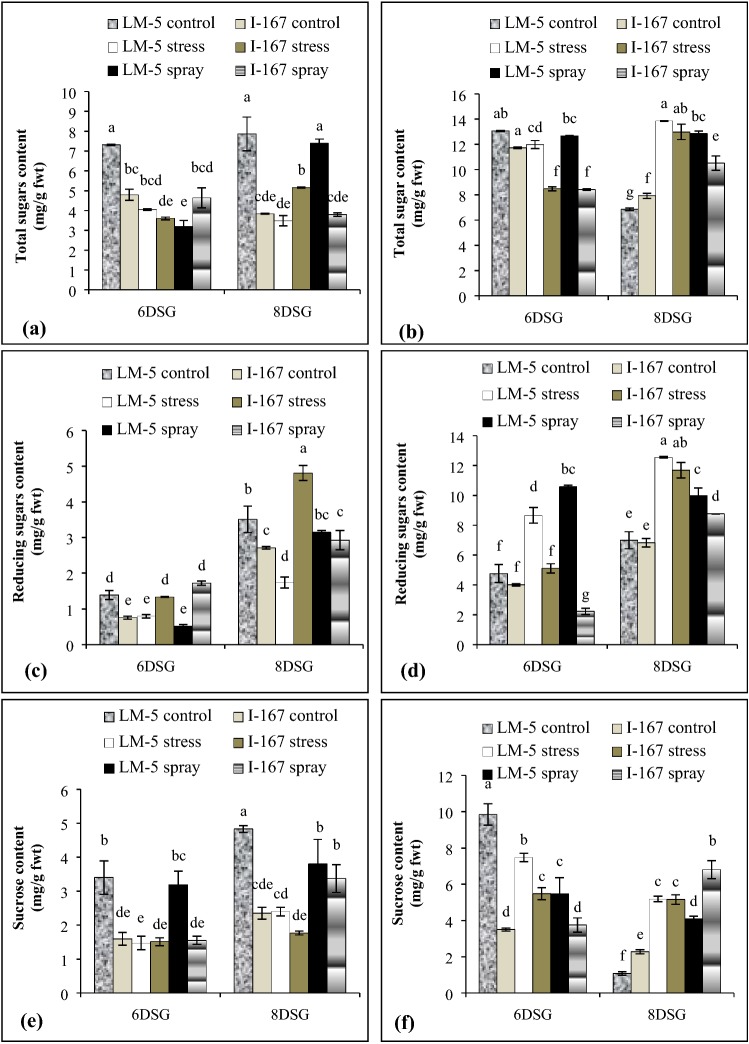
On exposure to water logging, TSS content decreased to a higher extent in the roots of LM 5 as compared to those of I 167 seedlings (Fig. 4a). Under water logging stress, the shoots of both genotypes showed a decrease in TSS content at 6 DSG stage which then increased at 8 DSG stage (Fig. 4b). Foliar application of KNO3 made the levels of total soluble sugars comparable to controls in the roots and shoots of both the genotypes, under water logging stress (Fig. 4a, b).
Fig. 4.
Effect of KNO3 on total soluble sugars, reducing sugars and sucrose content in the roots (a, c, e) and shoots (b, d, f) of LM-5 and I-167 seedlings respectively, under water logging conditions. Error bars represent standard deviations. Bars with different alphabets represent significant differences at p < 0.05
The content of reducing sugars decreased in the hypoxic roots of LM 5 seedlings but increased in those of I 167 at both the growth stages (Fig. 4c). Its content also increased in the shoots of both the genotypes (Fig. 4d). KNO3 treatment optimized the reducing sugar content by increasing it in the hypoxic roots of LM 5 seedlings while reducing it in those of I 167 (Fig. 4c). Foliar spray of KNO3 resulted in decreased reducing sugar content in shoots of both the genotypes at 8 DSG stage (Fig. 4d).
Sucrose content decreased in the roots and shoots of hypoxic LM 5 seedlings. However, it remained unaffected in the roots but increased in the shoots of water logged I 167 seedlings (Fig. 4e, f). Foliar spray of KNO3 increased sucrose content in roots of both the genotypes under hypoxia (Fig. 4e). It reduced the sucrose content in the shoots of LM 5 seedlings at both the growth stages, while only at 6 DSG in I 167 seedlings, under low oxygen conditions (Fig. 4f).
Discussion
The relatively higher PDC and ALDH activities in the waterlogged I 167 seedlings as compared to those of LM 5 (Fig. 1a, c) showed an adaptation of the relatively tolerant genotype in sustaining plant growth under anaerobic conditions by enhancing the synthesis of acetaldehyde and its faster conversion to less toxic acetate ions. The nitrate ions, generated from potassium nitrate foliar spray, acted as an alternate acceptor of electrons under anaerobiosis that helped in the oxidation of NADH coupled to the synthesis of ATP as was suggested by Carvalho et al. (2018). The regenerated NAD+ would be utilized for the continuation of the glycolytic pathway. In general, the decreased ALDH activity in the roots of both genotypes after KNO3 application was in parallel with their reduced PDC activities. It can be suggested that the reduced formation of the substrate (acetaldehyde), in the presence of nitrate ions down regulated the activity of ALDH in the roots of maize seedlings (Fig. 1).
The higher decrease in invertase activity in the roots of tolerant genotype might help in conserving carbon and ATP supplies that were needed for survival under hypoxia as was suggested by Zeng et al. (1999). The supplementation of nitrate ions, through the foliar spray, would have increased the production of NAD+ with the concurrent synthesis of ATP as was proposed by Carvalho et al. (2018) that favored the induction of AI and AKI activities in roots and shoots of the maize genotypes (Fig. 2) and enhanced the funneling of hexoses into the glycolytic pathway. The role of nitrate ions in increasing the activities of invertase isoforms, under flooded conditions, has been reported earlier (Carvalho et al. 2018).
The decrease in SS (synthesis) and SPS activities of seedlings under water logging stress (Fig. 3a, c) thus showed that the hypoxic roots do not utilize their limited energy for biosynthetic purposes. They would rather prefer glycolytic pathway for production of energy. A decrease in SPS activity under anaerobic conditions was also reported by Kuai et al. (2016). The unaltered SS activity in I 167 shoots at 6 DSG suggested that biosynthesis of sucrose via SS activity was not affected by one day of water logging stress. Prolonged water logging conditions (up to 8 DSG) were required to influence the SS activity of these seedlings. It was further inferred that KNO3, being an alternate acceptor of electrons, improved the energy status of the cells by promoting biosynthetic pathways via increased SS and SPS activities of the seedlings (Fig. 3c, d). The regulation of SPS activity of plants by nitrate ions has been reported in literature (Commichau et al. 2006).
The increased activity of one of the principal anaerobic proteins, SS (breakdown) (Fig. 3e), accompanied with a significant reduction in AI and AKI activities (Fig. 2) might favor energy conservation in the hypoxic roots of I 167 seedlings. As KNO3 treatment decreased the SS (breakdown) activity of the seedlings, it may be inferred that nitrate signaling, in fact, up regulated the synthesis of sucrose through induction of SS (synthesis) and SPS activities rather than its cleavage by SS (breakdown) activity.
The increased TSS content of I 167 roots at 8 DSG (Fig. 4a, b) could be an adaptive response towards stress conditions that might help I 167 in sustaining glycolytic and fermentative pathways with consistent production of ATP. This provided a carbohydrate based tolerance mechanism to I 167 genotype. Carbohydrate starvation has been reported as one of the possible consequences of low oxygen stress (Crawford and Braendle 1996) and might be responsible for the susceptibility shown by LM 5 seedlings. In the foliar sprayed seedlings, the reduced TSS content of the shoots at 8 DSG (Fig. 4b) showed that KNO3 treatment induced the effective translocation of sugars toward the roots in waterlogged seedlings. Besides, the diversion of carbon source towards the synthesis of organic acids (Stitt 1999; Lawlor 2002) during assimilation of supplemented nitrate ions might be responsible for the reduced levels of total sugars in the shoots (Fig. 4b).
The higher content of reducing sugars in waterlogged I 167 roots (Fig. 4c) correlated well with their increased SS activity (cleavage) under stress conditions (Fig. 3e). The increased content of reducing sugars in waterlogged roots of I 167 would thus be helpful in sustaining energy production, under hypoxia. The higher reducing sugar content of shoots accompanied with their lower content in roots (Fig. 4c, d) showed the poor translocation of sugars in LM 5 seedlings that might be responsible for the poor performance of LM 5 roots under waterlogged conditions. A reduction in the supply of carbohydrates to the roots due to inhibition of phloem transport has been reported to be responsible for the accumulation of assimilates in the shoots of waterlogged plants (Irfan et al. 2010). KNO3 treatment mitigated the effects of water logging and tended to shift the levels of reducing sugars towards control by inducing their translocation towards the sink organs.
The unchanged sucrose content in the hypoxic roots of I 167 seedlings would be responsible for their higher tolerance capacity than LM 5 genotype (Fig. 4e). It has been cited in literature that the genotypes which maintain their sugar status under extreme conditions have better chances of stress survival (Kumutha et al. 2008). Since, sucrose acts as a preferential source of carbon, under low oxygen conditions (Bouny and Saglio 1996), the increased sucrose content in the waterlogged roots of I 167 and LM 5, upon KNO3 application (Fig. 4e), enhanced the respiratory efficiency of these seedlings under low oxygen conditions. The unaltered sucrose content of I 167 shoots (Fig. 4f) might be due to the notably higher SPS activity of the water logged seedlings (Fig. 3d). After KNO3 application, the further enhancement in sucrose content of I 167 shoots at 8 DSG, (Fig. 4f) might be due to their remarkably higher SS (synthesis) and SPS activities than those of LM 5 seedlings (Fig. 3). Sucrose synthesis in plants has been suggested to be favored by nitrate ions (Tischner 2000). Besides, sucrose induced transcription and post translational activation of nitrate reductase has been observed in plants (Klein et al. 2000). This showed that nitrate ions modulate their own reduction by regulating sucrose concentration through sucrose metabolizing enzymes. These results thus showed that KNO3 enhanced seedling survival under water logging conditions and directed the carbohydrates towards biosynthetic pathways in I 167 seedlings. In foliar treated LM 5 seedlings, the declined sucrose contents of the shoots might be a result of their improved translocation towards the roots for enhancing the glycolytic efficiency of the hypoxic tissues thereby improving growth efficiency of the susceptible genotype.
It may thus be suggested that nitrate ions induced a network of reactions for improving water logging tolerance of maize seedlings. It acted as an alternate electron acceptor and thus decreased the activities of fermentative enzymes. It funneled the sugars into the glycolytic pathway by inducing acid and alkaline invertase activities in the tissues. It induced sucrose synthesizing enzymes and directed the hexoses towards biosynthetic pathways.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aggarwal PK, Kalra N, Chander S, Pathak H. Infocrop: a dynamic simulation model for the assessment of crop yields, losses due to pests, and environmental impact of agro-ecosystems in tropical environments. I. Model description. Agric Syst. 2006;89:1–25. doi: 10.1016/j.agsy.2005.08.001. [DOI] [Google Scholar]
- Bouny M, Saglio P. Glycolytic flux and hexokinase activities in anoxic maize root tips acclimated by hypoxic pretreatment. Plant Physiol. 1996;111:187–194. doi: 10.1104/pp.111.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho PAD, Oliveria LEMD, Domiciano D, Carvalho JND, Prodente DDO, Guimaraes RJ. Effect of nitrogen source and oxygen deficiency on carbon metabolism and antioxidant system of rubber tree plants (Hevea spp.) Aust J Crop Sci. 2018 doi: 10.21475/ajcs.18.12.01.pne774. [DOI] [Google Scholar]
- Crawford RMM, Braendle R. Oxygen deprivation stress in a changing environment. J Exp Bot. 1996;47:145–159. doi: 10.1093/jxb/47.2.145. [DOI] [Google Scholar]
- Commichau FM, Forchhammer K, Stulke J. Regulatory links between carbon and nitrogen metabolism. Curr Opin Microbiol. 2006;9:167–172. doi: 10.1016/j.mib.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Dey PM. Changes in the foms of invertase during germination of mungbean. Phytochemistry. 1986;25:51–53. doi: 10.1016/S0031-9422(00)94499-6. [DOI] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Robers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Gascon S, Lampen JO. Purification of the internal invertase of yeast. J Biol Chem. 1968;243:1567–1572. [PubMed] [Google Scholar]
- Habibzadeh F, Sorooshzadeh A, Pirdashti H, Modarres-Sanavy SAM. Alleviation of waterlogging damage by foliar applications of nitrogen compounds and tricyclazole in canola. Aust J Crop Sci. 2013;7:401–406. [Google Scholar]
- Hossain MA, Uddin SN. Mechanisms of water logging tolerance in wheat: morphological and metabolic adaptations under hypoxia or anoxia. Aust J Crop Sci. 2011;5:1094–1101. [Google Scholar]
- Irfan M, Hayat S, Hayat Q, Afroz S, Ahmad A. Physiological and biochemical changes in plants under water logging. Protoplasma. 2010;241:3–17. doi: 10.1007/s00709-009-0098-8. [DOI] [PubMed] [Google Scholar]
- Jain R, Singh SP, Singh A, Singh S, ChandraA SS. Response of foliar application of nitrogen compounds on sugarcane grown under water logging stress. Sugar Tech. 2016;18:433–436. doi: 10.1007/s12355-015-0406-x. [DOI] [Google Scholar]
- Kato-Naguchi H. Evaluation of the importance of lactate for the activation of ethanolic fermentation in the lettuce in anoxia. Plant Physiol. 2000;109:28–33. doi: 10.1034/j.1399-3054.2000.100105.x. [DOI] [Google Scholar]
- Kaur K, Gupta AK, Kaur N. Effect of water deficit on carbohydrate status and enzymes of carbohydrate metabolism in seedlings of wheat cultivars. Indian J Biochem Biophys. 2007;44:223–230. [PubMed] [Google Scholar]
- Ke D, Yahia E, Mateos M, Kader AA. Ethanolic fermentation of Bartlett pears as influenced by ripening stage and atmospheric composition. J Am Soc Hort Sci. 1994;119:976–982. doi: 10.21273/JASHS.119.5.976. [DOI] [Google Scholar]
- Kerr PS, Kalt-Torres W, Huber SC. Resolution of two molecular forms of sucrose-phosphate synthase from maize, soyabean and spinach leaves. Planta. 1987;170:515–519. doi: 10.1007/BF00402985. [DOI] [PubMed] [Google Scholar]
- Klein D, Morcuende R, Stitt M, Krapp A. Regulation of nitrate reduction expression in leaves by nitrate and nitrogen metabolism is completely overridden when sugars fall below a critical level. Plant, Cell Environ. 2000;23:863–871. doi: 10.1046/j.1365-3040.2000.00593.x. [DOI] [Google Scholar]
- Kuai J, Chen Y, Wang Y, Meng Y, Chen B, Zhao W, Zhou Z. Effect of water logging on carbohydrate metabolism and the quality of fiber in cotton (Gossypium hirsutum L.) Plant Sci. 2016 doi: 10.3389/fpls-2016.00877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumutha D, Sairam RK, Ezhilmathi K, Chinnusamy V, Meena RC. Effect of water logging on carbohydrate metabolism in pigeon pea (Cajanus cajan L.): upregulation of sucrose synthase and alcohol dehydrogenase. Plant Sci. 2008;175:706–716. doi: 10.1016/j.plantsci.2008.07.013. [DOI] [Google Scholar]
- Lawlor DW. Carbon and nitrogen assimilation in relation to yield: mechanisms are the key to understanding production systems. J Exp Bot. 2002;53:773–787. doi: 10.1093/jexbot/53.370.773. [DOI] [PubMed] [Google Scholar]
- Liu F, Cui X, Horner HT, Weiner H, Schnable PS. Mitochondrial aldehyde dehydrogenase activity is required for male fertility in maize. Plant Cell. 2001;13:1063–1078. doi: 10.1105/tpc.13.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manik SMN, Pengilley G, Dean G, Field B, Shabala S, Zhou M. Soil and crop management practices to minimize the impact of water logging on crop productivity. Front Plant Sci. 2019;10:140. doi: 10.3389/fpls.2019.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N. A photometric adaptation of the Somogoyi method for the determination of glucose. J Biol Chem. 1944;153:375–380. [Google Scholar]
- Ren B, Zhang J, Li X, Fan X, Dong S, Liu P, Zhao B. Effects of water logging on the yield and growth of summer maize under field conditions. Can J Plant Sci. 2014;94:23–31. doi: 10.4141/cjps2013-175. [DOI] [Google Scholar]
- Rocha M, Licausi F, Arau jo WL, Nunes-Nesi A, Sodek L, Fernie AR, Van Dongen JT. Glycolysis and the tricarboxylic acid cycle are linked by alanine aminotransferase during hypoxia induced by water logging of Lotus japonicas. Plant Physiol. 2010;152:1501–1513. doi: 10.1104/pp.109.150045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasidharan R, Hartman S, Liu Z, Martopawiro S, Sajeev N, Veen HV, Yeung E, Voesenek LAC. Signal dynamics and interactions during flooding stress. Plant Physiol. 2018;176:1106–1117. doi: 10.1104/pp.17.01232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairam RK, Kumutha D, Ezhilmathi K, Deshmukh PS, Srivastava GC. Physiology and biochemistry of water logging tolerance in plants. Biol Plant. 2008;52:401–412. doi: 10.1007/s10535-008-0084-6. [DOI] [Google Scholar]
- Statista (2019) World corn production by country 2018/19. https://www.statista.comstatistics/254292/global-corn-production-by-country. Accessed 10 Sept 2019
- Stitt M. Nitrate regulation of metabolism and growth. Curr Opin Plant Biol. 1999;2:178–186. doi: 10.1016/S1369-5266(99)80033-8. [DOI] [PubMed] [Google Scholar]
- Tischner R. Nitrate uptake and reduction in higher and lower plants. Plant Cell Environ. 2000;23:1005–1024. doi: 10.1046/j.1365-3040.2000.00595.x. [DOI] [Google Scholar]
- Zanandrea I, Alves JD, Deuner S, Goulart PDFP, Henrique PDC, Silveira NM. Tolerance of Sesbania virgata plants to flooding. Aust J Bot. 2009;57:661–669. doi: 10.1071/BT09144. [DOI] [Google Scholar]
- Zeng Y, Avigne WT, Koch KE. Rapid repression of maize invertase by low oxygen: invertase/sucrose synthase balance, sugar signaling potential and seedling survival. Plant Physiol. 1999;121:599–608. doi: 10.1104/pp.121.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]