Abstract
The rapid emergence and dissemination of methicillin-resistant Staphylococcus aureus (MRSA) strains poses a major threat to public health. MRSA possesses an arsenal of secreted host-damaging virulence factors that mediate pathogenicity and blunt immune defenses. Panton–Valentine leukocidin (PVL) and α-toxin are exotoxins that create lytic pores in the host cell membrane. They are recognized as being important for the development of invasive MRSA infections and are thus potential targets for antivirulence therapies. Here, we report the high-resolution X-ray crystal structures of both PVL and α-toxin in their soluble, monomeric, and oligomeric membrane-inserted pore states in complex with n-tetradecylphosphocholine (C14PC). The structures revealed two evolutionarily conserved phosphatidylcholine-binding mechanisms and their roles in modulating host cell attachment, oligomer assembly, and membrane perforation. Moreover, we demonstrate that the soluble C14PC compound protects primary human immune cells in vitro against cytolysis by PVL and α-toxin and hence may serve as the basis for the development of an antivirulence agent for managing MRSA infections.
Keywords: methicillin-resistant Staphylococcus aureus (MRSA), toxin, virulence factor, structural biology, drug discovery, antivirulence therapy, MRSA, pore-forming toxin, structure-based drug design, virulence factor, phosphatidylcholine, Panton-Valentine leukocidin (PVL), alpha-toxin, leukocidin ED (LukED)
Introduction
Infection with Staphylococcus aureus can cause severe and devastating illness and is one of the leading causes of death by any infectious agent in the United States (1, 2). S. aureus is notorious for its ability to acquire genetic determinants of antibiotic resistance and virulence that enhance fitness and pathogenicity (3, 4). Methicillin-resistant S. aureus (MRSA)2 now accounts for >60% of S. aureus isolates in United States intensive care units, severely restricting antibiotic treatment options (2). MRSA also spreads rapidly among healthy individuals in the community, causing predominantly skin and soft tissue infections and life-threatening infections, including bacteremia, endocarditis, osteomyelitis, and necrotizing pneumonia (2). Disturbingly, MRSA can live in the biofilm state (5, 6), and it has long been recognized that biofilms increase resistance to antimicrobial agents and the host immune response (7). MRSA is currently treated with vancomycin, clindamycin, linezolid, and daptomycin (8), but resistance to these “last-resort” antibiotics has been reported (9–13). For these reasons, the World Health Organization identifies MRSA as one of six “high-priority” pathogens that pose an enormous threat to public health (14). Thus, new therapeutics with novel mechanisms of action are desperately needed to combat this high-threat pathogen.
USA300 is the most prevalent strain of MRSA in the United States and represents a growing threat in both community and healthcare settings (15). Its heightened virulence and severity are related to the production of a mixture of cytolytic pore-forming exotoxins that mediate virulence and impair host immune defenses (3, 16). The pharmacological targeting of these cytotoxins has been recognized as a promising new therapeutic approach to reducing morbidity and mortality associated with MRSA infection. Leukocidins and α-toxin, secreted by S. aureus as water-soluble, monomeric polypeptides, constitute the α-hemolysin subfamily of β-barrel pore-forming toxins (17). Five different bipartite leukocidins have been described, including Panton–Valentine leukocidin (PVL), leukocidin ED (LukED), two γ-hemolysins (HlgAB and HlgCB), and leukocidin AB (LukAB; also known as LukGH), each of which consists of two distinct polypeptides referred to as the S and F subunits (reviewed in Ref. 18). Their cellular tropism and species specificity are determined by the S subunits LukS-PV, LukE, HlgA, HlgC, and LukA (19–21). The S and F subunits and single-component α-toxin share a unique modular structure consisting of the amino latch and prestem regions and the β-sandwich and rim domains (see Fig. 1A) (22–26). The X-ray crystal structures of the membrane-inserted pore oligomer forms of α-toxin, HlgAB, and LukGH and of the membrane surface-bound prepore heterooctamer forms of HlgAB and HlgCB have been determined (27–30). These structures, and supporting biochemical and genetic data (26, 31–33), suggest that members of this subfamily share a common mechanism of cytolytic action (reviewed in Refs. 34 and 35). The cytolytic process begins with the binding of soluble toxin monomers to a cell surface receptor (21, 36). The membrane-bound monomers then associate to form a nonlytic, oligomeric prepore. Finally, the translocation of the prestem regions across the membrane results in the bilayer-spanning β-barrel pore structure and consequent membrane permeabilization and cell lysis.
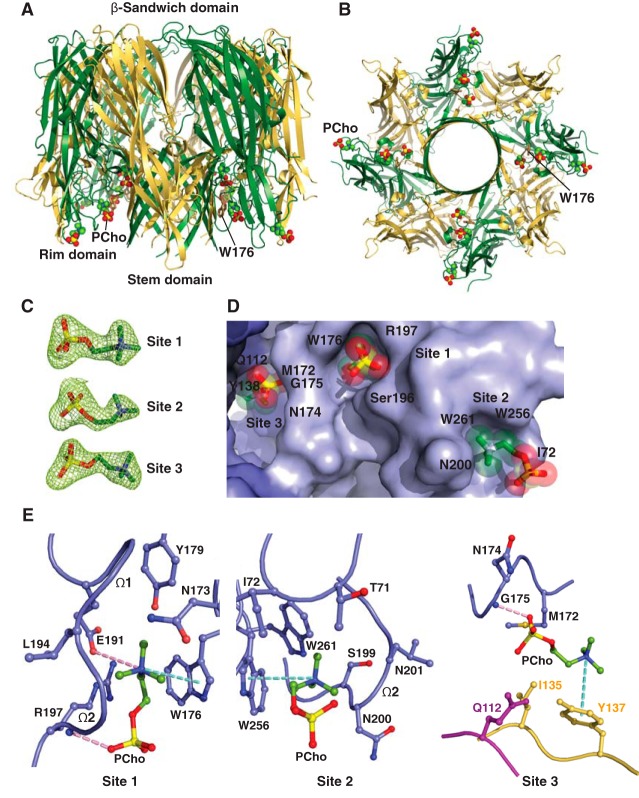
Figure 1.
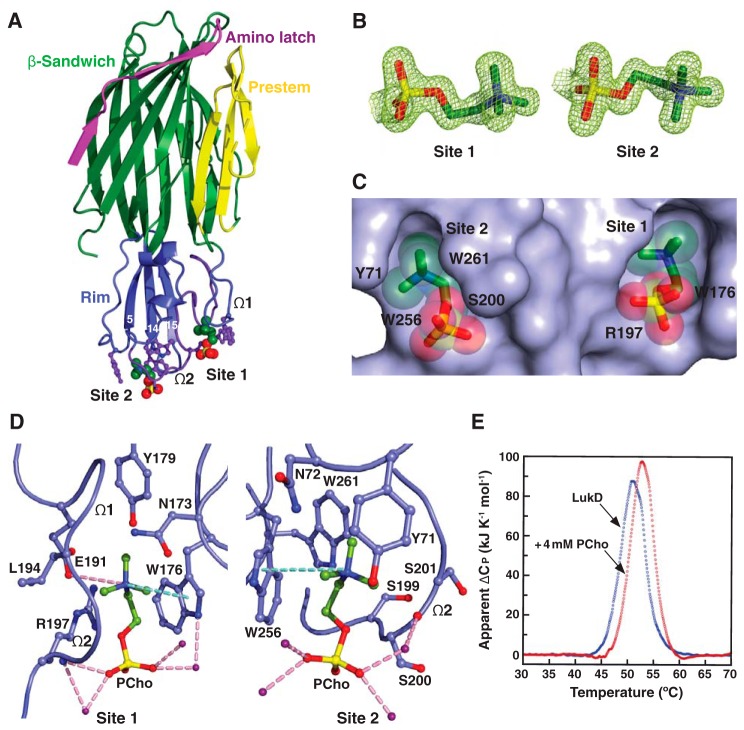
Structural basis for C14PC binding to LukD. A, cocrystal structure of C14PC bound to LukD shown from a side view perpendicular to the membrane plane, with the PCho moieties and the side chains of key binding site residues displayed as CPK spheres and in ball-and-stick format, respectively. The amino latch (magenta) and prestem (yellow) regions and the β-sandwich (green) and rim (blue) domains are indicated. The two binding sites are labeled, as are the β-strands that compose the rim domain and the Ω1 and Ω2 loops. B, 2Fo − Fc omit electron density map (green mesh) for the two PCho moieties at 1.5σ contour level. C, surface representation of the C14PC-LukD complex viewed parallel to the membrane, with the PCho moieties shown in stick format with transparent CPK spheres. The locations of key binding site residues are indicated. D, close-up view of the two adjacent PCho binding pockets, with residues that make direct side-chain contacts with the PCho moieties shown in ball-and-stick format. Cation-π interactions are represented as green dotted lines. Hydrogen bonds and salt bridges are shown as pink dotted lines, and water molecules are shown as purple spheres. E, DSC thermograms of LukD in the absence (Tm = 51.0 °C) and presence (Tm = 52.8 °C) of PCho.
MRSA strains that harbor the phage-encoded PVL have been linked to highly virulent and severe community-acquired skin infections (37), as well as necrotizing pneumonia and lethal necrotizing fasciitis (38). The role of PVL production in the pathogenesis of MRSA was demonstrated in both a rabbit model of necrotizing pneumonia and humanized mouse models of skin infection and pneumonia (39–41). PVL induces leukocyte destruction and tissue necrosis through interaction with the complement receptors C5aR and C5L2 (42–45). PVL, in conjunction with HlgAB, contributes to MRSA biofilm-mediated killing of neutrophils (46). On the other hand, the chromosomally encoded α-toxin lyses epithelial and endothelial cells, red blood cells, lymphocytes, and monocytes by targeting its receptor, the metalloprotease ADAM10 (36, 47). The elevated expression of α-toxin in the USA300 clone and in historic human epidemic strains correlates with increased pathogenicity in mouse models of skin and soft tissue infection, pneumonia, and sepsis (48, 49). α-Toxin also plays a role in biofilm formation by clinical MRSA isolates (50). Moreover, LukED relies on the chemokine receptor CCR5 to kill T lymphocytes, macrophages, and dendritic cells, as well as CXCR1 and CXCR2 to kill leukocytes (19, 51). Inhibition of the interaction between LukED and CCR5 has been shown to block cytotoxicity and attenuate S. aureus infection in mice (19). Together, these cytotoxins can modulate phagocytic cell functions via their specific receptors and contribute to MRSA immune evasion and disease pathogenesis. As such, the discovery and development of new antivirulence agents that protect from the combined immune cytolytic activities of this subfamily of pore-forming toxins is of the utmost importance.
There is considerable evidence pointing to the role of phosphatidylcholine (PC) in the mechanism of pore formation by these toxins. PC is an absolute requirement for pore formation by α-toxin, HlgAB, and HlgCB and has been shown to inhibit their cytolytic effects (52–56). Particularly, crystallographic studies revealed the presence of single, highly conserved phosphocholine (PCho) binding sites on the rim domains of the monomeric F subunit HlgB and the α-toxin protomer in the heptameric pore complex (22, 57). These binding sites have been shown by mutational analysis to be required for membrane targeting and cytolytic function of the two toxins (32, 58). It is generally accepted that α-toxin and the F subunits LukD, LukF-PV, LukB, and HlgB also function in cell attachment through the engagement of their rim domains with the PC headgroup in the plasma membrane of target cells (52, 54, 57). In this report, we demonstrate that the soluble, monomeric, and oligomeric pore forms of both PVL and α-toxin deploy two distinct modes to recognize and bind the PC-containing membrane and suggest a novel molecular mechanism for PC-dependent pore formation by members of this subfamily. Furthermore, we find that the soluble compound n-tetradecylphosphocholine (C14PC) effectively inhibits the cytolysis of primary human immune cells by PVL, α-toxin, and LukED in vitro, thus demonstrating the potential utility of this antivirulence agent alone or in combination with antibiotics against MRSA.
Results and discussion
C14PC binds to the rim domain of LukD at two adjacent but distinct sites
To better understand the molecular basis for the recognition of PCho by the leukocidin F subunits, we determined the crystal structures of LukD with and without C14PC at 1.5 and 1.75 Å resolution, respectively (Table 1). C14PC was selected in the present study as a PC mimic for its high micellization efficiency due to low critical micelle concentration. The two protein structures are closely similar, with an r.m.s.d. for Cα atoms of 0.69 Å. The rim domain forms an antiparallel, three-stranded open-face β-sandwich toppled by two surface-exposed consecutive Ω loops (residues 180–194, Ω1 and 195–202, Ω2) (Fig. 1A). Two PCho moieties that bind to opposite sides of the Ω2 loop were unexpectedly discovered upon examination of the difference electron density map in the C14PC-bound structure (Fig. 1B). The average B-factor for these two moieties is 22 Å2, and that for the surrounding solvent molecules and protein atoms is 21 Å2. The two binding sites are ∼16 Å apart (Fig. 1C). The PCho moiety at the first binding site (site 1) is lodged into a concave pocket similar to one in HlgB (PDB code 3LKF). This pocket is formed by two extended segments (residues 171–173 and 176–179, respectively) and the Ω1–Ω2 junction (residues 191–197) (Fig. 1, A and D). The quaternary ammonium group of the PCho moiety engages in a cation–π interaction with Trp176 while forming a salt bridge to Glu191 (3.79 Å) (Fig. 1D). Its N-methyl and methylene groups are in van der Waal contact (<4.0 Å) with the main-chain atoms of Asn173, Glu191, Leu194, and Gly195 and with the side chains of Asn173, Trp176, Tyr179, and Glu191. Furthermore, the phosphate group is hydrogen-bonded through its O2 oxygen to the main-chain amide of Arg197 (2.85 Å) on one side of the pocket opening, and the side-chain of this residue also wraps around the three other oxygens (Fig. 1D). In addition, three water molecules form hydrogen bonds to the O2 and O3 oxygens.
Table 1.
X-ray data collection and refinement statistics
| LukD, no ligand | LukD, C14PC-bound | LukF-PV, C14PC-bound | α-ToxinH35A, C14PC-bound | PVL, heterooctamer, C14PC-bound | α-Toxin, heptamer, C14PC-bound | α-ToxinH35A, heptamer, C14PC-bound | |
|---|---|---|---|---|---|---|---|
| PDB code | 6U33 | 6U2S | 6U3F | 6U3T | 6U3Y | 6U49 | 6U4P |
| Data collection | NSLS X4C | MacCHESS-F1 | SSRL 9-2 | SSRL 9-2 | SSRL 9-2 | SSRL 9-2 | SSRL 9-2 |
| Cell dimensions | |||||||
| a, b, c (Å) | 123.6, 48.7, 66.7 | 149.2, 37.8, 77.9 | 49.3, 49.3, 266.9 | 136.2, 136.2, 166.0 | 139.1, 139.1, 246.8 | 150.4, 135.0, 132.5 | 151.2, 134.6, 130.7 |
| α, β, γ (degrees) | 90.0, 120.8, 90.0 | 90.0, 119.8, 90.0 | 90.0, 90.0, 120.0 | 90.0, 90.0, 90.0 | 90.0, 90.0, 90.0 | 90.0, 91.5, 90.0 | 90.0, 91.8, 90.0 |
| Wavelength (Å) | 0.979 | 0.977 | 0.979 | 0.979 | 0.979 | 0.886 | 0.979 |
| Space group | C2 | C2 | P3221 | I422 | I422 | C2 | C2 |
| Resolution (Å) | 57.3–1.75 (1.78–1.75)a | 67.6–1.50 (1.53–1.50) | 89.0–1.78 (1.82–1.78) | 43.8–2.80 (2.87–2.80) | 38.7–2.04 (2.08–2.04) | 132.4–2.35 (2.39–2.35) | 37.8–2.50 (2.56–2.50) |
| Rmeas | 0.055 (0.297) | 0.054 (0.270) | 0.035 (0.109) | 0.135 (0.912) | 0.098 (0.981) | 0.101 (0.548) | 0.061 (0.515) |
| I/σI | 15.8 (3.7) | 9.7 (4.4) | 18.9 (20.5) | 5.4 (2.4) | 6.6 (2.1) | 7.2 (2.1) | 10.2 (2.4) |
| Completeness (%) | 92.6 (58.7) | 97.8 (84.7) | 99.7 (98.2) | 99.8 (99.1) | 99.9 (98.7) | 94.5 (96.6) | 96.5 (98.1) |
| Multiplicity | 3.0 (2.3) | 3.2 (3.0) | 9.4 (9.2) | 12.7 (10.4) | 12.8 (8.9) | 2.8 (2.8) | 3.4 (3.4) |
| CC½ (%) | 98.4 (93.8) | 98.9 (96.1) | 99.9 (99.7) | 95.6 (71.6) | 95.6 (75.4) | 92.5 (71.5) | 96.8 (86.4) |
| No. of unique reflections | 32,045 (1,505) | 59,415 (3,824) | 37,358 (2,428) | 19,666 (1,262) | 76,882 (3,741) | 103,631 (7,554) | 87,452 (6,406) |
| Refinement statistics | |||||||
| Resolution (Å) | 57.3–1.75 | 67.6–1.50 | 89.0–1.78 | 43.8–2.80 | 38.7–2.04 | 132.4–2.35 | 37.8–2.50 |
| (1.79–1.75) | (1.54–1.50) | (1.83–1.78) | (2.86–2.80) | (2.09–2.04) | (2.41–2.35) | (2.56–2.50) | |
| Rwork | 0.175 (0.231) | 0.162 (0.216) | 0.179 (0.154) | 0.197 (0.295) | 0.182 (0.242) | 0.194 (0.261) | 0.212 (0.289) |
| Rfree | 0.217 (0.286) | 0.188 (0.245) | 0.210 (0.195) | 0.246 (0.381) | 0.225 (0.269) | 0.239 (0.314) | 0.249 (0.372) |
| r.m.s.d. | |||||||
| Bond lengths (Å) | 0.019 | 0.019 | 0.017 | 0.003 | 0.015 | 0.020 | 0.011 |
| Bond angles (degrees) | 1.9 | 1.9 | 2,4 | 1.0 | 2.2 | 2.3 | 2.0 |
| Average B-factor (Å2) | |||||||
| Protein | 15.2 | 14.3 | 21.5 | 51.4 | 33.1 | 26.5 | 51.4 |
| C14PC | 26.1 | 51.5 | 113.5 | 60.9 | 68.6 | 102.1 | |
| Water | 34.1 | 39.3 | 37.6 | 59.4 | 47.5 | 40.1 | 60.0 |
| Ion | 35.7 | 47.8 | 119.5 | 70.8 | 73.8 | 120.0 | |
| Ramachandran plot | |||||||
| Favored (%) | 95.6 | 95.5 | 95.5 | 94.4 | 96.9 | 96.0 | 94.0 |
| Allowed (%) | 4.4 | 4.5 | 4.5 | 5.6 | 3.1 | 4.0 | 6.0 |
| Disallowed (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
a Values for the highest-resolution shell are shown in parentheses.
Immediately adjacent to site 1 is a novel second binding site (site 2), where the PCho moiety occupies a shallow surface pocket that is framed by the C-terminal half of the Ω2 loop (residues 198–202) and the β14–β15 loop (residues 257–260) and flanked by the side chains of Tyr71, Asn72, Trp256, and Trp261 (Fig. 1, A and D). The quaternary ammonium group is sandwiched between the aromatic rings of Tyr71 and Trp256 through cation–π interactions, and the two indole rings of the latter residue and Trp261 interact with each other in an edge-to-face fashion to engage the N-methyl and methylene groups, which also make contacts with the main-chain atoms of Ser199, Ser200, and Ser201 and with the side chain of Asn72 (Fig. 1D). The phosphate group is secured by a water-mediated hydrogen bonding interaction with the main-chain carbonyl of Ser200 (O2–H2O = 2.53 Å and H2O–O = 2.76 Å), whose Cα and Cβ atoms pack against the O1, O2, and O4 oxygens (Fig. 1D).
The highly complementary interactions between the two adjacent binding sites and the PCho moieties are ostensibly important for specific recognition and binding. The buried solvent-accessible surface area of PCho is 262 Å2 at site 1 and 231 Å2 at site 2, which correspond to ∼77 and 69% of the unbound PCho surface area, respectively. The side chains of the conserved Trp176-Arg197 and Ser200-Trp256-Trp261 residues, seen below, that define site 1 and site 2, respectively, become more ordered upon binding to C14PC. This side-chain flexibility could allow these two adjacent, largely preformed pockets to efficiently accommodate the PCho moieties that have distinct binding poses and residue interactions (Fig. 1, C and D). Consistent with this argument, in differential scanning calorimetry (DSC) experiments, LukD (10 μm) was found to unfold in a single cooperative transition, with a midpoint melting temperature (Tm) of 51.0 °C, whereas this Tm value was shifted to 52.8 °C in the presence of PCho (4 mm), representing the enhanced thermal stability that accompanies complex formation (Fig. 1E). Thus, our results suggest a revised mode of PC recognition and membrane targeting by the rim domain loops.
Binding mode of C14PC to the rim domain of LukF-PV
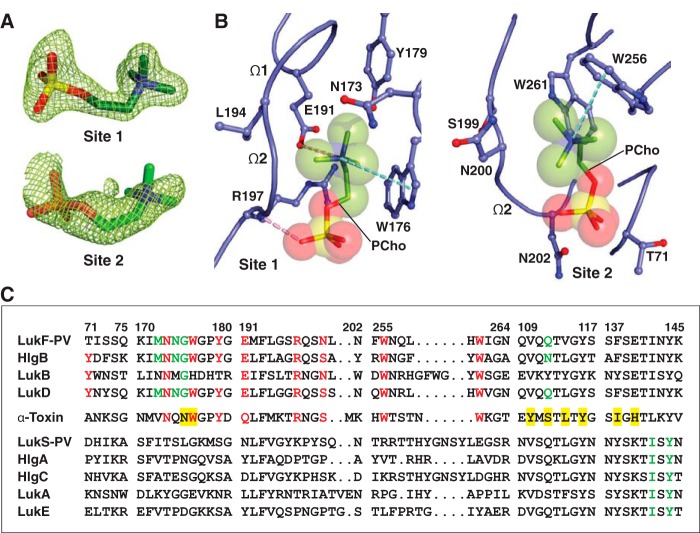
To validate this binding mode, we co-crystallized LukF-PV with C14PC and solved its structure at 1.78 Å resolution (Table 1). In effect, PCho moieties engage the aforementioned two adjacent binding pockets on the rim domain surface (Fig. 2, A and B). At site 1, the quaternary ammonium group of the PCho moiety forms both a cation–π interaction with Trp176 and a salt bridge to Glu191 (3.84 Å); its N-methyl and methylene groups interact with both the main-chain atoms of Leu194 and Gly195 and the side chains of Asn173, Trp176, Tyr179, Glu191, and Arg197, and the phosphate group is held in place by a hydrogen bond between its O2 oxygen and the main-chain amide of Arg197 (2.72 Å), along with the side chain of this residue lying against the O2 and O3 oxygens (Fig. 2B). At site 2, the quaternary ammonium group participates in a cation–π interaction with Trp256 (Fig. 2B). Further contacts are made between the N-methyl and methylene groups and both the main-chain atoms of Ser199, Asn200, and Leu201 and the side chains of Asn200, Trp256, and Trp261. Polar interactions are also observed between the phosphate and both the main-chain atom of Asn200 and the side chain of Asn202 (Fig. 2B).
Figure 2.
Structure of C14PC-bound Luk-PV. A, 2Fo − Fc electron density map shown as green mesh around the two PCho moieties contoured at 1.5σ (site 1) and 1.0σ (site 2). B, molecular interactions in the C14PC-LukF-PV complex, with the PCho moieties shown in stick format with transparent CPK spheres. The side chains of residues that make direct contacts with the PCho moieties are shown in ball-and-stick format. The two binding sites and the Ω1 and Ω2 loops are labeled. Cation-π interactions are represented as green dotted lines. Hydrogen bonds and salt bridges are shown as pink dotted lines. C, sequence alignment for members of the α-hemolysin toxin subfamily around the regions of the three PCho-binding sites (see “Results” for details). Four segments of the rim domain (residues 71–75, 170–180, 191–202, and 255–264) and two segments of the stem domain (residues 109–117 and 137–145) are delineated by spaces and numbered according to the mature LukF-PV protein. Conserved residues at the two binding sites on the rim domain are highlighted in red. Conserved residues that constitute the interprotomer binding sites on the PVL heterooctamer and the α-toxin heptamer are highlighted with a green and yellow background, respectively.
The solvent-accessible surface area of PCho buried by the LukF-PV interaction comprises 264 Å2 (79%) at site 1 and 214 Å2 (63%) at site 2. DSC measurements reveal that the Tm of LukF-PV increased from 50.3 to 52.3 °C when it was bound to PCho. We note that the PCho moiety at site 2 has considerably higher average B-factor and poorer electron density than that at site 1 (70 Å2 as compared with 31 Å2), suggesting that the former moiety is less tightly bound and exhibits greater spatial or temporal disorder. In LukD, the aromatic side chain of Tyr71 contributes to the cation–π binding interaction at site 2 (see Fig. 1D), whereas the corresponding residue in LukF-PV (Thr71) cannot make this interaction (Fig. 2C), likely accounting for the lower-affinity binding site. The critical functional role of this affinity difference is highlighted by the observation that replacement of Thr71 with a tyrosine endows LukF-PV with the ability to bind human erythrocytes and acquire hemolytic activity when combined with the S subunit of HlgAB (33). Therefore, the elaborate structural features of the two distinct, adjacent PCho-binding sites on the leukocidin F subunits may be explained by a selective pressure for membrane PC itself acting as their cell surface receptor.
C14PC binding by monomeric α-toxinH35A
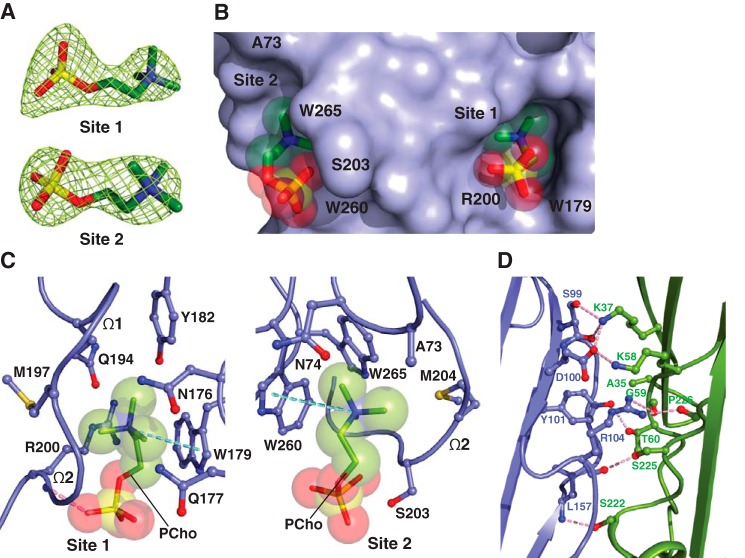
To discern the mechanism in the attachment of α-hemolysin subfamily members to host cells, we determined the 2.80 Å crystal structure of C14PC in complex with the oligomerization-defective His35→Ala mutant of α-toxin (α-toxinH35A) (59) (Table 1). The asymmetric unit contains two nearly identical protein monomers (r.m.s.d. for Cα atoms of 0.44 Å), each bound to two PCho moieties (Fig. 3A). These moieties occupy the two adjacent binding pockets described above (Fig. 3B). At site 1, which is similar to that on the α-toxin protomer in the heptameric pore complex (57), the quaternary ammonium group of the PCho moiety makes a cation–π interaction with Trp179 (Fig. 3C). Its N-methyl and methylene groups are surrounded by the main-chain atoms of Met197 and Lys198 and by the side chains of Asn176, Gln177, Trp179, Tyr182, Gln194, Met197, and Arg200. Importantly, the O2 oxygen of the phosphate group establishes a strong hydrogen bond to the main-chain amide of Arg200 (2.64 Å) that also makes side-chain contacts with the O2 and O4 oxygens (Fig. 3C). At site 2, the quaternary ammonium group forms a cation–π interaction with Trp260, and the N-methyl and methylene groups interact with the main-chain atoms of Gly202, Ser203, and Met204 and with the side chains of Ala73, Asn74, and Trp265 (Fig. 3B). The phosphate group is clearly visible in the electron density map, although the fine detail of the oxygens is not clear. There are contacts of 3.19 Å between the phosphate and Ser203 and of 3.62 Å between the phosphate and Trp260 (Fig. 3C). Upon binding to α-toxinH35A, PCho buries 268 Å2 (79%) and 203 Å2 (61%) of its solvent-accessible surface area at site 1 and site 2, respectively. DSC analysis shows that the addition of PCho increased the Tm of α-toxinH35A from 50.8 to 52.4 °C. We also observed that the average B-factor for the PCho moiety at site 2 is significantly higher than that at site 1 (112 Å2 as compared with 75 Å2). As discussed in the preceding section, the decreased affinity of site 2 for PCho may arise from the presence of an alanine at position 73 (corresponding to LukD Tyr71) (Fig. 2C).
Figure 3.
The two adjacent PC-binding pockets on monomeric α-toxinH35A. A, 2Fo − Fc electron density map (green mesh) for the two PCho moieties contoured at 1.2σ (site 1) and 1.0σ (site 2). B, surface representation of the C14PC-α-toxinH35A complex viewed parallel to the membrane, with the PCho moieties shown in stick format with transparent CPK spheres. The two binding sites are labeled. The locations of key binding site residues are indicated. C, close-up view of the two adjacent PCho-binding pockets, with the PCho moieties displayed in stick format with transparent CPK spheres. Residues that make direct side chain contacts with the PCho moieties are shown in ball-and-stick format. The Ω1 and Ω2 loops are labeled. Cation-π interactions are represented as green dotted lines. Hydrogen bonds are shown as pink dotted lines. D, close-up view of the interface between the two independent α-toxinH35A monomers (blue and green, respectively) in the asymmetric unit. Hydrogen bonds and salt bridges near the His35→Ala mutation site are depicted as pink dotted lines.
Closer examination of the positions and conformations of the two PCho moieties in the superimposed cocrystal structures of C14PC with α-toxinH35A, LukD, and LukF-PV revealed remarkable similarities. There are few differences in the positions of the five key binding site amino acid side chains (Trp179, Arg200, Ser203, Trp260, and Trp265 in α-toxin; equivalent to Trp176, Arg197, Ser/Asn200, Trp256, and Trp261 in LukD and LukF-PV) in these structures. The three Trp side chains provide two important anchor points for locating the PCho moieties in the two adjacent binding sites, and the Arg and Ser/Asn residues are critical determinants in the binding of the two phosphate groups. Evidently, PC recognition specificity is achieved by a combination of stacking and hydrogen-bonding interactions and van der Waals contacts. Our study shows that membrane PC serves as the common receptor for α-toxin and the leukocidin F subunits, in agreement with previous observations (52, 54, 57). The presence of the two adjacent PC-binding sites on the toxin monomer is consistent with the estimated cross-sectional areas of the PC-bound rim domain (∼150 Å2) and one PC molecule (∼70 Å2) (60).
Intermolecular contacts between the above two α-toxinH35A monomers comprising the crystal asymmetric unit are formed by residues in the β-sandwich domain (Fig. 3D). Comparison of the conformation of these contact residues with their interprotomeric equivalents in the unliganded and C14PC-bound heptamers of WT α-toxin (PDB code 7AHL; see Fig. 5) reveals no local conformational changes involving the main-chain or side-chain atoms. Superposition of the α-toxinH35A dimer onto two adjacent promoters in the above two WT toxin heptamers yields overall Cα r.m.s.d. values of 0.99 and 0.95 Å, respectively, indicating their structural similarity. Dimer interfaces have similar buried surface area values, from 2,061 to 2,171 Å2. It is also important to note that the crystal structure of unliganded α-toxinH35A (PDB code 4YHD) lacks the aforementioned intermolecular contacts between six independent monomers in the asymmetric unit. In this structure, both the amino latch and prestem regions have well-defined density with the exception of the six-residue prestem loop and pack against the β-sandwich core of the protein. By contrast, these two regions are apparently disordered in the C14PC-bound structure. Our results suggest that α-toxinH35A may be trapped in a PC-bound dimeric state, which may represent an on-pathway intermediate in the assembly of the heptameric pore complex.
Figure 5.
Structure of C14PC bound to the α-toxin heptamer. A and B, ribbon representation of C14PC in complex with α-toxin heptamer shown from the side (A) and cytoplasmic (B) views. The PCho moieties and the side chain of Trp179 are shown as CPK spheres and in stick format, respectively. The amino latch region and the β-sandwich, rim, and stem domains are colored as in Fig. 1A. C, 2Fo − Fc electron density map (green mesh) contoured at 1.0σ for residues Asn178, Trp179, and Gly180 and the three PCho moieties in a single protomeric unit. D, surface representation of the three partially overlapping PCho-binding pockets on a single protomeric unit viewed from the cytoplasmic side, with PCho moieties shown in stick format with transparent CPK spheres. The three binding pockets are labeled. The locations of key binding site residues are indicated. E, molecular interactions in the heptameric α-toxin-C14PC complex, with the PCho moieties in a single protomeric unit displayed in ball-and-stick format. Residues that make direct side-chain contacts with the PCho moieties are shown in ball-and-stick format colored in blue for protomer A, in purple for protomer E, and in yellow for protomer F. The Ω1 and Ω2 loops are labeled. Cation-π interactions are represented as green dotted lines. Hydrogen bonds and salt bridges are shown as pink dotted lines. F, close-up view of interprotomer interactions in the triangle region in the crystal structure of the C14PC-bound α-toxinH35A heptamer. Protomers A, B, and C are colored blue, green, and yellow, respectively.
Given their expected importance in membrane targeting, the five key PC-binding site residues are highly conserved or invariant in both α-toxin and the leukocidin F subunits but are absent in the S subunits, with the exception of a histidine at position 176 in LukB (Fig. 2C). Of particular importance, LukB exists as a soluble heterodimeric complex with LukA (61). This finding is consistent with the central role of the conserved Trp176 of the three other F subunits in their binding to the PC bilayer (22, 52, 54) (this study). We therefore propose that the binding of the F subunit to the PC-rich membrane is allosterically coupled to heterodimerization with its S subunit counterpart. Likewise, membrane binding by α-toxin, mediated by PC and/or ADAM10, irrevocably commits the monomers to dimerization. The remarkable high degree of conservation of the two adjacent PC-binding sites among α-toxin and the F subunits reflects a strong selective pressure on the ability of these two sites to help anchor toxin monomers to the cell surface and to form intermolecular contacts that prime the ensuing formation of the oligomeric, membrane-inserted pore complex.
In summary, the bivalent rim domain interaction with PC provides a mechanism by which soluble toxin monomers can recognize and target the PC-containing membrane, thereby promoting dimer-nucleated pore assembly. The relatively low affinity of PC-mediated binding may facilitate subsequent establishment of the final geometry of the oligomeric pore complex, which we discuss below. α-Toxin and the leukocidin S subunits also bind their cognate proteinaceous receptors (19–21, 47), and these interactions likely work in concert with the PC-targeting mechanism to modulate toxin binding, pore formation, and cytotoxicity. Finally, and most importantly, structural elucidation of the two conserved, adjacent PC-binding pockets on α-toxin and the leukocidin F subunits will guide the rational development of PC analogs as decoy receptors that prevent the cytotoxin from binding to susceptible cells.
Structure of the C14PC-bound PVL heterooctamer
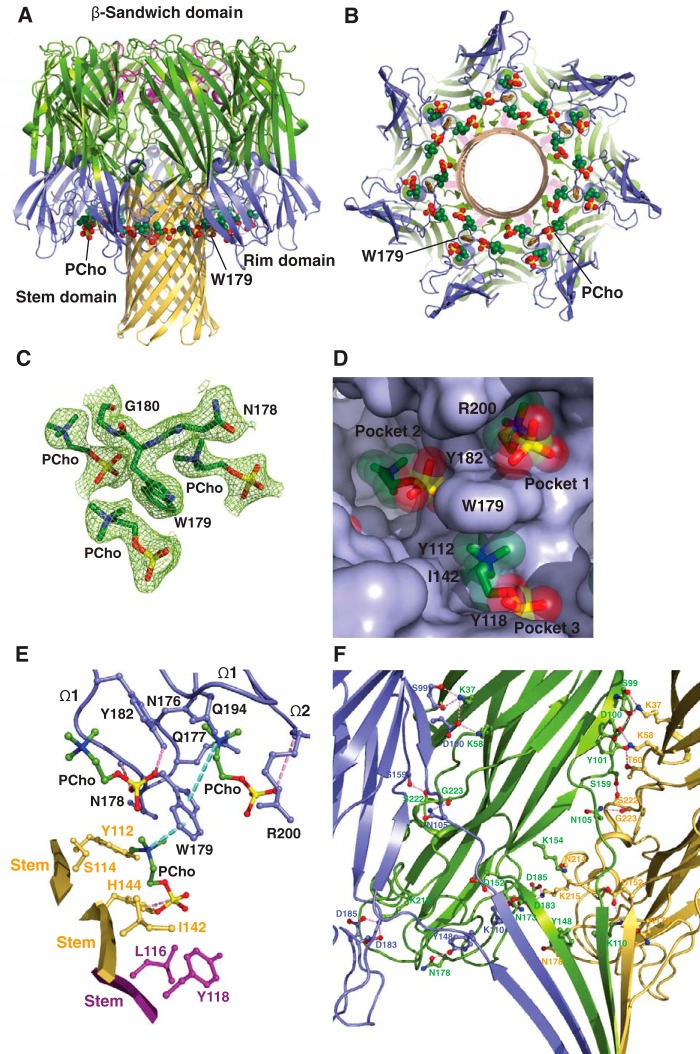
In light of previous studies suggesting that PC plays a crucial role in the assembly and function of the α-toxin heptamer (54, 57), we co-crystallized the LukS-PV and LukF-PV proteins with C14PC in the presence of n-octyl-β-glucoside. The structure of the complex was solved at 2.04 Å resolution by molecular replacement (Table 1). The asymmetric unit contains one LukF-PV/LukS-PV heterodimer and a single LukS-PV molecule. The heterodimer interacts with three crystallographic 4-fold symmetry-related copies of itself to generate a heterooctamer (Fig. 4, A and B). In this β-barrel pore complex, four LukF-PV protomers (denoted A, C, E, and G) and four LukS-PV protomers (B, D, F, and H) are arranged in an alternating fashion around the central axis of the pore, in which the stem domain folds into an antiparallel β-barrel composed of 16 β-strands. We could not discern electron density corresponding to the bottom third of the stem domain in our structure. Two distinct interfaces between neighboring protomers involve residues that are distributed among the amino latch region and the β-sandwich and stem domains and bury 2,644 and 1,902 Å2 of solvent-accessible surface area, respectively. The electron density map revealed clearly the presence of PCho moieties at three distinct binding sites on each of the four protomeric units of the PVL heterooctamer (Fig. 4, C and D). The two adjacent binding sites are essentially the same as those on the above-described toxin monomer, whereas the other, novel site lies at the interface between the rim domain of a LukF-PV protomer (e.g. protomer A) and the proximal stem domain regions of protomers G and H. The average B-factor for the three PCho moieties is significantly higher than that for the surrounding residues (60 Å2 as compared with 31 Å2), possibly due to greater disorder and/or subunitary occupancy. Superposition of the PVL heterooctamer bound to C14PC onto the unliganded HlgAB (PDB code 3B07) and LukGH (PDB code 4TW1) heterooctamers yields Cα r.m.s.d. values of 0.67 and 1.14 Å, respectively, suggesting that the PVL pore does not undergo large conformational changes upon binding to C14PC.
Figure 4.
Crystal structure of C14PC in complex with the PVL heterooctamer. A and B, ribbon representation of the C14PC-bound PVL heterooctamer shown from the side (A) and cytoplasmic (B) views. The PCho moieties and the side chain of Trp176 are displayed as CPK spheres and in stick format, respectively. The LukF-PV and LukS-PV subunits are colored green and yellow, respectively. The β-sandwich, rim, and stem domains are indicated. C, 2Fo − Fc omit electron density map contoured at 1.0σ shown as green mesh around the three PCho moieties in a single protomeric unit. D, surface representation of the three PCho-binding pockets on a single protomeric unit viewed from the cytoplasmic side, with the PCho moieties shown in stick format with transparent CPK spheres. The three binding sites are labeled. The locations of key binding site residues are indicated. E, close-up view of the PCho moieties in the three binding pockets on a single protomeric unit. The side chains of residues that make direct contacts with the PCho moieties are shown in ball-and-stick format colored in blue for protomer A, in magenta for protomer G, and in yellow for protomer H. The Ω1 and Ω2 loops are labeled. Cation-π interactions are depicted as green dotted lines. Hydrogen bonds and salt bridges are represented as pink dotted lines.
The three PCho-binding sites on a single protomeric unit are contained within a water-accessible crevice between the inner surface of the rim domain and the upper portion of the stem domain (Fig. 4, A and B). As noted above, the two adjacent sites correspond to those on the rim domain of monomeric LukF-PV (Fig. 4D; see Fig. 2B), differing only in the presence of more stabilizing molecular contacts at site 2 on the heterooctamer. Specifically, the quaternary ammonium group of the PCho moiety makes a cation–π interaction with Trp256, and the indole ring of this residue establishes an edge-to-face interaction with the indole ring of Trp261 to pack against the N-methyl and methylene groups, which are also in contact with the main-chain atoms of Thr71, Ser199, Asn200, and Leu201 and with the side chain of Ile72 (Fig. 4E). At site 3, the aromatic ring of Tyr137 of protomer H forms a cation–π interaction with the quaternary ammonium group and stacks against the N-methyl and methylene groups that are also lined with the side chain of Ile135 of protomer H (Fig. 4E). Furthermore, the O3 oxygen of the phosphate group hydrogen-bonds to the main-chain amide of Gly175 of protomer A (2.65 Å), and the O1 and O3 oxygens engage both the main-chain atoms of Asn174 and Gly175 of protomer A and the side chains of Met172 of protomer A and Gln112 of protomer G (Fig. 4E). The solvent-accessible surface area of PCho buried upon complex formation is 258 Å2 (77%) at site 1, 189 Å2 (57%) at site 2, and 224 Å2 (65%) at site 3.
Our results suggest that multivalent binding of the PVL heterooctamer to PC on the membrane surface leads to localized alterations in the lipid bilayer and thus promotes the insertion of amphipathic β-hairpins to produce the β-barrel piercing the bilayer. Critical residues Tyr137 of LukS-PV and Gly175 of LukF-PV at site 3 are invariant in the leukocidin S and F subunits, respectively (Fig. 2C), underscoring their functional importance. Furthermore, three similar PC-binding pockets also exist in protomers of the C14PC-bound α-toxin heptamer described below.
Binding mode of C14PC to the α-toxin heptamer
To evaluate the binding of the α-toxin heptamer to the PC headgroup in a membrane-mimicking environment, we determined the crystal structure of its complex with C14PC at 2.35 Å resolution (Table 1). In this structure, three PCho moieties are bound to each of the seven protomeric units in the water-accessible crevice between the rim and stem domains (Fig. 5, A and B). The indole ring of Trp179 mediates three-way interactions with these three moieties (Fig. 5C). Their conformations are clearly defined in three partially overlapping but distinct binding pockets of the crevice (Fig. 5D). One pocket corresponds to site 1 on the toxin monomer described above, whereas the other two are novel heptamer-specific binding sites (see below). The average B-factor for the three PCho moieties is 60 Å2, and for surrounding protein atoms, it is 33 Å2. The structure of the C14PC-bound heptamer is very similar to that of the unliganded heptamer (PDB code 7AHL; r.m.s.d. for Cα atoms of 0.48 Å), with only minor changes in the positions of side chains involved in direct contact with C14PC. The pairwise r.m.s.d. values among protomers A–G in the heptamer span a range from 0.13 to 0.17 Å for Cα atoms. The PCho moieties at each of the three binding sites have essentially identical conformation and orientation in each of the seven protomeric units, with average r.m.s.d. values of 0.34 Å for the first pocket, 0.32 Å for the second pocket, and 0.41 Å for the third pocket. For this reason, the following structural analysis of these binding pockets applies to all of the protomeric units.
The first pocket, defined by Trp179 and Arg200, is the same as that on monomeric α-toxinH35A (see Fig. 3), albeit the hydrogen bond between the phosphate group of the PCho moiety and the main-chain amide of Arg200 is considerably longer and weaker in the latter (Fig. 5E). The second pocket lined by all four residues on strand β12 of the rim domain snugly accommodates the PCho moiety (Fig. 5, D and E). It mediates a network of van der Waals contacts involving both the main-chain atoms of Gly180 and Pro181 and the aromatic rings of Trp179 and Tyr182, forming hydrogen bonds via its hydroxyl group toward the O3 oxygen of the phosphate group (2.69 Å) and via its O2 oxygen with the main-chain amide of Gly180 (2.84 Å) while in the cis rotamer.
The third pocket is located at the interface between the rim domain of protomer A and the proximal stem domain regions of protomers E and F (Fig. 5E), in contrast to the other pockets that are constituted solely by residues from the rim domain. The third pocket is formed by residues Asn178 and Trp179 from the rim domain of protomer A; by Leu116 and Tyr118 from the stem domain of protomer E; and by Tyr112, Ser114, Ile142, Gly143, and His144 from the stem domain of protomer F (Fig. 5E). The indole ring of Trp179 is situated to produce a cation–π interaction with the quaternary ammonium group of the PCho moiety (Fig. 5E). The N-methyl and methylene groups participate in extensive contacts with the main-chain atoms of Gly143 and Asn178 and with the side chains of Tyr112, Ser114, and Ile142. The PCho moiety is further stabilized by a hydrogen bond between the O3 oxygen of the phosphate group and the ND1 atom of His144 (2.78 Å) and by contacts between the O1, O2, and O3 oxygens and the side chains of Leu116, Tyr118, and His144 (Fig. 5E). The solvent-accessible surface areas buried upon binding of the PCho moieties to the first, second, and third pockets are 260 A2 (76%), 207 A2 (60%), and 285 A2 (83%), respectively.
These results strengthen the hypothesis that multivalent binding of the PC bilayer by the α-toxin heptamer may help overcome the energetic barrier to deformation of the membrane during assembly of the β-barrel pore lining, thereby driving the conversion of the prepore to the transmembrane pore complex. Indeed, replacement of Trp179 and Arg200 with alanines in α-toxin is known to lead to an arrested prepore state in which only the top half of the cytolytic β-barrel pore has formed (26). Together with analysis of intermediate stages of the α-toxin assembly process with engineered disulfide bonds (34), our study also suggests that the interaction between the α-toxin prepore and the PC headgroup may induce a large conformational change in the prestem region, which is essential for pore formation.
Structure of the α-toxinH35A heptamer in complex with C14PC
In the α-toxin pore structure, His35 is located in the crucial interprotomeric contact region (27), and nonconservative replacements at this position (including H35A) have been shown to abolish heptamer formation and thus cytolytic activity and lethal toxicity (62–64). In light of our findings that the PC bilayer binding might promote both the oligomerization of α-toxin monomers and the structural rearrangements that accompany the prepore-to-pore conversion, we hypothesized that a high concentration of C14PC could facilitate the assembly of the α-toxinH35A pore complex. To directly test this hypothesis, we have determined the structure of the α-toxinH35A heptamer crystallized in the presence of 25 mm C14PC at 2.5 Å resolution (Table 1 and Fig. 5F); it is worth noting the use of 5 mm C14PC for the crystallization of the α-toxinH35A monomer (see Fig. 3 and “Experimental procedures”). In this mutant pore complex, PCho moieties bind in the first and second pockets described above on the rim domain of each protomer. In essence, the C14PC-bound structures of the α-toxinH35A and WT heptamers are nearly identical, with r.m.s.d. values of 0.04–1.42 Å over 2,051 Cα atoms. The positions and conformations of the two PCho moieties are also similar. However, C14PC does not bind to the aforementioned interprotomer pocket on the α-toxinH35A pore, whereas B-factors for this mutant pore are considerably higher than those for the WT one (24–201 Å2 as compared with 13–73 Å2), consistent with the pronounced effect of the H35A mutation on cytotoxicity (59). These results support our hypothesis that the PC-rich membrane acts as a critical effector of oligomerization and pore formation by α-toxin.
In summary, despite their different subunit composition and stoichiometry, α-toxin and the leukocidins likely follow an evolutionarily conserved PC-dependent pore assembly pathway, involving the initial membrane binding of toxin monomers and membrane-dependent dimerization and oligomerization, followed by the prepore-to-pore transition and membrane perforation. It should be stressed that our crystallographic results demonstrate that the interactions between PCho and the oligomeric pore forms of α-toxin and PVL differ considerably. Importantly, atomic-level insight of the toxin oligomer-PC interactions obtained here will facilitate the development of PC analogs that inhibit pore formation and thus block the immune cytolytic effects of this subfamily of proteins.
Inhibition of the cytotoxicity of LukED, PVL, and α-toxin by C14PC
The presence of the conserved PC binding sites in the leukocidins and α-toxin (see above) suggests that PC mimetic compounds may interfere with toxin-mediated killing of primary human immune cells. Therefore, flow cytometry experiments were conducted to first evaluate the ability of C14PC to diminish the cytolytic activity of LukED in Jurkat cells expressing CCR5. This Jurkat cell line has been shown to be susceptible to the toxin (19). LukED at a concentration of 2.5 μg/ml resulted in ∼80% lysis of Jurkat cells within 1 h at 37 °C (Fig. 6A). We found that C14PC inhibited the lysis in a concentration-dependent manner, with an IC50 value between 15 and 25 μm (Fig. 6A). In sharp contrast, PCho did not show appreciable inhibitory activity up to 0.5 mm. We conclude that C14PC produces effective toxin inhibition by presenting multiple copies of the PC headgroup on its micellar surface, in accordance with previous observations (54).
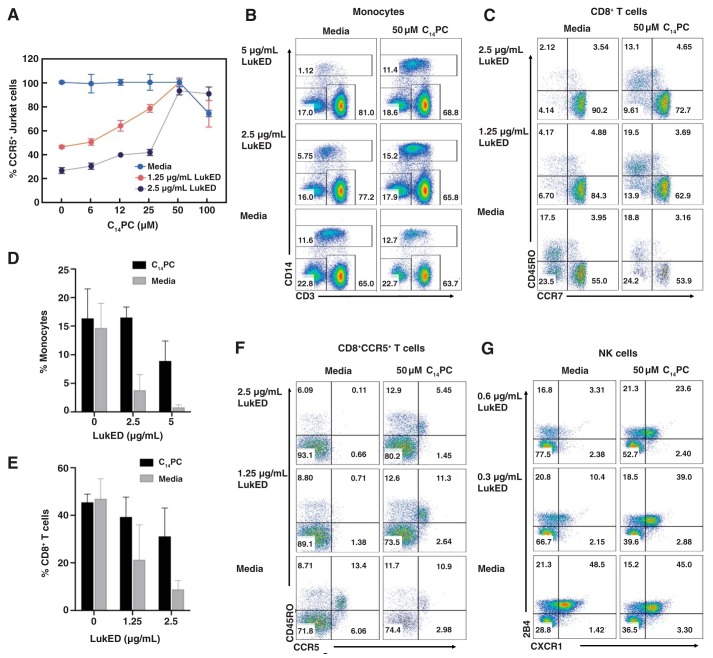
Figure 6.
C14PC protects target cells from lysis by LukED. A, titration of the cytotoxicity of LukED by C14PC. CCR5+ Jurkat cells were challenged with different concentrations of LukED in the absence and presence of C14PC (6–100 μm). Cell viability was determined by flow cytometry and normalized to that in the medium control. Data are representative of at least three independent experiments, and values are expressed in the mean of triplicate measurements ± S.E. B, protective effect of C14PC against LukED-mediated killing of primary human monocytes. PBMCs were challenged with LukED in the presence and absence of 50 μm C14PC. Monocyte subsets were identified as CD3−CD14+ after gating on live PBMCs as determined by flow cytometry. Data are representative of two independent experiments. Percentages of cells in each quadrant are indicated. C, protective effect of C14PC against LukED-mediated killing of CD8+ effector memory T cells (CCR7−CD45RO+ and CCR7−CD45RO−). PBMCs were challenged with LukED in the presence and absence of 50 μm C14PC. Live CD3+CD8+ T cell subsets were gated and analyzed for the expression of CCR7 and CD45RO by flow cytometry. Data are representative of two independent experiments using blood from different donors. D and E, bar graphs showing inhibition of the LukED-induced lysis of monocytes (D) and CD8+ effector memory T cells (E) by 50 μm C14PC. Error bars, S.E. F, protective effect of C14PC against LukED-mediated killing of CD8+CCR5+ T cells. PBMCs were challenged with LukED in the presence and absence of 50 μm C14PC. Live CD3+CD8+ T cell subsets were gated and analyzed for the expression of CCR5 and CD45RO by flow cytometry. Data are representative of two independent experiments. G, protective effect of C14PC against LukED-mediated killing of NK cells. PBMCs were challenged with LukED in the presence and absence of 50 μm C14PC. PBMCs were first gated on live CD3−HLA-DR− cells, and the proportion of CXCR1+2B4+ NK cells was analyzed by flow cytometry. Data are representative of two independent experiments.
To investigate the protective effects of C14PC on LukED-induced lysis of primary human leukocytes expressing CCR5 and CXCR1 in vitro, LukED at concentrations of 2.5 and 5 μg/ml was first preincubated with 50 μm C14PC at 4 °C and was subsequently added to peripheral blood mononuclear cells (PBMCs) labeled with specific cell surface markers. After 1–1.5 h at 37 °C, the cells were stained with fixable viability dye eFluor 506 and analyzed by flow cytometry. Inhibition of LukED by C14PC was assessed by determining the relative abundance of viable cells after challenge with the toxin or medium. As expected, CD14+ monocytes were significantly absent by 2.5 and 5 μg/ml of LukED (Fig. 6D), whereas pretreatment with 50 μm C14PC produced a 70–90% protective effect against monocyte lysis (Fig. 6, B and D). Likewise, 50 μm C14PC blocked the lysis of CD8+ effector memory T cells by 50–75% (Fig. 6, C and E) and of CD8+CCR5+ T cells by 50–95% (Fig. 6F). Moreover, 50 μm C14PC also rescued 50–85% of NK cells (Fig. 6G), which are highly susceptible to LukED due to their surface expression of CXCR1 (19). These results demonstrate that C14PC confers target cell protection by blocking the interaction between LukED and membrane PC.
We next sought to assess the ability of C14PC to abrogate the cytolytic activities of PVL and α-toxin using the in vitro cell viability assay described above. The addition of 2 and 10 ng/ml PVL led to 85–95% lysis of monocytes after 1.5 h of incubation at 37 °C (Fig. 7, A and B). Pretreatment with 100 μm C14PC suppressed the lysis by 90% (Fig. 7, A and B). Similarly, α-toxin at concentrations of 30 and 100 ng/ml caused 75–90% lysis of monocytes and ∼50% lysis of CD3+ T cells after incubation at 37 °C for 24 h, whereas 100 μm C14PC caused 75–90% reduction of the lytic activity (Fig. 7, C and D). We conclude that C14PC is a broad-spectrum small-molecule inhibitor of LukED, PVL, and α-toxin and that membrane PC contributes to the mechanism of their cytolytic action.
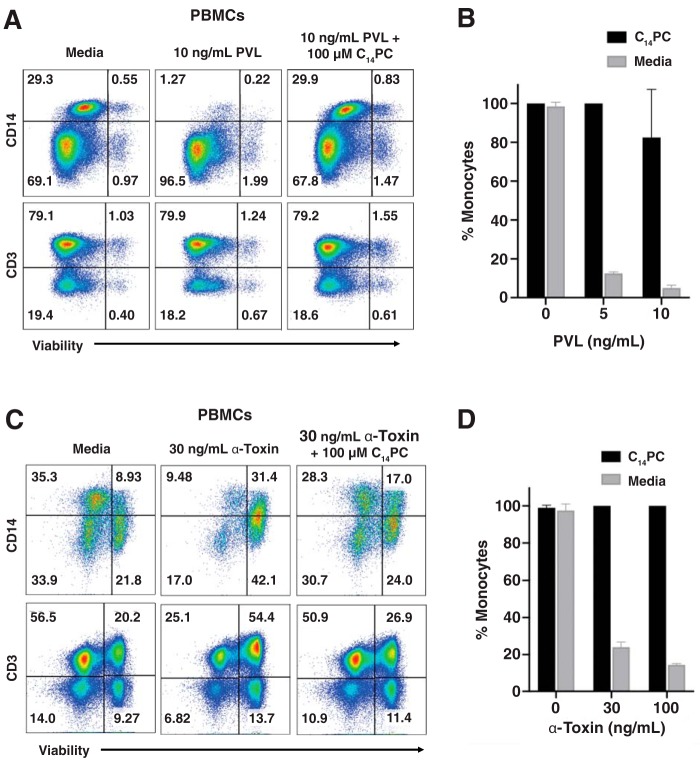
Figure 7.
C14PC protects primary human monocytes from lysis by both PVL and α-toxin. A, protective effect of C14PC against PVL-mediated killing of monocytes. PBMCs were challenged with PVL in the presence and absence of 100 μm C14PC. Monocyte subsets were gated based on the forward scatter and side scatter parameters and then analyzed for the expression of CD14+ and CD3+, respectively. Cell viability was determined by flow cytometry and normalized to that in the medium control. Data are representative of two independent experiments using blood from different donors. Percentages of cells in each quadrant are indicated. B, bar graph showing inhibition of the PVL-induced lysis of monocytes by 100 μm C14PC. Error bars, S.E. C, protective effect of C14PC against α-toxin–mediated killing of monocytes. PBMCs were challenged with α-toxin in the presence and absence of 100 μm C14PC. Monocyte subsets were identified and quantified as described in A. Data are representative of two independent experiments using blood from different donors. D, bar graph showing inhibition of the α-toxin–induced lysis of monocytes by 100 μm C14PC. Error bars, S.E.
Implication for MRSA drug discovery
The high prevalence of highly pathogenic MRSA is creating a crisis in modern healthcare due to the limited therapeutic options available, the toll of severe disease and mortality it inflicts, and the enormous cost of inpatient care to which it contributes (3, 4). The ability of MRSA to form biofilms on necrotic tissues and medical devices is also an important virulence mechanism that complicates infections (5, 6). As antibiotic resistance continues to emerge, disarming the major virulence mechanisms of MRSA strains has the potential to become an alternative therapeutic approach aimed at limiting host tissue damage while aiding immune clearance. The α-hemolysin subfamily of cytotoxins represents a prime target for antivirulence drug development, owing to their critical roles in inactivating host immune defenses, destroying tissue barriers, and modulating inflammatory responses (3, 16). mAbs targeting α-toxin have been shown to prevent human lung cell injury in vitro and protect experimental animals against lethal S. aureus pneumonia (65). Several such mAbs are currently in clinical trials, including mAbs MEDI4893 and KBSA301 (Refs. 66–69). Given the variability of MRSA immune evasion determinants, such single-target drugs are most likely to be inadequate to achieve a therapeutic effect (68). Our structural elucidation of the two conserved, adjacent PCho-binding pockets on the rim domains of α-toxin and the leukocidin F subunits will guide the rational development of PC analogs that prevent cytotoxin assembly and pore formation in the susceptible cell membrane, thereby blocking the cytolytic effects of this subfamily of proteins. Using a combined structural biology and pharmacological approach, we have been able to demonstrate that C14PC is a novel broad-spectrum inhibitor of PVL, LukED, and α-toxin in vitro. In light of the safety of miltefosine (hexadecylophosphocline, C16PC), an oral drug used for the treatment of leishmaniasis (70), we expect that C14PC will likewise be well-tolerated in humans. Considering its conserved mechanism of action and low production costs, C14PC may provide the basis for the development of prophylactic and therapeutic agents that reduce the virulence of MRSA infection.
Experimental procedures
Chemicals
All chemicals used were of analytical grade. Unless otherwise indicated, chemicals were purchased from Sigma–Aldrich. Detergents were from Anatrace.
Cloning and protein purification
The full-length LukD (residues 1–301), LukE (residues 1–283), LukF-PV (residues 1–301), LukS-PV (residues 1-284), and α-toxin (residues 1–293) constructs, excluding their signal peptides, were subcloned individually into a modified pET3a vector (Novagen). Site-directed mutagenesis was carried out using the Kunkel method. All constructs were verified by DNA sequencing. E. coli BL21(DE3)pLysS cells transformed with each plasmid were grown at 37 °C in Luria-Bertani medium until the A600 was between 0.6 and 0.8. IPTG was then added to a final concentration of 0.5 mm, and incubation was continued for 24 h at 16 °C. Cells were harvested by centrifugation; suspended in 50 mm sodium acetate buffer, pH 5.4, 25% sucrose, 5 mm EDTA, and 5 mm DTT; and lysed at 4 °C using an Avestin Emulsiflex C3 homogenizer. Inclusion bodies were isolated by centrifugation, washed twice with the same buffer, and subsequently incubated overnight at 4 °C in 50 mm sodium acetate buffer, pH 5.4, 5 mm DTT, and 6 m guanidine HCl or 8 m urea. Insoluble material was removed by centrifugation, and the protein solution was then dialyzed for 2 days at 4 °C against three changes of buffer A (50 mm sodium acetate, pH 5.4, and 1 mm EDTA). After removal of the insoluble material by centrifugation, the refolded recombinant toxin was loaded onto a CM-Sepharose CL-6B column equilibrated with buffer A and eluted using a linear gradient from 0 to 1 m NaCl. Fractions containing the recombinant toxin were pooled, dialyzed against buffer A, concentrated, and loaded onto a GE Mono S 5/50 GL equilibrated with buffer A, and the toxin was eluted using a linear gradient from 0 to 0.5 m NaCl. The toxin was further purified using size-exclusion chromatography on a GE Superdex 200 10/300 GL equilibrated with 50 mm sodium acetate, pH 5.4, 100 mm NaCl. Fractions containing the toxin were pooled, concentrated to ∼20 mg/ml, and stored at −80 °C until use. The concentration of the toxin in purified preparations was determined through UV absorbance measurements.
Crystallization
All crystallization experiments were performed at room temperature using the hanging-drop vapor diffusion method by mixing 1 μl of protein solution with an equal volume of precipitant solution. Crystals of LukD were grown from protein at 12 mg/ml in 10 mm sodium acetate, pH 5.4, and precipitant solution (20% PEG MME 2000, 10 mm NiCl2, and 0.1 m Tris-HCl, pH 8.5). For data collection, the crystals were cryoprotected with 15% glycerol in the mother liquor and then flash-cooled in liquid nitrogen. The C14PC-LukD complex was crystallized from protein at 10 mg/ml in 10 mm sodium acetate, pH 5.4, 10 mm C14PC, and 30 mm n-octyl-β-d-glucoside (βOG) and precipitant solution (28% PEG 400, 0.2 m MgCl2, and 0.1 m HEPES, pH 7.5). The crystals were flash-cooled by plunging directly into liquid nitrogen. Crystals of LukF-PV complexed with C14PC were grown from protein at 10 mg/ml in 10 mm sodium acetate, pH 5.4, 10 mm C14PC, and 30 mm βOG and precipitant solution (2.6 m ammonium sulfate, 5% PEG 400, and 0.1 m HEPES, pH 8.5). The crystals were flash-cooled in liquid nitrogen. The C14PC–α-toxinH35A complex was crystallized from protein at 10 mg/ml in 10 mm sodium acetate, pH 5.4, 5 mm C14PC, 40 mm βOG, and 0.4 mm deoxy-Big CHAP and precipitant solution (1.5 m ammonium sulfate, 0.25 m potassium sodium tartrate, and 0.1 m sodium citrate, pH 6.0). The crystals were transferred into stabilizing solution (2.25 m ammonium sulfate, 5% glycerol, 20 mm C14PC, and 0.1 m sodium citrate, pH 6.0) and then allowed to equilibrate against 3 m ammonium sulfate for 1 h at room temperature prior to flash-freezing in liquid nitrogen. The PVL heterooctamer in complex with C14PC was crystallized from LukF-PV at 6.7 mg/ml and LukS-PV at 6.3 mg/ml in 10 mm sodium acetate, pH 5.4, 15 mm C14PC, and 40 mm βOG and precipitant solution (0.16 m magnesium formate). The crystals were transferred into dehydrating solution (2.7 m ammonium sulfate, and 20 mm C14PC) and then allowed to equilibrate against 3 m ammonium sulfate for 3 h at room temperature prior to flash-freezing in liquid nitrogen. Crystals of the α-toxin heptamer-C14PC complex were grown from protein at 8 mg/ml in 10 mm sodium acetate, pH 5.4, 15 mm C14PC, and 30 mm βOG and precipitant solution (2 m ammonium sulfate, 0.2 m potassium sodium tartrate, and 0.1 m sodium citrate, pH 6.0). The crystals were flash-frozen in liquid nitrogen. The α-toxinH35A heptamer in complex with C14PC was crystallized from protein at 10 mg/ml in 10 mm sodium acetate, pH 5.4, 25 mm C14PC, and 40 mm βOG and precipitant solution (1.9 m ammonium sulfate, 0.25 m potassium sodium tartrate, and 0.1 m sodium citrate, pH 5.2). The crystals were flash-cooled in liquid nitrogen.
Data collection and structure determination
Diffraction data were collected at 100 K at beamline X4C at the National Synchrotron Light Source at Brookhaven National Laboratory, at the Cornell High Energy Synchrotron Source (CHESS) beamline F1, and at the Stanford Synchrotron Radiation Lightsource (SSRL) beamline 9-2. The diffraction data were processed with HKL-2000 (71). Initial phases were determined by molecular replacement using Phaser (72) with respective models of HlgB (PDB code 1LKF), LukF-PV (1PVL), α-toxinH35A (4YHD), the HlgAB heterooctamer (3B07), and the α-toxin heptamer (7AHL). Refinement was carried out in Refmac5 (73), alternating with manual rebuilding and adjustment in COOT (74). Coordinates for C14PC were generated using LibCheck (75). TLS refinement was performed in Refmac5 (76). Crystallographic data and refinement statistics are summarized in Table 1.
Structural analyses
Model quality was judged using the programs Rampage, Procheck, and Sfcheck (77–79). Protein-ligand contacts for the toxin-C14PC complex structures were analyzed using the program COOT (80). The r.m.s.d. values were calculated using the program SuperPose (81). Molecular and solvent-accessible surfaces were calculated with the AREAIMOL program (82) from the CCP4 suite (83). PyMOL (DeLano Scientific) was used to render structure figures.
Differential scanning calorimetry
Protein thermal stability was determined by differential scanning calorimetry (DSC) using a Nano-DSC model 602000 calorimeter (TA Instruments). Protein solutions in buffer A (20 mm sodium acetate, pH 5.8, and 50 mm NaCl) in the presence and absence of 4 mm PCho were subjected to a temperature increase of 1 °C/min from 0 to 100 °C under a pressure of 3 atm, and the evolution of heat was recorded as a differential power between reference (buffer A) and sample (10 μm protein in buffer A) cells. The resulting thermograms (after buffer subtraction) were used to derive thermal transition midpoints (Tm values). Fitting to the two-state scaled model provided in NanoAnalyze software was used to obtain a Tm value. The experiments were repeated two times with consistent results.
Isolation of human peripheral blood mononuclear cells
Blood samples were obtained from healthy, consenting donors as buffy coats (New York Blood Center) and leukopaks (AllCells, Alameda, CA). Human PBMCs were isolated from peripheral blood by density gradient centrifugation using Ficoll-Paque Plus (GE Life Sciences).
Cytolysis inhibition assay
Flow cytometry was used to assay permeabilization of the plasma membrane (pore formation) by LukED, PVL, and α-toxin in Jurkat cells and primary human immune cells as described previously (84). Briefly, C14PC (6–100 μm) was preincubated individually with different concentrations of the LukD and LukF-PV F subunits and α-toxin in a V-bottom 96-well plate for 30 min at 4 °C. These mixtures were then added to prestained PBMCs and incubated with the cognate LukE and LukS-PV S subunits for 1–1.5 h and with α-toxin for 24 h in a humidified 5% CO2 incubator at 37 °C. The cytotoxin-treated cells were stained with a viability dye and analyzed by FACS. IC50 values were calculated using GraphPad Prism by fitting data to single-slope dose-response curves constrained to 0 and 100% values.
Staining and FACS analysis
PBMCs were differentially stained with specific cell surface markers prior to intoxication to identify distinct cell populations. Antibodies used for flow cytometric staining included CD3-Alexa 532 (clone UCHT1) (eBioscience, San Diego, CA), CD4-Brilliant Violet 570, CD8-Pacific Blue, CD45RO-APCCy7, CD14-Alexa 700, CD27-PeCy7, CD244 (2B4)-Percp Cy5.5, CXCR1-APC (Biolegend, San Diego, CA), CCR5-PE (BD Biosciences), and CCR7-FITC (R&D Systems, Minneapolis, MN). After intoxication, cells were collected, washed with PBS, and stained with fixable viability dye eFluor 506 (eBioscience). Data were acquired on a BD LSRFortessa X-20 instrument (BD Biosciences) using FACSDiva software and the iQue Screener PLUS (Intellicyt) using ForeCyt Software or SP6800 Spectral Analyzer (Sony Biotechnology, CA). Data analysis was performed using FlowJo software (TreeStar Inc, Ashland, OR). Statistical analysis was performed using GraphPad Prism 8 software.
Data availability
All data are contained within the article. The atomic coordinates and structure factors (codes 6U33, 6U2S, 6U3F, 6U3T, 6U3Y, 6U49, and 6U4P) have been deposited in the Protein Data Bank.
Author contributions
J. L., L. K., D. U., and M. L. data curation; J. L. software; J. L., L. K., D. U., and M. L. formal analysis; J. L. and M. L. validation; J. L. visualization; J. L., L. K., D. U., and M. L. methodology; J. L., D. U., and M. L. writing-original draft; V. J. T. and M. L. resources; D. U. and M. L. investigation; M. L. conceptualization; M. L. supervision; M. L. funding acquisition; M. L. project administration; M. L. writing-review and editing.
Acknowledgments
We thank the beamline personnel at the Cornell High Energy Synchrotron Source and the Stanford Synchrotron Radiation Lightsource for data collection; J. Cai for participation and assistance in the early stage of the project; M. Zhang and Q. Li for technical assistance; and J. Nunberg, N. Kallenbach, and J. Lu for comments on the manuscript.
This work was supported by National Institutes of Health Grant AI094599 (to M. L.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- MRSA
- methicillin-resistant Staphylococcus aureus
- PVL
- Panton–Valentine leukocidin
- C14PC
- n-tetradecylphosphocholine
- LukED
- leukocidin ED
- LukAB
- leukocidin AB
- HlgAB
- γ-hemolysin
- PC
- phosphatidylcholine
- PCho
- phosphocholine
- Cα
- alpha carbon
- r.m.s.d.
- root mean square deviation
- PDB
- Protein Data Bank
- CPK
- space-filling calotte
- DSC
- differential scanning calorimetry
- PBMC
- peripheral blood mononuclear cell
- βOG
- n-octyl-β-d-glucoside.
References
- 1. Lowy F. D. (1998) Staphylococcus aureus infections. N. Engl. J. Med. 339, 520–532 10.1056/NEJM199808203390806 [DOI] [PubMed] [Google Scholar]
- 2. Klevens R. M., Morrison M. A., Nadle J., Petit S., Gershman K., Ray S., Harrison L. H., Lynfield R., Dumyati G., Townes J. M., Craig A. S., Zell E. R., Fosheim G. E., McDougal L. K., Carey R. B., et al. (2007) Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298, 1763–1771 10.1001/jama.298.15.1763 [DOI] [PubMed] [Google Scholar]
- 3. Otto M. (2010) Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Annu. Rev. Microbiol. 64, 143–162 10.1146/annurev.micro.112408.134309 [DOI] [PubMed] [Google Scholar]
- 4. Chambers H. F., and Deleo F. R. (2009) Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 7, 629–641 10.1038/nrmicro2200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jones S. M., Morgan M., Humphrey T. J., and Lappin-Scott H. (2001) Effect of vancomycin and rifampicin on meticillin-resistant Staphylococcus aureus biofilms. Lancet 357, 40–41 10.1016/S0140-6736(00)03572-8 [DOI] [PubMed] [Google Scholar]
- 6. Otto M. (2008) Staphylococcal biofilms. Curr. Top. Microbiol. Immunol. 322, 207–228 10.1007/978-3-540-75418-3_10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bowler P. G. (2018) Antibiotic resistance and biofilm tolerance: a combined threat in the treatment of chronic infections. J. Wound Care 27, 273–277 10.12968/jowc.2018.27.5.273 [DOI] [PubMed] [Google Scholar]
- 8. Liu C., Bayer A., Cosgrove S. E., Daum R. S., Fridkin S. K., Gorwitz R. J., Kaplan S. L., Karchmer A. W., Levine D. P., Murray B. E., Rybak M. J., Talan D. A., and Chambers H. F. (2011) Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin. Infect. Dis. 52, 285–292 10.1093/cid/cir034 [DOI] [PubMed] [Google Scholar]
- 9. Liu C., Bayer A., Cosgrove S. E., Daum R. S., Fridkin S. K., Gorwitz R. J., Kaplan S. L., Karchmer A. W., Levine D. P., Murray B. E., Rybak J. R., Talan D. A., Chambers H. F., and Infectious Diseases Society of America (2011) Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 52, e18–e55 10.1093/cid/ciq146 [DOI] [PubMed] [Google Scholar]
- 10. Han L. L., McDougal L. K., Gorwitz R. J., Mayer K. H., Patel J. B., Sennott J. M., and Fontana J. L. (2007) High frequencies of clindamycin and tetracycline resistance in methicillin-resistant Staphylococcus aureus pulsed-field type USA300 isolates collected at a Boston ambulatory health center. J. Clin. Microbiol. 45, 1350–1352 10.1128/JCM.02274-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mendes R. E., Sader H. S., Deshpande L. M., Diep B. A., Chambers H. F., and Jones R. N. (2010) Characterization of baseline methicillin-resistant Staphylococcus aureus isolates recovered from phase IV clinical trial for linezolid. J. Clin. Microbiol. 48, 568–574 10.1128/JCM.01384-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mangili A., Bica I., Snydman D. R., and Hamer D. H. (2005) Daptomycin-resistant, methicillin-resistant Staphylococcus aureus bacteremia. Clin. Infect. Dis. 40, 1058–1060 10.1086/428616 [DOI] [PubMed] [Google Scholar]
- 13. Wilson P., Andrews J. A., Charlesworth R., Walesby R., Singer M., Farrell D. J., and Robbins M. (2003) Linezolid resistance in clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 51, 186–188 10.1093/jac/dkg104 [DOI] [PubMed] [Google Scholar]
- 14. Willyard C. (2017) The drug-resistant bacteria that pose the greatest health threats. Nature 543, 15 10.1038/nature.2017.21550 [DOI] [PubMed] [Google Scholar]
- 15. Diekema D. J., Richter S. S., Heilmann K. P., Dohrn C. L., Riahi F., Tendolkar S., McDanel J. S., and Doern G. V. (2014) Continued emergence of USA300 methicillin-resistant Staphylococcus aureus in the United States: results from a nationwide surveillance study. Infect. Control Hosp. Epidemiol. 35, 285–292 10.1086/675283 [DOI] [PubMed] [Google Scholar]
- 16. Diep B. A., Gill S. R., Chang R. F., Phan T. H., Chen J. H., Davidson M. G., Lin F., Lin J., Carleton H. A., Mongodin E. F., Sensabaugh G. F., and Perdreau-Remington F. (2006) Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367, 731–739 10.1016/S0140-6736(06)68231-7 [DOI] [PubMed] [Google Scholar]
- 17. Gouaux E., Hobaugh M., and Song L. (1997) α-Hemolysin, γ-hemolysin, and leukocidin from Staphylococcus aureus: distant in sequence but similar in structure. Protein Sci. 6, 2631–2635 10.1002/pro.5560061216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alonzo F. 3rd, and Torres V. J. (2014) The bicomponent pore-forming leucocidins of Staphylococcus aureus. MMBR 78, 199–230 10.1128/MMBR.00055-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alonzo F. 3rd, Kozhaya L., Rawlings S. A., Reyes-Robles T., DuMont A. L., Myszka D. G., Landau N. R., Unutmaz D., and Torres V. J. (2013) CCR5 is a receptor for Staphylococcus aureus leukotoxin ED. Nature 493, 51–55 10.1038/nature11724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gauduchon V., Werner S., Prévost G., Monteil H., and Colin D. A. (2001) Flow cytometric determination of Panton-Valentine leucocidin S component binding. Infect. Immun. 69, 2390–2395 10.1128/IAI.69.4.2390-2395.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spaan A. N., van Strijp J. A. G., and Torres V. J. (2017) Leukocidins: staphylococcal bi-component pore-forming toxins find their receptors. Nat. Rev. Microbiol. 15, 435–447 10.1038/nrmicro.2017.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Olson R., Nariya H., Yokota K., Kamio Y., and Gouaux E. (1999) Crystal structure of staphylococcal LukF delineates conformational changes accompanying formation of a transmembrane channel. Nat. Struct. Biol. 6, 134–140 10.1038/5821 [DOI] [PubMed] [Google Scholar]
- 23. Pédelacq J. D., Maveyraud L., Prévost G., Baba-Moussa L., González A., Courcelle E., Shepard W., Monteil H., Samama J. P., and Mourey L. (1999) The structure of a Staphylococcus aureus leucocidin component (LukF-PV) reveals the fold of the water-soluble species of a family of transmembrane pore-forming toxins. Structure 7, 277–287 10.1016/S0969-2126(99)80038-0 [DOI] [PubMed] [Google Scholar]
- 24. Guillet V., Roblin P., Werner S., Coraiola M., Menestrina G., Monteil H., Prévost G., and Mourey L. (2004) Crystal structure of leucotoxin S component: new insight into the Staphylococcal β-barrel pore-forming toxins. J. Biol. Chem. 279, 41028–41037 10.1074/jbc.M406904200 [DOI] [PubMed] [Google Scholar]
- 25. Nocadello S., Minasov G., Shuvalova L., Dubrovska I., Sabini E., Bagnoli F., Grandi G., and Anderson W. F. (2016) Crystal structures of the components of the Staphylococcus aureus leukotoxin ED. Acta Crystallogr. D Struct. Biol. 72, 113–120 10.1107/S2059798315023207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sugawara T., Yamashita D., Kato K., Peng Z., Ueda J., Kaneko J., Kamio Y., Tanaka Y., and Yao M. (2015) Structural basis for pore-forming mechanism of staphylococcal α-hemolysin. Toxicon 108, 226–231 10.1016/j.toxicon.2015.09.033 [DOI] [PubMed] [Google Scholar]
- 27. Song L., Hobaugh M. R., Shustak C., Cheley S., Bayley H., and Gouaux J. E. (1996) Structure of staphylococcal α-hemolysin, a heptameric transmembrane pore. Science 274, 1859–1866 10.1126/science.274.5294.1859 [DOI] [PubMed] [Google Scholar]
- 28. Badarau A., Rouha H., Malafa S., Logan D. T., Håkansson M., Stulik L., Dolezilkova I., Teubenbacher A., Gross K., Maierhofer B., Weber S., Jägerhofer M., Hoffman D., and Nagy E. (2015) Structure-function analysis of heterodimer formation, oligomerization, and receptor binding of the Staphylococcus aureus bi-component toxin LukGH. J. Biol. Chem. 290, 142–156 10.1074/jbc.M114.598110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yamashita K., Kawai Y., Tanaka Y., Hirano N., Kaneko J., Tomita N., Ohta M., Kamio Y., Yao M., and Tanaka I. (2011) Crystal structure of the octameric pore of staphylococcal γ-hemolysin reveals the β-barrel pore formation mechanism by two components. Proc. Natl. Acad. Sci. U.S.A. 108, 17314–17319 10.1073/pnas.1110402108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yamashita D., Sugawara T., Takeshita M., Kaneko J., Kamio Y., Tanaka I., Tanaka Y., and Yao M. (2014) Molecular basis of transmembrane β-barrel formation of staphylococcal pore-forming toxins. Nat. Commun. 5, 4897 10.1038/ncomms5897 [DOI] [PubMed] [Google Scholar]
- 31. Valeva A., Pongs J., Bhakdi S., and Palmer M. (1997) Staphylococcal α-toxin: the role of the N-terminus in formation of the heptameric pore: a fluorescence study. Biochim. Biophys. Acta 1325, 281–286 10.1016/S0005-2736(96)00266-0 [DOI] [PubMed] [Google Scholar]
- 32. Walker B., and Bayley H. (1995) Key residues for membrane binding, oligomerization, and pore forming activity of staphylococcal α-hemolysin identified by cysteine scanning mutagenesis and targeted chemical modification. J. Biol. Chem. 270, 23065–23071 10.1074/jbc.270.39.23065 [DOI] [PubMed] [Google Scholar]
- 33. Yokota K., and Kamio Y. (2000) Tyrosine 72 residue at the bottom of rim domain in LukF crucial for the sequential binding of the staphylococcal γ-hemolysin to human erythrocytes. Biosci. Biotechnol. Biochem. 64, 2744–2747 10.1271/bbb.64.2744 [DOI] [PubMed] [Google Scholar]
- 34. Kawate T., and Gouaux E. (2003) Arresting and releasing Staphylococcal α-hemolysin at intermediate stages of pore formation by engineered disulfide bonds. Protein Sci. 12, 997–1006 10.1110/ps.0231203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dal Peraro M., and van der Goot F. G. (2016) Pore-forming toxins: ancient, but never really out of fashion. Nat. Rev. Microbiol. 14, 77–92 10.1038/nrmicro.2015.3 [DOI] [PubMed] [Google Scholar]
- 36. Berube B. J., and Bubeck Wardenburg J. (2013) Staphylococcus aureus α-toxin: nearly a century of intrigue. Toxins 5, 1140–1166 10.3390/toxins5061140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lina G., Piémont Y., Godail-Gamot F., Bes M., Peter M. O., Gauduchon V., Vandenesch F., and Etienne J. (1999) Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 29, 1128–1132 10.1086/313461 [DOI] [PubMed] [Google Scholar]
- 38. Gillet Y., Issartel B., Vanhems P., Fournet J. C., Lina G., Bes M., Vandenesch F., Piémont Y., Brousse N., Floret D., and Etienne J. (2002) Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 359, 753–759 10.1016/S0140-6736(02)07877-7 [DOI] [PubMed] [Google Scholar]
- 39. Diep B. A., Chan L., Tattevin P., Kajikawa O., Martin T. R., Basuino L., Mai T. T., Marbach H., Braughton K. R., Whitney A. R., Gardner D. J., Fan X., Tseng C. W., Liu G. Y., Badiou C., et al. (2010) Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc. Natl. Acad. Sci. U.S.A. 107, 5587–5592 10.1073/pnas.0912403107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tseng C. W., Biancotti J. C., Berg B. L., Gate D., Kolar S. L., Müller S., Rodriguez M. D., Rezai-Zadeh K., Fan X., Beenhouwer D. O., Town T., and Liu G. Y. (2015) Increased susceptibility of humanized NSG mice to panton-valentine leukocidin and Staphylococcus aureus skin infection. PLoS Pathog. 11, e1005292 10.1371/journal.ppat.1005292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Prince A., Wang H., Kitur K., and Parker D. (2017) Humanized mice exhibit increased susceptibility to Staphylococcus aureus pneumonia. J. Infect. Dis. 215, 1386–1395 10.1093/infdis/jiw425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Löffler B., Hussain M., Grundmeier M., Brück M., Holzinger D., Varga G., Roth J., Kahl B. C., Proctor R. A., and Peters G. (2010) Staphylococcus aureus Panton-Valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 6, e1000715 10.1371/journal.ppat.1000715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Woodin A. M. (1960) Purification of the two components of leucocidin from Staphylococcus aureus. Biochem. J. 75, 158–165 10.1042/bj0750158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ward P. D., and Turner W. H. (1980) Identification of staphylococcal Panton-Valentine leukocidin as a potent dermonecrotic toxin. Infect. Immun. 28, 393–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Spaan A. N., Henry T., van Rooijen W. J. M., Perret M., Badiou C., Aerts P. C., Kemmink J., de Haas C. J. C., van Kessel K. P. M., Vandenesch F., Lina G., and van Strijp J. A. G. (2013) The staphylococcal toxin Panton-Valentine leukocidin targets human C5a receptors. Cell Host Microbe 13, 584–594 10.1016/j.chom.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 46. Bhattacharya M., Berends E. T. M., Chan R., Schwab E., Roy S., Sen C. K., Torres V. J., and Wozniak D. J. (2018) Staphylococcus aureus biofilms release leukocidins to elicit extracellular trap formation and evade neutrophil-mediated killing. Proc. Natl. Acad. Sci. U.S.A. 115, 7416–7421 10.1073/pnas.1721949115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wilke G. A., and Bubeck Wardenburg J. (2010) Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus α-hemolysin-mediated cellular injury. Proc. Natl. Acad. Sci. U.S.A. 107, 13473–13478 10.1073/pnas.1001815107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bubeck Wardenburg J., and Schneewind O. (2008) Vaccine protection against Staphylococcus aureus pneumonia. J. Exp. Med. 205, 287–294 10.1084/jem.20072208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. DeLeo F. R., Kennedy A. D., Chen L., Bubeck Wardenburg J., Kobayashi S. D., Mathema B., Braughton K. R., Whitney A. R., Villaruz A. E., Martens C. A., Porcella S. F., McGavin M. J., Otto M., Musser J. M., and Kreiswirth B. N. (2011) Molecular differentiation of historic phage-type 80/81 and contemporary epidemic Staphylococcus aureus. Proc. Natl. Acad. Sci. U.S.A. 108, 18091–18096 10.1073/pnas.1111084108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Anderson M. J., Schaaf E., Breshears L. M., Wallis H. W., Johnson J. R., Tkaczyk C., Sellman B. R., Sun J., and Peterson M. L. (2018) α-Toxin contributes to biofilm formation among Staphylococcus aureus wound isolates. Toxins (Basel) 10, 157 10.3390/toxins10040157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Reyes-Robles T., Alonzo F. 3rd, Kozhaya L., Lacy D. B., Unutmaz D., and Torres V. J. (2013) Staphylococcus aureus leukotoxin ED targets the chemokine receptors CXCR1 and CXCR2 to kill leukocytes and promote infection. Cell Host Microbe 14, 453–459 10.1016/j.chom.2013.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Watanabe M., Tomita T., and Yasuda T. (1987) Membrane-damaging action of staphylococcal α-toxin on phospholipid-cholesterol liposomes. Biochim. Biophys. Acta 898, 257–265 10.1016/0005-2736(87)90065-4 [DOI] [PubMed] [Google Scholar]
- 53. Noda M., Kato I., Hirayama T., and Matsuda F. (1980) Fixation and inactivation of staphylococcal leukocidin by phosphatidylcholine and ganglioside GM1 in rabbit polymorphonuclear leukocytes. Infect. Immun. 29, 678–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Valeva A., Hellmann N., Walev I., Strand D., Plate M., Boukhallouk F., Brack A., Hanada K., Decker H., and Bhakdi S. (2006) Evidence that clustered phosphocholine head groups serve as sites for binding and assembly of an oligomeric protein pore. J. Biol. Chem. 281, 26014–26021 10.1074/jbc.M601960200 [DOI] [PubMed] [Google Scholar]
- 55. Ferreras M., Höper F., Dalla Serra M., Colin D. A., Prévost G., and Menestrina G. (1998) The interaction of Staphylococcus aureus bi-component γ-hemolysins and leucocidins with cells and lipid membranes. Biochim. Biophys. Acta 1414, 108–126 10.1016/S0005-2736(98)00160-6 [DOI] [PubMed] [Google Scholar]
- 56. Potrich C., Bastiani H., Colin D. A., Huck S., Prévost G., and Dalla Serra M. (2009) The influence of membrane lipids in Staphylococcus aureus γ-hemolysins pore formation. J. Membr. Biol. 227, 13–24 10.1007/s00232-008-9140-6 [DOI] [PubMed] [Google Scholar]
- 57. Galdiero S., and Gouaux E. (2004) High resolution crystallographic studies of α-hemolysin-phospholipid complexes define heptamer-lipid head group interactions: implication for understanding protein-lipid interactions. Protein Sci. 13, 1503–1511 10.1110/ps.03561104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Monma N., Nguyen V. T., Kaneko J., Higuchi H., and Kamio Y. (2004) Essential residues, W177 and R198, of LukF for phosphatidylcholine-binding and pore-formation by staphylococcal γ-hemolysin on human erythrocyte membranes. J. Biochem. 136, 427–431 10.1093/jb/mvh140 [DOI] [PubMed] [Google Scholar]
- 59. Liang X., Yan M., and Ji Y. (2009) The H35A mutated α-toxin interferes with cytotoxicity of Staphylococcal α-toxin. Infect. Immun. 77, 977–983 10.1128/IAI.00920-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nagle J. F., and Tristram-Nagle S. (2000) Lipid bilayer structure. Curr. Opin. Struct. Biol. 10, 474–480 10.1016/S0959-440X(00)00117-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. DuMont A. L., Yoong P., Liu X., Day C. J., Chumbler N. M., James D. B., Alonzo F. 3rd, Bode N. J., Lacy D. B., Jennings M. P., and Torres V. J. (2014) Identification of a crucial residue required for Staphylococcus aureus LukAB cytotoxicity and receptor recognition. Infect. Immun. 82, 1268–1276 10.1128/IAI.01444-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Menzies B. E., and Kernodle D. S. (1994) Site-directed mutagenesis of the α-toxin gene of Staphylococcus aureus: role of histidines in toxin activity in vitro and in a murine model. Infect. Immun. 62, 1843–1847 10.1128/IAI.62.5.1843-1847.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Krishnasastry M., Walker B., Braha O., and Bayley H. (1994) Surface labeling of key residues during assembly of the transmembrane pore formed by Staphylococcal α-hemolysin. FEBS Lett. 356, 66–71 10.1016/0014-5793(94)01240-7 [DOI] [PubMed] [Google Scholar]
- 64. Jursch R., Hildebrand A., Hobom G., Tranum-Jensen J., Ward R., Kehoe M., and Bhakdi S. (1994) Histidine residues near the N terminus of Staphylococcal α-toxin as reporters of regions that are critical for oligomerization and pore formation. Infect. Immun. 62, 2249–2256 10.1128/IAI.62.6.2249-2256.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ragle B. E., and Bubeck Wardenburg J. (2009) Anti-α-hemolysin monoclonal antibodies mediate protection against Staphylococcus aureus pneumonia. Infect. Immun. 77, 2712–2718 10.1128/IAI.00115-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Le V. T., Tkaczyk C., Chau S., Rao R. L., Dip E. C., Pereira-Franchi E. P., Cheng L., Lee S., Koelkebeck H., Hilliard J. J., Yu X. Q., Datta V., Nguyen V., Weiss W., Prokai L., et al. (2016) Critical role of α-toxin and protective effects of its neutralization by a human antibody in acute bacterial skin and skin structure infections. Antimicrob. Agents Chemother. 60, 5640–5648 10.1128/AAC.00710-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hua L., Cohen T. S., Shi Y., Datta V., Hilliard J. J., Tkaczyk C., Suzich J., Stover C. K., and Sellman B. R. (2015) MEDI4893* Promotes survival and extends the antibiotic treatment window in a Staphylococcus aureus immunocompromised pneumonia model. Antimicrob. Agents Chemother. 59, 4526–4532 10.1128/AAC.00510-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sause W. E., Buckley P. T., Strohl W. R., Lynch A. S., and Torres V. J. (2016) Antibody-based biologics and their promise to combat Staphylococcus aureus infections. Trends Pharmacol. Sci. 37, 231–241 10.1016/j.tips.2015.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yu X. Q., Robbie G. J., Wu Y., Esser M. T., Jensen K., Schwartz H. I., Bellamy T., Hernandez-Illas M., and Jafri H. S. (2017) safety, tolerability, and pharmacokinetics of medi4893, an investigational, extended-half-life, anti-Staphylococcus aureus α-toxin human monoclonal antibody, in healthy adults. Antimicrob. Agents Chemother. 61, e01020–16 10.1128/AAC.01020-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dorlo T. P., Balasegaram M., Beijnen J. H., and de Vries P. J. (2012) Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J. Antimicrob. Chemother. 67, 2576–2597 10.1093/jac/dks275 [DOI] [PubMed] [Google Scholar]
- 71. Otwinowski Z., and Minor W. (1997) Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 10.1016/S0076-6879(97)76066-X [DOI] [PubMed] [Google Scholar]
- 72. McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., and Read R. J. (2007) Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 10.1107/S0021889807021206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Murshudov G. N., Vagin A. A., and Dodson E. J. (1997) Refinement of macromolecular structures by the maximum-likehood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 10.1107/S0907444996012255 [DOI] [PubMed] [Google Scholar]
- 74. Emsley P., and Cowtan K. (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 10.1107/S0907444904019158 [DOI] [PubMed] [Google Scholar]
- 75. Vagin A. A., Steiner R. A., Lebedev A. A., Potterton L., McNicholas S., Long F., and Murshudov G. N. (2004) REFMAC5 dictionary: organization of prior chemical knowledge and guidelines for its use. Acta Crystallogr. D Biol. Crystallogr. 60, 2184–2195 10.1107/S0907444904023510 [DOI] [PubMed] [Google Scholar]
- 76. Winn M. D., Isupov M. N., and Murshudov G. N. (2001) Use of TLS parameters to model anisotropic displacements in macromolecular refinement. Acta Crystallogr. D Biol. Crystallogr. 57, 122–133 10.1107/S0907444900014736 [DOI] [PubMed] [Google Scholar]
- 77. Lovell S. C., Davis I. W., Arendall W. B. 3rd, de Bakker P. I., Word J. M., Prisant M. G., Richardson J. S., and Richardson D. C. (2003) Structure validation by Cα geometry: φ,ψ and Cβ deviation. Proteins 50, 437–450 10.1002/prot.10286 [DOI] [PubMed] [Google Scholar]
- 78. Vaguine A. A., Richelle J., and Wodak S. J. (1999) SFCHECK: a unified set of procedures for evaluating the quality of macromolecular structure-factor data and their agreement with the atomic model. Acta Crystallogr. D Biol. Crystallogr. 55, 191–205 10.1107/S0907444998006684 [DOI] [PubMed] [Google Scholar]
- 79. Laskowski R. A., MacArthur M. W., Moss D. S., and Thornton J. M. (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26, 283–291 10.1107/S0021889892009944 [DOI] [Google Scholar]
- 80. Emsley P., Lohkamp B., Scott W. G., and Cowtan K. (2010) Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 10.1107/S0907444910007493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Maiti R., Van Domselaar G. H., Zhang H., and Wishart D. S. (2004) SuperPose: a simple server for sophisticated structural superposition. Nucleic Acids Res. 32, W590–W594 10.1093/nar/gkh477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lee B., and Richards F. M. (1971) The interpretation of protein structures: estimation of static accessibility. J. Mol. Biol. 55, 379–400 10.1016/0022-2836(71)90324-X [DOI] [PubMed] [Google Scholar]
- 83. Winn M. D., Ballard C. C., Cowtan K. D., Dodson E. J., Emsley P., Evans P. R., Keegan R. M., Krissinel E. B., Leslie A. G., McCoy A., McNicholas S. J., Murshudov G. N., Pannu N. S., Potterton E. A., Powell H. R., et al. (2011) Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 10.1107/S0907444910045749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Alonzo F. 3rd, Benson M. A., Chen J., Novick R. P., Shopsin B., and Torres V. J. (2012) Staphylococcus aureus leucocidin ED contributes to systemic infection by targeting neutrophils and promoting bacterial growth in vivo. Mol. Microbiol. 83, 423–435 10.1111/j.1365-2958.2011.07942.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are contained within the article. The atomic coordinates and structure factors (codes 6U33, 6U2S, 6U3F, 6U3T, 6U3Y, 6U49, and 6U4P) have been deposited in the Protein Data Bank.