Abstract
Growth Differentiation Factor 15 (GDF15), also known as NSAID activated gene-1 (NAG-1), is associated with a large number of biological processes and diseases, including cancer and obesity. GDF15 is synthesized as pro-GDF15, is dimerized, and is cleaved and secreted into the circulation as a mature dimer GDF15. Both the intracellular GDF15 and the circulating mature GDF15 are implicated in biological processes, such as energy homeostasis and body weight regulation. Although there have been many studies on GDF15, GFRAL, a member of the glial-derived neurotropic factor receptor α family, has only been recently identified as a receptor for mature GDF15. In this review, we focused on cancer and energy homeostasis along with obesity and body weight, and the effect of the identification of the GDF15 receptor in these investigations. In addition, the therapeutic potential of GDF15 as a pharmacological agent in obesity and other metabolic diseases was discussed.
Keywords: Growth Differentiation Factor 15, non-steroidal anti-inflammatory drug activated gene-1, obesity, cancer prevention, GFRAL receptor complex, appetite suppression, energy metabolism, aging, survival
1. Introduction
In the last two decades, Growth Differentiation Factor 15 (GDF15 has received considerable attention by the medical field because of its multiple roles in several diseases, including cancer, cardiovascular disease, and obesity. GDF15 has been identified by several groups using different cloning strategies (Baek & Eling, 2006). GDF15 was named as NSAID activated gene-1 (NAG-1) (Baek, Kim, Nixon, Wilson, & Eling, 2001), macrophage inhibitory cytokine-1 (MIC-1) (Bootcov, et al., 1997), placental transformation growth factor-β (PTGFB) (P. X. Li, et al., 2000), and prostate derived factor (PDF) (Paralkar, et al., 1998). Recently, based on the crystal structures of the mature GDF15 and the mature GDF15-GFRAL extracellular domain complex, GDF15 was reclassified as a member of the Glial cell-derived neurotropic factor (GDNF) family with activity dependent on RET and not the TGF-β receptors (Hsu, et al., 2017). For this review article, we will refer to this protein as GDF15 that was initially characterized as a divergent member of the TGF-β superfamily (Hsiao, et al., 2000).
Similar to many other proteins, GDF15 is regulated at the level of transcription, translation, and even translocation in the cell. It is synthesized as a pro-GDF15 dimer in the cytoplasm, and subsequently, cleaved and secreted as the mature dimer GDF15. In addition, the pro-peptide GDF15, a cleavage product and unprocessed pro-GDF15 dimer, can bind to the extracellular matrix that can act as a deposit site (Bauskin, et al., 2005). The circulating serum levels of only the mature GDF15 can be easily measured and are very low in humans; however, they are dramatically increased in a large number of diseases, including cancer, cardiovascular disease, liver and kidney diseases, and tissue damage. In addition, the serum levels of GDF15 are very high during pregnancy; the placenta exhibits high levels of GDF15 (P. X. Li, et al., 2000). Age, smoking, stress, and environmental factors are other risk factors that may increase GDF15 levels, and thus, GDF15 has been proposed as a biomarker for many diseases and is considered a marker for all-cause mortality (Wiklund, et al., 2010). Indeed, increased serum levels of mature GDF15 are associated with the progression and prognosis of the diseases, such as cardiovascular diseases, diabetes, cancer, and many others (Bauskin, et al., 2006; T. Kempf, et al., 2012; X. Wang, Chen, & Zhang, 2016). GDF15 exhibits multifunctional and diverse biological activities associated with these diseases as reported in the literature; however, many of these studies are conflicting. For example, GDF15 has been reported to inhibit and enhance tumor development and progression as summarized in a recent review (X. Wang, Baek, & Eling, 2013). With the identification of GFRAL as the receptor for mature GDF15 and novel findings in several other recent publications, a careful review and reanalysis of published findings on GDF15 is necessary.
2. Biochemistry, molecular characterization, and regulation of expression
The human GDF15 gene is located in chromosome 19 and its cDNA has been isolated from mouse and canine (Hsiao, et al., 2000; Yamaguchi, Whitlock, et al., 2008). The promoter of human GDF15 has been characterized and possesses binding sites for several transcriptional factors, including p53, EGR-1, CREB, Sp1, CHOP, ER stress, and ATF3. GDF15 expression is also increased by the peroxisome proliferator-activated receptor (PPAR) γ ligands (Baek, Wilson, Hsi, & Eling, 2003; Chintharlapalli, Papineni, Baek, Liu, & Safe, 2005; Yamaguchi, Cekanova, et al., 2008) and the PI3K/AKT/GSK- 3β pathways (Baek, Kim, Nixon, DiAugustine, & Eling, 2004; Baek, et al., 2003; S.-H. Lee, et al., 2008; Yamaguchi, Lee, Eling, & Baek, 2004). The expression of GDF15 can also be regulated at the epigenetic level (Kadowaki, et al., 2012). Research, to date, has demonstrated that GDF15 expression is induced not only during diseases but can also be increased by NSAIDs (Baek, Wilson, Lee, & Eling, 2002) and a large number of compounds known to prevent the development of cancers (Baek, Kim, Jackson, et al., 2004; Lee, Cekanova, & Baek, 2008; Lee, Kim, Yamaguchi, Eling, & Baek, 2005; Lee, et al., 2006; Nualsanit, et al., 2012). In basal conditions, human GDF15 transcripts are highly expressed in placenta and at lower levels in the colon, kidney, and prostate (Paralkar, et al., 1998). Canine GDF15 is highly expressed in the liver and lung (Yamaguchi, Whitlock, et al., 2008), whereas human GDF15 is not expressed in the liver. On the other hand, mouse GDF15 is highly expressed in the liver and moderately in the kidney (Hsiao, et al., 2000), indicating a different distribution of basal GDF15 expression among species. However, phylogenetic tree analysis indicated that canine GDF15 is more closely related to that of the mouse than human in terms of tissue distribution.
Human GDF15 is synthesized as pro-GDF15, which then dimerizes through cysteine residues to form pro-GDF15 dimer, It is then cleaved at an RXXR site, forming a 112 amino acid C-terminal dimeric protein and a pro-peptide (X. Wang, et al., 2013). Based on GenBank database analysis, the RXXR sequence is conserved in human, mouse, rat, canine, and chimpanzee GDF15. The carboxy-terminal domain of GDF-15 contains the characteristic seven conserved cysteine residues necessary for the formation of the cysteine knot and the single interchain disulfide bond. The mature GDF15 contains two additional cysteine residues that form a fourth intrachain disulfide bond which is not found in the members of TGF-β superfamily (Hsu, et al., 2017). The crystal structures of the GDF15 and GDF15-GRAL receptor complex have been recently reported (Hsu, et al., 2017).
The mature dimeric protein is secreted into the extracellular matrix and can be found in human blood. Experimental evidence has clearly confirmed that the secreted mature dimer exhibited biological activity, but the biological activity of the pro-peptide and pro- GDF15 monomer and/or dimer, that is probably secreted, has not been well investigated. Although, pro-GDF15 is present both inside and outside of the cells, its fate and biological activity is unclear. With an effort to elucidate the biological activity of GDF15, we had recently reported that pro-GDF15 accumulates in the nucleus and contributes to transcriptional regulation (Min, Liggett, et al., 2016). These findings demanded the need for further elucidation of the molecular mechanisms which regulate the production, modification, secretion, and nuclear translocation of GDF15, which presumably affect its biologic activity inside and outside the cell. GDF15 is classified as a moonlighting protein that exhibits multiple biological functions depending on its location (Min, Lee, & Baek, 2016; Joo Heon Yoon, Ryu, & Baek, 2018).
3. Maturation and secretion of GDF15/NAG-1
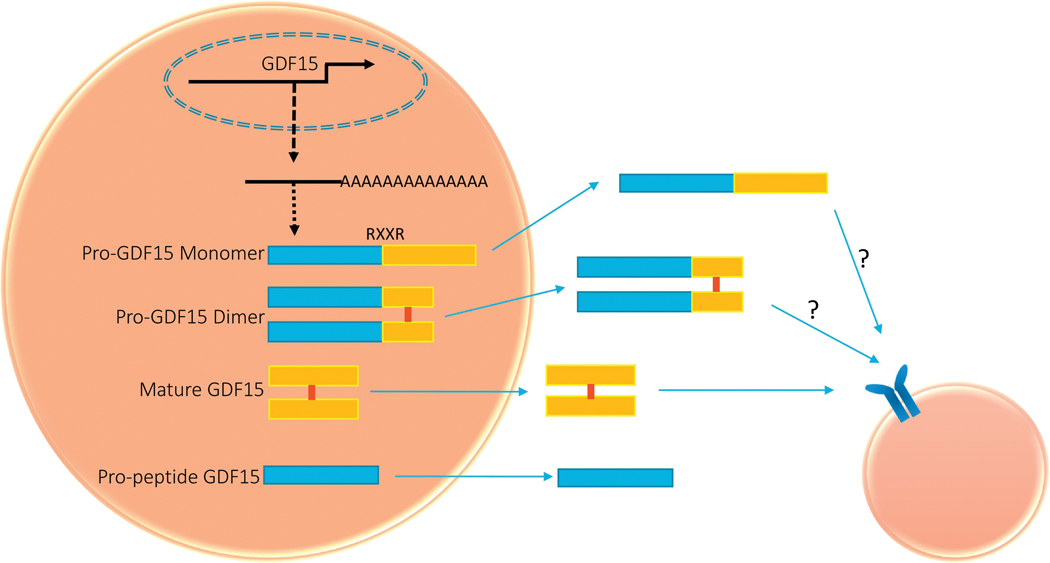
GDF15 was initially identified as a member of the TGF-β superfamily based on the similarity of its amino acid sequence to other members of this family of proteins (Bootcov et. al., 2007). Similar to other members of the TGF-β superfamily, GDF15 is formed as a full-length dimer protein and is cleaved at an RXXR site with the secretion of a mature dimeric protein that is assumed to be the active form of GDF15. The pro- GDF15 consists of 167 amino acids and contains an N-linked glycosylation site at amino acid position 70. It undergoes dimerization by a specific disulfide linkage to form the pro-GDF15 dimeric precursor (Bauskin, et al., 2000). The pro-protein dimer undergoes proteolytic cleavage catalyzed by furin-like protease at the amino acid target sequence RXXR to release C-terminal dimeric mature GDF15. The mature dimer and the pro-GDF15 are then secreted into the extracellular matrix (Figure 1). The pro-protein selectively binds to extracellular matrix and contributes to the latent storage in the stroma (Bauskin et al., 2005). GDF15 may exist in multiple forms within the cell: the pro- GDF15 monomer (~40 kDa), the pro-GDF15 dimer (~80 kDa), and the mature dimer (~30 kDa) (Figure 1). In addition, the GDF15 precursor protein contains an N-terminal signal peptide, important for its intracellular trafficking and secretion. Recently, pro- GDF15 has been identified as a substrate for PACE4, a subtilisin-like endoprotease that is overexpressed in prostate cancer. Inhibition of PACE4 reduced the serum levels of the mature GDF15 but increased the intracellular levels of the pro-GDF15 (Couture, et al., 2017). Moreover, proprotein convertase subtilisin/kexin type (PCSK) 3, 5, 6 are involved in GDF15 cleavage to produce mature GDF15 (J. J. Li, et al., 2018). However, whether these PCSK isoforms are essential for GDF15 maturation remains unclear, and inactivation of all three PCSK enzymes is likely needed to definitively address this question.
Figure 1.
Schematic diagram of various GDF15 proteins. GDF15 is synthesized in the cytoplasm with pro-GDF15 monomer, which then forms pro-GDF15 dimer. All the forms indicated are probably secreted into extracellular matrix, and, at least, mature GDF15 binds to receptor. The details are provided in the main text.
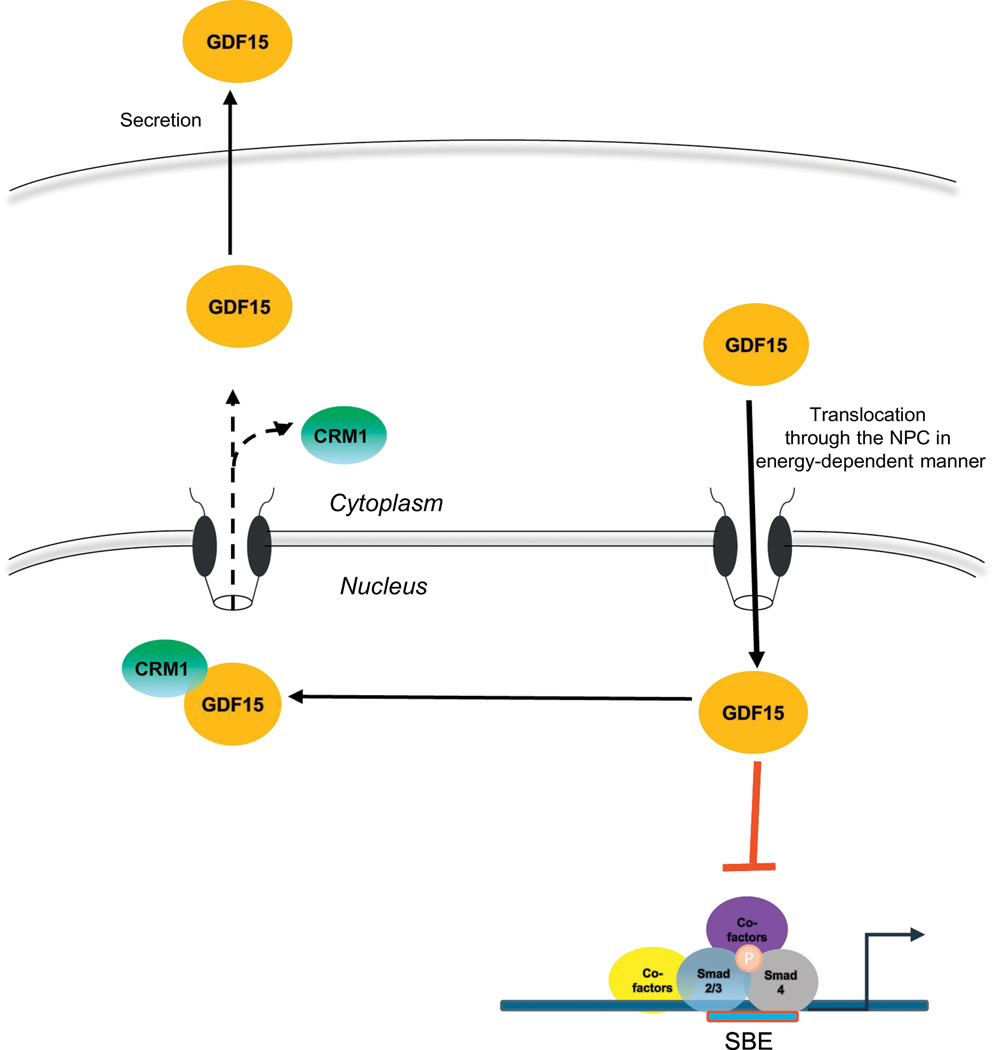
Pro-GDF15 contains a nuclear localization signal and nuclear export signal in its peptide sequence. Mature GDF15 is secreted out of the cell by an unknown secretory pathway. We recently reported that pro-GDF15 accumulates in the nucleus and controls transcriptional regulation (Min, Liggett, et al., 2016). Our results suggest that the cleavage of pro-GDF15 to its mature form and its subsequent secretion is dependent on translocation into the nucleus. Cytoplasmic pro-GFD15 is recognized by import machinery and is translocated into the nucleus via the nuclear pore complex in an energy-dependent manner. In the nucleus, pro-GDF15 interrupts the DNA binding capacity of the Smad complex leading to attenuation of TGF-β1-induced SMAD signaling pathway, thereby altering cell migration and invasion (Figure 2). Further analysis of nuclear pro-GDF15 revealed that pro-GDF15 also modifies Hippo pathway in cancer cells, thereby inhibiting cell migration and metastasis (unpublished data). Nuclear pro-GDF15 interacts with CRM1 to export out of the nucleus. Interestingly, blocking the nuclear export of pro-GDF15 by CRM1 inhibitor results in the inhibition of the secretion of mature GDF15 to the extracellular matrix. These results suggest that GDF15 must pass through the nucleus before its secretion via vesicle formation. These findings warrant further study of the molecular mechanisms by which this protein translocates from the nucleus to the extracellular matrix, affecting biological activity through protein production, modification, and translocation. Our publication is the first study demonstrating the critical importance of GDF15 nuclear translocation in secretion of its mature dimer and the first report confirming the biological activity for pro-GDF15 in the nucleus. Because GFRAL has been identified as the receptor for mature GDF15 and is involved in energy homeostasis, it is necessary to elucidate whether nuclear pro- GDF15 exhibits biological activities different from the GFRAL-mediated effects.
Figure 2.
GDF15 translocation in the cells. GDF15 is translocated into the nucleus, and then, exported to cytoplasm. Finally, GDF15 is secreted into the extracellular matrix. CRM1 (green) participates in GDF15 translocation from nucleus to cytoplasm, whereas importin may participate in GDF15 translocation from cytoplasm to nucleus. Nuclear GDF15 may inhibit SMAD activity that regulates several genes associated with metastasis.
4. Identification and characterization of the mature GDF15 receptor
A number of studies have reported that various TGF-β receptors are activated by GDF15 but the results of these studies could be compromised by the presence of a TGF-β contaminants in the recombinant GDF15 preparations and resulting in inconsistent findings (Olsen, Skjærvik, Størdal, Sundan, & Holien, 2017). Moreover, direct binding of GDF15 to known TGF-β receptors could not be confirmed, and led to search for the receptor for the secreted form of GDF15 by four independent groups (Emmerson, et al., 2017; Hsu, et al., 2017; Shannon E. Mullican & Rangwala, 2018; L. Yang, et al., 2017). The GDF15 receptor, a member of the glial-cell-derived neurotropic factor family (GDNF), is GDNF family receptor α-like (GFRAL) protein, which is a transmembrane protein that binds exclusively to GDF15 with high affinity. GFRAL binds to the tyrosine kinase co-receptor, RET, and binding of GDF15 to this complex induces phosphorylation of RET as well as intracellular signaling molecules, AKT, ERK1/2, and phospholipase C (PLCγ) (Shannon E. Mullican & Rangwala, 2018; L. Yang, et al., 2017). The signaling pathway for GDF15/GFRAL is still not fully understood and requires additional studies to correlate the GDF15 receptor to the biological activities attributed to mature GDF15. The crystal structure of GDF15 has been solved, which revealed disulfide-bonding configuration, not reported for other TGF-β family members. The crystal structure of the GDF15/GFRAL has also been elucidated, which shows the molecular interactions between specific amino acid residues of GDF15 and GFRAL. The molecular basis for GDF15 activity is via GFRAL/RET and not via TGF-β signaling (Hsu, et al., 2017).
The expression of GFRAL is essentially limited to the CNS, particularly in the brainstem of mice, rats, monkeys, and humans (S. E. Mullican, et al., 2017) in the area postrema (AP) with inconsistent results of expression in the nucleus of solitary tract (NTS). The co-receptor, RET, was expressed in the AP and NTS with additional expression observed in several regions of the hypothalamus (Hsu, et al., 2017). The expression of GFRAL was undetectable in peripheral tissues except for testis (S. E. Mullican, et al., 2017; L. Yang, et al., 2017). Furthermore, the expression of GFRAL was very low or undetectable in 30 cell lines (S. E. Mullican, et al., 2017), many of which were reported to exhibit a biological response after treatment with GDF15. The absence of the GDF15 receptor in peripheral tissue and in these cell lines coupled with the problem of contamination in rGDF15 preparations render the validity of many of these findings uncertain.
5. Role of GDF15 in cancer: Pro-cancer and Anti-cancer activities
Transgenic mice (GDF15Tg+/BL6, C57/BL6 background, and GDF15Tg+/FVB, FVB background) expressing human GDF15 have been developed by our group, (Baek, et al., 2006) and we have found that GDF15Tg+/BL6and GDF15Tg+/FVB mice are resistant to chemically-and genetically-induced intestinal polyp and lung tumor formation, respectively. Approximately 50% reduction in polyps was observed after azoxymethane treatment of GDF15Tg+/BL6mice, and we observed a 40% decrease in polyp formation in the intestine by crossing GDF15Tg+/BL6mice with ApcMin+ mice, as compared to control littermates. We have also found that the overexpressed human GDF15 protein in GDF15Tg+/FVB mice inhibited the formation of induced lung tumors and lung inflammation via downregulation of the p38MAPK signaling pathway, and induced apoptosis through the activation of caspase 3/7 (Cekanova, et al., 2009). A number of other studies, including ours, have described an anti-tumorigenic function for GDF15, in which it induces apoptosis and may negatively affect tumor growth (Baek, Kim, et al., 2001; Baek, et al., 2006; Chintharlapalli, et al., 2005; Jutooru, Chadalapaka, Chintharlapalli, Papineni, & Safe, 2009; Kelly, Scott Lucia, & Lambert, 2009; Tsui, et al., 2015; H. Yang, Filipovic, Brown, Breit, & Vassilev, 2003). These results have indicated that GDF15 acts as a tumor suppressor gene in colorectal and lung tumorigenesis (Baek & Eling, 2006). In contrast, many studies have observed major upregulation of GDF15 mRNA and protein expression in cancer biopsies (Nakamura, et al., 2003; Senapati, et al., 2010). Many studies have suggested GDF15 expression per se in cancer but do not elucidate the mechanism of GDF15-induced tumorigenesis. During cancer development and progression, the role of GDF-15 has remained controversial, depending on the tumor entity and models investigated. Below are some examples of contradictory results for GDF15, including GDF15 functional role in tumorigenesis.
5–1. Lung cancer:
GDF15 is downregulated in non-small cell lung cancer in vivo and in vitro and correlates with poor survival. Overexpression of GDF15 significantly represses NSCLC cell proliferation and induces apoptosis. Further mechanistic studies have revealed that GDF15 expression is subjected to epigenetic repression mediated by EZH2 in NSCLC. Inhibition of EZH2 expression prevented its binding to the GDF15 promoter region and reduced the trimethylation modification pattern of H3K27; an increase in GDF15 expression was observed (Lu, et al., 2018). An anticancer compound, Taiwanin A, also exhibited GDF15 induction in human lung carcinoma (Harn, et al., 2014). These findings are consistent with a tumor suppressor role for GDF15 in non-small cell lung cancer. Together, GDF15 is a direct target of EZH2 and, as a regulator of proliferation, might serve as a candidate prognostic biomarker and target for novel therapies in human NSCLC. We have also found that the overexpressed GDF15 protein in GDF15Tg+/FVB mice (FVB background) inhibited the formation of urethane-induced lung tumors mediated by reduced lung inflammation via downregulation of the p38 MAPK signaling pathway, and induced apoptosis through the activation of caspase 3/7 (Cekanova, et al., 2009). In contrast, GDF15 has been reported to facilitate A549 cell proliferation via GCN5-dependent KLF5 acetylation (Zhao, et al., 2018). They reported an increase in GDF15 expression in the tumors based on immunohistochemistry, and decrease in tumor formation in A549 cells after silencing of GDF15 as examined by a xenograft tumor assay. It should be noted that A549 cells do not express the GDF15 receptor. Taken together, these findings suggested that GDF15 functions as a tumor suppressor or tumor promoter in NSCLC, depending on the method and cell context.
5.2. Colon cancer:
GDF-15 has been reported as an effective biomarker in patients with metastatic colorectal cancer with the same sensitivity as CEA (Vocka, et al., 2016), and in vitro assay suggested that GDF15 promotes EMT and metastasis in colorectal cancer cells (C. Li, et al., 2015). In contrast to the results from in vitro assays, studies conducted in GDF15 transgenic mice (GDF15-Tg) and GDF15 knock-out mice consistently demonstrated anti-tumorigenic activity associated with expression of this protein (Baek, et al., 2006; Zimmers, Gutierrez, & Koniaris, 2010). These results indicated that GDF15 is a potential tumor suppressor gene at least, in part, in an animal model commonly used in colorectal cancer investigations (Baek & Eling, 2006).
5.3. Prostate cancer:
Like other cancer patients, prostate cancer patients also exhibit high levels of GDF15 in their serum (J. Li, Veltri, Yuan, Christudass, & Mandecki, 2015). Further, combining analysis of GDF15 with PSA in patient serum provides a more promising diagnostic tool for prostate cancer patients (Bansal, et al., 2017). The activation of kinase signaling pathway by GDF15 have also been reported in prostate cell culture system, indicating that GDF15 seems to affect several kinase pathways, including EGFR, pAKT, and FAK-RhoA, in prostate cancer cells (Mimeault, Johansson, & Batra, 2012). However, the expression of GDF15 receptor needs to be validated in these studies. In contrast, studies from the TRAMP mice with deleted germ line GDF15 gene reported increased local tumor growth, resulting in decreased survival consistent with an overall protective role for GDF15 in early primary tumor development. However, in advancing prostate disease, GDF15 overexpression may promote local invasion and metastatic spread (Yasmin Husaini, et al., 2015).
5.4. Breast cancer:
GDF15 promotes the acquisition of cancer stem cell-like properties in breast cancers (Sasahara, et al., 2017), and confers resistance to HER2- targeted therapy in breast cancer (Joshi, Brown, Griner, & Nahta, 2011). In addition, GDF15 promotes EMT and invasiveness of breast cancers through insulin-like growth factor-1 receptor (IGF-1R) signaling and transcription factor, FoxM1, upregulation (Peake, Eze, Yang, Castellino, & Nahta, 2017). These data suggested that the GDF15 contributes to breast cancer progression, in part, by activating signaling pathways that control EMT and cellular invasion. In contrast, it has been reported that oncogenic protein, YAP, promotes metastasis of breast cancer cells by repressing GDF15 transcription, indicating that GDF15 plays a role in anti-metastasis (T. Wang, et al., 2018). The anti-cancer activity of several anti-cancer compounds, including 5F-203 and prodigiosin (2-methyl-3-pentyl-6-methoxyprodigiosene), is mediated, in part, by GDF15 expression (Martinez, et al., 2006; Soto-Cerrato, Viñals, Lambert, Kelly, & Pérez- Tomás, 2007).
5.5. Cervical Cancer:
Recently, GDF15 has been reported to be involved in cervical carcinogenesis, activating the PI3K/AKT and MAPK/ERK signaling pathways in complex with ErbB2, which then alters the expression of cell cycle regulators, including p21, CDK2/4, and CyclinD1/E1, and ultimately promotes cell proliferation in human cervical cancer (S. Li, Ma, Zheng, & Zhang, 2018). These results suggested that GDF15 activates the PI3K/AKT and MAPK/ERK signaling pathways primarily via ErbB2 in cervical cancer. There has been no study indicating anti-cancer activity of GDF15 in cervical cancer.
5.6. Brain cancer:
Glioblastoma multiforme (GBM) is a grade IV astrocytoma with a median survival of 12 months despite current multi-modal treatment options. Similar to other cancers, the data have been contradictory for brain cancer. There have been several studies that suggest that GDF15 may act as an oncogene. Increased GDF15 levels have been found in the blood of glioblastoma patients and endogenous levels of GDF15 contribute to proliferation and immune escape of malignant gliomas in an immunocompetent host (Roth, et al., 2010). In addition, patients with glioblastoma and higher levels of GDF15 in cerebrospinal fluid exhibited a poorer survival (Shnaper, et al., 2009) supporting a pro-tumorigenic role for GDF15 in brain cancer. In contrast, GDF15 expression in glioblastoma cell lines is significantly lower than that in benign glioma cells and normal human astrocytes (Kadowaki, et al., 2012). In GBM, MG132 exhibited increased phosphorylation of the p38 MAPK pathway, and the induction of GDF15 by p38 MAPK is a potential contributor to the anti-glioblastoma activity of proteasome inhibitors (Shimizu, et al., 2013). Since PI3K/AKT and Smad2/3 signaling cascades exhibit opposing effects in GDF15-induced glioblastoma cell apoptosis, the different results of GDF15 in brain cancer may be due to the opposite effects of PI3K/AKT and Smad2/3 signaling cascades. Lower levels of Smad3 may lead to the loss of apoptosis sensitivity in response to GDF15 in some glioblastoma cell lines (Z. Zhang, et al., 2014).
5.7. Pro-cancer and anti-cancer activities of GDF15:
The role of GDF15 is contradictory in some cancers as summarized in Table 1. In general, the anti-tumorigenic effect or prevention of tumor development was observed in transgenic mice expressing GDF15. For studies in mice deficient in GDF15 the results have been consistent. However, TRAMP mice expressing mGDF15 under the control of macrophage specific colony stimulating factor-1 promoter exhibited smaller prostate tumors and an increase in metastases (Y. Husaini, et al., 2012). Transgenic mice exhibit an increase in life span whether maintained on a regular diet or a high fat diet; at 90 weeks of age, no tumors were detected (Wang et al., 2014). Similarly, obese mice, treated for 1 year with adenovirus expressing hGDF15, showed no detrimental effects of the GDF15 expression (Xiong et al., 2017). In contrast, most of the results for a pro-cancer activity for GDF15 are from in vitro experiments that use cultured cells. The absence of expression of the GDF15 receptor, GFRAL, in these cells and contamination of commercial preparation of mature GDF15 raises questions on the validity of some of these findings. In other studies, the endogenous formation of GDF15 was reduced by the use of short hairpin GDF15 constructs resulting in an increase in biological activity associated with its pro-cancer effects (C. Li, et al., 2015). The exclusive effect of the constructs on GDF15 biosynthesis needs to be confirmed. Some possible explanations of the contradictory in vitro activity include: 1) GDF15 may have different functions in different cancer cells, 2) an unidentified activity of pro-GDF15 or other forms of GDF15 in cancer cells, and 3) the presence of active monomers of GDF15 in cancer cells. The current GDF15 antibodies do not distinguish between the monomeric and dimeric GDF15. Therefore, additional investigations need to be conducted to elucidate the role of GDF15 in cancer.
Table 1.
Summary of GDF15’s role in cancer. Each reference includes functional study of GDF15 either in vivo or in vitro. Anti, anti-proliferation activity; Pro, Cell Promotion.
| In Vitro vs In Vivo | Role | Use of Recombinant GDF15 | Reference | |
|---|---|---|---|---|
| Lung Cancer | Urethane-induced lung tumorigenesis model in GDF15 Transgenic mice | Anti | No | (Cekanova, et al., 2009) |
| SPCA1 and H1299 cells | Anti | No | (Lu, et al., 2018) | |
| A549 and H460 cells | Anti | No | (Harn, et al., 2014) | |
| H460 cells | Anti | No | (Kadara, Schroeder, Lotan, Pisano, & Lotan, 2006) | |
| A549 cells | Pro | No | (Zhao, et al., 2018) | |
| Colorectal cancer | HT29 and SW480 cells | Pro | Yes | (C. Li, et al., 2015) |
| HCT-116 cells | Anti | No | (Baek, Kim, Nixon, Wilson, & Eling, 2001) | |
| GDF15 knock-out/MIN mouse | Anti | No | (Zimmers, Gutierrez, & Koniaris, 2010) | |
| Azoxymethane-induced mouse model and MIN mouse model in GDF15 Tg mice | Anti | No | (Baek, et al., 2006) | |
| Prostate Cancer | WPE1-NB26 cells/Immunohistochemistry | Pro | No | (Mimeault, Johansson, & Batra, 2012) |
| TRAMP mice model | Anti/Pro | No | (Yasmin Husaini, et al., 2015) | |
| Breast Cancer | BT474 breast cancer cells/ Immunohistochemistry | Pro | Yes | (Peake, Eze, Yang, Castellino, & Nahta, 2017) |
| MCF7/BT474/BT20 breast cancer cells. | Pro | Yes | (Sasahara, et al., 2017) | |
| MCF7 | Anti | No | (Martinez, et al., 2006) | |
| MCF7/MDA-MB-231 | Anti | No | (Soto-Cerrato, Vin als, Lambert, Kelly, & Pe rez- Toma s, 2007 | |
| Cervical cancer | HeLa/HT-3 cervical cancer cells | Pro | Yes | (S. Li, Ma, Zheng, & Zhang, 2018) |
| Brain Cancer | SMA-560 Brain cancer cells | Pro | Yes | (Roth, et al., 2010) |
| U87 brain cancer cells | Anti | No | (Shimizu, et al., 2013) |
6. GDF15 and the regulation of body weight
The regulation of body weight and energy homeostasis by GDF15 is now supported and confirmed by a considerable amount of experimental and clinical data. GDF15’s effect on mice body weight was first observed in 2006, with ubiquitous tissue expression of the full-length human GDF15 protein (hGDF15) and circulating levels of the mature or secreted hGDF15 (20–50 ng/mL) (Baek, et al., 2006). Expression of hGDF15 has profound biological effects on body weight and fat content as determined by Piximus image analysis. A comparison between wild-type littermates and transgenic male mice at 11 weeks of age is shown in Table 2. Despite these changes in body weight no difference in food consumption was observed (Baek, et al., 2006; Chrysovergis, et al., 2014).
Table 2.
Positive and Negative Health Effects of GDF15 observed in mice.
| Health effects | Methods | Positive vs Negative | References |
|---|---|---|---|
| Longer life span | Tg | Positive | (Wang, et al., 2014) |
| Lower body weight | Tg | Positive | (Baek, et al., 2006; Chrysovergis, et al., 2014; Wang, et al., 2014) |
| KO | Positive | (Tsai, et al., 2013) | |
| rGDF15 | (Mullican, et al., 2017) | ||
| Lower adipose tissue weights-WAT, BAT, eWAT, iWAT | Tg | Positive | (Chrysovergis, et al., 2014) |
| Improved glucose tolerance and insulin sensitivity | KO | Positive | (Tran, Yang, Gardner, & Xiong, 2018) |
| Tg | Positive | (Chrysovergis, et al., 2014) | |
| Lower LDL and Lower total cholesterol | rGDF15 | Positive | (Ackermann, Bonaterra, Kinscherf, & Schwarz, 2018) |
| Tg | Positive | (Chrysovergis, et al., 2014) | |
| Inflammation | Tg | Positive: lower expression of inflammatory cytokines, IL-18, IL-1β,TNFα, KC, IL-6, MCP-1. | (J. M. Kim, et al., 2013) |
| Tg | Positive: Reduced Lysozyme in Tg mice | (Cekanova, et al., 2009) | |
| GDF15 KO/ApoE KO | Negative: Involved in atherosclerotic lesion progression | (Bonaterra, et al., 2012) | |
| Lower macrophage infiltration into fat, liver | KO | Positive | (K. H. Kim, et al., 2018) |
| Lower leptin levels | Tg | Positive | (J. M. Kim, et al., 2013) |
| Change/increase in energy homeostasis with shift from carbohydrate to lipid metabolism | Ad-GDF15 | Positive | (M. Zhang, Sun, Qian, & Tang, 2018) |
| Inhibits tumor development- Intestinal and Lung cancer | Tg | Positive | (Baek, et al., 2006; Cekanova, et al., 2009) |
| Protects against cardiac hypertrophy (enlarged heart) | Tg | Positive | (Y. Zhang, et al., 2017) |
| Increase in tramp prostate mice metastasis | KO | Negative | (Y. Husaini, et al., 2012) |
Subsequent studies with the transgenic mice confirmed the lower body weight and further characterized this phenotype. The ubiquitous expression of hGDF15 in mice did not decrease food consumption but did improve glucose tolerance, insulin sensitivity, and lowered body weight, insulin, glucose levels, serum IGF-1 levels, leptin levels, and adipose tissue (Chrysovergis, et al., 2014). It also reduced the inflammatory response, increased oxidative metabolism, and energy expenditure (J. M. Kim, et al., 2013). The expression of hGDF15 protected the mice from glucose intolerance, prevented lower insulin sensitivity, prevented liver steatosis, and prevented obesity in genetically induced and high fat diet-induced obese models (Chrysovergis, et al., 2014; J. M. Kim, et al., 2013; X. Wang, et al., 2014). In addition, the lifespan of the hGDF15 transgenic mice is significantly longer for mice on either normal or high-fat diet (X. Wang, et al., 2014) suggesting that GDF15 is a regulator of mammalian longevity acting as a survival factor.
A major advancement in understanding the biological activity of mature GDF15 was the identification of the GDF15 receptor (GFRAL). Four independent groups identified the GFRAL, a member of GDNF-family (GDNF), as the receptor for the secreted or mature form of GDF15 (Emmerson, et al., 2017; Hsu, et al., 2017; S. E. Mullican, et al., 2017; L. Yang, et al., 2017). After binding of GDF15 to GFRAL, it forms a complex with RET and triggers the phosphorylation of RET, ERK, ribosomal protein S6 (pS6), and PLCγ. Administration of GDF15 to wild-type mice and rats results in reduced body weight, fat, serum leptin levels, and serum insulin levels; along with lower food intake and blood glucose, thus shifting the metabolism from carbohydrate to lipid oxidation. These responses were reported for wild-type mice and were not found in GFRAL deficient mice (GFRAL−/−). GFRAL (−/−) mice did not differ from wild-type mice in terms of body weight or fat content when on a regular diet and have similar energy expenditure (Shannon E. Mullican & Rangwala, 2018; L. Yang, et al., 2017). Higher body weight and fat content were observed as expected, when GFRAL (−/−) mice were placed on a high- fat diet (S. E. Mullican, et al., 2017); however, other investigators have not observed these differences (Emmerson, et al., 2017). Based on results from these investigations, the GFRAL receptor is clearly required for the body weight decrease, food intake reduction, and the metabolic effects observed after the pharmacological administration of mature GDF15 (Emmerson, et al., 2017; Hsu, et al., 2017; S. E. Mullican, et al., 2017; L. Yang, et al., 2017) or after treatment with cisplatin which increased serum levels of GDF15 (Hsu, et al., 2017). The results from other studies (V. W.-W. Tsai, et al., 2013) and from studies with GFRAL (−/−) mice support the hypothesis that the reduction in food, intake mediated by activation of GFRAL, is responsible for the decrease in body weight when GDF15 is administrated. Furthermore, the rats and mice treated with rGDF15 lost the same amount of body weight and fat, supporting the hypothesis that a decrease in food intake is responsible for lower bodyweight (S. E. Mullican, et al., 2017; L. Yang, et al., 2017).
Mice deficient in GDF15 have been reported to exhibit a modest increase in body weight and fat content as compared to wild-type mice. Female GDF15 (−/−) mice, but not male GDF15 (−/−) mice, exhibited an increase in food intake and lower total energy expenditure (V. W.-W. Tsai, et al., 2013). Furthermore, little differences in body weight, glucose and insulin tolerances, fat content, and energy expenditure were observed between controls and GDF15 (−/−) mice fed with a high-fat diet for 6 weeks (Chung, et al., 2017). In another study, wild type and GDF15 (−/−) male mice had similar weights; however, compared to wild-type mice, the male GDF15 (−/−) exhibited a greater weight gain, higher glucose levels, and higher insulin levels on a high-fat diet (Tran, Yang, Gardner, & Xiong, 2018). Similar results were obtained for female mice but the difference between the wild type and GDF15 (−/−) female mice were smaller. For male mice, oxygen consumption and heat production were lower in the GDF15 (−/−) male mice, indicating a lower metabolic rate, whereas a lower metabolic rate was reported in GDF15 (−/−) female mice; however, the difference is not statistically significant. These findings are consistent with the low concentration (0.42 pM) (Chung, et al., 2017) of serum levels of GDF15 in wild-type mice on a regular diet. This concentration is below the ED50 reported for activation of the GFRAL receptor [1 × 10−10 M (L. Yang, et al., 2017), 10 pM (Hsu, et al., 2017), 0.4 nM (S. E. Mullican, et al., 2017)]. Modest differences in weight and responses between wild-type mice and GDF15 (−/−) mice suggest that regulation of body weight due to GDF15 is not a normal mechanism of weight control but only occurs under conditions of stress, which causes increase in expression of GDF15.
Besides experiments using transgenic and/or knock-out mice, xenograft mouse model with engineered tumor cells expressing GDF15 and mice injected with recombinant GDF15 (rGDF15) have been used to increase the GDF15 serum levels in mice. Johnen et al. (2007) reported that mice with xenografts of DU-145 prostate tumor cells, expressing GDF15, exhibited a lower body weight, less adipose tissue, and decrease in food intake (Johnen, 2007). Obese C57BL6 with xenograft of B16/F10 melanoma cells lost weight as the tumor grew and the serum levels of GDF15 increased with no changes in food intake (Chrysovergis, et al., 2014). The expression of lipolysis genes in the white adipose tissue (WAT) and the expression of thermogenic genes in brown adipose tissue (BAT) were increased from mice with xenografts expressing GDF15 (Chrysovergis, et al., 2014). Based on these investigations, GDF15 appears to be a regulator of body weight mediated by decreased food intake and/or increased oxidative metabolism.
6.1. Conflicting results using transgenic versus knockout mice in terms of anti-obesity
Contradictory results for the relative importance of food intake/appetite reduction and oxidative metabolism/energy expenditure in weight reduction are apparent from published studies. This important issue needs to be resolved to fully understand the modulation of body weight by GDF15. Most of the conflicting data are observed when comparing results obtained after GDF15 administration to results from transgenic mice expressing GDF15. In general, appetite reduction is observed after administration of rGDF15 in mice, but it was not observed in transgenic mice. Changes in oxidative metabolism are observed in GDF15 transgenic mice, but not consistently reported after GDF15 administration as discussed below.
6.1.1. Transgenic mice
For ubiquitous expressing transgenic mice, food consumption, expressed as per mouse or per body weight, was the same for wild-type mice fed with either both regular or high- fat diets (Chrysovergis, et al., 2014). This phenomenon was seen in two different strains of mice, C57BL6 and FVB mice. In a study on non-alcoholic fatty liver disease or NASH, mice expressing full-length hGDF15 with a liver-specific promoter and with serum levels between 3 and 50 ng/mL were very similar to ubiquitous expressing transgenic mice in terms of metabolic characteristics, food intake, and protection of the liver from inflammatory steatosis. Furthermore, an increase in the expression of endogenous mouse GDF15 reduced steatohepatitis in a mouse model of NASH. GDF15 also suppresses the expression of fibrosis related genes (Tgfb1, Col1a1, Timp1, and Acta2) and osteospontin in the liver of NASH mice, whereas GDF15 deficient mice showed enhanced NASH phenotypes (K. H. Kim, et al., 2018). In another study, GDF15 was characterized as a heart-derived hormone which regulates body growth (T. Wang, et al., 2017). These studies on transgenic mice, expressing GDF15, indicate altered metabolism rather than appetite suppression as critical for weight reduction. It is not clear why appetite suppression is not observed in the transgenic GDF15 models. One speculative explanation is an adaptive response of the neural circuit that suppresses appetite in the mouse models.
In contrast to these studies, investigations with a transgenic mouse expressing full-length mouse GDF15 with a macrophage-specific expression, controlled by c-fms promoter (expression in the spleen), reported lower food intake or appetite suppression. These mice exhibited a lower body weight and fat content, improved glucose tolerance, and increased insulin sensitivity but no changes in energy expenditure as compared to syngeneic control mice, indicating that appetite suppression is responsible for the lower body weight (Johnen, 2007; Macia, et al., 2012).
6.1.2. GDF15 (−/−) mice
Skeletal MKO (muscle specific knockout of Crif1) mice with altered mitochondrial unfolded protein response exhibit higher levels of GDF15 and are protected against obesity, have better insulin sensitivity, increased energy expenditure, and oxidative metabolism without any change in food consumption as compared to control mice. Metabolic phenotype as seen in transgenic mice was not observed in GDF15 deficient mice that had been crossed with the MKO mice (MKO/GDF15 (−/−)) (Chung, et al., 2017). Thus, protection against obesity and the metabolic phenotypes in MKO mice is dependent on the induction of GDF15 in skeletal muscle. GDF15 has been characterized as a myomitokine regulating systemic energy homeostasis (Chung, et al., 2017).
6.1.3. Administration of rGDF15 to the mice
Administration of GFD15 into mice can produce responses similar to those observed in transgenic mice. Administration of GDF15 to lean or obese mice and to other species (rats and monkeys) increased the serum levels of GDF15 in mice. In all these investigations, a consistent decrease in body weight and adipose tissue, and a reduction in food intake were observed that were, in general, both dose and time- dependent (Johnen, 2007). These responses, however, were not observed in the GFRAL (−/−) mice (Hsu, et al., 2017; S. E. Mullican, et al., 2017; L. Yang, et al., 2017). The critical role of the activation of GFRAL receptor by the administered GDF15 in appetite suppression and weight reduction is firmly established by these studies. In two studies, one with obese mice and the second with lean mice, administration of rGDF15 did not alter energy expenditure but decreased the respiratory exchange ratio (RER), indicating a shift from carbohydrate to lipid metabolism. Injection of recombinant GDF15 (rGDF15) every other day for 3 weeks into obese mice resulted in increased oxygen consumption and CO2 production, increased energy expenditure, reduced body weight, fat content, liver, and eWAT steatosis. Expression of lipolytic and oxidative metabolism genes in the liver, iWAT, and eWAT were increased (Chung, et al., 2017). Furthermore, these GDF15 treated mice exhibited reduced body weight, fat content, and liver/ eWAT steatosis with no changes in food intake or activity. Obese mice either feeding on an HFD or Ob/Ob mice exhibited a shift to lipid metabolism and increased oxidative metabolism after chronic treatment with rGDF15. GDF15 treatment for 34 days in mice fed a control diet or HFD caused the following effects: reduction in body weight (greater reduction in mice fed with HFD), reduction in fat mass (more reduction in fat mass in mice fed with HFD), a reduction in food intake, increase in energy expenditure, increase in thermogenesis, and improved glucose and insulin tolerance. Treatment with rGDF15 also lowered pro-inflammatory, cytokine levels in tissue and serum, lowered macrophages infiltration in adipose tissue, and reduced liver steatosis in mice fed with HFD (Tsai et al., 2018.) Treatment of obese mice on a high-fat diet increased energy expenditure and increased the expression of UCP1 a key regulator of thermogenesis in inguinal fat (V. W.-W. Tsai, et al., 2013). These findings obtained by administration of GDF15 are in agreement with previously published data with mice expressing hGDF15 (Chrysovergis, et al., 2014; J. M. Kim, et al., 2013; X. Wang, et al., 2014).
6.2. Biochemical mechanism of GDF15 in anti-obesity
The underlying changes involved in the biochemical processes and signaling pathways altered by the increased expression of GDF15 in transgenic mice responsible for weight reduction and metabolic phenotype related to appetite suppression are poorly understood. One focus of research to address this issue was the NLRP3 inflammasome that plays an important regulatory role in obesity-induced insulin resistance (Stienstra, et al., 2010; Vandanmagsar, et al., 2011). The levels of IL-18 and Il-1β, that are associated with obesity and insulin resistance, are lower in the white adipose tissue (WAT) of transgenic mice ubiquitously expressing GDF15 (X. Wang, et al., 2014). These mice fed with either a regular or high-fat diet exhibited reduced caspase-1 and inflammasome activity that regulates the levels of IL-18 and 1L-1β. The transgenic mice exhibited lower levels of macrophage infiltration into liver and adipose tissues and reduced levels of inflammatory cytokines. The lower NLRP3 inflammasome activity explains, in part, the improved insulin sensitivity, lower body weight, and protection against inflammatory steatosis observed in the transgenic mice expressing GDF15 (Chrysovergis, et al., 2014; X. Wang, et al., 2014). In the WAT of young and old transgenic mice, suppression of the IGF-1 and insulin/mTOR signaling pathways has been reported and is linked to improved insulin sensitivity and an increase in lifespan. In other studies, GDF15 has been reported to inhibit growth hormone signaling resulting in a lower level of IGF-1 which is related to the anti-cancer activity of GDF15 (T. Wang, et al., 2017; X. Wang, Chrysovergis, Bienstock, Shim, & Eling, 2012).
A second major investigative research is on energy homeostasis. Mice with ubiquitous hGDF15 expression show no differences in adipocyte differentiation in white adipose tissue (WAT) between wild-type and transgenic mice; however, the expression of genes related to lipolysis was higher in the transgenic mice (Chrysovergis, et al., 2014). In the brown fat (BAT), an increase in the expression of thermogenesis and oxidative metabolism genes has been observed in the transgenic mice fed either with a regular or high-fat diet. Such increases in expressions of genes related to lipolysis, oxidative metabolism, and thermogenesis were observed in mice at both 30 and 95 weeks of age (X. Wang, et al., 2014). An increase in lipolysis and fatty acid oxidation was observed in the livers, eWAT (epididymal white fat), and extensor muscle (EDL) of MKO mice (Chung, et al., 2017). The increase in the expression of lipolysis proteins in the WAT results in lower triglyceride content and smaller adipocyte size as observed in the GDF15 transgenic mice fed with either a regular or HFD. The increased expression of oxidative metabolism proteins in BAT and eWAT increases fatty acid oxidation, and hence, serum levels of free fatty acids and triglycerides are not higher in the GDF15 transgenic mice as expected due to increased lipolysis. The net result of the increase in oxidative metabolism is a decrease in body weight and adipose tissue without a reduction in food intake.
6.3. Other molecules involved in anti-obesity activity
Increased lipolysis, metabolic activity, and energy expenditure can lower body weight, improve insulin sensitivity, and resistance to obesity. The transcriptional co-regulator TRIP-Br2 controls fat storage by the regulation of lipolysis, metabolic activity, and energy expenditure (Liew, et al., 2013). TRIP-Br2 deficient mice do not have appetite suppression but exhibit upregulation of genes related to lipolysis, oxidative metabolism, and thermogenesis in adipose tissue and are resistant to obesity. They also have improved glucose tolerance, insulin sensitivity, and increased fat lipolysis and thermogenesis. The metabolic characteristics of the TRIP-Br2 deficient mice are very similar if not identical to the GDF15 transgenic mice (Chrysovergis, et al., 2014; X. Wang, et al., 2014) and the MKO mice (Chung, et al., 2017), indicating that a decrease in body weight is not exclusively dependent on appetite suppression. The shift from carbohydrate to lipid metabolism, and fatty acid oxidation, observed after administration of GDF15 and in the transgenic mouse models, may contribute to cachexia observed during chronic diseases, such as cancer. Excessive fatty acid oxidation can induce muscle atrophy observed during cachexia (Fukawa, et al., 2016). The shift from carbohydrate to lipid metabolism after rGDF15 treatment was not observed in the GFRAL (−/−) mice, suggesting that the metabolic effects observed in transgenic mice and after rGDF15 treatment was dependent on the activation of GFRAL. Central regulation of oxidative metabolism is supported by ample experimental evidence (Berthoud, Lenard, & Shin, 2011; Clapham & Miller, 2011) and the lipolytic and mitochondrial-oxidation pathways are β3 adrenergic regulated (Collins, 2012). Thus, the mechanism of how activation of the GFRAL receptor in the brain increases oxidative metabolism is still unknown and needs to be confirmed by future studies.
Except for the studies conducted by Macia et al. (Macia, et al., 2012), transgenic mice expressing GDF15 have exhibited a lower body weight and generally have the same metabolic characteristics as a mouse treated with GDF15, except that food intake is the same as wild-type littermates, and energy expenditure is increased and shifted from carbohydrate to lipid metabolism. Since the food consumption is the same between transgenic and control mice, and comparison of transgenic mice to wild-type mice is, in essence, a paired feeding experiment, changes in oxidative metabolism must be responsible for the metabolic phenotype. These results also suggest that the increase in oxidative metabolism observed after administration of rGDF15 is not likely due to the decrease in food consumption (V. W.-W. Tsai, et al., 2013). One difference between studies reporting altered oxidative metabolism versus those reporting no changes in oxidative metabolism is the duration of treatment. Changes in oxidative metabolism were observed after generation of chronic high levels of serum GDF15 (Chrysovergis, et al., 2014; Chung, et al., 2017; V. W.-W. Tsai, et al., 2013), while the absence of altered oxidative metabolism was observed with acute treatment (Hsu, et al., 2017; Johnen, 2007; Macia, et al., 2012; L. Yang, et al., 2017). The difficulties and complexity in evaluation of energy metabolism in mice may provide an explanation for the inconsistent findings on energy expenditure (Tschöp, et al., 2011). The most consistent results were obtained by analyzing the expression of gene and protein related to lipolysis, thermogenesis, and oxidative metabolism and the shift from carbohydrate to lipid metabolism. High-fat diet has been shown to alter biochemical processes related to metabolism and could alter the response to GDF15. For example, NLRP3 inflammasome activity is higher in mice on high-fat diet (HFD) (Stienstra, Tack, Kanneganti, Joosten, & Netea, 2012; Vandanmagsar, et al., 2011) and obesity increases the expression of TRIP-Br2 in visceral fat (Liew, et al., 2013) (Figure 3).
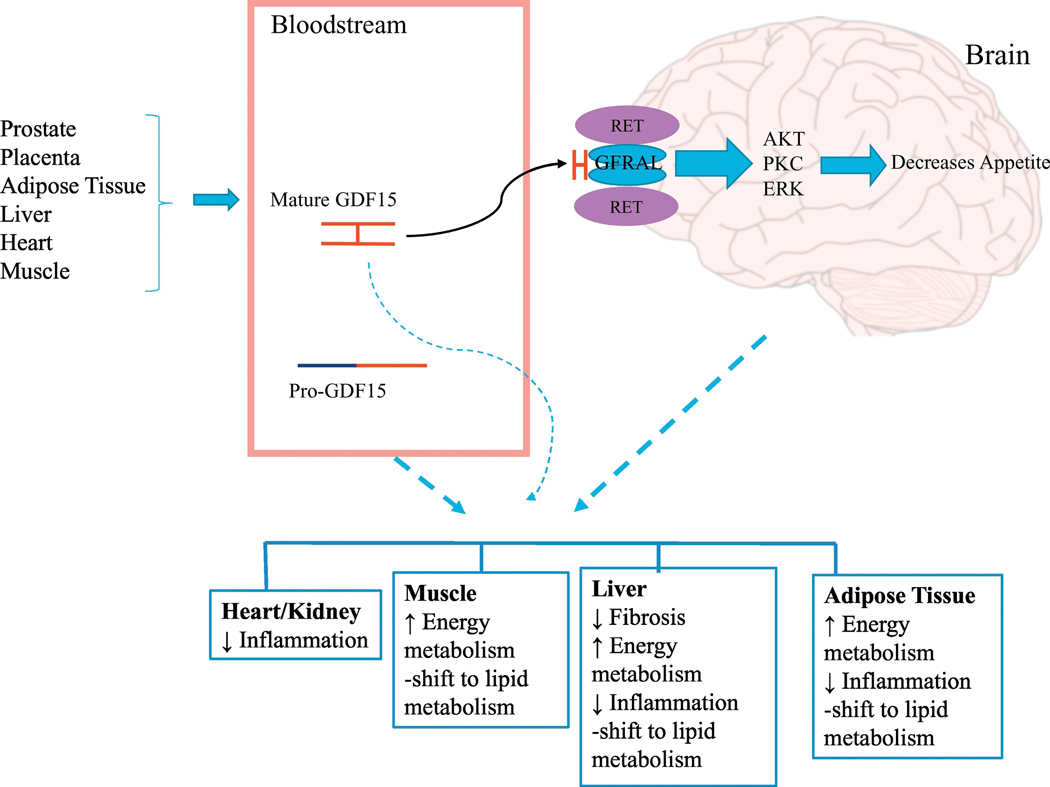
Figure 3.
GDF15 effects on obesity. Some tissues express GDF15 and secrete mature GDF15 into the bloodstream. In the brain, mature GDF15 binds to GFRAL/RET receptor and controls food appetite via certain unknown kinase signaling. In addition, pro-GDF15 and mature GDF15 may affect several metabolic pathways, independent of GFRAL/RET receptor pathway. The details are provided in the main text.
7. Biological activities independent of mature GDF15
Previous studies have confirmed that secreted mature dimer present in the circulating blood suppresses appetite and alters energy homeostasis by the activation of GFRAL- RET receptor complex located in specific regions of the brainstem. This activity of mature GDF15 could be associated with diseases, such as obesity and cachexia associated with cancer [reviewed in (Shannon E. Mullican & Rangwala, 2018)] and reduction in chronic inflammation (J. M. Kim, et al., 2013; V. W. W. Tsai, Husaini, Sainsbury, Brown, & Breit, 2018; X. Wang, et al., 2014). A diverse number of GDF15 biological activities have been reported previously and is reportedly mediated via activation of SMADs. Although GDF15 has been investigated as a divergent member of the TGF-β superfamily, a recent report demonstrated that mature GDF15 does not bind to any known TGF-β receptors (Hsu, et al., 2017). Considering the many contradictory results, the specific expression of the receptor, GFRAL, in the brain, the lack of GFRAL expression in frequently used cellular systems, and the contamination of some preparations of rGDF15 with TGF-β1 requires a careful reanalysis of these publication results.
During the formation of mature GDF15 from endogenous sources, in transgenic animals and in cellular system expressing the full-length GDF15, the secretion of mature GDF15 dimer can be accompanied by the release of the pro-GDF15 dimer, and possibly, the GDF15 pro-peptide (Bauskin, et al., 2005; Bauskin, et al., 2010). No information has been reported on the circulating levels of pro-GDF15 dimer or pro-GDF15 with its biological activity, a potential receptor. This field may be a fruitful area of investigation to explain some effects observed in animals and in cells designed to express full-length GDF15. The activation of the GFRAL-RET receptor by pro-GDF15 has not been reported in the literature as per our knowledge.
Multiple forms of GDF15 are present in cells, and their roles and interactions with cellular systems remain to be determined. We recently reported that pro-GDF15 is translocated into the nucleus by an energy dependent process (Min, Liggett, et al., 2016). Inside the nucleus pro-GDF15 inhibits DNA binding of the SMAD complex to the promoter region of TGF- β target gene regulating the transcriptional activity of the SMAD pathway. For example, GDF15 has been reported to suppress TGF-β1 mediated cell invasion and cell migration (Min, Liggett, et al., 2016) by this mechanism, a finding that provides some clues as to how GDF15 may reduce the development and progression of cancer. These findings indicate a need for further study of the molecular mechanisms by which the intracellular forms of GDF15 can modulate biological processes independent of the GFRAL receptor. The nuclear-cytoplasmic shuttling of pro-GDF15 may permit interactions with regulatory processes inside of cells.
8. Therapeutic potential for GDF15/GFRAL in weight homeostasis
Obesity:
Several studies on regulation of energy homeostasis clearly indicate a potential anti-obesity pharmacological approach based on the activation of the GFRAL receptor present in the brain. Injections of mature rGDF15 for approximately 1 week have been reported to suppress appetite and lower body weight (Johnen, 2007; S. E. Mullican, et al., 2017; L. Yang, et al., 2017). Administration of murine mature GDF15 to mice at a dose of 0.5 μg/g body weight per day for 7 days results in a serum concentration of ~10 ng/mL. After 14 days to 21 weeks of treatment, a significant body weight reduction, reduction in food intake, increase in energy expenditure, improved glucose tolerance, and reduction in inflammatory cytokines was observed (V. W. W. Tsai, et al., 2018). Similarly, the daily administration of human mature GDF15 via adenovirus system to mice, rats, and obese monkeys lowered the body weight and improved the metabolic profile with a limitation that the native GDF15 reportedly has a short half-life of 3 hours (Xiong, et al., 2017). The pharmaceutical industry has created fusions between mature GDF15 and either human serum albumin (HSA) or the Fc part of immunoglobulin (Fc) to increase the half-life from hours to days. Treatment of either lean or obese mice with these fusion proteins exhibited reduced appetite, lower body weight, improved metabolic parameters shifting toward lipid oxidation (Emmerson, et al., 2017; Hsu, et al., 2017; S. E. Mullican, et al., 2017; Xiong, et al., 2017). Administration of a Fc-GDF15 protein weekly for 6 weeks to obese cynomolgus monkeys led to reduced body weight, lower food consumption, improved insulin and glucose levels, and lower triglyceride level in blood, which indicates increase in lipid metabolism (Xiong, et al., 2017). A single injection of an HSA-GDF15 fusion protein into obese monkeys was sufficient to maintain the decrease in appetite and body weight for 4 weeks (Shannon E. Mullican & Rangwala, 2018) suggesting that these fusion GDF15 proteins could be used for chronic treatments.
In addition to the use of mature GDF15 or modified GDF15 fusion proteins as pharmaceutical drugs, some consideration should be given to the development of drugs which increase the expression of GDF15 in tissues. The promoter region of GDF15 was investigated and several transcriptional regulation sites have been identified (Baek, Horowitz, & Eling, 2001). A large number of agents, including dietary chemicals and chemicals known to have anti-oxidant or anti-cancer activity, increase the expression of GDF15 (Eling, Baek, Shim, & Lee, 2006; J. H. Yoon & Baek, 2005). Compounds that increase the stability of mRNA prolonging the half-life of GDF15 mRNA also increase the expression of GDF15 (Martinez, et al., 2006). Among those, many phytochemicals with anti-obesity property increase GDF15 expression at the transcription level, including capsaicin (Lee, Richardson, Dashwood, & Baek, 2012), resveratrol (Baek, Wilson, & Eling, 2002), and conjugated linoleic acids (Lee, et al., 2006). Since these compounds have been associated with anti-obesity, these drugs could be modified to generate better GDF15 inducer.
The in vitro studies with intestinal cells have revealed that the most potent inducer of GDF15 expression is sulindac sulfide, a drug that inhibits intestinal tumor development in mice (K. S. Kim, et al., 2002). The reduction of polyps in APC/Min mice after treatment with prodrug sulindac requires GDF15, indicating an increased expression of mGDF15 (Zimmers, et al., 2010). It should be possible to design drugs that specifically induce the expression of GDF15 with limited side effects.
Anorexia/cachexia:
The increase in the circulating levels of GDF15 during diseases, particular cancer and, most notably, prostate cancer is a cause of anorexia (loss of appetite) and cachexia (extreme weight loss and muscle wasting) (Lerner, et al., 2016). The findings in experimental animals described in the previous sections clearly support the hypothesis for the circulating mature GDF15, and the subsequent activation of the receptor GFRAL is considered to be responsible for these effects. Several pharmaceutical approaches can be considered to treat or reverse these deleterious effects of GDF15. One approach is to develop GDF15 specific antibodies that can be injected to sequester, and hence, lower the concentration of mature GDF15. Administration of a specific GDF15 antibody in mice with high levels of GDF15 in the blood reversed the weight loss and diminished the fat and muscle mass caused by xenograft tumors expressing GDF15 (Johnen, 2007; Lerner, et al., 2016). The characterization of GFRAL as the GDF15 receptor offers additional targets for drug development. Injection of a fusion GDF15 protein (Fc-GDF15) into wild type mice led to decrease in body weight and food intake; however, these responses were not observed in GFRAL knockout mice. Treatment of rats with a mouse monoclonal anti-GFRAL antibody blocked the weight loss and food intake effects of Fc-GDF15. This result indicates that GRAL is a promising target for drug development and show that anti- GDF15 antibodies can inhibit the effect of the native protein and fusion constructs (Emmerson, et al., 2017). The GFRAL signaling pathway, that is only poorly understood, is a potential target for development of drugs for treating or preventing the deleterious effects of high GDF15 levels.
Based on the secretion pathway of GDF15, inhibiting GDF15 secretion may not facilitate anorexia/cachexia. GDF15 has been known to translocate from cytoplasm to nucleus, followed by secretion to the extracellular matrix (Min, Lee, et al., 2016; Min, Liggett, et al., 2016). A critical problem related to dynamic proteins, like GDF15, is the paucity of information on why these proteins have multiple functions. Thus, elucidating dynamic protein, like GDF15, could help to define GDF15 as a target to treat many diseases, as well as address another critical gap in current research including, transcriptional/translational regulation of a secreted protein. Thus, retaining GDF15 in the nucleus or cytoplasm by some means may help cancer patients with respect to anorexia/cachexia. Furthermore, a recent study suggested that GDF15 in the nucleus inhibited Smad pathways that are involved in the cell migration and metastasis (Min, Liggett, et al., 2016). Therefore, it is a very attractive approach that could be explored to retain GDF15 inside of the cells in cancer patients.
9. Therapeutic potential of GDF15 in other diseases
Inflammation:
Transgenic mice expressing GDF15 exhibit a weaker inflammatory response to LPS as compared to wild type mice (J. M. Kim, et al., 2013). In addition, the GDF15 mice exhibit a lower basal serum level of inflammatory cytokines and lower NLRP3 inflammasome activity (X. Wang, et al., 2014). Mice that express GDF15 expression have reduced nonalcoholic steatohepatitis, an inflammatory condition in the liver (K. H. Kim, et al., 2018), while mice deficient in GDF15 are more susceptible to LPS induced heart and renal injury (Abulizi, et al., 2017). Thus, GDF15 may exhibit therapeutic potential for the treatment of inflammatory related diseases.
Cardiovascular diseases:
Acute myocardial infarction (AMI) is a major cause of morbidity and mortality worldwide. GDF15 appears to play a role in cardiovascular diseases, such as heart failure (Wollert, et al., 2007), cardiac hypertrophy (Dominguez-Rodriguez, Abreu-Gonzalez, & Avanzas, 2011), and coronary heart disease (CHD) (Tibor Kempf, et al., 2009). Transgenic mice expressing GDF15 are resistant to the ischemia reperfusion injury that occurs during heart transplantation suggesting a potential therapeutic utility of GDF15 in heart replacement (Y. Zhang, et al., 2017).
Diabetes:
GDF15 is clearly a regulator of energy homeostasis as discussed in the text above. Transgenic mice expressing GDF15 and treatment of experimental animal with mature GDF15 regulates energy metabolism and body weight. In these experiments an increase in glucose tolerance and increase insulin sensitivity was observed. Furthermore, the inflammasome NLRP3 that contributes to insulin resistance in obesity is lowered by the expression of GDF15. These metabolic effects coupled with the reduction in body weight and fat content makes GDF15 as an attractive candidate for use in the treatment of type 2 diabetes.
Fibrosis:
Non-alcoholic steatohepatitis (NASH) is associated with obesity and insulin resistance. In KO-GDF15 mice the pathology of NASH is increased including fibrosis of the liver while in transgenic mice expressing GDF15, the expression of fibrosis related genes are suppressed in vitro and in the livers of mice in vivo. In transgenic mice expressing GDF15 the pathology and metabolic deterioration was suppressed. Serum GDF15 level is associated with a risk of advanced fibrosis among NAFLD subjects independently of age, gender, BMI, insulin resistance and low skeletal muscle mass (Koo, et al., 2018). This increase in GDF15 may be a protective mechanism to prevent progression of the disease. These finding suggest that GDF15’s suppression of fibrosis may be a suitable candidate or target for drug development
Potential Risks:
The literature is awash with a diverse and large number of biological activities reported to be mediated by GDF15. This is likely the result of increase in the expression of GDF15 in tissues and the increase in serum levels associated with a diverse and large number of diseases and stimuli. The results from these studies indicate that GDF15 can exhibit positive and deleterious health effects and frequently, because GDF15 was first characterized as a member of the TGF-β superfamily, an analogy is often made to the pleiotropic activities of the TGF-β. The results from in vitro investigations, which rely heavily on using GDF15 protein preparations, must be analyzed carefully because of the uncertainty of the GDF15 purity, quality, and reported contamination with TGF-β1 (Olsen, et al., 2017). Experiments where rGDF15 is injected into mice and the effects observed also suffer from the same problem and the findings need to be cautiously considered. Investigations with endogenous or engineered GDF15 expression in cells do not exhibit this problem but expression of the receptor, GFRAL, has not been confirmed in any rodent or human cell line (S. E. Mullican, et al., 2017; L. Yang, et al., 2017). Thus, biological responses observed with these cells are not GFRAL receptor mediated, but could be due to different effects of intracellular GDF15 forms that may have on intracellular function (Y. Zhang, et al., 2017). Although co-receptor, RET, is widely expressed in rodent tissue, the expression of GFRAL is essential exclusively in the hindbrain. Biological responses of GDF15 reported in the peripheral tissue of experimental animals need to be linked to activation of a receptor, only present in the hindbrain or indirectly to receptor mediated biological changes associated with the regulation of energy homeostasis. For many of these studies, it appears the assumption or hypothesis of the investigation is that increased GDF15 level is assumed to be a cause of the disease. The serum levels of GDF15 have been proposed as a marker for “all cause mortally” (Wiklund, et al., 2010). However transgenic mice expressing hGDF15 exhibit an increase in life span when fed with either a regular or high fat diet, and no increase in formation of tumors or other pathology effects were observed up to 90 weeks of age (X. Wang, et al., 2014). Obese mice injected with hGDF15 expressing virus for 1 year exhibited no detrimental effects of GDF15 expression in the 19 tissues examined (Xiong, et al., 2017). The expression of GDF15 is upregulated by tumor suppressor pathways, for example, p53 and its expression is increased by a large number of chemicals with disease preventative activity (X. Wang, et al., 2013). It seems reasonable to hypothesize that GDF15 expression may increase during the early stages of disease, as a protective mechanism, and cells may later develop tolerance, downregulation, or mutation of GDF15-affected pathways. Presented in Table 3 is a summary of the biological activities observed in GDF15 transgenic mice as compared to wild-type mice. Only few deleterious health effects of GDF15 have been reported. TRAMP mice, a model for prostate cancer, was crossed with mice expressing GDF15 to give a Tramp mouse expressing GDF15. Although GDF15 slowed cancer development and increased survival, an increase in metastases was noted (Y. Husaini, et al., 2012). All of the other difference reported in the literature or observed in this laboratory between the GDF15 mice and wild type mice are responses that are a positive improvement to health. These findings are consistent in terms of the increase in GDF15 levels as a stress survival response rather than as a cause of a disease or deleterious response. With these contradictions in mind, a careful analysis of the benefits of GDF15 as a drug and the potential risk needs to be done.
Table 3.
Body weigh changes between GDF15 (NAG-1) transgenic and control mice.
| control mice | GDF15 Tg mice | p value | |
|---|---|---|---|
| Total weight (g) | 22.2± 0.4 | 17.2±0.2 | 0.0003 |
| Fat (g) | 3.6±0.03 | 2.6±0.1 | 0.0001 |
| % Fat | 16.3±0.2 | 14.6±0.2 | 0.006 |
| Lean tissue (g) | 18.6±0.4 | 15.1±0.2 | 0.0008 |
Values are the mean ± SE
10. Summary and Conclusion
Our understanding of GDF15 biological activities has been significantly improved by the recent characterization of the GFRAL as GDF15 receptor. However, several important questions still remain to be answered. Considerable evidence has been published which have confirmed that the levels of circulating mature GDF15 dimer, a non-homeostatic regulator of body weight activate the GFRAL/RET circuit located in specific regions of brainstem. Activation of the neural circuit regulates food intake and energy expenditure, and hence, body weight. Unanswered questions are what is the nature of the receptor signaling, and how this neural network alters appetite and energy expenditure of peripheral tissues, such as brown adipose tissue, liver, and muscle, that are important in energy homeostasis. Investigations with mice expressing GDF15 indicate the importance of energy homeostasis in mediating a reduction in body weight and adipose tissue weight. The regulation of body weight by GDF15 occurs only during condition of stress, injury, or diseases when GDF15 levels are increased. Thus, the increase in GDF15 expression can be considered as a survival response.
The mature GDF15 dimer, pro-GDF15, and the pro-peptide GDF15 fragment can be secreted into the circulation, but little information is available on the serum levels of the pro-GDF15/pro-peptide GDF15 or its biological activity. Does the pro-GDF15/pro- peptide GDF15 possess biological activity, or does it, in some way, participate in the biological activity of the mature GDF15, or is this peptide just cellular garbage? This is a question that requires further investigation in order to potentially elucidate the multiple biological processed associated with GDF15, some of which cause deleterious effects. GDF15 is reported to exhibit anti- and pro-tumorigenic activities and other effects that are difficult to rationalize because of the specific expression of GFRAL in the brain. However, reports showing the accumulation of full-length GDF15 in the nucleus and subsequent inhibition of transcriptional regulation of the Smad pathway and probably other pathways could elucidate the reduction in tumors observed in mice expressing GDF15. This as an area that requires further study to elucidate the potential therapeutic use of GDF15. The published findings as summarize in this article have provided the basis to develop the pharmaceutical approaches necessary for the successful use of GDF15 as a therapeutic agent in obesity and other related metabolic disorders.
Acknowledgments:
We thank Dr. Jeanelle Martinez for careful reading and useful comments in this manuscript. We are also grateful to Kali Chrysovergis and Justin Kosak for the productive discussions, their efforts, and dedication for investigating GDF15 at NIEHS. This work was supported, in part, by the NIH, NIEHS Intramural Research Program (T.E.E) Z01- ES010016–14. This work was also supported by the Research Institute for Veterinary Science, and BK21 PLUS Program for Creative Veterinary Science Research Center, Seoul National University, and by the National Research Foundation of Korea (NRF) grant funded by the Korea government (NRF- 2018R1A2B2002923) to S.J.B.
Footnotes
Conflict of interest statement: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Seung Joon Baek, Bldg 81 Rm 413, Laboratory of Signal Transduction, College of Veterinary Medicine, Seoul National University, 1 Gwanak-ro, Gwanak-gu, Seoul 08826, Korea.
Thomas Eling, NIEHS/NIH, 111 TW Alexander Dr. Bldg. 101 F-095, Research Triangle Park, NC 27709.
References
- Abulizi P, Loganathan N, Zhao D, Mele T, Zhang Y, Zwiep T, Liu K, & Zheng X (2017). Growth Differentiation Factor-15 Deficiency Augments Inflammatory Response and Exacerbates Septic Heart and Renal Injury Induced by Lipopolysaccharide. Scientific Reports, 7, 1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adela R, & Banerjee SK (2015). GDF-15 as a target and biomarker for diabetes and cardiovascular diseases: a translational prospective. J. Diabetes Res, 2015, 490842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek SJ, & Eling TE (2006). Changes in gene expression contribute to cancer prevention by COX inhibitors. Prog Lipid Res, 45, 1–16. [DOI] [PubMed] [Google Scholar]
- Baek SJ, Horowitz JM, & Eling TE (2001). Molecular cloning and characterization of human nonsteroidal anti-inflammatory drug-activated gene promoter. Basal transcription is mediated by Sp1 and Sp3. J Biol Chem, 276, 33384–33392. [DOI] [PubMed] [Google Scholar]
- Baek SJ, Kim JS, Jackson FR, Eling TE, McEntee MF, & Lee SH (2004). Epicatechin gallate-induced expression of NAG-1 is associated with growth inhibition and apoptosis in colon cancer cells. Carcinogenesis, 25, 2425–2432. [DOI] [PubMed] [Google Scholar]
- Baek SJ, Kim JS, Nixon JB, DiAugustine RP, & Eling TE (2004). Expression of NAG- 1, a transforming growth factor-beta superfamily member, by troglitazone requires the early growth response gene EGR-1. J Biol Chem, 279, 6883–6892. [DOI] [PubMed] [Google Scholar]
- Baek SJ, Kim KS, Nixon JB, Wilson LC, & Eling TE (2001). Cyclooxygenase inhibitors regulate the expression of a TGF-beta superfamily member that has proapoptotic and antitumorigenic activities. Mol Pharmacol, 59, 901–908. [PubMed] [Google Scholar]
- Baek SJ, Okazaki R, Lee SH, Martinez J, Kim JS, Yamaguchi K, Mishina Y, Martin DW, Shoieb A, McEntee MF, & Eling TE (2006). Nonsteroidal anti-inflammatory drug-activated gene-1 over expression in transgenic mice suppresses intestinal neoplasia. Gastroenterology, 131, 1553–1560. [DOI] [PubMed] [Google Scholar]
- Baek SJ, Wilson LC, & Eling TE (2002). Resveratrol enhances the expression of non- steroidal anti-inflammatory drug-activated gene (NAG-1) by increasing the expression of p53. Carcinogenesis, 23, 425–434. [DOI] [PubMed] [Google Scholar]
- Baek SJ, Wilson LC, Hsi LC, & Eling TE (2003). Troglitazone, a peroxisome proliferator-activated receptor gamma (PPAR gamma) ligand, selectively induces the early growth response-1 gene independently of PPAR gamma. A novel mechanism for its anti-tumorigenic activity. J Biol Chem, 278, 5845–5853. [DOI] [PubMed] [Google Scholar]
- Baek SJ, Wilson LC, Lee CH, & Eling TE (2002). Dual function of nonsteroidal anti- inflammatory drugs (NSAIDs): inhibition of cyclooxygenase and induction of NSAID- activated gene. J Pharmacol Exp Ther, 301, 1126–1131. [DOI] [PubMed] [Google Scholar]
- Bansal N, Kumar D, Gupta A, Chandra D, Sankhwar SN, & Mandhani A (2017).Relevance of MIC-1 in the Era of PSA as a Serum Based Predictor of Prostate Cancer: A Critical Evaluation. Scientific Reports, 7, 16824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauskin AR, Brown DA, Junankar S, Rasiah KK, Eggleton S, Hunter M, Liu T, Smith D, Kuffner T, Pankhurst GJ, Johnen H, Russell PJ, Barret W, Stricker PD, Grygiel JJ, Kench JG, Henshall SM, Sutherland RL, & Breit SN (2005). The propeptide mediates formation of stromal stores of PROMIC-1: role in determining prostate cancer outcome. Cancer Res, 65, 2330–2336. [DOI] [PubMed] [Google Scholar]
- Bauskin AR, Brown DA, Kuffner T, Johnen H, Luo XW, Hunter M, & Breit SN (2006). Role of macrophage inhibitory cytokine-1 in tumorigenesis and diagnosis of cancer. Cancer Res, 66, 4983–4986. [DOI] [PubMed] [Google Scholar]
- Bauskin AR, Jiang L, Luo XW, Wu L, Brown DA, & Breit SN (2010). The TGF-beta superfamily cytokine MIC-1/GDF15: secretory mechanisms facilitate creation of latent stromal stores. J Interferon Cytokine Res, 30, 389–397. [DOI] [PubMed] [Google Scholar]
- Bauskin AR, Zhang HP, Fairlie WD, He XY, Russell PK, Moore AG, Brown DA, Stanley KK, & Breit SN (2000). The propeptide of macrophage inhibitory cytokine (MIC-1), a TGF-beta superfamily member, acts as a quality control determinant for correctly folded MIC-1. EMBO J, 19, 2212–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud H-R, Lenard NR, & Shin AC (2011). Food reward, hyperphagia, and obesity. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 300, R1266–R1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootcov MR, Bauskin AR, Valenzuela SM, Moore AG, Bansal M, He XY, Zhang HP, Donnellan M, Mahler S, Pryor K, Walsh BJ, Nicholson RC, Fairlie WD, Por SB, Robbins JM, & Breit SN (1997). MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci U S A, 94, 11514–11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cekanova M, Lee SH, Donnell RL, Sukhthankar M, Eling TE, Fischer SM, & Baek SJ (2009). Nonsteroidal anti-inflammatory drug-activated gene-1 expression inhibits urethane-induced pulmonary tumorigenesis in transgenic mice. Cancer Prev Res (Phila Pa), 2, 450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintharlapalli S, Papineni S, Baek SJ, Liu S, & Safe S (2005). 1,1-Bis(3’-indolyl)-1-(p- substitutedphenyl)methanes are peroxisome proliferator-activated receptor gamma agonists but decrease HCT-116 colon cancer cell survival through receptor- independent activation of early growth response-1 and nonsteroidal anti- inflammatory drug-activated gene-1. Mol Pharmacol, 68, 1782–1792. [DOI] [PubMed] [Google Scholar]
- Chrysovergis K, Wang X, Kosak J, Lee SH, Kim JS, Foley JF, Travlos G, Singh S, Baek SJ, & Eling TE (2014). NAG-1/GDF-15 prevents obesity by increasing thermogenesis, lipolysis and oxidative metabolism. Int J Obes (Lond), 38, 1555–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HK, Ryu D, Kim KS, Chang JY, Kim YK, Yi H-S, Kang SG, Choi MJ, Lee SE, Jung S-B, Ryu MJ, Kim SJ, Kweon GR, Kim H, Hwang JH, Lee C-H, Lee S-J, Wall CE, Downes M, Evans RM, Auwerx J, & Shong M (2017). Growth differentiation factor 15 is a myomitokine governing systemic energy homeostasis. The Journal of Cell Biology, 216, 149–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE, & Miller C (2011). A thermodynamic framework for understanding temperature sensing by transient receptor potential (TRP) channels. Proceedings of the National Academy of Sciences of the United States of America, 108, 19492–19497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S (2012). β-Adrenoceptor Signaling Networks in Adipocytes for Recruiting Stored Fat and Energy Expenditure. Frontiers in endocrinology, 2, 102–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture F, Sabbagh R, Kwiatkowska A, Desjardins R, Guay S-P, Bouchard L, & Day R (2017). PACE4 Undergoes an Oncogenic Alternative Splicing Switch in Cancer. Cancer Research, 77, 6863–6879. [DOI] [PubMed] [Google Scholar]
- Dominguez-Rodriguez A, Abreu-Gonzalez P, & Avanzas P (2011). Relation of Growth- Differentiation Factor 15 to Left Ventricular Remodeling in ST-Segment Elevation Myocardial Infarction. The American Journal of Cardiology, 108, 955–958. [DOI] [PubMed] [Google Scholar]
- Eling TE, Baek SJ, Shim M, & Lee CH (2006). NSAID activated gene (NAG 1), a modulator of tumorigenesis. J Biochem Mol Biol, 39, 649–655. [DOI] [PubMed] [Google Scholar]
- Emmerson PJ, Wang F, Du Y, Liu Q, Pickard RT, Gonciarz MD, Coskun T, Hamang MJ, Sindelar DK, Ballman KK, Foltz LA, Muppidi A, Alsina-Fernandez J, Barnard GC, Tang JX, Liu X, Mao X, Siegel R, Sloan JH, Mitchell PJ, Zhang BB, Gimeno RE, Shan B, & Wu X (2017). The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat Med, 23, 1215–1219. [DOI] [PubMed] [Google Scholar]
- Frimodt-Møller M, von Scholten BJ, Reinhard H, Jacobsen PK, Hansen TW, Persson FI, Parving H-H, & Rossing P (2018). Growth differentiation factor-15 and fibroblast growth factor-23 are associated with mortality in type 2 diabetes – An observational follow-up study. PLoS One, 13, e0196634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukawa T, Yan-Jiang BC, Min-Wen JC, Jun-Hao ET, Huang D, Qian C-N, Ong P, Li Z, Chen S, Mak SY, Lim WJ, Kanayama H. o., Mohan RE, Wang RR, Lai JH, Chua C, Ong HS, Tan K-K, Ho YS, Tan IB, Teh BT, & Shyh-Chang N (2016).Excessive fatty acid oxidation induces muscle atrophy in cancer cachexia. Nature Medicine, 22, 666. [DOI] [PubMed] [Google Scholar]
- Harn H-J, Chuang H-M, Chang L-F, Huang A, Hsieh S-T, Lin S-Z, Chou C-W, Kuo YH, & Chiou T-W (2014). Taiwanin A targets non-steroidal anti-inflammatory drug- activated gene-1 in human lung carcinoma. Fitoterapia, 99, 227–235. [DOI] [PubMed] [Google Scholar]
- Hsiao EC, Koniaris LG, Zimmers-Koniaris T, Sebald SM, Huynh TV, & Lee SJ (2000). Characterization of growth-differentiation factor 15, a transforming growth factor beta superfamily member induced following liver injury. Mol Cell Biol, 20, 3742–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu J-Y, Crawley S, Chen M, Ayupova DA, Lindhout DA, Higbee J, Kutach A, Joo W, Gao Z, Fu D, To C, Mondal K, Li B, Kekatpure A, Wang M, Laird T, Horner G, Chan J, McEntee M, Lopez M, Lakshminarasimhan D, White A, Wang S-P, Yao J, Yie J, Matern H, Solloway M, Haldankar R, Parsons T, Tang J, Shen WD, Alice Chen Y, Tian H, & Allan BB (2017). Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature, 550, 255. [DOI] [PubMed] [Google Scholar]
- Husaini Y, Lockwood GP, Nguyen TV, Tsai VW-W, Mohammad MG, Russell PJ, Brown DA, & Breit SN (2015). Macrophage Inhibitory Cytokine-1 (MIC- 1/GDF15) Gene Deletion Promotes Cancer Growth in TRAMP Prostate Cancer Prone Mice. PLoS One, 10, e0115189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husaini Y, Qiu MR, Lockwood GP, Luo XW, Shang P, Kuffner T, Tsai VW, Jiang L, Russell PJ, Brown DA, & Breit SN (2012). Macrophage Inhibitory Cytokine-1 (MIC-1/GDF15) Slows Cancer Development but Increases Metastases in TRAMP Prostate Cancer Prone Mice. PLoS One, 7, e43833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnen H (2007). Tumor-induced anorexia and weight loss are mediated by the TGF-[beta] superfamily cytokine MIC-1. Nat. Med, 13, 1333–1340. [DOI] [PubMed] [Google Scholar]
- Joshi JP, Brown NE, Griner SE, & Nahta R (2011). Growth differentiation factor 15 (GDF15)-mediated HER2 phosphorylation reduces trastuzumab sensitivity of HER2- overexpressing breast cancer cells. Biochemical Pharmacology, 82, 1090–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutooru I, Chadalapaka G, Chintharlapalli S, Papineni S, & Safe S (2009). Induction of apoptosis and nonsteroidal anti-inflammatory drug-activated gene 1 in pancreatic cancer cells by a glycyrrhetinic acid derivative. Mol Carcinog, 48, 692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki M, Yoshioka H, Kamitani H, Watanabe T, Wade PA, & Eling TE (2012). DNA methylation-mediated silencing of nonsteroidal anti-inflammatory drug- activated gene (NAG-1/GDF15) in glioma cell lines. Int J Cancer, 130, 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JA, Scott Lucia M, & Lambert JR (2009). p53 controls prostate-derived factor/macrophage inhibitory cytokine/NSAID-activated gene expression in response to cell density, DNA damage and hypoxia through diverse mechanisms. Cancer Letters, 277, 38–47. [DOI] [PubMed] [Google Scholar]
- Kempf T, Guba-Quint A, Torgerson J, Magnone MC, Haefliger C, Bobadilla M, & Wollert KC (2012). Growth differentiation factor 15 predicts future insulin resistance and impaired glucose control in obese nondiabetic individuals: results from the XENDOS trial. Eur J Endocrinol, 167, 671–678. [DOI] [PubMed] [Google Scholar]
- Kempf T, Sinning J-M, Quint A, Bickel C, Sinning C, Wild PS, Schnabel R, Lubos E, Rupprecht HJ, Münzel T, Drexler H, Blankenberg S, & Wollert KC (2009). Growth-Differentiation Factor-15 for Risk Stratification in Patients With Stable and Unstable Coronary Heart Disease. Circulation : Cardiovascular Genetics, 2, 286–292. [DOI] [PubMed] [Google Scholar]
- Kim JM, Kosak JP, Kim JK, Kissling G, Germolec DR, Zeldin DC, Bradbury JA, Baek SJ, & Eling TE (2013). NAG-1/GDF15 transgenic mouse has less white adipose tissue and a reduced inflammatory response. Mediators Inflamm, 2013, 641851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Kim SH, Han DH, Jo YS, Lee Y. h., & Lee M-S (2018). Growth differentiation factor 15 ameliorates nonalcoholic steatohepatitis and related metabolic disorders in mice. Scientific Reports, 8, 6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS, Baek SJ, Flake GP, Loftin CD, Calvo BF, & Eling TE (2002). Expression and regulation of nonsteroidal anti-inflammatory drug-activated gene (NAG-1) in human and mouse tissue. Gastroenterology, 122, 1388–1398. [DOI] [PubMed] [Google Scholar]
- Koo BK, Um SH, Seo DS, Joo SK, Bae JM, Park JH, Chang MS, Kim JH, Lee J, Jeong W-I, & Kim W (2018). Growth differentiation factor 15 predicts advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease. Liver International, 38, 695–705. [DOI] [PubMed] [Google Scholar]
- Lambrecht S, Smith V, De Wilde K, Coudenys J, Decuman S, Deforce D, De Keyser F, & Elewaut D (2014). Growth Differentiation Factor 15, a Marker of Lung Involvement in Systemic Sclerosis, Is Involved in Fibrosis Development but Is not Indispensable for Fibrosis Development. Arthritis & Rheumatology, 66, 418–427. [DOI] [PubMed] [Google Scholar]
- Lee S-H, Bahn JH, Choi CK, Whitlock NC, English AE, Safe S, & Baek SJ (2008). ESE-1/EGR-1 pathway plays a role in tolfenamic acid-induced apoptosis in colorectal cancer cells. Mol Cancer Ther, 7, 3739–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Cekanova M, & Baek SJ (2008). Multiple mechanisms are involved in 6- gingerol-induced cell growth arrest and apoptosis in human colorectal cancer cells. Mol Carcinog, 47, 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Kim JS, Yamaguchi K, Eling TE, & Baek SJ (2005). Indole-3-carbinol and 3,3’-diindolylmethane induce expression of NAG-1 in a p53-independent manner. Biochem Biophys Res Commun, 328, 63–69. [DOI] [PubMed] [Google Scholar]
- Lee SH, Richardson RL, Dashwood RH, & Baek SJ (2012). Capsaicin represses transcriptional activity of beta-catenin in human colorectal cancer cells. J Nutr Biochem, 23, 646–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Yamaguchi K, Kim JS, Eling TE, Safe S, Park Y, & Baek SJ (2006). Conjugated linoleic acid stimulates an anti-tumorigenic protein NAG-1 in an isomer specific manner. Carcinogenesis, 27, 972–981. [DOI] [PubMed] [Google Scholar]
- Lerner L, Tao J, Liu Q, Nicoletti R, Feng B, Krieger B, Mazsa E, Siddiquee Z, Wang R, Huang L, Shen L, Lin J, Vigano A, Chiu MI, Weng Z, Winston W, Weiler S, & Gyuris J (2016). MAP3K11/GDF15 axis is a critical driver of cancer cachexia. Journal of cachexia, sarcopenia and muscle, 7, 467–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Wang J, Kong J, Tang J, Wu Y, Xu E, Zhang H, & Lai M (2015). GDF15 promotes EMT and metastasis in colorectal cancer. Oncotarget, 7, 860–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Veltri RW, Yuan Z, Christudass CS, & Mandecki W (2015). Macrophage Inhibitory Cytokine 1 Biomarker Serum Immunoassay in Combination with PSA Is a More Specific Diagnostic Tool for Detection of Prostate Cancer. PLoS One, 10, e0122249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ, Liu J, Lupino K, Liu X, Zhang L, & Pei L (2018). Growth Differentiation Factor 15 Maturation Requires Proteolytic Cleavage by PCSK3, −5, and −6. Molecular and Cellular Biology, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li PX, Wong J, Ayed A, Ngo D, Brade AM, Arrowsmith C, Austin RC, & Klamut HJ (2000). Placental transforming growth factor-beta is a downstream mediator of the growth arrest and apoptotic response of tumor cells to DNA damage and p53 overexpression. J Biol Chem, 275, 20127–20135. [DOI] [PubMed] [Google Scholar]
- Li S, Ma Y-M, Zheng P-S, & Zhang P (2018). GDF15 promotes the proliferation of cervical cancer cells by phosphorylating AKT1 and Erk1/2 through the receptor ErbB2. Journal of Experimental &Clinical Cancer Research, 37, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew CW, Boucher J, Cheong JK, Vernochet C, Koh H-J, Mallol C, Townsend K, Langin D, Kawamori D, Hu J, Tseng Y-H, Hellerstein MK, Farmer SR, Goodyear L, Doria A, Blüher M, Hsu SIH, & Kulkarni RN (2013). Ablation of TRIP-Br2, a regulator of fat lipolysis, thermogenesis and oxidative metabolism, prevents diet-induced obesity and insulin resistance. Nature Medicine, 19, 217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, He X, Su J, Wang J, Liu X, Xu K, De W, Zhang E, Guo R, & Shi YE (2018). EZH2-Mediated Epigenetic Suppression of GDF15 Predicts a Poor Prognosis and Regulates Cell Proliferation in Non-Small-Cell Lung Cancer. Molecular therapy. Nucleic acids, 12, 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia L, Tsai VW-W, Nguyen AD, Johnen H, Kuffner T, Shi Y-C, Lin S, Herzog H, Brown DA, Breit SN, & Sainsbury A (2012). Macrophage inhibitory cytokine 1 (MIC-1/GDF15) decreases food intake, body weight and improves glucose tolerance in mice on normal & obesogenic diets. PLoS One, 7, e34868–e34868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez JM, Sali T, Okazaki R, Anna C, Hollingshead M, Hose C, Monks A, Walker NJ, Baek SJ, & Eling TE (2006). Drug-induced expression of nonsteroidal anti- inflammatory drug-activated gene/macrophage inhibitory cytokine-1/prostate- derived factor, a putative tumor suppressor, inhibits tumor growth. J Pharmacol Exp Ther, 318, 899–906. [DOI] [PubMed] [Google Scholar]
- Mimeault M, Johansson SL, & Batra SK (2012). Pathobiological Implications of the Expression of EGFR, pAkt, NF-κB and MIC-1 in Prostate Cancer Stem Cells and Their Progenies. PLoS One, 7, e31919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KW, Lee SH, & Baek SJ (2016). Moonlighting proteins in cancer. Cancer Lett, 370, 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KW, Liggett JL, Silva G, Wu WW, Wang R, Shen RF, Eling TE, & Baek SJ (2016). NAG-1/GDF15 accumulates in the nucleus and modulates transcriptional regulation of the Smad pathway. Oncogene, 35, 377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullican SE, Lin-Schmidt X, Chin CN, Chavez JA, Furman JL, Armstrong AA, Beck SC, South VJ, Dinh TQ, Cash-Mason TD, Cavanaugh CR, Nelson S, Huang C, Hunter MJ, & Rangwala SM (2017). GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat Med, 23, 1150–1157. [DOI] [PubMed] [Google Scholar]
- Mullican SE, & Rangwala SM (2018). Uniting GDF15 and GFRAL: Therapeutic Opportunities in Obesity and Beyond. Trends in Endocrinology & Metabolism, 29, 560–570. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Scorilas A, Stephan C, Yousef GM, Kristiansen G, Jung K, & Diamandis EP (2003). Quantitative analysis of macrophage inhibitory cytokine-1 (MIC-1) gene expression in human prostatic tissues. Br J Cancer, 88, 1101–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nualsanit T, Rojanapanthu P, Gritsanapan W, Lee SH, Lawson D, & Baek SJ (2012). Damnacanthal, a noni component, exhibits antitumorigenic activity in human colorectal cancer cells. J Nutr Biochem, 23, 915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen OE, Skjærvik A, Størdal BF, Sundan A, & Holien T (2017). TGF-β contamination of purified recombinant GDF15. PLoS One, 12, e0187349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paralkar VM, Vail AL, Grasser WA, Brown TA, Xu H, Vukicevic S, Ke HZ, Qi H, Owen TA, & T DD. (1998). Cloning and characterization of a novel member of the transforming growth factor-β/ bone morphogenetic protein family. J Biol Chem, 273, 13760–13767. [DOI] [PubMed] [Google Scholar]
- Peake BF, Eze SM, Yang L, Castellino RC, & Nahta R (2017). Growth differentiation factor 15 mediates epithelial mesenchymal transition and invasion of breast cancers through IGF-1R-FoxM1 signaling. Oncotarget, 8, 94393–94406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth P, Junker M, Tritschler I, Mittelbronn M, Dombrowski Y, Breit SN, Tabatabai G, Wick W, Weller M, & Wischhusen J (2010). GDF-15 Contributes to Proliferation and Immune Escape of Malignant Gliomas. Clinical Cancer Research, 16, 3851–3859. [DOI] [PubMed] [Google Scholar]
- Sasahara A, Tominaga K, Nishimura T, Yano M, Kiyokawa E, Noguchi M, Noguchi M, Kanauchi H, Ogawa T, Minato H, Tada K, Seto Y, Tojo A, & Gotoh N (2017). An autocrine/paracrine circuit of growth differentiation factor (GDF) 15 has a role for maintenance of breast cancer stem-like cells. Oncotarget, 8, 24869–24881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senapati S, Rachagani S, Chaudhary K, Johansson SL, Singh RK, & Batra SK (2010). Overexpression of macrophage inhibitory cytokine-1 induces metastasis of human prostate cancer cells through the FAK-RhoA signaling pathway. Oncogene, 29, 1293–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Kadowaki M, Yoshioka H, Kambe A, Watanabe T, Kinyamu HK, & Eling TE (2013). Proteasome inhibitor MG132 induces NAG-1/GDF15 expression through the p38 MAPK pathway in glioblastoma cells. Biochemical and Biophysical Research Communications, 430, 1277–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shnaper S, Desbaillets I, Brown DA, Murat A, Migliavacca E, Schluep M, Ostermann S, Hamou M-F, Stupp R, Breit SN, de Tribolet N, & Hegi ME (2009). Elevated levels of MIC-1/GDF15 in the cerebrospinal fluid of patients are associated with glioblastoma and worse outcome. International journal of cancer, 125, 2624–2630. [DOI] [PubMed] [Google Scholar]
- Soto-Cerrato V, Viñals F, Lambert JR, Kelly JA, & Pérez-Tomás R (2007). Prodigiosin induces the proapoptotic gene NAG-1 via glycogen synthase kinase-3β activity in human breast cancer cells. Molecular Cancer Therapeutics, 6, 362–369. [DOI] [PubMed] [Google Scholar]
- Stienstra R, Joosten LAB, Koenen T, van Tits B, van Diepen JA, van den Berg SAA, Rensen PCN, Voshol PJ, Fantuzzi G, Hijmans A, Kersten S, Müller M, van den Berg WB, van Rooijen N, Wabitsch M, Kullberg B-J, van der Meer JWM, Kanneganti T, Tack CJ, & Netea MG (2010). The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell metabolism, 12, 593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienstra R.Tack, Cees J, Kanneganti T-D, Joosten Leo A. B., & Netea Mihai G. (2012). The Inflammasome Puts Obesity in the Danger Zone. Cell metabolism, 15, 10–18. [DOI] [PubMed] [Google Scholar]
- Tran T, Yang J, Gardner J, & Xiong Y (2018). GDF15 deficiency promotes high fat diet- induced obesity in mice. PLoS One, 13, e0201584–e0201584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai VW-W, Macia L, Johnen H, Kuffner T, Manadhar R, Jørgensen SB, Lee-Ng KKM, Zhang HP, Wu L, Marquis CP, Jiang L, Husaini Y, Lin S, Herzog H, Brown DA, Sainsbury A, & Breit SN (2013). TGF-b superfamily cytokine MIC-1/GDF15 is a physiological appetite and body weight regulator. PLoS One, 8, e55174–e55174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai VWW, Husaini Y, Sainsbury A, Brown DA, & Breit SN (2018). The MIC- 1/GDF15-GFRAL Pathway in Energy Homeostasis: Implications for Obesity, Cachexia, and Other Associated Diseases. Cell metabolism, 28, 353–368. [DOI] [PubMed] [Google Scholar]
- Tschöp MH, Speakman JR, Arch JRS, Auwerx J, Brüning JC, Chan L, Eckel RH, Farese RV Jr., Galgani JE, Hambly C, Herman MA, Horvath TL, Kahn BB, Kozma SC, Maratos-Flier E, Müller TD, Münzberg H, Pfluger PT, Plum L, Reitman ML, Rahmouni K, Shulman GI, Thomas G, Kahn CR, & Ravussin E (2011). A guide to analysis of mouse energy metabolism. Nature methods, 9, 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui KH, Hsu SY, Chung LC, Lin YH, Feng TH, Lee TY, Chang PL, & Juang HH (2015). Growth differentiation factor-15: a p53- and demethylation-upregulating gene represses cell proliferation, invasion, and tumorigenesis in bladder carcinoma cells. Sci Rep, 5, 12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandanmagsar B, Youm Y-H, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, & Dixit VD (2011). The NLRP3 inflammasome instigates obesity- induced inflammation and insulin resistance. Nature Medicine, 17, 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vocka M, Langer D, Petrtyl J, Kalousova M, Zima T, Hanus T, & Petruzelka LB (2016). Growth differentiation factor 15 (GDF-15) as potential serum biomarkers in patients with metastatic colorectal cancer. Journal of Clinical Oncology, 34, e15098–e15098. [Google Scholar]
- Wang T, Liu J, McDonald C, Lupino K, Zhai X, Wilkins BJ, Hakonarson H, & Pei L (2017). GDF15 is a heart-derived hormone that regulates body growth. EMBO molecular medicine, 9, 1150–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Mao B, Cheng C, Zou Z, Gao J, Yang Y, Lei T, Qi X, Yuan Z, Xu W, & Lu Z (2018). YAP promotes breast cancer metastasis by repressing growth differentiation factor-15. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease, 1864, 1744–1753. [DOI] [PubMed] [Google Scholar]
- Wang X, Baek SJ, & Eling TE (2013). The diverse roles of nonsteroidal anti- inflammatory drug activated gene (NAG-1/GDF15) in cancer. Biochem Pharmacol, 85, 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chen LL, & Zhang Q (2016). Increased Serum Level of Growth Differentiation Factor 15 (GDF-15) is Associated with Coronary Artery Disease. Cardiovasc Ther, 34, 138–143. [DOI] [PubMed] [Google Scholar]
- Wang X, Chrysovergis K, Bienstock RJ, Shim M, & Eling TE (2012). The H6D variant of NAG-1/GDF15 inhibits prostate xenograft growth in vivo. The Prostate, 72, 677–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Chrysovergis K, Kosak J, Kissling G, Streicker M, Moser G, Li R, & Eling TE (2014). hNAG-1 increases lifespan by regulating energy metabolism and insulin/IGF-1/mTOR signaling. Aging, 6, 690–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiklund FE, Bennet AM, Magnusson PK, Eriksson UK, Lindmark F, Wu L, Yaghoutyfam N, Marquis CP, Stattin P, Pedersen NL, Adami HO, Gronberg H, Breit SN, & Brown DA (2010). Macrophage inhibitory cytokine-1 (MIC- 1/GDF15): a new marker of all-cause mortality. Aging cell, 9, 1057–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollert KC, Kempf T, Lagerqvist B, Lindahl B, Olofsson S, Allhoff T, Peter T, Siegbahn A, Venge P, Drexler H, & Wallentin L (2007). Growth Differentiation Factor 15 for Risk Stratification and Selection of an Invasive Treatment Strategy in Non–ST-Elevation Acute Coronary Syndrome. Circulation, 116, 1540–1548. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Walker K, Min X, Hale C, Tran T, Komorowski R, Yang J, Davda J, Nuanmanee N, Kemp D, Wang X, Liu H, Miller S, Lee KJ, Wang Z, & Véniant MM (2017). Long-acting MIC-1/GDF15 molecules to treat obesity: Evidence from mice to monkeys. Science Translational Medicine, 9, eaan8732. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Cekanova M, McEntee MF, Yoon JH, Fischer SM, Renes IB, Van Seuningen I, & Baek SJ (2008). Peroxisome proliferator-activated receptor ligand MCC-555 suppresses intestinal polyps in ApcMin/+ mice via extracellular signal- regulated kinase and peroxisome proliferator-activated receptor-dependent pathways. Mol Cancer Ther, 7, 2779–2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K, Lee SH, Eling TE, & Baek SJ (2004). Identification of nonsteroidal anti- inflammatory drug-activated gene (NAG-1) as a novel downstream target of phosphatidylinositol 3-kinase/AKT/GSK-3beta pathway. J Biol Chem, 279, 49617–49623. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Whitlock NC, Liggett JL, Legendre AM, Fry MM, & Baek SJ (2008). Molecular characterisation of canine nonsteroidal anti-inflammatory drug-activated gene (NAG-1). Vet J, 175, 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Filipovic Z, Brown D, Breit SN, & Vassilev LT (2003). Macrophage inhibitory cytokine-1: a novel biomarker for p53 pathway activation. Mol Cancer Ther, 2, 1023–1029. [PubMed] [Google Scholar]
- Yang L, Chang CC, Sun Z, Madsen D, Zhu H, Padkjaer SB, Wu X, Huang T, Hultman K, Paulsen SJ, Wang J, Bugge A, Frantzen JB, Norgaard P, Jeppesen JF, Yang Z, Secher A, Chen H, Li X, John LM, Shan B, He Z, Gao X, Su J, Hansen KT, Yang W, & Jorgensen SB (2017). GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat Med, 23, 1158–1166. [DOI] [PubMed] [Google Scholar]
- Yoon JH, & Baek SJ (2005). Molecular targets of dietary polyphenols with anti- inflammatory properties. Yonsei Med J, 46, 585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Ryu J, & Baek SJ (2018). Moonlighting Activity of Secreted Inflammation- Regulatory Proteins. Yonsei medical journal, 59, 463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Moszczynski LA, Liu Q, Jiang J, Zhao D, Quan D, Mele T, McAlister V, Jevnikar A, Baek SJ, Liu K, & Zheng X (2017). Over-expression of growth differentiation factor 15 (GDF15) preventing cold ischemia reperfusion (I/R) injury in heart transplantation through Foxo3a signaling. Oncotarget, 8, 36531–36544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Wu L, Wang J, Li G, Feng D, Zhang B, Li L, Yang J, Ma L, & Qin H (2014). Opposing Effects of PI3K/Akt and Smad-Dependent Signaling Pathways in NAG-1- Induced Glioblastoma Cell Apoptosis. PLoS One, 9, e96283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Li Y, Qiu W, He F, Zhang W, Zhao D, Zhang Z, Zhang E, Ma P, Liu Y, Ma L, Yang F, Wang Y, & Shu Y (2018). C5a induces A549 cell proliferation of non-small cell lung cancer via GDF15 gene activation mediated by GCN5-dependent KLF5 acetylation. Oncogene, 37, 4821–4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmers TA, Gutierrez JC, & Koniaris LG (2010). Loss of GDF-15 abolishes sulindac chemoprevention in the ApcMin/+ mouse model of intestinal cancer. J Cancer Res Clin Oncol, 136, 571–576. [DOI] [PubMed] [Google Scholar]