Abstract
To address the need for a quantitative approach to the measurement of cleaning effectiveness, related to biologically derived surface contamination, three commercially available adenosine triphosphate (ATP) test systems were used to collect multiple samples measured in relative light units (RLUs) from 27 elementary schools in the southwestern United States before and after a standardized cleaning protocol. The database consisted of 6480 ATP measurements from four critical surfaces (student desktops, cafeteria tabletops, and restroom sinks and stall doors). Data was assessed according to ranges of ATP values before and after cleaning. Results showed the potential for such data to provide the basis for a standardized approach to the measurement of cleaning effectiveness, based on detection and quantification of pollutant loads of biological origin, across critical surfaces in school building environments. It is anticipated that verification of this data in school buildings across different geographic and climatic regions will lead to the establishment of “acceptable” ranges of ATP values that can be used as a practice-based approach to improving cleaning practices and contributing to healthier school environments.
Keywords: adenosine triphosphate, clean, disease, schools, surface contamination
INTRODUCTION
School environments are a particular concern related to adverse health effects, as well as the potential for poor indoor environmental quality (IEQ), which can compromise the learning process of students. According to a U.S. Government Accountability Office report,(1) schools in unsatisfactory condition can be found in every part of the country. More than 14 million children are being taught in school buildings needing significant repairs to restore them to good overall condition. To compound this problem, the definition of a “clean” environment in educational facilities is lacking at present. Meeting the challenge in setting practitioner-based cleaning protocols today must be based on the relationship between our understanding of the term “clean” and its relationship to the health of occupants. Today, the professional cleaning industry has moved toward the approach of “cleaning for health first and appearance second,” based on three decades of investigation and research that has characterized the building ecosystem, in addition to the assessment of occupant exposures and potential health risks.
Cleanliness requirements for commercial or public buildings, or specific operations within such buildings, often require facilities to be kept in a clean and sanitary condition, typically determined by visual inspection. Rarely are quantitative measurements of the surfaces and materials that are cleaned and sanitized required in support of the visual assessment. This is unfortunate, as cleaning practices and procedures may be adequate according to an eyeball assessment but inadequate for the efficient removal of unwanted and less visible substances such as biological residue pollutants. As a result, ecological sub-compartments (reservoirs) have been identified that can collect and retain pollutants that without adequate cleaning (physical removal of biologically derived surface soiling/cleanliness residuals invisible to the naked eye) can become active disseminators (sources) of potentially infectious agents.
Centers for Disease Control and Prevention (CDC) guidance on influenza in schools states that school staff should “encourage routine surface cleaning through education, policy, and the provision of supplies,” and to “routinely clean surfaces and objects that are touched often.”(2) School classrooms, locker rooms, cafeterias, and related areas must be cleaned and sanitized to prevent environmental contamination and contact transmission of potentially infectious agents. Similarly, rest-rooms must be cleaned and monitored for effectiveness to reduce exposure and resultant student illnesses from a variety of pathogens.
A recent review of the significance of fomites (inanimate surfaces) in the spread of disease indicates that level of cleanliness is one of a number of primary factors involved in the spread of viral disease in crowded indoor establishments, including schools.(3) Increased efforts at improved cleaning of floors and desks in schools have been shown to reduce upper respiratory symptoms.(4) The reduction of airborne pollutants through effective cleaning practices and, hence, reduced occupant exposures and health risks has been demonstrated in a long-term cleaning effectiveness study.(5) More recent studies collectively indicate that enhanced hygiene in schools and targeted cleaning of biological residual contamination related to frequent contact points result in reduced illnesses tied into bacterial contamination reservoirs (MRSA, Shigella outbreaks), reduced sick building syndrome symptoms, and reduced absenteeism due to infectious illness.(6–10)
Adenosine triphosphate (ATP) has historically been used successfully as an important marker for the detection and quantification of pollutant loads of biological origin and overall cleanliness in the food production and food service industries, and it has been recently confirmed as a representative marker for detection of levels of surface contamination in hospital settings.(11,12) Previous research has also identified the use of ATP marker as a feasible, rapid, real-time, quantitative, and economical approach to the measurement of cleaning effectiveness on a variety of surfaces and materials in school buildings.(13) ATP is the energy driver for biological systems and can be measured through an enzymatic luciferin/luciferase reaction detected and quantified as bioluminescence. The method converts ATP into a light signal that is measured by an instrument that provides a quantitative measurement of ATP from biomass in relative light units (RLUs). Thus, after cleaning and a drop in biomass, there is a corresponding decrease in measureable ATP.
The aim of the research reported here was to develop a standardized approach to the measurement of cleaning effectiveness (based on detection and quantification of pollutant loads of biological origin) using ATP as representative marker across critical surfaces recognized as posing health risks in school building environments.
METHODS
Sampling Methods and Materials Used
In this study, three of the most widely used and commercially available ATP rapid detection systems were chosen to gather data on surface contamination in schools. The three systems used were as follows: NovaLUM (Charm Sciences, Inc., Lawrence, Kans.); Clean-Trace (3M, St. Paul, Minn.); and ATP Hygiene Monitoring System (Hygiena, Camarillo, Calif.).
The first step undertaken was to conduct a pilot study in two school buildings to ensure reliability of the ATP systems in field conditions (data not shown). The pilot study revealed that a statistically significant reduction in ATP measures could be demonstrated with a sample size of 10 samples per surface type.
Prior to field testing, the ATP systems were checked according to their own calibration procedures. Each ATP system is factory calibrated. The systems also have separate calibration control kits for periodic calibration checks, along with blank checks. Sampling was done for each system according to the manufacturer's sampling protocol, and ATP readings in RLUs were those read directly from each test system's instrument. Multiple blanks were run through the course of this study for QA/QC purposes.
In addition, replicate organism detection and counting agar plates (RODAC) were used in parallel with the ATP systems for the detection of culturable bacteria on surfaces as a direct measure of improved sanitization. RODAC plates (Item #823002; Carolina Biological, Burlington, N.C.) contained trypticase soy agar, with lecithin and polysorbate 80 as disinfectant neutralizers.
Selection of School Buildings and Sampling Approach
Thirty-five elementary schools from a 70-school district in the southwestern United States were randomly selected to be available for the surface sampling effort. Determination of the number of schools necessary for the study was based on initial measurement in an 8-school subset of the 35 schools. The eight schools were sampled using the three ATP systems and RODAC plates to gather initial data on surface contamination in the schools according to the protocols discussed below. Based on resultant data, power analyses revealed the adequate number of schools necessary for assessment of surface contamination pre- and post-cleaning to be between 25 and 30 schools. The final number of schools included in this study was 27.
All schools were studied 1 week at a time, which allowed testing critical surfaces in each school over 3 different days within the week. Thus, the 27-school study spanned a 30-week period within the 2009–2010 academic year.
Cleaning and Sampling Protocol
Standardized pre-cleaning sampling (all ATP and RODAC) was completed on each surface before routine school- scheduled cleaning had occurred in each area. Pre-cleaning sampling was conducted after lunch activities had taken place in the cafeteria area and at the end of the school day for the classroom and restroom areas. Based on the pilot study (discussed above) as well as a review of current optimal approaches to surface cleaning practices, a standardized cleaning protocol was used to prepare the surfaces for the post-cleaning measurements.
All cleaning was done by a school district project team trained to ensure that the protocol was followed precisely in each school building, thus eliminating school-to-school variability among cleaning personnel. Prior to the post-cleaning samples being taken, cleaning was conducted on the study surface by the following protocol: (1) test surfaces were first wiped with a microfiber towel and then sprayed with an EPA-registered one-step cleaner/disinfectant, routinely used in the school district, covering the desired area; (2) the surface was then wiped with another microfiber cloth until dry, after which post-cleaning ATP and RODAC samples were collected directly adjacent to locations where the corresponding pre- cleaning samples were taken.
For sample collection, clean and disinfected neoprene templates (25-cm2 sample area) were used as the areas for swabbing for ATP from all surfaces—student desktops, cafeteria tabletops, restroom sink edges, and stall doors. As with the ATP sampling templates, the surface area of each RODAC agar plate was approximately 25 cm2.
For each school, two fifth-grade classrooms were selected for ATP and RODAC sampling. Ten total desk surfaces were selected for sampling each day. In the cafeterias, five tables were selected and divided into two halves for a total of 10 cafeteria sampling surfaces each day. For bathroom areas, two restrooms in each school were selected (one girls and one boys); a total of 10 sink areas and 10 stall doors were selected for sampling each day. Thus, for each school on a given day, and using a single ATP system, there were a total of 80 ATP samples and 80 bacterial (RODAC) samples collected. Thus, in one week in one school, there were 240 ATP (120 pre, 120 post) and 80 RODAC samples (40 pre, 40 post, sampled on one day of the week) collected. In 27 weeks there was a total of 6480 ATP samples collected (2160 for each ATP system: 1080 pre, 1080 post) and 2160 RODAC samples collected (1080 pre, 1080 post).
Care was taken to ensure that surfaces sampled on any given day were adjacent to but not from the same spot sampled on the previous sampling day. The order of use of each ATP system was rotated within a week throughout the sampling period to avoid any systematic effects related to a specific weekday (e.g., caused by occupancy patterns and cleaning routines practiced in the schools).
Building Characterization
In addition to ATP and bacterial data, a comprehensive approach to building characterization was also used to explore other factors that may influence results pertaining to cleaning. School and classroom characterization checklists noting information related to building type, construction materials, age, ventilation systems, designated cleaning regimens within the schools, moisture damage (pre- and existing), and so on, were recorded for each school. Whereas data was screened to avoid any abnormalities that could influence the assessment of cleaning effectiveness, this article focuses on ATP and bacterial data and includes only the surface types and materials in the analyses.
Statistical Analyses
The study protocol was designed to measure differences in ATP readings (using the three different ATP methods in parallel) before and after cleaning on selected surfaces. Analyses of pre- and post-cleaning data from critical surfaces focused on the reduction of measured RLU values afforded by the cleaning (as well as percent reduction) from each of the three ATP systems. Likewise, to observe whether a significant reduction in ATP readings by the three test systems would correspond to a meaningful decrease in total aerobic bacteria, pre- and post-cleaning levels and percent reductions of total culturable aerobic bacteria were assessed by means of RODAC plates for the selected surfaces and locations.
SPSS statistical software version 17.0 (IBM, Armonk, N.Y.) was used for all analyses. First, the distributions were studied, as well as descriptive statistics including mean, median, standard deviation (SD), and percentile values of the pre- and post-cleaning measurements for each method. Log-transformed values, 5% trimmed mean, and SD for log-transformed data were calculated. Percent reduction was calculated for each method as pre-cleaning minus post-cleaning divided by pre-cleaning times 100%.
RESULTS AND DISCUSSION
For the ATP pre- and post-cleaning measurements, across all surfaces, log-normal distributions were reached when the lowest and highest 2.5% pre-cleaning readings were trimmed. For post-cleaning measurements, log-normal distributions were reached for ATP1, ATP2, and RODAC when the lowest and highest 2.5% of the readings were trimmed. For ATP3 post-cleaning, log-normal distribution was reached when the lowest and highest 5% of the readings were trimmed.
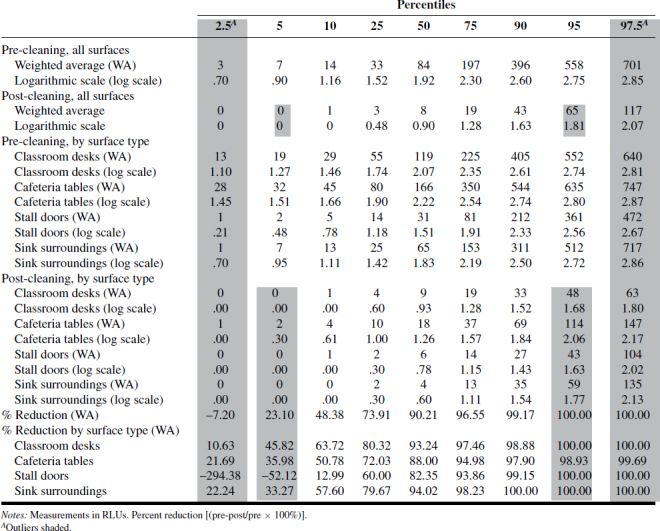
A summary of the ATP and RODAC data is presented in Tables I–IV. Tables I–III show ATP data as RLUs and percent reduction in terms of 2.5, 5, 10, 25, 50, 75, 90, 95, and 97.5 percentiles across “all surfaces,” as well as individual surfaces. Based on those values, as well as values expressed as log-transformed data, there was consistency between ATP1 and ATP2 test systems where RLU values were highest for classroom desks and cafeteria tables, followed by sink surfaces and then toilet stall doors. The ATP3 test system data results were less consistent and did not follow the ATP1 and ATP2 results as to ranking of most-to-least contaminated of the four surfaces examined. The reason for this inconsistency should be further examined for more reliable assessment of surface-specific cleanliness.
TABLE II. ATP2 Measurements and Percent Reduction.
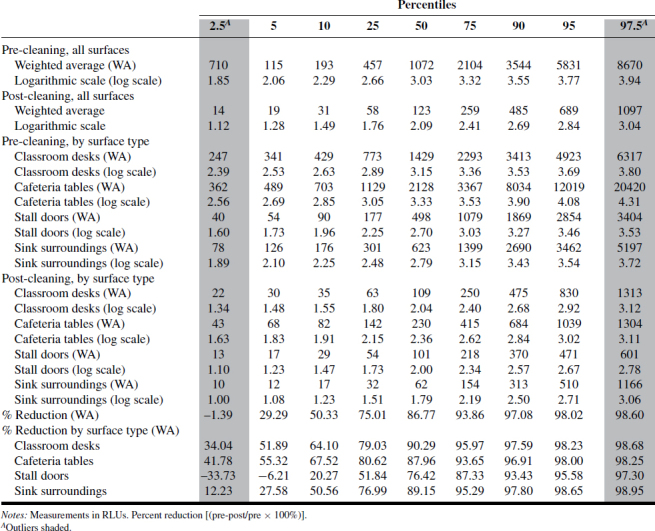 |
TABLE I. ATP1 Measurements and Percent Reduction.
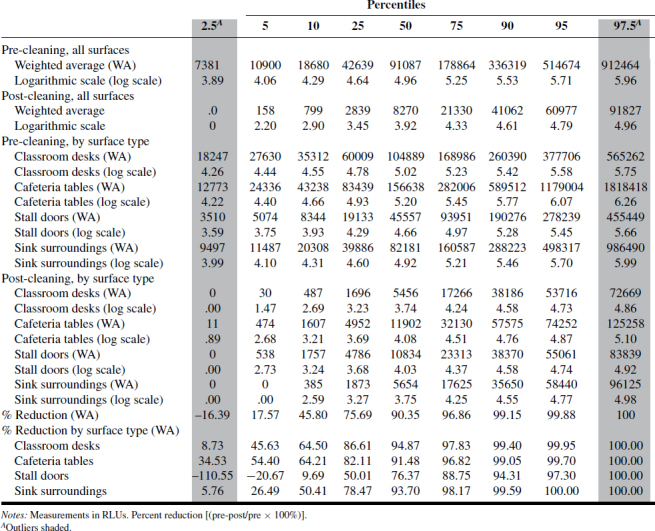 |
TABLE IV. RODAC Measurements and Percent Reduction.
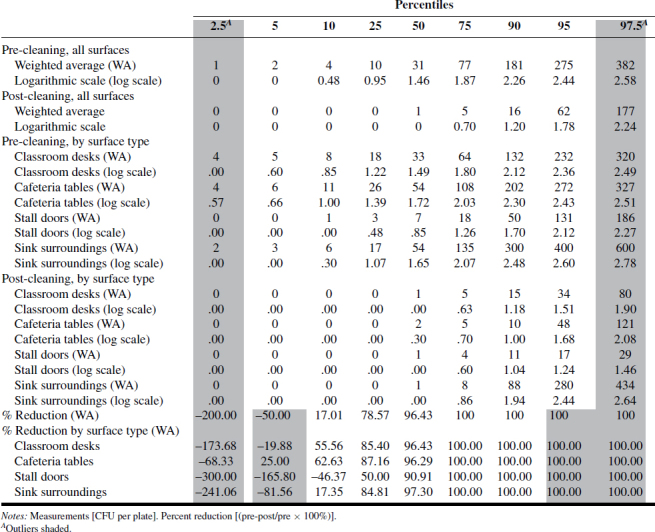 |
TABLE III. ATP3 Measurements and Percent Reduction.
 |
The distribution of RLU values shown for ATP1 and ATP2 represents 4320 measurements (2160 pre-cleaning, 2160 post-cleaning) and provides for the first time a view of the extent of biologically associated surface contamination across multiple school buildings, as well as its statistically derived distribution. Those values were derived on the basis of an acceptably rigorous cleaning protocol, in conjunction with a standardized method of ATP testing.
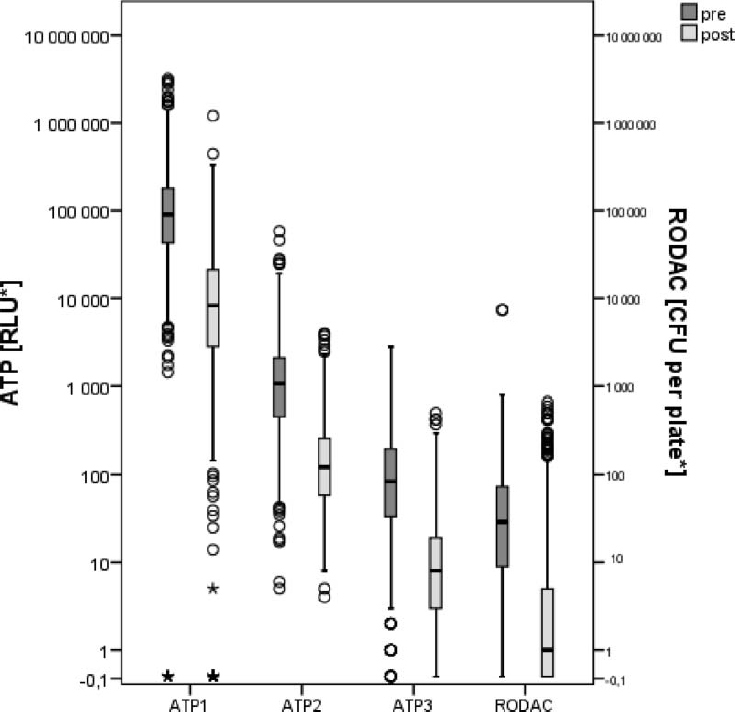
Compared with pre-clean measurements, post-cleaning data was approximately one order of magnitude (or 90%) lower than pre-cleaning data, as shown in Figure 1. Analysis of the 6480 ATP samples demonstrated the potential for ATP sampling to serve as a means of defining and quantifying cleaning effectiveness based on a percent reduction approach. While percent reductions for “all surfaces” appear consistent across the three ATP methods, the data for individual surfaces are less so, indicating a need for individual values for specific critical surfaces.
FIGURE 1. Box plots for collective all surfaces pre- and post-cleaning results for ATP and RODAC data. Note:
 ATP and RODAC values are each sampled for 25 cm2 surface area.
ATP and RODAC values are each sampled for 25 cm2 surface area.
Tables I–IV provide the log-normal distribution data for all the ATP and RODAC data. Using this data to generate “typical” ranges of pre- and post-ATP or RODAC data can be conducted based on a statistical approach often used to describe such distributions. Since about 95% of values drawn from a normal distribution are within two SD away from the mean and about 68% of the values are within one SD, we calculated typical ranges for log-transformed data based on 5% trimmed mean ± 2SD. In most cases, typical range is approximately ±1 log from the mean. Defining what may be acceptable as a reasonable range of values in schools can be based on a more rigorous criteria derived statistically. The range corresponding to a value less than (or equal to) +1SD from the 5% trimmed mean was calculated and referred to as a reasonable range, i.e., values that are not extraordinarily high. These typical and reasonable ranges are presented in Tables V (pre-cleaning) and VI (post-cleaning) as log-transformed data.
TABLE V. Typical Ranges for Pre-Cleaning Log-Transformed ATP and RODAC.
| 5% Trimmed Mean | SD | ±2SD Typical Range | ≤+1SD Reasonable RangeA | |
|---|---|---|---|---|
| ATP1 | ||||
| Pre-cleaning, all surfaces | 4.96 | 0.50 | 3.97–5.96 | ≤5.46 |
| Pre-cleaning, by surface type | ||||
| Classroom desks | 5.01 | 0.36 | 4.28–5.73 | ≤5.37 |
| Cafeteria tables | 5.19 | 0.46 | 4.27–6.12 | ≤5.65 |
| Stall doors | 4.62 | 0.51 | 3.60–5.65 | ≤5.13 |
| Sink surroundings | 4.91 | 0.47 | 3.97–5.85 | ≤5.38 |
| ATP2 | ||||
| Pre-cleaning, all surfaces | 2.99 | 0.52 | 1.95–4.02 | ≤3.51 |
| Pre-cleaning, by surface type | ||||
| Classroom desks | 3.13 | 0.36 | 2.41–3.85 | ≤3.49 |
| Cafeteria tables | 3.32 | 0.41 | 2.51–4.14 | ≤3.73 |
| Stall doors | 2.65 | 0.53 | 1.59–3.71 | ≤3.18 |
| Sink surroundings | 2.82 | 0.47 | 1.88–3.75 | ≤3.29 |
| ATP3 | ||||
| Pre-cleaning, all surfaces | 1.91 | 0.57 | 0.77–3.05 | ≤2.48 |
| Pre-cleaning, by surface type | ||||
| Classroom desks | 2.04 | 0.48 | 1.09–3.00 | ≤2.52 |
| Cafeteria tables | 2.21 | 0.40 | 1.41–3.01 | ≤2.61 |
| Stall doors | 1.53 | 0.61 | 0.32–2.74 | ≤2.14 |
| Sink surroundings | 1.80 | 0.54 | 0.71–2.89 | ≤2.35 |
| RODAC | ||||
| Pre-cleaning, all surfaces | 1.41 | 0.69 | 0.02–2.80 | ≤2.10 |
| Pre-cleaning, by surface type | ||||
| Classroom desks | 1.49 | 0.54 | 0.42–2.56 | ≤2.03 |
| Cafeteria tables | 1.70 | 0.53 | 0.64–2.76 | ≤2.23 |
| Stall doors | 0.87 | 0.61 | 0.00–2.08 | ≤1.48 |
| Sink surroundings | 1.56 | 0.80 | 0.00–3.15 | ≤2.35 |
Reasonable range to be interpreted as values that are not extraordinarily high.
TABLE VI. Typical Ranges for Post-Cleaning Log-Transformed ATP and RODAC.
| 5% Trimmed Mean | SD | ±2SD Typical Range | ≤+1SD Reasonable RangeA | |
|---|---|---|---|---|
| ATP1 | ||||
| Post-cleaning, all surfaces | 3.87 | 0.98 | 1.91–5.83 | ≤4.85 |
| Post-cleaning, by surface type | ||||
| Classroom desks | 3.70 | 1.02 | 1.66–5.74 | ≤4.72 |
| Cafeteria tables | 4.05 | 0.85 | 2.36–5.75 | ≤4.90 |
| Stall doors | 3.99 | 0.88 | 2.22–5.76 | ≤4.87 |
| Sink surroundings | 3.69 | 1.09 | 1.51–5.86 | ≤4.77 |
| ATP2 | ||||
| Post-cleaning, all surfaces | 2.08 | 0.48 | 1.13–3.04 | ≤2.56 |
| Post-cleaning, by surface type | ||||
| Classroom desks | 2.08 | 0.45 | 1.18–2.98 | ≤2.53 |
| Cafeteria tables | 2.37 | 0.36 | 1.65–3.10 | ≤2.74 |
| Stall doors | 2.02 | 0.43 | 1.16–2.88 | ≤2.45 |
| Sink surroundings | 1.84 | 0.51 | 0.83–2.85 | ≤2.34 |
| ATP3 | ||||
| Post-cleaning, all surfaces | 0.89 | 0.57 | 0.00–2.02 | ≤1.46 |
| Post-cleaning, by surface type | ||||
| Classroom desks | 0.90 | 0.51 | 0.00–1.91 | ≤1.41 |
| Cafeteria tables | 1.25 | 0.49 | 0.27–2.23 | ≤1.74 |
| Stall doors | 0.75 | 0.51 | 0.00–1.78 | ≤1.26 |
| Sink surroundings | 0.67 | 0.60 | 0.00–1.87 | ≤1.27 |
| RODAC | ||||
| Post-cleaning, all surfaces | 0.34 | 0.60 | 0.00–1.54 | ≤0.94 |
| Post-cleaning, by surface type | ||||
| Classroom desks | 0.33 | 0.53 | 0.00–1.38 | ≤0.85 |
| Cafeteria tables | 0.35 | 0.54 | 0.00–1.43 | ≤0.89 |
| Stall doors | 0.27 | 0.46 | 0.00–1.19 | ≤0.73 |
| Sink surroundings | 0.47 | 0.79 | 0.00–2.05 | ≤1.26 |
Reasonable range to be interpreted as values that are not extraordinarily high.
While ATP does not directly monitor viruses in an environment, it does measure a mixture of biological forms that indicate human cellular material, along with that from a variety of bacteria and fungi. Such material includes epithelium from upper respiratory mucus membranes (mouth, throat, nasal passages), and from saliva and exudates and associated material from coughs and sneezes from persons with viral as well as bacterial infections. Viruses are associated with living cells as viruses need them to replicate. Again, ATP is an overall generic marker of biological contamination, and it allows us to monitor potential viral contamination (from viral infections) indirectly.
The ATP/RODAC data comparison from this study reveals a reduction of culturable bacteria concomitant with ATP reduction after cleaning. An example of RODAC (culturable bacteria data) compared with the ATP data for a specific surface type (and broken down into surface material) is provided in Figure 2. The data display the microbial reduction as compared with the ATP values.
FIGURE 2. Median ATP [RLU*] and RODAC [CFU per plate*] readings for bathroom/stall door samples by material. Note: ATP and RODAC values are each sampled for 25-cm2 surface area.
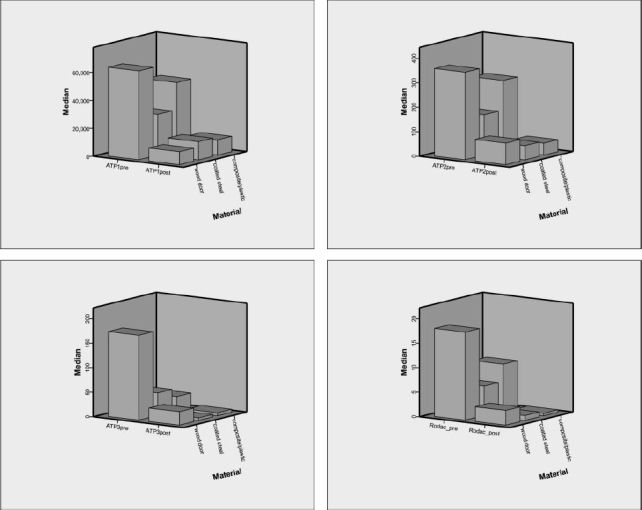
Table VII shows correlation coefficients between log-transformed ATP and RODAC values. There are statistically significant, moderate correlations between pre-cleaning ATP and bacterial counts (Pearson correlations 0.22–0.29). However, post-cleaning correlations between ATP and RODAC were weaker and not statistically significant (Pearson correlations 0.03–0.19), and in general, higher correlations were observed between different ATP systems (pre-cleaning 0.31–0.45, post-cleaning 0.19–0.34). The reason for relatively weak/moderate correlations between different systems could be partly related to the systems themselves, but also, it appears there can be considerable differences in ATP levels across surfaces. Thus, a sample taken adjacent to another sample could have a vastly different reading. It is therefore prudent that assessment of cleaning effectiveness is based on large enough sample size (i.e., in this study 10 samples per surface type per school). Outliers (values that are extremely high or low) should be omitted as indicated inTables I–IV.
TABLE VII. Pearson Correlation Coefficients Between Log-Transformed ATP and RODAC Readings.
| ATP1 | ATP2 | ATP3 | RODAC | |
|---|---|---|---|---|
| Pre-cleaning | ||||
| ATP1 | 1 | .445A | .321A | .290A |
| ATP2 | .445A | 1 | .312A | .274A |
| ATP3 | .321A | .312A | 1 | .219A |
| RODAC | .290A | .274A | .219A | 1 |
| Post-cleaning | ||||
| ATP1 | 1 | .336A | .193A | .010 |
| ATP2 | .336A | 1 | .231A | .110 |
| ATP3 | .193A | .231A | 1 | .084 |
| RODAC | .010 | .110 | .084 | 1 |
Correlation is significant at the 0.01 level (two-tailed).
While the cleanliness of surfaces in school buildings and the standardization of cleaning effectiveness are critical to the building ecosystem, it is recognized that they are inextricably linked to other fundamental indicators of IEQ as well as other building operation and maintenance practices, such as moisture control and mechanical systems. In this regard, dampness and mold problems, as well as building airflow and ventilation have been shown as important for student exposures to airborne biological, particulate, and volatile chemical pollutants, and perhaps academic performance.(14,15) Thus, it remains prudent that when researching surface cleanliness in school buildings, that cleanliness data and the overall health of the school environment should be interpreted relative to various factors, including data collected on temperature and humidity, ventilation, settled dust, and moisture intrusion.
The objective of the study was not to demonstrate a definitive link between disease transmission potential and ATP residuals on surfaces. Rather, it was to exhibit the usefulness of ATP as a problem-solving, relatively simple, rapid, and affordable marker for the routine detection of residual contamination of biological origin on surfaces. ATP is an excellent candidate marker for the monitoring of biologically derived surface soiling/cleanliness, since most surfaces collect and retain soils, dusts, and various deposits containing a myriad of particles and fragments of biological origin, including those from plants (such as pollens, fibers, dusts, and so on) and animals (skin cells, dander, insect parts, secretions, exudates, and so on), as well as microorganisms that are environmentally ubiquitous. ATP monitoring, however, is not appropriate for the determination of the presence or reduction of specific non-biological pollutants that are recognized and regulated human health hazards such as lead, asbestos, and chemical residues, among others.
Based on the extensive measurements taken on critical surface areas of the participant schools, the data generated in this study allow for the initial establishment of typical ranges of ATP values that can be expected in school environments. It is anticipated that such ranges can be established for each surface type, and that those may be used in a standardized and routine approach to the monitoring of cleaning effectiveness in school buildings.
CONCLUSION
The data generated in this study provides a basis for understanding the distribution of ATP RLU values that can be expected pre- and post-cleaning of critical surfaces in an average school building. While all three ATP test systems generated meaningful data showing distinct differences in RLU values pre- and post-cleaning on a variety of materials and surfaces, variability seen related to the three test systems’ readouts of RLU values emphasizes the need for industry to work toward standardization of RLU readings across all test systems. Reasonable range values from this study have been established and may be factored into a standardized approach to the routine monitoring of cleaning effectiveness based on detection and quantification of pollutant loads of biological origin in school buildings. Data also revealed a reduction of culturable bacteria coincident with ATP reduction after cleaning. Specific ATP values/ranges appear to be a function of both surface material type and location within a school building, and further study in school buildings across the United States is expected to confirm unique values for such critical compartments of the school building ecosystem.
ACKNOWLEDGMENTS
This study was supported by a collaborative grant from the Cleaning Industry Research Institute International (Albany, N.Y.) and ISSA, The Worldwide Cleaning Industry Association (Lincolnwood, Ill.). We express our immense gratitude to the facility management personnel of the participating school district in the southwestern United States. We also thank Alesia Bailey and Randy Smith (University of Tulsa, Tulsa, Okla.), and Keith Leese and Cathy Richmond (LRC Indoor Research & Testing, Cary, N.C.), for their dedicated efforts, and without whom this project would not have been successful.
REFERENCES
- 1.General Accounting Office (GAO) : “School Facilities: America's Schools Reporting Differing Conditions.” Document#: GAO/HEHS-96-148. Report to Congressional Requesters, B-272038. Washington, D.C.: GAO, June 14, 1996. [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) : “Guidance for School Administrators to Help Reduce the Spread of Seasonal Influenza in K-12 Schools.” Available at http://www.cdc.gov/flu/school/guidance.htm (accessed October 21, 2012).
- 3.Boone S.A., and Germa C.P.: Significance of fomites in the spread of respiratory and enteric viral disease. Appl. Environ. Microbiol. 73(6): 1687–1696 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walinder R., Norback D., Wieslander G., Smedje G., Erwall C., and Venge P.: Nasal patency and lavage biomarkers in relation to settled dust and cleaning routines in schools. Scand. J. Work Environ. Health 25(2): 137–143 (1999). [DOI] [PubMed] [Google Scholar]
- 5.Franke D.L., Cole E.C., Leese K.E., Foarde K.K., and Berry M.A.: Cleaning for improved indoor air quality: An initial assessment of effectiveness. Indoor Air 7: 41–54 (1997). [Google Scholar]
- 6.Higashiyama M., Ito T., Han X., et al. : Trial to control an outbreak of Panton-Valentine leukocidin-positive methicillin-resistant Staphylococcus aureus at a boarding school in Japan. Am. J. Infect. Control 39: 868–865 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Hostetler K., Lux M., Shelley K., Drummond J., and Laguna P.: MRSA as a health concern in athletic facilities. J. Environ. Health 74: 18–25 (2011). [PubMed] [Google Scholar]
- 8.Nandrup-Bus I.: Comparative studies of hand disinfection and handwashing procedures as tested by pupils in intervention programs. Am. J. Infect. Control 39: 450–455 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Zhang X., Zhao Z., Nordquist T., Larsson L., Sebastian A., and Norback D.: A longitudinal study of sick building syndrome among pupils in relation to microbial components in dust in schools in China. Sci. Total Environ. 409: 5263–5259 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Schulte J., Williams L., Jawaid A., et al. : How we didn't clean up until we washed our hands: Shigellosis in an elementary and middle school in North Texas. South. Med. J. 105: 1–4 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Cunningham A., Rajagopal R., Lauer J., and Allwood P.: Assessment of hygienic quality of surfaces in retail food service establishments based on microbial counts and real-time detection of ATP. J. Food Protect. 74(4): 686–690 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Sherlock O., O'Connell N., Creamer E., and Humphreys H.: Is it really clean? An evaluation of the efficacy of four methods for determining hospital cleanliness. J. Hosp. Infect. 72(2): 140–1466 (2009). [DOI] [PubMed] [Google Scholar]
- 13.Cole E.C., Shaughnessy R.J., Haverinen-Shaughnessy U., and Leese K.E.: Measurement of Cleaning Effectiveness in School Buildings: Evaluation of a Candidate Standard Method. In Proceedings Indoor Air 2011. Santa Cruz, Calif.: ISIAQ, 2011. [Google Scholar]
- 14.Shaughnessy R.J., Haverinen-Shaughnessy U., Nevalainen A., and Moschandreas D.: A preliminary study on the association between ventilation rates in classrooms and student performance. Indoor Air 16(6): 465–468 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Haverinen-Shaughnessy U., Moschandreas D., and Shaughnessy R.: Association between sub-standard classroom ventilation rates and students' academic achievement. J. Indoor Air 21(2): 121–131 (2011). [DOI] [PubMed] [Google Scholar]


