Abstract
This study aimed to examine the effects of lower limb blood flow restriction (BFR) performed during 3-a-side futsal game training on aerobic and anaerobic performance of futsal players. Twelve male futsal players were randomized into two groups (n = 6); both groups performed ten sessions of the 3-a-side game every other day in half of a futsal court; but one group trained under BFR conditions. Pneumatic cuffs used for the BFR group were inflated to 110% leg systolic blood pressure and increased by 10% after each two completed sessions. Before and after the training sessions subjects completed a series of tests to assess aerobic and anaerobic performances along with changes in blood lactate and anabolic and catabolic hormones. All aerobic and anaerobic performance variables improved in both group after training, however improvements in mean power (12.2%, p = 0.03), run time to fatigue (TTF), (7.1%, p = 0.02) and running economy (RE), (-22.7%, p = 0.01) were significantly greater in the BFR group. There were also significant increases in growth hormone (p = 0.01), testosterone to cortisol ratio at first session (p = 0.01) and rate of lactate removal (p = 0.01) at last session in the BFR group compared to the non-BFR group. Small-sided game (SSG) training with the addition of BFR because of accumulated metabolites and hormonal changed leads to substantially greater increases in performance than SSGs training alone.
Key points.
Small-sided game with BFR leads to greater acute increases in GH and testosterone than SSGs training alone.
Small-sided game while BFR result in removing lactate faster than SSGs training alone.
Small-sided game with BFR leads to greater increases in performance than SSGs training alone.
Key words: Blood flow restriction, growth hormone, lactate removal, small sided game, testosterone to cortisol ratio
Introduction
Futsal is a competitive ball sport consisting of short duration sprints interspersed with short recovery periods (Naser et al., 2017). The repeated high-intensity nature of futsal requires players to develop high-levels of both long duration exercise and short duration exercise capacity (Castagna et al., 2009). Indeed, previous research reported that professional players can develop maximum oxygen consumption (VO2max) values in excess of 60 ml·kg-1·min-1 to cope with the games physical requirements (Alvarez et al., 2009; Castagna et al., 2009). Futsal players typically cover 3-5 km during a game and more than 50% of the time is performed at a high intensity (Castagna et al., 2009; Naser et al., 2017). A previous study has reported players achieve a mean blood lactate concentration of 5.3 mmol·L-1 and spend ~80% of actual playing time at exercise intensities higher than 85% of their maximum heart rate (HRmax) (Barbero-Alvarez et al., 2008).
To date, there is little research investigating the actual training of futsal players (Barbieri et al., 2016; Berdejo-del-Fresno et al., 2015). Traditionally, training has focused on a combination of generic team-sport exercises, aimed at improving repeated sprint ability and long duration exercise capacity and simulated game play to enhance skill and techniques (Barbieri et al., 2016). A recent trend in team sports has seen an increase in the use of small-sided game (SSG) to not only improve the players technical and tactical aspects but also provide a time-efficient method for improving players physical condition (Amani-Shalamzari et al., 2019b; Halouani et al., 2014b). Compared to generic training the use of SSGs provides the added benefit of allowing for more training time spent developing game relevant skills and tactical abilities while simultaneously providing game specific fitness (Amani-Shalamzari et al., 2019b; Engel et al., 2018; Helgerud et al., 2001).
Given time constraints, coaches are constantly looking for exercises that may further enhance the effectiveness of the available training sessions. One that has received recent attention is the use of limb blood flow restriction (BFR) (Pope et al., 2013). Tourniquets or pneumatic cuffs are used to occlude or restrict venous return and restrict arterial blood flow during exercise with BFR (Pope et al., 2013). Using BFR during training reduces the amount of oxygen and nutrients supplied to active muscle thereby increasing hypoxic conditions and placing the muscle under greater metabolic stress (Tanimoto et al., 2005). Recently research suggests that occlusion, when used in conjunction with training, may provide greater gains in both aerobic and anaerobic performance (de Oliveira et al., 2016). Previous research has also suggested that BFR training may cause increases the levels of hormones related to muscle hypertrophy like growth hormone (GH) and insulin-like growth factor-1 (IGF-1) (Abe et al., 2005; Takano et al., 2005b). The majority of early research has utilized BFR in conjunction with low-intensity aerobic (<40% VO2max) or resistance training protocols (<30% one repetition maximum (1RM)) and reported enhancements in oxidative enzymes, VO2max, exercise time to exhaustion and increased muscle, hypertrophy and strength (Abe et al., 2010; Scott et al., 2017). More recent research has investigated the use of BFR in conjunction with higher-load training (de Oliveira et al., 2016; Pope et al., 2013; Tanimoto et al., 2005). One study reported no added benefit from the addition of BFR on aerobic capacity (Laurentino et al., 2008), while other studies have reported greater improvements in muscle strength (Cook et al., 2014), VO2max and anaerobic running capacity (de Oliveira et al., 2016) and also sprint performance (Behringer et al., 2017). Given the potential benefits for both aerobic and anaerobic performance characteristics and the relative ease of its application in training situations, it seems plausible that BFR may provide a valuable additional training methodology for futsal players, especially when used in conjunction with SSG play.
Adding BFR to exercise increases the metabolic load (internal exercise load), which leads to further metabolic adaptations such as lactate maintenance during exercise. Due to the specificity principle of training for train based on muscle involvement pattern in the futsal, we hypothesis that adding BFR to 3-a-side game which is predominantly dependent on the aerobic system leads to increase the exercise metabolic load and as well increased lactate tolerance, which players often encounter during the futsal games. Therefore, the aim of the current study was to investigate the utility of BFR used in combination with SSG training for enhancing the long and short duration exercise capacities of futsal players. We hypothesized that high metabolic pressure during the BFR may cause to improve performance in futsal players.
Methods
Experimental approach to the problem
The study was a control-trial in which subjects were randomly assigned to either an experimental (BFR n = 6) or control group (non-BFR n = 6) for ten sessions of game-specific futsal training.
Participants
Twelve male futsal players aged 23 ± 2 years, body height 1.74 ± 0.05 m, body weight 67.5 ± 6.8 kg, BMI 22.2 ± 2.0 kg/m2, with at least five years’ experience in futsal (played at National League Second Division) volunteered to participate in this study. All subjects were in healthiness without any orthopedic, neuromuscular or cardiovascular diseases and provided written informed consent before testing. They excluded from the study if they had more than one session absentee. The study was a controlled trial in which subjects were randomly assigned to either an experimental (BFR n = 6) or control group (non-BFR n = 6) for ten sessions of game-specific futsal training. In order to estimate the number of participants needed in the study, a sample size calculation was performed using G*Power Software version 3.1.9.6 (Düsseldorf, Germany) (Faul et al., 2007) for a repeated measures ANOVA (within factors), using an α rejection criterion of 0.05 and 0.95 (1-β) power, and an average correlation of rho = 0.50 between the repeated measures for 4 within subject measurements to detect a medium effect (f = 0.5). The power calculation indicated that a minimum of 12 participants was required to be able to find such an effect. All experimental procedures were approved by the Ethics committee of Sport Sciences Research Institute of Iran with code IR.SSRI.REC.1396.187 and were conducted in accordance with the Declaration of Helsinki. The researcher explained the risks and benefits of the study to participants and obtained written informed consent prior to initial assessments.
The strength, muscle activation, and hormones (IGF-1 and myostatin) data have been published (Amani-Shalamzari et al., 2019a), but complementary data on aerobic and anaerobic performance, blood lactate concentration, and Hormones (Testosterone, GH, and cortisol) have not previously been analysed or reported.
Test procedures
Subjects completed a series of physiological and performance tests over three days, one week before and after a 3-week (10 sessions) training intervention. A schematic showing the testing timeline is shown in Figure 1. Physiological tests were performed in a temperature-controlled laboratory environment and consisted of a VO2max test followed by a time to fatigue (TTF) test on day two followed by a Wingate 30-second maximal test (WANT) on day three. On Day one, subjects initially completed an incremental speed test on a motorized treadmill (Pulsar hp Cosmos, Nussdorf-Traustein, Germany) set at a 1% inclination for determination of VO2max. The treadmill test commenced at 10 km·h-1 and progressed with speed increments of 1 km·h-1 every 3 min until subjects reached voluntary exhaustion. Throughout testing respiratory gases were measured using an alpha gas standard calibrated metabolic system (MetaLyzer3B, CORTEX, Leipzig, Germany). VO2max was calculated as the highest oxygen consumed over a 30 s period. VO2max was confirmed when 3 or more of the following criteria were met: (1) a plateau in VO2 despite an increase in running speed; (2) a respiratory exchange ratio (RER) higher than 1.2; (3) peak heart rate at least equal to 90% of the age-predicted maximum; and/or (4) visible exhaustion (Bayati et al., 2011). Each subject's velocity at VO2max (vVO2max) was identified from the respiratory measures as the minimum velocity at which the recorded VO2max was initially obtained. Also a measure of running economy (RE) was identified using the mean absolute oxygen cost (L·min-1) measured during the final 2 min of the 11-km·h-1 running stage. This running economy occurs below a respiratory exchange ratio of 1.0 that is between 7 and 12 km·h-1 dependent on each individual’s running ability (Paton et al., 2017).
Figure 1.
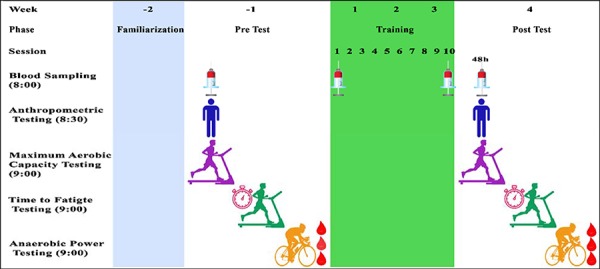
Schematic overview of the study timeline.
On next day, the incremental test subjects completed a treadmill TTF at their previously determined vVO2max. Subjects commenced the TTF test at 60% of their vVO2max, and then progressively increased to 100% vVO2max over a 30-45 s period. Total run duration was taken from the moment speed equaled the target vVO2max until subjects volitional exhaustion. For comparison, both pre and post-training vVO2max tests were performed at the same pre-training vVO2max velocity. On the following day, subjects performed a 30-s Wingate test on a stationary bicycle ergometer (Model 894E, Monark, Vansbro, Sweden) to obtain measures of peak power (PP), average power (AP) and minimum power (MP). Subjects commenced the WANT test with a 4-min warm-up performed at 60 revolutions per minute (RPM) against basket weight only (=60W). On completion of the warm-up subjects were immediately given a 5 s countdown to the automatically controlled beginning of the test. During the test subjects pedaled as fast as they could for 30 s while remaining seated. The resistance load for the Wingate test was set equivalent to 7.5% of each subjects’ body weight. Players were verbally encouraged during all tests to perform to their maximum ability. As reported the aerobic and anaerobic performance and physiological parameters are time-of-day dependent, the tests were repeated in the same time in pre- and post-test (Chtourou and Souissi, 2012).
Training protocol
Subjects suspended all other generic and futsal specific training for the duration of the study intervention period. Futsal training for both BFR and non-BFR groups consisted of 3-a-side SSG play on a 20 × 20 m hard surface pitch. Three-a-side is an effective and specific SSG for improving aerobic endurance fitness, as its physiological responses are similar to high intensity aerobic exercises (Halouani et al., 2014a). All subjects completed a total of ten supervised training sessions over three weeks with at least 48 h between sessions. Games consisted of periods of 3 min of activity interspersed with 2 min passive recovery. The total number of repetitions (reps) performed increased from sessions 1-9 (4 reps for sessions 1-3, 6 reps for sessions 4-7, 8 reps for sessions 8-9). The tenth session was performed at the same intensity of the first session to allow for physiological comparisons. All games were styled free touch with the ball always made promptly available if it went out of play. All games were played against opposing teams under the same experimental condition; e.g. BFR vs. BFR and non-BFR vs. non-BFR for all training sessions.
During training sessions, the BFR group wore right and left leg pneumatic cuffs (13 cm width, Ghamatpooyan, Tehran, Iran) placed on the upper-thighs immediately inferior to the gluteal fold. In the first week training, cuffs were inflated to 110% of each leg individual's systolic blood pressure (SBP). Leg SBP was considered about 1.2 fold of arm SBP (Sareen et al., 2012). Resting blood pressure was measured in a sitting position by a digital manometer (Omron M6 Comfort, Omron Healthcare, Kyoto, Japan) before the training workout. Cuff pressure was then progressively increased by 10% following each two completed training sessions. Cuffs remained inflated for each 3 min training period and were immediately deflated for the subsequent 2 min recovery period. Players sat down on a bench and inflated the cuff by a gauge, when the target pressure was reached, players performed three squats to ensure that the exact pressure was reached and then the gauge was cut off. Heart rate during all training sessions was recorded using a short-range telemetry device (Polar RC3, Polar Electro Oy, Kempele, Finland). The rate of perceived exertion (RPE) was measured immediately after each exercise set using the Borg non-linear (6-20) rating scale. In the first and last training sessions (1 and 10) fingertip blood lactate measures (Lactate Scout+, Cosmos Sirius, Germany) were taken immediately on completion of game play and at 5- and 12-min post play. All lactate measurements were taken with the occlusion cuffs removed.
Hormonal analysis
Before commencement of the training sessions (at baseline), immediately after the first session (acute response) and last training sessions (acute response to training) and 48 h after the last training session (adaptation) venous blood samples were drawn for hormonal analysis. Blood sample (5 cc) were taken from an antecubital vein and collected in ethylenediaminetetraacetic (EDTA) tubes. In the second and third blood sampling the data modified based on the plasma volume changes according to the formula of Dill and Costill (1974). Blood samples were immediately centrifuged (4° C, 3000 rpm) for 10 min after collection to isolate serum, and were then stored at -20° C until subsequent batch analysis. Serum samples were analyzed by enzyme immunoassay for free testosterone (testosterone kit IBL, RE52151, Hamburg, Germany), cortisol (cortisol kit IBL, RE52061, Hamburg, Germany) and GH (growth hormone kit IBL, MG59121, Hamburg, Germany). The inter- and intra-assay coefficient of variations (CV) of hormone measurement was 5.5% and 3.1% (testosterone), 3.4% and 2.6% (cortisol) and 8.1% and 4.9% (GH).
Statistical analyses
All simple group data are presented as mean ± standard deviation (SD). The magnitude of group effects on performance and physiological measures were determined using a made for purpose spreadsheet (Hopkins, 2006). The mean effect and the 95% confidence limits (CL) were determined using the unequal-variance, t-statistic computed for change scores between the experimental (BFR) and control (non-BFR) groups. Measures were log transformed to reduce bias arising from non-uniformity of error and back-transformed to obtain changes in means as percent values. The spreadsheet also provides the associated effect size (ES) statistic for changes within and between groups. The effect size thresholds of <0.2, 0.2-0.5, 0.5-0.8 and >0.8 were considered as trivial, small, moderate and large effects respectively, in accordance with the recommendations of Cohen (Paton et al., 2017). Also significance based analysis was performed (SPSS statistical software, version 19) to determine differences within and between groups. A paired sample t-test was used to analyze differences in measured parameters and hormone levels intra groups. A repeated measure analysis of variance (ANOVA) with factors for time and group was used to determine differences in blood lactate accumulation. Thresholds for significance for all test was set at alpha = 0.05. Figure 2 and 3 were prepared in GraphPad Prism (Version 7.03, GraphPad Software).
Figure 2.
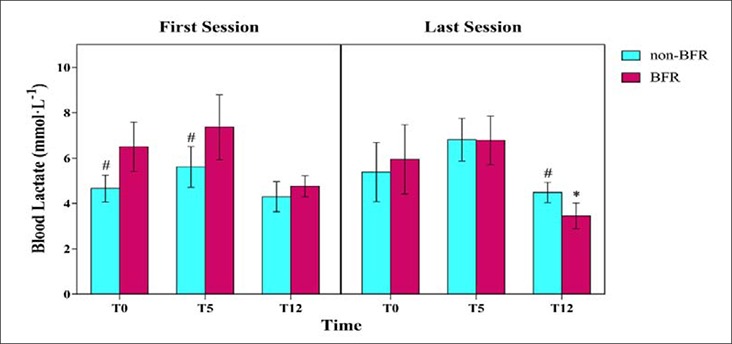
Blood lactate level at three times (T0, T5, T12) after the first and last session. BFR, blood flow restriction; T0, immediately; T5, 5 min later; T12, 12 min later; * significant difference with first session (p < 0.05); # significant difference with BFR group (p < 0.05).
Figure 3.
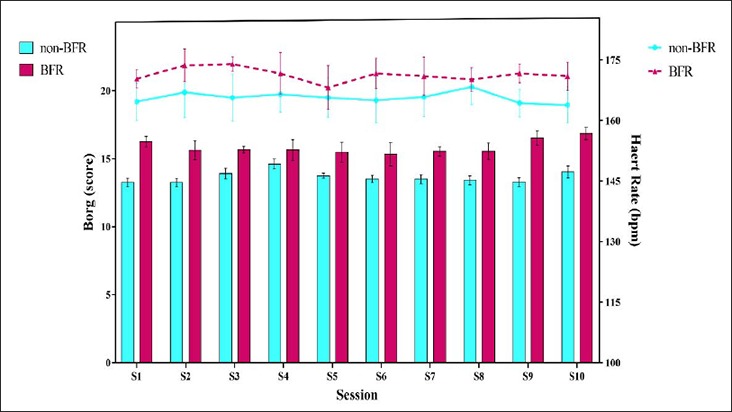
Heart rate and Borg scale during all sessions. BFR, blood flow restriction.
Results
Performance measures
Table 1 shows the measured aerobic and anaerobic performance variables and magnitude of change pre- to post-training for the two groups. Both BFR and non-BFR groups showed substantial increases in VO2max (p = 0.03), and vVO2max (p = 0.03) following the training period; however the overall between-group differences were deemed small (ES = 0.22, 0.28 respectively) and not significant (p > 0.05). There were significant differences between groups in TTF (p = 0.02) with a moderate effect (ES = 0.66) and RE (p = 0.01) with a large negative effect size (ES = -1.3) in favor of the BFR group (Table 1). TTF and RE improved significantly in the BFR group (p = 0.01) and non-significantly (p = 0.37) in the non-BFR group respectively.
Table 1.
Anaerobic and aerobic variables (pre and post training) for BFR and non-BFR groups. Data are means ± SD.
| Variables | BFR | non-BFR | Group difference | |||||
|---|---|---|---|---|---|---|---|---|
| Pre- training |
Post- training |
Δ Change (%) |
Pre- training |
Post- training |
Δ Change (%) |
P value | ES | |
| Aerobic tests (Treadmill) | ||||||||
| VO2max (ml·kg-1·min-1) | 44.0 ± 5.7 | 48.7 ± 5.3 | 11.1 ± 4.1 | 37.1 ± 5.2 | 39.8 ± 6.6 | 6.8 ± 3.7 | 0.11 | 0.22 |
| vVO2max (km·h-1) | 15.8 ± 0.9 | 16.4 ± 1.1 | 4.2 ± 3.2 | 14.9 ± 0.7 | 15.3 ± 0.9 | 2.2 ± 1.7 | 0.22 | 0.28 |
| TTF (s) | 259.8 ± 15.4 | 285.8 ± 14.5 | 10.0 ± 5.0 | 244.2 ± 12.9 | 253.5 ± 11.4 | 3.9 ± 2.2 | 0.04# | 0.76 |
| O2 Cost (L·min-1) | 0.23 ± 0.04 | 0.18 ± 0.03 | -22.7 ± 10.3 | 0.20 ± 0.02 | 0.19 ± 0.01 | -4.2± 11.31 | 0.01# | -1.32 |
| Anaerobic tests (WANT) | ||||||||
| Peak power (W·kg-1) | 10.0 ± 1.5 | 11.2 ± 1.8 | 12.7 ± 7.9 | 10.6 ± 1.0 | 11.1 ± 1.1 | 4.8 ± 5.1 | 0.08 | 0.55 |
| Average power (W·kg-1) | 7.0 ± 0.5 | 7.8 ± 0.4 | 12.2 ± 6.7 | 7.2 ± 0.5 | 7.4 ± 0.5 | 1.7 ± 6.4 | 0.03# | 1.32 |
| Minimum power (W·kg-1) | 3.9 ± 0.4 | 4.9 ± 0.8 | 24.3 ± 17.0 | 3.9 ± 0.8 | 4.1 ± 0.6 | 7.2 ± 11.4 | 0.09 | 0.86 |
BFR, blood flow restriction; TTF, run time to fatigue; ES, effect size.
# Significant difference between groups (p < 0.05);
* Significant difference within group (p < 0.05).
The Wingate test results showed that AP increased significantly (p = 0.01) in the BFR group and non-significantly (p = 0.51) in non-BFR group. The resultant change between the groups was significant (p = 0.03) and considered as a large effect (ES = 1.3) in favor of the BFR group. Peak power also increased significantly in the BFR group (p = 0.01) and in the non-BFR group (p = 0.01). The resultant change between the groups was not significant (p = 0.08) and considered as a moderate effect (ES = 0.55). Similarly, the MP increased significantly (p = 0.02) in the BFR group and non-significantly (p = 0.17) in the non-BFR group and also the change between the groups was not significant, although the changes considered a large effect (ES = 0.86) (Table 1).
The result for blood lactate concentration after the first and last session training are shown in Figure 2. In the first session, there was a significantly higher blood lactate concentration immediately (p = 0.01) and 5 min later (p = 0.03) in the BFR group compare to the non-BFR group. The ANOVA showed there was a significant group × time interaction effect (p = 0.04) for blood lactate. The blood lactate levels decreased in both groups at 12 min later; at the last session, blood lactate levels were significant (p = 0.01) reduced in the BFR group (3.45 ± 0.57 mmol·L-1) compare to the non-BFR group (4.48 ± 0.45 mmol·L-1) (Figure 2).
RPE and heart rate
Figure 3 presents the mean of RPE and HR during the 10 training sessions. In all training sessions, RPE and HR were significantly greater (p ≤ 0.01) in the BFR group than in the non-BFR group.
Hormones
Testosterone, GH, and cortisol data are presented in Table 2. GH levels increased in both groups but was substantially greater in the BFR group, so the differences between the two groups were significant at the first session (p = 0.02). In favor to the BFR group, GH levels at the response to the last training session increased non-significantly in both groups. At the end of the protocol, increases in GH resting levels were non-significant.
Table 2.
Data of hormones in BFR and non-BFR groups. Data are means ± SD.
| Variables | Group | Baseline | Acute 1 | Acute 2 | Adaptation |
|---|---|---|---|---|---|
|
Growth hormone (ng/mL) |
BFR | 0.83 ± 0.12 | 8.56 ± 3.17*# | 2.53 ± 1.32* | 1.10 ± 0.36 |
| non-BFR | 0.87 ± 0.05 | 3.92 ± 1.47* | 1.68 ± 0.07* | 0.89 ± 0.06 | |
|
Testosterone (ng/mL) |
BFR | 5.52 ± 0.67 | 8.55 ± 1.43*# | 7.52 ± 0.96* | 6.63 ± 0.97 |
| non-BFR | 4.75 ± 0.73 | 6.08 ± 1.29* | 6.33 ± 0.58* | 5.55 ± 0.99 | |
|
Cortisol (ng/mL) |
BFR | 108.83 ± 15.77 | 185.82 ± 13.74* | 156.83 ± 42.47* | 112.80 ± 28.73 |
| non-BFR | 109.27 ± 25.45 | 194.02 ± 11.07* | 153.48 ± 35.49 | 104.80 ± 12.15 | |
|
Ratio (%) |
BFR | 0.051 ± 0.006 | 0.046 ± 0.007# | 0.050 ± 0.011 | 0.061 ± 0.011* |
| non-BFR | 0.051 ± 0.01 | 0.032 ±0.007* | 0.044 ± 0.014 | 0.053 ± 0.010 |
BFT, blood flow restriction; baseline, before getting pre-testing; Acute1, immediately after first session; Acute 2, immediately after last session; adaptation, 48 h after last training session.
* intragroup significant differences with Baseline at p < 0.05;
# intergroup significant differences at p < 0.05.
The levels of testosterone and cortisol increased significantly immediately after the first session and the last session in both groups (p < 0.05) except cortisol at last session, but the difference at testosterone level (p = 0.04, ES = 0.37) and testosterone to cortisol ratio (p = 0.01, ES = 0.51) after the first session was significant between both groups. Ten sessions of futsal training with and without BFR did not change the resting levels of both hormones (p > 0.05), although a slight increase was observed in the testosterone levels so that the resting testosterone to cortisol ratio was significantly increased only in the BFR group (p = 0.01).
Discussion
The main aim of the study was to investigate the effects of a 3-week SSG training combined with BFR on the aerobic and anaerobic performance of futsal players. The findings revealed that applying BFR increased the internal training load (HR and RPE) and resulted in small additional but potentially meaningful improvements in several aspects of aerobic and anaerobic performance. There was a significant increase in the serum levels of the GH and testosterone and, cortisol immediately after SSG with and without BFR, but 48 h after the last training session, no significant changes were observed in their levels. However, increased the testosterone to cortisol ratio was significant in the BFR group.
The findings of this study showed that the physical stress of a game with additional BFR was higher than a game without BFR which supported by previous studies (Amani-Shalamzari et al., 2019c; Park et al., 2010). It wasprobably that the mean RPE and HR in the BFR group was higher probably due to the lower oxygen delivery conditions provided for active muscles while BFR (Tanimoto et al., 2005). The RPE values reported during training without BFR were partly light to somewhat hard, but when played under BFR, the RPE values increased to hard and very hard. The increased heart rate in the BFR group was consistent with previous BFR studies (86% HRmax for BFR group and 83% HRmax for non-BFR group) and was likely due to a reduction in the stroke volume because of a reduction in venous return (Takano et al., 2005a; Renzi et al., 2010).
Following the first training session, there were significantly greater blood lactate levels in the BFR group compared to the non-BFR group. It seemed therefore that BFR increased metabolic stress by impairing oxygen availability and thus more energy was derived from the anaerobic system with a resultant increase in blood lactate (Teixeira et al., 2018). The higher lactate levels following BFR were supported by the findings of previous studies which reported that lactate was higher during resistance exercise in a BFR group than a non-BFR group (Takarada et al., 2000; Teixeira et al., 2018). During the last training session, decreased lactate levels were observed in both groups but significantly more so in the BFR trained group. Training with BFR has previously been shown to improve onset blood lactate accumulation (de Oliveira et al., 2016). In the other hand, exercise training increases lactate removal by increasing hepatic capacity for gluconeogenesis as well as increasing lactate transport capacity and reducing glycogenolysis in muscle (Stallknecht et al., 1998). Therefore, it seemed that training with BFR facilitates muscular adaptations, leading to a greater lactate clearance and potentially faster recovery of futsal players. The improved lactate clearance may be also related to the elevated testosterone levels observed in the BFR group since previous research has shown testosterone induces an increase in both monocarboxylate transporter-1 (MCT1) and MCT4 proteins and their cell membrane content in skeletal muscle (Enoki et al., 2006).
During a 3-a-side game training, futsal players performed numerous short sprints, and other high-intensity activities continuously (Aguiar et al., 2013), so this format led to improved anaerobic indices, PP, AP and MP in favor of the BFR group. Of the measured anaerobic indexes in our study, only the changes in AP differed significantly between the two groups, as the exposure of players to extreme metabolic acidosis during the BFR training likely resulted in increased lactate tolerance and ultimately the improved AP in the Wingate test. An improvement in AP is important for futsal players, because the lactic-anaerobic system is the pre-dominant energy system used in futsal. Therefore, BFR trained players can tolerate higher blood lactate and perform more activities under high pressure in futsal.
Increases in VO2max occurred in both groups after training with somewhat larger increases in the BFR group but without any significant differences between groups. The increase in VO2max seen in both groups was similar to the increases reported by Paton et al. (2017) following eight sessions running of the treadmill at 80% of peak running velocity in BFR and control groups with only trivial (ES = 0.18) differences between groups. Also, in another similar study using repeated sessions of low-intensity cycle training with and without BFR, (de Oliveira et al., 2016) reported increases of 5.6 ± 4.2% in the BFR group and 0.4 ± 4.7% in the non-BFR training group. It seems that the intensity of exercise plays a role in the increase of VO2max. Despite the similar increase in VO2max and vVO2max in both groups, RE and TTF only significantly increased in the BFR group. In fact, the submaximal oxygen cost was reduced by ~22% and TTF increased by 7% in the BFR group, while both variables in the non-BFR group remained unchanged. It is possible that the, improvement in TTF was related to an increased RE as a result of local muscular adaptation. It was reported that increased red cell mass and improved skeletal muscle buffer capacity were involved in improving RE (Barnes and Kilding., 2015). The results of this study showed elevated testosterone and GH concentration may also be involved in improvement of RE, because the GH-IGF-1 axis is involved in the physiologic elevation of hemoglobin (Burtscher et al., 2010), and testosterone stimulates erythropoiesis by stimulating erythropoietin (Bachman et al., 2014), and also inducing an increase in MCT1 and MCT4 protein (Enoki et al., 2006). Consistent with the present research, it has previously been reported that GH increases after exercise with BFR (Abe et al., 2006). One of the potential enhancement mechanisms associated with BFR for functional and physiological adaptations is metabolic acidosis (Takarada et al., 2000; Pope et al., 2013). The local accumulation of metabolites is important for endocrine activation because this can stimulate hypothalamic-pituitary axis through activation of group III and group IV afferent fibers (Pierce et al., 2006), and lead to increased cortisol and GH. BFR reduced intramuscular oxygen delivery (Tanimoto et al., 2005), and decreased venous clearance of metabolites (Inagaki et al., 2011) which leads to increased metabolic acidosis; this situation creates intracellular swelling promoting an anabolic response (Loenneke et al., 2012). Another potential mechanism is the additional pressure caused by BFR in contractile mechanics results in enhanced growth factor expression (Pope et al., 2013). At the end of the protocol, the serum GH levels were increased insignificantly in both groups.
In the present study, a significant increase in testosterone and the testosterone to cortisol ratio was observed at the first session of work with BFR. Greater physiological pressure with BFR increased the levels of cortisol. However, a greater increase in testosterone level caused a significant increase in the ratio. One study reported no significant increases in testosterone levels following light resistance exercise with BFR and attributed it to the low load of exercise (Reeves et al., 2006). It is apparent that the intensity of the game in this study was higher than used in previous research. Therefore, the higher exercise load likely produced a greater cortisol and testosterone response. Also, studies have shown the high-intensity exercises that producing lactate lead to stimulates testosterone release from Leydig cells in the rat testis via increasing cAMP production (Lin et al., 2001; Lu et al., 1997). So elevated lactate levels in the present study could be a reason for the increased level of testosterone after exercise. At the end of the training period, the testosterone levels increased in both groups, and levels of cortisol remained constant. Due to the greater increase in testosterone levels in the BFR group, their ratio was increased. Therefore, the greater pressure on the BFR training, with more overcompensation during the recovery period, improved the observed aerobic and anaerobic performances.
There are several limitations to this study. Firstly, maybe one believes applying the pressurized cuffs during the game which possibility would limit futsal activities, but in practice, there was not any limitation to perform them. Also, as the first research our goal was to accurately control the pressure of cuff in order to having generalizability and now, we can recommend players can use the higher cuff pressures. Secondly, due to the number of players in the team, the small sample size maybe effective on the observed changes.
Conclusion
The efficiency of SSG is obvious in promoting simultaneous tactical and physical fitness in team sports (Amani-Shalamzari et al., 2019b). Therefore, coaches are recommended to add BFR to SSG to increase the internal training load and provide substantial improvements in both aerobic and anaerobic markers of performance. Applying BFR to SSG training, also increase the internal load of training elicits acute increases in GH and testosterone, and provides additional benefits to promote the aerobic and anaerobic performance.
Acknowledgements
The authors would like to acknowledge the players who participated in this study. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The experiments comply with the current laws of the country in which they were performed. The authors have no conflict of interest to declare.
Biographies

Sadegh AMANI-SHALAMZARI
Employment
Assistant professor at the Kharazmi University
Degree
PhD
Research interests
Blood Flow Restriction, Small Sided Game, Futsal/Football Fitness.
E-mail: amani_sadegh@khu.ac.ir

Ali SARIKHANI
Employment
Kharazmi University
Degree
MSc
Research interests
Blood Flow Restriction, Small Sided Game
E-mail: ali_sarikhani1993@yahoo.com

Carl PATON
Employment
Associate professor at the Eastern Institute of Technology
Degree
PhD
Research interests
Performance monitoring and enhancement in sports performance
E-mail: cpaton@eit.ac.nz

Hamid RAJABI
Employment
Professor at the Kharazmi University
Degree
PhD
Research interests
Neuromuscular physiology and fatigue.
E-mail: hrajabi@hotmail.com

Mahdi BAYATI
Employment
Assistant professor at the Sport Sciences Research Institute
Degree
PhD
Research interests
High-intensity interval training, Training monitoring.
E-mail: m.bayati@ssrc.ac.ir

Pantelis T. NIKOLAIDIS
Employment
Lecturer at the Hellenic Army Academy
Degree
PhD
Research interests
Exercise Physiology and Exercise
Testing.
E-mail: pademil@hotmail.com

Beat KNECHTLE
Employment
Professor at the Institute of Primary Care
Degree
MD
Research interests
Cardiology and exercise physiology.
E-mail: beat.knechtle@hispeed.ch
References
- Abe T., Fujita S., Nakajima T., Sakamaki M., Ozaki H., Ogasawara R., Sugaya M., Kudo M., Kurano M., Yasuda T., Sato Y., Ohshima H., Mukai C., Ishii N. (2010) Effects of Low-Intensity Cycle Training with Restricted Leg Blood Flow on Thigh Muscle Volume and VO2MAX in Young Men. Journal of Sports Science and Medicine 9, 452-458. [PMC free article] [PubMed] [Google Scholar]
- Abe T., Kearns C.F., Sato Y. (2006) Muscle size and strength are increased following walk training with restricted venous blood flow from the leg muscle, Kaatsu-walk training. Journal of Applied Physiology 100, 1460-1466. [DOI] [PubMed] [Google Scholar]
- Abe T., Yasuda T., Midorikawa T., Sato Y., Kearns C.F., Inoue K., Koizumi K., Ishii N. (2005) Skeletal muscle size and circulating IGF-1 are increased after two weeks of twice daily "KAATSU" resistance training. International Journal of KAATSU Training Research 1, 6-12. [Google Scholar]
- Aguiar M.V., Botelho G.M., Goncalves B.S., Sampaio J.E. (2013) Physiological responses and activity profiles of football small-sided games. Journal of Strength and Conditioning Research 27, 1287-1294. [DOI] [PubMed] [Google Scholar]
- Alvarez J.C., D'Ottavio S., Vera J.G., Castagna C. (2009) Aerobic fitness in futsal players of different competitive level. Journal of Strength and Conditioning Research 23, 2163-2166. [DOI] [PubMed] [Google Scholar]
- Amani-Shalamzari S., Farhani F., Rajabi H., Abbasi A., Sarikhani A., Paton C., Bayati M., Berdejo-Del-Fresno D., Rosemann T., Nikolaidis P.T., Knechtle B. (2019a) Blood Flow Restriction During Futsal Training Increases Muscle Activation and Strength. Frontiers in Physiology 10, 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amani-Shalamzari S., Khoshghadam E., Doniaee A., Parnow A., Bayati M., Clemente F.M. (2019b) Generic vs. small-sided game training in futsal: Effects on aerobic capacity, anaerobic power and agility. Physiology & behavior 204, 347-354. [DOI] [PubMed] [Google Scholar]
- Amani-Shalamzari S., Rajabi S., Rajabi H., Gahreman D.E., Paton C., Bayati M., Rosemann T., Nikolaidis P.T., Knechtle B. (2019c) Effects of Blood Flow Restriction and Exercise Intensity on Aerobic, Anaerobic, and Muscle Strength Adaptations in Physically Active Collegiate Women. Frontiers in Physiology 10, 810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachman E., Travison T.G., Basaria S., Davda M.N., Guo W., Li M., Connor Westfall J., Bae H., Gordeuk V., Bhasin S. (2014) Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: evidence for a new erythropoietin/hemoglobin set point. The Journals of Gerontology: Series A 69, 725-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbero-Alvarez J.C., Soto V.M., Barbero-Alvarez V., Granda-Vera J. (2008) Match analysis and heart rate of futsal players during competition. Journal of Sports Sciences 26, 63-73. [DOI] [PubMed] [Google Scholar]
- Barbieri R.A., Zagatto A.M., Milioni F., Barbieri F.A. (2016) Specific futsal training program can improve the physical performance of futsal players. Sport Sciences for Health 12, 247-253. [Google Scholar]
- Barnes K.R., Kilding A.E. (2015) Strategies to improve running economy. Sports Medicine 45, 37-56. [DOI] [PubMed] [Google Scholar]
- Bayati M., Farzad B., Gharakhanlou R., Agha-Alinejad H. (2011) A practical model of low-volume high-intensity interval training induces performance and metabolic adaptations that resemble 'all-out' sprint interval training. Journal of Sports Science and Medicine 10, 571-576. [PMC free article] [PubMed] [Google Scholar]
- Behringer M., Behlau D., Montag J.C.K., McCourt M.L., Mester J. (2017) Low-Intensity Sprint Training With Blood Flow Restriction Improves 100-m Dash. Journal of Strength and Conditioning Research 31, 2462-2472. [DOI] [PubMed] [Google Scholar]
- Berdejo-del-Fresno D., Moore R., Laupheimer M.W. (2015) VO2max changes in English futsal players after a 6-week period of specific small-sided games training. American Journal of Sports Science and Medicine 3, 28-34. [Google Scholar]
- Burtscher M., Gatterer H., Faulhaber M., Gerstgrasser W., Schenk K. (2010) Effects of intermittent hypoxia on running economy. International Journal of Sports Medicine 31, 644-650. [DOI] [PubMed] [Google Scholar]
- Castagna C., D'Ottavio S., Granda Vera J., Barbero Alvarez J.C. (2009) Match demands of professional Futsal: a case study. Journal of Science and Medicine in Sport 12, 490-494. [DOI] [PubMed] [Google Scholar]
- Chtourou H., Souissi N. (2012) The effect of training at a specific time of day: a review. Journal of Strength and Conditioning Research 26, 1984-2005. [DOI] [PubMed] [Google Scholar]
- Cook C.J., Kilduff L.P., Beaven C.M. (2014) Improving strength and power in trained athletes with 3 weeks of occlusion training. International Journal of Sports Physiology and Performance 9, 166-172. [DOI] [PubMed] [Google Scholar]
- de Oliveira M.F., Caputo F., Corvino R.B., Denadai B.S. (2016) Short-term low-intensity blood flow restricted interval training improves both aerobic fitness and muscle strength. Scandinavian Journal of Medicine & Science in Sports 26, 1017-1025. [DOI] [PubMed] [Google Scholar]
- Dill B.D., Costill D.L. (1974) Calculation of percentage changes in volume of blood, plasma, and red cell in dehydration. Journal of Applied Physiology 37:247-248. [DOI] [PubMed] [Google Scholar]
- Engel F.A., Ackermann A., Chtourou H., Sperlich B. (2018) High-Intensity Interval Training Performed by Young Athletes: A Systematic Review and Meta-Analysis. Frontiers in Physiology 9, 1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoki T., Yoshida Y., Lally J., Hatta H., Bonen A. (2006) Testosterone increases lactate transport, monocarboxylate transporter (MCT) 1 and MCT4 in rat skeletal muscle. The Journal of physiology 577, 433-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.G., Buchner A. (2007) G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods 39, 175-191. [DOI] [PubMed] [Google Scholar]
- Halouani J., Chtourou H., Dellal A., Chaouachi A., Chamari K. (2014a) Physiological responses according to rules changes during 3 vs. 3 small-sided games in youth soccer players: stop-ball vs. small-goals rules. Journal of Sports Sciences 32, 1485-1490. [DOI] [PubMed] [Google Scholar]
- Halouani J., Chtourou H., Gabbett T., Chaouachi A., Chamari K. (2014b) Small-sided games in team sports training: a brief review. Journal of Strength and Conditioning Research 28, 3594-3618. [DOI] [PubMed] [Google Scholar]
- Helgerud J., Engen L.C., Wisloff U., Hoff J. (2001) Aerobic endurance training improves soccer performance. Medicine and Science in Sports and Exercise 33, 1925-1931. [DOI] [PubMed] [Google Scholar]
- Hopkins W. (2006) Spreadsheets for analysis of controlled trials, with adjustment for a subject characteristic. Sportscience 46-50. [Google Scholar]
- Inagaki Y., Madarame H., Neya M., Ishii N. (2011) Increase in serum growth hormone induced by electrical stimulation of muscle combined with blood flow restriction. European Journal of Applied Physiology 111, 2715-2721. [DOI] [PubMed] [Google Scholar]
- Laurentino G., Ugrinowitsch C., Aihara A.Y., Fernandes A.R., Parcell A.C., Ricard M., Tricoli V. (2008) Effects of strength training and vascular occlusion. International Journal of Sports Medicine 29, 664-667. [DOI] [PubMed] [Google Scholar]
- Lin H., Wang S.W., Wang R.Y., Wang P.S. (2001) Stimulatory effect of lactate on testosterone production by rat Leydig cells. Journal of Cellular Biochemistry 83, 147-154. [DOI] [PubMed] [Google Scholar]
- Loenneke J.P., Fahs C.A., Rossow L.M., Abe T., Bemben M.G. (2012) The anabolic benefits of venous blood flow restriction training may be induced by muscle cell swelling. Medical Hypotheses 78, 151-154. [DOI] [PubMed] [Google Scholar]
- Lu S.S., Lau C.P., Tung Y.F., Huang S.W., Chen Y.H., Shih H.C., Tsai S.C., Lu C.C., Wang S.W., Chen J.J., Chien E.J., Chien C.H., Wang P.S. (1997) Lactate and the effects of exercise on testosterone secretion: evidence for the involvement of a cAMP-mediated mechanism. Medicine and Science in Sports and Exercise 29, 1048-1054. [DOI] [PubMed] [Google Scholar]
- Naser N., Ali A., Macadam P. (2017) Physical and physiological demands of futsal. Journal of Exercise Science and Fitness 15, 76-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Kim J.K., Choi H.M., Kim H.G., Beekley M.D., Nho H. (2010) Increase in maximal oxygen uptake following 2-week walk training with blood flow occlusion in athletes. European Journal of Applied Physiology 109, 591-600. [DOI] [PubMed] [Google Scholar]
- Paton C.D., Addis S.M., Taylor L.A. (2017) The effects of muscle blood flow restriction during running training on measures of aerobic capacity and run time to exhaustion. European Journal of Applied Physiology 117, 2579-2585. [DOI] [PubMed] [Google Scholar]
- Pierce J.R., Clark B.C., Ploutz-Snyder L.L., Kanaley J.A. (2006) Growth hormone and muscle function responses to skeletal muscle ischemia. Journal of Applied Physiology 101,1588-1595. [DOI] [PubMed] [Google Scholar]
- Pope Z.K., Willardson J.M., Schoenfeld B.J. (2013) Exercise and blood flow restriction. Journal of Strength and Conditioning Research 27, 2914-2926. [DOI] [PubMed] [Google Scholar]
- Reeves G.V., Kraemer R.R., Hollander D.B., Clavier J., Thomas C., Francois M., Castracane V.D. (2006) Comparison of hormone responses following light resistance exercise with partial vascular occlusion and moderately difficult resistance exercise without occlusion. Journal of Applied Physiology 101, 1616-1622. [DOI] [PubMed] [Google Scholar]
- Renzi C.P., Tanaka H., Sugawara J., (2010) Effects of leg blood flow restriction during walking on cardiovascular function. Medicine and Science in Sports and Exercise 42,726-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sareen P., Saxena K., Sareen B., Taneja B. (2012) Comparison of arm and calf blood pressure. Indian Journal of Anaesthesia 56, 83-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott B.R., Peiffer J.J., Goods P.S.R. (2017) The Effects of Supplementary Low-Load Blood Flow Restriction Training on Morphological and Performance-Based Adaptations in Team Sport Athletes. Journal of Strength and Conditioning Research 31, 2147-2154. [DOI] [PubMed] [Google Scholar]
- Stallknecht B., Vissing J., Galbo H. (1998) Lactate production and clearance in exercise. Effects of training. A mini-review. Scandinavian Journal of Medicine & Science in Sports 8, 127-131. [DOI] [PubMed] [Google Scholar]
- Takano H., Morita T., Iida H., Asada K., Kato M., Uno K., Hirose K., Matsumoto A., Takenaka K., Hirata Y., Eto F., Nagai R., Sato Y., Nakajima T. (2005a) Hemodynamic and hormonal responses to a short-term low-intensity resistance exercise with the reduction of muscle blood flow. European Journal of Applied Physiology 95, 65-73. [DOI] [PubMed] [Google Scholar]
- Takano H., Morita T., Iida H., Kato M., Uno K., Hirose K., Matsumoto A., Takenaka K., Hirata Y., Furuichi T., Eto F., Nagai R., Sato Y., Nakajima T. (2005b) Effects of low-intensity "KAATSU" resistance exercise on hemodynamic and growth hormone responses. International Journal of KAATSU Training Research 1, 13-18. [Google Scholar]
- Takarada Y., Nakamura Y., Aruga S., Onda T., Miyazaki S., Ishii N. (2000) Rapid increase in plasma growth hormone after low-intensity resistance exercise with vascular occlusion. Journal of Applied Physiology 88, 61-65. [DOI] [PubMed] [Google Scholar]
- Tanimoto M., Madarame H., Ishii N. (2005) Muscle oxygenation and plasma growth hormone concentration during and after resistance exercise: Comparison between "KAATSU" and other types of regimen. International Journal of KAATSU Training Research 1, 51-56. [Google Scholar]
- Teixeira E.L., Barroso R., Silva-Batista C., Laurentino G.C., Loenneke J.P., Roschel H., Ugrinowitsch C., Tricoli V. (2018) Blood flow restriction increases metabolic stress but decreases muscle activation during high-load resistance exercise. Muscle & Nerve 57, 107-111. [DOI] [PubMed] [Google Scholar]


