Abstract
The use of Boston type 1 keratoprosthesis (BKPro) has significantly increased worldwide. It is no longer considered a procedure of last resort but a reasonable option for patients with otherwise poor prognosis for a traditional penetrating keratoplasty. BKPro was approved by the Food and Drug Administration in 1992 for bilateral severe corneal blindness due to multiple corneal transplant failure. Over the years, indications have extended beyond recurrent immunologic rejection to include other conditions such as chemical injury and other causes of bilateral limbal stem cell deficiency, extensive corneal neovascularization, neurotrophic corneas and hypotony, among others. Numerous advances in the design of the BKPro, improvement of preoperative, intraoperative and postoperative management have resulted in favorable outcomes and a reduction in postoperative complications. Accordingly, many studies have shown that implantation of this device is highly effective in restoring vision with very good short-term outcomes. However, due to the lifetime risk of sight-threatening complications after BKPro implantation, a longer follow-up period should provide outcomes that are more realistic. In this review, the authors examined only the results of publications with an average of at least 2 years of follow-up. The overall intermediate to long-term visual outcomes and retention rate in BKPro seem to be favorable. However, autoimmune diseases and cicatrizing conditions continue to show a higher incidence of postoperative complications that require further management.
Keywords: Boston keratoprosthesis, corneal transplantation, limbal stem cell deficiency
Introduction
There are approximately 36 million individuals who are blind worldwide, and corneal disease is the fifth leading cause of blindness following cataract, uncorrected refractive error, glaucoma and age-related macular degeneration.1 Corneal transplantations including penetrating keratoplasty (PKP), anterior lamellar keratoplasty or endothelial keratoplasty are excellent procedures for restoring vision. According to the 2018 EBAA (Eye Bank Association of America) statistical report, the most common indication for any corneal transplantation in the United States is endothelial failure while keratoconus and keratitis are the most common indications in other regions.2,3 Although corneal graft survival rates are quite high in these conditions, the survival rates in eyes with high-risk characteristics are significantly lower and in some cases so poor that traditional keratoplasty is not even indicated, underscoring the need for other forms of treatment. In addition, the scarcity of donor corneas in many regions of the world prevents traditional keratoplasty to have a significant impact on corneal blindness where the prevalence is the highest.
With the goal to bring an answer to this unmet need, Prof. Claes Dohlman developed the Boston keratoprosthesis type 1 (BKPro) at the Massachusetts Eye and Ear Infirmary. Since its initial FDA approval in 1992, its use has increased continuously for the last two decades not only in the USA but also in other countries. As of January 2019, over 19,000 devices were implanted worldwide making it the most commonly used artificial cornea in the history of the field.4 The focus of this review is to examine current indications and management of the BKPro with a focus on intermediate to long-term outcomes.
Design Development: Evolution of the Boston KPro Type 1
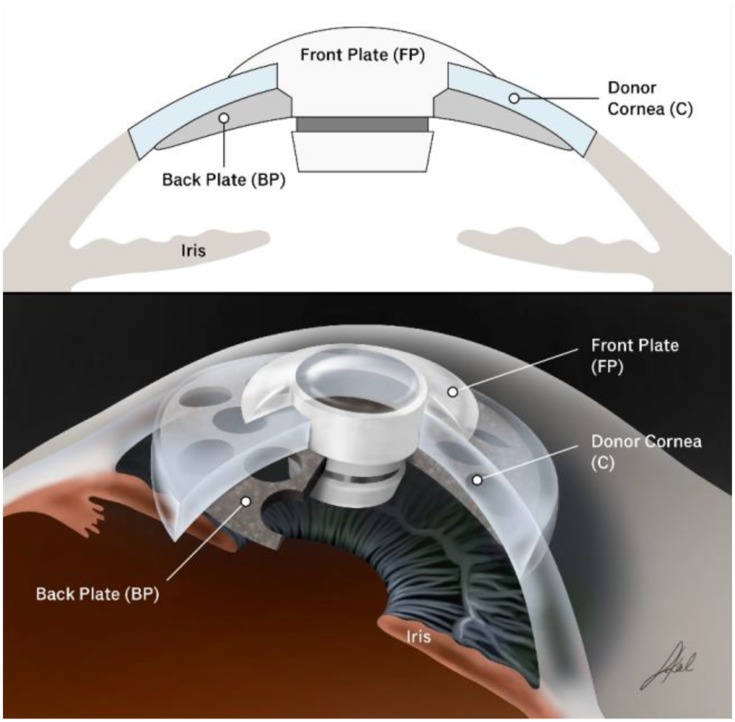
The BKPro was first introduced by Dr. Dohlman in the 1970s. It is a collar button design, consisting of a front plate with an optical stem, a corneal allograft button and a back plate (Figure 1).The front plate is made of medical-grade polymethylmethacrylate (PMMA). The radius of curvature of the optical surface, which is 3.5–3.7 mm in central diameter and 5 mm including the front plate, determines the power of the BKPro. The BKPro is available in single standard pseudophakic power and customized aphakic for various axial lengths (range 16–31 mm in increments of 1 mm).5 The edge of the front plate is carefully refined in the manufacturing process to prevent foreign body sensation and ensure a smooth blend between the PMMA and donor cornea. The central stem consists of an intraocular portion and a locking interface. The intraocular portion has a flat interface to allow the passage of light rays without bending. The locking interface is the area where back plate is secured. The original design had two and a half turn threads for screwing the back plate in place. However, in 2003, a titanium locking ring was added to secure the back plate in position and prevent later intraocular unscrewing of the plate.6. In 2007, the newer stem with threadless design was introduced. It was expected to eliminate corneal graft damage during screwing, be easier to use by surgeons and make inexpensive manufacture of the device possible by molding rather than machining.7
Figure 1.
Type I Boston keratoprosthesis.
The back plate continued to evolve over the past two decades. Early iterations of the back plate consisted of a solid 8 mm PMMA. However, the high incidence (over 50%) of sterile keratolysis observed with this model was thought to be secondary to decreased nutritional support from the aqueous humor to the donor cornea. This observation led to the development of a fenestrated back plate. Sixteen round holes (1.17 mm diameter each) in an 8.5 mm sized back plate and eight round holes (1.3 mm diameter each) in a 7.0 mm sized back plate were added to the design allowing aqueous to reach the graft. This modification resulted in a decrease in keratolysis to approximately 10% of cases.6,8 Currently, the back plate is available in two materials, the original PMMA and a newer titanium model. PMMA is an inert and well-tolerated material with long-term safe intraocular use. Titanium also provides excellent tissue tolerance in biological implants and has many special properties, including high resistance to corrosion, lightness and strength.9,10 These characteristics allow the titanium back plate to be thinner (titanium back plate has an edge thickness of 0.25 mm compared to 0.8 mm central and 0.6 mm peripheral thickness in the PMMA back plate). Titanium is nonmagnetic, therefore patients can be subjected to magnetic resonance imaging. Moreover, the titanium back plate can be colored via electrochemical anodization to improve cosmesis.11 In 2014, the click-on version was introduced including a titanium backplate that clicks onto the stem without the need for a locking ring. Initially, the main advantage of the titanium back plate was thought to be a reduction in retroprosthetic membrane (RPM) formation. Todani and colleagues reported a decreased frequency in RPM formation from 31.2% with the PMMA back plate to 13% with titanium at 6-month follow-up.12 However, a case-matched control study by Talati and colleagues reported no statistically significant difference in the frequency of visually significant RPM in titanium and PMMA back plate groups at 12 months (35% and 30%, respectively).13 Additionally, Taniguchi and colleagues recently reported that neither the material nor the size of the BKPro back plate had a significant impact on angle anatomy.14
A low-cost model of artificial cornea, the Auro KPro, based on the same design as the Boston KPro tye is currently manufactured by Aurolab in India. Although evidence is still relatively limited, outcomes appear comparable to those of the BKPro.15 In 2019, the FDA approved a new BKPro model: the Lucia keratoprosthesis. This design reduced manufacturing costs and offers a single titanium back plate 7.75 mm in diameter. In addition, the radial petaloid shape of the back plate holes and anodization to a brown color help improve cosmetic appearance.16
Indications
Nowadays, indications for the BKPro have been expanded beyond its initial indication of multiple graft failure to include primary implantation in patients that are not candidates for PKP such as those with bilateral limbal stem cell deficiency, hypotony and silicon oil-filled eyes, extensive corneal neovascularization and neurotrophic keratitis. Several studies over the past decade clearly highlight the importance of the underlying condition and ocular comorbidities in postoperative BKPro outcomes.17 In general, better visual outcomes were common in non-cicatrizing conditions, while patients with autoimmune disease and preexisting glaucoma presented a less favorable prognosis.
Multiple graft failure remains the most common indication for the BKPro in many long-term studies with 50–65% of eyes achieving a visual acuity of 20/200 or better at 3 years follow-up and a device retention rate of 80–87.8% at the last follow-up.18–22 When to repeat a graft or move to BKPro is a decision that varies from surgeon to surgeon. In a meta-analysis including 26 studies Ahmad and colleagues concluded that the likelihood of maintaining 20/200 or better at 2 years was significantly higher after BKPro implantation (80% of eyes) compared with repeat PKP (42% of eyes).23 Additionally, the probability of maintaining a clear graft was 47% compared with a BKPro retention rate of 75% at 5 years. The rate of glaucoma progression at 3 years was not significantly different between the groups (25% after repeat PK and 30% in BKPro).
Recent evidence suggests that results of BKPro implantation may be more favorable when performed as a primary corneal procedure. Several studies have shown that eyes receiving a primary BKPro achieve better best-corrected visual acuity outcomes when compared with eyes implanted with BKPro after multiple graft failure.18,24,25 Retention rates of BKPro devices were not statistically significant in primary and secondary implantation.
Boston keratoprosthesis has been considered as an option in selected cases of pediatric corneal disease given the suboptimal postoperative visual outcomes and low percentage of clear grafts achieved after pediatric keratoplasty. The more intense immune response leads to much higher rejection rates in children than adults and the challenges of amblyopia treatment and visual rehabilitation after keratoplasty in young children cannot be understated.26 In theory, the BKPro has the potential advantages of quickly restoring visual acuity due to lack of postoperative astigmatism and aphakia compensation as well as eliminating the risk of allograft rejection. However, the data in this patient population is limited with one study by Fung and colleagues showing poor visual results in pediatric BKPro and high rate of postoperative complications including low device retention.27 As a result, BKPro is not generally recommended for pediatric use. It is possible that age of implantation is an important predictor of outcomes and that older children may fair better than infants but evidence is reduced to a few successful cases and more data is needed to confirm this observation.
The use of BKPro in patients with unilateral disease or good vision in the contralateral eye is still controversial for some surgeons. Kosker and colleagues reported almost 50% of patients receiving the device achieved the minimum visual acuity required for binocular functioning.28 Similarly, two other studies demonstrated improvement of vision-related quality of life after BKPro implantation in patients with good preoperative visual acuity in the contralateral eye.29,30 The authors, therefore, propose that BKPro implantation is reasonable for patients with good vision in the fellow eye. In contrast, due to the indefinite risk of postoperative complications associated with BKPro devices, some surgeons recommend that patients with good vision in one eye should not undergo BKPro implantation. Supporting this argument, one study showed that patients with good vision in the contralateral eye were more likely to have postoperative complications in the BKPro eye than patients that were blind in the contralateral eye.31
Intermediate to Long-Term Outcomes
Many studies have reported favorable short-term outcomes of BKPro type-I implantation with an average of 80% retention and more than 70% of eyes achieving a visual acuity of 20/200 or better at 2 years of follow-up.32–34 However, the long-term risk of complications can cause an attrition of vision over time; therefore, longer follow-up periods should provide us with a more realistic perspective of keratoprosthesis outcomes. Table 1 summarizes the most relevant studies with intermediate (2–5 years) and long-term (>5 years) outcomes. Results by specific indications are summarized in Table 2.
Table 1.
Intermediate to Long-Term Results of BKPro Implantation from Single and Multicenter Case Series Studies
| Author /Year /Origin |
Cohort | N=eyes | Mean Follow-Up Time [Range] (Months) | Primary BKPro | Indication | Preoperative BCVA ≥ 20/200 | Postoperative BCVA ≥ 20/200 | Anatomical Retention at Last Follow-Up | Complications |
|---|---|---|---|---|---|---|---|---|---|
| Chang35 2015 USA |
1°BKPro in nonimmune disease | 43 | 39 [12–72] | 100% | Aniridia 25.58%, Neurotrophic-keratopathy 13.9%, Infectious keratitis 11.6% |
12% | 72% at 2 yrs, 66% at last follow-up | 81% | RPM 51.2%, Worsened glaucoma 32.6%, De novo glaucoma 14%, Corneal melt 18.6%, Endophthalmitis 4.7%, CME 9.3%, Sterile vitritis 14% |
| Driver18 2018 USA |
1° BKPro vs previous PKP (2° BKPro) | 67 vs 183 | 42±24.5 [1.9–92.8] vs 38.4 ± 28.3 [0.2–119.2] | 28.6% | Corneal vascularization 43.3%, SJS 22.4%, Chemical burn 22.4% |
3% vs 6.7% | 78–87% vs 56–67% at 6 yrsǂ | 89.6% vs 87.8% | RPM 40.3% vs 51.2%, PED 37.3% vs 27.4%, Elevated IOP 31.3% vs 17.7%ǂ |
| Kang25 2017 USA |
1° BKPro vs previous PKP (2° BKPro) | 28 vs 28 | 60±25.2 vs 60±34.8 | 50% | Chemical injury 29%, Aniridia 25%, Autoimmune 18% |
4% vs 0% | 64–71% vs 29–44% at 5 yrsǂ | 0.032 vs 0.034 per eye-year | RPM 46% vs 35%, New onset glaucoma 36% vs 14%, Post op-glaucoma surgery 29% vs 14%, Endophthalmitis 11% vs 14% |
| Greiner20 2011 USA |
N/A | 40 | 33.6±16.5 [5–72] | N/A | Graft failure 47.5%, Chemical injury 25%, Aniridia 12.5% |
5% | 29% at 4 yrs, 50% at last follow-up | 80% | RPM 55%, Elevated IOP 40%, De novo glaucoma 27.5%, Worsened glaucoma 22.5%, Endophthalmitis 12.5%, Corneal melt 15% |
| Lekhanont21 2014 Thailand |
≥4-year follow-up | 47 | 64.9±15.2 [48–88] | 40.5% | PBK 21.4%, Chemical injury 19.1%, SJS 19.1% |
0% | 42.9% at 6 yrs, 42.9% at last follow-up | 80.9% | RPM 52.4%, New glaucoma 30.9%, Worsen glaucoma 30.9%, Corneal melt 23.8%, Infectious keratitis 21.4%, Endophthalmitis 11.9%, RD 4.7% |
| Samarawickrama36 2017 UK |
≥1-year follow-up | 43 | 28.4±12.9 [12–56] | 5% | Bullous keratopathy 56%, LSCD 15%, Corneal ectasia 10% |
LogMAR 2.22± 0.36 | N/A (improved 46%, same 31%) | 92% | Worsened glaucoma 33%, RPM 21%, Removal BKPro 10%, CME 8%, RD 8%, Infectious keratitis 8%, Endophthalmitis 5%, Hypotony 8% |
| Aravena19 2018 USA |
≥5-year follow-up | 58 | 82.8±20.5 [57–145] | 24% | Graft failure 56.9%, Chemical injury 13.8%, Aniridia 6.9% |
5% | 82% at 8 yrs, 85% at last follow-up; | 94.8% | RPM 51.3%, PED 43.1%, Sterile corneal melt 25.9%, Increase IOP 24.1%, CME 20.7%, RD 15.5%, Sterile vitritis 12.1%, Endophthalmitis 0% |
| Srikumaran37 2014 Multicenter |
Multicenter | 139 | 46.7±26 [6 wk −8.7 yrs] | 27.3% | OSD 23%, Congenital corneal abnormalities 12.9%, Bullous keratopathy and KCN 35.3% |
10.8% | 44% at last follow-up | 67% at 7 yrs | RPM 49.7%, Glaucoma requiring surgery 21.6%, Sterile corneal necrosis 19.5%, RD 18.6%, Endophthalmitis 15.5%, PED 8.2%, Corneal infiltrate 3.4% |
Notes: ǂStatistically significant difference. N/A: Not available-either the respective values were not reported or reported in other formats.
Abbreviations: BCVA, best corrected distance visual acuity; BKpro, Boston keratoprosthesis; PKP, penetrating keratoplasty; SJS, Steven’s Johnson syndrome; LSCD, limbal stem cell deficiency; OSD, ocular surface diseases; PBK, pseudophakic bullous keratopathy; KCN, keratoconus; RPM, retroprosthetic membrane; PED, persistent epithelial defect; IOP, intraocular pressure; CME, chronic macular edema; RD, retinal detachment.
Table 2.
Results of Boston Keratoprosthesis Case Series for Specific Indications
| Author /Year /Country |
Cohort | N=Eyes | Mean Follow-Up Time [Range] (Months) | Primary BKPro | Indication | Preoperative BCVA ≥20/200 | Postoperative BCVA ≥ 20/200 | Anatomical Retention Rate at Last Follow-Up | Complications |
|---|---|---|---|---|---|---|---|---|---|
| Homayounfar38 2017, USA |
Age ≥ 75 years | 44 | 27.12 [0.8–72] | 47.7% | Graft failure 52.3%, Corneal scar 18.2%, LSCD 18.2% |
0% | 45.45% at last follow-up | 89% | RPM 45.5%, CME 13.6%, Elevated IOP 9.1%, Kpro-replacement 11.4%, Microbial keratitis 9.1%, Endophthalmitis 6.8%, Sterile vitritis 6.8% |
| Fung27 2018 Canada |
Children ≥16 years | 11 | 26.7 [6.5–85] | 55% | Aniridia 45%, Peters type II 45% |
0% | N/A (worse 55%, same 27%) | 36% | RPM 82%, Corneal melt 45%, RD 45%, Infectious keratitis with endophthalmitis 27% |
| Aravena39 2016 USA |
LSCD vs non-LSCD | 54 vs 95 | 37.1±26.4 vs 38.2± 28.9 | 40.7% vs 2.1% | Graft failure 25.9%, Chemical injury 20.4%, SJS 18.5% |
7% vs 9% | 76% vs 51% at 2 yrsǂ, 85% vs 46% at 5 yrsǂ | 94.4% vs 86.3% | RPM 37% vs 54%ǂ, PED 50% vs 28%ǂ, Sterile stromal necrosis 22% vs 10%, Corneal infiltrate 20% vs 9%, Endophthalmitis 0% vs 2.1%, BKPro removed 22.2% vs 20% |
| Goins40 2016 USA |
OSD (no vs mild to moderate vs severe) | 75 | 41.4 [0.8–82.8] | 20% | No OSD 49.3%, Mild to moderate OSD 20%, Severe OSD 30.6% |
26.7% | N/A (probability of improve vision at 5 yrs 53%) | 85.3% | RPM 29.7% vs 40% vs 34.8%, Endophthalmitis 5.4% vs 0% vs 21.7% ǂ, Ulcerative keratitis 5.4% vs 6.7% vs 39.1%ǂ |
| Sayegh41 2008 USA |
SJS (BKPro I, II) |
TBKpro I 6 | 43.2±18 [10.2–67.2] | 44% | SJS 94%, TEN 6% |
0% | 50% at last follow-up, (BCVA>20/40 31.25%) | N/A | RPM 56%, Tissue melt and leak 25%, New glaucoma 19%, Endophthalmitis 0%, |
| Palioura42 2013 USA |
MMP (BKPro I, II) | TBKpro I 8 | 38.4±25.2 | 50% | MMP 100% | 0% | 12.5% at last follow-up | 37.5% | RPM 62.5%, Extrusion 62.5%, RD 37.5%, Endophthalmitis 0% |
| Alexander43 2015 Multicentre |
Non-SJS vs SJS | 182 vs 27 | 29.3 vs 17.6ǂ | 23.1% vs 66.7%ǂ | Graft failure 56.2%, LSCD 12.4% |
5% vs 0% | 63% vs 96% at last follow-upǂ | 84% vs 55%ǂ | RPM 49% vs 37%, Corneal necrosis 8.3% vs 59.3%ǂ, PED 24.3 vs 59.3%ǂ, Endophthalmitis 2.2% vs 0% |
| Shah44 2018 USA |
Aniridia | 46 | 54±19.2 [24–88.8] | 26% | Aniridia 100% | 7% | N/A (43.5% BCVA improve ≥ 2 lines at last follow-up) | 87% | RPM 61%, New onset or progression of glaucoma 26% |
| Salvador-Culla 45 2016 Dominican- Republic |
Severe ocular burn | 42 | 40.2±24.4 [6–98] | N/A | Severe ocular burn | 0% | 58% at 3yrs, 77% at 5 yrs | 91.4% at 2 yrs | PCO 52.4%, Glaucoma 33.3%, RPM 11.9%, Corneal melting 31%, RD 4.8%, Vitritis 4.8%, Endophthalmitis 2.4% |
| Shanbhag46 2018 USA |
Chemical injury (systemic review) |
106 | 24.99±14 | N/A | Alkali and acid burn | N/A | 64.15% at last follow-up | 88.8% at 2 yrs | Valve implantation 10.8%, CPC 4.9%, Removal of KPro 11.76%, Glue application 4.9%, Surgeries for RD 3.9% |
| Brown47 2014 USA |
HSV vs HZV | 5 vs 4 | 48.4 vs 50.5 | 0% vs 25% | - | 20% vs 25% | 100% vs 25% at last follow-upǂ | 100% vs 25%ǂ | Wound dehiscence 0% vs 75%ǂ, Microbial keratitis 0% vs 75%ǂ, Glaucoma escalation 40% vs 0%, Endophthalmitis 0% vs 50%, CME 0% vs 25% |
| Fry48 2018 USA |
HSV vs controlled | 11 vs 138 | 43.6±24.2 vs 41.2±30.0 | 9% vs 32% | Control group; Graft failure 68.8% | 9% vs 8% | 60% vs 62% at 3 yrs | 64% vs 91%ǂ | RPM 45.5% vs 48.8%, PED 63.6% vs 34.1%, CME 45.5% vs 12.3%ǂ, Corneal infiltrate 27.3% vs 12.3%, Elevated IOP 27.3% vs 15.2%, Sterile vitritis 18.2% vs 9.4% |
Notes: ǂStatistically significant difference. N/A: Not available-either the respective values were not reported or reported in other formats.
Abbreviations: BCVA, best-corrected distance visual acuity; BKpro, Boston keratoprosthesis; SJS, Steven’s Johnson syndrome; TEN, toxic epidermal necrolysis; LSCD, limbal stem cell deficiency; OSD, ocular surface diseases; MMP, mucous membrane pemphigoid; HSV, herpes simplex viral infection; HZV, herpes zoster viral infection; RPM, retroprosthetic membrane; PCO, posterior capsular opacification; PED, persistent epithelial defect; IOP, intraocular pressure; CME, chronic macular edema; RD, retinal detachment.
Visual Outcomes
An improvement in visual acuity was observed in the majority of eyes after BKPro implantation (Table 1). Postoperative BCVA ≥20/200 ranged between 44% and 85% at last follow-up.19–21,35,37 Two studies reported 46–54.8% of the eyes achieving visual acuity improvement at the last follow-up visit.21,36 Specific indications tended to have a significant impact on outcomes. In patients with LSCD, Aravena and colleagues reported 76% of the eyes achieving postoperative BCVA of 20/200 or better at 3-year follow-up.39 Similarly, several publications showed that BCVA improved to ≥20/200 in 64–67% and ≥20/60 in 55% of eyes implanted with BKPro for chemical injury.46,49 However, in autoimmune associated-LSCD, including SJS and MMP, less than 50% of eyes maintained BCVA ≥ 20/200.41,42 For patients with aniridia, two studies reported that 43.5–65% of eyes improved vision from baseline.44,50 Finally, Fry and colleagues reported 60% of the eyes with BCVA of 20/200 or better at 3 years in patients with herpes simplex keratitis.48 In contrast, patients with herpes zoster infection achieved BCVA ≥20/200 only in 25% of eyes.47
Anatomical Retention
Overall the intermediate to long-term retention rates of BKPro implantation are favorable, ranging from 80% to 94.8% at last follow-up visit (range 24.1–82.8 months; Table 1). Understanding the definition of retention for each study is important, as some of the highest retention rates do not consider replacement as failure while other studies do. One of the largest multicenter studies including 139 eyes demonstrated a retention rate to 67% at 7 years of follow-up.37 Similarly, a meta-analysis reported a combined retention rate of 88% and 74% at 2 and 5 years, respectively.51
Retention rates also seemed to be affected by the primary indication for BKPro implantation. In agreement with short-term studies, better retention rates were observed in non-cicatrizing conditions, ranging from 77% to 97.3%.19,28,35,38,40,44,50 In contrast, in autoimmune-associated cicatrizing conditions such as SJS or MMP, retention rates were significantly lower (range 37.5–55%).42,43 Alexander and colleagues reported that most retention failures in SJS were due to sterile corneal necrosis (78%; 14/18 eyes). In comparison, retention in eyes with nonautoimmune forms of LSCD was better, ranging from 64% to 100% at last follow-up.39,40,48,49,52 Of note, the two studies reporting 100% retention in chemical injury, had an incidence of KPro replacement of 10% and 22%. Interestingly, more than 70% of eyes had previous surface surgery, including limbal stem cell transplantation or mucous membrane grafting highlighting that preparation of the ocular surface in severely compromised eyes is an important step that increases keratoprosthesis success.49,52
Postoperative Complications
Retroprosthetic Membrane
Retroprosthetic membrane (RPM) is the most common complication after BKPro implantation. The incidence of RPM in the studies included in this review varied between 18% and 55%.(Table 1). Incidence of RPM seems to be higher in the eyes with infectious keratitis or aniridia.53 Besides interfering with vision, some studies postulate that RPM is associated with corneal melt, chronic hypotony and retinal detachment.54,55 Almost 50% of eyes with RPM required some form of treatment. Seventy percent of RPMs requiring treatment are successfully treated with Nd:YAG laser, whereas 30% of residual eyes require surgical intervention (either KPro replacement or pars plana membranectomy).37
Sterile Keratolysis
Sterile keratolysis or corneal melting can lead to exposure of the back plate, leakage of aqueous and frank extrusion of the device. Overall, our report found an incidence of corneal melting of 11% to 25.9%. (Table 1). The highest incidence was found in eyes with autoimmune disease (25–62.5%).41–43 and chemical injury (22–40%).46,49,52 Persistent epithelial defect (PED) is also associated with a higher risk of sterile corneal necrosis and infectious keratitis in patients with BKPro.41,52,56 Moreover, there is evidence that RPM is associated with an increased risk of keratolysis, possibly due to obstruction of nutritional support from the aqueous humor, and data show that the greater the thickness of the RPM the higher the risk.55 Development of corneal melt could be minimized by optimizing the ocular surface. The use of well-fitted soft contact lenses helps improve corneal hydration reducing dellen formation, epithelial defects and keratolysis.57 Toxicity from multiple eye drops and mechanical trauma or exposure from eyelid abnormalities should be considered and corrected. In autoimmune patients with active inflammation, systemic immunosuppressive therapy might be necessary. In early cases, the use of anticollagenase agents such as topical medroxyprogesterone or oral tetracycline might be tried.58 If an aqueous leak is present cyanoacrylate glue can be helpful. Moreover, other treatment modalities including conjunctival flap, donor corneal lamellar graft with or without buccal mucosal graft, or crescenteric amniotic membrane graft have been reported.59–61 Whereas in some cases, KPro replacement is needed (Figure 2).
Figure 2.
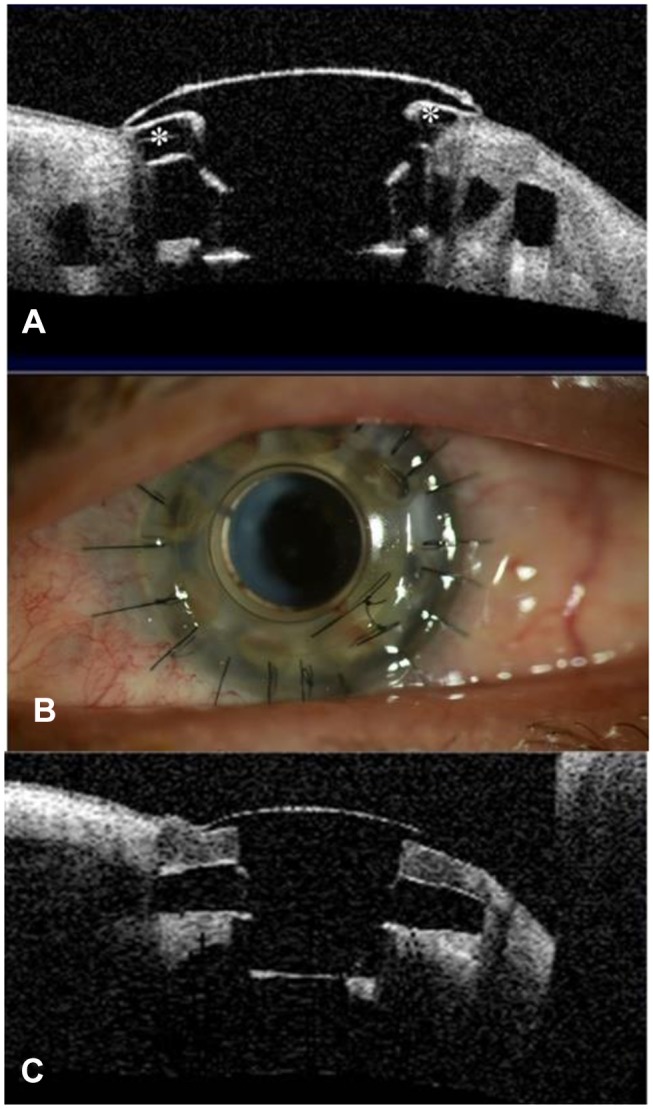
Patient with implanted type 1 Boston keratoprosthesis. (A) Anterior segment OCT showing sterile corneal melt. There is evidence of back plate exposure (*). (B) One week after graft replacement without removing the keratoprosthesis for the treatment of corneal melt. (C) Postoperative anterior segment OCT showing good graft apposition with the KPro optical stem in the same patient.
Glaucoma-Associated Complications
Glaucoma is the leading cause of permanent visual loss in BKPro.51 Prevalence of preexisting glaucoma varied from 33.3% to 89.3%62 Our report found that 20.2–40% of eyes had intraocular pressure (IOP) elevation, 14–36% developed de-novo glaucoma and 13–33% had glaucoma progression (Table 1). Due to preexisting corneal pathology, it is difficult to accurately evaluate IOP, optic nerve and visual fields before BKPro surgery. Moreover, glaucoma is especially challenging to diagnose, monitor, and treat after BKPro implantation. While likely multifactorial, serial anterior segment OCT after BKPro implantation showed that progressive angle closure plays an important role in KPro associated glaucoma.63,64 Moreover, early and aggressive treatment should be considered because of the rapid disease progression observed in BKPro patients. Glaucoma drainage device (GDD) placement and endoscopic or transscleral diode cyclophotocoagulation are highly effective modalities used for IOP control in most publications.19,65-67 Most studies advocate prior GDD surgery or concurrent BKPro and GDD implantation in eyes with preexisting glaucoma.67,68 Lentis and colleagues demonstrated that eyes receiving BKPro plus GDD implantation had significantly lower IOP than eyes receiving BKPro alone with no significant difference in the rate of serious vision-threatening complications.69 Combined pars plana vitrectomy and GDD implantation is a suitable alternative that has shown good efficacy in IOP control with a decreased risk of complications70,71 (Figure 3).
Figure 3.
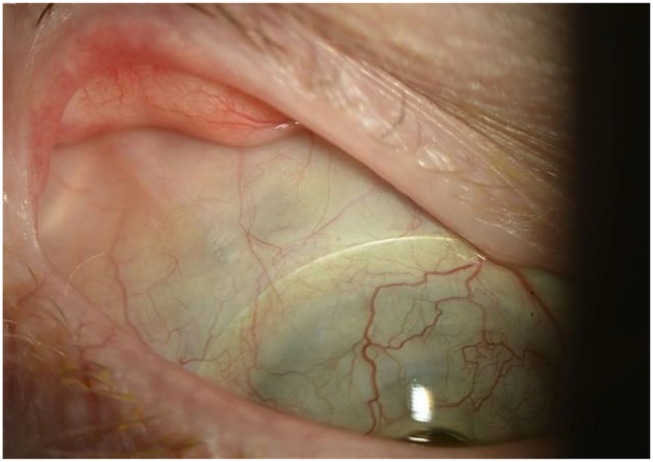
Slit-lamp photograph of a patient with BKPro I implant combined with pars plana glaucoma drainage device (GDD) 8 years ago. This approach allowed good contact lens fitting and eliminated friction between the contact lens and plate/tube of GDD. Kontur lens base curve 8.9 and diameter 16 mm is in place.
Infectious Keratitis and Endophthalmitis
Similar to any other human implantable prosthetic device, infection is a concern in patients with BKPro. But uniquely to keratoprosthesis is that the device is in contact with the normal microbial flora on the ocular surface and the sterile anterior chamber. This characteristic combined with its lack of biointegration makes potential intraocular infection a life-long risk. In addition, the already compromised ocular surface, chronic steroid use and continuous contact lens further increase the risk of corneal infection. Rates of infectious keratitis and endophthalmitis vary among studies ranging from 3.4% to 21.4% and from 0% to 15.5%, respectively (Table 1). Patients with autoimmune disease and chemical injury have been found to have a higher risk.40,49,72 Other associated-risk factors for endophthalmitis were found to be postoperative infectious keratitis, glaucoma drainage device erosion and non-compliance with antibiotic prophylaxis.73 Daily prophylactic antibiotics are the standard of care after BKPro implantation. However, there are no standardized regimens of prophylactic antibiotic and the drugs used vary by surgeon. The use of 1.4% vancomycin or 1% chloramphenicol with 0.1%Trimethoprim sulfate-Polymyxin B (TMP-PMB) or fluoroquinolone is recommended for high-risk patients.74 While most common isolated microorganisms are still gram-positive bacteria, fungal and uncommon fluoroquinolone-resistant organisms have been reported.75,76 The use of 2.5–5% povidone-iodine at each clinic visit or antifungal prophylaxis twice daily for a week every 3 months in high-risk patients (immunocompromised, chronic steroid use, exposure to warm, humid or agricultural environment and previous fungal infection) is encouraged by some expert opinions.74 Continuous bandage contact lens wear may protect corneal tissue from dehydration and prevent dellen formation. On the other hand, it may increase colonization of infectious organisms and biofilm formation. Therefore, regular disinfection or changing of the bandage lens is recommended.77
Vitreoretinal Complications
A recent systematic review reported a combined incidence of vitreoretinal complications, including retinal/choroidal detachment, choroidal/suprachoroidal/vitreous hemorrhage, cystoid macular edema, choroidal effusion, and epiretinal/preretinal membrane of 27.5%.51 Vitreoretinal complications appear to be more common within the first year after BKPro implantation. Choroidal detachments are usually associated with postoperative hypotony with an incidence of 2–16.9% and usually resolve without surgical intervention.78–80 Retinal detachment is one of the most common vitreoretinal complications after BKPro implantation with an incidence of 4.76–15.5% (Table 1). Most retinal detachment cases amenable to surgical repair with good anatomical outcomes.81,82 However, visual results are not as favorable. The frequency of macular-related complications including epiretinal membrane or cystoid macular edema is also not uncommon (11–35% and 10.8–14%, respectively) with some cases requiring surgical intervention.78,82 Chronic cystoid macular edema typically responds poorly to treatment.78
Future of BKPro
There is overwhelming evidence pointing towards the prevention and treatment of glaucoma as a major priority in BKpro. There is a clear need to develop alternative methods for reliable IOP measurement.67,83 Promising results of telemetric IOP monitoring in 12 patients implanted with an intraocular sensor in the ciliary sulcus at the time of BKPro surgery were recently published by Ender and colleagues showing good safety and tolerability of the device as well as successful telemetric IOP assessment.84 Similarly, plans to integrate a pressure sensor (micro-optomechanical pressure sensor) in the BKPro device are underway.85 In addition to better IOP monitoring, several publications advocate the use of anterior segment optical coherence tomography (OCT), 3D-optic nerve OCT, optic nerve head imaging and digital planimetric analysis of Goldman visual field, to better assess structural and functional glaucomatous change after BKPro implantation.62,63,86
Many modalities are also postulated to prevent other BKPro-related complications. The lack of biointegration has long been considered one of the greatest BKPro downsides leading to a continuous risk of intraocular infection. To address this issue, surface modification of the PMMA optic stem is being studied, with encouraging results showing improved biocompatibility and biointegration between the donor tissue and the device.95 The use of antibiotic-slow releasing contact lenses has been proposed as an alternative for infection prophylaxis.87,88 The use of collagen-crosslinked tissue as the carrier graft has been proposed to reduce the risk of corneal melt and a multicenter randomized clinical trial is underway to evaluate its benefits in patients considered to be high risk for this complication.89 A minimally invasive flex-BKPro is being studied for non-penetrating implantation to reduce intraocular complications in selected patients.90 Finally, wide-angle and ultra-widefield fundus imaging may improve visualization of the peripheral retina.91
Due to the limited availability of fresh human donor tissue worldwide, many studies have focused on an alternative substitute for fresh donor cornea that can reduce the cost associated with fresh tissue procurement, storage, and transportation. The use of frozen carrier grafts offers similar clinical outcomes with no differences in device retention, visual rehabilitation, or rates of complications when compared to fresh corneal donors.92 Furthermore, the sterilization technique by gamma ray radiation has been successfully used as carrier for both BKPro and fresh cornea as well, allowing long-time storage and easy transport at room temperature.93 Additionally, xenograft transplantation, limited in the past due to risk of graft failure, host immune reaction and cross-species diseases has recently found a renewed interest. Mohammad and colleagues proposed a method to decellularize and sterilize porcine corneas which were then successfully transplanted into rabbit eyes.94 However, further research is needed to determine if it would be applicable for human use.
Preliminary results of a new keratoprosthesis design, the Lux KPro have been recently reported.96 It is used for eyes with severe cicatricial ocular surface diseases. The design is a 3-piece design with a PMMA optic, titanium back plate, and also a titanium sleeve to take advantage of titanium’s superior tissue biocompatibility. The ocular surface is covered with mucus membrane. It is considered for patients that are not candidates for the type 1 BKPro and have poor eyelids and no tooth precluding implantation of a type 2 BKPro or an osteo-odonto keratoprosthesis.
Conclusion
The BKPro is the most commonly used artificial cornea. The evolution of its design during the past two decades not only improved outcomes but also resulted in expanded indications. Intermediate and long-term outcomes are good but the risk of blinding complications after implantation remains, making frequent lifetime monitoring and treatment a must. Current efforts focused on increasing accessibility of the device as well as research directed to improving biointegration and IOP monitoring among other areas will hopefully result in better long-term outcomes and a greater impact in corneal blindness.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221–e34. doi: 10.1016/S2214-109X(17)30393-5 [DOI] [PubMed] [Google Scholar]
- 2.Mathews PM, Lindsley K, Aldave AJ, Akpek EK. Etiology of global corneal blindness and current practices of corneal transplantation: a focused review. Cornea. 2018;37(9):1198–1203. doi: 10.1097/ICO.0000000000001666 [DOI] [PubMed] [Google Scholar]
- 3.Matthaei M, Sandhaeger H, Hermel M, et al. Changing indications in penetrating keratoplasty: a systematic review of 34 years of global reporting. Transplantation. 2017;101(6):1387–1399. doi: 10.1097/TP.0000000000001281 [DOI] [PubMed] [Google Scholar]
- 4.Chodosh J FDA approval obtained for the Boston Keratoprosthesis type I Lucia design. BOSTON KPro news; July 2019.
- 5.Ilhan-Sarac O, Akpek EK. Current concepts and techniques in keratoprosthesis. Curr Opin Ophthalmol. 2005;16(4):246–250. doi: 10.1097/01.icu.0000172829.33770.d3 [DOI] [PubMed] [Google Scholar]
- 6.Traish AS, Chodosh J. Expanding application of the Boston type I keratoprosthesis due to advances in design and improved post-operative therapeutic strategies. Semin Ophthalmol. 2010;25(5–6):239–243. doi: 10.3109/08820538.2010.518895 [DOI] [PubMed] [Google Scholar]
- 7.Dohlman CH, Harissi-Dagher M, Graney J. The Boston Keratoprosthesis: a new threadless design. Digit J Ophthalmol. 2007;13(3). [Google Scholar]
- 8.Harissi-Dagher M, Khan BF, Schaumberg DA, Dohlman CH. Importance of nutrition to corneal grafts when used as a carrier of the Boston Keratoprosthesis. Cornea. 2007;26(5):564–568. doi: 10.1097/ICO.0b013e318041f0a6 [DOI] [PubMed] [Google Scholar]
- 9.Tan XW, Riau A, Shi ZL, et al. In vitro effect of a corrosive hostile ocular surface on candidate biomaterials for keratoprosthesis skirt. Br J Ophthalmol. 2012;96(9):1252–1258. doi: 10.1136/bjophthalmol-2012-301633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong Y, Yang J, Wang L, et al. An improved biofunction of titanium for keratoprosthesis by hydroxyapatite-coating. J Biomater Appl. 2014;28(7):990–997. doi: 10.1177/0885328213490312 [DOI] [PubMed] [Google Scholar]
- 11.Paschalis EI, Chodosh J, Spurr-Michaud S, et al. In vitro and in vivo assessment of titanium surface modification for coloring the backplate of the Boston keratoprosthesis. Invest Ophthalmol Vis Sci. 2013;54(6):3863–3873. doi: 10.1167/iovs.13-11714 [DOI] [PubMed] [Google Scholar]
- 12.Todani A, Ciolino JB, Ament JD, et al. Titanium back plate for a PMMA keratoprosthesis: clinical outcomes. Graefes Arch Clin Exp Ophthalmol. 2011;249(10):1515–1518. doi: 10.1007/s00417-011-1684-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talati RK, Hallak JA, Karas FI, de la Cruz J, Cortina MS. Retroprosthetic membrane formation in Boston Keratoprosthesis: a case-control-matched comparison of titanium versus PMMA backplate. Cornea. 2018;37(2):145–150. doi: 10.1097/ICO.0000000000001462 [DOI] [PubMed] [Google Scholar]
- 14.Taniguchi EV, Paschalis EI, Crnej A, et al. The role of the back plate in angle anatomy with the Boston type I keratoprosthesis. Cornea. 2017;36(9):1096–1101. doi: 10.1097/ICO.0000000000001248 [DOI] [PubMed] [Google Scholar]
- 15.Basu S, Serna-Ojeda JC, Senthil S, Pappuru RR, Bagga B, Sangwan V. The aurolab keratoprosthesis (KPro) versus the Boston type I Kpro: 5-year clinical outcomes in 134 cases of bilateral corneal blindness. Am J Ophthalmol. 2019;205:175–183. doi: 10.1016/j.ajo.2019.03.016 [DOI] [PubMed] [Google Scholar]
- 16.Bakshi SK, Paschalis EI, Graney J, Lucia CJ. Beyond: development of an affordable keratoprosthesis. Cornea. 2019;38(4):492–497. doi: 10.1097/ICO.0000000000001880 [DOI] [PubMed] [Google Scholar]
- 17.Ahmad S, Akpek EK, Gehlbach PL, Dunlap K, Ramulu PY. Predictors of visual outcomes following Boston type 1 keratoprosthesis implantation. Am J Ophthalmol. 2015;159(4):739–747. doi: 10.1016/j.ajo.2014.12.024 [DOI] [PubMed] [Google Scholar]
- 18.Driver TH, Aravena C, Duong HNV, et al. Outcomes of the Boston type I keratoprosthesis as the primary penetrating corneal procedure. Cornea. 2018;37(11):1400–1407. doi: 10.1097/ICO.0000000000001735 [DOI] [PubMed] [Google Scholar]
- 19.Aravena C, Yu F, Aldave AJ. Long-term visual outcomes, complications, and retention of the Boston type I keratoprosthesis. Cornea. 2018;37(1):3–10. doi: 10.1097/ICO.0000000000001405 [DOI] [PubMed] [Google Scholar]
- 20.Greiner MA, Li JY, Mannis MJ. Longer-term vision outcomes and complications with the Boston type 1 keratoprosthesis at the University of California, Davis. Ophthalmology. 2011;118(8):1543–1550. doi: 10.1016/j.ophtha.2010.12.032 [DOI] [PubMed] [Google Scholar]
- 21.Lekhanont K, Thaweesit P, Muntham D, Chuckpaiwong V, Vongthongsri A. Medium-term outcomes of boston type 1 keratoprosthesis implantation in Bangkok, Thailand. Cornea. 2014;33(12):1312–1319. doi: 10.1097/ICO.0000000000000265 [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Wang C, Liu Q, Wang Z, Gao M. Comparison of the clinical efficacy of Boston keratoprosthesis type I and repetitive penetrating keratoplasty for refractory keratopathy. J Craniofac Surg. 2020;31(2):e194–e199. doi: 10.1097/SCS.0000000000006164 [DOI] [PubMed] [Google Scholar]
- 23.Ahmad S, Mathews PM, Lindsley K, et al. Boston type 1 keratoprosthesis versus repeat donor keratoplasty for corneal graft failure: a systematic review and meta-analysis. Ophthalmology. 2016;123(1):165–177. doi: 10.1016/j.ophtha.2015.09.028 [DOI] [PubMed] [Google Scholar]
- 24.Fadous R, Levallois-Gignac S, Vaillancourt L, Robert MC, Harissi-Dagher M. The Boston Keratoprosthesis type 1 as primary penetrating corneal procedure. Br J Ophthalmol. 2015;99(12):1664–1668. doi: 10.1136/bjophthalmol-2014-306161 [DOI] [PubMed] [Google Scholar]
- 25.Kang KB, Karas FI, Rai R, et al. Five year outcomes of Boston type I keratoprosthesis as primary versus secondary penetrating corneal procedure in a matched case control study. PLoS One. 2018;13(2):e0192381. doi: 10.1371/journal.pone.0192381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang C, O’Hara M, Mannis MJ. Primary pediatric keratoplasty: indications and outcomes. Cornea. 2009;28(9):1003–1008. doi: 10.1097/ICO.0b013e3181a186c0 [DOI] [PubMed] [Google Scholar]
- 27.Fung SSM, Jabbour S, Harissi-Dagher M, et al. Visual outcomes and complications of type I Boston keratoprosthesis in children: a retrospective multicenter study and literature review. Ophthalmology. 2018;125(2):153–160. doi: 10.1016/j.ophtha.2017.07.009 [DOI] [PubMed] [Google Scholar]
- 28.Kosker M, Suri K, Rapuano CJ, et al. Long-term results of the Boston keratoprosthesis for unilateral corneal disease. Cornea. 2015;34(9):1057–1062. doi: 10.1097/ICO.0000000000000495 [DOI] [PubMed] [Google Scholar]
- 29.Cortina MS, Hallak JA. Vision-related quality-of-life assessment using NEI VFQ-25 in patients after Boston keratoprosthesis implantation. Cornea. 2015;34(2):160–164. doi: 10.1097/ICO.0000000000000310 [DOI] [PubMed] [Google Scholar]
- 30.Pineles SL, Ela-Dalman N, Rosenbaum AL, Aldave AJ, Velez FG. Binocular visual function in patients with Boston type I keratoprostheses. Cornea. 2010;29(12):1397–1400. doi: 10.1097/ICO.0b013e3181da58d0 [DOI] [PubMed] [Google Scholar]
- 31.Shanbhag SS, Saeed HN, Colby KA, Chodosh J. Comparative outcomes of Boston keratoprosthesis type 1 implantation based on vision in the contralateral eye. Cornea. 2018;37(11):1408–1413. doi: 10.1097/ICO.0000000000001721 [DOI] [PubMed] [Google Scholar]
- 32.Aldave AJ, Kamal KM, Vo RC, Yu F. The Boston type I keratoprosthesis: improving outcomes and expanding indications. Ophthalmology. 2009;116(4):640–651. doi: 10.1016/j.ophtha.2008.12.058 [DOI] [PubMed] [Google Scholar]
- 33.Bradley JC, Hernandez EG, Schwab IR, Mannis MJ. Boston type 1 keratoprosthesis: the university of california davis experience. Cornea. 2009;28(3):321–327. doi: 10.1097/ICO.0b013e31818b8bfa [DOI] [PubMed] [Google Scholar]
- 34.Ciolino JB, Belin MW, Todani A, Al-Arfaj K, Rudnisky CJ. Boston keratoprosthesis type 1 study G. Retention of the Boston keratoprosthesis type 1: multicenter study results. Ophthalmology. 2013;120(6):1195–1200. doi: 10.1016/j.ophtha.2012.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang HY, Luo ZK, Chodosh J, Dohlman CH, Colby KA. Primary implantation of type I Boston keratoprosthesis in nonautoimmune corneal diseases. Cornea. 2015;34(3):264–270. doi: 10.1097/ICO.0000000000000357 [DOI] [PubMed] [Google Scholar]
- 36.Samarawickrama C, Strouthidis N, Wilkins MR. Boston keratoprosthesis type 1: outcomes of the first 38 cases performed at Moorfields Eye Hospital. Eye (Lond). 2018;32(6):1087–1092. doi: 10.1038/s41433-018-0016-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Srikumaran D, Munoz B, Aldave AJ, et al. Long-term outcomes of boston type 1 keratoprosthesis implantation: a retrospective multicenter cohort. Ophthalmology. 2014;121(11):2159–2164. doi: 10.1016/j.ophtha.2014.05.030 [DOI] [PubMed] [Google Scholar]
- 38.Homayounfar G, Grassi CM, Al-Moujahed A, Colby KA, Dohlman CH, Chodosh J. Boston keratoprosthesis type I in the elderly. Br J Ophthalmol. 2017;101(4):514–518. doi: 10.1136/bjophthalmol-2015-307868 [DOI] [PubMed] [Google Scholar]
- 39.Aravena C, Bozkurt TK, Yu F, Aldave AJ. Long-term outcomes of the Boston type I keratoprosthesis in the management of corneal limbal stem cell deficiency. Cornea. 2016;35(9):1156–1164. doi: 10.1097/ICO.0000000000000933 [DOI] [PubMed] [Google Scholar]
- 40.Goins KM, Kitzmann AS, Greiner MA, et al. Boston type 1 keratoprosthesis: visual outcomes, device retention, and complications. Cornea. 2016;35(9):1165–1174. doi: 10.1097/ICO.0000000000000886 [DOI] [PubMed] [Google Scholar]
- 41.Sayegh RR, Ang LP, Foster CS, Dohlman CH. The Boston keratoprosthesis in Stevens-Johnson syndrome. Am J Ophthalmol. 2008;145(3):438–444. doi: 10.1016/j.ajo.2007.11.002 [DOI] [PubMed] [Google Scholar]
- 42.Palioura S, Kim B, Dohlman CH, Chodosh J. The Boston keratoprosthesis type I in mucous membrane pemphigoid. Cornea. 2013;32(7):956–961. doi: 10.1097/ICO.0b013e318286fd73 [DOI] [PubMed] [Google Scholar]
- 43.Alexander JK, Basak SK, Padilla MD, Yu F, Aldave AJ. International outcomes of the Boston type I keratoprosthesis in Stevens-Johnson syndrome. Cornea. 2015;34(11):1387–1394. doi: 10.1097/ICO.0000000000000619 [DOI] [PubMed] [Google Scholar]
- 44.Shah KJ, Cheung AY, Holland EJ. Intermediate-term and long-term outcomes with the Boston type 1 keratoprosthesis in aniridia. Cornea. 2018;37(1):11–14. doi: 10.1097/ICO.0000000000001412 [DOI] [PubMed] [Google Scholar]
- 45.Salvador-Culla B, Kolovou PE, Arzeno L, Martinez S, Lopez MA. Boston Keratoprosthesis Type 1 in chemical burns. Cornea. 2016;35(6):911–916. doi: 10.1097/ICO.0000000000000837 [DOI] [PubMed] [Google Scholar]
- 46.Shanbhag SS, Saeed HN, Paschalis EI, Chodosh J. Boston keratoprosthesis type 1 for limbal stem cell deficiency after severe chemical corneal injury: a systematic review. Ocul Surf. 2018;16(3):272–281. doi: 10.1016/j.jtos.2018.03.007 [DOI] [PubMed] [Google Scholar]
- 47.Brown CR, Wagoner MD, Welder JD, et al. Boston keratoprosthesis type 1 for herpes simplex and herpes zoster keratopathy. Cornea. 2014;33(8):801–805. doi: 10.1097/ICO.0000000000000164 [DOI] [PubMed] [Google Scholar]
- 48.Fry M, Aravena C, Yu F, Kattan J, Aldave AJ. Long-term outcomes of the Boston type I keratoprosthesis in eyes with previous herpes simplex virus keratitis. Br J Ophthalmol. 2018;102(1):48–53. doi: 10.1136/bjophthalmol-2017-310186 [DOI] [PubMed] [Google Scholar]
- 49.Phillips DL, Hager JL, Goins KM, et al. Boston type 1 keratoprosthesis for chemical and thermal injury. Cornea. 2014;33(9):905–909. doi: 10.1097/ICO.0000000000000204 [DOI] [PubMed] [Google Scholar]
- 50.Hassanaly SI, Talajic JC, Harissi-Dagher M. Outcomes following Boston type 1 keratoprosthesis implantation in aniridia patients at the University of Montreal. Am J Ophthalmol. 2014;158(2):270–6 e1. doi: 10.1016/j.ajo.2014.05.009 [DOI] [PubMed] [Google Scholar]
- 51.Priddy J, Bardan AS, Tawfik HS, Liu C. Systematic review and meta-analysis of the medium- and long-term outcomes of the Boston type 1 keratoprosthesis. Cornea. 2019;38:1465–1473. doi: 10.1097/ICO.0000000000002098 [DOI] [PubMed] [Google Scholar]
- 52.Magalhaes FP, Hirai FE, de Sousa LB, de Oliveira LA. Boston type 1 keratoprosthesis outcomes in ocular burns. Acta Ophthalmol. 2013;91(6):e432–e436. doi: 10.1111/aos.12083 [DOI] [PubMed] [Google Scholar]
- 53.Rudnisky CJ, Belin MW, Todani A, et al. Risk factors for the development of retroprosthetic membranes with Boston keratoprosthesis type 1: multicenter study results. Ophthalmology. 2012;119(5):951–955. doi: 10.1016/j.ophtha.2011.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dokey A, Ramulu PY, Utine CA, et al. Chronic hypotony associated with the Boston type 1 keratoprosthesis. Am J Ophthalmol. 2012;154(2):266–71.e1. doi: 10.1016/j.ajo.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 55.Sivaraman KR, Hou JH, Allemann N, de la Cruz J, Cortina MS. Retroprosthetic membrane and risk of sterile keratolysis in patients with type I Boston Keratoprosthesis. Am J Ophthalmol. 2013;155(5):814–822. doi: 10.1016/j.ajo.2012.11.019 [DOI] [PubMed] [Google Scholar]
- 56.Sejpal K, Yu F, Aldave AJ. The Boston keratoprosthesis in the management of corneal limbal stem cell deficiency. Cornea. 2011;30(11):1187–1194. doi: 10.1097/ICO.0b013e3182114467 [DOI] [PubMed] [Google Scholar]
- 57.Harissi-Dagher M, Beyer J, Dohlman CH. The role of soft contact lenses as an adjunct to the Boston keratoprosthesis. Int Ophthalmol Clin. 2008;48(2):43–51. doi: 10.1097/IIO.0b013e318169511f [DOI] [PubMed] [Google Scholar]
- 58.Hicks CR, Crawford GJ. Melting after keratoprosthesis implantation: the effects of medroxyprogesterone. Cornea. 2003;22(6):497–500. doi: 10.1097/00003226-200308000-00001 [DOI] [PubMed] [Google Scholar]
- 59.Chan CC, LoVerde L, Qiang J, Nordlund ML, Holland EJ. Incidence, risk factors, and surgical management of Boston type 1 keratoprosthesis corneal melts, leaks, and extrusions. Cornea. 2016;35(8):1049–1056. doi: 10.1097/ICO.0000000000000911 [DOI] [PubMed] [Google Scholar]
- 60.Tay E, Utine CA, Akpek EK. Crescenteric amniotic membrane grafting in keratoprosthesis-associated corneal melt. Arch Ophthalmol. 2010;128(6):779–782. [DOI] [PubMed] [Google Scholar]
- 61.Ziai S, Rootman DS, Slomovic AR, Chan CC. Oral buccal mucous membrane allograft with a corneal lamellar graft for the repair of Boston type 1 keratoprosthesis stromal melts. Cornea. 2013;32(11):1516–1519. doi: 10.1097/ICO.0b013e3182a480f5 [DOI] [PubMed] [Google Scholar]
- 62.Ali MH, Dikopf MS, Finder AG, et al. Assessment of glaucomatous damage after Boston keratoprosthesis implantation based on digital planimetric quantification of visual fields and optic nerve head imaging. Cornea. 2018;37(5):602–608. doi: 10.1097/ICO.0000000000001544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang JJ, Allemann N, Cruz Jde L, Cortina MS. Serial analysis of anterior chamber depth and angle status using anterior segment optical coherence tomography after boston keratoprosthesis. Cornea. 2013;32(10):1369–1374. doi: 10.1097/ICO.0b013e3182a0cff5 [DOI] [PubMed] [Google Scholar]
- 64.Qian CX, Hassanaly S, Harissi-Dagher M. Anterior segment optical coherence tomography in the long-term follow-up and detection of glaucoma in Boston type I keratoprosthesis. Ophthalmology. 2015;122(2):317–325. doi: 10.1016/j.ophtha.2014.08.007 [DOI] [PubMed] [Google Scholar]
- 65.Nguyen P, Chopra V. Glaucoma management in Boston keratoprosthesis type I recipients. Curr Opin Ophthalmol. 2014;25(2):134–140. doi: 10.1097/ICU.0000000000000035 [DOI] [PubMed] [Google Scholar]
- 66.Rivier D, Paula JS, Kim E, Dohlman CH, Grosskreutz CL. Glaucoma and keratoprosthesis surgery: role of adjunctive cyclophotocoagulation. J Glaucoma. 2009;18(4):321–324. doi: 10.1097/IJG.0b013e3181815485 [DOI] [PubMed] [Google Scholar]
- 67.Vajaranant TS, Liu J, Wilensky J, Cortina MS, Aref AA. Innovative approaches to glaucoma management of Boston keratoprosthesis type 1. Curr Ophthalmol Rep. 2016;4(3):147–153. doi: 10.1007/s40135-016-0102-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crnej A, Paschalis EI, Salvador-Culla B, et al. Glaucoma progression and role of glaucoma surgery in patients with Boston keratoprosthesis. Cornea. 2014;33(4):349–354. doi: 10.1097/ICO.0000000000000067 [DOI] [PubMed] [Google Scholar]
- 69.Lenis TL, Chiu SY, Law SK, Yu F, Aldave AJ. Safety of concurrent Boston type I keratoprosthesis and glaucoma drainage device implantation. Ophthalmology. 2017;124(1):12–19. doi: 10.1016/j.ophtha.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 70.Li JY, Greiner MA, Brandt JD, Lim MC, Mannis MJ. Long-term complications associated with glaucoma drainage devices and Boston keratoprosthesis. Am J Ophthalmol. 2011;152(2):209–218. doi: 10.1016/j.ajo.2011.01.034 [DOI] [PubMed] [Google Scholar]
- 71.Huh ES, Aref AA, Vajaranant TS, de la Cruz J, Chau FY, Cortina MS. Outcomes of pars plana glaucoma drainage implant in Boston type 1 keratoprosthesis surgery. J Glaucoma. 2014;23(1):e39–e44. doi: 10.1097/IJG.0b013e31829e55f8 [DOI] [PubMed] [Google Scholar]
- 72.Nouri M, Terada H, Alfonso EC, Foster CS, Durand ML, Dohlman CH. Endophthalmitis after keratoprosthesis: incidence, bacterial causes, and risk factors. Arch Ophthalmol. 2001;119(4):484–489. doi: 10.1001/archopht.119.4.484 [DOI] [PubMed] [Google Scholar]
- 73.Robert MC, Moussally K, Harissi-Dagher M. Review of endophthalmitis following Boston keratoprosthesis type 1. Br J Ophthalmol. 2012;96(6):776–780. doi: 10.1136/bjophthalmol-2011-301263 [DOI] [PubMed] [Google Scholar]
- 74.Behlau I, Martin KV, Martin JN, et al. Infectious endophthalmitis in Boston keratoprosthesis: incidence and prevention. Acta Ophthalmol. 2014;92(7):e546–e555. doi: 10.1111/aos.12309 [DOI] [PubMed] [Google Scholar]
- 75.Barnes SD, Dohlman CH, Durand ML. Fungal colonization and infection in Boston keratoprosthesis. Cornea. 2007;26(1):9–15. doi: 10.1097/01.ico.0000224650.19837.25 [DOI] [PubMed] [Google Scholar]
- 76.Chan CC, Holland EJ. Infectious endophthalmitis after Boston type 1 keratoprosthesis implantation. Cornea. 2012;31(4):346–349. doi: 10.1097/ICO.0b013e31821eea2f [DOI] [PubMed] [Google Scholar]
- 77.Foulks GN. Prolonging contact lens wear and making contact lens wear safer. Am J Ophthalmol. 2006;141(2):369–373. doi: 10.1016/j.ajo.2005.08.047 [DOI] [PubMed] [Google Scholar]
- 78.Goldman DR, Hubschman JP, Aldave AJ, et al. Postoperative posterior segment complications in eyes treated with the Boston type I keratoprosthesis. Retina. 2013;33(3):532–541. doi: 10.1097/IAE.0b013e3182641848 [DOI] [PubMed] [Google Scholar]
- 79.Rishi P, Rishi E, Koundanya VV, Mathur G, Iyer G, Srinivasan B. Vitreoretinal complications in eyes with boston keratoprosthesis type I. Retina. 2016;36(3):603–610. doi: 10.1097/IAE.0000000000000740 [DOI] [PubMed] [Google Scholar]
- 80.Moshiri A, Safi M, Morse LS, et al. Posterior segment complications and impact on long-term visual outcomes in eyes with a type 1 Boston keratoprosthesis. Cornea. 2019;38(9):1111–1116. doi: 10.1097/ICO.0000000000001983 [DOI] [PubMed] [Google Scholar]
- 81.Ray S, Khan BF, Dohlman CH, D’Amico DJ. Management of vitreoretinal complications in eyes with permanent keratoprosthesis. Arch Ophthalmol. 2002;120(5):559–566. doi: 10.1001/archopht.120.5.559 [DOI] [PubMed] [Google Scholar]
- 82.Kiang L, Sippel KC, Starr CE, et al. Vitreoretinal surgery in the setting of permanent keratoprosthesis. Arch Ophthalmol. 2012;130(4):487–492. doi: 10.1001/archophthalmol.2011.1115 [DOI] [PubMed] [Google Scholar]
- 83.Estrovich IE, Shen C, Chu Y, et al. Schiotz tonometry accurately measures intraocular pressure in Boston type 1 keratoprosthesis eyes. Cornea. 2015;34(6):682–685. doi: 10.1097/ICO.0000000000000406 [DOI] [PubMed] [Google Scholar]
- 84.Enders P, Hall J, Bornhauser M, et al. Telemetric intraocular pressure monitoring after Boston keratoprosthesis surgery. Ophthalmology. 2019;126(2):322–324. doi: 10.1016/j.ophtha.2018.09.028 [DOI] [PubMed] [Google Scholar]
- 85.Hui P-C, Chodosh J, Dohlman CH, Paschalis EI. Integrating a pressure sensor in the Boston KPro. Boston KPro news. July 2019.
- 86.Khoueir Z, Jassim F, Braaf B, et al. Three-dimensional optical coherence tomography imaging for glaucoma associated with Boston keratoprosthesis type I and II. J Glaucoma. 2019;28(8):718–726. doi: 10.1097/IJG.0000000000001280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carreira AS, Ferreira P, Ribeiro MP, et al. New drug-eluting lenses to be applied as bandages after keratoprosthesis implantation. Int J Pharm. 2014;477(1–2):218–226. doi: 10.1016/j.ijpharm.2014.10.037 [DOI] [PubMed] [Google Scholar]
- 88.Ciolino JB, Stefanescu CF, Ross AE, et al. In vivo performance of a drug-eluting contact lens to treat glaucoma for a month. Biomaterials. 2014;35(1):432–439. doi: 10.1016/j.biomaterials.2013.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Robert MC, Arafat SN, Ciolino JB. Collagen cross-linking of the Boston keratoprosthesis donor carrier to prevent corneal melting in high-risk patients. Eye Contact Lens. 2014;40(6):376–381. doi: 10.1097/ICL.0000000000000081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Paschalis EI, Pineda R, Gonzalez-Andrades M, et al. A New Pre-Descemet’s Keratoprosthesis. Boston KPro news. 2017. September. [Google Scholar]
- 91.Kornberg DL, Yannuzzi NA, Klufas MA, D’Amico DJ, Orlin A, Kiss S. Ultra-widefield imaging of posterior segment pathology in the setting of the Boston keratoprosthesis. Retina. 2016;36(6):1101–1110. doi: 10.1097/IAE.0000000000000833 [DOI] [PubMed] [Google Scholar]
- 92.Muzychuk AK, Robert MC, Dao S, Harissi-Dagher M. Boston keratoprosthesis type 1: a randomized controlled trial of fresh versus frozen corneal donor carriers with long-term follow-up. Ophthalmology. 2017;124(1):20–26. doi: 10.1016/j.ophtha.2016.07.019 [DOI] [PubMed] [Google Scholar]
- 93.Gonzalez-Andrades M, Sharifi R, Islam MM, et al. Improving the practicality and safety of artificial corneas: pre-assembly and gamma-rays sterilization of the Boston Keratoprosthesis. Ocul Surf. 2018;16(3):322–330. doi: 10.1016/j.jtos.2018.04.002 [DOI] [PubMed] [Google Scholar]
- 94.Islam MM, Sharifi R, Gonzalez-Andrades M. Finding an optimal substitute for human donor corneas. Boston KPro news. July 2019.
- 95.Sharifi R, Mahmoudzadeh S, Islam MM, et al. Poly(methyl methacrylate): covalent functionalization of PMMA surface with L-3,4-Dihydroxyphenylalanine (L-DOPA) to enhance its biocompatibility and adhesion to corneal tissue (Adv. Mater. Interfaces 1/2020). Adv Mater Interfaces. 2020;7(1):2070001. doi: 10.1002/admi.202070001 [DOI] [Google Scholar]
- 96.Bakshi SK, Graney J, Paschalis EI, et al. Design and outcomes of a novel keratoprosthesis: addressing unmet needs in end-stage cicatricial corneal blindness. Cornea. Epub 2019 8. [DOI] [PubMed] [Google Scholar]