Abstract
Despite the presence of many hematological prognostic indexes, clinical course and overall survival are often highly variable even within the same patient subgroup. Recent studies suggest that simple, cost-effective, low-risk tests such as neutrophil to lymphocyte ratio (NLR) and lymphocyte to monocyte ratio (LMR) may be used to evaluate the prognosis. Their role has been well confirmed in diffuse large B-cell lymphoma (DLBCL), Hodgkin lymphoma (HL) and multiple myeloma (MM), but until now the prognostic significance of NLR and LMR in leukemias has not been widely reported. In this article, we analyze the literature data on prognostic value of NLR and LMR in haematological malignancies in the context of classic prognostic factors and clinical course.
Keywords: neutrophil to lymphocyte ratio, lymphocyte to monocyte ratio, non-Hodgkin lymphoma, Hodgkin lymphoma, multiple myeloma
Introduction
Complete blood count (CBC) is an inexpensive and easy to perform diagnostic test, widely used in everyday clinical practice. It is of great importance in diagnostics and monitoring of different medical conditions, not only hematological ones. Although used for years, new applications of CBC are still being discovered. Recently, numerous studies focused on proportion of different types of leukocytes in various medical conditions. Neutrophil to lymphocyte ratio (NLR) and lymphocyte to monocyte ratio (LMR) seem most valuable parameters.1
NLR has already proved itself useful in prognostics of infectious diseases,2 inflammatory conditions,3 surgical emergencies,4 postoperative complications5 and as a bone loss index in postmenopausal women.1,6 It can also be used for mortality stratification in major cardiac events.7,8 Several studies have revealed the significance of NLR as prognostic factor in patients with solid tumors such as gastric cancer,9 breast cancer,10 head and neck cancers,11 hepatic cell carcinoma,12 lung cancer,13 esophageal cancer,14 melanoma.15 Similarly, application of LMR as a prognostic factor is under close investigation. There is a strong evidence for its significance in formulating the prognosis in cardiovascular diseases and solid tumors.16 While the prognostic role of NLR and LMR is undeniable in many solid tumors, it is still unclear in many types of leukemias and lymphomas. The reasons why NLR and LMR can be used as prognostic factors, remain speculative.
Leukocytes Involvement in Tumor Pathogenesis
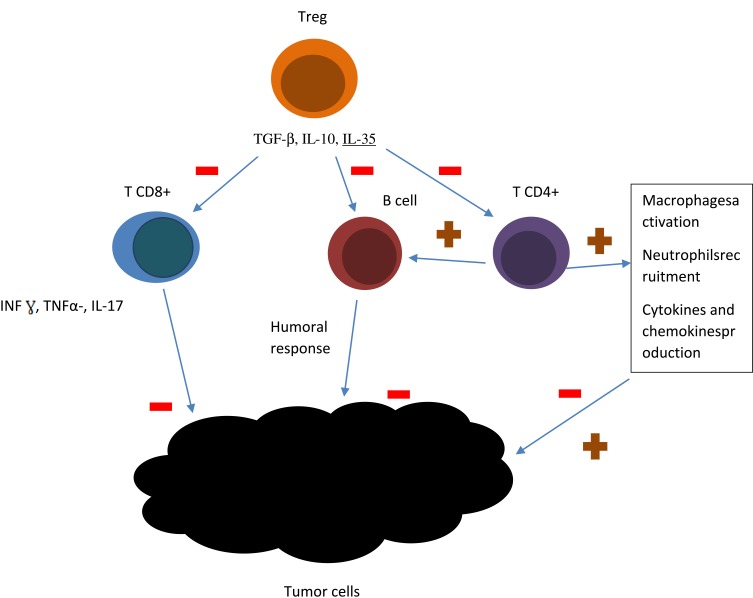
Malignant tumors are encircled by matrix and stromal cells, such as fibroblasts and myofibroblasts, neuroendocrine cells, adipose cells, immune and inflammatory cells, the blood and lymphatic vascular networks. These elements form tumor microenvironment, a dynamic, mysterious and complicated background, linked with every step of tumorigenesis. Nowadays, oncologists pay a lot of attention to tumor microenvironment, as it significantly influences therapeutic response and clinical outcome.17 Hematological malignancies are characterized by slightly different microenvironment than the one of solid tumors. The presence of a prominent number of intratumoral immune cells influences both antitumor immunity and immunodeficiency.18 T cells are divided into CD4+ T (helper T cells, Th) and CD8+ T (cytotoxic T cells, Tc) cells. IFN-γ, TNF-α, and IL17, which present antitumor effect are mainly produced by CD8+T cells.18 In patients with B-cell lymphomas T cells typically comprise up to 50% of the intratumoral cells. The frequency and location of T cells in biopsy specimens are predictive of the patient outcome.19 Higher intratumor CD8+ cells level generally predicts better outcome in patients with lymphomas.20 CD4+ T cells regulate immune response by enhancing the production of antibodies by neighboring B cells, macrophages activity stimulation, recruiting neutrophils, eosinophils, basophils and stimulation of cytokines and chemokines production.21 T CD4+ population comprises of different subpopulations: T regulatory cells, T helper cells, Th17 cells, T FH cells. T reg is a particularly important subpopulation, in the context of host immune response. Elevated numbers of Treg is often detected in peripheral blood and biopsy specimens in patients with cancer. Treg cells are highly prevalent in lymphoma biopsy material and are believed to suppress antitumor immunity by suppression of other CD4+ and CD8+ T-cell populations.22 CD8+ and CD4+ lymphocytes interaction is of importance in inducting tumor-cell apoptosis.16 Low amount of lymphocytes infiltrating the tumor can promote relapse or metastasis.23 The presence of lymphopenia usually signifies the severity of the process and facilitates cancer cell escape from the immune of tumor-infiltrating lymphocytes (TILs).24 Numerous studies have showed that tumor cells escape by expression of PD-L1 and junction with PD-1 on lymphocytes.23 There is an increasing evidence for contribution of B cells to tumor response. There are different subsets of B cells, contributing to both pro- as well as antitumor immune responses.25 B cells are marked by different antigens depending on physiological state, CD19 and CD20 are mainly expressed by pre-B cells, immature B cells, and plasma cells, IgM, IgD, and CR1 mark mature B cells; IgM, IgD, IgA, IgG are mainly expressed in memory B cells.18 Figure 1 presents schematic model of lymphocytes involvement in tumor pathogenesis.
Figure 1.
A schematic model showing lymphocytes involvement in tumor pathogenesis. T CD8+ cells produce IFN-γ, TNF-α, and IL17, what stimulates antitumor effect. CD4+ T: enhance the production of antibodies by neighboring B cells, activate macrophages, recruit neutrophils, eosinophils, basophils and stimulate the production of cytokines and chemokines production. T regulatory cells suppress antitumor immunity by suppression of other CD4+ and CD8+ T-cell populations. The figure is the authors' interpretation based on references.18,21,22
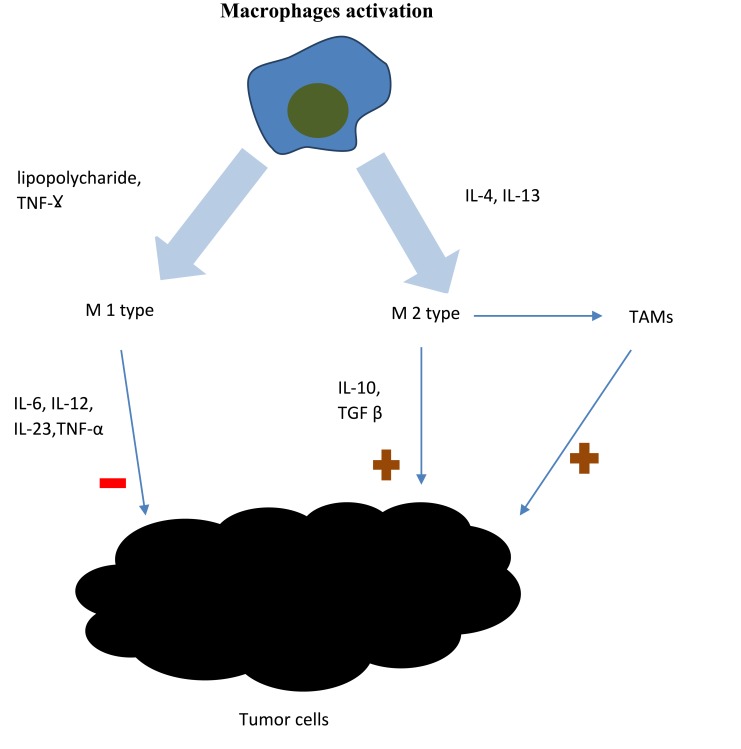
Cells of the monocyte lineage are crucial to the innate immune response. Their main function is to serve as the first line of resistance against microbes, but also to activate adaptive immune responses. There are two types of immunological responses: immune response-1 and immune response-2, of which the second one is prognostically unfavorable in malignant tumors. Many genes, whose products take part in immunologic response-2 are expressed by peripheral blood monocytes. There is an association between their expression level and cancer prognosis. Additionally, monocyte chemoattractant protein plays important role in modulating tumorigenesis.19,26 TNF α and IL-1, which are also secreted from monocytes, are associated with poor prognosis in cancer patients.27 Thus, monocytes play an important role in tumor microenvironment and might be considered as markers of a prolific tumor burden. There are two types of macrophages activation: M1 and M2 activation, depending on the type of stimulation. M1 activation is stimulated by lipopolysaccharide and IFN-γ. M1 macrophages infiltrate the tumor microenvironment in response to inflammatory signals mentioned above and release proinflammatory cytokines and chemokines, which promote the differentiation of T and NK cells. M2 activation is stimulated by IL-4 and IL-13. Macrophages can differentiate into a tumor-associated macrophages (TAMs).18 TAMs influence tumor cells as well as the tumor microenvironment. TAMs stimulate tumor cells proliferation, migration and genetic instability and promote angiogenesis and lymphoangiogenesis, which facilitates metastasis.18,28 It was proved in B-cell non-Hodgkin lymphomas and in Hodgkin lymphoma that higher density of intratumoral macrophages is associated with progression and poor prognosis.29 CD68 is a marker expressed on macrophages that correlates with overall survival. High number of CD68-expressing cells was associated with poorer survival in Farinha et al study.30 While the benefits of neutrophil actions are undeniable in the context of infection or trauma, their effects in the context of oncogenesis seem problematic.31 Figure 2 presents macrophages involvement in tumor pathogenesis. Neutrophils exhibit anti-tumor activity, but also suppress the cytolytic activity of immune cells such as lymphocytes.32 They secrete multiple cytokines such as: interleukin-2 (IL-2), interleukin-10 (IL-10), tumor necrosis factor α (TNF α), which are well known promotors of tumorigenesis. This may be one of the reasons why upwards of 15% malignancies worldwide are initiated by infections.33
Figure 2.
Macrophages involvement in tumor pathogenesis. There are two types of macrophages activation: M1 and M2 activation. M1 activation is stimulated by lipopolysaccharide and IFN-γ. M1 macrophages infiltrate the tumor microenvironment and release factors, which promote the differentiation of T and NK cells. M1 macrophages present antitumorigenic effect. M2 activation is stimulated by IL-4 and IL-13. M2 macrophages present pro-tumourigenic effect. M2 macrophages can differentiate into tumor-associated macrophages (TAMs), which stimulate tumor cells proliferation, migration and genetic instability and promote angiogenesis and lymphoangiogenesis. The figure is the authors' interpretation based on references.18,28
In summary, the main cells, promoting immunodeficiency are regulatory T (Treg) lymphocytes marked by Foxp3+, myeloid-derived suppressor cells (MDSC) marked by HMGB1 and M2 macrophages marked by CD163+.17,34-36 Granulocytes may also promote cancer development by inducing alterations in stromal cells and through the expression of cytokines, such as hematopoietic growth factor (HGF) and granulocyte colony-stimulating factor (G-CSF).37–39 IL-10 and TGF-β are antitumor cytokines, which paradoxically can promote immunosuppression, by activation of Treg cells.
High NLR reflects a decrease in the number of lymphocytes and an elevated number of neutrophils in tumor microenvironment. The LMR is calculated by dividing the absolute lymphocyte counts by the absolute monocyte counts from the blood test. The absolute neutrophil count might serve as a marker of systemic inflammation, which provides favorable environment for the development of malignant tumors. In contrast, the absolute lymphocyte count reflects immunosuppression, which is associated with poor outcome in a number of solid and hematological malignancies.40 What follows from the above considerations, NLR and LMR present biological rationale, as they reflect the interaction between tumor microenvironment and host’s immunologic response.32 Numerous studies have proved that inflammation plays a crucial role in tumor initiation, growth and progression.41 There exists plenty of evidence that systemic inflammatory response is a determinant of prognosis in patients with malignant neoplasms. Inflammatory response is reflected by agents such as: elevated C-reactive protein (CRP) level, hypoalbuminemia or high white cell, neutrophil and platelet counts.16 As our understanding of the inflammatory microenvironment of cancer has improved, many inflammatory indicators including NLR, platelet to lymphocyte ratio, or CRP are under investigation as diagnostic and prognostic biomarkers of cancer.
NLR and LMR in B-Cell Lymphomas
Diffuse Large B Cell Lymphoma
Diffuse large B cell lymphoma (DLBCL) is the most common type of lymphoma among adults which accounts for 30–58% of non-Hodgkin lymphomas. The staging has been established according to Ann-Arbor system. Currently, International Prognostic Index (IPI) is prevalent in formulating the diagnosis. The IPI score is calculated taking into account: patient’s age, serum lactate dehydrogenase level, Eastern Cooperative Oncology Group (ECOG) performance score, disease stage, and the number of extranodal disease localizations.42 Approximately 60–70% patients can be cured, using currently available regimens (most of all R-CHOP), other patients fail to respond to treatment or present poor long term OS. Highly heterogenic course of the disease justifies the necessity of usage of new, more precise prognostic factors.43
Prognostic significance of NLR in diffuse large B cell lymphoma has been demonstrated in numerous studies, the largest of which is meta-analysis by Mu et al41 2515 patients, who took part in eleven trials published before September 2017, were included in the study. All patients were treated with R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone). It was demonstrated that high NLR is a significant indicator for poor overall survival (OS)(HR 1.826, 95% CI 1.238–2.692) and progression-free survival (PFS)(HR 1.591, 95% CI 1.124–2.252) and this may be used as an independent risk factor in DLBCL. The association between mortality risk and NLR was non-linear. High pretreatment NLR was associated with elder age, advanced Ann Arbor stage, higher incidence rate of B symptoms, more frequent bone marrow involvement and higher lactate dehydrogenase (LDH). There was no association between NLR and sex, international prognostic index (IPI) score or extra nodal disease.41
On the contrary, in Wang et al meta-analysis,43 performed on less numerous group (9 studies, 2297 patients) extranodal disease and IPI score corresponded with NLR value. The presence of B group symptoms was not associated with NLR. Identically, as in Mu’s at al. study, NLR was a significant indicator for poor OS (HR = 1.84, 95% CI = 1.52–2.22, p<0.001) and poor PFS (HR = 1.64, 95% CI = 1.36–1.98, p<0.001). High NLR was also associated with Ann Arbor stage and LDH level, and was not related to sex, age or ECOG score.41,43 These results are in accordance with previous findings regarding DLBCL. Feng et al44 proved that low absolute lymphocyte count (ALC) is an adverse prognostic sign in DLBCL.44 Annibali et al45 revealed that NLR is an independent prognostic factor and it can help to separate patients with low/intermediate IPI (IPI<3).45
In contrast to the studies mentioned above, Azuma et al46 failed to reveal predictive value of NLR. The study was performed on 530 patients with de novo DLBCL. The cutoff for NLR was estimated 5.2 and it was not associated with OS and PFS. Authors suggest that National Comprehensive Cancer Network - International Prognostic Index (NCCN-IPI) might be the most powerful predictor in patients with DLBCL, treated with rituximab. A hypothesis has been made that NLR might not be good to reflect the outcome of lymphoid malignancies, as tumor-infiltrating lymphocytes (TILs) (which are associated with better prognosis) number might be decreased.46 “Derived” NLR is a variant of NLR and is also a prognostic factor in DLBCL. It denotes the difference between absolute leukocyte count and absolute neutrophil count divided by absolute neutrophil count. High derived NLR also represents poor prognostic factor in DLBCL.47
Similarly, the usage of LMR in DLBCL outcome assessment might be promising. Wanget al.23 investigated the prognostic value of NLR, LMR, PLR (platelet to lymphocyte ratio) and agreed an association of these factors with the expression of CD163+ M2 TAM (tumor associated macrophages) and PD-1 (programmed death cell 1)+TILs in tumor microenvironment. It turned out that lower LMR, higher NLR, CD163+ M2 tumor-associated macrophages (TAM) higher than 9.5% and PD1+ TIL lower than 4.5 cells per high power field were associated with worst OS and PFS.23 Previously, LMR was negatively correlated with TAM infiltration in tumor microenvironment.48 Higher number of TAM, derived from monocytes inhibit antitumor immunity. LMR < 2.71 was a negative prognostic marker for predicting OS (HR,1.658;95% CI,1.930–2.703; p=0.042) and PFS (HR,1.528; 95% CI, 1.006–2.315; p=0.049).23
Follicular Lymphoma
Follicular lymphoma (FL) is the most common indolent non-Hodgkin lymphoma in Western countries. It is a heterogeneous disease with a varying prognosis. The management of FL is dependent on patient and disease features. Most patients need treatment after 3–4 years after formulating the diagnosis. Numerous tools are available for risk stratification.49 Follicular Lymphoma International Prognostic Index (FLIPI) score is most frequently used to classify patients into risk groups: low, intermediate and high risk group.50 The factors used to calculate FLIPI comprise: age, Ann Arbor stage, hemoglobin level, number of nodal areas and serum LDH level.51 As FLIPI does not always reflect patient’s survival in the age of modern therapy, new prognostic strategies, for example based on tumor microenvironment parameters, are needed.26 As it is believed that serum leucocyte levels reflect tumor microenvironment, studies has been conducted, considering the relationship of absolute monocyte count (AMC) as well as absolute lymphocyte count (ALC) and FL patient’s outcomes. Wilcox et al52 found a positive correlation between OS and AMC (AMC cut off value has been calculated 0·57 × 109 cells/l).52 In contrast, Watanabe et al53 found no such association with cut off value of 0·34 × 109 cells/l.53 As for ALC, Siddiqui et al54 reported that an ALC ≤ 1·0 × 109 cells/l represented poor prognostic parameter for OS in FL, most of all in patients with Grade 1 or 2 disease.54 Recently, Marcheselli et al55 proved that only AMC is a powerful predictor of PFS, and maybe OS in FL patients, treated with combination chemotherapy regimens, containing rituximab. AMC could be used as simple predictive factor, independently of the treatment regimen. This also can be merged with other factors that determine the IPI and FLIPI.55
In comparison with DLBCL, there is less evidence for prognostic value of NLR in FL. Lee et al26 performed retrospective cohort study, in which 88 patients with FL took part. LMR and NLR were evaluated as valuable prognostic factors. The best cut-off values were 3.20 for LMR and 2.18 for LMR. High LMR at diagnosis was associated with superior PFS (HR 0.31, 95% CI 0.13 to 0.71), as well as high NLR at relapse was associated with poorer post progression survival (HR 1.24, 95% CI 1.04 to 1.49). Authors conclude that LMR and NLR values might be used alongside with FLIPI, to achieve credible prognostic information.26 Belotti et al56 revealed that FL patients with LMR above 2 had longer time to treatment compared with those with LMR below 2. 2-year PFS in patients treated with rituximab was superior in the LMR above 2 group.56 Kumagai et al57 evaluated the significance of ALC/AMC ratio in FL patients treated with rituximab-containing chemotherapy. It has been revealed that decreased ALC/AMC ratio was associated with inferior PFS (HR 2.714; 95% CI 1.060–6.948; p= 0.037) and was an independent poor prognostic factor. ALC/AMC ratio might be useful in selection of candidates for “watch and wait strategy” among FL patients.57
Mantle Cell Lymphoma
Mantle cell lymphoma (MCL) is a type of B-cell non-Hodgkin lymphoma, representing approximately 7–9% lymphomas in Europe. MCL is characterized by the translocation t (11;14), which results in cyclin D1 overexpression. Patients median age is 60 years old, strong male predominance is observed.58 Patients are generally diagnosed in stage III/IV of the disease and present with lymphadenopathy, blood and bone marrow involvement and splenomegaly.59 In most patients with MCL the therapy is applied at the time of diagnosis, but a subset of patients with indolent MCL might be managed using “watch and wait” strategy.60
In risk stratification, International Prognostic Index (IPI) has been used initially for patients with MCL but it has not been very discriminatory, in particular considering the low risk patients. Consequently, Mantle cell International Prognostic Index (MIPI), was developed by the European MCL Network.58 Prognostic factors for shorter overall survival (OS), according to MIPI are: elderly age, worse ECOG score, higher LDH, and a higher white blood cell count at diagnosis. In Hoster et al61 study patients have been classified according to MIPI into low risk (median OS not reached), intermediate risk (median OS 51 months), and high risk groups (median OS 29 months).61 Although MIPI provides important prognostic data, it is not always sufficient in evaluation of clinical course of the disease.32 MIPI has been introduced for advanced staged patients, in pre-rituximab era.62 Ki-67 index exhibits some additional discriminatory application, as well as gene expression profiling.58 For example, SOX11 expression is believed to identify low-risk, indolent cases.60
Little attention has been drawn to host immunity and tumor microenvironment prognostic factors in MCL. Koh et al63 demonstrated that the AMC, at the time of diagnosis, is an independent prognostic factor for OS in MCL and is associated with poor clinical outcome.63 Then, more precise prognostic significance of AMC in MCL patients has been demonstrated by Von Hohenstaufen et al.64 Authors combined AMC and beta-2-microglobulin values with MIPI, which resulted in more accurate prognostic risk classification.64 Goy et al65 used ALC and AMC in combination, instead of solely AMC. Superior ALC/AMC (cut off calculated >2), after induction therapy, was associated with longer overall survival in MCL.65 In contrary, in George et al66 study, the connection between AMC and the outcome was not observed.32,66
The first and the only study aimed at investigating NLR prognostic significance in MCL was published by Haydaroglu et al.32 The absolute monocyte count (AMC), NLR, PLR at diagnosis of mantle cell lymphoma has been evaluated in 96 patients. AMC>/=580, NLR >/=2,43 and PLR>/=120,85 turned out to be negative prognostic factors for 5-year PFS. However, NLR has not be found as an independent risk factor for PFS and OS.32 Also, prognostic value of peripheral blood lymphocyte subsets counts has been thoroughly analyzed.62 One and only research considering the subject was carried out on 68 MCL patients. The parameters measured by peripheral blood flow cytometry were established as follows: absolute CD4+ T cell counts (ACD4C), CD8+ T cell counts (ACD8C), nature killer cell counts, and CD4/CD8. Those values were compiled with clinical parameters and long-term outcomes. High AMC, low ACD4C and low CD4/CD8 ratio were found to be poor prognostic factors for OS. It was reported that low ACD4C was a significant predictor of unfavorable OS. The 3-year OS in patients with high ACD4C was 70%, whereas OS in patients with low ACD4C was calculated as 36%. No such difference was noticed in OS between patients with low and high ACD8C (42% vs 59%). The 3-year OS among patients with high CD4/CD8 ratio amounted to 60%, while OS among patients with low CD4/CD8 ratio was 25%.62 Another study, comparing T cell subgroups in lymph node biopsy in patients with MCL and patients with reactive lymph nodes, revealed that higher levels of T cells, CD8+ T cells, and most of all CD4+ T cells, correlated with indolent MCL.Cell counts were decreased in more aggressive tumors. High CD4/CD8 ratio was associated with favorable OS.67 This is in accordance with Zhang et al study. On the other hand, Zhou et al68 showed that low absolute natural killer cells value predict lower OS in MCL patients.68
Primary Central Nervous System Lymphoma
Primary central nervous system lymphoma (PCNSL) is a rare neoplasm, with high fatality rate. The population with higher risk of falling ill is elderly people and immunosuppressed patients, for example HIV-infected or on immunosuppressant medications.There are two prognostic indexes, widely used in PCNSL. The International Extranodal Lymphoma Study Group (IELSG) developed a prognostication, in which unfavorable factors are: age above 60, poor ECOG score, elevated serum LDH level, elevated CSF protein level and involvement of deep brain structure. Accordingly, patients are divided into three groups, in which two-year survival is estimated to be 15 to 80%.69 The second system has been developed in Memorial Sloan Kettering Cancer Center and incorporates only age and Karnofsky Performance Scale score. Patients are also classified into three risk categories.70 The serum LDH level is an approved prognostic marker, other serum biomarkers are under investigation.71 It appears that low absolute lymphocyte count at diagnosis seems to be a poor prognostic factor.72 NLR is also under investigation. In Jung et al33 study, 39 patients with primary central nervous system lymphoma were divided into a high NLR group and a low NLR group. The cut-off value was established for 2. The low NLR group exhibited better response to induction of chemotherapy compared with the high NLR group. The high NLR group also revealed a significantly worse 3-year OS (HR 2.64, 95% CI 1.06–6.60; p=0.038) and a worse 3-year PFS (HR 2.41, 95% CI 1.07–5.42; p=0.034). Furthermore, pre-treatment high values of PLR and RDW were also poor prognostic markers. What is interesting, is the impact of systemic inflammation on development of PCNSL.33 As we know central nervous system is devoid of lymphoid aggregation. The role of NLR, PLR and RDW suggests that systemic inflammation could affect PCNSL. In Montesinos-Rongen’s opinion, original cell of PCNSL develops outside the central nervous system, but survives only in its immunodeficient environment.73 In contrary to the studies mentioned above, Le at al.74 reported that neither neutrophils, lymphocyte, or platelets counts nor their ratios (neutrophil-to-lymphocyte ratio and neutrophil-to-platelets ratio) predicted OS. Interestingly, the only factor associated with OS was pretreatment hemoglobin. The presence of anemia predicted poorer survival (HR, 0.77; 95% CI, 0.67–0.88; p < 0.001).74
NLR and LMR in T and NK-Cell Lymphomas
Primary Cutaneous T-Cell Lymphoma
Primary cutaneous T-cell lymphoma (PCTCL) is a heterogenous group of non-Hodgkin’s lymphomas (NHL) which accounts for two-thirds of cutaneous lymphomas. The most common variant of CTCL is mycosis fungoides (MF), accounting for over half of all cases.75 In its early stages, the disease tends to be indolent, with little effect on patient’s life expectancy, however a subset of patients undergo rapid progression and extracutaneous spread of T cells. It is crucial to identify individuals with high risk of progression.76 Traditionally, overall clinical characteristics, such as skinstage (T) or histologic features for example large cell transformation (LCT), identify patients with poor prognosis. It is worth emphasizing that there is great heterogeneity in these subclasses of PCL. Moreover, in everyday medical practice, there are no genetic markers available, that could identify subsets of patients in a more accurate way.77
What follows from the above considerations, is that new prognostic markers are needed in MF. A complete blood count is one of the tests considered useful in making the prognosis. Elevated absolute lymphocyte count and eosinophilia are associated with disease progression and disease-specific death.76 Changes in the T-cell population can also be correlated with the risk of disease progression. In Abeni et al78 study, the number of CD8+ T cells below 600/mL has been associated with a worse prognosis in MF patients. Moreover, exceedingly high risk of death was reported for patients with leukocytes> or = 9000 and CD8+ < 600 cells microL.78
Cengiz et al79 revealed that high NLR at diagnosis of MF represents a simple, poor prognostic factor. NLR higher than 2.85 could be used for identifying high-risk patients. In advanced stages of the disease high NLR was associated with high beta-2-microglobulin level.79 On the contrary, Eren et al80 found no significant difference in treatment demand, time to treatment, progression in stage and time to progression in stage in patients with NLR>/=2 and NLR<2.80 The recent prospective study by Uysal et al81 considered screening tests in patients with early-staged mycosis fungoides. No differences have been detected between NLR value in different staging groups. It may be possible that NLR could be a prognostic marker only in patients with advanced disease.81 In 2019, Vonderheid et al82 found no association between NLR and MF prognosis. What is interesting, the authors reported prognostic significance of serum copper in CTCL patients. Copper level higher than normal was associated with an increased risk of disease progression and shortened disease-specific survival for patients with patch or plaque phase MF.82
Peripheral T-Cell Lymphoma
Peripheral T-cell lymphoma, unspecified (PTCLU) is a group of heterogeneous diseases that cannot be further classified into any other category according to World Health Organization classification.83 It encompasses a group of lymphomas with generally poor prognosis, with a 5-year survival rate of approximately 32%.84 In PTCLU Ann Arbor score, PIT (Prognostic Index for PTCLU) score, IPI score, IPTCLP (International Peripheral T cell Lymphoma Project Score) and modified PIT are used to prognose the outcome.85 It seems that NLR also exhibits prognostic significance. In Beltran et al86 study NLR>/=4 was associated with worse OS after adjustment for the PIT (HR 4.30, 95% CI 1.90–9.69; p < 0.001) and IPI score (HR 2.60, 95% CI 1. 12–6.04; p = 0.03).86 Kaito et al proposed a new prognostic index in PTCLU comprising neutrophil to lymphocyte ratio, lactate dehydrogenase and albumin. Its main goal is to predict the effect of first-line chemotherapy. Authors measured time to treatment failure rate (TTF) after 1 year from diagnosis. It was calculated as 71.4% in patients with score 0 (no adverse factor), 31.8% with score 1 (1 adverse factor) and 4.5% with score 2 (2–3 adverse factors). The prognostic power of this model was superior to PIT power. Authors conclude that patients with scores of 1 and 2, responding to chemotherapy should be perfect candidates for upfront stem cell transplantation. As for LMR, Cencini et al87 proved that patients with lower ALC/AMC ratios (ALC/AMC < 2) had shorter OS and PFS. They also combined ALC/AMC ratio and PTCL-U score, which resulted in the division for 3 groups with different prognosis.87
Extranodal Natural Killer/T Cell Lymphoma
Extranodal natural killer/T cell lymphoma (ENKTL) is a highly aggressive lymphoma derived from mature NK- and T-cells. ENKTL can be pathologically divided into two types, nasal and non-nasal ENKTL.The majority of ENKTL occur in the upper aerodigestive tract. It can also be situated in skin, testis and salivary glands. IPI and PINK (prognostic index for NK/T cell lymphoma) are useful prognostic models, verified in numerous studies.88 Zhou study evaluated prognostic value of derived NLR (dNLR) in 33 newly diagnosed ENKTL patients. Patients with dNLR ≥3.6 presented shorter OS and PFS than patients with dNLR<3.6. Low absolute lymphocyte count was also adverse prognostic marker.89
T-Lymphoblastic Lymphoma
T-lymphoblastic lymphoma (T-LBL) is a neoplasm of immature T-cell lineage (T-LBL) T-LBL is more common in children than in adults and concerns mostly boys. The pathologic characteristics of LBL are similar to those of acute lymphoblastic leukemia (ALL),90 The difference between acute lymphoblastic leukemia is that in LBL bone marrow involvement is below 25% (or 20% according to WHO).91 Lately, after the adoption of pediatric-derived, lymphoblastic leukemia-like protocols the survival in T-LBL has improved significantly. Due to rarity of the disease, prognostication still remains a challenge.92 Although many of T-LBL prognostic factors have been reported, such as age above 30–40, elevated LDH, bone marrow involvement, stage IV, B symptoms, or early CNS invasion, these need to be furtherly elaborated on. Meiwei et al93 reported that Ki-67≥75%, elevated LDH, pleural effusion and no OR after chemotherapy affected survival.93 IPI score is frequently used. Feng et al observed the outcome of 75 newly diagnosed adult patients with T-lymphoblastic lymphoma (T-LBL) in correlation to LMR, NLR and PLR values. Patients with LMR</=2,8, NLR >/=3,3 and PLR>/=200 had inferior PFS and inferior OS. Authors proposed a “complete blood count score” model, comprised of LMR, NLR and PLR. 3-year OS was 84%, 53% and 30% for low-, intermediate- and high-risk patients. Prognosis established using this model was much more accurate than when using IPI score.94
NLR and MLR in Hodgkin’s Lymphoma
Hodgkin’s lymphoma is a neoplastic disease characterized by the presence of cancerous Reed-Sternberg cells and Hodgkin cells in the tumor background. Patients are commonly diagnosed in their 20s-30s.HL is highly curable with combination chemotherapy, radiation or combined modality treatment, even in advanced stages of the disease. The Ann Arbor staging system with Cotswolds modification is widely used. It is based on whether the involved lymph nodes are situated on one or both sides of the diaphragm, the number of sites involved, whether there is contiguous extranodal involvement or disseminated extranodal disease, and whether typical systemic symptoms (B symptoms) or bulky disease are present.95 Fluorodeoxyglucose (FDG) positron emission tomography (PET) and computed tomography (CT) should be performed. PET scans are interpreted using simple, reproducible criteria workshopped in Deauville, France. In 2014, the Lugano classification modernized staging for lymphomas, as fluorodeoxyglucose (FDG) positron emission tomography (PET) and computed tomography (CT) was incorporated into standard staging. The Lugano staging is not yet universally accepted.96 Prognostication in HL seems to be of great importance, especially considering the information, that at least 10–20% of patients are under- or overtreated. Both under- and overtreatment might result in serious adverse events, including patient’s death, due to disease relapse or unnecessary toxic treatment.97
Most recent study, considering prognostic role of NLR in Hodgkin’s lymphoma has been published by Dogan et al.98 A cut-off value 4.23 for NLR was used to predict the outcome. High NLR values were associated with poorer treatment response rate. The sensitivity and specificity of this index was estimated as 60% and 65%.98 Romano et al99 compared NLR values of patients with HL and healthy individuals (5.0 vs 1.6). Higher NLR was associated with advanced stage, increased neutrophil count, reduced lymphocyte count and higher levels of markers of systemic inflammation. PFS at 60 months was 86.6% vs 70.1% in patients with NLR>/=6 and NLR<6. It was stated that NLR is a predictor of PFS in HL patients independently of the stage at diagnosis. Authors propose that integration of PET-2 scan, NLR and LMR values could be used to establish new prognostic system in HL.99 In Koh’s et al100 study it was proved that NLR values above 4.3 predicted poorer OS in advanced cHL.100 Marcheselli et al101 determined that NLR above 6 was associated with poorer PFS and OS in every stage of cHL.101 Risk stratification in early-staged HL in the era of de-escalation treatment is more important than ever. It seems crucial to identify patients who cannot go under treatment de-escalation due to high relapse risk. Positron emission tomography (PET) is widely used, but is considered as not sufficient. Reddy et al102 made an retrospective analysis, to determine whether NLR or PLR present predictive significance. 338 patients with stage I and II classical Hodgkin lymphoma (cHL) were included. Cut off points were appointed: 6.4 for NLR and 266.2 for PLR. Both NLR and PLR were associated with worse FFP (freedom from progression). Moreover, there was association between adverse pre-treatment factors such as bulky disease, presence of B symptoms, stage II disease, and NLR and PLR values. Unfortunately, both NLR and PLR failed to predict relapse.102
The prognostic value of LMR in HL has been controversial for quite a long time. Few studies have proved that correlation between LMR and survival outcomes does exist, other reporter otherwise.103 Gu et al16 meta-analysis on prognostic value of LMR in different cancer types, found that LMR at diagnosis predicted poorer cancer-specific survival and PFS in HL.16 In 2019, Fung Lee et al meta-analysis was released. The study concerns prognostic value of LMR in HL. Eight retrospective studies were involved. Low LMR was associated with poorer OS and PFS, which might be due to more aggressive HL nature or poorer treatment tolerance. Lymphopenia is a proven prognostic factor in HL, which is convergent with LMR prognostic significance.104 Table 1 summarizes evidence for prognostic significance of NLR in lymphomas. A summary of the LMR data is presented in Table 2.
Table 1.
Evidence for Prognostic Significance of NLR in Lymphomas
| Disease | Study | Area | Number of Patients | Results |
|---|---|---|---|---|
| DLBCL | Mu et al meta-analysis41 |
USA, China, Korea, Austria, Croatia | 2515 | NLR is an indicator for poor OS (HR 1.826, 95% CI 1.238–2.692) and poor PFS (HR 1.591, 95% CI 1.124–2.252). |
| Wang et al meta-analysis43 | USA, Taiwan, Korea, Austria, China | 2297 | NLR is an indicator for poor OS (HR = 1.84, 95% CI = 1.52–2.22, p<0.001) and poor PFS (HR = 1.64, 95% CI = 1.36–1.98, p<0.001). | |
| Annibali et al.45 | Italy | 505 | Patients with NLR<3.5 had higher 4-year OS probability than patients with NLR ratio ≥3.5 (86% vs 64%) and higher 4-year EFS probability than patients with NLR ratio ≥3.5 (76% vs 48%). | |
| Azuma et al.46 | Japan | 530 | No prognostic significance of NLR | |
| FL | Lee et al.26 | Hong Kong | 88 | High LMR (>3.20) at diagnosis was associated with superior PFS (HR 0.31, 95% CI 0.13 to 0.71). |
| MCL | Haydaroglu et al.32 | Turkey | 96 | The group with a NLR ≥ 2.43 had poorer 5-year PFS (21.0 ± 3.81, 95% CI = 13.53–28.47, P < 0.001) and poorer 5-year OS (31.0 ± 1.54, 95% CI = 27.96–34.03, P < 0.001). |
| PCNSL | Jung et al.33 | Korea | 62 | worse 3-year overall survival (OS) (42.5 vs 71.2%; p=0.031) and a worse 3-year progression-free survival (PFS) (37.3 vs 60.1%; p=0.028) in high NLR group |
| Le et al.74 | France | 182 | No prognostic significance of NLR | |
| MF | Cengiz et al.79 | Turkey | 119 | ANC/ALC ratios of 2.85 or higher at diagnosis were positively correlated with elevated beta-2-microglobulin, advanced disease stage, and disease progression |
| Eren et al.80 | Turkey | 117 | no association between the NLR and treatment demand, time to treatment, progression in stage, and TTP in stage in MF patients | |
| Uysal et al.81 | Turkey | 112 | no correlation between clinical responsiveness and NLR | |
| Vonderheid et al.82 | USA | 98 | NLR not significantly associated with prognosis | |
| PTCLU | Beltran et al.86 | Peru | 83 | NLR ≥ 4 was associated with worse OS (HR 3.96, 95% CI 1.92–8.17; p < 0.001). |
| ENKTL | Zhou et al.89 | China | 33 | Patients with high dNLR (≥3.6) revealed significantly shorter OS (P=0.001) and PFS (P=0.008) than those with low dNLR |
| T-LBL | Feng et al.94 | China | 75 | NLR ≥3.3 correlated with inferior PFS and OS, when compared with patients with NLR <3.3 (PFS: 7.5 and 39.5 months, p = 0.001; OS: 24.5 and 54 months, p = 0.05) |
| HL | Dogan et al.98 | Turkey | 232 | High NLR values were significantly related to disease stage, early-stage risk scoring and response to the treatment. |
| Romano et al.99 | Italy | 180 | PFS at 60 months was 86.6% versus 70.1% in patients with NLR ≥ 6 or NLR < 6 | |
| Koh et al.100 | Korea | 312 | high ANC/ALC ratio (≥4.3) correlated with poor OS (P < 0.001) | |
| Marcheselli et al.101 | Italy, Israel | 990 | Patients with NLR >6 had a worse PFS and OS compared to those with NLR ≤6 (84% vs 75% and 92% vs 88%, at 5 years; HR of 1.65 and 1.82). | |
| Reddy et al.102 | USA | 338 | Two‐year FFP for patients with NLR ≥6·4 was 82·2% vs 95·7% with NLR <6·4 (P < 0·001). |
Abbreviations: DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; MCL, mantle cel lymphoma’ PCNSL, primary central nervous system lymphoma; MF, mycosis fungoides; PTCLU, peripheral T-cell lymphoma, unspecified; ENKTL, extranodular natural killer/T-cell lymphoma; T-LBL, T-lymphoblastic lymphoma; HL, Hodgkin’s lymphoma; OS, overall survival; PFS, progression-free survival; EFS, event-free survival; FFP, freedom from progression; dNLR, derived NLR.
Table 2.
Evidence for Prognostic Significance of LMR in Hematological Malignancies
| Disease | Study | Number of Patients | Area | NLR Influence on OS | NLR Influence on PFS/EFS |
|---|---|---|---|---|---|
| DLBCL | Wang et al23 | 355 | China | LMR < 2.71 was a negative prognostic marker for OS (HR,1.658;95% CI,1.930–2.703; p=0.042) and a negative prognostic marker for PFS (HR,1.528; 95% CI, 1.006–2.315; p=0.049) | |
| FL | Lee et al26 | 88 | Hong Kong | high LMR (>3.20) at diagnosis was associated with superior PFS (HR of 0.31 (95% CI 0.13 to 0.71)) | |
| Belotti et al56 | 132 | Italy | 2-year PFS was superior in the LMR > 2 group | ||
| Kumagai et al57 | 99 | Japan | a decreased LMR was a significant poor prognostic factor | ||
| MCL | Goy et al65 | 96 | USA | An elevated ALC/AMC >2 is associated with improved OS in MCL. | |
| HL | Romano et al99 | 180 | Italy | PFS at 60 months was 86.6% vs 70.1% in patients with NLR ≥ 6 or NLR < 6. | |
| Fung Lee et al meta-analysis104 | 3319 | USA, Italy, Israel, Hungary, Korea, Greece, Serbia | low LMR was associated with a significantly poorer OS (HR 2.66, 95% CI 1.67, 4.26; P = 0.014; I2 = 62.5%; p = 0.014). LMR was associated with poorer PFS (HR 2.19, 95% CI 1.46, 3.29, P < 0.001; I2 = 52.2%; p = 0.079) | ||
| MM | Romano et al. | 208 | Italy | Patients with LMR < 3.6 had shorter PFS than those with LMR ≥ 3.6 (18.5 vs 40.5 months, p = 0.0003). | |
Abbreviations: DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; MCL, mantle cell lymphoma; PCNSL, primary central nervous system lymphoma; MF, mycosis fungoides; PTCLU, peripheral T-cell lymphoma, unspecified; ENKTL, extranodular natural killer/T-cell lymphoma; T-LBL, T-lymphoblastic lymphoma; HL, Hodgkin’s lymphoma; MM, multiple myeloma; OS, overall survival; PFS, progression-free survival; EFS, event-free survival.
NLR and MLR in Multiple Myeloma
Multiple myeloma (MM) is a neoplastic disease classified as a monoclonal gammopathy (plasma cell dyscrasia). Its essence is an uncontrolled proliferation of B cells at the final stage of differentiation, ie after the immunoglobulin heavy chain class switch recombination. Plasmocytes produce monoclonal protein globulins or its fragments105 Multiple myeloma accounts for approximately 1–2% of all malignant tumors and 10–15% of hematological malignancies.106 Durie and Salmon were creators of the first staging system in MM.107 It was revised in 2006.106 The Durie-Salmon classification (DSS) uses serum immunoglobulin M-component level, urine light chain M-component level, hemoglobin value, calcium concentration and the number of bone lesions to estimate tumor mass and predict survival. It 2005, Greippet al., introduced International Staging System (ISS). Patient classification and stratification is based solely on albumin and beta-microglobulin level.108 ISS overcame many limitations of Durie-Salmon staging system. It is easy to compute, provides more equal distribution among risk subgroups and presumably, is better correlated with post transplantation outcomes.109 It is currently assumed that International Staging System (ISS) is the most accurate to evaluate the prognosis in multiple myeloma. However, clinical course of MM is so highly variable, that clinicians need even more precise prognostic information.110 Cytogenetics is good prognostic biomarker, but its cost and convenience limits its application. Thus, the systemic inflammation markers, such as NLR and LMR are recently under close investigation in MM.111
Two meta-analyses considering the prognostic significance of NLR in MM were published in 2018. Zeng et al111 demonstrated poor OS (HR: 1.73, 95% CI: 1.23–2.44; P = 0.002) and PFS (HR: 1.74, 95% CI: 1.11–2.73; P = 0.015) when pretreatment NLR was elevated. Moreover, NLR was associated with ISS, isotype of light chain and response to treatment.111 Mu et al110 founded linear association between increased NLR and risk of mortality in MM patients. There was a linear association between NLR and ISS staging.110 Kim et al112 proposed ‘MPI analysis’, consisted of NLR pretreatment, platelet count and CRP. One point is assigned each for high NLR pretreatment, low PLT and high CRP level. MPI might be effective in predicting the survival of newly diagnosed MM patients undergoing active treatment. Proposed risk categories were stratified in: low (0 points), intermediate (1 point) and high (2–3 points). Median overall survival corresponded with risk stratification. What is more, prognostic significance of proposed index is significant regardless of age, renal function and novel agent treatment. It might be useful complementary to ISS in MM patients.112
It seems that NLR and LMR might have direct impact on choosing the most appropriate treatment regimen. As we know, MM patients can be treated with regimens consisting of proteasome inhibitor, IMiDs or both. Some authors claim that, high NLR is a poor prognostic factor only in patients treated with thalidomide and lenalidomide. On the other side low LMR predicts lower OS in all treatment groups.113 Dosani et al114 noticed that NLR and LMR do not always correlate with each other and might have different biological significance.114 All in all, NLR and LMR were confirmed as prognostic markers in patients treated with novel agents. Thus, high-risked patients (with NLR>/=2 or LMR<3.6) should be addressed to regimens containing both proteasome inhibitor and IMiDs.113 Elevated derived NLR level is an independent adverse prognostic factor for transplantation-ineligible MM patients. In Lee et al study, complete response rate was lower in the high derived NLR group in comparison to the low derived NLR group. The 2-year OS rate were: 72.2 in high derived NLR group and 84.7% in low derived NLR group.115 The data summary is presented in Tables 2 and 3 for LMR and NLR, respectively.
Table 3.
Evidence for Prognostic Significance of NLR in Multiple Myeloma, Leukemias and Myeloproliferative Neoplasms
| Disease | Study | Area | Number of Patients | NLR Influence on OS | NLR Influence on PFS/EFS |
|---|---|---|---|---|---|
| MM | Zeng et al meta-analysis111 | Turkey, Korea, China, Italy, USA | 1886 | poor OS (HR: 1.73, 95% CI: 1.23–2.44; P = 0.002) and poor PFS (HR: 1.74, 95% CI: 1.11–2.73; P = 0.015) when pretreatment NLR elevated |
|
| Mu et al110 meta-analysis | Turkey, China, Korea, Italy, USA | 1971 | increased NLR predicted poorer OS (HR 2.084, 95% CI: 1.341–3.238) and PFS(HR 1.029, 95% CI: 1.016–1.042) | ||
| Lee et al115 | 38 clinical centers | 176 | High dNLR was an independent poor prognostic factor for OS (hazard ratio 2.217, 95% CI 1.015–4.842; p = 0.0458) in transplantation-ineligible patients with MM. | ||
| RR-AML | Mushtaq et al.116 | USA | 63 | Median OS in patients with NLR of 3 or more was 3.4 months (95% CI 3.2–3.7) vs 9.2 months (95% CI 7.1–11.3) in those with NLR <3 (P=0.040). | |
| CLL | Chiarenza et al.117 | Italy | 400 | NLR ≥ 3.0, predicted a good prognosis in CLL patients | |
| PMF | Lucijanic et al.119 | Croatia | 102 | higher- NLR (HR=2.76; p=0.004) predicted poorer survival | |
Abbreviations: MM, multiple myeloma; RR-AML, relapsed/refractory acute myeloid leukemia; CLL, chronic lymphocytic leukemia; PMF, primary myelofibrosis; OS, overall survival; PFS, progression-free survival; EFS, event-free survival; dNLR-derived NLR.
NLR and LMR in Leukemias
Until now the prognostic significance of NLR and LMR in leukemias has not been widely reported. There is only one paper, evaluating the role of NLR in formulating the prognosis in acute leukemia patients. Mushtaq et al116 explored the association of NLR, overall survival (OS) and response to treatment in patients with relapsed/refractory acute myeloid leukemia (RR-AML). 63 adults with RR-AML were included. Mean NLR was 1.54, 11% patients had NLR of 3 or more. High NLR was an independent poor prognostic factor. Median OS in patients with NLR of 3 or more was 3.4 months (95% CI 3.2–3.7) vs 9.2 months (95% CI 7.1–11.3) in patients with NLR <3.116
Similarly, the issue of NLR and LMR significance in chronic lymphocytic leukemia, has been rarely raised. Chiarenza et al117 evaluated the prognostic role of blood neutrophils, monocytes and non-neoplastic lymphocytes in 400 CLL patients at diagnosis. To achieve this goal, absolute neutrophil count (ANC), absolute monocyte count (AMC), absolute T-lymphocyte count (ALC-CD3+), NLR and NMR (neutrophil to monocyte ratio) were evaluated. Compared to healthy individuals, CLL patients showed increased AMC value and increase in the ALC-CD3+ value. There was no difference in ANC. The median NLR ratio was higher in CLL patients compared to healthy individuals. NMR median value was higher in early stage compared to advanced stage (8.0, 6.7, 5.1 in Binet A, B, C).
No correlation has been found between NLR, NMR and genetic aberrations. Higher NLR and NMR were associated with the absence of serum prognostic markers, such as CD38, and CD49d.NLR and NMR were higher in untreated patients than treated ones (median NLR 2.42 vs 1.95; NMR 8.0 vs 7.0). Low NLR and NMR ratio were markers of increased risk to symptomatic progression. A strongest positive prognostic factor in CLL was displayed by NMR value.117 The data summary is presented in Table 3.
NLR and LMR in Myeloproliferative Neoplasms
Myeloproliferative neoplasms comprise polycythemia vera (PV), essential thrombocythemia (ET), primary myelofibrosis (PMF), chronic myeloid leukemia (CML), chronic neutrophilic leukemia, chronic eosinophilic leukemia-not otherwise specified and myeloproliferative neoplasm, unclassifiable.118 There exists little evidence for the prognostic significance of NLR and LMR in myeloproliferative neoplasms. Lucijanic et al119 investigated the prognostic value of NLR and PLR in PMF. NLR and PLR were higher in patients with PMF than in healthy individuals. Higher NLR was associated with Janus-kinase-2 (JAK2)-mutation, wild-type-Calreticulin (CALR), older age and higher leukocytes, higher hemoglobin, larger spleen size. No association with C-reactive-protein (CRP) value has been found. Higher NLR (HR=2.76; p=0.004) and lower PLR (HR=1.99; p=0.042) were independent markers of poor survival.119
Thrombosis leads to higher morbidity and mortality in PV and ET. Kocak et al120 evaluated the relation between NLR values and thrombosis risk in myeloproliferative neoplasms mentioned above. No associations have been found.120 In contrast, Zhou et al121 proved that NLR at diagnosis in ET is a valuable parameter for future thrombotic events.121 Hacibekiroglu et al122 evaluated the inflammation parameters in Philadelphia negative myeloproliferative neoplasia (PV, PMF, ET) patients and its impact on thrombosis. No significant difference has been found between thrombosis history and NLR value.122 It seems that in myeloproliferative malignancies, oxidative stress is of great importance as a mechanism of tumorigenesis.123–125 This reflects an imbalance between overproduction of reactive oxygen species and the cellular antioxidant defense. High levels of oxidative stress trigger cellular signaling pathways responsible for chronic inflammation, involved in tumorigenesis.126,127 NLR and LMR may reflect chronic inflammation in myeloproliferative neoplasms. Table 3 summarizes evidence for prognostic significance of NLR in myeloproliferative neoplasms.
Conclusions
Despite the presence of numerous hematological prognostic indexes, clinical progress and survival are often highly varied even within the same patient subgroup. New prognostic strategies are urgently needed, in order to use the appropriate therapies and to personalize the treatment intensity. Recent studies suggest that simple, cost-effective, low-risk tests, such as cell count or its ratios at diagnosis may be used to evaluate the prognosis. NLR and LMR are tumor microenvironment biomarkers that can be used as prognostic factors, not only in solid tumors, but as well in hematological malignancies. The role of NLR and LMR has been well confirmed in several hematologic disorders such as DLBCL, HL and MM. However, there is still little evidence for its significance in other abnormalities of hematopoietic system. Particularly, the prognostic significance of these parameters in acute and chronic leukemias and also in myeloproliferative neoplasms, could be an interesting field of research. The evaluation of the exact clinical significance of NLR and LMR can help to improve known prognostic indexes, in order to make prognosis in hematological malignancies more precise.
Funding Statement
This study was funded by the research grant of Medical University of Lublin [DS176].
Disclosure
The authors declare that there is no conflict of interest regarding the publication of this article.
References
- 1.Forget P, Khalifa C, Defour JP, et al. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res Notes. 2017;10(1):12. doi: 10.1186/s13104-016-2335-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell CD, Parajuli A, Gale HJ, et al. The utility of peripheral blood leucocyte as biomarkers in infectious diseases: a systematic review and meta-analysis. J Infect. 2019;78(5):339–348. doi: 10.1016/j.jinf.2019.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarikaya M, Dogan Z, Ergul B, et al. Neutrophil-to-lymphocyte ratio as a sensitive marker in diagnosis of celiac disease. Ann Gastroenterol. 2014;27:431–432. [PMC free article] [PubMed] [Google Scholar]
- 4.Hajibandeh S, Hobbs N, Mansour M, Mansour M. Neutrophil-to-lymphocyte ratio predicts acute appendicitis and distinguishes between complicated and uncomplicated appendicitis: a systematic review and meta-analysis. Am J Surg. 2020;219(1):154–163. doi: 10.1016/j.amjsurg.2019.04.018 [DOI] [PubMed] [Google Scholar]
- 5.Vulliamy P, McCluney S, Mukherjee S, et al. Postoperative elevation of the neutrophil: lymphocyte ratio predicts complications following esophageal resection. World J Surg. 2016;40(6):1397–1403. doi: 10.1007/s00268-016-3427-z [DOI] [PubMed] [Google Scholar]
- 6.Liu W, Huang Z, Tang S, et al. An evaluation of homocysteine, C-reactive protein, lipid levels, neutrophils to lymphocyte ratio in postmenopausal osteopenic women. Gynecol Endocrinol. 2016;32(6):446–448. doi: 10.3109/09513590.2015.1126711 [DOI] [PubMed] [Google Scholar]
- 7.Gibson PH, Croal BL, Cuthbertson BH, et al. Preoperative neutrophil-lymphocyte ratio and outcome from coronary artery bypass grafting. Am Heart J. 2007;154(5):995–1002. doi: 10.1016/j.ahj.2007.06.043 [DOI] [PubMed] [Google Scholar]
- 8.Dong CH, Wang ZM, Chen SY, et al. Neutrophil to lymphocyte ratio predict mortality and major adverse cardiac events in acute coronary syndrome: a system atic review and meta-analysis. Clin Biochem. 2018;52:131–136. doi: 10.1016/j.clinbiochem.2017.11.008 [DOI] [PubMed] [Google Scholar]
- 9.Sun J, Chen X, Gao P, et al. Can the neutrophil to lymphocyte ratio be used to determine gastric cancer treatment outcomes? A systematic review and meta-analysis. Dis Markers. 2016:7862469. doi: 10.1155/2016/7862469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ethier JL, Desautels D, Templeton A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 2017;19(1):2. doi: 10.1186/s13058-016-0794-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu Y, Wang H, Yan A, et al. Pretreatment neutrophil to lymphocyte ratio in determining the prognosis of head and neck cancer: a meta-analysis. BMC Cancer. 2018;18(1):383. doi: 10.1186/s12885-018-4230-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng J, Cai J, Li H, et al. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as prognostic predictors for hepatocellular carcinoma patients with various treatments: a meta-analysis and systematic review. Cell Physiol Biochem. 2017;44(3):967–981. doi: 10.1159/000485396 [DOI] [PubMed] [Google Scholar]
- 13.Yu Y, Qian L, Cui J. Value of neutrophil-to-lymphocyte ratio for predicting lung cancer prognosis: a meta-analysis of 7,219 patients. Mol Clin Oncol. 2017;7(3):498–506. doi: 10.3892/mco.2017.1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pirozzolo G, Gisbertz SS, Castoro C, et al. Neutrophil-to-lymphocyte ratio as prognostic marker in esophageal cancer: a systematic review and meta-analysis. J Thorac Dis. 2019;11(7):3136–3145. doi: 10.21037/jtd.2019.07.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding Y, Zhang S, Qiao J. Prognostic value of neutrophil-to-lymphocyte ratio in melanoma: evidence from a PRISMA-compliant meta-analysis. Medicine (Baltimore). 2018;97(30):e11446. doi: 10.1097/MD.0000000000011446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu L, Li H, Chen L, et al. Prognostic role of lymphocyte to monocyte ratio for patients with cancer: evidence from a systematic review and meta-analysis. Oncotarget. 2016;7(22):31926–31942. doi: 10.18632/oncotarget.7876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang M, Zhao J, Zhang L, et al. Role of tumor microenvironment in tumorigenesis. J Cancer. 2017;8(5):761–773. doi: 10.7150/jca.17648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ansell SM, Vonderheide RH. Cellular Composition of the Tumor Microenvironment. Am Soc Clin Oncol Educ Book. 2013;e91–e97. doi: 10.1200/EdBook_AM.2013.33.e91 [DOI] [PubMed] [Google Scholar]
- 19.Dave SS, Wright G, Tan B, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med. 2004;351:2159–2169. doi: 10.1056/NEJMoa041869 [DOI] [PubMed] [Google Scholar]
- 20.Wahlin BE, Sander B, Christensson B, et al. CD8+ T-cell content in diagnostic lymph nodes measured by flow cytometry is a predictor of survival in follicular lymphoma. Clin Cancer Res. 2007;13:388–397. doi: 10.1038/bcj.2011.53 [DOI] [PubMed] [Google Scholar]
- 21.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1016/0091-6749(94)90332-8 [DOI] [PubMed] [Google Scholar]
- 22.Yang ZZ, Novak AJ, Stenson MJ, et al. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood. 2006;107:3639–3646. doi: 10.1182/blood-2005-08-3376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Gao K, Lei W, et al. Lymphocyte-to-monocyte ratio is associated with prognosis of diffuse large B-cell lymphoma: correlation with CD163 positive M2 type tumor-associated macrophages, not PD-1 positive tumor-infiltrating lymphocytes. Oncotarget. 2017;8(3):5414–5425. doi: 10.18632/oncotarget.14289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi: 10.1093/jnci/dju124 [DOI] [PubMed] [Google Scholar]
- 25.Peiling T, Hiroyuki K, Edwin J, Hanash SM. The emerging role of B cells in tumor immunity. Cancer Res. 2016;76(19):5597–5601. doi: 10.1158/0008-5472.CAN-16-0431 [DOI] [PubMed] [Google Scholar]
- 26.Lee SF, Luque-Fernandez MA. Prognostic value of lymphocyte-to-monocyte ratio and neutrophil-to-lymphocyte ratio in follicular lymphoma: a retrospective cohort study. BMJ Open. 2017;7(11):e017904. doi: 10.1136/bmjopen-2017-017904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Q, Hu T, Zheng E, et al. Prognostic role of the lymphocyte-to-monocyte ratio in colorectal cancer: an up-to-date meta-analysis. Medicine (Baltimore). 2017;96(22):e7051. doi: 10.1097/MD.0000000000007051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mantovani A, Marchesi F, Malesci A, et al. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416. doi: 10.1038/nrclinonc.2016.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–265. doi: 10.1002/path.1027 [DOI] [PubMed] [Google Scholar]
- 30.Farinha P, Masoudi H, Skinnider BF, et al. Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL). Blood. 2005;106:2169–2174. doi: 10.1182/blood-2005-04-1565 [DOI] [PubMed] [Google Scholar]
- 31.Singel KL, Segal BH. Neutrophils in the tumor microenvironment: trying to heal the wound that cannot heal. Immunol Rev. 2016;273(1):329–343. doi: 10.1111/imr.12459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haydaroglu SH. Can the prognosis of mantle cell lymphoma be predicted by simple CBC counts? Medicine (Baltimore). 2019;98(30):e16180. doi: 10.1097/MD.0000000000016180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung J, Lee H, Yun T, et al. Prognostic role of the neutrophil-to-lymphocyte ratio in patients with primary central nervous system lymphoma. Oncotarget. 2017;8(43):74975–74986. doi: 10.18632/oncotarget.20480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baruch K, Rosenzweig N, Kertser A, et al. Breaking immune tolerance by targeting Foxp3(+) regulatory T cells mitigates Alzheimer’s disease pathology. Nat Commun. 2015;6:7967. doi: 10.1038/ncomms8967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parker KH, Sinha P, Horn LA, et al. HMGB1 enhances immune suppression by facilitating the differentiation and suppressive activity of myeloid-derived suppressor cells. Cancer Res. 2014;74(20):5723–5733. doi: 10.1158/0008-5472.CAN-13-2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butt AQ, Mills KH. Immunosuppressive networks and checkpoints controlling antitumor immunity and their blockade in the development of cancer immunotherapeutics and vaccines. Oncogene. 2014;33(38):4623–4631. doi: 10.1038/onc.2013.432 [DOI] [PubMed] [Google Scholar]
- 37.Dobrenis K, Gauthier LR, Barroca V, et al. Granulocyte colony-stimulating factor off-target effect on nerve outgrowth promotes prostate cancer development. Int J Cancer. 2015;136:982–988. doi: 10.1002/ijc.29046 [DOI] [PubMed] [Google Scholar]
- 38.Hu P, Wang G, Shen M, et al. Intratumoral polymorphonuclear granulocyte is associated with poor prognosis in squamous esophageal cancer by promoting epithelial-mesenchymal transition. Future Oncol. 2015;11:771–783. doi: 10.2217/fon.14.306 [DOI] [PubMed] [Google Scholar]
- 39.Yoshimura T, Imamichi T, Weiss JM, et al. Induction of monocyte chemoattractant proteins in macrophages via the production of granulocyte/macrophage colony-stimulating factor by breast cancer cells. Front Immunol. 2016;8:1524. doi: 10.3389/fimmu.2016.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beltran BE, Castro D, De La Cruz-vargas JA, et al. The neutrophil‐lymphocyte ratio is prognostic in patients with early stage aggressive peripheral T cell lymphoma. Br J Haematol. 2019;184(4):650–653. doi: 10.1111/bjh.15141 [DOI] [PubMed] [Google Scholar]
- 41.Mu S, Ai L, Fan F, et al. Prognostic role of neutrophil-to-lymphocyte ratio in diffuse large B cell lymphoma patients: an updated dose-response meta-analysis. Cancer Cell Int. 2018;18:119. doi: 10.1186/s12935-018-0609-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tilly H, Gomes da Silva M, Vitolo U, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(5):v116–v125. doi: 10.1093/annonc/mdv304 [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Zhou X, Liu Y, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in diffuse large B-cell lymphoma: a meta-analysis. PLoS One. 2017;12(4):e0176008. doi: 10.1371/journal.pone.0176008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng JH, Wang ZJ, Guo XP, et al. Prognostic significance of absolute lymphocyte count at diagnosis of diffuse large B-cell lymphoma: a meta-analysis. Int J Hematol. 2012;95(2):143–148. doi: 10.1007/s12185-011-0993-6 [DOI] [PubMed] [Google Scholar]
- 45.Annibali O, Hohaus S, Marchesi F, et al. The neutrophil/lymphocyte ratio ≥3.5 is a prognostic marker in diffuse large B-cell lymphoma: a retrospective analysis from the database of the Italian regional network ‘Rete Ematologica del Lazio per i Linfomi’ (RELLI). Leuk Lymphoma. 2019;26(5). doi: 10.1080/10428194.2019.1633628 [DOI] [PubMed] [Google Scholar]
- 46.Azuma Y, Nakaya A, Fujita S, et al. Neutrophil-to-lymphocyte ratio (NLR) fails to predict outcome of diffuse large B cell lymphoma. Leuk Res Rep. 2019;25(12):100173. doi: 10.1016/j.lrr.2019.100173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Troppan K, Deutsch A, Gerger A, et al. The derived neutrophil to lymphocyte ratio is an independent prognostic factor in patients with diffuse large B-cell lymphoma. Br J Cancer. 2014;110:369–374. doi: 10.1038/bjc.2013.763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koh YW, Kang HJ, Park C, et al. The ratio of the absolute lymphocyte count to the absolute monocyte count is associated with prognosis in Hodgkin’s lymphoma: correlation with tumor-associated macrophages. Oncologist. 2012;17:871–880. doi: 10.1634/theoncologist.2012-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matasar MJ, Luminari S, Barr PM, et al. Follicular lymphoma: recent and emerging therapies, treatment strategies, and remaining unmet needs. Oncologist. 2019;24(11):e1236–e1250. doi: 10.1634/theoncologist.2019-0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Federico M, Bellei M, Marcheselli L, et al. Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol. 2009;10(27):4555–4562. doi: 10.1200/JCO.2008.21.3991 [DOI] [PubMed] [Google Scholar]
- 51.Solal‐Celigny P, Roy P, Colombat P, et al. Follicular Lymphoma International Prognostic Index. Blood. 2004;104:1258–1265. doi: 10.1182/blood-2003-12-4434 [DOI] [PubMed] [Google Scholar]
- 52.Wilcox RA, Ristow K, Habermann TM, et al. absolute monocyte count is associated with overall survival in patients newly diagnosed with follicular lymphoma. Leuk Lymphoma. 2012;53:575–580. doi: 10.3109/10428194.2011.637211 [DOI] [PubMed] [Google Scholar]
- 53.Watanabe R, Tomita N, Kishimoto K. Absolute monocyte count in follicular lymphoma patients treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. Leuk Res. 2013;37:1208–1212. doi: 10.1016/j.leukres.2013.07.015 [DOI] [PubMed] [Google Scholar]
- 54.Siddiqui M, Ristow K, Markovic SN, et al. Absolute lymphocyte count predicts overall survival in follicular lymphomas. Br J Haematol. 2006;134:596–601. doi: 10.1111/j.1365-2141.2006.06232.x [DOI] [PubMed] [Google Scholar]
- 55.Marcheselli L, Bari A, Anastasia A, et al. Prognostic roles of absolute monocyte and absolute lymphocyte counts in patients with advanced‐stage follicular lymphoma in the rituximab era: an analysis from the FOLL05 trial of the Fondazione Italiana Linfomi. Br J Haematol. 2015;169:544–551. doi: 10.1111/bjh.13332 [DOI] [PubMed] [Google Scholar]
- 56.Belotti A, Doni E, Bolis S, et al. Peripheral blood lymphocyte/monocyte ratio predicts outcome in follicular lymphoma and in diffuse large B-cell lymphoma patients in the rituximab era. Clin Lymphoma Myeloma Leuk. 2015;15(4):208–213. doi: 10.1016/j.clml.2014.10.001 [DOI] [PubMed] [Google Scholar]
- 57.Kumagai S, Tashima M, Fujikawa J, et al. Ratio of peripheral blood absolute lymphocyte count to absolute monocyte count at diagnosis is associated with progression-free survival in follicular lymphoma. Int J Hematol. 2014;99(6):737–742. doi: 10.1007/s12185-014-1576-0 [DOI] [PubMed] [Google Scholar]
- 58.Vose JM. Mantle cell lymphoma: 2017 update on diagnosis risk-stratification, and clinical management. Am J Hematol. 2017;92(8):806–813. doi: 10.1002/ajh.24797 [DOI] [PubMed] [Google Scholar]
- 59.Tiemann M, Schrader C, Klapper W, et al. Histopathology, cell proliferation indices and clinical outcome in 304 patients with mantle cell lymphoma (MCL): a clinicopathological study from the European MCL Network. Br J Haematol. 2005;131:29–38. doi: 10.1111/j.1365-2141.2005.05716.x [DOI] [PubMed] [Google Scholar]
- 60.Cohen JB, Zain JM, Kahl BS. Current approaches to mantle cell lymphoma: diagnosis, prognosis, and therapies. Am Soc Clin Oncol Educ Book. 2017;37:512–525. doi: 10.14694/EDBK_175448 [DOI] [PubMed] [Google Scholar]
- 61.Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced mantle cell lymphoma. Blood. 2008;111:558–565. doi: 10.1182/blood-2007-06-095331 [DOI] [PubMed] [Google Scholar]
- 62.Zhang XY, Xu J, Zhu HY, et al. Negative prognostic impact of low absolute CD4+ T cell counts in peripheral blood in mantle cell lymphoma. Cancer Sci. 2016;107(10):1471–1476. doi: 10.1111/cas.13020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koh YW, Shin SJ, Park C, et al. Absolute monocyte count predicts overall survival in mantle cell lymphomas: correlation with tumour-associated macrophages. Hematol Oncol. 2014;32(4):178–186. doi: 10.1002/hon.2106 [DOI] [PubMed] [Google Scholar]
- 64.von Hohenstaufen KA, Conconi A, de Campos CP, et al. Prognostic impact of monocyte count at presentation in mantle cell lymphoma. Br J Haematol. 2013;162:465–473. doi: 10.1111/bjh.12409 [DOI] [PubMed] [Google Scholar]
- 65.Goy A, Feldman T, Leslie LA, et al. Prognostic value of the absolute lymphocyte to monocyte (ALC/AMC) ratio on overall survival among patients with mantle cell lymphoma. J Clin Oncol. 2017;35(15_suppl):e19030. doi: 10.1200/JCO.2017.35.15_suppl.e19030 [DOI] [Google Scholar]
- 66.George A, Prince HM, Szer J, et al. Prognostic impact of monocyte count at presentation in mantle cell lymphoma. Br J Haematol. 2014;164:890–893. doi: 10.1111/bjh.12683 [DOI] [PubMed] [Google Scholar]
- 67.Nygren L, Wasik AM, Baumgartner‐Wennerholm S, et al. T‐cell levels are prognostic in mantle cell lymphoma. Clin Cancer Res. 2014;20:6096–6104. doi: 10.1158/1078-0432.CCR-14-0889 [DOI] [PubMed] [Google Scholar]
- 68.Zhou XH, Zhang XY, Liang JH, et al. Low absolute NK cell counts in peripheral blood are associated with inferior survival in patients with mantle cell lymphoma. Cancer Biomark. 2019;24(4):439–447. doi: 10.3233/cbm-182193 [DOI] [PubMed] [Google Scholar]
- 69.Ferreri AJ, Blay JY, Reni M, et al. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003;21:266–272. doi: 10.1200/JCO.2003.09.139 [DOI] [PubMed] [Google Scholar]
- 70.Abrey LE, Ben-Porat L, Panageas KS, et al. Primary central nervous system lymphoma: the memorial sloan-kettering cancer center prognostic model. J Clin Oncol. 2006;24:5711–5715. doi: 10.1200/JCO.2006.08.2941 [DOI] [PubMed] [Google Scholar]
- 71.Rimas V, Lukas MD, Stupp R, et al. Primary central nervous system lymphoma—PART 1: epidemiology, diagnosis, staging, and prognosis. Oncology (Williston Park). 2018;32(1):17–22. [PubMed] [Google Scholar]
- 72.Jang JE, Kim YR, Kim SJ, et al. A new prognostic model using absolute lymphocyte count in patients with primary central nervous system lymphoma. Eur J Cancer. 2016;57:127–135. doi: 10.1016/j.ejca.2016.01.016 [DOI] [PubMed] [Google Scholar]
- 73.Montesinos-Rongen M, Kuppers R, Schlűter D, et al. Primary central nervous system lymphomas are derived from germinal-center B cells and show a preferential usage of the V4-34 gene segment. Am J Pathol. 1999;155:2077–2086. doi: 10.1016/S0002-9440(10)65526-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Le M, Garcilazo Y, Ibáñez-Juliá MJ, et al. Pretreatment hemoglobin as an independent prognostic factor in primary central nervous system lymphomas. Oncologist. 2019;24(9):e898–e904. doi: 10.1634/theoncologist.2018-0629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lovgren M, Scarisbrick J. Update on skin directed therapies in mycosis fungoides. Chin Clin Oncol. 2018;8(1). doi: 10.21037/cco.2018.11.03 [DOI] [PubMed] [Google Scholar]
- 76.Dulmage B, Geskin L, Guitart J, et al. The biomarker landscape in mycosis fungoides and Sézary syndrome. Exp Dermatol. 2017;26(8):668–676. doi: 10.1111/exd.13261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Olsen E. Evaluation, diagnosis, and staging of cutaneous lymphoma. Dermatol Clin. 2015;33(4):643–654. doi: 10.1016/j.det.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 78.Abeni D, Frontani M, Sampogna F, et al. Circulating CD8+ lymphocytes, white blood cells, and survival in patients with mycosis fungoides. Br J Dermatol. 2005;153(2):324–330. doi: 10.1111/j.1365-2133.2005.06755.x [DOI] [PubMed] [Google Scholar]
- 79.Cenguz FP, Emiroglu N, Ozkaya DB, et al. Prognostic evaluation of neutrophil/lymphocyte ratio in patients with mycosis fungoides. Ann Clin Lab Sci. 2017;47:25–28. [PubMed] [Google Scholar]
- 80.Eren R, Nizam N, Doğu MH, et al. Evaluation of neutrophil-lymphocyte ratio in patients with early-stage mycosis fungoides. Ann Hematol. 2016;95(11):1853–1857. doi: 10.1007/s00277-016-2779-7 [DOI] [PubMed] [Google Scholar]
- 81.Uysal I, Akdoğan N, Falay M, Şahin B, Özet G, Yalçın B. Screening tests in patients with early-stage mycosis fungoides. Turk J Dermatol. 2019;13:1–7. doi: 10.4274/tdd.galenos.2018.3699 [DOI] [Google Scholar]
- 82.Vonderheid EC, Martinez AR. Prognostic significance of serum copper in patients with cutaneous T-cell lymphoma. Clin Lymphoma Myeloma Leuk. 2019;19(4):228–238. doi: 10.1016/j.clml.2018.12.020 [DOI] [PubMed] [Google Scholar]
- 83.Broccoli A, Zinzani PL. Peripheral T-cell lymphoma, not otherwise specified. Blood. 2017;129(9):1103–1112. doi: 10.1182/blood-2016-08-692566 [DOI] [PubMed] [Google Scholar]
- 84.Li Q, Gao S, Ma J, et al. A lower ALC/AMC ratio is associated with poor prognosis of peripheral T-cell lymphoma-not otherwise specified. Leuk Res. 2018;73:5–11. doi: 10.1016/j.leukres.2018.07.020 [DOI] [PubMed] [Google Scholar]
- 85.Patel M. Peripheral T cell lymphoma. Indian J Med Res. 2018;147(5):439–441. doi: 10.4103/ijmr.IJMR_1849_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Beltran BE, Aguilar C, Quiñones P, et al. The neutrophil-to-lymphocyte ratio is an independent prognostic factor in patients with peripheral T-cell lymphoma, unspecified. Leuk Lymphoma. 2016;57(1):58–62. doi: 10.3109/10428194.2015.1045897 [DOI] [PubMed] [Google Scholar]
- 87.Cencini E, Fabbri A, Sicuranza A, et al. Prognostic significance of lymphocyte/monocyte count and neutrophil/lymphocyte count in peripheral T cell lymphoma. Leuk Res. 2019;77:5–7. doi: 10.1016/j.leukres.2018.12.008 [DOI] [PubMed] [Google Scholar]
- 88.Kim SJ, Yoon DH, Jaccard A, et al. A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: a multicentre, retrospective analysis. Lancet Oncol. 2016;17(3):389–400. doi: 10.1016/S1470-2045(15)00533-1 [DOI] [PubMed] [Google Scholar]
- 89.Zhou X, Sun X, Zhao W, et al. Prognostic significance of peripheral blood absolute lymphocyte count and derived neutrophil to lymphocyte ratio in patients with newly diagnosed extranodal natural killer/T-cell lymphoma. Cancer Manag Res. 2019;11:4. doi: 10.2147/CMAR.S193397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ryu IH, Cho IS, Ryu AJ, et al. Long-term survival after T-cell lymphoblastic lymphoma treated with one cycle of hyper-CVAD regimen. Cancer Res Treat. 2015;47(1):115–119. doi: 10.4143/crt.2013.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cortelazzo S, Ferreri A, Hoelzer D, et al. Lymphoblastic lymphoma. Crit Rev Oncol Hematol. 2017;113:304–317. doi: 10.1016/j.critrevonc.2017.03.020 [DOI] [PubMed] [Google Scholar]
- 92.Xi Y, Li J, Zhang P, et al. Upregulation of miRNA-17 and miRNA-19 is associated with unfavorable prognosis in patients with T-cell lymphoblastic lymphoma. Exp Mol Pathol. 2015;99(2):297–302. doi: 10.1016/j.yexmp.2015.07.012 [DOI] [PubMed] [Google Scholar]
- 93.Hu Meiwei MS, Wang H, Wang L, Yang M, Lou Y, Jin J. Outcome of adult T-lymphoblastic lymphoma depends on ALL-type chemotherapy, prognostic factors, and performance of allogeneic hematopoietic stem cell transplantation. Medicine. 2018;97(28):e11374. doi: 10.1097/MD.0000000000011374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Feng X, Li L, Wu J, et al. Complete blood count score model integrating reduced lymphocyte-monocyte ratio, elevated neutrophil-lymphocyte ratio, and elevated platelet-lymphocyte ratio predicts inferior clinical outcomes in adult T-Lymphoblastic lymphoma. Oncologist. 2019;24(11):e1123–e1131. doi: 10.1634/theoncologist.2018-0789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ansell S. Hodgkin lymphoma: 2018 update on diagnosis, risk‐stratification, and management. Am J Hematol. 2018;93(5):704–715. doi: 10.1002/ajh.25071 [DOI] [PubMed] [Google Scholar]
- 96.Shanbhag S, Ambinder RF. Hodgkin lymphoma: a review and update on recent progress. CA Cancer J Clin. 2018;68(2):116–132. doi: 10.3322/caac.21438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Diehl V. Hematology: are macrophages the bad guys in Hodgkin lymphoma? Nat Rev Clin Oncol. 2010;7:301–302. doi: 10.1038/nrclinonc.2010.71 [DOI] [PubMed] [Google Scholar]
- 98.Dogan A, Demircioglu S. Assessment of the neutrophil-lymphocyte ratio in classic hodgkin lymphoma patients. Pak J Med Sci. 2019;35(5):1270–1275. doi: 10.12669/pjms.35.5.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Romano A, Parrinello NL, Vetro C, et al. Prognostic meaning of neutrophil to lymphocyte ratio (NLR) and lymphocyte to monocyte ration (LMR) in newly diagnosed Hodgkin lymphoma patients treated upfront with a PET-2 based strategy. Ann Hematol. 2018;97(6):1009–1018. doi: 10.1007/s00277-018-3276-y [DOI] [PubMed] [Google Scholar]
- 100.Koh YW, Kang HJ, Park C, et al. Prognostic significance of the ratio of absolute neutrophil count to absolute lymphocyte count in classic Hodgkin lymphoma. Am J Clin Pathol. 2012;138(6):846–854. doi: 10.1309/AJCPO46GFKGNXCBR [DOI] [PubMed] [Google Scholar]
- 101.Marcheselli R, Bari A, Tadmor T, et al. Neutrophil-lymphocyte ratio at diagnosis is an independent prognostic factor in patients with nodular sclerosis Hodgkin lymphoma: results of a large multicenter study involving 990 patients. Hematol Oncol. 2017;35(4):561–566. doi: 10.1002/hon.2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reddy JP, Hernandez M, Gunther JR. Pre‐treatment neutrophil/lymphocyte ratio and platelet/lymphocyte ratio are prognostic of progression in early stage classical Hodgkin lymphoma. Br J Haematol. 2018;180(4):545–549. doi: 10.1111/bjh.15054 [DOI] [PubMed] [Google Scholar]
- 103.Romano A, Vetro C, Donnarumma D, et al. Early interim 2-(1)fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to peripheral blood lymphocyte/monocyte ratio at diagnosis in classical Hodgkin’s lymphoma. Haematologica. 2012;97:e21–e23. doi: 10.3324/haematol.2012.064576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee SF, Ng TY, Spika D, et al. Prognostic value of lymphocyte-monocyte ratio at diagnosis in Hodgkin lymphoma: a meta-analysis. BMC Cancer. 2019;19(1). doi: 10.1186/s12885-019-5552-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dmoszyńska A, Usnarska-Zubkiewicz L, Walewski J, et al. Zalecenia Polskiej Grupy Szpiczakowej dotyczące rozpoznawaniai leczenia szpiczaka plazmocytowego oraz innych dyskrazji plazmocytowych na rok 2017. Acta Haematol Pol. 2017;48(2):55–103. doi: 10.1016/j.achaem.2017.05.003 [DOI] [Google Scholar]
- 106.Dmoszyńska A. Szpiczak Plazmocytowy I Inne Gammapatie Monoklonalne, Red. A Dmoszyńska T.Robak I.Hus. [W:] Podstawy Hematologii. Lublin: Czelej; 2015. [Google Scholar]
- 107.Durie BG, Salmon SE. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975;36(3):842–854. doi: [DOI] [PubMed] [Google Scholar]
- 108.Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23(15):3412–3420. doi: 10.1200/JCO.2005.04.242 [DOI] [PubMed] [Google Scholar]
- 109.Hari PN, Zhang MJ, Roy V, et al. Is the international staging system superior to the durie-salmon staging system? A comparison in multiple myeloma patients undergoing autologous transplant. Leukemia. 2009;23(8):1528–1534. doi: 10.1038/leu.2009.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mu S, Ai L, Fan F, et al. Prognostic role of neutrophil–lymphocyte ratio in multiple myeloma: a dose–response meta-analysis. Onco Targets Ther. 2018;11:499–507. doi: 10.2147/OTT.S153146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zeng Q, Liu Z, Li Q, Liu T. Prognostic value of neutrophil to lymphocyte ratio and clinicopathological characteristics for multiple myeloma: a meta-analysis. Medicine (Baltimore). 2018;97(41):e12678. doi: 10.1097/MD.0000000000012678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kim DS, Yu ES, Kang KW, et al. Myeloma prognostic index at diagnosis might be a prognostic marker in patients newly diagnosed with multiple myeloma. Korean J Intern Med. 2017;32(4):711–721. doi: 10.3904/kjim.2016.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Romano A, Laura Parrinello N, Cerchione C, et al. The NLR and LMR ratio in newly diagnosed MM patients treated upfront with novel agents. Blood Cancer J. 2017;7(12):649. doi: 10.1038/s41408-017-0019-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dosani T, Covut F, Beck R, et al. Significance of the absolute lymphocyte/monocyte ratio as a prognostic immune biomarker in newly diagnosed multiple myeloma. Blood Cancer J. 2017;7(6):e579. doi: 10.1038/bcj.2017.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee GW, Park SW, Go SI, et al. The Derived neutrophil-to-lymphocyte ratio is an independent prognostic factor in transplantation ineligible patients with multiple myeloma. Acta Haematol. 2018;140:146–156. doi: 10.1159/000490488 [DOI] [PubMed] [Google Scholar]
- 116.Mushtaq MU, Chaudhary SG, Murthy G, et al. Prognostic significance of neutrophil-to-lymphocyte ratio in relapsed/refractory acute myeloid leukemia. Blood. 2018;132(S1):5245. doi: 10.1073/pnas.1902375116 [DOI] [Google Scholar]
- 117.Chiarenza A, Di Raimondo F, Parrinello NL, et al. Neutrophil to T-Lymphocyte Ratio (NLR) and Neutrophil to Monocyte Ratio (NMR) are promising biomarkers in chronic lymphocytic leukemia prognostic assessment. Blood. 2017;130(S1):2277. doi: 10.1182/blood.V130.Suppl_1.2277.2277 [DOI] [Google Scholar]
- 118.Barbui T, Thiele J, Gisslinger H, et al. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: document summary and in-depth discussion. Blood Cancer J. 2018;8(2):15. doi: 10.1038/s41408-018-0054-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lucijanic M, Cicic D, Stoos-Veic T, et al. Elevated Neutrophil-to-lymphocyte-ratio and platelet-to-lymphocyte ratio in myelofibrosis: inflammatory biomarkers or representatives of myeloproliferation itself? Anticancer Res. 2018;38(5):3157–3163. doi: 10.21873/anticanres.12579 [DOI] [PubMed] [Google Scholar]
- 120.Kocak MZ, Dağlı M, Ünlü A. The ratio of platelet/lymphocyte, the ratio of neutrophil/lymphocyte and some haemogram parameters related to thrombosis in essential thrombocytosis and polycythaemia vera. Biomed Res. 2017;28(7):3036–3039. [Google Scholar]
- 121.Zhou D, Chen W, Cheng H, et al. Clinico-hematological profile and thrombotic/hemorrhagic events in 150 chinese patients with essential thrombocythemia. Leuk Res. 2018;69:1–6. doi: 10.1016/j.leukres.2018.03.013 [DOI] [PubMed] [Google Scholar]
- 122.Hacibekiroglu T, Akinci S, Basturk A, et al. Evaluation of inflammation parameters in philadelphia negative chronic myeloproliferative neoplasia patients. Asian Pac J Cancer Prev. 2015;16(13):5159–5162. doi: 10.7314/apjcp.2015.16.13.5159 [DOI] [PubMed] [Google Scholar]
- 123.Gaman AA, Moisa C, Diaconu ACmGaman MA, Gaman MA. Crosstalk between oxidative stress, chronic inflammation and disease progression in essential thrombocythemia. Rev Chim. 2019;70(10):3486–3489. doi: 10.37358/RC.19.10.7581 [DOI] [Google Scholar]
- 124.Moisa C, Gaman MA, Diaconu CC, Assani AD, Gaman AM. The evaluation of oxidative stress in patients with essential thrombocythemia treated with risk-adapted therapy. Arch Balk Med Union. 2018;53(4):529–534. doi: 10.31688/ABMU.2018.53.4.07 [DOI] [Google Scholar]
- 125.Pascu EG, Gaman MA, Moisa C, Gaman AM. Oxidative Stress and BCR-ABL1 Transcript Levels in Chronic Myeloid Leukemia: an Intricate Relationship. Rev Chim. 2019;70(9):3193–3196. doi: 10.37358/RC.19.9.7514 [DOI] [Google Scholar]
- 126.Moisă C, Găman MA, Pascu EG. The role of oxidative stress in essential thrombocythemia. Arch Balk Med Union. 2018;53(1):70–75. [Google Scholar]
- 127.Moisa C, Gaman MA, Diaconu CC, Gaman AM. Oxidative stress levels, JAK2V617F mutational status and thrombotic complications in patients with essential thrombocythemia. Rev Chim. 2019;70(8):2822–2825. doi: 10.37358/RC.19.8.7435 [DOI] [Google Scholar]