Significance
At high latitudes, about 10% of the population suffers from depression in winter. Although it has become a serious public health issue, its underlying mechanism remains unknown. Interestingly, animals also show depression-like behavior in winter, and small teleosts have emerged as powerful models for the study of complex brain disorders. Here, we show that medaka exhibit decreased sociability and increased anxiety-like behavior under winter-like conditions. Using metabolomic and transcriptomic analyses, we found changes in multiple signaling pathways involved in depression, including the NRF2 antioxidant pathway. Chemical genomics and targeted mutation of the NRF2 gene revealed that seasonal changes in the NRF2 pathway regulate winter depression-like behavior. This study provides insights into the understanding and treatment of seasonally regulated affective disorders.
Keywords: seasonal affective disorder, winter depression, medaka, circadian rhythm, chemical genomics
Abstract
Seasonal changes in the environment lead to depression-like behaviors in humans and animals. The underlying mechanisms, however, are unknown. We observed decreased sociability and increased anxiety-like behavior in medaka fish exposed to winter-like conditions. Whole brain metabolomic analysis revealed seasonal changes in 68 metabolites, including neurotransmitters and antioxidants associated with depression. Transcriptome analysis identified 3,306 differentially expressed transcripts, including inflammatory markers, melanopsins, and circadian clock genes. Further analyses revealed seasonal changes in multiple signaling pathways implicated in depression, including the nuclear factor erythroid-derived 2-like 2 (NRF2) antioxidant pathway. A broad-spectrum chemical screen revealed that celastrol (a traditional Chinese medicine) uniquely reversed winter behavior. NRF2 is a celastrol target expressed in the habenula (HB), known to play a critical role in the pathophysiology of depression. Another NRF2 chemical activator phenocopied these effects, and an NRF2 mutant showed decreased sociability. Our study provides important insights into winter depression and offers potential therapeutic targets involving NRF2.
In temperate zones, organisms are exposed to dynamic annual changes in the environment, including day length, temperature, and rainfall. To better adapt to these seasonal changes, animals change their physiology and behavior, such as their ability to reproduce, hibernate, and molt (1). Several environmental and nutritional factors, however, influence seasonality. For example, goats are generally considered seasonal breeders and mate during the fall. This seasonality is obvious at high latitudes (>35°) but less marked at low latitudes. Indeed, when seasonally breeding goats are transferred from high latitudes to the tropics, they are no longer seasonal and start breeding all year round (2). In contrast, rats are believed to be nonseasonal animals. However, Fischer 344 rats exhibit clear seasonality when their nutrition is restricted (3). Thus, differences between seasonal and nonseasonal animals appears to be a measure of the strength of their seasonal responses. Since seasonal changes in physiology and behavior are fundamental to an organism, it is plausible that all living species have the potential for seasonal adaptation. Although less obvious, humans are sometimes considered seasonal as seasonal changes in birth rates and the occurrence of infectious, heart, and cerebrovascular diseases have been reported (4, 5).
Seasonal affective disorder (SAD) is a subtype of depression characterized by recurrent episodes that manifest annually, generally in winter (also known as winter depression) (6). Typical symptoms of SAD include low mood, lethargy, sleep problems, disrupted circadian rhythms, social withdrawal, decreased libido, and changes in appetite and body weight (5). Interestingly, seasonal changes in the environment can lead to similar depression- and anxiety-like behaviors in animals (7). We recently reported robust seasonal changes in the behavior of medaka fish (Oryzias latipes) (8). Under winter conditions, medaka are infertile, relatively immobile, exhibit lower light sensitivity, as well as changes in color perception. Notably, seasonal changes in color perception have also been reported in humans (9). Furthermore, patients with SAD show lower light sensitivity in winter compared to healthy subjects as measured by the electroretinogram, and this decreased retinal sensitivity, along with depressed mood, are normalized in summer or following bright light therapy (10). Most behavioral abnormalities are not caused by single gene mutations and are likely the result of dysregulation in multiple brain structures and neural pathways. Therefore, both forward and reverse genetic approaches are quite difficult. We aim to overcome these challenges and address the underlying mechanisms of seasonally regulated behaviors using more global approaches, including metabolomic and transcriptomic analyses of the whole brain of medaka fish. Furthermore, we used a chemical genomics approach in vivo. Understanding the molecular basis of these behaviors has important implications for both the basic pathology of SAD and the possible therapeutic intervention for depressive behaviors.
Results
Medaka Are Less Social in Winter.
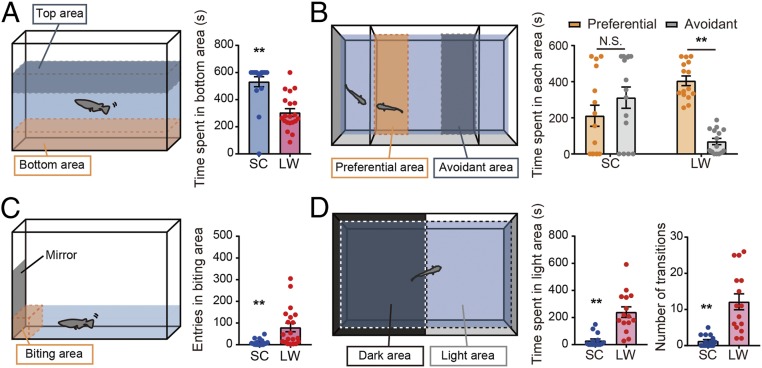
Medaka show striking differences in behavior depending on the season. Fish maintained in summer-like conditions are fertile, active, and swim throughout the tank, while those kept under winter-like conditions are infertile, relatively immobile, and remain at the bottom of the tank (8). For the emergence of these summer or winter phenotypes, both photoperiod and temperature are important in medaka fish. In the present study, we used adult male medaka maintained in conditions mimicking winter (short day and cool temperature [SC]: 10-h light/14-h dark; 10 °C) and divided them into two groups: one remained in SC, while the other group was transferred into summer-like conditions (long day and warm temperature [LW]: 14-h light/10-h dark; 26 °C). Two weeks after the transfer, behavioral tests were performed. We first examined behavior using a novel tank test. The time spent in the bottom area of the tank was significantly shorter in medaka kept under LW than those in SC conditions (Fig. 1A). Since medaka are highly social animals, we next performed a three-chamber sociability test (Fig. 1B). Medaka in LW conditions spent more time in the "preferential area" exhibiting interest in other individuals, whereas medaka kept under SC conditions swam randomly and failed to show a preference for other fish (Fig. 1B). Decreased sociability under SC conditions was confirmed in a mirror biting test. The number of approaches to the mirror was significantly decreased in SC compared with LW conditions (Fig. 1C). We further examined anxiety-like behavior using a light–dark tank test (Fig. 1D). Medaka kept under LW spent more time in the "light area" compared to medaka in SC conditions (Fig. 1D). The frequency of entering the light area was also significantly higher in medaka maintained in LW conditions (Fig. 1D). Similar results were observed in female medaka, demonstrating that these behaviors were not sex specific (SI Appendix, Fig. S1). These results indicate that, regardless of sex, medaka are less social and show more anxiety-like behaviors in winter- compared to summer-like conditions.
Fig. 1.
Medaka are less social and show more anxiety-like behaviors under winter-like conditions. (A) Schematic of the novel tank test (Left). The time spent in bottom area was shorter in medaka kept under LW conditions than those kept under SC conditions (Welch’s t test, **P < 0.01, mean ± SEM, and n = 18–20) (Right). (B) Schematic of the tank for the three-chamber sociability test (Left). Medaka kept under LW conditions spent more time in the preferential area, whereas medaka kept under SC conditions failed to show any interest in other fish (two-way ANOVA and Sidak’s multiple comparison test. **P < 0.01, N.S. is not significant, mean ± SEM, and n = 15) (Right). (C) Schematic of the mirror biting test (Left). The numbers of crossings into the mirror contact zone (outlined) were significantly decreased under SC than LW conditions. (Welch’s t test, **P < 0.01, mean ± SEM, and n = 17–20) (Right). (D) Schematic of the tank for the light–dark test (Left). Medaka in LW conditions spent more time in the light area than medaka kept under SC conditions. Also, medaka kept under LW conditions showed significantly more transitions between light and dark areas. (Welch’s t test, **P < 0.01, mean ± SEM, and n = 15) (Right).
Seasonal Changes in Depression-Associated Metabolites.
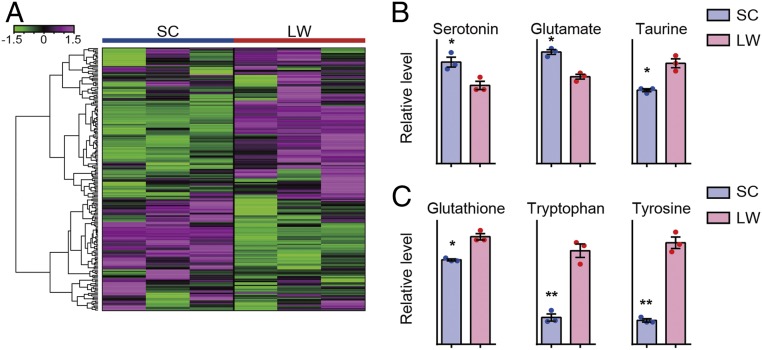
To characterize the metabolomic landscape of medaka maintained in conditions mimicking winter and summer, we examined whole brain metabolites by capillary electrophoresis-mass spectrometry (CE-MS). Adult male medaka kept under SC conditions either remained in SC or were transferred into LW conditions. Two weeks after the transfer, whole brains were collected in the middle of the light phase. Metabolomics measured a total of 204 metabolites (Fig. 2A and SI Appendix, Table S1) and identified seasonal changes in 68 metabolites (P < 0.05, Welch’s t test, and n = 3) (SI Appendix, Table S2). Serotonin has been considered the most important monoamine responsible for depression ("monoamine hypothesis") (11). Higher serotonin levels were observed in the medaka brain in SC compared to LW conditions (Fig. 2B) Unfortunately, our metabolomics analysis could not distinguish other monoamines (i.e., noradrenaline vs. 6-hydroxydopamine and dopamine vs. octopamine) (SI Appendix, Table S2). A glutamatergic N-methyl-d-aspartate (NMDA) receptor antagonist, ketamine, acts as an antidepressant (12). We observed significantly higher levels of glutamate under SC conditions (Fig. 2B). Taurine is thought to act as a glutamate antagonist and has antidepressant activity (13). We found reduced taurine levels in medaka brains in SC conditions (Fig. 2B). Emerging evidence also indicates the involvement of the inflammatory response and oxidative stress in depression ("inflammation hypothesis") (14–16). We observed decreased levels of the antioxidant glutathione (GSH) as well as the amino acids tryptophan and tyrosine in SC conditions (Fig. 2C).
Fig. 2.
Brain metabolites reflect depression-like status in winter-like conditions. (A) Hierarchical clustering of 204 measured metabolites. The color scale at the left represents normalized signal intensity. (B) Metabolomic measurements of the neurotransmitters, serotonin, glutamate, and taurine. (Welch’s t test, *P < 0.05, mean ± SEM, and n = 3). (C) Metabolomic measurements of the antioxidant glutathione and the amino acids tryptophan and tyrosine (Welch’s t test, *P < 0.05, **P < 0.01, mean ± SEM, and n = 3).
Seasonal Changes in Circadian and Inflammatory Pathways.
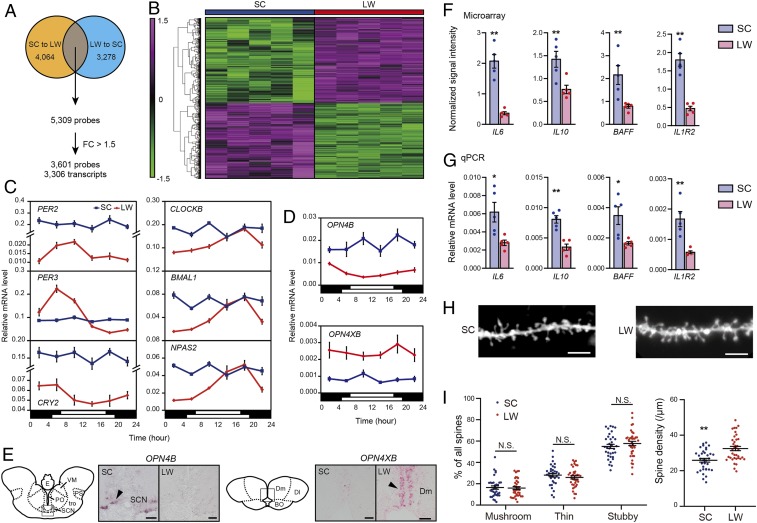
We next performed microarray analysis to examine the seasonal transcriptional landscape of the medaka brain. To minimize the number of false positives, we performed two sets of experiments: SC to LW and LW to SC. In the SC-to-LW experiment, adult male medaka maintained in SC conditions either remained in SC or were transferred into LW conditions. By contrast, in the LW-to-SC experiment, adult male medaka kept under LW conditions either remained in LW or were transferred into SC conditions. In both experiments, 2 wk after transfer, whole brains were collected in the middle of the light phase. Microarray analysis identified 5,309 probes in common between the two experiments (Moderated t test, P < 0.05, false discovery rate [FDR] < 0.05, SC to LW: n = 6; LW to SC: n = 5). Of these, 3,601 probes were identified by a 1.5-fold cutoff that represents 3,306 transcripts (Fig. 3 A and B and SI Appendix, Table S3). Disrupted circadian rhythms, such as abnormal phase and diminished amplitude, are often observed in SAD ("circadian rhythm hypothesis") (17, 18). The microarray analyses identified several differentially expressed genes, including those encoding melanopsin photopigments OPN4B and OPN4XB and circadian clock genes PER2, PER3, and CRY2 (SI Appendix, Table S3). These results were confirmed by qPCR (two-way ANOVA, P < 0.05) and/or in situ hybridization (Fig. 3 C–E) (SI Appendix, SI Text). In addition to the clock genes above, we performed qPCR analysis on CLOCKB, BMAL1, and NPAS2 and discovered that they were also differentially expressed (two-way ANOVA and P < 0.05) (Fig. 3C). Interestingly, expression of these circadian clock genes was no longer rhythmic, or their amplitude was significantly reduced in winter- compared to summer-like conditions (Fig. 3C and SI Appendix, Table S4).
Fig. 3.
Disruption of circadian rhythms and elevation of inflammatory markers in winter-like conditions. (A) Two sets of microarray experiments identified 5,309 differential probes between SC and LW conditions. Further analysis using a fold-change (FC) cutoff of 1.5 identified 3,601 probes representing 3,306 transcripts. (B) Clustered organization of 3,601 probes between SC and LW conditions. Data were normalized over the entire data set. The color scale at the Left represents the normalized signal intensity. (C) Temporal expression profiles of circadian clock genes under SC (blue) and LW (red) conditions verified by qPCR. The bars at the bottom of each graph represent the lighting conditions (two-way ANOVA, P < 0.05, mean ± SEM, and n = 4 to 5). Note that for PER2 and CRY2, the y axis values are different for SC and LW conditions. (D and E) Seasonal changes in OPN4B and OPN4XB expressions validated by qPCR (D) and in situ hybridization (E). The black arrow indicates the hybridization signal. (Scale bar, 40 μm) (two-way ANOVA, P < 0.05, mean ± SEM, and n = 4 to 5). BO: bulbus olfactorius; Dl: pars lateralis of the dorsal telencephalic area; Dm: pars medialis of the dorsal telencephalic area; E: epiphysis; PO: nucleus preopticus; PS: nucleus pretectalis superficialis; SCN: suprachiasmatic nucleus; tro: tractus opticus; VM: nucleus ventromedialis thalami. (F and G) Seasonal changes in the cytokines and cytokine receptor measured by microarray (F) and qPCR (G) (Welch’s t test, *P < 0.05, **P < 0.01, mean ± SEM, and n = 5). (H) Representative images of DiI-labeled dendritic spines within the DI. (Scale bar, 5 μm.) (I) Spine morphology and density within the DI, analogous to the mammalian hippocampus, in SC and LW conditions (Welch’s t test, **P < 0.01, N.S. is not significant, mean ± SEM, and n = 34 and 37 dendritic segments analyzed for SC and LW conditions, respectively).
In addition to circadian clock genes, we discovered up-regulation of several inflammatory markers, including cytokines (e.g., fish specific interleukin-6 [IL6: M17], IL10, tumor necrosis factor ligand superfamily member 13B (also known as B-cell activating factor [BAFF]), and a cytokine receptor (interleukin-1 receptor type 2 [IL1R2]) under SC conditions (Fig. 3 F and G). The inflammatory response and oxidative stimuli have been shown to change spine density in several brain regions, including the hippocampus ("neuroplasticity hypothesis") (15, 16). We, therefore, analyzed spine morphology in the medaka brain using DiOlistic labeling with the lipophilic dye 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) (SI Appendix, SI Text). We examined the pars lateralis of the dorsal telencephalic area (Dl), which is considered analogous to the mammalian hippocampus (19). Although the percentage of each spine type (i.e., mushroom, thin, and stubby) did not show statistical differences, an overall decrease in spine density in the medaka brain was observed in the Dl region in SC compared with LW conditions (Fig. 3 H and I).
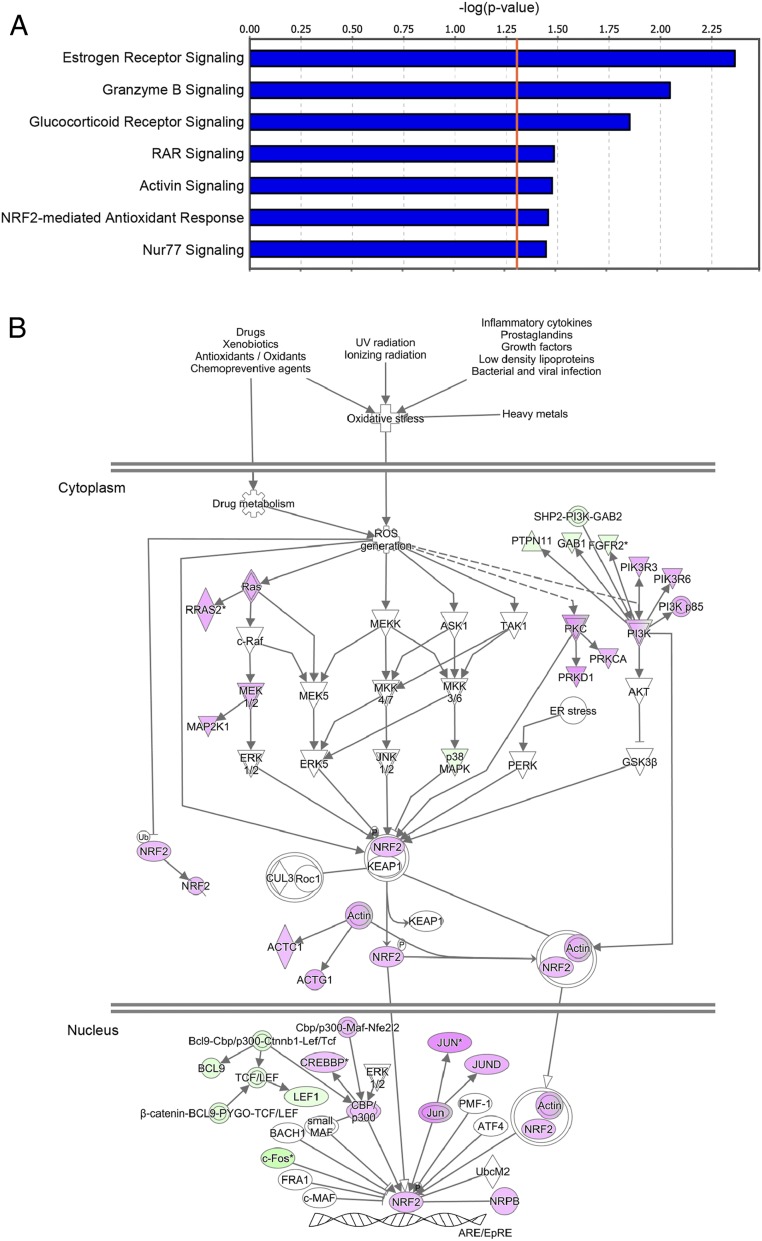
Ingenuity pathway analysis (IPA) also identified multiple signaling pathways previously implicated in depression (Fig. 4A), including estrogen receptor signaling (20), glucocorticoid receptor signaling (21), activin signaling (22), and the NRF2-mediated antioxidant response (23) (Fig. 4B and SI Appendix, Figs. S2–S6).
Fig. 4.
Inactivation of multiple signaling pathways including the anti-inflammatory pathway under winter-like conditions. (A) Canonical signaling pathways significantly inactivated under winter-like conditions. The x axis shows the inverse logarithm of the Fisher’s exact P value corrected for multiple comparisons using the Benjamini–Hochberg test. The orange line indicates the threshold of significance (P < 0.05). (B) Inactivation of the NRF2-mediated antioxidant response under winter-like conditions. Genes highlighted in magenta and green are summer-induced and summer-repressed genes, respectively.
Drug Screening to Treat Winter Behavior.
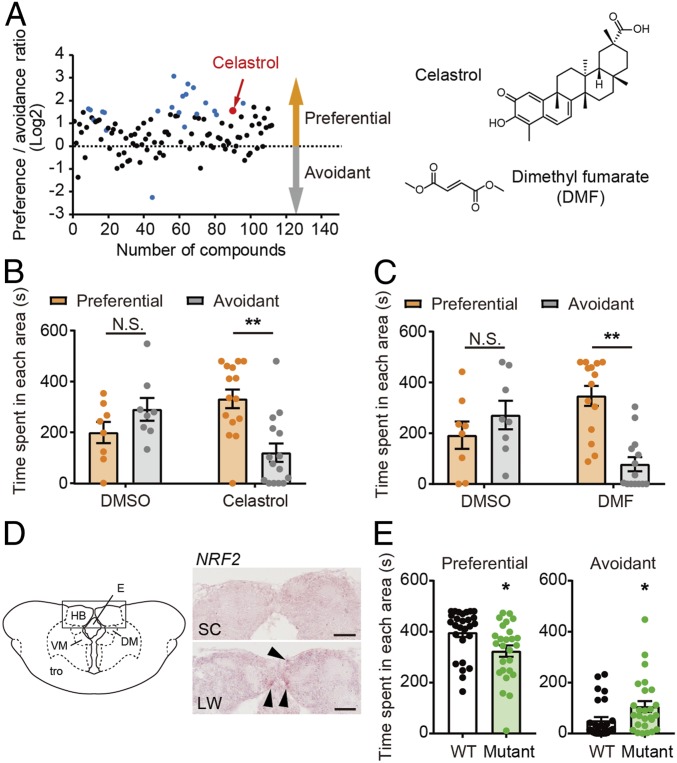
To further understand the underlying mechanism of winter behavior, we took a chemical genomics approach and screened an in-house library of existing drugs (SI Appendix, Table S5). We employed the three-chamber sociability test for in vivo chemical screening of medaka kept under winter-like conditions. Adult male medaka maintained in SC conditions were treated with each drug at the final concentration of 1 μM for 24 h before the behavioral test was conducted (n = 8). From this first screen of 112 existing drugs, we obtained 21 potential hit compounds (P < 0.05) (Fig. 5A and SI Appendix, Table S5 and Fig. S7). These compounds were then reexamined by different experimenters using eight fish (second screening). The drugs that passed both the first and the second screenings were further examined using 15 fish (third screening). Of the 112 existing drugs tested, the traditional Chinese medicine celastrol was the only compound that had consistent effects and passed all three screens (Fig. 5 A and B and SI Appendix, Fig. S7). Celastrol has been shown to activate NRF2 signaling (24, 25). Consistent with this, we also observed activation of the NRF2 antioxidant pathway under summer-like conditions in our transcriptome analysis (Fig. 4). Furthermore, microarray analysis between celastrol-treated and DMSO-treated (control) medaka kept under winter-like conditions revealed the induction of several NRF2 target genes, including GST omega-1, glutathione peroxidase, prostaglandin reductase 1, proteasome subunit α type-6 and β type-7 and c-x-c chemokine receptor type 2 (Moderated t test, P < 0.05, FDR < 0.05, and n = 4) (SI Appendix, Table S6). We hypothesized that other structurally different NRF2 activators, such as dimethyl fumarate (DMF), should have similar effects as celastrol. Indeed, treatment with DMF increased sociability thereby mimicking summer-like behavior under winter-like conditions (Fig. 5C). When we examined localization of NRF2 mRNA within the medaka brain, its expression was observed in the ventral HB as well as the dorsal HB (Fig. 5D). The teleost ventral HB is anatomically homologous to the mammalian lateral HB (LHb) (26). The LHb is well conserved across species, receives neural input from the limbic forebrain, and has major projections to monoaminergic (i.e., dopamine and serotonergic) systems. It also plays a critical role in the pathophysiology of depression (26–28).
Fig. 5.
Drug screening treating winter behavior. (A) Of 112 existing drugs, 21 potential hits (blue and red dots) were detected in the three-chamber sociability test (first screen, P < 0.05, and n = 8). Celastrol (red dot) was the only drug that passed the first, second, and third screenings. The y axis shows the log2 ratio between the time spent in the preferential area and the avoidant area. (B) Celastrol-treated medaka spent more time in the preferential area, while dimethyl sulfoxide (DMSO)-treated medaka did not under winter-like conditions (two-way ANOVA and Sidak’s multiple comparison test. **P < 0.01, N.S. is not significant, mean ± SEM, and n = 8–15). (C) Structurally distinct NRF2 activator, DMF, rescued winter behavior by increasing sociability (two-way ANOVA and Sidak’s multiple comparison test. **P < 0.01, N.S. is not significant, mean ± SEM, and n = 8–14). (D) Expression of NRF2 in the HB examined by in situ hybridization. The black arrow indicates the hybridization signal. (Scale bar, 50 μm.) HB: habenula; E: epiphysis; DM: nucleus dorsomedialis thalami, VM: nucleus ventromedialis thalami; tro: tractus opticus. (E) Genome-edited NRF2 mutant spent less time in the preferential area and more time in the avoidant area under summer-like conditions (*P < 0.05, mean ± SEM, and n = 26 to 27).
To further examine the role of NRF2 in winter depression-like behavior, we generated a genome-edited NRF2 mutant using the CRISPR/Cas9 system. We crossed an injected G0 founder with a wild-type fish to obtain F1 heterozygotes, and then F1 heterozygotes were crossed to generate F2 mutant and wild-type siblings. NRF2 mutant medaka had 4-bp deletion in exon 2 of the NRF2 gene, and this deletion caused a frame shift, introducing a premature stop codon upstream of the DNA binding and dimerization domains (SI Appendix, Fig. S8). We did not observe any developmental, morphological, or behavioral abnormalities in the mutant fish under normal housing conditions. We then examined sociability under summer-like conditions using the three-chamber sociability test. Mutant medaka were less social and spent less time in the preferential area (Fig. 5E). Although we attempted to examine the effect of celastrol on mutant fish under winter-like conditions, many NRF2 mutant fish died, suggesting that they are highly sensitive to environmental changes.
Discussion
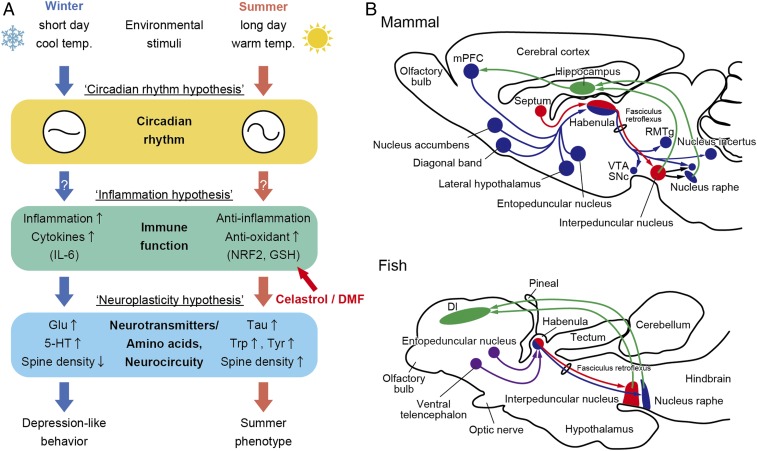
Seasonal changes in the environment may lead to depression- and anxiety-like behaviors in both humans and animals. However, the underlying mechanisms remain unknown. In the present study, we demonstrated that short days and cool temperatures mimicking winter conditions decreased sociability and increased anxiety-like behavior in medaka (Figs. 1 and 6A).
Fig. 6.
Conservation of the HB pathway and possible mechanisms that cause winter behavior. (A) Hypothetical model of the underlying mechanisms regulating seasonal changes in behaviors. (B) Conservation of HB pathways in mammals and fish. Schematic of sagittal sections of rodent and fish brains. Red shows medial (mammal) and dorsal (fish) HB circuitries, while blue shows lateral (mammal) and ventral (fish) HB circuitries. Green shows feedback projections to the hippocampus and medial prefrontal cortex. Although the entopeduncular nucleus and ventral telencephalon send projections to fish HB, exact targets remain unclear (purple). mPFC: medial prefrontal cortex; VTA: ventral tegmental area; SNc: substantia nigra, pars compacta; RMTg: rostromedial tegmental nucleus; Dl: pars lateralis of the dorsal telencephalic area. Modified from Aizawa et al. (26) and Mizumori and Baker (28).
Bright light therapy is an effective treatment for patients with SAD, whereas decreased light exposure is often a risk factor (10). Melanopsin is involved in circadian photoentrainment, and its gene variants have been linked to SAD (29). Interestingly, we found differential expression of the melanopsin homologs OPN4B and OPN4XB (Fig. 3) between medaka kept under winter- and summer-like conditions. Candidate gene studies for SAD also reported SNPs in circadian clock genes, including PER2, PER3, BMAL1, CLOCK, NPAS2, and CRY2 (30–34). We demonstrated a significant decrease in the amplitude of circadian clock gene rhythms in medaka kept under winter-like conditions compared to those in summer-like conditions (Figs. 3 and 6A). These results are consistent with the hypothesis that disruption of circadian rhythms is associated with SAD (18).
Growing evidence points to a role for the immune system in the pathophysiology of depression, specifically, the inflammatory response and oxidative stress (14–16). In human patients and animal models, depression is accompanied by a decrease in antioxidants (e.g., GSH) and amino acids (e.g., tryptophan and tyrosine) and an increase in inflammatory markers in the brain (14–16). We discovered lower levels of GSH, tryptophan, and tyrosine in the brain of medaka kept under winter-like conditions (Fig. 2). We also demonstrated higher expression of inflammation markers, including cytokines (IL6, IL10, and BAFF) and the cytokine receptor (IL1R2) in the brain under winter-like conditions (Figs. 3 and 6A). It has been reported that the inflammatory cytokine IL-6 induces IL-10 production (35) and hence, BAFF production in the microglia (36). Several lines of evidence have indicated that inflammatory cytokines influence the monoamine serotonin as well as the excitatory amino acid glutamate (15). We also detected seasonal changes in serotonin and glutamate (Fig. 2). Although low serotonin levels are considered to be associated with depression, higher serotonin levels were observed in the medaka brain in winter-like compared to summer-like conditions. The ratio between serotonin metabolite 5-hydorxyindoleacetic acid (5-HIAA) and serotonin are typically used as a measure of synaptic transmission, but 5-HIAA was not detected in our analysis. In this case, serotonin turnover may be decreased, which suggests dysfunction of serotonergic neuronal activity. Due to its rapid antidepressant actions, the glutamatergic NMDA receptor antagonist ketamine has attracted attention in the mental health field (37). On the contrary, increased levels of glutamate and reduced taurine have been reported in the brains of patients with depression (38, 39). Consistent with this, we observed increased glutamate and reduced taurine levels in the brain of medaka kept under winter-like conditions (Fig. 2). In addition to the impact of cytokines on neurotransmitter systems, inflammation influences synaptic plasticity (16). Indeed, decreased spine density associated with depression was also observed in the medaka brain in the Dl region, which is considered to be homologous to the mammalian hippocampus (Figs. 3 and 6B).
Canonical pathway analysis identified winter-induced inactivation of multiple biological networks known to be associated with depression (Fig. 4A). Consistent with the antidepressive effects of endogenous estrogen in both men and women (40), transcriptome analysis revealed inactivation of estrogen receptor signaling under winter-like conditions. We also observed inactivation of activin signaling under winter-like conditions. An enriched environment activates the activin pathway and enhances cognitive performance and affective behaviors, while impairment of this pathway results in anxiety and depression (22). We also detected inactivation of the retinoic acid receptor (RAR) and glucocorticoid receptor signaling under winter-like conditions. These results are consistent with the notion that RAR signaling is involved in the modulation of the hypothalamic–pituitary–adrenal (HPA) axis (41). Dysregulation in the HPA axis has been demonstrated in a wide spectrum of psychiatric disorders (21). Although hyperactivity of the HPA axis is generally associated with depression, its hypoactivity may result in fatigue and tiredness (42). It is interesting that hyperactivity of the HPA axis is rarely observed in patients with SAD, unlike many other psychiatric conditions (43).
NRF2 is a transcriptional activator of antioxidant genes involved in anti-inflammatory processes in the brain. Reduced levels of GSH are associated with depression, and NRF2 induces GSH (23). Of note, we found inactivation of NRF2 signaling, reduction of GSH, and induction of inflammatory markers in medaka kept under winter-like conditions (Figs. 2–4). Orphan nuclear receptor NR4A1 (Nur77) is involved in the regulation of the inflammatory response (44). Therefore, inactivation of the Nur77 signaling pathway under winter-like conditions might be modulating the inflammatory response.
To further understand the underlying mechanism of winter depression-like behavior, we used a chemical genomics approach and discovered that the traditional Chinese medicine celastrol rescues winter behavior in medaka (Fig. 5). Celastrol is currently used for treatment of inflammatory diseases and cancer and has multiple molecular targets, including nuclear factor κB and heat shock protein 90 (45). Furthermore, increasing evidence suggests that celastrol exerts its anti-inflammatory effects by activating the NRF2 antioxidant pathway (24, 25). Importantly, the structurally distinct NRF2 activator, DMF, also rescued winter behavior, and a CRISPR/Cas9-generated NRF2 mutant showed decreased sociability under summer-like conditions (Fig. 5). Similarly, it has been reported that Nrf2-null mice show depressive-like behaviors, and that, in mouse models of depression, treatment with a NRF2 agonist shows antidepressive effects (46). Thus, NRF2 activators have been proposed as potential drug candidates for depression (23). It is particularly interesting to note that NRF2 expression is observed in the HB (Fig. 5). Although fish do not have a defined prefrontal cortex (PFC), the HB is evolutionarily highly conserved and connects the limbic forebrain and monoaminergic system (Fig. 6B). Recently, the LHb has emerged as a key brain region in the pathophysiology of depression and has been shown to mediate the antidepressive actions of ketamine in rat and mouse models of depression (37). Furthermore, deep brain stimulation has been used to regulate neural activity of the LHb in treatment-resistant depressed patients (47). It should be noted that recent studies in mice also demonstrated that the periHB nucleus, adjacent to the LHb, mediates the light-mediated mood alteration (48).
Treatment of multifactorial psychiatric disorders with just one drug, or monotherapy, is often insufficient, and comedication therapy is typically more effective. Therefore, there is an urgent need for new therapeutic targets and more effective therapies (49). Animal models play an essential role in the discovery of new drugs in virtually all fields of medicine. Small teleosts, such as zebrafish and medaka, are emerging models for the study of complex brain disorders and are becoming popular and more powerful models in pharmacogenetic studies. Despite the obvious differences between fish and humans, many features of their central nervous system are highly conserved, including neurotransmitters, their receptors and transporters, enzymes for their synthesis and degradation, as well as the neurons that underlie their function. Moreover, antipsychotics affect swimming patterns via conserved molecular targets and pathways that affect activity patterns in mammals (50, 51). Importantly, animal models of psychiatric disorders are required to satisfy three important criteria: i) face, ii) construct, and iii) predictive validities (52). i) Face validity refers to how similar the phenotypes are between an animal model and the human disorder. Although there are obvious behavioral differences between fish and humans, our previous and current studies of medaka kept under winter-like conditions demonstrated significant parallels with SAD symptoms, such as decreased locomotor and reproductive activity, lower light sensitivity, decreased sociability, and increased anxiety-like behavior (8). ii) Construct validity refers to how a mechanism is shared between an animal model and the human disorder. Our metabolomic, transcriptomic, and morphological analyses demonstrated seasonal changes at multiple levels, including metabolites (e.g., neurotransmitters, antioxidant, and amino acids), gene expression (e.g., circadian clock genes, cytokines, and cytokine receptor), and neuroplasticity (e.g., spine density). These phenotypes fit well with proposed hypotheses for depression including the circadian rhythm hypothesis, the inflammation hypothesis, and the neuroplasticity hypothesis (Fig. 6A). iii) Predictive validity refers to how medications similarly effect the behavior of an animal model and the disorder. For example, the antidepressant fluoxetine, a selective serotonin reuptake inhibitor, has been suggested to improve SAD. When we examined the effect of a 2-wk fluoxetine treatment of medaka kept under winter-like conditions, medaka moved to the top of the tank in the open field tank test, reflecting reduced anxiety-like behavior (SI Appendix, Fig. S9A). However, this treatment had no influence on behavior in the three-chamber sociability test (SI Appendix, Fig. S9B). This partial effect of fluoxetine might explain the view that there is not enough evidence to support the use of fluoxetine for the treatment of SAD (53). As mentioned above, SAD symptoms typically appear during fall to winter and disappear during spring to summer. A seasonal change in the environment from winter to spring is the ultimate treatment for SAD, and this appears to be true in medaka fish. Depression is considered an adaptation to a harsh environment, and to our knowledge, none of the currently available animal models for depression exhibit spontaneous recurrent mood episodes. Given the striking parallels between patients with SAD and medaka kept under winter-like conditions, we believe that our present findings provide insights into the understanding and treatment of seasonally regulated affective disorders. However, there exist clear differences between fish and mammals. For example, eyes are the only photoreceptive organ to reset the circadian clock in mammals, while light acts directly on peripheral clocks in fish (54). Since SAD-like symptoms have recently been reported in the diurnal primate, Rhesus macaque (55), future comparative studies on other organisms, such as macaques, are, indeed, important to better understand the evolution of vertebrate seasonal adaptation and seasonal changes in physiology and mood in humans.
Materials and Methods
Animals.
Adult wild-type medaka were obtained from a local dealer (Fuji 3A project, Nagoya) and used for our behavioral studies since homogeneous inbred strains seemed inappropriate for understanding heterogeneous complex behaviors. Medaka were kept under SC (10-h light/14-h dark and 10 °C) or LW (14-h light/10-h dark and 26 °C) conditions using housing systems (MEITO system MH-R1600V, Meito Suien, Nagoya and LP-30LED-8CTAR, NK system, Osaka). Animals were treated in accordance with the guidelines of Nagoya University and the National Institutes of Natural Sciences. All experimental protocols were approved by the Animal Experiment Committee of Nagoya University and the National Institutes of Natural Sciences.
Behavioral Tests.
For the novel tank test, fish were introduced into a new clear acrylic tank (W 20 × D 4.5 × H 15 cm) filled with water to a height of 12 cm, and behaviors were recorded for 12 min by video camera (Panasonic HC-V230M and JVC Kenwood GZ-F117). The first and last minutes of the 12-min recording were removed, and the remaining 10 min were used for analysis. The top and bottom areas (20-cm W × 4.5-cm D × 2.4-cm H) were defined as the areas adjacent to the water surface and the bottom of the chamber, respectively, and time spent in each area was measured. For the three-chamber sociability test, plastic chambers were used (15-cm W × 10-cm D × 5.5-cm H) that were divided by plastic partitions into three sections (two side chambers and a middle chamber). On the day of the experiment, a test fish and an unfamiliar fish were released into the middle (9-cm W × 10-cm D) and side chambers (3-cm W × 10-cm D), respectively, while the other side chamber was empty. Opaque partitions were used initially, preventing the test fish from seeing the unfamiliar fish during the 10-min adaptation. After exchanging the opaque partitions for clear ones, behaviors were recorded for 10 min by a video camera. From the 10-min recording, 8 min were used for the analysis, deducting 1 min after and 1 min before the stop of the recording. The preferential or avoidant area was defined as the area (3-cm W × 10-cm D) adjacent to both side chambers, and time spent in each area was measured. For the mirror biting test, fish were introduced into a clear acrylic tank (20-cm W × 4.5-cm D × 15-cm H) filled with water to a height of 2 cm. After a 10-min habituation period, mirrors (4.5-cm W × 9-cm H) were placed on either side of the tank. Behaviors were recorded for 12 min by video camera, and as above, the middle 10 min of the recording record were used for analysis. The biting area was defined as the area (1-cm W × 4.5-cm D × 2-cm H) adjacent to the mirrors, and time spent in these areas was measured. For the light–dark tank test, a plastic tank (15-cm W × 10-cm D × 5.5-cm H) divided into two sections of equal size, by covering the bottom and side areas with white- or black-colored opaque tape, was used. The fish were put into the dark area, allowing them to move freely between the dark and the light areas for 15 min. Time spent in the light area and the total number of transitions from one area to the other area were measured. Data were analyzed by the behavioral analysis software Smart (Panlab Harvard Apparatus).
Drug Administration.
Chemical library stocks were initially prepared at 10 mM in DMSO. The day before the behavioral assay, 150 μL of 10-mM chemical stock solutions were added to 1.5 L of fish water in order to dilute the drug to the final concentration of 1 μM. This results in the final DMSO concentration of 0.1% in control and drug-treated fish. Fish (n = 4 per tank) were exposed to each compound for 24 h until the behavioral test. Since some of the celastrol-treated medaka died after the first screening of celastrol at a concentration of 1 μM, potentially due to hepatic and cardiac toxicity (56), we examined the effects of celastrol at a lower concentration of 0.2 μM in the second and third screenings. Effects of DMF were examined at the concentration of 52 μM according to Kulkarni et al. (57). Effects of daily administration of fluoxetine for 2 wk were examined at the concentration of 0.8 μM according to Ziv et al. (58).
Metabolomics.
Whole brains of medaka kept under SC and LW conditions were used. For each sample, eight brains were pooled. Metabolomics analysis was conducted by Human Metabolome Technologies (59). Each frozen sample was homogenized in acetonitrile, and metabolites were extracted. CE–MS was performed using an Agilent CE–MS system equipped with a time-of-flight mass spectrometer system. Identified metabolites were quantified by comparing their peak areas with those of authentic standards using MasterHands ver.2.17.1.11 software developed by Keio University.
Microarray Experiments.
Medaka microarrays (Custom Gene Expression Microarray 4 × 44 K, Agilent Technologies), which contain more than 31,000 probes, were used for these experiments. For SC and LW conditions, whole brains were collected from five to six animals. Total RNA was prepared from each fish using a RNeasy tissue kit (QIAGEN). Complementary DNA (cDNA) synthesis and complementary RNA (cRNA) labeling reactions were performed with the Low Input Quick Amp Labeling Kit (Agilent Technologies). Labeled cRNA was purified with RNeasy mini spin columns (QIAGEN) and hybridized using the Gene Expression Hybridization Kit (Agilent Technologies). After washing with Gene Expression Wash Buffer (Agilent Technologies), the glass slide was scanned on a Microarray Scanner (Agilent Technologies). Canonical pathway analysis was performed using IPA by the Chemical Evaluation and Research Institute, Japan to determine the functional pathways represented by the identified genes. To minimize false positives and negatives and to maximize the detection of biologically meaningful information, the following criteria were used to extract the data; i) t test, P < 0.05, FDR < 0.05, FC > 1.5, and flag present in both SC and LW conditions, ii) t test, P < 0.01, FDR < 0.01, FC = 1.0–1.5, and flag present in both SC and LW conditions, iii) t test, P < 0.01, FDR < 0.01, FC > 3.0, and flag present in either SC or LW condition. To evaluate the effect of celastrol on gene expression, whole brain samples were collected 24 h after the administration of DMSO or celastrol under SC conditions (t test, P < 0.05, FDR < 0.05, FC > 1.5, and n = 4).
Quantitative PCR.
cDNA was synthesized by reverse transcription of total RNA (200 ng) using ReverTra Ace (Toyobo) and oligo-dT primers. Samples contained SYBR Premix Ex Taq II (Takara), gene-specific primers at 0.4 μM (SI Appendix, Table S7), and 2 µL of synthesized cDNA in a 20-µL volume. qPCR was performed on an Applied Biosystems 7500 Real-Time PCR System or an Applied Biosystems QuantStudio 3 Real-Time PCR System using the following conditions: 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 34 s. House-keeping gene RPL7 was used as an internal RNA control. To determine the rhythmicity of the gene expression, the Jonckheere–Terpstra–Kendall (JTK_CYCLE) algorithm was used for qPCR data with a Bonferroni adjusted P value cutoff P < 0.01 (60). (SI Appendix, Table S4).
Analysis of Spine Density.
DiOlistic labeling with a lipophilic dye DiI was performed to visualize the dendritic spines within the pars lateralis of the Dl. DiI-coated tungsten particles (1.6 μm in diameter; Bio-Rad) were prepared following the previous studies (61, 62). Experimental details are described in the SI Appendix.
In Situ Hybridization.
Expression of OPN4B and NRF2 was analyzed using digoxygenin-labeled RNA probes. To analyze expression of OPN4XB, in situ hybridization was performed using RNAscope (Advanced Cell Diagnostics) following the manufacturer’s guidelines. Experimental details are described in the SI Appendix.
Genome Editing Using the CRISPR/Cas9 System.
Template DNA for the synthesis of single guide RNA (sgRNA) was PCR amplified from the sgRNA expression vector (pDR274; Addgene plasmid 42,250) with the forward primer TAATACGACTCACTATAGGACCTCGGTGCCAGGCGAGGTTTTAGAGCTAGAAATAGC and the reverse primer AAAAGCACCGACTCGGTGCC. Amplified DNA was purified using the QIAquick PCR Purification Kit (QIAGEN). Purified DNA was used as a template, and the AmpliScribe T7-Flash Transcription Kit (Epicentre) was used to synthesize the sgRNA. The Cas9 expression vector (hCas9; gifted by Professor Zhang of the Massachusetts Institute of Technology) was linearized with NotI, and the capped Cas9 mRNA was synthesized using the mMESSAGE mMACHINE SP6 Transcription Kit (Thermo Fisher Scientific). Both RNAs were purified using the RNeasy MinElute Cleanup Kit (QIAGEN). A mixed solution of the sgRNA (50 ng/µL) and Cas9 mRNA (100 ng/µL) were microinjected into one-cell stage embryos using a fine glass needle. NRF2 mutant medaka were genotyped using PCR primers 5′-GTGCACAATGGCTTCCAACTC-3′ and 5′-GGGAGGATGGGTTTTGTGTGA-3′, and amplicons were analyzed on a microchip electrophoresis system (MCE-202 MultiNA; Shimazu). Mutant alleles were identified by heteroduplex mobility assay and direct sequencing of the PCR product (63).
Statistical Analysis.
Microarray data were analyzed using the GeneSpring software (Agilent Technologies). Differentially expressed genes were identified by a moderated t test (P < 0.05), followed by the Benjamini–Hochberg FDR < 0.05.
Data Availability.
The microarray data are available in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) database, http://www.ncbi.nlm.nih.gov/ (accession no. GSE124988). All other data and associated protocols used for this study are available in the main paper and the SI Appendix. Materials used for this study will be available upon requests to the corresponding author.
Supplementary Material
Acknowledgments
We thank Drs. Kuniaki Saito, Akihiro Mouri, and Kazuo Kunisawa for helpful discussions. This work was supported, in part, by a Japan Society for Promotion of Science (JSPS) KAKENHI “Grant-in-Aid for Specially Promoted Research” (26000013), “Grant-in-Aid for Scientific Research (S)” (19H05643), and by the Human Frontier Science Program Research Grant (RGP0030/2015). WPI-ITbM was supported by the World Premier International Research Center Initiative, Ministry of Eduction, Culture, Sports, Science and Technology (MEXT), Japan.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The microarray data are available in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) database, http://www.ncbi.nlm.nih.gov/ (accession no. GSE124988). Materials used for this study will be available upon requests to the corresponding author.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2000278117/-/DCSupplemental.
References
- 1.Nakane Y., Yoshimura T., Photoperiodic regulation of reproduction in vertebrates. Annu. Rev. Anim. Biosci. 7, 173–194 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Fatet A., Pellicer-Rubio M. T., Leboeuf B., Reproductive cycle of goats. Anim. Reprod. Sci. 124, 211–219 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Yasuo S., et al. , Differential response of type 2 deiodinase gene expression to photoperiod between photoperiodic Fischer 344 and nonphotoperiodic Wistar rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R1315–R1319 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Foster R. G., Roenneberg T., Human responses to the geophysical daily, annual and lunar cycles. Curr. Biol. 18, R784–R794 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Wirz-Justice A., Seasonality in affective disorders. Gen. Comp. Endocrinol. 258, 244–249 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Rosenthal N. E., et al. , Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Arch. Gen. Psychiatry 41, 72–80 (1984). [DOI] [PubMed] [Google Scholar]
- 7.Dulcis D., Jamshidi P., Leutgeb S., Spitzer N. C., Neurotransmitter switching in the adult brain regulates behavior. Science 340, 449–453 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Shimmura T., et al. , Dynamic plasticity in phototransduction regulates seasonal changes in color perception. Nat. Commun. 8, 412 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welbourne L. E., Morland A. B., Wade A. R., Human colour perception changes between seasons. Curr. Biol. 25, R646–R647 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Lavoie M. P., et al. , Evidence of a biological effect of light therapy on the retina of patients with seasonal affective disorder. Biol. Psychiatry 66, 253–258 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Andrews P. W., Bharwani A., Lee K. R., Fox M., Thomson J. A. Jr, Is serotonin an upper or a downer? The evolution of the serotonergic system and its role in depression and the antidepressant response. Neurosci. Biobehav. Rev. 51, 164–188 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Kavalali E. T., Monteggia L. M., How does ketamine elicit a rapid antidepressant response? Curr. Opin. Pharmacol. 20, 35–39 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu G. F., et al. , Antidepressant effect of taurine in chronic unpredictable mild stress-induced depressive rats. Sci. Rep. 7, 4989 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maes M., Galecki P., Chang Y. S., Berk M., A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 676–692 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Miller A. H., Raison C. L., The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 16, 22–34 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wohleb E. S., Franklin T., Iwata M., Duman R. S., Integrating neuroimmune systems in the neurobiology of depression. Nat. Rev. Neurosci. 17, 497–511 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Lewy A. J., Sack R. L., Singer C. M., White D. M., Hoban T. M., Winter depression and the phase-shift hypothesis for bright light’s therapeutic effects: History, theory, and experimental evidence. J. Biol. Rhythms 3, 121–134 (1988). [DOI] [PubMed] [Google Scholar]
- 18.Wirz-Justice A., Chronobiology and mood disorders. Dialogues Clin. Neurosci. 5, 315–325 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto N., et al. , A new interpretation on the homology of the teleostean telencephalon based on hodology and a new eversion model. Brain Behav. Evol. 69, 96–104 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Maney D. L., Polymorphisms in sex steroid receptors: From gene sequence to behavior. Front. Neuroendocrinol. 47, 47–65 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pariante C. M., Lightman S. L., The HPA axis in major depression: Classical theories and new developments. Trends Neurosci. 31, 464–468 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Link A. S., Zheng F., Alzheimer C., Activin signaling in the pathogenesis and therapy of neuropsychiatric diseases. Front. Mol. Neurosci. 9, 32 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maes M., et al. , New drug targets in depression: Inflammatory, cell-mediated immune, oxidative and nitrosative stress, mitochondrial, antioxidant, and neuroprogressive pathways. And new drug candidates–Nrf2 activators and GSK-3 inhibitors. Inflammopharmacology 20, 127–150 (2012). [DOI] [PubMed] [Google Scholar]
- 24.Seo W. Y., et al. , Celastrol induces expression of heme oxygenase-1 through ROS/Nrf2/ARE signaling in the HaCaT cells. Biochem. Biophys. Res. Commun. 407, 535–540 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Divya T., Dineshbabu V., Soumyakrishnan S., Sureshkumar A., Sudhandiran G., Celastrol enhances Nrf2 mediated antioxidant enzymes and exhibits anti-fibrotic effect through regulation of collagen production against bleomycin-induced pulmonary fibrosis. Chem. Biol. Interact. 246, 52–62 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Aizawa H., Amo R., Okamoto H., Phylogeny and ontogeny of the habenular structure. Front. Neurosci. 5, 138 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Y., Wang H., Hu J., Hu H., Lateral habenula in the pathophysiology of depression. Curr. Opin. Neurobiol. 48, 90–96 (2018a). [DOI] [PubMed] [Google Scholar]
- 28.Mizumori S. J. Y., Baker P. M., The lateral habenula and adaptive behaviors. Trends Neurosci. 40, 481–493 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roecklein K. A., et al. , A missense variant (P10L) of the melanopsin (OPN4) gene in seasonal affective disorder. J. Affect. Disord. 114, 279–285 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johansson C., et al. , Circadian clock-related polymorphisms in seasonal affective disorder and their relevance to diurnal preference. Neuropsychopharmacology 28, 734–739 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Kim H. I., et al. , Association of CLOCK, ARNTL, and NPAS2 gene polymorphisms and seasonal variations in mood and behavior. Chronobiol. Int. 32, 785–791 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Kovanen L., Donner K., Kaunisto M., Partonen T., CRY1 and CRY2 genetic variants in seasonality: A longitudinal and cross-sectional study. Psychiatry Res. 242, 101–110 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Partonen T., et al. , Three circadian clock genes Per2, Arntl, and Npas2 contribute to winter depression. Ann. Med. 39, 229–238 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Zhang L., et al. , A PERIOD3 variant causes a circadian phenotype and is associated with a seasonal mood trait. Proc. Natl. Acad. Sci. U.S.A. 113, E1536–E1544 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheng W. S., Hu S., Kravitz F. H., Peterson P. K., Chao C. C., Tumor necrosis factor alpha upregulates human microglial cell production of interleukin-10 in vitro. Clin. Diagn. Lab. Immunol. 2, 604–608 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li K., Yu W., Cao R., Zhu Z., Zhao G., Microglia-mediated BAFF-BAFFR ligation promotes neuronal survival in brain ischemia injury. Neuroscience 363, 87–96 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Yang Y., et al. , Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature 554, 317–322 (2018b). [DOI] [PubMed] [Google Scholar]
- 38.Hashimoto K., Sawa A., Iyo M., Increased levels of glutamate in brains from patients with mood disorders. Biol. Psychiatry 62, 1310–1316 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Perry T. L., et al. , Hereditary mental depression and Parkinsonism with taurine deficiency. Arch. Neurol. 32, 108–113 (1975). [DOI] [PubMed] [Google Scholar]
- 40.Xu Y., et al. , Study of sex differences in duloxetine efficacy for depression in transgenic mouse models. Front. Cell. Neurosci. 11, 344 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu P., et al. , All-trans retinoic acid-induced hypothalamus-pituitary-adrenal hyperactivity involves glucocorticoid receptor dysregulation. Transl. Psychiatry 3, e336 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nieman L. K., Dynamic evaluation of adrenal hypofunction. J. Endocrinol. Invest. 26 (suppl. 7), 74–82 (2003). [PubMed] [Google Scholar]
- 43.James S. P., et al. , The dexamethasone suppression test in seasonal affective disorder. Compr. Psychiatry 27, 224–226 (1986). [DOI] [PubMed] [Google Scholar]
- 44.McMorrow J. P., Murphy E. P., Inflammation: A role for NR4A orphan nuclear receptors? Biochem. Soc. Trans. 39, 688–693 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Kannaiyan R., Shanmugam M. K., Sethi G., Molecular targets of celastrol derived from thunder of God vine: Potential role in the treatment of inflammatory disorders and cancer. Cancer Lett. 303, 9–20 (2011). [DOI] [PubMed] [Google Scholar]
- 46.Martín-de-Saavedra M. D., et al. , Nrf2 participates in depressive disorders through an anti-inflammatory mechanism. Psychoneuroendocrinology 38, 2010–2022 (2013). [DOI] [PubMed] [Google Scholar]
- 47.Sartorius A., et al. , Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol. Psychiatry 67, e9–e11 (2010). [DOI] [PubMed] [Google Scholar]
- 48.Fernandez D. C., et al. , Light affects mood and learning through distinct retina-brain pathways. Cell 175, 71–84.e18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akil H., et al. , Treatment resistant depression: A multi-scale, systems biology approach. Neurosci. Biobehav. Rev. 84, 272–288 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalueff A. V., Stewart A. M., Gerlai R., Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 35, 63–75 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rennekamp A. J., Peterson R. T., 15 years of zebrafish chemical screening. Curr. Opin. Chem. Biol. 24, 58–70 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kato T., Kasahara T., Kubota-Sakashita M., Kato T. M., Nakajima K., Animal models of recurrent or bipolar depression. Neuroscience 321, 189–196 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Thaler K., et al. , Second-generation antidepressants for seasonal affective disorder. Cochrane Database Syst. Rev. 12, CD008591 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Whitmore D., Foulkes N. S., Sassone-Corsi P., Light acts directly on organs and cells in culture to set the vertebrate circadian clock. Nature 404, 87–91 (2000). [DOI] [PubMed] [Google Scholar]
- 55.Qin D., et al. , The first observation of seasonal affective disorder symptoms in Rhesus macaque. Behav. Brain Res. 292, 463–469 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Chen S. R., et al. , A mechanistic overview of triptolide and celastrol, natural products from Tripterygium wilfordii Hook F. Front. Pharmacol. 9, 104 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kulkarni P., et al. , Novel zebrafish EAE model: A quick in vivo screen for multiple sclerosis. Mult. Scler. Relat. Disord. 11, 32–39 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Ziv L., et al. , An affective disorder in zebrafish with mutation of the glucocorticoid receptor. Mol. Psychiatry 18, 681–691 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sugiura Y., Taguchi R., Setou M., Visualization of spatiotemporal energy dynamics of hippocampal neurons by mass spectrometry during a kainate-induced seizure. PLoS One 6, e17952 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hughes M. E., Hogenesch J. B., Kornacker K., JTK_CYCLE: An efficient nonparametric algorithm for detecting rhythmic components in genome-scale data sets. J. Biol. Rhythms 25, 372–380 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seabold G. K., Daunais J. B., Rau A., Grant K. A., Alvarez V. A., DiOlistic labeling of neurons from rodent and non-human primate brain slices. J. Vis. Exp. 41, 2081 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sherazee N., Alvarez V. A., DiOlistics: Delivery of fluorescent dyes into cells. Methods Mol. Biol. 940, 391–400 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen J., et al. , Efficient detection, quantification and enrichment of subtle allelic alterations. DNA Res. 19, 423–433 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The microarray data are available in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) database, http://www.ncbi.nlm.nih.gov/ (accession no. GSE124988). All other data and associated protocols used for this study are available in the main paper and the SI Appendix. Materials used for this study will be available upon requests to the corresponding author.