Significance
It is commonly thought that human locomotor development stems from a single precursor behavior, consisting of alternating flexor–extensor movements, such as kicking or stepping on ground. According to this view, kicking and stepping are identical movement patterns generated by the same neural mechanisms. Here we show that the neuromuscular modules of neonatal kicking and stepping are different, presumably related to different neural mechanisms. Kicking involves an adult-like number of temporal activation patterns, whose association with specific sets of muscles varies across movements. Ground-stepping involves a limited number of activation patterns, each associated with a stable muscle synergy. Since neonatal kicking and ground-stepping seem to anticipate subsequent developmental changes of locomotion in human babies, they might represent distinct locomotor antecedents.
Keywords: pattern generation, modular control, development, stepping
Abstract
Mature locomotion involves modular spinal drives generating a set of fundamental patterns of motoneuron activation, each timed at a specific phase of locomotor cycles and associated with a stable muscle synergy. How locomotor modules develop and to what extent they depend on prior experience or intrinsic programs remains unclear. To address these issues, we herein leverage the presence at birth of two types of locomotor-like movements, spontaneous kicking and weight-bearing stepping. The former is expressed thousands of times in utero and postnatally, whereas the latter is elicited de novo by placing the newborn on the ground for the first time. We found that the neuromuscular modules of stepping and kicking differ substantially. Neonates kicked with an adult-like number of temporal activation patterns, which lacked a stable association with systematic muscle synergies across movements. However, on the ground neonates stepped with fewer temporal patterns but all structured in stable synergies. Since kicking and ground-stepping coexist at birth, switching between the two behaviors may depend on a dynamic reconfiguration of the underlying neural circuits as a function of sensory feedback from surface contact. We tracked the development of ground-stepping in 4- to 48-mo-old infants and found that, after the age of 6 mo, the number of temporal patterns increased progressively, reaching adult-like conformation only after independent walking was established. We surmise that mature locomotor modules may derive by combining the multiple patterns of repeated kicking, on the one hand, with synergies resulting from fractionation of those revealed by sporadic weight-bearing stepping, on the other hand.
In vertebrates, spinal networks show functional modularity at different levels of organization (1–8). At the motor output, muscle coordination results from the modular engagement of motoneuron pools, such that groups of muscles (muscle synergies) share common temporal patterns of activation (9). Computational methods such as nonnegative matrix factorization (NNMF) (10) can be applied to the electromyographic (EMG) activities to recover the statistical structure of neural drive to muscles from the variability of muscle activations (8, 9, 11, 12). Neurophysiological studies in frogs (2, 13, 14), mice (15), rats (16, 17), cats (18), and monkeys (8) support the idea that the temporal activation patterns and the muscle synergies derived from EMG recordings reflect the output of spinal networks of premotor interneurons. Indeed, the modular EMG organization is preserved after complete spinal cord lesions in animals (19–21) and humans (22, 23).
Modularity has received special attention for locomotion, since its neural infrastructure is highly conserved across vertebrates (24, 25). Thus, it has been shown that the fundamental locomotor patterns of mammals and birds are very similar (26). They include four main sequential activations of muscle synergies timed at a specific phase of the step cycle, corresponding to limb touch-down, propulsion, lift-off, and swing (7, 11, 15, 17, 22, 27). While the neuromuscular modules of adult locomotion have been thoroughly investigated (9, 17, 20, 22, 28, 29), there is still limited knowledge about their development since birth (11, 21, 30, 31). Here we consider the development of human locomotion.
Newborn babies express two types of locomotor-like movements: They step on the ground if supported (ground-stepping) (11, 30–36) and they kick spontaneously in air (also defined as air-stepping) (32, 33, 36, 37). Both types of coordinated movements involve rhythmic leg flexion–extension, often characterized by left–right alternation of limbs (32, 33). According to a commonly held view, neonatal kicking and stepping are identical movement patterns generated by the same neural mechanisms (32, 35–37). We challenge this view starting from the following premises. Spontaneous kicking is produced thousands of times before birth, and it has been interpreted as an adaptive locomotor program for frequent changes of the intrauterine position of the fetus (38). Moreover, kicking movements persist over several months after birth (32, 38). Instead, weight-bearing stepping is evoked by the pediatrician at the well-baby examination for the first and often the only time before the initiation of voluntary walking, and it generally disappears a few weeks after birth unless trained (32, 33). Moreover, stepping is triggered by the contact with the support surface and involves antigravity activity. Instead, sensory inputs are not necessary for triggering spontaneous movements; these involve limited feedback about axial limb load and hip extension, two critical signals for phase transitions during ground-stepping (25, 39, 40). Finally, work in other limbed vertebrates shows that the neonatal spinal cord can generate a variety of different motor activities, depending on the stimulation conditions (4, 15, 41–44), and it is likely that human neonates can do the same (38, 45).
We hypothesize that kicking and ground-stepping in human neonates may be two distinct locomotor precursors, reflecting the influence of prior experience and intrinsic developmental programs to a different extent and resulting from different combinations of feedforward and feedback signals. If so, mature locomotion might stem from distinct neonatal precursors (36), each one characterized by a different set of features that might be combined during later development (46). To address these issues, we compared the motor patterns of ground-stepping and kicking in full-term neonates (median age 2 d postpartum). In addition, we tracked the development of ground-stepping in 4- to 48-mo-old children. Motor patterns were investigated by applying NNMF to the EMG activities of leg muscles, which result from lumbosacral motoneuronal activations.
Results
Ground-stepping was tested in neonates held upright on a walkway (n = 33) or a treadmill at different speeds (n = 21) (Fig. 1 and SI Appendix, Supplementary Methods). Upon contacting the surface, all recorded neonates stepped forward, but over a very limited range of speeds. Progression speed was 0.05 ± 0.02 m/s (mean ± SD) across all subjects of the group stepping on the walkway. On the treadmill, neonates stepped from 0.03 m/s to 0.15 m/s, but about half of all steps were performed at 0.05 m/s and only 25% of all steps were at a higher speed (SI Appendix, Fig. S1). On the treadmill, neonates performed bouts (sequences) of up to 20 (median = 7) consecutive steps alternating between the left and right limb, separated by <3 s. Supine neonates (n = 11) were also able to step on a vertical treadmill when gently pushed against its surface (SI Appendix, Fig. S2).
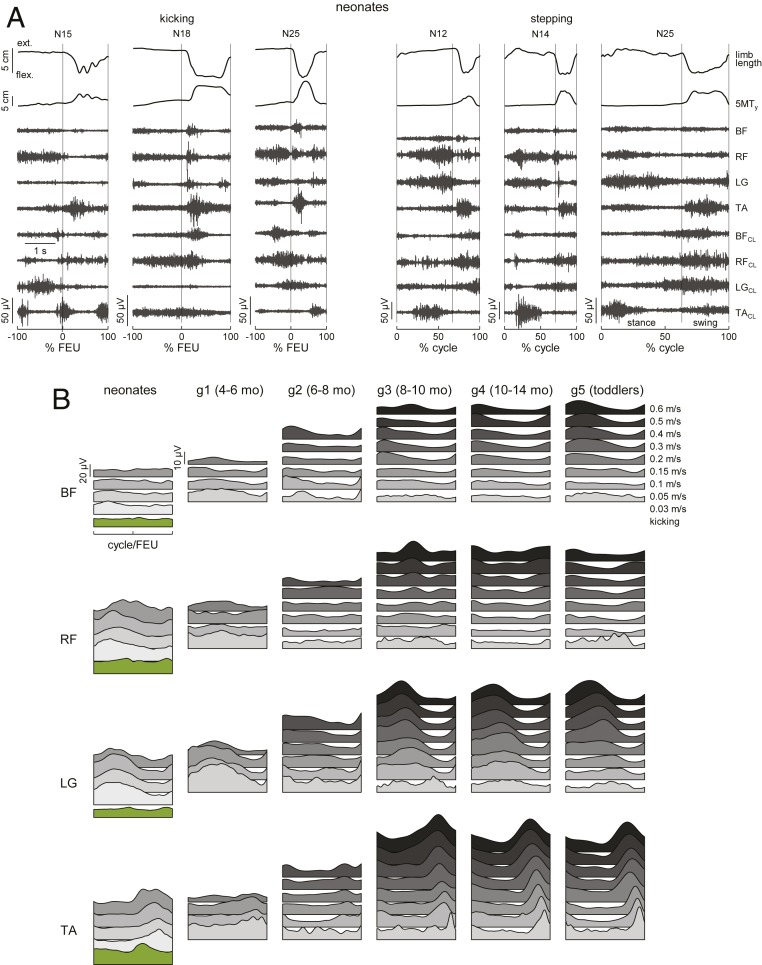
Fig. 1.
Recorded EMG profiles during stepping and kicking. (A) Examples of foot motion and raw EMGs in different neonates. Limb length, distance between GT and LM markers; 5MTy, y coordinate of 5MT-marker; CL, contralateral leg muscles. Stepping includes stance and swing (vertical line marks transition). Kicking includes a flexion–extension unit (FEU) and a time-epoch just preceding and of the same duration as the FEU. Time scale for all panels in the leftmost panel. (B) Ensemble-averaged (across all cycles of all subjects of each group) rectified, filtered EMG profiles aligned with stance onset (stepping), or −100% FEU (kicking) are plotted over a normalized time base. Bottom to Top: Kicking in neonates (green), stepping at increasing treadmill speeds (grayscale). Data refer to pooled cycles from all neonates kicking supine and all children stepping on treadmill. g1, 4- to 6-mo infants; g2, 6 to 8 mo; g3, 8 to 10 mo; g4, 10 to 14 mo; g5, toddlers, 12 to 15 mo (numbers of subjects in SI Appendix, Supplementary Methods).
Spontaneous kicking (SI Appendix, Fig. S3) was recorded while neonates (n = 18) were placed supine on a table or neonates (n = 17) were held vertically in air. Kinematics of kicking qualitatively resembled that of ground-stepping, consistent with previous observations (32, 36, 37). Thus, both behaviors involved quasi-synchronous flexion of hip, knee, and ankle, followed by a forward swing and extension of the limb. Kicking involved alternate flexion–extension of left and right limbs (74% of cases), unilateral (24%), or simultaneous bilateral (2%) flexion–extension. Neonates performed bouts of up to 29 (median = 5) consecutive alternating kicks separated by <3 s. The range of peak foot velocity of kicking largely overlapped that of ground-stepping (SI Appendix, Fig. S4A), as did the range of changes of effective limb length, denoting the extent of whole limb flexion–extension (SI Appendix, Fig. S4B). Thus, both neonate ground-stepping and spontaneous kicking displayed key kinematic signatures of locomotor-like behavior (32).
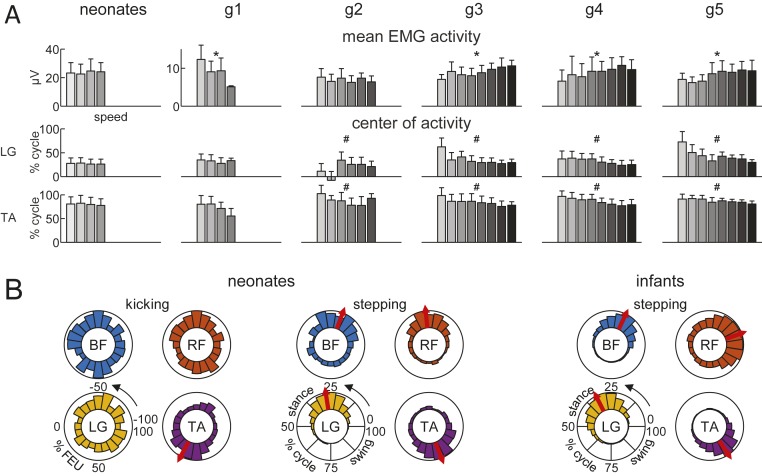
The fundamental distinction between the two behaviors emerged clearly when considering detailed muscle activities. We observed considerable variability in the time-varying profiles of EMG activity across individual cycles for both ground-stepping and kicking (Fig. 1A). Yet, for ground-stepping but not for kicking, systematic modulations of activity were evident in the ensemble averages of rectified, filtered EMG profiles aligned with cycle onset, after normalizing all data to the cycle duration (Fig. 1B). Thus, during ground-stepping, several thigh (e.g., biceps femoris [BF], rectus femoris [RF]) and leg muscles (e.g., gastrocnemius lateralis [LG]) were coactivated with a quasi-sinusoidal waveform over most of the ipsilateral stance phase, while limb flexors (e.g., tibialis anterior [TA]) were mostly active during ipsilateral swing. Flexor–extensor coactivations tended to peak around midstance, contributing to stiffening of the limb and exerting vertical forces, supporting part of the body weight (31). Instead, activation of the TA tended to peak around midswing, contributing to lifting the foot off the ground together with hip flexors. Since neither the mean EMG amplitude [ANOVA, F(3, 485) = 1.99, P = 0.11, n = 488 step-cycles in 21 neonates] nor the EMG timing (circular parametric Watson–Williams test, P > 0.48) depended significantly on treadmill speed (Fig. 2A), we pooled the data across speeds to compute the center of activity of EMGs (Fig. 2B). We found that the distribution of the center of activity was narrowly tuned relative to the step cycle (Rayleigh test, P < 0.001), indicating a relatively consistent timing of peak activation. The center of activity occurred at 19% ± 18% (mean ± SD), 27% ± 16%, 27% ± 11%, and 81 ± 16% of the step cycle for the BF, RF, LG, and TA, respectively.
Fig. 2.
Relationship of EMG with speed and cycle. (A) Mean (+SD) EMG across BF, RF, LG, and TA muscles (Upper) and center of activity of average rectified EMG for LG and TA (Lower) versus treadmill speed (same speeds as in Fig. 1B, range 0.03 to 0.6 m/s). An asterisk (*) (one-way ANOVA) and pound sign (#) (Watson–Williams multisample test for equal means) denote significant (P < 0.05) differences across speeds. (B) Polar histograms of center of activity for kicking and treadmill-stepping versus normalized cycles discretized in 20 sectors. Black arrows: Progression time, with angle that varies from 0 to 360° corresponding to 0 and 100% cycle for stepping, and to −100% and 100% FEU for kicking. Bar height denotes the percentage of cycles whose center of activity is located in the corresponding sector. Red arrows: Resultant (circular mean) center of activity (Rayleigh test for nonuniform circular distributions, P < 0.05). Data refer to pooled cycles from neonates kicking supine and children stepping on treadmill, where center of activity was identifiable (Rayleigh test, P < 0.05). In B, data from all infants of g1 to g5 were pooled together.
For kicking, despite the prominent EMG activity of both flexor and extensor muscles during each flexion–extension cycle (Fig. 1A), the ensemble averages aligned with cycle onset did not show appreciable modulations (Fig. 1B). This depended on the fact that muscle activations varied conspicuously from cycle to cycle. The center of activity of EMG occurred at −19% ± 44% (mean ± SD), −63% ± 43%, −75% ± 43%, and 34 ± 38% of the kick cycle for the BF, RF, LG, and TA, respectively. The center of activity was uniformly distributed (Rayleigh test, P > 0.05, n = 270 kick-cycles in 17 neonates) irrespective of whether EMG traces were aligned with flexion onset (Fig. 2B) or extension onset of each cycle, except for the TA. The center of activity of this ankle flexor was more narrowly distributed when aligned with flexion onset (Rayleigh test, P < 0.001) than with extension onset (P = 0.063). However, in both cases, the timing variability of the TA was roughly comparable to that of the other muscles during kicking, and more than twice as large as that during ground-stepping.
Cluster Analysis of Activation Patterns and Muscle Synergies from Single Cycles.
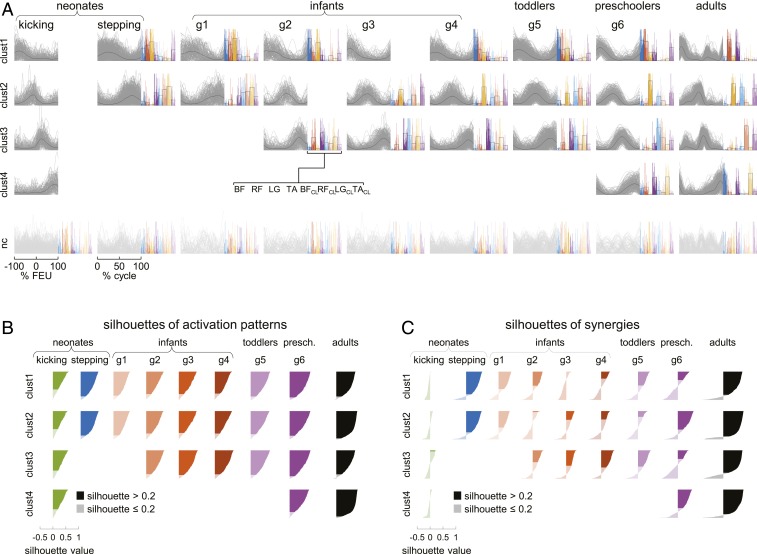
To describe the statistical structure of neural drive to muscles, we applied NNMF to the EMG activity of bilateral muscles (11). Given the large intercycle variability of muscle activations, we applied NNMF to EMGs recorded in each single cycle of each participant. We used cluster analysis to identify similar activation patterns and similar muscle synergies across cycles. The optimal clustering solutions were determined using the silhouette method (47), which is based on the comparison of within-cluster distances (cluster tightness) with between-cluster distances (cluster separation). The method shows which items are placed well within their cluster and which ones lie somewhere in between clusters.
This analysis applied to a group of adults (n = 16) walking on a treadmill at 1.1 m/s yielded four optimal clusters of activation patterns (Fig. 3 A and B), each recruiting a stable set of muscles as shown by the associated clusters of muscle synergies (Fig. 3 A and C). Ninety-two percent of all activation patterns (n = 3,471) and 85% of all muscle synergies were above the silhouette threshold (S > 0.2), indicating reliable clustering. The activation patterns did not differ appreciably when extracted from a smaller set of muscles (n = 8) (Fig. 3A) or a larger set (n = 22) (SI Appendix, Fig. S5). The timing and shape of these patterns, as well as the associated muscle synergies, obtained with cluster analysis were very similar to those reported in previous publications using different methods (11, 29).
Fig. 3.
Cluster analysis of computed neuromuscular modules of bilateral EMGs for kicking and stepping on treadmill. (A, Upper) Clusters of activation patterns (S > 0.2) from single cycles of all subjects of each group in gray, average patterns in black. Corresponding synergies weights (S > 0.2) for single cycles in color, average values as empty bars. Patterns and synergies are plotted only if S > 0.2 in >15% of cases. (Lower) Not-clustered (nc, S ≤ 0.2) activation patterns (light gray) and associated weights. Silhouettes of activation patterns (B) and synergies weights (C) ranked in decreasing order for the single kicks or steps of A (below-threshold silhouettes in light color). Kicking cycles included −100% ÷ 100% FEU. g6, preschoolers, 24 to 48 mo.
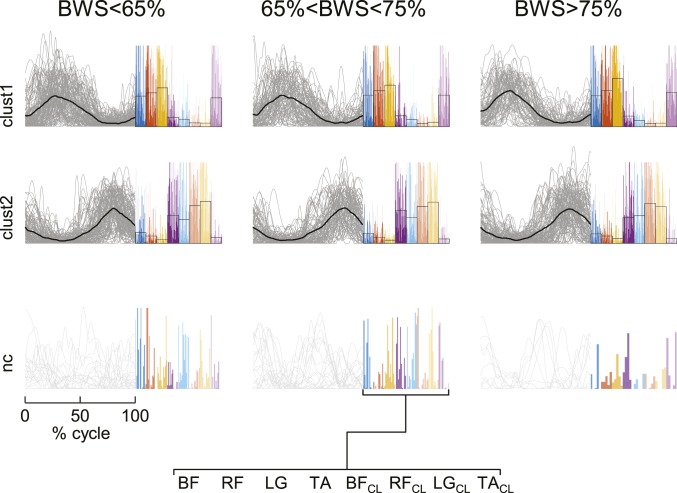
However, in neonates we found two optimal clusters of activation patterns for ground-stepping, each associated with a stable muscle synergy (Fig. 3), in agreement with previous findings obtained by averaging EMGs across step cycles (11). Eighty-seven percent of all activation patterns (n = 1,178) and 77% of all muscle synergies were above silhouette threshold. Also for neonates, the activation patterns did not differ appreciably when extracted from 8 muscles (Fig. 3A) or 22 muscles (SI Appendix, Fig. S5). Nor did the stepping patterns change appreciably as a function of body load (Fig. 4). This was shown in experiments (n = 27 neonates) in which the pediatrician holding the upright child under the armpits varied the support force from trial to trial. Overall, neonates in these experiments supported from 7% up to 67% of their body weight. Nevertheless, the waveforms of the two main activation patterns were very similar across the three groups of body-weight support of Fig. 4. Thus, the median scalar-product similarity was 0.99 (5th to 95th percentiles 0.983, 0.998).
Fig. 4.
Cluster analysis of computed neuromuscular modules for stepping on walkway at different levels of body weight supported by the neonate. Range of supported body weight (BWS) is 33 to 65% (mean = 57%, n = 85 strides), 65 to 75% (mean = 70%, n = 63 strides), and 75 to 93% (mean = 81%, n = 67 strides), from left to right. Same format as in Fig. 3A.
Strikingly, cluster analysis applied to kicking cycles yielded four optimal clusters of activation patterns, instead of two clusters as in ground-stepping (Fig. 3 A and B). Of kicking activation patterns (n = 895), 67% were above the silhouette threshold. However, none of the clusters of kicking patterns was associated with a corresponding cluster of muscle synergies (Fig. 3C), indicating that each instantiation of a kicking pattern could engage a different combination of muscles. Indeed, only 1% of the muscle synergies were above the silhouette threshold and were clustered reliably. This observation is consistent with the uniform distribution of the center of activity of most EMGs reported above.
Specular results were obtained by clustering muscle synergies instead of activation patterns: Both clusters of ground-stepping synergies but none of kicking synergies were associated with a set of consistent activation patterns (SI Appendix, Fig. S6).
Validation of Cluster Analysis.
We wondered whether the clustering algorithm might have identified clusters in the absence of an underlying structure. To address this concern, we generated multiple sets of randomized activation patterns, and clustered them as before. We compared the original clusters of kicking and stepping with the clusters obtained by randomization. Both the silhouettes and the normalized Hubert’s γ-statistics were significantly higher for the original clusters than for the random clusters (P < 0.05), thus rejecting the null hypothesis that the original activation patterns were randomly structured.
Significance of the Activation Patterns.
We also verified that the structure of the activation patterns found in neonates did not result from a bias intrinsic to the extraction method. To this end, we applied the cluster analysis to structureless data obtained by randomly reshuffling all EMG samples, independently for each muscle. We found that the silhouettes of the activation patterns of the simulated data were above threshold in only 8% of cases for kicking and 7% of cases for stepping.
Spatial Decomposition of Muscle Activities over Data Ensembles.
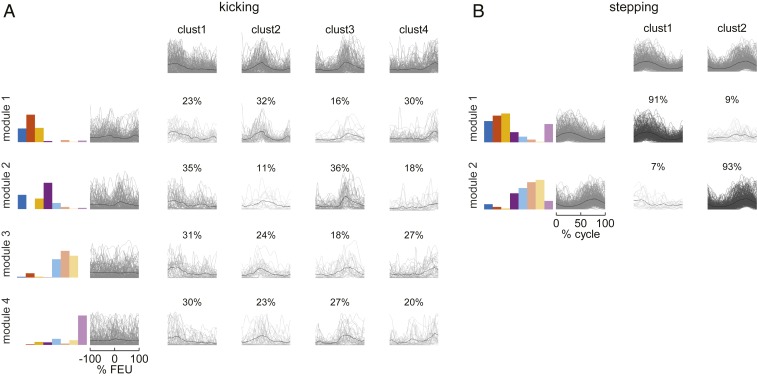
Cluster analysis showed that, unlike the activation patterns of stepping, those of kicking were not consistently associated with specific muscle synergies. However, one cannot exclude the possibility that even for kicking there exists an underlying statistical structure of muscle synergies across movements, but this is independent of any systematic association with activation patterns. Then, if kicking and stepping represented distinct locomotor-like behaviors, one would expect that the muscle synergies extracted from the data ensemble of kicking were significantly different from those of stepping. To test this hypothesis, we computed muscle synergies with NNMF over ensembles of cycles and individuals, instead of cycle by cycle as in the previous cluster analysis. By varying the number of synergies from one to seven, we found that the percent of variance accounted for (VAF) by the synergies was significantly lower for kicking than for stepping [ANOVA, F(1, 139) = 21.31, P < 0.001], indicating that the dimensionality of muscle synergies was higher for kicking than for stepping. Then, we used a bootstrap approach to test whether there were significant differences between the synergies of kicking and those of stepping for the nine possible combinations of synergies ranging from two to four. We found that, for seven of nine comparisons, the synergies from stepping and kicking cycles had at least one nonshared dimension, since one or more principal angles between the subspaces spanned by each set were significantly larger than expected from noise and sampling variability (P < 0.05). Overall, these results indicate that, even assuming the existence of fixed muscle synergies, the synergies for kicking and stepping were significantly different. Importantly, as in the case of the previous analysis from single cycles, the fixed synergies extracted over the ensemble of cycles and individuals were also associated with consistent activation patterns for stepping but not for kicking. Indeed, the activation patterns were quasi-uniformly distributed across clusters for kicking, whereas they were concentrated for stepping (Fig. 5).
Fig. 5.
Cluster analysis of the activation patterns computed from the spatial decomposition of muscle activities over data ensembles in neonates. (A) Results for kicking by considering four modules. (B) Results for stepping by considering two modules. Each column plots for each module (from top to bottom) the activation patterns that fit each cluster derived from spatial decomposition, along with the corresponding percentage of cases.
Development of Stepping Modules.
We tracked the development of ground-stepping in six groups of children at different developmental stages, including infants unable to step unsupported (g1 to g4, 4- to 14-mo-old), toddlers who had just started walking independently (g5, 12 to 15 mo), and preschoolers (g6, 24 to 48 mo). They were tested on a walkway (n = 39) or a treadmill at different speeds (n = 38). All (but one) infants younger than 8 mo had not practiced stepping before the recording sessions. Most children were able to step over a wide range of treadmill speeds (SI Appendix, Fig. S1), showing adaptation of EMG activity to speed changes. Thus, both the mean EMG amplitude (ANOVA, all P < 0.001, n = 38 children) and the EMG timing (circular parametric Watson–Williams test, all P < 0.03) depended significantly on speed (Fig. 2A), except for the mean EMG amplitude of g2 (P = 0.09) and the EMG timing of g1 (P > 0.13) that did not depend significantly on speed.
Two optimal clusters of activation patterns were still associated with ground stepping in 4- to 6-mo-old infants, while a third cluster appeared at 6 to 8 mo, and a fourth cluster roughly comparable to that of adults appeared at about 24 mo in preschoolers (Fig. 3 A and B). On average, 84% of all activation patterns and 43% of all muscle synergies were above silhouette threshold (Fig. 3C).
Reconstruction of Muscle Activities for Stepping Children from Kicking Patterns.
As previously noticed, the activation patterns of neonate kicking poorly resembled those of neonate ground-stepping, consistent with the hypothesis that these neonatal behaviors may be subserved by partly different mechanisms. However, the patterns of neonate kicking tended to resemble the stepping patterns of children at later developmental stages (Fig. 3A). Thus, the scalar-product similarity between the kicking patterns and the stepping patterns increased significantly as a function of the ordinal developmental stage from neonate to preschoolers (linear regression, r = 0.83, P = 0.02). In particular, the similarity between the kicking patterns and the stepping patterns of preschoolers was high (0.93).
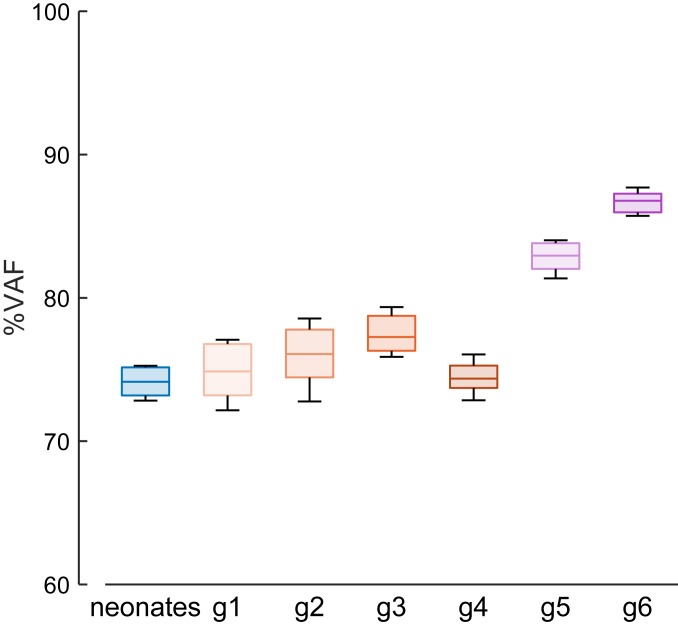
To explore further the possibility that kicking might anticipate some features of subsequent locomotor development, we quantified the extent to which the muscle activities of ground-stepping at different developmental stages could be reconstructed starting from the neonate kicking patterns. To this end, we cross-fitted with bootstrapping the EMG activities of the step cycles of the participants in each group of children with the kicking patterns (Fig. 6). We found that the VAF of reconstruction increased significantly with developmental stage (linear regression, r = 0.83, P = 0.02). In particular, the median VAF (86.4%, 5th to 95th percentiles 85.6%, 86.9%) of reconstructing the EMGs of preschoolers (g6) with the kicking patterns of neonates was not significantly (P > 0.05) different from the median VAF (86.3%, 5th to 95th percentiles 85.6%, 86.9%) of reconstructing the EMGs of g6 with g6 patterns. In contrast, the activation patterns of neonate ground-stepping did not reconstruct well the EMGs of either neonate kicking (median VAF = 54.3%, 5th to 95th percentiles 51.9%, 56.4%) or stepping children of any group (all VAF < 60%), with no significant trend with developmental stage (linear regression, r = 0.23, P = 0.62).
Fig. 6.
Reconstruction of EMG activities of stepping children using neonate kicking patterns. Box-and-whisker plots of median and 5th to 95th percentiles (over 100 bootstrap iterations) of the VAF of the reconstruction of the EMGs of each group of children (neonates, g1 to g6); the whiskers extend to the lowest and highest values.
Muscle Synergies of Older Children as Fractionated Muscle Synergies of Neonate Stepping.
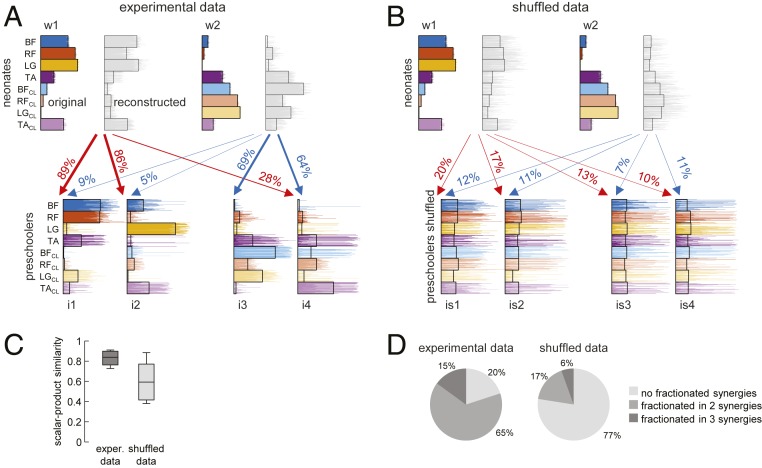
As we remarked earlier, in contrast to the activation patterns of neonate kicking, the patterns of neonate ground-stepping were consistently associated with stable muscle synergies, as was the case for the patterns of later developmental stages (Fig. 3). We asked whether and how the muscle synergies of neonate ground-stepping might be related to those of older children. Stepping neonates exhibited a good deal of muscle coactivation that tended to decrease during development, resulting in more fractionated muscle activations (Fig. 1B). This fractionation might arise from a split of each neonate muscle synergy into two or more synergies during development, as it occurs following plastic neural reorganization after a stroke in adults (48). Fractionation of muscle activity would be consistent with the increasing dimensionality of the stepping modules from neonates to older children. To test this hypothesis, we split with bootstrapping the muscle synergies of neonate ground-stepping to best fit either the experimental synergies of preschoolers (Fig. 7A) or the random synergies obtained by shuffling the experimental ones (Fig. 7B). We found that the median scalar-product similarity with the original was significantly (P < 0.05) higher for the former than for the latter (Fig. 7C). Moreover, the percentage of the neonate synergies that were split in the synergies of preschoolers was 80% for the experimental data but only 23% for the shuffled data (Fig. 7D).
Fig. 7.
Muscle synergies of preschoolers explained as fractionations of synergies of ground-stepping neonates. (A) The procedure identified synergies of preschoolers as fractionations of neonate synergies in the indicated percentages, since the synergies identified as fractionations could be linearly combined to reconstruct the corresponding neonate synergies. (B) The same procedure applied to random synergies obtained by shuffling the experimental ones. (C) Box-and-whisker plots for median and 5th to 95th percentiles (over 100 bootstrap iterations) of scalar-product similarity between original and reconstructed synergies. (D) Overall percentages of neonate synergies significantly split in the synergies of preschoolers or shuffled data.
Discussion
We found that neonatal kicking and ground-stepping involved neuromuscular modules with different complexity and flexibility. We also found that the activation patterns of neonate kicking resembled the stepping patterns of older children, so that preschoolers’ EMGs were reconstructed well using kicking patterns. On the other hand, the activation patterns of neonate ground-stepping poorly resembled the patterns of preschoolers, but fractionation of the muscle synergies of ground-stepping neonates matched the synergies of preschoolers. In the following, we discuss the possibility that neonatal kicking and ground-stepping may represent distinct locomotor precursors, since each of these behaviors appears to anticipate a subset of features that characterize later development.
Kicking involved complex and flexible modules, consisting of an adult-like number of temporal activation patterns, whose association with specific sets of muscles varied considerably across movements. The variable linkage of temporal patterns with specific muscle groups is consistent with the view that the recruitment of muscle synergies within the locomotor networks is downstream of the spinal interneurons generating the activation patterns (8, 14, 49, 50). It is also consistent with the view that the spinal circuitry operates as a dynamical system whose variable states can generate different muscle synergies (51). Complexity and flexibility of spontaneous pattern generation in human neonates are reminiscent of features revealed at early developmental stages in different types of animal preparations (15, 41, 42, 44). Thus, in newborn rats, spontaneous motor activity involves a variable association of muscle synergies with each movement (52). Furthermore, the muscle synergies evoked in the isolated spinal cord of neonatal rats depend strongly on the applied pharmacological agent (41) or the neural pathway stimulated electrically (44). Interestingly, four temporal patterns similar to those we found here for neonatal kicking were revealed by NNMF applied to cycle-averaged firing rates of hundreds of motoneurons recorded in the isolated, pharmacologically stimulated spinal cord of neonatal mouse during locomotor-like activity (15). Therefore, multiple flexor and extensor modules are already present at birth in rodents (4, 15, 24) and humans (present study).
Spontaneous movements such as kicking show some degree of developmental continuity between prenatal and postnatal behavior (38). Their frequent occurrence in variable forms allows exploring a wide range of limb kinematics and kinetics (45). Variation and flexibility of healthy spontaneous movements contrast with the movement stereotypy typical of developmental motor disorders (38, 45). In animal models, sensory feedback resulting from spontaneous activity has been shown to tune motor commands and reflexes, and facilitate the organization of neural circuits at spinal and supraspinal levels by establishing maps of sensorimotor connections that progressively become stable with age (3, 52). In particular, spontaneous movements drive maturation of pattern generation by integrating multiple sensory inputs (3, 45, 53).
In contrast with kicking, neonatal ground-stepping involved only two temporal activation patterns, each associated with a stable muscle synergy, one for limb extension during stance and the other one for limb flexion during swing. The present results confirm previous results obtained on average data of newborns stepping on a walkway (11), and extend the observation to the analysis of single cycles and to stepping on a treadmill at different speeds. In infants older than about 6 mo of age, we found that the number of temporal patterns associated with ground-stepping increased progressively with age, reaching adult-like conformation only after independent walking was established. Similarly, newborn rodents show a simple alternation between flexors and extensors at a time when they are mainly crawling (25, 42). The mature, four-phase patterns emerge during the first few weeks when pups start supporting themselves while walking (42).
In contrast with spontaneous kicking, weight-bearing stepping is experienced for the first time after birth. Therefore, it reflects intrinsic locomotor functions independent of prior experience with that specific behavior. The question is why neonatal stepping does not utilize the same four-phase patterns exhibited by kicking and by the isolated spinal cord in animal models. One possible explanation is that the sensory signals generated by the contact with the support surface, present in ground-stepping but not in kicking or in the isolated spinal cord, interact with central locomotor generators and constrain the expression of neuromuscular modules limiting their number and shaping their patterns, at least in altricial animals (such as humans and rodents) who are unable to walk independently soon after birth.
Since kicking and ground-stepping coexist at birth, switching between the two behaviors may depend on a dynamic reconfiguration of the underlying neural circuits (54) as a function of sensory feedback from surface contact (20, 23, 39, 40, 49, 51). There might be two distinct neural circuits, one for kicking and another for stepping, which are separately activated depending on feedforward and feedback signals, or a single, more complex neural circuit that can perform both types of movements switching types of patterns based on the presence of load feedback.
Because neonatal kicking and ground-stepping appear to anticipate subsequent developmental changes of locomotion in human babies, we suggest that they may be locomotor precursors (36). Kicking showed activation patterns with a similar dimensionality and waveform as those of more mature locomotion, while stepping showed stable muscle synergies whose fractionation could account for the synergies of older children. These two sets of features, multiple patterns and stable synergies, might be combined during development, eventually leading to intentional, unsupported walking, in parallel with the growing role of supraspinal control and a better integration of feedback and feedforward signals (11, 33, 34). Gradual, age-related combination of synergies with patterns might occur during the first months after birth, a critical developmental period of heightened neural plasticity (45, 55). The progressive increase of the number of neuromuscular modules during ground-stepping after about 6 mo of age might be also related to evolving body postures of the infant (36, 45). Postural feedback from skin, muscle, tendon, and vestibular receptors might trigger adaptive changes in the spinal interneuronal circuitry, adding or tuning modules so that they are tailored to the limb and body biomechanics of the growing individual (12). Distinct sensory signals are processed separately in dedicated spinal subcircuits (56), and the effects of proprioceptive and tactile stimuli change drastically during development (57).
In sum, our results are compatible with the hypothesis that neonatal kicking and stepping reflect a preparation for the adult (32). However, these behaviors might also reflect transient adaptations to the current environment and context, not necessarily related to later adaptations (58). Indeed, the distinction between innate and learned behavior may be moot, since “most, if not all, of the motor patterns available at birth are subject to maturation and are modified substantially through learning” (59).
Irrespective of the extent to which neonatal kicking and stepping are direct antecedents of mature locomotion, they represent an overt manifestation of the development of the neuronal networks underlying locomotor-like movements (58). Thus, our quantitative identification of the neonatal motor patterns may prove helpful for diagnosis in children at risk for neuromotor disorders (38). Timely diagnosis for such children is crucial, since medical interventions can be much more effective if they are started early in development (60).
Materials and Methods
All experiments were in accordance with the World Medical Association Declaration of Helsinki for medical research involving human subjects. The experiments were approved by the Research Ethics Committees of Azienda Sanitaria Locale (Local Health Centre) Roma C (protocol CEI/15843 study 609 and protocol 27593 study 38.15), Santa Lucia Foundation (protocol CE/AG4/PROG.341-01), and Veltischev Research and Clinical Institute for Pediatrics of the Pirogov Russian National Research Medical University (protocol 14/18). A parent for the child and all adult subjects provided informed written consent to participate in the study after the nature and possible consequences of the study were explained. Detailed protocols, recording and analysis procedures are available in SI Appendix, Supplementary Methods.
Briefly, kinematics was recorded by monitoring markers attached at the hip (greater trochanter, GT), knee (lateral femur epicondyle, LE), ankle (lateral malleolus, LM), and fifth metatarsophalangeal joint (5MT). The instantaneous distance between the GT and LM markers defined the effective limb length, denoting the variable extent of flexion–extension. Tangential (3D) foot velocity was computed from the instantaneous position of the 5MT. In all children, surface EMG activities were recorded bilaterally from the RF, BF, TA, and LG. In 10 neonates, we also recorded bilaterally from the gluteus maximus (GM), tensor fascia latae (TFL), adductor longus (Add), vastus lateralis (VL), vastus medialis (VM), gastrocnemius medialis (MG), and soleus (Sol). In all adults, we recorded bilaterally from the gluteus medius (Gmed), TFL, semitendinosus (ST), BF, RF, VL, VM, MG, LG, Sol, and TA. EMG data were filtered and rectified. For each muscle, we calculated the center of activity over a step or kick cycle using circular statistics. Basic neuromuscular modules were extracted from bilateral EMG time-varying profiles of each single cycle for all participants using the NNMF algorithm (10). Before applying NNMF, we subtracted the minimum over the cycle from each EMG profile and normalized the EMG amplitude to the maximum computed over all cycles of a given participant and condition. To identify similar activation patterns across cycles, all activation patterns extracted from single cycles of all subjects of each group and condition were pooled together and partitioned in k mutually exclusive patterns using the k-means algorithm (13). We determined the optimal number of clusters in the range 2 to 20 using the silhouette method (47).
Data Availability.
Deidentified source data of all figures are deposited at https://zenodo.org/record/3666098.
Supplementary Material
Acknowledgments
We thank Marika Cicchese, Nadia Dominici, Michael J. MacLellan, Vito Mondì, Daniela Morelli, and Tiziana Silei for help with some experiments; and Auke Ijspeert, Antonella Maselli, and Richard Poppele for comments and suggestions. This work was supported by the Italian Ministry of Health (Ricerca corrente, Istituto di Ricovero e Cura a Carattere Scientifico Fondazione Santa Lucia); Italian Space Agency (Grants I/006/06/0 and 2019-11-U.0); Italian University Ministry (Progetti di Rilevante Interesse Nazionale Grants 2015HFWRYY and 2017CBF8NJ_005); and the Russian Foundation for Basic Research (Grant 18-015-00187).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: Deidentified source data of all figures are deposited at https://zenodo.org/record/3666098.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1920984117/-/DCSupplemental.
References
- 1.Grillner S., Neurobiological bases of rhythmic motor acts in vertebrates. Science 228, 143–149 (1985). [DOI] [PubMed] [Google Scholar]
- 2.Bizzi E., Mussa-Ivaldi F. A., Giszter S., Computations underlying the execution of movement: A biological perspective. Science 253, 287–291 (1991). [DOI] [PubMed] [Google Scholar]
- 3.Petersson P., Waldenström A., Fåhraeus C., Schouenborg J., Spontaneous muscle twitches during sleep guide spinal self-organization. Nature 424, 72–75 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Hägglund M., et al. , Optogenetic dissection reveals multiple rhythmogenic modules underlying locomotion. Proc. Natl. Acad. Sci. U.S.A. 110, 11589–11594 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ampatzis K., Song J., Ausborn J., El Manira A., Separate microcircuit modules of distinct v2a interneurons and motoneurons control the speed of locomotion. Neuron 83, 934–943 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Levine A. J., et al. , Identification of a cellular node for motor control pathways. Nat. Neurosci. 17, 586–593 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ting L. H., et al. , Neuromechanical principles underlying movement modularity and their implications for rehabilitation. Neuron 86, 38–54 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takei T., Confais J., Tomatsu S., Oya T., Seki K., Neural basis for hand muscle synergies in the primate spinal cord. Proc. Natl. Acad. Sci. U.S.A. 114, 8643–8648 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bizzi E., Cheung V. C. K., d’Avella A., Saltiel P., Tresch M., Combining modules for movement. Brain Res. Rev. 57, 125–133 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee D. D., Seung H. S., Learning the parts of objects by non-negative matrix factorization. Nature 401, 788–791 (1999). [DOI] [PubMed] [Google Scholar]
- 11.Dominici N., et al. , Locomotor primitives in newborn babies and their development. Science 334, 997–999 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Bizzi E., Cheung V. C., The neural origin of muscle synergies. Front. Comput. Neurosci. 7, 51 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saltiel P., Wyler-Duda K., d’Avella A., Tresch M. C., Bizzi E., Muscle synergies encoded within the spinal cord: Evidence from focal intraspinal NMDA iontophoresis in the frog. J. Neurophysiol. 85, 605–619 (2001). [DOI] [PubMed] [Google Scholar]
- 14.Hart C. B., Giszter S. F., A neural basis for motor primitives in the spinal cord. J. Neurosci. 30, 1322–1336 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machado T. A., Pnevmatikakis E., Paninski L., Jessell T. M., Miri A., Primacy of flexor locomotor pattern revealed by ancestral reversion of motor neuron identity. Cell 162, 338–350 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tresch M. C., Bizzi E., Responses to spinal microstimulation in the chronically spinalized rat and their relationship to spinal systems activated by low threshold cutaneous stimulation. Exp. Brain Res. 129, 401–416 (1999). [DOI] [PubMed] [Google Scholar]
- 17.Wenger N., et al. , Spatiotemporal neuromodulation therapies engaging muscle synergies improve motor control after spinal cord injury. Nat. Med. 22, 138–145 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemay M. A., Grill W. M., Modularity of motor output evoked by intraspinal microstimulation in cats. J. Neurophysiol. 91, 502–514 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Roh J., Cheung V. C., Bizzi E., Modules in the brain stem and spinal cord underlying motor behaviors. J. Neurophysiol. 106, 1363–1378 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desrochers E., Harnie J., Doelman A., Hurteau M. F., Frigon A., Spinal control of muscle synergies for adult mammalian locomotion. J. Physiol. 597, 333–350 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Q., Logan D., Giszter S. F., Motor primitives are determined in early development and are then robustly conserved into adulthood. Proc. Natl. Acad. Sci. U.S.A. 116, 12025–12034 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivanenko Y. P., et al. , Temporal components of the motor patterns expressed by the human spinal cord reflect foot kinematics. J. Neurophysiol. 90, 3555–3565 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Minassian K., Hofstoetter U. S., Dzeladini F., Guertin P. A., Ijspeert A., The human central pattern generator for locomotion: Does it exist and contribute to walking? Neuroscientist 23, 649–663 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Kiehn O., Decoding the organization of spinal circuits that control locomotion. Nat. Rev. Neurosci. 17, 224–238 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grillner S., El Manira A., Current principles of motor control, with special reference to vertebrate locomotion. Physiol. Rev. 100, 271–320 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Catavitello G., Ivanenko Y., Lacquaniti F., A kinematic synergy for terrestrial locomotion shared by mammals and birds. eLife 7, e38190 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santuz A., et al. , Modular organization of murine locomotor pattern in the presence and absence of sensory feedback from muscle spindles. J. Physiol. 597, 3147–3165 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Ivanenko Y. P., Poppele R. E., Lacquaniti F., Five basic muscle activation patterns account for muscle activity during human locomotion. J. Physiol. 556, 267–282 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clark D. J., Ting L. H., Zajac F. E., Neptune R. R., Kautz S. A., Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J. Neurophysiol. 103, 844–857 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ivanenko Y. P., et al. , Changes in the spinal segmental motor output for stepping during development from infant to adult. J. Neurosci. 33, 3025–3036 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sylos-Labini F., et al. , Foot placement characteristics and plantar pressure distribution patterns during stepping on ground in neonates. Front. Physiol. 8, 784 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thelen E., Fisher D. M., Newborn stepping: An explanation for a “disappearing” reflex. Dev. Psychol. 18, 760–775 (1982). [Google Scholar]
- 33.Yang J. F., Stephens M. J., Vishram R., Infant stepping: A method to study the sensory control of human walking. J. Physiol. 507, 927–937 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forssberg H., Ontogeny of human locomotor control. I. Infant stepping, supported locomotion and transition to independent locomotion. Exp. Brain Res. 57, 480–493 (1985). [DOI] [PubMed] [Google Scholar]
- 35.Domellöf E., Rönnqvist L., Hopkins B., Functional asymmetries in the stepping response of the human newborn: A kinematic approach. Exp. Brain Res. 177, 324–335 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Adolph K. E., Robinson S. R., “The road to walking: What learning to walk tells us about development” in Oxford Handbook of Developmental Psychology, Zelazo P. D., Ed. (Oxford University Press, 2013), vol. 1, pp. 403–443. [Google Scholar]
- 37.Barbu-Roth M., et al. , Air stepping in response to optic flows that move toward and away from the neonate. Dev. Psychobiol. 56, 1142–1149 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Prechtl H. F. R., Continuity of Neural Functions from Prenatal to Postnatal Life (Blackwell, Oxford, UK, 1984). [Google Scholar]
- 39.Duysens J., Clarac F., Cruse H., Load-regulating mechanisms in gait and posture: Comparative aspects. Physiol. Rev. 80, 83–133 (2000). [DOI] [PubMed] [Google Scholar]
- 40.Pearson K. G., Generating the walking gait: Role of sensory feedback. Prog. Brain Res. 143, 123–129 (2004). [DOI] [PubMed] [Google Scholar]
- 41.Kiehn O., Kjaerulff O., Spatiotemporal characteristics of 5-HT and dopamine-induced rhythmic hindlimb activity in the in vitro neonatal rat. J. Neurophysiol. 75, 1472–1482 (1996). [DOI] [PubMed] [Google Scholar]
- 42.Clarac F., Brocard F., Vinay L., The maturation of locomotor networks. Prog. Brain Res. 143, 57–66 (2004). [DOI] [PubMed] [Google Scholar]
- 43.Marder E., Rehm K. J., Development of central pattern generating circuits. Curr. Opin. Neurobiol. 15, 86–93 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Klein D. A., Patino A., Tresch M. C., Flexibility of motor pattern generation across stimulation conditions by the neonatal rat spinal cord. J. Neurophysiol. 103, 1580–1590 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hadders-Algra M., Early human motor development: From variation to the ability to vary and adapt. Neurosci. Biobehav. Rev. 90, 411–427 (2018). [DOI] [PubMed] [Google Scholar]
- 46.Hamburger V., Some aspects of the embryology of behavior. Q. Rev. Biol. 38, 342–365 (1963). [DOI] [PubMed] [Google Scholar]
- 47.Rousseeuw P. J., Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 20, 53–65 (1987). [Google Scholar]
- 48.Cheung V. C., et al. , Muscle synergy patterns as physiological markers of motor cortical damage. Proc. Natl. Acad. Sci. U.S.A. 109, 14652–14656 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grillner S., Biological pattern generation: The cellular and computational logic of networks in motion. Neuron 52, 751–766 (2006). [DOI] [PubMed] [Google Scholar]
- 50.McCrea D. A., Rybak I. A., Organization of mammalian locomotor rhythm and pattern generation. Brain Res. Rev. 57, 134–146 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hultborn H., State-dependent modulation of sensory feedback. J. Physiol. 533, 5–13 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blumberg M. S., Coleman C. M., Gerth A. I., McMurray B., Spatiotemporal structure of REM sleep twitching reveals developmental origins of motor synergies. Curr. Biol. 23, 2100–2109 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Donovan M. J., Wenner P., Chub N., Tabak J., Rinzel J., Mechanisms of spontaneous activity in the developing spinal cord and their relevance to locomotion. Ann. N. Y. Acad. Sci. 860, 130–141 (1998). [DOI] [PubMed] [Google Scholar]
- 54.Marder E., O’Leary T., Shruti S., Neuromodulation of circuits with variable parameters: Single neurons and small circuits reveal principles of state-dependent and robust neuromodulation. Annu. Rev. Neurosci. 37, 329–346 (2014). [DOI] [PubMed] [Google Scholar]
- 55.Berardi N., Sale A., Maffei L., Brain structural and functional development: Genetics and experience. Dev. Med. Child Neurol. 57 (suppl. 2), 4–9 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Arber S., Motor circuits in action: Specification, connectivity, and function. Neuron 74, 975–989 (2012). [DOI] [PubMed] [Google Scholar]
- 57.Iizuka M., Kiehn O., Kudo N., Development in neonatal rats of the sensory resetting of the locomotor rhythm induced by NMDA and 5-HT. Exp. Brain Res. 114, 193–204 (1997). [DOI] [PubMed] [Google Scholar]
- 58.Oppenheim R. W., The neuroembryological study of behavior: Progress, problems, perspectives. Curr. Top. Dev. Biol. 17, 257–309 (1982). [DOI] [PubMed] [Google Scholar]
- 59.Grillner S., Wallén P., Innate versus learned movements—A false dichotomy? Prog. Brain Res. 143, 3–12 (2004). [DOI] [PubMed] [Google Scholar]
- 60.Novak I., et al. , Early, accurate diagnosis and early intervention in cerebral palsy: Advances in diagnosis and treatment. JAMA Pediatr. 171, 897–907 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified source data of all figures are deposited at https://zenodo.org/record/3666098.