Abstract
Although insulin analogs have markedly improved glycemic control for people with diabetes, glycemic excursions still cause major health problems and complications. In particular, the narrow therapeutic window of current insulin therapy makes it extremely difficult to maintain normoglycemia without risking severe hypoglycemia. Currently, there are no FDA-approved insulin therapeutics whose bioactivity is regulated by blood glucose levels. This review discusses recent progress on developing glucose-responsive insulin (GRI) bioconjugates without the need of exogenous matrices. Through this approach, tremendous efforts have been made over the years to demonstrate the promise of better glycemic control and reduced risk of hypoglycemia. Last, we discuss future directions of GRI development with a goal to maximize the glucose responsiveness.
Keywords: bioconjugate, glucose-responsive insulin, glycemic control, hypoglycemia
Despite considerable efforts to develop advanced insulin therapeutics over many years, the treatment of type 1 diabetes (T1D) remains challenging because it relies on both insulin therapies and patient self-regulation. The design and creation of fast-acting insulin (Humalog and Novolog) and long-lasting insulin (Lantus, Levemir and Tresiba) allows people with T1D to achieve much better glycemic control.1 Nonetheless, current insulin therapy still causes hypoglycemia if overdosed. This is a major risk because hypoglycemia can lead to acute complications such as loss of consciousness, coma, and death.2 Fear of these dreaded complications leads to insufficient insulin administration and inadequately treated diabetes. The resulting chronic hyperglycemia causes complications such as blindness, kidney failure, and heart disease.3 To address this unmet clinical need, the concept of glucose-responsive insulin (GRI) delivery was introduced in 1979 by Brownlee and Cerami.4 Ideally, a GRI is a drug that precisely delivers the required amount of insulin and, by responding to the circulating local glucose levels, maintains normal glucose levels throughout the day.5 There are three distinct clinical advantages of injecting such insulin whose activity is modulated in vivo by circulating blood glucose levels. First, errors in underdosing insulin would be markedly reduced because GRI would be released from a depot when blood glucose levels are high. Second, errors in overdosing insulin would be markedly reduced because GRI would be inactivated when blood glucose levels start to decline, thus reducing the risk of hypoglycemia. Finally, by reducing the degree of both hyper- and hypoglycemia, GRI could markedly reduce the magnitude and frequency of glycemic excursions.
Currently, most GRI systems being explored utilize polymeric biomaterials that embed insulin in a matrix that contains glucose-responsive elements, such as glucose-binding proteins (GBPs), glucose oxidase (GOx), and phenylboronic acids (PBAs), which can regulate the insulin release rate via changes in the matrix structure, polymer degradation, or glucose binding competition.6 GBPs are a group of natural carbohydrate-binding proteins. The most commonly used GBP for insulin delivery is concanavalin A (ConA) due to its four high-affinity glucose binding sites. Therefore, ConA has been used as a crosslinker for glucose-containing biopolymer formation.7 At high glucose levels, free glucose competes for the binding sites, breaks the polymer linkage and leads to insulin release. Glucose oxidase (GOx) is an enzyme that catalyzes the conversion of glucose into gluconic acid while generating hydrogen peroxide. By integrating GOx into a polymer matrix, acid-sensitive groups can produce a glucose-triggered response (volume change or matrix degradation), leading to insulin release from the matrices. Recently, Yu et al utilized the local hypoxia environment created by the GOx enzymatic conversion to achieve glucose responsiveness8 and broadened the application scope of GOx. Last but not least, PBAs, unlike the previous proteins, are a class of small molecules that bind 1,2- or 1,3-cis diols to form a negatively charged complex. PBAs can bind to glucose as well as other sugar sources; however, glucose is by far the most prevalent physiological diol. To achieve GRI delivery, PBAs are often incorporated into the cross-linked polymeric matrix, which swells due to the negatively charged complex with glucose and releases insulin.9 All these delivery systems require either matrices or administration devices (eg, microneedles) to encapsulate insulin that would be released upon elevated glucose conditions.
In contrast to the encapsulation approaches, insulin can be modified with other motifs to achieve glucose-responsive properties without the need of exogenous matrices or materials. However, due to the small size of insulin, only a limited amount of bioconjugation approaches are available without disrupting the bioactivity.10 For this reason, there are fewer reported examples of GRI through bioconjugation approaches than through matrix-based approaches. In this review, we will discuss four approaches to achieve glucose responsiveness using modified insulin molecules without the need of exogenous matrices. In the end, we will discuss future directions for developing GRI molecules using unexplored mechanistic designs that may push the field further.
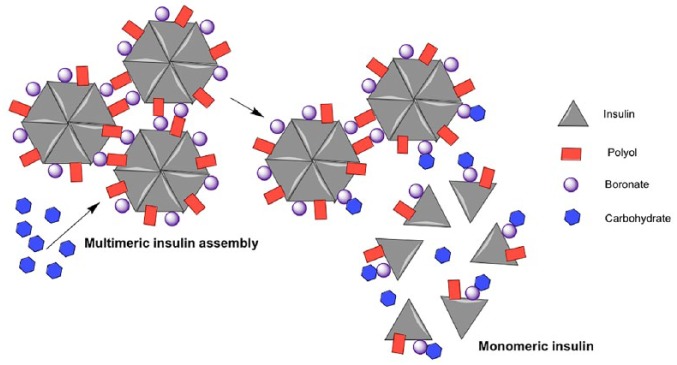
Multimeric Insulin Assembly Through PBA-Diol Crosslinking
Researchers from Novo Nordisk published a seminal work in 2005 to use insulin bioconjugation to achieve glucose-responsive properties.11 Insulin intrinsically forms hexamers, which consist of three sets of dimers coordinated by two zinc ions. This work utilized the reversible binding between PBA and diols to control insulin multimeric assembly (Figure 1). Specifically, both the PBA group and diol moiety (from glucosamine) were conjugated to the B29 lysine of insulin. The B29 conjugated groups do not interfere with insulin’s hexamer formation and bioactivity but further promote multiassembly of hexamers as demonstrated by size-exclusion chromatography. This assembly can be dissociated using 50 mM sorbitol to yield insulin hexamers. Under the same condition, glucose cannot dissociate the multiassembly. Since sorbitol binds to PBA 100-fold stronger than glucose (Kd: 0.1 mM vs 10 mM), it is likely that the insulin multiassembly is too strong to dissociate for glucose. The investigators further showed that 100 mM glucose can dissociate a weaker insulin assembly that is formed by a 1:1 mixture of the bioconjugated insulin and unmodified insulin. This result indicated that this system can be glucose responsive if the interactions between hexamers are optimized. Though 100 mM (1800 mg/dl) glucose is much higher than the physiological glucose range, this work was the first demonstration of a GRI design without the need of external matrices, paving the way for subsequent GRI designs.
Figure 1.
Schematic representation of multimeric insulin assembly through PBA-diol crosslinking.
PBA-Modified Aliphatic Insulin Conjugate
The Anderson and Langer lab at MIT reported the use of a PBA-modified aliphatic insulin molecule to achieve glucose responsiveness.12 This concept is built upon the clinical success of insulin detemir as a long-acting insulin. The myristic group on B29 lysine from insulin detemir enables the insulin to bind to and be sequestered by circulating serum albumin. As a result, insulin detemir has an onset of action of 1-2 hours, with a peak action at 3-9 hours and duration lasting up to 20-24 hours.13 The interaction between the aliphatic chain and serum albumin is due to hydrophobic interactions and it was therefore proposed that the terminal insertion of PBA may regulate this binding in a glucose-dependent manner (Figure 2). In a no glucose situation, PBA remains uncharged and the interaction between insulin and albumin is strong. When glucose concentration increases, PBA becomes negatively charged, which then weakens the hydrophobic interaction and leads to insulin release. In this paper, Ins-PBA-F, a fluoro-PBA-modified aliphatic insulin conjugate, was demonstrated to respond faster to a glucose challenge compared to insulin detemir in vivo. Furthermore, compared to insulin detemir, an equal dose of Ins-PBA-F is less potent in normoglycemic range in healthy mice. These results demonstrated the in vivo glucose-responsive property of Ins-PBA-F. However, the investigators did not observe a glucose-dependent interaction between albumin and Ins-PBA-F; therefore, the molecular mechanism behind this glucose-responsive property is still unclear. Nonetheless, this work demonstrates insulin conjugates can achieve in vivo glucose responsiveness without the use of external matrices.
Figure 2.
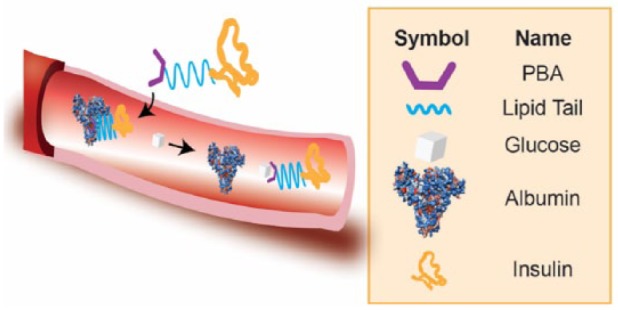
Schematic representation of PBA-modified aliphatic insulin conjugate.
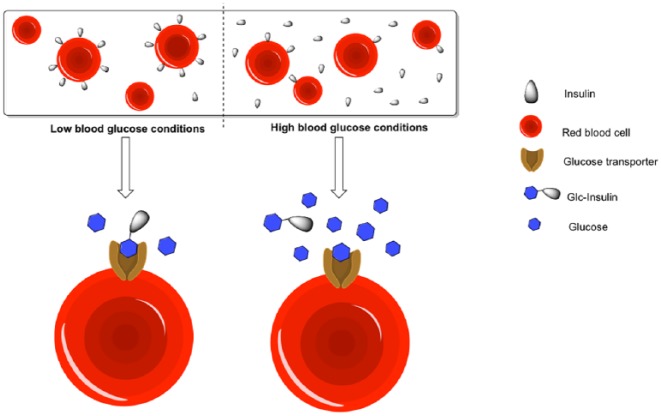
Red Blood Cell-Bound Insulin Conjugate
Recently, Wang et al utilized a new concept to achieve in vivo glucose responsiveness through red blood cells.14 The investigators showed that the glucose-modified insulin conjugate (Glc-Insulin) can bind to the glucose transporters on the surface of red blood cells in a glucose-dependent manner (Figure 3). To evaluate the in vivo performance, Glc-Insulin was mixed with red blood cells before intravenous injections. In streptozotocin-treated diabetic mice, serum insulin levels increased after the glucose challenge indicating a GRI release from the red blood cells. On the other hand, while Glc-Insulin injections led to hypoglycemia in healthy mice, the Glc-Insulin-red blood cell complexes did not cause hypoglycemia suggesting reduced insulin activity. Noted, intravenous injections are required in this system instead of the clinically relevant subcutaneous injections most likely due to the use of red blood cells. Nonetheless, this is an elegant demonstration of using endogenous cells to achieve glucose responsiveness.
Figure 3.
Schematic representation of red blood cell-bound insulin conjugate.
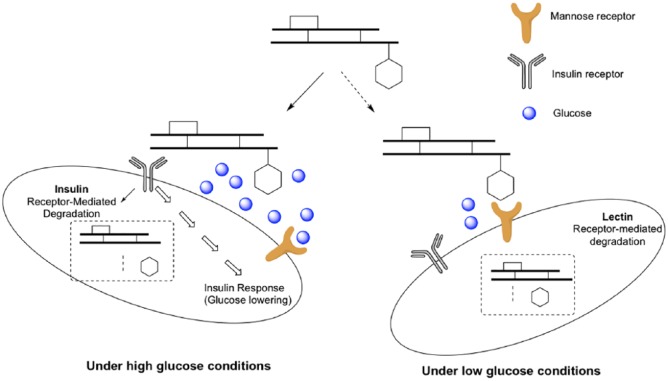
Glucose-Responsive Insulin Through Mannose Receptor Interactions
Researchers at Merck Research Laboratories recently published a series of GRI conjugates based on the glucose-dependent interaction between GRI and the mannose receptor C-type 1 (MR).15,16 In this design, native insulin was acylated on both the N-terminal of the A-chain and B29 lysine with mannose ligands to generate MK-2640 (Figure 4). Acylation on the N-terminal A-chain of insulin is unusual because this modification is well known to reduce insulin bioactivity.17 Indeed, MK-2640 has a 15-fold reduced binding affinity toward the insulin receptor (IR) compared to native insulin. In this case, MK-2640 binds to MR 2-fold stronger than IR so that the glucose-dependent binding property of MR can be used for glucose-responsive properties. Using a surface plasmon resonance assay, glucose has an IC50 of 8 mM (144 mg/dl) to disrupt the MK-2640/MR interaction. To confirm the in vivo glucose responsiveness, the Merck team performed glycemic clamp studies in dogs to measure the serum insulin levels at various blood glucose concentrations. While the concentration of native insulin remained the same in various glucose levels (not glucose-responsive), a glucose-dependent increase of serum MK-2640 levels was observed from 80 mg/dl to 280 mg/dl glucose levels. This is the first demonstration of glucose-dependent serum insulin levels for GRI conjugates in animal models of diabetes. Merck recently published clinical evaluations of MK-2640 in healthy and T1D volunteers.18 Unlike the preclinical result, the authors were not able to demonstrate a glucose-dependent insulin clearance. A significant glucose-dependent increase of glucose infusion rate was observed, which is the first demonstration in humans.
Figure 4.
Schematic representation of GRI through mannose receptor interactions.
Future Directions
The development of insulin has come a long way since its discovery in 1921. Yet, development of novel insulin molecules continues in multiple directions.19 First, efforts to find alternative routes, such as oral administration, for insulin delivery are persistently explored—although these efforts have had little clinical success. Second, with the clinical advance of closed-loop insulin delivery systems, ultrafast-acting insulin formulations are being developed to maximize the performance of such systems. Last but not least, GRI delivery is an area of interest for researchers in both academic and industry settings. Most of the efforts are in creating polymeric matrices that can respond to elevated glucose levels and trigger insulin release. This review focuses on the use of insulin bioconjugates without exogenous matrices to achieve glucose responsiveness. Four independent examples were discussed that utilized either insulin self-assembly properties or endogenous proteins to achieve glucose-dependent properties. The aim of the GRI technologies is to offer better glycemic control without the increased risk of hypoglycemia. While still in the early discovery phase, these examples pave the road for future novel GRI bioconjugates. Currently, the Merck candidate MK-2640 is the most advanced case with phase 1 trial results published in 2018.18 However, the promising preclinical work does not fully translate into humans as only pharmacodynamic (PD) glucose responsiveness (indicated by glucose infusion rate) was observed but not pharmacokinetic (PK) glucose responsiveness (indicated by insulin clearance). It was further noted that the clearance of MK-2640 was saturated or nearly so at relevant plasma insulin concentrations, which may contribute to poor clinical translation of glucose-responsive PK. The result suggests that GRI bioconjugates designed through a second partner (mannose receptor, albumin, red blood cells or other future mechanisms) need to be carefully evaluated for clinical translation.
The major challenge to design novel GRI bioconjugates is the glucose sensing mechanism. PBA is currently the only nonsugar small molecule that has been used for GRI designs. While the Kd of PBA and glucose interaction fits well for serum glucose levels, the poor selectivity for PBA (10-fold stronger affinity to fructose than glucose) raises concerns. One future direction to address the selectivity concern is to use bidentate PBA as a selective glucose sensor (Figure 5A).20,21 PBAs typically tend to bind to diols from furanose forms due to the preferred geometry, which results in stronger affinity toward fructose. However, the bidentate PBAs can recognize two sets of diols from pyranose forms and therefore provide high selectivity for glucose over fructose. Another strategy is to use de novo glucose sensors to generate GRIs. For example, the lab of Anthony Davis developed a series of synthetic lectin molecules that can specifically bind glucose but no other sugars (Figure 5B).22 These cage-like molecules can potentially be conjugated on insulin molecules for GRI development. Recently, Novo Nordisk licensed this technology from Ziylo Ltd, a start-up company spun out from the Davis lab, which further demonstrates pharma’s interest on GRI. There are also ongoing efforts to develop glucose-dependent chemical reactions. Recently, Jensen et al published a patent application on glucose-sensitive hydrazone reactions (Glucose-sensitive peptide hormones, WO2018115462A1). The investigators observed a glucose-responsive hydrazone dissociation property. Such hydrazone linkers may be conjugated to insulin molecule for GRI designs. Another challenge for GRI bioconjugates is that it may be extremely difficult to reach the same performance as pancreatic beta cells (7-10-fold increased insulin secretion in response to 2-fold glucose increase). Indeed, the intrinsic limitation of a single molecule is that it’s hard to mimic the complicated cellular signaling in beta cells. One future direction to maximize the GRI performance is to combine orthogonal designs to achieve additive or even cooperative effects to mimic the response of beta cells. The GRI bioconjugates can further be coupled with the use of glucose-responsive matrices or closed-loop insulin delivery systems for optimal results.
Figure 5.
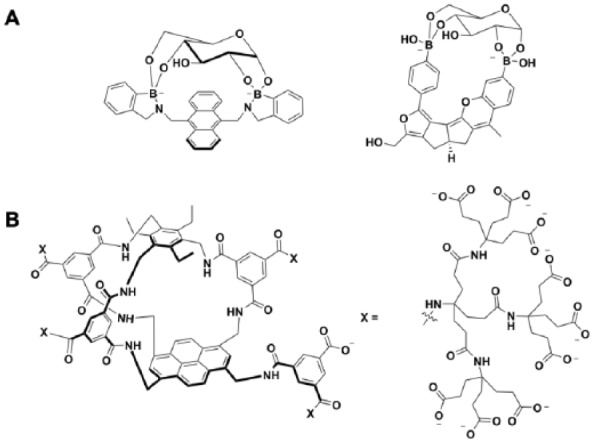
Selective glucose binders. (A) Bidentate PBAs can selectively bind pyranoses such as glucose. (B) Synthetic lectins developed by the lab of Anthony Davis.
In summary, significant progress was made to validate the concept of GRI bioconjugates. The promise for better glycemic control, reduced risk of hypoglycemia, and increased quality of life attracts research efforts to develop GRI technologies. Our increasing knowledge about novel insulin molecules and the insulin/insulin receptor interactions in the past few years may provide new areas of research toward GRI designs.23-25 Nearly a century after the discovery of insulin, the pursuit for the perfect insulin is still ongoing.
Acknowledgments
We thank Drs Sanjoy Dutta, Simon Fisher, James Mu, Jonathan Rosen, Nobert Tennagels, and Matthias Urmann for helpful discussion on the topic of glucose-responsive insulin.
Footnotes
Abbreviations: Con A, concanavalin A; GBP, glucose-binding protein; GOx, glucose oxidase; GRI, glucose-responsive insulin; IR, insulin receptor; MR, mannose receptor C-type 1; PBA, phenylboronic acid; PD, pharmacodynamics; PK, pharmacokinetics; T1D, type 1 diabetes.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: DHCC is a scientific consultant for Merck & Co and receives funding supports from Sanofi.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: JDRF Strategic Research Agreement (3-SRA-2015-120-Q-R), American Diabetes Association Junior Faculty Development Award (1-16-JDF-018), and Driving Out Diabetes Initiative at University of Utah to DHCC.
ORCID iD: Danny Hung-Chieh Chou  https://orcid.org/0000-0001-9110-614X
https://orcid.org/0000-0001-9110-614X
References
- 1. Berenson DF, Weiss AR, Wan ZL, Weiss MA. Insulin analogs for the treatment of diabetes mellitus: therapeutic applications of protein engineering. Ann N Y Acad Sci. 2011;1243:E40-E54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hypoglycemia: a pitfall of insulin therapy. West J Med. 1983;139:688-695. [PMC free article] [PubMed] [Google Scholar]
- 3. Brownlee M, Hirsch IB. Glycemic variability: a hemoglobin A1c-independent risk factor for diabetic complications. JAMA. 2006;295:1707-1708. [DOI] [PubMed] [Google Scholar]
- 4. Brownlee M, Cerami A. A glucose-controlled insulin-delivery system: semisynthetic insulin bound to lectin. Science. 1979;206:1190-1191. [DOI] [PubMed] [Google Scholar]
- 5. Bakh NA, Cortinas AB, Weiss MA, et al. Glucose-responsive insulin by molecular and physical design. Nat Chem. 2017;9:937. [DOI] [PubMed] [Google Scholar]
- 6. Rege NK, Phillips NF, Weiss MA. Development of glucose-responsive “smart” insulin systems. Curr Opin Endocrinol Diabetes Obes. 2017;24:267-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zion TC, Zarur A, Ying JY. Stimuli-responsive systems for controlled drug delivery. US Patent US7531191 B2. 2009. [Google Scholar]
- 8. Yu J, Zhang Y, Ye Y, et al. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc Natl Acad Sci USA. 2015;112:8260-8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matsumoto A, Ishii T, Nishida J, Matsumoto H, Kataoka K, Miyahara Y. A synthetic approach toward a self-regulated insulin delivery system. Angew Chem. 2012;51:2124-2128. [DOI] [PubMed] [Google Scholar]
- 10. Mayer JP, Zhang F, DiMarchi RD. Insulin structure and function. Biopolymers. 2007;88:687-713. [DOI] [PubMed] [Google Scholar]
- 11. Hoeg-Jensen T, Havelund S, Nielsen PK, Markussen J. Reversible insulin self-assembly under carbohydrate control. J Am Chem Soc. 2005;127:6158-6159. [DOI] [PubMed] [Google Scholar]
- 12. Chou DH, Webber MJ, Tang BC, et al. Glucose-responsive insulin activity by covalent modification with aliphatic phenylboronic acid conjugates. Proc Natl Acad Sci USA. 2015;112:2401-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DeWitt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: scientific review. JAMA. 2003;289:2254-2264. [DOI] [PubMed] [Google Scholar]
- 14. Wang C, Ye Y, Sun W, et al. Red blood cells for glucose-responsive insulin delivery. Adv Mater. 2017;29. [DOI] [PubMed] [Google Scholar]
- 15. Kaarsholm NC, Lin S, Yan L, et al. Engineering glucose responsiveness into insulin. Diabetes. 2018;67:299-308. [DOI] [PubMed] [Google Scholar]
- 16. Yang R, Wu M, Lin S, et al. A glucose-responsive insulin therapy protects animals against hypoglycemia. JCI Insight. 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen D, Disotuar MM, Xiong X, Wang Y, Chou DH-C. Selective N-terminal functionalization of native peptides and proteins. Chem Sci. 2017;8:2717-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krug AW, Visser SAG, Tsai K, et al. Clinical evaluation of MK-2640: an insulin analog with glucose-responsive properties. Clin Pharmacol Ther. 2019;105:417-425. [DOI] [PubMed] [Google Scholar]
- 19. Zaykov AN, Mayer JP, DiMarchi RD. Pursuit of a perfect insulin. Nat Rev Drug Discov. 2016;15:425. [DOI] [PubMed] [Google Scholar]
- 20. James TD, Sandanayake KRAS, Shinkai S. A glucose-selective molecular fluorescence sensor. Angew Chem Int Edit. 1994;33:2207-2209. [Google Scholar]
- 21. Yang W, He H, Drueckhammer DG. Computer-guided design in molecular recognition: design and synthesis of a glucopyranose receptor this work was supported by the National Institutes of Health (grant DK5523402). Angewandte Chemie. 2001;40:1714-1718. [PubMed] [Google Scholar]
- 22. Ke C, Destecroix H, Crump MP, Davis AP. A simple and accessible synthetic lectin for glucose recognition and sensing. Nat Chem. 2012;4:718-723. [DOI] [PubMed] [Google Scholar]
- 23. Menting JG, Whittaker J, Margetts MB, et al. How insulin engages its primary binding site on the insulin receptor. Nature. 2013;493:241-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Menting JG, Yang Y, Chan SJ, et al. Protective hinge in insulin opens to enable its receptor engagement. Proc Natl Acad Sci USA. 2014;111:E3395-E3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Menting JG, Gajewiak J, MacRaild CA, et al. A minimized human insulin-receptor-binding motif revealed in a Conus geographus venom insulin. Nat Struct Mol Biol. 2016;23:916-920. [DOI] [PubMed] [Google Scholar]