Traditional continuous glucose monitoring (CGM) systems require multiple, user-conducted sensor calibrations daily to account for the drift in sensor performance.1 The emerging of factory-calibrated CGM systems such as Abbott FreeStyle Libre 2 (FSL2)2 and Dexcom G6 (G6) CGM3 eliminates the need for user calibration and benefits patients by removing the inconvenience and taking away a significant source of error from sensor calibration.1 Factory calibration shifts the burden to manufacturers to improve sensor stability and reduce process variability to achieve consistent individual sensor behavior.4 During the first day of operation, CGM sensors are also susceptible to the host immune reaction to insertion-induced wound, while the sensors are still stabilizing from a packaging state to the subcutaneous environment.5 Past studies have shown increased mean absolute relative difference (MARD) during the first day of sensor operation, which improves over wear duration.3
This study compares the performance of FSL2 and G6 CGM during the first day of sensor operation. The study compared the performance of each system to venous plasma glucose reference measurements. Twenty-five subjects at one clinical research site in the United States wore one FSL2 sensor on the back of the upper arm and one G6 sensor on the abdomen. Both sensors were used with no additional manual calibrations. Subjects underwent venous Yellow Spring Instrument 2300 Stat Plus Glucose Analyzer (YSI) sampling every 15 minutes for a total of 8 hours.
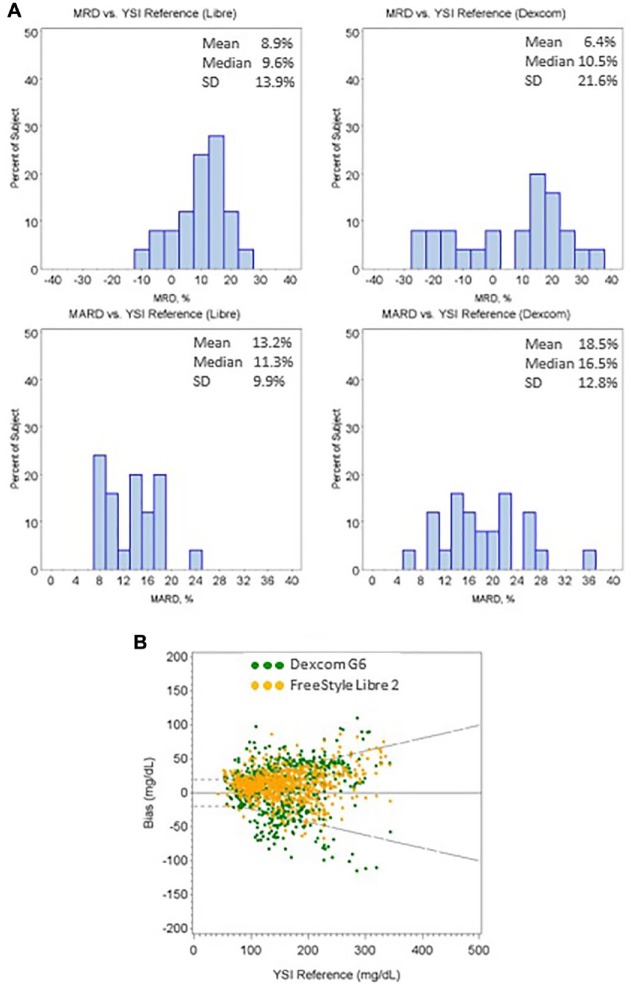
The study showed that 64.3% of G6 results and 84.8% of FSL2 results are within the 20%/20 mg/dL, while 5.3% of G6 results and 1.2% of FSL2 results are outside of 40%/40 mg/dL from the YSI reference. Figure 1(a) shows the histograms of mean relative difference (MRD) and MARD of individual sensor from both CGM systems. G6 shows 18.5% MARD (6.4% MRD) while FSL2 has 13.2% MARD (8.9% MRD). Sensors from G6 showed wider spread in both MARD and MRD compared to sensors from FSL2. Figure 1(b) shows the system agreement plot between the two systems against YSI reference.
Figure 1.
(a) Histograms of individual sensor MRD and MARD of both FreeStyle Libre 2 and Dexcom G6 CGM systems; (b) The Bland–Altman difference plot of the two CGM systems versus the YSI reference.
CGM, continuous glucose monitoring; MARD, mean absolute relative difference; MRD, mean relative difference.
Dash lines are the ±20%/±20 mg/dL limits.
The performance of CGM sensors during first day after implant could be challenged due to host immune reaction to the sensor insertion. It is further complicated for factory-calibrated CGM systems where confirmatory fingerstick test is not required. G6 system has indicated 87.8% results within 20%/20 mg/dL from reference and 11.5% MARD in the pivotal study3 from first day of sensor wear; it is, however, showing significantly worse results in this study when evaluated immediately following insertion. Measurement noise resulting from host immune reaction is difficult to be filtered through advanced algorithm; therefore, proper sensor insertion is critical to ensure the accuracy of measurement during the early insertion period. The commercial G6 system uses an automatic sensor applicator supported by a clinical study with similar sample size6 while the pivotal study used a manual applicator.3 It is not clear whether the new applicator contributes to the higher number of G6 sensors experiencing early measurement noise.
These results significantly differ from the pivotal study. The observed difference in individual sensor MARDs and MRDs from G6 could potentially be caused by the variability in immune response, potentially due to automatic applicator or manufacturing process where only a limited number of calibration codes are available to a large batch of sensors. New studies that are required to identify whether this is only an early-phase problem or could last well beyond the first day are vital to patient safety for using the factory-calibrated system.
Acknowledgments
We are deeply indebted to the research participants. Our sincere thanks to all the staff of Clinical Trials of Texas.
Footnotes
Declaration of Conflicting Interests: The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by Abbott Diabetes Care.
References
- 1. Vaddiraju S, Burgess DJ, Tomazos I, Jain FC, Papadimitrakopoulos F. Technologies for continuous glucose monitoring: current problems and future promises. J Diabetes Sci Technol. 2010;4(6):1540-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016;388(10057):2254-2263. [DOI] [PubMed] [Google Scholar]
- 3. Wadwa RP, Laffel LM, Shah VN, Garg SK. Accuracy of a factory-calibrated, real-time continuous glucose monitoring system during 10 days of use in youth and adults with diabetes. Diabetes Technol Ther. 2018;20(6):395-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Udo H, Budiman ES. Factory-calibrated continuous glucose sensors: the science behind the technology. Diabetes Technol Ther. 2017;19(S2):S44-S50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Helton KL, Ratner BD, Wisniewski NA. Biomechanics of the sensor-tissue interface-effects of motion, pressure, and design on sensor performance and the foreign body response-part I: theoretical framework. J Diabetes Sci Technol. 2011;5(3):632-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shah VN, Laffel LM, Wadwa RP, Garg SK. Performance of a factory-calibrated real-time continuous glucose monitoring system utilizing an automated sensor applicator. Diabetes Technol Ther. 2018;20(6):429-433. [DOI] [PMC free article] [PubMed] [Google Scholar]