Abstract
Insulin infusion pump, continuous glucose monitoring (CGM), and insulin infusion set (IIS) have been developed to be increasingly feasible for people with type 1 diabetes (T1D). Several recently approved CGMs are transitioning from 7-day to 10-day wear time without the need for fingerprick recalibration. Nevertheless, studies and improvements on IIS, a critical part of insulin pump therapy, have been limited. In particular, the recommended wear time of IIS is still 2-3 days, which can hardly match the current duration of CGM for potential closed-loop system development. It is generally believed that both the inserted catheter and the subsequent infused insulin drug could induce particular subcutaneous tissue response and skin-related complications at the infusion site. In certain cases, poor glycaemic control, increased risk of hypoglycemia, and serious cosmetic impact on people with diabetes were observed. Skin complication has also been attributed as an important factor resulting users to discontinue insulin pump therapy. This article provides the rare systematic review of IIS induced subcutaneous tissue responses and skin complications, including the impacts from the inserted catheters, the subcutaneous infused insulin, and the adhesive or tape used to immobilize the catheter. The FDA’s recommendation for the frequency of IIS change was further discussed. Future studies on this topic are required to further understand the IIS-related problems, and future strategies could be developed accordingly to significantly reduce the incidence of these problems, extend the wear time, and increase the acceptance of insulin pump based therapy.
Keywords: continuous subcutaneous insulin infusion, infusion catheter, insulin infusion set, skin complications, type 1 diabetes, tissue response
Type 1 diabetes (T1D), a disease characterized by a complete deficiency in insulin resulting from autoimmune destruction of pancreatic beta cells, affects approximately 20 million people worldwide, and this number keeps on increasing roughly 3% each year.1-3 For people with T1D, insulin therapy has been demonstrated as an effective pharmaceutical treatment option to lower blood glucose.3,4 To achieve superior glycemic control, replacement therapy that mimics the insulin secretion profile from healthy people is highly desired yet hardly achieved. Orally delivered insulin could closely mimic the physiological path of pancreatic insulin and significantly enhance patient compliance with its ease of administration.5,6 However, oral delivery of insulin still faces a major challenge due to various gastrointestinal barriers to drug absorption.6 Although numerous strategies have been developed to overcome these barriers and improve the stability and bioavailability of oral insulin.7-9 Unfortunately, no commercial oral insulin products are yet available to address the clinical hurdles.9 Up to now, clinical insulin therapy is available as an injectable formulation which can be administered via multiple daily injections (MDI) and continuous subcutaneous insulin infusion (CSII).10,11 Compared with the painful and repetitive MDI, CSII shows the advantages including better glycemic control, a lower dose of administered insulin and improved health-related quality of life and thus has been recommended by national guidelines as a therapeutic option.12-15
As an emerging type of CSII, closed-loop CSII systems have been recently introduced where a patient will wear an infusion set linked to a pump for insulin delivery and a continuous glucose sensor inserted at a second site for glycemic monitoring. In the past several decades, many sophisticated and advanced continuous glucose monitors (CGMs) have been developed, which can continuously capture blood glucose fluctuations and therefore enable complete tracking of blood glucose trends over time.16-20 Many CGMs have been approved to use for up to 3-7 days postimplantation but constant finger-prick was required to periodically recalibrate the sensor.21-23 Recently (September 27, 2017), Food and Drug Administration (FDA) approved the FreeStyle Libre Flash system from Abbott as a stand-alone glucose monitoring device, which can be used for up to 10 days without requiring fingerstick blood calibrations.24 Soon afterward (March 27, 2018), Dexcom G6 CGM, a head-to-head competing technology from Dexcom was also approved by FDA, which can be worn for up to 10 days with zero fingerstick blood testing.25
For all types of insulin pumps (except for OmniPod), an insulin infusion set (IIS) is indispensable to connect the insulin reservoir in the pump with the infusion site in the people with diabetes.26,27 Typically, IIS is based on thin, soft, and flexible plastic tubing. One end of the tubing is steel or Teflon catheter, which is inserted through the skin with a given angle and remains in the subcutaneous (SC) tissue for insulin delivery. The other end is connected to the insulin reservoir of the pump via a Luer-lock or a proprietary connector. Thus, insulin from the reservoir can be pumped through the tubing and the inserted catheter into the SC tissue to control blood glucose according to patients’ demand.
IIS is a key component of an insulin pump system and its failure has been frequently observed in clinical practice. Research and/or publications on the IIS are quite limited, particularly when compared with the numerous studies on the CGMs.27,28 Majority discussions on the IIS actually appeared on internet blogs and forums, where patients considered IIS as a major concern when using the insulin pumps.29,30 Common issues occurred to adult and adolescent patients with IIS include catheter occlusions or kinking,4,31 loosened adhesives,32 infections at the infusion sites,33 lipohypertrophy,31,34 and local skin reactions such as redness, swelling, itching, and so on.35,36 These potential complications can be generally categorized into infusion set problems such as kinking and blockage, and infusion site problems such as skin-related complications.31 In the last three decades, the infusion set problems have been well investigated and improved thanks to the improvements in contemporary insulin pump technology and increasing options in IIS, such as various lengths, materials (steel or Teflon), diameters, and designs to meet the individual patient requirements.27 Nevertheless, little progress has been made in understanding and resolving the infusion site problems. To avoid skin-related complications and ensure a stable and reliable efficacy of the infused insulin, the infusion catheter and infusion site still have to be changed every 2-3 days (the same recommended duration of use since 1983).37-39
It is widely recognized that both the inserted catheter and the subsequent infused insulin drug could induce particular SC tissue response and skin-related complications at the infusion site.35,40 Some cases may influence glycaemic control and increase the risk of hypoglycemia as well as have a cosmetic impact on the patients.36,37,39 Moreover, some studies indicate that skin complication is an important factor resulting users to discontinue insulin pump therapy.27,35 To the best of our knowledge, no systematic review of the SC tissue responses in patients with diabetes has been reported to date, which will be the main focus of this article. We summarize the published literature about inflammatory response and related skin complications at the catheter insertion site based on three major factors, the catheter, the infused insulin, and the adhesive or tape to immobilize the catheter. We further discuss the FDA’s recommendation for the frequency of IIS change. Last, we provide perspective on future research on insulin infusion catheters that should be focused on exploring the specific tissue response at the catheter insertion site and strategies prolonging the catheter wear time.
Inflammatory Response Caused by the Catheter
SC tissue is an important target for drug delivery. For people with diabetes treated either by MDI or CSII, SC tissue of different anatomical regions, including the arm, abdomen, thigh, and buttocks, can be used for insulin adminstration.41-44 In particular, the abdomen is recommended as the preferred insertion site for the IIS since this region is not only convenient and comfortable for catheter insertion but also enables rapid insulin absorption.44,45 Among several clinical studies where patients received CSII, inflammation at the infusion sites has been frequently observed. For example, Mecklenburg et al reported that the inflammation at the infusion site was the main reason (53%) for discontinuing insulin pump therapy reported by adult patients on CSII.46 Clinical data from a group of 177 patients followed for up to 5 years showed that the most common reason reported by patients for terminating CSII therapy was discomfort, irritation, or inflammation at the infusion site.47 In a contrastive study, 48% (24 of 50) of people with diabetes treated with CSII reported inflammation at the infusion site while it was only 12% (6 of 50) in daily insulin injecting patients.48 According to the self-reported data from 116 CSII-treated patients for 518 patient years, a total of 134 cases of SC inflammation at the infusion site were reported.49 It should be noted that all these clinical data were symptom-based and there is limited research on why inflammation was observed following the catheter insertion.
In general, tissue exposure to biomaterial implants, for example, insulin infusion catheters, triggers a foreign body response, which is a stepwise process consisting of inflammatory events, wound healing and, if not resolved, end-stage tissue fibrosis and scarring.50,51 For insulin therapy, insertion of an infusion catheter into the SC tissue will inevitably introduce a local trauma. This trauma can induce an acute inflammatory response and subsequently affect local blood flow and metabolism in the SC tissue.52,53 Moreover, in contrast to inserting a needle for seconds into the skin in MDI therapy, an inserted infusion catheter will indwell in SC tissue for several days. Tissue reaction during the catheter indwelling period has rarely been studied, but it is similar to an implanted CGM that has been numerously studied,54-56 a sustained local irritation occurs and further causes increased production of proinflammatory cytokines in the interstitial fluid and possible foreign body response. In the case of CGMs, tissue reactions to the implanted sensors (ie, inflammation, wound healing, and fibrosis) can lead to the migration and accumulation of inflammatory cells at the sensor implantation site, which creates a metabolic barrier to retard glucose diffusion to the implanted sensor and decreases glucose availability, thereby compromising the accuracy and lifespan of implanted sensor.57,58 Similarly for the implanted catheter, one would expect, again few scientific evidence has been reported, that the inflammation-induced alteration in the local SC microenvironment after the catheter insertion could affect the insulin absorption and compromise the wear time of the infusion catheter.
To further understand the tissue response after SC catheter insertion, histological information with skin biopsies from people with diabetes will be extremely helpful, though obtaining tissue samples from pump users could be difficult. Furthermore, future histological studies on animals receiving the catheters are required to obtain more insights into the SC inflammation issue.
Infections and Other Complications Caused by the Catheter
In the early years of CSII therapy, infection at the infusion site was a common complication and always resulted in a termination of CSII.59,60 The high frequency of infections typically resulted from inadequate disinfection before catheter insertion and a low-frequency change of the IIS. The IIS indwells at the same site for several days and the insertion site beneath the adhesive is typically warm and humid. This provides an ideal condition for bacterial growth. Majority cases of infusion site infections were reported to be caused by Staphylococcus.61 Infections caused by other pathogens (eg, Streptococcus or Rhizomucor) have also been found.62,63 In clinical practice, most of these infections were mild and did not require antibiotic treatment. So far there were only two cases, extremely rare though, reporting serious systemic complications caused by IIS-related infections, including one incident of acute bacterial endocarditis64 and one incident of toxic-shock syndrome.65 In the past few years, with increasing hygienic precautions and improved training experience with insulin pumps, the skin infection has been less frequently observed in CSII therapy.
Other skin-related complications that frequently observed in insulin pump therapy involve both acute and chronic adverse effects. Commonly seen acute skin reactions include bruising, redness, swelling, and erythema, and typically occur when the catheter was kept at the same insertion site for over 2-3 days.37,66 A pilot investigation has examined the link of IIS-related events to the prolonged use of catheters beyond the recommended duration.66 It was found that the skin problems, such as itching, bruising, swelling, and pain, started to occur in a measurable amount on the 3rd day of catheter use and were reported to be significant by 40% patients on the 5th day of use. Recent data from a prospective randomized crossover study with 2×3-month observation periods with 22 T1D patients using a Teflon catheter showed that significantly more skin-related adverse events such as erythema, rash, pain, reddening, irritation, hemorrhage were observed with 4-day use than that with 2-day use.37
Unlike the acute adverse reaction where the inserted catheter is considered as a major cause, chronic side effects such as scars, nodules, and lipohypertrophy, are recognized to be associated with both the inserted catheter and the subsequent presence of insulin in the SC tissue.36,37,66 These chronic effects can be observed in both adults and children even though the catheter set is changed every 3 days following the manufacturer’s recommendation. An across-sectional study of dermatological side effects in 50 children and adolescents with T1D who were using CSII therapy for more than 6 months showed that 94% had scars with a diameter less than 3 mm, 66% had erythema not associated with nodules, 62% had SC nodules, and 42% had lipohypertrophy (Figure 1).35 A recent report based on 54 T1D patients on CSII therapy aged between 3 and 20 years also indicated the prevalence of these dermatological complications.67
Figure 1.
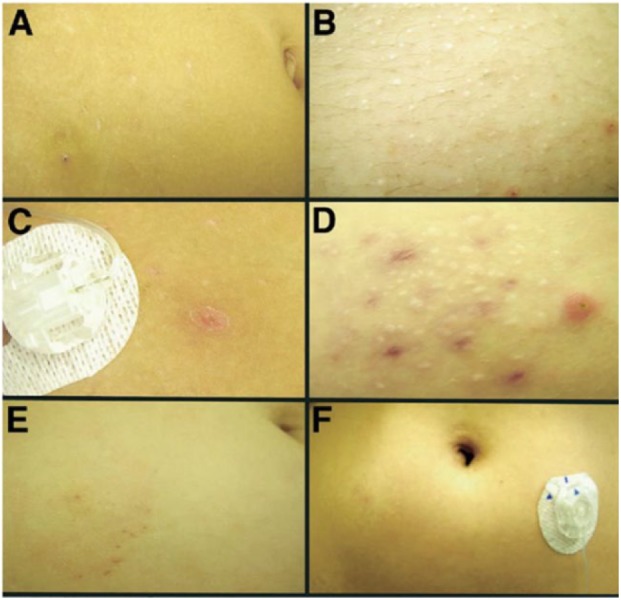
Photographs of skin-related complications at the CSII sites. (A) Hypopigmented scars and bruise. (B) Multiple hypopigmented scars and two areas of erythema. (C) Single erythematous nodule. (D) Multiple areas of erythema, erythematous nodules, and larger hypopigmented scars. (E) Epidermal abrasion caused by infusion set tape. (F) Bilateral lipohypertrophy. Reprinted with permission from Conwell et al,35 © 2008 by Elsevier Ltd.
As discussed above, the inserted catheter is a major cause of the skin problems despite being approved for the intended SC use. There is a variety of IISs currently available for patients to choose from; however, it is unclear whether changing catheter types will significantly resolve the complication issues. Some comparative studies on different types of IISs have been reported, while most of them focused on the impact of different catheter materials on the duration of usage,68 glucose control,69 and insulin absorption.70 To date, no systematic studies have been performed to evaluate the influence of different catheters on skin-related complications under controlled conditions. A recent review article summarized the available published studies on the different catheter materials in CSII and pointed out that no clear statement could be made on the pro or con for a steel versus a Teflon catheter when it comes to local skin reactions and insulin absorption at the infusion site.40
Complications Caused by the Infused Insulin
Both lipohypertrophy and lipoatrophy are common tissue response mainly associated with the SC bolus and continuous insulin infusion. Insulin-induced lipoatrophy presents as the loss of SC fat at the site of insulin injection and occurs more often in people with diabetes with MDI than CSII.33 Since the introduction of highly purified recombinant human insulin over recent years, lipoatrophy has been extremely rare in insulin pump-treated patients.35,71 Lipohypertrophy usually presents as soft dermal nodules within the normal surface epidermis with fat cell hypertrophy, which, however, remains the most commonly occurred cutaneous complication during CSII (Figure 1F). In an across-sectional study of dermatological side effects in 50 children and adolescents with T1D who were using CSII therapy for more than 6 months, it was found that the occurrence rate of lipohypertrophy at the infusion site was 42%.35 In another similar study of dermatological side effects and complications in preschool-age and school-age children, lipohypertrophy showed a high incidence during CSII (45% in preschool-age children and 47% in school-age children).36 According to a self-reported questionnaire completed by subjects with T1D under CSII, the average occurrence rate of lipohypertrophy at the infusion site was 26.1%, and this incidence became more often in those with a longer history of CSII therapy.31 Lipohypertrophic areas have been clinically found to be coincident with impaired insulin absorption and deteriorative glycemic control in patients with long-term insulin pump therapy.72,73
Complications Caused by Adhesives
For IIS systems, adhesives have been employed to immobilize the catheters on the skin. There is also an increasing practice on using tapes to immobilize infusion lines to the skin that connect the infusion catheter to the pump. These actions are based on the concerns that without fixing the infusion lines with tapes, any tug or movement of the infusion line can be directly transferred to the catheter, leading to loosened adhesives, skin irritation, redness, bruising around inserted site and insulin leakage through the skin trauma.67 Nevertheless, the extensive use of adhesives and tapes may cause allergies or contact dermatitis. This skin problem, although rare, has been reported during the CSII therapy.74,75 On a very rare occasion, the contact dermatitis persists despite changing the type of adhesives. This may be attributed to the different responses to adhesives among the pump users.
The Longevity Issue of IIS
It is well known that the FDA recommended the continuous use of IIS for only 2-3 days at the insertion site. However, in practice, this recommendation was originally developed based on anecdotal case reports. In 1983, the Centers for Disease Control and Prevention (CDC) reported a case study of an 11-year-old girl with T1D using CSII who frequently did not change the pump infusion site for 10 days, resulting in a Staphylococcus aureus abscess at the insertion site and toxic shock syndrome.65 At that time this concern had not been verified in any randomized controlled trial, and there was no specific procedural guideline available to minimize the risk of SC infections in CSII users. So FDA adopted CDC’s Guideline for Prevention of Intravascular Infections to formulate their “2-3 days” recommendation.76 Since then, both the catheter manufacturers and insulin manufacturers recommend changing infusion sets and infusion sites after 2-3 days of use to avoid skin and other undesired side effects in CSII therapy. This recommendation is still in use today although IIS has experienced great evolution in the last few decades.
Despite the “2-3 days” recommendation in place, many pump users do not adhere to it according to the self-reported questionnaires31 and diabetes forums.77,78 In practice, pump users often keep their IIS inserted for several days (3-5 days) or even longer (7-10 days). Typically, they do not change the IIS until the self-monitored blood glucose level starts to rise or visually adverse event starts to occur at the infusion site. There are also pump users reporting that they have to change the IIS more frequently (even daily or every 12 hours). The reason for such interindividual difference among patients is still not clear.
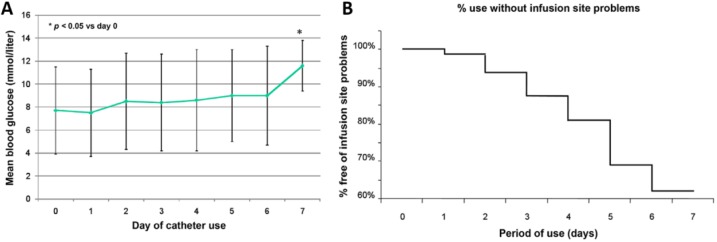
To our knowledge, investigation on the longevity of the inserted catheter is extremely limited. In a pilot study to assess the optimal frequency for changing catheters in insulin pump therapy, 12 patients with T1D were asked to continuously wear an IIS with a Teflon catheter for up to 5 days.66 The results indicated a consistent increase in mean daily blood glucose levels with the duration of catheter usage (that from 135 mg/dL on Day 1 to 162 mg/dL on Day 5). In addition, clinically relevant adverse events also started to occur during the first three days and their incidence increased further with longer use (Figure 2). The authors concluded that infusion catheters should only be used for 2-3 days to avoid adverse events and potential metabolic deterioration. In another similar study, a double-blind, randomized, crossover trial was conducted with 20 T1D patients using an IIS without change for up to 100 hours.39 The results indicated that from Day 2 to Day 5 of the IIS use, the daily average glucose level increased from 123 to 164 mg/dL, and the daily maximum glucose increased from 208 to 243 mg/dL. Deteriorated glycemic control was observed even after increasing the total daily insulin dose from 49 to 55 U. The authors suggested that the optimal duration of IIS use is 2 days. In a more recent web-based study with 243 participating adults with T1D, it was found that the fasting blood glucose levels increased with the duration of IIS use (from 126 mg/dL on Day 1 to 133 mg/dL on Day 3 to 147 mg/dL on Day 5).38
Figure 2.
(A) Mean daily blood glucose of the patients during the insertion of catheters. (B) Kaplan–Meyer curve for patients free from IIS treatment-induced adverse events. Reprinted with permission from Schmid et al,66 © 2010 by Diabetes Technology Society.
To further understand the longevity of inserted IIS and its relation to the observed deteriorated glycemic control or adverse reaction, the histological study should be followed, though obtaining samples could be difficult as previously discussed. In addition, IIS systems have experienced a major update on the tubing and catheter materials, and studies on the up-to-date IISs are desirable.
Conclusion
With fast-paced development of insulin pump and closed-loop infusion systems, the CGM and CSII will become more and more feasible for people with T1D in the near future. Several recently FDA-approved CGMs are transitioning from 7-day to 10-day wear without the need for recalibration through fingerpricks. However, as a critical part of insulin pump therapy, the studies on the IIS is still limited. Many IIS-related problems have been reported since the 1980s, while little progress has been made over the last three decades in understanding and solving these problems. In particular, the proper use of IIS in CSII therapy is still 2-3 days, which can hardly match the current duration of CGM for potential closed-loop system development. The investigation to develop IIS with longer duration of use is challenging but highly desirable. This will require further study to understand SC tissue response to the inserted catheter and major factors contributing to the short-term in vivo use (patient samples will be invaluable here). Based on this knowledge, future strategies could be developed to significantly reduce the incidence of IIS-related problems, extend the wear time, and increase the acceptance of insulin pump based therapy.
Footnotes
Abbreviations: CDC, Centers for Disease Control and Prevention; CGM, continuous glucose monitor; CSII, continuous subcutaneous insulin infusion; FDA, US Food and Drug Administration; IIS, insulin infusion set; MDI, multiple daily injections; SC, subcutaneous; T1D, type 1 diabetes.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (DP2DK111910), the Juvenile Diabetes Research Foundation (1-SRA-2018-620-S-B, 2-SRA-2017-429-S-B), and the National Science Foundation (DMR-1809229).
References
- 1. American Diabetes Association. Standards of medical care in diabetes—2016 abridged for primary care providers. Clin Diabetes. 2016;34:3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. Atlanta, GA: Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 3. Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383:69-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pickup JC. Insulin-pump therapy for type 1 diabetes mellitus. N Engl J Med. 2012;366:1616-1624. [DOI] [PubMed] [Google Scholar]
- 5. Williams SJ, Wang Q, MacGregor RR, et al. Adhesion of pancreatic beta cells to biopolymer films. Biopolymers. 2009;91:676-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nguyen TX, Huang L, Gauthier M, et al. Recent advances in liposome surface modification for oral drug delivery. Nanomedicine. 2016;11:1169-1185. [DOI] [PubMed] [Google Scholar]
- 7. Wang Q, Cheng H, Peng H, et al. Non-genetic engineering of cells for drug delivery and cell-based therapy. Adv Drug Deliv Rev. 2015;91:125-140. [DOI] [PubMed] [Google Scholar]
- 8. Gu Z, Aimetti AA, Wang Q, et al. Injectable nano-network for glucose-mediated insulin delivery. ACS Nano. 2013;7:4194-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Banerjee A, Ibsen K, Brown T, et al. Ionic liquids for oral insulin delivery. PNAS. 2018;115:7296-7301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Misso ML, Egberts KJ, Page M, et al. Cochrane review: continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Evid Based Child Health. 2010;5:1726-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoogma R, Hammond P, Gomis R, et al. Comparison of the effects of continuous subcutaneous insulin infusion (CSII) and NPH-based multiple daily insulin injections (MDI) on glycaemic control and quality of life: results of the 5-nations trial. Diabet Med. 2006;23:141-147. [DOI] [PubMed] [Google Scholar]
- 12. Bruttomesso D, Costa S, Baritussio A. Continuous subcutaneous insulin infusion (CSII) 30 years later: still the best option for insulin therapy. Diabetes Metab Res Rev. 2009;25:99-111. [DOI] [PubMed] [Google Scholar]
- 13. Monami M, Lamanna C, Marchionni N, et al. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in type 1 diabetes: a meta-analysis. Acta Diabetol. 2010;47:77-81. [DOI] [PubMed] [Google Scholar]
- 14. Pańkowska E, Błazik M, Dziechciarz P, et al. Continuous subcutaneous insulin infusion vs. multiple daily injections in children with type 1 diabetes: a systematic review and meta-analysis of randomized control trials. Pediatr Diabetes. 2009;10:52-58. [DOI] [PubMed] [Google Scholar]
- 15. Benkhadra K, Alahdab F, Tamhane SU, et al. Continuous subcutaneous insulin infusion versus multiple daily injections in individuals with type 1 diabetes: a systematic review and meta-analysis. Endocrine. 2017;55:77-84. [DOI] [PubMed] [Google Scholar]
- 16. Bode B, Battelino T. Continuous glucose monitoring. Int J Clin Pract. 2010;64:11-15. [DOI] [PubMed] [Google Scholar]
- 17. Tamborlane WV, Beck RW, Bode BW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464-1476. [DOI] [PubMed] [Google Scholar]
- 18. Bailey TS, Chang A, Christiansen M. Clinical accuracy of a continuous glucose monitoring system with an advanced algorithm. J Diabetes Sci Technol. 2014;9:209-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Christiansen M, Bailey T, Watkins E, et al. A new-generation continuous glucose monitoring system: improved accuracy and reliability compared with a previous-generation system. Diabetes Technol Ther. 2013;15:881-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen C, Zhao XL, Li ZH, et al. Current and emerging technology for continuous glucose monitoring. Sensors. 2017;17:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Langendam M, Luijf YM, Hooft L, et al. Continuous glucose monitoring systems for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2012;1:CD008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rodbard D. Continuous glucose monitoring: a review of successes, challenges, and opportunities. Diabetes Technol Ther. 2016;18:S3-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ray PP. Continuous glucose monitoring: a systematic review of sensor systems and prospects. Sensor Rev. 2018;38:420-437. [Google Scholar]
- 24. Food and Drug Administration. FDA approves first continuous glucose monitoring system for adults not requiring blood sample calibration. 2017. Available at: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm577890.htm.
- 25. Dexcom. FDA authorizes marketing of the new Dexcom G6 CGM eliminating the need for fingerstick blood testing for people with diabetes. 2018. Available at: https://provider.dexcom.com/industry-news/fda-authorizes-marketing-new-dexcom-g6-cgm-eliminating-need-fingerstick-blood-testing.
- 26. Pisano M. Overview of insulin and non-insulin delivery devices in the treatment of diabetes. P T. 2014;39:866-873. [PMC free article] [PubMed] [Google Scholar]
- 27. Heinemann L, Krinelke L. Insulin infusion set: the Achilles heel of continuous subcutaneous insulin infusion. J Diabetes Sci Technol. 2012;6:954-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heinemann L, Walsh J, Roberts R. We need more research and better designs for insulin infusion sets. J Diabetes Sci Technol. 2014;8:199-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. TuDiabetes Forum. When do you change infusion sets? 2014. Available at: https://forum.tudiabetes.org/t/when-do-you-change-infusion-sets/14235.
- 30. Diabetes UK. Infusion set problems.= 2018. Available at: https://www.diabetes.co.uk/forum/threads/infusion-set-problems.135929/#post-1697591.
- 31. Pickup JC, Yemane N, Brackenridge A, et al. Nonmetabolic complications of continuous subcutaneous insulin infusion: a patient survey. Diabetes Technol Ther. 2014;16:145-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pozzilli P, Battelino T, Danne T, et al. Continuous subcutaneous insulin infusion in diabetes: patient populations, safety, efficacy, and pharmacoeconomics. Diabetes Metab Res Rev. 2016;32:21-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Richardson T, Kerr D. Skin-related complications of insulin therapy. Am J Clin Dermatol. 2003;4:661-667. [DOI] [PubMed] [Google Scholar]
- 34. Radermecker RP, Piérard GE, Scheen AJ. Lipodystrophy reactions to insulin. Am J Clin Dermatol. 2007;8:21-28. [DOI] [PubMed] [Google Scholar]
- 35. Conwell LS, Pope E, Artiles AM, et al. Dermatological complications of continuous subcutaneous insulin infusion in children and adolescents. J Pediatr. 2008;152:622-628. [DOI] [PubMed] [Google Scholar]
- 36. Schober E, Rami B. Dermatological side effects and complications of continuous subcutaneous insulin infusion in preschool-age and school-age children. Pediatr Diabetes. 2009;10:198-201. [DOI] [PubMed] [Google Scholar]
- 37. Pfützner A, Sachsenheimer D, Grenningloh M, et al. Using insulin infusion sets in CSII for longer than the recommended usage time leads to a high risk for adverse events: results from a prospective randomized crossover study. J Diabetes Sci Technol. 2015;9:1292-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sampson Perrin AJ, Guzzetta RC, Miller KM, et al. A web-based study of the relationship of duration of insulin pump infusion set use and fasting blood glucose level in adults with type 1 diabetes. Diabetes Technol Ther. 2015;17:307-310. [DOI] [PubMed] [Google Scholar]
- 39. Thethi TK, Rao A, Kawji H, et al. Consequences of delayed pump infusion line change in patients with type 1 diabetes mellitus treated with continuous subcutaneous insulin infusion. J Diabetes Complications. 2010;24:73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Heinemann L. Insulin infusion sets: a critical reappraisal. Diabetes Technol Ther. 2016;18:327-333. [DOI] [PubMed] [Google Scholar]
- 41. Frid A, Hirsch L, Gaspar R, et al. The Third Injection Technique Workshop in Athens (TITAN). Diabetes Metab. 2010;36:S19-S29. [DOI] [PubMed] [Google Scholar]
- 42. Kalra S, Mithal A, Sahay R, et al. Indian injection technique study: injecting complications, education, and the health care professional. Diabetes Ther. 2017;8:659-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zanfardino A, Iafusco D, Piscopo A, et al. Continuous subcutaneous insulin infusion in preschool children: butt or tummy, which is the best infusion set site? Diabetes Technol Ther. 2014;16:563-566. [DOI] [PubMed] [Google Scholar]
- 44. Frid AH, Kreugel G, Grassi G, et al. New insulin delivery recommendations. Mayo Clinic Proc. 2016;91:1231-1255 [DOI] [PubMed] [Google Scholar]
- 45. Frid A, Hirsch L, Gaspar R, et al. New injection recommendations for patients with diabetes. Diabetes Metab. 2010;36:S3-S18. [DOI] [PubMed] [Google Scholar]
- 46. Mecklenburg RS, Benson EA, Benson JW, Jr, et al. Long-term metabolic control with insulin pump therapy: report of experience with 127 patients. N Engl J Med. 1985;313:465-468. [DOI] [PubMed] [Google Scholar]
- 47. Guinn TS, Bailey GJ, Mecklenburg RS. Factors related to discontinuation of continuous subcutaneous insulin-infusion therapy. Diabetes Care. 1988;11:46-51. [DOI] [PubMed] [Google Scholar]
- 48. van Faassen I, Razenberg PP, Simoons-Smit AM, et al. Carriage of Staphylococcus aureus and inflamed infusion sites with insulin-pump therapy. Diabetes Care. 1989;12:153-156. [DOI] [PubMed] [Google Scholar]
- 49. Chantelau E, Spraul M, Mühlhauser I, et al. Long-term safety, efficacy and side-effects of continuous subcutaneous insulin infusion treatment for type 1 (insulin-dependent) diabetes mellitus: a one centre experience. Diabetologia. 1989;32:421-426. [DOI] [PubMed] [Google Scholar]
- 50. Ward WK. A review of the foreign-body response to subcutaneously-implanted devices: the role of macrophages and cytokines in biofouling and fibrosis. J Diabetes Sci Technol. 2008;2:768-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dondossola E, Holzapfel BM, Alexander S, et al. Examination of the foreign body response to biomaterials by nonlinear intravital microscopy. Nat Biomed Eng. 2017;1:0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pachler C, Ikeoka D, Plank J, et al. Subcutaneous adipose tissue exerts proinflammatory cytokines after minimal trauma in humans. Am J Physiol Endocrinol Metab. 2007;293:e690-e696. [DOI] [PubMed] [Google Scholar]
- 53. Højbjerre L, Skov-Jensen C, Kaastrup P, et al. Effect of steel and Teflon infusion catheters on subcutaneous adipose tissue blood flow and infusion counter pressure in humans. Diabetes Technol Ther. 2009;11:301-306. [DOI] [PubMed] [Google Scholar]
- 54. Zhang ES, Cao ZQ. Coated glucose sensors dodge recalibration. Nat Biomed Eng. 2018;2:881-882. [DOI] [PubMed] [Google Scholar]
- 55. Klueh U, Frailey JT, Qiao Y, et al. Cell based metabolic barriers to glucose diffusion: macrophages and continuous glucose monitoring. Biomaterials. 2014;35:3145-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xie X, Doloff JC, Yesilyurt V, et al. Reduction of measurement noise in a continuous glucose monitor by coating the sensor with a zwitterionic polymer. Nat Biomed Eng. 2018;2:894-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Klueh U, Liu Z, Feldman B, et al. Metabolic biofouling of glucose sensors in vivo: role of tissue microhemorrhages. J Diabetes Sci Technol. 2011;5:583-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Novak MT, Reichert WM. Modeling the physiological factors affecting glucose sensor function in vivo. J Diabetes Sci Technol. 2015;9:993-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mecklenburg RS, Benson EA, Benson JW, et al. Acute complications associated with insulin infusion pump therapy: report of experience with 161 patients. JAMA. 1984;252:3265-3269. [PubMed] [Google Scholar]
- 60. Kostev K, Rockel T, Rosenbauer J, Rathmann W. Risk factors for discontinuation of insulin pump therapy in pediatric and young adult patients. Prim Care Diabetes. 2014;8:346-351. [DOI] [PubMed] [Google Scholar]
- 61. Lenhard MJ, Reeves GD. Continuous subcutaneous insulin infusion: a comprehensive review of insulin pump therapy. Arch Intern Med. 2001;161:2293-2300. [DOI] [PubMed] [Google Scholar]
- 62. Nowakowska M, Jarosz-Chobot P, Polańska J, et al. Bacterial strains colonizing subcutaneous catheters of personal insulin pumps. Pol J Microbiol. 2007;56 (4):239-243. [PubMed] [Google Scholar]
- 63. Wickline CL, Cornitius TG, Butler T. Cellulitis caused by Rhizomucor pusillus in a diabetic patient receiving continuous insulin infusion pump therapy. South Med J. 1989;82:1432-1434. [DOI] [PubMed] [Google Scholar]
- 64. Teutsch SM, Herman WH, Dwyer DM, et al. Mortality among diabetic patients using continuous subcutaneous insulin-infusion pumps. N Engl J Med. 1984;310:361-368. [DOI] [PubMed] [Google Scholar]
- 65. Centers for Disease Control. Toxic-shock syndrome in a patient using a continuous subcutaneous insulin infusion pump-Idaho. MMWR Morb Mortal Wkly Rep. 1983;32:404-406. [PubMed] [Google Scholar]
- 66. Schmid V, Hohberg C, Borchert M, et al. Pilot study for assessment of optimal frequency for changing catheters in insulin pump therapy-trouble starts on day 3. J Diabetes Sci Technol. 2010;4:976-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Binder E, Lange O, Edlinger M, et al. Frequency of dermatological side effects of continuous subcutaneous insulin infusion in children and adolescents with type 1 diabetes. Exp Clin Endocrinol Diabetes. 2015;123:260-264. [DOI] [PubMed] [Google Scholar]
- 68. Johansson UB, Adamson U, Lins PE, et al. Patient management of long-term continuous subcutaneous insulin infusion. J Adv Nurs. 2005;51:112-118. [DOI] [PubMed] [Google Scholar]
- 69. Reichert D. Realität der Insulinpumpentherapie: Daten von 1142 Patienten aus 40 DSPen. Diabetes Stoffwechsel Herz. 2013;22:367-363. [Google Scholar]
- 70. Patel PJ, Benasi K, Ferrari G, et al. Randomized trial of infusion set function: steel versus Teflon. Diabetes Technol Ther. 2014;16:15-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ampudia-Blasco FJ, Hasbum B, Carmena R. A new case of lipoatrophy with lispro insulin in insulin pump therapy: is there any insulin preparation free of complications? Diabetes Care. 2003;26:953-954. [DOI] [PubMed] [Google Scholar]
- 72. Chowdhury TA, Escudier V. Poor glycaemic control caused by insulin induced lipohypertrophy. BMJ. 2003;327:383-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Johansson U-B, Amsberg S, Hannerz L, et al. Impaired absorption of insulin aspart from lipohypertrophic injection sites. Diabetes Care. 2005;28:2025-2027. [DOI] [PubMed] [Google Scholar]
- 74. Saccabusi S, Boatto G, Asproni B, et al. Sensitization to methyl methacrylate in the plastic catheter of an insulin pump infusion set. Contact Dermat. 2001;45:47-48. [DOI] [PubMed] [Google Scholar]
- 75. Jolanki R, Kanerva L, Estlander T, et al. Allergic contact dermatitis from phenoxyethoxy ethylacrylates in optical fiber coating, and glue in an insulin pump set. Contact Dermat. 2001;45:36-37. [DOI] [PubMed] [Google Scholar]
- 76. Simmons BP, Hooton TM, Wong ES, et al. Guidelines for prevention of intravascular infections. Infect Control Hosp Epidemiol. 1982;3:61-72. [Google Scholar]
- 77. TuDiabetes Forum. Infusion site change frequency. 2016. Available at: https://forum.tudiabetes.org/t/infusion-site-change-frequency/57736.
- 78. Diabetes UK. How long can you leave a infusion set really in? 2013. Available at: https://www.diabetes.co.uk/forum/threads/how-long-can-you-leave-a-infusion-set-really-in.43134/.