Abstract
Background:
Integrated personalized diabetes management (iPDM) is a digitally supported therapeutic concept to improve patient-physician interaction to overcome the aspects of clinical inertia. Integrated personalized diabetes management can support decision making and improve therapeutic outcomes of suboptimally controlled persons with insulin-treated type 2 diabetes (T2D). In this paper, we report the results of an analysis of the PDM-ProValue study program on the effectiveness and perceived benefit of this approach, with a focus on how physicians used and assessed the digital tools provided for the iPDM process.
Materials and Methods:
The study program included two 12-month, prospective, controlled, cluster-randomized multicenter trials. A total of 101 practices participated with 907 patients. Practices were cluster-randomized to an intervention group and a control group. Digital tools for data visualization and analysis applied were used. HCP were asked to assess the use, relevance, and usefulness of the tools.
Results:
A clear preference was stated for the visual overview over more statistically complex analyses. A total of 83% of the participants rated a high relevance of the “daily profile,” 81% of the “total profile,” and 68% the “risk illustrated by traffic light symbols” for the therapy decision. The overall iPDM process was very favorably rated with respect to structuredness and potential for personalized treatment and well accepted among health care professionals (HCP).
Conclusions:
Embedding digital tools in a structured process (iPDM) were proved to provide a benefit for insulin-treated T2D patients and their physicians. These results offer insight for further development and improvement of the tools and add information on how to overcome clinical inertia.
Keywords: type 2 diabetes, personalized diabetes management, digital tools, insulin therapy regimen, process improvement
Introduction
Diagnostic and therapeutic treatment options for patients with diabetes have markedly improved in the last few decades.1,2 However, many studies came to the conclusion that these new opportunities did not lead to substantial improvement in glycemic control.3-5
One reason for the lack of progress is the phenomenon of clinical inertia, which has been described as a lack of initiation or intensification of an antidiabetic therapy as intensively as recommended in guidelines for persons with diabetes. Some patients find it difficult to act adequately upon data provided by self-monitoring of blood glucose (SMBG) and to implement therapeutic recommendations in their everyday life.6-8
It has been shown previously that SMBG based on the individual situation of the patient combined with adequate analysis of diagnostic data can support therapy optimization, desirable behavioral changes, and thus lead to improved clinical outcomes.9-12 A reciprocity in patient and physician’s perceptions of the other’s controlling interpersonal behavior is also useful for better diabetes control.13
Integrated personalized diabetes management (iPDM) is a digitally supported structured, six-step concept which combines structured SMBG, use of diabetes data management software, collaborative patient-physician communication, and support of therapeutic decision-making in an iterative six-step, structured intervention process14 (Figure 1). Such a patient-centered and personalized approach for the treatment of people with type 2 diabetes (T2D) was also recommended recently by international guidelines.15,16
Figure 1.
Integrated personalized diabetes management process (a) and schematic display of daily profiles from the digital tool used in the study (b). (a) The iterative integrated personalized diabetes management process starts with an initial assessment of the patient status and a demand-oriented education/training. Subsequently, blood glucose data are collected according to a structured, therapy adapted regimen, followed by electronic documentation and systematic data analysis. In the next step, the current treatment is reviewed and adapted individually when indicated, and finally, the treatment effectiveness is assessed at the patient’s next visit. The process is then run through again. (b) Schematic overview of Accu-Chek Smart Pix showing an example for the glycemic risk traffic lights on the left and the daily self-monitoring of blood glucose profile on the right.
The PDM-ProValue study program previously showed that iPDM improves therapeutic outcomes of suboptimally controlled patients with insulin-treated T2D.17 Overall, iPDM improved the use of diagnostic data leading to better glycemic control, enabled more timely treatment adjustments (indicating reduced clinical inertia), and increased patient adherence and treatment satisfaction among patients and physicians.
Here, we report the results of an analysis of the PDM-ProValue study program on how physicians used and assessed the digital tools provided for the iPDM process, and also how physicians and medical staff rated the iPDM approach in terms of process quality and potential for personalized treatment.
Methods
The PDM-ProValue Study Program
Study design
To evaluate the efficacy and benefit of iPDM in patients with insulin-treated T2D, two parallel 12-month, prospective, cluster-randomized, multi-center controlled trials were conducted in Germany.15,18 Altogether, 101 study centers, both general practitioner (GP) and diabetes specialized practices (DSP), were randomized to an intervention arm that utilized iPDM and to a control arm that continued with usual care. A total of 907 patients (diagnosed T2D, age ≥18 years, HbA1c ≥7.5% measured during the last six weeks prior to study inclusion, subcutaneous insulin therapy for greater than or equal to six months) were enrolled and eligible. Trials were registered with ClinicalTrials.gov NCT02268929 (PDM-ProValueGP) and NCT02156349 (PDM-ProValueDSP).
The results reported here originate from the pooled data of both studies. However, as only the patients in the iPDM group were using the digital tools, the respective data presented here are only covering this patient group (n = 414) in 53 centers.
Patients and physicians in the intervention group (iPDM) followed the structured iPDM process (Figure 1). The physicians reviewed and assessed their experience with the digital tools utilized.
Physicians received training based on a structured curriculum (four one-hour sessions, including video instruction programs and role-play exercises). Patients were treated with usual care under the boundary conditions (guideline-conform therapeutic and diagnostic measures cleared for reimbursement) recommended provided by the German statutory health insurance with six scheduled visits throughout the 12-month study period. Physicians were free to use their judgment in their treatment choices.
Digital Tools Used in the PDM-ProValue Study
Diabetes management system
Most digital features utilized in the PDM-ProValue study were part of the Accu-Chek Smart Pix diabetes management system. This system consists of a device reader to download the data from connected devices and a standalone software solution which provides systematic electronic evaluation and visualization of blood glucose measurement results and therapy data (eg, insulin deliveries/dosages). The data are presented as a report with several elements that can be selected, including graphics, tables, and statistics (Figure 1; Table 1).
Table 1.
Overview of Digital Tools Used in the Integrated Personalized Diabetes Management Arm of the PDM-ProValue Study Program.
| (Digital) iPDM tools | Function | Tested for relevance | Tested for usefulness |
|---|---|---|---|
| Glycemic risk traffic light | Data interpretation tool to provide fast risk assessment in three specific areas (hypo-/hyperglycemia, values in target range) | x | x |
| BGM compliance monitor | Provide patient with individual, therapy adapted SMBG measuring schemes and assess the patient adherence to the provided scheme | x | x |
| Handout with BGM recommendation for patient | Take-home printout summary as patient reminder | x | |
| Daily profile | Blood glucose data visualization over course of a day | x | x |
| Weekly profile | Blood glucose data visualization over course of a week | x | x |
| Total profile | Data visualization over a selected period of time | x | x |
| Statistical tool: percentage of values in target range | Graphical overview of percentage of values in, above, or below target range in given time frame | x | x |
| Statistical tool: mean blood glucose level and standard deviation, LBGI/HBGI | Statistical analyses providing insight in quality of glycemic control | x |
Abbreviations: BGM, blood glucose monitoring; iPDM, integrated personalized diabetes management; LBGI/HBGI, low blood glucose index/high blood glucose index; SMBG, self-monitoring of blood glucose.
Glycemic risk traffic lights and compliance monitor
The study sites also received a software add-on with an extended functional scope: a risk traffic light add-in and a blood glucose monitoring (BGM) compliance monitor. The glycemic risk assessment tool allowed for fast visual traffic-light style evaluation (red-yellow-green) in three specific areas of glycemic control: hypoglycemia, mean blood glucose values in target range, and glycemic variability. To assess the adherence of patients to the structured testing regime recommended by the physician, the BGM compliance monitor was developed for study purpose only and introduced in the software. Physicians selected the recommended testing regime from the software tool which then used specific algorithms to calculate the adherence based on the actual SMBG measurements (Table 1).
Evaluation of the PDM Tools
Physicians were asked to assess several aspects of the digital tools described above. At each study visit, the rating was entered in a Likert type scale directly in the electronic Case Report Form for each patient.
In addition to the assessment on an individual patient level, some aspects were also investigated on a study center level at the end of the study. Data were statistically analyzed in a descriptive manner.
Table 2 provides an overview of the analyses reported here.
Table 2.
Overview of Physicians’ Ratings on the Digital Tools Used in the Study.
| Relevance on patient level | Relevance of the tool for decision-making on therapy adaptations at month 12 | Figure 2(a) |
| Relevance on patient level over time | Relevance of the tool for decision making on therapy adaptations over course of the study | Figure 2(b) |
| Usefulness on patient level | Usefulness of the tool for communication with the patient at month 12 | Figure 3(a) |
| Usefulness on patient level over time | Usefulness of the tool for communication with the patient | Figure 3(b) |
| Frequency of use on study site level | Frequency of use of the tool at each study site assessed at month 12 | Figure 4 |
| Process quality | Assessment of the structuredness of processes and its fitness for personalization | Table 3 |
| Acceptance by medical staff | Rating of the acceptance of iPDM by physician and medical staff | Table 3 |
Abbreviation: iPDM, integrated personalized diabetes management.
Results
Assessment of the Digital Tools by Study Physicians
Decision making on therapy adaptations for each patient
At each visit, physicians assessed the relevance of the provided information originating from the digital tools for their respective therapy decision for each patient. The “daily profile” and the “total profile” were rated as most relevant for the therapy decision by the physicians, with 80.7% and 75.4% at month 12, respectively, when taking together the positive categories “deciding (meaning that treatment decisions were mainly based on the information of this tool),” “very high,” or “high” relevance (Figure 2a). The “daily profile” was considered to be most relevant for decision making, provided the highest share of ratings in the category “deciding” at month 12 (29.0%). Throughout the course of the study, the relevance of the daily profile as a “deciding” tool increased markedly (from 18.6% at week 3 to 29.0% at month 12, Figure 2b).
Figure 2.
(a) Relevance of the digital tools concerning therapy adaptations at month 12 rated by physicians (personalized diabetes management group only, n = 440, answers obtained from n = 414 patients). (b) Development of the relevance of the digital tools rated by physicians as “deciding” for their decisions on therapy adaptations over the study course (personalized diabetes management group only, n = 440, answers obtained from n = 414 patients).
Altogether, four tools were favorably rated (relevance “deciding,” “high,” or “very high” relevance of >50%) at month 12: in addition to “daily profile” and “total profile,” these were the “risk illustrated by traffic light symbols” (67.0%) and the “BGM compliance monitor” (63.3%). On the other side, two more sophisticated statistical reports were considered as “not relevant” for more than a fifth of the patients: “percentage of values in target range” (21.7%) and “low blood glucose index/high blood glucose index (LBGI/HBGI)” (23.2%).
Communicating With the Patient
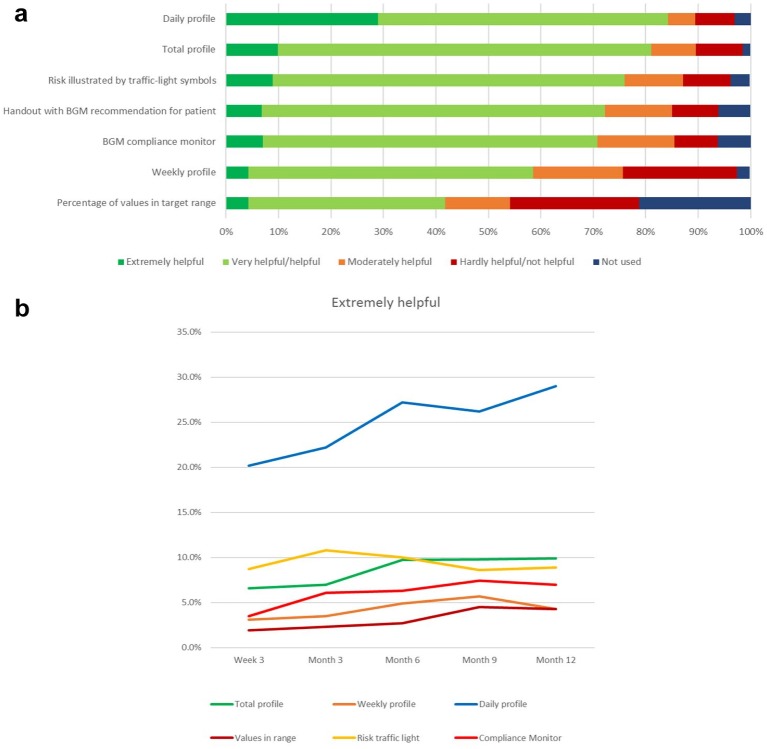
Another set of questions explored the usefulness of the digital tools for the communication with the patient. Again, daily and total profile were rated most favorably, now both over 80% in the categories helpful/very helpful or extremely helpful, with the daily profile again gathering the most ratings in the highest category (Figure 3a).
Figure 3.
(a) Usefulness of the integrated personalized diabetes management tools for the dialog with the patient at month 12 rated by physicians (percentage of patients) (personalized diabetes management group only, n = 440, answers obtained from n = 414 patients). (b) Development of the usefulness of the integrated personalized diabetes management tools rated by physicians as “extremely helpful” for their dialog with the patient over the study course (personalized diabetes management group only, n = 440, answers obtained from n = 414 patients).
The handout with BGM testing scheme recommendations and the patient’s current percentage of values in target range which had been printed out by the center for the patient at each visit achieved over 70% positive ratings (combined categories “extremely helpful,” “very helpful,” or “helpful” category). The information on the percentage of patient values in target range achieved rather low ratings. Physicians stated that they did not use this information for over 20% of their patients.
Over the course of the study, the ratings for most tools remained largely the same, with only the daily profile showing a similar improvement in the highest category “extremely helpful,” from 19% to 29% (Figure 3b).
Frequency of use by participating practices
Frequency of the iPDM tools used by each participating practice was assessed at the end of the study. Risk traffic light symbols and reports on SMBG data over time (total/weekly/daily profile) were stated to be used “always” or “most of the time,” with 84.9% of the answers in this area, followed by the SMBG compliance monitor (Figure 4). More complex statistical reports like standard error/mean blood glucose level or LBGI/HBGI were less often used: the latter was stated to be used “occasionally,” “rarely,” or “never” in over 60.4% of the answers.
Figure 4.
Frequency of use of different tools on a center level, integrated personalized diabetes management group (n = 53).
Optimization of the treatment process
The improvement of process structuredness by the implementation of iPDM was appreciated by the physicians: 57% judged the process as “much better” or “better” structured, respectively, than without iPDM (Table 3). For the patient-specific therapy personalization or personalization, similar results were obtained: 54% of patients stated that the option to personalize the treatment is “much better” or “better,” respectively after the introduction of iPDM.
Table 3.
Structuredness of Processes and Personalization, and Overall Rating of the Integrated Personalized Diabetes Management by Physician and Medical Staff.
| Ratinga |
||||
|---|---|---|---|---|
| Much better/better | Better | A bit better | Unchanged | |
| Structuredness of process | 57% | 23% | 21% | |
| Personalization | 54% | 25% | 21% | |
Assessed at center level in the integrated personalized diabetes management group (n = 53).
Overall rating
The acceptance of the iPDM process was high among both physicians and medical staff: altogether, 89% of the centers confirmed “very high” or “high/rather high” acceptance of this integrated concept at physician level and even 92% of the medical staff did so.
Both physicians and medical staff involved in the study conduction were asked for their acceptance of the iPDM approach. For 58.5% of the physicians and 52% of the medical staff, acceptance was high or very high, and only 11.3% of physicians and 7.7% of medical staff stated medium or low acceptance (Figure 5).
Figure 5.
Overall rating of the integrated personalized diabetes management approach by physician and medical staff at study end (n = 53).
Discussion
Personalized care planning leads to improvements in people’s capability to self-manage their diabetes when compared to usual care.19 It also can improve certain indicators of physical and psychological health status. The PDM-ProValue study program provides guidance and hints on how to move forward with the ever present demand for personalization and digital transformation of health care to optimize resource consumption while at the same time achieve relevant improvement of outcomes for patients and physicians.
The key outcome of the PDM-ProValue study program was the improvement of glycemic control and a range of patient and physician reported outcomes17 In addition, significant differences between the iPDM and the usual care group in the frequency of a broad range of therapy recommendations by the treating physician were observed. The iPDM approach seems to better activate and empower the patient-physician team in overcoming clinical inertia, the phenomenon of lack in therapeutic progress as described earlier.
Based on this successful proof of concept, the first implementation projects are already underway. A project funded by the European Institute for Innovation and Technology Health (EIT Health), for instance, is laying the groundwork to initiate value-based concepts based on iPDM in European countries.20
To further investigate the key to the success of the iPDM approach, and to learn about patient and physician preferences in order to further improve the concept for future use, the digital tools utilized for the iPDM intervention were assessed in this study and then reported in this article.
Relevance and Usefulness of iPDM Tools
Not surprisingly, iPDM reports and tools which provide a quick overview of the blood glucose data and allow for easy visualization were appreciated by physicians to be most relevant for decision making and most useful for the therapy discussion with the patient. These tools were most importantly the “daily profile” (providing a cumulative view of the daily patterns), followed by other overview tools as the “total profile” (providing an overview over all data in a selected time frame), the risk (for potentially harmful glycemic events, eg, hypo- and hyperglycemia) illustrated by traffic light symbols and the BGM compliance monitor.
While for most tools, the relevance or usefulness was described with the categories “helpful” or “very helpful,” physicians used the category “deciding” (indicating an outstanding role for decision making) for the daily profile in over 25% of the patients. Also, the “daily profile” appreciation as relevant for therapy adaptation and useful for discussion clearly rose throughout the course of the study, indicating an increasing recognition of its value by using the tool. Physicians seem to base their conclusion on the daily profile and get more confident in doing so as the study proceeds. The daily profile seems to best provide a clue to what is going on with the patient’s glycemic values, thereby serving as a starting point for the consideration of therapy changes.
The frequency of tool usage documented at center level (not focusing on the individual patient) shows that the digital tool used most frequently was the risk traffic light symbol, followed by the glucose profiles over time.
Interestingly, while the overview displays precede the risk traffic tool in the assessment of relevance and usefulness, risk traffic light was used more frequently on a center level. This may reflect the benefit of a simple and easy to understand data display (as a traffic light style visualization) to discuss the glycemic status with the patient, while at the same time, the decision of the physician was based on more detailed findings as provided in the daily overview.
The tools rated the least favorable both for the relevance for decision making and discussion with the patient were the statistical information on HBGI and LBGI and the time in target range. This observation was confirmed by the frequency of use data, where statistical parameters end up as the tools used least frequently. Obviously, this information has only limited benefits for decision making on therapy adaptations and the discussion with the patient. However, it might prove more important when it comes to document outcomes or monitor the disease progression.
Process Quality: Assessment of Structuredness and Personalization
The categories “structuredness” and “personalization” were introduced for the physicians as a measure for the quality of the iPDM therapy process. Both items were recorded at a center level in the iPDM group at the end of the study. Structuredness related to the perception of a visit, as opposed to a more freewheeling and unstructured sequence of actions or discussion. Personalization was intended to reflect the potential to adapt a therapy process on the needs and requirements of an individual patient, in the sense of personalized medicine. Both features were rated favorably by the physicians. Integrated personalized diabetes management seems to be helpful to both introduce a process providing meaningful guidance and better personalize the treatment. Although in the study setting the physicians were trained on how to conduct the iPDM cycle steps and how to apply the PDM tools used in the study, the introduction of iPDM in general does not require dedicated training activities above an informative level. Rather, it is important to develop physicians’ mindset with respect to patient centered communication and participative decision making, which is inherently supported by iPDM.
Together, the observed improvements might be a good basis to support the physician in better understanding and more efficiently taking action in the therapy process—a contribution to overcome clinical inertia.
The acceptance of iPDM, and thus the potential for a successful implementation, was overall “high” or “very high” for both physicians and medical staff—exceeding 50% for both groups, and together with the “rather high” category, acceptance neared or exceeded 90%. The study inclusion criteria required that physicians had no prior experience with the use of PDM-type IT tools, so these findings show that users with low experience are willing to follow such a rather complex setting. Some restraint toward highest acceptance most likely originates from the artificial study situation. Health care professionals might be willing to accept rather complex requirements for documentation and conduction in a study setting, however, the transfer in clinical routine asks for a much more intuitive and more hands-off approach. Here, we see a big opportunity to improve digital tools in a holistic setting, which does not require the physician or patient to take complex setup steps or tick boxes of a workflow, but which rather allows for intuitive yet meaningful guidance.
Limitations of the study were for one of the cluster-randomized settings for which the rationale has been reported elsewhere and which may result in unintended effects on center level.21 For the investigation presented here, reporting of results is limited to the intervention group, as the control group had not received the PDM tools. The questionnaires on the PDM tools focused largely on the physicians, so we do not know whether patients would agree with their assessment. Finally, no comparable data from other studies are available to potentially enhance the validity of our results.
In conclusion, the results regarding usefulness, structuredness, and personalization of the iPDM process show that physicians (and also their medical staff) appreciate the benefits of the assessed digital tools and accept the implied iPDM approach. The majority of the practitioners planned to further use the BGM profiles and statistical parameters as well as the risk traffic light symbols.
Perceived usefulness and good usability are the key elements of technology acceptance.22 The easy-to-use and well-established components of the iPDM cycle do not require physicians to learn and operate with new sophisticated technologies, and the basic tools and methods of iPDM are already broadly available.
The iPDM concept used in the PDM ProValue study program provides remarkable answers for real-world outpatient settings. The iPDM approach is easy to implement and the costs are low. Thus, this approach could also be transferred to technically remote population groups or to countries without developed health systems. The iPDM approach has the potential to support the implementation of the ADA/EASD guidelines in everyday diabetes care.
Conclusion
The PDM-ProValue study program has shown that embedding digital tools in a structured process (iPDM) provides a benefit for insulin-treated patients with T2D and their physicians. Integrated personalized diabetes management was well accepted by the physicians who observed the improvement in structuredness and personalization of the process and stated preferences for the observed relevance of the tools for therapeutic decision making and the usefulness for a patient-physician dialog. These results offer insight for further development and improvement of the tools and add information on how to overcome clinical inertia, thus providing valuable information and guidance on how to best apply iPDM to provide optimal personalized care for people with diabetes.
Acknowledgments
The authors wish to thank Dr Angelika Müller, Roche Diabetes Care, Mannheim/Germany and Dr Karin Kreuel, Cologne/Germany, for critical discussions, review, and editorial assistance in developing the manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: BK and WS received research funding from Roche Diabetes Care Deutschland GmbH. Lutz Heinemann has received research funding and consulting fees from Dexcom, Roche Diabetes Care, Medtronic, Integrity Ltd., and Sanofi. JW and AM are employees of Roche Diabetes Care Deutschland GmbH. IV and HK are employees of Roche Diabetes Care GmbH.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by an unrestricted grant from Roche Diabetes Care Deutschland GmbH, Germany.
ORCID iDs: Lutz Heinemann  https://orcid.org/0000-0003-2493-1304
https://orcid.org/0000-0003-2493-1304
Bernhard Kulzer  https://orcid.org/0000-0001-9120-4479
https://orcid.org/0000-0001-9120-4479
References
- 1. Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med. 2013;368(17):1613-1624. doi: 10.1056/NEJMsa1213829 [DOI] [PubMed] [Google Scholar]
- 2. Jacob L, Waehlert L, Kostev K. Changes in type 2 diabetes mellitus patients in German primary care prior to (2006) and after (2010, 2014) launch of new drugs. J Diabetes Sci Technol. 2016;10(2):414-420. doi: 10.1177/1932296815607860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Egede LE, Gebregziabher M, Echols C, Lynch CP. Longitudinal effects of medication nonadherence on glycemic control. Ann Pharmacother. 2014;48(5):562-570. doi: 10.1177/1060028014526362. [DOI] [PubMed] [Google Scholar]
- 4. Currie CJ, Peyrot M, Morgan CLet al. The impact of treatment noncompliance on mortality in people with type 2 diabetes. Diabetes Care. 2012;35(6):1279-1284. doi: 10.2337/dc11-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wild H. The economic rationale for adherence in the treatment of type 2 diabetes mellitus. Am J Manag Care. 2012;18(3 suppl):S43-S48. [PubMed] [Google Scholar]
- 6. O’Connor PJ, Sperl-Hillen JAM, Johnson PE, Rush WA, Biltz G. Clinical inertia and outpatient medical errors. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety. Vol. 2 Rockville, MD: Agency for Healthcare Research and Quality/Department of Defense; 2005:309-321. [PubMed] [Google Scholar]
- 7. Zafar A, Davies M, Azhar A, Khunti K. Clinical inertia in management of T2DM. Prim Care Diabetes. 2010;4(4):203-207. [DOI] [PubMed] [Google Scholar]
- 8. Silva DD, Bosco AA. An educational program for insulin self-adjustment associated with structured self-monitoring of blood glucose significantly improves glycemic control in patients with type 2 diabetes mellitus after 12 weeks: a randomized, controlled pilot study. Diabetol Metab Syndr. 2015;7:2. doi: 10.1186/1758-5996-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Polonsky WH, Fisher L, Schikman CHet al. A structured self-monitoring of blood glucose approach in type 2 diabetes encourages more frequent, intensive, and effective physician interventions: results from the STeP study. Diabetes Technol Ther. 2011;13(8):797-802. doi: 10.1089/dia.2011.0073. [DOI] [PubMed] [Google Scholar]
- 10. Polonsky WH, Jelsovsky Z, Panzera S, Parkin CG, Wagner RS. Primary care physicians identify and act upon glycemic abnormalities found in structured, episodic blood glucose monitoring data from non-insulin-treated type 2 diabetes. Diabetes Technol Ther. 2009;11(5):283-291. doi: 10.1089/dia.2008.0087 [DOI] [PubMed] [Google Scholar]
- 11. Kempf K, Kruse J, Martin S. ROSSO-in-praxis follow-up: long-term effects of self-monitoring of blood glucose on weight, hemoglobin A1c, and quality of life in patients with type 2 diabetes mellitus. Diabetes Technol Ther. 2012;14(1):59-64. doi: 10.1089/dia.2011.0116. [DOI] [PubMed] [Google Scholar]
- 12. Siegel E, Sturm D, Franke Det al. Selbstmonitoring beim typ-2-diabetes – Ein Praxis-Leitfaden. Der Hausarzt. 2013;50(3):26-30. [Google Scholar]
- 13. Auerbach SM, Clore JN, Kiesler DJet al. Relation of diabetic patients’ health-related control appraisals and physician-patient interpersonal impacts to patients’ metabolic control and satisfaction with treatment. J Behav Med. 2002;25(1):17-31. [DOI] [PubMed] [Google Scholar]
- 14. Ceriello A, Barkai L, Christiansen JSet al. Diabetes as a case study of chronic disease management with a personalized approach: the role of a structured feedback loop. Diabetes Res Clin Pract. 2012;98(1):5-10. doi: 10.1016/j.diabres.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 15. Inzucchi SE, Bergenstal RM, Buse JBet al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58(3):429-442. doi: 10.1007/s00125-014-3460-0. [DOI] [PubMed] [Google Scholar]
- 16. Davies MJ, D’Alessio DA, Fradkin Jet al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669-2701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kulzer B, Daenschel W, Daenschel Iet al. Integrated personalized diabetes management improves glycemic control in patients with insulin-treated type 2 diabetes: results of the PDM-ProValue study program. Diabetes Res Clin Pract. 2018;144:200-212. doi: 10.1016/j.diabres.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 18. Kulzer B, Daenschel W, Daenschel Iet al. Integrated personalized diabetes management (PDM): design of the ProValue studies: prospective, cluster-randomized, controlled, intervention trials for evaluation of the effectiveness and benefit of PDM in patients with insulin-treated type 2 diabetes. J Diabetes Sci Technol. 2016;10(3):772-781. doi: 10.1177/1932296815617487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Coulter A, Entwistle VA, Eccles A, Ryan S, Shepperd S, Perera R. Personalised care planning for adults with chronic or long-term health conditions. Cochrane Database Syst Rev. 2015;3:CD010523. doi: 10.1002/14651858.CD010523.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. https://www.eithealth.eu/en_US/ipdm-go Accessed April 4, 2019.
- 21. Klonoff DC, Blonde L, Cembrowski Get al. Consensus report: the current role of self-monitoring of blood glucose in non-insulin-treated type 2 diabetes. J Diabetes Sci Technol. 2011;5(6):1529-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nedopil C, Schauber C, Glende S. A collection of characteristics and requirements of primary, secondary, and tertiary users of AAL solutions, and a guideline for user-friendly AAL design. http://www.aal-europe.eu/wp-content/uploads/2015/02/AALA_Knowledge-Base_YOUSE_online.pdf. Accessed January 12, 2019.