Abstract
SARS-CoV-2 infection is mild in the majority of individuals but progresses into severe pneumonia in a small proportion of patients. The increased susceptibility to severe disease in the elderly and individuals with co-morbidities argues for an initial defect in anti-viral host defense mechanisms. Long-term boosting of innate immune responses, also termed “trained immunity,” by certain live vaccines (BCG, oral polio vaccine, measles) induces heterologous protection against infections through epigenetic, transcriptional, and functional reprogramming of innate immune cells. We propose that induction of trained immunity by whole-microorganism vaccines may represent an important tool for reducing susceptibility to and severity of SARS-CoV-2.
Netea and colleagues argue that we may be able to prevent or decrease the severity of SARS-CoV-2 infection through certain clinically approved live vaccines that “train” the innate immune system to be broadly vigilant against viral infection.
Main Text
From an Emerging Pathogen to Pandemic
In December 2019, health authorities in Wuhan, China, identified a series of pneumonia cases of unknown etiology that were linked to the city’s seafood market. The spread of the infection and the subsequent health emergency led to mobilization of healthcare authorities, medical professionals, and research communities. This resulted in rapid discovery of the pathogen as a novel coronavirus, development of diagnostic tools, and steps for treatment protocols. This new pathogen was called severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), and the disease has been termed coronavirus disease 2019 (COVID-19). Transmission of SARS-CoV-2 occurs mainly via respiratory droplets, similar to the spread of influenza. The estimated basic reproduction number (R0) and serial interval are 2.2 and 5–6 days, respectively, a doubling time of the number of infected subjects every 3 days. The clinical spectrum of SARS-CoV-2 ranges from asymptomatic disease to mild upper respiratory tract infection symptoms (fever, sore throat, cough, and fatigue) and severe pneumonia with respiratory failure and death (Huang et al., 2020).
Since the first reports of cases in Wuhan, SARS-CoV-2 spread rapidly throughout the world, and on March 11, 2020, the World Health Organization (WHO) declared the coronavirus outbreak a pandemic. Millions of people have already been infected, and more than 100,000 individuals have died. Despite all preventive measures, the number of cases is still rising, with Europe and the United States being the hotspot of the pandemic but with increasing numbers of cases in other countries and continents. Epidemiological data show that the elderly and those with co-morbidities (diabetes, obesity, and cardiovascular, respiratory, renal, and lung diseases) are most susceptible to COVID-19 and more likely to suffer from the most severe disease complications. Interestingly, young children, including infants who are more susceptible to other infections, have milder symptoms and less severe COVID-19.
Host-Pathogen Interaction during SARS-CoV-2 Infection
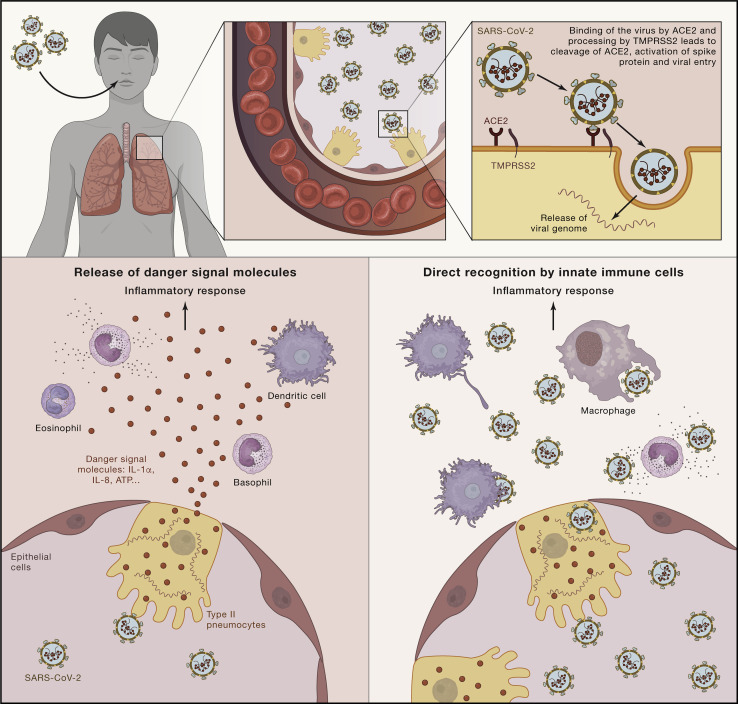
One very important aspect in improving the outcome of patients with COVID-19 is understanding the mechanisms leading to increased severity and mortality. The first event after inhalation of SARS coronaviruses is invasion of epithelial cells and type II pneumocytes through binding of the SARS spike protein to angiotensin-converting enzyme 2 (ACE2) receptors (Figure 1 ; Kuba et al., 2005). This complex is proteolytically processed by transmembrane protease serine 2 (TMPRSS2), leading to cleavage of ACE2 and activation of the spike protein (Glowacka et al., 2011), thereby facilitating viral entry into the target cell. It has been suggested that cells in which both ACE2 and TMPRSS2 are expressed are most susceptible to entry by coronaviruses from the SARS family, among which is the virus described to cause SARS (SARS-CoV) (Shulla et al., 2011) and, most likely, also SARS-CoV-2. Viral entry and cell infection trigger the host’s immune response, and an inflammatory cascade is initiated by innate immune cells. The receptor and signaling mechanisms actually responsible for induction of inflammatory mediators, such as cytokines or chemokines, by SARS-CoV-2 have not yet been identified. However, two possible mechanisms can be envisaged; one is represented by release of danger signal molecules, such as certain cytokines (e.g., interleukin-1α [IL-1α] and IL-8) or ATP, and a second may involve a different recognition pathway mediated in professional immune cells by known pattern recognition receptors, such as Toll-like receptors (TLRs) (Figure 1). Indeed, it has been shown that SARS-CoV is recognized by TLR3 and TLR4 that induce an inflammatory reaction through both MyD88 (Sheahan et al., 2008) and TRIF-mediated pathways (Totura et al., 2015), and a similar process may be hypothesized for SARS-CoV-2. Similarly, activation of the inflammasome and the IL-1β pathway by SARS-CoV (Shi et al., 2019) is also likely to play an important role in pathogenesis; this hypothesis is supported by recent transcriptional identification of the IL-1 pathway as being strongly upregulated in COVID-19 patients (Ong et al., 2020). Induction of innate immune responses is a crucial step in the pathophysiology of the disease. On one hand, it triggers the anti-viral host defense mechanisms necessary for elimination of infection, but on the other hand, it may contribute to hyperinflammation and tissue damage during the later stages of the disease in a minority of patients.
Figure 1.
Description of Host-Pathogen Interactions between SARS-CoVs and the Host Immune System
The first event after inhalation of SARS-CoV is invasion of epithelial cells and type II pneumocytes through binding of the virus to angiotensin-converting enzyme 2 (ACE-2) receptors through the spike protein expressed on the surface of the virus. The complex formed is processed by TMPRSS2 leading to cleavage of ACE-2 and activation of the spike protein, facilitating viral entry into the target cell. Viral entry and cell infection trigger the immune response. Two possible mechanisms of initiation of the inflammatory cascade can be considered. One is release of danger signal molecules, such as certain cytokines (such as IL-1α or IL-8) or ATP. The second involves different recognition pathways mediated by immune cells that initiate the inflammatory response.
The major clinical complication in patients with COVID-19 is respiratory failure due to local hyperinflammation and acute respiratory distress syndrome (ARDS). The pathophysiology of these complications has strong similarities to other severe viral lung infections, such as influenza, and other infections caused by coronaviruses (SARS and Middle East respiratory syndrome [MERS]). An important mechanism mediating lung pathology in these infections is a cytokine storm leading to the so-called “macrophage activation syndrome” (MAS) (Channappanavar and Perlman, 2017, Huang et al., 2005, Wong et al., 2004). Indeed, an increasing number of very recent studies argue for an increased systemic inflammatory reaction in patients with severe SARS-CoV-2 infection. Circulating concentrations of proinflammatory cytokines, such as IL-6, tumor necrosis factor (TNF), MCP1, MIP1A, and IP10, are increased in COVID-19 patients in ICU when compared to those who do not need treatment in the ICU, although the concentrations of some of these cytokines are only moderately increased (Huang et al., 2020). This strong increase in systemic inflammation is associated with endothelial dysfunction, reflected by elevated d-dimers (Zhou et al., 2020) and hyperactive CCR6+Th17+ T cells locally in the lung (Xu et al., 2020). This is supported by a recent study that described the sequence of immune cell population changes in one patient with mild to moderate COVID-19 who recovered from infection (Thevarajan et al., 2020). In this patient, the increase in systemic concentrations of proinflammatory cytokines was minimal, even during days 7–9, when the patient was symptomatic. This suggests that a mild course of infection is associated with few systemic inflammatory effects.
These data therefore suggest that an exuberant innate immune response in the later part of the disease is the main immune dysregulation in patients with severe, but not mild, COVID-19 infection. However, new studies suggest that immune dysfunction in COVID-19 is more complex. In this respect, lymphopenia is one of the most important immune features of the disease that is associated with severity, with CD4 and CD8 lymphocytes being defective (Qin et al., 2020). Known age-related differences in the immune function of T cell and B cells could lead to insufficient control of viral replication, increasing disease severity. Consistent with this, a recent study shows hyperinflammation of the immune response in COVID-19 patients, characterized by high cytokine production capacity of circulating monocytes despite the severity of the disease (which is different from bacterial sepsis) and high systemic concentrations of proinflammatory cytokines (Giamarellos-Bourboulis et al., 2020). These data suggest that while sustained innate immune function leads to hyperinflammation, lymphocyte numbers decline and that their function may be defective.
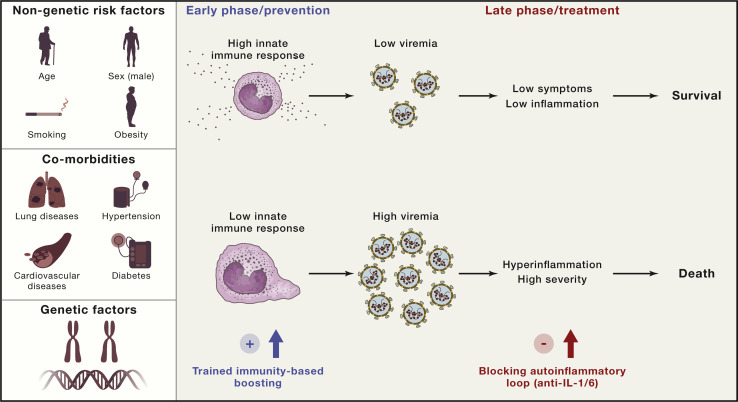
It can thus be postulated that, when someone with a potent immune response encounters the SARS-CoV-2 virus, strong activation of local innate and adaptive immune responses keeps viremia under control, leading to low systemic inflammation and recovery. In contrast, in individuals with a defective immune response because of old age or co-morbidities, unrestricted viral replication leads to high concentrations of the virus, which, in turn, triggers hyperinflammation and severe complications such as ARDS and death (Figure 2 ). It could therefore be argued that a rational immune-based therapy should be adapted according to the phase of the disease. In the uninfected host, the strategy should be to boost the innate immune responses to enable viral replication to be kept under control. In the later phase of the disease, the aim should be to interrupt the hyper-inflammatory loop to prevent severe complications. We therefore argue that induction of innate immune reprogramming (trained immunity) before infection may be a rational approach for boosting antiviral responses and thus preventing infection. Treatment with inhibitors of the IL-1/IL-6 pathway has been proposed to be useful in management of hyperinflammation in severely ill patients with COVID-19, and clinical trials with this strategy are underway.
Figure 2.
Innate Immune Dysregulation in the Pathophysiology of COVID-19
Genetic and non-genetic risk factors as well as the presence of co-morbidities determine the efficacy of host defense mechanisms. In the early phase of infection, a potent host defense leads to suppression of viral replication, which subsequently leads to low levels of inflammation, less severe symptoms, and a good prognosis. If host defense mechanisms are defective, then they can lead to massive viral replication, systemic hyperinflammation, high severity of disease, and, ultimately, death.
Reprogramming Innate Immune Responses: Trained Immunity and Viral Infections
Innate immune responses are the first line of defense against invading pathogens. Myeloid (e.g., neutrophils, monocytes, and macrophages) and lymphoid innate immune cells (e.g., natural killer [NK] and γδT cells) contribute to activation of host defense during infection, and they are able to eliminate the vast majority of microorganisms that infect us every day. Only when this first line of defense is overwhelmed by large numbers of pathogens or very invasive microorganisms is the adaptive immune response (T and B cells) activated. For a long time, it was believed that only cells of the adaptive immune system can mount immunological memory and protect against recurrent infection. This property of lymphocytes is the basis of vaccine efficacy against specific infections.
However, an increasing number of studies show that innate immune cells can also display adaptive characteristics after certain infections or vaccines, a property that is functionally similar to building immunological memory; this process has been termed trained immunity (Netea et al., 2020). Greater protection against reinfection, a de facto innate immune memory, has also been reported in plants and invertebrates, which lack an adaptive immune system. Studies performed in mice defective in functional adaptive immune responses, such as Scid or Rag1 −/− mice, also show that partial protection from reinfection can be mediated by innate immune cells (Kleinnijenhuis et al., 2012). Finally, long-term functional changes with increased antimicrobial function of innate immune cells has been demonstrated in humans after vaccination with live vaccines (Kleinnijenhuis et al., 2012).
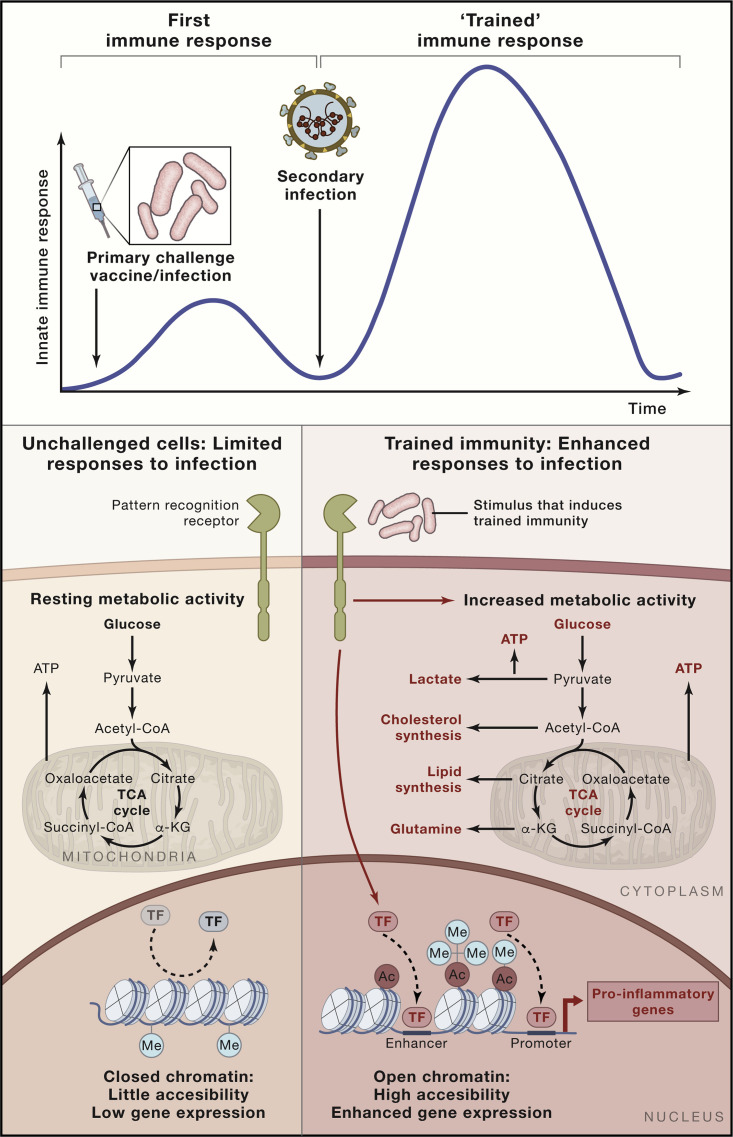
The molecular mechanisms responsible for long-term reprogramming of innate immune cells, such as monocytes, macrophages, and NK cells, are epigenetic in nature. An initial challenge of innate immune cells by vaccines or living pathogens during infection leads to activation of intracellular pathways that subsequently lead to upregulation of proinflammatory gene transcription. After the initial stimulus is eliminated or subsides, gene transcription returns to baseline. Transcription of genes important for host defense and production of proinflammatory cytokines is subsequently increased more potently when monocytes or macrophages are re-challenged with the same or a different stimulus (Netea et al., 2020). To allow this to happen, the cell machinery must grant access to the regions of the genome that contain the target sequences and the regulatory elements of the genes involved in these processes. This is regulated through durable epigenetic modifications, which allow unfolding of the chromatin and accessibility of transcription factors to the promoter and enhancer regions of the involved immune-related genes (Netea et al., 2020). The persistence of epigenetic modifications permits cells to remain in a “trained” functional state and allows increased accessibility to proinflammatory genes, facilitating faster and increased responsiveness after re-challenge. Metabolic processes leading to selective accumulation or depletion of certain metabolites of the central metabolism regulate this process because they function as co-regulatory molecules for epigenetic enzymes (Figure 3 ).
Figure 3.
Trained Immunity Mechanisms and Improvement of Anti-viral Host Defense
Trained immunity is defined as an enhanced innate immune response to different pathogens after an initial challenge, such as vaccination or infection. Certain microbial ligands capable of binding specific pattern recognition receptors are able to induce durable metabolic and epigenetic changes in innate immune cells. This reprogramming of the metabolic and epigenetic landscape of the cell allows quick accessibility of transcription factors to the promoter and enhancer regions of pro-inflammatory genes upon restimulation, facilitating gene expression. The increased metabolic activity of the cell affords fast supply of the energy and metabolites necessary to mount a robust immune response upon restimulation. Combination of these epigenetic and metabolic effects affords increased responsiveness upon secondary stimulation with the same or a different ligand and can even protect against a subsequent infection.
An important aspect relates to the durability of the trained immunity response, which is ensured at two levels: through effects at the level of bone marrow precursors (central innate immune memory) and at the level of tissue macrophages (peripheral innate immune memory). While transcriptional, epigenetic, and functional changes have been identified in circulating myeloid cells months after induction of trained immunity, these cells have a lifespan of only days in the circulation. This paradox has been explained by the finding that this process also takes place at the level of myeloid bone marrow progenitors, leading to a myelopoiesis bias and release of monocytes with a heightened preparedness to respond to pathogens (Mitroulis et al., 2018). Importantly, a second level at which trained immunity is induced is represented by the local environments in the tissue and, in the case of respiratory infections, in the lung. Experimental studies have demonstrated that alveolar or lung macrophages can also undergo long-term reprogramming after infection. A gammaherpesvirus infection can protect against an allergic response (Machiels et al., 2017), while adenovirus infection induced remodeling in alveolar macrophages and subsequently induced more pronounced anti-bacterial immunity (Yao et al., 2018). This suggests that induction of trained immunity can also provide protection from infections in which local host defense mechanisms are important in specific organs, such as the lung.
The vaccine that has probably been best studied for induction of trained immunity is the tuberculosis (TB) vaccine bacillus Calmette-Guérin (BCG). BCG is a live attenuated strain derived from an isolate of Mycobacterium bovis and is one of the most widely used vaccines in the world, with more than 130 million infants receiving this vaccine every year. Although developed as a vaccine against tuberculosis, numerous studies have shown its ability to induce potent protection against other infectious diseases, so-called off-target or non-specific effects (NSEs). Several epidemiological studies in low-income countries have shown an association between neonatal BCG vaccination and a profound reduction in neonatal mortality (Benn et al., 2013). Two meta-analyses performed at the request of the WHO have concluded that BCG vaccination is very likely to have important beneficial NSEs, although the quality of some of the studies was questioned, and it was concluded that more research is warranted (Higgins et al., 2016). In addition, experimental studies suggest that BCG may protect against viral pathogens, including respiratory syncytial virus, human papilloma virus, and herpes simplex virus (Freyne et al., 2015, Moorlag et al., 2019). Moreover, studies performed in mice provide evidence of an effect of intranasal BCG administration on secondary viral infections. In two separate studies, BCG reduced viral titers of influenza A virus compared with a control, which resulted in reduced inflammation and lung injury (Mukherjee et al., 2017, Spencer et al., 1977). Furthermore, enhanced resistance against various other viruses in BCG-vaccinated mice has been described, including herpes simplex virus type 1 (Floc’h and Werner, 1976) and 2 (Starr et al., 1976), Sendai virus (Ishihara et al., 1987), and ectromelia virus (Suenaga et al., 1978).
BCG vaccination of human healthy volunteers enhances production of pro-inflammatory cytokines such as IL-1β, TNF-α, and IL-6 by monocytes upon ex vivo stimulation with unrelated pathogens (Kleinnijenhuis et al., 2012). Moreover, we found that, among human healthy volunteers who received a yellow fever vaccine as a model of a viral human infection, those who had received the BCG vaccine 1 month earlier had less viremia and improved anti-viral responses compared with individuals who received a placebo (Arts et al., 2018). Only limited studies have assessed the protective heterologous effects of BCG vaccination in adults. A recent study in adolescents in South Africa, investigating the impact of BCG vaccination on M. tuberculosis infection, has recorded the incidence of other infections to assess the safety of the vaccination. While this was not a planned endpoint in the trial, it is interesting to observe that individuals vaccinated with BCG recorded a 73% reduction in respiratory tract infections compared with non-vaccinated individuals (Nemes et al., 2018). A small study in Indonesia in the elderly reported that multiple doses of BCG vaccination resulted in a significant reduction in the number of respiratory tract infections compared with a placebo (Wardhana et al., 2011). A clinical trial in Japan found a reduced risk of pneumonia upon BCG vaccination in previously tuberculin-negative elderly people (Ohrui et al., 2005).
Induction of Trained Immunity: a Tool against COVID-19
The discovery that BCG can induce trained immunity and offers protection against heterologous infections suggests that BCG vaccination may offer protection against respiratory tract infections, including viral infections. We therefore hypothesize that induction of trained immunity in general, and by BCG vaccination in particular, might be a potent preventive measure against SARS-CoV-2 infection and/or might reduce disease severity. The administration of the BCG vaccine in immunocompetent adults is safe, even in latently TB-infected adults and in those with prior BCG vaccination (Hatherill et al., 2014). In a randomized controlled trial that compared BCG revaccination with a novel TB vaccine and with a placebo, no vaccine-related serious adverse events were observed in the 312 patients in the BCG group (Nemes et al., 2018). Based on publicly available data on BCG vaccination and reported COVID-19 cases, several preliminary studies suggest that countries without universal policies of BCG vaccination (Italy, the Netherlands, Spain, the United States, and Ecuador) are more prone to be severely affected compared with neighboring countries with universal and long-standing BCG vaccination policies, which would be consistent with the idea that BCG protects the vaccinated elderly population. These data are, however, observational and prone to confounding issues such as limited testing and reporting in many countries; hence, they provide at most indirect support for a protective effect of BCG (Dayal and Gupta, 2020, Green et al., 2020, Miller et al., 2020).
A randomized controlled trial provides the highest quality of evidence for this research question. Given the immediate threat of the SARS-CoV-2 epidemic, trials should be designed as pragmatic studies with a highly feasible primary endpoint that can be measured continuously (on a weekly basis). This allows the most rapid identification of a beneficial outcome that would allow the community to also benefit from the intervention if and as soon as it has been demonstrated to be effective. Such trials could be first initiated in populations at high risk of infection or those at high risk of mortality; hospital staff caring for COVID-19 patients are in the first category, while elderly individuals or patients with co-morbidities are in the second. Although immunosenescence may result in less effective responses in the elderly, a recent study demonstrated largely preserved innate immune function, while adaptive immune responses (especially interferon γ [IFNγ]/IL-22 production) are defective (Ter Horst et al., 2016). These findings suggest that an effective trained immunity response after vaccination may also occur in the elderly.
Based on these data, trials assessing the efficacy of BCG vaccination in healthcare workers have been started in the Netherlands and Australia and are being prepared in Denmark, the United States, France, Uruguay, and South Africa. In addition, a randomized clinical trial in which hospitalized elderly patients without immunosuppression have been vaccinated at hospital discharge with a placebo or BCG is ongoing in Greece. The primary study endpoint is the time until the first infection. Enrollment has finished, and results are anticipated by October 2020. Moreover, another clinical study will assess the capacity of the VPM1002 vaccine to protect against COVID-19. VPM1002 is a BCG strain that was genetically modified to improve its immunogenicity by replacing the urease C-encoding gene with the listeriolysin-encoding gene from Listeria monocytogenes (Nieuwenhuizen et al., 2017). Finally, in Tanzania and Uganda, an RCT is under preparation that will randomize 2,000 older adults (>50 years) under chronic care for conditions like hypertension and diabetes.
In addition to BCG, induction of trained immunity by other vaccinations or microbial stimuli might be envisaged to protect against COVID-19. The measles vaccine and oral polio vaccine (OPV) have also been shown to provide protection against heterologous infections and mortality (for a review, see Goodridge et al., 2016). Based on these data, it is justifiable to also evaluate these vaccines for induction of a non-specific boost of anti-viral mechanisms against COVID-19. Similarly, intranasal administration of the TLR3 ligand poly(IC:LC) has been shown to protect against SARS in experimental models (Kumaki et al., 2017) and could be tested against infection with SARS-CoV-2.
Boosting or Inhibiting the Immune Response in COVID-19 Patients
In relation to the modulatory approaches presented here, we propose using strategies that can improve the anti-viral responses of the host (namely, trained immunity) to protect against infection with SARS-CoV-2. Induction of trained immunity with BCG vaccination results in a more effective cytokine response; cytokines important for anti-viral effects, including IL-1β, TNF, and IFNs, are more readily produced. Importantly, however, these cytokines are also present in high circulating concentrations in patients with severe forms of the disease and can contribute to ARDS (Channappanavar and Perlman, 2017). Inflammatory Th1/Th17 responses (Wu and Yang, 2020) may also contribute to lung injury in the late phase of the disease. Therefore, the theoretical possibility that induction of trained immunity could have deleterious effects by boosting or inhibiting innate immune responses needs to be considered.
The answer to this question relies on the pathophysiological stage of the disease when these two approaches are initiated. In healthy individuals who are vaccinated with BCG and in whom the innate antimicrobial mechanisms would be boosted by trained immunity, this is inferred to lead to decreased viremia, faster viral elimination as well as decreased inflammation, fewer symptoms, and quicker recovery. In this context, both the circulating concentrations of inflammatory cytokines and the inflammatory Th1/Th17 responses would be induced less strongly. In contrast, an initial defective antiviral response in some individuals at risk (e.g., the elderly) probably leads to high viremia, deleterious systemic inflammation, and severe disease. Breaking the loop of systemic inflammation in these patients (for example, by blocking the IL-1 pathway with anakinra or IL-6 function with tocilizumab) could have life-saving effects in some of these patients.
We thus propose that induction of trained immunity is an important host-directed approach in COVID-19 patients, which might lead to improved anti-viral host defense as well as decreased systemic inflammation, ultimately leading to a better chance of a favorable outcome (Figure 1).
Conclusions and Perspectives
The COVID-19 pandemic is the most serious global health crisis since the Spanish flu in 1918 and probably the most important global crisis since the end of the Second World War. In such difficult situations in which hundreds of thousands of patients have already been infected and thousands have died already, rapid mobilization not only of healthcare authorities and the medical community but also of the entire strength of the research community is needed to identify the best methods for diagnosis, prevention, and treatment of patients.
One important component of the pathophysiology of COVID-19 is dysregulation of inflammatory responses, and here we provided an overview of these processes and proposed a rationale for host-directed therapy based on induction of trained immunity in populations at risk.
Several very important questions need to be tackled urgently regarding SARS-CoV-2 infection. First, joint efforts should be made to decipher in detail the pathophysiology of the disease. While at this stage there are several similarities with other severe respiratory viral infections such as influenza, which help us to propose a first battery of immunomodulatory agents to be tested (such as anakinra or tocilizumab), there are also important differences and, most likely, additional pathways involved. Second, understanding in detail the pathophysiology of the disease will ultimately lead to better design of new trained immunity approaches, and large studies are needed to demonstrate clinically relevant benefits and identify subgroups with the highest medical gain.
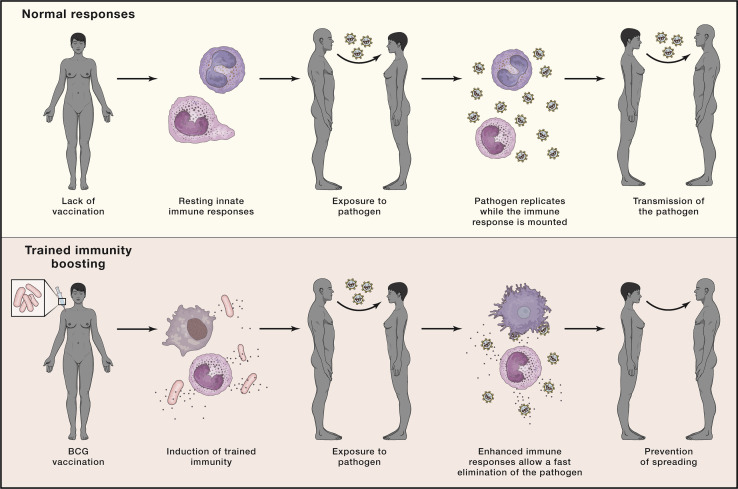
Finally, clinical trials testing the capacity of BCG to protect against COVID-19 have been initiated in several countries. However, BCG may not be the only vaccine that may have such positive heterologous effects; new recombinant MTB-based vaccines, such as VPM1002, or other vaccines, such as the measles vaccine and OPV, may have similar effects and are also considered for clinical trials. Such an approach to vaccination using trained immunity, even if successful, will only provide partial protection for a limited period of time. Therefore, induction of trained immunity while useful, is only a bridge toward development of a specific vaccine against SARS-CoV-2, which is the most important tool for controlling the pandemic. However, employing trained immunity boosters of the host defense, even if effective for a limited period of time, might contribute to reducing the spread of the infection in the first phases of a pandemic and are an important tool in the fight against a rapidly spreading emerging pathogen (Figure 4 ).
Figure 4.
Trained Immunity Can Be Used to Prevent the Spread of New Infections
Boosting the host defense by induction of trained immunity induces a durable state of activation in the cells of the innate immune system. In this scenario, these cells would offer faster and increased responsiveness upon contact with a new pathogen, decreasing the severity of the infection and limiting its transmission.
One important aspect that should be underlined for all components of research regarding COVID-19 is collaboration. In a severe crisis, such as the SARS-CoV-2 pandemic, full exchange of methodologies and protocols as well as collaboration within clinical trials is crucial for increasing the chances to identify new tools for diagnosis and therapy and improving the outcomes of our patients.
Acknowledgments
M.G.N. was supported by an ERC advanced grant (833247) and a Spinoza grant of the Netherlands Association for Scientific Research. R.v.C. is supported by the National Institutes of Health (R01AI145781) and European Union (RIA2018CO-2514-PROTID).
References
- Arts R.J.W., Moorlag S.J.C.F.M., Novakovic B., Li Y., Wang S.-Y., Oosting M., Kumar V., Xavier R.J., Wijmenga C., Joosten L.A.B. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe. 2018;23:89–100.e5. doi: 10.1016/j.chom.2017.12.010. [DOI] [PubMed] [Google Scholar]
- Benn C.S., Netea M.G., Selin L.K., Aaby P. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends Immunol. 2013;34:431–439. doi: 10.1016/j.it.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayal D., Gupta S. Connecting BCG Vaccination and COVID-19: Additional Data. MedRxiv. 2020 doi: 10.1101/2020.04.07.20053272. [DOI] [Google Scholar]
- Floc’h F., Werner G.H. Increased resistance to virus infections of mice inoculated with BCG (Bacillus calmette-guérin) Ann. Immunol. (Paris) 1976;127:173–186. [PubMed] [Google Scholar]
- Freyne B., Marchant A., Curtis N. BCG-associated heterologous immunity, a historical perspective: intervention studies in animal models of infectious diseases. Trans. R. Soc. Trop. Med. Hyg. 2015;109:52–61. doi: 10.1093/trstmh/tru197. [DOI] [PubMed] [Google Scholar]
- Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N., Damoraki G., Gkavogianni T., Adami M.E., Katsaounou P. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020 doi: 10.1016/j.chom.2020.04.009. Published online April 17, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowacka I., Bertram S., Müller M.A., Allen P., Soilleux E., Pfefferle S., Steffen I., Tsegaye T.S., He Y., Gnirss K. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011;85:4122–4134. doi: 10.1128/JVI.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodridge H.S., Ahmed S.S., Curtis N., Kollmann T.R., Levy O., Netea M.G., Pollard A.J., van Crevel R., Wilson C.B. Harnessing the beneficial heterologous effects of vaccination. Nat. Rev. Immunol. 2016;16:392–400. doi: 10.1038/nri.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C.M., Fanucchi S., Fok E.T., Moorlag S.J.C.F.M., Dominguez-Andres J., Negishi Y., Joosten L.A.B., Netea M.G., Mhlanga M.M. COVID-19: A model correlating BCG vaccination to protection from mortality implicates trained immunity. MedRxiv. 2020 doi: 10.1101/2020.04.10.20060905. [DOI] [Google Scholar]
- Hatherill M., Geldenhuys H., Pienaar B., Suliman S., Chheng P., Debanne S.M., Hoft D.F., Boom W.H., Hanekom W.A., Johnson J.L. Safety and reactogenicity of BCG revaccination with isoniazid pretreatment in TST positive adults. Vaccine. 2014;32:3982–3988. doi: 10.1016/j.vaccine.2014.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.P.T., Soares-Weiser K., López-López J.A., Kakourou A., Chaplin K., Christensen H., Martin N.K., Sterne J.A.C., Reingold A.L. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. BMJ. 2016;355:i5170. doi: 10.1136/bmj.i5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K.J., Su I.J., Theron M., Wu Y.C., Lai S.K., Liu C.C., Lei H.Y. An interferon-γ-related cytokine storm in SARS patients. J. Med. Virol. 2005;75:185–194. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara C., Mizukoshi N., Iida J., Kato K., Yamamoto K., Azuma I. Suppression of Sendai virus growth by treatment with N alpha-acetylmuramyl-L-alanyl-D-isoglutaminyl-N epsilon-stearoyl-L-lysine in mice. Vaccine. 1987;5:295–301. doi: 10.1016/0264-410x(87)90155-1. [DOI] [PubMed] [Google Scholar]
- Kleinnijenhuis J., Quintin J., Preijers F., Joosten L.A.B., Ifrim D.C., Saeed S., Jacobs C., van Loenhout J., de Jong D., Stunnenberg H.G. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA. 2012;109:17537–17542. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaki Y., Salazar A.M., Wandersee M.K., Barnard D.L. Prophylactic and therapeutic intranasal administration with an immunomodulator, Hiltonol® (Poly IC:LC), in a lethal SARS-CoV-infected BALB/c mouse model. Antiviral Res. 2017;139:1–12. doi: 10.1016/j.antiviral.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machiels B., Dourcy M., Xiao X., Javaux J., Mesnil C., Sabatel C., Desmecht D., Lallemand F., Martinive P., Hammad H. A gammaherpesvirus provides protection against allergic asthma by inducing the replacement of resident alveolar macrophages with regulatory monocytes. Nat. Immunol. 2017;18:1310–1320. doi: 10.1038/ni.3857. [DOI] [PubMed] [Google Scholar]
- Miller A., Reandelar M.J., Fasciglione K., Roumenova V., Li Y., Otazu G.H. Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: an epidemiological study. MedRxiv. 2020 doi: 10.1101/2020.03.24.20042937. [DOI] [Google Scholar]
- Mitroulis I., Ruppova K., Wang B., Chen L.S., Grzybek M., Grinenko T., Eugster A., Troullinaki M., Palladini A., Kourtzelis I. Modulation of Myelopoiesis Progenitors Is an Integral Component of Trained Immunity. Cell. 2018;172:147–161.e12. doi: 10.1016/j.cell.2017.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorlag S.J.C.F.M., Arts R.J.W., van Crevel R., Netea M.G. Non-specific effects of BCG vaccine on viral infections. Clin. Microbiol. Infect. 2019;25:1473–1478. doi: 10.1016/j.cmi.2019.04.020. [DOI] [PubMed] [Google Scholar]
- Mukherjee S., Subramaniam R., Chen H., Smith A., Keshava S., Shams H. Boosting efferocytosis in alveolar space using BCG vaccine to protect host against influenza pneumonia. PLoS ONE. 2017;12:e0180143. doi: 10.1371/journal.pone.0180143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemes E., Geldenhuys H., Rozot V., Rutkowski K.T., Ratangee F., Bilek N., Mabwe S., Makhethe L., Erasmus M., Toefy A., C-040-404 Study Team Prevention of M. Tuberculosis infection with H4:IC31 vaccine or BCG revaccination. N. Engl. J. Med. 2018;379:138–149. doi: 10.1056/NEJMoa1714021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea M.G., Domínguez-Andrés J., Barreiro L.B., Chavakis T., Divangahi M., Fuchs E., Joosten L.A.B., van der Meer J.W.M., Mhlanga M.M., Mulder W.J.M. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020 doi: 10.1038/s41577-020-0285-6. Published online March 4, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuizen N.E., Kulkarni P.S., Shaligram U., Cotton M.F., Rentsch C.A., Eisele B., Grode L., Kaufmann S.H.E. The recombinant bacille Calmette-Guérin vaccine VPM1002: Ready for clinical efficacy testing. Front. Immunol. 2017;8:1147. doi: 10.3389/fimmu.2017.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohrui T., Nakayama K., Fukushima T., Chiba H., Sasaki H. [Prevention of elderly pneumonia by pneumococcal, influenza and BCG vaccinations] Nippon Ronen Igakkai Zasshi. 2005;42:34–36. doi: 10.3143/geriatrics.42.34. [DOI] [PubMed] [Google Scholar]
- Ong E.Z., Chan Y.F.Z., Leong W.Y., Lee N.M.Y., Kalimuddin S., Mohamed S., Mohideen H., Chan K.S., Tan A.T., Bertoletti A. A dynamic immune response shapes COVID-19 progression. Cell Host Microbe. 2020 doi: 10.1016/j.chom.2020.03.021. Published online April 30, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. Published online March 12, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T., Morrison T.E., Funkhouser W., Uematsu S., Akira S., Baric R.S., Heise M.T. MyD88 is required for protection from lethal infection with a mouse-adapted SARS-CoV. PLoS Pathog. 2008;4:e1000240. doi: 10.1371/journal.ppat.1000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C.S., Nabar N.R., Huang N.N., Kehrl J.H. SARS-Coronavirus Open Reading Frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discov. 2019;5:101. doi: 10.1038/s41420-019-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulla A., Heald-Sargent T., Subramanya G., Zhao J., Perlman S., Gallagher T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J. Virol. 2011;85:873–882. doi: 10.1128/JVI.02062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer J.C., Ganguly R., Waldman R.H. Nonspecific protection of mice against influenza virus infection by local or systemic immunization with Bacille Calmette-Guérin. J. Infect. Dis. 1977;136:171–175. doi: 10.1093/infdis/136.2.171. [DOI] [PubMed] [Google Scholar]
- Starr S.E., Visintine A.M., Tomeh M.O., Nahmias A.J. Effects of immunostimulants on resistance of newborn mice to herpes simplex type 2 infection. Proc. Soc. Exp. Biol. Med. 1976;152:57–60. doi: 10.3181/00379727-152-39327. [DOI] [PubMed] [Google Scholar]
- Suenaga T., Okuyama T., Yoshida I., Azuma M. Effect of Mycobacterium tuberculosis BCG infection on the resistance of mice to ectromelia virus infection: participation of interferon in enhanced resistance. Infect. Immun. 1978;20:312–314. doi: 10.1128/iai.20.1.312-314.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter Horst R., Jaeger M., Smeekens S.P., Oosting M., Swertz M.A., Li Y., Kumar V., Diavatopoulos D.A., Jansen A.F.M., Lemmers H. Host and Environmental Factors Influencing Individual Human Cytokine Responses. Cell. 2016;167:1111–1124.e13. doi: 10.1016/j.cell.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevarajan I., Nguyen T.H.O., Koutsakos M., Druce J., Caly L., van de Sandt C.E., Jia X., Nicholson S., Catton M., Cowie B. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat. Med. 2020;26:453–455. doi: 10.1038/s41591-020-0819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totura A.L., Whitmore A., Agnihothram S., Schäfer A., Katze M.G., Heise M.T., Baric R.S. Toll-like receptor 3 signaling via TRIF contributes to a protective innate immune response to severe acute respiratory syndrome coronavirus infection. MBio. 2015;6:e00638. doi: 10.1128/mBio.00638-15. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardhana D., Datau E.A., Sultana A., Mandang V.V., Jim E. The efficacy of Bacillus Calmette-Guerin vaccinations for the prevention of acute upper respiratory tract infection in the elderly. Acta Med. Indones. 2011;43:185–190. [PubMed] [Google Scholar]
- Wong C.K., Lam C.W.K., Wu A.K.L., Ip W.K., Lee N.L.S., Chan I.H.S., Lit L.C.W., Hui D.S.C., Chan M.H.M., Chung S.S.C., Sung J.J. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Yang X.O. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 2020 doi: 10.1016/j.jmii.2020.03.005. S1684-1182(20)30065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Jeyanathan M., Haddadi S., Barra N.G., Vaseghi-Shanjani M., Damjanovic D., Lai R., Afkhami S., Chen Y., Dvorkin-Gheva A. Induction of Autonomous Memory Alveolar Macrophages Requires T Cell Help and Is Critical to Trained Immunity. Cell. 2018;175:1634–1650.e17. doi: 10.1016/j.cell.2018.09.042. [DOI] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]