Significance
The electrocatalytic conversion of CO2 to value-added products, especially valuable multicarbon products, is a pathway toward sustainable formation of chemicals and fuels typically derived from fossil fuels, while mitigating CO2 emissions. Fundamental understanding and development of more efficient catalysts for this reaction require deep investigation into structures with high intrinsic activity, which are limited at present. This work comprehensively investigates a dynamic copper nanoparticle ensemble catalyst that significantly improves intrinsic activity of copper for multicarbon formation. Through concerted ex situ and in situ characterization techniques, it illustrates an electrochemically induced fusion of copper nanoparticles that result in a catalytically active disordered structure, motivating closer study of disordered metal nanostructures for C–C coupling electrocatalysis.
Keywords: electrocatalysis, nanomaterials, CO2 reduction, structure dynamics
Abstract
Promotion of C–C bonds is one of the key fundamental questions in the field of CO2 electroreduction. Much progress has occurred in developing bulk-derived Cu-based electrodes for CO2-to-multicarbons (CO2-to-C2+), especially in the widely studied class of high-surface-area “oxide-derived” copper. However, fundamental understanding into the structural characteristics responsible for efficient C–C formation is restricted by the intrinsic activity of these catalysts often being comparable to polycrystalline copper foil. By closely probing a Cu nanoparticle (NP) ensemble catalyst active for CO2-to-C2+, we show that bias-induced rapid fusion or “electrochemical scrambling” of Cu NPs creates disordered structures intrinsically active for low overpotential C2+ formation, exhibiting around sevenfold enhancement in C2+ turnover over crystalline Cu. Integrating ex situ, passivated ex situ, and in situ analyses reveals that the scrambled state exhibits several structural signatures: a distinct transition to single-crystal Cu2O cubes upon air exposure, low crystallinity upon passivation, and high mobility under bias. These findings suggest that disordered copper structures facilitate C–C bond formation from CO2 and that electrochemical nanocrystal scrambling is an avenue toward creating such catalysts.
Structural evolution of materials under operation is a topic of emergent importance in the field of heterogeneous catalysis (1). Since nanoscale catalysts are highly susceptible to structural changes due to their high surface area and energetics, growing awareness of this phenomenon has led to deeper understanding of catalysis from a spectrum of works in both gas and liquid phase (1–4). The catalytic relevance of such dynamics to CO2 electrocatalysis has recently been highlighted in copper-based systems (5–8).
Understanding the structural requirements of copper to promote CO2 electroconversion to multicarbon products (CO2-to-C2+) is crucial for the development of catalyst materials in the field. With copper as the sole material to demonstrate CO2-to-C2+ at reasonable rate and efficiency (9), a host of high-activity copper catalysts have shown promise in achieving high C2+ activity/selectivity in bulk product formation rates (10–17). However, the intrinsic CO2-to-C2+ activity (e.g., μAC2+/cm2Cu) of many of these catalysts only marginally improves against a typical copper foil surface thus far, suggesting that nominal activity enhancements are inextricable from the modulation of extrinsic factors, such as local pH or mass transport, through catalyst microstructure (9). Hence, despite many investigations into the structural and/or compositional origin of CO2-to-C2+ (14, 15, 18–21), only few reports identify a core structural variable that correlates with and directly influences the intrinsic CO2-to-C2+ activity (22). We ascribe this discrepancy largely to two difficulties: 1) enriching the active surface on bulk-processed copper catalysts and 2) the tendency for materials to transform under electrochemical conditions.
Recently, we reported the structural transformation of a copper nanoparticle (Cu NP) ensemble into cuboidal structures, coupled with selective electroconversion of CO2 to multicarbons at lowered overpotentials (23). The observed coalescence of NPs toward distinct shape formation and desirable catalytic performance, in contrast to conventional sintering and deactivation, warrants a close study of the electrochemical evolution process. In light of both the prevalence of structural dynamics on copper nanocatalysts and the importance of understanding structural effects on intrinsic activity, such work may enable directed evolution strategies as new avenues to electrocatalyst development for CO2 utilization.
In this work, we probe the structural transformation process of the previously reported Cu NP ensembles under CO2 electroreducing conditions. By closely tracking the material state over electrolysis, an ex situ structural signature (i.e., single crystalline oxide nanocubes) is identified and confirmed to be unique to the C2+ active catalyst. This signature is determined to result from oxidation after removal of bias; hence, a chemical passivation technique is employed to impede this oxidation. Subsequent passivated ex situ characterization reveals a second signature: low crystallinity or enriched disorder, boosting the intrinsic C2+ activity by sevenfold over crystalline counterparts. Further in situ characterization suggests that the disordered state arises in part due to a high mobility of the initial state, resulting in a rapid transformation on a time scale of seconds. Together, these three lines of characterization illustrate a phenomenon we term “electrochemical scrambling,” in which cathodic bias induces the rapid fusion of nanocrystals into an active disordered state, highly susceptible to concurrent oxidation and crystallization upon exposure to ambient conditions. These findings motivate the closer study of disordered surfaces as structural characteristics responsible for enhancing CO2-to-C2+ turnover and raise the prospect of structural dynamics as an approach to catalyst design.
Results and Discussion
Colloidally synthesized Cu NPs (7 nm) were spread on carbon paper and biased in CO2 electroreducing conditions for multicarbon production in an aqueous H-cell environment (0.1 M KHCO3). The observed change by ex situ characterization is rapid and striking, with the appearance of cubic structures on the order of seconds and the predominance of these structures on the order of minutes (Fig. 1A and SI Appendix, Fig. S1). More negative potentials resulted in a more rapid evolution process (SI Appendix, Fig. S2). Regardless, the evolution of NPs under these biases eventually resulted in identical catalysts by both their morphology and catalytic activity (SI Appendix, Fig. S3).
Fig. 1.
Ex situ electron microscopy of the Cu NP ensemble evolution timeline. (A) SEM after removing the electrode at various time points of bias. (Scale bars, 100 nm.) (B) Schematic of the structural evolution. (C) TEM after 50 s (Left) and 3,600 s (Right) of bias. (Scale bars, 50 nm.) (D) HRTEM (Scale bar, 10 nm) of a single Cu2O cube at 50 s with fast Fourier transform showing Cu2O reflections (Scale bar, 2 nm−1) and a selected region at higher magnification (Scale bar, 2 nm). Electrochemical conditions are −0.6 V vs. RHE in 0.1 M KHCO3 at 1 atm CO2.
Qualitatively, the evolution is described by the loss of original NPs, being gradually replaced by cubic and cuboidal structures (Fig. 1B). The formation of cubes (Fig. 1C) is surprising given that the original NPs are spherical and exhibit fivefold twinned Cu (SI Appendix, Fig. S4). Furthermore, high-resolution transmission electron microscopy (HRTEM) reveals that these cubes are single-crystalline Cu2O (Fig. 1D). These observations are beyond the irregularly shaped structures typically expected from NP coalescence (24, 25). The copper appears to redistribute over the course of evolution, such that at intermediary time points when many particles have transformed the resultant cubes are smaller and more uniformly spread. Indeed, the 10- to 20-nm particles at later times are characterized as monocrystalline Cu2O cuboids as well (SI Appendix, Fig. S5). The prevalence of these structures with high crystallinity is confirmed on the ensemble by X-ray diffraction, most clearly seen at early time points (SI Appendix, Fig. S6). The Cu2O cubes grow with no preferred orientation on the graphitic support (SI Appendix, Fig. S7), suggesting the absence of interaction with the support as part of the process.
To quantitatively assess the evolution of metal/oxide phase distribution, ex situ Cu K-edge X-ray absorption near-edge structure (XANES) experimental spectra were least-square linear combination-fitted (LCF) using component phases. The XANES of the original Cu NPs was used alongside Cu0 and Cu2O standards for a three-component LCF at early time points (Fig. 2A). The Cu NPs are suitable as a phase component as they are observed at those times by microscopy, and their XANES exhibits unique features poorly accounted for by other standard Cu phases (SI Appendix, Fig. S8). The relative phase portions were extracted from the XANES spectra as the evolution proceeds (SI Appendix, Fig. S9).
Fig. 2.
Ex situ Cu K-edge XANES spectroscopy of catalysts. (A) Three standards used in this work: Cu, Cu2O, and 7-nm Cu NPs. (B) XANES time series over electrolysis (−0.6 V vs. RHE) time ex situ with arrows indicating Cu2O features. (C) Shift in composition tracked by LCF. (D) Catalytic selectivity for C1 and C2+ products in samples O, L, and P (error bars 1 SD, n = 3) at −0.8 V vs. RHE. (E) XANES of O, L, and P after 1 h at −0.8 V vs. RHE. (Inset) The correlation of phase composition and C2+ selectivity. (F) Scheme summarizing the catalytic relevance of the Cu2O structures observed uniquely in sample O.
Cursory analysis of the time series (Fig. 2B) shows a rapid increase in intensity of the rising edge 1s → 4px,y shake-down feature (∼8,984 eV) and a fingerprint crest (∼9,015 eV) indicative of Cu2O (26). Quantitative tracking by LCF more visibly demonstrates that the early loss of Cu NPs is counterbalanced by a rapid growth of Cu2O cubes (Fig. 2C). Beyond this critical period of Cu2O formation ending around 600 s, most of the steady-state structure is characterized by the retention of cuboidal Cu2O crystals. At more negative potentials, the phase evolution is accelerated but results in a similar final phase distribution (SI Appendix, Fig. S10). The minor metallic Cu phase is mainly found at the junctions of aggregates of Cu2O cubes and less frequently in polycrystalline particles with no distinct morphology (SI Appendix, Fig. S11). Thus, ex situ XANES further confirms that the evolution of Cu NPs results in Cu2O cuboidal rich structures as first identified using ex situ electron microscopy.
To evaluate the catalytic relevance of the appearance of Cu2O cuboids, we considered two methods of varying the original evolved Cu NP system (sample O). First, larger 14-nm Cu NPs (sample L) were synthesized to controllably change the starting state of the material (SI Appendix, Fig. S12). The characteristic cube formation is absent for these larger particles under similar electrochemical conditions (SI Appendix, Fig. S13). Furthermore, the phase distribution remains mostly metallic throughout electrolysis (SI Appendix, Fig. S14). Second, we also aimed to disrupt the evolution process of the original NPs by applying a voltammetric pulse perturbation program. The perturbation is applied at frequent intervals throughout the critical period of evolution, interrupting the transformation with a mild oxidation pulse (SI Appendix, Fig. S15). As a result, despite beginning with the same NPs, the pulsed electrode (sample P) consists of larger, more irregular morphologies compared to sample O (SI Appendix, Fig. S16).
A substantial reduction of C2+ formation is observed for samples L and P relative to O (Fig. 2D and SI Appendix, Fig. S17), in conjunction with a more metallic composition by XANES (Fig. 2E and SI Appendix, Figs. S14 and S18) and more polycrystalline aggregates of Cu as opposed to cuboidal oxides (SI Appendix, Fig. S19). As such, we find that the catalytic variants lacking C2+ selectivity also have lower Cu2O signature in their ex situ composition (Fig. 2 E, Inset and SI Appendix, Table S1). These ex situ single-crystalline cuboidal Cu2O structures are therefore a signature for catalytically enhanced C2+ formation. Variations of the evolution pathway, either by modifying the initial state or by perturbing the evolution process, shift the distribution of catalyst structures toward polycrystalline NPs with a high proportion of metallic Cu postreaction, correlated with lower activity for C2+ formation (Fig. 2F).
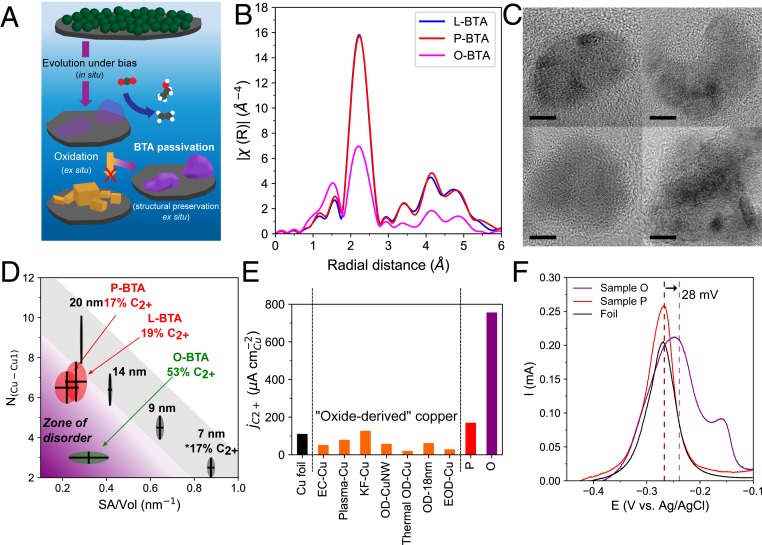
However, formation of Cu2O is thermodynamically disfavored under CO2 reducing conditions (27, 28), suggesting that the Cu2O cubes should not have formed in situ. Indeed, when Cu2O nanocubes are directly grown on the carbon support, activity is strictly limited to hydrogen evolution and C1 formation at potentials where sample O makes majority multicarbons (SI Appendix, Fig. S20). Furthermore, Cu2O electrochemically grown on carbon, after CO2 electrolysis, is dominated by metallic Cu consisting of small particles and broken cubes with their facets destroyed (SI Appendix, Fig. S21). Hence, neither crystalline cubic Cu2O itself nor its reduced equivalent is the active phase in the transformed Cu NP ensemble, despite the above ex situ observations. These findings suggest that a different structure, most likely metallic, is formed in situ and is uniquely active for CO2-to-C2+. Moreover, the structure readily and distinctively oxidizes to the single-crystal Cu2O cubic signature at open circuit. To identify this structure, we employed a chemical immersion passivation method using benzotriazole (BTA), which is known to prevent copper oxidation (Fig. 3A) (29). Immersion into a BTA solution immediately postelectrolysis coordinates BTA to the surface, preventing the formation of Cu2O single crystals and preserving more of the underlying metallic portion as characterized by XANES, X-ray diffraction, scanning transmission electron microscopy coupled with electron energy loss spectroscopy (STEM-EELS), and X-ray photoelectron spectroscopy (XPS) (SI Appendix, Figs. S22–S25).
Fig. 3.
Ex situ elucidation of the disordered C–C active surface. (A) BTA passivation diverts oxidation of the active catalyst for characterization. (B) R-space EXAFS of BTA-passivated O, L, and P after 1 h at −0.8 V vs. RHE. (C) HRTEMs of O-BTA. (Scale bar, 5 nm.) (D) Relationship between SA/Vol and NCu-Cu1, showing unusually low coordination of O-BTA. Error bars are 1 SD (see SI Appendix, Supplementary Discussion S1 for details on SA/Vol and SI Appendix, Figs. S26 and S28 for EXAFS fit details). Isolated 7-nm NPs catalytic activity from previous work at −0.8 V vs. RHE (23). (E) Specific ECSA-normalized C2+ partial current density at −0.8 V vs. RHE for foil (14), high-performance oxide-derived catalysts from literature (see SI Appendix, Fig. S36 for details), P, and O. (F) Desorption of underpotentially deposited Pb as an indicator for adsorbate binding strength, where sample O exhibits anodic shifts (peaks at −0.25 and −0.16 V vs. Ag/AgCl) associated with its disordered surface. Dashed lines represent centroids for each anodic stripping voltammogram.
Cu K-edge extended X-ray absorption fine structure (EXAFS) was then used to probe the difference between the three catalysts when oxidation is inhibited by BTA (O-BTA, L-BTA, and P-BTA). A clear feature of O-BTA is the lower scattering amplitude from the first Cu shell of the metallic phase at 2.1 Å, with its subsequent shells following a similar trend (Fig. 3B). These EXAFS spectra were then fit in q-space to derive a numerical description of the coordination environment (SI Appendix, Fig. S26). The EXAFS-derived fitting parameter NCu-Cu1 is used here as a quantitative descriptor of this empirical scattering amplitude difference. According to the fits (SI Appendix, Table S2), O-BTA exhibits a considerably lower NCu-Cu1 (3.0) compared to the other catalysts L-BTA (NCu-Cu1 = 6.8) and P-BTA (NCu-Cu1 = 6.5). To contextualize this trend, a series of NPs with varying size and well-defined crystallinity were employed as standards (SI Appendix, Fig. S27). The EXAFS-derived NCu-Cu1 is expected to decrease for smaller-sized NPs (SI Appendix, Figs. S28 and S29) by the rise in the surface area-to-volume ratio (SA/Vol); however, NCu-Cu1 for O-BTA is unexpectedly low given its size of structures (∼20 nm). Furthermore, all three BTA-passivated samples are composed of similarly sized nanostructures (SI Appendix, Fig. S30) and therefore the difference in SA/Vol does not sufficiently explain the difference in NCu-Cu1 between O-BTA and L/P-BTA.
Low NCu-Cu1 can result not only from having a higher portion of atoms with missing neighbors but also by local atomic disorder, which leads to destructive interference of the scattered X-rays (30). A chemical effect should also be considered—the imperfectly passivating effect of BTA results in some remnant oxide as evidenced by the rise in amplitude at 1.5 Å, which would partially contribute to a lower observed NCu-Cu1. Nevertheless, crystalline domains in O-BTA are difficult to detect by HRTEM (Fig. 3C), in contrast with L/P-BTA where extended metallic domains are easily identified (SI Appendix, Fig. S31). Nanobeam electron diffraction (NBED) (SI Appendix, Fig. S32) suggests a relative lack of crystallinity in O-BTA as well. Meanwhile, control experiments on model Cu-based nanocrystal systems (i.e., single nanocrystals and the original fivefold twinned NPs) confirm that BTA coordination alone is insufficient to disrupt the inherent crystallinity of a Cu nanostructure (SI Appendix, Figs. S33 and S34). Therefore, despite some limitations of the BTA immersion technique, the overall implication of a disordered active structure is considered the most likely interpretation of these results.
These observations indicate that O-BTA achieves a unique state of structural disorder characterized by a low EXAFS-derived NCu-Cu1 at low SA/Vol (Fig. 3D; calculation details in SI Appendix, Supplementary Discussion S1). L/P-BTA exhibit similarly low SA/Vol but higher NCu-Cu1, indicating that higher crystalline order is linked to their lower C2+ selectivity. Furthermore, low NCu-Cu1 achieved by high SA/Vol in a crystalline state is likewise insufficient to attain high C2+ selectivity at low overpotentials, as evidenced by the activity of isolated small Cu NPs (Fig. 3D) (23). These findings strongly suggest that the electrochemical “scrambling” of the original NPs creates a disordered active surface (low NCu-Cu1 with low SA/Vol) catalytically favorable for CO2-to-multicarbons. Previous works have conjectured C2+-boosting effects of tuning binding strength of key intermediates, especially *CO, on diverse and undercoordinated copper surfaces (15, 21, 31). Accordingly, a host of high-activity oxide-derived copper catalysts may present such disordered surface sites. However, the direct identification/quantitation of disorder in these catalysts and its effect on binding has not emerged, likely because the large volume involved of the necessary copper oxides as precursors (e.g., oxidized Cu foils) yields a large proportion of bulk atoms that are catalytically irrelevant but spectroscopically obtrusive for ensemble characterization. Furthermore, because such OD-Cu catalysts tend to have high roughness factors, they may present a complex mix of disordered/undercoordinated active sites and catalytically inactive copper surfaces, one possible explanation for the lack of intrinsic activity increase previously observed (9).
To quantify the enhancement of intrinsic C–C activity owing to the disordered Cu resulting from electrochemical NP scrambling, we estimated the electrochemical surface area (ECSA)-normalized specific C2+ activity (μA/cm2Cu) of the catalysts using lead underpotential deposition to measure Cu ECSA (SI Appendix, Fig. S35 and Supplementary Discussion S2). Sample O exhibits a considerable sevenfold increase in C2+ activity to that of a Cu foil, whereas the polycrystalline sample P exhibits only a minor increase (Fig. 3E). OD-Cu catalysts (10, 14–16) that show high performance by typically presented metrics such as F.E. (SI Appendix, Fig. S36) demonstrate similar or lower intrinsic activity to sample P or Cu foil as well (9). In contrast, the enrichment of disorder on sample O is directly responsible for a higher C–C dimerization activity per copper surface available, ranging from 6- to 40-fold enhancement over a representative spread of OD-Cu catalysts (Fig. 3E and SI Appendix, Fig. S37) at moderate overpotentials [−0.8 V vs. the reversible hydrogen electrode (RHE)].
The high degree of disorder exhibited by sample O is expected to result in a large concentration of strong binding sites for the key intermediate *CO, thereby increasing the probability and rate of C–C dimerization. Desorption of underpotentially deposited Pb adatoms sheds some light into the Cu surface (Fig. 3F). Sample P and Cu foil both exhibit anodic stripping of Pb in the same potential region of around −0.27 V vs. Ag/AgCl. Meanwhile, sample O exhibits Pb desorption peaks (−0.25 and −0.16 V vs. Ag/AgCl) that are quite anodically shifted compared to sample P or Cu foil (−0.27 V vs. Ag/AgCl), resulting in a +28-mV shift in the centroid of the anodic stripping wave. In particular, the salient component at −0.16 V is not observed in the crystalline Cu samples in this work or in previous studies of Pb underpotential stripping on polycrystalline Cu surfaces composed of common low-index facets or on single-crystal Cu (32, 33). This indicates that sample O contains a prevalence of Cu surfaces that should have stronger electrochemical binding to adsorbates, with a lower presence of the sites on the crystalline counterpart (i.e., Cu foil) that exhibit weaker binding. The anodic shift is drastically diminished when the catalyst oxidizes to the characteristic single-crystal Cu2O cubes, where the reduced equivalent is not disordered as before (SI Appendix, Fig. S38). It is also observed in a greatly reduced manner on a representative OD-Cu catalyst (SI Appendix, Fig. S38), which explains the low intrinsic C2+ activity resulting from their large portion of inactive Cu surfaces. Hence, enhanced C–C activity in sample O stems from the concentration of structural disorder, which creates surfaces of increased electrochemical binding strength to catalytic intermediates.
To determine whether any signature of the evolution can be observed in situ, we used liquid cell electrochemical (EC) XANES and (S)TEM to directly visualize their formation from the starting NP ensemble (Fig. 4A). Upon reductive bias, the EC-XANES shows a rapid (<40 s) transition to metallic Cu (Fig. 4B), which develops its characteristic oxidized feature after exposure to ambient conditions (SI Appendix, Fig. S39). This confirms that the phase of the in situ active structure is Cu0, formed almost immediately upon bias, and that Cu2O only develops afterward at open circuit.
Fig. 4.
In situ characterization of the Cu NP ensemble evolution under bias. (A) Configurations used for in situ EC-XANES and EC-(S)TEM. (B) EC-XANES with the initial (top) scan at open circuit and the remainder during bias at -0.6 V vs. RHE. (C) EC-HAADF-STEM of the 7-nm Cu NP system taken over a 10-s biasing period (frames from Movie S1; scale bars, 100 nm). (D) Ex situ TEM of the EC-STEM electrode after biasing, showing the expected NP-to-cube evolution (scale bar, 100 nm), with NBED showing single-crystal Cu2O (Scale bar, 2 nm−1). (E) Unified description of electrochemically scrambled nanocrystals with disordered surfaces that enhance CO2-to-C2+, vs. relatively inactive polycrystalline Cu nanocrystals.
NP evolution was also visually tracked by EC–high-angle annular dark-field (HAADF)–STEM (SI Appendix, Fig. S40). An electrochemical potential of −0.6 V vs. the internal reference electrode (C/Pt) was selected to induce electrochemical evolution and cathodic current, but without rupturing the cell from vigorous gas product formation. Based on water-splitting cyclic voltammetry control experiments within the cell, this potential is negative of the onset potential for the hydrogen evolution reaction (SI Appendix, Fig. S41). Within a second of bias, ∼20-nm structures appear to grow from the 7-nm NPs (Fig. 4C, Movie S1, and SI Appendix, Fig. S42). When the EC-TEM cell is then dried at ambient conditions, cubic structures are commonly found on the electrodes, which are characterized as single-crystal Cu2O (Fig. 4D and SI Appendix, Fig. S43), confirming that catalytically relevant structures have formed for the biasing period of EC-TEM. In contrast, larger (15- to 20-nm) NPs do not undergo much structural change even after 60 s of bias (Movie S2 and SI Appendix, Fig. S44), as expected from the postelectrolysis structures of sample L that indicate minimal movement (SI Appendix, Fig. S13). These in situ results confirm that the evolution process unique to sample O occurs rapidly, on the order of seconds. Further resolution of the structural details of these systems was limited due to the NPs’ dynamic nature (SI Appendix, Supplementary Discussion S3 and Movies S3 and S4).
In conjunction with our ex situ observations, we find that electrochemical bias induces rapid fusion of an NP ensemble into a disordered metallic steady state (sample O) with high C2+ selectivity and intrinsic C–C activity, which we term scrambling (Fig. 4E). This relative loss of atomic order is likely what triggers a complete “recrystallization” to a single crystalline Cu2O nanocube on air exposure. The effect is largest for small nanocrystals, owing to their higher energy increasing the tendency for free atomic migration upon bias as represented by their high mobility. In contrast, larger NPs scramble to a lesser extent, while redox pulses allow periods of oxide crystallization to interrupt the scrambling process, resulting in samples L/P exhibiting more typical polycrystalline Cu structures surrounded by oxide shells. The disorder-derived active surfaces arising from scrambling exhibits increased electrochemical binding strength, which potentially translates to a higher population of strongly bound *CO. This effect may be related to previous reports of disorder-induced microstrain modulating electrocatalytic activity (34). Additionally, relaxing the geometric constraint of crystallinity may allow configurations that alleviate the undesired repulsive interactions of a high density of bound *CO (35), comparable to the creation of optimal geometries in enzymatic systems. The sum of these effects potentially contributes to the overpotential reduction toward C–C coupling in the scrambled nanocrystal system. Catalysts exhibiting a lower degree of disorder (e.g., samples L/P) thus are only able to yield comparable C2+ activity at elevated potentials (−0.9 V and beyond) (SI Appendix, Fig. S45).
The mechanism of disorder development in the scrambled NP system should stem from the structural details of the original NP. For instance, the fivefold twinned 7-nm NP structure contains a high proportion of atoms at grain boundaries, where disorder-forming processes such as premelting are observed in model crystal systems (36). The disorder may also be intrinsic to the effect of reducing an oxide shell, as is present on the original NPs and has been suggested previously (18). However, since not every reduced oxide can achieve the same catalytic activity (as shown by the Cu2O cubes as-grown), the NP fusion involved in the scrambled system is likely to be critical to preserving such characteristics. For example, as the fusion occurs within the NP ensemble, the presence of a high grain boundary density may result in pinned crystallite junctions that impede boundary migration and crystal growth as evolution proceeds (37). Additional factors not presently investigated, such as the behavior of surface ligands under bias, may also play a role. Eventually, unraveling the atomistic details of the electrochemical nanocrystal scrambling process will contend with the complex interplay among an ensemble of particles composed of nanoscale-twinned Cu cores, thin Cu2O shells, and surrounding ligands, under biased conditions. Additionally, the question of how it is maintained raises an intriguing opportunity to understand the structural requirements for its sustenance, in the context of translating such insights to other materials. We expect more advanced operando techniques as well as cryo–X-ray absorption spectroscopy (XAS) (38) to yield important new insights, presenting opportunities for designing catalysts with the necessary characteristics to attain similarly active states.
In conclusion, we have comprehensively probed the dynamic fusion of Cu NPs under CO2 reducing conditions to form multicarbon-active scrambled nanocrystals. These structures are described by three distinct signatures: ex situ formation of single-crystal oxide cubes, low coordination and crystallinity after passivation, and high mobility observed in situ. Together, these observations suggest an enriched disorder in the copper phase with substantially enhanced intrinsic CO2-to-C2+ compared to crystalline Cu, motivating closer investigation into the atomic details of low-crystallinity Cu surface motifs and their preservation throughout. Moreover, we emphasize that a full understanding of active catalysts, which are more likely to be structurally unstable, will require ex situ, quasi-in situ, and operando techniques explored in concert.
Materials and Methods
NP Synthesis and Electrode Fabrication.
Seven-nanometer copper NPs were synthesized as previously reported (23). For larger NPs, size was controlled by tuning the mole ratio of tetradecylphosphonic acid to copper(I) acetate (CuAc), where higher ratio resulted in larger particles. Specifically, to synthesize 7-, 9-, 14-, and 20-nm NPs, ratios of 0.5, 0.7, 1, and 1.25, respectively, were used while maintaining the absolute concentration of CuAc (1 mmol).
NP concentrations by mass of copper were measured by inductively coupled plasma optical emission spectroscopy, after which 68.9 μg of copper was deposited on 1-cm2 area of carbon paper (Sigracet 29AA; Fuel Cell Store) to make working electrodes. Larger NPs were deposited in a similar manner with identical mass loading.
For electrochemically grown Cu2O nanocubes, a square-wave voltammetric pulse program was employed as reported previously. Cu2O nanocubes were directly grown on carbon paper (Sigracet 29AA) (39).
For copper foil-derived electrodes, copper foil (99.9999%; Alfa Aesar) was electropolished in 85% H3PO4 at 3 V for 3 min against a copper counterelectrode. Electrochemically cycled oxide-derived copper was then fabricated following the method previously reported for EC–Cu (14).
Electrochemical Evolution of NPs and Catalytic Activity Measurements.
The customized electrochemical setup used to carry out bias-induced evolution of NPs and catalytic testing has been described previously (23). All electrochemical measurements were made against a 3 M KCl Ag/AgCl reference electrode (World Precision Instruments). For all biasing experiments, the bias was applied directly without a prior linear sweep. Evolution was typically carried out by directly applying −1.2 V vs. Ag/AgCl, while catalytic results were measured at −1.45 V vs. Ag/AgCl. These potentials were considered equivalent by the steady-state structure made, as elaborated upon in SI Appendix, Fig. S3. All chronoamperometry was corrected with 85% of the solution resistance (Biologic), with the remaining 15% corrected afterward. Gas and liquid products were measured by a gas chromatograph [equipped with a thermal conductivity detector and a flame ionization detector with methanizer (SRI)] and NMR with water suppression (Bruker Avance 500), respectively.
For pulsed preparation of sample P, the potential was varied between −1.2 V vs. Ag/AgCl and 0.0 V vs. Ag/AgCl with periods of 2.5 s each. This pulse program was repeated 240 times, for a total of 1200 s of bias, before the potential was immediately switched to −1.45 V vs. Ag/AgCl for catalytic testing.
Passivation with BTA (Sigma-Aldrich 99%) was conducted after electrolysis by removing the electrode from the cell and, within a short period time, immersing the electrode in a premade 10 mL bath of BTA dissolved in CO2-saturated 0.1 M KHCO3. The amount of BTA was set to a mole ratio of 1000 mol BTA to 1 mol Cu (the maximum concentration shown in SI Appendix, Fig. S22).
Lead underpotential deposition was conducted immediately postelectrolysis in a solution of 0.1 M NaClO4, 10 mM HClO4, and 3 mM Pb(II)(ClO4)2. Cyclic voltammetry in the Pb underpotential region was conducted at 10 mV/s six times, for which the cycles were confirmed consistent and the fifth scan was reported. The potentials are referenced against a 1.0 M KCl Ag/AgCl electrode.
Ex Situ Structural Characterization.
Electrodes were directly imaged by SEM (Ultra 55-FESEM), and samples were crushed into a fine powder for powder X-ray diffraction (Bruker D8). For TEM sample preparation, the carbon paper electrode was crushed into a fine powder and dropcast on a TEM grid, typically lacey carbon, from a dispersion in toluene. Low magnification TEM (Hitachi H-7650) was conducted at 100 kV, while HRTEM and STEM-EELS (Tecnai F20 UT) were conducted at 200 kV. NBED, along with additional HRTEM (JEOL 2100-F), were also conducted at 200 kV. XPS (K-Alpha ; Thermo Scientific) was measured using an Al Ka source with spectra collected at a pass energy of 20 eV and step size of 0.05 eV.
XAS.
XAS experiments were conducted at the Advanced Light Source X-Ray Fluorescence Microprobe beamline 10.3.2 following previously reported procedures for Cu NPs (40). All data were recorded in fluorescence using quick-XAS (QXAS) mode [where the Si(111) is scanned on the fly], using a seven-element Ge solid-state fluorescence detector. Energy calibration was performed using a Cu foil (first derivative maximum set at 8980.48 eV) (41). For ex situ measurements, electrodes were biased in the typical manner and removed for measurement under ambient conditions. Cu K-edge XANES data processing (deadtime correction, deglitching, energy calibration, preedge background substraction, and postedge normalization), and least-square linear combination fits (LCF), were conducted with the LabVIEW custom software available at https://sites.google.com/lbl.gov/lbnl-als-1032/software-download. In addition, LCFs were performed in Athena for corroboration; the variation of phase contributions between these two programs did not exceed 5% in all cases.
EXAFS data reduction was performed in Athena and EXAFS fitting was performed in Artemis according to previous procedures (40). Both programs are part of Demeter package 0.9.26 (42). For EXAFS data fitting of the various Cu samples, two FEFF models of Cu metal and Cu2O were generated. They were made according to the well-known crystal structure (both face-centered cubic with respect to Cu) of the bulk materials. All Cu NPs samples were fit using the first three single scattering paths and the collinear triple scattering path to the fourth shell from Cu metal, as well as the first O single scattering path in Cu2O. Addition of the first Cu single scattering path in Cu2O was required to fit the samples containing a more prominent oxide phase.
In situ XAS was conducted in a custom-designed polyether ether ketone electrochemical cell. A working electrode was fabricated by depositing Cu NPs over a 1-cm2 area of carbon paper (Sigracet 29AA), which was pressed against a 300-μm Kapton window. The cell was filled and constantly purged at under 1 sccm with 0.1 M KHCO3 kept under CO2 saturation. Open circuit voltage was measured during purging, and XANES scans were taken after the cell was completely filled. To begin electrochemical evolution, −1.3 V vs. Ag/AgCl was applied vs. the 3 M KCl Ag/AgCl reference electrode with a glassy carbon counter electrode. Due to the rapid structure evolution under bias, the XANES spectral range was shortened so that each spectrum took only ∼40 s, increasing temporal resolution for qualitative study. Depending on the desired duration of bias, 1 to 20 QXAS lines were measured before electrolysis was stopped and the cell disassembled under ambient conditions.
In Situ Electron Microscopy.
All in situ electron microscopy was conducted at 200 kV (2100-F; JEOL). Commercial electrochemical TEM chips (XL P7 carbon electrochemical chip; Hummingbird) were used with a 500-nm silicon nitride spacer (Hummingbird), such that the total assembly included 100 nm of silicon nitride and 500 nm of liquid volume. All biasing leads were carbon-coated Pt as supplied by the manufacturer.
To create working electrodes, a 2-μL droplet of a dilute (25 μg/mL) solution of Cu NPs in hexanes was spread over the viewing area of the chip. The droplet was allowed to evaporate, after which the full cell was assembled. Three hundred microliters of ethanol were first purged through, after which 1 to 1.5 mL of 0.1 M KHCO3 or pure H2O were purged at 10 μL/min in order to wet and fill up the entire cell. Control experiments were conducted with Pt/C particles (20 wt % nominal; Alfa Aesar) using a similar loading strategy, without ethanol purging.
Electrochemical testing was conducted in a specially designed biasing holder (Hummingbird) with a floating potentiostat (Gamry Interface 1000), using a three-electrode configuration. The biasing potential was set at −0.6 V vs. the carbon pseudoreference electrode, at which chronoamperometry using a typical cell produced ∼500 nA of reductive current. Higher currents/potentials (e.g., >1 μA at −0.8 V vs. the carbon pseudoreference) were deemed unsuitable due to violent ruptures of the electrochemical cell, while significantly lower currents (i.e., order of 10 to 100 nA) failed to reproduce the cubic oxide structures postelectrolysis in favor of adventitious side reactions such as the growth of polycrystalline copper.
During bias, electrolyte purging was halted. Real-time videos were recorded using a screen capture program at five frames per second, matching both the raster rate of the STEM beam over the viewing frame and the exposure time of the camera during bright-field TEM. After electrochemical imaging experiments, the holder was disconnected from the potentiostat. The cell was then directly disassembled and the working chip rinsed gently with H2O before drying under N2. The chip was then imaged by TEM and NBED (JEOL 2100-F) under vacuum conditions.
Data Availability.
All data analyzed in this work are included in the main text and SI Appendix.
Supplementary Material
Acknowledgments
This work was supported by Director, Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences, & Biosciences Division, of the US Department of Energy under Contract DE-AC02-05CH11231, FWP CH030201 (Catalysis Research Program). TEM, SEM, and XPS were conducted using facilities at the National Center for Electron Microscopy and Imaging and Nanofabrication facilities at the Molecular Foundry. Work at the Molecular Foundry was supported by the Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under Contract DE-AC02-05CH11231. We thank Hao Zhang for her help in conducting XAS experiments. The Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under Contract DE-AC02-05CH11231. This work made use of the facilities at the NMR Facility, College of Chemistry, University of California, Berkeley. Inductively coupled plasma optical emission spectrometry was supported by the Microanalytical Facility, College of Chemistry, University of California, Berkeley. D.K. and S.Y. acknowledge support from the Samsung Scholarship. C.X. and Q.K. acknowledge support from the Suzhou Industrial Park fellowship.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1918602117/-/DCSupplemental.
References
- 1.Tao F. F., Crozier P. A., Atomic-scale observations of catalyst structures under reaction conditions and during catalysis. Chem. Rev. 116, 3487–3539 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Tao F., et al. , Reaction-driven restructuring of Rh-Pd and Pt-Pd core-shell nanoparticles. Science 322, 932–934 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Cui C., Gan L., Heggen M., Rudi S., Strasser P., Compositional segregation in shaped Pt alloy nanoparticles and their structural behaviour during electrocatalysis. Nat. Mater. 12, 765–771 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Beermann V., et al. , Real-time imaging of activation and degradation of carbon supported octahedral Pt-Ni alloy fuel cell catalysts at the nanoscale using in situ electrochemical liquid cell STEM. Energy Environ. Sci. 12, 2476–2485 (2019). [Google Scholar]
- 5.Huang J., et al. , Potential-induced nanoclustering of metallic catalysts during electrochemical CO2 reduction. Nat. Commun. 9, 3117 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim Y.-G. G., Baricuatro J. H., Javier A., Gregoire J. M., Soriaga M. P., The evolution of the polycrystalline copper surface, first to Cu, (111) and then to Cu(100), at a fixed CO2RR potential: A study by operando EC-STM. Langmuir 30, 15053–15056 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Gunathunge C. M., et al. , Spectroscopic observation of reversible surface reconstruction of copper electrodes under CO2 reduction. J. Phys. Chem. C 121, 12337–12344 (2017). [Google Scholar]
- 8.Weng Z., et al. , Active sites of copper-complex catalytic materials for electrochemical carbon dioxide reduction. Nat. Commun. 9, 415 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nitopi S., et al. , Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Mistry H., et al. , Highly selective plasma-activated copper catalysts for carbon dioxide reduction to ethylene. Nat. Commun. 7, 12123 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li C. W., Ciston J., Kanan M. W., Electroreduction of carbon monoxide to liquid fuel on oxide-derived nanocrystalline copper. Nature 508, 504–507 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Jiang K., et al. , Metal ion cycling of Cu foil for selective C–C coupling in electrochemical CO2 reduction. Nat. Catal. 1, 111–119 (2018). [Google Scholar]
- 13.Hoang T. T. H., Ma S., Gold J. I., Kenis P. J. A., Gewirth A. A., Nanoporous copper films by additive-controlled electrodeposition: CO2 reduction catalysis. ACS Catal. 7, 3313–3321 (2017). [Google Scholar]
- 14.Lum Y., Yue B., Lobaccaro P., Bell A. T., Ager J. W., Optimizing C–C coupling on oxide-derived copper catalysts for electrochemical CO2 reduction. J. Phys. Chem. C 121, 14191–14203 (2017). [Google Scholar]
- 15.Handoko A. D., et al. , Mechanistic insights into the selective electroreduction of carbon dioxide to ethylene on Cu2O-derived copper catalysts. J. Phys. Chem. C 120, 20058–20067 (2016). [Google Scholar]
- 16.Kwon Y., Lum Y., Clark E. L., Ager J. W., Bell A. T., CO2 electroreduction with enhanced ethylene and ethanol selectivity by nanostructuring polycrystalline copper. ChemElectroChem 3, 1012–1019 (2016). [Google Scholar]
- 17.Wang H., et al. , Rapid and scalable synthesis of cuprous halide-derived copper nano-architectures for selective electrochemical reduction of carbon dioxide. Nano Lett. 19, 3925–3932 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Eilert A., et al. , Subsurface oxygen in oxide-derived copper electrocatalysts for carbon dioxide reduction. J. Phys. Chem. Lett. 8, 285–290 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Hahn C., et al. , Engineering Cu surfaces for the electrocatalytic conversion of CO2: Controlling selectivity toward oxygenates and hydrocarbons. Proc. Natl. Acad. Sci. U.S.A. 114, 5918–5923 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren D., Fong J., Yeo B. S., The effects of currents and potentials on the selectivities of copper toward carbon dioxide electroreduction. Nat. Commun. 9, 925 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng X., Jiang K., Fan S., Kanan M. W., A direct grain-boundary-activity correlation for CO electroreduction on Cu nanoparticles. ACS Cent. Sci. 2, 169–174 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H., Li Y., Koper M. T. M., Calle-Vallejo F., Bond-making and breaking between carbon, nitrogen, and oxygen in electrocatalysis. J. Am. Chem. Soc. 136, 15694–15701 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Kim D., Kley C. S., Li Y., Yang P., Copper nanoparticle ensembles for selective electroreduction of CO2 to C2-C3 products. Proc. Natl. Acad. Sci. U.S.A. 114, 10560–10565 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manthiram K., Beberwyck B. J., Alivisatos A. P., Enhanced electrochemical methanation of carbon dioxide with a dispersible nanoscale copper catalyst. J. Am. Chem. Soc. 136, 13319–13325 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Goodman E. D., Schwalbe J. A., Cargnello M., Mechanistic understanding and the rational design of sinter-resistant heterogeneous catalysts. ACS Catal. 7, 7156–7173 (2017). [Google Scholar]
- 26.Tranquada J. M., Heald S. M., Moodenbaugh A. R., X-ray-absorption near-edge-structure study of La2-x(Ba,Sr)xCuO4-y superconductors. Phys. Rev. B Condens. Matter 36, 5263–5274 (1987). [DOI] [PubMed] [Google Scholar]
- 27.Beverskog B., Puigdomenech I., Revised pourbaix diagrams for copper at 25 to 300°C. J. Electrochem. Soc. 144, 3476 (1997). [Google Scholar]
- 28.Lum Y., Ager J. W., Stability of residual oxides in oxide-derived copper catalysts for electrochemical CO2 reduction investigated with 18 O labeling. Angew. Chem. Int. Ed. Engl. 57, 551–554 (2018). [DOI] [PubMed] [Google Scholar]
- 29.Finšgar M., Milošev I., Inhibition of copper corrosion by 1,2,3-benzotriazole: A review. Corros. Sci. 52, 2737–2749 (2010). [Google Scholar]
- 30.Teo B. K., Chen H. S., Wang R., Antonio M. R., EXAFS of glassy metallic alloys: Amorphous and crystalline MoNi. J. Non-Cryst. Solids 58, 249–274 (1983). [Google Scholar]
- 31.Cavalca F., et al. , Nature and distribution of stable subsurface oxygen in copper electrodes during electrochemical CO2 reduction. J. Phys. Chem. C 121, 25003–25009 (2017). [Google Scholar]
- 32.Brisard G. M., Zenati E., Gasteiger H. A., Marković N. M., Ross P. N., Underpotential deposition of lead on Cu(100) in the presence of chloride: Ex-situ low-energy electron diffraction, auger electron spectroscopy, and electrochemical studies. Langmuir 13, 2390–2397 (1997). [Google Scholar]
- 33.Kim C., Möller T., Schmidt J., Thomas A., Strasser P., Suppression of competing reaction channels by Pb adatom decoration of catalytically active Cu surfaces during CO2 electroreduction. ACS Catal. 9, 1482–1488 (2019). [Google Scholar]
- 34.Kühl S., et al. , Concave curvature facets benefit oxygen electroreduction catalysis on octahedral shaped PtNi nanocatalysts. J. Mater. Chem. A Mater. Energy Sustain. 7, 1149–1159 (2019). [Google Scholar]
- 35.Eren B., et al. , Activation of Cu(111) surface by decomposition into nanoclusters driven by CO adsorption. Science 351, 475–478 (2016). [DOI] [PubMed] [Google Scholar]
- 36.Alsayed A. M., Islam M. F., Zhang J., Collings P. J., Yodh A. G., Premelting at defects within bulk colloidal crystals. Science 309, 1207–1210 (2005). [DOI] [PubMed] [Google Scholar]
- 37.Holm E. A., Foiles S. M., How grain growth stops: A mechanism for grain-growth stagnation in pure materials. Science 328, 1138–1141 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Bergmann A., et al. , Reversible amorphization and the catalytically active state of crystalline Co3O4 during oxygen evolution. Nat. Commun. 6, 8625 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo X., Lv W., Li X.-Y., Additive-free shape-invariant nano-to-micron size-tuning of Cu2O cubic crystals by square-wave voltammetry. J. Phys. Chem. C 118, 11062–11077 (2014). [Google Scholar]
- 40.Kim D., Becknell N., Yu Y., Yang P., Room-temperature dynamics of vanishing copper nanoparticles supported on silica. Nano Lett. 17, 2732–2737 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Kraft S., Stümpel J., Becker P., Kuetgens U., High resolution x-ray absorption spectroscopy with absolute energy calibration for the determination of absorption edge energies. Rev. Sci. Instrum. 67, 681–687 (1996). [Google Scholar]
- 42.Ravel B., Newville M., ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analyzed in this work are included in the main text and SI Appendix.