Significance
Among vertebrates, pregnancy has evolved more than 150 times independently. A fundamental problem for pregnancy to evolve is inadvertent rejection of the embryo when being recognized as foreign tissue by the vertebrate’s adaptive immune system. We show that the unique evolution of male pregnancy in pipefishes and seahorses coincided with a genomic modification of one arm of the adaptive immune system. Our findings indicate a trade-off between immunological tolerance and embryo rejection to accompanying the emergence of male pregnancy. That syngnathids survive in an ocean of microbes despite their drastically modified immune defense suggests an unexpected immunological flexibility. Our results may improve the understanding of immune-deficiency diseases and call for a reassessment of vertebrate immunity.
Keywords: immunological tolerance, major histocompatibility complex, male pregnancy, seahorse, comparative genomics
Abstract
A fundamental problem for the evolution of pregnancy, the most specialized form of parental investment among vertebrates, is the rejection of the nonself-embryo. Mammals achieve immunological tolerance by down-regulating both major histocompatibility complex pathways (MHC I and II). Although pregnancy has evolved multiple times independently among vertebrates, knowledge of associated immune system adjustments is restricted to mammals. All of them (except monotremata) display full internal pregnancy, making evolutionary reconstructions within the class mammalia meaningless. Here, we study the seahorse and pipefish family (syngnathids) that have evolved male pregnancy across a gradient from external oviparity to internal gestation. We assess how immunological tolerance is achieved by reconstruction of the immune gene repertoire in a comprehensive sample of 12 seahorse and pipefish genomes along the “male pregnancy” gradient together with expression patterns of key immune and pregnancy genes in reproductive tissues. We found that the evolution of pregnancy coincided with a modification of the adaptive immune system. Divergent genomic rearrangements of the MHC II pathway among fully pregnant species were identified in both genera of the syngnathids: The pipefishes (Syngnathus) displayed loss of several genes of the MHC II pathway while seahorses (Hippocampus) featured a highly divergent invariant chain (CD74). Our findings suggest that a trade-off between immunological tolerance and embryo rejection accompanied the evolution of unique male pregnancy. That pipefishes survive in an ocean of microbes without one arm of the adaptive immune defense suggests a high degree of immunological flexibility among vertebrates, which may advance our understanding of immune-deficiency diseases.
Pregnancy is the most dramatic form of parental investment, protecting embryos from extreme temperatures, anoxia, osmotic stress, and predation at the cost of fewer young, less effective dispersal, and high-energy demand (1, 2). Although this trait requires multiple anatomical and physiological changes (3) involving development, morphology, osmoregulation, endocrinology, and immunology (4, 5), it has evolved independently in more than 150 vertebrate lineages. A fundamental problem for pregnancy to evolve is the rejection of the embryo that is recognized as foreign tissue by the vertebrate’s adaptive immune system, as it displays alleles also from the other parent. Modulation of the immune system to tolerate foreign protein signatures of the embryonic tissue, in turn, is conflicting with the maintenance of immunological vigilance toward pathogens (6).
Being mammals themselves, researchers have almost exclusively focused on mammalian pregnancy to assess the key adaptations for pregnancy evolution. In vertebrates, the unique diversity of the classic major histocompatibility complex (MHC) class I and II genes (7–9) plays a key role for self/nonself-recognition. While in mammals an initial inflammation seems crucial for embryo implantation (10), during pregnancy mammals prevent an immunological rejection of the embryo with tissue layers of specialized fetal cells, the trophoblasts (11–13). Trophoblasts do not express MHC II (14–16) and thus prevent antigen presentation to maternal T-helper (Th) cells (17), which otherwise would trigger an immune response against nonself. Additionally, expression of classic MHC I genes (HLA-A, -B, and -D) is down-regulated (18). These immunological adaptations are mediated by a cross-talk between the placental trophoblasts and uterine immune cells, in particular natural killer cells and regulatory T cells (Tregs) (19, 20). Tregs maintain self-tolerance by suppressing inflammatory Th1 immune responses (21), as implied by the fact that Treg deficiencies evoke miscarriage (6).
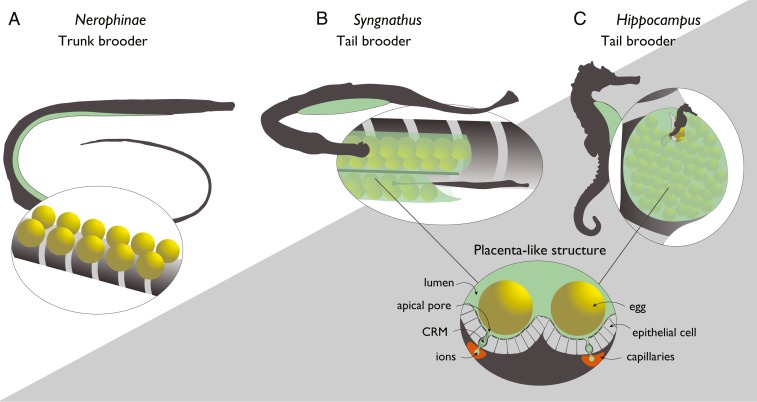
In order to gain insight into the successive evolution of pregnancy and the corresponding molecular cooption of genes and pathways, comparative studies along well-resolved phylogenetic clades are essential. The lack of transitional stages renders mammals unsuitable to reconstruct critical steps for the evolution of pregnancy. Fishes show the greatest diversity in pregnancy evolution, which makes them pivotal for the assessment of the evolution of vertebrate pregnancy (5). Pregnancy has evolved in six teleost orders (Lophiiformes, Beloniformes, Cyprinodontiforames, Scopaeniformes, Perciformes, and Syngnathiformes) (22). Among those, we selected the Syngnathiformes as an ideal taxonomic group to reconstruct possible genomic modifications underlying pregnancy evolution. Species in this family display a gradient of male pregnancy, ranging from external attachment of the eggs to the belly (in the subfamily Nerophinae) to additional external protection via skin flaps (in Doryrhamphus, Oosthethus, and Solegnathiinae), with evolution of internal gestation via brood pouches in Syngnathus (inverted brood pouch) and Hippocampus (sealed brood pouch) as the most advanced states of full internal pregnancy (23, 24) (Fig. 1). In the latter two genera, the fertilized eggs (and later the hatched embryos) become engulfed and effectively integrated by parental tissue, and are supplied with nutrients, oxygen, and parental immunity via a placenta-like organ (1, 25, 26). This gradient in parental investment provides a unique opportunity to analyze the concomitant changes in the vertebrate immune system, and to test whether adaptations similar to mammalian pregnancy assure immunological tolerance. We hypothesized that immunological tolerance toward embryonic tissue is correlated to a genomic modification of the adaptive immune system. We focused particularly on the MHC I and MHC II pathways due to their key roles in immunological tolerance, a hypothesis that was also based on preliminary transcriptomic data in one genus (27).
Fig. 1.
Morphology of brood pouches of subfamily Nerophinae (A), and of the genera Syngnathus (B), and Hippocampus (C) and display of the placenta-like structures in syngnathids (only Syngnathus and Hippocampus). The placenta-like structure with lumen, apical pore, CRM (cells rich in mitochondria), ions, epithelial cells, capillaries, and the egg are drawn after figure 1 of ref. 28.
Here we present comprehensive genome data on 12 representative species of the Syngnathiformes covering a broad range of parental reproductive investment. By assessing their immune gene repertoire, we reconstructed the evolutionary acquisition of immunological tolerance within this unique lineage. As a nonmutually exclusive explanation, we also assessed whether immune gene regulation contributes to immunological tolerance in syngnathids, similar to mammals, and measured differential gene expression during male pregnancy in Syngnathus typhle using comparative transcriptomics to assess whether immunological tolerance can also be achieved by gene regulation in syngnathids, similar to mammals.
Genome Size Evolution in Syngnathiformes
We selected one species, S. typhle, to obtain a high coverage and contiguous genome assembled to a high-quality draft stage sufficient to achieve gene repertoire completeness using a combination of paired-end and mate-pair libraries. The genomes of 11 additional species were assembled to draft stage (SI Appendix, Table S2). Based on the whole-genome datasets, including the already published genomes of Syngnathus scovelli and Hippocampus comes (29, 30), Bayesian phylogenetic analyses (SI Appendix, Table S3) places the origin of the Syngnathiformes clade at 80 Mya (SI Appendix, Fig. S1). Surprisingly, the Syngnathiformes lineage contains species with very divergent genome sizes, spanning from 347 Mbp (Syngnathus rostellatus) to 1.8 Gbp (Entelurus aequoreus) (Table 1). Syngnathiformes species lacking male pregnancy—namely Fistularia tabacaria, Mullus surmuletus, Dactylopterus volitans, Aeoliscus strigatus, and Macroramphorus scolopax—displayed larger genomes than both genera with full male pregnancy (i.e., all Hippocampus and Syngnathus species). In contrast, the Nerophinae pipefishes with external male pregnancy, specifically Nerophis ophidion and E. aequoreus, have significantly larger genomes (Table 1). Concordantly, during 50 million y of evolution, transposable elements have expanded in Nerophinae, most likely explaining the large genome sizes within this subfamily (SI Appendix, Fig. S2 and Table S4).
Table 1.
Summary of species, estimated genome sizes, and assembly statistics
| Species | Name | Estimated genome size (Mbp) | Assembly size (Mbp) | N50 scaffold (Mbp) | N50 contig | BUSCO complete (%) |
| Aeoliscus strigatus | Jointed razorfish | 403 | 381 | 115.8 | 15.9 | 89.6 |
| Dactylopterus volitans | Flying Gurnard | 499 | 577 | 17.1 | 8.3 | 74.1 |
| Doryramphus dactylophorus | Banded pipefish | 651 | 619 | 75.2 | 27.6 | 87.1 |
| Entelurus aequoreus | Snake pipefish | 1834 | 557 | 3.9 | 3.4 | 21.6 |
| Fistularia tabacaria | Bluespotted cornetfish | 762 | 593 | 107.2 | 17.7 | 90.8 |
| Hippocampus comes* | Tiger tail seahorse | NA | 494 | 2034.5 | 39.6 | 89.4 |
| Hippocampus kuda | Yellow seahorse | 478 | 445 | 31.2 | 10.4 | 83.9 |
| Hippocampus whitei | White´s seahorse | 461 | 433 | 40.8 | 10.3 | 86.0 |
| Macroramphorus scolopax | Longspine snipefish | 507 | 418 | 41.8 | 13.4 | 86.1 |
| Mullus surmuletus | Surmulet | 569 | 469 | 17.2 | 7.2 | 73.8 |
| Nerophis ophidion | Straightnose pipefish | 1581 | 976 | 6.8 | 5.2 | 33.6 |
| Syngnathus rostellatus | Nilsson´s pipefish | 347 | 283 | 87.6 | 14.9 | 89.0 |
| Syngnathus scovelli* | Gulf pipefish | NA | 307 | 12400.1 | 27.8 | 85.8 |
| Syngnathus typhle | Broadnosed pipefish | NA | 315 | 3047.0 | 25.8 | 88.8 |
NA, not applicable.
Already published genomes.
Modification of MHC II Pathways in Syngnathus and Hippocampus
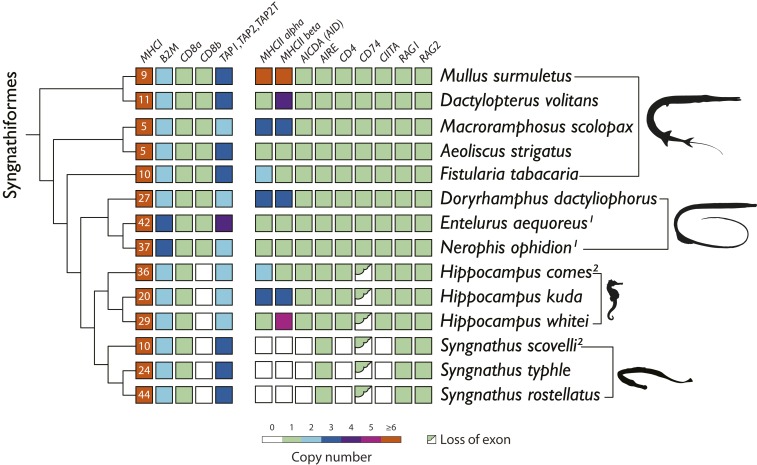
In order to correlate the modification of adaptive immunity with the degree of male pregnancy, a set of key genes involved in adaptive immunity was analyzed from the assembled genomes presented here along with two previously published syngnathid genomes (29, 30) (SI Appendix, Table S2). MHC I and MHC II are essential for the recognition process of nonself-peptides by presenting them to CD8+ and CD4+ T cells, respectively. In line with our hypothesis, all fully pregnant species (i.e., genera Syngnathus and Hippocampus) underwent considerable modifications of their adaptive immune system characterized by losses or changes of key genes of the MHC II pathway (Fig. 2; details on ortholog search and analyses can be found in the SI Appendix, sections 5.1. and 5.5.11).
Fig. 2.
Remodeling and loss of key genes of the MHC class I and II pathways among 14 species of Syngnathiformes. Species are arranged according to their phylogeny (SI Appendix, Fig. S1). The copy numbers of the most important genes of the MHC I pathway (MHC I, B2M, CD8a, CD8b and TAP1, TAP2, TAP2T) and MHC II pathway (MHC IIα, MHC IIβ, AICDA [AID], AIRE, CD4, CD74, CIITA, RAG1, RAG2) are displayed. Superscript “1” specifies large fragmented genomes; superscript “2” specifies previously published genomes. White boxes indicate loss of genes and half green boxes indicate loss of exons.
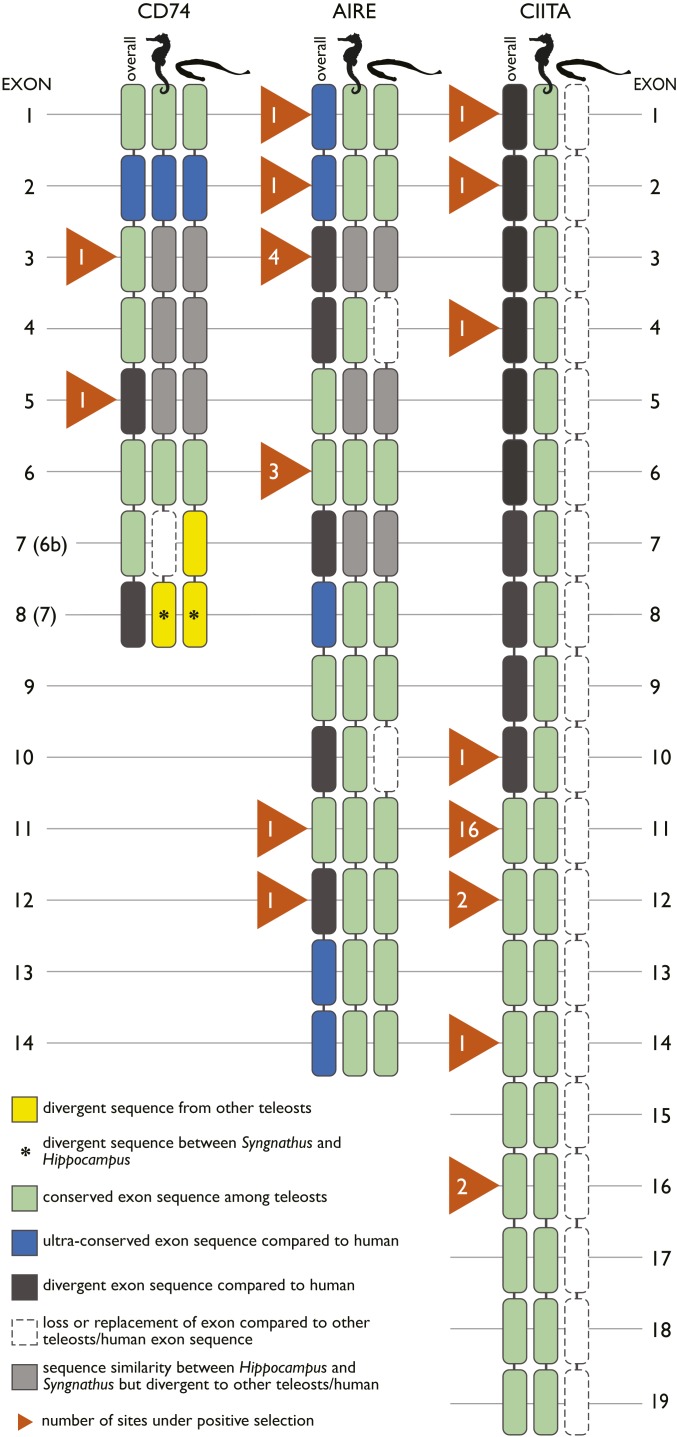
The invariant chain of MHC II (CD74), preventing premature peptide binding of MHC II, displayed a divergent exon 3 in Syngnathus and Hippocampus compared to both mammals and other teleosts (Fig. 3). Additionally, Hippocampus had a sequence substitution of exon 6b, while Syngnathus displayed a divergent exon compared to other fish and human. Both exons 3 and 6b are located in the protein region protruding into the endosomal lumen. Several lines of evidence suggest that these losses are impairing functions of CD74. In human, exon 3 of CD74 covers the region associating with MHC II (CLIP) [amino acids 108 to 124 in SI Appendix, Dataset 1: 10CD74_clean (31)]. Exon 6b is annotated as Thyroglobulin type I repeats, which are proposed cysteine protease inhibitors (this exon consists of six conserved cysteine residues) and are implicated in delaying the degradation of internalized antigens subsequently preserving epitopes for antigen presentation (32, 33).
Fig. 3.
Domain-level alignment of three genes (CD74, AIRE, CIITA) critical for the MHC class II pathway with lost or divergent exons in Hippocampus and Syngnathus compared to other vertebrate sequences. For each exon, sequence divergence to other teleosts (yellow), human (34), or sequence conservation other teleosts (green), human (blue) are shown. Exon loss is indicated in white and sequence conservation between Hippocampus and Syngnathus but divergence to other teleosts/humans in gray. Orange triangles indicate the number of sites under positive selection. An ansterisk (*) indicates yellow exon 6b and 7 of CD74 also diverge between Syngnathus and Hippocampus.
As the most drastic change in gene repertoire, all Syngnathus species have lost the genes encoding the classic MHC II α- and β-chains, implying that the presentation of antigens to the T cell receptor on CD4+ T lymphocytes is disabled (Fig. 2). This is supported by a loss of CD4, mediating successful receptor binding and activation of CD4+ T lymphocytes—AICDA, responsible for the unique receptor diversity of the antibodies and CIITA, the MHC II transactivator—which control the expression of MHC II genes in antigen-presenting cells. The only canonical gene of the MHC II pathway remaining in the Syngnathus genomes was the autoimmune regulator (35), driving negative selection on self-recognizing T cells (36). While leading and trailing exons of AIRE were well conserved among all investigated Syngnathiformes species compared to reference sequences from other fish families, several other exons diverged markedly or homologous sequences were not found [Fig. 3 and SI Appendix, Dataset 1: 13AIRE_exon_overview (31)]. In the genera Syngnathus and Hippocampus, exons 3, 4, 5, 6, and 12 of AIRE were lost or substituted with very divergent sequences that could not be aligned. Putative loss of MHC II-related function of the AIRE transcription factor is further emphasized by the lack of expression in various S. typhle tissues, which could result in insufficient negative selection of T cells in the thymus (36). Overall, our findings suggest that the MHC class II pathway was lost in Syngnathus.
The situation in Hippocampus was more complex. Similar modifications as in Syngnathus for the CD74 gene were observed in terms of a divergent exon 3, and in a substitution of exon 6b, Importantly, no loss of the MHC II genes as in all three Syngnathus species was observed. However, in Hippocampus, the MHC II gene sequences, in particular of the β-copy, were highly distinct from other functional MHC II genes found in species with functional MHC class II such as zebrafish, seabass, salmon, and guppy [SI Appendix, Dataset 1: 29_MHCII_beta_complete (31)]. In parallel, small sections of some CIITA exons displayed substitutions compared to both other teleosts and mammals. This is in line with findings for AIRE in both Hippocampus and Syngnathus, where several exons were either lost or diverged markedly compared to other teleosts, indicating most likely an alternative function not related to MHC II (Fig. 3). Moreover, the tertiary structure of MHC IIβ genes of Hippocampus was predicted to lack two critical cysteine bridges that are essential to form the peptide-binding pocket of the MHC II molecule [SI Appendix, Dataset 1: 29MHCII_beta_complete (31)]. In line with these findings, in Hippocampus we identified positive selection in sequences of genes that were lost in Syngnathus (AIRE, CD4, and CIITA), which may suggest neo- or subfunctionalization (SI Appendix, Table S11).
A closer examination of the invariant chain encoding gene CD74 also suggests that the evolution of adaptive immunity has taken distinct routes in the two sister genera Syngnathus and Hippocampus. A shared relaxed selection on CD74 in the common ancestor of syngnathids resulted in a loss-of-function by either sequence substitution in the Hippocampus or divergence in the Syngnathus for exon 6b, accompanied with a divergent exon 3 in both lineages. While subsequently several core genes of the MHC II pathway were lost in Syngnathus, genes of the MHC II pathway were under positive selection in Hippocampus [Fig. 3 and SI Appendix, Table S11 and Dataset 1, gene alignments (31)] and showed clear sequence divergence compared to other teleosts and humans (Fig. 3). Different scenarios may explain the observed pattern in the MHC II pathway of Hippocampus.
First, the sequence divergence of the MHC II core genes in contrast to other teleosts and the signs of positive selection could indicate that in Hippocampus the MHC II genes were taking over alternative or novel functions. CD74 is pivotal for a functional MHC II pathway, as supported by an impaired assembly and surface expression of MHC II and a defective antigen presentation in invariant chain knockout mice (37). While generally the CLIP of CD74 (exon 3) associates with MHC II, the remaining exons of CD74 act as chaperone, transporting MHC II to the loading compartment. The loss of exon 6b in Hippocampus could indicate a compromised loading process. Accordingly, the MHC II system in Hippocampus is likely to be less efficient in contrast to other vertebrates, which may suffice to permit the evolution of full male pregnancy.
Second, the MHC II pathway may not be compromised in its function despite the lost and diverged exons of CD74 over a functional rearrangement of the immune system. However, this is less likely, as mice with transgenic expression of a truncated CD74 protein lacking the CLIP region (the part of the gene that diverges from other teleosts in Hippocampus) could not pursue MHC II trafficking (38) (Fig. 3 and SI Appendix, Table S11).
Modifications of the MHC I Pathway under Pregnancy
In Gadiformes (cod-like fishes) an independent loss of the MHC class II pathway was recently reported, and the observed diversification of MHC I genes was hypothesized to compensate for the loss of a functional MHC II pathway (39, 40). Accordingly, we assessed MHC I copy number in syngnathids using the most conserved exon 4 of the MHC I gene and found it to be higher in all species displaying male pregnancy (the Nerophinae with external male pregnancy [27 to 42 copies], Hippocampus [20 to 36 copies], and Syngnathus [24 to 44 copies] with full male pregnancy) compared to species without pregnancy (5 to 10 copies) (Fig. 2). While all identified MHC I sequences in Syngnathiformes are part of the U lineage (41), the distinct cluster of syngnathid MHC I sequences supports a potential coevolution of MHC I with male pregnancy (SI Appendix, Figs. S9 and S10). These lineage-specific MHC I variants likely increase the ligand repertoire and suggest a possible function within the cross-presentation pathway, in contrast to Atlantic cod, where cross-presentation could be hindered due to loss of the entire CD74, a gene with crucial function in the MHC I cross-presentation pathway (42). Moreover, key genes of the MHC I pathway, such as β2-Microglobulin (B2M, important for the availability of MHC I light-chain proteins) and CD8 (responsible for activation of CD8+ T lymphocytes), were under positive selection in syngnathids, similar to RAG1 that facilitates V(D)J recombination and TAP1/TAP2 that function as heterodimers in the transport of antigens (SI Appendix, Table S11). This supports a shift from the MHC II to MHC I cross-presentation pathway as all of the latter genes (CD8, RAG1, and TAP1/TAP2) also have important functions in the MHC I cross-presentation pathway. The identification of marked positive-selection signals support an interpretation of the MHC I pathway to coevolve with male pregnancy. The expanded MHC I repertoire is likely to be linked to the simultaneous loss/rearrangement of the MHC II pathway and may compensate its function/deficiency over the MHC I cross-presentation pathway.
Pregnancy requires special physiological adaptations to assure the oxygen supply to the growing embryo. In line with these expectations, the repertoire of hemoglobin genes encoding oxygen transport show signs of coevolution with male pregnancy. All syngnathids have lost the hemoglobin gene alpha 6, while those genera with full male pregnancy, Syngnathus and Hippocampus, have also lost the alpha 5 gene. Conversely, fully pregnant species have gained alpha 1 and alpha 2 hemoglobin genes (SI Appendix, Figs. S14–S16). It is tempting to speculate that the shift in hemoglobin gene repertoire indicates selection for more effective oxygen transfer from father to offspring in male pregnancy evolution.
Modulation of Gene Expression during Pregnancy
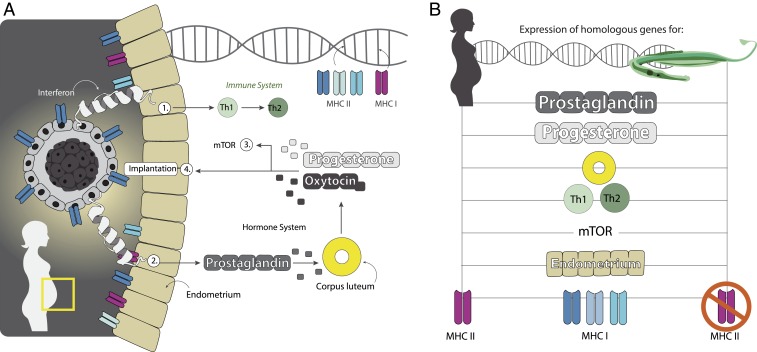
Next, we assessed whether or not the evolution of immunological tolerance required the cooption of similar genes and physiological processes in female and male pregnancy. To do so, we analyzed global gene-expression patterns using RNA sequencing in our model species S. typhle in brood pouch tissues during pouch development and pregnancy. At the same time, this approach assessed whether the evolution of immunological tolerance in male pregnancy was also achieved by immune gene regulation as in mammals, in addition to the identified changes in gene repertoire. We examined the gene-expression profiles of male undeveloped brood pouch tissues (control) against developed pouch tissue from mature and receptive males (43); pouch tissue at early- and at late-pregnancy genes with a false-discovery rate-corrected P value of <0.05, as determined by the cuffdiff algorithm, were categorized as differentially expressed (44). All differentially expressed genes were searched for potential functions via homology, using reported functions in female pregnancy of mammals, in the squamate reptile Chalcides ocellatus (45) and in male pregnancy of S. scovelli (29) and Hippocampus abdominalis (46). A total of 141 genes were significantly up- or down-regulated during male pregnancy in S. typhle and S. scovelli (29). The direction of expression in differentially expressed genes correlated between S. typhle and S. scovelli (R2 = 0.767), implying that up- or down-regulation during pregnancy was mostly consistent in both pipefish species. In particular, this was the case for the four genes with the strongest up-regulation during pregnancy (MYOC, HCEA, LS-12, APOA1) and for the two genes that showed the most massive down-regulation during pregnancy (STX2 and MSXC). Several genes known to be differentially expressed in the pregnancy of the seahorse H. abdominalis also showed expression changes in the pregnancy of the pipefish S. typhle (SI Appendix, Table S12). We identified 116 genes covering important pathways in human female pregnancy as differentially expressed during male pregnancy in S. typhle. These were involved in the prostaglandin, mammalian target of rapamycin (mTOR), and progesterone pathways, in corpus luteum degradation, parent–embryo transport, placenta development, conceptus implantation, and embryo growth (Fig. 4 and SI Appendix, section 7.3 and Tables S12 and S13).
Fig. 4.
Cooption of genes known from female human pregnancy in syngnathid male pregnancy. (A) Human female pregnancy and the most important immunological, developmental, and endocrinological pathways involved. (B) Genes involved in those pathways with an established role in human pregnancy change their expression also during pipefish pregnancy.
In summary, these findings suggest that the independent evolutionary trajectories of female and male pregnancy in two vertebrate classes have resulted in expression changes in highly overlapping sets of genes coding for pathways with similar functions (SI Appendix, Tables S12 and S13). Apparently, the convergent evolution of male and female pregnancy has coopted a similar set of genes and involves similar physiological pathways. Functional tests using visualization of gene expression over in situ hybridization and gene knockdowns will permit further investigation of the molecular basis of male pregnancy evolution and its mechanistic similarity to female pregnancy in the future.
Immune Gene Expression and Male Pregnancy
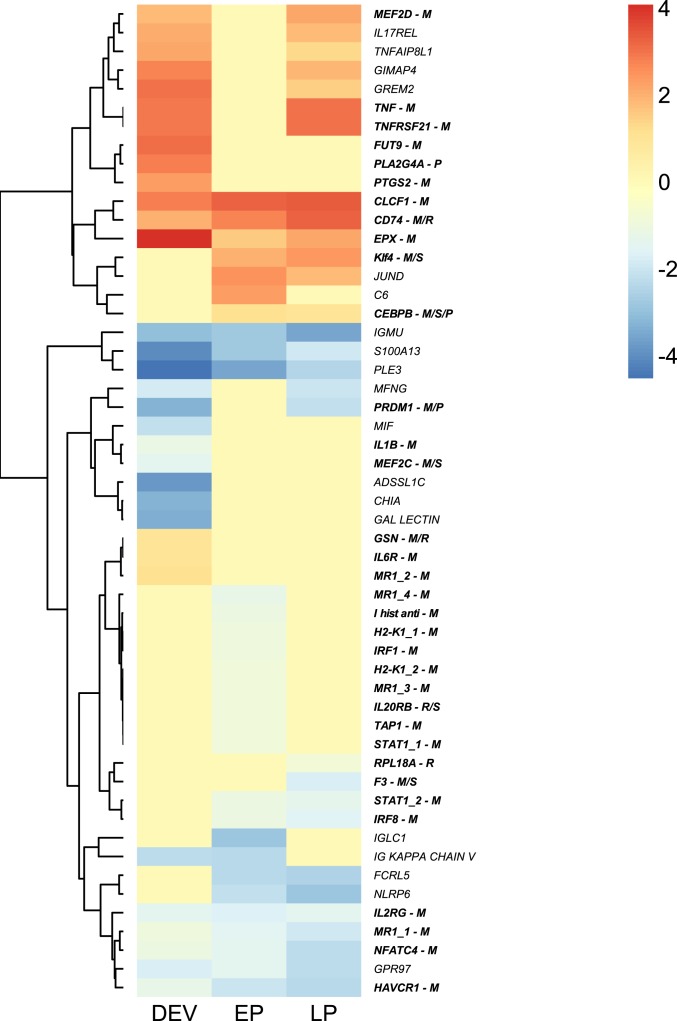
Next, we focused on immune gene-expression changes that accompany the modification of the MHC II pathway and the MHC I gene repertoire expansion. We analyzed the differential expression of immune genes that are either known to have a function in female pregnancy in mammals or known to also show expression changes in pregnancy of reptile, of seahorse, or of another pipefish species [in bold italics below; in the parenthesiss M indicates a gene known to have a function in mammals, R in the reptile C. ocellatus (45), S in the seahorse H. abdominalis (46), and P in the pipefish S. scovelli (29)] (SI Appendix, Table S12) or those with a fold-change > 2 (220 genes in total, including 30 immune genes) (only in italics) (Fig. 5 and SI Appendix, Table S13).
Fig. 5.
Heatmap displaying expression changes (log2 fold-change) of S. typhle immune genes during pouch development (DEV), early pregnancy (EP), and late pregnancy (LP). Homologs of genes marked with (M) possess known function (bold) in mammals (M), designation (R) denotes differential expression in the squamate reptile C. ocellatus (R), in the seahorse H. abdominalis (S), or in the pipefish species S. scovelli (P).
Collectively, the observed gene-expression changes during male pregnancy contribute to immunological tolerance during pregnancy already apparent from the gene repertoire. In particular, we identified expression changes of proinflammatory Th1 and antiinflammatory Th2 responses and a simultaneous down-regulation of the MHC I pathway during pregnancy, which resemble the expression changes during mammalian pregnancy. An inflammation response was suggested to be important for successful implantation in mammalian pregnancy (10). The key genes mediating this specific inflammation at implantation in mammals—IL6R (M/P), TNF (M), and PTGS2 (M) (10, 47)—were up-regulated during pouch development in pipefish. Here, other inflammation responses dropped [down-regulation of proinflammatory interleukins IL1B (M) and IL2RG (M), and S100A13 (interleukin secretion gene); the proinflammatory cytokine MIF; FHL2 involved in inflammation response; ADSSL1C involved in antimicrobial peptide synthesis; the antimicrobial peptide PLE3; JUND involved in LPS response; and up-regulation of GSN (M/R) that binds to LPS].
Simultaneously, lymphocyte maturation and proliferation were suppressed through the down-regulation of CHIA and MEF2C (M/S) (maturation of B cells and important in mammalian embryo development), the up-regulation of GIMAP4 that enhances lymphocyte apoptosis, and the up-regulation of the transcriptional repressor PRDM1 (M/P) that initiates in mammals a lineage-restricted progenitor cell population contributing to placental growth and morphogenesis (48). Consistent with a shift from Th1 to Th2 immune responses during mammalian pregnancy, CEBPB (M/S/P), which represses Th1 but facilitates Th2 immune response, was up-regulated during pipefish male pregnancy. This coincided with expression dynamics of EPX (M) mediating eosinophil activity and promoting mammalian placental development (49). Lymphocyte maturation and proliferation remained consistently repressed during pregnancy as indicated by down-regulations of RPL18A (R) (activation of T cell proliferation [Th1]), of FCRL5 (enhancing B cell development), of the proinflammatory interleukin IL2RG (R/S), and of the interleukin secretion genes S100A13 and IL20R (R/S).
During late pregnancy only, GPR97 and MFNG (both responsible for B cell differentiation) were down-regulated along with the genes NFATC4 and HAVCR1, which are involved in T cell maturation. Few genes involved in Th1 immune response were up-regulated during pregnancy [TNF (M), CLCF1 (M), KLF4 (M/S), and TNFRSF21 (M)]. In female pregnancy, those genes were shown to have additional functions: TNF (M) mediates placental development and implantation (10, 50), CLCF1 (M) is responsible for the onset of labors at term [a process resembling inflammation (51)], and KLF4 (M/S) is key for the maintenance of gestation (52). The two inflammation genes, PLA2G4A (P) and IL17REL, were up-regulated during pouch development but not during pregnancy. In summary, inflammation responses during male pregnancy could be overlapping with previously identified expression patterns of homologous genes responsible for the regulation of inflammation during egg implantation and female pregnancy.
Analogous to human pregnancy where CASP3 (M/S) modifies the MHC class I pathway (53, 54) to support immunological tolerance (55), CASP3 (M/S) was up-regulated during pipefish pregnancy. Throughout early mammalian pregnancy, TAP1 (M) is increasingly expressed on placenta-specific trophoblasts and plays an important role in preventing maternal immune attacks toward the embryos (56). Such up-regulation of TAP1 (M) was also identified in pipefish. Mammalian trophoblasts only express nonclassic nondiverse MHC I genes that will not induce a nonself-rejection reaction against the embryo (57, 58). In pipefish, a series of MHC I pathway genes that are involved in antigen recognition, presentation, and processing were down-regulated during pregnancy, such as F10 (M), H2-K1 (M), IRF1 (M), IRF8 (M), MR1 (M), FUT9 (M), and the Ig chains (Ig κ chain V, Ig μ chain C, IGLC1). In humans, the silencing of CD74 (M/R) during pregnancy is key for maintaining the acceptance of the semiallogenic embryo (59). The up-regulation of CD74 (M/R) is puzzling as almost all other genes of the MHC II pathway are absent in S. typhle. As key exons of CD74 are diverged or substituted in Syngnathus, the up-regulation of CD74 during pregnancy rather suggests a change of function for CD74 in the evolution of male pregnancy. This suggests that consistent with female pregnancy, antigen recognition over the MHC I pathway could also be down-regulated during pipefish male pregnancy.
Discussion
Although pregnancy is widespread among the vertebrates, very little is known on the immunological modifications that are required to prevent embryo rejection other than within the class mammalia. Here, we present a major modification of the immune system associated with increasing investment into pregnancy in the fish family of pipefishes and seahoreses that not only entailed gene-expression changes during pregnancy but also coincided with major alterations of the gene repertoire of both MHC pathways. While the identified rearrangement and loss of the core genes of the MHC II pathway is consistent with an adaptive explanation to modulate the immune system so as to prevent immunological rejection of the embryo, demonstrating causality would require future functional validation. While in Syngnathus the genomic knockout of the MHC II pathway must have resulted in a loss-of-function, in Hippocampus the situation remains inconclusive. Knockdowns in closely related teleost species of the CD74 exon that was substituted in Hippocampus would be needed to illuminate its impact on the MHC II pathway.
One of the most unexpected findings was that even within a single fish family, the rearrangement of the MHC II pathway differed between the genera Hippocampus and Syngnathus. At the same time, this demonstrates both a strong selection for reduction of immunological vigilance displayed by the MHC class II pathway during pregnancy evolution and a remarkable flexibility of the vertebrate immune system in general. Because this unique fish family displays male pregnancy, any of the immunological adaptations are also not compounded by the sex per se (60) [i.e., the fact that the female sex through provisioning of eggs usually needs a more competent immune system under conventional sex roles, referred to as Bateman’s principle (61)].
Within the evolution of vertebrate immune systems, the crucial role of the MHC II pathway for the recognition of pathogenic epitopes is commonly considered essential (8). The almost complete loss of the MHC II pathway in Syngnathus emphasizes that the vertebrate immune system has a much higher degree of functional flexibility than previously assumed, in line with recent findings in the Gadiformes lineage (34, 39). The complete loss of classic MHC IIα and -β genes in all cod-like fishes (Gadiformes) coincides with truncation of CD4 and loss of CD74 (39, 40). While in that taxonomic group, the selection regime leading to MHC class II pathway loss is still elusive, we provide evidence among Syngnathiformes that modification and loss of adaptive immune genes and pathways is associated with the evolution of male pregnancy, which potentially selected for immunological tolerance. As Syngnathids and Gadiformes are only distantly related (SI Appendix, Fig. S1), losses and divergence of key genes of the MHC II pathway in each of those groups represent independent evolutionary events, likely driven by different selection factors.
The loss of gut-associated lymphatic tissues (GALT), the spleen (62), and the immune genes (CD4, MHC II, AICDA, CIITA) in Syngnathus represent critical pathways that are attacked by the HIV (CD4+ T cells, GALT). As a natural “knockout” for the MHC class II pathway, Syngnathus may thus become instrumental in the future as a model for research on natural or disease-related immune deficiencies.
Materials and Methods
We have sequenced and assembled 12 Syngnathiformes genomes (SI Appendix, Table S2) and annotated the genome of S. typhle. We generated a time-calibrated phylogeny of Syngnathiformes (SI Appendix, section 3 and Fig. S1). To search for shifts in the optima of genome size in the different lineages, we applied the Ornstein–Uhlenbeck process using the Syngnathiformes phylogeny and the genome sizes of the species (SI Appendix, section 4.1 and Fig. S2). To investigate potential reasons for differences in genome size, a library of repeated elements was created (SI Appendix, section 4.2 and Table S4). For immune, pregnancy, and hemoglobin genes, translated query sequences, either as whole sequences and for MHC and hemoglobin also split into individual exons, were used as input in a TBLASTN search toward the scaffold from the assembled draft genomes. For MHC I and MHC II searches, unitigs were used due to large copy numbers (SI Appendix, section 5.1). Upon gene alignments, gene trees were generated with RAxML (v8.2.10.) (SI Appendix, section 5.2) and local gene synteny was explored for MHC II, AID, CD4, and CIITA (SI Appendix, section 5.4). We assessed site-specific, positive selection and gene-wide selection across the whole tree and with the syngnathids as the foreground branches (SI Appendix, section 6 and Table S11). To assess which genes are differentially expressed during male pregnancy and during the development of the brood pouch tissue, we sequenced the transcriptome of S. typhle in different tissue types (undeveloped brood pouch, developed pouch, pouch at early pregnancy, pouch at late pregnancy). Differential gene expression was calculated pairwise against the undeveloped pouch and all differentially expressed genes were searched for potential functions via homology (SI Appendix, section 7).
Supplementary Material
Acknowledgments
We thank all participants of previous fish genomics meetings where this project was initiated and discussed repeatedly; Kjetill S. Jakobsen, Walter Salzburger, Ralf Schneider, Jamie Parker, Isabel Tanger, Hinrich Schulenburg, and the pipefish group for input and specific comments on this manuscript; and Susanne Landis @scienstration for artwork. This project was funded by grants from the German Research Foundation and a European Research Council Starting grant (malePREG) (to O.R.). Sequencing library creation and high-throughput sequencing was carried out at the Norwegian Sequencing Centre, University of Oslo (http://www.sequencing.uio.no), Norway and McGill University and Genome Quebec Innovation Centre, Canada. Computational work was performed on the Abel Supercomputing Cluster (Norwegian Metacenter for High Performance Computing and the University of Oslo) operated by the Research Computing Services group at USIT, the University of Oslo IT-department (https://www.uio.no/tjenester/it/forskning/).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the European Nucleotide Archive, https://www.ebi.ac.uk/ena (accession no. PRJEB32126). Gene alignments for positive selection analyses can be found at Figshare: doi:10.6084/m9.figshare.11499360.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1916251117/-/DCSupplemental.
References
- 1.Ripley J. L., Foran C. M., Direct evidence for embryonic uptake of paternally-derived nutrients in two pipefishes (Syngnathidae: Syngnathus spp.). J. Comp. Physiol. B 179, 325–333 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Shine R., “Ecological influences on the evolution of vetebrate viviparity” in Complex Organismal Functions, Integration and Evolution in Vertebrates, Wake D. B., Roth G., Eds. (Wiley, New York, 1989). [Google Scholar]
- 3.Gittlemann J. L., The phylogeny of parental care in fishes. Anim. Behav. 29, 936–941 (1981). [Google Scholar]
- 4.Wourms J. P., Viviparity: The maternal-fetal relationship in fishes. Am. Zool. 21, 473–515 (1981). [Google Scholar]
- 5.Wourms J. P., Lombardi J., Reflection on the evolution of piscine viviparity. Am. Zool. 32, 276–293 (1992). [Google Scholar]
- 6.La Rocca C., Carbone F., Longobardi S., Matarese G., The immunology of pregnancy: Regulatory T cells control maternal immune tolerance toward the fetus. Immunol. Lett. 162, 41–48 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Cooper M. D., Alder M. N., The evolution of adaptive immune systems. Cell 124, 815–822 (2006). [DOI] [PubMed] [Google Scholar]
- 8.Flajnik M. F., Kasahara M., Origin and evolution of the adaptive immune system: Genetic events and selective pressures. Nat. Rev. Genet. 11, 47–59 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janeway C. A., Travers P., Walport M., Shlomchik M. J., Eds., Immunobiology (Garland Science, New York, 2001). [Google Scholar]
- 10.Chavan A. R., Griffith O. W., Wagner G. P., The inflammation paradox in the evolution of mammalian pregnancy: Turning a foe into a friend. Curr. Opin. Genet. Dev. 47, 24–32 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Erlebacher A., Why isn’t the fetus rejected? Curr. Opin. Immunol. 13, 590–593 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Sharma S., Natural killer cells and regulatory T cells in early pregnancy loss. Int. J. Dev. Biol. 58, 219–229 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moffett A., Loke C., Immunology of placentation in eutherian mammals. Nat. Rev. Immunol. 6, 584–594 (2006). [DOI] [PubMed] [Google Scholar]
- 14.von Boehmer H., Kisielow P., Self-nonself discrimination by T cells. Science 248, 1369–1373 (1990). [DOI] [PubMed] [Google Scholar]
- 15.Jiang H., Chess L., Regulation of immune responses by T cells. N. Engl. J. Med. 354, 1166–1176 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Cooper M. D., Herrin B. R., How did our complex immune system evolve? Nat. Rev. Immunol. 10, 2–3 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Zheng J., Johnson M. L., Redmer D. A., Reynolds L. P., Estrogen and progesterone receptors, cell proliferation, and c-fos expression in the ovine uterus during early pregnancy. Endocrinology 137, 340–348 (1996). [DOI] [PubMed] [Google Scholar]
- 18.Hiby S. E., et al. , Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J. Exp. Med. 200, 957–965 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Croy B. A., et al. , Imaging of vascular development in early mouse decidua and its association with leukocytes and trophoblasts. Biol. Reprod. 87, 125 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erlebacher A., Immunology of the maternal-fetal interface. Annu. Rev. Immunol. 31, 387–411 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Wing K., Sakaguchi S., Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat. Immunol. 11, 7–13 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Avise J., Evolutionary Perspectives on Pregnancy (Columbia University Press, 2013). [Google Scholar]
- 23.Vincent A., Ahnesjö I., Berglund A., Rosenqvist G., Pipefishes and seahorses: Are they all sex role reversed? Trends Ecol. Evol. 7, 237–241 (1992). [DOI] [PubMed] [Google Scholar]
- 24.Wilson A. B., Vincent A., Ahnesjö I., Meyer A., Male pregnancy in seahorses and pipefishes (family Syngnathidae): Rapid diversification of paternal brood pouch morphology inferred from a molecular phylogeny. J. Hered. 92, 159–166 (2001). [DOI] [PubMed] [Google Scholar]
- 25.Partridge C., Shardo J., Boettcher A., Osmoregulatory role of the brood pouch in the euryhaline Gulf pipefish, Syngnathus scovelli. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 147, 556–561 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Roth O., Klein V., Beemelmanns A., Scharsack J. P., Reusch T. B. H., Male pregnancy and biparental immune priming. Am. Nat. 180, 802–814 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Haase D., et al. , Absence of major histocompatibility complex class II mediated immunity in pipefish, Syngnathus typhle: Evidence from deep transcriptome sequencing. Biol. Lett. 9, 20130044 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harlin-Cognato A., Hoffman E. A., Jones A. G., Gene cooption without duplication during the evolution of a male-pregnancy gene in pipefish. Proc. Natl. Acad. Sci. U.S.A. 103, 19407–19412 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Small C. M., et al. , The genome of the Gulf pipefish enables understanding of evolutionary innovations. Genome Biol. 17, 258 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin Q., et al. , The seahorse genome and the evolution of its specialized morphology. Nature 540, 395–399 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roth O., et al. , Syngnathiformes immune gene alignments Figshare. https://figshare.com/articles/Syngnathiformes_immune_gene_alignments/11499360. Deposited 27 March 2020.
- 32.Stumptner P., Benaroch P., Interaction of MHC class II molecules with the invariant chain: Role of the invariant chain (81-90) region. EMBO J. 16, 5807–5818 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schröder B., The multifaceted roles of the invariant chain CD74—More than just a chaperone. Biochim. Biophys. Acta 1863, 1269–1281 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Blackburn D. G., Evolution of vertebrate viviparity and specializations for fetal nutrition: A quantitative and qualitative analysis. J. Morphol. 276, 961–990 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Tine M., et al. , European sea bass genome and its variation provide insights into adaptation to euryhalinity and speciation. Nat. Commun. 5, 5770 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson M. S., Su M. A., Aire and T cell development. Curr. Opin. Immunol. 23, 198–206 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viville S., et al. , Mice lacking the MHC class II-associated invariant chain. Cell 72, 635–648 (1993). [DOI] [PubMed] [Google Scholar]
- 38.Matza D., et al. , Invariant chain induces B cell maturation in a process that is independent of its chaperonic activity. Proc. Natl. Acad. Sci. U.S.A. 99, 3018–3023 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Star B., et al. , The genome sequence of Atlantic cod reveals a unique immune system. Nature 477, 207–210 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malmstrøm M., et al. , Evolution of the immune system influences speciation rates in teleost fishes. Nat. Genet. 48, 1204–1210 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Grimholt U., et al. , A comprehensive analysis of teleost MHC class I sequences. BMC Evol. Biol. 15, 32 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Basha G., et al. , A CD74-dependent MHC class I endolysosomal cross-presentation pathway. Nat. Immunol. 13, 237–245 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Betancur-R R., et al. , The tree of life and a new classification of bony fishes. PLoS Curr. 5, 10.1371/ecurrents.tol.53ba26640df0ccaee75bb165c8c26288 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trapnell C., et al. , Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562–578 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brandley M. C., Young R. L., Warren D. L., Thompson M. B., Wagner G. P., Uterine gene expression in the live-bearing lizard, Chalcides ocellatus, reveals convergence of squamate reptile and mammalian pregnancy mechanisms. Genome Biol. Evol. 4, 394–411 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whittington C. M., Griffith O. W., Qi W., Thompson M. B., Wilson A. B., Seahorse brood pouch transcriptome reveals common genes associated with vertebrate pregnancy. Mol. Biol. Evol. 32, 3114–3131 (2015). [DOI] [PubMed] [Google Scholar]
- 47.Geisert R., Fazleabas A., Lucy M., Mathew D., Interaction of the conceptus and endometrium to establish pregnancy in mammals: Role of interleukin 1β. Cell Tissue Res. 349, 825–838 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mould A., Morgan M. A., Li L., Bikoff E. K., Robertson E. J., Blimp1/Prdm1 governs terminal differentiation of endovascular trophoblast giant cells and defines multipotent progenitors in the developing placenta. Genes Dev. 26, 2063–2074 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knudsen U. B., Uldbjerg N., Rechberger T., Fredens K., Eosinophils in human cervical ripening. Eur. J. Obstet. Gynecol. Reprod. Biol. 72, 165–168 (1997). [DOI] [PubMed] [Google Scholar]
- 50.Chwalisz K., et al. , Cervical ripening with the cytokines interleukin 8, interleukin 1 β and tumour necrosis factor α in guinea-pigs. Hum. Reprod. 9, 2173–2181 (1994). [DOI] [PubMed] [Google Scholar]
- 51.Khanjani S., et al. , NF-κB regulates a cassette of immune/inflammatory genes in human pregnant myometrium at term. J. Cell. Mol. Med. 15, 809–824 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blanchon L., et al. , Activation of the human pregnancy-specific glycoprotein PSG-5 promoter by KLF4 and Sp1. Biochem. Biophys. Res. Commun. 343, 745–753 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Joyce M. M., et al. , Pig conceptuses increase uterine interferon-regulatory factor 1 (IRF1), but restrict expression to stroma through estrogen-induced IRF2 in luminal epithelium. Biol. Reprod. 77, 292–302 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Murphy S. P., et al. , Interferon gamma in successful pregnancies. Biol. Reprod. 80, 848–859 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jeyasuria P., Wetzel J., Bradley M., Subedi K., Condon J. C., Progesterone-regulated caspase 3 action in the mouse may play a role in uterine quiescence during pregnancy through fragmentation of uterine myocyte contractile proteins. Biol. Reprod. 80, 928–934 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clover L. M., Sargent I. L., Townsend A., Tampé R., Redman C. W. G., Expression of TAP1 by human trophoblast. Eur. J. Immunol. 25, 543–553 (1995). [DOI] [PubMed] [Google Scholar]
- 57.Zhang X., et al. , Ovarian stimulation retards postimplantation development and alters global gene expression profile of blastocysts in mouse. Fertil. Steril. 93, 2770–2773 (2010). [DOI] [PubMed] [Google Scholar]
- 58.Bainbridge D. R., Ellis S. A., Sargent I. L., The short forms of HLA-G are unlikely to play a role in pregnancy because they are not expressed at the cell surface. J. Reprod. Immunol. 47, 1–16 (2000). [DOI] [PubMed] [Google Scholar]
- 59.Przybyl L., et al. , Cd74-dysregulation of macrophage-trophoblastic interactions in the preeclamptic placenta. Hypertension 66, A042 (2015). [Google Scholar]
- 60.Roth O., Scharsack J. P., Keller I., Reusch T. B. H., Bateman’s principle and immunity in a sex-role reversed pipefish. J. Evol. Biol. 24, 1410–1420 (2011). [DOI] [PubMed] [Google Scholar]
- 61.Rolff J., Bateman’s principle and immunity. Proc. Biol. Sci. 269, 867–872 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsunaga T., Rahman A., What brought the adaptive immune system to vertebrates?—The jaw hypothesis and the seahorse. Immunol. Rev. 166, 177–186 (1998). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.