1. Introduction
The capitol of Hubei province in China, Wuhan, became the center of an outbreak of pneumonia of unknown cause in December 2019 [1]. This outbreak of pneumonia was the emergence of the novel coronavirus (SARS-CoV-2 later named coronavirus disease 2019 or COVID-19) and continues summing cases in every continent in only a few months [1]. The World Health Organization (WHO) situation report 58 [2], indicated that, as of the 18th of March, there had been 191,127 confirmed cases and 7,807 deaths around the world. Due to its significant impact, the emergence of this novel coronavirus has been declared a pandemic [1]. The executive governmental branch of the U.S. has recently invoked the Defense Production Act to increase the domestic production of medical supplies necessary for fighting the current pandemic [3]. The purpose of this invocation will likely be use to drive private businesses to increase U.S. production of Personal Protective Equipment (PPE) and other critical medical supplies and devices. The Food and Drug Administration (FDA) reported that the COVID-19 outbreak would likely impact the medical product supply chain, including potential disruptions to supply or shortages of critical medical products in the U.S [4].
Additive manufacturing (i.e., 3D Printing) is uniquely well positioned to support the shortage of critical medical devices [4]. Advancements in additive manufacturing techniques and development of antimicrobial polymers, offer the possibility of printing and customizing a wide range of medical devices. The critical limitation for the use of polymeric materials to additively manufacture critical medical devices is the material contamination by bacteria and viruses [5]. Previous investigations have shown strong evidence of the use of different forms of copper as a biocidal agent [5–12] and the use of copper nanocomposites to enhance the antimicrobial properties of polymers used in the development of medical devices [9,11,12].
Several international efforts, such as the Open Source COVID19 Medical Supplies Group (International) and Hack the Pandemic (Copper3D Inc) have made significant progress using additive manufacturing to develop critical medical devices. The purpose of the current manuscript is to provide a perspective of the role of additive manufacturing in the COVID-19 pandemic with an emphasis in the mechanism of action and applications of antimicrobial polymers for the development of critical medical devices.
2. Theoretical mechanisms of the enhanced antimicrobial behavior of additive manufacturing polymers
The development of an affective antimicrobial polymer for additive manufacturing seems increasingly critical due to the extensive used of polymers in the prototyping of critical medical devices. It has been suggested [12] that the addition of nanoparticles of copper to polymers and the resulting antimicrobial properties have promising applications to the development of medical devices associated to bacterial growth [12]. Previous investigations have used copper nanocomposites to enhance the antimicrobial properties of polymers used in injection molding and additive manufacturing to develop medical device [9,11] Currently, the most commonly used polymer in additive manufacturing is polylactic acid. Polylactic acid has been described as the main commodity polymer derived from annually renewable resources, such as corn [13]. Thus, the use of a renewable resource to produce antimicrobial polymers for additive manufacturing could significantly assist the current medical product supply chain disruptions involving the manufacturing of critical medical devices in austere clinical settings.
A recent publication in The New England Journal of Medicine by van Doremalen et al. [8], suggested that copper was more effective than Stainless Steel in reducing the COVID-19 virus viability, predicted decay, and Half-Life reduction. Specifically, using a Bayesian regression model, the authors reported that after exposure to a copper surface the median Half-Life reduction for the COVID-19 virus occurred at 0.774 hours (C.I. = 0.427–1.19) and no viable COVID-19 virus was measured after 4 hours. Stainless Steel, however, resulted in less desirable results showing a median Half-life reduction at 5.63 hours (C.I. = 4.59–6.86) with viable virus detected up to 72 hours. Similarly, the polymer, polypropylene, showed a low median Half-life reduction at 6.81 (C.I. = 5.62–8.17) hours with viable virus also detected up to 72 hours [8]. Thus, standard polymers have the potential problem of promoting COVID-19 virus viability for up to 3 days, while copper surfaces reduce viral viability to only 4 hours.
The development of an affective antimicrobial polymer for additive manufacturing seems increasingly critical due to the extensive used of polymers in the prototyping of critical medical devices. It has been suggested [12] that the addition of nanoparticles of copper to polymers and the resulting antimicrobial properties have promising applications to the development of medical devices associated to bacterial growth [12]. Furthermore, previous investigations have use copper nanocomposites to enhance the antimicrobial properties of polymers used in injection molding and additive manufacturing to develop medical devices [9,11].
The strong biocidal effects of copper found by van Doremalen et al. [8], are supported by previous investigations [6,7] that have examined the viral deactivation properties of copper oxide particles infused in textiles. Borkow et al. [7], found that the addition of copper oxide into respiratory protective face masks resulted in potent anti-influenza properties against human influenza A (H1N1) and avian influenza (H9N2) without altering their physical barrier properties. Furthermore, Borkow [6] examined the capacity of copper oxide-containing filters to neutralize viruses in suspension and found that these filters resulted in a significant reduction of the infectious viral titers, ranging from 1.1 log10 to 4.6 log10 for Yellow Fiber, Influenza A virus, Measles, Respiratory Cynsytial, Parainfluence 3, HIV-1, Adenovirus type 1 and Cytomegalovirus [6].
The porous configuration of 3D printed plastic parts (6–8 μm) [14] and the difficulties to sterilize them [15] can complicate the use of additive manufacturing for the development of critical medical devices, especially those exposed to large microbial loads [15]. However, the use of commercially available antimicrobial materials [11] and the implementation of printing specifications settings resulting in fused extruded layers, can stop molecules down to 0.000282 μm, significantly smaller than viruses, such as the virus associated to COVID-19 (0.03 ± 0.01 μm) [16]. The recent elaboration of thermoplastics blends with antimicrobial copper nanocomposites is a direct and practical approach to produce antimicrobial thermoplastics [11]. The antimicrobial properties of copper, has been enhanced by two main factors [9,12]. The first, is reducing the size of the copper particles to the nanoscale (10 nm) [10] increasing the volume of copper that can be added to a given solution or matrix as well as increasing the total surface area of the particles releasing a higher amount of metal ions [9,12]. The second is incorporation of copper nanoparticles into polymer matrices [9,12]. Copper nanoparticles on a polymer structure present a stronger antimicrobial effect than microparticles or metal surfaces by facilitating the adsorption of microorganisms on the polymer surface triggering the diffusion of water through the polymer matrix [12]. In turn, water with dissolved oxygen reaches the surface of embedded copper nanoparticles allowing the corrosion processes to take affect by releasing copper ions. Copper ions reach the composite surface damaging the microorganism cell membrane allowing the metal ions to enter the cell and damage DNA, RNA, and other biomolecules [12,17]. The copper ions and associated hydroxyl radicals produces DNA denaturalization damaging helical structures [17]. This DNA and RNA damage has been shown to deactivate viruses [17]. Copper oxide affected free viruses, virions being formed within the cytoplasm of cells during the cell exposure to copper, and virions prior to their budding from the cells (Figure 1) [12,17]. Furthermore, a previous investigation [11] showed that a commercially available antimicrobial additive manufacturing polymer (PLACTIVETM 1% copper nanoparticles composite, Copper3D, Santiago, Chile) was up to 99.99% effective against Methicillin-resistant Staphylococcus aureus and Escherichia coli. Thus, the unprecedented need of biocidal polymers during a pandemic and the high accessibility of additive manufacturing equipment and materials can drive the implementation of this technology to revolutionize the manufacturing of critical medical devices when the supply chain is insufficient [9].
Figure 1.
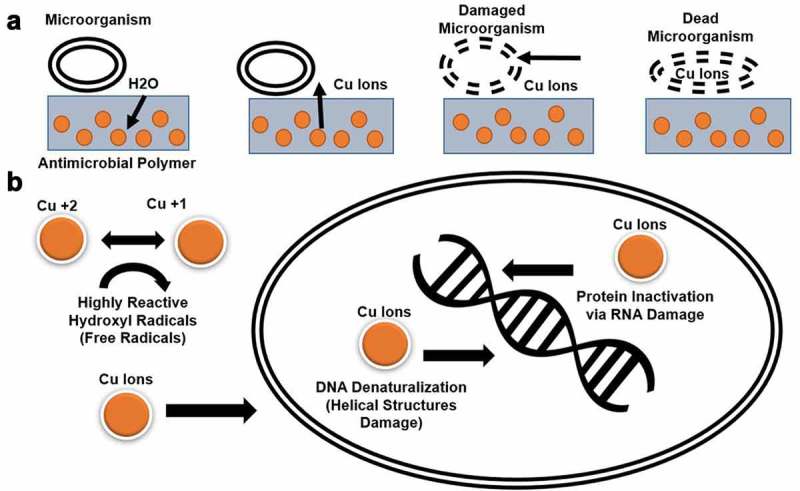
Theoretical mechanisms for the enhanced antimicrobial behavior of additive manufacturing polymers. (a) Copper nanoparticles on a polymer structure present a stronger antimicrobial effect than microparticles or metal surfaces. Antimicrobial polymers facilitate the process of attaching the microorganism on the polymer surface triggering the diffusion of water through the polymer matrix. Water with dissolved oxygen reaches the surface of embedded metal nanoparticles allowing dissolution or corrosion processes releasing metal ions; metal ions reach the composite surface damaging the bacteria membrane. Afterward, metal ions can diffuse into the interior of the microorganism. (b) The antimicrobial mechanisms of nanoparticles of copper consist in producing cell membrane damage via copper ions that damage polyunsaturated fatty acid compromising the structure of the cell membrane and producing leakage of mobile cellular solutes resulting in cell death. The redox cycling between Cu2+ and Cu1+ can catalyze the production of highly reactive hydroxyl radicals, which can subsequently damage cell membrane lipids, proteins, DNA, RNA, and other biomolecules. Once copper and associated hydroxyl radicals are inside of the cell, it produces DNA denaturalization damaging helical structures. Copper also damage and alter proteins acting as a protein inactivator via RNA, useful to deactivate a wide range of viruses.
3. Manufacturing of critical medical devices during a pandemic
COVID-19 patients are expected to experience pneumonia-like symptoms such as difficulty in breathing. The appropriate supply of devices aimed to provide supportive care and PPE will be critical in the next few weeks as the virus spread to a greater percentage of the population [4]. The use of additive manufacturing and antimicrobial polymers can be used in the prototyping of critical medical devices to accelerate the production of the final device (i.e., connectors for ventilators) or as a final product (i.e., face masks).
As this pandemic develops, providing oxygen to patients experiencing severe symptoms will be critical. It is expected that mechanical ventilators will play an important role in the treatment of COVID-19 [8]. Hospitals in the U.S. are currently expecting an unprecedented number of new COVID-19 cases. The shortage of mechanical ventilators and health workers needed to operate them, may lead to a catastrophic scenario. A previous investigation [18] showed that a single ventilator could be quickly modified to ventilate four simulated adults for a limited time. The study showed significant potential for the expanded use of a single ventilator during cases of disaster surge involving multiple casualties with respiratory failure. The study used a customized connector made of other readily available connective pieces to ventilate four lung simulators for 12 hours [18]. The main limitations of using a customized connector assembled with several connective pieces is the inability of reducing the size of the connector to minimize dead space volume [18]. Furthermore, repurposing connectors from other medical devices, can result in air leakage and infectious complications from sharing one ventilator. The use of antimicrobial polymers can facilitate the prototyping and clinical testing of these connectors with the objective of accelerating the production of the final product using conventional manufacturing methods, such as injection molding. The final production of these connectors could effectively expand the use of a single machine to ventilate four simulated adults experiencing respiratory failure due to COVID-19. The customization of the design can assist to minimize dead space volume and prevent air leakage due to unnecessary connections. During critical situations when doctors need to take life-or-death decisions due to the lack of ventilator, the use of additive manufacturing would provide a feasible alternative for sharing the use of a single ventilator. Table 1 includes a list of open-source critical medical devices prototypes, including the ‘H Connector’ for standard ventilator and the antimicrobial NanoHack 2.0 face mask among others.
Table 1.
Examples of open source critical medical devices.
| Description | Source | \Limitations |
|---|---|---|
| NanoHack Protective Mask: Provides basic protection for from airborne particles. |
Files: https://copper3d.com/hackthepandemic/ https://3dprint.nih.gov/discover/3dpx-013667 |
-Proof-of-concept design -Requires a proprietary antimicrobial material -Last resort device, intended for the general public use -Difficult to sterilize |
| H Connector for Ventilators: Expanded use of a single ventilator to ventilate four simulated adults | Files: https://copper3d.com/hconnector/ | -Proof of concept design -Need mechanical characterization data -Only compatible with 22 mm tubbing -Difficult to sterilize |
| Prusa Protective Face Shield: Provides protection from large splashes. | Files: https://www.prusaprinters.org/prints/25857-prusa-protective-face-shield-rc1 | -Requires third-party shield -Difficult to sterilize -Requires the use of antimicrobial polymer |
| Reanimation valve: Connects to a Venturi Oxygen mask to regulate the percentage of oxygen delivery. | Files: https://grabcad.com/library/respirator-free-reanimation-venturi-s-valve-1 | -Proof of concept design -Need mechanical characterization data -Difficult to sterilize |
| Hands-Free Door Opener: Attaches to door handle to prevent microbial contamination. | Files: https://www.materialize.com/en/hands-free-door-opener | -Requires third-party hardware -Difficult to sterilize |
Surgical masks are the most commonly used PPE by the general population, as well as health care workers. Surgical masks are effective in blocking large-particle droplets, but do not filter or block small particles in the air. The main reason, surgical masks do not provide complete protection is due to the loose fit between the surface of the mask and face allowing the entrance from small particle and large droplets. The Centers for Disease Control and Prevention (CDC) recommends to safely discard used masks in a plastic bag and put it in the trash [19]. The last and crucial step to safely discard these masks is hand washing [19]. Previous published research [7] has suggested that the high viral load remaining in surgical masks can be a source of viral transmission both to the health care worker wearing the mask and to patients [7]. This may happen when healthcare workers touch their mask and then fail to wash their hands properly or when they dispose of the mask without proper safe disposal precautions [7]. Thus, the used of additive manufacturing using antimicrobial polymers to develop reusable face masks (Table 1) can significantly reduce the viral load remaining on the mask protecting the end-users from contamination during prolonged mask wearing [7].
4. Conclusion
In conclusion, additive manufacturing is uniquely well positioned to support the shortage of critical medical devices [4]. Advancements in the development of commercially available antimicrobial polymers for additive manufacturing, offer the possibility of rapid prototyping a wide range of critical medical devices. The strong scientific evidence provided by previous investigations about the biocidal effects of copper nanocomposites and the enhanced antimicrobial behavior of these composites in polymers, provides an alternative for the rapid prototyping of critical medical devices during a pandemic. Furthermore, the proposed theoretical cellular mechanism presented in the current perspective, provides a potential pathway to the potential inactivation of the COVID-19 virus on surfaces of critical medical devices manufactured with antimicrobial polymers.
5. Expert opinion
The current limitations of using additive manufacturing to produce critical medical devices is the slow production time, verification of the quality of the print, and compliance with regulatory entities. The slow production time of additive manufacturing can be offset by the flexibility of using and transport a raw material to austere environments for final production. The industrial production of antimicrobial polymers and additive manufacturing of on-demand critical medical devices are described in Figure 2. The ability to produce antimicrobial polymers using a renewable source that can be stored and transported as a raw material could significantly assist the current medical product supply chain disruptions involving the manufacturing of critical medical devices in austere clinical settings. It can be argued that current state of additive manufacturing may not be mature enough to develop a final product ready to be implemented in a clinical setting. However, the true benefits of accessible additive manufacturing techniques, such as fused deposition modeling, during a fast-developing pandemic are the ability to produce functional complex geometries both quickly and at low cost. These functional complex geometries and the rapid prototyping of experimental medical devices accelerates the developing process of a final product of a medical device and its implementation. The use of more sophisticated additive manufacturing methods and materials, such as Selective Laser Sintering and Polyamide 12 powdered thermoplastic polymers embedded with copper nanoparticles composite would significantly improve the durability facilitating the implementation of antimicrobial medical devices in clinical settings.
Figure 2.
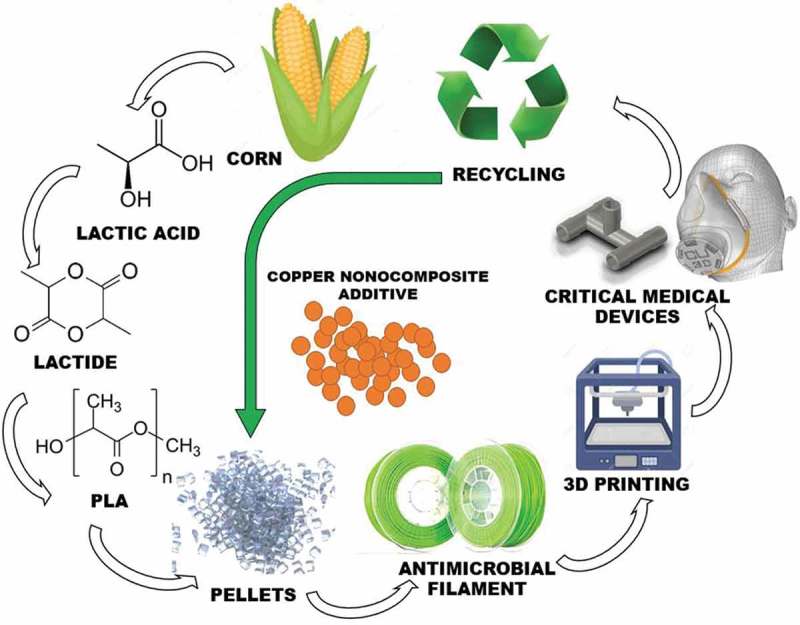
The manufacturing process of antimicrobial critical medical devices using an antimicrobial polymer. The process starts with corn fermentation (corn to Lactic Acid), condensation (Lactide) and polymerization (Polylactic acid; PLA). The addition of copper nanocomposite additive to pellets at different concentrations allows the development of a multipurpose antimicrobial filament. The recyclable characteristics of this filament facilitate the production of new antimicrobial medical devices in austere environments.
The use of additive manufacturing of critical medical devices has facilitated efforts of international groups, such as the Open Source COVID19 Medical Supplies Group (International) and Hack the Pandemic (Copper3D Inc) to respond to disruptions to the medical product supply chain by providing alternative options to access critical medical devices. As additive manufacturing technology matures and continues to permeate varied industries and products, it is apparent that some level of regulation must be developed to ensure consistent quality and reproducibility across fabrication types, manufacturers and materials. A recent FDA Draft Guidance titled ‘Technical Considerations for Additive Manufactured Devices’ [20] serves as a first step toward defining government policy regarding the use of additive manufacturing for critical medical devices. These regulatory efforts validate the use of additive manufacturing to rapid prototype critical medical devices and accelerate the process of final production and implementation. It is feasible that within a 5-year period, additive manufacturing and the use of antimicrobial polymers will play a crucial role in the development of on-demand and implementation of antimicrobial critical medical devices in clinical settings.
Funding Statement
This paper was funded by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number [R01NS114282] and the NASA Nebraska Space Grant (Federal Award #NNX15AI09H).
Article highlights
A recent publication in The New England Journal of Medicine by van Doremalen et al., suggested that copper was more effective than Stainless Steel in reducing the COVID-19 virus viability, predicted decay, and Half-Life reduction.
Copper oxide show biocidal effects for a wide range of viruses, including those causing Yellow Fiber, Influenza A virus, Measles, Respiratory Cynsytial, Parainfluence 3, HIV-1, Adenovirus type 1 and Cytomegalovirus.
Copper nanoparticles on a polymer structure present a stronger antimicrobial effect than microparticles or metal surfaces.
The copper ions and associated hydroxyl radicals produces DNA denaturalization damaging helical structures deactivating viruses.
The strong scientific evidence provided by previous investigations about the biocidal effects of copper nanocomposites and the enhanced antimicrobial behavior of these composites in polymers, provides an alternative for the rapid prototyping of critical medical devices during a pandemic.
The proposed theoretical cellular mechanism presented in the current perspective, provides a potential pathway for the inactivation of the COVID-19 virus on surfaces of critical medical devices manufactured with antimicrobial polymers.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.WHO Coronavirus disease (COVID-19) pandemic. 2020. [cited 2020 March22]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 2.WHO Coronavirus disease 2019 (COVID-19) situation report – 58. 2020. [cited 2020 March20]. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200318-sitrep-58-covid-19.pdf?sfvrsn=20876712_2
- 3.FEMA The Defense Production Act of 1950, as Amended (50 U.S.C. App. 2061 et seq.). 1950. [cited 2020 March20]. Available from: https://www.fema.gov/media-library/assets/documents/15666
- 4.FDA Coronavirus (COVID-19) supply chain update. 2020. [cited 2020 March22]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-supply-chain-update
- 5.Gonzalez-Henriquez CM, Sarabia-Vallejos MA, Rodriguez Hernandez J.. Antimicrobial polymers for additive manufacturing. Int J Mol Sci. 2019. March 10;20:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borkow G, Sidwell RW, Smee DF, et al. Neutralizing viruses in suspensions by copper oxide-based filters. Antimicrob Agents Chemother. 2007. July;51(7):2605–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Crucial paper describing the ability of copper oxide in neutralizing viruses.
- 7.Borkow G, Zhou SS, Page T, et al. A novel anti-influenza copper oxide containing respiratory face mask. PloS One. 2010. June 25;5(6):e11295. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Significant research finding about the strong efficacy of copper-oxide added to protective equipment to inactivate different influenza viruses.
- 8.van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020. March 17;382382(16):1564–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Crucial research study showing quantitative data of the COVID-19 virus viability and half-life on different surfaces.
- 9.Palza H, Nunez M, Bastias R, et al. In situ antimicrobial behavior of materials with copper-based additives in a hospital environment. Int J Antimicrob Agents. 2018. June;51(6):912–917. [DOI] [PubMed] [Google Scholar]
- 10.Palza H, Quijada R, Delgado K.. Antimicrobial polymer composites with copper micro- and nanoparticles: effect of particle size and polymer matrix. J Bioact Compat Polym. 2015;30(4):366–380. [Google Scholar]
- 11.Zuniga J. 3D printed antibacterial prostheses. Appl Sci. 2018;8(9):1651. [Google Scholar]; • Significant findings of the application and effectiveness of an antimicrobial polymer to develop antimicrobial medical devices.;
- 12.Palza H. Antimicrobial polymers with metal nanoparticles. Int J Mol Sci. 2015. January 19;16(1):2099–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Council NR Commodity polymers from renewable resources: polylactic acid. 2001. 2020. [cited 2020 Apr]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK44131/
- 14.Chuang KCG, Joseph E, Draper RD, et al. Additive manufacturing and characterization of ultem polymers and composites. 2015. [cited 2020 March22]. Available from: https://ntrs.nasa.gov/search.jsp?R=20160001352
- 15.Kondor S, Grant CG, Liacouras P, et al. On demand additive manufacturing of a basic surgical kit. J Med Devices. 2013;7(3):030916-030916-030912. [Google Scholar]
- 16.Barnard DL, Kumaki Y. Recent developments in anti-severe acute respiratory syndrome coronavirus chemotherapy. Future Virol. 2011;6(5):615–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gadi B, Jeffrey G. Copper as a biocidal tool. Curr Med Chem. 2005;12(18):2163–2175. [DOI] [PubMed] [Google Scholar]
- 18.Neyman G, Irvin CB. A single ventilator for multiple simulated patients to meet disaster surge. Acad Emerg Med. 2006. November;13(11):1246–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.FDA Personal protective equipment for infection control. 2020. [cited 2020 March20]. Available from: https://www.fda.gov/medical-devices/general-hospital-devices-and-supplies/personal-protective-equipment-infection-control
- 20.FDA Technical considerations for additive manufactured devices. 2016. [cited 2020 Apr]. Available from: http://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm499809.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- WHO Coronavirus disease (COVID-19) pandemic. 2020. [cited 2020 March22]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- WHO Coronavirus disease 2019 (COVID-19) situation report – 58. 2020. [cited 2020 March20]. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200318-sitrep-58-covid-19.pdf?sfvrsn=20876712_2
- FEMA The Defense Production Act of 1950, as Amended (50 U.S.C. App. 2061 et seq.). 1950. [cited 2020 March20]. Available from: https://www.fema.gov/media-library/assets/documents/15666
- FDA Coronavirus (COVID-19) supply chain update. 2020. [cited 2020 March22]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-supply-chain-update
- Council NR Commodity polymers from renewable resources: polylactic acid. 2001. 2020. [cited 2020 Apr]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK44131/
- Chuang KCG, Joseph E, Draper RD, et al. Additive manufacturing and characterization of ultem polymers and composites. 2015. [cited 2020 March22]. Available from: https://ntrs.nasa.gov/search.jsp?R=20160001352
- FDA Personal protective equipment for infection control. 2020. [cited 2020 March20]. Available from: https://www.fda.gov/medical-devices/general-hospital-devices-and-supplies/personal-protective-equipment-infection-control
- FDA Technical considerations for additive manufactured devices. 2016. [cited 2020 Apr]. Available from: http://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm499809.pdf


