Abstract
To develop a new therapeutic strategy against thyroid cancer (TC), the expression of both substance P (SP) and neurokinin-1 receptor (NK-1R) must be demonstrated in TC cells. This study aims to examine by immunohistochemistry the localization of SP and the NK-1R in human TC samples (papillary, follicular, medullary, anaplastic) in metastasis and in healthy thyroid samples. SP and the NK-1R were expressed in all normal and TC samples. In healthy glands, SP was located in follicular cells (nucleus) and colloid, and NK-1R in follicular cells (cytoplasm) and stroma. In TC samples, SP was visualized in follicular cells (nucleus and cytoplasm), stroma and colloid, and NK-1R in follicular cells (cytoplasm), stroma and colloid. A semiquantitative scoring system (Allred Unit Scoring System) was applied. The expression (Allred total score) of SP and NK-1R was weaker in normal thyroid glands than in TC. In comparison with TC samples, a lower intensity/proportion of SP (nucleus and cytoplasm of follicular cells; stroma) was observed in normal samples. By contrast, in the colloid of TC samples the presence of SP was lower than in normal samples. In comparison with TC samples, the presence of the NK-1R in the cytoplasm of follicular cells and colloid was lower in normal thyroid samples, whereas the expression of this receptor in the stroma was higher. The results reported in this study suggest that the NK-1R could be a new target for the treatment of TC and use of the NK-1R antagonists could serve as a new anti-TC therapeutic strategy.
Key words: Immunohistochemistry, NK-1 receptor, nucleus, SP, tachykinin, thyroid gland
Introduction
Substance P (SP), an undecapeptide belonging to the tachykinin family of peptides, is widely distributed throughout the whole body. The neurokinin-1 receptor (NK-1R, which also shows a widespread distribution throughout the body), the NK-2R and the NK-3R mediate the biological actions exerted by tachykinins (SP, neurokinin A, neurokinin B). SP has the highest affinity for the NK-1R and hence the biological actions (e.g., neurogenic inflammation, pain, mitogenesis, regulation of the cardiovascular system) exerted by SP are mainly mediated by this receptor. SP is released from nerve endings and has also been located in human non-neuronal cell types.1,2
SP not only acts as a neurotransmitter but could also act, in cancer cells, as a functional regulator in an autocrine and/or paracrine manner.1,3,4 Many data confirm the involvement of the SP/NK-1R system in cancer: i) both SP and the NK-1R have been located in peri- and intra-tumoral blood vessels and in tumor cells;4,5 ii) at nanomolar concentration, SP induces mitogenesis in many human cancer cells;2,4,6-18 iii) SP increases the phosphorylation of Akt, exerting an antiapoptotic effect,19 and iv) SP induces the migration of cancer cells promoting invasion/metastasis: this is mediated by the Rho-associated coiled-coil kinase (ROCK) signalling pathway which modulates the myosin regulatory light chain protein; the phosphorylation of this protein (SP increases its phosphorylation) promotes changes in cellular shape, including blebbing; these changes are essential for cancer cell movement, spreading and infiltration.20 This is very important, since the prevention of metastasis is a major goal in cancer treatment (over 90% of cancer deaths are derived from the development of metastases).4 It is known that NK-1R antagonists inhibit the above effects mediated by SP and, in addition, promote the apoptosis of human cancer cells.4,7-11,13,15 These findings mean that the SP/NK-1R system plays an important role in cancer progression, that the NK-1R is an important target for cancer treatment and that NK-1R antagonists may act as broad-spectrum antitumor drugs.4,7,10,11,13,21 For this reason, in order to develop new therapeutic strategies, it is crucial to demonstrate the presence in tumor cells of SP and the NK-1R. In this sense, this work aims to study by immunohistochemistry the presence of SP/NK-1R in human thyroid cancer (TC) and in healthy thyroid samples. In a patient with medullary TC, a high level of SP in plasma has been previously reported.22 Moreover, only in one case of twenty-seven cases of medullary TC the presence of SP was demonstrated by immunohistochemistry,23 whereas in other study the expression of NK-1R was reported in 10/12 medullary TC samples.5 Thus, based on this poor and partial knowledge (only medullary TC was previously studied), here we try to demonstrate for the first time the immunolocalization of SP and NK-1R in four human TC types (papillary, follicular, anaplastic, metastasis) and in healthy samples.
In humans, TC is the most prevalent endocrine malignancy.24 Differentiated thyroid carcinoma (DTC), which includes papillary and follicular cancer, comprises the vast majority (> 90%) of all TC. TC includes types ranging from indolent localized papillary carcinomas to the fulminant and lethal anaplastic disease.25 The most frequent subtype is the DTC, derived from epithelial cells, and includes papillary (PTC, 80%), follicular (FTC, 11%) and other less frequent histologic subtypes [e.g., Hürthle cells, insular, poorly differentiated TC (PDTC), follicular variant of PTC, tall cell carcinoma]. The risk of developing PTC is higher in patients suffering from Hashimoto’s thyroiditis. This TC type is more aggressive than others PTC.26 The medullary TC (MTC), derived from the calcitoninproducing parafollicular cells (C cells) of the thyroid gland, represents 5-10% of all TCs,27 whereas the anaplastic TC (ATC), a highly aggressive tumor, is present in only 2% of the patients, this being one of the most lethal human tumors with a mean survival of six months after diagnosis. There are other subtypes even less frequent, such as lymphomas or sarcomas from the thyroid gland.28 Distant metastases (half of them are detectable at initial stages of the disease) are observed in about 15% of DTC patients.29 Unfortunately, the incidence of TC continues to rise rapidly worldwide, especially in women: it has been predicted that in 2019, PTC would have become the third most common cancer in women.30 Recently, a study has been published on thyroid cancer incidence trends in USA and the authors concluded that incidence trends have been mainly shaped by changes in professional guideline recommendations.31 Thus, new therapeutic strategies must be urgently developed. One of these could be the use of NK-1R antagonists and hence, to develop in the future this new therapeutic strategy, it is first needed to know whether the SP/NK-1R system is expressed or not in human TC. This is the main goal of this work.
Materials and Methods
Thyroid tissue samples were collected from patients diagnosed with TC and surgically treated with curative intent. We studied 135 samples from 26 cases (ten males and 16 females). There were 9 cases of FTC, 9 cases of PTC (two of them with Hürthle cell variant and one with follicular variant), 2 cases of MTC, 2 cases of ATC and 4 cases of undifferentiated metastasis TC (MeTC). Healthy thyroid samples were also taken from individuals of both sexes. This research was approved by the Badajoz University School of Medicine Ethical Committee (Badajoz, Spain). Informed consents were obtained from the patients and both protocol and procedure of this work were performed under the ethical guidelines and legal recommendations of both Spanish and European Union laws. The study was conducted according to the Declaration of Helsinki.
Four tissue samples were picked from each case studied: the first for SP, the second for NK-1R, the third for hematoxylin-eosin and the forth was used as negative control (the primary antibody was omitted, being replaced by non-immune serum: no immunostaining was observed) (Figures 1B and 2B). Samples of human lung cancer, in which the presence of SP and NK-1R was previously demonstrated by immunohistochemistry,16 were used as positive control. Immunoreactivity, as previously reported,16 was found in lung cancer sections in the expected location of the targeted antigen. Samples were fixed in 4% formaldehyde and embedded in paraffin blocks. Then, adequate tissue sections (4 μm) were obtained for DAKO Autostainer Instruments, mounted on slides and finally each sample was identified. Sections were deparaffinised with xylene and rehydrated through a series of ethanol. Then, heat-induced epitope retrieval using PT Link module and EnVision Flex protocols on an automated system (Autostainer Link) was performed according to manufacturer’s instructions (DAKO, Santa Clara, USA). For antigen retrieval, the sections were boiled in citrate buffer (2.94 g/L sodium citrate, pH 6.0) for 15 min and subsequently cooled to 30ºC. Endogenous peroxidase activity was blocked by incubating the sections in methanol with 1.5% H2O2 for 2 min. After washing with phosphate-buffered saline (PBS), the sections were incubated with 10% non-immune horse serum for 15 minutes. Sections were incubated overnight at 4ºC with 1/1,000 diluted anti-NK-1R antibody (SAB-4502913, Sigma-Aldrich, Madrid, Spain) or 1/2,000 diluted anti-SP antibody (S-1542, Sigma-Aldrich). Sections were washed in PBS and incubated in biotinylated horse anti-mouse antibody (1/500) (Vector Laboratories Inc., Burlingham, CA, USA) with peroxidase- streptavidin conjugate (1/400) (Immunotech Laboratories, Monrovia, CA, USA) for 30 min. The sections were washed in phosphate-citrate buffer (pH 5.8), and SP and NK-1R were visualized by light microscopy with diaminobenzidine (DAB) reagent (0.06% 3, 3 diaminobenzidine tetrahydrochloride and 0.03% H2O2 in phosphate-citrate buffer). TC samples were evaluated by an independent pathologist. In each slide, ten representative high-power microscopic fields were evaluated using a 40x objective. The presence or absence of immunoreactivity for SP/NK-1R in follicular and endothelial cells, colloid and stroma was studied, as well as the int ensity o f the i mmu noreacti vit y and whether or not t he immunoreactivity was localized in the nucleus and/or the cytoplasm of cells. In order to decrease subjectivity in the observation of the immunoreactivity, a semiquantitative scoring system (Allred Unit Scoring System) was applied.32 This is a method for scoring the immunostaining signals from 0 to 8. Moreover, the proportion score (PS) that represents the estimated proportion of positive tumor cells on the entire section has been also assigned (ranging from 0 to 5), as well as the intensity score (IS) estimating the average staining intensity of positive tumor cells (ranging from 0 to 3). The PS and IS are summed to obtain the total score (TS), from 0 to 8.
Results
The SP/NK-1R system in the normal thyroid gland
In the follicular cells of healthy samples (compared to TC samples), a lower SP intensity and proportion regarding the nucleus (Allred media TS: 5) and cytoplasm (Allred media TS: 0) were observed (Figure 1A). No SP-immunoreactivity was observed in the stroma (Allred media TS: 0) and, in the colloid, the immunoreactivity was medium (Allred media TS: 6). NK-1R-immunoreactivity was observed in follicular cells (Allred media TS: 4 (cytoplasm); Allred media TS: 0 (nucleus)) (Figure 2A). In stroma a weak immunoreactivity was observed (Allred media TS: 2) and no immunoreactivity in the colloid (Allred media TS: 0) (for NK-1R) was found. No immunoreactivity for SP and NK-1R was observed in endothelial cells.
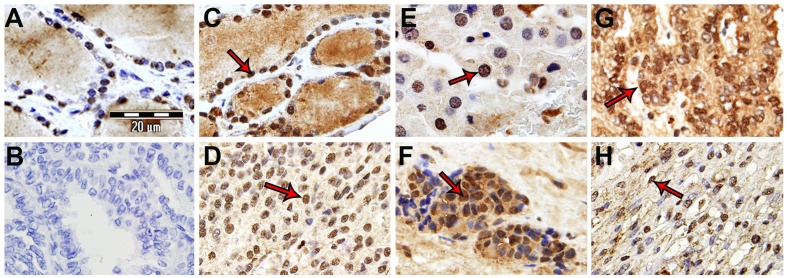
Figure 1.
Thyroid samples: SP expression showing an intense nuclear pattern (arrows). A) Healthy thyroid. B) Negative control. C) PTC. D) FTC. E) Hürthle cell variant TC carcinoma. F) MTC. G) MeTC. H) ATC.
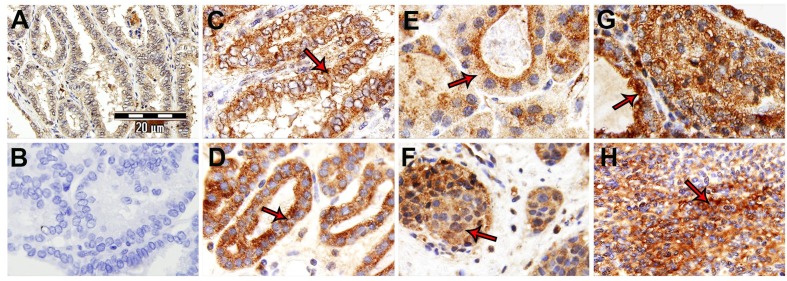
Figure 2.
Thyroid samples: NK-1R expression showing a predominantly cytoplasmic pattern (arrows). A) Healthy thyroid. B) Negative control. C) PTC. D) FTC. E) Hürthle cell variant TC carcinoma. F) MTC. G) MeTC. H) ATC.
The SP/NK-1R system in TC: SP immunoreactivity
Table 1 shows, according to the Allred Unit Scoring System, the results found for SP in TC samples (stroma, colloid, nucleus/cytoplasm of follicular cells). The peptide was observed in the nucleus of all follicular cells (high intensity and proportion) showing different TS ranges (Allred media TS: 5.69), whereas cytoplasm staining (low intensity and proportion) was observed in 53.8% of these cells (Allred media TS: 2.42).
In PTC (case number: 1-9) (Table 1), an intense immunoreactivity was observed in the nucleus of the follicular cells, whereas a lower proportion and intensity staining was visualized in the cytoplasm of these cells, stroma and colloid (Figure 1C). Moreover, the presence of SP in Hürthle cell variant follicular carcinoma was also studied. Hürthle cells show a granular cytoplasm due to mitochondrial accumulation and the nucleus shows an apparent nucleolus. The periphery of the nucleus showed an intense SP staining, whereas in the stroma, colloid and cytoplasm of the Hürthle cells a low immunoreactivity was observed (Figure 1E). In an FTC variant arising from Hashimoto’s thyroiditis, an intense immunoreactivity was observed in the nucleus of follicular cells that are mixed with a prominent lymphocytic infiltration. A low immunoreactivity was observed in the stroma, colloid and cytoplasm of follicular cells. In FTC samples (case number: 10-17) (Table 1), a strong staining in the nuclei of follicular cells was observed, whereas the cytoplasm of these cells, colloid and stroma showed a poor immunoreactivity (Figure 1D). In MTC samples (case number: 18-20) (Table 1), an intense immunoreactivity was found in the nucleus of follicular cells, whereas the cytoplasm showed a moderate intensity (Figure 1F). In the stroma and colloid of MTC samples, a very low immunoreactivity was visualized. In MeTC (case number: 23-25) (Table 1), the highest intensity in immunoreactivity was observed in the nucleus of follicular cells, whereas the cytoplasm of these cells, stroma and colloid showed an intense immunoreactivity (Figure 1G). ATC (case number: 21 and 22) (Table 1) showed an intense immunoreactivity in the nuclei of follicular cells, whereas in the stroma, colloid and cytoplasm of follicular cells a low SP-immunoreactivity was visualized according to the Allred Unit Scoring System (Figure 1H).
Finally, no immunoreactivity for SP was observed in the small blood vessels of TC samples; however, in some large blood vessels observed in FTC samples the immunoreactivity was exclusively found in endothelial cells (nucleus: high intensity; cytoplasm: moderate intensity) (Figure 3).
The SP/NK-1R system in TC: NK-1R immunoreactivity
In Table 2, the NK-1R immunostaining is shown according to the Allred Unit Scoring System. An intense/highly proportioned cytoplasmic staining was observed in 93.3% of the samples and no nuclear staining in 88.5% of them. In healthy thyroid glands, a cytoplasmic staining was observed but lower (intensity and proportion) than that found in TC; no nuclear staining was observed (Figure 2A).
Table 1.
SP immunoreactivity in human TC samples.
| Cytoplasm | Nucleus | Stroma | Colloid | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Sex | Age | Type | Proportion | Intensity | Allred | Proportion | Intensity | Allred | Proportion | Intensity | Allred | Proportion | Intensity | Allred |
| 1 | M | 59 | PTC | 2 | 2 | 4 | 3 | 3 | 6 | 2 | 3 | 5 | 2 | 4 | 6 |
| 2 | F | 48 | PTC | 0 | 0 | 0 | 2 | 2 | 4 | 0 | 0 | 0 | 4 | 2 | 6 |
| 3 | M | 39 | PTC | 2 | 1 | 3 | 2 | 1 | 3 | 2 | 2 | 4 | 3 | 2 | 5 |
| 4 | M | 51 | PTC | 0 | 0 | 0 | 4 | 3 | 7 | 3 | 1 | 4 | 4 | 2 | 6 |
| 5 | F | 21 | PTC | 0 | 0 | 0 | 4 | 3 | 7 | 2 | 1 | 3 | 3 | 2 | 5 |
| 6 | M | 31 | PTC | 2 | 2 | 4 | 2 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | F | 41 | PTC | 4 | 3 | 7 | 2 | 3 | 5 | 0 | 0 | 0 | 4 | 2 | 6 |
| 8 | F | 39 | PTC | 0 | 0 | 0 | 3 | 4 | 7 | 0 | 0 | 0 | 1 | 1 | 2 |
| 9 | M | 57 | PTC | 0 | 0 | 0 | 2 | 3 | 5 | 0 | 0 | 0 | 1 | 1 | 2 |
| 10 | F | 40 | FTC | 0 | 0 | 0 | 4 | 2 | 6 | 0 | 0 | 0 | 0 | 0 | 0 |
| 11 | F | 73 | FTC | 3 | 1 | 4 | 4 | 4 | 8 | 0 | 0 | 0 | 4 | 2 | 6 |
| 12 | F | 76 | FTC | 0 | 0 | 0 | 2 | 3 | 5 | 3 | 1 | 4 | 0 | 0 | 0 |
| 13 | F | 32 | FTC | 0 | 0 | 0 | 2 | 2 | 4 | 1 | 2 | 3 | 1 | 1 | 3 |
| 14 | M | 28 | FTC | 0 | 0 | 0 | 4 | 3 | 7 | 0 | 0 | 0 | 3 | 1 | 4 |
| 15 | F | 63 | FTC | 5 | 1 | 6 | 5 | 3 | 8 | 2 | 1 | 3 | 0 | 0 | 0 |
| 16 | F | 11 | FTC | 0 | 0 | 0 | 4 | 2 | 6 | 0 | 0 | 0 | 3 | 1 | 4 |
| 17 | F | 26 | FTC | 0 | 0 | 0 | 4 | 3 | 7 | 0 | 0 | 0 | 3 | 2 | 5 |
| 18 | M | 50 | MTC | 2 | 1 | 3 | 4 | 3 | 7 | 4 | 1 | 5 | 0 | 0 | 0 |
| 19 | M | 74 | MTC | 0 | 0 | 0 | 1 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20 | F | 75 | MTC | 3 | 2 | 5 | 4 | 3 | 7 | 0 | 0 | 0 | 0 | 0 | 0 |
| 21 | F | 72 | ATC | 3 | 2 | 5 | 2 | 1 | 3 | 3 | 3 | 6 | 0 | 0 | 0 |
| 22 | F | 63 | ATC | 2 | 1 | 3 | 3 | 2 | 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| 23 | M | 34 | MeTC | 2 | 2 | 4 | 4 | 3 | 7 | 2 | 2 | 4 | 4 | 2 | 6 |
| 24 | M | 57 | MeTC | 5 | 1 | 6 | 2 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 25 | F | 39 | MeTC | 4 | 2 | 6 | 5 | 3 | 8 | 3 | 1 | 4 | 0 | 0 | 0 |
ATC, anaplastic TC; F, female; FTC, follicular TC; M, male; MTC, medullary TC; MeTC, metastasis TC; PTC, papillary TC. Nucleus/cytoplasm data are referred to follicular cells.
In PTC (case number: 1-9) (Table 2), an intense expression of NK-1R was observed in the cytoplasm of follicular cells (Figure 2C), whereas the colloid showed a moderate intensity. No immunoreactivity for SP was observed in the stroma and nuclei of follicular cells.
In FTC (case number: 10-17) (Table 2), a high expression of NK-1Rs was observed in the cytoplasm of follicular cells; in the nuclei of these cells and in the stroma, low or no immunoreactivity was observed, whereas in the colloid the immunoreactivity was moderate (Figure 2D). In a FTC variant arising from Hashimoto’s thyroiditis, NK-1R-immunoreactivity was found in the cytoplasm of follicular cells, whereas in the stroma, colloid and nuclei of these cells a low or no immunoreactivity was visualized. In MeTC (case number: 23-26) (Table 2), a high NK-1R immunoreactivity was observed in the cytoplasm of follicular cells, whereas in the stroma, colloid and nuclei of these cells a low or no immunoreactivity was observed (Figure 2G). In the cytoplasm of Hürthle cells, NK-1R immunoreactivity was observed (Figure 2E). In MTC samples (case number: 18-20) (Table 2), C cells showed a cytoplasm intensely stained; in contrast, no immunoreactivity was observed in the rest of the tissue (Figure 2F). In ATC (case number: 21 and 22) (Table 2), a complete disruption of the follicular thyroid architecture was found and in general the immunoreactivity for NK-1R was low (Figure 2H). Finally, no immunoreactivity for the NK-1R was observed in blood vessels.
Figure 3.
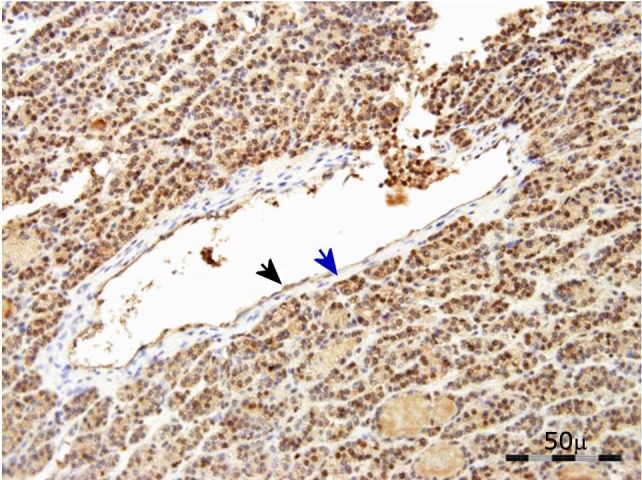
SP expression in large blood vessels (FTC sample). Immunoreactive nucleus in the endothelial cell (black arrow). No immunoreactivity in tunica media/intima (blue arrow).
Table 2.
NK-1R immunoreactivity in human TC samples.
| Cytoplasm | Nucleus | Stroma | Colloid | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Sex | Age | Type | Proportion | Intensity | Allred | Proportion | Intensity | Allred | Proportion | Intensity | Allred | Proportion | Intensity | Allred |
| 1 | M | 59 | PTC | 3 | 3 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 2 | 5 |
| 2 | F | 48 | PTC | 4 | 3 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 1 | 6 |
| 3 | M | 39 | PTC | 5 | 2 | 7 | 0 | 0 | 0 | 2 | 1 | 3 | 3 | 2 | 5 |
| 4 | M | 51 | PTC | 3 | 2 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 4 |
| 5 | F | 21 | PTC | 3 | 2 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | M | 31 | PTC | 2 | 3 | 5 | 0 | 0 | 0 | 2 | 1 | 3 | 0 | 0 | 0 |
| 7 | F | 41 | PTC | 4 | 3 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 2 | 5 |
| 8 | F | 39 | PTC | 4 | 3 | 7 | 0 | 0 | 0 | 2 | 1 | 3 | 4 | 2 | 6 |
| 9 | M | 57 | PTC | 3 | 2 | 5 | 0 | 0 | 0 | 2 | 1 | 3 | 0 | 0 | 0 |
| 10 | F | 40 | FTC | 4 | 3 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 3 |
| 11 | F | 73 | FTC | 4 | 3 | 7 | 0 | 0 | 0 | 4 | 2 | 6 | 4 | 3 | 7 |
| 12 | F | 76 | FTC | 3 | 3 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 | 6 |
| 13 | F | 32 | FTC | 2 | 3 | 5 | 0 | 0 | 0 | 1 | 2 | 3 | 3 | 2 | 5 |
| 14 | M | 28 | FTC | 4 | 2 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 15 | F | 63 | FTC | 3 | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 16 | F | 11 | FTC | 4 | 2 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 17 | F | 26 | FTC | 5 | 3 | 8 | 0 | 0 | 0 | 1 | 1 | 2 | 3 | 2 | 5 |
| 18 | M | 50 | MTC | 4 | 3 | 7 | 0 | 0 | 0 | 3 | 1 | 4 | 0 | 0 | 0 |
| 19 | M | 74 | MTC | 3 | 3 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20 | F | 75 | MTC | 5 | 2 | 7 | 3 | 2 | 5 | 2 | 1 | 3 | 0 | 0 | 0 |
| 21 | F | 72 | ATC | 2 | 2 | 4 | 2 | 2 | 4 | 0 | 0 | 0 | 3 | 2 | 5 |
| 22 | F | 63 | ATC | 2 | 1 | 3 | 2 | 2 | 4 | 0 | 0 | 0 | 3 | 2 | 5 |
| 23 | M | 34 | MeTC | 5 | 3 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 24 | M | 57 | MeTC | 5 | 3 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 3 |
| 25 | F | 39 | MeTC | 4 | 3 | 7 | 0 | 0 | 0 | 2 | 2 | 4 | 2 | 1 | 3 |
| 26 | F | 39 | MeTC | 5 | 3 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 1 | 4 |
ATC, anaplastic TC; F, female; FTC, follicular TC; M, male; MTC, medullary TC; MeTC, metastasis TC; PTC, papillary TC. Nucleus/cytoplasm data are referred to follicular cells.
Statistical analysis
In comparison to controls and in order to analyze changes regarding the immunostaining observed in TC samples, the Wilcoxon test for paired samples was performed (Tables 3 and 4). The 5% (P<0.05) level was considered significant. The software used was SPSS Statistics 19.3. In TC, no sex or age differences were found regarding the proportion/intensity of SP or NK-1R.
Comparing the presence of nuclear SP immunostaining in TC and normal follicular cells, it was demonstrated that the number of TC cells expressing the peptide was higher than that observed in normal cells (P<0.000), whereas the cytoplasmic expression of SP in TC was more than two-fold higher than in normal ones (P<0.001). In stroma, the immunostaining in TC was twice that of healthy cases (P<0.002), whereas in the colloid of the healthy tissue there was more SP than in TC samples (P<0.008).
The number of TC follicular cells expressing NK-1Rs in the cytoplasm was higher than that observed in normal cells (P<0.000), whereas no statistical difference was found between TC and normal follicular cells, regarding the nuclear expression of NK-1R (P<0.102). In the stroma, the number of cells expressing the NK-1R (in TC samples) was lower, in comparison with that found in healthy samples (P<0.010); in the colloid of TC samples NK-1R immunoreactivity was observed, but this was not found in normal samples (P<0.001).
Discussion
For the first time, the immunolocalization of SP and NK-1R in human normal thyroid glands has been described as well as in four types of TC samples (ATC, FTC, MeTC and PTC). SP and the NK- 1R have been observed in all TC and healthy samples studied. The main finding of this work is that the expression of both SP/NK-1R was higher in TC samples than in healthy ones. Our data increase the knowledge of the SP/NK-1R system in TC, since only the presence of SP/NK-1R in MTC had previously been reported.5,23 However, it is important to note that here we observed SP in all normal and MTC samples studied; in contrast, previous studies reported SP immunoreactivity in 1 case of 27 MTC23 and immunoreactivity for NK-1R in 10 cases of 12 MTC.5
The presence of SP/NK-1R in the nucleus/cytoplasm of TC follicular cells is in agreement with the results found, in previous studies, in other human cancer cells: kerastocystic odontogenic tumors,33 gastric tumor,18,34 larynx carcinoma,35 oral squamous tumor,36 small and non-small lung cancer,16 melanoma15 and breast cancer.17 In large blood vessels of FTC samples, SP has been observed in the nucleus of endothelial cells. This is in agreement with previous works reporting the presence of SP in the nucleus of these cells located in placenta, decidua, trophoblasts of fetal membranes and chorionic villi (cytotrophoblast and syncytiotrophoblast). 1 Moreover, SP was also located in the nuclei of Hofbauer cells and myocytes and in vascular anomalies the expression of both SP and NK-1R was reported.2,37 It is also known that in tumors, both SP and NK-1R were found in intra- and peritumoral blood vessels and, in fact, during neoangiogenesis, both tissue innervation and the expression of NK-1 receptors were increased.4,5 It has been demonstrated that NK-1R antagonists (via the NK-1R) exert an antiangiogenic action, since they inhibited tumor neoangiogenesis.38,39
As previously suggested,2,34 the presence of SP in the nuclei of TC cells (e.g., follicular cells) indicates that the peptide could act as an epigenetic factor, regulating gene expression in these cells. In fact, SP regulates transcription factors and proto-oncogenes (e.g., NF-kB, c-fos, c-jun, AP-1, hypoxia-inducible factor (HIF-1α), cmyc) that are involved in inflammation (NF-kB), cell cycle progression and cellular transformation (c-myc), angiogenesis/vasculogenesis (HIF-1α), cellular differentiation (c-fos, c-jun, AP-1), apoptosis.4,6,40,41 The presence of SP in the cytoplasm of normal and TC follicular cells suggests that the peptide is released, exerting autocrine, paracrine and/or endocrine actions.4,8 Moreover, the presence of SP in the colloid of normal and TC samples supports the release and storage of the peptide into the colloid, whereas the presence of the NK-1R in the colloid of TC samples, but not in normal thyroid samples, suggests that it is related to the turnover of tumor cells which is higher in TC cells than in healthy cells.
NK-1R is involved in the viability of cancer cells and it has been suggested that, because the signal mediated by SP is crucial for tumor cells, these cells overexpress the receptor.4,8,14,16 SP could be released from TC cells and, via the NK-1R, to facilitate the proliferation of TC cells (autocrine mechanism),4,8,18,42,43 since in human cancer cells it is known that SP induces mitogenesis.4,8,12-14,44 Thus, SP (via the NK-1R) activates members of the mitogenactivated protein kinase (MAPK) cascade [e.g., extracellular signal- regulated kinases 1 and 2 (ERK1/2) is translocated into the nucleus, promoting cell proliferation]. To activate the MAPK cascade, the presence of a functional EGFR kinase domain is required6,45 and it is also known that SP increases the phosphorylation/ activity of protein kinase B (which it is inhibited by NK-1R antagonists), suppressing apoptosis.19,46,47 SP also promotes the migration/invasion of cancer cells,42 this being an important prerequisite for cancer progression and hence membrane blebbing (this is mediated by the SP/NK-1R system) is crucial in cell spreading and migration.48
Table 3.
Comparison of the SP immunoexpression in TC and healthy thyroid samples (Allred media). Wilcoxon Test with bilateral asymptotic significance.
| Immunostaining | Thyroid cancer (Allred media) | Healthy thyroid samples (Allred media) | P |
|---|---|---|---|
| Cytoplasm (follicular cells) | 2.42 [0.00-7.00] | 0.00 [0.00-0.00] | <0.001 |
| Nucleus (follicular cells) | 5.69 [3.00-8.00] | 4.00 [4.00-4.00] | <0.000 |
| Stroma | 1.88 [0.00-6.00] | 0.00 [0.00-0.00] | <0.002 |
| Colloid | 2.53 [0.00-6.00] | 4.00 [4.00-4.00] | <0.008 |
Table 4.
Comparison of the NK-1R immunoexpression in TC and healthy thyroid samples (Allred media). Wilcoxon Test with bilateral asymptotic significance.
| Immunostaining | Thyroid cancer (Allred media) | Healthy thyroid samples (Allred media) | P |
|---|---|---|---|
| Cytoplasm (follicular cells) | 6.19 [3.00-8.00] | 4.00 [4.00-4.00] | <0.000 |
| Nucleus (follicular cells) | 0.50 [0.00-5.00] | 0.00 [0.00-0.00] | <0.102 |
| Stroma | 1.15 [0.00-6.00] | 2.00 [2.00-2.00] | 0.010 |
| Colloid | 2.53 [0.00-7.00] | 0.00 [0.00-0.00] | <0.001 |
The possible release of SP from TC cells suggests that the peptide could exert a paracrine action on endothelial cells expressing the NK-1R, since SP could induce the proliferation of the latter cells promoting neovascularization and hence promoting the development of the tumor.4 Moreover, the tumor mass could also release SP into the blood (endocrine mechanism), increasing the plasma level of the peptide. This is supported by a high plasma level of SP observed in a patient with MTC.22 This is very important since the increased SP level could promote development of the paraneoplasic syndrome (thrombosis, emotional stress, pruritus, malnutrition). Platelets express NK-1R, SP induces thrombosis and NK-1R antagonists decrease the thrombus formation.49 Thus, the release of SP from the tumor mass can induce thrombophilia because the risk of thrombosis is increased. An increase in the plasma level of SP has been related to emotional stress (anxiety and depression) and hence the release of SP from the TC tumor mass could induce depression because the peptide could trigger cancer progression by establishing a cross-talk between the limbic system (emotional stress) and the TC tumor mass and vice versa. The high level of SP in blood could be related to pruritus, since it is known that the peptide induces pruritus and that NK-1R antagonists improve it.50
The SP/NK-1R system is also involved in energy production (glycolysis) and it is known that the glycolytic rate is higher in cancer cells than in normal ones and that, by means of the glycogen breakdown, cancer cells augment their metabolism and hence the size of the tumor mass also increase.4,51 It has been suggested that the glycolytic function is linked to the number of NK-1R expressed by the cell: tumor cells express more NK-1Rs than normal cells and, for this reason, the glycolytic rate is higher in the tumor cells.4
During the last 10 years, efforts have been made to investigate the molecular pathways and critical alterations involved in the tumorigenesis of TC.52,53 Consensus guidelines recommend that patients with TC undergo a surgical procedure (full thyroidectomy), whereas adjuvant radioiodine treatment (131I) is indicated for patients showing a higher risk for disease recurrence/mortality. In general, recurrence is treated with surgery, radioactive iodine and external beam radiation.54 A high dose of levothyroxine is administered to suppress serum thyrotropin and in this way the stimulation of possible remaining tumor cells is blocked. Thus, there are numerous therapeutic strategies to treat TC. A novel strategy could be the use of NK-1R antagonists, since the SP/NK-1R system is expressed in TC. The expression/secretion of peptides (e.g., SP) by tumor cells opens new therapeutic possibilities to improve both diagnosis and treatment of tumors.
In summary, this work shows the immunolocalization of SP and NK-1R in human normal thyroid and TC samples. Here, a semiquantitative scoring system (Allred Unit Scoring System) was applied and hence the subjective interpretation of the results, after the application of the immunohistochemical technique, was considerably decreased. This is important since the methodology developed in this work can be potentially applied in routine cases. In healthy thyroid, SP was located in follicular cells (nucleus) and colloid, whereas in TC the peptide was observed in follicular cells (nucleus and cytoplasm), stroma and colloid. In normal thyroid, NK- 1R was visualized in follicular cells (cytoplasm) and stroma, whereas in TC the receptor was found in follicular cells (cytoplasm), stroma and colloid. The expression (Allred TS score) of SP and NK-1R was weaker in normal thyroid glands than in TC. In comparison with normal thyroid samples, a higher intensity/proportion of SP (nucleus and cytoplasm of follicular cells; stroma) was observed in TC samples. By contrast, in the colloid of normal thyroid glands the presence of SP was higher than in TC. In comparison with normal thyroid samples, the presence of the NK-1R in the cytoplasm of follicular cells and colloid was higher in TC samples, whereas the expression of this receptor in the stroma was lower. Our study increases knowledge of the SP/NK-1R system in normal thyroid samples and four types of TC (ATC, FTC, metastasis TC and PTC). The results reported in this study suggest that, in addition to other emerging therapeutic strategies against TC [e.g., immunotherapy (monoclonal antibodies and immune checkpoint blockade, adoptive immunotherapy), targeting chemokine receptors, inhibitors of the MAPK and PI3K pathways],55-57 the NK-1R could be a new target for the treatment of TC and that the use of NK-1R antagonists could serve as a new anti-TC therapeutic strategy.
Acknowledgements
The authors wish to thank Dr. Manuel Limón (Head of the Department of Pathology, Mérida Hospital, Badajoz, Spain) and Dr. Rafael Martínez (Head of the Department of Pathology, Virgen de Valme Hospital, Sevilla, Spain) for their generous help; Mr. Javier Muñoz (University of Seville, Spain), Ms. Esther Rosso and Mr. Francisco Jesus Fuentes for their technical assistance and Ms. Diane Haun (University of Utah, USA) for reviewing the English. This work has been supported by “Programa XI: Financiación de Unidades de Excelencia de la Universidad de Salamanca” (Spain).
References
- 1.Muñoz M, Pavón A, Rosso M, Salinas-Martín MV, Pérez A, Carranza A, et al. Immunolocalization of NK-1 receptor and substance P in human normal placenta. Placenta 2010;31:649-51. doi: 10.1016/j.placenta.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Muñoz M, Carranza A, Pavón A, Anderson G, Coveñas R. Immunolocalization of substance P and NK-1 receptor in Hofbauer cells in human normal placenta. Microsc Res Tech 2013;76:1310-3. doi: 10.1002/jemt.22303. [DOI] [PubMed] [Google Scholar]
- 3.García-Recio S, Fuster G, Fernández-Nogueira P, Pastor-Arroyo EM, Park SY, Mayordomo C, et al. Substance P autocrine signaling contributes to persistent HER2 activation that drives malignant progression and drug resistance in breast cancer. Cancer Res 2013;73:6424-34. doi: 10.1158/0008-5472.CAN-12-4573. [DOI] [PubMed] [Google Scholar]
- 4.Muñoz M, Coveñas R, Esteban F, Redondo M. The substance P/NK-1 receptor system: NK-1 receptor antagonists as anticancer drugs. J Biosci 2015;40:441-63. doi: 10.1007/s12038-015-9530-8. [DOI] [PubMed] [Google Scholar]
- 5.Hennig IM, Laissue JA, Horisberger U, Reubi JC. Substance- P receptors in human primary neoplasms: tumoral and vascular localization. Int J Cancer 1995; 61: 786-92. doi: 10.1002/ijc. 2910610608. [DOI] [PubMed] [Google Scholar]
- 6.Luo W, Sharif TR, Sharif M. Substance P-induced mitogenesis in human astrocytoma cells correlates with activation of the mitogen-activated protein kinase signaling pathway. Cancer Res 1996;56:4983-91. PMID: 8895754. [PubMed] [Google Scholar]
- 7.Muñoz M, Coveñas R. NK-1 receptor antagonists: a new paradigm in pharmacological therapy. Curr Med Chem 2011;18:1820-31. doi: 10.2174/092986711795496746. [DOI] [PubMed] [Google Scholar]
- 8.Muñoz M, Coveñas R. Involvement of substance P and the NK-1 receptor in cancer. Peptides 2013;48:1-9. doi: 10.1016/j.peptides.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Muñoz M, Coveñas R. Involvement of substance P and the NK-1 receptor in pancreatic cancer. World J Gastroenterol 2014; 20: 2321-34. doi: 10.3748/wjg.v20.i9.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muñoz M, Rosso M. The NK-1 receptor antagonist aprepitant as a broad-spectrum antitumor drug. Invest New Drugs 2010;28:187-93. doi: 10.1007/s10637-009-9218-8. [DOI] [PubMed] [Google Scholar]
- 11.Muñoz M, Pérez A, Rosso M, Zamarriego C, Rosso R. Antitumoral action of the neurokinin-1 receptor antagonist L- 733,060 on human melanoma cell lines. Melanoma Res 2004;14:183-8. doi: 10.1097/01.cmr.0000129376.22141.a3. [DOI] [PubMed] [Google Scholar]
- 12.Muñoz M, Rosso M, Pérez A, Coveñas R, Rosso M, Zamarriego C, et al. Antitumoral action of the neurokinin-1- receptor antagonist L-733,060 and mitogenic action of substance P on human retinoblastoma cell lines. Invest Ophthalmol Vis Sci 2005;46:2567-70. doi:10.1167/iovs.04-1530. [DOI] [PubMed] [Google Scholar]
- 13.Muñoz M, Rosso M, Pérez A, Coveñas R, Rosso M, Zamarriego C, et al. The NK1 receptor is involved in the antitumoural action of L-733,060 and in the mitogenic action of substance P on neuroblastoma and glioma cell lines. Neuropeptides 2005;39:427-32. doi: 10.1016/j.npep.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Muñoz M, Rosso M, Coveñas R. A new frontier in the treatment of cancer: NK-1 receptor antagonists. Curr Med Chem 2010;17:504-16. doi: 10.2174/092986710790416308. [DOI] [PubMed] [Google Scholar]
- 15.Muñoz M, Rosso M, Robles-Frías MJ, Salinas-Martín MV, Rosso R, González-Ortega A, et al. The NK-1 receptor is expressed in human melanoma and is involved in the antitumor action of the NK-1 receptor antagonist aprepitant on melanoma cell lines. Lab Invest 2010;90:1259-69. doi: 10.1038/labinvest.2010.92. [DOI] [PubMed] [Google Scholar]
- 16.Muñoz M, González-Ortega A, Rosso M, Robles-Frías MJ, Carranza A, Salinas-Martín MV, et al. The substance P/neurokinin- 1 receptor system in lung cancer: focus on the antitumor action of neurokinin-1 receptor antagonists. Peptides 2012;38:318-25. doi: 10.1016/j.peptides.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 17.Muñoz M, González-Ortega A, Salinas-Martín MV, Carranza A, García-Recio S, Almendro V, et al. The neurokinin-1 receptor antagonist aprepitant is a promising candidate for the treatment of breast cancer. Int J Oncol 2014;45:1658-72. doi: 10.3892/ijo.2014.2565. [DOI] [PubMed] [Google Scholar]
- 18.Rosso M, Robles-Frías MJ, Coveñas R, Salinas-Martín MV, Muñoz M. The NK-1 receptor is expressed in human primary gastric and colon adenocarcinomas and is involved in the antitumor action of L-733,060 and the mitogenic action of substance P on human gastrointestinal cancer cell lines. Tumor Biol 2008;29:245-54. doi: 10.1159/000152942. [DOI] [PubMed] [Google Scholar]
- 19.Akazawa T, Kwatra SG, Goldsmith LE, Richardson MD, Cox EA, Sampson JH, et al. A constitutively active form of neurokinin 1 receptor and neurokinin 1 receptor-mediated apoptosis in glioblastomas. J Neurochem 2009;109:1079-86. doi: 10.1111/j.1471-4159.2009.06032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meshki J, Douglas SD, Hu M, Leeman SE, Tuluc F. Substance P induces rapid and transient membrane blebbing in U373MG cells in a p21-activated kinase-dependent manner. PLoS One 2011;6:e25332. doi: 10.1371/journal.pone.0025332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gillespie E, Leeman SE, Watts LA, Coukos JA, O’Brien MJ, Cerda SR, et al. Truncated neurokinin-1 receptor is increased in colonic epithelial cells from patients with colitis associated cancer. Proc Natl Acad Sci USA 2011;108:17420-5. doi: 10.1073/pnas.1114275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skrabanek P, Cannon D, Dempsey J, Kirrane J, Neligan M, Powell D. Substance P in medullary carcinoma of the thyroid. Experientia 1979;35:1259-60. doi: 10.1007/bf01963325. [DOI] [PubMed] [Google Scholar]
- 23.Holm R, Sobrinho-Simões M, Nesland JM, Gould VE, Johannessen JV. Medullary carcinoma of the thyroid gland: an immunocytochemical study. Ultrastruct Pathol 1985;8:25-41. doi: 10.3109/01913128509141506. [DOI] [PubMed] [Google Scholar]
- 24.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 25.Sherman SI. Thyroid carcinoma. Lancet 2003;361:501-11.doi 10.1016/s0140-6736(03)12488-9. [DOI] [PubMed] [Google Scholar]
- 26.Kim KW, Park YJ, Kim EH, Park SY, Park DJ, Ahn SH, et al. Elevated risk of papillary thyroid cancer in Korean patients with Hashimoto's thyroiditis. Head Neck. 2011;33 691-5. doi: 10.1002/hed.21518. [DOI] [PubMed] [Google Scholar]
- 27.Pusztaszeri MP, Sadow PM, Faquin WC. CD117: a novel ancillary marker for papillary thyroid carcinoma in fine-needle aspiration biopsies. Cancer Cytopathol 2014;122:596-603. doi: 10.1002/cncy.21437. [DOI] [PubMed] [Google Scholar]
- 28.Ragazzi MA, Ciarrocchi V, Sancisi G, Gandolfi A, Bisagni A, Piana S. Update on anaplastic thyroid carcinoma: morphological, molecular, and genetic features of the most aggressive thyroid cancer. Int J Endocrinol 2014;2014:790834. doi: 10.1155/2014/790834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haugen BR, Sherman SI. Evolving approaches to patients with advanced differentiated thyroid cancer. Endocr Rev 2013;34:439-55. doi: 10.1210/er.2012-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aschebrook-Kilfoy B, Schechter RB, Shih YC, Kaplan EL, Chiu BC, Angelos P, et al. The clinical and economic burden of a sustained increase in thyroid cancer incidence. Cancer Epidemiol Biomarkers Prev 2013;22:1252-9. doi: 10.1158/1055-9965.EPI-13-0242. [DOI] [PubMed] [Google Scholar]
- 31.Pereira M, Williams VL, Johnson JH, Valderrábano P. Thyroid cancer incidence trends in the United States: association with changes in professional guideline recommendations. Thyroid 2020. doi: 10.1089/thy.2019.0415. [DOI] [PubMed] [Google Scholar]
- 32.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 1998;11:155-68. PMID: 9504686. [PubMed] [Google Scholar]
- 33.González-Moles MA, Mosqueda-Taylor A, Esteban F, Gil-Montoya JA, Díaz-Franco MA, Delgado M, et al. Cell proliferation associated with actions of the substance P/NK-1 receptor complex in keratocystic odontogenic tumours. Oral Oncol 2008;44:1127-33. doi: 10.1016/j.oraloncology.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Muñoz M, Rosso M, Carranza A, Coveñas R. Increased nuclear localization of substance P in human gastric tumor cells. Acta Histochem 2017;119:337-42. doi: 10.1016/ j.acthis.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Esteban F, González-Moles MA, Castro D, del Martín-Jaén M, Redondo M, Ruiz-Avila I, et al. Expression of substance P and neurokinin-1-receptor in laryngeal cancer: linking chronic inflammation to cancer promotion and progression. Histopathology 2009;54:258-60. doi: 10.1111/j.1365-2559.2008.03193.x. [DOI] [PubMed] [Google Scholar]
- 36.Brener S, González-Moles MA, Tostes D, Esteban F, Gil-Montoya JA, Ruiz-Avila I, et al. A role for the substance P/NK- 1 receptor complex in cell proliferation in oral squamous cell carcinoma. Anticancer Res 2009;29:2323-9. PMID: 19528498. [PubMed] [Google Scholar]
- 37.Ortiz-Prieto A, Bernabeu-Witte J, Zulueta-Dorado T, Lorente-Lavirgen AI, Muñoz M. Immunolocalization of substance P and NK-1 receptor in vascular anomalies. Arch Dermatol Res 2017;309:97-102. doi: 10.1007/s00403-016-1707-y. [DOI] [PubMed] [Google Scholar]
- 38.Berger M, Neth O, Ilmer M, Garnier A, Salinas-Martín MV, de Agustín Asencio JC, et al. Hepatoblastoma cells express truncated neurokinin-1 receptor and can be growth inhibited by aprepitant in vitro and in vivo. J Hepatol 2014; 60: 985-94. doi: 10.1016/j.jhep.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 39.Guha S, Eibl G, Kisfalvi K, Fan RS, Burdick M, Reber H, et al. Broad-spectrum G protein-coupled receptor antagonist, [DArg1, DTrp5,7,9, Leu11] SP: a dual inhibitor of growth and angiogenesis in pancreatic cancer. Cancer Res 2005; 65: 2738-45. doi: 10.1158/0008-5472.CAN-04-3197. [DOI] [PubMed] [Google Scholar]
- 40.Koh YH, Tamizhselvi R, Bhatia M. Extracellular signal-regulated kinase 1/2 and c-Jun NH2-terminal kinase, through nuclear factor-kappaB and activator protein-1, contribute to caerulein-induced expression of substance P and neurokinin-1 receptors in pancreatic acinar cells. J Pharmacol Exp Ther 2010;332:940-8. doi: 10.1124/jpet.109.160416. [DOI] [PubMed] [Google Scholar]
- 41.Walczak-Drzewiecka A, Ratajewski M, Wagner W, Dastych J. HIF-1alpha is up-regulated in activated mast cells by a process that involves calcineurin and NFAT. J Immunol 2008;181: 1665-72. doi: 10.4049/jimmunol.181.3.1665. [DOI] [PubMed] [Google Scholar]
- 42.Feng F, Yang J, Tong L, Yuan Y, Tian Y, Hong L, et al. Substance P immunoreactive nerve fibres are related to gastric cancer differentiation status and could promote proliferation and migration of gastric cancer cells. Cell Biol Int 2011;35:623-9. doi: 10.1042/CBI20100229. [DOI] [PubMed] [Google Scholar]
- 43.Friess H, Zhu Z, Liard V, Shi X, Shrikhande SV, Wang L, et al. Neurokinin-1 receptor expression and its potential effects on tumor growth in human pancreatic cancer. Lab Invest 2003;83:731-42. doi: 10.1097/01.lab.0000067499.57309.f6. [DOI] [PubMed] [Google Scholar]
- 44.Ma J, Yuan S, Cheng J, Kang S, Zhao W, Zhang J. Substance P promotes the progression of endometrial adenocarcinoma. Int J Gynecol Cancer 2016;26:845-50. doi: 10.1097/IGC. 0000000000000683. [DOI] [PubMed] [Google Scholar]
- 45.Mitsuhashi M, Ohashi Y, Schichijo S, Christian C, Sudduth-Klinger J, Harrowe G, et al. Multiple intracellular signalling pathways of the neuropeptide SP receptor. J Neurosci Res 1992;32:437-43. doi:10.1002/jnr.490320315. [DOI] [PubMed] [Google Scholar]
- 46.Nakajima Y, Tsuchida K, Negishi M, Ito S, Nakanishi S. Direct linkage of three tachykinin receptors to stimulation of both phosphatidylinositol hydrolysis and cyclic AMP cascades in transfected Chinese hamster ovary cells. J Biol Chem 1992;267:2437-42. [PubMed] [Google Scholar]
- 47.Takeda Y, Blount P, Sachais BS, Hershey AD, Raddatz R, Krause J. Ligand binding kinetics of substance P and neurokinin A receptors stably expressed in Chinese hamster ovary cells and evidence for differential stimulation of inositol 1, 4, 5-triphosphate and cyclic AMP second messenger responses. J Neurochem 1992;59:740-5. doi: 10.1111/j.1471-4159.19 92.tb09430.x. [DOI] [PubMed] [Google Scholar]
- 48.Meshki J, Douglas SD, Lai JP, Schwartz L, Kilpatrick LE, Tuluc F. Neurokinin 1 receptor mediates membrane blebbing in HEK293 cells through a Rho/Rho-associated coiled-coil kinase-dependent mechanism. J Biol Chem 2009;284:9280-9. doi: 10.1074/jbc.M808825200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones S, Tucker KL, Sage T, Kaiser WJ, Barrett NE, Lowry PJ, et al. Peripheral tachykinins and the neurokinin receptor NK1 are required for platelet thrombus formation. Blood 2008;111:605-12. doi:10.1182/blood-2007-07-103424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ständer S, Siepmann D, Herrgott I, Sunderkötter C, Luger TA. Targeting the neurokinin receptor 1 with aprepitant: a novel antipruritic strategy. PLoS One 2010;5:e10968. doi: 10.1371/journal.pone.0010968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Medrano S, Gruenstein E, Dimlich RV. Substance P receptors on human astrocytoma cells are linked to glycogen breakdown. Neurosci Lett 1994;167:14-8. doi: 10.1016/0304-3940(94)91017-0. [DOI] [PubMed] [Google Scholar]
- 52.Alonso-Gordoa T, Díez JJ, Durán M, Grande E. Advances in thyroid cancer treatment: latest evidence and clinical potential. Ther Adv Med Oncol 2015;7:22-38. doi: 10.1177/17588340 14551936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu Y, Wang H, Chen E, Xu Z, Chen B, Lu G. Candidate microRNAs as biomarkers of thyroid carcinoma: a systematic review, meta-analysis, and experimental validation. Cancer Med 2016;5:2602-14. doi: 10.1002/cam4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee JW, Lee SM, Lee DH, Kim YJ. Clinical utility of 18FFDG PET/CT concurrent with 131I therapy in intermediate-tohigh- risk patients with differentiated thyroid cancer: dual-center experience with 286 patients. J Nucl Med 2013;54:1230-6. doi: 10.2967/jnumed.112.117119. [DOI] [PubMed] [Google Scholar]
- 55.Ma M, Lin B, Wang M, Liang X, Su L, Okose O, et al. Immunotherapy in anaplastic thyroid cancer. Am J Transl Res 2020;12:974-88. [PMC free article] [PubMed] [Google Scholar]
- 56.Coperchini F, Croce L, Marinò M, Chiovato L, Rotondi M. Role of chemokine receptors in thyroid cancer and immunotherapy. Endocr Relat Cancer 2019;26:R465-78. doi: 10.1530/ERC-19-0163. [DOI] [PubMed] [Google Scholar]
- 57.Laetitia G, Sven S, Fabrice J. Combinatorial therapies in thyroid cancer: an overview of preclinical and clinical progresses. Cells 2020;9pii E830. doi: 10.3390/cells9040830. [DOI] [PMC free article] [PubMed] [Google Scholar]