Background
The most common clinical diagnosis in adults with bilateral renal cysts, hypertension and chronic kidney disease is autosomal dominant polycystic kidney disease, which is caused by a mutation in the gene that encodes polycystin-1 (PKD1) or polycystin-2. (PKD2) (1). The diagnosis may be difficult when there are variations in the severity of renal disease or in the presentation of extra-renal disease, especially when there is no family history. Other conditions can present with bilateral renal cysts in adults. One of these conditions is a syndrome that includes Hereditary Angiopathy, Nephropathy, Aneurysms, and muscle Cramps (HANAC syndrome), which is caused by a mutation in the gene (COL4A1) that encodes the α1 isoform of type IV collagen (2, 3).
Objective
To illustrate how advanced molecular testing can distinguish between these 2 conditions.
Case Series
Other physicians referred us a 67-year-old man because his chronic kidney disease was progressing (serum creatinine 176.8 μmol/L or 2 mg/dL). He had an abdominal ultrasound 13 years earlier that documented bilateral renal cysts, which were later confirmed by CT imaging. His medical history included stage 1 hypertension controlled with an ACE inhibitor but no family history of kidney cysts. This information was consistent with autosomal dominant polycystic kidney disease.
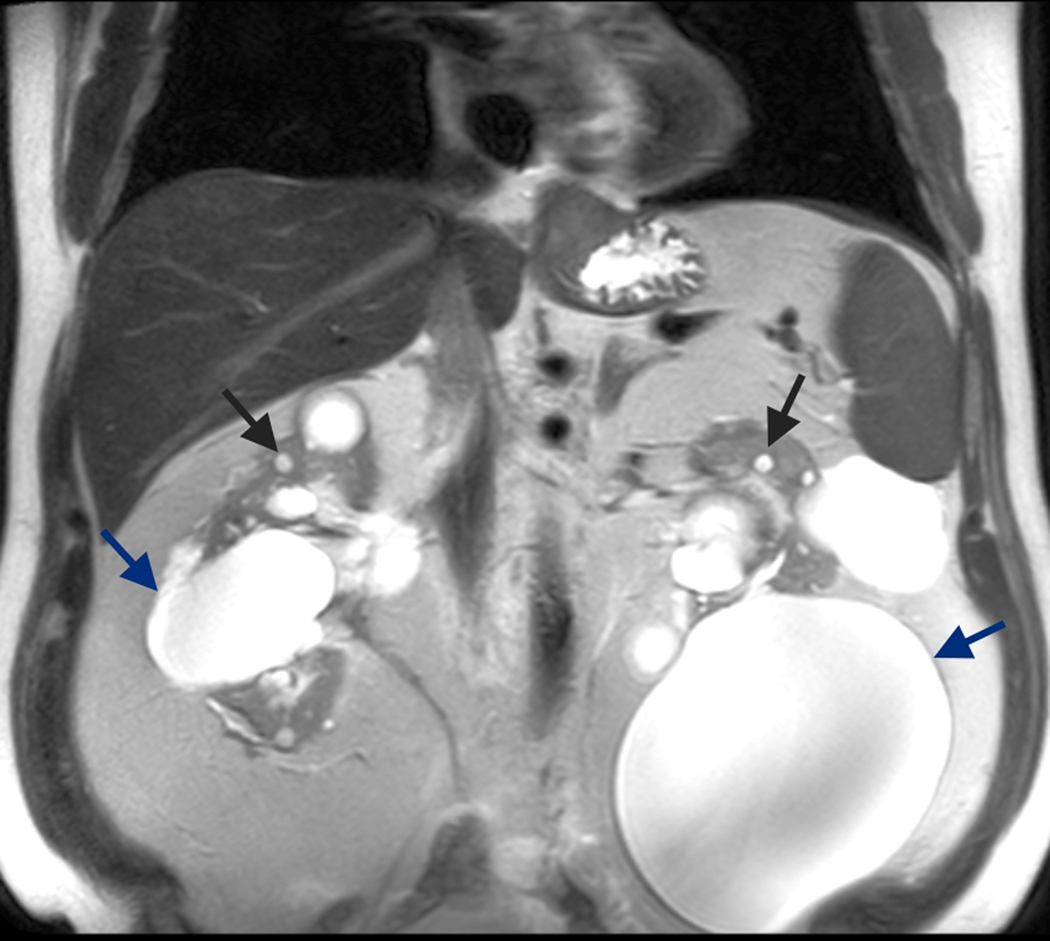
However, a recent MRI showed only minimally enlarged kidneys, relatively well-preserved intervening renal parenchyma, and large dominant cysts projecting from the renal cortex [Figure 1]. In contrast, MRIs of age-matched kidneys with autosomal dominant polycystic disease typically show substantially enlarged kidneys with more uniform cysts distributed throughout the renal parenchyma. In addition, our patient’s history included recurrent retinal hemorrhages with tortuosity of the retinal arteries that was first recognized at age 13 and similar retinal findings in his father and paternal aunt, a pattern suggesting autosomal dominant inheritance. Our patient also had a 10-year history of generalized myalgias with a normal rheumatologic evaluation that included blood levels of muscle enzymes. These findings made us suspect HANAC syndrome, and we used whole exome sequencing to identify a heterozygous variant in the causative gene for HANAC syndrome (a deleterious mutation that is predicted to replace glycine with arginine at protein position 486 in exon 23, cDNA nucleotide position 1456 of COL4A1). We confirmed the variant by Sanger sequencing. We did not find any deleterious mutations in PKD1, PKD2, or in any of the 116 other genes that have been associated with cystic kidney disease.
Figure 1.
Coronal T2 MRI of the first patient’s abdomen showing bilateral mildly enlarged kidneys with multiple small cysts (black arrows) and dominant large cortical cysts (blue arrows) projecting from the renal cortex.
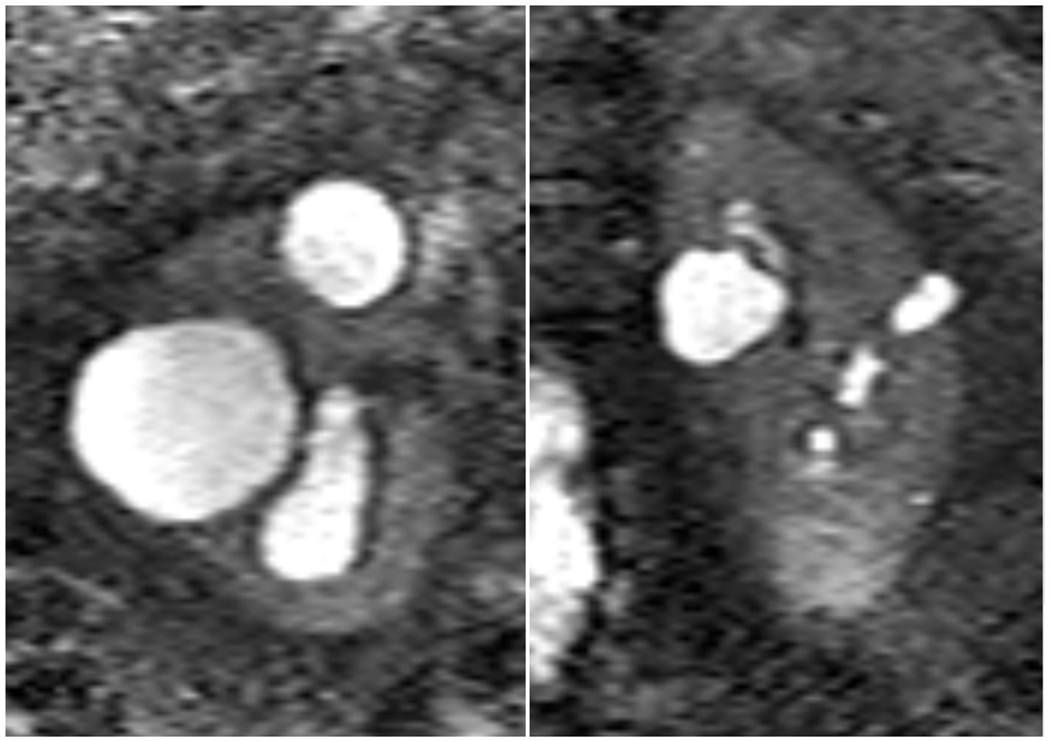
We performed whole exome sequencing on 17 additional patients with polycystic kidney disease who were part of a NIH-sponsored study of kidney volume progression in autosomal dominant polycystic kidney disease but were known not to have a mutation in PKD1 or PKD2, the two autosomal dominant polycystic kidney disease genes (4). We identified 1 additional patient with a heterozygous variant in the causative gene for HANAC syndrome. When compared with the variant in the first patient, this variant was different (a deleterious mutation in COL4A1 that is predicted to substitute tryptophan for arginine at position 538). This second patient had borderline enlarged kidneys with a cyst pattern that was similar to the pattern in our first patient. [Figure 2]. This patient also had minimal progression of total kidney volume during 12 years of follow up and a history of intracranial aneurysm. As a result, we think he has a HANAC-like syndrome rather than autosomal dominant polycystic kidney disease.
Figure 2.
Coronal T2 MRI of the second patient’s abdomen showing bilateral mildly enlarged kidneys with several small to moderate cysts and relatively preserved renal parenchyma.
Discussion
The autosomal dominant genetic conditions that result in enlarged cystic kidneys include autosomal dominant polycystic kidney disease, tuberous sclerosis, von Hippel-Lindau syndrome, and hepatocyte nuclear factor 1ß-related disease. Based on the experience we report here, we believe that COL4A1 mutations causing HANAC syndrome should be included in the differential diagnosis of bilateral cystic kidney disease. Given the inherent complexity of detecting mutations in PKD1 (5) and the fact that 8–15% of patients with autosomal dominant polycystic kidney disease have no detectable mutation in either PKD1 or PKD2, genetic diagnosis is not routinely pursued in these patients. However, some patients with atypical presentations might benefit from these genomic methods. For example, the data that we obtained from whole exome sequencing prompted us to screen our first patient for intracranial aneurysms and to inform his prognosis, which affected our plan for long-term management. Moreover, these methods can ensure that appropriate patients are selected for clinical trials.
Acknowledgments:
The authors thank the patients for their participation in the study. The authors are grateful for the support from the Yale Center for Mendelian Genomics (NIH U54 HG006504). This work was supported by the American Society of Nephrology Ben J Lipps fellowship (2015–17) and the Polycystic Kidney Disease foundation fellowship (2017–19) to AG, and NIH grants for the George M. O’Brien Kidney Center at Yale (P30 DK079310) and DK100592 to SS and the Baltimore PKD Center (P30 DK090868) and DK095036 to TW.
Footnotes
Conflict of interest: None
Contributor Information
Ashima Gulati, Departments of Internal Medicine and Pediatrics, Division of Nephrology, Yale University School of Medicine, New Haven, Connecticut, USA.
Kyongtae T. Bae, Department of Radiology, University of Pittsburgh School of Medicine, Pittsburgh, PA.
Stefan Somlo, Departments of Internal Medicine (Nephrology) and Genetics, Yale University School of Medicine, New Haven, Connecticut, USA.
Terry Watnick, Department of Medicine, Division of Nephrology, University of Maryland School of Medicine.
References
- 1.Pei Y, Watnick T. Diagnosis and screening of autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis 2010; 17:140–52. PMID: 20219617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plaisier E, Gribouval O, Alamowitch S, Mougenot B, Prost C, Verpont MC, et al. COL4A1 mutations and hereditary angiopathy, nephropathy, aneurysms, and muscle cramps. N Engl J Med 2007; 357:2687–95. PMID: 18160688 [DOI] [PubMed] [Google Scholar]
- 3.Plaisier E, Chen Z, Gekeler F, Benhassine S, Dahan K, Marro B, et al. Novel COL4A1 mutations associated with HANAC syndrome: a role for the triple helical CB3[IV] domain. Am J Med Genet A 2010; 152A:2550–5. PMID: 20818663 [DOI] [PubMed] [Google Scholar]
- 4.Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF Jr, et al. ; CRISP Investigators. Volume progression in polycystic kidney disease. N Engl J Med 2006; 354:212–30. PMID: 16707749 [DOI] [PubMed] [Google Scholar]
- 5.Song X, Haghighi A, Iliuta IA, Pei Y. Molecular diagnosis of autosomal dominant polycystic kidney disease [published online ahead of print August 13, 2017]. Expert Rev Mol Diagn. 2017; 17:885–95. PMID: 28724316 [DOI] [PubMed] [Google Scholar]