Abstract
Immune system abnormalities have been widely reported among children with autism spectrum disorder (ASD), which may increase the risk of childhood infections. The Study to Explore Early Development (SEED) is a multisite case-control study of children aged 30–69 months, born in 2003–2006. Cases are children previously diagnosed and newly identified with ASD enrolled from education and clinical settings. Children with a previously diagnosed non-ASD developmental condition were included in the developmental delay/disorder (DD) control group. The population (POP) control group included children randomly sampled from birth certificates. Clinical illness from infection during the first 28 days (“neonatal,” from medical records) and first three years of life (caregiver report) in cases was compared to DD and POP controls; and between cases with and without regression. Children with ASD had greater odds of neonatal (OR = 1.8; 95%CI: 1.1, 2.9) and early childhood infection (OR = 1.7; 95%CI: 1.5, 1.9) compared to POP children, and greater odds of neonatal infection (OR = 1.5; 95%CI: 1.1, 2.0) compared to DD children. Cases with regression had 1.6 times the odds (95%CI: 1.1, 2.3) of caregiver-reported infection during the first year of life compared to cases without regression, but neonatal infection risk and overall early childhood infection risk did not differ. Our results support the hypothesis that children with ASD are more likely to have infection early in life compared to the general population and to children with other developmental conditions. Future studies should examine the contributions of different causes, timing, frequency, and severity of infection to ASD risk.
Keywords: childhood infection, temperature dysregulation, autism spectrum disorder, developmental disabilities, autism regression
Lay Summary:
We looked at infections during early childhood in relation to autism spectrum disorder (ASD). We found that children with ASD were more likely to have an infection in the first 28 days of life and before age three compared to children with typical development. Children with ASD were also more likely than children with other developmental delays or disorders to have an infection in the first 28 days of life.
Introduction
Autism spectrum disorder (ASD) is characterized by persistent difficulties with social communication and restricted and repetitive behaviors and interests. Research to date suggests that ASD etiology is multifactorial and includes both genetic and environmental components [Lyall et al., 2017]. Infection, inflammation, and immune activation may be related elements in the ASD causal pathway. Studies have shown that children with ASD have increased incidence of autoimmune disorders and altered levels of immune mediators suggesting ongoing immune dysfunction [Estes & McAllister, 2015;Singh, 2009]. Other studies indicate an association between the expression of genes related to immune function and ASD [Korvatska, Van de Water, Anders, & Gershwin, 2002].
Additionally, previous studies have found a link between levels of viral [Gentile et al., 2014b;Nicolson, Gan, Nicolson, & Haier, 2007] and bacterial [Nicolson et al., 2007] seromarkers and ASD, and between viral seromarkers and autism [Singh, 2009; Lintas, Altieri, Lombardi, Sacco, & Persico, 2010]. ASD has been associated with increased diagnosis of ear infections, risk of diarrhea and the common cold, and rates of infection during the first 30 days of life [Atladóttir, Schendel, Lauritsen, Henriksen, & Parner, 2012;Rosen, Yoshida, & Croen, 2007;Atladóttir, Henriksen, Schendel, & Parner, 2012;Adams et al., 2016; Rosenhall, Nordin, Sandström, Ahlsén, & Gillberg, 1999; Inscoe & Bones, 2016]. However, several studies found no association between childhood infection and either ASD or autism [Burbelo et al., 2013;Ajamian, Kosofsky, Wormser, Rajadhyaksha, & Alaedini, 2013;D’Souza, Fombonne, & Ward, 2006;Gentile et al., 2014a;Satterfield, Garcia, Gurrieri, & Schwartz, 2010; Lintas et al., 2011;Libbey et al., 2007;Rosen et al., 2007]. Overall, the data available on occurrence of infections in children with ASD are conflicting and limited by small sample sizes, inadequate comparison groups, or lack of well-defined ASD diagnostic criteria. Previous studies also did not examine whether associations between childhood infection and ASD varied across behavioral or developmental subgroups of children with ASD.
The current study of childhood infection and ASD used data from the Study to Explore Early Development (SEED) to address these gaps in study design. SEED is a multisite case-control study that combines a large sample size, multiple comparison groups, a diverse sample, and cohorts of cases and controls that are well defined based on rigorous assessments [Schendel et al., 2012]. Our primary aim was to examine whether children with ASD were more likely to have experienced clinical illness from infection during the neonatal period and the first three years of life than typically developing children or children with other non-ASD developmental delays or disorders. Using a non-ASD developmentally delayed/disordered control group was intended to differentiate the effects of ASD from those of neurodevelopmental disorders more generally and to control for recall bias. We also assessed whether the association varied according to the age at which clinical illness occurred. Additionally, we explored whether children with ASD who experience a loss in previously acquired language or social skills (i.e., regression, which occurs in approximately 32% of all children with ASD) [Barger, Campbell, & McDonough, 2013;0zonoff et al., 2010] are more likely than those without regression to have experienced clinical infection during the neonatal period or first three years of life.
Methods
SEED Study Design and Enrollment
The SEED case-control study enrolled children born from September 1, 2003 through August 31, 2006 at one of six sites across the country. To be eligible, children must have been born and resided in a study catchment area at first contact. Only those with a caregiver aged at least 18 years who lived with and cared for the child from six months of age and spoke either English (all sites) or Spanish (two sites) were enrolled. Demographics of the study population have been described [DiGuiseppi et al., 2016]. To ensure the appropriate use of validated instruments, enrolled children were 30.0–68.9 months old at the time of clinical evaluation, i.e., the age range for which the measures were normed. Study methods have been detailed elsewhere [Schendel et al., 2012]. Institutional Review Boards at the Centers for Disease Control and Prevention and each SEED site approved the study. All procedures were performed in accordance with the ethical standards of the institutional and national research committees and with the 1964 Helsinki declaration, and its later amendments or comparable ethical standards.
Cases and Controls
All eligible families were administered the Social Communication Questionnaire (SCQ) [Rutter, Bailey, & Lord, 2003a] to screen for children with undiagnosed ASD [Allen, Silove, Williams, & Hutchins, 2007;Lee, David, Rusyniak, Landa, & Newschaffer, 2007]. Only those children assessed in person were administered the Mullen Scales of Early Learning (MSEL) test [Mullen, 1995]. Children with a previous diagnosis of ASD, SCQ score ≥ 11, or ASD symptoms observed during the MSEL test were administered the ADOS [Lord, Rutter, Dilavore, & Risi, 1999;Gotham, Risi, Pickles, & Lord, 2007] and ADI-R [Lord, Rutter, & Le Couteur, 1994;Rutter, Le Couteur, & Lord, 2003b];children who met established cutoff scores were classified as ASD (cases). Children with incomplete or indeterminate developmental assessments (n = 569) were classified as “possible cases” and excluded from this analysis. Children with a previously diagnosed non-ASD developmental delay/disorder, an SCQ< 11 and no symptoms of ASD demonstrated during administration of the Mullen were classified as DD without ASD characteristics (DD controls). Children who met criteria for a full ASD evaluation but did not meet case criteria (n = 306) were classified as DD with ASD characteristics and were excluded from this analysis. Children in the ASD and DD groups were recruited from educational and clinical settings serving children with developmental disabilities or other disorders. Children from the general population (POP) were randomly sampled from birth certificates and were included in the POP control group if they did not have a developmental delay/disorder and had a SCQ score < 11. Detailed methods for classification of cases and controls have previously been described [Wiggins et al., 2015].
Regression
Regression in children with ASD was defined as any loss of language or social skills noted on the ADI-R that persisted at least 3 months. Children with ASD who did not experience either a loss of language or loss of social skills persisting three or more months were considered not to have regression.
Exposures
Clinical infections were ascertained in two ways: (a) report by the primary caregiver of infection diagnosed by a doctor or other healthcare professional before the child’s third birthday and (b) diagnosis of infection, fever or hypothermia recorded in neonatal medical records. We included neonatal hypothermia only if documented after the first 24 hours of life, which generally indicates an infection, usually septicemia [Kumar, Shearer, Kumar, & Darmstadt, 2009]. Low temperatures in the first day of life are more often the consequence of environmental factors, including actions taken by medical staff, or neonatal characteristics, such as low birthweight and preterm delivery [Smales & Kime, 1978], and were excluded from this analysis.
Data Collection:
Caregiver interview (CGI).
Each child’s primary caregiver (the biological mother in 98% of enrolled families) completed the CGI, which included questions about family, child and household characteristics. Caregivers were asked if their child had ever had an infection or other condition diagnosed by a doctor or other healthcare professional between birth and age three years. A list of infections and other conditions was provided as a prompt. Respondents were also instructed to report any disorders not included on the list, which were recorded as free text. Two SEED investigators, including a pediatrician, categorized all reported conditions as infection (fungal, bacterial, viral, other defined infection, or probable infection of unknown origin [e.g., ear infection]) or no infection. A third investigator reviewed responses with discordant categorization between the initial two investigators. Children with a CGI considered to be complete based on study completion criteria were included in the analyses of caregiver-reported infections.
For each reported infection, caregivers were asked whether it occurred before the child’s first birthday (first year of life), between the child’s first and second birthday (second year of life), between the child’s second and third birthday (third year of life), or during multiple time periods. Because of the instrument design, if the child had multiple occurrences of the same type of infection during any single year of life (e.g., two ear infections before the first birthday), the infection was only captured once for that year.
Neonatal medical records.
Information on infections occurring during the period from birth to the time the child was discharged was abstracted from medical records from the child’s hospital of delivery or any hospital to which the child was directly transferred. Abstractors matched disorders in medical records to a previously defined list of infections and other disorders. Those not found on this list were collected as open text responses. Neonatal infections and disorders were classified as infection or no infection as described above. Abnormally low temperatures (hypothermia defined as skin and axillary temperatures <35.5 °C and rectal temperatures <36.0 °C) and high temperatures (fever defined as skin and axillary temperatures >37.5 °C and rectal temperatures >38.0 °C) were also collected. Infections and abnormal temperatures documented to have occurred within the first 28 days of life were considered neonatal events. All children with a medical record abstraction were included in the analyses regarding neonatal record data.
Covariates.
We assessed variables known or likely to be associated with both case status and childhood infection as potential confounders. Maternal race/ethnicity, maternal education, maternal age at birth, child sex, child age at enrollment, current household income, number of children <18 years old living in the household, duration of breast feeding, and maternal psychiatric conditions were collected from CGI reports and categorized as shown in Table 1. Gestational age and birthweight were obtained from birth records. Selected congenital malformations were collected from abstracted neonatal medical records and categorized as “none” vs. “any.” “Any” congenital malformation included malformations with a prevalence over 3.0 per 10,000 live births in the United States and a known association with infection risk either due to the condition itself or its treatment (e.g., hypospadias, Down syndrome, pulmonary valve atresia and/or stenosis, cleft palate, and/or lip) [Parker et al., 2010;Mai et al., 2015;Centers for Disease Control and Prevention, 2017]. We also considered the number of days from birth to child’s neonatal hospital discharge, truncated at 28 days, as a potential mediator or confounder of the relationship between ASD and neonatal infection.
Table 1.
Maternal and Child Characteristics by Case Status (Children with Autism Spectrum Disorder vs. General Population Controls and Children with Non-ASD Developmental Delays or Disorders Controls (n = 2445); and Among Children with ASD by Regression Status (Regression vs. No Regression) (n = 672)
| ASD (n = 699) |
POP (n = 980) |
DD (n = 766) |
ASD With Regression (n = 178) |
ASD Without Regression (n = 494) |
|
|---|---|---|---|---|---|
| Avg(sd) | Avg(sd) | Avg(sd) | Avg(sd) | Avg(sd) | |
| Child age at enrollment (mo) | 55.7(6.9) | 55.6(7.6) | 55.7(7.4) | 54.7(7.0) | 56.1(6.8) |
| Birthweight (gm) | 3177(771) | 3321(639) | 3126(862) | 3247(641) | 3144(822) |
| Maternal age at birth (yr) | 31.6(5.5) | 31.9(5.4) | 32.2(5.5) | 30.6(5.1) | 31.9(5.6) |
| Child sex | n(%) | n(%) | n(%) | n(%) | n(%) |
| Female | 127(18.2) | 457(46.6) | 290(37.9) | 34(19.1) | 88(17.8) |
| Male | 572(81.8) | 523(53.4) | 476(62.1) | 144(80.9) | 406(82.2) |
| Maternal education | |||||
| Less than Bachelors | 333(48.0) | 341(34.9) | 294(38.5) | 84(47.2) | 238(48.7) |
| Bachelors | 212(30.5) | 346(35.5) | 256(33.6) | 56(31.5) | 148(30.3) |
| Graduate | 149(21.5) | 289(29.6) | 213(27.9) | 38(21.3) | 103(21.1) |
| Maternal race/ ethnicity | |||||
| White Non-Hispanic | 386(55.7) | 683(70.5) | 497(65.5) | 95(53.4) | 273(55.9) |
| Other Non-Hispanic | 222(32.0) | 203(20.9) | 169(22.3) | 55(30.9) | 160(32.8) |
| Hispanic/Latino/Latina | 85(12.3) | 83(8.6) | 93(12.3) | 28(15.7) | 55(11.3) |
| Household income | |||||
| < $50,000 | 266(39.5) | 267(28.0) | 228(31.4) | 81(46.3) | 177(37.6) |
| $50,000–$90,000 | 216(32.1) | 329(34.5) | 263(36.2) | 50(28.6) | 157(33.3) |
| $90,000 + | 191(28.4) | 357(37.5) | 235(32.4) | 44(25.1) | 137(29.1) |
| Enrollment site | |||||
| CA | 108(15.5) | 152(15.5) | 124(16.2) | 28(15.7) | 75(15.2) |
| CO | 141(20.2) | 199(20.3) | 132(17.2) | 38(21.3) | 89(18.0) |
| GA | 138(19.7) | 181(18.5) | 165(21.5) | 28(15.7) | 109(22.1) |
| MD | 108(15.5) | 143(14.6) | 104(13.6) | 34(19.1) | 74(15.0) |
| NC | 103(14.7) | 172(17.6) | 156(20.4) | 23(12.9) | 75(15.2) |
| PA | 101(14.4) | 133(13.6) | 85(11.1) | 27(15.2) | 72(14.6) |
| Number of children in home | |||||
| 1 | 141(20.4) | 127(13.1) | 135(17.8) | 36(20.3) | 103(21.1) |
| 2 | 329(47.5) | 498(51.4) | 347(45.8) | 79(44.6) | 238(48.8) |
| 3 | 148(21.4) | 250(25.8) | 196(25.9) | 46(26.0) | 93(19.1) |
| 4+ | 74(10.7) | 93(9.6) | 79(10.4) | 16(9.0) | 54(11.1) |
| Maternal psychiatric diagnosisa | |||||
| Yes | 224(32.0) | 211(21.5) | 202(26.4) | 70(39.3) | 146(29.6) |
| No | 431(61.7) | 692(70.6) | 489(63.8) | 96(53.9) | 320(64.8) |
| Missing | 44(6.3) | 77(7.9) | 75(9.8) | 12(6.7) | 28(5.7) |
| Congenital malformationsb | |||||
| Yes | 21(3.0) | 12(1.2) | 45(5.9) | 1(0.6) | 20(4.0) |
| No | 678(97.0) | 968(98.8) | 721(94.1) | 177(99.4) | 474(96.0) |
| Gestational age | |||||
| Preterm (<37 months) | 114(16.4) | 93(9.5) | 164(21.5) | 26(14.8) | 82(16.7) |
| Early term (37–38 months) | 182(26.2) | 276(28.3) | 213(27.9) | 44(25.0) | 132(26.8) |
| Full term (39+ months) | 399(57.4) | 605(62.1) | 386(50.6) | 106(60.2) | 278(56.5) |
| Duration of breastfeeding | |||||
| Never Breastfed | 98(14.0) | 97(9.9) | 97(12.7) | 22(12.4) | 73(14.8) |
| 1–3 months | 147(21.0) | 143(14.6) | 145(18.9) | 36(20.2) | 111(22.5) |
| 4–6 months | 116(16.6) | 164(16.7) | 119(15.5) | 31(17.4) | 76(15.4) |
| 7–12 months | 146(20.9) | 284(29.0) | 179(23.4) | 37(20.8) | 101(20.4) |
| 13+ months | 95(13.6) | 216(22.0) | 137(17.9) | 29(16.3) | 62(12.6) |
| Missing | 97(13.9) | 76(7.8) | 89(11.6) | 23(12.9) | 71(14.4) |
| Days to discharge (neonatal records only) | ASD (n = 594) | POP (n = 771) | DD (n = 594) | ASD with regression (n = 147) | ASD without regression (n = 423) |
| ≤1 | 56(9.4) | 90(11.7) | 67(11.3) | 15(10.2) | 37(8.7) |
| 2 | 237(39.9) | 351(45.5) | 199(33.5) | 61(41.5) | 167(39.5) |
| 3–4 | 148(24.9) | 189(24.5) | 145(24.4) | 41(27.9) | 101(23.9) |
| 5–28+ | 96(16.2) | 75(9.7) | 133(22.4) | 12(8.2) | 81(19.1) |
| Missing | 57(9.6) | 66(8.6) | 50(8.4) | 18(12.2) | 37(8.7) |
Abbreviations: ASD = Autism spectrum disorder; POP = General population; DD = Non-ASD developmental delays or disorders.
Numbers may not add to totals due to missing data (all variables missing <5% data except for those shown).
Maternal psychiatric condition defined as ever diagnosed with anxiety, bipolar disorder, depression, eating disorder, obsessive compulsive disorder, personality disorder, schizophrenia, self-injurious behavior, sleep disorder, or attempted suicide.
Congenital malformations include: hypospadias, Down syndrome, pulmonary valve atresia and/or stenosis, cleft palate and/or lip, coarctation of the aorta, gastroschisis, atrioventricular septal defect/endocardial cushion defect, Tetralogy of Fallot, rectal and large intestinal atresia/stenosis, spina bifida without anencephaly, small intestinal atresia/stenosis, and transposition of the great arteries.
Analysis
Odds ratios (OR) and 95% confidence intervals (95%CI) were calculated using mixed effects multivariable logistic regression generalized estimating equation models that accounted for correlation by enrollment site. All models assumed an independent correlation structure. Outcomes were defined as ASD vs. POP or ASD vs. DD;and among cases, regression vs. no regression. It could not be determined whether infections recorded in neonatal medical records were the same infections reported by the caregiver as occurring during a child’s first year. Therefore, infections identified from the CGI and from medical records were analyzed separately. Exposures of interest included any CGI-reported infection, CGI-reported age of infection (compared to those who never had an infection) and, from neonatal medical records, any infection, neonatal hypothermia, and neonatal fever.
Model Building
A base model examining each exposure in cases vs. controls included adjustment for child sex and maternal race/ethnicity, education, and age at child’s birth. Associations of potential confounders with outcome and exposure were assessed using chi-square or t-tests accounting for unequal variance using the Satterthwaite method. Variables associated with both the exposure and outcome of interest at P < .20 were tested and retained in the final model if the OR estimate of interest changed by ≥10%. For all analyses, P-values <.05 were considered statistically significant. All analyses were performed using SAS 9.4.
Exploratory and Sensitivity Analyses
Neonatal infection, hypothermia and birthweight.
Although hypothermia in the first day of life is more often the consequence of environmental factors or neonatal characteristics, e.g., low birthweight, it may also be a marker of infection. We explored whether hypothermia was associated with neonatal infection or low birthweight in the first day of life using Fisher’s exact test.
Length of hospital stay.
We explored estimates of the association between ASD and neonatal infections before and after adjustment for length of hospital stay. Because nearly 9% of children with a neonatal medical record were missing data on days to discharge, these analyses were restricted to those with complete data on hospital stay.
Preterm birth.
As preterm birth is a risk factor for both ASD and other developmental disorders, we examined the association between ASD cases vs. DD controls and neonatal infections stratified by preterm (<37 months) and nonpreterm birth (37+ months).
In-person assessment.
All individuals suspected to have ASD were required to complete an in-person developmental assessment to validate ASD classification; however, individuals could have received a classification of DD or POP without an in-person assessment. Because of potential bias due to differences between those who did and did not complete an in-person visit, we performed a sensitivity analysis restricting our sample to children assessed in person.
Results
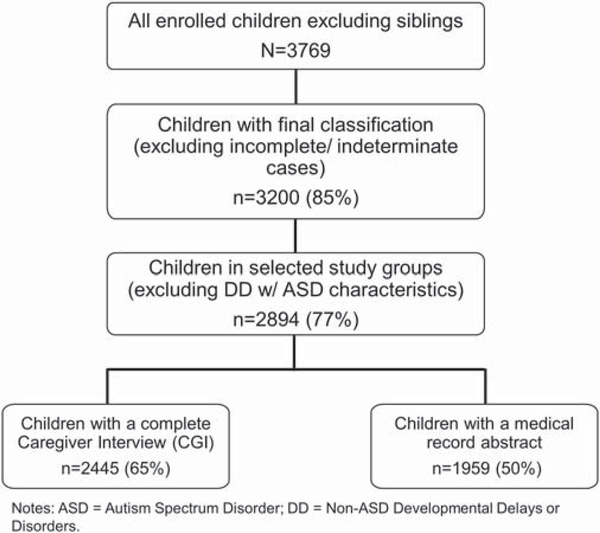
Of the 3769 children enrolled in SEED, 2445 children met all inclusion criteria for this analysis (Fig. 1), consisting of 699 ASD, 980 POP, and 766 DD children. Among ASD cases, 672 (96%) had ADI-R regression data. Of those, 178 (26%) had indicators of regression and 494 (74%) did not. We included 1959 children with a neonatal medical record abstraction in analyses of neonatal exposures.
Figure 1.
Flow diagram of children included in seed analyses of childhood infection and autism spectrum disorder.
Characteristics of Cases and Controls
Selected characteristics of cases and controls are shown in Table 1. In addition to group differences previously described [Diguiseppi et al., 2016;Wiggins et al., 2015], children with ASD were less likely to have been breastfed more than 12 months compared to DD and POP children, and mothers of children with ASD were more likely to have a psychiatric diagnosis compared to DD and POP group mothers. Children in the ASD group had lower mean birthweight and were more likely to be preterm and to have a congenital malformation than POP children. Compared to DD children, children with ASD were more often full-term and less likely to have a congenital malformation. Children with ASD were less likely than DD children but more likely than POP children to remain in the hospital more than 5 days after birth.
Infections in ASD vs. POP.
ASD cases were at significantly higher odds of having an infection during the first three years of life and during the neonatal period compared to POP controls, after adjustment for sociodemographic and other confounding variables (Table 2). Cases were also significantly more likely than POP controls to have an infection during the first year of life only, third year of life only, and during multiple time periods. In addition, children with ASD were more likely to experience hypothermia during the neonatal period compared to their POP counterparts.
Table 2.
Odds of Having an Infection during the First Three Years of Life and during the Neonatal Period by Case Status (ASD vs. POP controls and ASD vs. DD controls)
| Caregiver interviews | ASD (n = 699) n (%) | POP (n = 980) n (%) | Crude OR (95% CI) | Adjusted ORa (95% CI) |
|---|---|---|---|---|
| Ever infection | 359(51.4) | 396(40.4) | 1.56(1.36–1.79)e | 1.66(1.47–1.87)e |
| Age of infectionb | ||||
| Never infection | 340(49.3) | 584(60.0) | REF | REF |
| Infection only in first year of life | 72(10.4) | 71(7.3) | 1.74(1.11–2.74)e | 1.76(1.14–2.71)e |
| Infection only in second year of life | 45(6.5) | 65(6.7) | 1.19(0.60–2.34) | 1.35(0.67–2.74) |
| Infection only in third year of life | 40(5.8) | 56(5.7) | 1.23(1.01–1.50)e | 1.32(1.14–1.53)e |
| Infection during multiple time periods | 193(28.0) | 198(20.3) | 1.67(1.45–1.94)e | 1.78(1.57–2.02)e |
| Neonatal records | ASD (n = 594) | POP (n = 771) | Crude OR (95% CI) | Adjusted ORa (95% CI) |
| Ever infectionc | 29(4.9) | 17(2.2) | 2.28(1.25–4.16)e | 1.80(1.12–2.90)e |
| Low temperature | 9(1.5) | 2(0.3) | 5.92(1.90–18.44)e | 6.30(1.65–24.08)e |
| High temperature | 20(3.4) | 24(3.1) | 1.08(0.84–1.41) | 1.29(0.90–1.87) |
| Caregiver interviews | ASD (n = 699) | DD (n = 766) | Crude OR (95% CI) | Adjusted ORa (95% CI) |
| Ever infection | 359(51.4) | 386(50.4) | 1.04(0.82–1.32) | 1.05(0.83–1.33) |
| Age of infectionb | ||||
| Never infection | 340(49.3) | 380(49.6) | REF | REF |
| Infection only in first year of life | 72(10.4) | 73(9.5) | 1.10(0.72–1.69) | 1.17(0.77–1.77) |
| Infection only in second year of life | 45(6.5) | 47(6.1) | 1.07(0.74–1.54) | 1.08(0.67–1.76) |
| Infection only in third year of life | 40(5.8) | 34(4.4) | 1.31(0.84–2.07) | 1.44(0.88–2.37) |
| Infection during multiple time periods | 193(28.0) | 232(30.3) | 0.93(0.80–1.08) | 0.90(0.79–1.04) |
| Neonatal records | ASD (n = 594) | DD (n = 594) | Crude OR (95% CI) | Adjusted ORa (95% CI) |
| Ever infectiond | 29(4.9) | 25(4.2) | 1.17(0.92–1.48) | 1.46(1.05–2.03)e |
| Low temperature | 9(1.5) | 3(0.5) | 3.03(0.94–9.81) | 2.88(0.83–10.05) |
| High temperature | 20(3.4) | 28(4.7) | 0.70(0.49–1.01) | 0.70(0.47–1.03) |
Abbreviations: ASD = Autism spectrum disorder; POP = General population; DD = Non-ASD developmental delays or disorders; OR = Odds ratio.
All models adjusted for child sex, maternal education, maternal race/ethnicity, and maternal age at birth, and accounted for correlation by enrollment site.
Age of infection is a five-level variable; OR’s are calculated separately for each age category, comparing children in that category to those who never had an infection; age of infection missing for 9 ASD, 0 DD, and 6 POP children.
Additional adjustment for: Child birthweight.
Additional adjustment for: Family income and child’s gestational age.
Statistically significant.
Infections in ASD vs. DD.
Compared to DD controls, cases were more likely to have an infection during the neonatal period, in adjusted analyses (Table 2). However, cases and DD controls did not differ in their likelihood of having a caregiver-reported infection during the first three years of life or in the likelihood of having abnormal temperatures during the neonatal period.
Infections in ASD cases with vs. without regression.
In the ASD group, children with and without regression did not differ significantly for any of the explored exposures, except that cases with regression had significantly higher odds of having an infection during the first year of life in adjusted analysis (Table 3).
Table 3.
Odds of Having an Infection during the First Three Years of Life and during the Neonatal Period for Children with ASD with Regression vs. Children with ASD without Regression
| Caregiver interviews | Regression (n = 178) | No regression (n = 494) | Crude OR (95% CI) | Adjusted ORa (95% CI) |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Ever infection | 94(52.8) | 252(51.0) | 1.07(0.81–1.43) | 1.04(0.74–1.46) |
| Age of infectionb | ||||
| Never infection | 84(47.5) | 242(49.8) | REF | REF |
| Infection only in first year of life | 25(14.1) | 44(9.1) | 1.64(1.27–2.12)d | 1.60(1.14–2.26)d |
| Infection only in second year of life | 9(5.1) | 35(7.2) | 0.74(0.33–1.65) | 0.67(0.27–1.68) |
| Infection only in third year of life | 6(3.4) | 33(6.8) | 0.52(0.13–2.19) | 0.47(0.09–2.39) |
| Infection during multiple time periods | 53(29.9) | 132(27.2) | 1.16(0.87–1.54) | 1.16(0.86–1.55) |
| Neonatal records | Regression (n = 147) | No regression (n = 423) | Crude OR (95% CI) | Adjusted ORa (95% CI) |
| Ever infectionc | 6(4.1) | 23(5.4) | 0.74(0.26–2.12) | 0.95(0.32–2.87) |
| Low temperaturec | 2(1.4) | 7(1.7) | 0.82(0.10–6.86) | 0.97(0.11–8.99) |
| High temperature | 8(5.4) | 10(2.4) | 2.38(1.08–5.22)d | 2.21(0.97–5.02) |
Abbreviations: ASD = Autism spectrum disorder; OR = Odds ratio.
All models adjusted for child sex, maternal education, maternal race/ethnicity, and maternal age at birth, and accounted for correlation by enrollment site.
Age of infection is a five-level variable; OR’s are calculated separately for each age category, comparing children in that category to those who never had an infection; age of infection missing for 1 child with ASD with regression and 8 children with ASD without regression.
Additional adjustment for: Child birthweight.
Statistically significant.
Exploratory and sensitivity analyses.
Neonatal hypothermia in the first day of life was associated with low birthweight (P = 0.0004) but not neonatal infection (P = 0.0535). We compared estimates of the association between neonatal infection and ASD with and without adjustment for length of hospital stay, and saw no substantive differences in the magnitude, direction, or statistical significance of the estimates compared to either POP or DD controls (Supporting Information Table S1). We also saw no difference in the likelihood of neonatal infection among children with ASD compared to DD when stratified by preterm (OR: 1.31;95%CI: 0.65–2.67) and nonpreterm birth (OR: 1.38;95%CI: 0.75–2.55).
We performed a sensitivity analysis that excluded children without an in-person assessment (n = 83 DD, 89 POP). Relative to both control groups, the associations between infection and ASD were similar in direction, magnitude, and statistical significance (Supporting Information Table S2).
Discussion
Our findings suggest that children with ASD are more likely to have an infection in the neonatal period compared to typically developing (POP) children and to children with non-ASD developmental disorders (DD), and in the first three years of life compared to typically developing children. The association between ASD and infection was similar in magnitude whether based on caregiver report (first three years) or medical records (neonatal period), when compared to POP controls. During the neonatal period, children with ASD were also more likely to have a hypothermic event compared to the POP group. The association of ASD with neonatal infection when compared to DD children was substantially smaller than when compared to POP children, and there was no evidence that caregiver-reported infection risk before age three differed in the ASD vs. DD groups.
Our findings that children with ASD were at higher odds of having an infection before age three compared to POP children is similar to other studies. Atladóttir et al. [2010] found that children hospitalized for infection were more likely to be diagnosed with ASD compared to those hospitalized for a noninfectious reason. Atladóttir, Schendel, et al. [2012] also found that children who had either an inpatient or outpatient hospital contact for any infection were at increased risk of subsequent ASD diagnosis. However, both studies also found that children with hospital contact for noninfectious somatic disease were more likely to receive an ASD diagnosis, suggesting that hospital contact itself was associated with ASD diagnosis, rather than specifically those children with infection-related events. In contrast, Rosen et al. [2007] found no association between ASD and ever having an infection and found slightly lower odds of infection among ASD cases in the first two years of life.
Half of ASD and DD group children and over 40% of POP group children had at least one caregiver-reported diagnosis of infection by age three. This is likely to be an underestimate. In a case-control study of ASD and early infection, 97% of all children had at least one medically diagnosed infection by age two [Rosen et al., 2007] and in a UK-based national cohort study, 100% of children had one or more symptoms of infection by 57 months, of whom 97% sought medical care [Hay et al., 2005]. Unfortunately, our study did not have access to postnatal medical records to validate caregiver-reported infection rates. Future studies should consider using both caregiver reports and medical records as sources for data collection.
Children with ASD were more likely than either control group to have a neonatal infection and more likely than POP children to have a neonatal hypothermic event and an infection during the first year of life. These findings suggest the increased likelihood of infection in the first year of life among children with ASD may be driven by an increased rate of infection during the neonatal/early postnatal period. At birth, the central nervous system is not fully developed and is susceptible to damage by immune responses to infection, such as cytokine production and inflammation [Libbey, Sweeten, McMahon, & Fujinami, 2005]. Rosen et al. [2007] found no difference between cases and controls in rates of infection from the first month through the first year of life but found slightly higher odds of having an infection in the first 30 days of life among individuals with ASD. In addition, behavioral changes parallel to ASD have been seen in animal models after viral infection shortly after birth [Hornig, Weissenböck, Horscroft, & Lipkin, 1999]. Our findings could also be the result of a continued in utero infection. Infection and fever in utero are potentially important factors in the development of ASD [Brucato et al., 2017;Hornig et al., 2018;Zerbo et al., 2015] and are being examined by another forthcoming SEED paper. From an etiological standpoint, the first 30 days of life could be particularly important for environmental effects, including infection or thermodysregulation, on neurodevelopment among children with ASD and should be evaluated in future studies.
We found only one other epidemiological study to date that has examined infection in relation to regression status in ASD. Christopher et al. [2004] reported no difference in risk of perinatal infections between children with ASD with and without regression but did not explore postnatal infections. Among children with ASD, we found that those with regression were more likely to have an infection during the first year of life only. Since postnatal infections were collected from caregiver report, this finding may be due to caregivers of children with ASD and regression more often recalling their child’s past infections. Confidence intervals were wide for all comparisons due to the small number of affected children; thus, we could not exclude important differences between groups for other measured exposures. If true, this finding in addition to associations found between children with ASD vs. POP and DD controls, supports the potential importance of early life exposures on neurodevelopment among children with ASD. Our study identified regression through use of the ADI-R; other tools for regression identification might have yielded different results. Future studies might consider validating our findings using a different measure of regression in ASD.
Although our sample size ensured adequate power to detect differences in infections between groups, it was too small to adequately examine specific types of infections, particularly fungal, and bacterial infections, which may be etiologically important. We also did not have sufficient data to evaluate the severity of infection. Future analyses that incorporate data from subsequent phases of SEED will be important contributions to this work. Future studies should also examine childhood infections in the context of how and with what they are treated.
The CGI questionnaire did not allow for calculation of total numbers of infections. Nevertheless, we were able to collect detailed data on childhood infections from multiple postnatal time periods using caregiver report. In using a questionnaire that asked specifically about diagnosed disorders, this study focused on symptomatic infections diagnosed by a healthcare professional and their relation to ASD. Therefore, neither asymptomatic infections nor minor infections that did not result in a medical care visit were captured. Asymptomatic and minor infections may also be associated with ASD development. A study conducted by Lintas et al. [2010] found polyomavirus, a typically asymptomatic infection, more frequently in the brains of patients with ASD compared to controls without ASD. Asymptomatic infections induce an immune response, even though disease is not present, and could still affect neurodevelopment. Our study was not designed to explore how asymptomatic infections might relate to ASD. Further studies testing the hypothesis that immune response affects neurodevelopment among individuals with ASD are warranted.
We explored whether length of hospital stay after birth influenced the association between autism and neonatal infection. Children with ASD were more likely to have longer stays in the hospital before discharge than POP children, which could provide more opportunities to diagnose and document infection, biasing results away from the null. A longer hospital stay could also have increased the risk of acquiring infection, thus potentially explaining the higher risk in this group. However, length of hospital stay did not appear to contribute significantly as either a confounder or a mediator of the relationships studied. Further, the fact that children with ASD were less likely to stay five days or more in the hospital compared to DD controls, yet still had a significantly greater risk of neonatal infection, suggests identified differences were not solely attributable to length of hospitalization.
We performed a sensitivity analysis restricted to children assessed in person. Mothers of SEED children without an in-person assessment were more likely to be of minority race and lower education [Bradley et al., 2017], both of which are associated with a higher burden of infection [Centers for Disease Control and Prevention, 2005]. By including all DD and POP children whether or not assessed in person, the association between ASD and childhood infection may have been biased towards the null due to differences in sociodemographic or other characteristics; but also could have been biased away from the null, if children in the ASD group had more complete information on infection history. However, we saw no differences in the magnitude, direction, or significance of effect estimates in these sensitivity analyses.
One of the strengths of SEED is its use of a consistent, rigorous clinical diagnostic protocol to evaluate, and classify children with ASD and its inclusion of children with ASD who had not previously received an ASD diagnosis. Another strength is the racially, ethnically, and socioeconomically diverse study population from multiple catchment areas, which increased the external validity and generalizability of our results. To include a diverse population, children with ASD and DD were identified from multiple clinical and educational organizations that serve populations with developmental disabilities, while POP controls were randomly sampled from birth certificates. Due to our recruitment approach, some families identified for inclusion in the study were not located or could not be contacted. Such families may not have met a priori criteria for eligibility into the study, which required that families reside in the catchment area at both the time of birth and enrollment. However, the difference in recruitment strategies may have introduced some selection bias into our study. At one study site that had data on all nonresponders, nonresponse was associated with younger maternal age, lower maternal education, and non-white race, but was not associated with any perinatal factors [unpublished data]. Of those children enrolled in the SEED study, we excluded 35% who did not complete the study or the CGI questionnaire. Differences between families who did and did not meet completion criteria have been thoroughly described and discussed in Bradley et al. [2017]. Briefly, lower study and CGI completion were associated with lower maternal education, younger age, and non-white race, which may have influenced our results as these characteristics are also associated with a higher risk of childhood infection [Centers for Disease Control and Prevention, 2005].
This study used two methods to ascertain infections, both of which have potential strengths and limitations. The caregiver interview enabled us to capture infections throughout the first three years of life but was subject to recall bias, if caregivers of children with ASD and DD were more likely to recall childhood infections because of their child’s diagnoses. However, use of two control groups (DD and POP) allowed us to differentiate between an association with ASD or with neurodevelopmental disorders in general and provided a means of controlling for recall bias. By using neonatal medical records, we were able to examine the likelihood of infection without potential recall bias, although only for the period of initial neonatal hospitalization as we did not have medical record data for the remaining first three years of life. Neonatal medical record abstractors were not blinded to child’s case status, though case status would not have been apparent in the medical records. All abstractors were thoroughly trained and used standardized forms to abstract neonatal records. Quality control was carried out on 5% of medical record abstractions, 100% of which had a ≥90% reliability [Schendel et al., 2012]. Therefore, although infections reported by caregivers during their interview were subject to recall bias, the fact that we saw similar associations based on review of medical records supports the validity of our findings.
Overall, our results support the hypothesis that children with ASD are more likely to have an infection compared to POP and DD children though there were fewer, smaller differences in children with ASD compared to DD children. Further, our study showed that childhood infection appears to be unrelated to ASD regression status except possibly for infections occurring in the first year of life. Future studies on ASD should focus on examining thermodysregulation during the neonatal period, the impact of the immune response to symptomatic and asymptomatic infection on neurodevelopment, and the association of different causes, timing, frequency, and severity of infections on risk for ASD. Subsequent research should work to minimize the effects of recall bias on the investigation of childhood infection and ASD and further examine infection in relation to regression status in children with ASD using larger samples.
Supplementary Material
Supporting Information Table S1 Odds of Having an Infection during the Neonatal Period by Case Status (ASD vs. POP Controls and ASD vs. DD Controls) comparing Models with and without Adjustment for Hospital Stay among Children with Complete Information on Number of Days of Hospital Stay after Birth.
Supporting Information Table S2 Sensitivity Analysis of the Odds of Having an Infection during the First Three Years of Life or during the Neonatal Period by Case Status (ASD vs. POP Controls and ASD vs. DD Controls) comparing All Children regardless of Clinic Visit and Those with a Clinic Visit Only.
Acknowledgments
This research was supported by the Centers for Disease Control and Prevention (CDC), Centers for Autism and Developmental Disabilities Research, Study to Explore Early Development through six cooperative agreements: Cooperative Agreement Number U10DD000180, Colorado School of Public Health/University of Colorado, School of Medicine; Cooperative Agreement Number U10DD000181, Kaiser Foundation Research Institute (CA);Cooperative Agreement Number U10DD000182, University of Pennsylvania; Cooperative Agreement Number U10DD000183, Johns Hopkins University; Cooperative Agreement Number U10DD000184, University of North Carolina at Chapel Hill; and Cooperative Agreement Number U10DD000498, Michigan State University. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Footnotes
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
REFERENCES
- Adams DJ, Susi A, Erdie-Lalena CR, Gorman G, Hisle-Gorman E, Rajnik M, … Nylund CM (2016). Otitis media and related complications among children with autism spectrum disorders. Journal of Autism and Developmental Disorders, 46(5), 1636–1642. [DOI] [PubMed] [Google Scholar]
- Ajamian M, Kosofsky BE, Wormser GP, Rajadhyaksha AM, & Alaedini A. (2013). Serologic markers of Lyme disease in children with autism. Journal of the American Medical Association, 309(17), 1771–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen CW, Silove N, Williams K, & Hutchins P. (2007). Validity of the social communication questionnaire in assessing risk of autism in preschool children with developmental problems. Journal of Autism and Developmental Disorders, 37(7), 1272–1278. [DOI] [PubMed] [Google Scholar]
- Atladóttir HO, Henriksen TB, Schendel DE, & Parner ET (2012). Using maternally reported data to investigate the association between early childhood infection and autism spectrum disorder: the importance of data source. Paediatric and Perinatal Epidemiology, 26(4), 373–385. [DOI] [PubMed] [Google Scholar]
- Atladóttir HO, Schendel DE, Lauritsen MB, Henriksen TB, & Parner ET (2012). Patterns of contact with hospital for children with an autism spectrum disorder: A Danish register-based study. Journal of Autism and Developmental Disorders, 42(8), 1717–1728. [DOI] [PubMed] [Google Scholar]
- Atladóttir HO, Thorsen P, Schendel DE, Østergaard L, Lemcke S, & Parner ET (2010). Association of hospitalization for infection in childhood with diagnosis of autism spectrum disorders: A Danish cohort study. Archives of Pediatrics and Adolescent Medicine, 164(5), 470–477. [DOI] [PubMed] [Google Scholar]
- Barger BD, Campbell JM, & McDonough JD (2013). Prevalence and onset of regression within autism spectrum disorders: A meta-analytic review. Journal of Autism and Developmental Disorders, 43(4), 817–828. [DOI] [PubMed] [Google Scholar]
- Bradley CB, Browne EN, Alexander AA, Collins J, Dahm JL, Diguiseppi C, et al. (2017). Demographic and operational factors predicting study completion in a multi-site case-control study of preschool children. American Journal of Epidemiology, 187(3), 592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brucato M, Ladd-Acosta C, Li M, Caruso D, Hong X, Kaczaniuk J, … Wang X. (2017). Prenatal exposure to fever is associated with Autism Spectrum Disorder in the Boston Birth Cohort. Autism Research, 10(11), 1878–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbelo PD, Swedo SE, Thurm A, Bayat A, Levin AE, Marques A, & Iadarola MJ (2013). Lack of serum antibodies against Borrelia burgdorferi in children with autism. Clinical and Vaccine Immunology, 20(7), 1092–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2005). Racial disparities in nationally notifiable diseases - United States, 2002. Morbidity and Mortality Weekly News, 54(1), 9–11. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Division of birth defects and developmental disabilities: Data & statistics (NCBDDD). Retrieved February 5, 2017, from https://www.cdc.gov/ncbddd/birthdefects/data.html
- Christopher JA, Sears L, Williams P, Oliver J, & Hersh J. (2004). Familial, medical and developmental patterns of children with autism and a history of language regression. Journal of Developmental and Physical Disabilities, 16, 163–170. [Google Scholar]
- Diguiseppi CG, Daniels JL, Fallin DM, Rosenberg SA, Schieve LA, Thomas KC, … Schendel DE (2016). Demographic profile of families and children in the Study to Explore Early Development (SEED): Case-control study of autism spectrum disorder. Disability and Health Journal, 9(3), 544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza Y, Fombonne E, & Ward BJ (2006). No evidence of persisting measles virus in peripheral blood mononuclear cells from children with autism spectrum disorder. Pediatrics, 118(4), 1664–1675. [DOI] [PubMed] [Google Scholar]
- Estes ML, & McAllister AK (2015). Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nature Reviews: Neuroscience, 16(8), 469–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile I, Zappulo E, Bonavolta R, Maresca R, Messana T, Buonomo AR, … Bravaccio C. (2014a). Prevalence and titre of antibodies to cytomegalovirus and epstein-barr virus in patients with autism spectrum disorder. In Vivo, 28(4), 621–626. [PubMed] [Google Scholar]
- Gentile I, Zappulo E, Bonavolta R, Maresca R, Riccio MP, Buonomo AR, … Bravaccio C. (2014b). Exposure to Varicella Zoster Virus is higher in children with autism spectrum disorder than in healthy controls. Results from a case-control study. In Vivo, 28(4), 627–631. [PubMed] [Google Scholar]
- Gotham K, Risi S, Pickles A, & Lord C. (2007). The autism diagnostic observation Schedule: Revised algorithms for improved diagnostic validity. Journal of Autism and Developmental Disorders, 37(4), 613–627. [DOI] [PubMed] [Google Scholar]
- Hay AD, Heron J, Ness A, & ALSPAC study team. (2005). The prevalence of symptoms and consulations in pre-school children in the Avon Longitudinal Study of Parents and Children (ALSPAC): A prospective cohort study. Family Practice, 22(4), 367–374. [DOI] [PubMed] [Google Scholar]
- Hornig M, Bresnahan MA, Che X, Schultz AF, Ukaigwe JE, Eddy ML, … Lipkin WI (2018). Prenatal fever and autism risk. Molecular Psychiatry, 23, 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornig M, Weissenbock H, Horscroft N, & Lipkin WI (1999). An infection-based model of neurodevelopmental damage. Proceedings of the National Academy of Sciences of the United States of America, 96(21), 12102–12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inscoe JR, & Bones C. (2016). Additional difficulties associated with aetiologies of deafness: outcomes from a parent questionnaire of 540 children using cochlear implants. Cochlear Implants International, 17(1), 21–30. [DOI] [PubMed] [Google Scholar]
- Korvatska E, Van de Water J, Anders TF, & Gershwin ME (2002). Genetic and immunologic considerations in autism. Neurobiology of Disease, 9(2), 107–125. [DOI] [PubMed] [Google Scholar]
- Kumar V, Shearer JC, Kumar A, & Darmstadt GL (2009). Neonatal hypothermia in low resource settings: A review. Journal of Perinatology, 29(6), 401–412. [DOI] [PubMed] [Google Scholar]
- Lee LC, David AB, Rusyniak J, Landa R, & Newschaffer CJ (2007). Performance of the social communication questionnaire in children receiving preschool special education services. Research in Autism Spectrum Disorders, 1(2), 126–138. [Google Scholar]
- Libbey JE, Coon HH, Kirkman NJ, Sweeten TL, Miller JN, Lainhart JE, … Fujinami RS (2007). Are there altered antibody responses to measles, mumps, or rubella viruses in autism. Journal of Neurovirology, 13(3), 252–259. [DOI] [PubMed] [Google Scholar]
- Libbey JE, Sweeten TL, McMahon WM, & Fujinami RS (2005). Autistic disorder and viral infections. Journal of Neurovirology, 11(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Lintas C, Altieri L, Lombardi F, Sacco R, & Persico AM (2010). Association of autism with polyomavirus infection in postmortem brains. Journal of Neurovirology, 16(2), 141–149. [DOI] [PubMed] [Google Scholar]
- Lintas C, Guidi F, Manzi B, Mancini A, Curatolo P, & Persico AM (2011). Lack of infection with XMRV or other MLV-related viruses in blood, post-mortem brains and paternal gametes of autistic individuals. PLoS One, 6(2), e16609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, Dilavore PC, & Risi S. (1999). Autism Diagnostic Observation Schedule-WPS (ADOS-WPS). Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Lord C, Rutter M, & Le Couteur A. (1994). Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism Developmental Disorder, 24, 659–685 [DOI] [PubMed] [Google Scholar]
- Lyall K, Croen L, Daniels J, Fallin MD, Ladd-Acosta C, Lee BK, … Newschaffer C. (2017). The changing epidemiology of autism spectrum disorders. Annual Review of Public Health, 38, 81–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai CT, Isenburg J, Langlois PH, Alverson C, Gilboa SM, Rickard R, et al. (2015). Population-based birth defects data in the United States, 2008 to 2012: Presentation of state-specific data and descriptive brief on variability of prevalence. Birth defects research. Part A: Clinical and molecular. Teratology, 103(11), 972–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen E, & AGS. (1995). Mullen scales of early learning. Circle Pines, MN: American Guidance Service, Inc. [Google Scholar]
- Nicolson GL, Gan R, Nicolson NL, & Haier J. (2007). Evidence for Mycoplasma ssp., Chlamydia pneunomiae, and human herpes virus-6 coinfections in the blood of patients with autistic spectrum disorders. Journal of Neuroscience Research, 85(5), 1143–1148. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Iosif AM, Baguio F, Cook IC, Hill MM, Hutman T, et al. (2010). A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child and Adolescent Psychiatry, 49(3), 256–266. [PMC free article] [PubMed] [Google Scholar]
- Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, et al. (2010). Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Research. Part A: Clinical and molecular. Teratology, 88(12), 1008–1016. [DOI] [PubMed] [Google Scholar]
- Rosen NJ, Yoshida CK, & Croen LA (2007). Infection in the first 2 years of life and autism spectrum disorders. Pediatrics, 119(1), e61–e69. [DOI] [PubMed] [Google Scholar]
- Rosenhall U, Nordin V, Sandström M, Ahlsén G, & Gillberg C. (1999). Autism and hearing loss. Journal of Autism and Developmental Disorders, 29(5), 349–357. [DOI] [PubMed] [Google Scholar]
- Rutter ML, Bailey A, & Lord C. (2003a). Social communication questionnaire (SCQ): Manual. Western Psychological Services. [Google Scholar]
- Rutter ML, Le Couteur A, & Lord C. (2003b). ADI-R: The autism diagnostic interview-revised. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Satterfield BC, Garcia RA, Gurrieri F, & Schwartz CE (2010). PCR and serology find no association between xenotropic murine leukemia virus-related virus (XMRV) and autism. Molecular Autism, 1(1), 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendel DE, Diguiseppi C, Croen LA, Fallin MD, Reed PL, Schieve LA, … Yeargin-Allsopp M. (2012). The study to explore early development (SEED): A multisite epidemiologic study of autism by the Centers for Autism and Developmental Disabilities Research and Epidemiology (CADDRE) network. Journal of Autism and Developmental Disorders, 42(10), 2121–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VK (2009). Phenotypic expression of autoimmune autistic disorder (AAD): A major subset of autism. Annals of Clinical Psychiatry, 21(3), 148–161. [PubMed] [Google Scholar]
- Smales OR, & Kime R. (1978). Thermoregulation in babies immediately after birth. Archives of Disease in Childhood, 53(1), 58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins LD, Reynolds A, Rice CE, Moody EJ, Bernal P, Blaskey L, … Levy SE (2015). Using standardized diagnostic instruments to classify children with autism in the study to explore early development. Journal of Autism and Developmental Disorders, 45(5), 1271–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbo O, Qian Y, Yoshida C, Grether JK, Van de Water J, & Croen LA (2015). Maternal infection during pregnancy and autism spectrum disorders. Journal of Autism and Developmental Disorders, 45(12), 4015–4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Table S1 Odds of Having an Infection during the Neonatal Period by Case Status (ASD vs. POP Controls and ASD vs. DD Controls) comparing Models with and without Adjustment for Hospital Stay among Children with Complete Information on Number of Days of Hospital Stay after Birth.
Supporting Information Table S2 Sensitivity Analysis of the Odds of Having an Infection during the First Three Years of Life or during the Neonatal Period by Case Status (ASD vs. POP Controls and ASD vs. DD Controls) comparing All Children regardless of Clinic Visit and Those with a Clinic Visit Only.