Abstract
Background
Printed educational materials are widely used passive dissemination strategies to improve the quality of clinical practice and patient outcomes. Traditionally they are presented in paper formats such as monographs, publication in peer‐reviewed journals and clinical guidelines.
Objectives
To assess the effect of printed educational materials on the practice of healthcare professionals and patient health outcomes.
To explore the influence of some of the characteristics of the printed educational materials (e.g. source, content, format) on their effect on professional practice and patient outcomes.
Search methods
For this update, search strategies were rewritten and substantially changed from those published in the original review in order to refocus the search from published material to printed material and to expand terminology describing printed materials. Given the significant changes, all databases were searched from start date to June 2011. We searched: MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), HealthStar, CINAHL, ERIC, CAB Abstracts, Global Health, and the EPOC Register.
Selection criteria
We included randomised controlled trials (RCTs), quasi‐randomised trials, controlled before and after studies (CBAs) and interrupted time series (ITS) analyses that evaluated the impact of printed educational materials (PEMs) on healthcare professionals' practice or patient outcomes, or both. We included three types of comparisons: (1) PEM versus no intervention, (2) PEM versus single intervention, (3) multifaceted intervention where PEM is included versus multifaceted intervention without PEM. There was no language restriction. Any objective measure of professional practice (e.g. number of tests ordered, prescriptions for a particular drug), or patient health outcomes (e.g. blood pressure) were included.
Data collection and analysis
Two review authors undertook data extraction independently, and any disagreement was resolved by discussion among the review authors. For analyses, the included studies were grouped according to study design, type of outcome (professional practice or patient outcome, continuous or dichotomous) and type of comparison. For controlled trials, we reported the median effect size for each outcome within each study, the median effect size across outcomes for each study and the median of these effect sizes across studies. Where the data were available, we re‐analysed the ITS studies and reported median differences in slope and in level for each outcome, across outcomes for each study, and then across studies. We categorised each PEM according to potential effects modifiers related to the source of the PEMs, the channel used for their delivery, their content, and their format.
Main results
The review includes 45 studies: 14 RCTs and 31 ITS studies. Almost all the included studies (44/45) compared the effectiveness of PEM to no intervention. One single study compared paper‐based PEM to the same document delivered on CD‐ROM. Based on seven RCTs and 54 outcomes, the median absolute risk difference in categorical practice outcomes was 0.02 when PEMs were compared to no intervention (range from 0 to +0.11). Based on three RCTs and eight outcomes, the median improvement in standardised mean difference for continuous profession practice outcomes was 0.13 when PEMs were compared to no intervention (range from ‐0.16 to +0.36). Only two RCTs and two ITS studies reported patient outcomes. In addition, we re‐analysed 54 outcomes from 25 ITS studies, using time series regression and observed statistically significant improvement in level or in slope in 27 outcomes. From the ITS studies, we calculated improvements in professional practice outcomes across studies after PEM dissemination (standardised median change in level = 1.69). From the data gathered, we could not comment on which PEM characteristic influenced their effectiveness.
Authors' conclusions
The results of this review suggest that when used alone and compared to no intervention, PEMs may have a small beneficial effect on professional practice outcomes. There is insufficient information to reliably estimate the effect of PEMs on patient outcomes, and clinical significance of the observed effect sizes is not known. The effectiveness of PEMs compared to other interventions, or of PEMs as part of a multifaceted intervention, is uncertain.
Plain language summary
Printed educational materials: effects on professional practice and healthcare outcomes
Medical journals and clinical practice guidelines are common channels to distribute scientific information to healthcare providers, as they allow a wide distribution at relatively low costs. Delivery of printed educational materials is meant to improve healthcare professionals' awareness, knowledge, attitudes, and skills, and ultimately improve professional practice and patients' health outcomes. Results of this review suggest that printed educational materials slightly improve healthcare professional practice compared to no intervention, but a lack of results prevent any conclusion on their impact on patient outcomes.
Summary of findings
Summary of findings for the main comparison. Printed educational material vs. no intervention.
| Printed educational material vs. no intervention | |||
| Patient or population: healthcare professionals (physicians in 9/10 studies) Settings: multiple settings Intervention: printed educational material Comparison: no intervention | |||
| Outcomes* | Standard median effect size | No of participants (studies) | Quality of the evidence (GRADE) |
| Categorical measure of professional practice Absolute risk difference across various outcomes Mean follow‐up: 6 months | 0.02 higher (range from 0.00 to +0.11) | 294,937 (7 studies) | ⊕⊕⊝⊝ low1, 2, 3 |
| Continuous measure of professional practice Standardised mean difference across various outcomes Mean follow‐up: 9 months | 0.13 higher (range from ‐0.16 to +0.36) | 297 (3 studies) | ⊕⊝⊝⊝ very low3,4,5 |
| * Where studies reported more than one measure of each endpoint, the primary measure (as defined by the authors of the study) or the median measure was abstracted. For categorical measures, we calculated the odds ratio between the intervention of interest and the control intervention. For continuous endpoints, we calculated standardised mean difference by dividing the mean score difference of the intervention and comparison groups in each study by the pooled estimate standard deviation for the two groups | |||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
1 Unclear sequence generation.
2 Unclear addressing of incomplete outcome data.
3 Imprecision of the observed effect ‐ the analyses used do not allow computing confidence intervals to support an evaluation of the precision of the estimate. However, most of the median effect sizes of the individual studies included were imprecise.
4 Inadequate allocation concealment. 5 Inconsistency: one study measured a deterioration in outcomes whereas the other two showed improvements.
Background
Description of the condition
Most research findings are not making their way into practice in a timely fashion despite the considerable resources devoted to health sciences research (Graham 2006). Recommendations are frequently not applied in practice and many patients do not benefit from evidence‐based research (Grol 2001; Schuster 2005).
Description of the intervention
Printed educational materials (PEMs) are probably one of the most common approaches to translate research findings into clinical practice (Bero 1998). This review focuses on the passive dissemination of PEMs, defined as the distribution of published or printed recommendations for clinical care including clinical practice guidelines, monographs, and publications in peer‐reviewed journals, delivered personally or through mass mailing (Grimshaw 2003).
How the intervention might work
PEMs have the potential to improve the care received by patients by promoting clinical practice of proven benefit and discouraging ineffective procedures (Woolf 1999). Given that PEMs are familiar, accessible, inexpensive, and convenient to use, they could be a cost‐effective intervention within healthcare settings (Grimshaw 2004; Grimshaw 2006).
Potential factors influencing the implementation of PEMs can be derived from various theories on quality‐improvement and implementation of change in health care (Grol 2007; Gross 2001; Stergiou‐Kita 2010). Cognitive theories suggest that PEMs should take into account healthcare professionals' decision processes and learning styles to support their decisions in practice better. Educational and adult learning theories propose that change is driven by the desire to learn and be professionally competent, suggesting that PEMs should be linked to professionals' needs and motivation, define personal targets for improvement and contain individual 'learning plans' related to desired performance. Attitudinal and motivational theories suggest that PEMs should address professionals' attitudes, beliefs, perceived social norms, and experienced control related to desired performance to influence their motivations to change. Professional development theories emphasise the importance of professional loyalty, pride, consensus, and that change be endorsed by a professional body; thus, PEMs should incorporate these elements and define professional standards for the desired behaviour. Social influence theories suggest that the content or message of the PEMs be endorsed or reinforced by recognised leaders in their field. Literature on communication design might also be useful to appraise some of the more visual aspects of PEMs (Ancker 2007; Rosenbaum 2010).
The persuasive communication theory proposes five input variables that may possibly affect communication effectiveness: source, message, channel, receiver, and destination (Wilson 2010). For the purpose of this review, we chose to focus on the three variables to characterise the intervention itself, namely source, message and channel. In addition, to acknowledge the possible importance of PEMs' visual aspects to explain their effectiveness, we added a variable that we labelled 'format'. With regards to source, we considered credibility and proximity of the source. Source credibility influences the extent to which a message is believed (Tseng 1999; Wathen 2002), so that PEMs that are endorsed by a credible organisation, such as a national professional organisation might have more impact on practice. Proximity of the source to the target audience (i.e. when the information is locally tailored to the audience) can also affect health behaviour change more positively than can targeted, personalised, or generic interventions (Revere 2001). For channel, we considered the mode, frequency, and duration of PEM delivery. The mode of delivery must be appropriate to the target audience ‐ widest audiences should be reached via mass communication and local audiences via personalised channels (Marriott 2000). Frequently delivered PEMs that lead to a more frequent exposure of the professional to the message, following principles of persuasive communication, might be more effective to improve professional practice performance (Davis 2009; McGuire 1989). For message, we considered the PEM's clinical area, type of targeted behaviour, purpose, level of evidence, and educational component. Compatibility of PEMs with existing beliefs, for example, if PEM's purpose is to increase an established management, could possibly increase their acceptability to users (Rogers 1995), but evidence has demonstrated that clinical recommendations that are more compatible with clinician beliefs were less effective to change professional practice, which is likely to be because of ceiling effects (Foy 2002). Evidence‐based recommendations are better followed in practice than recommendations that are not based on scientific evidence (Foy 2002; Grol 1998). For format, we considered format, appearance, and length. Shorter and simpler documents have the potential to facilitate more effective and efficient uptake of key information, as professionals often do not have time to screen, organise, and appraise new scientific literature (Grandage 2002; Marriott 2000; Wang 2009).
Why it is important to do this review
The first version of the present review on the effectiveness of the passive dissemination of PEMs included nine studies comparing PEMs to no intervention and it concluded that this strategy had little impact on professional practice (Freemantle 1997). These results were then supported by another broader review of 44 reviews covering a wide range of interventions that concluded that passive dissemination is generally ineffective (NHS 1999). These early results led researchers to use PEMs as a control condition for evaluating the impacts of more complex and intensive quality improvement interventions (e.g. Jain 2006; Maiman 1988; Mettes 2010), instead of evaluating PEMs per se. However, subsequent reviews (Grimshaw 2004; Hakkennes 2008) and the first update of the present review published in 2008 showed that PEMs led to modest, but significant, improvement in professional practice (Farmer 2008). The first update included nine randomised controlled trials (RCTs) comparing PEMs to no intervention and observed a median absolute improvement in performance of 4.3% for categorical process outcomes (six studies: Bearcroft 1994; Beaulieu 2004; Bjornson 1990; Croudace 2003; Kottke 1989; Oakeshott 1994) and a relative improvement of 13.6% for continuous process outcomes (three studies: Azocar 2003; Denig 1990; Oakeshott 1994).
Since the last update, several new studies of the passive dissemination of PEMs have been published, but no other review on the effectiveness of this strategy to improve any professional behaviour has, to our knowledge, been done. Several reviews have studied the passive dissemination of PEMs alongside other types of quality improvement strategies to improve specific behaviours, such as antibiotic prescribing (Arnold 2005), use of imaging (French 2010), management of diabetes (de Belvis 2009; Seitz 2011), or psychiatric care (Weinmann 2007). However, these reviews included few studies that compared the passive dissemination of PEMs to no intervention, limiting conclusions on their effectiveness.
In addition, the small number of trials included in the first update prevented exploration of which PEM characteristics were associated with greater effectiveness. The larger number of studies gathered through this second update should allow us to assess the impact of potential effect modifiers of PEMs (to then suggest strategies to optimise them). It should also allow us to generalise the review conclusions to a larger set of conditions.
Objectives
To assess the effect of PEMs on the practice of healthcare professionals and patient health outcomes.
To explore the influence of some of the characteristics of the PEMs (e.g. mode of delivery, source of information, format) on their effect on professional practice and patient outcomes.
To address the first objective, we included the following types of comparisons: (1) PEM only compared to no intervention, (2) PEM only versus single intervention, and (3) multifaceted intervention where PEM is included versus multifaceted intervention without PEM.
To address the second objective, we classified each included intervention according to potential effect modifiers related the source of the PEMs, the channel used for their delivery, the message, and their format.
Methods
Criteria for considering studies for this review
Types of studies
RCTs, quasi‐randomised studies, controlled before and after studies (CBAs) and interrupted time series (ITS) analyses were included. For CBAs, we considered only the trials that used contemporaneous data collection (i.e. pre‐ and post‐intervention periods for study and control sites are the same); that selected appropriate control site for studies using second site as controls (i.e. study and control sites are comparable with respect to dominant reimbursement system, level of care, setting of care, and academic status); and that used a minimum number of sites (i.e. there was a minimum of two intervention sites and two control sites). We used two criteria for inclusion of studies with an ITS design: a clearly defined point in time when the intervention occurred, and at least three data points before and three after the intervention. We included studies published in all languages.
Types of participants
Any healthcare professionals provided with PEMs to improve their practice or patient outcomes, or both. We included studies in which the participants were students and healthcare professionals only if we could separate the outcomes from students and qualified healthcare professionals.
Types of interventions
We included studies of the distribution of published or printed recommendations for clinical care and evidence to inform practice, comprising clinical practice guidelines, journal articles, and monographs. We included PEMs delivered personally (i.e. addressed to a specific individual), through mass mailings, or passively delivered through broader communication channels (e.g. printable documents available on the Internet, mass media). Interventions to provide increased access to electronically retrievable information were considered to be outside of the scope of this review.
We included multifaceted interventions that comprised PEM only if they were compared to the same multifaceted intervention without the studied PEM.
Types of outcome measures
Any objective measure either of professional practice (e.g. the number of tests ordered, prescriptions for a particular drug) or of patient health outcomes (e.g. blood pressure, complications after surgery). Studies that only reported the impact of PEMs on healthcare professionals' attitudes, awareness, knowledge, or opinions were excluded.
Search methods for identification of studies
Electronic searches
Primary studies and related systematic reviews were identified using the following bibliographic databases, sources and approaches.
Databases
MEDLINE, OVID (1948 to June 2011)
EMBASE, OVID (1947 to June 2011)
The Cochrane Central Register of Controlled Trials (CENTRAL)
Cumulative Index to Nursing and Allied Health Literature CINAHL, EbscoHost (1980 to June 2011)
The EPOC Specialised Register, Reference Manager
CAB Abstracts, EbscoHost (1973 to June 2011)
ERIC: Educational Resources Information Center, Wilson (1966 to June 2011)
Global Health, CAB Direct (1973 to June 2011)
HealthStar, OVID (1999 to June 2011)
Strategy
For this update, the original search strategy was revised and refined to describe the concept of PEMs better and to incorporate methodological changes for identifying non‐RCT study designs such as CBA and ITS. Given the significant changes to the strategy, each database was searched from its start date to June 2011. The finalised strategies reflect an iterative development process whereby results of test strategies written by the EPOC Trials Search Co‐ordinator were screened by authors for relevance. Based on this feedback, terms were added to or deleted from the final strategies. Strategies for this update are in Appendix 1; the original strategy is in Appendix 2.
The search strategy included both controlled vocabulary terms and keywords. One portion of the search was a focused keyword search using high‐value phrases such as printed educational materials, or print intervention,print/written material in proximity to education terms; results from this portion of the strategy were not combined with methodological filters and all citations were screened. The second part of the strategy used Medical Subject Headings (MeSH) for continuing education and in‐service training and combined these concepts with terms describing health professionals and a broad array of synonyms for print material. This strategy also incorporated two methodological search filters ‐ the Cochrane RCT Sensitivity/Precision Maximizing Filter (cf. Cochrane Handbook for Systematic Reviews of Interventions Chapter 6.4d); and the EPOC Filter. Strategies were developed for OVID MEDLINE and were translated for other databases.
Searching other resources
Additional information was identified as follows:
reviewed reference lists of included studies, relevant systematic reviews, or other publications;
conducted cited reference searches in ISI Web of Science/Web of Knowledge.
Data collection and analysis
Selection of studies
Two review authors (from LC, AGi, MF, AGr, LC) independently screened the titles and abstract of all the retrieved reports to assess which studies met the inclusion criteria. We then retrieved full‐text copies of all papers that were either potentially relevant or for which the inclusion criteria were not clear in the title or abstract. Any disagreements on selection were resolved by discussion among the review authors and arbiters (JG, FL).
Data extraction and management
For multi‐arm studies, we selected the intervention groups as those that could be included in a pair‐wise comparison of intervention groups that, if investigated alone, would meet the criteria for including studies in the review. Where more than two arms met these inclusion criteria, we selected the most intensive intervention among the experimental arms.
Two review authors extracted outcome data independently (from LC, JO, LN) and disagreements were resolved by discussion between the review authors and arbiters (AGi, ST). New in this update, we gathered the actual PEMs to allow a better description of their characteristics. For the extraction of the data on the characteristics of the studies and interventions, we used a modified version of the EPOC data collection checklist. A single review author initially extracted the data and a second review author double‐checked the extracted data (MS, LC). All modifications proposed by the second review author to the initial extraction were verified by a third review author (JO). Disagreements were resolved by discussion between the review authors and arbiters (AGi).
We categorised each PEM according to potential effects modifiers by reading the study report and by assessing, where available, the PEM itself (Appendix 3). We chose the characteristics (effect modifiers) that we hypothesised would be most important in explaining differences in the effectiveness of the PEM. Effects modifiers related either to the source of the PEMs, the channel used to deliver them, their message, or their format, as described hereafter:
Source
Source of information: researchers/clinicians, university, local expert body, national professional expert body, national government expert body, local clinicians, international professional expert body, international government expert body (Tseng 1999; Wathen 2002).
Endorsement: endorsed by an official source, not endorsed (Marriott 2000; Wathen 2002).
Tailoring: tailored to individuals based on diagnostic, behavioural, or motivational characteristics; tailored to groups of individuals; personalised but not tailored; generic (Baker 2010; Bull 2001; Kreuter 1996; Revere 2001).
Channel
Mode of delivery: publication in peer‐reviewed journal, passive dissemination, direct mailing, mass mailing, media, hand delivery (Grol 1998).
Frequency of delivery: once, twice, three times, more than three times, indeterminate (Davis 2009).
Duration of delivery: once, one to three months, four to six months, over six months, indeterminate.
Message
Clinical area: e.g. cardiovascular disease, antibiotic treatment, hypertension, diabetes, oestrogen replacement therapy, statin therapy, chest radiography, prostheses, orthopaedic surgery (Marriott 2000; Grol 2003).
Type of targeted behaviour: prescribing/treatment, financial, general management of a problem, diagnosis, procedures, referrals, test ordering, surgery, patient education/advice, clinical prevention, screening, reporting, professional‐patient communication, record keeping, discharge planning (Arnold 2005).
Purpose: initiation of new management, stopping the introduction of new management, increase of established management, cessation of established management, reduction of established management, modification of management (Foy 2002; Grol 1998; Rogers 1995).
Level of evidence: system, summary, systematic review of RCTs, clinical practice guidelines, other synthesis, original RCT, original non‐RCT study, expert opinion (Burgers 2003a; Foy 2002; Grol 1998; Haynes 2007).
Educational component: continuing professional development credits to recipients, delivered as part of a formal education programme, clear statement that intended for education, no evidence of educational component (Davis 2009).
Format
Format: publication of RCT in peer‐reviewed journal, quick reference of clinical practice guidelines, full clinical practice guidelines, newsletter/bulletin, manual of article reprints, other (Grandage 2002).
Appearance: black and white with figures/tables, enhanced communication format (Bull 2001; Hoffman 2004).
Length: more than two pages, two pages or less (Wang 2009).
A single review author initially categorised each PEM and a second review author double‐checked the categories chosen (MS, LC). All modifications proposed by the second review author to the initial classification were verified by a third review author (JO). Disagreements were resolved by discussion between the review authors and arbiters (AGi).
We contacted the primary authors of the studies to complete missing data relative to outcomes, study design, and mode of delivery. We also asked them for the actual PEM that had been evaluated within the study if it was unavailable within the report and could be not found on the Internet.
Assessment of risk of bias in included studies
At least two review authors (from AGi, NB, JO, SG) assessed the risk of bias for each included study using the criteria described in the EPOC module (see 'Additional information', 'Assessment of methodological quality' under Group Details). We resolved any discrepancies in quality ratings by discussion among review authors. We contacted the primary authors of the studies to complete missing data regarding sequence generation and allocation concealment.
Unit of analysis issues
We noted whether studies randomised healthcare providers or clusters of providers, such as practices. If the analysis did not allow for clustering of healthcare providers, we recorded a unit of analysis error, as such analysis tends to overestimate the precision of the effect of treatment (Donner 2001).
Dealing with missing data
When required information to perform the calculations on an outcome was missing, this outcome was not included in the analyses.
Data synthesis
We structured data analysis using the statistical methods developed by Grimshaw and colleagues (Grimshaw 2004). Studies were grouped according to study design (ITS or controlled studies), type of end point (professional practice or patient outcome, continuous or dichotomous) and type of comparison (PEM only versus no intervention or PEM only versus other intervention). For studies where the quantitative data were absent or insufficient to calculate effect sizes, we presented the qualitative data as presented by the authors and conducted a descriptive analysis of the effectiveness of the included PEMs. The hypothesised direction of effect differed between studies, with some studies expecting an increase in end point and others expecting a decrease. In all cases, the effect size was standardised so that a positive difference between post‐intervention percentages or means was a favourable end point.
Interrupted time series
Descriptive statistics for each study were tabulated, and we re‐analysed the results where possible. For the purpose of re‐analysis, data on individual observations over time were derived from tables of results or graphs presented in the original study, by reading the corresponding values from the images. This approach shows good consistency between data derived from graphs and those explicitly reported in papers (Grilli 2002).
Following recommendations of Ramsay and colleagues (Ramsay 2003), time regression analyses were used to re‐analyse the results of each study. We first identified the best statistical model to use by testing for autocorrelation using the Durbin‐Watson statistic. We then compared the results of the autoregressive integrated moving average (ARIMA) model and segmented linear regression analysis. We found the ARIMA model to be more appropriate in only two of the 54 included outcomes, and both models gave comparable results in these cases, so we decided to use only the segmented linear regression model.
We estimated two effect sizes: (1) the change in the level, which tells us the short‐term change in the level of the outcome immediately after the introduction of the PEM and (2) the change in the slope (trend) of the regression line, which estimates the effect size with increasing time after the PEM intervention as the per cent change in level at each time point. If the PEM had an effect, it may have produced a change in level, a change in slope, or both. Using the estimated change in level and its standard error, we calculated standardised effect sizes by dividing each estimated change in level by its standard error. We used these standardised changes in level to calculate median level differences for each study, and then for each type of outcome (professional practice or patient outcomes).
Studies with a control group (C‐RCT, RCT and CBA)
Where studies reported more than one measure of each end point, the primary measure (as defined by the authors of the study) or the median measure was abstracted. For example, if the study reported multiple dichotomous professional practice variables, and none of them was denoted the primary variable, then the effect sizes of all the variables were ranked and the median value was taken. For dichotomous end points, we calculated the median absolute risk difference (ARD) between the intervention of interest and the control intervention. The ARD represents the difference in end point between intervention and control group (intervention minus control). A positive ARD indicates that performance improved more in the group that received the PEM than in the control group (e.g. an ARD of 0.11 indicates an absolute improvement in compliance with the targeted behaviours of 11%). For continuous end points, we calculated standardised mean difference (SMD) by dividing the mean score difference of the intervention and comparison groups in each study by the pooled estimate standard deviation for the two groups. For dichotomous and continuous end points, we constructed the 95% confidence intervals (CI) according to the recommendations of Review Manager 5 (RevMan 2011). When no baseline was reported, we considered groups to be similar prior to the intervention. When the baseline was different for the two groups, we extracted a qualitative quote from the primary study report on the effectiveness of the intervention and on any confounding factors when available.
Analyses were carried out using the SAS software package (version 9.2), and Review Manager (version 5.1) (RevMan 2011). We interpreted P < 0.05 as indicating statistical significance.
Subgroup analysis and investigation of heterogeneity
We considered the PEM characteristics that were listed previously (Data extraction and management) as potential sources of heterogeneity to explain variations in the results of the included studies. We prepared box plots (displaying median effect sizes, interquartile ranges, and outliers) and visually explored the size of the observed effects in relationship to each of these characteristics. Based on the work of various authors outlined in the Background section (How the intervention might work), we hypothesised that endorsement (Tseng 1999; Wathen 2002), tailoring (Revere 2001), increased frequency (Davis 2009; McGuire 1989), better quality of evidence (Foy 2002; Grol 1998), educational component, enhanced communication format, and shorter length (Grandage 2002; Marriott 2000; Wang 2009) would enhance the PEM effectiveness. We did not have a priori hypotheses for the other potential effect modifiers.
Results
Description of studies
Results of the search
We identified 3715 potentially relevant reports, of which we excluded 3101 based on their titles and abstracts. The complete texts of the remaining 614 reports were retrieved and screened against our inclusion criteria. This second detailed screening led to the exclusion of 564 reports, leaving 50 included reports of 45 studies. One study was published in three reports (Avorn 1983), and three studies were published in two reports (Azocar 2003; Kajita 2010; Perria 2007). Fifty‐two PEM interventions were evaluated by the 45 included studies (some studies evaluated more than one PEM).
Included studies
Twenty‐nine new studies were added to this review since the previous update, and four studies were removed. Two studies were removed because they did not use a PEM on its own as the intervention (PEM combined with one or multiple co‐interventions versus no intervention). One study was removed and combined to an included study because they were, in fact, two reports on the same RCT (Avorn 1983). A study that had been included as a CBA trial was removed from this review, because the report did not provide pre‐intervention data, and the authors of the primary study did not answer our requests for this information (Steffensen 1997).
A total of 45 studies were included, comprising eight C‐RCTs (Bearcroft 1994; Dormuth 2004; Jousimaa 2002, Kajita 2010, Oakeshott 1994; Perria 2007, Tsuji 2009, Watson 2001) and six RCTs (Avorn 1983, Azocar 2003; Beaulieu 2004; Bjornson 1990; Denig 1990; Kottke 1989). Thirty‐one studies used ITS (Austin 2003; Austin 2004A; Austin 2004B; Austin 2005; Barbaglia 2009; Black 2002; Buyle 2010; Coopersmith 2002; Fijn 2000; Fonarow 2009; Fukuda 2009; Guay 2007; Haas 2004; Hersh 2004; Jackevicius 2001; Jameson 2010; Juurlink 2004; Kabir 2007; Lam 2009; Majumdar 2003; Majumdar 2004; Mason 1998/99; Mason 2001; Matowe 2002; Meyer 2007; Roberts 2007; Santerre 1996; Shah 2008; Stafford 2004; Wang 2005; Weiss 2011).
Almost all the included studies addressed comparison group #1 (PEM only compared to no intervention); only one included study addressed comparison group #2 (PEM only versus single intervention) and it compared paper‐based PEM to PEM on CD‐ROM (Jousimaa 2002). No studies addressed comparison group #3 (multifaceted intervention where PEM is included versus multifaceted intervention without PEM).
Most of the included studies took place in North America (12 in Canada, 11 in the US and one in both countries). We also included 18 studies conducted in Europe (including 11 in the UK), two in Japan, and one in Brazil. Ten studies took place in general or family medicine practices, nine in outpatient (ambulatory) settings, six in hospitals, three in mixed settings, one in a municipal health centre, and one in a managed behavioural healthcare organisation. The clinical settings of 15 studies were unclear; rather, participants were selected from within a specific geographic region. In most studies (42/45), participants were physicians. In three studies, participants combined physicians and nurses, physicians and pharmacists, or psychologists. In one study, the participants were nurses, public health nurses, and allied health professionals in the field of community health. It was unclear which type of health professionals participated in the remaining study.
Description of printed educational materials
Most studies (36/45) evaluated a single PEM. Two studies evaluated simultaneously several PEMs (respectively 12 and 11 distinct PEMs) that presented similar characteristics (Dormuth 2004; Weiss 2011), and three ITS studies assessed more than two or three PEMs with very similar characteristics (Austin 2005; Hersh 2004; Wang 2005). Because we did not have the data required to analyse the effectiveness of each of these PEMs separately, we considered the effectiveness of the combined PEMs as they were a single intervention. Lastly, a few ITS studies evaluated the impact of multiple distinct PEMs that were delivered successively over time, by looking at the trends before and after each of the delivered PEM. For instance, a single study evaluated the impact of four PEMs (Fonarow 2009) and three studies evaluated two PEMs each (Haas 2004; Kabir 2007; Majumdar 2003). PEMs evaluated using ITS designs were different from those evaluated with RCT designs. These PEMs were more homogenous regarding their source, endorsement, and format, as they were generally reports of an RCT published in a peer review journal. They also often target prescribing. PEMs tested by means of RCT designs were more diverse. In the following section, we describe the characteristics of these 52 PEMs.
PEM characteristics (potential effect modifiers)
Source
Various sources produced the studied PEMs (Table 2). Twenty‐four PEMs were produced by researchers or clinicians. Fourteen PEMs were produced by national professional expert bodies, such as the Women's Health Initiative, the College des Médecins du Quebec, the Society for Obstetrics and Gynecology, or the Royal College of Radiologists. Four PEMs came from local expert bodies.
1. Characteristics of the included interventions: source and channel of information.
| Study (PEM) | Source of information * | Endorsement | Tailoring ¶ | Mode of delivery¥ | Frequency of delivery | Duration of delivery |
| Austin 2003 (HERS trial report) | 1 | yes | 4 | 1 | indeterminate | indeterminate |
| Austin 2004‐A (WHI trial report) | 1 | yes | 4 | 1 | indeterminate | indeterminate |
| Austin 2004‐B (ALLHAT trial report) | 1 | yes | 4 | 1 | indeterminate | indeterminate |
| Austin 2005 | 1 | yes | 4 | 1 | indeterminate | indeterminate |
| Avorn 1983 | 2 | unclear | 2 | 3 | 4 times | 4‐6 months |
| Azocar 2003 | 1; 3 | unclear | 1 | 3 | once | once |
| Barbaglia 2009 (WHI trial report) | 1 | yes | 4 | 1 | indeterminate | indeterminate |
| Bearcroft 1994 | 1 | unclear | 1 | 3 | once | once |
| Beaulieu 2004 | 4 | yes | 3 | 4 | twice | 1‐3 months |
| Bjornson 1990 | 3 | unclear | 1 | 3 | once | once |
| Black 2002 (EHC‐OM bulletin) | 4 | yes | 4 | 2 | indeterminate | indeterminate |
| Buyle 2010 | 3 | yes | 4 | 2 | indeterminate | indeterminate |
| Coopersmith 2002 | 6 | unclear | 2 | 7 | once | once |
| Denig 1990 | 5 | yes | 3 | 3 | once | once |
| Dormuth 2004 | 2 | yes | 3 | 4 | 4 times | > 6 months |
| Fijn 2000 | 4 | yes | 4 | 2 | indeterminate | indeterminate |
| Fonarow 2009 (MIRACL trial report) | 1 | yes | 4 | 1 | indeterminate | indeterminate |
| Fonarow 2009 (PROVE‐IT TIMI 22 trial report) | 1 | yes | 4 | 1 | indeterminate | indeterminate |
| Fonarow 2009 (ACC‐AHA‐STEMI guidelines) | 4 | yes | 4 | 2 | indeterminate | indeterminate |
| Fonarow 2009 (AHA‐AHA‐NS guidelines) | 4 | yes | 4 | 2 | indeterminate | indeterminate |
| Fukuda 2009 | 4 | yes | 4 | 2 | indeterminate | indeterminate |
| Guay 2007 (WHI publication) | 1 | yes | 4 | 1 | indeterminate | indeterminate |
| Haas 2004 (HERS publication) | 1 | yes | 4 | 1 | indeterminate | indeterminate |
| Haas 2004 (WHI publication) | 1 | yes | 4 | 1 | indeterminate | indeterminate |
| Hersh 2004 (WHI; HERS; HERSIII publications) | 1 | yes | 4 | 1 | indeterminate | indeterminate |
| Jackevicius 2001 | 1 | yes | 4 | 1 | indeterminate | indeterminate |
| Jameson 2010 | 4 | yes | 4 | 2 | indeterminate | indeterminate |
| Jousimaa 2002 | 4 | unclear | 4 | 3 | once | once |
| Juurlink 2004 | 1 | yes | 4 | 1 | indeterminate | indeterminate |
| Kabir 2007 (LIFE publication) | 1 | yes | 4 | 1 | indeterminate | indeterminate |
| Kabir 2007 (ALLHAT publication) | 1 | yes | 4 | 1 | indeterminate | indeterminate |
| Kabir 2007 (VALUE trial report) | 1 | yes | 4 | 1 | indeterminate | indeterminate |
| Kajita 2012 | 9 | unclear | 4 | 3 | once | once |
| Kottke 1989 | 9 | unclear | 4 | 7 | once | once |
| Lam 2009 | 1 | yes | 4 | 1 | indeterminate | indeterminate |
| Majumdar 2003 (HOPE trial report) | 1 | yes | 4 | 1 | indeterminate | indeterminate |
| Majumdar 2003 (RALES trial report) | 1 | yes | 4 | 1 | indeterminate | indeterminate |
| Majumdar 2004 (WHI trial report) | 1 | yes | 4 | 1 | indeterminate | indeterminate |
| Mason 1998 | 9 | yes | 4 | 4 | once | once |
| Mason 2001 (EHC‐OM bulletin) | 4 | yes | 4 | 2 | once | once |
| Matowe 2002 | 4 | yes | 4 | 4 | once | once |
| Meyer 2007 | 1; 3 | yes | 4 | 7 | once | once |
| Oakeshott 1994 | 4 | yes | 4 | 4 | once | once |
| Perria 2007 | 1; 3 | unclear | 4 | 3 | once | once |
| Roberts 2007 | 4 | unclear | 4 | 2 | indeterminate | indeterminate |
| Santerre 1996 | 4 | yes | 4 | 2 | indeterminate | indeterminate |
| Shah 2008 | 1 | yes | 4 | 1 | indeterminate | indeterminate |
| Stafford 2004 (ALLHAT trial report) | 1 | yes | 4 | 1 | indeterminate | indeterminate |
| Tsuji 2009 | 1 | no | 4 | 7 | once | once |
| Wang 2005 (ADA guidelines; ATP III trial report) | 4 | yes | 4 | 2 | indeterminate | indeterminate |
| Watson 2001 | 3 | no | 4 | 3 | once | once |
| Weiss 2011 | 3 | yes | 4 | 4 | once | once |
* Source of information: 1 = researchers / clinicians; 2 = university; 3 = local expert body; 4 = national professional expert body; 5 = national government expert body; 6 = local clinicians; 7 = international professional expert body; 8 = international government expert body; 9 = unclear.
¶ Tailoring: 1 = tailored to individuals based on diagnostic, behavioural, or motivational characteristics; 2 = tailored to groups of individuals; 3 = personalised, but not tailored (person's name on the information); 4 = generic; 5 = unclear.
¥ Mode of delivery: 1 = publication in peer‐reviewed journal; 2 = passive dissemination; 3 = direct mailing; 4 = mass mailing; 5 = media; 6 = hand delivery; 7 = unclear.
Three‐quarters of the studied PEMs (39/52) were endorsed, for example by a college of physicians, corporate source, or other key stakeholder. A large proportion of the endorsed PEMs (22/39) were peer‐reviewed journal publications that were considered to be endorsed.
Three PEMs were tailored to participating professionals and two were tailored to groups of individuals. Three PEMs were personalised, that is the recipient's name appeared on the printed information (Beaulieu 2004; Denig 1990; Dormuth 2004). However, most were generic, without any tailoring (44/52).
Channel
Thirty‐three PEMs were disseminated passively, of which 23 by publication in a peer‐reviewed journal (Table 2). The frequency and duration of exposure of professionals to these documents was indeterminate. Nine PEMs were disseminated actively through direct mailing, eight of which were delivered only once and the last one four times during a four‐ to six‐month period. Six PEMs were disseminated through mass mailing, with variable frequencies and durations of delivery: four were delivered once, one was delivered twice, and the other consisted in a series of evidence‐based bulletins mailed out regularly over a three‐year period. Four PEMs were delivered a single time through a mode that is unclear in the reviewed study. No PEMs were disseminated solely by electronic means, but those that were disseminated passively probably used electronic dissemination channels, such as the journal's website in the case of the articles published in scientific journals.
Message
The PEMs covered a broad range of clinical areas, including cardiovascular diseases (10 PEMs), oestrogen replacement therapy for menopausal women (eight PEMs), hypertension (five PEMs), and diabetes (four PEMs) (Table 3).
2. Characteristics of the included interventions: message.
| Study (PEM) | Clinical area | Type of targeted behaviour* | Purpose¥ | Level of evidence⇕ | Educational component ∆ |
| Austin 2003 (HERS trial report) | Oestrogen replacement therapy for menopausal women | 1 | 5 | 6 | 4 |
| Austin 2004‐A (WHI trial report) | Oestrogen replacement therapy for menopausal women | 1 | 5 | 6 | 4 |
| Austin 2004‐B (ALLHAT trial report) | Hypertension | 1 | 6 | 6 | 4 |
| Austin 2005 | Cardiovascular disease | 1 | 6 | 6 | 4 |
| Avorn 1983 | No specific clinical area | 1; 2 | 6 | 4 | 3 |
| Azocar 2003 | Depression | 1; 3; 4; 5 | 6 | 4 | 3 |
| Barbaglia 2009 (WHI trial report) | Oestrogen replacement therapy for menopausal women | 1 | 5 | 6 | 4 |
| Bearcroft 1994 | Chest radiography | 5; 6; 7 | 5 | 4 | 4 |
| Beaulieu 2004 | Stable angina | 1 | 6 | 4 | 3 |
| Bjornson 1990 | Cardiovascular disease | 1 | 6 | 6 | 4 |
| Black 2002 (EHC‐OM bulletin) | Glue ear surgery | 5; 8 | 5 | 2 | 4 |
| Buyle 2010 | Antibiotic treatment | 1; 2 | 6 | 4 | 4 |
| Coopersmith 2002 | Central venous catheter insertion | 3 | 6 | 9 | 3 |
| Denig 1990 | Antispasmodic drugs for Irritable bowel syndrome and renal colic spasms | 1 | 6 | 9 | 4 |
| Dormuth 2004 | No specific clinical area | 1 | 6 | 2 | 4 |
| Fijn 2000 | Antithrombotic therapy | 1 | 3 | 4 | 4 |
| Fonarow 2009 (MIRACL trial report) | Cardiovascular disease | 1 | 3 | 6 | 4 |
| Fonarow 2009 (PROVE‐IT TIMI 22 trial report) | Cardiovascular disease | 1 | 3 | 6 | 4 |
| Fonarow 2009 (ACC‐AHA‐STEMI guidelines) | Cardiovascular disease | 1; 3; 4; 5; 6; 7; 8; 9; 10; 11; 15 | 6 | 4 | 4 |
| Fonarow 2009 (AHA‐AHA‐NS guidelines) | Cardiovascular disease | 1; 3; 4; 5; 6; 7; 8; 9; 10; 11; 15 | 6 | 4 | 4 |
| Fukuda 2009 | Breast‐conserving surgery | 1; 8 | 3 | 4 | 4 |
| Guay 2007 (WHI publication) | Oestrogen replacement therapy for menopausal women | 1 | 5 | 6 | 4 |
| Haas 2004 (HERS publication) | Oestrogen replacement therapy for menopausal women | 1 | 5 | 6 | 4 |
| Haas 2004 (WHI publication) | Oestrogen replacement therapy for menopausal women | 1 | 5 | 6 | 4 |
| Hersh 2004 (WHI; HERS; HERSIII publications) | Oestrogen replacement therapy for menopausal women | 1 | 5 | 6 | 4 |
| Jackevicius 2001 | Cardiovascular disease | 1 | 3 | 6 | 4 |
| Jameson 2010 | Orthopaedic surgery | 1 | 3 | 4 | 4 |
| Jousimaa 2002 | Unclear | 16 | 3 | 4 | 4 |
| Juurlink 2004 | Cardiovascular disease | 1 | 3 | 6 | 4 |
| Kabir 2007 (LIFE publication) | Hypertension | 1 | 6 | 6 | 4 |
| Kabir 2007 (ALLHAT publication) | Hypertension | 1 | 6 | 6 | 4 |
| Kabir 2007 (VALUE trial report) | Hypertension | 1 | 6 | 6 | 4 |
| Kajita 2012 | Osteoporosis prevention | 3; 9; 10 | 6 | 9 | 4 |
| Kottke 1989 | Smoking cessation | 9 | 3 | 9 | 4 |
| Lam 2009 | Diabetes | 1 | 5 | 6 | 4 |
| Majumdar 2003 (HOPE trial report) | Cardiovascular disease | 1 | 3 | 6 | 4 |
| Majumdar 2003 (RALES trial report) | Cardiovascular disease | 1 | 3 | 6 | 4 |
| Majumdar 2004 (WHI trial report) | Oestrogen replacement therapy for menopausal women | 1 | 5 | 6 | 4 |
| Mason 1998 | Depression | 1 | 3 | 2 | 4 |
| Mason 2001 (EHC‐OM bulletin) | Glue ear surgery | 5; 8 | 5 | 2 | 4 |
| Matowe 2002 | X‐rays examination | 7 | 6 | 4 | 4 |
| Meyer 2007 | Pneumonia | 1 | 5 | 4 | 4 |
| Oakeshott 1994 | X‐rays examination | 6; 7 | 6 | 4 | 4 |
| Perria 2007 | Diabetes | 3 | 6 | 4 | 4 |
| Roberts 2007 | Protheses | 3 | 6 | 4 | 4 |
| Santerre 1996 | Caesarean section | 5 | 5 | 4 | 4 |
| Shah 2008 | Diabetes | 1 | 4 | 3 | 4 |
| Stafford 2004 (ALLHAT trial report) | Hypertension | 1 | 3 | 6 | 4 |
| Tsuji 2009 | Depression | 1; 4 | 3 | 4 | 3 |
| Wang 2005 (ADA guidelines; ATP III trial report) | Diabetes | 3; 12 | 3 | 4 | 4 |
| Watson 2001 | Inflammation | 1 | 3 | 4 | 4 |
| Weiss 2011 | Antibiotic treatment | 1 | 5 | 8 | 3 |
* Type of targeted behaviour: 1 = prescribing/treatment; 2 = financial (resource use); 3 = general management of a problem; 4 = diagnosis; 5 = procedures; 6 = referrals; 7 = test ordering; 8 = surgery; 9 = patient education/advice; 10 = clinical prevention service; 11 = screening; 12 = reporting; 13 = professional‐patient communication; 14 = record keeping; 15 = discharge planning; 16 = unclear.
∡ Purpose: 1 = initiation of management (e.g. introduction of new technology); 2 = stopping introduction of new management; 3 = increase of established management; 4 = cessation of established management; 5 = reduction of established management; 6 = modification of management (e.g. increased management in one activity, reduction in another).
⇕ Level of evidence: 1 = system (computerised decision support services); 2 = summary (evidence‐based textbooks); 3 = systematic review of RCTs; 4 = clinical practice guidelines developed through formal consensus process; 5 = other synthesis; 6 = original RCT; 7 = original study not RCT; 8 = expert opinion; 9 = unclear.
∆ Educational component: 1 = continuing professional development (CPD) credits to recipients of PEMs; 2 = PEM delivered within a formal education programme; 3 = clear statement in in the study that the PEM is intended for education; 4 = no clear educational component.
¶ Tailoring: 1 = tailored to individuals based on diagnostic, behavioural, or motivational characteristics; 2 = tailored to groups of individuals; 3 = personalised, but not tailored (person's name on the information); 4 = generic; 5 = unclear.
Most PEMs (38/52) targeted a single type of clinical behaviour and 13 addressed two or more behaviours. Thirty‐nine PEMs targeted providers' prescribing or treatment behaviour, eight targeted the general management of a problem, and six addressed procedures. There were five PEMs for test ordering, five regarding referrals, five directed at surgery, four targeted at patient education/advice, and four on diagnoses. Three PEMs targeted clinical prevention services; two covered screening and two discharge planning; and a single PEM was aimed at reporting.
Almost all the PEMs (51/52) were intended to modify an already established management, either to increase it (16 PEMs), to decrease it (15 PEMs), or to increase management in one activity and reduce it in another activity (20 PEMs). A single PEM was intended to cease an established practice.
The level of evidence used was clear for 48 PEMs, including 20 clinical practice guidelines developed through a formal consensus process, 22 original RCTs, four summaries, and one systematic review of RCTs. A single PEM was based on expert opinion.
Only six PEMs specified that they were intended for educational purposes; most were unclear in that respect.
Format
Twenty‐three PEMs consisted of a publication in a peer‐reviewed journal, and were thus printed in black and white with figures or tables (Table 4). Most of these (22/23) were longer than two pages: one was two pages long, and the length was not specified for one.
3. Characteristics of the included interventions: format.
| Study (PEM) | Format* | Appearance§ | Length¥ |
| Austin 2003 (HERS trial report) | 1 | 1 | 1 |
| Austin 2004‐A (WHI trial report) | 1 | 1 | 1 |
| Austin 2004‐B (ALLHAT trial report) | 1 | 1 | 1 |
| Austin 2005 | 1 | 1 | 1 |
| Avorn 1983 | 2 | 3 | 3 |
| Azocar 2003 | 2 | 3 | 2 |
| Barbaglia 2009 (WHI trial report) | 1 | 1 | 1 |
| Bearcroft 1994 | 3 | 3 | 3 |
| Beaulieu 2004 | 2 | 3 | 2 |
| Bjornson 1990 | 1 | 1 | 1 |
| Black 2002 (EHC‐OM bulletin) | 4 | 1 | 1 |
| Buyle 2010 | 4 | 3 | 3 |
| Coopersmith 2002 | 6 | 3 | 1 |
| Denig 1990 | 4 | 1 | 3 |
| Dormuth 2004 | 4 | 2 | 1 |
| Fijn 2000 | 3 | 3 | 3 |
| Fonarow 2009 (MIRACL trial report) | 1 | 1 | 1 |
| Fonarow 2009 (PROVE‐IT TIMI 22 trial report) | 1 | 1 | 1 |
| Fonarow 2009 (ACC‐AHA‐STEMI guidelines) | 3 | 1 | 1 |
| Fonarow 2009 (AHA‐AHA‐NS guidelines) | 3 | 1 | 1 |
| Fukuda 2009 | 3 | 3 | 3 |
| Guay 2007 (WHI publication) | 1 | 1 | 1 |
| Haas 2004 (HERS publication) | 1 | 1 | 1 |
| Haas 2004 (WHI publication) | 1 | 1 | 1 |
| Hersh 2004 (WHI; HERS; HERSIII publications) | 1 | 1 | 1 |
| Jackevicius 2001 | 1 | 1 | 1 |
| Jameson 2010 | 3 | 3 | 3 |
| Jousimaa 2002 | 3 | 3 | 1 |
| Juurlink 2004 | 1 | 1 | 1 |
| Kabir 2007 (LIFE publication) | 1 | 1 | 1 |
| Kabir 2007 (ALLHAT publication) | 1 | 1 | 1 |
| Kabir 2007 (VALUE trial report) | 1 | 1 | 1 |
| Kajita 2012 | 3 | 3 | 3 |
| Kottke 1989 | 5 | 3 | 1 |
| Lam 2009 | 1 | 1 | 1 |
| Majumdar 2003 (HOPE trial report) | 1 | 1 | 1 |
| Majumdar 2003 (RALES trial report) | 1 | 1 | 1 |
| Majumdar 2004 (WHI trial report) | 1 | 1 | 1 |
| Mason 1998 | 4 | 1 | 1 |
| Mason 2001 (EHC‐OM bulletin) | 4 | 1 | 1 |
| Matowe 2002 | 3 | 3 | 3 |
| Meyer 2007 | 3 | 3 | 3 |
| Oakeshott 1994 | 3 | 3 | 2 |
| Perria 2007 | 3 | 3 | 3 |
| Roberts 2007 | 3 | 1 | 1 |
| Santerre 1996 | 3 | 3 | 3 |
| Shah 2008 | 1 | 1 | 1 |
| Stafford 2004 (ALLHAT trial report) | 1 | 1 | 1 |
| Tsuji 2009 | 6 | 3 | 3 |
| Wang 2005 (ADA guidelines; ATP III trial report) | 1 | 1 | 1 |
| Watson 2001 | 3 | 3 | 3 |
| Weiss 2011 | 3 | 2 | 1 |
* Format: 1 = publication of RCT in peer‐reviewed journal; 2 = quick reference of clinical guidelines; 3 = full clinical guidelines; 4 = newsletter/bulletin; 5 = manual of peer‐reviewed clinical article reprints; 6 = other.
§ Appearance: 1 = black and white, with a few figures/tables; 2 = enhanced communication format (colour, picture, or figure); 3 = unclear.
¥ Length: 1 = more than two pages; 2 = two pages or less; 3 = unclear.
Sixteen PEMs consisted of full sets of evidence‐based guidelines, and their appearance was not specified except for four of them: three were published in black and white and the other consisted of 11 two‐page highly graphic colour‐PEM presenting with clinical information on diagnosis and recommendations on antibiotic treatments (Weiss 2011).
Six PEMs were newsletters or bulletins: four were published in black and white, one in colour, and the format of one was unclear. The coloured one consisted of 12 issues of an evidence‐based drug therapy series in an enhanced communication format named Therapeutics Letter (Dormuth 2004).
Three PEMs were brief summaries of clinical guidelines and one PEM was a black and white manual of peer‐reviewed clinical article reprints.
Excluded studies
The complete list of excluded full‐text papers assessed for eligibility can be found in Appendix 4. Among the 564 excluded studies, 381 studies were excluded due to ineligible study design, 28 studies due to ineligible study participants, 144 studies were excluded due to non‐PEM intervention, and 17 studies due to inappropriate outcomes.
Reasons for exclusion for 27 studies are found in the excluded studies table (Excluded studies). Five studies were excluded due to ineligible study design (Kulkarni 1998; Martino 2011; Mollon 2009; Morse 2009; Ozgun 2010). Five studies were excluded for not having objective outcomes (Evans 2010; Hunskaar 1996; Jackevicius 1999; Mockiene 2011; Richardson 2002). One study was excluded because pre‐intervention data was not provided (Steffensen 1997). Two studies were excluded for not having PEM as an intervention (Fontaine 2006; Perez‐Jauregui 2008). One study was excluded due to the intervention being aimed at patients rather than health care professionals (Janmeja 2009). One study was excluded because it focused on evaluating the validity of the guideline rather than the effectiveness to change professional practice (Kocher 2003). Twelve studies were excluded for not reporting data from comparison groups (Bishop 2010; Croudace 2003; Emslie 1993; Engers 2005Ferrari 2005; Hazard 1997; Jain 2006; Maiman 1988; Majumdar 2008; Mettes 2010; Schwartz 2007; Simon 2007). Six of these studies included multi‐faceted comparisons and it was difficult to determine the effectiveness of PEM (Bishop 2010; Croudace 2003; Engers 2005; Hazard 1997; Jain 2006; Mettes 2010).
Risk of bias in included studies
Among the 14 RCTs included in this review, we found the randomisation process to be appropriate in eight studies and the concealment of allocation to be appropriate in 10 studies (Figure 1). All studies, except for two, reported appropriate means to blind outcome assessment. There was a low risk of attrition bias in seven studies, but the risk was unclear in the other seven RCTs. Inappropriate protection against contamination (high risk in three studies, unclear in three studies) was the main bias for the RCTs. A potential unit of analysis error was identified in one C‐RCT in which the analyses did not account for clustering (Bearcroft 1994).
1.
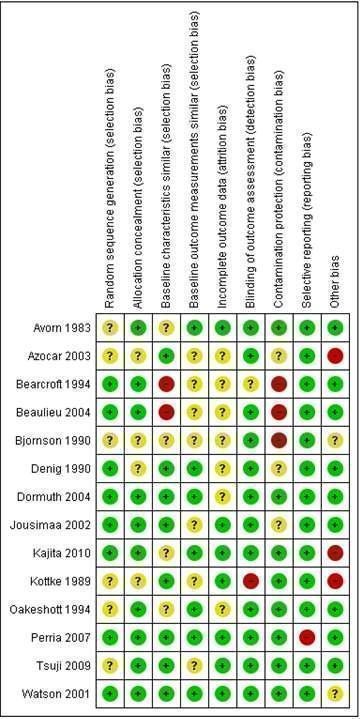
Risk of bias summary: review authors' judgements about each risk of bias item for each included RCT study.
Thirty‐one of the included studies were ITS designs. Most had low risk of the intervention affecting data collection and low risks of detection, attrition, and reporting biases (Figure 2). For four ITS studies, there were high risks that the intervention effects were affected by other changes happening at the same time as the intervention. This risk was low in four studies, but unclear in the remaining 23. The direction of the intervention effect was only specified in 10 studies. A single study scored all items appropriately (Black 2002).
2.
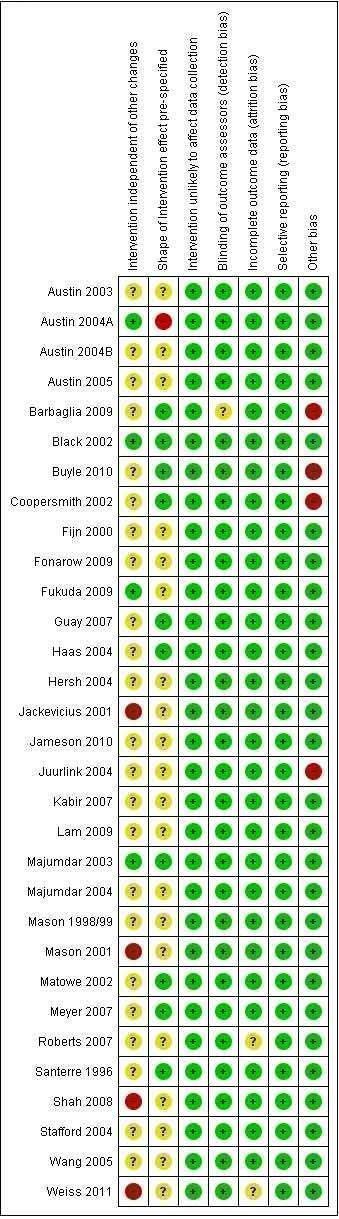
Risk of bias summary: review authors' judgements about each risk of bias item for each included ITS study.
Effects of interventions
See: Table 1
Comparison group #1: PEM only compared to no intervention
Professional practice outcomes
Seventy‐three categorical professional practice outcomes were evaluated within nine RCTs that compared PEM to no intervention. Data from seven of these studies (69 outcomes) were available for re‐analysis. The median ARD across all outcomes from these studies was 0.02 (range 0 to 0.11) (Table 5), indicating a 2% absolute improvement in professional practice in groups that received PEMs compared to groups that received no intervention. In five of the seven studies, the observed median effect was statistically significant. A unit of analysis error was observed in one study (Bearcroft 1994), and so we cannot estimate the statistical significance of the effects reported in that study. Four outcomes from three studies could not be included in this analysis because of incomplete data sets. Among these, three were reported by study authors to have improved after exposure of study participants to a PEM intervention (Beaulieu 2004; Dormuth 2004), whereas it is difficult to draw any conclusions from the report for the fourth outcome (Bjornson 1990).
4. Comparison group #1, RCT design ‐ categorical, professional practice outcomes. Standard median effect size across all studies in this table = 0.02.
| Study | Outcome | Control (n/N) | Experimental (n/N) | Absolute risk difference (95% CI)* | Standard median effect size | ||
| Pre | Post | Pre | Post | ||||
| Tsuji 2009 | Prescription of an antidepressant at the first appointment with the clinician | NA | 100/114 | NA | 119/120 | 0.11 (0.05 to 0.18) | 0.11 |
| Oakeshott 1994 | Relevant positive findings at radiology | 9/21 | 10/21 | 9/22 | 10/22 | ‐0.02 (‐0.32 to 0.28) | 0.02 |
| Radiological request forms giving physical findings | 14/21 | 12/21 | 13/22 | 13/22 | 0.02 (‐0.28 to 0.31) | ||
| Proportion of radiology requests conforming to the guidelines | 16/21 | 15/21 | 16/22 | 18/22 | 0.1 (‐0.15 to 0.36) | ||
| Bjornsson 1990 | Complete change of therapy | NA | 1/288 | NA | 4/288 | 0.01 (‐0.00 to 0.03) | 0.01 |
| Bearcroft 1994 | X‐ray requests not meeting guideline requirements∡ | NA | 87/1059 | NA | 78/1362 | 0.02 | 0.045 |
| X‐ray requests with inadequate patient history∡ | NA | 164/1059 | NA | 148/1362 | 0.05 | ||
| Recorded clinical diagnosis | NA | 454/1059 | NA | 668/1362 | 0.06 | ||
| Reported smoking history | NA | 258/1059 | NA | 382/1362 | 0.04 | ||
| Dormuth 2004 | Newly treated patients receiving the analysis drugs (metronidazole/amoxicillin or tetracycline) | 20/134,245 | 10/137,742 | 7/153,561 | 9/157,743 | 0 (‐0.00 to 0.00) | 0 |
| Newly treated patients receiving the analysis drugs (ASA/ibuprofen/naproxen) | 116/136,589 | 121/142,610 | 100/156,390 | 131/161,168 | 0 (‐0.00 to 0.00) | ||
| Newly treated patients receiving the analysis drug (isosorbide dinitrate) | 7/142,091 | 4/131,571 | 7/160,368 | 7/144,926 | 0 (‐0.00 to 0.00) | ||
| Newly treated patients receiving the analysis drug (thiazide diuretics) | 114/141,176 | 50/131,588 | 104/156,544 | 69/148,488 | 0 (‐0.00 to 0.00) | ||
| Newly treated patients receiving the analysis drug (inhaled corticosteroids) | 13/138,165 | 4/140,163 | 15/150,533 | 11/154,274 | 0 (‐0.00 to 0.00) | ||
| Newly treated patients receiving the analysis drug (calcium‐channel blockers)* | 141,107/141,176 | 131,541/131,588 | 156,457/156,544 | 148,450/148,488 | 0 (‐0.00 to 0.00) | ||
| Newly treated patients receiving the analysis drug (long‐acting benzodiazepines)* | 141,806/141,967 | 133,804/133,995 | 154,554/154,719 | 147,960/148,121 | 0 (‐0.00 to 0.00) | ||
| Newly treated patients receiving the analysis drug (hormones)* | 133,333/133,403 | 134,904/134,991 | 147,656/147,745 | 147,381/147,487 | 0 (‐0.00 to 0.00) | ||
| Newly treated patients receiving the analysis drug (calcium‐channel blockers)* | 132,461/132,512 | 139,870/139,935 | 150,298/150,358 | 152,025/152,082 | 0 (‐0.00 to 0.00) | ||
| Newly treated patients receiving the analysis drug (clonazepam/alprazolam/diazepam)* | 129,906/129,951 | 139,796/139,836 | 148,318/148,381 | 152,844/152,891 | 0 (‐0.00 to 0.00) | ||
| Newly treated patients receiving the analysis drug (finasteride)* | 136,681/136,691 | 129,769/129,775 | 152,183/152,195 | 142,379/142,392 | 0 (‐0.00 to 0.00) | ||
| Perria 2007 | Metabolic control | 196/2232 | 230/2232 | 169/2190 | 222/2190 | 0 (‐0.02 to 0.02) | 0 |
| Macrovascular complications | 244/2232 | 277/2232 | 235/2190 | 257/2190 | ‐0.01 (‐0.03 to 0.01) | ||
| Microvascular complications | 112/2232 | 105/2232 | 98/2190 | 108/2190 | 0 (‐0.01 to 0.01) | ||
| Kajita 2010 | Education on milk and dairy product ‐ young | 4/49 | 6/49 | 4/51 | 8/51 | 0.03 (‐0.10 to 0.17) | 0.04 |
| Education on milk and dairy product ‐ post | 19/49 | 20/49 | 16/51 | 26/51 | 0.1 (‐0.09 to 0.30) | ||
| Education on milk and dairy product ‐ elderly | 18/49 | 20/49 | 16/51 | 23/51 | 0.04 (‐0.15 to 0.24) | ||
| Education on soy product ‐ young | 14/49 | 20/49 | 19/51 | 27/51 | 0.12 (‐0.07 to 0.32) | ||
| Education on soy product ‐ post | 16/49 | 20/49 | 20/51 | 28/51 | 0.14 (‐0.05 to 0.33) | ||
| Education on soy product ‐ elderly | 16/49 | 20/49 | 19/51 | 26/51 | 0.1 (‐0.09 to 0.30) | ||
| Education on calcium Intake ‐ young | 25/49 | 22/49 | 22/51 | 29/51 | 0.12 (‐0.07 to 0.31) | ||
| Education on calcium intake ‐ post | 26/49 | 23/49 | 23/51 | 33/51 | 0.18 (‐0.01 to 0.37) | ||
| Education on calcium Intake ‐ Elderly | 23/49 | 21/49 | 22/51 | 29/51 | 0.14 (‐0.05 to 0.33) | ||
| Education on calcium supplement ‐ young | 0/49 | 0/49 | 0/51 | 1/51 | 0.02 (‐0.03 to 0.07) | ||
| Education on calcium supplement ‐ post | 0/49 | 0/49 | 0/51 | 2/51 | 0.04 (‐0.03 to 0.10) | ||
| Education on calcium supplement ‐ elderly | 0/49 | 0/49 | 0/51 | 2/51 | 0.04 (‐0.03 to 0.10) | ||
| Education on vitamin D intake ‐ young | 0/49 | 0/49 | 1/51 | 2/51 | 0.04 (‐0.03 to 0.10) | ||
| Education on vitamin D intake ‐ post | 0/49 | 0/49 | 1/51 | 2/51 | 0.04 (‐0.03 to 0.10) | ||
| Education on vitamin D intake ‐ elderly | 0/49 | 0/49 | 0/51 | 1/51 | 0.02 (‐0.03 to 0.07) | ||
| Education on magnesium intake ‐ young | 1/49 | 0/49 | 1/51 | 2/51 | 0.04 (‐0.03 to 0.10) | ||
| Education on magnesium intake ‐ post | 0/49 | 0/49 | 1/51 | 2/51 | 0.04 (‐0.03 to 0.10) | ||
| Education on magnesium intake ‐ elderly | 0/49 | 0/49 | 0/51 | 1/51 | 0.02 (‐0.03 to 0.07) | ||
| Education on isoflavone intake ‐ young | 2/49 | 4/49 | 3/51 | 5/51 | 0.02 (‐0.10 to 0.13) | ||
| Education on isoflavone intake ‐ post | 2/49 | 5/49 | 3/51 | 8/51 | 0.05 (‐0.08 to 0.19) | ||
| Education on brisk walking ‐ elderly | 14/49 | 10/49 | 19/51 | 25/51 | 0.29 (0.11 to 0.46) | ||
| Education on high‐impact training ‐ young | 2/49 | 4/49 | 2/51 | 10/51 | 0.11 (‐0.02 to 0.25) | ||
| Education on high‐impact training ‐ post | 2/49 | 5/49 | 2/51 | 9/51 | 0.07 (‐0.06 to 0.21) | ||
| Education on high‐impact training ‐ elderly | 2/49 | 5/49 | 2/51 | 11/51 | 0.11 (‐0.03 to 0.25) | ||
| Education on low‐impact training ‐ elderly | 4/49 | 2/49 | 8/51 | 12/51 | 0.19 (0.07 to 0.32) | ||
| Education on being active in everyday life ‐ elderly | 0/49 | 2/49 | 1/51 | 2/51 | 0 (‐0.08 to 0.08) | ||
| Education on strengthening of back muscles ‐ elderly | 0/49 | 1/49 | 2/51 | 3/51 | 0.04 (‐0.04 to 0.11) | ||
| Education on exposure to sunlight ‐ young | 6/49 | 5/49 | 4/51 | 2/51 | ‐0.06 (‐0.16 to 0.04) | ||
| Education on exposure to sunlight ‐ post | 6/49 | 4/49 | 4/51 | 2/51 | ‐0.04 (‐0.14 to 0.05) | ||
| Education on exposure to sunlight ‐ elderly | 5/49 | 4/49 | 4/51 | 2/51 | ‐0.04 (‐0.14 to 0.05) | ||
| Education on maintenance of appropriate weight ‐ young | 8/49 | 12/49 | 15/51 | 12/51 | ‐0.01 (‐0.18 to 0.16) | ||
| Education on maintenance of appropriate weight ‐ post | 8/49 | 12/49 | 14/51 | 12/51 | ‐0.01 (‐0.18 to 0.16) | ||
| Education on maintenance of appropriate weight ‐ elderly | 7/49 | 11/49 | 13/51 | 10/51 | ‐0.03 (‐0.19 to 0.13) | ||
| Education on do not start smoking ‐ young | 8/49 | 6/49 | 9/51 | 3/51 | ‐0.06 (‐0.18 to 0.05) | ||
| Education on do not start smoking ‐ post | 8/49 | 6/49 | 8/51 | 4/51 | ‐0.04 (‐0.16 to 0.07) | ||
| Education on stop smoking ‐ young | 5/49 | 2/49 | 6/51 | 4/51 | 0.04 (‐0.05 to 0.13) | ||
| Education on stop smoking ‐ post | 5/49 | 1/49 | 5/51 | 3/51 | 0.04 (‐0.04 to 0.11) | ||
| Education on stop smoking ‐ elderly | 5/49 | 1/49 | 5/51 | 3/51 | 0.04 (‐0.04 to 0.11) | ||
| Education on alcohol drinking ‐ elderly | 7/49 | 8/49 | 11/51 | 10/51 | 0.03 (‐0.12 to 0.18) | ||
| Education for elderly subjects with a history of falls ‐ elderly | 30/49 | 23/49 | 24/51 | 23/51 | 0.06 (‐0.13 to 0.26) | ||
| Education on total body exercise including balance ‐ post | 10/49 | 8/49 | 8/51 | 8/51 | 0.05 (‐0.08 to 0.19) | ||
| Education on total body exercise including balance ‐ elderly | 15/49 | 13/49 | 11/51 | 13/51 | 0.09 (‐0.07 to 0.25) | ||
| Education on modification of behaviour after examination of risk factors ‐ post | 15/49 | 10/49 | 15/51 | 10/51 | 0.03 (‐0.12 to 0.18) | ||
| Education on modification of behaviour after examination of risk factors ‐ elderly | 20/49 | 18/49 | 22/51 | 18/51 | 0.09 (‐0.09 to 0.27) | ||
| Education on environmental Improvement ‐ post | 14/49 | 10/49 | 17/51 | 10/51 | 0.03 (‐0.12 to 0.18) | ||
| Education on environmental Improvement ‐ elderly | 20/49 | 19/49 | 26/51 | 19/51 | 0.09 (‐0.10 to 0.27) | ||
| Beaulieu 2004 | Antiplatelets prescription | Quote: "we observed an overall increase of 10% in the prescribing rates for antiplatelet agents and beta blockers from 1997 to 1999, and a smaller overall increase in the prescribing rates for hypolipaemic drugs. However, for hypolipaemic drugs these increases were not distributed equally among patient age groups: greater increases were seen for patients aged greater than or equal to 70 years (Figure 2b)" (improvement) | |||||
| Hypolipaemics prescription (β‐blockers) | |||||||
| Bjornsson 1990 | Partial change of therapy | Quote: "a total of five (0.9%) of the physicians in the two groups switched their patients to both hydralazine and isosorbide (full change); another 23 (4.05) switched them to at least one of the drugs or discontinued prazosin (partial change)" (indeterminate) | |||||
| Dormuth 2004¥ | Newly treated patients receiving the analysis drug (cimetidine) | Quote: "a significant change was observed in the proportion of newly treated patients receiving the analysis drugs as first‐line therapy. The preference for the analysis drugs was 1.3 times more in the predicted direction in the intervention group of physicians than in the control group (95% confidence interval [CI] 1.13 ‐ 1.52)" (improvement) | |||||
* Results were transformed so that a positive difference in outcomes between groups could be interpreted as an improvement in outcome.
¥ Baseline measures not comparable.
∡ Confidence intervals are not included due to a unit of analysis error.
Thirteen continuous professional practice outcomes were compared to no intervention within five of the included RCTs. We had the complete data to calculate effect sizes for three studies (eight outcomes). We calculated a 0.13 improvement in the standard median effect size for these outcomes (range ‐0.16 to 0.36) (Table 6). In two of the eight outcomes the observed effect size was statistically significant. For the two other RCTs, the data set was incomplete and we were unable to re‐analyse the results ‐ study authors reported an improvement in outcomes after exposure of participants to the PEM in one instance (Avorn 1983) and no effect in the other (Azocar 2003).
5. Comparison group #1, RCT design ‐ continuous, professional practice outcomes. Standard median effect size across all studies in this table = 0.13.
| Study | Outcome | Control | Experimental | Standard effect size (95% CI)* | Standard median effect size | ||||
| N | Pre mean (SD) | Post mean (SD) | N | Pre mean (SD) | Post mean (SD) | ||||
| Denig 1990 | Antispasmodic prescription ‐ undesirable antispasmodics (IBS)* | 90 | 28.2 (31.6) | 29 (28.3) | 96 | 27.2 (38.2) | 25.6 (33.6) | 0.11 (‐0.18; 0.40) | 0.13 |
| Antispasmodic prescription ‐ all antispasmodics (IBS)* | 90 | 124.9 (88.2) | 130.4 (101.2) | 96 | 116.5 (92.7) | 115.7 (97.5) | 0.15 (‐0.14; 0.44) | ||
| Kottke 1989 | Average proportion patients asked by physicians if they smoke | 17 | NA | 51.4 (24.9) | 22 | NA | 61 (29) | ‐0.34 (‐0.29; 0.98) | 0.36 |
| Proportion of patients asked by physicians to quit smoking for each physician | 17 | NA | 39.7 (14.2) | 22 | NA | 54.9 (20) | 0.84 (0.18; 1.50) | ||
| Proportion of smoking patients who were asked to set a quit date for each physician | 17 | NA | 5.4 (17.3) | 22 | NA | 9.6 (19.5) | 0.22 (‐0.41; 0.86) | ||
| Proportion of smoking patients who were given a follow‐up appointment for each physician | 17 | NA | 3.8 (5.5) | 22 | NA | 6.9 (10.1) | 0.36 (‐0.28; 1.00) | ||
| Smoking patients who received supportive materials | 17 | NA | 10.6 (7.7) | 22 | NA | 36.4 (15.7) | 1.96 (1.18; 2.75) | ||
| Watson 2001 | Prescription of 3 recommended NSAIDS relative to total NSAID prescribing (mean in all practices) (%) | 36 | 79 (4.9) | 81.2 (3.7) | 36 | 77 (7.6) | 80.3 (7.2) | ‐0.16 (‐0.32; 0.26) | ‐0.16 |
| Avorn 1983 | Mean number of units prescribed / physician (All three drugs) | 140 | 5415 (NA) | 4921 (NA) | 132 | 5875 (NA) | 5071 (NA) | NA | NA |
| Quote: "a significant difference was found in the postintervention prescribing pattern of the face‐to‐face group as compared with those of the other physicians in the study in terms of units of medication (number of tablets or capsules) prescribed for the three target‐drugs groups" (improvement) | |||||||||
| Azocar 2003 | Guidelines adherence (Combined outpatient) | Quote: "there were no group differences in the probability of receiving medication (overall adjusted probability = 0.61), psychotherapy (overall adjusted probability = 0.49), or combined treatment (overall adjusted probability = 0.50). Given the possibility that patients received services from clinicians outside of the study, either concurrently or subsequently within the index episode, further analyses assessed the effect that guideline dissemination had on the receipt of any mental health service type. This analysis also showed no dissemination effects, indicating an equal likelihood of receiving psychotherapy, medication management, or combined outpatient care, intermediate care (eg. day treatment or residential treatment) and inpatient care within the index episode" (no effect) | |||||||
| Guideline adherence (continuation of treatment. i.e. more than 180 days of treatment) | Quote: "finally, there were no differences in the delivery of continuation treatment across dissemination group despite the fact that this practice is heavily emphasized in UBH, AHCPR, and APA treatment guidelines. Only 19% of study patients received continuation care" (no effect) | ||||||||
| Guideline adherence (documentation of a mental health or substance abuse comorbidity) | Quote: "detection of comorbid substance use disorders by study clinicians was low, with only 0.6% documenting the detection of substance abuse or dependence where actual rates are to be approximately 15%" (no effect) | ||||||||
| Guideline adherence (documentation of medical condition inducing depression) | Quote: "detection of depression due to medical problems by clinicians, using Mood Disorder Due to a Medical Condition of the Diagnostic and Statistical Manual Fourth Edition (DSM IV) diagnosis code as a proxy, also was remarkably low at 0.4%" (no effect) | ||||||||
* Results were transformed so that a positive difference in outcomes between group could be interpreted as an improvement in outcome.
We included 62 professional practice outcomes from the 31 ITS studies (Table 7). The minimum amount of observations needed for re‐analysis was missing for eight of these outcomes. Two of these missing outcomes (rates of lipid‐lowering agent use for all patients and for patients initiating treatment) came from the Fonarow 2009 study, for which we were able to re‐analyse nine other outcomes. Therefore, we were able to re‐analyse 54 outcomes from 25 studies using time series regressions. For 27 of these outcomes (from 16 ITS studies) we calculated statistically significant improvements in slopes or levels, or both, between the periods before PEM and after PEM disseminations. For 11 of these outcomes (extracted from seven ITS studies) we calculated a significant improvement in one measure and a deterioration in the other between the periods before and after the disseminations of the PEM (Austin 2003; Fonarow 2009; Haas 2004; Majumdar 2003; Mason 1998/99; Roberts 2007; Shah 2008). We found a significant deterioration in both the slope and level between the periods before and after PEM disseminations in a single outcome from the Roberts 2007 study.
6. Comparison group #1, ITS design, professional practice outcomes. Data were re‐analysed with segmented regression statistical model. P value < 0.0001:***, < 0.001: **, ≤ 0.05: *, > 0.05: NS. Standardardised median change in level across all studies in this table = 1.69.
| ID | Outcome | Change in slope (SE) | Level | ||
| Change in level of outcome (SE) | Standardised change in level of outcome (change/SE) | Median change in level | |||
| Austin 2003 | Per cent of female patients over 65 receiving ERT Rx before and after HERS study∡ | 0.18 (0.03)*** | ‐0.81 (0.27)* | ‐2.98* | 0.45 |
| Austin 2003 | Incidence of female patients over 65 receiving ERT Rx before and after HERS study∡ | 228 (24)*** | 726.92 (188)** | 3.88** | |
| Austin 2004A | Total number of claims for clonidine in Ontario for women 65 years of age and older | 6.3 (11) (NS) | 102.39 (31)* | 3.28* | 1.57 |
| Austin 2004A | Total number of claims for clonidine in Ontario for men 65 years of age and older | 5.2 (3.3) (NS) | ‐1.41 (10) (NS) | ‐0.14 (NS) | |
| Austin 2004B | Relative market share of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers∡ | ‐0.81 (0.74) (NS) | 8.89 (1.92)*** | 4.62*** | 3.23 |
| Austin 2004B | Relative market share of β‐blockers∡ | ‐0.34 (0.57) (NS) | ‐0.66 (1.65) (NS) | 0.40 (NS) | |
| Austin 2004B | Relative market share of thiazide‐type diuretics | ‐0.32 (0.39) (NS) | 10.45 (1)*** | 9.99*** | |
| Austin 2004B | Relative market share of calcium channel blockers∡ | ‐0.10 (0.44) (NS) | 2.14 (1.2) (NS) | 1.84 (NS) | |
| Austin 2005 | Statin prescribing (atorvastatin 80 mg/day) | 73 (9.6)*** | 366.28 (3) *** | 10.59*** | 5.36 |
| Austin 2005 | Statin prescribing (pravastatin 40 mg/day)∡ | ‐87 (73) (NS) | ‐41.14 (31) (NS) | 0.14 (NS) | |
| Black 2002 | Mean number of surgery per 10,000 children aged under 10 years for 13 health districts∡ | 13.9 (4.0)* | 9.89 (11.6) (NS) | 0.85 (NS) | 0.85 |
| Buyle 2010 | Monthly ratio of intravenous versus total fluoroquinolone consumption, in daily defined doses per 1000 bed days | ‐0.10 (0.19) (NS) | ‐4.95 (2.4) * | ‐2.07* | ‐2.07 |
| Coopersmith 2002 | Monthly rate per 1000 central venous catheter days of catheter‐related bloodstream infections (BSI)∡ | ‐0.01 (0.02) (NS) | 0.52 (0.24) * | 2.13* | 2.13 |
| Fonarow 2009 ‐ MIRACL | Rates (%) of lipid‐lowering agent use for all patients | ‐0.50 (0.10)*** | 3.49 (0.85)** | 4.13** | 0.91 |
| Fonarow 2009 ‐ MIRACL | Rates (%) of lipid‐lowering agent use for patients initiating treatment | ‐0.04 (0.14) (NS) | 0.69 (0.76) (NS) | 0.91 (NS) | |
| Fonarow 2009 ‐ MIRACL | Rate (%) of lipid‐lowering agent use for patent continuing treatment | 0.14 (0.15) (NS) | ‐4.02 (0.79) ** | ‐5.06** | |
| Fonarow 2009 ‐ ACC‐AHA NS | Rates (%) of lipid‐lowering agent use for all patients | 0.34 (0.08)** | 0.02 (0.52) (NS) | 0.04 (NS) | ‐1.57 |
| Fonarow 2009 ‐ ACC AHA NS | Rates (%) of lipid‐lowering agent use for patients initiating treatment | 0.23 (0.10)* | ‐1.93 (0.61)* | ‐3.18* | |
| Fonarow 2009 ‐ PROVE‐IT TIMI 22 | Rates (%) of lipid‐lowering agent use for all patients | 0.43 (0.32) (NS) | 6.24 (0.95)*** | 6.55*** | 4.63 |
| Fonarow 2009 ‐ PROVE‐IT TIMI 22 | Rate (%) of lipid‐lowering agent use for patent continuing treatment | ‐0.14 (0.37) (NS) | 5.35 (1.2)** | 4.63** | |
| Fonarow 2009 ‐ PROVE‐IT TIMI 22 | Rates (%) of lipid‐lowering agent use for patients initiating treatment | ‐0.14 (0.37) (NS) | 5.35 (1.2)** | 4.63** | |
| Fonarow 2009 ‐ ACC‐AHA STEMI Guideline | Rate (%) of lipid‐lowering agent use for patent continuing treatment | 0.23 (0.10)* | ‐1.93 (0.61)* | ‐3.18* | ‐3.18 |
| Guay 2007 | Total number of HRT prescriptions dispensed per month∡ | ‐30 (89) (NS) | ‐695.38 (193)** | 3.61** | 3.61 |
| Haas 2004 ‐ HERS study | Percentage of women reporting hormone use∡ | 0.58 (0.17)* | ‐3.95 (2.09) (NS) | ‐1.89 (NS) | ‐1.89 |
| Haas 2004 ‐ WHI study | Percentage of women reporting hormone use∡ | 1.5 (0.24)*** | 4.16 (2.0) * | 2.08* | 2.08 |
| Jackevicius 2001 | All statin prescriptions | 0.52 (0.04)*** | 2.39 (0.52) *** | 4.58*** | 4.58 |
| Jameson 2010 | Use of low‐molecular‐weight‐heparin (LMWH) | 0.63 (0.17)* | 1.92 (1.2) (NS) | 1.64 (NS) | 1.64 |
| Juurlink 2004 | Rate of spironolactone Rx | 12 (0.92)*** | 62.43 (4.4) (NS) | 14.26*** | 14.26 |
| Kabir 2007 ‐ LIFE trial | New prescriptions of atenolol | 0.07 (0.07) (NS) | ‐0.39 (0.5) (NS) | ‐0.82 (NS) | 1.60 |
| Kabir 2007 ‐ LIFE trial | New prescriptions of losartan | ‐0.04 (0.05) (NS) | 0.71 (0.2) * | 4.01* | |
| Kabir 2007 ‐ ALLHAT | New prescription of angiotensin converting enzyme (ACE) inhibitors | 0.33 (0.11)* | 0.91 (0.82) (NS) | 1.12 (NS) | 0.41 |
| Kabir 2007 ‐ ALLHAT | New prescriptions of thiazide‐type diuretics | ‐0.05 (0.11) (NS) | ‐0.22 (0.73) (NS) | ‐0.31 (NS) | |
| Kabir 2007 ‐ VALUE trial | New prescription of valsartan∡ | 0.0014 (0.05) (NS) | ‐0.01 (0.34) (NS) | ‐0.03 (NS) | ‐0.41 |
| Kabir 2007 ‐ VALUE trial | New prescriptions of calcium channel blockers | 0.14 (0.07) (NS) | ‐0.42 (0.54) (NS) | ‐0.78 (NS) | |
| Lam 2009 | Rate of statin use per 1000 diabetic haemodialysis patients | ‐8.2 (4.6) (NS) | 39.52 (22.5) (NS) | ‐1.76 (NS) | ‐1.76 |
| Majumdar 2003‐ HOPE | Number of prescriptions of ramipril in Canada | 37 (1.9)*** | ‐37.45 (6.82)* | ‐5.49* | ‐4.21 |
| Majumdar 2003‐ HOPE | Number of prescriptions of ramipril in the US | 18 (3.1)** | ‐29.25 (10)* | ‐2.92* | |
| Majumdar 2003‐ RALES | Number of prescriptions of Spironolactone in Canada | 7.83 (1.7)* | 8.16 (5.4) (NS) | 1.53 (NS) | 1.73 |
| Majumdar 2003‐ RALES | Number of prescriptions of spironolactone in the US | 5.3 (2.4)* | 14.12 (7.3) (NS) | 1.93 (NS) | |
| Majumdar 2004 | Number of prescriptions for postmenopausal hormone therapy∡ | 1.2 (2.8) (NS) | 9.87 (8.63) (NS) | 1.14 (NS) | 3.70 |
| Majumdar 2004 | Number of prescriptions for postmenopausal hormone therapy (Premarin)∡ | 0.37 (0.06)** | 0.86 (0.19)* | 4.54* | |
| Majumdar 2004 | Number of prescriptions for postmenopausal hormone therapy (Pempro)∡ | 0.33 (0.08)* | 1.40 (0.25)** | 5.53** | |
| Majumdar 2004 | Number of prescriptions for postmenopausal hormone therapy (lower dose Premarin and Pempro)∡ | ‐0.05 (0.05) (NS) | 0.15 (0.12) (NS) | 1.24 (NS) | |
| Majumdar 2004 | Number of prescription for postmenopausal hormone therapy (all other formulations)∡ | 0.44 (0.05)*** | 0.52 (0.14)* | 3.70* | |
| Mason 1998‐99 | Prescription of antidepressants (selective serotonin reuptake inhibitors, SSRIs)∡ | ‐3584 (683)*** | 4092 (3318) (NS) | 1.23 (NS) | 2.17 |
| Mason 1998‐99 | Prescription of antidepressants (tricyclic) | ‐984 (414)* | 5901 (1897) * | 3.11* | |
| Mason 2001 | Use of surgery for glue ear∡ | 0.05 (0.01)* | 0.39 (0.08) *** | 4.83*** | 4.83 |
| Matowe 2002 | Total number of imaging requests from general practice∡ | 11 (18) (NS) | ‐71.70 (162) (NS) | ‐0.44 (NS) | ‐0.44 |
| Meyer 2007 | Antimicrobial use density∡ | ‐2.15 (0.81) (NS) | 386 (123)* | 3.14* | 3.14 |
| Roberts 2007 | Rate (%) of use of uncemented prostheses∡ | ‐1.6 (0.51)* | ‐5.47 (1.8)* | ‐3.01* | ‐1.89 |
| Roberts 2007 | Rate (%) of use of hybrid prostheses of all hips implanted | 2.8 (0.77)* | ‐1.87 (2.6) (NS) | ‐0.74 (NS) | |
| Shah 2008 | Number of new users of thiazolidinedione (rosiglitazone or pioglitazone) | 28 (1.81)*** | ‐218.07 (31)*** | 6.96*** | 6.96 |
| Stafford 2004 | Number of α‐blockers prescriptions dispensed ‐ all α‐blockers (both newly dispensed and refills)∡ | 0.14 (0.01)*** | 0.06 (0.07) (NS) | 0.87 (NS) | 0.87 |
| Weiss 2011 | Monthly prescribing rates (no. of prescriptions/1000 inhabitants) for all antibiotics in Quebec relative to the rest of Canada∡ | ‐0.16 (0.10) (NS) | 2.98 (1.5) (NS) | 2.00 (NS) | 2.00 |
| Barbaglia 2009 | Prevalence of HRT use in women | Quote: "annual increases in the prevalence of HT use in all age group (Fig. 1) were found from 1998 up to 2002 when prevalence levels peaked, especially in the youngest age group (11.0%) and in the group aged 55 to 59 years (10.1%). A sudden reversal and similar progressive decrease were observed in all age groups and for all educational levels. Five years after publication of the WHI results, the prevalence of HT users was 1.2% in 50‐ to 54‐year‐old women (a decrease of 89.1% with respect to 2002; 57% within the subsequent 2 y), 1.4% (overall 87.5%; 61% in the first 2 y) in 55‐ to 59‐year‐old women, 0.6% (‐84.6%) in 60‐ to 64‐year‐old women, and 0.3% (‐66.0%) in 64‐ to 69‐year‐old women" (improvement) | |||
| Fijn 2000 | Proportion of patients newly prescribed antithrombotic therapy after having a diagnosis of ischaemic heart disease | Quote: "the introduction of national guidelines increased the chance of antithrombotic prescribing by a factor of 1.4. The present findings indicate that for about 65% of all newly diagnosed IHD patients, antithrombotics are initiated within 6 months after diagnosis" (improvement) | |||
| Fonarow 2009 ‐ ACC‐AHA STEMI Guideline | Rates (%) of lipid‐lowering agent use for all patients | Quote: "although each successive quarter and year showed an increase in lipid‐lowering medication treatment rates from 1998 to 2006, the rate of increase was larger in the earlier periods of the study (Figure 2). Although the slope differences of monthly discharge rates before and after each publication date of interest showed decreases, the greatest jump in lipid‐lowering medication use was observed in month 72, corresponding to the publication on the PROVE IT‐TIMI 22. Each publication time point of interest before month 72 showed no significant upward jumps in treatment. However, beginning with month 72, each of the 2 remaining time points demonstrated a significantly greater absolute increase in the use of lipid‐lowering agents at discharge (month 72, 6.0%, P b .0001, and month 77, 5.7%, P b .0001) than otherwise would have been expected. Multivariate logistic modeling qualitatively confirmed that the upward change in the level of medication use (or jump) at each time point was independent of other variables predictive of lipid‐lowering medication use" (improvement) | |||
| Fonarow 2009 ‐ ACC‐AHA STEMI Guideline | Rates (%) of lipid‐lowering agent use for patients initiating treatment | Quote: "most patients being prescribed lipid lowering medications at hospital discharge were newly initiated on therapy during hospitalization for AMI, and the large increase in use of lipid‐lowering medications at discharge is not merely resulting from a substantial increase in preadmission use of lipid‐lowering medications over time" (improvement) | |||
| Fukuda 2009 | Use of breast‐conserving surgery | Quote: "the proportion of BCS use increased from 26.4% before guideline publication to 59.9% after guideline publication in Japan. The percentage of patients receiving BCS almost doubled between the two time periods (P<0.001)" (improvement) | |||
| Hersh 2004 | Prescriptions of all forms of hormone therapy per year | Quote: "following the release of WHI and HERS II in July 2002, hormone therapy prescriptions declined in successive months through July 2003 (Figure 1 and FIGURE 2). Based on data from July 2003, hormone therapy prescriptions declined by 38% (95% CI, 37%‐39%) overall relative to months prior to July 2002, with a decline of 74% (95%CI, 73%‐75%) for Prempro. The percentage of women aged 50 to 74 years taking hormone therapy increased from 33% to 42% between 1995 and 2001. By July 2003, this exposure had declined to 28% of women in this age group" (improvement) | |||
| Santerre 1996 | Proportion of vaginal birth after C‐section in 55 hospitals | Quote: "the parameter estimates on the guideline binary variable in the regression models indicate that the ACOG guideline resulted in a 31% increase in the VBAC rate at the typical hospital in Massachusetts. That percentage increase amounts to a permanent 5.6 percentage point increase in the VBAC rate when evaluated at the mean VBAC rate of 17.92% for the sample" (improvement) | |||
| Wang 2005 | LDL cholesterol control for diabetes visits relative to CHD visits | Quote: "in 1995, the rate of LDL cholesterol control was 4% higher for diabetes visits than for CHD (Fig. 1B). LDL cholesterol control increased for both diseases over time (but at a slower speed for diabetes) to 44% of diabetes visits and 55% of CHD visits in 2004, with an absolute difference of 11%. Despite the publication of new guidelines in 1998 and 2001, diabetes lagged behind CHD visits in LDL cholesterol control after 1998" (no effect) | |||
∡ Results were transformed so that a positive difference in outcomes between groups could be interpreted as an improvement in outcome.
Data from these ITS studies allowed us to calculate an overall improvement in professional practice outcomes across studies immediately after the introduction of the PEM, with a standardized median change in level of 1.69 (range from ‐6.96 to +14.26). This increase in level at the time of the introduction of the PEM is a standardized value and so it has no units. Standardization was performed by dividing the change in level by the standard deviation of the change in level. In a study such as Mason 2001, where the study authors measured a standard deviation of 0.08 for the observed change in level, the 1.69 increase would represent a 0.13 increase in the number of procedures per 1000 habitants under 15 years old right after the introduction of the PEM, here a bulletin. Among the eight outcomes that we could not re‐analyse, seven were reported by study authors to have improved after exposure of the study participants to the PEMs (Barbaglia 2009; Fijn 2000; Fonarow 2009; Fukuda 2009; Hersh 2004; Santerre 1996), whereas one was reported not to have been affected by the PEM intervention (Wang 2005) (Table 7).
Patient health outcomes
A single categorical patient outcome was evaluated in an RCT (Tsuji 2009) and gave an ARD of 0.13 (Table 8), indicating an improvement of 13% in this outcome in the group exposed to the PEM only compared to the no intervention group. Another RCT reported five continuous patient health outcomes: one significantly improved while the others were not significantly changed in the group exposed to the PEM only compared to the no intervention group (Table 9). Overall, we calculated an overall median standardised effect size of ‐0.14 across these five outcomes. Two ITS studies reported four patient health outcomes, and our calculations showed an overall standardised median improvement in level of 3.79 across these four outcomes (Table 10).
7. Comparison group #1, RCT design ‐ categorical, patient outcomes.
| Study | Outcome | Control (n/N) | Experimental (n/N) | Absolute risk difference (95% CI) | ||
| Pre | Post | Pre | Post | |||
| Tsugi 2009 | Clinical remission | NA | 65/114 | NA | 84/120 | 0.13 (0.01 to 0.25) |
8. Comparison group #1, RCT design ‐ continuous, patient outcome.
| Study | Outcome | Control | Experimental | Standard effect size (95% CI) | Standard median effect size | ||||
| N | Pre mean (SD) | Post mean (SD) | N | Pre mean (SD) | Post mean (SD) | ||||
| Kottke 1989 | Proportion of patients who agreed to quit smoking for each physician | 17 | NA | 17.1 (8.1) | 22 | NA | 25.5 (12.9) | 0.74 (0.09; 1.40) | ‐0.14 |
| Proportion of patients who reported an attempt to quit smoking (more than 24 hours without smoking) | 17 | NA | 44.4 (12.6) | 22 | NA | 44 (9.6) | ‐0.04 (‐0.67; 0.60) | ||
| Duration of smoking cessation (in days) | 17 | NA | 74.2 (35.8) | 22 | NA | 66.7 (63.1) | ‐0.14 (‐0.77; 0.50) | ||
| Number of months patients have attempted to quit (patient report) | 17 | NA | 8.2 (2.0) | 22 | NA | 7.8 (1.2) | ‐0.25 (‐0.88; 0.39) | ||
| Proportion patients who reported not smoking at the time of interview for each physician | 17 | NA | 14.3 (6.5) | 22 | NA | 12 (7.4) | ‐0.32 (‐0.96; 0.32) | ||
9. Comparison group #1, ITS design, patient outcomes. Data were re‐analysed with segmented regression statistical model. P value < 0.0001:***, < 0.001: **, ≤ 0.05: *, > 0.05: NS. Standard median change in level across all studies in this table = 3.79.
| ID | Outcome | Change in slope (SE) | Level | ||
| Change in level of outcome (SE) | Standardised change in level of outcome (change/SE) | Median change in level | |||
| Jameson 2010 | Complications from hip or knee replacement surgeries (venous thromboembolic events; VTE)∡ | ‐0.01 (0.02) (NS) |
‐0.30 (0.16) (NS) | ‐1.91 (NS) | ‐1.82 |
| Jameson 2010 | Complications from hip or knee replacement surgeries (thrombocytopenia; TCP)∡ | 0.01 (0.01) (NS) | ‐0.07 (0.04) (NS) | ‐1.73 (NS) | |
| Juurlink 2004 | Rate of hospital admission for hyperkalaemia for patients with heart failure | 0.53 (0.08)*** | 3.63 (0.37)*** | 9.94*** | 9.41 |
| Juurlink 2004 | Rate of in‐hospital death owing to hyperkalaemia for heart failure patients | 0.05 (0.03) (NS) | 0.64 (0.07)*** | 8.87*** | |
∡ Results were transformed so that a positive difference in outcomes between groups could be interpreted as an improvement in outcome.
Comparison group #2: PEM only versus single intervention
A single RCT compared a group exposed to a PEM (paper‐based guidelines) to a group exposed to another type of intervention (computerised guidelines). This study measured nine categorical professional outcomes, none of which showed significant changes between groups. The standardised median ARD across all these outcomes was ‐0.02 (Table 11).
10. Comparison group #2, RCT design ‐ categorical, professional practice outcomes.
| Study | Outcome | Control (n/N) | Experimental (n/N) | Absolute risk difference (95% CI) | Standard median effect size | ||
| Pre | Post | Pre | Post | ||||
| Jousimaa 2002 | Proportion of consultation decision compliant with guidelines (laboratory examinations) | NA | 1372/1529 | NA | 1481/1640 | 0.01 (‐0.02 to 0.03) | ‐0.02 |
| Proportion of consultation decision compliant with guidelines (radiological examinations) | NA | 1416/1518 | NA | 1504/1604 | 0 (‐0.01 to 0.02) | ||
| Proportion of consultation decision compliant with guidelines (physical examinations) | NA | 1461/1545 | NA | 1494/1610 | ‐0.02 (‐0.03 to ‐0.00) | ||
| Proportion of consultation decision compliant with guidelines (other examinations) | NA | 248/307 | NA | 235/314 | ‐0.06 (‐0.12 to 0.01) | ||
| Proportion of consultation decision compliant with guidelines (procedures) | NA | 140/171 | NA | 152/196 | ‐0.04 (‐0.13 to 0.04) | ||
| Proportion of consultation decision compliant with guidelines (physiotherapy) | NA | 83/103 | NA | 77/98 | ‐0.02 (‐0.13 to 0.09) | ||
| Proportion of consultation decision compliant with guidelines (non‐pharmacological treatments) | NA | 110/122 | NA | 80/92 | ‐0.03 (‐0.12 to 0.05) | ||
| Proportion of consultation decision compliant with guidelines (pharmacological treatment) | NA | 1350/1568 | NA | 1391/1654 | ‐0.02 (‐0.04 to 0.00) | ||
| Proportion of consultation decision compliant with guidelines (referrals) | NA | 1508/1578 | NA | 1619/1684 | 0.01 (‐0.01 to 0.02) | ||
Effects modifiers
We prepared box plots to explore whether various PEM characteristics might influence their effectiveness to change professional practice. Visual inspection of these graphs suggests that some characteristics may have more potential to influence effectiveness. For example, we observed that effectiveness varied more among the following categories: source of information (Figure 3), tailoring (Figure 4), clinical areas (Figure 5), type of targeted behaviour (Figure 6), purpose (Figure 7), level of evidence (Figure 8), and format (Figure 9). Visual inspection of the bar graphs also suggested that PEMs' effectiveness does not vary much depending on the mode, frequency, or duration of delivery (Figure 10; Figure 11; Figure 12, respectively). Some potential effect modifiers did not vary across the studied PEMs. For instance, most of the PEMs were endorsed (Figure 13), most did not specify any educational component (Figure 14), they were generally all black and white with a few figures and tables (appearance, Figure 15), and most were longer than two pages (length, Figure 16). This lack of variability prevents any conclusion on the importance of these characteristics to determine PEMs' effectiveness. For RCT studies, there were often only one or two studies in each category of effect modifier (Figure 3; Figure 13; Figure 4; Figure 10; Figure 11; Figure 12; Figure 5; Figure 6; Figure 7; Figure 8; Figure 14; Figure 9; Figure 15; Figure 16). For example, there is a range of clinical areas that are covered by the included PEMs, but only a few studies tested PEMs in each clinical area (Figure 5). For ITS studies, PEMs were more homogenous and many studies were thus grouped within the same characteristic with only a few studies that tested PEMs with different characteristics (Figure 3; Figure 13; Figure 4; Figure 10; Figure 11; Figure 12; Figure 5; Figure 6; Figure 7; Figure 8; Figure 14; Figure 9; Figure 15; Figure 16; ). For example, almost all the PEMs tested with ITS study designs were tailored (Figure 4).
3.
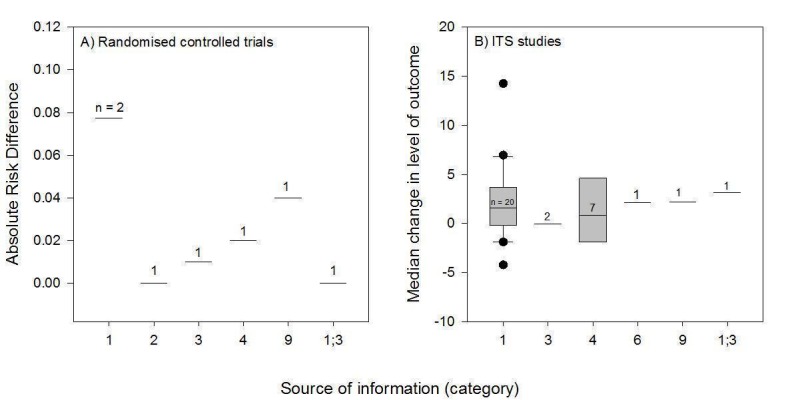
Potential effect modifier ‐ source of information. Legend: 1 = researchers/clinicians; 2 = university; 3 local expert body; 4 = national professional expert body; 5 = national government expert body; 6 = local clinicians; 7 = international expert body; 8 = international government expert body; 9 = unclear.
4.
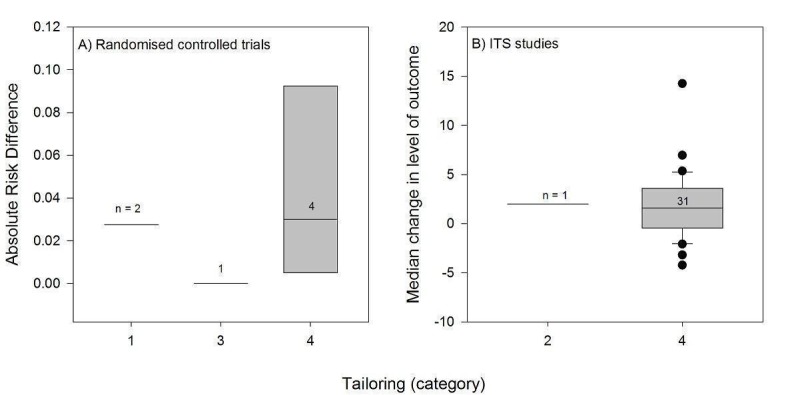
Potential effect modifier ‐ tailoring. Legend: 1 = tailored to individuals based on diagnostic, behavioural, or motivational characteristics; 2 = tailored to groups of individuals; 3 = personalised, but not tailored (person's name on the information); 4 = generic; 5 = unclear.
5.
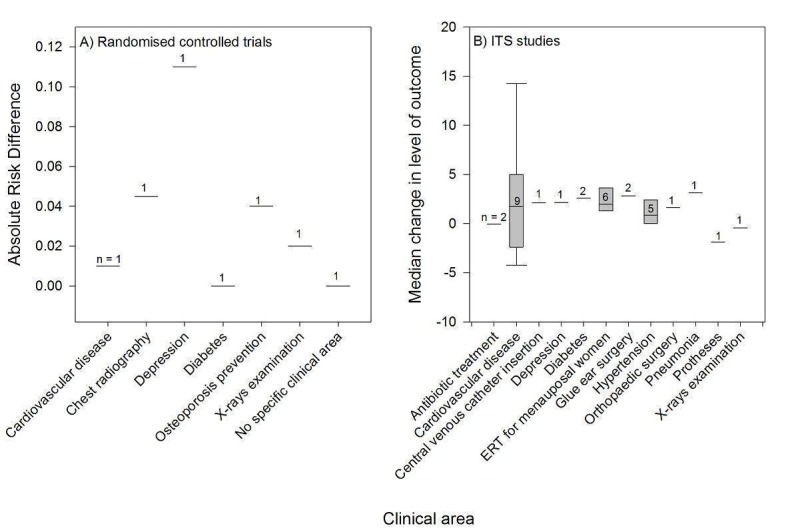
Potential effect modifier ‐ clinical area. Legend: ERT = Oestrogen‐replacement therapy.
6.
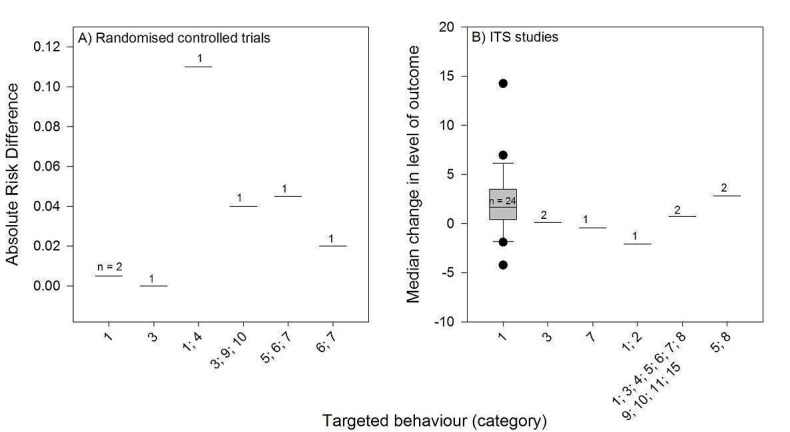
Potential effect modifier ‐ type of targeted behaviour. Legend: 1 = prescribing/treatment; 2 = financial (resource use); 3 = general management of a problem; 4 = diagnosis; 5 = procedures; 6 = referrals; 7 = test ordering; 8 = surgery; 9 = patient education/advice; 10 = clinical prevention service; 11 = screening; 12 = reporting; 13 = professional‐patient communication; 14 = record keeping; 15 = discharge planning; 16 = unclear.
7.
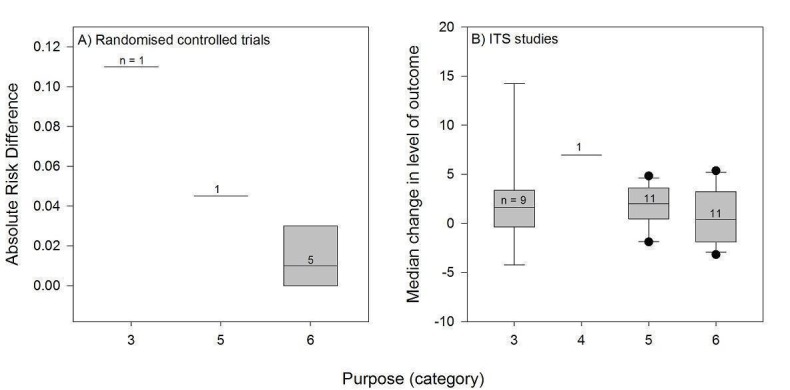
Potential effect modifier ‐ purpose. Legend: 1 = initiation of management (e.g. introduction of new technology); 2 = stopping introduction of new management; 3 = increase of established management; 4 = cessation of established management; 5 = reduction of established management; 6 = modification of management (e.g. increased management in one activity, reduction in another).
8.
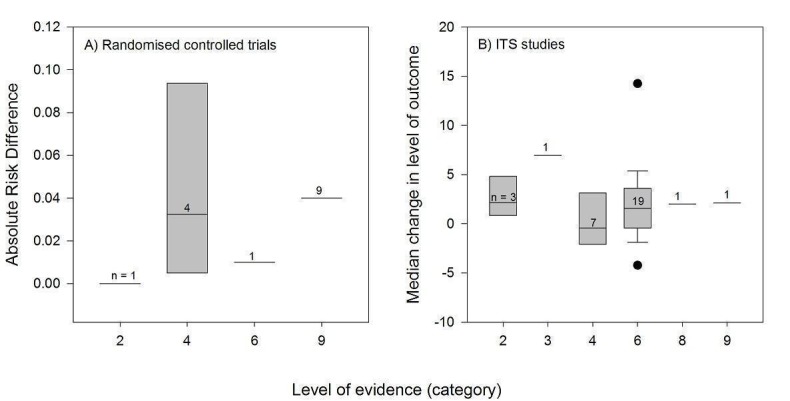
Potential effect modifier ‐ Level of evidence. Legend: 1 = system (computerised decision support); 2 = summaries (evidence‐based textbook); 3 = systematic review of RCTs; 4 = clinical practice guidelines developed through formal consensus process; 5 = other synthesis; 6 = original RCT; 7 = original studies not RCT; 8 = expert opinion; 9 = unclear.
9.
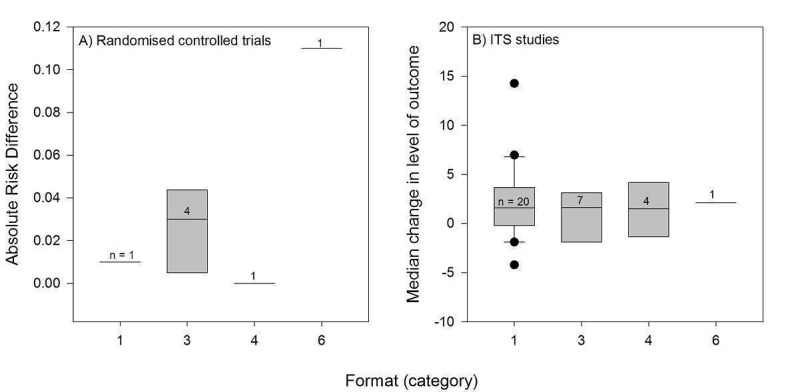
Potential effect modifier ‐ format. Legend: 1 = publication of RCT results in peer‐reviewed journal; 2 = quick reference of clinical guidelines; 3 = full clinical guidelines; 4 = newsletter or bulletin; 5 = manual of peer‐reviewed clinical article reprints; 6 = other.
10.
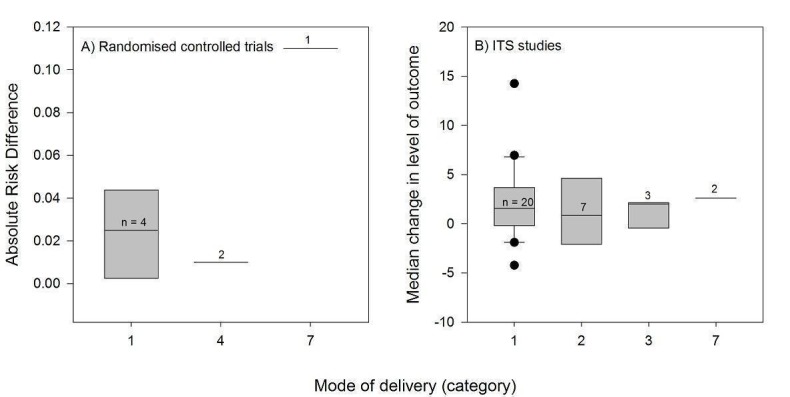
Potential effect modifier ‐ Mode of delivery. Legend: 1 = publication in peer‐reviewed journal; 2 = passive dissemination; 3 = direct mailing; 4 = mass mailing; 5 = media; 6 = hand delivery; 7 = unclear
11.
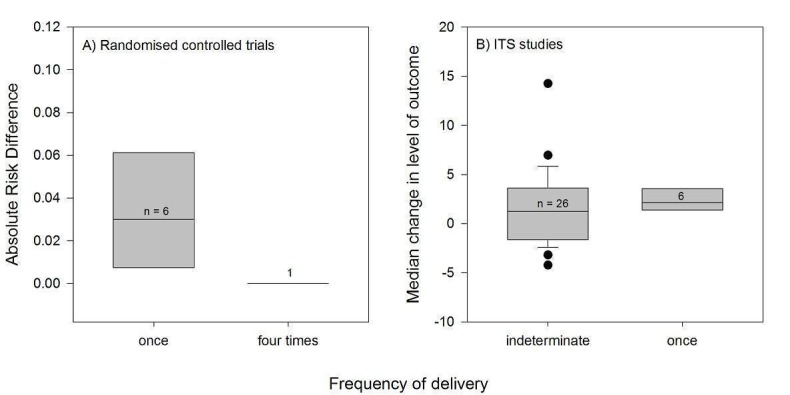
Potential effect modifier ‐ frequency of delivery (once, twice, 3 times, more than 3 times, indeterminate).
12.
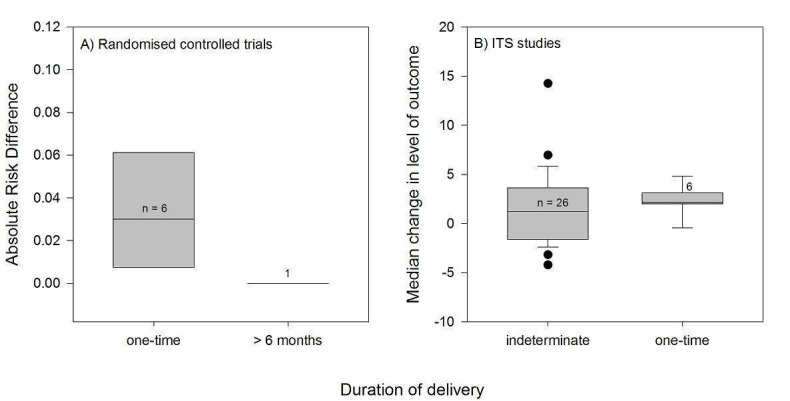
Potential effect modifier ‐ duration of delivery (once, 1‐3 months, 4‐6 months, over 6 months, indeterminate).
13.
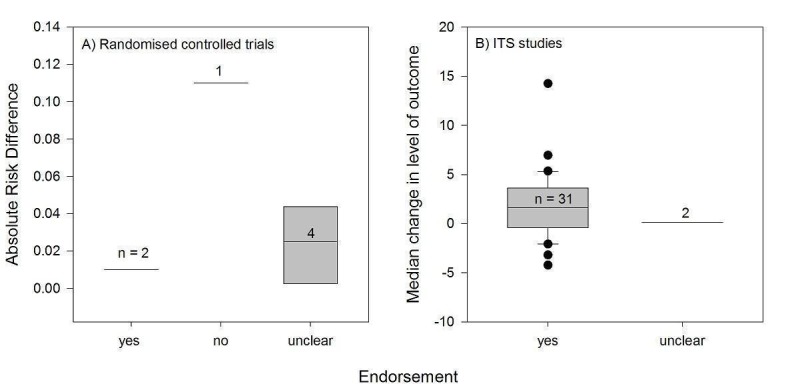
Potential effect modifier ‐ endorsement (yes, no, unclear).
14.
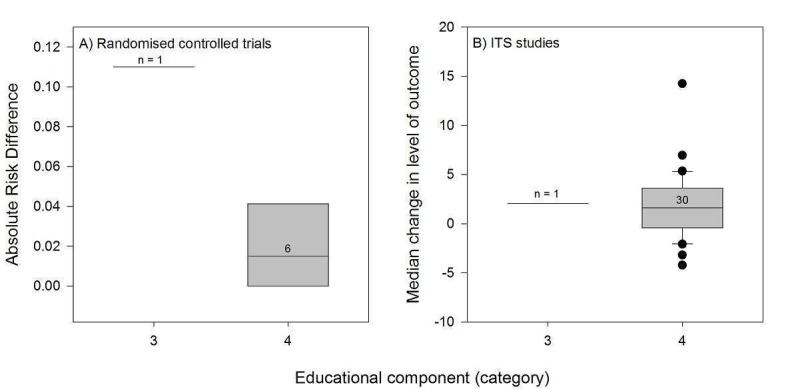
Potential effect modifier ‐ educational component. Legend: 1 = continuing professional development (CPD) credits to recipients of PEMs; 2 = PEM delivered within a formal education programme; 3 = clear statement in the study that the PEM is intended for education; 4 = no clear educational component.
15.
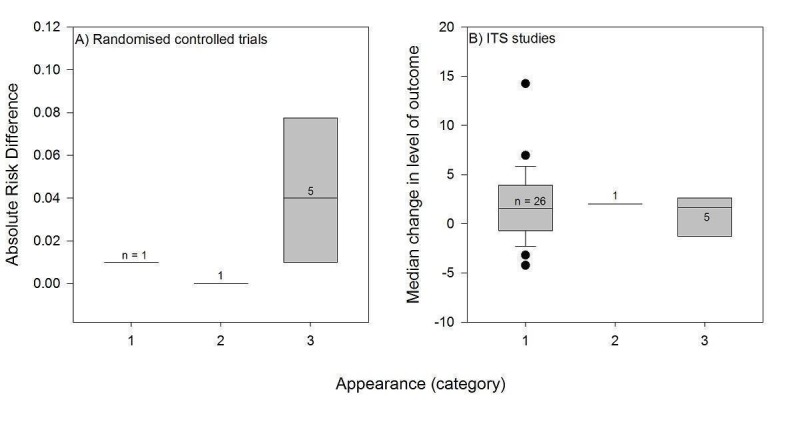
Potential effect modifier ‐ appearance. Legend: 1 = black and white, with a few figures or tables; 2 = enhanced communication format (colour, picture, or figure); 3 = unclear.
16.
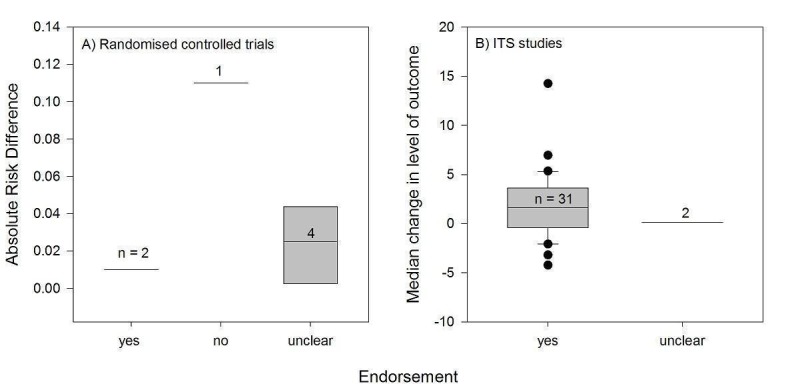
Potential effect modifier ‐ endorsement (yes, no, unclear).
Discussion
Summary of main results
Overall, the results of this review suggest that when used alone, and compared to a 'no intervention' control, PEMs can have a small beneficial effect on professional practice outcomes. Various measures of effectiveness have been gathered through this review process, and although some analyses present limitations regarding internal or external validity, overall they make a good case that PEMs can have an impact on professional practice.
More weight should be given to the data collected from the 14 RCTs. For categorical outcomes, the overall effect size (ARD 0.02) was derived from a larger set of studies (seven studies) and outcomes (69 outcomes). Thus, we can have more confidence in the median effect size estimated from this larger data set. These results are somewhat reflected in the descriptive results from the RCTs that could not be re‐analysed: study authors reported improvements in three out of the five studies. For continuous outcomes, re‐analysis allowed calculating a relatively important standardised effect size of 0.13, but it was derived from only three studies. When taken individually, continuous outcomes did not show consistent statistical differences among them (two out of eight were statistically significant).
Data collected from ITS studies represent a larger data set that is prone to important risks of bias, especially since the studies were conducted retrospectively, often without pre‐specifying the expected effect of the intervention. Nevertheless, results from these studies were consistent across studies and support the conclusions gathered from more robust study designs, that PEMs can change professional practice outcomes. Results of ITS were more positive that results of RCTs, which could be a function of the design, or that different types of PEMs are being evaluated in different designs. For instance, ITS are more likely to be evaluated in peer‐review publications, and are prone to more important publication bias as high‐profile papers are likely to be chosen.
Because we cannot ascribe CIs to the observed effect sizes, we cannot rule out the possibility that these effects might have occurred by chance alone. Clinical significance of the observed effect sizes is unknown, but they fall below the range of effects of other quality improvement systematic reviews that reported median effect sizes ranging from 0.04 to 0.09 for categorical professional outcomes and from 10% to 16% for continuous outcomes (Flodgren 2011; Forsetlund 2009; Jamtvedt 2006; O'Brien 2007; Shojania 2010). Clinical significance of the observed 13% improvement in continuous outcomes may be easier to judge if, for instance, we consider the results of Denig 1990: an 11% improvement corresponded in this case to a change from 27 defined daily doses (DDDs) of undesirable antispasmodic per 1000 prescriptions before the PEM delivery to 26 DDDs/1000 prescriptions after the PEM delivery.
Insufficient information was collected to make a conclusion on effectiveness of PEMs to improve patient outcomes.
A few characteristics of the PEMs seem promising to increase their impact on professional practice, but the limited number of studies prevents any conclusion. These findings are exploratory should be interpreted with caution.
Overall completeness and applicability of evidence
Although PEMs were distributed to many types of healthcare professionals, participants in the included studies were generally physicians (90% physicians only, 6% mix of health professionals, 4% unclear).Therefore, the findings of our review need to be confirmed for other types of professionals. The included studies were performed in developed countries (almost all in North America and Europe), primarily in outpatient practices and in some hospitals. The applicability of the observed results to other settings is unknown.
Compared to previous reviews of clinical practice guidelines (Grimshaw 2004), we have included more diverse types of PEMs, including full clinical guidelines, guideline summaries, publications in peer‐reviewed journals, bulletins, or newsletters. Therefore, our results can be generalised to a broader category of PEMs. More studies are needed to draw conclusions on many of the potential effect modifiers that we have decided to study. For instance, most PEMs were are not explicit about their educational intent, so it is difficult from the set of included studies to evaluate whether an intervention developed specifically as educational would be more efficient.
Even though PEMs are often used as an add‐on to a single or multifaceted intervention, no evidence can be used to support this practice as we were not able to find any studies comparing the addition of a PEM to another intervention compared to the intervention alone. To improve the applicability of this review, we chose to exclude the many studies that compared multifaceted intervention including PEMs to a 'no intervention' control, as these comparisons do not allow isolation of the 'PEM effect' from the effect of the other interventions.
We did not restrict our review to specific outcomes or clinical areas, allowing for a greater number of included studies. Thus, were able to review and pool a relatively wide variety of professional practice outcomes. This breadth of outcomes also allows for generalisability to any clinical situation. However, a relatively small number of studies looked at patient outcomes. Our inclusion criteria (any objective measure of professional practice or patient outcomes) also led to the exclusion of many educational interventions that are typically evaluated with non‐clinical outcomes (e.g. knowledge, attitudes). The benefits of PEMs should be interpreted in the context of their costs and span of coverage. Unfortunately, no studies undertook a formal economic evaluation of the effects of the PEMs.
The level and slope estimates were evaluated with time series analyses, from a limited number of data points considering that this type of analysis would be best performed with a minimum of 50 to 100 data points (Chatfield 2001; Lagarde 2012). Thus, the pooling of level differences is also prone to substantial imprecision.
Quality of the evidence
The methodological quality of the 14 RCTs was variable; the proportion of quality criteria met varied from two to eight out of nine. The items 'Random sequence generation' and 'Allocation concealment' were evaluated as having unclear or high risk of bias in 43% and 36% of studies, respectively, resulting in risks of selection bias for these studies, and possibly leading to an overestimation of effects (Wood 2008). This is likely to be a consequence of the randomisation issues, as only four RCTs reported comparable baseline data. As we included only objective outcomes, it was to be expected that most studies would have blinded assessment of outcomes (12 of 14 outcomes). The completeness of outcome data was unclear in many RCTs (seven of 14 RCTs), which is likely to be because of the inclusion of older trials published before the CONSORT statement, and these can often make study interpretation difficult (Higgins 2011). To limit this potential attrition bias, we were able to get additional information by contacting the authors directly. Most of the reviewed RCTs were clustered (nine of 14 RCTs), avoiding contamination problems so that changes in the comparison group could be more dependably ascribed to the intervention effect. However, the risk of contamination bias was uncertain in two C‐RCTs, and one C‐RCT did not take into account clustering in analysis potentially leading to a unit of analysis error.
Inclusion of ITS studies allows considerably more experimental studies to be reviewed, with the drawback and challenge of having to weight methodological quality in the review conclusions. As mentioned earlier, ITS studies are conducted retrospectively, often without pre‐specifying the expected effect of the intervention, or acknowledging the presence of a secular trend. It is still important to include these studies since finding an equivalent control group of practitioners who is not exposed can be challenging when recommendations are disseminated widely on a national level ‐ or when consensus recommendations are directed at the entire population of practitioners (Kanouse 1995). We avoided the problem of inappropriate analyses in reviewed ITS studies by re‐analysing all the results using times series regressions (Ramsay 2003).
Quality assessment items were not consistently described in all the included studies, suggesting that there remains room for improvement in the level of reporting on quality assessment criteria in publications.
Potential biases in the review process
Our approach focused on the observed effect sizes and does not consider statistical significance or weight by study size. However, it provides information on the effect size of the intervention, which is more informative than the vote counting approach. It is also possible that our review suffered from publication bias, so the reader should consider the possibility that we are overestimating the effectiveness of the intervention.
We were often limited by missing information from the primary studies. For instance, frequency of the PEM delivery was generally not reported in primary studies, and the messages and formats of the PEMs were not clearly and consistently described across the primary literature. To complete the missing information, we attempted to obtain a copy of the actual PEM tested within each study; despite our best efforts, we were not able to obtain copies of all the PEMs and some information remained missing.
Agreements and disagreements with other studies or reviews
The findings from this review differ from the last update of this Cochrane review that concluded that "PEMs when used alone may have a beneficial effect on process outcomes but not on patient outcomes" (Farmer 2008). Inclusion of more studies in the present update may have led to more conservative estimates of effect. The results are not yet stable and further research might be needed. Before this review, Grimshaw and colleagues (Grimshaw 2004) had conducted the most comprehensive systematic review on the effectiveness of guideline dissemination and implementation strategies. Their review results concur with the present work, as they found that PEMs have a moderate effect across health conditions.
Authors' conclusions
Implications for practice.
PEMs are a commonly used method of disseminating information to healthcare professionals. They can be distributed to large numbers of healthcare professionals and are relatively inexpensive. Studies of the effects of PEMs generally show modest, but potentially important, improvements in professional practice. Only a few studies have shown small deteriorations of uncertain clinical significance. Those interested in using PEMs should be aware of the potentially small effects and limitations of the current evidence. Further, there is preliminary evidence about how to optimise educational materials.
Implications for research.
Authors of future primary studies are encouraged to provide a detailed description of the PEM studied and to publish it with their report to allow further message and format analysis. This would allow for replication, comparison across studies, and more robust analyses of effect modifiers. Future studies should also consider evaluating head‐to‐head comparisons of PEMs with different characteristics.
More PEM versus control two‐arm studies are needed to obtain a more definite answer on the effectiveness of PEMs to improve professional practice. More research is also required to address the effectiveness of PEMs on improving patient outcomes.
Studies should be sufficiently powered to detect smaller effects.
Quasi‐experimental designs such as ITS may increasingly be used for evaluating PEMs and other interventions for change in healthcare practice, given their low cost, convenience, and value for informing policy decisions. However, it is important that appropriate statistical methods be used to analyse time series data, preferably time series regression models.
In many studies, PEMs serve as a control group rather than an intervention of interest, or some studies used PEMs alongside other interventions for investigating additive effects of interventions. Future intervention studies examining the effect of PEMs should consider the impact of PEMs on their own.
Economic evaluations of PEMs are needed. Future studies should provide information about the resources required for development, dissemination, and implementation of PEMs (Grimshaw 2004).
We chose to describe some PEMs' characteristics that may have affected their effectiveness, based on broader categories of the persuasive communication theory (source, channel, and message). However, each of these characteristics could only be evaluated in a limited number of included studies. This prevented any conclusion on the relative importance of these potential effect modifiers to improve professional practice, and calls for more research on the characteristics of PEM that truly lead to a change in behaviour.
What's new
| Date | Event | Description |
|---|---|---|
| 6 March 2015 | Amended | Standard median effect size range corrected in the summary of findings table |
History
Protocol first published: Issue 3, 2003 Review first published: Issue 3, 2008
| Date | Event | Description |
|---|---|---|
| 2 April 2013 | Amended | Edits to contact details |
| 10 September 2012 | New search has been performed | Review has been updated |
| 10 September 2012 | New citation required but conclusions have not changed | New authors, now has 45 studies. |
| 16 June 2011 | Amended | Minor edits |
| 18 February 2009 | Amended | Minor edits |
| 12 November 2008 | Amended | Minor changes |
| 23 April 2008 | Amended | Converted to new review format. |
Notes
This review replaces the reviews that is now withdrawn by Freemantle et al (Freemantle 1997) and is an update of the review by Farmer et al (Farmer 2008).
The first version of the review (Freemantle 1997) considered the following comparisons: (1) PEMs against a non‐intervention control and (2) multifaceted intervention plus PEMs versus PEMs alone. In the subsequent version (Farmer 2008), we modified the proposed comparisons to separate the effect of PEM from the effect of other interventions. We thus do not include any more studies that compare PEMs with PEMs as part of a multifaceted intervention, but will compare PEMs as part of a multifaceted intervention versus multifaceted interventions not including PEMs.
In the present update of the review, we excluded three studies that had been previously included because they compared PEM as part of a multifaceted intervention to a control condition, which is not one of the studied comparisons. Croudace 2003 compared PEM plus educational meeting to usual care, and Hazard 1997 compared PEM plus reminder at the point of care to the delayed intervention. Also, two reports that were included as distinct studies in the previous version of the review have been included as two reports of the same study in this version (Avorn 1983). The CBA that had been included in both the previous updates of this review (Steffensen 1997) was removed because of a lack of pre‐intervention data.
Acknowledgements
We would like to acknowledge the contributions of all the people who helped during the review process: Ms. Laurie Carignan and Ms. Michele Shemilt contributed to study selection and data extraction, Mr. Lawrence Ndoh and Ms. Yasmin Banu contributed to data extraction, Ms. Sandy Gill and Ms. Norma Brown contributed to risk of bias evaluations, Ms. Angela Barbara contributed to data analysis and writing of the first draft, Mr. Jamie O'Donnell contributed to data extraction, risk of bias evaluation, data analysis and helped coordinate the study. We would like to acknowledge the help of Dr Robby Nieuwlaat for translation of articles. We would like to acknowledge the help from the librarians at the Centre Hospitalier Saint‐François d'Assise, Ms. Michelle Petitclerc and Mrs. Jacinte Biron. We would also like to acknowledge the authors from previous versions of the review: Dr. Lisa Bero, Dr. Nick Freemantle, Dr. Andy Oxman, Ms. Lucile Turcot, Dr. Emma Harvey, and Dr Jessie McGowan. We would like to thank the peer reviewers, and EPOC editors who provided input, particularly Dr. Gillian Leng who was the referee editor for this review.
A special thank you is extended to the primary study authors who responded to our requests for more information: Dr. Francisca Azocar, Dr. Marie‐Dominic Beaulieu, Kim Gans, Dr. Rowland Hazard, Dr. John Holden, Dr. Eileen Kaner, Dr. Thomas Kottke, Dr. Karmeen Kulkarni, Dr. Sumit Majumdar, Dr. James Mason, Dr. Sujivia Ratnaike, Dr. Stephen Soumerai, Dr. Peter Austin, Dr. Francesc Macià, Dr. Franky Buyle, Dr. Gregg Fonarow, Dr Simon Jameson, Dr. Zubair Kabir, Dr Kathleen Bennett, Dr. Amit Garg, Dr. Sumit Majumdar, Dr. Elisabeth Meyer, Dr. Baiju Shah, and Dr. Karl Weiss.
Thank you to Dr. Craig Ramsay for helpful comments on the statistical analysis of the ITS studies.
Appendices
Appendix 1. Search strategies 2011
MEDLINE(R) <1948 to present> 1 (guideline? and (impact or influence)).ti. (638) 2 (impact and guideline?).ti. (532) 3 (effect$ and guideline?).ti. (886) 4 (impact and bulletin?).ti. (7) 5 (impact and publication?).ti. (97) 6 (impact and disseminat$).ti. (75) 7 (guideline and (notification or notify$)).ti. (2) 8 (publication and evidence).ti. (48) 9 (guideline? and disseminat$).ti. (109) 10 drug utilization/ and publication.ti,ab. (93) 11 education, dental, continuing/ or education, medical, continuing/ or education, nursing, continuing/ or education, pharmacy, continuing/ (41329) 12 11 and patient education as topic/ (940) 13 *Physician's practice patterns/ and *practice guidelines as topic/ (1011) 14 *Family practice/ and *practice guidelines as topic/ (456) 15 *primary health care/ and *practice guidelines as topic/ (345) 16 publication.ti. and physician's practice patterns/ (34) 17 (publication and (influenc$ or impact or chang$ or prescribing or physician? behavio?r?)).ti. (141) 18 publication.ti. and practice guidelines as topic/ (60) 19 or/1‐10,12‐18 (4514) 20 19 not "publication bias".ti. [Strategy A ] (4492) 21 print$ education$.ti,ab. (86) 22 ((print or printed) adj2 intervention?).ti,ab. (60) 23 ((allied health$ or counsel?or? or doctor? or nurse or nurses or physician? or physiotherapist? or therapist? or dentist? or pharmacist? or health$ worker? or health$ staff) adj2 (pamphlet? or booklet? or poster? or brochure? or written material? or printed or print)).ti,ab. (93) 24 paper‐based education$.ti,ab. (4) 25 (postal adj4 guideline?).ti,ab. (19) 26 (spiral bound or bound copy or bound copies).ti,ab. (10) 27 or/21‐26 [Strategy B: Keyword‐‐screen without filters] (265) 28 education, dental, continuing/ or education, medical, continuing/ or education, nursing, continuing/ or education, pharmacy, continuing/ (41329) 29 (continuing adj (medical or nursing or pharma$ or dental$ or physician? or doctor? or surg$) adj2 education$).ti,ab. (3981) 30 (continuing education$ adj2 (medical or nursing or pharma$ or dental$ or physician? or doctor? or surg$)).ti,ab. (743) 31 CME.ti,ab. (3055) 32 or/28‐31 [Continuing Medical Education CME] (44488) 33 education, professional/ or education, continuing/ or education, professional, retraining/ (9345) 34 ((train$ or educat$) adj2 (clinical competenc$ or practitioner? or practice? or general practi$ or family doctor?)).ti,ab. (9123) 35 Education Department, Hospital/ (209) 36 (continuing adj2 education$).ti,ab. (13514) 37 (professional adj2 (development$ or education$ or retrain$ or skill? enhanc$ or (skill? adj2 improv$) or training or upgrade? or upgrading)).ti,ab. (8465) 38 (professional adj2 (education$ or training)).ti,ab. (4836) 39 or/33‐38 [Cont Education General/Professional Dev General] (37565) 40 exp Physicians/ or exp Nurses/ or "Internship and Residency"/ or Preceptorship/ or Clinical Competence/ (205019) 41 (exp Allied Health Personnel/ not Animal Technicians/) or (exp Health Occupations/ not exp Veterinary Medicine/) (1174652) 42 exp Medical Staff/ or exp Nursing Staff/ or Pharmacists/ or Laboratory Personnel/ or exp Dentists/ or exp Dental Staff/ (93842) 43 exp Health Facility Administrators/ (9604) 44 (counsel?or? or dental aide or dental aides or dental hygienist? or dentist? or dietetic? or dietician? or doctor? or general practitioner? or health$ professional? or hospitalist? or medical aide? or medical aides or medical technician? or nurse or nurses or nutritionist? or orthodontist? or pediatric$ or paediatric$ or pharmacist? or physician? or physiotherapist? or psychiatrist? or psychiatric? aide or psychiatric aides or psychologist? or practitioner? or rheumatologist? or surgeon? or therapist?).ti. (330556) 45 (internship? or intern? or resident? or residency or residencies).ti. (25238) 46 or/40‐45 [Health Professionals] (1516683) 47 ((print or printed or paper) adj2 (display? or document? or education$ material? or format? or portfolio or material? or media or medium? or workshop? material?)).ti,ab. (2704) 48 ((print or printed) adj5 (format or formats)).ti,ab. (128) 49 (printed adj4 (diagram? or text)).ti,ab. (117) 50 (paper adj5 format?).ti,ab. (298) 51 (book? or booklet? or brochure? or bulletin? or handout? or hand‐out? or "hard copy" or hardcopy or "hard copies" or hardcopies or monograph$ or paper‐based or "paper copy" or "paper copies" or print‐based or pamphlet? or poster?).ti,ab. (31033) 52 (written material? or written teaching or written learning).ti,ab. (669) 53 (mail$ adj2 (information or guideline? or publication? or protocol? or practice guideline or therap$ guideline? or prescrib$ guideline or article or articles or research or result? or study or studies or journal? or copy or copies)).ti,ab. (716) 54 exp books/ or manuals as topic/ or reference books/ or textbooks as topic/ or broadsides as topic/ or pamphlets/ (21585) 55 posters as topic/ (33) 56 or/47‐55 [Print Materials ] (53646) 57 Guideline adherence/ (14605) 58 ((guideline? or best practice? or evidence or EBM) adj2 (adher$ or apply$ or application or disseminat$ or implement$ or introduc$ or publication or release or uptake)).ti,ab. (9552) 59 ((publication or published) adj2 (guideline? or protocol?)).ti,ab. (4002) 60 or/57‐58 [GL Adherence] (22663) 61 Guidelines as Topic/ or Practice guidelines as Topic/ (87965) 62 exp Evidence‐based practice/ (45475) 63 (evidence based adj2 (practice? or practitioner? or medicine or medical or treatment? or therap$ or nurse or nurses or nursing or dentist$ or healthcare or care)).ti,ab. (16859) 64 (applied learning or knowledge transfer$ or knowledge translation).ti,ab. (928) 65 or/62‐64 [Evidence Based Medicine/Knowledge transfer‐translation‐‐EBM/KT] (53167) 66 exp patient care management/ or comprehensive health care/ or critical pathways/ or "delivery of health care"/ or "delivery of health care, integrated"/ or health care reform/ or dentist's practice patterns/ or disease management/ or medication reconciliation/ or medication therapy management/ or nurse's practice patterns/ or patient care team/ or nursing, team/ or patient‐centered care/ or "quality of health care"/ (467245) [Patient Care] 67 (randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti. (713091) 68 exp animals/ not humans.sh. (3598690) 69 "comment on".cm. or systematic review.ti. or literature review.ti. or editorial.pt. or meta‐analysis.pt. or news.pt. or review.pt. [This line is not found in Cochrane Handbook; added by TSC to exclude irrelevant publication types] (2404378) 70 67 not (or/68‐69) [Cochrane RCT Filter 6.4.d Sens/Precision Maximizing] (560727) 71 intervention?.ti. or (intervention? adj6 (clinician? or collaborat$ or community or complex or DESIGN$ or doctor? or educational or family doctor? or family physician? or family practitioner? or financial or GP or general practice? or hospital? or impact? or improv$ or individuali?e? or individuali?ing or interdisciplin$ or multicomponent or multi‐component or multidisciplin$ or multi‐disciplin$ or multifacet$ or multi‐facet$ or multimodal$ or multi‐modal$ or personali?e? or personali?ing or pharmacies or pharmacist? or pharmacy or physician? or practitioner? or prescrib$ or prescription? or primary care or professional$ or provider? or regulatory or regulatory or tailor$ or target$ or team$ or usual care)).ab. (114937) 72 (hospital$ or patient?).hw. and (study or studies or care or health$ or practitioner? or provider? or physician? or nurse? or nursing or doctor?).ti,hw. (614765) 73 demonstration project?.ti,ab. (1704) 74 (pre‐post or "pre test$" or pretest$ or posttest$ or "post test$" or (pre adj5 post)).ti,ab. (47930) 75 (pre‐workshop or post‐workshop or (before adj3 workshop) or (after adj3 workshop)).ti,ab. (434) 76 trial.ti. or ((study adj3 aim?) or "our study").ab. (447008) 77 (before adj10 (after or during)).ti,ab. (299382) 78 ("quasi‐experiment$" or quasiexperiment$ or "quasi random$" or quasirandom$ or "quasi control$" or quasicontrol$ or ((quasi$ or experimental) adj3 (method$ or study or trial or design$))).ti,ab,hw. [ML] (82551) 79 ("time series" adj2 interrupt$).ti,ab,hw. [ML] (598) 80 (time points adj3 (over or multiple or three or four or five or six or seven or eight or nine or ten or eleven or twelve or month$ or hour? or day? or "more than")).ab. (6197) 81 pilot.ti. (29268) 82 Pilot projects/ [ML] (65924) 83 (clinical trial or controlled clinical trial or multicenter study).pt. [ML] (560102) 84 (multicentre or multicenter or multi‐centre or multi‐center).ti. (22040) 85 random$.ti,ab. or controlled.ti. (590536) 86 (control adj3 (area or cohort? or compare? or condition or design or group? or intervention? or participant? or study)).ab. not (controlled clinical trial or randomized controlled trial).pt. [ML] (322561) 87 "comment on".cm. or review.ti,pt. or randomized controlled trial.pt. [ML] (2454941) 88 (rat or rats or cow or cows or chicken? or horse or horses or mice or mouse or bovine or animal?).ti. (1215743) 89 exp animals/ not humans.sh. [ML] (3598690) 90 *experimental design/ or *pilot study/ or quasi experimental study/ [EM] (16605) 91 ("quasi‐experiment$" or quasiexperiment$ or "quasi random$" or quasirandom$ or "quasi control$" or quasicontrol$ or ((quasi$ or experimental) adj3 (method$ or study or trial or design$))).ti,ab. [EM] (82551) 92 ("time series" adj2 interrupt$).ti,ab. [EM] (598) 93 (or/71‐86) not (or/87‐89) [EPOC Methods Filter ML 1.9] (1714043) 94 or/71‐77,80‐81,84‐85,88,90‐92 [EPOC Methods Filter EM 1.9‐2.3] (3103200) Combinations 95 32 and 56 [CME & Print] (750) 96 (39 and 46 and 56) not 95 [Print & CE & Health Professionals] (248) 97 (56 and 60) not (or/95‐96) [Print & GL Adherence] (383) 98 (56 and 61) not (or/95‐97) [Print & GL as Topic] (933) 99 (56 and 65) not (or/95‐98) [Print and EBM/KT] (476) 100 (56 and 66 and 46) not (or/95‐99) [Print & Patient Care & Health Professionals] (1598) 101 (or/95‐100) not 27 [Strategy C] (4337) Results 102 (20 or 101) and 70 [RCT results Strategies A, C] (727) 103 (101 and 93) not (or/69,102) [EPOC results Strategies A, C] (1265) 104 or/21‐26 [Strategy B: Keyword‐‐screen without filters] (265)
Ovid HealthStar <1999 to May 2011> Strategy was identical to that for OVID MEDLINE; number of results are below. 95 32 and 56 [CME & print] (359) 96 (39 and 46 and 56) not 95 [CE & health pro & print] (145) 97 (56 and 60) not (or/95‐96) [print & GL adherence] (307) 98 (56 and 61) not (or/95‐97) [print & GL as topic] (159) 99 (56 and 65) not (or/95‐98) [print and EBM/KT] (176) 100 (56 and 66 and 46) not (or/95‐99) [print & pt care & health pro] (1053) 101 (or/95‐100) not 27 [results before filters] (2166) 102 ((20 or 101) and 70) [RCT results, Strategies A, C ] (353) 103 (101 and 93) not (or/69,102) [EPOC results, Strategies A, C] (711) 104 or/21‐26 [KW screen without filters] (161)
EMBASE Classic + EMBASE (OVID) <1947 to 2011 June 10> 1 print$ education$.ti,ab. (97) 2 ((print or printed) adj2 intervention?).ti,ab. (62) 3 ((allied health$ or counsel?or? or doctor? or nurse or nurses or physician? or physiotherapist? or therapist? or dentist? or pharmacist? or health$ worker? or health$ staff) adj2 (pamphlet? or booklet? or poster? or brochure? or written material? or printed or print)).ti,ab. (115) 4 paper‐based education$.ti,ab. (2) 5 (postal adj4 guideline?).ti,ab. (46) 6 or/1‐5 [KW screen without filters] (313) 7 (continuing adj (medical or nursing or pharma$ or dental$ or physician? or doctor? or surg$) adj2 education$).ti,ab. (5103) 8 (continuing education$ adj2 (medical or nursing or pharma$ or dental$ or physician? or doctor? or surg$)).ti,ab. (873) 9 CME.ti,ab. (5190) 10 or/7‐9 [CME] (10058) 11 *vocational education/ (4170) 12 continuing education/ (24423) 13 ((train$ or educat$) adj2 (clinical competenc$ or practitioner? or practice? or general practi$ or family doctor?)).ti,ab. (10677) 14 (continuing adj2 education$).ti,ab. (16727) 15 (professional adj2 (development$ or education$ or retrain$ or skill? enhanc$ or (skill? adj2 improv$) or training or upgrade? or upgrading)).ti,ab. (10595) 16 (professional adj2 (education$ or training)).ti,ab. (6130) 17 or/11‐16 [CE general] (59211) 18 *residency education/ or *clinical competence/ (20700) 19 exp *physician/ or exp *paramedical personnel/ or exp *dentistry/ or exp *preventive dentistry/ or *dental surgery/ or *medical staff/ (313208) 20 exp *nursing discipline/ or *nursing/ or exp *nurse/ (157423) 21 *optometry/ or *podiatry/ or *medical psychology/ or *serology/ (11643) 22 *psychiatry/ or *child psychiatry/ (41816) 23 (counsel?or? or dental aide or dental aides or dental hygienist? or dentist? or dietetic? or dietician? or doctor? or general practitioner? or health$ professional? or hospitalist? or medical aide? or medical aides or medical technician? or nurse or nurses or nutritionist? or orthodontist? or pediatric$ or paediatric$ or pharmacist? or physician? or physiotherapist? or psychiatrist? or psychiatric? aide or psychiatric aides or psychologist? or practitioner? or rheumatologist? or surgeon? or therapist?).ti. (387185) 24 (internship? or intern? or resident? or residency or residencies).ti. (28985) 25 or/18‐24 [health professionals] (766023) 26 ((print or printed or paper) adj2 (display? or document? or education$ material? or format? or portfolio or material? or media or medium? or workshop? material?)).ti,ab. (3403) 27 ((print or printed) adj5 (format or formats)).ti,ab. (163) 28 (printed adj4 (diagram? or text)).ti,ab. (124) 29 (paper adj5 format?).ti,ab. (377) 30 (book? or booklet? or brochure? or bulletin? or handout? or hand‐out? or "hard copy" or hardcopy or "hard copies" or hardcopies or monograph$ or paper‐based or "paper copy" or "paper copies" or print‐based or pamphlet? or poster?).ti,ab. (46075) 31 (written material? or written teaching or written learning).ti,ab. (810) 32 (mail$ adj2 (information or guideline? or publication? or protocol? or practice guideline or therap$ guideline? or prescrib$ guideline or article or articles or research or result? or study or studies or journal? or copy or copies)).ti,ab. (1275) 33 *medical illustration/ (1657) 34 *book/ (4738) 35 or/26‐34 [Print material KW & SH] (56537) 36 ((guideline? or best practice? or evidence or EBM) adj2 (adher$ or apply$ or application or disseminat$ or implement$ or introduc$ or publication or release or uptake)).ti,ab. (12014) 37 ((publication or published) adj2 (guideline? or protocol?)).ti,ab. (5244) 38 or/36‐37 [GL adherence] (16761) 39 exp *evidence based practice/ (27747) 40 (evidence based adj2 (practice? or practitioner? or medicine or medical or treatment? or therap$ or nurse or nurses or nursing or dentist$ or healthcare or care)).ti,ab. (20366) 41 (applied learning or knowledge transfer$ or knowledge translation).ti,ab. (1026) 42 or/39‐41 [EBM/KT] (41720) 43 *patient care/ (35908) 44 exp *nursing assessment/ (10475) 45 *patient care planning/ or *primary health care/ or *progressive patient care/ or *health care delivery/ or *integrated health care system/ or *health care policy/ or *disease management/ or *managed care/ or *medication therapy management/ or *patient selection/ or *health care quality/ or *rapid response team/ or *clinical pathways/ (173413) 46 or/43‐45 [patient care] (214605) 47 intervention?.ti. or (intervention? adj6 (clinician? or collaborat$ or community or complex or DESIGN$ or doctor? or educational or family doctor? or family physician? or family practitioner? or financial or GP or general practice? or hospital? or impact? or improv$ or individuali?e? or individuali?ing or interdisciplin$ or multicomponent or multi‐component or multidisciplin$ or multi‐disciplin$ or multifacet$ or multi‐facet$ or multimodal$ or multi‐modal$ or personali?e? or personali?ing or pharmacies or pharmacist? or pharmacy or physician? or practitioner? or prescrib$ or prescription? or primary care or professional$ or provider? or regulatory or regulatory or tailor$ or target$ or team$ or usual care)).ab. (140986) 48 (hospital$ or patient?).hw. and (study or studies or care or health$ or practitioner? or provider? or physician? or nurse? or nursing or doctor?).ti,hw. (1201558) 49 demonstration project?.ti,ab. (2043) 50 (pre‐post or "pre test$" or pretest$ or posttest$ or "post test$" or (pre adj5 post)).ti,ab. (63052) 51 (pre‐workshop or post‐workshop or (before adj3 workshop) or (after adj3 workshop)).ti,ab. (533) 52 trial.ti. or ((study adj3 aim?) or "our study").ab. (570480) 53 (before adj10 (after or during)).ti,ab. (385889) 54 ("quasi‐experiment$" or quasiexperiment$ or "quasi random$" or quasirandom$ or "quasi control$" or quasicontrol$ or ((quasi$ or experimental) adj3 (method$ or study or trial or design$))).ti,ab,hw. [ML] (123459) 55 ("time series" adj2 interrupt$).ti,ab,hw. [ML] (680) 56 (time points adj3 (over or multiple or three or four or five or six or seven or eight or nine or ten or eleven or twelve or month$ or hour? or day? or "more than")).ab. (7492) 57 pilot.ti. (36582) 58 Pilot projects/ [ML] (46805) 59 (clinical trial or controlled clinical trial or multicenter study).pt. [ML] (0) 60 (multicentre or multicenter or multi‐centre or multi‐center).ti. (28288) 61 random$.ti,ab. or controlled.ti. (710884) 62 (control adj3 (area or cohort? or compare? or condition or design or group? or intervention? or participant? or study)).ab. not (controlled clinical trial or randomized controlled trial).pt. [ML] (467730) 63 "comment on".cm. or review.ti,pt. or randomized controlled trial.pt. [ML] (1865950) 64 (rat or rats or cow or cows or chicken? or horse or horses or mice or mouse or bovine or animal?).ti. (1487767) 65 exp animals/ not humans.sh. [ML] (1668982) 66 *experimental design/ or *pilot study/ or quasi experimental study/ [EM] (3285) 67 ("quasi‐experiment$" or quasiexperiment$ or "quasi random$" or quasirandom$ or "quasi control$" or quasicontrol$ or ((quasi$ or experimental) adj3 (method$ or study or trial or design$))).ti,ab. [EM] (106818) 68 ("time series" adj2 interrupt$).ti,ab. [EM] (680) 69 (or/47‐62) not (or/63‐65) [EPOC Methods Filter ML 1.9] (2652569) 70 or/47‐53,56‐57,60‐61,64,66‐68 [EPOC Methods Filter EM 1.9‐2.3] (4156041) 71 controlled clinical trial/ or controlled study/ or randomized controlled trial/ [EM] (3590624) 72 (book or conference paper or editorial or letter or review).pt. not randomized controlled trial/ [Per BMJ Clinical Evidence filter] (3504869) 73 (random sampl$ or random digit$ or random effect$ or random survey or random regression).ti,ab. not randomized controlled trial/ [Per BMJ Clinical Evidence filter] (38712) 74 (animal$ not human$).sh,hw. (3563374) 75 71 not (or/72‐74) [Trial filter per BMJ CLinical Evidence] (2332014) 76 10 and 35 [CME & print] (177) 77 (17 and 25 and 35) not 76 [CE & health pro & print] (205) 78 (35 and 38) not (or/76‐77) [print & guideline adherence] (264) 79 (35 and 42) not (or/76‐78) [print & EBM] (413) 80 (35 and 46 and 25) not (or/76‐79) [print & patient care & health pro] (191) 81 (or/76‐80) not 6 [results excluding Keyword results] (1227) 82 guideline? and (impact or influence)).ti. (770) 83 (impact and guideline?).ti. (639) 84 (effect$ and guideline?).ti. (1033) 85 (impact and bulletin?).ti. (11) 86 (impact and publication?).ti. (116) 87 (impact and disseminat$).ti. (91) 88 (guideline and (notification or notify$)).ti. (2) 89 (publication and evidence).ti. (51) 90 (guideline? and disseminat$).ti. (123) 91 (publication and (influenc$ or impact or chang$ or prescribing or physician? behavio?r?)).ti. (177) 92 *drug utilization/ and publication.ti,ab. (22) 93 *clinical practice/ and *practice guidelines/ (892) 94 publication.ti. and *clinical practice/ (15) 95 publication.ti. and *practice guidelines/ (100) 96 *general practice/ and *practice guidelines/ (328) 97 *primary health care/ and *practice guidelines/ (221) 98 or/82‐97 [] (3675) 99 ((81 or 98) and 75) [RCT results] (685) 100 (81 and 70) not 102 [EPOC Filter results] (485) 101 or/1‐5 [Keyword Results] (313)
The Cochrane Library(Wiley) Search Date: 10 June 2011#1 (print* education*):ti or (print* education*):ab #2 ((print or printed) NEAR/2 intervention):ti,ab #3 ((allied health* or counsellor or counselor or doctor or nurse or nurses or physician or physiotherapist or therapist or dentist or pharmacist or health* worker or health* staff) NEAR/2 (pamphlet or booklet or poster or brochure or written material or printed or print)):ti,ab #4 paper‐based education*:ti,ab #5 (postal near/4 guideline):ti,ab #6 (#1 OR #2 OR #3 OR #4 OR #5) #7 MeSH descriptor Education, Dental, Continuing, this term only #8 MeSH descriptor Education, Medical, Continuing, this term only #9 MeSH descriptor Education, Nursing, Continuing, this term only #10 MeSH descriptor Education, Pharmacy, Continuing, this term only #11 (continuing NEXT (medical or nursing or pharma* or dental* or physician or doctor or surg*) NEAR/2 education*):ti,ab #12 (continuing education* NEAR/2 (medical or nursing or pharma* or dental* or physician or doctor or surg*)):ti,ab #13 CME:ti,ab. #14 (#7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13) #15 MeSH descriptor Education, Professional, this term only #16 MeSH descriptor Education, Continuing, this term only #17 MeSH descriptor Education, Professional, Retraining, this term only #18 ((train* or educat*) near/2 (clinical competenc* or practitioner or practice or general practi* or family doctor)):ti,ab #19 MeSH descriptor Education Department, Hospital, this term only #20 (continuing near/2 education*):ti,ab #21 (professional NEAR/2 (development* or education* or retrain* or skill enhanc* or (skill near/2 improv*) or training or upgrade or upgrading)):ti,ab #22 (professional near/2 (education* or training)):ti,ab #23 (#15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22) #24 MeSH descriptor Physicians explode all trees #25 MeSH descriptor Nurses explode all trees #26 MeSH descriptor Internship and Residency, this term only #27 MeSH descriptor Preceptorship, this term only #28 MeSH descriptor Clinical Competence, this term only #29 MeSH descriptor Allied Health Personnel explode all trees #30 MeSH descriptor Animal Technicians explode all trees #31 (#29 AND NOT #30) #32 MeSH descriptor Health Occupations explode all trees #33 MeSH descriptor Veterinary Medicine explode all trees #34 (#32 AND NOT #33) #35 MeSH descriptor Medical Staff explode all trees #36 MeSH descriptor Nursing Staff explode all trees #37 MeSH descriptor Pharmacists, this term only #38 MeSH descriptor Laboratory Personnel, this term only #39 MeSH descriptor Dentists explode all trees #40 MeSH descriptor Dental Staff explode all trees #41 MeSH descriptor Health Facility Administrators explode all trees #42 (counsellor or counselor or dental aide or dental aides or dental hygienist or dentist or dietetic or dietician or doctor or general practitioner or health* professional or hospitalist or medical aide or medical aides or medical technician or nurse or nurses or nutritionist or orthodontist or pediatric* or paediatric* or pharmacist or physician or physiotherapist or psychiatrist or psychiatric aide or psychiatric aides or psychologist or practitioner or rheumatologist or surgeon or therapist):ti #43 (internship or intern or resident or residency or residencies):ti #44 (#24 OR #25 OR #26 OR #27 OR #28 OR #31 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43) #45 ((print or printed or paper) near/2 (display or document or education* material or format or portfolio or material or media or medium or workshop material)):ti,ab #46 ((print or printed) near/5 (format or formats)):ti,ab #47 (printed near/4 (diagram or text)):ti,ab #48 (paper near/5 format):ti,ab #49 (book or booklet or brochure or bulletin or handout or hand‐out or "hard copy" or hardcopy or "hard copies" or hardcopies or monograph* or paper‐based or "paper copy" or "paper copies" or print‐based or pamphlet or poster):ti,ab #50 (written material or written teaching or written learning):ti,ab #51 (mail* near/2 (information or guideline or publication or protocol or practice guideline or therap* guideline or prescrib* guideline or article or articles or research or result or study or studies or journal or copy or copies)):ti,ab #52 MeSH descriptor Books explode all trees #53 MeSH descriptor Manuals as Topic, this term only #54 MeSH descriptor Reference Books, this term only #55 MeSH descriptor Textbooks as Topic, this term only #56 MeSH descriptor Broadsides as Topic, this term only #57 MeSH descriptor Pamphlets, this term only #58 MeSH descriptor Posters as Topic, this term only #59 (#45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58) #60 MeSH descriptor Guideline Adherence, this term only #61 ((guideline or best practice or evidence or EBM) near/2 (adher* or apply* or application or disseminat* or implement* or introduc* or publication or release or uptake)):ti,ab #62 ((publication or published) near/2 (guideline or protocol)):ti,ab #63 (#60 OR #61 OR #62) #64 MeSH descriptor Guidelines as Topic, this term only #65 MeSH descriptor Practice Guidelines as Topic, this term only #66 (#64 OR #65) #67 MeSH descriptor Evidence‐Based Practice explode all trees #68 (evidence based near/2 (practice or practitioner or medicine or medical or treatment or therap* or nurse or nurses or nursing or dentist* or healthcare or care)):ti,ab #69 (applied learning or knowledge transfer* or knowledge translation):ti,ab #70 (#67 OR #68 OR #69) #71 MeSH descriptor Patient Care Management explode all trees #72 MeSH descriptor Quality of Health Care, this term only #73 (#71 OR #72) #74 (#14 AND #59) #75 (#23 AND #44 AND #59) #76 (#59 AND #63) #77 (#59 AND #66) #78 (#59 AND #70) #79 (#59 AND #73 AND #44) #80 (( #74 OR #75 OR #76 OR #77 OR #78 OR #79 ) AND NOT #6)
CAB Abstracts (via CAB Direct) Search date: 14 June 2011S# Query & results S81 S53 and S80 13 S80 S75 or S76 or S77 or S78 or S79 337884 S79 TI (( “control* N1 clinical” or “control* N1 group*” or “control* N1 trial*” or “control* N1 study” or “control* N1 studies” or “control* N1 design*” or “control* N1 method*” ))or AB (( “control* N1 clinical” or “control* N1 group*” or “control* N1 trial*” or “control* N1 study” or “control* N1 studies” or “control* N1 design*” or “control* N1 method*” ))114905 S78 TI controlled or AB controlled 104753 S77 TI random* or AB random* 162227 S76 TI (( “clinical study” or “clinical studies” ))or AB (( “clinical study” or “clinical studies” ))12941 S75 TI ((multicent* n2 design*) or (multicent* n2 study) or (multicent* n2 studies) or (multicent* n2 trial*))or AB ((multicent* n2 design*) or (multicent* n2 study) or (multicent* n2 studies) or (multicent* n2 trial*))5402 S74 S53 and S73 31 S73 S54 or S55 or S56 or S57 or S58 or S59 or S60 or S61 or S62 or S63 or S64 or S65 or S66 or S67 or S68 or S69 or S70 or S71 or S72 461900 S72 TI ((time points n3 over) or (time points n3 multiple) or (time points n3 three) or (time points n3 four) or (time points n3 five) or (time points n3 six) or (time points n3 seven) or (time points n3 eight) or (time points n3 nine) or (time points n3 ten) or (time points n3 eleven) or (time points n3 twelve) or (time points n3 month*) or (time points n3 hour*) or (time points n3 day*) or (time points n3 "more than"))or AB ((time points n3 over) or (time points n3 multiple) or (time points n3 three) or (time points n3 four) or (time points n3 five) or (time points n3 six) or (time points n3 seven) or (time points n3 eight) or (time points n3 nine) or (time points n3 ten) or (time points n3 eleven) or (time points n3 twelve) or (time points n3 month*) or (time points n3 hour*) or (time points n3 day*) or (time points n3 "more than"))1219 S71 TI ((control w3 area) or (control w3 cohort*) or (control w3 compar*) or (control w3 condition) or (control w3 group*) or (control w3 intervention*) or (control w3 participant*) or (control w3 study))or AB ((control w3 area) or (control w3 cohort*) or (control w3 compar*) or (control w3 condition) or (control w3 group*) or (control w3 intervention*) or (control w3 participant*) or (control w3 study))87090 S70 TI ((multicentre or multicenter or multi‐centre or multi‐center))or AB ((multicentre or multicenter or multi‐centre or multi‐center))7471 S69 TI random* OR controlled 22117 S68 TI ((trial or (study n3 aim) or "our study"))or AB ((trial or (study n3 aim) or "our study"))171932 S67 TI ((pre‐workshop or preworkshop or post‐workshop or postworkshop) or (before n3 workshop) or (after n3 workshop))or AB ((pre‐workshop or preworkshop or post‐workshop or postworkshop) or (before n3 workshop) or (after n3 workshop))8874 S66 TI ((demonstration project OR demonstration projects OR preimplement* or pre‐implement* or post‐implement* or postimplement*))or AB ((demonstration project OR demonstration projects OR preimplement* or pre‐implement* or post‐implement* or postimplement*))840 S65 TX (intervention n6 clinician*) or (intervention n6 community) or (intervention n6 complex) or (intervention n6 design*) or (intervention n6 doctor*) or (intervention n6 educational) or (intervention n6 family doctor*) or (intervention n6 family physician*) or (intervention n6 family practitioner*) or (intervention n6 financial) or (intervention n6 GP) or (intervention n6 general practice*) Or (intervention n6 hospital*) or (intervention n6 impact*) Or (intervention n6 improv*) or (intervention n6 individualize*) Or (intervention n6 individualise*) or (intervention n6 individualizing) or (intervention n6 individualising) or (intervention n6 interdisciplin*) or (intervention n6 multicomponent) or (intervention n6 multi‐component) or (intervention n6 multidisciplin*) or (intervention n6 multi‐disciplin*) or (intervention n6 multifacet*) or (intervention n6 multi‐facet*) or (intervention n6 multimodal*) or (intervention n6 multi‐modal*) or (intervention n6 personalize*) or(intervention n6 personalise*) or (intervention n6 personalizing) or (intervention n6 personalising) or (intervention n6 pharmaci*) or (intervention n6 pharmacist*) or (intervention n6 pharmacy) or (intervention n6 physician*) or (intervention n6 practitioner*) Or (intervention n6 prescrib*) or (intervention n6 prescription*) or (intervention n6 primary care) or (intervention n6 professional*) or (intervention* n6 provider*) or (intervention* n6 regulatory) or (intervention n6 regulatory) or (intervention n6 tailor*) or (intervention n6 target*) or (intervention n6 team*) or (intervention n6 usual care) 19452 S64 TI ((collaborativ* or collaboration* or tailored or personalised or personalized))or AB ((collaborativ* or collaboration* or tailored or personalised or personalized))19849 S63 TI pilot 5667 S62 AB "before‐and‐after" 171176 S61 TI time series or AB time series 9749 S60 AB ( before* n10 during or before n10 after ) 18 S59 TI ((time point*) or (period* n4 interrupted) or (period* n4 multiple) or (period* n4 time) or (period* n4 various) or (period* n4 varying) or (period* n4 week*) or (period* n4 month*) or (period* n4 year*))or AB ((time point*) or (period* n4 interrupted) or (period* n4 multiple) or (period* n4 time) or (period* n4 various) or (period* n4 varying) or (period* n4 week*) or (period* n4 month*) or (period* n4 year*)) 116965 S58 TI (( quasi‐experiment* or quasiexperiment* or quasi‐random* or quasirandom* or quasi control* or quasicontrol* or quasi* W3 method* or quasi* W3 study or quasi* W3 studies or quasi* W3 trial or quasi* W3 design* or experimental W3 method* or experimental W3 study or experimental W3 studies or experimental W3 trial or experimental W3 design* ))or AB (( quasi‐experiment* or quasiexperiment* or quasi‐random* or quasirandom* or quasi control* or quasicontrol* or quasi* W3 method* or quasi* W3 study or quasi* W3 studies or quasi* W3 trial or quasi* W3 design* or experimental W3 method* or experimental W3 study or experimental W3 studies or experimental W3 trial or experimental W3 design* ))25483 S57 TI pre w7 post or AB pre w7 post 12418 S56 TI ((comparative N2 study) or (comparative N2 studies) or (evaluation study) or (evaluation studies ))or AB ((comparative N2 study) or (comparative N2 studies) or (evaluation study) or (evaluation studies ))22749 S55 TI ((pre‐test* or pretest* or posttest* or post‐test* or preimplement* or pre‐implement*))or AB ((pre‐test* or pretest* or posttest* or post‐test* or preimplement* or pre‐implement*))3636 S54 TI (( intervention* or multiintervention* or multi‐intervention* or postintervention* or post‐intervention* or preintervention* or pre‐intervention* ))or AB (( intervention* or multiintervention* or multi‐intervention* or postintervention* or post‐intervention* or preintervention* or pre‐intervention* ))72421 S53 S52 not S11 116 S52 S47 or S48 or S49 or S51 117 S51 S36 and S25 and S50 34 S50 DE "patient care" OR DE "home care" OR DE "hospice care" OR DE "hospital care" OR DE "intensive care" OR DE "long term care" OR DE "postoperative care" OR DE "preoperative care" OR DE "self care" 7996 S49 S36 and S42 67 S48 S21 and S25 and S36 41 S47 S15 and S36 13 S46 S43 or S44 or S45 16843 S45 TI ((applied learning or knowledge transfer* or knowledge translation))or AB ((applied learning or knowledge transfer* or knowledge translation))600 S44 TI ((evidence based N2 practice) or (evidence based N2 practitioner) or (evidence based N2 medicine) or (evidence based N2 medical) or (evidence based N2 treatment) or (evidence based N2 therap*) or (evidence based N2 nurse) or (evidence based N2 nurses) or (evidence based N2 nursing) or (evidence based N2 dentist*) or (evidence based N2 healthcare) or (evidence based N2 care))or AB ((evidence based N2 practice) or (evidence based N2 practitioner) or (evidence based N2 medicine) or (evidence based N2 medical) or (evidence based N2 treatment) or (evidence based N2 therap*) or (evidence based N2 nurse) or (evidence based N2 nurses) or (evidence based N2 nursing) or (evidence based N2 dentist*) or (evidence based N2 healthcare) or (evidence based N2 care))1169 S43 DE "guidelines" 15215 S42 S37 or S38 or S39 or S40 or S41 2006 S41 TI ((publication N2 guideline) or (publication N2 protocol) or (published N2 guideline) or (published N2 protocol))or AB ((publication N2 guideline) or (publication N2 protocol) or (published N2 guideline) or (published N2 protocol))120 S40 TI ((EBM N2 adher*) or (EBM N2 apply*) or (EBM N2 application) or (EBM N2 disseminat*) or (EBM N2 implement*) or (EBM N2 introduc*) or (EBM N2 publication) or (EBM N2 release) or (EBM N2 uptake))or AB ((EBM N2 adher*) or (EBM N2 apply*) or (EBM N2 application) or (EBM N2 disseminat*) or (EBM N2 implement*) or (EBM N2 introduc*) or (EBM N2 publication) or (EBM N2 release) or (EBM N2 uptake))13 S39 TI ((evidence N2 adher*) or (evidence N2 apply*) or (evidence N2 application) or (evidence N2 disseminat*) or (evidence N2 implement*) or (evidence N2 introduc*) or (evidence N2 publication) or (evidence N2 release) or (evidence N2 uptake))or AB ((evidence N2 adher*) or (evidence N2 apply*) or (evidence N2 application) or (evidence N2 disseminat*) or (evidence N2 implement*) or (evidence N2 introduc*) or (evidence N2 publication) or (evidence N2 release) or (evidence N2 uptake))1377 S38 TI ((best practice N2 adher*) or (best practice N2 apply*) or (best practice N2 application) or (best practice N2 disseminat*) or (best practice N2 implement*) or (best practice N2 introduc*) or (best practice N2 publication) or (best practice N2 release) or (best practice N2 uptake))or AB ((best practice N2 adher*) or (best practice N2 apply*) or (best practice N2 application) or (best practice N2 disseminat*) or (best practice N2 implement*) or (best practice N2 introduc*) or (best practice N2 publication) or (best practice N2 release) or (best practice N2 uptake))115 S37 TI ((guideline N2 adher*) or (guideline N2 apply*) or (guideline N2 application) or (guideline N2 disseminat*) or (guideline N2 implement*) or (guideline N2 introduc*) or (guideline N2 publication) or (guideline N2 release) or (guideline N2 uptake))or AB ((guideline N2 adher*) or (guideline N2 apply*) or (guideline N2 application) or (guideline N2 disseminat*) or (guideline N2 implement*) or (guideline N2 introduc*) or (guideline N2 publication) or (guideline N2 release) or (guideline N2 uptake))408 S36 S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 96152 S35 (DE "books" OR DE "textbooks" OR DE "monographs" OR DE "posters" OR DE "handbooks") 2748 S34 TI ((mail* N2 information) or (mail* N2 guideline) or (mail* N2 publication) or (mail* N2 protocol) or (mail* N2 practice guideline) or (mail* N2 therap* guideline) or (mail* N2 prescrib* guideline) or (mail* N2 article) or (mail* N2 articles) or (mail* N2 research) or (mail* N2 result) or (mail* N2 study) or (mail* N2 studies) or (mail* N2 journal) or (mail* N2 copy) or (mail* N2 copies))or AB ((mail* N2 information) or (mail* N2 guideline) or (mail* N2 publication) or (mail* N2 protocol) or (mail* N2 practice guideline) or (mail* N2 therap* guideline) or (mail* N2 prescrib* guideline) or (mail* N2 article) or (mail* N2 articles) or (mail* N2 research) or (mail* N2 result) or (mail* N2 study) or (mail* N2 studies) or (mail* N2 journal) or (mail* N2 copy) or (mail* N2 copies))293 S33 TI ((written material or written teaching or written learning))or AB ((written material or written teaching or written learning))or AB ((written material or written teaching or written learning))or AB ((written material or written teaching or written learning))58 S32 TI ((book or booklet or brochure or bulletin or handout or hand‐out or "hard copy" or hardcopy or "hard copies" or hardcopies or monograph* or paper‐based or "paper copy" or "paper copies" or print‐based or pamphlet or poster))or AB ((book or booklet or brochure or bulletin or handout or hand‐out or "hard copy" or hardcopy or "hard copies" or hardcopies or monograph* or paper‐based or "paper copy" or "paper copies" or print‐based or pamphlet or poster))92972 S31 TI (paper N5 format) or AB (paper N5 format) 53 S30 TI ((printed N4 diagram) or (printed N4 text))or AB ((printed N4 diagram) or (printed N4 text))14 S29 TI ((print N5 format) or (print N5 formats) or (printed N5 format) or (printed N5 formats)) or AB ((print N5 format) or (print N5 formats) or (printed N5 format) or (printed N5 formats)) 30 S28 TI ((paper N2 display) or (paper N2 document) or (paper N2 education* material) or (paper N2 format) or (paper N2 portfolio) or (paper N2 material) or (paper N2 media) or (paper N2 medium) or (paper N2 workshop material))or AB ((paper N2 display) or (paper N2 document) or (paper N2 education* material) or (paper N2 format) or (paper N2 portfolio) or (paper N2 material) or (paper N2 media) or (paper N2 medium) or (paper N2 workshop material))844 S27 TI ((printed N2 display) or (printed N2 document) or (printed N2 education* material) or (printed N2 format) or (printed N2 portfolio) or (printed N2 material) or (printed N2 media) or (printed N2 medium) or printed N2 workshop material))or AB ((printed N2 display) or (printed N2 document) or (printed N2 education* material) or (printed N2 format) or (printed N2 portfolio) or (printed N2 material) or (printed N2 media) or (printed N2 medium) or (printed N2 workshop material))117 S26 TI ((print N2 display) or (print N2 document) or (print N2 education* material) or (print N2 format) or (print N2 portfolio) or (print N2 material) or (print N2 media) or (print N2 medium) or (print N2 workshop material))or AB ((print N2 display) or (print N2 document) or (print N2 education* material) or (print N2 format) or (print N2 portfolio) or (print N2 material) or (print N2 media) or (print N2 medium) or (print N2 workshop material))309 S25 S22 OR S23 OR S24 29715 S24 (DE "physicians" OR DE "general practitioners" OR DE "pediatricians" OR DE "surgeons" OR DE "nurses" OR DE "nursing" OR DE "health care workers" OR DE "dentists" OR DE "medical auxiliaries") 16364 S23 TI (internship or intern or resident or residency or residencies) 3010 S22 TI (counsellor or dental aide or dental aides or dental hygienist or dentist or dietetic or dietician or doctor or general practitioner or health* professional or hospitalist or medical aide or medical aides or medical technician or nurse or nurses or nutritionist or orthodontist or pediatric* or paediatric* or pharmacist or physician or physiotherapist or psychiatrist or psychiatric aide or psychiatric aides or psychologist or practitioner or rheumatologist or surgeon or therapist) 15228 S21 S16 or S17 or S18 or S19 or S20 3285 S20 TI ((professional N2 education*) or (professional N2 training))or AB ((professional N2 education*) or (professional N2 training))1082 S19 TI ((professional N2 development*) or (professional N2 education*) or (professional N2 retrain*) or (professional N2 skill enhanc*) or (professional N2 training) or (professional N2 upgrade) or (professional N2 upgrading))or AB ((professional N2 development*) or (professional N2 education*) or (professional N2 retrain*) or (professional N2 skill enhanc*) or (professional N2 training) or (professional N2 upgrade) or (professional N2 upgrading))1630 S18 TI (continuing N2 education*) or AB (continuing N2 education*) 909 S17 TI ((educat* N2 clinical competenc*) or (educat* N2 practitioner) or (educat* N2 practice) or (educat* N2 general practi*) or (educat* N2 family doctor))or AB ((educat* N2 clinical competenc*) or (educat* N2 practitioner) or (educat* N2 practice) or (educat* N2 general practi*) or (educat* N2 family doctor))525 S16 TI ((train* N2 clinical competenc*) or (train* N2 practitioner) or (train* N2 practice) or (train* N2 general practi*) or (train* N2 family doctor))or AB ((train* N2 clinical competenc*) or (train* N2 practitioner) or (train* N2 practice) or (train* N2 general practi*) or (train* N2 family doctor))400 S15 S12 or S13 or S14 496 S14 TI CME or AB CME 317 S13 TI ((continuing education* N2 medical) or (continuing education* N2 nursing) or (continuing education* N2 pharma*) or (continuing education* N2 dental*) or (continuing education* N2 physician) or (continuing education* N2 doctor) or (continuing education* N2 surg*))or AB ((continuing education* N2 medical) or (continuing education* N2 nursing) or (continuing education* N2 pharma*) or (continuing education* N2 dental*) or (continuing education* N2 physician) or (continuing education* N2 doctor) or (continuing education* N2 surg*))211 S12 TI ((continuing N1 medical N2 education*) or (continuing N1 nursing N2 education*) or (continuing N1 pharma* N2 education*) or (continuing N1 dental* N2 education*) or (continuing N1 physician N2 ducation*) or (continuing N1 doctor N2 education*) or (continuing N1 surg* N2 education*))or AB ((continuing N1 medical N2 education*) or (continuing N1 nursing N2 education*) or (continuing N1 pharma* N2 education*) or (continuing N1 dental* N2 education*) or (continuing N1 physician N2 education*) or (continuing N1 doctor N2 education*) or (continuing N1 surg* N2 education*))207 S11 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 115 S10 TI ((spiral bound or bound copy or bound copies))or AB ((spiral bound or bound copy or bound copies))40 S9 TI (postal N4 guideline) or AB (postal N4 guideline) 1 S8 TI paper‐based education* or AB paper‐based education* 2 S7 TI ((allied health* N2 written material) or (counsel?or N2 written material) or (doctor N2 written material) or (nurse N2 written material) or (nurses N2 written material) or (physician N2 written material) or (physiotherapist N2 written material) or (therapist N2 written material) or (dentist N2 written material) or (pharmacist N2 written material) or (health* worker N2 written material) or (health* staff N2 written material))or AB ((allied health* N2 written material) or (counsel?or N2 written material) or (doctor N2 written material) or (nurse N2 written material) or (nurses N2 written material) or (physician N2 written material) or (physiotherapist N2 written material) or (therapist N2 written material) or (dentist N2 written material) or (pharmacist N2 written material) or (health* worker N2 written material) or (health* staff N2 written material))1 S6 TI ((allied health* N2 brochure) or (counsel?or N2 brochure) or (doctor N2 brochure) or (nurse N2 brochure) or (nurses N2 brochure) or (physician N2 brochure) or (physiotherapist N2 brochure) or (therapist N2 brochure) or (dentist N2 brochure) or (pharmacist N2 brochure) or (health* worker N2 brochure) or (health* staff N2 brochure))or AB ((allied health* N2 brochure) or (counsel?or N2 brochure) or (doctor N2 brochure) or (nurse N2 brochure) or (nurses N2 brochure) or (physician N2 brochure) or (physiotherapist N2 brochure) or (therapist N2 brochure) or (dentist N2 brochure) or (pharmacist N2 brochure) or (health* worker N2 brochure) or (health* staff N2 brochure))1 S5 TI ((allied health* N2 poster) or (counsel?or N2 poster) or (doctor N2 poster) or (nurse N2 poster) or (nurses N2 poster) or (physician N2 poster) or (physiotherapist N2 poster) or (therapist N2 poster) or (dentist N2 poster) or (pharmacist N2 poster) or (health* worker N2 poster) or (health* staff N2 poster))or AB ((allied health* N2 poster) or (counsel?or N2 poster) or (doctor N2 poster) or (nurse N2 poster) or (nurses N2 poster) or (physician N2 poster) or (physiotherapist N2 poster) or (therapist N2 poster) or (dentist N2 poster) or (pharmacist N2 poster) or (health* worker N2 poster) or (health* staff N2 poster))1 S4 TI ((allied health* N2 booklet) or (counsel?or N2 booklet) or (doctor N2 booklet) or (nurse N2 booklet) or (nurses N2 booklet) or (physician N2 booklet) or (physiotherapist N2 booklet) or (therapist N2 booklet) or (dentist N2 booklet) or (pharmacist N2 booklet) or (health* worker N2 booklet) or (health* staff N2 booklet))or AB ((allied health* N2 booklet) or (counsel?or N2 booklet) or (doctor N2 booklet) or (nurse N2 booklet) or (nurses N2 booklet) or (physician N2 booklet) or (physiotherapist N2 booklet) or (therapist N2 booklet) or (dentist N2 booklet) or (pharmacist N2 booklet) or (health* worker N2 booklet) or (health* staff N2 booklet))1 S3 TI ((allied health* N2 pamphlet) or (counsel?or N2 pamphlet) or (doctor N2 pamphlet) or (nurse N2 pamphlet) or (nurses N2 pamphlet) or (physician N2 pamphlet) or (physiotherapist N2 pamphlet) or (therapist N2 pamphlet) or (dentist N2 pamphlet) or (pharmacist N2 pamphlet) or (health* worker N2 pamphlet) or (health* staff N2 pamphlet))or AB ((allied health* N2 pamphlet) or (counsel?or N2 pamphlet) or (doctor N2 pamphlet) or (nurse N2 pamphlet) or (nurses N2 pamphlet) or (physician N2 pamphlet) or (physiotherapist N2 pamphlet) or (therapist N2 pamphlet) or (dentist N2 pamphlet) or (pharmacist N2 pamphlet) or (health* orker N2 pamphlet) or (health* staff N2 pamphlet))1 S2 TI ((print N2 intervention) or (printed N2 intervention))or AB ((print N2 intervention) or (printed N2 intervention))27 S1 TI print* education* or AB print* education* 45
CINAHL (Ebsco)
| # | Query 14June 2011 |
| S150 | S149 and S111 |
| S149 | S148 not (S122 OR S120) |
| S148 | S123 or S124 or S125 or S126 or S127 or S128 or S129 or S130 or S131 or S134 or S137 or S140 or S142 or S144 or S146 or S147 |
| S147 | TI (publication and (influenc* or impact or chang* or prescribing or physician behavio#r)) |
| S146 | S145 and S138 |
| S145 | TI publication |
| S144 | S143 and S139 |
| S143 | (MM "Primary Health Care") |
| S142 | S141 and S139 |
| S141 | (MH "Family Practice") |
| S140 | S138 and S139 |
| S139 | (MH "Practice Guidelines") |
| S138 | (MH "Practice Patterns") |
| S137 | S135 and S136 |
| S136 | (MH "Patient Education") |
| S135 | (MH "Education, Medical, Continuing") OR (MH "Education, Nursing, Continuing") |
| S134 | S133 and S132 |
| S133 | TI publication or AB publication |
| S132 | (MH "Drug Utilization") |
| S131 | TI (guideline and disseminat*) |
| S130 | TI (publication and evidence) |
| S129 | TI (guideline and (notification or notify*)) |
| S128 | TI (impact and disseminat*) |
| S127 | TI (impact and publication) |
| S126 | TI (impact and bulletin) |
| S125 | TI (effect* and guideline) |
| S124 | TI (impact and guideline) |
| S123 | TI (guideline and (impact or influence)) |
| S122 | S121 NOT S120 |
| S121 | S119 AND S104 |
| S120 | S119 AND S111 |
| S119 | S118 NOT S12 |
| S118 | S112 OR S113 OR S114 OR S115 OR S116 OR S117 |
| S117 | S56 AND S79 AND S43 |
| S116 | S56 AND S68 |
| S115 | S56 AND S64 |
| S114 | S56 AND S63 |
| S113 | S26 AND S43 AND S56 |
| S112 | S17 AND S56 |
| S111 | S105 or S106 or S107 or S108 or S109 or S110 |
| S110 | TI ( “control* N1 clinical” or “control* N1 group*” or “control* N1 trial*” or “control* N1 study” or “control* N1 studies” or “control* N1 design*” or “control* N1 method*” ) or AB ( “control* N1 clinical” or “control* N1 group*” or “control* N1 trial*” or “control* N1 study” or “control* N1 studies” or “control* N1 design*” or “control* N1 method*” ) |
| S109 | TI controlled or AB controlled |
| S108 | TI random* or AB random* |
| S107 | TI ( “clinical study” or “clinical studies” ) or AB ( “clinical study” or “clinical studies” ) |
| S106 | (MM "Clinical Trials+") |
| S105 | TI ( (multicent* n2 design*) or (multicent* n2 study) or (multicent* n2 studies) or (multicent* n2 trial*) ) or AB ( (multicent* n2 design*) or (multicent* n2 study) or (multicent* n2 studies) or (multicent* n2 trial*) ) |
| S104 | S80 or S81 or S82 or S83 or S84 or S85 or S86 or S87 or S88 or S89 or S90 or S91 or S92 or S93 or S94 or S95 or S96 or S97 or S98 or S99 or S100 or S101 or S102 or S103 |
| S103 | TI ( (time points n3 over) or (time points n3 multiple) or (time points n3 three) or (time points n3 four) or (time points n3 five) or (time points n3 six) or (time points n3 seven) or (time points n3 eight) or (time points n3 nine) or (time points n3 ten) or (time points n3 eleven) or (time points n3 twelve) or (time points n3 month*) or (time points n3 hour*) or (time points n3 day*) or (time points n3 "more than") ) or AB ( (time points n3 over) or (time points n3 multiple) or (time points n3 three) or (time points n3 four) or (time points n3 five) or (time points n3 six) or (time points n3 seven) or (time points n3 eight) or (time points n3 nine) or (time points n3 ten) or (time points n3 eleven) or (time points n3 twelve) or (time points n3 month*) or (time points n3 hour*) or (time points n3 day*) or (time points n3 "more than") ) |
| S102 | TI ( (control w3 area) or (control w3 cohort*) or (control w3 compar*) or (control w3 condition) or (control w3 group*) or (control w3 intervention*) or (control w3 participant*) or (control w3 study) ) or AB ( (control w3 area) or (control w3 cohort*) or (control w3 compar*) or (control w3 condition) or (control w3 group*) or (control w3 intervention*) or (control w3 participant*) or (control w3 study) ) |
| S101 | TI ( multicentre or multicenter or multi‐centre or multi‐center ) or AB random* |
| S100 | TI random* OR controlled |
| S99 | TI ( trial or (study n3 aim) or "our study" ) or AB ( (study n3 aim) or "our study" ) |
| S98 | TI ( pre‐workshop or preworkshop or post‐workshop or postworkshop or (before n3 workshop) or (after n3 workshop) ) or AB ( pre‐workshop or preworkshop or post‐workshop or postworkshop or (before n3 workshop) or (after n3 workshop) ) |
| S97 | TI ( demonstration project OR demonstration projects OR preimplement* or pre‐implement* or post‐implement* or postimplement* ) or AB ( demonstration project OR demonstration projects OR preimplement* or pre‐implement* or post‐implement* or postimplement* ) |
| S96 | (intervention n6 clinician*) or (intervention n6 community) or (intervention n6 complex) or (intervention n6 design*) or (intervention n6 doctor*) or (intervention n6 educational) or (intervention n6 family doctor*) or (intervention n6 family physician*) or (intervention n6 family practitioner*) or (intervention n6 financial) or (intervention n6 GP) or (intervention n6 general practice*) Or (intervention n6 hospital*) or (intervention n6 impact*) Or (intervention n6 improv*) or (intervention n6 individualize*) Or (intervention n6 individualise*) or (intervention n6 individualizing) or (intervention n6 individualising) or (intervention n6 interdisciplin*) or (intervention n6 multicomponent) or (intervention n6 multi‐component) or (intervention n6 multidisciplin*) or (intervention n6 multi‐disciplin*) or (intervention n6 multifacet*) or (intervention n6 multi‐facet*) or (intervention n6 multimodal*) or (intervention n6 multi‐modal*) or (intervention n6 personalize*) or(intervention n6 personalise*) or (intervention n6 personalizing) or (intervention n6 personalising) or (intervention n6 pharmaci*) or (intervention n6 pharmacist*) or (intervention n6 pharmacy) or (intervention n6 physician*) or (intervention n6 practitioner*) Or (intervention n6 prescrib*) or (intervention n6 prescription*) or (intervention n6 primary care) or (intervention n6 professional*) or (intervention* n6 provider*) or (intervention* n6 regulatory) or (intervention n6 regulatory) or (intervention n6 tailor*) or (intervention n6 target*) or (intervention n6 team*) or (intervention n6 usual care) |
| S95 | TI ( collaborativ* or collaboration* or tailored or personalised or personalized ) or AB ( collaborativ* or collaboration* or tailored or personalised or personalized ) |
| S94 | TI pilot |
| S93 | (MH "Pilot Studies") |
| S92 | AB "before‐and‐after" |
| S91 | AB time series |
| S90 | TI time series |
| S89 | AB ( before* n10 during or before n10 after ) or AU ( before* n10 during or before n10 after ) |
| S88 | TI ( (time point*) or (period* n4 interrupted) or (period* n4 multiple) or (period* n4 time) or (period* n4 various) or (period* n4 varying) or (period* n4 week*) or (period* n4 month*) or (period* n4 year*) ) or AB ( (time point*) or (period* n4 interrupted) or (period* n4 multiple) or (period* n4 time) or (period* n4 various) or (period* n4 varying) or (period* n4 week*) or (period* n4 month*) or (period* n4 year*) ) |
| S87 | TI ( ( quasi‐experiment* or quasiexperiment* or quasi‐random* or quasirandom* or quasi control* or quasicontrol* or quasi* W3 method* or quasi* W3 study or quasi* W3 studies or quasi* W3 trial or quasi* W3 design* or experimental W3 method* or experimental W3 study or experimental W3 studies or experimental W3 trial or experimental W3 design* ) ) or AB ( ( quasi‐experiment* or quasiexperiment* or quasi‐random* or quasirandom* or quasi control* or quasicontrol* or quasi* W3 method* or quasi* W3 study or quasi* W3 studies or quasi* W3 trial or quasi* W3 design* or experimental W3 method* or experimental W3 study or experimental W3 studies or experimental W3 trial or experimental W3 design* ) ) |
| S86 | TI pre w7 post or AB pre w7 post |
| S85 | MH "Multiple Time Series" or MH "Time Series" |
| S84 | TI ( (comparative N2 study) or (comparative N2 studies) or evaluation study or evaluation studies ) or AB ( (comparative N2 study) or (comparative N2 studies) or evaluation study or evaluation studies ) |
| S83 | MH Experimental Studies or Community Trials or Community Trials or Pretest‐Posttest Design + or Quasi‐Experimental Studies + Pilot Studies or Policy Studies + Multicenter Studies |
| S82 | TI ( pre‐test* or pretest* or posttest* or post‐test* ) or AB ( pre‐test* or pretest* or posttest* or "post test* ) OR TI ( preimplement*" or pre‐implement* ) or AB ( pre‐implement* or preimplement* ) |
| S81 | TI ( intervention* or multiintervention* or multi‐intervention* or postintervention* or post‐intervention* or preintervention* or pre‐intervention* ) or AB ( intervention* or multiintervention* or multi‐intervention* or postintervention* or post‐intervention* or preintervention* or pre‐intervention* ) |
| S80 | (MH "Quasi‐Experimental Studies") |
| S79 | S69 or S70 or S71 or S72 or S73 or S74 or S75 or S76 or S77 or S78 |
| S78 | (MH "Quality of Health Care") |
| S77 | (MH "Health Services Accessibility") |
| S76 | (MH "Patient Selection") |
| S75 | (MH "Practice Patterns") |
| S74 | (MH "Medication Reconciliation") |
| S73 | (MH "Disease Management") |
| S72 | (MH "Critical Path") |
| S71 | (MH "Patient Care Plans+") OR (MH "Nursing Care Plans+") OR (MH "Patient Centered Care") |
| S70 | (MH "Managed Care Programs+") |
| S69 | (MH "Health Care Delivery") OR (MH "Health Care Delivery, Integrated") OR (MH "Health Care Reform") |
| S68 | S65 or S66 or S67 |
| S67 | (MH "Evidence‐Based Dental Practice") OR (MH "Nursing Practice, Evidence‐Based") OR (MH "Physical Therapy Practice, Evidence‐Based") |
| S66 | TI ( (applied learning or knowledge transfer* or knowledge translation) ) or AB ( (applied learning or knowledge transfer* or knowledge translation) ) |
| S65 | TI ( (evidence based N2 practice) or (evidence based N2 practitioner) or (evidence based N2 medicine) or (evidence based N2 medical) or (evidence based N2 treatment) or (evidence based N2 therap*) or (evidence based N2 nurse) or (evidence based N2 nurses) or (evidence based N2 nursing) or (evidence based N2 dentist*) or (evidence based N2 healthcare) or (evidence based N2 care) ) or AB ( (evidence based N2 practice) or (evidence based N2 practioner) or (evidence based N2 medicine) or (evidence based N2 medical) or (evidence based N2 treatment) or (evidence based N2 therap*) or (evidence based N2 nurse) or (evidence based N2 nurses) or (evidence based N2 nursing) or (evidence based N2 dentist*) or (evidence based N2 healthcare) or (evidence based N2 care) ) |
| S64 | (MH "Practice Guidelines") |
| S63 | S57 or S58 or S59 or S60 or S61 or S62 |
| S62 | TI ( (publication N2 guideline) or (publication N2 protocol) or (published N2 guideline) or (published N2 protocol) ) or AB ( (publication N2 guideline) or (publication N2 protocol) or (published N2 guideline) or (published N2 protocol) ) |
| S61 | TI ( (EBM N2 adher*) or (EBM N2 apply*) or (EBM N2 application) or (EBM N2 disseminat*) or (EBM N2 implement*) or (EBM N2 introduc*) or (EBM N2 publication) or (EBM N2 release) or (EBM N2 uptake) ) or AB ( (EBM N2 adher*) or (EBM N2 apply*) or (EBM N2 application) or (EBM N2 disseminat*) or (EBM N2 implement*) or (EBM N2 introduc*) or (EBM N2 publication) or (EBM N2 release) or (EBM N2 uptake) ) |
| S60 | TI ( (evidence N2 adher*) or (evidence N2 apply*) or (evidence N2 application) or (evidence N2 disseminat*) or (evidence N2 implement*) or (evidence N2 introduc*) or (evidence N2 publication) or (evidence N2 release) or (evidence N2 uptake) ) or AB ( (evidence N2 adher*) or (evidence N2 apply*) or (evidence N2 application) or (evidence N2 disseminat*) or (evidence N2 implement*) or (evidence N2 introduc*) or (evidence N2 publication) or (evidence N2 release) or (evidence N2 uptake) ) |
| S59 | TI ( (best practice N2 adher*) or (best practice N2 apply*) or (best practice N2 application) or (best practice N2 disseminat*) or (best practice N2 implement*) or (best practice N2 introduc*) or (best practice N2 publication) or (best practice N2 release) or (best practice N2 uptake) ) or AB ( (best practice N2 adher*) or (best practice N2 apply*) or (best practice N2 application) or (best practice N2 disseminat*) or (best practice N2 implement*) or (best practice N2 introduc*) or (best practice N2 publication) or (best practice N2 release) or (best practice N2 uptake) ) |
| S58 | TI ( (guideline N2 adher*) or (guideline N2 apply*) or (guideline N2 application) or (guideline N2 disseminat*) or (guideline N2 implement*) or (guideline N2 introduc*) or (guideline N2 publication) or (guideline N2 release) or (guideline N2 uptake) ) or AB ( (guideline N2 adher*) or (guideline N2 apply*) or (guideline N2 application) or (guideline N2 disseminat*) or (guideline N2 implement*) or (guideline N2 introduc*) or (guideline N2 publication) or (guideline N2 release) or (guideline N2 uptake) ) |
| S57 | (MH "Guideline Adherence") |
| S56 | S44 or S45 or S46 or S47 or S48 or S49 or S50 or S51 or S52 or S53 or S54 or S55 |
| S55 | (MH "Posters") |
| S54 | (MH "Manuscripts") OR (MH "Pamphlets") OR (MH "Policy and Procedure Manuals") OR (MH "Reports") OR (MH "Print Materials") |
| S53 | (MH "Books+") |
| S52 | TI ( (mail* N2 information) or (mail* N2 guideline) or (mail* N2 publication) or (mail* N2 protocol) or (mail* N2 practice guideline) or (mail* N2 therap* guideline) or (mail* N2 prescrib* guideline) or (mail* N2 article) or (mail* N2 articles) or (mail* N2 research) or (mail* N2 result) or (mail* N2 study) or (mail* N2 studies) or (mail* N2 journal) or (mail* N2 copy) or (mail* N2 copies) ) or AB ( (mail* N2 information) or (mail* N2 guideline) or (mail* N2 publication) or (mail* N2 protocol) or (mail* N2 practice guideline) or (mail* N2 therap* guideline) or (mail* N2 prescrib* guideline) or (mail* N2 article) or (mail* N2 articles) or (mail* N2 research) or (mail* N2 result) or (mail* N2 study) or (mail* N2 studies) or (mail* N2 journal) or (mail* N2 copy) or (mail* N2 copies) ) |
| S51 | TI ( (written material or written teaching or written learning) ) or AB ( (written material or written teaching or written learning) ) |
| S50 | TI ( (book or booklet or brochure or bulletin or handout or hand‐out or "hard copy" or hardcopy or "hard copies" or hardcopies or monograph* or paper‐based or "paper copy" or "paper copies" or print‐based or pamphlet or poster) ) or AB ( (book or booklet or brochure or bulletin or handout or hand‐out or "hard copy" or hardcopy or "hard copies" or hardcopies or monograph* or paper‐based or "paper copy" or "paper copies" or print‐based or pamphlet or poster) ) |
| S49 | TI (paper N5 format) or AB (paper N5 format) |
| S48 | TI ( (printed N4 diagram) or (printed N4 text) ) or AB ( (printed N4 diagram) or (printed N4 text) ) |
| S47 | TI ( (print N5 format) or (print N5 formats) or (printed N5 format) or (printed N5 formats) ) or AB ( (print N5 format) or (print N5 formats) or (printed N5 format) or (printed N5 formats) ) |
| S46 | TI ( (paper N2 display) or (paper N2 document) or (paper N2 education* material) or (paper N2 format) or (paper N2 portfolio) or (paper N2 material) or (paper N2 media) or (paper N2 medium) or (paper N2 workshop material) ) or AB ( (paper N2 display) or (paper N2 document) or (paper N2 education* material) or (paper N2 format) or (paper N2 portfolio) or (paper N2 material) or (paper N2 media) or (paper N2 medium) or (paper N2 workshop material) ) |
| S45 | TI ( (printed N2 display) or (printed N2 document) or (printed N2 education* material) or (printed N2 format) or (printed N2 portfolio) or (printed N2 material) or (printed N2 media) or (printed N2 medium) or (printed N2 workshop material) ) or AB ( (printed N2 display) or (printed N2 document) or (printed N2 education* material) or (printed N2 format) or (printed N2 portfolio) or (printed N2 material) or (printed N2 media) or (printed N2 medium) or (printed N2 workshop material) ) |
| S44 | TI ( (print N2 display) or (print N2 document) or (print N2 education* material) or (print N2 format) or (print N2 portfolio) or (print N2 material) or (print N2 media) or (print N2 medium) or (print N2 workshop material) ) or AB ( (print N2 display) or (print N2 document) or (print N2 education* material) or (print N2 format) or (print N2 portfolio) or (print N2 material) or (print N2 media) or (print N2 medium) or (print N2 workshop material) ) |
| S43 | (S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42) |
| S42 | TI (internship or intern or resident or residency or residencies) |
| S41 | TI (counsellor or dental aide or dental aides or dental hygienist or dentist or dietetic or dietician or doctor or general practitioner or health* professional or hospitalist or medical aide or medical aides or medical technician or nurse or nurses or nutritionist or orthodontist or pediatric* or paediatric* or pharmacist or physician or physiotherapist or psychiatrist or psychiatric aide or psychiatric aides or psychologist or practitioner or rheumatologist or surgeon or therapist) |
| S40 | (MH "Health Facility Administrators") |
| S39 | (MH "Dental Auxiliaries+") |
| S38 | (MH "Dentists") |
| S37 | (MH "Laboratory Personnel") |
| S36 | (MH "Pharmacists") |
| S35 | (MH "Staff Nurses") OR (MH "Nursing Staff, Hospital") |
| S34 | (MH "Medical Staff+") |
| S33 | (MH "Health Occupations+") |
| S32 | (MH "Allied Health Personnel") OR (MH "Audiologists") OR (MH "Cardiopulmonary Technicians") OR (MH "Cardiovascular Technicians") OR (MH "Dental Auxiliaries+") OR (MH "Dialysis Technicians") OR (MH "Dietetic Technicians, Registered") OR (MH "Dietitians") OR (MH "Electroneurodiagnostic Technologists") OR (MH "Emergency Medical Technicians") OR (MH "Laboratory Personnel+") OR (MH "Medical Assistants") OR (MH "Occupational Therapists") OR (MH "Occupational Therapy Assistants") OR (MH "Ophthalmic Technologists") OR (MH "Orthopedic Technologists") OR (MH "Pharmacy Technicians") OR (MH "Physical Therapist Assistants") OR (MH "Physical Therapists") OR (MH "Physician Assistants") OR (MH "Radiology Personnel+") OR (MH "Respiratory Therapists") OR (MH "Speech‐Language Pathologists") OR (MH "Speech‐Language Pathology Assistants") OR (MH "Surgical Technologists") |
| S31 | (MH "Clinical Competence") |
| S30 | (MH "Preceptorship") |
| S29 | (MH "Internship and Residency") |
| S28 | (MH "Advanced Practice Nurses+") OR (MH "Nurse Administrators+") OR (MH "Nurse Anesthetists") OR (MH "Nurse Midwives") OR (MH "Emergency Nurse Practitioners") OR (MH "Gerontologic Nurse Practitioners") OR (MH "Practical Nurses") OR (MH "Nurses, Male") |
| S27 | (MH "Physicians+") |
| S26 | S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 |
| S25 | TI ( (professional N2 education*) or (professional N2 training) ) or AB ( (professional N2 education*) or (professional N2 training) ) |
| S24 | TI professional N2 skill N2 improv* or AB professional N2 skill N2 improv* |
| S23 | TI ( (professional N2 development*) or (professional N2 education*) or (professional N2 retrain*) or (professional N2 skill enhanc*) or (professional N2 training) or (professional N2 upgrade) or (professional N2 upgrading) ) or AB ( (professional N2 development*) or (professional N2 education*) or (professional N2 retrain*) or (professional N2 skill enhanc*) or (professional N2 training) or (professional N2 upgrade) or (professional N2 upgrading) ) |
| S22 | TI (continuing N2 education*) or AB (continuing N2 education*) |
| S21 | TI ( (educat* N2 clinical competenc*) or (educat* N2 practitioner) or (educat* N2 practice) or (educat* N2 general practi*) or (educat* N2 family doctor) ) or AB ( (educat* N2 clinical competenc*) or (educat* N2 practitioner) or (educat* N2 practice) or (educat* N2 general practi*) or (educat* N2 family doctor) ) |
| S20 | TI ( (train* N2 clinical competenc*) or (train* N2 practitioner) or (train* N2 practice) or (train* N2 general practi*) or (train* N2 family doctor) ) or AB ( (train* N2 clinical competenc*) or (train* N2 practitioner) or (train* N2 practice) or (train* N2 general practi*) or (train* N2 family doctor) ) |
| S19 | (MH "Refresher Courses") |
| S18 | (MH "Education, Continuing") |
| S17 | (S13 or S14 or S15 or S16) |
| S16 | TI CME or AB CME |
| S15 | TI ( (continuing education* N2 medical) or (continuing education* N2 nursing) or (continuing education* N2 pharma*) or (continuing education* N2 dental*) or (continuing education* N2 physician) or (continuing education* N2 doctor) or (continuing education* N2 surg*) ) or AB ( (continuing education* N2 medical) or (continuing education* N2 nursing) or (continuing education* N2 pharma*) or (continuing education* N2 dental*) or (continuing education* N2 physician) or (continuing education* N2 doctor) or (continuing education* N2 surg*) ) |
| S14 | TI ( (continuing N1 medical N2 education*) or (continuing N1 nursing N2 education*) or (continuing N1 pharma* N2 education*) or (continuing N1 dental* N2 education*) or (continuing N1 physician N2 education*) or (continuing N1 doctor N2 education*) or (continuing N1 surg* N2 education*) ) or AB ( (continuing N1 medical N2 education*) or (continuing N1 nursing N2 education*) or (continuing N1 pharma* N2 education*) or (continuing N1 dental* N2 education*) or (continuing N1 physician N2 education*) or (continuing N1 doctor N2 education*) or (continuing N1 surg* N2 education*) ) |
| S13 | (MH "Education, Medical, Continuing") OR (MH "Education, Nursing, Continuing") |
| S12 | (S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11) |
| S11 | TI postal N4 guideline or AB postal N4 guideline |
| S10 | TI paper‐based education or AB paper‐based education |
| S9 | TI ( (allied health* N2 print) or (counsellor N2 print) or (doctor N2 print) or (nurse N2 print) or (nurses N2 print) or (physician N2 print) or (physiotherapist N2 print) or (therapist N2 print) or (dentist N2 print) or (pharmacist N2 print) or (health* worker N2 print) or (health* staff N2 print) ) or AB ( (allied health* N2 print) or (counsellor N2 print) or (doctor N2 print) or (nurse N2 print) or (nurses N2 print) or (physician N2 print) or (physiotherapist N2 print) or (therapist N2 print) or (dentist N2 print) or (pharmacist N2 print) or (health* worker N2 print) or (health* staff N2 print) ) |
| S8 | TI ( (allied health* N2 printed) or (counsellor N2 printed) or (doctor N2 printed) or (nurse N2 printed) or (nurses N2 printed) or (physician N2 printed) or (physiotherapist N2 printed) or (therapist N2 printed) or (dentist N2 printed) or (pharmacist N2 printed) or (health* worker N2 printed) or (health* staff N2 printed) ) or AB ( (allied health* N2 printed) or (counsellor N2 printed) or (doctor N2 printed) or (nurse N2 printed) or (nurses N2 printed) or (physician N2 printed) or (physiotherapist N2 printed) or (therapist N2 printed) or (dentist N2 printed) or (pharmacist N2 printed) or (health* worker N2 printed) or (health* staff N2 printed) ) |
| S7 | TI ( (allied health* N2 written material) or (counsellor N2 written material) or (doctor N2 written material) or (nurse N2 written material) or (nurses N2 written material) or (physician N2 written material) or (physiotherapist N2 written material) or (therapist N2 written material) or (dentist N2 written material) or (pharmacist N2 written material) or (health* worker N2 written material) or (health* staff N2 written material) ) or AB ( (allied health* N2 written material) or (counsellor N2 written material) or (doctor N2 written material) or (nurse N2 written material) or (nurses N2 written material) or (physician N2 written material) or (physiotherapist N2 written material) or (therapist N2 written material) or (dentist N2 written material) or (pharmacist N2 written material) or (health* worker N2 written material) or (health* staff N2 written material) ) |
| S6 | TI ( (allied health* N2 brochure) or (counsellor N2 brochure) or (doctor N2 brochure) or (nurse N2 brochure) or (nurses N2 brochure) or (physician N2 brochure) or (physiotherapist N2 brochure) or (therapist N2 brochure) or (dentist N2 brochure) or (pharmacist N2 brochure) or (health* worker N2 brochure) or (health* staff N2 brochure) ) or AB ( (allied health* N2 brochure) or (counsellor N2 brochure) or (doctor N2 brochure) or (nurse N2 brochure) or (nurses N2 brochure) or (physician N2 brochure) or (physiotherapist N2 brochure) or (therapist N2 brochure) or (dentist N2 brochure) or (pharmacist N2 brochure) or (health* worker N2 brochure) or (health* staff N2 brochure) ) |
| S5 | TI ( (allied health* N2 poster) or (counsellor N2 poster) or (doctor N2 poster) or (nurse N2 poster) or (nurses N2 poster) or (physician N2 poster) or (physiotherapist N2 poster) or (therapist N2 poster) or (dentist N2 poster) or (pharmacist N2 poster) or (health* worker N2 poster) or (health* staff N2 poster) ) or AB ( (allied health* N2 poster) or (counsellor N2 poster) or (doctor N2 poster) or (nurse N2 poster) or (nurses N2 poster) or (physician N2 poster) or (physiotherapist N2 poster) or (therapist N2 poster) or (dentist N2 poster) or (pharmacist N2 poster) or (health* worker N2 poster) or (health* staff N2 poster) ) |
| S4 | TI ( (allied health* N2 booklet) or (counsellor N2 booklet) or (doctor N2 booklet) or (nurse N2 booklet) or (nurses N2 booklet) or (physician N2 booklet) or (physiotherapist N2 booklet) or (therapist N2 booklet) or (dentist N2 booklet) or (pharmacist N2 booklet) or (health* worker N2 booklet) or (health* staff N2 booklet) ) or AB ( (allied health* N2 booklet) or (counsellor N2 booklet) or (doctor N2 booklet) or (nurse N2 booklet) or (nurses N2 booklet) or (physician N2 booklet) or (physiotherapist N2 booklet) or (therapist N2 booklet) or (dentist N2 booklet) or (pharmacist N2 booklet) or (health* worker N2 booklet) or (health* staff N2 booklet) ) |
| S3 | TI ( (allied health* N2 pamphlet) or (counsellor N2 pamphlet) or (doctor N2 pamphlet) or (nurse N2 pamphlet) or (nurses N2 pamphlet) or (physician N2 pamphlet) or (physiotherapist N2 pamphlet) or (therapist N2 pamphlet) or (dentist N2 pamphlet) or (pharmacist N2 pamphlet) or (health* worker N2 pamphlet) or (health* staff N2 pamphlet) ) or AB ( (allied health* N2 pamphlet) or (counsellor N2 pamphlet) or (doctor N2 pamphlet) or (nurse N2 pamphlet) or (nurses N2 pamphlet) or (physician N2 pamphlet) or (physiotherapist N2 pamphlet) or (therapist N2 pamphlet) or (dentist N2 pamphlet) or (pharmacist N2 pamphlet) or (health* worker N2 pamphlet) or (health* staff N2 pamphlet) ) |
| S2 | TI ( (print N2 intervention) or (printed N2 intervention) ) or AB ( (print N2 intervention) or (printed N2 intervention) ) |
| S1 | TI print* education* or AB print* education* |
ERIC (Education Resources Information Center) via Wilson Limit: Journal TI: (printed educational material) OR (bulletin or brochure or pamphlet or poster ormonograph or booklet or journal article or hardcopy or guideline or print) OR ((disseminate or dissemination or distribute or distribution or mail or mailed or mailingor posted or postal) AND (impact or effect or effectiveness or efficacy or influence or alteror change)) AND KW: counsellor? or doctor? or nurs* or physician? or practitioner? or therapist? or dentist? or dental aide? or dental auxiliaries or surgeon? or health* workers or health*professionals or nutritionist? or pharmacist? or paediatrician? or psychologist? or psychiatrist?
EPOC register (Reference Manager) Truncation of all terms was automatic. ALL FIELDS: Print Intervention TITLE: disseminat or distribute or mail or posted or postal or sent or receive or distribution or bulletin or guideline or letter or publication or print or written or brochure or pamphlet or protocol or hardcop or research or printed or material AND ALL FIELDS: counsellor or doctor or physician or practitioner or nurse or therapist or psychologist or pscyhiatrist or dentist or dental or dietician or surgeon or healthcare worker or health care worker or nutritionist ALL FIELDS: effect or impact or influence or efficacy or alter or change Total 297
Global Health Database (via CAB Direct) Search Date: June 17, 2011 title:("dissemination" Or "disseminate" OR mail OR "distribution" OR "distribute" OR "protocol" OR "protocols" OR "guideline" OR "guidelines" OR "letter" OR "letters" OR "bulletin" OR "posted" OR "postal" OR "publication") AND title:("impact" OR "effect" OR "effectiveness" OR "efficacy") OR title:(("counsel" OR "dental aide" OR "dental aides" OR "dental hygienist" OR "dentist" OR "dietetic" OR "dietician" OR "doctor" OR "general practitioner" OR "healthcare professional" OR "health care professional" OR "hospitalist" OR "medical aide" OR "medical aides" OR "medical technician" OR "nurse" OR "nurse" OR "nutritionist" OR "orthodontist" OR "pediatrician" OR "paediatrician" OR "pharmacist" OR "physician" OR "physiotherapist" OR "psychiatrist" OR "psychiatric aide" OR "psychiatric aides" OR "psychologist" OR "practitioner" OR "rheumatologist" OR "surgeon" OR "therapist")) AND title:(("book" OR "books" OR "booklet" OR "booklets" OR "brochure" OR "brochures" OR "bulletin" OR "bulletins" OR "handout" OR "handouts" OR "hand‐out" Or "hand‐outs" OR "hard copy" OR "hardcopy" OR "hard copies" OR "hardcopies" OR "monographs" OR "monograph" OR "paper‐based" OR "paper copy" OR "paper copies" OR "print‐based" OR "pamphlet" OR "pamphlets" OR "poster" OR "posters" OR "guideline" OR "guidelines" OR "protocol" OR "protocols" OR "manual" OR "manuals")) OR title:(("counsel" OR "dental aide" OR "dental aides" OR "dental hygienists" OR "dentists" OR "dietetics" OR "dieticians" OR "doctors" OR "general practitioners" OR "healthcare professionals" OR "health care professionals" OR "hospitalists" OR "medical aide" OR "medical aides" OR "medical technicians" OR "nurse" OR "nurses" OR "nutritionists" OR "orthodontists" OR "pediatricians" OR "paediatricians" OR "pharmacists" OR "physicians" OR "physiotherapists" OR "psychiatrists" OR "psychiatric aide" OR "psychiatric aides" OR "psychologists" OR "practitioners" OR "rheumatologists" OR "surgeons" OR "therapists")) AND title:(("book" OR "books" OR "booklet" OR "booklets" OR "brochure" OR "brochures" OR "bulletin" OR "bulletins" OR "handout" OR "handouts" OR "hand‐out" Or "hand‐outs" OR "hard copy" OR "hardcopy" OR "hard copies" OR "hardcopies" OR "monographs" OR "monograph" OR "paper‐based" OR "paper copy" OR "paper copies" OR "print‐based" OR "pamphlet" OR "pamphlets" OR "poster" OR "posters" OR "guideline" OR "guidelines" OR "protocol" OR "protocols" OR "manual" OR "manuals")) OR title:(printed AND education) OR title:(printed AND intervention)
Appendix 2. Search strategy per original review
teaching materials/ OR ((education$ or teach$) adj (material$ or book$ or monograph$ or pamphlet$ or journal$ or guidelines$ or publication$ or serial$ or papers$ or information)).tw. OR education$ intervention$.tw. OR exp education/mt AND print$.tw.
Appendix 3. Listing of the printed educational material evaluated in the included studies
| Study / PEM label(s) | PEM description | Availability |
| Austin 2003 / HERS trial report |
Publication in peer‐reviewed journal: HERS: Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA 1998;280:605‐13 |
HERS is available |
| Austin 2004‐A / WHI trial report |
Publication in peer‐reviewed journal: Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288:321‐33 |
WHI is available |
| Austin 2004‐B / ALLHAT trial report |
Publication in peer‐reviewed journal: ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs. diuretic: the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002;288:2981‐97 |
ALLHAT is available |
| Austin 2005 / REVERSAL, PROVE IT–TIMI22 trials reports |
2 publications in peer‐reviewed journals: REVERSAL: Nissen SE, Tuzcu EM, Schoenhagen P, Brown BG, Ganz P, Vogel RA, Crowe T, Howard G, Cooper CJ, Brodie B, Grines CL, DeMaria AN, for the REVERSAL Investigators. Effect of intensive compared with moderate lipid‐lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 2004;291:1071–80 PROVEIT‐TIMI22: Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495‐504 |
REVERSAL, PROVE IT–TIMI22 are available |
| Avorn 1983 / FDA Bulletin | Bulletin patterned after the Federal Drug Administration Drug Bulletin describing alternatives to targeted drugs |
Not available |
| Azocar 2003 / UBH guidelines | US United Behavioral Health (UBH) best practice guidelines for the treatment of major depression | Not available |
| Barbaglia 2009 / WHI trial report |
Publication in peer‐reviewed journal: Writing Group for the Women’s Health Initiative Investigators. Risk and benefits of estrogens plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative Randomized Controlled Trial. JAMA 2002;288:321‐33 |
WHI is available |
| Beaulieu 2004 / Guidelines summary | 1 page summary of Quebec provincial guidelines (Canada) for anti‐anginal therapy | Not available |
| Bearcroft 1993/ UK guidelines | Guidelines for referrals for chest radiography for general practitioners | Not available |
| Bjornson 1990 / VA trial report | Publication in peer‐reviewed journal: Cohn JN, Archibald DG, Ziesche S, Franciosa JA, Harston WE, Tristani FE, Dunkman WB, Jacobs W, Francis GS, Flohr KH, Goldman S, Cobb FR, Shah PM, Saunders R, Fletcher RD, Loeb HS, Hughes VC, Baker B. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study. N Engl J Med 986;314:1547‐52 |
VA available |
| Black 2002 / EHC‐OM | EHC‐OM: National Health Service (NHS). The treatment of persistent glue ear in children. Effective Health Care (Bulletin) November 1992, Number 4 | EHC‐OM is available |
| Buyle 2010/ Belgian guidelines |
Belgian guidelines for sequential antibiotic therapy (intravenous to oral with fluoroquinolones) published in Pharmacotherapeutic Committee drug letter (October 2003) | Available |
| Coopersmith 2002/self‐study module |
10‐page self‐study module on risk factors and practice modifications involved in catheter‐related infections for registered nurses | Not available |
| Denig 1990/Dutch drug bulletin | Dutch drug bulletin Geneesmiddelenbulletin for physicians and pharmacists | Not available |
| Dormuth 2004/Canadian drug bulletin | 12 issues of the drug bulletin Therapeutics Letter | Not available |
| Fijn 2000/Dutch national recommendations | Dutch national recommendations on antithrombotic prophylaxis of ischaemic heart disease | Not available |
| Fonarow 2009/MIRACL, PROVE‐IT TIMI 22, AHA‐AHA‐NS and ACC‐AHA‐STEMI |
2 publications in peer‐reviewed journals: MIRACL: Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001;285:1711‐8 PROVEIT‐TIMI22: Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495‐504 2 guidelines: AHA‐AHA‐NS : ACC/AHA 2002 guideline update for the management of patients with unstable angina and non–ST‐segment elevation myocardial infarction ACC‐AHA‐STEMI: ACC/AHA guidelines for the management of patients with ST‐elevation myocardial infarction |
MIRACL, PROVE‐IT TIMI 22, AHA‐AHA‐NS, ACC‐AHA‐STEMI are available |
| Fukuda 2009/Japanese guidelines on breast cancer |
Japanese evidence‐based clinical practice guidelines for treatment of early‐stage breast cancer | Not available |
| Guay 2007 / WHI trial report |
Publication in peer‐reviewed journal: Writing Group for the Women’s Health Initiative Investigators. Risk and benefits of estrogens plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative Randomized Controlled Trial. JAMA 2002;288:321‐33 |
WHI is available |
| Haas 2004 / HERS and WHI trials reports |
2 publications in peer‐reviewed journals: HERS: Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA 1998;280:605‐13. WHI: Writing Group for the Women’s Health Initiative Investigators. Risk and benefits of estrogens plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative Randomized Controlled Trial. JAMA 2002;288:321‐33 |
HERS, WHI are available |
| Hersh 2004 / HERS, HERS II, WHI trials reports |
3 publications in peer‐reviewed journals: HERS: Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA 1998;280:605‐13 HERS II: Grady D, Herrington D, Bittner V, et al. Cardiovascular disease outcomes during 6.8 years of hormone therapy. JAMA 2002;288:49‐57 WHI: Writing Group for the Women’s Health Initiative Investigators. Risk and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 2002;288:321‐33 |
HERS, HERS II, WHI are available |
| Jackevicius 2001/4S trial report |
Publication in peer‐reviewed journal: Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383‐9 |
4S Available |
| Jameson 2010/ NICE guidelines for orthopaedic surgery |
The National Institute for Health and Clinical Excellence's recommendations and guideline on prophylaxis for venous thromboembolism in orthopaedic surgery | Not available |
| Jousimaa 2002 / Finnish guidelines |
Collection of Finnish clinical practice guidelines for primary and ambulatory care Evidence‐Based Medicine Guidelines (previously Physician's Desk Reference and Database) | Not available |
| Juurlink 2004 / RALES trial report | Publication in peer‐reviewed journal: Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999;341:709‐17 |
RALES is available |
| Kabir 2007 / LIFE, ALLHAT and VALUE trials reports |
3 publications in peer‐reviewed journals: LIFE: Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, deFaire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe‐Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H, LIFE Study Group. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 2002;359:995–1003 ALLHAT: ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs. diuretic: the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002;288:2981–97 VALUE: Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, Hua T, Laragh J, McInnes GT, Mitchell L, Plat F, Schork A, Smith B, Zanchetti A, VALUE trial group. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet 2004;363:2022–31 |
ALLHAT, VALUE, LIFE are available |
| Kajita 2012/ Japanese guidelines on osteoporosis |
Japanese evidence‐based guideline Evidence‐based guideline for the prevention of osteoporosis and osteoporotic fractures in community health | Not available |
| Kottke 1989/Smoking cessation booklet | Smoking cessation booklet Quit‐and‐win | Available |
| Lam 2009 / 4D trial report |
Publication in peer‐reviewed journal: Wanner C, Krane V, Marz W et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 2005;353:238–48 |
4D is available |
| Majumdar 2003/ HOPE and RALES trials reports |
2 studies published in peer‐reviewed journals: HOPE study published in:
Randomized Aldactone Evaluation Study (RALES):
|
HOPE and RALES trials publications are available Available |
| Majumdar 2004 / WHI trial report |
Publication in peer‐reviewed journal: Writing Group for the Women’s Health Initiative Investigators. Risk and benefits of estrogens plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative Randomized Controlled Trial. JAMA 2002;288:321‐33 |
WHI is available |
| Mason 1998 / EHC‐D |
EHC‐D: National Health Service (NHS). The treatment of depression in primary care. Effective Health Care (Bulletin) March 1993, Number 5 | EHC‐D is available |
| Mason 2001 / EHC‐OM |
EHC‐OM: National Health Service (NHS). The treatment of persistent glue ear in children. Effective Health Care (Bulletin) November 1992, Number 4 | EHC‐OM is available |
| Matowe 2002/ UK Royal college of radiologists guidelines |
Royal College of Radiologists. Making the Best Use of a Department of Radiology: Guidelines for Doctors. London: Royal College of Radiologists, 1998 | Not available |
| Meyer 2007/ German guidelines for the ICU | Guidelines on empirical antibiotic treatment in the Intensive Care Unit (ICU) | Not available |
| Oakeshott 1994/UK Royal college of radiologists guidelines | Royal College of Radiologists. Making the Best Use of a Department of Radiology: Guidelines for Doctors. London: Royal College of Radiologists, 1990 | Not available |
| Perria 2007/ Italian guidelines | Italian evidence‐based guidelines for the management of non‐complicated type 2 diabetes mellitus | Not available |
| Roberts 2007/NICE guidelines for primary hip replacement |
National Institute for Health and Clinical Excellence (NICE). Guidelines on the Selection of Prostheses for Primary Hip Replacement. London: NHS, April 2000 | Available |
| Santerre 1996/ACOG guidelines |
American College of Obstetricians and Gynecologists (ACOG) clinical management guidelines for vaginal birth after cesarean delivery (VBAC) | Not available |
| Shah 2008/Nissen and al. study report |
Publication in peer‐reviewed journal: Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. New Engl J Med 2007;356:2457–71 |
Available |
| Stafford 2004 / ALLHAT trial report |
Publication in peer‐reviewed journal: ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs. diuretic: the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002;288:2981‐97 |
ALLHAT is available |
| Tsuji 2009/Guidelines for physician depression |
Depression diagnosis and treatment guide for primary care physicians | Not available |
| Wang 2005/ADA and ATP III trials reports | ADA: American Diabetes Association (ADA) guidelines published in January 1998 advocated an LDL cholesterol goal under 100 mg/dl for patients with diabetes Publication in peer‐reviewed journal: ATP III: Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults: Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486–97 |
ATP III is available |
| Watson 2001/Guidelines for musculoskeletal disorder | Guidelines for the use of oral Non‐Steroidal Antiinflammatory drugs (NSAIDs) in the management of musculoskeletal disorders | Available: Watson M. The Development, Implementation And Evaluation Of Prescribing Guidelines In General Practice. 1998; PhD Thesis Algorithm |
| Weiss 2011/Quebec guidelines on antibiotics |
Eleven 2‐page graphic user‐friendly guidelines providing clinical information and antibiotic recommendations | Available |
Appendix 4. Full‐text papers assessed for eligibility but excluded from the review
Reference List
(1) Effective IEC approaches for Asia. JOICFP News 1995 Feb;(248):7. [PM: 12288395]
(2) Special report: the publication of new evidence and effect on physician prescribing behaviors. Technology Evaluation Center Assessment Program 2004 Dec;19(11):1‐3. [PM: 15651133]
(3) Standards on verbal orders rank high among common compliance problems. ED Management 2009 May;21(5):Suppl 1‐2. [PM: 19552346]
(4) Adam C, Rosser D, Manji M. Impact of introducing a sedation management guideline in intensive care. Anaesthesia 2006 Mar;61(3):260‐3. [PM: 16480351]
(5) Adams A, Ockene JK, Wheller EV, Hurley TG. Alcohol counseling: physicians will do it. Journal of General Internal Medicine 1998 Oct;13(10):692‐8. [PM: 9798817]
(6) Adank K, Barras JP, Biland L, Bollinger A, Galeazzi R, Kampf R, et al. Guidelines for the evaluation of the therapeutic effects of peripheral vasoactive drugs in arterial occlusive disease. Vasa 1981;10(4):337‐41. [PM: 7032111]
(7) Afghani B, Ngo T, Leu SY, Wu FL, Cecilio M, Aron‐Johnson P, et al. The effect of an interventional program on adherence to the american academy of pediatrics guidelines for palivizumab prophylaxis. Pediatric Infectious Disease Journal 2006 Nov;25(11):1019‐24. [PM: 17072124]
(8) Aghaie‐Jaladerany H, Cowell D, Geddes CC. The early impact of the United Kingdom Chronic Kidney Disease (CKD) guidelines on the number of new attendances at renal clinics. Scottish Medical Journal 2007 Nov;52(4):28‐31. [PM: 18092634]
(9) Akers L, Gordon JS, Andrews JA, Barckley M, Lichtenstein E, Severson HH. Cost effectiveness of changing health professionals' behavior: training dental hygienists in brief interventions for smokeless tobacco cessation. Preventive Medicine 2006 Dec;43(6):482‐7. [PM: 16920184]
(10) Al‐Dabagh H, Archer J, Newton M, Kwagyan J, Nunlee‐Bland G. Osteoporosis awareness protocol for patients with fragility fractures. Journal of the National Medical Association 2009 Feb;101(2):145‐50. [PM: 19378631]
(11) Alerany C, Campany D, Monterde J, Semeraro C. Impact of local guidelines and an integrated dispensing system on antibiotic prophylaxis quality in a surgical centre. Journal of Hospital Infection 2005 Jun;60(2):111‐7. [PM: 15866008]
(12) Amamra N, Touzet S, Colin C, Ponchon T. Impact of guidelines for endoscopy in patients with Barrett's esophagus: a multifaceted interventional study. Gastroenterologie Clinique et Biologique 2009 Jun;33(6‐7):470‐7. [PM: 19443156]
(13) Ammendolia C, Hogg‐Johnson S, Pennick V, Glazier R, Bombardier C. Implementing evidence‐based guidelines for radiography in acute low back pain: a pilot study in a chiropractic community. Journal of Manipulative & Physiological Therapeutics 2004 Mar;27(3):170‐9. [PM: 15129199]
(14) Ammerman A, Caggiula A, Elmer PJ, Kris‐Etherton P, Keyserling T, Lewis C, et al. Putting medical practice guidelines into practice: the cholesterol model. American Journal of Preventive Medicine 1994 Jul;10(4):209‐16. [PM: 7803063]
(15) Amodio‐Groton M, Sinnett MJ, Culshaw D, Leviton IM. Antibiotic guidelines for community‐acquired pneumonia: Are they effective and do physicians comply? Drug Benefit Trends 2003;15(8):40‐6. [EM 2003371758]
(16) Anwar RM, Dhanji A, Fish A, Singh S. Impact of protocol‐based guidelines on the management and outcome of acute upper gastrointestinal hemorrhage in a district general hospital. Canadian Journal of Gastroenterology 2003 Feb;17(2):97‐100. [PM: 12605245]
(17) Applegate H, Kelley ML, Applegate BW, Jayasinghe IK, Venters CL. Clinical case study: pediatric residents' discussions of and interventions for children's behavioral and emotional problems. Journal of Pediatric Psychology 2003 Jul;28(5):315‐21. [PM: 12808008]
(18) Arabi YM, Haddad S, Tamim HM, Al‐Dawood A, Al‐Qahtani S, Ferayan A, et al. Mortality reduction after implementing a clinical practice guidelines‐based management protocol for severe traumatic brain injury. Journal of Critical Care 2010 Jun;25(2):190‐5. [PM: 19592201]
(19) Arenas MD, Alvarez‐Ude F, Gil MT, Moledous A, Malek T, Nunez C, et al. Implementation of 'K/DOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease' after the introduction of cinacalcet in a population of patients on chronic haemodialysis. Nephrology Dialysis Transplantation 2007 Jun;22(6):1639‐44. [PM: 17277339]
(20) Armon K, MacFaul R, Hemingway P, Werneke U, Stephenson T. The impact of presenting problem based guidelines for children with medical problems in an accident and emergency department. Archives of Disease in Childhood 2004 Feb;89(2):159‐64. [PM: 14736635]
(21) Armstrong EP, Langley PC. The impact of a disease management intervention on the treatment of infectious disease in a managed care organization. Disease Management 1999;2(3):61‐77. [EM 1999346560]
(22) Armstrong EP, Langley PC. Treatment of pneumonias in a managed care organisation. The impact of a treatment guideline promotion on physician prescribing behaviour. Disease Management and Health Outcomes 1999;6(3):159‐73. [EM 1999334207]
(23) Armstrong EP. Clinical and economic outcomes of an ambulatory urinary tract infection disease management program. American Journal of Managed Care 2001 Mar;7(3):269‐80. [PM: 11258144]
(24) Armstrong G, Blashki G, Joubert L, Bland R, Moulding R, Gunn J, et al. An evaluation of the effect of an educational intervention for Australian social workers on competence in delivering brief cognitive behavioural strategies: a randomised controlled trial. BMC Health Services Research 2010;10(304). [PM: 21050497]
(25) Asaro PV, Sheldahl AL, Char DM. Physician perspective on computerized order‐sets with embedded guideline information in a commercial emergency department information system. Annual Symposium Proceedings 2005;6‐10. [PM: 16778991]
(26) Assanasen S, Edmond M, Bearman G. Impact of 2 different levels of performance feedback on compliance with infection control process measures in 2 intensive care units. American Journal of Infection Control 2008 Aug;36(6):407‐13. [PM: 18675146]
(27) Atkinson MH, Menard HA, Kalish GH. Assessment of salsalate, a nonacetylated salicylate, in the treatment of patients with arthritis. Clinical Therapeutics 1995 Sep;17(5):827‐37. [PM: 8595635]
(28) Aubin M, Vezina L, Maziade J, Robitaille NM. Control of arterial hypertension: effectiveness of an intervention performed by family practitioners. Canadian Family Physician 1994 Oct;40:1742‐52. [PM: 7950469]
(29) Aucott JN, Pelecanos E, Dombrowski R, Fuehrer SM, Laich J, Aron DC. Implementation of local guidelines for cost‐effective management of hypertension. A trial of the firm system. Journal of General Internal Medicine 1996 Mar;11(3):139‐46. [PM: 8667090]
(30) Auleley GR, Ravaud P, Giraudeau B, Kerboull L, Nizard R, Massin P, et al. Implementation of the Ottawa ankle rules in France. A multicenter randomized controlled trial. JAMA 1997 Jun 25;277(24):1935‐9. [PM: 9200633]
(31) Avanzini F, Tognoni G, Alli C, Colombo F, Herxheimer A. How informed general practitioners manage mild hypertension: a survey of readers of drug bulletins in 7 countries. International Society of Drug Bulletins (ISDB). European Journal of Clinical Pharmacology 1996;49(6):445‐50. [PM: 8706768]
(32) Avila‐Aguero ML, Umana MA, Jimenez AL, Faingezicht I, Paris MM. Handwashing practices in a tertiary‐care, pediatric hospital and the effect on an educational program. Clinical Performance & Quality Health Care 1998 Apr;6(2):70‐2. [PM: 10180124]
(33) Bahrami M, Deery C, Clarkson JE, Pitts NB, Johnston M, Ricketts I, et al. Effectiveness of strategies to disseminate and implement clinical guidelines for the management of impacted and unerupted third molars in primary dental care, a cluster randomised controlled trial. British Dental Journal 2004 Dec 11;197(11):691‐6. [PM: 15592551]
(34) Bakemeier RF, Beck S, Murphy JR. Educational and consultative functions, topics, and methods of hospital general tumor conferences. Journal of Cancer Education 1995;9(4):217‐25. [PM: 7734286]
(35) Baker JA, Lovell K, Harris N. The impact of a good practice manual on professional practice associated with psychotropic PRN in acute mental health wards: an exploratory study. International Journal of Nursing Studies 2008 Oct;45(10):1403‐10. [PM: 18313059]
(36) Baker R, Reddish S, Robertson N, Hearnshaw H, Jones B. Randomised controlled trial of tailored strategies to implement guidelines for the management of patients with depression in general practice. British Journal of General Practice 2001 Sep;51(470):737‐41. [PM: 11593835]
(37) Baker R, Falconer SJ, Lambert PC. Randomised controlled trial of the effectiveness of feedback in improving test ordering in general practice. Scandinavian Journal of Primary Health Care 2003 Dec;21(4):219‐23. [PM: 14695072]
(38) Baker R, Fraser RC, Stone M, Lambert P, Stevenson K, Shiels C. Randomised controlled trial of the impact of guidelines, prioritized review criteria and feedback on implementation of recommendations for angina and asthma. British Journal of General Practice 2003 Apr;53(489):284‐91. [PM: 12879828]
(39) Ballard PE. New pamphlet enables dentists to take offensive. Journal ‐ Mississippi Dental Association 1973 Nov;29(4):‐13. [PM: 4587132]
(40) Banait G, Sibbald B, Thompson D, Summerton C, Hann M, Talbot S, et al. Modifying dyspepsia management in primary care: a cluster randomised controlled trial of educational outreach compared with passive guideline dissemination. British Journal of General Practice 2003 Feb;53(487):94‐100. [PM: 12817353]
(41) Bankole OO, Aderinokun GA, Denloye OO. Evaluation of a photo‐poster on nurses' perceptions of teething problems in South‐western Nigeria. Public Health 2005 Apr;119(4):276‐82. [PM: 15733687]
(42) Barazzoni F, Grilli R, Amicosante AM, Brescianini S, Marca MA, Baggi M, et al. Impact of end user involvement in implementing guidelines on routine pre‐operative tests. International Journal for Quality in Health Care 2002 Aug;14(4):321‐7. [PM: 12201191]
(43) Barner CW, Wylle‐Rosett J, Gans K. WAVE: a pocket guide for a brief nutrition dialogue in primary care. Diabetes Educator 2001 May;27(3):352‐8. [PM: 11912796]
(44) Barnes K, Itzkowitz S, Brown K. Teaching clinical management skills for genetic testing of hereditary nonpolyposis colorectal cancer using a Web‐based tutorial. Genetics in Medicine 2003 Jan;5(1):43‐8. [PM: 12544475]
(45) Barnes S. Preparing for surgery: providing the details. Journal of PeriAnesthesia Nursing 2001 Feb;16(1):31‐2. [PM: 11266642]
(46) Barry HC, Ebell MH, Shaughnessy AF, Slawson DC, Nietzke F. Family physicians' use of medical abstracts to guide decision making: style or substance? Journal of the American Board of Family Practice 2001 Nov;14(6):437‐42. [PM: 11757886]
(47) Bartholomew LK, Cushman WC, Cutler JA, Davis BR, Dawson G, Einhorn PT, et al. Getting clinical trial results into practice: design, implementation, and process evaluation of the ALLHAT Dissemination Project. Clinical Trials 2009 Aug;6(4):329‐43. [PM: 19587068]
(48) Baskin C, Seetharamu N, Mazure B, Vassallo L, Steinberg H, Kerpen H, et al. Effect of a CD‐ROM‐based educational intervention on resident knowledge and adherence to deep venous thrombosis prophylaxis guidelines. Journal of Hospital Medicine (Online) 2008 Jan;3(1):42‐7. [PM: 18257045]
(49) Beaulieu MD, Rivard M, Hudon E, Beaudoin C, Saucier D, Remondin M. Comparative trial of a short workshop designed to enhance appropriate use of screening tests by family physicians. CMAJ Canadian Medical Association Journal 2002 Nov 26;167(11):1241‐6. [PM: 12451077]
(50) Becker A, Leonhardt C, Kochen MM, Keller S, Wegscheider K, Baum E, et al. Effects of two guideline implementation strategies on patient outcomes in primary care: a cluster randomized controlled trial. Spine 2008 Mar 1;33(5):473‐80. [PM: 18317189]
(51) Bedlow AJ, Cliff S, Melia J, Moss SM, Seyan R, Harland CC. Impact of skin cancer education on general practitioners' diagnostic skills. Clinical & Experimental Dermatology 2000 Mar;25(2):115‐8. [PM: 10733633]
(52) Beilby J, Wutzke SE, Bowman J, Mackson JM, Weekes LM. Evaluation of a national quality use of medicines service in Australia: an evolving model. Journal of Evaluation in Clinical Practice 2006 Apr;12(2):202‐17. [PM: 16579830]
(53) Bekkering GE HHTvKMKDORBL. Effectiveness of an active intervention strategy for the implementation of the Dutch physiotherapy guideline on low back pain. Nederlands Tijdschrift Fysiotherapie 2005;115(3):62‐7.
(54) Bekkering GE, Hendriks HJ, van Tulder MW, Knol DL, Hoeijenbos M, Oostendorp RA, et al. Effect on the process of care of an active strategy to implement clinical guidelines on physiotherapy for low back pain: a cluster randomised controlled trial. Quality & Safety in Health Care 2005 Apr;14(2):107‐12. [PM: 15805455]
(55) Bekkering GE, van Tulder MW, Hendriks EJ, Koopmanschap MA, Knol DL, Bouter LM, et al. Implementation of clinical guidelines on physical therapy for patients with low back pain: randomized trial comparing patient outcomes after a standard and active implementation strategy. Physical Therapy 2005 Jun;85(6):544‐55. [PM: 15921475]
(56) Bell DS, Fonarow GC, Hays RD, Mangione CM. Self‐study from web‐based and printed guideline materials. A randomized, controlled trial among resident physicians. Annals of Internal Medicine 2000 Jun 20;132(12):938‐46. [PM: 10858176]
(57) Bellavance M, Rohlicek CV, Bigras JL, Cote JM, Paquet M, Lebel MH, et al. Palivizumab use among children with congenital heart disease in Quebec: Impact of Canadian guidelines on clinical practice. Paediatrics & Child Health 2006 Jan;11(1):19‐23. [PM: 19030237]
(58) Belongia EA, Knobloch MJ, Kieke BA, Davis JP, Janette C, Besser RE. Impact of statewide program to promote appropriate antimicrobial drug use. Emerging Infectious Diseases 2005 Jun;11(6):912‐20. [PM: 15963287]
(59) Benhamou D, Gross E, Brosseau M. Blood transfusion in adults: description of a quality assurance program. Annales Francaises d Anesthesie et de Reanimation 2001 Jan;20(1):57‐69. [PM: 11234582]
(60) Benjamin EM, Schneider MS, Hinchey KT. Implementing practice guidelines for diabetes care using problem‐based learning. A prospective controlled trial using firm systems. Diabetes Care 1999 Oct;22(10):1672‐8. [PM: 10526733]
(61) Beno L, Hinchman J, Kibbe D, Trowbridge F. Design and implementation of training to improve management of pediatric overweight. Journal of Continuing Education in the Health Professions 2005;25(4):248‐58. [PM: 16365897]
(62) Bernal‐Delgado E, Galeote‐Mayor M, Pradas‐Arnal F, Peiro‐Moreno S. Evidence based educational outreach visits: effects on prescriptions of non‐steroidal anti‐inflammatory drugs. Journal of Epidemiology & Community Health 2002 Sep;56(9):653‐8. [PM: 12177080]
(63) Berti A, Bregani ER, Manenti F, Pizzi C. Outcome of severely malnourished children treated according to UNICEF 2004 guidelines: a one‐year experience in a zone hospital in rural Ethiopia. Transactions of the Royal Society of Tropical Medicine & Hygiene 2008 Sep;102(9):939‐44. [PM: 18597802]
(64) Bertoni AG, Bonds DE, Chen H, Hogan P, Crago L, Rosenberger E, et al. Impact of a multifaceted intervention on cholesterol management in primary care practices: guideline adherence for heart health randomized trial. Archives of Internal Medicine 2009 Apr 13;169(7):678‐86. [PM: 19364997]
(65) Bertsche T, Bertsche A, Krieg EM, Kunz N, Bergmann K, Hanke G, et al. Prospective pilot intervention study to prevent medication errors in drugs administered to children by mouth or gastric tube: a programme for nurses, physicians and parents. Quality & Safety in Health Care 2010 Oct;19(5):e26. [PM: 20378618]
(66) Bialasiewicz AA, Breidenbach K, Ganesh A, Al‐Saeidi R, Ganguly SS, International Standards Organization. Conceiving and implementing an ISO 9001:2000 quality management system: quality improvement and efficiency increase over 3 years at the Dept. of Ophthalmology, Sultan Qaboos University in Oman. Ophthalmologe 2006 Oct;103(10):877‐87. [PM: 16821074]
(67) Bialasiewicz AA, Breidenbach K, Ganesh A, Al‐Saeidi R, Ganguly SS. Conceiving and implementing an ISO 9001:2000 quality management system. Quality improvement and efficiency increase over 3 years at the Dept. of Ophthalmology, Sultan Qaboos University in Oman. Ophthalmologe 2006;103(10):877‐87. [EM 2006521136]
(68) Biermann M, Pixberg MK, Dorr U, Dietlein M, Schlemmer H, Grimm J, et al. Guidelines on radioiodine therapy for differentiated thyroid carcinoma: impact on clinical practice. Nuclear‐Medizin 2005;44(6):229‐34. [PM: 16400382]
(69) Billi JE, Duran‐Arenas L, Wise CG, Bernard AM, McQuillan M, Stross JK. The effects of a low‐cost intervention program on hospital costs. Journal of General Internal Medicine 1992 Jul;7(4):411‐7. [PM: 1506947]
(70) Billings JA, Keeley A, Bauman J, Cist A, Coakley E, Dahlin C, et al. Merging cultures: palliative care specialists in the medical intensive care unit. Critical Care Medicine 2006 Nov;34(Suppl):S388‐93. [PM: 17057603]
(71) Bingham C, Costenbader K, Bender S, Duch D, Weinblatt M. Educating primary care providers on rheumatoid arthritis translates to changes in practice and referrals: Results from the RAPID continuing medical education program [Conference: American College of Rheumatology/Association of Rheumatology Health Professionals Annual Scientific Meeting, ACR/ARHP 10 Philadelphia, PA United States]. Arthritis and Rheumatism 2010. [EM 70380806]
(72) Bishop PB, Quon JA, Fisher CG, Dvorak MF. The Chiropractic Hospital‐based Interventions Research Outcomes (CHIRO) study: a randomized controlled trial on the effectiveness of clinical practice guidelines in the medical and chiropractic management of patients with acute mechanical low back pain. Spine Journal 2010 Dec;10(12):1055‐64. [PM: 20889389]
(73) Bittenbender P, Nortier N, O'Brien C, Oosterheert J, Smoes J. Emergencies happen, MERTs to the rescue! NASN school nurse 2009 Nov;24(6):268‐78. [PM: 20440943]
(74) Bjerrum L, Munck A, Gahrn‐Hansen B, Hansen MP, Jarboel D, Llor C, et al. Health Alliance for Prudent Prescribing, Yield and Use of Antimicrobial Drugs in the Treatment of Respiratory Tract Infections (HAPPY AUDIT). BMC Family Practice 2010;11(29). [PM: 20416034]
(75) Black RE. Communication for improved health services. Development Communication Report 1985;(51):1‐2. [PM: 12340537]
(76) Blais R, Laurier C, Pare M. Effect of feedback letters to physicians and pharmacists on the appropriate use of medication in the treatment of asthma. Journal of Asthma 2008 Apr;45(3):227‐31. [PM: 18415831]
(77) Bland DR, Dugan E, Cohen SJ, Preisser J, Davis CC, McGann PE, et al. The effects of implementation of the Agency for Health Care Policy and Research urinary incontinence guidelines in primary care practices. Journal of the American Geriatrics Society 2003 Jul;51(7):979‐84. [PM: 12834518]
(78) Blauer C, Schierz‐Hungerbuhler J, Trachsel E, Spirig R, Frei IA. A nurse‐led multidisciplinary malnutrition program to assess and treat patients with malnutrition or those at risk of malnutrition. Pflege 2008 Aug;21(4):225‐34. [PM: 18677686]
(79) Bleakney GM, McErlain S. Infant feeding guidelines: An evaluation of their effect on health professionals' knowledge and attitudes. Journal of Human Nutrition and Dietetics 1996;9(6):437‐50. [EM 1997041598]
(80) Block PC. Achieving effective cardiac care. ACC Current Journal Review 2004;13(7):16‐7. [EM 2004400227]
(81) Bobbio M, Malavasi P, Negri G, Salera M, Sapigni E, Carossino P, et al. The role of public health pharmacists in promoting information on benefits and risks of drugs: Evaluation of the impact of a new information strategy for primary care groups. Giornale Italiano di Farmacia Clinica 2011;25(1):12‐24. [EM 2011282726]
(82) Boddy J, Lai N, Lynch C, Shahzad SA, Doherty AP, Wallace DMA, et al. The role of active surveillance in the management of early prostate cancer and the impact of the NICE guidelines. British Journal of Medical and Surgical Urology 2010;3(3):101‐5. [EM 2010208033]
(83) Bolon MK, Arnold AD, Feldman HA, Goldmann DA, Wright SB. An antibiotic order form intervention does not improve or reduce vancomycin use. Pediatric Infectious Disease Journal 2005 Dec;24(12):1053‐8. [PM: 16371865]
(84) Bonetti D, Johnston M, Pitts NB, Deery C, Ricketts I, Bahrami M, et al. Can psychological models bridge the gap between clinical guidelines and clinicians' behaviour? A randomised controlled trial of an intervention to influence dentists' intention to implement evidence‐based practice. British Dental Journal 2003 Oct 11;195(7):403‐7. [PM: 14551633]
(85) Bonner A, MacCulloch P, Gardner T, Chase CW. A student‐led demonstration project on fall prevention in a long‐term care facility. Geriatric Nursing 2007 Sep;28(5):312‐8. [PM: 17923288]
(86) Bonson M, Phillips A. Designing and implementing an intravenous therapy book. Nursing Times 2006 Jul 18;102(29):34‐5. [PM: 16895248]
(87) Boolchand V, Olds G, Singh J, Singh P, Chak A, Cooper GS. Colorectal screening after polypectomy: a national survey study of primary care physicians. Annals of Internal Medicine 2006 Nov 7;145(9):654‐9. [PM: 17088578]
(88) Boonmak P, Boonmak S, Srichaipanha S, Poomsawat S. Knowledge and skill after brief ACLS training. Journal of the Medical Association of Thailand 2004 Nov;87(11):1311‐4. [PM: 15825705]
(89) Boonstra E, Lindbaek M, Khulumani P, Ngome E, Fugelli P. Adherence to treatment guidelines in primary health care facilities in Botswana. Tropical Medicine & International Health 2002 Feb;7(2):178‐86. [PM: 11841708]
(90) Boonstra E, Lindbaek M, Ngome E. Adherence to management guidelines in acute respiratory infections and diarrhoea in children under 5 years old in primary health care in Botswana. International Journal for Quality in Health Care 2005 Jun;17(3):221‐7. [PM: 15723818]
(91) Borntrager CF, Chorpita BF, Higa‐McMillan C, Weisz JR. Provider attitudes toward evidence‐based practices: are the concerns with the evidence or with the manuals? Psychiatric Services 2009 May;60(5):677‐81. [PM: 19411357]
(92) Botash AS, Galloway AE, Booth T, Ploutz‐Snyder R, Hoffman‐Rosenfeld J, Cahill L. Continuing medical education in child sexual abuse: cognitive gains but not expertise. Archives of Pediatrics & Adolescent Medicine 2005 Jun;159(6):561‐6. [PM: 15939856]
(93) Boufous S, Kelleher PW, Pain CH, Dann LM, Ieraci S, Jalaludin BB, et al. Impact of a chest‐pain guideline on clinical decision‐making. Medical Journal of Australia 2003 Apr 21;178(8):375‐80. [PM: 12697008]
(94) Bould MD, Hayter MA, Campbell D, Chandra D, Joo HS, Naik V. A cognitive aid for neonatal resuscitation: A randomized controlled trial [Conference 2009 CAS Annual Meeting Vancouver, BC Canada]. Canadian Journal of Anesthesia 2009;56:s16. [EM 70078698]
(95) Bould MD, Hayter MA, Campbell DM, Chandra DB, Joo HS, Naik VN. Cognitive aid for neonatal resuscitation: a prospective single‐blinded randomized controlled trial. British Journal of Anaesthesia 2009 Oct;103(4):570‐5. [PM: 19689979]
(96) Boursnell M, Prosser S. Putting children in the picture: improving responses to domestic violence in the emergency department. DEVELOPING PRACT 2001;(23):56‐63.
(97) Bowman MA, Russell NK, Boekeloo BO, Rafi IZ, Rabin DL. The effect of educational preparation on physician performance with a sexually transmitted disease‐simulated patient. Archives of Internal Medicine 1992 Sep;152(9):1823‐8. [PM: 1520049]
(98) Bradley WG, Anderson F, Gowda N, Miller RG, ALS CARE Study Group. Changes in the management of ALS since the publication of the AAN ALS practice parameter 1999. Amyotrophic Lateral Sclerosis & Other Motor Neuron Disorders 2004 Dec;5(4):240‐4. [PM: 15799554]
(99) Branch C, Kolovetsios T, Apampa B, Ndawula E. Compliance with prophylactic antibiotic guidelines during elective orthopaedic surgery in a foundation trust hospital: An audit [Conference: British Pharmaceutical Conference 2009 Manchester United Kingdom]. International Journal of Pharmacy Practice 2009;17(S2):B56‐7. [EM 70139969]
(100) Brattebo G, Hofoss D, Flaatten H, Muri AK, Gjerde S, Plsek PE. Effect of a scoring system and protocol for sedation on duration of patients' need for ventilator support in a surgical intensive care unit. BMJ 2002 Jun 8;324(7350):1386‐9. [PM: 12052813]
(101) Brimmer DJ, McCleary KK, Lupton TA, Faryna KM, Reeves WC. Continuing medical education challenges in chronic fatigue syndrome. BMC Medical Education 2009;9(70). [PM: 19954535]
(102) Britto MT, Schoettker PJ, Pandzik GM, Weiland J, Mandel KE. Improving influenza immunisation for high‐risk children and adolescents. Quality & Safety in Health Care 2007 Oct;16(5):363‐8. [PM: 17913778]
(103) Brown JB, Shye D, McFarland BH, Nichols GA, Mullooly JP, Johnson RE. Controlled trials of CQI and academic detailing to implement a clinical practice guideline for depression. Joint Commission Journal on Quality Improvement 2000 Jan;26(1):39‐54. [PM: 10677821]
(104) Brown R, Bratton SL, Cabana MD, Kaciroti N, Clark NM. Physician asthma education program improves outcomes for children of low‐income families. Chest 2004 Aug;126(2):369‐74. [PM: 15302719]
(105) Buchan H, Lourey E, D'Este C, Sanson‐Fisher R. Effectiveness of strategies to encourage general practitioners to accept an offer of free access to online evidence‐based information: a randomised controlled trial. Implementation Science 2009;4(68). [PM: 19840400]
(106) Buckley C, Sharrard H. Lithium monitoring for patients with learning disability: The role of the general practitioner. Quality in Primary Care 2003;11(4):329‐31. [EM 2004017977]
(107) Burke SM. Creating competency for home healthcare. Case management manual: a work‐in‐progress. Home Healthcare Nurse Manager 1999 Nov;3(6):2‐7. [PM: 10876502]
(108) Button D, Roe B, Webb C, Frith T, Colin‐Thome D, Gardner L. Consensus guidelines for the promotion and management of continence by primary health care teams: development, implementation and evaluation. NHS Executive Nursing Directorate. J ADV NURS 1998 Jan;27(1):91‐9. [PM: 9515613]
(109) Callegari S, Pini M, Andreoli L. Guidelines and clinical practice: anticoagulant therapy and cardioversion in atrial fibrillation. Giornale Italiano di Cardiologia 1999 Oct;29(10):1157‐63. [PM: 10546125]
(110) Callen J, Georgiou A, Prgomet M, Paoloni R, Westbrook J. A qualitative analysis of Emergency Department physicians' practices and perceptions in relation to test result follow‐up. Studies in Health Technology & Informatics 2010;160(Pt 2):1241‐5. [PM: 20841882]
(111) Calvo CB, Rubinstein A. Influence of new evidence on prescription patterns. Journal of the American Board of Family Practice 2002 Nov;15(6):457‐62. [PM: 12463291]
(112) Campina CS, Neto M, Trindade M. Beta‐lactam hypersensitivity: from guidelines to daily practice. Journal of Investigational Allergology & Clinical Immunology 2010;20(2):175‐6. [PM: 20461978]
(113) Campione JR, Sleath B, Biddle AK, Weinberger M. The influence of physicians' guideline compliance on patients' statin adherence: a retrospective cohort study. American Journal of Geriatric Pharmacotherapy 2005 Dec;3(4):229‐39. [PM: 16503318]
(114) Caniza MA, Maron G, Moore EJ, Quintana Y, Liu T. Effective hand hygiene education with the use of flipcharts in a hospital in El Salvador. Journal of Hospital Infection 2007 Jan;65(1):58‐64. [PM: 17147972]
(115) Cannon DS, Allen SN. A comparison of the effects of computer and manual reminders on compliance with a mental health clinical practice guideline. Journal of the American Medical Informatics Association 2000 Mar;7(2):196‐203. [PM: 10730603]
(116) Cauffman JG, Forsyth RA, Clark VA, Foster JP, Martin KJ, Lapsys FX, et al. Randomized controlled trials of continuing medical education: what makes them most effective? Journal of Continuing Education in the Health Professions 2002;22(4):214‐21. [PM: 12613056]
(117) Cave MT, Clandinin DJ. Revisiting the journal club. Medical Teacher 2007 May;29(4):365‐70. [PM: 17786752]
(118) Cayten CG, Staroscik R, Walker K, Morganroth J, Oler J. Impact of prehospital cardiac algorithms on ventricular fibrillation survival rates. Annals of Emergency Medicine 1981 Aug;10(8):432‐6. [PM: 7258758]
(119) Chalmers J. Implementation of guidelines for management of hypertension. Clinical & Experimental Hypertension (New York) 1999 Jul;21(5‐6):647‐57. [PM: 10423089]
(120) Chan JK, Sleat G, Sharma S, Phoenix G, Graham A. Gastroprotection in trauma patients receiving non‐steroidal anti‐inflammatory drugs. Surgeon Journal of the Royal Colleges of Surgeons of Edinburgh & Ireland 2010 Aug;8(4):206‐10. [PM: 20569940]
(121) Cheater FM, Baker R, Reddish S, Spiers N, Wailoo A, Gillies C, et al. Cluster randomized controlled trial of the effectiveness of audit and feedback and educational outreach on improving nursing practice and patient outcomes. Medical Care 2006 Jun;44(6):542‐51. [PM: 16708003]
(122) Cheng C, Umland E. Practical therapeutics: an innovative residency drug education curriculum. Family Medicine 2004 Apr;36(4):236‐8. [PM: 15057610]
(123) Cheng G, Wong HF, Chan A, Chui CH. The effects of a self‐educating blood component request form and enforcements of transfusion guidelines on FFP and platelet usage. Queen Mary Hospital, Hong Kong. British Committee for Standards in Hematology (BCSH). Clinical & Laboratory Haematology 1996 Jun;18(2):83‐7. [PM: 8866139]
(124) Chevalier C, Giral P, Oger C, Costedoat‐Rouchet G, Carzon M, Trutt B. Changes in biological test practices after publication of national guidelines for lipid management among patients under statin treatment. Revue d Epidemiologie et de Sante Publique 2005 Sep;53(4):393‐7. [PM: 16353514]
(125) Chitre MM, Hayes W. 3‐year results of a member and physician intervention to reduce risk associated with glucocorticoid‐induced osteoporosis in a health plan. Journal of Managed Care Pharmacy 2008 Apr;14(3):281‐90. [PM: 18439050]
(126) Chowdhury AK, Khan OF, Matin MA, Begum K, Galib MA. Effect of standard treatment guidelines with or without prescription audit on prescribing for acute respiratory tract infection (ARI) and diarrhoea in some thana health complexes (THCs) of Bangladesh. Bangladesh Medical Research Council Bulletin 2007 Apr;33(1):21‐30. [PM: 18246731]
(127) Coenen S, Dirven K, Michiels B, Denekens J, Van RP. Implementing a clinical practice guideline on acute cough in general practice: a Belgian experience with academic detailing. Medecine et Maladies Infectieuses 2005 Jun;35(Suppl 2):S97‐9. [PM: 15978401]
(128) Cohen SM, Kataoka‐Yahiro M. Provider adherence to clinical guidelines related to lipid‐lowering medications. Military Medicine 2010 Feb;175(2):122‐6. [PM: 20180482]
(129) Cole S, Raju M, Barrett J, Gerrity M, Dietrich A. The MacArthur Foundation Depression Education Program for Primary Care Physicians: background and rationale. General Hospital Psychiatry 2000 Sep;22(5):299‐358. [PM: 11203035]
(130) Coleman CI, Reddy P, Laster‐Bradley NM, Dorval S, Munagala B, White CM. Effect of practitioner education on adherence to asthma treatment guidelines. Annals of Pharmacotherapy 2003 Jul;37(7‐8):956‐61. [PM: 12841800]
(131) Coleman EA, Lord J, Heard J, Coon S, Cantrell M, Mohrmann C, et al. The Delta project: increasing breast cancer screening among rural minority and older women by targeting rural healthcare providers. ONCOL NURS FORUM 2003 Jul;30(4):669‐77. [PM: 12861326]
(132) Coll‐Vinent B, Pacheco G, Junyent M, Benito L, Hoyo J, Garcia A, et al. Impact of implementing common guidelines at different care levels in a healthcare area on the improvement of atrial fibrillation treatment. Revista Espanola de Cardiologia 2007 Apr;60(4):392‐403. [PM: 17521548]
(133) Comer JB, Emanuelsen KL, Drezner AD, Knauft RF. Developing guidelines for thrombolytic therapy. American Journal of Hospital Pharmacy 1984 Nov;41(11):2374‐7. [PM: 6548871]
(134) Concato J, Horwitz RI. Clinical practice guidelines and scientific evidence. JAMA 2009 Jul 8;302(2):145‐7. [PM: 19584341]
(135) Cook DA, Dupras DM, Thompson WG, Pankratz VS. Web‐based learning in residents' continuity clinics: a randomized, controlled trial. Academic Medicine 2005 Jan;80(1):90‐7. [PM: 15618102]
(136) Cooke M, Mattick RP, Walsh RA. Differential uptake of a smoking cessation programme disseminated to doctors and midwives in antenatal clinics. Addiction 2001 Mar;96(3):495‐505. [PM: 11255588]
(137) Cosmi E, Bevilacqua G, Maranghi L, Anceschi MM. The policy of antenatal corticosteroid administration in Italy vs. other European countries. Journal of Maternal‐Fetal & Neonatal Medicine 2004 Nov;16(Suppl 2):1‐3. [PM: 15590424]
(138) Costa A, Santos C, Ayres‐de‐Campos D, Costa C, Bernardes J. Access to computerised analysis of intrapartum cardiotocographs improves clinicians' prediction of newborn umbilical artery blood pH. BJOG: An International Journal of Obstetrics & Gynaecology 2010 Sep;117(10):1288‐93. [PM: 20618316]
(139) Couturier J, Isserlin L, Lock J. Family‐based treatment for adolescents with anorexia nervosa: a dissemination study. Brunner‐Mazel Eating Disorders Monograph Series 2010 May;18(3):199‐209. [PM: 20419524]
(140) Couzigou C, Lamory J, Salmon‐Ceron D, Figard J, Vidal‐Trecan GM. Short peripheral venous catheters: effect of evidence‐based guidelines on insertion, maintenance and outcomes in a university hospital. Journal of Hospital Infection 2005 Mar;59(3):197‐204. [PM: 15694976]
(141) Cowin L, Davies R, Estall G, Berlin T, Fitzgerald M, Hoot S. De‐escalating aggression and violence in the mental health setting. International Journal of Mental Health Nursing 2003 Mar;12(1):64‐73. [PM: 14685961]
(142) Creedon SA. Healthcare workers' hand decontamination practices: compliance with recommended guidelines. J ADV NURS 2005 Aug;51(3):208‐16. [PM: 16033588]
(143) Creedon SA. Health care workers' hand decontamination practices: an Irish study. Clinical Nursing Research 2006 Feb;15(1):6‐26. [PM: 16410620]
(144) Crits‐Christoph P, Gibbons MB, Ring‐Kurtz S, Gallop R, Present J. A pilot study of community‐friendly manual‐guided drug counseling. Journal of Substance Abuse Treatment 2009 Jul;37(1):8‐16. [PM: 19038525]
(145) Croudace T, Evans J, Harrison G, Sharp DJ, Wilkinson E, McCann G, et al. Impact of the ICD‐10 Primary Health Care (PHC) diagnostic and management guidelines for mental disorders on detection and outcome in primary care. Cluster randomised controlled trial. British Journal of Psychiatry 2003 Jan;182:20‐30. [PM: 12509314]
(146) Cullen W, Stanley J, Langton D, Kelly Y, Staines A, Bury G. Hepatitis C infection among injecting drug users in general practice: a cluster randomised controlled trial of clinical guidelines' implementation. British Journal of General Practice 2006 Nov;56(532):848‐56. [PM: 17132352]
(147) Cummings SR, Richard RJ, Duncan CL, Hansen B, Vander MR, Gerbert B, et al. Training physicians about smoking cessation: a controlled trial in private practice. Journal of General Internal Medicine 1989 Nov;4(6):482‐9. [PM: 2685206]
(148) Cummings SR, Coates TJ, Richard RJ, Hansen B, Zahnd EG, VanderMartin R, et al. Training physicians in counseling about smoking cessation. A randomized trial of the "Quit for Life" program. Annals of Internal Medicine 1989 Apr 15;110(8):640‐7. [PM: 2930094]
(149) Cunningham CM, Hanley GE, Morgan S. Patterns in the use of benzodiazepines in British Columbia: examining the impact of increasing research and guideline cautions against long‐term use. Health Policy 2010 Oct;97(2‐3):122‐9. [PM: 20413177]
(150) Currie JD, Chrischilles EA, Kuehl AK, Buser RA. Effect of a training program on community pharmacists' detection of and intervention in drug‐related problems. Journal of the American Pharmaceutical Association 1997 Mar;NS37(2):182‐91. [PM: 9069692]
(151) D'Escrivan T, Lemaire JS, Ivanov E, Boulo M, Soubrier S, Mille FX, et al. Surgical antimicrobial prophylaxis: compliance to guidelines and impact of targeted information program. Annales Francaises d Anesthesie et de Reanimation 2005 Jan;24(1):19‐23. [PM: 15661460]
(152) Dancey JE. From quality of publication to quality of care: translating trials to practice. Journal of the National Cancer Institute 2010 May 19;102(10):670‐1. [PM: 20410467]
(153) Danchaivijitr S, Pichiensatian W, Apisarnthanarak A, Kachintorn K, Cherdrungsi R. Strategies to improve hand hygiene practices in two university hospitals. Journal of the Medical Association of Thailand 2005 Dec;88(Suppl 10):S155‐60. [PM: 16850662]
(154) Danhauer JL, Johnson CE, Rotan SN, Snelson TA, Stockwell JS. National survey of pediatricians' opinions about and practices for acute otitis media and xylitol use. Journal of the American Academy of Audiology 2010 May;21(5):329‐46. [PM: 20569667]
(155) Dartnell JG, Allen B, McGrath KM, Moulds RF. Prescriber guidelines improve initiation of anticoagulation. Medical Journal of Australia 1995 Jan 16;162(2):70‐3. [PM: 7530800]
(156) David A, Banerjee S. Effectiveness of "palliative care information booklet" in enhancing nurses' knowledge. Indian Journal of Palliative Care 2010 Sep;16(3):164‐7. [PM: 21218007]
(157) Davies J, Hillman E. Eczema and prescribing. Community Practitioner 2008 Oct;81(10):36‐7. [PM: 18853887]
(158) Davis D, Galbraith R, American College of Chest Physicians Health and Science Policy Committee. Continuing medical education effect on practice performance: effectiveness of continuing medical education: American College of Chest Physicians Evidence‐Based Educational Guidelines. Chest 2009 Mar;135(Suppl 3):42S‐8S. [PM: 19265075]
(159) Davis J, Roberts R, Davidson DL, Norman A, Ogston S, Grimshaw JM, et al. Implementation strategies for a Scottish national epilepsy guideline in primary care: results of the Tayside Implementation of Guidelines in Epilepsy Randomized (TIGER) trial. Epilepsia 2004 Jan;45(1):28‐34. [PM: 14692904]
(160) De SG, Harvey KJ, Howard D, Mashford ML, Moulds RF. Improving the quality of antibiotic prescription patterns in general practice. The role of educational intervention. Medical Journal of Australia 1994 Apr 18;160(8):502‐5. [PM: 8170427]
(161) de VW, Schelvis M, Rustemeijer I, Bierens JJ. Self‐training in the use of automated external defibrillators: the same results for less money. Resuscitation 2008 Jan;76(1):76‐82. [PM: 17714851]
(162) Demoly P, Concas V, Urbinelli R, Allaert FA. Spreading and impact of the World Health Organization's Allergic Rhinitis and its impact on asthma guidelines in everyday medical practice in France. Ernani survey. Clinical & Experimental Allergy 2008 Nov;38(11):1803‐7. [PM: 18721255]
(163) Dempsey J. Falls prevention revisited: a call for a new approach. Journal of Clinical Nursing 2004 May;13(4):479‐85. [PM: 15086634]
(164) Denoeud L, Mazieres B, Payen‐Champenois C, Ravaud P. First line treatment of knee osteoarthritis in outpatients in France: adherence to the EULAR 2000 recommendations and factors influencing adherence. Annals of the Rheumatic Diseases 2005 Jan;64(1):70‐4. [PM: 15608302]
(165) Deuster S, Roten I, Muehlebach S. Implementation of treatment guidelines to support judicious use of antibiotic therapy. Journal of Clinical Pharmacy & Therapeutics 2010 Feb;35(1):71‐8. [PM: 20175814]
(166) Devine SG, Harrison SL, Buettner PG. Building capacity of maternity staff to discourage the use of sunlight therapy in the post‐partum period and infancy. Women & Birth: Journal of the Australian College of Midwives 2008 Sep;21(3):107‐11. [PM: 18653392]
(167) Devlin JW, Holbrook AM, Fuller HD. The effect of ICU sedation guidelines and pharmacist interventions on clinical outcomes and drug cost. Annals of Pharmacotherapy 1997 Jun;31(6):689‐95. [PM: 9184706]
(168) Dewar B, Tocher R, Watson W. Enhancing partnerships with relatives in care settings. Nursing Standard 2003 Jun 18;17(40):33‐9. [PM: 12854194]
(169) Dey P, Simpson CW, Collins SI, Hodgson G, Dowrick CF, Simison AJ, et al. Implementation of RCGP guidelines for acute low back pain: a cluster randomised controlled trial. British Journal of General Practice 2004 Jan;54(498):33‐7. [PM: 14965404]
(170) Dheda K, Crawford A, Hagan G, Roberts CM. Implementation of British Thoracic Society guidelines for acute exacerbation of chronic obstructive pulmonary disease: impact on quality of life. Postgraduate Medical Journal 2004 Mar;80(941):169‐71. [PM: 15016940]
(171) Di Spiezio SA, Spinelli M, Sosa Fernandez LM, Scognamiglio M, Lisbino M, Cirillo P, et al. Comparison of textbook versus educational video for training in office operative hysteroscopy: A randomized, controlled trial [Conference: 19th Annual Congress of the European Society for Gynaecological Endoscopy, ESGE Barcelona Spain]. Gynecological Surgery 2010;7:S46‐S47. [EM 70299246]
(172) di MP, Ban KM, Bartoloni A, Fowler KE, Saint S, Mannelli F. Assessing the sustainability of hand hygiene adherence prior to patient contact in the emergency department: A 1‐year postintervention evaluation. American Journal of Infection Control 2011 Feb;39(1):14‐8. [PM: 20965610]
(173) Dickerson M. Mandatory educational programs: a decentralized approach. Journal of Nursing Staff Development 1987;3(2):84‐6. [PM: 3647109]
(174) Didomenico RJ, Perez A, Schumann HM, Fontana DR, Kondos GT, Schumock GT. Impact of treatment guidelines on clinical and economic outcomes of acute decompensated heart failure. Annals of Pharmacotherapy 2008 Mar;42(3):327‐33. [PM: 18303142]
(175) Dietrich AJ, Olson AL, Sox CH, Winchell CW, Grant‐Petersson J, Collison DW. Sun protection counseling for children: primary care practice patterns and effect of an intervention on clinicians. Archives of Family Medicine 2000 Feb;9(2):155‐9. [PM: 10693733]
(176) Dijkema LM, Geelkerken RH, Brouwers PJ, de SP, van Det RJ. The number of carotid artery operations in the Twente Medical Spectrum before and after the publication of relevant international research on the benefits of the relief of carotid stenosis. Nederlands Tijdschrift voor Geneeskunde 1998 Sep 12;142(37):2043‐7. [PM: 9856210]
(177) Dijkstra RF, Niessen LW, Braspenning JC, Adang E, Grol RT. Patient‐centred and professional‐directed implementation strategies for diabetes guidelines: a cluster‐randomized trial‐based cost‐effectiveness analysis. Diabetic Medicine 2006 Feb;23(2):164‐70. [PM: 16433714]
(178) DiTusa L, Luzier AB, Jarosz DE, Snyder BD, Izzo JL, Jr. Treatment of hypertension in a managed care setting. American Journal of Managed Care 2001 May;7(5):520‐4. [PM: 11388131]
(179) Doherty SR, Jones PD, Davis L, Ryan NJ, Treeve V. Evidence‐based implementation of adult asthma guidelines in the emergency department: a controlled trial. Emergency Medicine Australasia 2007 Feb;19(1):31‐8. [PM: 17305658]
(180) Doig GS, Simpson F, Finfer S, Delaney A, Davies AR, Mitchell I, et al. Effect of evidence‐based feeding guidelines on mortality of critically ill adults: a cluster randomized controlled trial. JAMA 2008 Dec 17;300(23):2731‐41. [PM: 19088351]
(181) Dolovich L, Levine M, Tarajos R, Duku E. Promoting optimal antibiotic therapy for otitis media using commercially sponsored evidence‐based detailing: A prospective controlled trial. Drug Information Journal 1999;33(4):1067‐77. [EM 1999412071]
(182) Dolovich L, Sabharwal M, Agro K, Foster G, Lee A, McCarthy L, et al. The effect of pharmacist education on asthma treatment plans for simulated patients. Pharmacy World & Science 2007 Jun;29(3):228‐39. [PM: 17242854]
(183) Donovan JL, Schroeder WS, Tran MT, Foster K, Forrest A, Lee TB, et al. Assessment of eptifibatide dosing in renal impairment before and after in‐service education provided by pharmacists. Journal of Managed Care Pharmacy 2007 Sep;13(7):598‐606. [PM: 17874866]
(184) Douglas AE, Holley A, Udy A, Lipman J, Gomersall CD, Joynt GM, et al. Can learning to sustain life be BASIC? Teaching for the initial management of the critically ill in Australia and New Zealand. Anaesthesia & Intensive Care 2010 Nov;38(6):1043‐51. [PM: 21226436]
(185) Du Pen AR, Du PS, Hansberry J, Miller‐Kraybill B, Millen J, Everly R, et al. An educational implementation of a cancer pain algorithm for ambulatory care. Pain Management Nursing 2000 Dec;1(4):116‐28. [PM: 11709865]
(186) Dupont C, Touzet S, Colin C, Deneux‐Tharaux C, Rabilloud M, Clement HJ, et al. Incidence and management of postpartum haemorrhage following the dissemination of guidelines in a network of 16 maternity units in France. International Journal of Obstetric Anesthesia 2009 Oct;18(4):320‐7. [PM: 19733052]
(187) Durand M, Labarere J, Brunet E, Pons JC. Evaluation of a training program for healthcare professionals about breast‐feeding. European Journal of Obstetrics, Gynecology, & Reproductive Biology 2003 Feb 10;106(2):134‐8. [PM: 12551778]
(188) Dykes PC, Acevedo K, Boldrighini J, Boucher C, Frumento K, Gray P, et al. Clinical practice guideline adherence before and after implementation of the HEARTFELT (HEART Failure Effectiveness & Leadership Team) intervention. Journal of Cardiovascular Nursing 2005 Sep;20(5):306‐14. [PM: 16141775]
(189) Eakin EG, Brown WJ, Marshall AL, Mummery K, Larsen E. Physical activity promotion in primary care: bridging the gap between research and practice. American Journal of Preventive Medicine 2004 Nov;27(4):297‐303. [PM: 15488359]
(190) Eccles M, Steen N, Grimshaw J, Thomas L, McNamee P, Soutter J, et al. Effect of audit and feedback, and reminder messages on primary‐care radiology referrals: a randomised trial. Lancet 2001 May 5;357(9266):1406‐9. [PM: 11356439]
(191) Edlich RF, Wilder BJ, Silloway KA, Nichter LS, Bryant CA. Quality assessment of tetanus prophylaxis in the wounded patient. American Surgeon 1986 Oct;52(10):544‐7. [PM: 3767140]
(192) Elnour AA, Ellahham NH, Al Qassas HI. Raising the awareness of inpatient nursing staff about medication errors. Pharmacy World & Science 2008 Apr;30(2):182‐90. [PM: 17882532]
(193) Emerson CR, Antonopoulos MS, Marzella N, Grossman SS. Economic impact of implementing pneumonia treatment guidelines for intravenous to oral conversion. Hospital Pharmacy 2008;43(11):886‐94. [EM 2008574646]
(194) Emery J, Morris H, Goodchild R, Fanshawe T, Prevost AT, Bobrow M, et al. The GRAIDS Trial: a cluster randomised controlled trial of computer decision support for the management of familial cancer risk in primary care. British Journal of Cancer 2007 Aug 20;97(4):486‐93. [PM: 17700548]
(195) Emslie C, Grimshaw J, Templeton A. Do clinical guidelines improve general practice management and referral of infertile couples? BMJ 1993 Jun 26;306(6894):1728‐31. [PM: 8280213]
(196) Erramouspe J, Bailey JM, Cleveland KW, Casperson K, Hunt TL, Cady PS. Counter sampling combined with medical provider education: do they alter prescribing behavior? Consultant Pharmacist 2006 Aug;21(8):636‐42. [PM: 17076590]
(197) Evans AT, Rogers LQ, Peden JG, Jr., Seelig CB, Layne RD, Levine MA, et al. Teaching dietary counseling skills to residents: patient and physician outcomes. The CADRE Study Group. American Journal of Preventive Medicine 1996 Jul;12(4):259‐65. [PM: 8874689]
(198) Evans DW, Breen AC, Pincus T, Sim J, Underwood M, Vogel S, et al. The effectiveness of a posted information package on the beliefs and behavior of musculoskeletal practitioners: the UK Chiropractors, Osteopaths, and Musculoskeletal Physiotherapists Low Back Pain ManagemENT (COMPLeMENT) randomized trial. Spine 2010 Apr 15;35(8):858‐66. [PM: 20308941]
(199) Fang J, Alderman MH. Revascularization among patients with acute myocardial infarction complicated by cardiogenic shock and impact of American College of Cardiology/American Heart Association guidelines. American Journal of Cardiology 2004 Nov 15;94(10):1281‐5. [PM: 15541246]
(200) Fauci JM, Schneider KE, Whitworth JM, Subramaniam A, Erickson BK, Kim K, et al. Referral patterns and incidence of cervical intraepithelial neoplasia in adolescent and pregnant patients: the impact of the 2006 guidelines. Journal of Lower Genital Tract Disease 2011 Apr;15(2):124‐7. [PM: 21478699]
(201) Feder G, Griffiths C, Highton C, Eldridge S, Spence M, Southgate L. Do clinical guidelines introduced with practice based education improve care of asthmatic and diabetic patients? A randomised controlled trial in general practices in east London. BMJ 1995 Dec 2;311(7018):1473‐8. [PM: 8520339]
(202) Fender GR, Prentice A, Gorst T, Nixon RM, Duffy SW, Day NE, et al. Randomised controlled trial of educational package on management of menorrhagia in primary care: the Anglia menorrhagia education study. BMJ 1999 May 8;318(7193):1246‐50. [PM: 10231255]
(203) Fendler KJ, Gumbhir AK, Sall K. The impact of drug bulletins on physician prescribing habits in a health maintenance organization. Drug Intelligence & Clinical Pharmacy 1984 Jul;18(7‐8):627‐31. [PM: 6430661]
(204) Ferrando A, Pagano E, Scaglione L, Petrinco M, Gregori D, Ciccone G. A decision‐tree model to estimate the impact on cost‐effectiveness of a venous thromboembolism prophylaxis guideline. Quality & Safety in Health Care 2009 Aug;18(4):309‐13. [PM: 19651937]
(205) Fessler J, Gross J, Papendick H, Schubert I. Qualitative and economic impact of implementing GP guidelines in a medical practitioners' network. Zeitschrift fur Arztliche Fortbildung und Qualitatssicherung 2006;100(2):107‐12. [EM 2006183663]
(206) Fick DM, Maclean JR, Rodriguez NA, Short L, Heuvel RV, Waller JL, et al. A randomized study to decrease the use of potentially inappropriate medications among community‐dwelling older adults in a southeastern managed care organization. American Journal of Managed Care 2004 Nov;10(11 Pt 1):761‐8. [PM: 15623266]
(207) Figueiras A, Sastre I, Tato F, Rodriguez C, Lado E, Caamano F, et al. One‐to‐one versus group sessions to improve prescription in primary care: a pragmatic randomized controlled trial. Medical Care 2001 Feb;39(2):158‐67. [PM: 11176553]
(208) Finch RG, Metlay JP, Davey PG, Baker LJ. Educational interventions to improve antibiotic use in the community: Report from the International Forum on Antibiotic Resistance (IFAR) colloquium, 2002. Lancet Infectious Diseases 2004;4(1):44‐53. [EM 2004021363]
(209) Finkelstein JA, Davis RL, Dowell SF, Metlay JP, Soumerai SB, Rifas‐Shiman SL, et al. Reducing antibiotic use in children: a randomized trial in 12 practices. Pediatrics 2001 Jul;108(1):1‐7. [PM: 11433046]
(210) Finnerty M, Gregory C. Impact of a palliative care nursing educational program in a Veterans Administration medical center. J HOSP PALLIAT NURS 2001;12(6):370‐7.
(211) Fischer N, Steurer MA, Adams M, Berger TM, Swiss NN. Survival rates of extremely preterm infants (gestational age <26 weeks) in Switzerland: impact of the Swiss guidelines for the care of infants born at the limit of viability. Archives of Disease in Childhood Fetal & Neonatal Edition 2009 Nov;94(6):F407‐13. [PM: 19357122]
(212) Fitzgerald ST, Hill M, Santamaria B, Howard C, Jadack R. Diagnosis and management of asthma: occupational health nurses' awareness and use of national consensus practice guidelines. Aaohn journal 1996 Feb;44(2):78‐83. [PM: 8694979]
(213) Fitzpatrick KR, Pantle AC, McLaws ML, Hughes CF. Culture change for hand hygiene: clean hands save lives, part II. Medical Journal of Australia 2009 Oct 19;191(Suppl 8):S13‐7. [PM: 19835526]
(214) Flenady V, Macphail J, New K, Devenish‐Meares P, Smith J. Implementation of a clinical practice guideline for smoking cessation in a public antenatal care setting. Australian & New Zealand Journal of Obstetrics & Gynaecology 2008 Dec;48(6):552‐8. [PM: 19133042]
(215) Florence Z. Printed educational materials: effects on professional practice and healthcare outcomes. J ADV NURS 2001;65(6):1183‐4.
(216) Flottorp S, Havelsrud K, Oxman AD. Process evaluation of a cluster randomized trial of tailored interventions to implement guidelines in primary care‐‐why is it so hard to change practice? Family Practice 2003 Jun;20(3):333‐9. [PM: 12738704]
(217) Flynn ER, Wolf ZR, McGoldrick TB, Jablonski RA, Dean LM, McKee EP. Effect of three teaching methods on a nursing staff's knowledge of medication error risk reduction strategies. Journal of Nursing Staff Development 1996 Jan;12(1):19‐26. [PM: 8699272]
(218) Fontaine A, Mahe I, Bergmann JF, Fiessinger JN, Dhote R, Cohen P, et al. Effectiveness of written guidelines on the appropriateness of thromboprophylaxis prescriptions for medical patients: a prospective randomized study. Journal of Internal Medicine 2006 Oct;260(4):369‐76. [PM: 16961674]
(219) Fox J, Hendrickson S, Miller N, Parry C, Youngman D. A cooperative approach to standardizing care for patients with AMI or heart failure. Joint Commission Journal on Quality & Patient Safety 2006 Dec;32(12):682‐7. [PM: 17220157]
(220) Foy R, Penney GC, Grimshaw JM, Ramsay CR, Walker AE, MacLennan G, et al. A randomised controlled trial of a tailored multifaceted strategy to promote implementation of a clinical guideline on induced abortion care. BJOG: An International Journal of Obstetrics & Gynaecology 2004 Jul;111(7):726‐33. [PM: 15198764]
(221) Francis JJ, Grimshaw JM, Zwarenstein M, Eccles MP, Shiller S, Godin G, et al. Testing a TheoRY‐inspired MEssage ('TRY‐ME'): a sub‐trial within the Ontario Printed Educational Message (OPEM) trial. Implementation Science 2007;2(39). [PM: 18039363]
(222) Fransen J, Laan RF, Van Der Laar MA, Huizinga TW, Van Riel PL. Influence of guideline adherence on outcome in a randomised controlled trial on the efficacy of methotrexate with folate supplementation in rheumatoid arthritis. Annals of the Rheumatic Diseases 2004 Oct;63(10):1222‐6. [PM: 15361375]
(223) Franssen MT, Korevaar JC, van d, V, Boer K, Leschot NJ, Goddijn M. Management of recurrent miscarriage: evaluating the impact of a guideline. Human Reproduction 2007 May;22(5):1298‐303. [PM: 17317720]
(224) Freeborn DK, Shye D, Mullooly JP, Eraker S, Romeo J. Primary care physicians' use of lumbar spine imaging tests: effects of guidelines and practice pattern feedback. Journal of General Internal Medicine 1997 Oct;12(10):619‐25. [PM: 9346458]
(225) Freemantle N, Johnson R, Dennis J, Kennedy A, Marchment M. Sleeping with the enemy? A randomized controlled trial of a collaborative health authority/industry intervention to influence prescribing practice. British Journal of Clinical Pharmacology 2000 Feb;49(2):174‐9. [PM: 10671913]
(226) French JA, Stevenson CH, Eglinton J, Bailey JE. Effect of information about waiting lists on referral patterns of general practitioners. British Journal of General Practice 1990 May;40(334):186‐9. [PM: 2114133]
(227) Fretheim A, Oxman AD, Treweek S, Bjorndal A. Rational Prescribing in Primary Care (RaPP‐trial). A randomised trial of a tailored intervention to improve prescribing of antihypertensive and cholesterol‐lowering drugs in general practice [ISRCTN48751230. BMC Health Services Research 2003 Feb 27;3(1):5. [PM: 12657163]
(228) Friedl R, Hoppler H, Ecard K, Scholz W, Hannekum A, Ochsner W, et al. Multimedia‐driven teaching significantly improves students' performance when compared with a print medium. Annals of Thoracic Surgery 2006 May;81(5):1760‐6. [PM: 16631668]
(229) Frijling BD, Lobo CM, Hulscher ME, Akkermans RP, van Drenth BB, Prins A, et al. Intensive support to improve clinical decision making in cardiovascular care: a randomised controlled trial in general practice. Quality & Safety in Health Care 2003 Jun;12(3):181‐7. [PM: 12792007]
(230) Fukui T, Takashi O, Rahman M, Iino K, Uchitomi Y, Ogawa S, et al. Clinical effectiveness of evidence‐based guidelines for pain management of terminal cancer patients in Japan. Japan Medical Association Journal 2005;48(5):216‐23. [EM 2005325975]
(231) Ganeshalingam Y, Cooper C, Livingston G. Referral patterns and acetylcholinesterase inhibitor prescribing for cognitive impairment (1999‐2007): Impact of NICE guidelines. Psychiatric Bulletin 2008;32(7):265‐7. [EM 2008323129]
(232) Garcia JC, Ferreira Filho OF, Grion CM, Carrilho CM. Impact of the implementation of a therapeutic guideline on the treatment of nosocomial pneumonia acquired in the intensive care unit of a university hospital. Jornal Brasileiro De Pneumologia 2007 Apr;33(2):175‐84. [PM: 17724537]
(233) Gask L, Dowrick C, Dixon C, Sutton C, Perry R, Torgerson D, et al. A pragmatic cluster randomized controlled trial of an educational intervention for GPs in the assessment and management of depression. Psychological Medicine 2004 Jan;34(1):63‐72. [PM: 14971627]
(234) Gauthier M, Chevalier I, Gouin S, Lamarre V, Abela A. Ceftriaxone for refractory acute otitis media: impact of a clinical practice guideline. Pediatric Emergency Care 2009 Nov;25(11):739‐43. [PM: 19864968]
(235) Gaylis FD, Van SJ, Daneshvar MA, Gaylis GM, Gaylis JB, Sheela RB, et al. Preprinted Standardized Orders Promote Venous Thromboembolism Prophylaxis Compared With Traditional Handwritten Orders: An Endorsement of Standardized Evidence‐Based Practice. American Journal of Medical Quality 2010;25(6):449‐56. [EM 20805424]
(236) Gazarian M, Henry RL, Wales SR, Micallef BE, Rood EM, O'Meara MW, et al. Evaluating the effectiveness of evidence‐based guidelines for the use of spacer devices in children with acute asthma. Medical Journal of Australia 2001;174(8):394‐7. [EM 2001160350]
(237) Geraedts M, Selbmann HK, Meisner C. Effects of a regional intervention to promote asthma guidelines implementation. Gesundheitswesen 2002;64(5):235‐41. [EM 2002182551]
(238) Gerbert B, Bronstone A, Wolff M, Maurer T, Berger T, Pantilat S, et al. Improving primary care residents' proficiency in the diagnosis of skin cancer. Journal of General Internal Medicine 1998;13(2):91‐7. [EM 1998065598]
(239) Giardino AP, Burns KM, Giardino ER. The educational value of pediatric emergency transport: By design or by default? Pediatric Emergency Care 1993;9(5):275‐80. [EM 1993306392]
(240) Gibbins RL, Owens DR, Allen JC, Eastman L. Practical application of the European Field Guide in screening for diabetic retinopathy by using ophthalmoscopy and 35 mm retinal slides. Diabetologia 1998 Jan;41(1):59‐64. [PM: 9498631]
(241) Gifford DR, Mittman BS, Fink A, Lanto AB, Lee ML, Vickrey BG. Can a specialty society educate its members to think differently about clinical decisions? Results of a randomized trial. Journal of General Internal Medicine 1996 Nov;11(11):664‐72. [PM: 9120652]
(242) Gifford WA, Davies B, Graham ID, Lefebre N, Tourangeau A, Woodend K. A mixed methods pilot study with a cluster randomized control trial to evaluate the impact of a leadership intervention on guideline implementation in home care nursing. Implementation Science 2008;3(51). [PM: 19077199]
(243) Gildenhuys J, Lee M, Isbister GK. Does implementation of a paediatric asthma clinical practice guideline worksheet change clinical practice? International Journal of Emergency Medicine 2009 Apr;2(1):33‐9. [PM: 19390915]
(244) Gill JM, Mainous AG, III, Koopman RJ, Player MS, Everett CJ, Chen YX, et al. Impact of EHR‐based clinical decision support on adherence to guidelines for patients on NSAIDs: a randomized controlled trial. Annals of Family Medicine 2011 Jan;9(1):22‐30. [PM: 21242557]
(245) Girotti MJ, Fodoruk S, Irvine‐Meek J, Rotstein OD. Antibiotic handbook and pre‐printed perioperative order forms for surgical antibiotic prophylaxis: do they work? Canadian Journal of Surgery 1990 Oct;33(5):385‐8. [PM: 2224658]
(246) Glazier RH, Badley EM, Lineker SC, Wilkins AL, Bell MJ. Getting a Grip on Arthritis: an educational intervention for the diagnosis and treatment of arthritis in primary care. Journal of Rheumatology 2005 Jan;32(1):137‐42. [PM: 15630739]
(247) Goebel LJ. A peer review feedback method of promoting compliance with preventive care guidelines in a resident ambulatory care clinic. Joint Commission Journal on Quality Improvement 1997 Apr;23(4):196‐202. [PM: 9142611]
(248) Goff DC, Jr., Massing MW, Bertoni AG, Davis J, Ambrosius WT, McArdle J, et al. Enhancing quality of heart failure care in managed Medicare and Medicaid in North Carolina: results of the North Carolina Achieving Cardiac Excellence (NC ACE) Project. American Heart Journal 2005 Oct;150(4):717‐24. [PM: 16209973]
(249) Goldberg HI, Wagner EH, Fihn SD, Martin DP, Horowitz CR, Christensen DB, et al. A randomized controlled trial of CQI teams and academic detailing: can they alter compliance with guidelines? Joint Commission Journal on Quality Improvement 1998 Mar;24(3):130‐42. [PM: 9568553]
(250) Goldstein MK, Lavori P, Coleman R, Advani A, Hoffman BB. Improving adherence to guidelines for hypertension drug prescribing: cluster‐randomized controlled trial of general versus patient‐specific recommendations. American Journal of Managed Care 2005 Nov;11(11):677‐85. [PM: 16268751]
(251) Gonnah R, Hegazi MO, Hmdy I, Shenoda MM. Can a change in policy reduce emergency hospital admissions? Effect of admission avoidance team, guideline implementation and maximising the observation unit. Emergency Medicine Journal 2008 Sep;25(9):575‐8. [PM: 18723706]
(252) Gopal RG, Jeanes A, Russell H, Wilson D, Atere‐Roberts E, O'Sullivan D, et al. Effectiveness of short‐term, enhanced, infection control support in improving compliance with infection control guidelines and practice in nursing homes: a cluster randomized trial. Epidemiology & Infection 2009 Oct;137(10):1465‐71. [PM: 19257913]
(253) Gopinath RM, Suzuki NT. Clinical training program for staff pharmacists. Hospital Pharmacy 1985 Jun;20(6):395‐8. [PM: 10271921]
(254) Gora‐Harper ML, Hessel E, Shadick D. Effect of prescribing guidelines on the use of neuromuscular blocking agents. American Journal of Health‐System Pharmacy 1995 Sep 1;52(17):1900‐4. [PM: 8528853]
(255) Gordon DB, Jones HD, Goshman LM, Foley DK, Bland SE. A quality improvement approach to reducing use of meperidine. Joint Commission Journal on Quality Improvement 2000 Dec;26(12):686‐99. [PM: 11143208]
(256) Gorecki W, Grochowska E, Krysta M, Wojciechowski P, Taczanowska A, Stanek B. A prospective comparison of antibiotic usage in pediatric surgical patients: the safety, advantage, and effectiveness of the Surgical Infection Society guidelines versus a common practice. Journal of Pediatric Surgery 2002 Oct;37(10):1430‐4. [PM: 12378448]
(257) Gorton TA, Cranford CO, Golden WE, Walls RC, Pawelak JE. Primary care physicians' response to dissemination of practice guidelines. Archives of Family Medicine 1995 Feb;4(2):135‐42. [PM: 7842151]
(258) Gove S. Integrated management of childhood illness: conclusions. WHO Division of Child Health and Development. Bulletin of the World Health Organization 1997;75(Suppl 1):119‐28. [PM: 9529725]
(259) Grange F, Hedelin G, Halna JM, Grall JC, Kirstetter H, Guillaume JC, et al. Assessment of a general practitioner training campaign for early detection of cutaneous melanoma in the Haut‐Rhin department of France. Annales de Dermatologie et de Venereologie 2005 Dec;132(12 Pt 1):956‐61. [PM: 16446636]
(260) Grene N, Pointereau‐Bellanger A, Conort O, Fontan JE, Le MF, Madelaine I, et al. Multicenter study of the impact of prescription guidelines on the use of colony stimulating factors. Anti‐Cancer Drugs 2000 Feb;11(2):109‐15. [PM: 10789593]
(261) Griffith HM, Dickey L, Kamerow DB. Put prevention into practice: a systematic approach. Journal of Public Health Management & Practice 1995;1(3):9‐15. [PM: 10186631]
(262) Griffiths CE, Taylor H, Collins SI, Hobson JE, Collier PA, Chalmers RJ, et al. The impact of psoriasis guidelines on appropriateness of referral from primary to secondary care: a randomized controlled trial. British Journal of Dermatology 2006 Aug;155(2):393‐400. [PM: 16882180]
(263) Griggs WM, Morris RW, Runciman WB, Osborne GA, Paix AD. Trauma: development of a sub‐algorithm. Quality & Safety in Health Care 2005 Jun;14(3):e21. [PM: 15933295]
(264) Grimshaw JM, Zwarenstein M, Tetroe JM, Godin G, Graham ID, Lemyre L, et al. Looking inside the black box: a theory‐based process evaluation alongside a randomised controlled trial of printed educational materials (the Ontario printed educational message, OPEM) to improve referral and prescribing practices in primary care in Ontario, Canada. Implementation Science 2007;2(38). [PM: 18039362]
(265) Guadagnoli E, Normand SL, DiSalvo TG, Palmer RH, McNeil BJ. Effects of treatment recommendations and specialist intervention on care provided by primary care physicians to patients with myocardial infarction or heart failure. American Journal of Medicine 2004 Sep 15;117(6):371‐9. [PM: 15380493]
(266) Guay MP, Dragomir A, Pilon D, Moride Y, Perreault S. Changes in pattern of use, clinical characteristics and persistence rate of hormone replacement therapy among postmenopausal women after the WHI publication. Pharmacoepidemiology & Drug Safety 2007 Jan;16(1):17‐27. [PM: 16794994]
(267) Guest JF, Varney SJ, Diggle J. Impact of the British Thoracic Society chronic obstructive pulmonary disease guidelines on patients' health status, healthcare resource use and health‐related quality of life. Primary Care Respiratory Journal 2005 Oct;14(5):242‐51. [PM: 16701737]
(268) Gulati M, Patel S, Jaffe AS, Joseph AJ, Calvin JE, Jr. Impact of contemporary guideline compliance on risk stratification models for acute coronary syndromes in The Registry of Acute Coronary Syndromes. American Journal of Cardiology 2004 Oct 1;94(7):873‐8. [PM: 15464668]
(269) Gunderson EW, Levin FR, Owen P. Impact of a brief training on medical resident screening for alcohol misuse and illicit drug use. American Journal on Addictions 2008 Mar;17(2):149‐54. [PM: 18393059]
(270) Gunn J, Southern D, Chondros P, Thomson P, Robertson K. Guidelines for assessing postnatal problems: introducing evidence‐based guidelines in Australian general practice. Family Practice 2003 Aug;20(4):382‐9. [PM: 12876107]
(271) Gunter MJ, Brixner D, von WA, Carter S, Gregory C. Impact of a seizure disorder disease management program on patient‐reported quality of life. Disease Management 2004;7(4):333‐47. [PM: 15671790]
(272) Guterman JJ, Chernof BA, Mares B, Gross‐Schulman SG, Gan PG, Thomas D. Modifying provider behavior: a low‐tech approach to pharmaceutical ordering. Journal of General Internal Medicine 2002 Oct;17(10):792‐6. [PM: 12390556]
(273) Guterman SJ, VanRooyan MJ. Cost‐effective medicine: the financial impact that practice guidelines have on outpatient hospital charges in the emergency department. Journal of Emergency Medicine 1998 Mar;16(2):215‐9. [PM: 9543404]
(274) Haas JS, Kaplan CP, Gerstenberger EP, Kerlikowske K. Summaries for patients. Changes in the use of postmenopausal hormone replacement therapy after the publication of study results. Annals of Internal Medicine 2004 Feb 3;140(3):I50. [PM: 14757633]
(275) Hachemi M, Attof Y, Flamens C, Bastien O, Bouletreau P, Chambrier C, et al. Impact of the guidelines on clinical practice of artificial nutrition in intensive care unit after cardiovascular and thoracic surgery. Annales Francaises d Anesthesie et de Reanimation 2006 Oct;25(10):1034‐40. [PM: 17005359]
(276) Hagaman JT, Yurkowski P, Trott A, Rouan GW. Getting physicians to make "the switch": the role of clinical guidelines in the management of community‐acquired pneumonia. American Journal of Medical Quality 2005 Jan;20(1):15‐21. [PM: 15782751]
(277) Hak E, Hermens RP, Hoes AW, Verheij TJ, Kuyvenhoven MM, van Essen GA. Effectiveness of a co‐ordinated nation‐wide programme to improve influenza immunisation rates in The Netherlands. Scandinavian Journal of Primary Health Care 2000 Dec;18(4):237‐41. [PM: 11205093]
(278) Hall L, Eccles M, Barton R, Steen N, Campbell M. Is untargeted outreach visiting in primary care effective? A pragmatic randomized controlled trial. Journal of Public Health Medicine 2001 Jun;23(2):109‐13. [PM: 11450926]
(279) Hamley JH, MacGregor SH, Dunbar JA, Cromarty JA. Integrating clinical pharmacists into the primary health care team: a framework for rational and cost‐effective prescribing. Scottish Medical Journal 1997 Feb;42(1):4‐7. [PM: 9226769]
(280) Hammond CS, Wasson JH, Walker‐Corkery E, Fowler FJ, Barry MJ. A frequently used patient and physician‐directed educational intervention does nothing to improve primary care of prostate conditions. Urology 2001 Dec;58(6):875‐81. [PM: 11744449]
(281) Hamrin EK, Lindmark B. The effect of systematic care planning after acute stroke in general hospital medical wards. J ADV NURS 1990 Oct;15(10):1146‐53. [PM: 2258521]
(282) Hanbury A, Wallace L, Clark M. Use of a time series design to test effectiveness of a theory‐based intervention targeting adherence of health professionals to a clinical guideline. British Journal of Health Psychology 2009 Sep;14(Pt 3):505‐18. [PM: 18851769]
(283) Hannon R. Audit and feedback and educational outreach did not differ from printed educational materials for improving community nursing practice. Evidence‐Based Nursing 2007 Jan;10(1):26. [PM: 17218300]
(284) Harrington SS, Walker BL. A comparison of computer‐based and instructor‐led training for long‐term care staff. Journal of Continuing Education in Nursing 2002 Jan;33(1):39‐45. [PM: 15887359]
(285) Harris RH, MacKenzie TD, Leeman‐Castillo B, Corbett KK, Batal HA, Maselli JH, et al. Optimizing antibiotic prescribing for acute respiratory tract infections in an urban urgent care clinic. Journal of General Internal Medicine 2003 May;18(5):326‐34. [PM: 12795730]
(286) Hayes RP, Baker DW, Luthi JC, Baggett RL, McClellan W, Fitzgerald D, et al. The effect of external feedback on the management of medicare inpatients with congestive heart failure. American Journal of Medical Quality 2002 Nov;17(6):225‐35. [PM: 12487338]
(287) Hazard RG, Haugh LD, Reid S, McFarlane G, MacDonald L. Early physician notification of patient disability risk and clinical guidelines after low back injury: a randomized, controlled trial. Spine 1997 Dec 15;22(24):2951‐8. [PM: 9431632]
(288) Healey KM, Hoffman MA. Self‐instructional posters: one way to save time and money. Journal of Continuing Education in Nursing 1991 May;22(3):123‐5. [PM: 1907297]
(289) Helsper CW, van Essen GA, Bonten MJ, de Wit NJ. A support programme for primary care leads to substantial improvements in the effectiveness of a public hepatitis C campaign. Family Practice 2010 Jun;27(3):328‐32. [PM: 20223833]
(290) Helton KJ, Strife JL, Warner BW, Byczkowski TL, Donovan EF. The impact of a clinical guideline on imaging children with hypertrophic pyloric stenosis. Pediatric Radiology 2004 Sep;34(9):733‐6. [PM: 15316693]
(291) Hendricson WD, Wood PR, Hidalgo HA, Kromer ME, Parcel GS, Ramirez AG. Implementation of a physician education intervention. The Childhood Asthma Project. Archives of Pediatrics & Adolescent Medicine 1994 Jun;148(6):595‐601. [PM: 8193683]
(292) Henkel V, Althaus D, Hegerl U. Lessons from the Hampshire Depression Project. Lancet 2000 May 6;355(9215):1641. [PM: 10821382]
(293) Hepner KA, Rowe M, Rost K, Hickey SC, Sherbourne CD, Ford DE, et al. The effect of adherence to practice guidelines on depression outcomes. Annals of Internal Medicine 2007 Sep 4;147(5):320‐9. [PM: 17785487]
(294) Herrick DE, Lowe CR, Micalizzi JG, Kerby GS. The impact of an evidence‐based continuing education course on participant self‐efficacy and asthma educator‐certified (AE‐C) accreditation. [2011 American Academy of Allergy, Asthma and Immunology, AAAAI Annual Meeting San Francisco, CA]. Journal of Allergy and Clinical Immunology 2011;127(2 Suppl 1):AB67. [EM 70358984]
(295) Herschell AD, McNeil CB, Urquiza AJ, McGrath JM, Zebell NM, Timmer SG, et al. Evaluation of a treatment manual and workshops for disseminating, parent‐child interaction therapy. Administration & Policy in Mental Health 2009 Jan;36(1):63‐81. [PM: 19016321]
(296) Heyman E, Tyner R, Phipps C, Cave L, Owen DC. Is the hospital setting the place for teaching breast self‐examination? Cancer Nursing 1991 Feb;14(1):35‐40. [PM: 2013050]
(297) Higgins R, Hogg P. Patient preparation for diagnostic nuclear medicine imaging procedures: An analysis of ward nurse knowledge. Radiography 2002;8(3):139‐47. [EM 2002393734]
(298) Hill‐Smith I. Sharing resources to create a district drug formulary: a countywide controlled trial. British Journal of General Practice 1996 May;46(406):271‐5. [PM: 8762741]
(299) Hobolth L, Krag A, Malchow‐Moller A, Gancho V, Jensen SM, Bendtsen F. Adherence to guidelines in bleeding oesophageal varices and the effect on outcome‐comparison between a specialized unit and a community hospital [60th Annual Meeting of the American Association for the Study of Liver Diseases, 2009 Boston]. Hepatology 2009;50:442A. [EM 70073707]
(300) Hoeijenbos M, Bekkering T, Lamers L, Hendriks E, van TM, Koopmanschap M. Cost‐effectiveness of an active implementation strategy for the Dutch physiotherapy guideline for low back pain. Health Policy 2005 Dec;75(1):85‐98. [PM: 16298231]
(301) Holroyd BR, Wilson D, Rowe BH, Mayes DC, Noseworthy T. Uptake of validated clinical practice guidelines: experience with implementing the Ottawa Ankle Rules. American Journal of Emergency Medicine 2004 May;22(3):149‐55. [PM: 15138948]
(302) Holt RD, Rule DC, Basker RM, Davenport JC, Ralph JP, Murray JJ, et al. The influence on partial denture design of a teaching video for general dental practitioners. British Dental Journal 1994 May 21;176(10):379‐80. [PM: 8011375]
(303) Horwood C, Vermaak K, Rollins N, Haskins L, Nkosi P, Qazi S. Paediatric HIV management at primary care level: an evaluation of the integrated management of childhood illness (IMCI) guidelines for HIV. BMC Pediatrics 2009;9(59). [PM: 19772599]
(304) Hoskins G, Neville RG, McCowan C, Smith B, Clark RA, Ricketts IW. Scottish Asthma Management Initiative. Health Bulletin 2000 Nov;58(6):478‐88. [PM: 12813780]
(305) Hover AR, Cornwell V, Stevenson S, Sponenberg D. Evaluation of the American Academy of Pediatrics Principles on Management of Common Office Infections in a managed care setting. Missouri Medicine 2000 Dec;97(12):541‐4. [PM: 11138431]
(306) Hulscher ME, van Drenth BB, van der Wouden JC, Mokkink HG, van WC, Grol RP. Changing preventive practice: a controlled trial on the effects of outreach visits to organise prevention of cardiovascular disease. Quality in Health Care 1997 Mar;6(1):19‐24. [PM: 10166597]
(307) Humair JP, Cornuz J. A new curriculum using active learning methods and standardized patients to train residents in smoking cessation. Journal of General Internal Medicine 2003 Dec;18(12):1023‐7. [PM: 14687261]
(308) Hung CS, Lin JW, Hwang JJ, Tsai RY, Li AT. Using paper chart based clinical reminders to improve guideline adherence to lipid management. Journal of Evaluation in Clinical Practice 2008 Oct;14(5):861‐6. [PM: 19018919]
(309) Hunskaar S, Hannestad YS, Backe B, Matheson I. Direct mailing of consensus recommendations did not alter GPs' knowledge and prescription of oestrogen in the menopause. Scandinavian Journal of Primary Health Care 1996 Dec;14(4):203‐8. [PM: 8956447]
(310) Hutt E, Radcliff TA, Oman KS, Fink R, Ruscin JM, Linnebur S, et al. Impact of NHAP guideline implementation intervention on staff and resident vaccination rates. Journal of the American Medical Directors Association 2010 Jun;11(5):365‐70. [PM: 20511104]
(311) Hux JE, Melady MP, DeBoer D. Confidential prescriber feedback and education to improve antibiotic use in primary care: a controlled trial. CMAJ Canadian Medical Association Journal 1999 Aug 24;161(4):388‐92. [PM: 10478162]
(312) Ilett KF, Johnson S, Greenhill G, Mullen L, Brockis J, Golledge CL, et al. Modification of general practitioner prescribing of antibiotics by use of a therapeutics adviser (academic detailer). British Journal of Clinical Pharmacology 2000 Feb;49(2):168‐73. [PM: 10671912]
(313) Iregui M, Ward S, Clinikscale D, Clayton D, Kollef MH. Use of a handheld computer by respiratory care practitioners to improve the efficiency of weaning patients from mechanical ventilation. Critical Care Medicine 2002 Sep;30(9):2038‐43. [PM: 12352038]
(314) Jabbari H, Bakhshian F. Preventing nosocomial infection transmit in gynecology and midwifery service providers in Tabriz (experience of educational interventions). Medical Sciences Journal of Islamic Azad University 2008;18(4):e259‐64.
(315) Jackevicius CA, Chapman KR. Inhaler education for hospital‐based pharmacists: how much is required? Canadian Respiratory Journal 1999 May;6(3):237‐44. [PM: 10393285]
(316) Jacobs EM, Meulendijks CF, Elving L, van der Wilt GJ, Swinkels DW. Impact of the introduction of a guideline on the targeted detection of hereditary haemochromatosis. Netherlands Journal of Medicine 2005 Jun;63(6):205‐14. [PM: 16011012]
(317) Jafar TH, Hatcher J, Poulter N, Islam M, Hashmi S, Qadri Z, et al. Community‐based interventions to promote blood pressure control in a developing country: a cluster randomized trial. Annals of Internal Medicine 2009 Nov 3;151(9):593‐601. [PM: 19884620]
(318) Jain MK, Heyland D, Dhaliwal R, Day AG, Drover J, Keefe L, et al. Dissemination of the Canadian clinical practice guidelines for nutrition support: results of a cluster randomized controlled trial. Critical Care Medicine 2006 Sep;34(9):2362‐9. [PM: 16850001]
(319) Jamileh Y, Pedlar J. Effect of clinical guidelines on practice for extraction of lower third molars: study of referrals in 1997 and 2000. British Journal of Oral & Maxillofacial Surgery 2003 Dec;41(6):371‐5. [PM: 14614863]
(320) Janmeja AK, Mohapatra PR, Kumar M. The impact of "World Health Organization ‐ Government of India guidelines on chronic obstructive pulmonary diseases‐2003" on quality of life. Lung India 2009 Oct;26(4):102‐5. [PM: 20531989]
(321) Jans MP, Schellevis FG, Van HW, van Eijk JT. Improving general practice care of patients with asthma or chronic obstructive pulmonary disease: evaluation of a quality system. Effective Clinical Practice 2000 Jan;3(1):16‐24. [PM: 10788032]
(322) Jans MP, Schellevis FG, Le Coq EM, Bezemer PD, van Eijk JT. Health outcomes of asthma and COPD patients: the evaluation of a project to implement guidelines in general practice. International Journal for Quality in Health Care 2001 Feb;13(1):17‐25. [PM: 11330439]
(323) Jantti H, Kuisma M, Uusaro A. The effects of changes to the ERC resuscitation guidelines on no flow time and cardiopulmonary resuscitation quality: a randomised controlled study on manikins. Resuscitation 2007 Nov;75(2):338‐44. [PM: 17628319]
(324) Jardri R, Maron M, Pelta J, Thomas P, Codaccioni X, Goudemand M, et al. Impact of midwives' training on postnatal depression screening in the first week post delivery: a quality improvement report. Midwifery 2010 Dec;26(6):622‐9. [PM: 19211177]
(325) Jeba J, George R, Thangakunam B, Christopher DJ. Pain assessment and analgesic prescription for cancer patients in a medical ward: the influence of an educational intervention. National Medical Journal of India 2009 Jul;22(4):177‐80. [PM: 20120990]
(326) Jennett PA, Wilson TW, Hayton RC, Mainprize GW, Laxdal OE. Desirable behaviours in the office management of hypertension addressed through continuing medical education. Canadian Journal of Public Health 1989 Sep;80(5):359‐62. [PM: 2804866]
(327) Jiang SL, Ji XP, Zhang C, Wang XR, Zhang M, Zhang Y. Impact of Chinese guidelines for management of patients with acute myocardial infarction on outcomes of hospitalized patients. Chinese Medical Journal 2006 Jan 5;119(1):26‐31. [PM: 16454978]
(328) Joffres MR, Kamath TV, Williams GR, Casey J, Svenson LW. Impact of guidelines on health care use for the management of dyslipidemia in two Canadian provinces, Alberta and Nova Scotia, from 1990 to 2001. Canadian Journal of Cardiology 2004 Jun;20(8):767‐72. [PM: 15229757]
(329) Johnson CC, Martin M. Effectiveness of a physician education program in reducing consumption of hospital resources in elective total hip replacement. Southern Medical Journal 1996 Mar;89(3):282‐9. [PM: 8604457]
(330) Johnson DW, Craig W, Brant R, Mitton C, Svenson L, Klassen TP. A cluster randomized controlled trial comparing three methods of disseminating practice guidelines for children with croup [ISRCTN73394937. Implementation Science 2006;1(10). [PM: 16722541]
(331) Johnson E, Rolfs RT, Sauer BC, Porucznik CA. Utah's education campaign for prescription opioids [Conference: 25th Annual Meeting of the American Academy of Pain Medicine, AAPM Honolulu, HI United States]. Pain Medicine 2009;10(1):272‐3. [EM 70205027]
(332) Johnson RE, Campbell WH, Azevedo DJ, Christensen DB. Studying the impact of patient drug profiles in an HMO. Medical Care 1976 Oct;14(10):799‐807. [PM: 972558]
(333) Jones RH, Lydeard S, Dunleavey J. Problems with implementing guidelines: a randomised controlled trial of consensus management of dyspepsia. Quality in Health Care 1993 Dec;2(4):217‐21. [PM: 10132454]
(334) Jouclas VM. Manuals in the organization of nursing services. Revista Enfermagem Em Novas Dimensoes 1978 Jan;4(1):47‐52. [PM: 243953]
(335) Junghans C, Feder G, Timmis AD, Eldridge S, Sekhri N, Black N, et al. Effect of patient‐specific ratings vs conventional guidelines on investigation decisions in angina: Appropriateness of Referral and Investigation in Angina (ARIA) Trial. Archives of Internal Medicine 2007 Jan 22;167(2):195‐202. [PM: 17242322]
(336) Kahan NR, Kahan E, Waitman DA, Kitai E, Chintz DP. The tools of an evidence‐based culture: implementing clinical‐practice guidelines in an Israeli HMO. Academic Medicine 2009 Sep;84(9):1217‐25. [PM: 19707060]
(337) Kaneshiro M, Ho A, Chan M, Cohen H, Spiegel BM. Colonoscopy yields fewer polyps as the day progresses despite using social influence theory to reverse the trend. Gastrointestinal Endoscopy 2010 Dec;72(6):1233‐40. [PM: 21111873]
(338) Kattwinkel J, Nowacek GA, Cook LJ, Hurt H, Short JG. Perinatal outreach education. A continuation strategy for a basic program. American Journal of Perinatology 1984 Jul;1(4):335‐40. [PM: 6518071]
(339) Keay L, Stapleton F. Development and evaluation of evidence‐based guidelines on contact lens‐related microbial keratitis. Contact Lens & Anterior Eye 2008 Feb;31(1):3‐12. [PM: 18032091]
(340) Kennedy A, Nelson E, Reeves D, Richardson G, Roberts C, Robinson A, et al. A randomised controlled trial to assess the impact of a package comprising a patient‐orientated, evidence‐based self‐help guidebook and patient‐centred consultations on disease management and satisfaction in inflammatory bowel disease. Health Technology Assessment (Winchester, England) 2003;7(28):1‐113. [PM: 14567905]
(341) Kenyon S, Taylor DJ. The effect of the publication of a major clinical trial in a high impact journal on clinical practise: the ORACLE Trial experience. BJOG: An International Journal of Obstetrics & Gynaecology 2002 Dec;109(12):1341‐3. [PM: 12504968]
(342) Kerry S, Oakeshott P, Dundas D, Williams J. Influence of postal distribution of the Royal College of Radiologists' guidelines, together with feedback on radiological referral rates, on X‐ray referrals from general practice: a randomized controlled trial. Family Practice 2000 Feb;17(1):46‐52. [PM: 10673488]
(343) Khachi H, Burman M, Walters L, Sinha‐Ray R, Antoniou S, Mandal S. The impact of a multidisciplinary educational programme on the prescribing of oxygen in an acute trust [Conference: British Thoracic Society Winter Meeting 2010 London United Kingdom]. Thorax 2010;65:A113. [EM 70325941]
(344) Kim CJ, Kang DH. Utility of a Web‐based intervention for individuals with type 2 diabetes: the impact on physical activity levels and glycemic control. CIN: Computers, Informatics, Nursing 2006 Nov;24(6):337‐45. [PM: 17108753]
(345) Kim J, Chang S, Lee S, Jun E, Kim Y. An experimental study of students' self‐learning of the San‐Yin‐Jiao pressure procedure using CD‐ROM or printed materials. Journal of Nursing Education 2003 Aug;42(8):371‐6. [PM: 12938901]
(346) King S. Personalized medicine: what nurses need to know... Oncology Nursing Society 31st Annual Congress podium and poster abstracts. ONCOL NURS FORUM 2001;33(2):448.
(347) Kinmonth AL, Woodcock A, Griffin S, Spiegal N, Campbell MJ. Randomised controlled trial of patient centred care of diabetes in general practice: impact on current wellbeing and future disease risk. The Diabetes Care From Diagnosis Research Team. BMJ 1998 Oct 31;317(7167):1202‐8. [PM: 9794859]
(348) Kirby T. Atul Gawande‐‐making surgery safer worldwide. Lancet 2010 Sep 25;376(9746):1045. [PM: 20870085]
(349) Kirscht JP, Kirscht JL, Rosenstock IM. A test of interventions to increase adherence to hypertensive medical regimens. Health Education Quarterly 1981;8(3):261‐72. [PM: 7333851]
(350) Kirshbaum M. Translation to practice: a randomised, controlled study of an evidence‐based booklet for breast‐care nurses in the United Kingdom. Worldviews on Evidence‐Based Nursing 2008;5(2):60‐74. [PM: 18559019]
(351) Knox AB, Underbaake G, McBride PE, Mejicano GC. Organization development strategies for continuing medical education. Journal of Continuing Education in the Health Professions 2001;21(1):15‐23. [PM: 11291581]
(352) Kocher MS, Mandiga R, Murphy JM, Goldmann D, Harper M, Sundel R, et al. A clinical practice guideline for treatment of septic arthritis in children: efficacy in improving process of care and effect on outcome of septic arthritis of the hip. Journal of Bone & Joint Surgery ‐ American Volume 2003 Jun;85‐A(6):994‐9. [PM: 12783993]
(353) Korn R. The health information manager: the next generation. Journal of Ahima 1995 Oct;66(9):56‐9. [PM: 10151133]
(354) Koskinen H, Rautakorpi UM, Sintonen H, Honkanen P, Huikko S, Huovinen P, et al. Cost‐effectiveness of implementing national guidelines in the treatment of acute otitis media in children. International Journal of Technology Assessment in Health Care 2006;22(4):454‐9. [PM: 16984678]
(355) Kotagal UR, Robbins JM, Kini NM, Schoettker PJ, Atherton HD, Kirschbaum MS. Impact of a bronchiolitis guideline: a multisite demonstration project. Chest 2002 Jun;121(6):1789‐97. [PM: 12065340]
(356) Kotilampi M. The use of professional literature by nurses. Sairaanhoidon Vuosikirja 1972;9:58‐86. [PM: 4483501]
(357) Kreuter MW, Chheda SG, Bull FC. How does physician advice influence patient behavior? Evidence for a priming effect. Archives of Family Medicine 2000 May;9(5):426‐33. [PM: 10810947]
(358) Kwan T, Lin F, Ngai B, Loeb M. Vancomycin use in 2 Ontario tertiary care hospitals: a survey. Clinical & Investigative Medicine 1999 Dec;22(6):256‐64. [PM: 10664867]
(359) Labarere J, Bosson JL, Brion JP, Fabre M, Imbert B, Carpentier P, et al. Validation of a clinical guideline on prevention of venous thromboembolism in medical inpatients: a before‐and‐after study with systematic ultrasound examination. Journal of Internal Medicine 2004 Oct;256(4):338‐48. [PM: 15367177]
(360) Lagerlov P, Loeb M, Andrew M, Hjortdahl P. Improving doctors' prescribing behaviour through reflection on guidelines and prescription feedback: a randomised controlled study. Quality in Health Care 2000 Sep;9(3):159‐65. [PM: 10980076]
(361) Lamas GA, Pfeffer MA, Hamm P, Wertheimer J, Rouleau JL, Braunwald E. Do the results of randomized clinical trials of cardiovascular drugs influence medical practice? The SAVE Investigators. New England Journal of Medicine 1992 Jul 23;327(4):241‐7. [PM: 1535419]
(362) Landgren FT, Harvey KJ, Mashford ML, Moulds RF, Guthrie B, Hemming M. Changing antibiotic prescribing by educational marketing. Medical Journal of Australia 1988 Dec 5;149(11‐12):595‐9. [PM: 3200183]
(363) Lang E, Kastner S, Liebig K, Neundorfer B. Interventions for improvement of primary care in patients with low back pain: how effective are advice to primary care physicians on therapies and a multimodal therapy program arising out of cooperation of outpatient health care structures? Der Schmerz 2002 Feb;16(1):22‐33. [PM: 11845338]
(364) Lang WP, Farghaly MM, Woolfolk MW, Ziemiecki TL, Faja BW. Educating dentists about fissure sealants: effects on knowledge, attitudes, and use. Journal of Public Health Dentistry 1991;51(3):164‐9. [PM: 1920269]
(365) Langsford MJ, Dobbs FF, Morrison GM, Dance DA. The effect of introduction of a guideline on the management of vaginal discharge and in particular bacterial vaginosis in primary care. Family Practice 2001 Jun;18(3):253‐7. [PM: 11356730]
(366) Lanoir D, Chambrier C, Vergnon P, Meynaud‐Kraemer L, Wilkinson J, Mcpherson K, et al. Perioperative artificial nutrition in elective surgery: an impact study of French guidelines. Clinical Nutrition 1998 Aug;17(4):153‐7. [PM: 10205333]
(367) Lanoir D, Chambrier C, Vergnon P, Meynaud‐Kraemer L, Wilkinson J, Mcpherson K, et al. Perioperative artificial nutrition in elective surgery: An impact study of French guidelines. Clinical Nutrition 1998;17(4):153‐7. [EM 1998325930]
(368) LaPointe NM, DeLong ER, Chen A, Hammill BG, Muhlbaier LH, Califf RM, et al. Multifaceted intervention to promote beta‐blocker use in heart failure. American Heart Journal 2006 May;151(5):992‐8. [PM: 16644320]
(369) Lasby K, Dressler‐Mund D. Making the literature palatable at the bedside: Reference poster promotes oral feeding best practice. Advances in Neonatal Care 2011 Feb;11(1):17‐24. [PM: 21285652]
(370) Laurie‐Shaw B, Taylor W, Roach C. Focus on clinical best practices, patient safety and operational efficiency. Healthcare Quarterly 2006;10:50‐6. [PM: 17163118]
(371) Law M, Teplicky R, King S, King G, Kertoy M, Moning T, et al. Family‐centred service: moving ideas into practice. Child: Care, Health & Development 2005 Nov;31(6):633‐42. [PM: 16207220]
(372) Lazovich D, Curry SJ, Beresford SA, Kristal AR, Wagner EH. Implementing a dietary intervention in primary care practice: a process evaluation. American Journal of Health Promotion 2000 Nov;15(2):118‐25. [PM: 11194695]
(373) Lee E, McNally DL, Zuckerman IH. Evaluation of a physician‐focused educational intervention on medicaid children with asthma. Annals of Pharmacotherapy 2004 Jun;38(6):961‐6. [PM: 15122003]
(374) Lefevre F, Blum A, Bracard S, Regent D, Stines J, Guillemin F, et al. Impact of PACS implementation in a university center on radiology practice profile. Journal de Radiologie 2009 Sep;90(9 Pt 1):1046‐54. [PM: 19752808]
(375) Legare F, O'Connor AM, Graham ID, Wells GA, Jacobsen MJ, Elmslie T, et al. The effect of decision aids on the agreement between women's and physicians' decisional conflict about hormone replacement therapy. Patient Education & Counseling 2003 Jun;50(2):211‐21. [PM: 12781936]
(376) Lehmann DF, Guharoy R, Page N, Hirschman K, Ploutz‐Snyder R, Medicis J. Formulary management as a tool to improve medication use and gain physician support. American Journal of Health‐System Pharmacy 2007 Mar 1;64(5):464‐6. [PM: 17322158]
(377) Leichtnam‐Dugarin L, Carretier J, Delavigne V, Brusco S, Hoarau H, Philip T, et al. The SOR SAVOIR Patient, a project of patient information and education. Cancer Radiotherapie 2003 Jun;7(3):210‐2. [PM: 12834779]
(378) Leopold SS, Morgan HD, Kadel NJ, Gardner GC, Schaad DC, Wolf FM. Impact of educational intervention on confidence and competence in the performance of a simple surgical task. Journal of Bone & Joint Surgery ‐ American Volume 2005 May;87(5):1031‐7. [PM: 15866966]
(379) Letton AH, Bard M, Boyd JD, Crosson K, Ganz P, Loeb V, Jr., et al. Breakout III. Concerns of the patient and the public. Cancer 1990 May 15;65(Suppl 10):2411‐2. [PM: 2334882]
(380) Leung KY, Ling M, Tang GW. Use of hormone replacement therapy in the Hong Kong public health sector after the Women's Health Initiative trial. Maturitas 2005 Nov;52(3‐4):277‐85. [PM: 15950409]
(381) Lewis C, Amador EC, Ward BL, Russell RP. Directory of high blood pressure resources available to physicians. Maryland State Medical Journal 1984 Mar;33(3):216‐23. [PM: 6371394]
(382) Lewis CE, Freeman HE, Kaplan SH, Corey CR. The impact of a program to enhance the competencies of primary care physicians in caring for patients with AIDS. Journal of General Internal Medicine 1986 Sep;1(5):287‐94. [PM: 3772617]
(383) Lewis CE, Bursch B, Klau M, Konitsney D, Conrow S. Continuing medical education for AIDS: an organizational response. AIDS Education & Prevention 1993;5(3):263‐71. [PM: 8217478]
(384) Liaw ST, Sulaiman ND, Barton CA, Chondros P, Harris CA, Sawyer S, et al. An interactive workshop plus locally adapted guidelines can improve general practitioners asthma management and knowledge: a cluster randomised trial in the Australian setting. BMC Family Practice 2008;9(22). [PM: 18423050]
(385) Linden M, Westram A, Schmidt LG, Haag C. Impact of the WHO depression guideline on patient care by psychiatrists: a randomized controlled trial. European Psychiatry 2008 Sep;23(6):403‐8. [PM: 18583105]
(386) Little P, Roberts L, Blowers H, Garwood J, Cantrell T, Langridge J, et al. Should we give detailed advice and information booklets to patients with back pain? A randomized controlled factorial trial of a self‐management booklet and doctor advice to take exercise for back pain. Spine 2001 Oct 1;26(19):2065‐72. [PM: 11698879]
(387) Liu J, Ma JP, Huang JH, Wang L. A study of the influence of the guideline on the treatment of chronic congestive heart failure in the elderly. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue [Chinese Critical Care Medicine] 2010 Oct;22(10):606‐9. [PM: 20977844]
(388) Loeb M, Brazil K, Lohfeld L, McGeer A, Simor A, Stevenson K, et al. Effect of a multifaceted intervention on number of antimicrobial prescriptions for suspected urinary tract infections in residents of nursing homes: cluster randomised controlled trial. BMJ 2005 Sep 24;331(7518):669. [PM: 16150741]
(389) Loeches YB, de Lorenzo‐Caceres AA. Information leaflets for primary care patients. Atencion Primaria 2004 Feb 28;33(3):165. [PM: 14987506]
(390) Lomas J, Sisk JE, Stocking B. From evidence to practice in the United States, the United Kingdom, and Canada. Milbank Quarterly 1993;71(3):405‐10. [PM: 8413068]
(391) Lopes MHB, del Monte MCC, Barbosa M, Moromizato SS, Fayan ALN, de Souza EP, et al. Updating measures on universal precautions: a new approach methodology [Portuguese]. REV LAT AM ENFERMAGEM 2001;5(2):83‐91. [PMID: 9370759]
(392) Luciak‐Donsberger C, Piribauer F, Taylor NC. Enabling general physicians to perform periodontal screening during nationwide periodic health examinations. The Journal of Evidencebased Dental Practice 2008 Sep;8(3):186‐94. [PM: 18783766]
(393) Luitjes SH, Wouters MG, Franx A, Scheepers HC, Coupe VM, Wollersheim H, et al. Study protocol: Cost effectiveness of two strategies to implement the NVOG guidelines on hypertension in pregnancy: An innovative strategy including a computerised decision support system compared to a common strategy of professional audit and feedback, a randomized controlled trial. Implementation Science 2010;5(68). [PM: 20819222]
(394) Lundberg GD. Passive dissemination of printed educational materials in medicine has no or negligible effect on patient outcomes. Medscape journal of medicine 2008;10(11):255. [PM: 19099005]
(395) Macfarlane J, Holmes W, Gard P, Thornhill D, Macfarlane R, Hubbard R. Reducing antibiotic use for acute bronchitis in primary care: blinded, randomised controlled trial of patient information leaflet. BMJ 2002 Jan 12;324(7329):91‐4. [PM: 11786454]
(396) Mackain‐Bremner AA, Owens K, Wylde V, Bannister GC, Blom AW. Adherence to recommendations designed to decrease intra‐operative wound contamination. Annals of the Royal College of Surgeons of England 2008 Jul;90(5):412‐6. [PM: 18634740]
(397) Macmillan N. Rising to the information challenge. IDRC Reports 1996 Jan;23(4):18‐9. [PM: 12296168]
(398) Maffei FH, Sato AC, Torggler FF, Silva SC, Atallah A. Effect of the implementation of a guideline for venous thromboembolism prophylaxis in surgical patients. Revista Da Associacao Medica Brasileira 2009 Sep;55(5):587‐92. [PM: 19918661]
(399) Majumdar SR, Johnson JA, McAlister FA, Bellerose D, Russell AS, Hanley DA, et al. Multifaceted intervention to improve diagnosis and treatment of osteoporosis in patients with recent wrist fracture: a randomized controlled trial. CMAJ Canadian Medical Association Journal 2008 Feb 26;178(5):569‐75. [PM: 18299546]
(400) Malhotra A, Aziz K, Freston JW. Colorectal cancer screening: A retrospective study of compliance with guidelines in a university‐based primary care practice. Quality in Primary Care 2007;15(3):151‐5. [EM 2007321457]
(401) Mallet HP, Njikam A, Scouflaire SM. Evaluation of prescription practices and of the rational use of medicines in Niger. Sante 2001 Jul;11(3):185‐93. [PM: 11641083]
(402) Manchikanti L, Rivera JJ, Pampati V, Beyer C, Damron K, Barnhill RC. Effectiveness of clinical guidelines in interventional pain management. Pain Physician 2002 Apr;5(2):127‐32. [PM: 16902663]
(403) Marks MK, Hynson JL, Karabatsos G. Asthma: communication between hospital and general practitioners. Journal of Paediatrics & Child Health 1999 Jun;35(3):251‐4. [PM: 10404444]
(404) Marmion RM, Evans CF. Instant inservice: a poster program. Journal of Nursing Staff Development 1988;4(1):33‐4. [PM: 2450975]
(405) Marshall AL, Booth ML, Bauman AE. Promoting physical activity in Australian general practices: a randomised trial of health promotion advice versus hypertension management. Patient Education & Counseling 2005 Mar;56(3):283‐90. [PM: 15721970]
(406) Marshall WR, Rothenberger LA, Bunnell SL. The efficacy of personalized audiovisual patient‐education materials. Journal of Family Practice 1984 Nov;19(5):659‐63. [PM: 6208312]
(407) Martens JD, Winkens RA, van der Weijden T, de BD, Severens JL. Does a joint development and dissemination of multidisciplinary guidelines improve prescribing behaviour: a pre/post study with concurrent control group and a randomised trial. BMC Health Services Research 2006;6(145). [PM: 17081285]
(408) Martin CM, Doig GS, Heyland DK, Morrison T, Sibbald WJ, Southwestern Ontario Critical Care Research Network. Multicentre, cluster‐randomized clinical trial of algorithms for critical‐care enteral and parenteral therapy (ACCEPT). CMAJ Canadian Medical Association Journal 2004 Jan 20;170(2):197‐204. [PM: 14734433]
(409) Matowe L, Ramsay CR, Grimshaw JM, Gilbert FJ, Macleod MJ, Needham G. Effects of mailed dissemination of the Royal College of Radiologists' guidelines on general practitioner referrals for radiography: a time series analysis. Clinical Radiology 2002 Jul;57(7):575‐8. [PM: 12096854]
(410) Matthews JC, Johnson ML, Koelling TM. The impact of patient‐specific quality‐of‐care report cards on guideline adherence in heart failure. American Heart Journal 2007 Dec;154(6):1174‐83. [PM: 18035092]
(411) Maxwell AE, Jo AM, Chin SY, Lee KS, Bastani R. Impact of a print intervention to increase annual mammography screening among Korean American women enrolled in the National Breast and Cervical Cancer Early Detection Program. Cancer Detection & Prevention 2008;32(3):229‐35. [PM: 18799271]
(412) Mayer C, Andrusyszyn MA, Iwasiw C. Codman Award Paper: self‐efficacy of staff nurses for health promotion counselling of patients at risk for stroke. Axon 2005 Jun;26(4):14‐21. [PM: 16028726]
(413) McBee MJ. Pocket guide to ED orientation. Journal of Emergency Nursing 1996 Oct;22(5):446‐50. [PM: 8997972]
(414) McCabe M. Pharmacists should work with nurses in using educational tools. Hospital Management 1969 Jan;107(1):35‐7. [PM: 5782899]
(415) McClennan S, Worster A, MacMillan H. Caring for victims of intimate partner violence: a survey of Canadian emergency departments. CJEM Canadian Journal of Emergency Medical Care 2008 Jul;10(4):325‐8. [PM: 18652722]
(416) McDonnell M, Cullen A, Kiberd B, Mehanni M, Matthews T. A national model of care service for professionals dealing with sudden infant death. Irish Journal of Medical Science 1999 Oct;168(4):237‐41. [PM: 10624360]
(417) McDougal WS, Lunz ME, Hirst G. Postgraduate education: does it improve the knowledge base of practitioners with time? Journal of Urology 1998 Aug;160(2):502‐4. [PM: 9679913]
(418) McGregor AH, Dore CJ, Morris TP, Morris S, Jamrozik K. Function after spinal treatment, exercise and rehabilitation (FASTER): improving the functional outcome of spinal surgery. BMC Musculoskeletal Disorders 2010;11(17). [PM: 20102625]
(419) Mertz D, Koller M, Haller P, Lampert ML, Plagge H, Hug B, et al. Outcomes of early switching from intravenous to oral antibiotics on medical wards. Journal of Antimicrobial Chemotherapy 2009 Jul;64(1):188‐99. [PM: 19401304]
(420) Mettes TG, van der Sanden WJ, Bronkhorst E, Grol RP, Wensing M, Plasschaert AJ. Impact of guideline implementation on patient care: a cluster RCT. Journal of Dental Research 2010 Jan;89(1):71‐6. [PM: 19966044]
(421) Michel K, Valach L. Suicide prevention: spreading the gospel to general practitioners. British Journal of Psychiatry 1992 Jun;160:757‐60. [PM: 1617356]
(422) Miller L. Doctor's orders. Mental Health Today 2010 Mar;30. [PM: 20429111]
(423) Misrahi L, Colin C, Lanoir D, Chambrier C, Jolivet A, Bouletreau P. Economic impact of the consensus conference guidelines on postoperative artificial nutrition in the Rhone‐Alps region. Annales Francaises d Anesthesie et de Reanimation 1999 Feb;18(2):270‐9. [PM: 10207604]
(424) Mitchell ML, Courtney M. An intervention study to improve the transfer of ICU patients to the ward ‐‐ evaluation by family members. AUST CRIT CARE 2001;18(2):61.
(425) Mitchell ML, Courtney M. Improving transfer from the intensive care unit: the development, implementation and evaluation of a brochure based on Knowles' Adult Learning Theory. International Journal of Nursing Practice 2005 Dec;11(6):257‐68. [PM: 16255737]
(426) Mockiene V, Suominen T, Valimaki M, Razbadauskas A, Martinkenas A, Caplinskas S. The impact of an education intervention to change nurses' HIV‐related knowledge and attitudes in Lithuania: a randomized controlled trial. Journal of the Association of Nurses in AIDS Care 2011 Mar;22(2):140‐9. [PM: 21123087]
(427) Mollon DL, Fields WL. Is this the right patient? An educational initiative to improve compliance with two patient identifiers. Journal of Continuing Education in Nursing 2009 May;40(5):221‐7. [PM: 19489521]
(428) Moran WP, Chen GJ, Watters C, Poehling G, Millman F. Using a collaborative approach to reduce postoperative complications for hip‐fracture patients: a three‐year follow‐up. Joint Commission Journal on Quality & Patient Safety 2006 Jan;32(1):16‐23. [PM: 16514935]
(429) Morris AH, Orme J, Jr., Truwit JD, Steingrub J, Grissom C, Lee KH, et al. A replicable method for blood glucose control in critically Ill patients. Critical Care Medicine 2008 Jun;36(6):1787‐95. [PM: 18520641]
(430) Morrison J, Carroll L, Twaddle S, Cameron I, Grimshaw J, Leyland A, et al. Pragmatic randomised controlled trial to evaluate guidelines for the management of infertility across the primary care‐secondary care interface. BMJ 2001 May 26;322(7297):1282‐4. [PM: 11375232]
(431) Morse L, McDonald M. Failure of a poster‐based educational programme to improve compliance with peripheral venous catheter care in a tertiary hospital. A clinical audit. Journal of Hospital Infection 2009 Jul;72(3):221‐6. [PM: 19464755]
(432) Morton E, Tambor E, Rimer BK, Tessaro I, Farrell D, Siegler IC. Impact of National Cancer Institute revised mammography screening guidelines on women 40‐49. Womens Health Issues 1996 Sep;6(5):246‐54. [PM: 8870503]
(433) Murawski MM, Abdelgawad T. Exploration of the impact of preferred drug lists on hospital and physician visits and the costs to Medicaid. American Journal of Managed Care 2005 Jan;11(Special no.):35‐42. [PM: 15700908]
(434) Narasimhaswamy S, Vedi C, Xavier Y, Tseng CH, Shine D. Effect of implementing pain management standards. Journal of General Internal Medicine 2006 Jul;21(7):689‐93. [PM: 16808767]
(435) Noirot LA, Hollands JM, Reichley RM, Dunagan WC, Bailey TC. Long‐term effectiveness of an automated guideline adherence monitor for secondary prevention of acute myocardial infarction. Annual Symposium Proceedings/AMIA Symposium 2007;1061. [PM: 18694159]
(436) Nucci M, Landau M, Silveira F, Spector N, Pulcheri W. Application of the IDSA guidelines for the use of antimicrobial agents in neutropenic patients: impact on reducing the use of glycopeptides. Infection Control & Hospital Epidemiology 2001 Oct;22(10):651‐3. [PM: 11776354]
(437) O'Brien K, Wright J, Conboy F, Bagley L, Lewis D, Read M, et al. The effect of orthodontic referral guidelines: a randomised controlled trial. British Dental Journal 2000 Apr 8;188(7):392‐7. [PM: 10816930]
(438) O'Cathain A, Walters SJ, Nicholl JP, Thomas KJ, Kirkham M. Use of evidence based leaflets to promote informed choice in maternity care: randomised controlled trial in everyday practice. BMJ 2002 Mar 16;324(7338):643. [PM: 11895822]
(439) O'Connor PJ, Sperl‐Hillen J, Johnson PE, Rush WA, Crain AL. Customized feedback to patients and providers failed to improve safety or quality of diabetes care: a randomized trial. Diabetes Care 2009 Jul;32(7):1158‐63. [PM: 19366977]
(440) O'Hanrahan M. The educational value of printed information for hypertensive patients: an assessment. Irish Journal of Medical Science 1981;150(4).
(441) Overhage JM, Tierney WM, McDonald CJ. Computer reminders to implement preventive care guidelines for hospitalized patients. Archives of Internal Medicine 1996 Jul 22;156(14):1551‐6. [PM: 8687263]
(442) Owen PS, Golightly LK, MacLaren R, Ferretti KA, Badesch DB. Formulary management of recombinant factor VIIa at an academic medical center. Annals of Pharmacotherapy 2008 Jun;42(6):771‐6. [PM: 18477731]
(443) Ozgun H, Ertugrul BM, Soyder A, Ozturk B, Aydemir M. Peri‐operative antibiotic prophylaxis: adherence to guidelines and effects of educational intervention. International Journal Of Surgery 2010;8(2):159‐63. [PM: 20026001]
(444) Pagaiya N, Garner P. Primary care nurses using guidelines in Thailand: a randomized controlled trial. Tropical Medicine & International Health 2005 May;10(5):471‐7. [PM: 15860094]
(445) Palmer S, Bader MK, Qureshi A, Palmer J, Shaver T, Borzatta M, et al. The impact on outcomes in a community hospital setting of using the AANS traumatic brain injury guidelines. Americans Associations for Neurologic Surgeons. Journal of Trauma‐Injury Infection & Critical Care 2001 Apr;50(4):657‐64. [PM: 11303160]
(446) Paxton R, MacDonald F, Allott R, Mitford P, Proctor S, Smith M. Improving general practitioners' assessment and management of suicide risk. International Journal of Health Care Quality Assurance Incorporating Leadership in Health Services 2001;14(2‐3):133‐8. [PM: 11436749]
(447) Pazirandeh M. Does patient partnership in continuing medical education (CME) improve the outcome in osteoporosis management? Journal of Continuing Education in the Health Professions 2002;22(3):142‐51. [PM: 12227236]
(448) Perez‐Cuevas R, Reyes‐Morales H, Flores‐Hernandez S, Wacher‐Rodarte N. Effect of a clinical practice guideline for the management of diabetes type 2. Revista Medica del Instituto Mexicano del Seguro Social 2007 Jul;45(4):353‐60. [PM: 17949573]
(449) Perez A, Dennis RJ, Rodriguez B, Castro AY, Delgado V, Lozano JM, et al. An interrupted time series analysis of parenteral antibiotic use in Colombia. Journal of Clinical Epidemiology 2003 Oct;56(10):1013‐20. [PM: 14568634]
(450) Peterson GM, Drake CI, Jupe DM, Vial JH, Wilkinson S. Educational campaign to improve the prevention of postoperative venous thromboembolism. Journal of Clinical Pharmacy & Therapeutics 1999 Aug;24(4):279‐87. [PM: 10475986]
(451) Planas M, Penalva A, Burgos R, Puiggros C, Perez‐Portabella C, Espin E, et al. Guidelines for colorectal cancer: effects on nutritional intervention. Clinical Nutrition 2007 Dec;26(6):691‐7. [PM: 18029063]
(452) Premi J, Shannon SI. Randomized controlled trial of a combined video‐workbook educational program for CME. Academic Medicine 1993 Oct;68(Suppl 10):S13‐5. [PM: 8216618]
(453) Ray‐Coquard I, Philip T, de LG, Froger X, Suchaud JP, Voloch A, et al. A controlled "before‐after" study: impact of a clinical guidelines programme and regional cancer network organization on medical practice. British Journal of Cancer 2002 Feb 1;86(3):313‐21. [PM: 11875690]
(454) Rebbeck T, Maher CG, Refshauge KM. Evaluating two implementation strategies for whiplash guidelines in physiotherapy: a cluster randomised trial. Australian Journal of Physiotherapy 2006;52(3):165‐74. [PM: 16942451]
(455) Reid J. The Play@Home programme. Practising Midwife 2001 Feb;4(2):32‐5. [PM: 12026298]
(456) Richardson B, Kitchen G, Livingston G. The effect of education on knowledge and management of elder abuse: a randomized controlled trial. Age & Ageing 2002 Sep;31(5):335‐41. [PM: 12242194]
(457) Rieber U. Representation of pediatric nursing in the picture book. Kinderkrankenschwester 2008 Sep;27(9):368‐72. [PM: 18841638]
(458) Ripouteau C, Conort O, Lamas JP, Auleley GR, Hazebroucq G, Durieux P. Effect of multifaceted intervention promoting early switch from intravenous to oral acetaminophen for postoperative pain: controlled, prospective, before and after study. BMJ 2000 Dec 9;321(7274):1460‐3. [PM: 11110743]
(459) Rippon A. Caring is knowing. Nursing Standard 2010 Apr 7;24(31):26‐7. [PM: 20441031]
(460) Rood E, Bosman RJ, van der Spoel JI, Taylor P, Zandstra DF. Use of a computerized guideline for glucose regulation in the intensive care unit improved both guideline adherence and glucose regulation. Journal of the American Medical Informatics Association 2005 Mar;12(2):172‐80. [PM: 15561795]
(461) Rosier PK, Wall B, Discoe A. Posters: valuable educational tool. Journal of Continuing Education in Nursing 1989 Sep;20(5):238. [PM: 2507605]
(462) Roski J, Jeddeloh R, An L, Lando H, Hannan P, Hall C, et al. The impact of financial incentives and a patient registry on preventive care quality: increasing provider adherence to evidence‐based smoking cessation practice guidelines. Preventive Medicine 2003 Mar;36(3):291‐9. [PM: 12634020]
(463) Rossignol M, Allaert FA, Rozenberg S, Valat JP, Avouac B, Peres G, et al. Measuring the contribution of pharmacological treatment to advice to stay active in patients with subacute low‐back pain: a randomised controlled trial. Pharmacoepidemiology & Drug Safety 2005 Dec;14(12):861‐7. [PM: 15991263]
(464) Roth EJ, Plastaras CT, Mullin MS, Fillmore J, Moses ML. A simple institutional educational intervention to decrease use of selected expensive medications. Archives of Physical Medicine & Rehabilitation 2001 May;82(5):633‐6. [PM: 11346840]
(465) Rusnak M, Janciak I, Majdan M, Wilbacher I, Mauritz W, Australian Severe TBI Study Investigators. Severe traumatic brain injury in Austria VI: effects of guideline‐based management. Wiener Klinische Wochenschrift 2007 Feb;119(1‐2):64‐71. [PM: 17318752]
(466) Salahuddin M, Anwar MN. Study on effectiveness of guidelines and high dependency unit management on diabetic ketoacidosis patients. JPMI ‐ Journal of Postgraduate Medical Institute 2009;23(2):120‐3. [EM 2010579111]
(467) Sanders DS, Carter MJ, D'Silva J, James G, Bolton RP, Willemse PJ, et al. Percutaneous endoscopic gastrostomy: a prospective audit of the impact of guidelines in two district general hospitals in the United Kingdom. American Journal of Gastroenterology 2002 Sep;97(9):2239‐45. [PM: 12358239]
(468) Sanders KM, Satyvavolu A. Improving blood pressure control in diabetes: limitations of a clinical reminder in influencing physician behavior. Journal of Continuing Education in the Health Professions 2002;22(1):23‐32. [PM: 12004637]
(469) Sandrick K. Beyond RxD2: linking pharmacists doctors and nurses. Health Management Technology 1998 Nov;19(12):26‐8. [PM: 10187101]
(470) Sauro A, Scalzitti F, Buono N, Siringano R, Petrazzuoli F, Diodati G, et al. Spirometry is really useful and feasible in the GP's daily practice but guidelines alone are not. European Journal of General Practice 2005 Mar;11(1):29‐31. [PM: 15841063]
(471) Sayre MR, Cantrell SA, White LJ, Hiestand BC, Keseg DP, Koser S. Impact of the 2005 American Heart Association cardiopulmonary resuscitation and emergency cardiovascular care guidelines on out‐of‐hospital cardiac arrest survival. Prehospital Emergency Care 2009 Oct;13(4):469‐77. [PM: 19731159]
(472) Schaffner W, Ray WA, Federspiel CF, Miller WO. Improving antibiotic prescribing in office practice. A controlled trial of three educational methods. JAMA 1983 Oct 7;250(13):1728‐32. [PM: 6350633]
(473) Scheeres K, Wensing M, Mes C, Bleijenberg G. The impact of informational interventions about cognitive behavioral therapy for chronic fatigue syndrome on GPs referral behavior. Patient Education & Counseling 2007 Sep;68(1):29‐32. [PM: 17521842]
(474) Schlechter I. The ward manual‐‐an aid to practical work on the ward? Krankenpflege 1987 Feb;41(2):45‐9. [PM: 3102845]
(475) Schoenfeld P, Chey WD. An evidence‐based approach to clinical practice guidelines: diagnosis and treatment of irritable bowel syndrome. Clinical Gastroenterology & Hepatology 2003 Jul;1(4):322‐7. [PM: 15017675]
(476) Schuster RJ, Terwoord NA, Tasosa J. Changing physician practice behavior to measure and improve clinical outcomes. American Journal of Medical Quality 2006 Nov;21(6):394‐400. [PM: 17077421]
(477) Schwartz DN, Abiad H, DeMarais PL, Armeanu E, Trick WE, Wang Y, et al. An educational intervention to improve antimicrobial use in a hospital‐based long‐term care facility. Journal of the American Geriatrics Society 2007 Aug;55(8):1236‐42. [PM: 17661963]
(478) Schwartzberg JG, Guttman R. Effect of training on physician attitudes and practices in home and community care of the elderly. Archives of Family Medicine 1997 Sep;6(5):439‐44. [PM: 9305686]
(479) Selby ML, Riportella‐Muller R, Sorenson JR, Quade D, Luchok KJ. Increasing participation by private physicians in the EPSDT Program in rural North Carolina. Public Health Reports 1992 Sep;107(5):561‐8. [PM: 1410238]
(480) Sellier E, Labarere J, Bosson JL, Auvray M, Barrellier MT, Le HC, et al. Effectiveness of a guideline for venous thromboembolism prophylaxis in elderly post‐acute care patients: a multicenter study with systematic ultrasonographic examination. Archives of Internal Medicine 2006 Oct 23;166(19):2065‐71. [PM: 17060535]
(481) Seve P, Mackey J, Sawyer M, Lesimple T, de La FC, Broussolle C, et al. Impact of clinical practice guidelines on the management for carcinomas of unknown primary site: a controlled "before‐after" study. Bulletin du Cancer 2009 Apr;96(4):E7‐17. [PM: 19435692]
(482) Shah BR, Juurlink DN, Austin PC, Mamdani MM. New use of rosiglitazone decreased following publication of a meta‐analysis suggesting harm. Diabetic Medicine 2008 Jul;25(7):871‐4. [PM: 18644075]
(483) Shalansky SJ. Antibiotic interchange through educational interventions in a community hospital. American Journal of Hospital Pharmacy 1991 Dec;48(12):2655‐7. [PM: 1814215]
(484) Shekelle PG, Kravitz RL, Beart J, Marger M, Wang M, Lee M. Are nonspecific practice guidelines potentially harmful? A randomized comparison of the effect of nonspecific versus specific guidelines on physician decision making. Health Services Research 2000 Mar;34(7):1429‐48. [PM: 10737446]
(485) Shrake KL, Scaggs JE, England KR, Henkle JQ, Eagleton LE. Benefits associated with a respiratory care assessment‐treatment program: results of a pilot study. Respiratory Care 1994 Jul;39(7):715‐24. [PM: 10146052]
(486) Shulkin DJ, McGourty ME, Bourret J. Involving physicians in efforts to control pharmaceutical expenditures. Medical Interface 1995 Aug;8(8):85‐7. [PM: 10144777]
(487) Simon SR, Rodriguez HP, Majumdar SR, Kleinman K, Warner C, Salem‐Schatz S, et al. Economic analysis of a randomized trial of academic detailing interventions to improve use of antihypertensive medications. Journal of Clinical Hypertension 2007 Jan;9(1):15‐20. [PM: 17215654]
(488) Singer NJ, Willman J, Penzkowski D. Tools for meeting core measures for heart failure best practice [6th Annual Meeting of the American Association of Heart Failure Nurses, AAHFN 2010]. Heart and Lung 2010;39(4):364‐5. [EM 70238139]
(489) Singh R, Kishore J, Mathur RG, Mandal K, Puri S. The role of an information booklet on bio‐medical waste management for nurses. Nursing Journal of India 2002 Dec;93(12):271‐2. [PM: 12718116]
(490) Slora EJ, Steffes JM, Harris D, Clegg HW, Norton D, Darden PM, et al. Improving pediatric practice immunization rates through distance‐based quality improvement: a feasibility trial from PROS. Clinical Pediatrics 2008 Jan;47(1):25‐36. [PM: 17693592]
(491) Smabrekke L, Berild D, Giaever A, Myrbakk T, Fuskevag A, Ericson JU, et al. Educational intervention for parents and healthcare providers leads to reduced antibiotic use in acute otitis media. Scandinavian Journal of Infectious Diseases 2002;34(9):657‐9. [PM: 12374355]
(492) Smith AJ, Law CH, Khalifa MA, Hsieh ET, Hanna SS, Wright FC, et al. Multimodal CME for surgeons and pathologists improves colon cancer staging. Journal of Cancer Education 2003;18(2):81‐6. [PM: 12888381]
(493) Smith CJ, Gribbin J, Challen KB, Hubbard RB. The impact of the 2004 NICE guideline and 2003 General Medical Services contract on COPD in primary care in the UK. QJM 2008 Feb;101(2):145‐53. [PM: 18180254]
(494) Smith L, McClenahan J. User groups. View finders. Health Service Journal 1998 Feb 12;108(5591):33. [PM: 10176478]
(495) Smith M. A physician's reference booklet helps RNS. Nursing Management 1991 Jun;22(6):52. [PM: 2052254]
(496) Soler N, Ballester E, Martin A, Gobartt E, Miravitlles M, Torres A. Changes in management of chronic obstructive pulmonary disease (COPD) in primary care: EMMEPOC study. Respiratory Medicine 2010 Jan;104(1):67‐75. [PM: 20122630]
(497) Sondergaard J, Andersen M, Vach K, Kragstrup J, Maclure M, Gram LF. Detailed postal feedback about prescribing to asthma patients combined with a guideline statement showed no impact: a randomised controlled trial. European Journal of Clinical Pharmacology 2002 May;58(2):127‐32. [PM: 12012145]
(498) Sondergaard J, Foged A, Kragstrup J, Gaist D, Gram LF, Sindrup SH, et al. Intensive community pharmacy intervention had little impact on triptan consumption: a randomized controlled trial. Scandinavian Journal of Primary Health Care 2006 Mar;24(1):16‐21. [PM: 16464810]
(499) Spitz B, Fraker C, Meyer CP, Peterson T. Evolution of evidence‐based guidelines for home care: Wisconsin's experience. Home Healthcare Nurse 2007 May;25(5):327‐34. [PM: 17495563]
(500) Stamos TD, Shaltoni H, Girard SA, Parrillo JE, Calvin JE. Effectiveness of chart prompts to improve physician compliance with the National Cholesterol Education Program guidelines. American Journal of Cardiology 2001 Dec 15;88(12):1420‐3. [PM: 11741565]
(501) Suchyta MR, Dean NC, Narus S, Hadlock CJ. Effects of a practice guideline for community‐acquired pneumonia in an outpatient setting. American Journal of Medicine 2001 Mar;110(4):306‐9. [PM: 11239849]
(502) Suisted P, Seneviratne S, Burgess C. Management of cardiac failure ‐ Do practitioners follow guidelines? Journal of Pharmacy Technology 2005;21(1):16‐20. [EM 2005025106]
(503) Svarstad BL, Mount JK. Chronic benzodiazepine use in nursing homes: effects of federal guidelines, resident mix, and nurse staffing. Journal of the American Geriatrics Society 2001 Dec;49(12):1673‐8. [PM: 11844002]
(504) Swayne LC, Lam SC, Filippone AL, Ambrose RB. Credentialing of crossover privileges in fluoroscopy for nonradiologists. Radiology 1994 Jan;190(1):281‐2. [PM: 8259420]
(505) Swoboda S, Lichtenstern C, Ober MC, Taylor LA, Storzinger D, Michel A, et al. Implementation of practice guidelines for antifungal therapy in a surgical intensive care unit and its impact on use and costs. Chemotherapy 2009;55(6):418‐24. [PM: 19996586]
(506) Terry P, Pheley A, Williams D, Strickland S. The result of an educational intervention for physicians providing HIV‐antibody testing and counseling. Minnesota Medicine 1992 Apr;75(4):37‐9. [PM: 1584157]
(507) Teza SL, DeVito AV, Hiss RG. Resource manual to help your program meet the national standards. Diabetes Educator 1987 May;13:210‐28. [PM: 3646957]
(508) Thomas LG, Corwin EJ. The readability of printed education materials regarding hormone replacement therapy. Journal of the American Academy of Nurse Practitioners 1998 Oct;10(10):447‐52. [PM: 10085857]
(509) Thomas M, Gillespie W, Krauss J, Harrison S, Medeiros R, Hawkins M, et al. Focus group data as a tool in assessing effectiveness of a hand hygiene campaign. American Journal of Infection Control 2005 Aug;33(6):368‐73. [PM: 16061144]
(510) Thomas RE, Grimshaw JM, Mollison J, McClinton S, McIntosh E, Deans H, et al. Cluster randomized trial of a guideline‐based open access urological investigation service. Family Practice 2003 Dec;20(6):646‐54. [PM: 14701887]
(511) Thompson C, Kinmonth AL, Stevens L, Peveler RC, Stevens A, Ostler KJ, et al. Effects of a clinical‐practice guideline and practice‐based education on detection and outcome of depression in primary care: Hampshire Depression Project randomised controlled trial. Lancet 2000 Jan 15;355(9199):185‐91. [PM: 10675118]
(512) Thomson RG. Consensus publication guidelines: the next step in the science of quality improvement? Quality & Safety in Health Care 2005 Oct;14(5):317‐8. [PM: 16195562]
(513) Toh A, Mullin A, Grainger J, Uppal H. Indications for tonsillectomy: are we documenting them? Annals of the Royal College of Surgeons of England 2009 Nov;91(8):697‐9. [PM: 19909613]
(514) Tomasini J. A nursing care plan for dealing with anxiety. Soins; La Revue de Reference Infirmiere 1998 Dec;(631):16‐8. [PM: 10095741]
(515) Torrey WC, Drake RE, Dixon L, Burns BJ, Flynn L, Rush AJ, et al. Implementing evidence‐based practices for persons with severe mental illnesses. Psychiatric Services 2001 Jan;52(1):45‐50. [PM: 11141527]
(516) Tsuyuki RT, Ackman ML, Montague TJ, Clinical Quality Improvement Network Investigators. Effects of the 1994 Canadian Cardiovascular Society clinical practice guidelines for congestive heart failure. Canadian Journal of Cardiology 2002 Feb;18(2):147‐52. [PM: 11875584]
(517) Turnbull DA, Beilby JJ, Ziaian T, Qureshi F, Nelson M, Tonkin AL, et al. Disease management for hypertension: A pilot cluster randomized trial of 67 Australian general practices. Disease Management and Health Outcomes 2006;14(1):27‐35. [EM 2006082375]
(518) Turnbull F. Managing cardiovascular risk factors: the gap between evidence and practice. PLoS Medicine / Public Library of Science 2005 May;2(5):e131. [PM: 15916459]
(519) Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. New England Journal of Medicine 2008 Jan 17;358(3):252‐60. [PM: 18199864]
(520) Tyrer P. Practice‐based education did not increase recognition of depression by primary‐care physicians nor improve the outcome of depression. ACP Journal Club 2000;133(2):74.
(521) Uberfeldt E. Initial positive results of the German Nurses' Association's pamphlet action "Humanism in the hospital". Krankenpflege 1980 Jan;34(1):5‐6. [PM: 6767116]
(522) Vallano A, Llinares J, Amau JM, Martorell M, Girona L, Laporte JR. Impact of analgesic drug‐use guidelines for the management of postoperative pain: a drug utilization study. International Journal of Clinical Pharmacology & Therapeutics 2003 Apr;41(4):165‐70. [PM: 12712962]
(523) van Vliet EP, Steyerberg EW, Otten HJ, Rudolphus A, Knoester PD, Hoogsteden HC, et al. The effects of guideline implementation for proton pump inhibitor prescription on two pulmonary medicine wards. Alimentary Pharmacology & Therapeutics 2009 Jan;29(2):213‐21. [PM: 19006542]
(524) van Zwanenberg TD, Grant GB, Gregory DA. Can rational prescribing be assessed? Journal of the Royal College of General Practitioners 1987 Jul;37(300):308‐10. [PM: 3449633]
(525) van LA, van Schaik DJ, Dekker JJ, Beekman AT. Effectiveness of an intercultural module added to the treatment guidelines for Moroccan and Turkish patients with depressive and anxiety disorders. BMC Psychiatry 2011;11(13). [PM: 21247455]
(526) Verkaik R, Francke AL, van MB, Spreeuwenberg PM, Ribbe MW, Bensing JM. The effects of a nursing guideline on depression in psychogeriatric nursing home residents with dementia. International Journal of Geriatric Psychiatry 2011 Jul;26(7):723‐32. [PM: 21495077]
(527) Vernon SA, Ghosh G. Do locally agreed guidelines for optometrists concerning the referral of glaucoma suspects influence referral practice? Eye 2001 Aug;15(Pt 4):458‐63. [PM: 11767019]
(528) Viereck C, Boudes P. An analysis of the impact of FDA's guidelines for addressing cardiovascular risk of drugs for type 2 diabetes on clinical development. Contemporary Clinical Trials 2011 May;32(3):324‐32. [PM: 21266202]
(529) Vittoria PM. The quality use of nimodipine: Assessing the impact of consensus guidelines. Australian Journal of Hospital Pharmacy 1999;29(3):137‐41. [EM 1999229606]
(530) Vukic M, Negovetic L, Kovac D, Ghajar J, Glavic Z, Gopcevic A. The effect of implementation of guidelines for the management of severe head injury on patient treatment and outcome. Acta Neurochirurgica 1999;141(11):1203‐8. [PM: 10592121]
(531) Waldron T. Managed care's atypical response. New antipsychotics show promise, but are they getting into enough hands? Behavioral Healthcare Tomorrow 1999 Apr;8(2):28‐31. [PM: 10351297]
(532) Walker EA, Engel SS, Zybert PA. Dissemination of diabetes care guidelines: lessons learned from community health centers. Diabetes Educator 2001 Jan;27(1):101‐10. [PM: 11912611]
(533) Ward MM, Doebbeling BN, Vaughn TE, Uden‐Holman T, Clarke WR, Woolson RF, et al. Effectiveness of a nationally implemented smoking cessation guideline on provider and patient practices. Preventive Medicine 2003 Mar;36(3):265‐71. [PM: 12634017]
(534) Webb K. Nursing Times guide to care planning: nurse's little helper. Nursing Times 1987 May 6;83(18):39‐12. [PM: 3647442]
(535) West RR. Evidence based medicine overviews, bulletins, guidelines, and the new consensus. Postgraduate Medical Journal 2000 Jul;76(897):383‐9. [PM: 10878193]
(536) West YR, Kendrick BJL, Williamson DM. Evaluation of the impact of orthopaedic guidelines on referrals from primary care to a specialist department. Quality in Primary Care 2007;15(1):27‐31. [EM 2007125021]
(537) Wettermark B, Pehrsson A, Juhasz‐Haverinen M, Veg A, Edlert M, Tornwall‐Bergendahl G, et al. Financial incentives linked to self‐assessment of prescribing patterns: a new approach for quality improvement of drug prescribing in primary care. Quality in Primary Care 2009;17(3):179‐89. [PM: 19622268]
(538) Wight NE. Resources for physicians. Web sites, books, and organizations. Pediatric Clinics of North America 2001 Apr;48(2):539‐46. [PM: 11339172]
(539) Wijsman BP, Adrichem CM, Van't Hooft FM, Leuven JAG, Van der Laarse A, Smelt AHM. Diverse effects of the Dutch guidelines for a healthy diet on serum lipids of male patients with type IIA and type IIB hyperlipoproteinemia. European Journal of Internal Medicine 1993;4(2):115‐22. [EM 1993352011]
(540) Williams C, George L, Lowry M. A framework for patient assessment. Nursing Standard 1994 Jun 15;8(38):29‐33. [PM: 8080753]
(541) Williams JK, McCarthy AM, Bragadottir H, Reed D. School nurses' experiences, concerns, and knowledge of growth disorders in children: development of a monograph. Journal of School Nursing 2002 Feb;18(1):25‐32. [PM: 11853372]
(542) Williams KS, Crichton NJ, Roe B. Disseminating research evidence. A controlled trial in continence care. J ADV NURS 1997 Apr;25(4):691‐8. [PM: 9104664]
(543) Williams ML, Hill G, Jackson M. The impact of an acute myocardial infarction guideline and pathway on racial outcomes at a university hospital. Ethnicity & Disease 2006;16(3):653‐8. [PM: 16937601]
(544) Willis K, Singer M. Prevention as intervention: development of evidence based Lymphedema Risk Assessment Tool and practice guidelines... Oncology Nursing Society 31st Annual Congress podium and poster abstracts. ONCOL NURS FORUM 2001;33(2):406.
(545) Wishart GC, Chetty U. Introduction to sessions on guidelines and endocrine therapy, the influence of breast screening on number of mastectomies and the challenge between molecular science and traditional dogma in the treatment of breast cancer. Introduction to Session 6. Breast Cancer Research 2009;11(Suppl 3):S17. [PM: 20030868]
(546) Witt K, Knudsen E, Ditlevsen S, Hollnagel H. Academic detailing has no effect on prescribing of asthma medication in Danish general practice: a 3‐year randomized controlled trial with 12‐monthly follow‐ups. Family Practice 2004 Jun;21(3):248‐53. [PM: 15128684]
(547) Wojs DJ, Jr. What do you do if no one knows what to do? Journal of Trauma Nursing 2010 Apr;17(2):109‐11. [PM: 20559059]
(548) Wolters R, Wensing M, Klomp M, Lagro‐Jansen T, Weel C, Grol R. Effects of distance learning on clinical management of LUTS in primary care: a randomised trial. Patient Education & Counseling 2005 Nov;59(2):212‐8. [PM: 16257627]
(549) Wong EK, Truong PT, Kader HA, Nichol AM, Salter L, Petersen R, et al. Consistency in seroma contouring for partial breast radiotherapy: impact of guidelines. International Journal of Radiation Oncology, Biology, Physics 2006 Oct 1;66(2):372‐6. [PM: 16965989]
(550) Woodcock AJ, Kinmonth AL, Campbell MJ, Griffin SJ, Spiegal NM. Diabetes care from diagnosis: effects of training in patient‐centred care on beliefs, attitudes and behaviour of primary care professionals. Patient Education & Counseling 1999 May;37(1):65‐79. [PM: 10640121]
(551) Woolery‐Antill M. Granulocyte‐macrophage colony‐stimulating factor: a teaching program for staff and patients. Journal of Pediatric Oncology Nursing 1990 Apr;7(2):61. [PM: 2194506]
(552) Worrall CL, Anger BP, Simpson KN, Leon SM. Impact of a hospital‐acquired/ventilator‐associated/healthcare‐associated pneumonia practice guideline on outcomes in surgical trauma patients. Journal of Trauma ‐ Injury, Infection and Critical Care 2010;68(2):382‐6. [EM 2010128886]
(553) Wright J, Bibby J, Eastham J, Harrison S, McGeorge M, Patterson C, et al. Multifaceted implementation of stroke prevention guidelines in primary care: cluster‐randomised evaluation of clinical and cost effectiveness. Quality & Safety in Health Care 2007 Feb;16(1):51‐9. [PM: 17301206]
(554) Yealy DM, Auble TE, Stone RA, Lave JR, Meehan TP, Graff LG, et al. Effect of increasing the intensity of implementing pneumonia guidelines: a randomized, controlled trial. Annals of Internal Medicine 2005 Dec 20;143(12):881‐94. [PM: 16365469]
(555) Yeh ML, Chen HH, Liu PH. Effects of multimedia with printed nursing guide in education on self‐efficacy and functional activity and hospitalization in patients with hip replacement. Patient Education & Counseling 2005 May;57(2):217‐24. [PM: 15911196]
(556) Yeo GT, de Burgh SP, Letton T, Shaw J, Donnelly N, Swinburn ME, et al. Educational visiting and hypnosedative prescribing in general practice. Family Practice 1994 Mar;11(1):57‐61. [PM: 7913452]
(557) Yeoh BS, Taylor DM, Taylor SE. Education initiative improves the evidence‐based use of metoclopramide following morphine administration in the emergency department. Emergency Medicine Australasia 2009 Jun;21(3):178‐83. [PM: 19527276]
(558) Yeung JH, Cheung NK, Graham CA, Rainer TH. Reduced time on the spinal board‐effects of guidelines and education for emergency department staff. Injury 2006 Jan;37(1):53‐6. [PM: 16246337]
(559) Zack JE, Garrison T, Trovillion E, Clinkscale D, Coopersmith CM, Fraser VJ, et al. Effect of an education program aimed at reducing the occurrence of ventilator‐associated pneumonia. Critical Care Medicine 2002 Nov;30(11):2407‐12. [PM: 12441746]
(560) Zeliadt SB, Hoffman RM, Etzioni R, Gore JL, Kessler LG, Lin DW. Influence of publication of US and European prostate cancer screening trials on PSA testing practices. Journal of the National Cancer Institute 2011 Mar 16;103(6):520‐3. [PM: 21357307]
(561) Zernikow B, Hasan C, Hechler T, Huebner B, Gordon D, Michel E. Stop the pain! A nation‐wide quality improvement programme in paediatric oncology pain control. European Journal of Pain 2008 Oct;12(7):819‐33. [PM: 18222100]
(562) Zhang W, Shen X, Wang Y, Chen Y, Huang M, Zeng Q, et al. Antibiotic use in five children's hospitals during 2002‐2006: the impact of antibiotic guidelines issued by the Chinese Ministry of Health. Pharmacoepidemiology & Drug Safety 2008 Mar;17(3):306‐11. [PM: 18165944]
(563) Zidek W. Revised guidelines for antihypertensive treatment. What is relevant for the general practitioner? MMW Fortschritte der Medizin 2004 Jan 15;146(1‐2):37‐8. [PM: 18437867]
(564) Zimetbaum P, Reynolds MR, Ho KK, Gaziano T, McDonald MJ, McClennen S, et al. Impact of a practice guideline for patients with atrial fibrillation on medical resource utilization and costs. American Journal of Cardiology 2003 Sep 15;92(6):677‐81. [PM: 12972105]
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Austin 2003.
| Methods | Study design: ITS | |
| Participants | Physicians Clinical speciality: not clear Level of training: fully trained Setting/country: not clear/Canada |
|
| Interventions | 2 PEMs were studied, but only 1 respected our inclusion criteria for ITS studies that more than 3 points need to be available before and after the intervention, and that PEM was the HERS. The HERS study was published in 1998 and demonstrated that the risks associated with hormone therapy outweighed the benefits for women taking continuous oestrogen and progestin regimens | |
| Outcomes | 2 process outcomes (prescribing):
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | Unclear risk | No information is provided |
| Shape of Intervention effect pre‐specified ‐ ITS | Unclear risk | Quote, pg. 3241: "we examined patterns of prescriptions for estrogen replacement therapy (ERT) before and after publication of the Women's Health Initiative (WHI) study on July 17, 2002. We also examined trends around the publication of the Heart and Estrogen/progestin Replacement Study (HERS) in 1998" |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | COMMENT: the intervention (publication of the WHI study in 2002) did not affect either the source or method of data collection |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | The outcome was objective |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Low risk | Quote, pg. 3241: "we studied claims for ERT to Ontario's universal Drug Benefit program for seniors (ODB), which tracks medication use by all 1.3 million residents of Ontario older than 65 years" |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in the results section |
| Other bias ‐ ITS | Low risk | There was no evidence of other risks of bias |
Austin 2004A.
| Methods | Study design: ITS | |
| Participants | Physicians; Clinical specialty: Not clear; Level of training: Fully trained; Setting/Country: Not clear/Canada |
|
| Interventions | The PEM was the Women’s Health Initiative (WHI) trial, published on July 17, 2002, which concluded that overall health risks exceeded benefits from use of combined estrogen plus progestin among healthy postmenopausal women. | |
| Outcomes | 2 process outcomes (prescribing):
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | Low risk | Quote, pg. 193: "our study demonstrated a significant increase in incident clonidine use exceeding secular trends among elderly postmenopausal women" |
| Shape of Intervention effect pre‐specified ‐ ITS | High risk | Quote, pg. 193: "as women abandoned ERT, some may have initiated treatment with medications such as clonidine, for the treatment of menopausal hot flashes. There are limitations of our study. First, we were unable to determine the exact reason for initiating clonidine. Although clonidine is classified as an antihypertensive medication, it is not commonly used for hypertension" COMMENT: a rational explanation for the shape of intervention effect was not provided by the authors |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | Quote, pg. 191: "Retrospective, population‐based administrative database design" COMMENT: the intervention itself is unlikely to affect data collection |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | The outcome was objective |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Low risk | Quote, pg. 192: "we studied incident claims for clonidine to Ontario's universal Drug Benefit program for seniors (ODB), which tracks medication use by all 1.3 million residents of Ontario 65 years of age and older" COMMENT: data is collected pre‐ and post‐intervention from same province wide data base |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in the results section |
| Other bias ‐ ITS | Low risk | There was no evidence of other risks of bias |
Austin 2004B.
| Methods | Study design: ITS | |
| Participants | Physicians Clinical speciality: not clear Level of training: fully trained Setting/country: not clear/Canada |
|
| Interventions | The PEM was the ALLHAT, published on 18 December 2002, which concluded that thiazide‐type diuretics should be the first‐step antihypertensive therapy, compared with either calcium channel blockers or ACE inhibitors | |
| Outcomes | 4 process outcomes (prescribing):
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | Unclear risk | No information was provided |
| Shape of Intervention effect pre‐specified ‐ ITS | Unclear risk | Quote, pg. 44: "The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), published on December 18, 2002, concluded that thiazide‐type diuretics should be the first‐step antihypertensive therapy, compared with either calcium channel blockers (CCBs) or angiotensin converting enzyme (ACE) inhibitors. We examined trends in incident use of antihypertensive agents following publication of the ALLHAT trial" |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | The intervention (publication of the ALLHAT study in 2002) did not affect either the source or method of data collection |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | The outcome was objective |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Low risk | Quote, pg. 44: "we studied claims for antihypertensive agents that were submitted to the Ontario Drug Benefit (ODB) program between January 1, 1992, and April 30, 2003. The ODB program tracks prescriptions dispensed to all 1.3 million residents of Ontario older than 65 years.antihypertensive agents following publication of the ALLHAT trial" |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in the results section |
| Other bias ‐ ITS | Low risk | There was no evidence of other risks of bias |
Austin 2005.
| Methods | Study design: ITS | |
| Participants | Physicians Clinical speciality: not clear Level of training: fully trained Setting/country: not clear/Canada |
|
| Interventions | 2 PEMs are studied in this report: the REVERSAL trial, published on 3 March 2004, which demonstrated that for patients with CHD, intensive lipid‐lowering therapy reduced progression of coronary atherosclerosis compared with moderate therapy. 1 month later, the PROVE IT–TIMI22 trial (published on 8 April 2004) demonstrated that among patients who have recently had an ACS, an intensive lipid‐lowering statin regimen provided greater protection against death or major cardiovascular events than did a standard regimen. In both trials, standard therapy consisted of 40 mg/day of pravastatin, whereas intensive therapy consisted of 80 mg/day of atorvastatin. We compared the data before the 2 publications to the data after the 2 publications | |
| Outcomes | 2 process outcomes (prescribing):
|
|
| Notes | We looked at the combined effect of the 2 PEMs because of a lack of data to look at them separately. In this case, the 2 PEMs studied had similar characteristics, and we considered them as a whole (i.e. 1 PEM) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | Unclear risk | Quote, pg. 1300: "we were unable to account for temporal influences beyond the publication of the results of the trials. In particular, we were unable to account for changes in drug company promotion patterns." Quote, pg. 1300: "because of the study design and the relatively low monthly number of incident statin users, we were unable to definitively determine whether the trends that we observed were a result of an increase in the number of incident statin users who were being placed on high‐dose atorvastatin or whether they were because of prevalent statin users’ changing therapy" |
| Shape of Intervention effect pre‐specified ‐ ITS | Unclear risk | Quote, pg. 1297: "the objective of the present study was to examine the impact of the publication of these 2 trials on trends in intensive versus moderate statin therapy in the province of Ontario" COMMENT: the authors do not specify what the expected impact of the intervention is |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | Quote pg. 1297: "we studied claims for statins to Ontario's universal Drug Benefit program for seniors (ODB) between June 1, 1997 (the month atorvastatin was added to the ODB formulary), and September 30, 2004. The ODB tracks medication use by all 1.4 million residents of Ontario older than 65 years" COMMENT: data source and method of collection unchanged throughout study |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | The outcome was objective |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Low risk | The authors used the complete database of all prescription on Ontario, so there is no missing data |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in the results section |
| Other bias ‐ ITS | Low risk | There was no evidence of other risks of bias |
Avorn 1983.
| Methods | Study design: RCT Unit of allocation: physicians Stratification by: geographic location Type of comparison: PEM only vs. nothing:
Groups considered in review: A and B |
|
| Participants | Physicians Clinical speciality: not clear Level of training: fully trained Setting/country: not clear/US |
|
| Interventions | All members of the "print‐only" group received a letter announcing a pilot drug‐information programme. Half of this group then received a mailed copy of a PEM patterned after the Federal Drug Administration Drug Bulletin ("bulletin" ‐ 3 issues, mailed twice each) describing alternatives to targeted drugs. The other half of the "print‐only" group received these bulletins as well as 6 PEM ("unadvertisement") printed in colour, with illustrations and references | |
| Outcomes | 1 process outcome: mean number of units prescribed / physician (all 3 drugs) | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote, pg. 1460: "control and experimental interventions (described above) were then allocated randomly within each block" COMMENT: method of randomisation is not specified |
| Allocation concealment (selection bias) | Low risk | Quote, pg. 1460: "control and experimental interventions (described above) were then allocated randomly within each block" |
| Baseline characteristics similar (selection bias) | Unclear risk | Quote, pg. 1460: "the physicians in each of the study groups were comparable before the intervention in terms of the amount of the target drugs they prescribed through Medicaid, their type of specialty and their board certification" COMMENT: there are no data tables provided, neither is raw data provided in the text |
| Baseline outcome measurements similar (selection bias) | Low risk | Quote, pg. 1460: "the model thus controlled for differences in preintervention prescribing levels among individual physicians as well as for prescribing trends within the control group" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | While the authors do not give specific group‐by‐group drop‐out information, quote pg. 1460: "the dropout rates for each cause were found to be approximately equally divided among the three groups", total drop‐out was 5% overall (see drop‐out rates: pg. 1460, right column, first paragraph) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome was objective |
| Contamination protection (contamination bias) | Low risk | Quote, pg. 1460: "If a small town contained more than one physician from our sample, all physicians in that town were randomized as a cluster to prevent cross‐contamination of information" |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section were reported in the results section |
| Other bias | Low risk | There was no evidence of other risks of bias |
Azocar 2003.
| Methods | Study design: RCT Unit of allocation: physicians Type of comparison: PEM only vs. nothing
Groups considered in review: A and B |
|
| Participants | Psychologists, psychiatrists, Master's‐level therapists Clinical speciality: psychiatry and psychology Level of training: fully trained Setting/country: not clear/US |
|
| Interventions | The PEM consisted of the UBH best practice guidelines for the treatment of major depression compiled from guidelines from both the American Psychiatric Association and the Agency for Health Care Policy and Research as well as current research. The UBH guidelines consist of a 1‐page quick reference and an 8‐page reference booklet and recommend basic steps in the assessment and treatment of major depression. The PEM was mailed to the intervention group of providers (n = 132), specifically targeting a patient recently referred with a diagnosis of major depression | |
| Outcomes | 4 process outcomes:
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (2001 article), pg. 1015: "simple randomization was used" |
| Allocation concealment (selection bias) | Unclear risk | Quote (2001 article), pg. 1015: "simple randomization was used" |
| Baseline characteristics similar (selection bias) | Low risk | Quote (2001 article), pg. 1015: "the type of license was controlled for in all group comparisons because it was somewhat confounded by group assignment" |
| Baseline outcome measurements similar (selection bias) | Unclear risk | No information was provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote (2003 article), pg. 115: "in addition, patient noncompliance with treatment recommendations and patient dropout was not measured, yet they are factors that can significantly influence treatment length and efficiency. Furthermore, services provided but not billed to UBH such as medication management by primary care physicians could not be accounted for" COMMENT: Not enough information is provided on drop‐out rates in each group and on reasons for dropping out |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (2003 article), pg. 113: "guideline adherence was measured objectively using submitted claims and treatment plans provided by the clinicians" |
| Contamination protection (contamination bias) | Unclear risk | Quote (2003 article), pg. 1015: "simple randomization was used to give each clinician an equal chance of being assigned to each of the three groups…" COMMENT: professionals may have been allocated within a clinic or practice and it is possible that communication between intervention and control professionals could have occurred |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section were reported in the results section |
| Other bias | High risk | Quote (2003 article), pg. 115: "the small number of sessions delivered by study clinicians could have been due to the overrepresentation of psychiatrists in the sample and their delivering primarily monthly medication management services, rather than weekly psychotherapy", and "Furthermore, services provided but not billed to UBH such as medication management by primary care physicians could not be accounted for" |
Barbaglia 2009.
| Methods | Study design: ITS | |
| Participants | Physicians Clinical speciality: not clear Level of training: fully trained Setting/country: outpatient (e.g. ambulatory care provided by hospitals/specialists)/Spain |
|
| Interventions | The PEM was the WHI trial, published on 17 July 2002, which concluded that overall health risks exceeded benefits from use of combined oestrogen plus progestin among healthy postmenopausal women | |
| Outcomes | 4 process outcomes:
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | Unclear risk | No information was provided |
| Shape of Intervention effect pre‐specified ‐ ITS | Low risk | COMMENT: the authors describe how previous studies have shown decreases in HT use based on pharmacy data. They propose a study with direct report of HT use and a longer follow‐up period to better assess this trend |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | Data were collected during a breast screening programme that was not affected by the release of the trial |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Unclear risk | COMMENT: patients included in the study were interviewed at a breast cancer screening programme. The highly publicised nature of the WHI study suggests the possibility that the outcome assessor (patient) would be aware of the intervention |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Low risk | COMMENT: specific data on loss to follow‐up was not given for pre‐post‐intervention or by age group. However, a very small percentage was lost. Quote, pg. 1062: "we excluded 1,467 women (2.8%) from the analysis because of their inconsistencies in successive answers about HT use as well as 42 women (0.1%) who refused to complete the questionnaire" |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in results section |
| Other bias ‐ ITS | High risk | The primary outcome is not objective (self report) |
Bearcroft 1994.
| Methods | Study design: C‐RCT Unit of allocation: GP practices Type of comparison: PEM only vs. nothing
|
|
| Participants | Physicians Clinical speciality: general practice/family medicine Level of training: fully trained Setting/country: general practice/UK |
|
| Interventions | The PEM consisted of a mailed package including: guidelines for referrals for chest radiography that were advisory only and relevant background information. Guidelines for referrals for chest radiography were developed after a previous study involving the prospective analysis of 2017 consecutive chest radiograph referrals. The presenting indications were compared with the subsequent radiological findings and those indications with a particularly low yield were identified. These guidelines, therefore, were specifically relevant to local practice and they highlighted those groups of patients in whom, based on the previous study, significant abnormalities were uncommon. They were advisory only and included a general reminder that a good clinical history, together with a presumptive diagnosis, would allow a more helpful, accurate and patient‐specific report | |
| Outcomes | 4 process outcomes:
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote, pg. 56: "GP practices were allocated using a random number table into either the study or control group" |
| Allocation concealment (selection bias) | Low risk | COMMENT: the unit of allocation is by GP practice and allocation is performed on all units at the start of the study |
| Baseline characteristics similar (selection bias) | High risk | No baseline characteristics were reported |
| Baseline outcome measurements similar (selection bias) | Unclear risk | No information is provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | This is not specified: while it is implied by it being a prospective analysis of all GP requests for chest radiography, it is not specified whether any of the records were missing after baseline |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | While an attempt was made to blind the outcome assessors, quote, pg. 56: "the reporter was unaware from which group of GPs the request originated", this was not complete, quote, pg. 56: "the majority of the examinations performed were then reported by one of two radiologists (PWPB and JS)", and no quantification of this "majority" was provided |
| Contamination protection (contamination bias) | High risk | Quote, pg. 58: "in addition, there may have been crossfertilization between study and control groups as GPs meet professionally and socially. Such an effect would be conservative, leading to a reduction in the overall difference" |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section were reported in the results section |
| Other bias | Low risk | There is evidence of potential unit of analysis error |
Beaulieu 2004.
| Methods | Study design: RCT Unit of allocation: physicians Type of comparison: PEM only vs. nothing
Groups considered in review: A and B |
|
| Participants | Physicians Clinical speciality: general practice/family medicine, internal medicine, cardiology Level of training: fully trained Setting/country: mixed/Canada |
|
| Interventions | The PEM consisted of a 1‐page summary (developed by the College des Medecins du Quebec) of existing provincial guidelines for anti‐anginal therapy. This summary incorporated 3 key messages targeting the most problematic prescribing practices identified in our earlier cross‐sectional study, namely low prescribing rates for antiplatelet and hypolipaemic drugs and for β‐blockers in patients without apparent major contraindications. The key recommendations in the summary were: (i) to write a prescription for acetylsalicylic acid (aspirin) for patients with stable angina; (ii) to control serum cholesterol, with a target value for LDL cholesterol < 2.6 mmol/L; and (iii) to favour β‐blockers as the first choice for anti‐angina medication. Data on prescribing rates for the 3 targeted medication classes by physicians practicing in the same regions as the participating physicians were also included in the 1‐page summary | |
| Outcomes | 2 process outcomes:
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote, pg. 22: "the physicians identified in our previous study were randomly assigned, using computer‐generated random numbers, to one of three groups" |
| Allocation concealment (selection bias) | Low risk | COMMENT: the unit of allocation is by physician and allocation is performed on all units at the start of the study |
| Baseline characteristics similar (selection bias) | High risk | TABLE 1, pg. 24: "there was no significant difference in the distribution of the sexes and medical training amongst the study groups. There was a significant difference in the distribution of professional experience and mean number of patients in the database according to the physician's training amongst the groups" |
| Baseline outcome measurements similar (selection bias) | Unclear risk | No information was provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote, pg. 23: "of the 3293 physicians in our initial study, 967 (29.4%) were not in the database in 1999, hence were considered lost to follow‐up. Thus 2326 (70.6%) were available for the current study (Figure 1). Since our database was anonymous, it was impossible to track down what happened to those physicians" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome was objective |
| Contamination protection (contamination bias) | High risk | Quote, pg. 30: "contamination might have occurred between the study groups, either directly (physicians in the intervention groups sharing information with physicians in the control groups) or indirectly (uptake of the guideline messages through the communication channels of various stakeholders and CME activities). Such contamination is indicated by our survey of a subsample of the physicians.24 In this study, 90% of respondents, including physicians in the control group, were aware of the guidelines, and 75% had participated in at least one CME activity on the topic during the previous 6 months" |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section were reported in the results section |
| Other bias | Low risk | There was no evidence of other risks of bias |
Bjornson 1990.
| Methods | Study design: RCT Unit of allocation: physicians Type of comparison: PEM only vs. nothing
|
|
| Participants | Physicians Clinical speciality: general practice/family medicine, internal medicine, cardiology Level of training: fully trained Setting/country: mixed/US |
|
| Interventions | The PEM consisted of a mailed package that contained (1) a covering letter and questionnaire from the Drug‐Use Review coordinator, (2) the New England Journal of Medicine article (12 June 1976), which showed that patients who had the vasodilators hydralazine hydrochloride and isosorbide dinitrate added to their drug therapy had a lower mortality than those who had digoxin and diuretics; and (3) a drug history profile of a congestive heart failure patient based on a computer match of heart failure and the less effective therapy described in the VA study. The primary objective was to evaluate the Drug‐Use Review programme as an agent of change in physician prescribing practices after results of an RCT were published | |
| Outcomes | 2 process outcomes:
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was provided |
| Allocation concealment (selection bias) | Unclear risk | No information was provided |
| Baseline characteristics similar (selection bias) | Unclear risk | Quote, pg. 1543: "the physicians in the two groups were similar in terms of board certification, medical specialty, type and location of practice, sex ratio, medical school attended, and number of years of practice. The CHF patients represented by the two groups were well balanced in terms of age, sex ratio, and nursing home residency" COMMENT: there were no data tables provided, and raw data is not provided in the text |
| Baseline outcome measurements similar (selection bias) | Unclear risk | No information was provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Incomplete outcome data was only provided for the intervention group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome was objective |
| Contamination protection (contamination bias) | High risk | Quote, pg. 1543: "ninety‐five (67.4%) respondents in the intervention group indicated they were already aware of the VA study with 77 (54.6%) citing the New England Journal of Medicine article as the principal source of their knowledge (Table 1)" COMMENT: thus, perhaps the control group physicians were also aware of the study. Additionally, since the randomisation was not clustered by practice, physicians in the intervention group could have shared information with their colleagues in the control group |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section were reported in the results section |
| Other bias | Unclear risk | COMMENT: there was no information provided regarding from where the outcome data was being recorded. It may have come from Medicaid, a similar computer‐based record, or physician surveys |
Black 2002.
| Methods | Study design: ITS | |
| Participants | Not clear Clinical speciality: not clear Level of training: fully trained Setting/country: not clear/UK |
|
| Interventions | The PEM consisted of an NHS Effective Health Care bulletin (November 1992) on the treatment of glue ear in children (EHC‐OM bulletin). The bulletin reviewed the research evidence available at the time and recognised the benefits of surgery for children with severe glue ear (otitis media with effusion), but cautioned against overuse of surgery in children with milder forms of the condition that might resolve without any intervention. The stated primary aim of this paper was to ascertain whether or not the passive dissemination of national guidelines to typical service providers (district general hospitals as well as teaching hospitals) had any impact on clinical practice | |
| Outcomes | 1 process outcome: surgery rate for glue ear (mean number of surgery per 10,000 children aged under 10 years for 13 health districts) | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | Low risk | COMMENT: the authors present 5 possible alternative reasons that could contribute to the observed outcome and provide compelling arguments that these factors may have contributed, but that the intervention was effective. Reasons considered: statistical artefact, supply factors, demand factors, organisational changes in the NHS and broadly publicised adverse publicity |
| Shape of Intervention effect pre‐specified ‐ ITS | Low risk | COMMENT: the authors state that prior to the PEM, the rate of the surgery (primary outcome) was already declining, and that to demonstrate that the guidelines were effective: quote, pg. 121: "it would be necessary to show an acceleration in the decline. The primary aim of this paper is to ascertain whether or not the passive dissemination of national guidelines to typical service providers (district general hospitals as well as teaching hospitals) had any impact on clinical practice. Studies of such interventions in other areas have reported either no clinically significant effect or only a modest impact. If the guidelines were shown to have had an effect on this occasion, our secondary aim was to establish why this was so" |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | The intervention (publication of the Effective Health Care bulletin on childhood surgery for glue ear ‐ 1992) did not affect either the source or method of data collection |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | The outcome was objective |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Low risk | Quote, pg. 121‐122: "adjustments were made for shortfalls in the clinical coding in otolaryngology, which never exceeded a few percent in any year. It was assumed that failure to code procedures was not influenced by the procedure carried out. Intervention rates for surgery for OME were therefore adjusted according to the overall shortfall for the specialty" COMMENT: the authors do not provide numbers to support "a few percent"; however, it seems reasonable to infer that it is less than 10% |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in results section |
| Other bias ‐ ITS | Low risk | There was no evidence of other risks of bias |
Buyle 2010.
| Methods | Study design: ITS | |
| Participants | Physicians Clinical speciality: not clear Level of training: fully trained Setting/country: hospital/inpatient/Belgium |
|
| Interventions | The PEM consisted of guidelines for sequential antibiotic therapy (IV to PO with fluoroquinolones) published and disseminated in the local drug letter (October 2003), the official letter of the Pharmacotherapeutic Committee. This intervention was oriented towards all physicians (approximately 650) in the hospital | |
| Outcomes | 1 process outcome: usage of IV versus total fluoroquinolone | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | Unclear risk | No information was provided |
| Shape of Intervention effect pre‐specified ‐ ITS | Low risk | COMMENT: the authors describe the suitability of fluoroquinolones for IV to PO antibiotic switches and suggest that sequential therapy (which would be reflected by a decrease in the proportion of IV antibiotic out of total antibiotic use (IV + PO)) |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | The intervention (publication/dissemination of guideline in the local drug letter in October 2003) did not affect either the source or method of data collection |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | The outcome was objective |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Low risk | COMMENT: the reasons for loss to follow‐up were similar. The number lost was low and similarly distributed between groups (2/36 from control group; 5/45 in total from the 2 intervention groups) |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in results section |
| Other bias ‐ ITS | High risk | Quote, pg. 408‐409: "the IV/PO ratio may be an indicator for implementing sequential therapy but could be biased by confounding factors. An example of a possible confounding factor is the length of stay of the patients. Patients who are switched to an oral therapy could be discharged earlier as the oral therapy can easily be continued at home. In this case the IV/PO ratio will increase as we only look at the consumption in the hospital" |
Coopersmith 2002.
| Methods | Study design: ITS | |
| Participants | Physicians, nurses, critical care fellows Clinical speciality: not clear Level of training: fully trained Setting/country: hospital/inpatient/US |
|
| Interventions | The PEM consisted of a 10‐page self‐study module on risk factors and practice modifications involved in catheter‐related infections. The intervention was primarily targeted at registered nurses and provided actions to address specific risk factors. The stated purpose of the study was to determine whether an education initiative aimed at improving central venous catheter insertion and care could decrease the rate of primary bloodstream infections | |
| Outcomes | 1 process outcome: monthly rate per 1000 central venous catheter days of catheter‐related bloodstream infections | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | Unclear risk | No information provided |
| Shape of Intervention effect pre‐specified ‐ ITS | Low risk | Quote, pg. 59: "to determine whether a focused education initiative in a surgical/burn/trauma ICU could decrease the primary bloodstream infection rate" |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | The intervention (10‐page self‐study module about catheter‐related bloodstream infections) did not affect either the source or method of data collection |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | The outcome was objective |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Low risk | Quote, pg. 60: "all patients admitted to the ICU between January 1, 1998, and June 30, 1999, were followed prospectively by an infection control team and surveyed for bloodstream infections" COMMENT: while this implies complete data follow‐up, this is not specified |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in results section |
| Other bias ‐ ITS | High risk | Quote, pg. 63: "in a pre‐ and post observational, non randomized study, the ICU staff is not blinded to either the presence of or the recipients of the intervention. This raises the possibility of staff behaviour changes based upon the widespread knowledge of the measured outcome" |
Denig 1990.
| Methods | Study design: RCT Unit of allocation: physicians Stratification by: village or town Type of comparison: PEM only vs. nothing
|
|
| Participants | Physicians Clinical speciality: general practice/family medicine Level of training: fully trained Setting/country: general practice/The Netherlands |
|
| Interventions | The PEM consisted of a 'bulletin' that looked like a regular issue of the monthly Geneesmiddelenbulletin distributed by the Dutch government to all physicians and pharmacists. The bulletin used for the evaluation concerned the use of antispasmodic drugs for 2 kinds of spasms commonly seen in general practice, IBS and renal colic. The bulletin advised against (a) fixed combinations of antispasmodics with chlordiazepoxide, (b) PO/rectal butylscopolamine, and (c) fixed combinations of antispasmodics with metamizole. Recommended for renal colic were (d) diclofenac preparations. The objective was to evaluate the effects of a direct mailed drug bulletin on drug choice and prescribing practice in physicians | |
| Outcomes | 2 process outcomes:
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from correspondence with author: "The allocation was conducted by using envelopes drawn by a person who was not involved in the research project |
| Allocation concealment (selection bias) | Unclear risk | No information is provided |
| Baseline characteristics similar (selection bias) | Low risk | Quote, pg. 6: "the physicians participating in this study were similar to their colleagues in The Netherlands with regard to years in practice, size of practice, percentage of elderly patients, and sex distribution of patients (table 5.1). Moreover, there were no significant differences in these characteristics between the control and intervention groups of the study (t‐tests; P > 0.05)" |
| Baseline outcome measurements similar (selection bias) | Low risk | Quote, pg. 7: "...before the intervention, the study groups did not differ significantly in terms of knowledge, perceived drug utility, or stated prescription. Nor did a significant difference occur in actual prescribing between the intervention and control groups (Tables 5.2‐5.5). The physicians in both study groups who were interviewed, however, prescribed fewer antispasmodics in general as well as fewer undesirable antispasmodics than the physicians who did not agree to be interviewed but permitted the use of their prescribing data" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons are provided for the 25 withdrawal/ineligible participants who agreed to join, but did not form part of the group analysed, but no indication of the distribution between control and intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome was objective |
| Contamination protection (contamination bias) | Unclear risk | Quote, pg. 3: "physicians living in the same village or town were stratified into the control or intervention groups." From this quote it is UNCLEAR if a clustered approach was used to randomise participants |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section were reported in the results section |
| Other bias | Low risk | There was no evidence of other risks of bias |
Dormuth 2004.
| Methods | Study design: C‐RCT Unit of allocation: health areas Stratification by: number of physicians per area Type of comparison: PEM only vs. nothing
|
|
| Participants | Physicians Clinical speciality: general practice/family medicine Level of training: fully trained Setting/country: not clear/Canada |
|
| Interventions | The PEM consisted of 12 issues of the 'Therapeutics Letter' distributed between October 1994 and December 1997. Therapeutics Letter was a 2‐ to 4‐page colour‐printed bulletin mailed to most practicing physicians in British Columbia. Therapeutics Letter is a publication issued by the Therapeutics Initiative of the University of British Columbia. The letters included were those that had a clear message which could be predicted to result in a change to prescribing behaviour | |
| Outcomes | 12 process outcomes:
|
|
| Notes | ES not computable No intervention: increase of 10% in the number of patients with prescriptions PEM: decrease of 15% in the number of patients with prescriptions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote, pg. 1058: "one local health area in each pair was randomly selected and assigned (blindly by M.M. using the RAND function on Excel) to be in the control group" |
| Allocation concealment (selection bias) | Low risk | Quote, pg. 1058: "one local health area in each pair was randomly selected and assigned (blindly by M.M. using the RAND function in Excel) to be in the control group" |
| Baseline characteristics similar (selection bias) | Low risk | Quote, pg. 1059: "characteristics of the intervention and control physicians in 1991 are displayed in Table 2. The physicians and their patient populations were well balanced for these characteristics." TABLE 2, pg. 1058: "shows physician characteristics in 1994. Characteristics measured are percentage of general practitioners, mean age in years, percentage of men, mean number of visits from patients aged 66 years or more, mean age in years of patients aged 66 years or more and percentage of men/women/sex unknown of patients aged 66 years or more" COMMENT: the baseline characteristics of the intervention and control groups were reported and similar |
| Baseline outcome measurements similar (selection bias) | Low risk | Based on the large total number of prescriptions, baseline outcomes for the number of newly treated patients are similar across groups |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote, pg. 1058: "no requests to be excluded were received" COMMENT: the study does not specifically report on all physicians randomized by area at the beginning of the study remaining in the prescribing database throughout the study. Perhaps physicians retired, moved to a new area, or died |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome was objective |
| Contamination protection (contamination bias) | Low risk | Quote, pg. 1058: "the intervention and control groups were created by grouping an approximate 10% sample of prescribing physicians in 24 local health areas in a paired, cluster randomized design into 12 pairs based on the number of physicians in each area." such that all physicians within 1 local health area would be clustered |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section were reported in the results section |
| Other bias | Low risk | There was no evidence of other risks of bias |
Fijn 2000.
| Methods | Study design: ITS | |
| Participants | Physicians Clinical speciality: general practice/family medicine Level of training: fully trained Setting/country: not clear/The Netherlands |
|
| Interventions | The PEM consisted of revised independent Dutch national recommendations on antithrombotic prophylaxis of IHD were introduced in 1996. 2 peer‐reviewed clinical practice guidelines were issued: 1 by the Dutch Institute for Healthcare Improvement, a national scientific authority representing hospital specialists, and 1 by the Dutch Scientific Society of General Practitioners. At the same time, identical recommendations were presented by the Dutch Drug Bulletin Institute and the Health Insurance Fund Council. All of these recommend additional prophylactic antithrombotic therapy, preferably thrombocyte aggregation inhibitors, to existing rescue or maintenance therapy, or both, for acute and chronic IHD | |
| Outcomes | 1 process outcome: number of patient who were prescribed antithrombotic therapy after having a diagnosis of IHD | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | Unclear risk | No information was provided |
| Shape of Intervention effect pre‐specified ‐ ITS | Unclear risk | Quote, pg. 740: "All of these recommend additional prophylactic antithrombotic therapy, preferably thrombocyte aggregation inhibitors, to existing rescue and/or maintenance therapy for acute and chronic IHD." "this research will evaluate antithrombotic prescribing in newly diagnosed IHD patients in general practice" |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | The intervention did not affect the source (community pharmacies in the InterAction working group) or the method of data collection |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | The outcome was objective |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Low risk | The complete databases from 10 pharmacies were used |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in results section |
| Other bias ‐ ITS | Low risk | There was no evidence of other risks of bias |
Fonarow 2009.
| Methods | Study design: ITS | |
| Participants | Physicians Clinical speciality: not clear Level of training: fully trained Setting/country: hospital/inpatient/US |
|
| Interventions | The 4 PEMs were 2 published studies and 2 guidelines: 1. 4 April 2001, publication that statins produce early event reduction in ACS (MIRACL); 2. 22 March 2002, AHA/ACC Unstable Angina/Non‐STEMI guidelines recommending lipid‐lowering therapy before discharge in UA/non‐STEMI patients (ACC‐AHA‐NS); 3. 8 March 2004, publication that high‐dose statins superior in ACS to standard‐dose statins (PROVE IT‐TIMI 22); and 4. 4 August 2004, AHA/ACC STEMI guidelines recommending lipid‐lowering therapy before discharge in patients with STEMI (ACC‐AHA‐STEMI) | |
| Outcomes | 3 process outcomes:
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | Unclear risk | No information is provided |
| Shape of Intervention effect pre‐specified ‐ ITS | Unclear risk | Quote, pg. 186: "it has not been well studied to what extent utilization of lipid lowering medications in patients with AMI has changed in response to more recent published clinical trial evidence and updates to national guidelines. In this study, the National Registry for Myocardial Infarction (NRMI) 3, 4, and 5 was used to examine national trends in the use of lipid‐lowering medications at discharge in patients hospitalized for AMI from 1998 to 2006" |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | The interventions (MIRACL, ACC/AHA NSTEMI Guideline, PROVE IT‐TIMI 22, ACC/AHA STEMI Guideline) did not affect either the source or method of data collection |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | Outcome was objective |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Low risk | National registries were used all along the study |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in results section |
| Other bias ‐ ITS | Low risk | There was no evidence of other risks of bias |
Fukuda 2009.
| Methods | Study design: ITS | |
| Participants | Physicians Clinical speciality: surgery Level of training: fully trained Setting/country: hospital/inpatient/Japan |
|
| Interventions | The PEM consisted of evidence‐based clinical practice guidelines for treatment of early‐stage breast cancer in Japanese women published in July 1999. The guidelines recommended breast‐conserving surgery followed by radiotherapy for the majority of women with Stage I or II breast cancer | |
| Outcomes | 1 process outcome:
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | Low risk | Quote, pg. 373: "because of language barriers, several large clinical trials published in Western countries seemed to have less impact on knowledge of the effectiveness of BCS in Japan compared with the impact in English‐speaking countries. Before the publication of the Japanese guideline, therefore, it was possible that Japanese women might be unaware of this treatment choice" COMMENT: the authors make an argument that a language barrier (Japanese/English) may have limited passive dissemination from other countries |
| Shape of Intervention effect pre‐specified ‐ ITS | Unclear risk | Quote, pg. 373: "the aim of this study was to evaluate whether publication of clinical guidelines was associated with a change of treatment practices for breast cancer patients through the use of secondary administrative data from Japanese hospitals" |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | The intervention (evidence‐based clinical practice guidelines) did not affect either the source or method of data collection |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | The outcome was objective |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Low risk | The complete database of 10 teaching hospital in Japan was used for the study |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in results section |
| Other bias ‐ ITS | Low risk | There was no evidence of other risks of bias |
Guay 2007.
| Methods | Study design: ITS | |
| Participants | Physicians Clinical speciality: not clear Level of training: fully trained Setting/country: outpatient (e.g., ambulatory care provided by hospitals/specialists)/Canada |
|
| Interventions | The PEM was the WHI trial, published on 17 July 2002, which concluded that overall health risks exceeded benefits from use of combined oestrogen plus progestin among healthy postmenopausal women | |
| Outcomes | 1 process outcome: total number of HRT prescriptions dispensed per month | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | Unclear risk | No information was provided |
| Shape of Intervention effect pre‐specified ‐ ITS | Low risk | Quote, pg. 18: "from this perspective, the aim of our study is to evaluate the impact of the publication of the WHI study in the Quebecers population, and to estimate if the use of HRT did indeed change in accordance with the new guidelines" |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | The intervention (WHI study) did not affect either the source or method of data collection |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | The outcome was objective |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Low risk | The authors provide a thorough description of the proportions of patients removed from analysis by inclusion and exclusion criteria. There was a 10% difference between loss to follow‐up in pre‐WHI cohort (39% loss) and the post‐WHI cohort (49%), and the reasons for loss were similar. The cohorts were considerably different in absolute size, but this was attributable to the large difference in the time‐frame (16,560 patients, 3 years in pre‐WHI vs. 2067 women in 9 months post‐WHI) |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in results section |
| Other bias ‐ ITS | Low risk | There was no evidence of other risks of bias |
Haas 2004.
| Methods | Study design: ITS | |
| Participants | Physicians Clinical speciality: not clear Level of training: fully trained Setting/country: outpatient (e.g. ambulatory care provided by hospitals/specialists)/US |
|
| Interventions | 2 PEMs are studied in this report: 1. The HERS published in 1998 and 2. The WHI, published on 17 July 2002. These clinical trials demonstrated that the risks associated with hormone therapy outweighed the benefits for women taking continuous oestrogen and progestin regimens | |
| Outcomes | 2 process outcomes:
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | Unclear risk | No information was provided |
| Shape of Intervention effect pre‐specified ‐ ITS | Low risk | Quote, pg. 184: "we designed our analysis to examine whether the use of hormone therapy has changed among postmenopausal women as a result of the publication of the results from HERS and the WHI. We were also interested in examining whether patterns of use differ by patient characteristics. Because HERS examined the outcomes of older women, we hypothesized that there would be earlier and more substantial declines in hormone therapy use among this group. We also expected that there would be variation in use by race or ethnicity because white women may have better access to new information. Finally, because the WHI study results were specific to women taking continuous estrogen plus progestin, we hypothesized that hormone use would be more stable among women who had had hysterectomies because such women typically take only estrogen and may believe that the findings do not apply to them" |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | The interventions (HERS study; WHI Study) did not affect either the source or method of data collection |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | The outcome was objective |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Low risk | The San Francisco mammography registry was used |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in results section |
| Other bias ‐ ITS | Low risk | There was no evidence of other risks of bias |
Hersh 2004.
| Methods | Study design: ITS | |
| Participants | Physicians Clinical speciality: not clear Level of training: fully trained Setting/country: not clear/US |
|
| Interventions | 3 PEMs were studied in this report: 1. The HERS (August 1998), 2. HERS follow‐up (HERS II ‐ July 2002), and 3. The WHI (17 July 2002). HERS and HERS II concluded that postmenopausal hormone therapy with combination PO oestrogen/progestin offered no cardiovascular disease benefit among women with established disease. The oestrogen plus progestin trial of the WHI demonstrated that hormone therapy with an oestrogen/progestin combination caused increased risk of breast cancer and cardiovascular disease in postmenopausal women | |
| Outcomes | 1 process outcome: total number of prescriptions per year (before and after the publication of HERS ‐ August 1998) | |
| Notes | We looked at the combined effect of the 3 PEMs because of a lack of data to look at them separately. In this case, the 2 PEMs studied had similar characteristics, and we considered them as a whole (i.e. 1 PEM) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | Unclear risk | No information was provided |
| Shape of Intervention effect pre‐specified ‐ ITS | Unclear risk | Quote, pg. 48: "national trends in hormone therapy use since 1995 have not been reported, and the impact of recent evidence on hormone therapy prescriptions in subsequent months is unknown. Our objective was to describe these trends using national data on hormone therapy prescriptions and patient visits to physicians during which hormone therapy was prescribed" |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | The interventions (HERS study; HERS II; WHI Study) did not affect either the source or method of data collection |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | The outcome was objective |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Low risk | Data come from 2 nationally representative databases |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in results section |
| Other bias ‐ ITS | Low risk | There was no evidence of other risks of bias |
Jackevicius 2001.
| Methods | Study design: ITS | |
| Participants | Physicians Clinical speciality: internal medicine, cardiology, not specified Level of training: fully trained Setting/country: hospital/inpatient/Canada |
|
| Interventions | The PEM consisted of the 4S, published in 1994, which demonstrated that lipid lowering with simvastatin resulted in a clear and substantial decrease in total mortality and in fewer CHD events and less cardiovascular mortality when used in patients with CHD (history of angina or myocardial infarction) who also had high LDL‐cholesterol levels | |
| Outcomes | 1 process outcome: prescription for statin (all statins) | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | High risk | Quote, pg. 187: "it is impossible to separate the effects of the publication of 4S, the subsequent continuing education efforts, and the effects of marketing by the pharmaceutical industry. Therefore, the results of this study show the effects of the combined efforts among many different parties to promote appropriate medication prescribing with lipid‐lowering therapy in patients after AMI" |
| Shape of Intervention effect pre‐specified ‐ ITS | Unclear risk | Quote, pg. 183: "the use of statins in patients after AMI represents a proven innovation that is not complex to use, that has been endorsed by professional societies and practice guidelines, and that has been aggressively marketed by drug manufacturers. Analysis of the use of statins may provide us with information on the extent to which it is possible to change prescribing behaviour in a large population when strong clinical evidence and practice guidelines are combined with aggressive marketing" |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | The intervention (4S) did not affect either the source or method of data collection |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | The outcome was objective. |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Low risk | Quote, pg. 184: "all Ontario residents 65 years or older are covered under a comprehensive drug benefit plan. Each time a prescription is filled, a claim is submitted to the provincial government that contains the patient health insurance number and a unique drug identifier. The Ontario Myocardial Infarction Database provides data on all elderly patients treated for AMI in any Ontario hospital and records any prescriptions filled after hospital discharge" |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in results section |
| Other bias ‐ ITS | Low risk | There was no evidence of other risks of bias |
Jameson 2010.
| Methods | Study design: ITS | |
| Participants | Surgeons Clinical speciality: orthopaedic surgery Level of training: fully trained Setting/country: hospital/inpatient/UK |
|
| Interventions | The PEM consisted of a guideline on prophylaxis against venous thromboembolism produced by NICE in April 2007. The recommendations were that all orthopaedic inpatients be offered an LMWH for the duration of their stay in hospital, while high‐risk patients including all patients aged over 60 years should continue treatment for 4 weeks after discharge | |
| Outcomes | 1 process outcome: use of LMWH following a lower limb arthroplasty 2 patient outcomes:
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | Unclear risk | No information was provided |
| Shape of Intervention effect pre‐specified ‐ ITS | Unclear risk | Quote, pg. 124: "the early effect of the NICE guidelines has yet to be reported. This paper aims to examine their impact on the use of LMWH in patients undergoing arthroplasty of the lower limb in England and Wales, and to analyze the effect on the national rates of complications relating to venous thromboembolic prophylaxis" |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | The intervention (NICE guidelines on prophylaxis against venous thromboembolism) did not affect either the source or method of data collection |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | The outcome was objective |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Low risk | COMMENT: an exclusion criterion was described as "missing date of operation" in patient records and while the number and distribution between pre‐ and post‐guideline periods is not given, it is likely to be small and evenly distributed |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in results section |
| Other bias ‐ ITS | Low risk | There was no evidence of other risks of bias |
Jousimaa 2002.
| Methods | Study design: C‐RCT Unit of allocation: physician Type of comparison: paper‐based PEM vs. CD‐Rom PEM
|
|
| Participants | Physicians Clinical speciality: general practice/family medicine Level of training: newly qualified physicians in their last 2‐year training period (during which they work independently and are responsible for their own clinical decisions) Setting/country: general practice/Finland |
|
| Interventions | The PEM studied in this report was the Physician's Desk Reference and Database (now re‐named Evidence‐Based Medicine Guidelines), a collection of Finnish clinical practice guidelines. The over 1100 guidelines were written by GPs in cooperation with experts from other specialities | |
| Outcomes | 9 process outcomes:
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote, pg. 588: "students agreeing to participate in the study were randomized centrally using computer‐generated numbers to receive either computerized or textbook‐based guidelines" |
| Allocation concealment (selection bias) | Low risk | COMMENT: the unit of allocation is by physician and allocation is performed on all units at the start of the study |
| Baseline characteristics similar (selection bias) | Low risk | Quote, pg. 589: "the baseline characteristics of both study groups were similar (Table 1)" COMMENT: the baseline characteristics of the intervention and control groups were reported and similar |
| Baseline outcome measurements similar (selection bias) | Unclear risk | No information was provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The reasons for loss to study were similar and the proportions were similar, 6/72 = 8.3% in intervention and 3/67 = 4.5% in control group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote, pg. 589: "the anonymous patient records were then evaluated by one author (JJ, experienced primary care physician) blinded to the study group (computer or textbook, information searching or non‐information searching consultation)" COMMENT: the authors state explicitly that the primary outcome variables were assessed blindly |
| Contamination protection (contamination bias) | Unclear risk | COMMENT: professionals were possibly allocated within a clinic or practice and it is possible that communication between intervention and control professionals could have occurred |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section were reported in the results section |
| Other bias | Low risk | No evidence of other risks of bias |
Juurlink 2004.
| Methods | Study design: ITS | |
| Participants | Physicians Clinical speciality: not clear Level of training: fully trained Setting/country: mixed/Canada |
|
| Interventions | The PEM consisted of the RALES published in September 1999, which demonstrated that treatment with spironolactone substantially reduced morbidity and mortality in patients with severe heart failure | |
| Outcomes | 1 process outcome: rate of spironolactone prescription for patients with heart failure 2 patient outcomes:
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | Unclear risk | No information was provided |
| Shape of Intervention effect pre‐specified ‐ ITS | Unclear risk | Quote, pg. 543: "the Randomized Aldactone Evaluation Study (RALES) demonstrated that spironolactone significantly improves outcomes in patients with severe heart failure. Use of angiotensin‐converting–enzyme (ACE) inhibitors is also indicated in these patients. However, life‐threatening hyperkalemia can occur when these drugs are used together..." |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | The intervention (RALES) did not affect either the source or method of data collection |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | The outcome was objective |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Low risk | Quote, pg. 544: "we examined the computerized prescription records of the Ontario Drug Benefit Program, which records prescription drugs dispensed to all Ontario residents 65 years of age or older. The overall error rate in this database is less than 1 percent. Hospitalization records were obtained from the Canadian Institute for Health Information Discharge Abstract Database, which contains a record of all hospitalizations, including up to 16 diagnoses for each admission. Although the accuracy of coding in this database has not been established for all diagnoses, one recent study showed a positive predictive value of 90 to 96 percent for the diagnosis of heart failure." COMMENT: the authors establish that the databases used as sources are accurate and complete |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in results section |
| Other bias ‐ ITS | High risk | Quote, pg. 550: "indeed, many of the patients hospitalized for hyperkalemia may have died of another illness. The diagnostic coding for hyperkalemia has not been validated; moreover, many patients hospitalized for hyperkalemia may have also had volume contraction or renal insufficiency related to spironolactone therapy. In addition, we were unable to identify adverse outcomes that occurred before admission" |
Kabir 2007.
| Methods | Study design: ITS | |
| Participants | Physicians Clinical speciality: not clear Level of training: fully trained Setting/country: not clear/Ireland |
|
| Interventions | 3 PEMs were studied in this report: 1. the LIFE (2002), 2. the ALLHAT (18 December 2002), and 3. the VALUE (2004). The LIFE study showed that for a similar level of BP reduction losartan reduced events more than atenolol, a β‐adrenoceptor blocker. The ALLHAT trial confirmed that thiazides (chlorthalidone) controlled systolic BP as well as, and in elected subgroups better than both ACE inhibitors (lisinopril) and calcium channel blockers (amlodipine). However, the VALUE trial showed that the amlodipine‐based regimen significantly reduced BP further than valsartan, especially in the early period. Another feature common to all studies was a demonstration of the need for polypharmacy to achieve BP control | |
| Outcomes | 7 process outcomes:
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | Unclear risk | No information was provided |
| Shape of Intervention effect pre‐specified ‐ ITS | Unclear risk | Quote, pg. 382: "studies in Canada and the US have shown that such publications have influenced prescribing patterns. This study assesses such prescribing patterns in Ireland from January 2001 to July 2005, 12 months before and after the publication of the three major hypertension trials: LIFE, ALLHAT and VALUE" |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | The interventions (LIFE, ALLHAT, and VALUE studies) did not affect either the source or method of data collection |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | The outcome was objective |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Low risk | Data were collected from a regional database |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in results section |
| Other bias ‐ ITS | Low risk | There was no evidence of other risks of bias |
Kajita 2010.
| Methods | Study design: C‐RCT Unit of allocation: municipal health centres Type of comparison: PEM only vs. nothing
|
|
| Participants | Nurses, public health nurses, and allied health professionals in the field of community health Clinical speciality: community health Level of training: fully trained Setting/country: community‐based (e.g. community health centre, public health department)/Japan |
|
| Interventions | The intervention was the distribution of an evidence‐based guideline. The guideline was entitled "Evidence‐based guideline for the prevention of osteoporosis and osteoporotic fractures in community health", a purely evidence‐based practice guideline written in Japanese for the prevention of osteoporosis published in October 2004. This guideline was developed and formatted in accordance with recommendations for evidence‐based guidelines, according to formal assessment procedures specified in the Japanese version of the AGREE instrument | |
| Outcomes | 46 process outcomes, including implementation rate of evidence‐based health education items for osteoporosis prevention (see Table 5 for a complete list) | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote, pg. 2: "after the pre‐intervention assessment, the 100 centers were randomly allocated in a 1:1 ratio to the intervention and control group by a minimization method that defined region and city/town as stratification factors" |
| Allocation concealment (selection bias) | Low risk | Quote, pg. 2: "the allocation was performed by the controller of the trial (M. I.), who was not involved in the assessment as an evaluator" |
| Baseline characteristics similar (selection bias) | Unclear risk | Quote, pg. 4: "there were no significant differences between the intervention and control groups in municipality type, population, population aging rate, number of permanent health center staff, or the qualifications of the staff (physicians, public health nurses, nurses, dieticians, physical therapists, and clerks). There was no significant difference between the intervention and control groups in the implementation rate for osteoporosis screening or any type of health education or counseling before the intervention" COMMENT: numerical data to support this was not provided |
| Baseline outcome measurements similar (selection bias) | Low risk | Quote, pg. 4: "there was no significant difference in the overall score for the implementation status of evidence‐based health education items, as recommended by the guideline, between the intervention (median, 10; first and third quartiles: 3, 17) and control (median, 9; first and third quartiles: 1.5, 18.5) groups in the pre‐intervention assessment. The Table shows the implementation status of each health education item in these groups" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote, pg. 4: "all 100 municipal health centers completed the preintervention assessment. Of these, 3 centers declined to participate in the trial and 1 center was absorbed into another municipality (Figure 1). We performed the post‐intervention assessments for the remaining 96 centers (48 in the intervention group and 48 in the control group; 96% follow‐up rate)" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote, pg. 3: "the post‐intervention assessment was performed 1 year after the distribution of the guideline under blinded conditions in which the evaluators were unaware of the allocation" |
| Contamination protection (contamination bias) | Low risk | COMMENT: the unit of allocation was by institution (health centre) |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section were reported in the results section |
| Other bias | High risk | Quote, pg. 9: "the study did not use a double‐blind design because it was not possible to use a placebo guideline. Instead, we offered to reimburse the control centers for the cost for materials needed to revise their health education programs. Although only 3 centers claimed reimbursement, our offer may have increased the use of information other than the guideline in the control group and may have improved the evidence‐based status of the programs of the control centers, thereby decreasing the magnitude of differences in the outcome measures between the groups" |
Kottke 1989.
| Methods | Study design: RCT Unit of allocation: physicians Type of comparison: PEM only vs. nothing
Groups considered in review: A and B |
|
| Participants | Physicians Clinical speciality: general practice/family medicine Level of training: fully trained Setting/country: general practice/US |
|
| Interventions | The PEM studied in this report was a smoking cessation manual entitled 'Quit‐and‐Win' that could be used as an instructor's manual, as a self‐help guide, or as 1 part of a comprehensive intervention. The physicians were advised to give a copy to any patient who smoked. They were told that their supply of Quit‐and‐Win booklets would be replenished when required | |
| Outcomes | 5 process outcomes:
5 patient outcomes:
|
|
| Notes | 2 separate PEM analysis for all 10 points:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from correspondence with the author: "believe that we assigned the physicians using a computer random generator" |
| Allocation concealment (selection bias) | Unclear risk | No information was provided |
| Baseline characteristics similar (selection bias) | Low risk | TABLE 1 and quote, pg. 2103: "neither the mean age of the physicians, the size of the clinics nor the patient load…differed significantly among the three groups" COMMENT: even if professionals were well balanced, patients did not have all baseline characteristics similar |
| Baseline outcome measurements similar (selection bias) | Unclear risk | Outcomes were not collected at baseline |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The proportion of patient‐smokers was similar between groups, and the percentage reached at 1 year for follow‐up was similar. Quote, pg. 2103: "patients who either could not be contacted or refused to be interviewed were assumed to be continuing to smoke and were assumed not to have made any cessation attempts" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | This is a self‐report assessment by patients who were not blinded |
| Contamination protection (contamination bias) | Low risk | Quote, pg. 2102: "to prevent contamination from having physicians of the same practice in different trial groups, all physicians in the same practice were either moved to the most intense level of intervention to which any of them had been originally randomized or, if not yet randomized at the time this problem was discovered, added to the group to which their partner(s) had been randomized" |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section were reported in the results section |
| Other bias | High risk | COMMENT: the primary outcome measure was 102 question questionnaire for patients, making this outcome measure susceptible to LOW validity |
Lam 2009.
| Methods | Study design: ITS | |
| Participants | Physicians Clinical speciality: not clear Level of training: fully trained Setting/country: not clear/Canada |
|
| Interventions | The PEM studied in this report was "4D" (published 21 July 2005). The results showed that atorvastatin did not significantly reduce the primary end point of cardiovascular death, non‐fatal myocardial infarction, or stroke. In a secondary analysis, there was an unexpected increase in fatal strokes in the atorvastatin group compared with those receiving placebo. The trial investigators concluded that "in persons with type II diabetes mellitus who are receiving maintenance hemodialysis and have low‐density lipoprotein cholesterol values between 80 and 190 mg per deciliter (2.07 and 4.92 mmol/l), routine treatment with a statin to reduce the primary end point of death from cardiac causes, myocardial infarction, and stroke is not warranted" | |
| Outcomes | 1 process outcome: rate of statin use (age and sex standardised rate of statin use per 1000 diabetic haemodialysis patients) | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | Unclear risk | Quote, pg. 1174: "it was not possible to evaluate the extent to which other potential factors, such as pharmaceutical marketing, influenced prescribing patterns" |
| Shape of Intervention effect pre‐specified ‐ ITS | Unclear risk | Quote, pg. 1172: "one of the largest randomized controlled trials ever published in nephrology is Der Deutsche Diabetes Dialyse Studie (4D), which showed no beneficial effect of statins in diabetic patients receiving hemodialysis. We sought to determine whether there was a change in statin use among diabetic patients on dialysis after the publication of 4D" Quote, pg. 1177: "in this study, we specified the publication date of 4D (21 July 2005) as the primary time point to assess whether there was a change in prescribing practice" |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | The intervention (4D) did not affect either the source or the method of data collection |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | The outcome was objective |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Low risk | Quote, pg. 1177: "we used database codes with proven validity as detailed in Supplementary Appendix B. All of these data source have been successfully used in previous studies to examine prescribing rates of statins and a number of other medications in Ontario" COMMENT: 4 databases were used as sources in this report, all of which are comprehensive. Missing data were likely to be very low |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in results section |
| Other bias ‐ ITS | Low risk | There was no evidence of other risks of bias |
Majumdar 2003.
| Methods | Study design: ITS | |
| Participants | Physicians Clinical speciality: not clear Level of training: fully trained Setting/country: not clear/US and Canada |
|
| Interventions | 2 PEMs were studied in this report. The HOPE study demonstrated a 22% reduction in cardiovascular morbidity and mortality, and provided a new indication for ramipril. RALES compared spironolactone with placebo in patients with heart failure and demonstrated a 30% reduction in mortality | |
| Outcomes | 4 process outcomes:
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | Low risk | Quote, pg. 468: "To adjust for potential differences between Canadian and United States physicians in the adoption of published evidence, we examined the effect of the Randomized Aldactone Evaluation Study (RALES) on prescribing trends for spironolactone. This study compared spironolactone with placebo in patients with heart failure and demonstrated a 30% reduction in mortality. RALES was prereleased and published in the same year and the same journal as the HOPE study. Because spironolactone was not promoted by the pharmaceutical industry in either country, any observed differences in prescribing trends should be attributable mostly to a publication effect" |
| Shape of Intervention effect pre‐specified ‐ ITS | Low risk | Quote, pg. 468: "Therefore, we compared the prescribing trends for ramipril in Canada and the United States to test the hypotheses that publication of the HOPE study would increase the use of ramipril in both countries (publication effect), and that this increase would be greater in Canada (promotion effect)" |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | The interventions studied (HOPE; RALES) did not affect either the source or the method of data collection |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | The outcome was objective |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Low risk | Quote, pg. 468: "We used nationally representative drug dispensing information collected by IMS Health (IMS Health‐Canada and IMS Health‐America), which conducts research on prescribing patterns. Methods for data collection are identical in Canada and the United States. The IMS "CompuScript" database collects monthly dispensing records from a representative sample of retail pharmacies. The sample is drawn from 4800 pharmacies in Canada and 51,355 pharmacies in the United States, about two thirds of retail pharmacies" COMMENT: missing data, if any, were likely to be similar pre‐ and post‐intervention |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in results section |
| Other bias ‐ ITS | Low risk | There was no evidence of other risks of bias |
Majumdar 2004.
| Methods | Study design: ITS | |
| Participants | Physicians Clinical speciality: not clear Level of training: fully trained Setting/country: not clear/US |
|
| Interventions | The PEM was the WHI trial, published on 17 July 2002, which concluded that overall health risks exceeded benefits from use of combined oestrogen plus progestin among healthy postmenopausal women | |
| Outcomes | 5 process outcomes:
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | Unclear risk | No information was provided |
| Shape of Intervention effect pre‐specified ‐ ITS | Unclear risk | Quote, pg. 1983: "to examine pharmaceutical industry response to the WHI E+P [oestrogen plus progestin] results by analyzing promotional expenditures for hormone therapy before and after July 2002" |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | The intervention (WHI study) did not affect either source or method of data collection |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | The outcome was objective |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Low risk | Quote, pg. 1984: "we used nationally representative databases published by IMS Health (Plymouth Meeting, Pa), an independent pharmaceutical research company, to describe national trends in hormone therapy prescription and promotion. Information on prescriptions was obtained from the NPA, which we have described in detail elsewhere" COMMENT: missing data, if any, is likely to be similar pre‐ and post‐intervention |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in results section |
| Other bias ‐ ITS | Low risk | There was no evidence of other risks of bias |
Mason 1998/99.
| Methods | Study design: ITS | |
| Participants | Physicians Clinical speciality: general practice/family medicine Level of training: fully trained Setting/country: general practice/UK |
|
| Interventions | The PEM studied in this report was an "Effective Health Care" bulletin questioning the cost effectiveness of prescribing SSRIs was distributed to all GPs by the chief medical officer. Original distribution of the bulletin to all GPs occurred in March 1993. We examined the effect of this intervention on prescribing in English primary care using time‐series analysis | |
| Outcomes | 2 process outcomes:
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | Unclear risk | Quote, pg. 122: "the Effective Health Care Bulletin, and related article in the BMJ published at the same time, were the first scientific reports to question the widespread switch to SSRIs. These sparked considerable interest in the media, and also considerable activity from medical and pharmaceutical advisors in the NHS" |
| Shape of Intervention effect pre‐specified ‐ ITS | Unclear risk | A specific null hypothesis is not provided. Quote pg. 120: "we examined the effect of this intervention on prescribing in English primary care using time series analysis" |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | The Effective Health Care Bulletin (the intervention) did not affect the data source (Prescriptions Pricing Authority) or the method of data collection |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | The outcome was objective |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Low risk | Quote, pg. 120: "these data reflect the total number of prescriptions reimbursed for antidepressants on a quarterly basis" COMMENT: if a patient does not seek or receive reimbursement, this data could be missed, but this is unlikely to be affected by the publication of the PEMs |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in the results section |
| Other bias ‐ ITS | Low risk | There was no evidence of other risks of bias |
Mason 2001.
| Methods | Study design: ITS | |
| Participants | Physicians Clinical speciality: not clear Level of training: fully trained Setting/country: not clear/UK |
|
| Interventions | An NHS Effective Health Care bulletin (November 1992) on the treatment of glue ear in children (EHC‐OM bulletin) was distributed nationally to NHS decision makers in 1992. Based on systematic review, the bulletin concluded that surgery should be restricted to children with an extended period of substantial hearing impairment, with persistence and severity established by watchful waiting | |
| Outcomes | 1 process outcome: use of surgery for glue ear (mean number of procedures per 1000 habitants under 15 years old for 14 regions) | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | High risk | Quote, pg. 1097: "the change cannot be attributed to the bulletin alone, which was commissioned because of preexisting concerns about appropriate use of the procedure. Its publication received coverage in the medical and academic press,4 possibly encouraging doctors to examine their own practices and bring about behavioural change" |
| Shape of Intervention effect pre‐specified ‐ ITS | Unclear risk | Quote, pg. 1096: "based on systematic review, the bulletin concluded that surgery should be restricted to children with an extended period of substantial hearing impairment, with persistence and severity established by watchful waiting. We evaluated surgery rates before and after distribution of the bulletin" |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | The intervention (Effective Health Care bulletin) did not affect either the source or the method of data collection |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | The outcome was objective |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Low risk | Quote, pg. 1096: "quarterly numbers of D151 procedures—insertion of a ventilation tube through the tympanic membrane — performed in children aged under 15 in England from 1989 to 1996 were obtained from the hospital episodes system. We calculated per capita regional and national rates for this procedure" COMMENT: missing data, if any, were likely to be similar pre‐ and post‐intervention |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in the results section |
| Other bias ‐ ITS | Low risk | There was no evidence of other risks of bias |
Matowe 2002.
| Methods | Study design: ITS | |
| Participants | Physicians Clinical speciality: radiology Level of training: fully trained Setting/country: general practice/UK |
|
| Interventions | To evaluate the effect of postal dissemination of the third edition of the RCR guidelines on GP referral for radiography. The RCR guidelines were introduced to encourage appropriate use of diagnostic radiology and reduce the use of clinically unhelpful examinations. Between 1989 and 1998 4 editions of these guidelines were produced and a large number of copies distributed by mail to primary care. The current edition of the guideline includes 285 individual recommendations | |
| Outcomes | 1 process outcome: total number of x‐ray referrals | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | Unclear risk | No information was provided |
| Shape of Intervention effect pre‐specified ‐ ITS | Low risk | COMMENT: the authors specifically refer to reductions in x‐ray requests found by other studies and propose an ITS study of longer duration to improve the detection of the effect. They verified if other guidelines were disseminated independent of this study, and they also evaluated the effect of guidelines for 18 radiology examinations |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | The intervention did not affect the data source (hospital radiology department records), and sources and methods of data collection were the same before and after the intervention |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | The outcome was objective |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Low risk | Quote, pg. 576: "data were abstracted from the computerized administrative systems of two radiology departments serving over 90% of general practices in the region" COMMENT: missing data from GPs not using these radiology departments is not considered but it is not a high proportion (10%) |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in the results section |
| Other bias ‐ ITS | Low risk | There was no evidence of other risks of bias |
Meyer 2007.
| Methods | Study design: ITS | |
| Participants | Physicians Clinical speciality: general practice/family medicine Level of training: fully trained Setting/country: outpatient (e.g. ambulatory care provided by hospitals/specialists)/Germany |
|
| Interventions | Revised guidelines on empirical antibiotic treatment in the ICU: the written guidelines on empirical antibiotic treatment in the ICU were revised in December 2003 upon publication of the study by Chastre et al (Chastre 2003). and with respect to the local resistance situation. This change of empirical therapy was performed by a multidisciplinary team consisting of the intensive care specialist responsible for the ward and an infection control physician, and occasionally included also a microbiologist and a pharmacist | |
| Outcomes | 1 process outcome: antibiotic use density (AD; expressed as defined daily doses per 1000 patient‐days) | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | Unclear risk | No information was provided |
| Shape of Intervention effect pre‐specified ‐ ITS | Low risk | Quote, pg. 1148: "to evaluate the impact of an intervention to reduce the duration of antibiotic treatment for pneumonia in a neurosurgical intensive care unit (ICU). The usage of antibiotics and the resultant costs were examined using interrupted time series analysis while resistance and device‐associated infection rates are also described" |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | The intervention (written guidelines) did not affect the source or method of data collection |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | The outcome was objective |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Low risk | Quote, pg. 1149: "monthly data on antimicrobial usage and costs of antibiotics were obtained from the computerized pharmacy database" COMMENT: missing data, if any, is likely similar pre‐ and post‐intervention |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in the results section |
| Other bias ‐ ITS | Low risk | There was no evidence of other risks of bias |
Oakeshott 1994.
| Methods | Study design: C‐RCT Unit of allocation: practices Stratification by: number of partners and number of radiographic examinations requested Type of comparison: PEM only vs. nothing
|
|
| Participants | Physicians Clinical speciality: general practice/family medicine Level of training: fully trained (e.g., consultant) Setting/country: general practice/UK |
|
| Interventions | The PEM studied in this report consisted of the guidelines for examinations of the chest, limbs and joints, and spine taken from the RCR guidelines. The RCR guidelines aimed to encourage more appropriate use of diagnostic radiology and so reduce the use of clinically unhelpful x‐rays. The guidelines were printed verbatim on 2 sides of a sheet of A4 paper, which was then plasticised | |
| Outcomes | 3 process outcomes:
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Author could not confirm the method to generate the sequence (P. Oakeshott, personal communication) |
| Allocation concealment (selection bias) | Low risk | COMMENT: the unit of allocation is by physician and allocation was performed on all units at the start of the study |
| Baseline characteristics similar (selection bias) | Unclear risk | No report in text or tables of provider characteristics |
| Baseline outcome measurements similar (selection bias) | Low risk | COMMENT: we judge that no important difference is present across the study groups |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information was provided |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote, pg. 197: "conformity was assessed by P 0 and J W who were unaware which practices had been sent the guidelines" |
| Contamination protection (contamination bias) | Low risk | Quote, pg. 197: "practices were stratified by number of partners and number of radiographic examinations requested, and randomized into two groups" |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section were reported in the results section |
| Other bias | Low risk | There was no evidence of other risks of bias |
Perria 2007.
| Methods | Study design: C‐RCT Unit of allocation: GPs Type of comparison: PEM only vs. nothing
Groups considered in review: A and B |
|
| Participants | Physicians Clinical speciality: general practice/family medicine Level of training: fully trained (e.g., consultant) Setting/country: general practice/Italy |
|
| Interventions | The PEM studied in this report was an evidence‐based guideline for the management of non‐complicated type 2 diabetes mellitus. The source guideline was a French guideline entitled "Stratégie de prise en charge du patient diabétique de type 2 à l'exclusion de la prise en charge des complications" published by ANAES, which was then translated, updated, and adapted for Italian GPs | |
| Outcomes | 3 process outcomes:
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote, pg. 4: "our randomization sequences was computer‐generated" |
| Allocation concealment (selection bias) | Low risk | Quote, pg. 4: "randomization was performed by a researcher not involved in the study and who was blind to the identity of the practices" |
| Baseline characteristics similar (selection bias) | Low risk | Baseline information is provided in Table 1 and there are no important differences between study groups |
| Baseline outcome measurements similar (selection bias) | Low risk | The generalised estimating equation model was to used account for baseline differences |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | COMMENT: intervention arm 2 (passive dissemination) and the control group had similar numbers of missing data |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome was objective |
| Contamination protection (contamination bias) | Low risk | Quote, pg. 4: "GPs who accepted to take part in the study, were assigned by simple random allocation by the REXSCO [21] software, which assigns to same‐practice partners a nil probability of being randomized, thus minimizing the chances of participant contamination" |
| Selective reporting (reporting bias) | High risk | Quote, pg. 7: "as results showed the non‐effectiveness of the intervention strategy, we did not perform any economic evaluation or carry out analysis on participant sub‐clusters" COMMENT: all relevant primary outcomes were reported |
| Other bias | Low risk | No evidence of other risks of bias |
Roberts 2007.
| Methods | Study design: ITS | |
| Participants | Physicians Clinical speciality: prosthetic care Level of training: fully trained Setting/country: outpatient (e.g. ambulatory care provided by hospitals/specialists)/UK |
|
| Interventions | The PEM studied in this report was the Technology Appraisal Guidance No. 2 ‐ Guidance on the selection of protheses for primary total hip replacements (April 2000). TAG No. 2 contained a recommendation that cemented protheses be used | |
| Outcomes | 2 process outcomes:
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | Unclear risk | No information was provided |
| Shape of Intervention effect pre‐specified ‐ ITS | Unclear risk | Quote, pg. 864: "in April 2000, NICE published the Technology Appraisal Guidance (TAG) No. 2 ‐ ‘Guidance on the selection of prostheses for Primary Total Hip Replacements. […] As more than five years have passed since the publication of these guidelines, we decided to review the effect it has had, and the extent to which the guidelines have influenced clinical practice and contracting" |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | The intervention (NICE Technology Appraisal Guideline 2) did not affect either the source or method of data collection |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | The outcome was objective |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Unclear risk | Quote, pg. 865: "since the beginning of 1990, and with the agreement of all consultant orthopaedic surgeons in the region, all primary total hip and knee replacements (THR, TKR) performed throughout the Trent region were recorded prospectively" COMMENT: it is unlikely that there would be a difference in missing data before and after implementation of the intervention |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in the results section |
| Other bias ‐ ITS | Low risk | There was no evidence of other risks of bias |
Santerre 1996.
| Methods | Study design: ITS | |
| Participants | Physicians Clinical speciality: obstetrics and gynaecology Level of training: fully trained Setting/country: not clear/US |
|
| Interventions | In October 1988, the ACOG issued a physician practice guideline stating that a prior caesarean section was no longer a reason for performing a repeat section | |
| Outcomes | 1 process outcome: vaginal birth after previous caesarean section | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | Unclear risk | No information was provided |
| Shape of Intervention effect pre‐specified ‐ ITS | Low risk | Quote, pg. 317: "the ACOG guideline essentially states that a previous birth by cesarean is no longer a good reason for doing one again in the future. Consequently, if guidelines are effective at altering practice patterns, a noticeable increase in the VBAC rate should be detected after 1988 when the ACOG guideline was established" |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | The intervention (ACOG guidelines) did not affect either the source or method of data collection |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | The outcome was objective |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Low risk | The data set came from 55 Massachusetts hospitals from 1987 to 1991 |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in the results section |
| Other bias ‐ ITS | Low risk | There was no evidence of other risks of bias |
Shah 2008.
| Methods | Study design: ITS | |
| Participants | Physicians Clinical speciality: not clear Level of training: fully trained Setting/country: outpatient (e.g. ambulatory care provided by hospitals/specialists)/Canada |
|
| Interventions | The PEM studied in this report was the publication "Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes." New England Journal of Medicine, May 21, 2007. This meta‐analysis suggested an increased risk of myocardial infarction associated with rosiglitazone compared with active comparator or placebo | |
| Outcomes | 1 process outcome: number of new users of thiazolidinedione (rosiglitazone or pioglitazone) | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | High risk | Quote, pg. 873: "several other studies of cardiovascular risk with thiazolidinediones were reported throughout 2007, which may have contributed to the overall decline in their use" |
| Shape of Intervention effect pre‐specified ‐ ITS | Unclear risk | Quote, pg. 871: "we sought to determine whether physicians’ choices of glucose‐lowering medications changed in the immediate aftermath of the publication of the meta‐analysis" |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | The intervention (publication of report on rosiglitazone) did not affect either the source or the method of data collection |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | The outcome was objective |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Low risk | Quote, pg. 871: "we examined prescription claims in the Ontario Drug Benefits (ODB) programme database, which contains records of all prescription medications dispensed to Ontario residents aged ≥ 65 years. We restricted our analysis to people aged ≥ 66 years (approximate n = 1.5 million), purposefully excluding the first year of eligibility to avoid incomplete medication records" |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in the results section |
| Other bias ‐ ITS | Low risk | There was no evidence of other risks of bias |
Stafford 2004.
| Methods | Study design: ITS | |
| Participants | Physicians Clinical speciality: not clear Level of training: fully trained Setting/country: outpatient (e.g. ambulatory care provided by hospitals/specialists)/US |
|
| Interventions | The PEM studied in this report was the ALLHAT, published on 18 December 2002. In April 2000, the results that involved the study's doxazosin mesylate arm led to early termination of this arm owing to results that indicated an increased risk associated with use of the α‐blocker doxazosin mesylate compared with diuretics | |
| Outcomes | 1 process outcome: number of α‐blockers prescriptions dispensed (in millions) ‐ all α‐blockers, (both newly dispensed and refills) | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | Unclear risk | Quote, pg. 61: "because there are multiple simultaneous influences, it is difficult to establish a primary influencing factor on the significant decline in physician prescribing of α‐blockers. Nevertheless, our findings are clearly consistent with ALLHAT early termination results having a significant impact on α‐blocker use. Declining pharmaceutical industry promotion also may have contributed further to decreased α‐blocker use. The lack of an abrupt and more pronounced decline in prescribing shortly after the ALLHAT results, however, suggests slow and potentially incomplete diffusion of information from this clinical trial" |
| Shape of Intervention effect pre‐specified ‐ ITS | Unclear risk | Quote, pg. 55: "our analytic goals were 2‐fold: to describe patterns of α‐blocker use before and after the April 2000 publication of the early ALLHAT results and to examine whether these clinical trial results or alternative influences were associated with changes in α‐blocker prescribing that occurred in this time frame" |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | The intervention (publication of ALLHAT) did not affect either the source or the method of data collection |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | The outcome was objective |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Low risk | 2 databases were used as sources of prescribing information pre‐ and post‐intervention. Missing data, if any, were likely to be similar pre‐ and post‐intervention |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in the results section |
| Other bias ‐ ITS | Low risk | There was no evidence of other risks of bias |
Tsuji 2009.
| Methods | Study design: C‐RCT Unit of allocation: physician Stratification by: healthcare unit size and geographic location Type of comparison: PEM only vs. nothing
|
|
| Participants | Physicians Clinical speciality: general practice/family medicine Level of training: fully trained Setting/country: general practice/Brazil |
|
| Interventions | The PEM studied in this report was a depression‐specific guide, adapted from rigorous previously published guidelines, which provided brief and objective educational information regarding the effects of depression on patient daily living, strategies for improving adherence to treatment, and guidelines for the therapeutic management planning using standardised antidepressants in primary care | |
| Outcomes | 1 process outcome: preccscription of an antidepressant at the first appointment with the clinician 1 patient outcome: clinical remission (proportion of patients with depression severity of less than 8 points on Hamilton Rating Scale for Depression Severity) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was provided |
| Allocation concealment (selection bias) | Low risk | COMMENT: the unit of allocation was by physician and allocation was performed on all units at the start of the study |
| Baseline characteristics similar (selection bias) | Low risk | Quote, pg. 223: "clinician and patient baseline characteristics were comparable in the experimental and control groups (Tables 1 and 2)" |
| Baseline outcome measurements similar (selection bias) | Unclear risk | Baseline outcomes were not reported for this RCT |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote, pg. 223: "dichotomous end points (withdrawals, appropriate treatment and 16‐week clinical remission) were analyzed using the adjusted chi‐square approach." Withdrawals were quantified by group and reason, quote. pg. 223: "There were a total of 36 study withdrawals, 13 (10.8%) in the intervention arm and 23 (20.2%) in the usual care arm (intracluster coefficient correlation = 0.032, P = 0.153). Nine subjects (7.5%) in the intervention arm and 19 (16.7%) in the usual care arm withdrew (P = 0.122). Eight subjects, four (3.3%) in the intervention arm and four (3.5%) in the usual care arm, worsened and were withdrawn (P = .949)" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote, pg. 222: "investigators were blind to the treatment assignment of the clinicians and to which clinician the patient was assigned" and, "at 16‐week depression severity, as measured by the HAM‐D scale, was evaluated at a mental health facility by two independent evaluators who were blind to treatment allocation" |
| Contamination protection (contamination bias) | Low risk | Quote, pg. 222: "to avoid cross‐contamination of clinicians, sensitization of patients and for administrative reasons eight clinicians were stratified by basic healthcare unit size and geographical area and randomized to use either usual care or a treatment guide in treating depression" |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section were reported in the results section |
| Other bias | Low risk | There was no evidence of other risks of bias |
Wang 2005.
| Methods | Study design: ITS | |
| Participants | Physicians Clinical speciality: general practice/family medicine Level of training: fully trained Setting/country: outpatient (e.g. ambulatory care provided by hospitals/specialists)/US |
|
| Interventions | 2‐ PEMs were studied in this report. The ADA guidelines published in January 1998 advocated an LDL cholesterol goal under 100 mg/dL for patients with diabetes. The second PEM was the third report entitled ATP III published by the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (May 2001) that designated diabetes as a CHD risk equivalent, with the same LDL cholesterol goal of under 100 mg/dL | |
| Outcomes | 2 process outcomes:
|
|
| Notes | We looked at the combined effect of the 2 PEMs because of a lack of data to look at them separately. In this case, the 2 PEMs studied were very similar, and we characterised them as a whole (i.e. 1 PEM) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | Unclear risk | No information was provided |
| Shape of Intervention effect pre‐specified ‐ ITS | Unclear risk | Quote, pg. 2942: "The publication of the ADA and ATP III guidelines provides an opportunity to assess the effect of guideline changes on LDL cholesterol reporting and control for diabetes visits" |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | The interventions (ADA guidelines and ATP III guidelines) did not affect either the source or the method of data collection |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | The outcome was objective |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Low risk | Quote, pg. 2942: "we used the National Disease and Therapeutic Index (NDTI) (3), an ongoing survey of U.S. office‐based physicians conducted by IMS Health providing nationally representative diagnostic and treatment data, to analyze the national trends of LDL cholesterol reporting and control for diabetes and CHD visits by year between 1995 and 2004" |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in the results section |
| Other bias ‐ ITS | Low risk | There was no evidence of other risks of bias |
Watson 2001.
| Methods | Study design: C‐RCT Unit of allocation: practices Stratification by: size (number of GPs) and fund holding status Type of comparison: PEM only vs. nothing
Groups considered in review: A and B |
|
| Participants | Physicians Clinical speciality: general practice/family medicine Level of training: fully trained Setting/country: general practice/UK |
|
| Interventions | The PEM studied in this report was a locally developed guideline for the use of PO NSAIDs in the management of musculoskeletal disorders. NSAIDs were selected as the subject of the guidelines because they are associated with high volume and cost prescribing, significant morbidity and mortality, and considerable variation in practice. The guidelines were developed to promote awareness of NSAID prescribing issues and were informed by literature reviews of their relative effectiveness and safety | |
| Outcomes | 1 process outcome: prescription of 3 recommended NSAIDS relative to total NSAID prescribing (mean in all practices) (%) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (MC Watson, PhD Thesis), pg. 89‐90: "randomization commenced with the blinded selection of one of these cards. The practice undergoing randomization was then allocated to the study group corresponding to the number on the card. The second practice was then randomized to the group on the second selected card (without replacement of the first card), and so on" |
| Allocation concealment (selection bias) | Low risk | COMMENT: the unit of allocation is by practice and allocation is performed on all units at the start of the study |
| Baseline characteristics similar (selection bias) | Low risk | Quote, pg. 210: "the 20 participating practices did not differ appreciably from other practices in Avon in terms of size or dispensing status, although fewer had fund holding status (Table 1)" COMMENT: the baseline characteristics of the intervention and control groups were reported and similar |
| Baseline outcome measurements similar (selection bias) | Low risk | Quote, pg. 209: "analysis of covariance adjusting for baseline was performed using Stata" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | COMMENT: missing outcome measures were unlikely to bias the results because a registry was used in its entirety |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome was objective |
| Contamination protection (contamination bias) | Low risk | Quote, pg. 208: "practices in Avon, England, that used the Egton Medical Information Systems Ltd (EMIS) computer system (n=51) were invited to participate. Of these, 20 (39%) were randomized" |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section were reported in the results section |
| Other bias | Unclear risk | Quote, pg. 210: "ceiling effects will therefore have limited the magnitude of change possible" |
Weiss 2011.
| Methods | Study design: ITS | |
| Participants | Physicians, pharmacists Clinical speciality: general practice/family medicine Level of training: guidelines were distributed both to physicians and to residents in training, but prescribing data collected could only be from fully trained physicians Setting/country: outpatient (e.g. ambulatory care provided by hospitals/specialists)/Canada |
|
| Interventions | In 2004, the Quebec Medication Council (Conseil du Medicament du Quebec, Quebec City), with the help of designated physicians and pharmacists, issued a first series of guidelines targeting the most common infectious conditions in the outpatient setting. Eleven 2‐page highly graphic guidelines providing clinical information (diagnosis, investigation) and antibiotic recommendations were published and sent to all physicians (including medical residents), and pharmacists in January 2005. Emphasis was placed not only on proper antibiotic regimens but also on not using antibiotics when viral infections were suspected and on prescribing the shortest possible duration of treatment. A letter signed by all key stakeholders in Quebec (Minister of Health, College of Physicians, College of Pharmacists, and Medical associations) accompanied the initial mailing explaining the reasons supporting the process and the importance of prescribing antibiotics appropriately. The main objective of this study was to assess the impact of a multipronged, mostly Web‐based education strategy on the per capita number and cost of antibiotic prescriptions in the province of Quebec and to compare the trends with those the other 9 Canadian provinces | |
| Outcomes | 1 process outcome: monthly prescribing rates (number of prescriptions/1000 inhabitants) for all antibiotics in Quebec relative to the rest of Canada | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Intervention independent of other changes ‐ ITS | High risk | Quote, pg. 6: "this study has a number of limitations; we did not take into account samples given to physicians, but they represent a very small percentage of the total amount of antibiotics, and filling an antibiotic prescription at a community pharmacy does not guarantee that the patient will finish the entire treatment. The Quebec antibiotic guidelines were produced in a period when health care professionals, government authorities, and perhaps the population as a whole were highly aware of the risks associated with antibiotic overuse (C. difficile infections). Thus, external factors besides the guidelines themselves may have influenced antibiotic prescribing practices" |
| Shape of Intervention effect pre‐specified ‐ ITS | Unclear risk | Quote, pg. 2: "the main objective of this study was to assess the impact of a multipronged, mostly Web‐based education strategy on the per capita number and cost of antibiotic prescriptions in the province of Quebec and to compare the trends with those the other 9 Canadian provinces" |
| Intervention unlikely to affect data collection ‐ ITS | Low risk | The intervention (education guidelines) did not affect either the source or the method of data collection |
| Blinding of outcome assessors (detection bias) ‐ ITS All outcomes | Low risk | The outcome was objective |
| Incomplete outcome data (attrition bias) ‐ ITS All outcomes | Unclear risk | Quote, pg. 2: "the province of Quebec, Canada (2009 population, 7.8 million) has a universal health care insurance program in which medical visits, required investigations, and treatments (whether outpatient or inpatient) are provided free of charge to all citizens. In 1997, the Quebec government instituted a universal drug plan in which everybody has to be covered by either private insurance obtained through his or her employer (57% of the population) or by the public plan (43% of the population). Other provinces have similar drug plans, but not as extensive as that in Quebec" COMMENT: data for Quebec were likely to be complete, but no information was specified for the other provinces |
| Selective reporting (reporting bias) ‐ ITS | Low risk | All relevant outcomes in the methods section were reported in the results section |
| Other bias ‐ ITS | Low risk | There was no evidence of other risks of bias |
4D: Der Deutsche Diabetes Dialyse Studie; 4S: Scandinavian Simvastatin Survival Study; ACC: American College of Cardiology; ACE: angiotensin‐converting enzyme; ACOG: American College of Obstetricians and Gynecologists; ACS: acute coronary syndrome; ADA: American Diabetes Association; AGREE: Appraisal of Guidelines for Research and Evaluation; AHA: American Heart Association; ALLHAT: Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial; ANAES: Agence Nationale d'Accréditation et d'Evaluation en Santé; ASA: aspirin; ATP: Adult Treatment Panel; BP: blood pressure; CHD: coronary heart disease; CME: continuing medical education; C‐RCT: cluster randomised controlled trial; ERT: oestrogen replacement therapy; ES: effect size; GP: general practitioner; HERS: Heart and Estrogen/progestin Replacement Study; HOPE: Heart Outcomes and Prevention Evaluation; HRT: hormone replacement therapy; HT: hormone therapy; IBS: irritable bowel syndrome; ICU: intensive care unit; IHD: ischaemic heart disease; ITS: interrupted time series; IV: intravenous; LDL: low‐density lipoprotein; LIFE: Losartan Intervention for Endpoint; LMWH: low molecular weight heparin; MIRACL: Myocardial Ischemia Reduction with Acute Cholesterol Lowering; NDTI: National Disease and Therapeutic Index; NEJM: New England Journal of Medicine; NHS: National Health Service (UK); NICE: National Institute for Health and Clinical Excellence; NPA: National Prescription Audit Plus; NSAID: non‐steroidal anti‐inflammatory drug; ODB: Ontario's universal Drug Benefit program; PEM: printed educational material; PO: oral; PROVE IT‐TIMI22: Pravastatin or Atorvastatin Evaluation and Infection Therapy– Thrombolysis In Myocardial Infarction 22; RALES: Randomized Aldactone Evaluation Study; RCR: Royal College of Radiologists; RCT: randomised controlled trial; REVERSAL: Reversal of Atherosclerosis With Aggressive Lipid Lowering; SSRI: selective serotonin reuptake inhibitor; STEMI: ST‐elevation myocardial infarction; THR: total hip replacement; TKR: total knee replacement; UBH: United Behavioral Health; VA: Veterans Administration; VALUE: Valsartan Anti‐hypertensive Long‐term Use Evaluation; VBAC: vaginal births after caesarean; WHI: Women's Health Initiative.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bishop 2010 | The comparison studied is not included: multifaceted intervention comprising PEM + reminder vs. usual care |
| Croudace 2003 | The comparison studied is not included: multifaceted intervention comprising PEM + educational meeting vs. usual care |
| Emslie 1993 | The comparison studied is not included: PEM + reminder vs. usual care |
| Engers 2005 | The comparison studied is not included: multifaceted intervention comprising PEM + workshop vs. no intervention |
| Evans 2010 | Outcomes are not objective (knowledge test) |
| Ferrari 2005 | PEM only vs. usual care + information sheet |
| Fontaine 2006 | The intervention was a reminder |
| Hazard 1997 | The comparison studied is not included: multifaceted intervention comprising PEM + patient‐mediated reminder vs. usual care |
| Hunskaar 1996 | Outcomes were not objective |
| Jackevicius 1999 | Outcomes were not objective |
| Jain 2006 | The comparison studied was not included (PEM was used as control): PEM as part of a multifaceted intervention vs. PEM |
| Janmeja 2009 | The intervention was addressed at patients and not at healthcare professionals |
| Kocher 2003 | This study aimed to evaluate the validity of the guideline, and not its effectiveness to change professional practice |
| Kulkarni 1998 | Study design |
| Maiman 1988 | The comparison studied is not included (PEM is used as control): PEM + tutorial vs. PEM |
| Majumdar 2008 | The comparison studied was not included: PEM + reminder vs. usual care |
| Martino 2011 | Study design |
| Mettes 2010 | The comparison studied was not included (PEM is used as control): PEM + multifaceted intervention vs. PEM |
| Mockiene 2011 | The outcome was not objective |
| Mollon 2009 | Study design |
| Morse 2009 | Study design |
| Ozgun 2010 | Study design |
| Perez‐Jauregui 2008 | The intervention is a reminder. |
| Richardson 2002 | Outcomes were not objective |
| Schwartz 2007 | The comparison is not included: PEM + conference vs. usual care |
| Simon 2007 | The comparison was not included: PEM + academic detailing vs. no intervention |
| Steffensen 1997 | The report of this study did not provide pre‐intervention data |
PEM: printed educational material.
Characteristics of ongoing studies [ordered by study ID]
Shah 2010.
| Trial name or title | Evaluation of a toolkit to improve cardiovascular disease screening and treatment for people with type 2 diabetes: protocol for a cluster‐randomized pragmatic trial |
| Methods | Study design: C‐RCT Unit of allocation: practice Stratification by: health region Type of comparison: PEM only vs. nothing
|
| Participants | Physicians Clinical speciality: general practice/family medicine; Level of training: not clear |
| Interventions | The cardiovascular disease toolkit was packaged in a brightly coloured box with CDA branding. The contents included an introductory letter from the Chair of the practice guidelines' Dissemination and Implementation Committee; an 8‐page summary of selected sections of the practice guidelines targeted towards primary care physicians; a 4‐page synopsis of the key guideline elements pertaining to cardiovascular disease risk; a small double‐sided laminated card with a simplified algorithm for cardiovascular risk assessment, vascular protection strategies and screening for cardiovascular disease; and a pad of tear‐off sheets for patients with a cardiovascular risk self‐assessment tool and a list of recommended risk reduction strategies. In the intervention group, the Toolkit was mailed with the Spring 2009 edition of Canadian Diabetes, a newsletter from the CDA which provides practical information on diagnosis and treatment issues associated with diabetes that is sent quarterly to all primary care physicians in Canada. The content of this edition of the newsletter did not pertain to cardiovascular risk screening or treatment. Both the Toolkit and Canadian Diabetes were packaged together in a large mailing envelope. The control group received Canadian Diabetes alone in its usual shrink wrap packaging, and will receive the Toolkit with the Spring 2010 edition of the newsletter |
| Outcomes | The primary outcome will be that the patient is receiving a statin. Secondary outcomes will include 1) the receipt of an angiotensin converting enzyme inhibitor or angiotensin receptor blocker, 2) various intermediate measures (A1c, BP, LDL‐cholesterol, total‐/HDL‐cholesterol ratio, body mass index, and waist circumference), and 3) clinical inertia (the failure to change therapy in response to an abnormal A1c, BP, or cholesterol reading) |
| Starting date | Spring 2010 |
| Contact information | Baiju Shah University of Toronto, Toronto, Ontario, Canada Email: baiju.shah@ices.on.ca |
| Notes | ‐ |
Zwarenstein 2007.
| Trial name or title | The OPEM trial to narrow the evidence‐practice gap with respect to prescribing practices of general and family physicians: a C‐RCT, targeting the care of individuals with diabetes and hypertension in Ontario, Canada |
| Methods | Study design: C‐RCT Unit of allocation: practices Type of comparison: PEM only vs. nothing
Groups considered in review: A and D |
| Participants | Physicians Clinical speciality: general practice/family medicine Level of training: not clear |
| Interventions | The authors aim to conduct 3 replicates of the trial to cover the 3 evidence‐practice gaps over a 9‐month period (3 successive mail outs of informed). They plan to test the effects of short (directive) and long (discursive) PEMs compared with no PEM on the clinical practices of primary care physicians, and on related patient outcomes. In the first replicate (ACE inhibitors, hypertension treatment, and cholesterol‐lowering agents for diabetes), the first intervention group will receive a copy of informed with both the short, directive, evidence‐based outsert stapled to the lower‐left quarter of the front page, and the longer 2‐page insert focusing on the same topic as the outsert. The second intervention group will receive the identical informed, with only the above‐mentioned outsert. The third intervention group will receive the identical copy of informed with the above‐mentioned insert. The control group will receive the identical informed only, without the insert or the outsert. The healthcare topic shared by the insert and outsert will not be covered elsewhere in that issue of informed. For the second replicate (retinal screening in patients with diabetes), in addition to the short, directive outsert and the longer, explanatory insert, a reminder note will be added that physicians could give to their patients to supplement the verbal reminder that physicians are encouraged to give. Because it is not clear whether this patient‐held reminder to make an appointment with their eye‐care provider is any more effective than the verbal reminder that physicians will be encouraged to give, those physicians receiving an outsert to receive a pad of the patient‐aimed reminder slips will be randomised. For the third replicate (using thiazides as first‐line treatment for hypertension), 2 different short directive outsert messages will be used (in addition to the long, explanatory insert message). The OPEM team will develop the first outsert message, whereas a team of psychologists with experience in knowledge implementation and the use of psychological theories will develop the second outsert message. With the addition of a theory‐based outsert, it will be possible to determine whether a message that is based on psychological theory, specifically on the Theory of Planned Behaviour, will be more effective in changing clinical behaviour towards more evidence‐based practice than a message that is based on 'standard' methods, which are uninformed by an explicit theoretical basis |
| Outcomes |
|
| Starting date | |
| Contact information | Merrick Zwarenstein Institute for Clinical Evaluative Sciences, Toronto, Canada Email: merrick.zwarenstein@ices.on.ca |
| Notes |
ACE: angiotensin‐converting enzyme; ALLHAT: Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial; C‐RCT: cluster randomised controlled trial; HDL: high‐density lipoprotein; ITS: interrupted time series; LDL: low‐density lipoprotein; ODB: Ontario's universal Drug Benefit; OPEM: Ontario Printed Educational Message; PEM: printed educational material.
Contributions of authors
MF developed the search strategy. AGi, MF, AGr, FL, and JG identified the eligible studies. AGi and ST participated in data extraction; AGi, ST and SMK participated in data analysis; and AGi wrote the first draft of the review report. All authors revised the first draft and the final version of the review report.
Sources of support
Internal sources
Institute of Population Health, University of Ottawa, Canada.
Canadian Foundation for Innovation ‐ Canadian Research Chair, Canada.
Centre de Recherche, Centre Hospitalier Universitaire de Québec, Canada.
Health Services Research Unit, University of Aberdeen, UK.
University of Leeds, UK.
Department of Medical Education, University of Washington, USA.
Ottawa Hospital Research Institute, Canada.
Health Information Research Unit, McMaster University, Canada.
External sources
NIHR Cochrane Review Incentive Scheme 2011, UK.
The Wellcome Trust and Chief Scientist Office, Scottish Executive Health Department, UK.
CCOHTA'S 2004 Health Technology Assessment Capacity Building Grants Program, Canada.
Canadian Institutes for Health Research, Canada.
Knowledge Translation Canada Research Network, Canada.
Declarations of interest
JG is author of one of the included studies.
Edited (no change to conclusions)
References
References to studies included in this review
Austin 2003 {published data only}
Austin 2004A {published data only}
- Austin PC, Mamdani MM, Tu K. The impact of the Women's Health Initiative study on incident clonidine use in Ontario, Canada. Canadian Journal of Clinical Pharmacology 2004;11(2):e191‐4. [PubMed] [Google Scholar]
Austin 2004B {published data only}
Austin 2005 {published data only}
- Austin PC, Mamdani MM. Impact of the pravastatin or avorstatin evaluation and infection therapy‐thrombolysis in myocardial infarction. Circulation 2005;112(9):1296‐300. [DOI] [PubMed] [Google Scholar]
Avorn 1983 {published data only}
- Avorn J, Soumerai SB. Improving drug‐therapy decisions through educational outreach. A randomized controlled trial of academically based "detailing". New England Journal of Medicine 1983;308(24):1457‐63. [DOI] [PubMed] [Google Scholar]
- Soumerai SB, Avorn J. Economic and policy analysis of university‐based drug "detailing". Medical Care 1986; Vol. 24, issue 4:313‐31. [DOI] [PubMed]
- Soumerai SB, Avorn J. Predictors of physician prescribing change in an educational experiment to improve medication use. Medical Care 1987; Vol. 25, issue 3:210‐21. [DOI] [PubMed]
Azocar 2003 {published data only}
- Azocar F, Cuffel B, Goldman W, McCarter L. The impact of evidence‐based guideline dissemination for the assessment and treatment of major depression in a managed behavioral health care organization. Journal of Behavioral Health Services and Research 2003;30(1):109‐18. [DOI] [PubMed] [Google Scholar]
- Azocar F, Cuffel BD, Goldman W, McCulloch J. Best practices: dissemination of guidelines for the treatment of major depression in a managed behavioral health care network. Psychiatric Services 2001; Vol. 52, issue 8:1014‐6. [DOI] [PubMed]
Barbaglia 2009 {published data only}
Bearcroft 1994 {published data only}
- Bearcroft PWP, Small JH, Flower CDR. Chest radiography guidelines for general practitioners: a practical approach. Clinical Radiology 1994;49(1):56‐8. [DOI] [PubMed] [Google Scholar]
Beaulieu 2004 {published data only}
- Beaulieu M‐D, Brophy J, Jacques A, Blais R, Battista R, Lebeau R. Drug treatment of stable angina pectoris and mass dissemination of therapeutic guidelines: a randomized controlled trial. QJM: monthly journal of the Association of Physicians 2004;97(1):21‐31. [DOI] [PubMed] [Google Scholar]
Bjornson 1990 {published data only}
- Bjornson DC, Rector TS, Daniels CE, Wertheimer AI, Snowdon DA, Litman TJ. Impact of a drug‐use review program intervention on prescribing after publication of a randomized clinical trial. American Journal of Hospital Pharmacy 1990;47(7):1541‐6. [PubMed] [Google Scholar]
Black 2002 {published data only}
- Black N, Hutchings A. Reduction in the use of surgery for glue ear: did national guidelines have an impact?. Quality and Safety in Health Care 2002;11(2):121‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buyle 2010 {published data only}
Coopersmith 2002 {published data only}
Denig 1990 {published data only}
- Denig P, Haaijer‐Ruskamp FM, Zijsling DH. Impact of a drug bulletin on the knowledge, perception of drug utility, and prescribing behavior of physicians. DICP: the annals of pharmacotherapy 1990;24(1):87‐93. [DOI] [PubMed] [Google Scholar]
Dormuth 2004 {published data only}
- Dormuth CR, Maclure M, Bassett K, Jauca C, Whiteside C, Wright JM. Effect of periodic letters on evidence‐based drug therapy on prescribing behaviour: a randomized trial. CMAJ: Canadian Medical Association Journal 2004;171(9):1057‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fijn 2000 {published data only}
Fonarow 2009 {published data only}
Fukuda 2009 {published data only}
Guay 2007 {published data only}
Haas 2004 {published data only}
Hersh 2004 {published data only}
Jackevicius 2001 {published data only}
Jameson 2010 {published data only}
Jousimaa 2002 {published data only}
- Jousimaa J, Makela M, Kunnamo I, MacLennan G, Grimshaw JM. Primary care guidelines on consultation practices: the effectiveness of computerized versus paper‐based versions. A cluster randomized controlled trial among newly qualified primary care physicians. International Journal of Technology Assessment Health Care 2002; Vol. 18, issue 3:586‐96. [PubMed]
Juurlink 2004 {published data only}
Kabir 2007 {published data only}
- Kabir Z, Feely J, Bennett K. Primary care prescribing patterns in Ireland after the publication of large hypertension trials. British Journal of Clinical Pharmacology 2007; Vol. 64, issue 3:381‐5. [DOI] [PMC free article] [PubMed]
Kajita 2010 {published data only}
- Kajita E, Nakatani Y, Tamaki J, Ito N, Iki M. Evidence‐based assessment of the effectiveness of a clinical practice guideline for prevention of osteoporosis and osteoporotic fracture. IOF World Congress on Osteoporosis and 10th European Congress on Clinical and Economic Aspects of Osteoporosis and Osteoarthritis, IOF WCO ‐ ECCEO 10; 2010 May 5‐8; Florence, Italy. 2010.
- Nakatani Y, Tamaki J, Komatsu M, Iki M, Kajita E. Effect of distributing an evidence‐based guideline for prevention of osteoporosis on health education programs in municipal health centers: a randomized controlled trial. Journal of Epidemiology 2012; Vol. 22, issue 2:103‐12. [DOI] [PMC free article] [PubMed]
Kottke 1989 {published data only}
- Kottke TE, Brekke ML, Solberg LI, Hughes JR. Use smoking intervention by physicians. Doctors Helping Smokers, Round I. Journal of the American Medical Association 1989;261(14):2101‐6. [PubMed] [Google Scholar]
Lam 2009 {published data only}
Majumdar 2003 {published data only}
- Majumdar SR, McAlister FA, Soumerai SB. Synergy between publication and promotion: comparing adoption of new evidence in Canada and the United States. American Journal of Medicine 2003;115(6):467‐72. [DOI] [PubMed] [Google Scholar]
Majumdar 2004 {published data only}
- Majumdar SR, Almasi EA, Stafford RS. Promotion and prescribing of hormone therapy after report of harm by the Women's Health Initiative. Journal of the American Medical Association 2004;292(16):1983‐8. [DOI] [PubMed] [Google Scholar]
Mason 1998/99 {published data only}
- Mason J, Freemantle N, Young P. The effect of the distribution of Effective Health Care Bulletins on prescribing selective serotonin reuptake inhibitors in primary care. Health Trends 1998/99;30:120‐2. [Google Scholar]
Mason 2001 {published data only}
- Mason J, Freemantle N, Browning G. Impact of effective health care bulletin on treatment of persistent glue ear in children: time series analysis. BMJ 2001;323(7321):1096‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matowe 2002 {published data only}
- Matowe L, Ramsay CR, Grimshaw JM, Gilbert FJ, Macleod MJ, Needham G. Effects of mailed dissemination of the Royal College of Radiologists' guidelines on general practitioner referrals for radiography: a time series analysis. Clinical Radiology 2002;57(7):575‐8. [DOI] [PubMed] [Google Scholar]
Meyer 2007 {published data only}
Oakeshott 1994 {published data only}
- Oakeshott P, Kerry SM, Williams JE. Randomized controlled trial of the effect of the Royal College of Radiologists' guidelines on general practitioners' referrals for radiographic examination. British Journal of General Practice 1994;44(382):197‐200. [PMC free article] [PubMed] [Google Scholar]
Perria 2007 {published data only}
- Perria C, Group IS. Strategies for the introduction and implementation of a guideline for the treatment of type 2 diabetics by general practitioners (GPs) of the Lazio region of Italy (IMPLEMEG study): protocol for a cluster randomised controlled trial. BMC Health Services Research 2004; Vol. 4, issue 1:13. [DOI] [PMC free article] [PubMed]
- Perria C, Mandolini D, Guerrera C, Jefferson T, Billi P, Calzini V, et al. Implementing a guideline for the treatment of type 2 diabetics: results of a cluster ‐ randomized controlled trial (C‐RCT). BMC Health Services Research 2007; Vol. 7, issue 79. [DOI] [PMC free article] [PubMed]
Roberts 2007 {published data only}
Santerre 1996 {published data only}
- Santerre RE. The effect of the ACOG guideline on vaginal births after cesarean. Medical Care Research and Review 1996;53(3):315‐29. [DOI] [PubMed] [Google Scholar]
Shah 2008 {published data only}
Stafford 2004 {published data only}
Tsuji 2009 {published data only}
- Tsuji SR, Atallah AN, Aranha FC, Tonhom AP, Siqueira AC, Jr, et al. Cluster randomized clinical trial (ISRCTN23732000) to evaluate the effectiveness of a diagnosis recognition and treatment guide for depressive disorders in primary care [MEDLINE]. Journal of Evaluation in Clinical Practice 2009; Vol. 15, issue 1:222‐5. [DOI] [PubMed]
Wang 2005 {published data only}
Watson 2001 {published data only}
Weiss 2011 {published data only}
References to studies excluded from this review
Bishop 2010 {published data only}
- Bishop PB, Quon JA, Fisher CG, Dvorak MF. The Chiropractic Hospital‐based Interventions Research Outcomes (CHIRO) study: a randomized controlled trial on the effectiveness of clinical practice guidelines in the medical and chiropractic management of patients with acute mechanical low back pain. The Spine Journal 2010; Vol. 10, issue 12:1055‐64. [DOI] [PubMed]
Croudace 2003 {published data only}
- Croudace T, Evans J, Harrison G, Sharp DJ, Wilkinson E, McCann G, et al. Impact of the ICD‐10 primary health care (PHC) diagnostic and management guidelines for mental disorders on detection and outcome in primary care. British Journal of Psychiatry 2003;182:20‐30. [DOI] [PubMed] [Google Scholar]
Emslie 1993 {published data only}
- Emslie C, Grimshaw J, Templeton A. Do clinical guidelines improve general practice management and referral of infertile couples?. BMJ 1993; Vol. 306, issue 6894:1728‐31. [DOI] [PMC free article] [PubMed]
Engers 2005 {published data only}
- Engers AJ, Wensing M, Tulder MW, Timmermans A, Oostendorp RA, Koes BW, et al. Implementation of the Dutch low back pain guideline for general practitioners: a cluster randomized controlled trial. Spine 2005;30(6):559‐600. [DOI] [PubMed] [Google Scholar]
Evans 2010 {published data only}
- Evans DW, Breen AC, Pincus T, Sim J, Underwood M, Vogel S, et al. The effectiveness of a posted information package on the beliefs and behavior of musculoskeletal practitioners: the UK Chiropractors, Osteopaths, and Musculoskeletal Physiotherapists Low Back Pain ManagemENT (COMPLeMENT) randomized trial. Spine 2010; Vol. 35, issue 8:858‐66. [DOI] [PubMed]
Ferrari 2005 {published data only}
- Ferrari R, Rowe BH, Majumdar SR, Cassidy JD, Blitz S, Wright SC, et al. Simple educational intervention to improve the recovery from acute whiplash: results of a randomized, controlled trial. Academic Emergency Medicine 2005;12(8):699‐706. [DOI] [PubMed] [Google Scholar]
Fontaine 2006 {published data only}
Hazard 1997 {published data only}
- Hazard RG, Haugh LD, Reid S, McFarlane G, MacDonald L. Early physician notification of patient disability risk and clinical guidelines after low back injury: a randomized controlled trial. Spine 1997;22(24):2951‐8. [DOI] [PubMed] [Google Scholar]
Hunskaar 1996 {published data only}
Jackevicius 1999 {published data only}
Jain 2006 {published data only}
Janmeja 2009 {published data only}
- Janmeja AK, Mohapatra PR, Kumar M. The impact of "World Health Organization ‐ Government of India guidelines on chronic obstructive pulmonary diseases‐2003" on quality of life. Lung India 2009; Vol. 26, issue 4:102‐5. [DOI] [PMC free article] [PubMed]
Kocher 2003 {published data only}
- Kocher MS, Mandiga R, Murphy JM, Goldmann D, Harper M, Sundel R, et al. A clinical practice guideline for treatment of septic arthritis in children: efficacy in improving process of care and effect on outcome of septic arthritis of the hip. Journal of Bone & Joint Surgery, American Volume 2003; Vol. 85A, issue 6:994‐9. [PubMed]
Kulkarni 1998 {published data only}
- Kulkarni K, Castle G, Gregory R, Holmes A, Leontos C, Powers M, et al. Nutrition practice guidelines for type 1 diabetes mellitus positively affect dietician practices and patient outcomes. The Diabetes Care and Education Dietetic Practice Group. Journal of the American Dietetic Association 1998;98(1):62‐70. [DOI] [PubMed] [Google Scholar]
Maiman 1988 {published data only}
Majumdar 2008 {published data only}
- Majumdar SR, Johnson JA, McAlister FA, Bellerose D, Russell AS, Hanley DA, et al. Multifaceted intervention to improve diagnosis and treatment of osteoporosis in patients with recent wrist fracture: a randomized controlled trial. CMAJ: Canadian Medical Association Journal 2008; Vol. 178, issue 5:569‐75. [DOI] [PMC free article] [PubMed]
Martino 2011 {published data only}
Mettes 2010 {published data only}
Mockiene 2011 {published data only}
- Mockiene V, Suominen T, Valimaki M, Razbadauskas A, Martinkenas A, Caplinskas S. The impact of an education intervention to change nurses' HIV‐related knowledge and attitudes in Lithuania: a randomized controlled trial. The Journal of the Association of Nurses in AIDS Care : JANAC. 2011; Vol. 22, issue 2:140‐9. [DOI] [PubMed]
Mollon 2009 {published data only}
Morse 2009 {published data only}
Ozgun 2010 {published data only}
Perez‐Jauregui 2008 {published data only}
Richardson 2002 {published data only}
Schwartz 2007 {published data only}
Simon 2007 {published data only}
- Simon SR, Rodriguez HP, Majumdar SR, Kleinman K, Warner C, Salem‐Schatz S, et al. Economic analysis of a randomized trial of academic detailing interventions to improve use of antihypertensive medications. Journal of Clinical Hypertension (Greenwich, Conn.) 2007; Vol. 9, issue 1:15‐20. [DOI] [PMC free article] [PubMed]
Steffensen 1997 {published data only}
- Steffensen FH, Sorensen HT, Olesen F. Impact of local evidence‐based clinical guidelines ‐ a Danish intervention study. Family Practice 1997;14(3):209‐15. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Shah 2010 {published data only}
- Shah BR, Bhattacharyya O, Yu C, Mamdani M, Parsons JA, Straus SE, et al. Evaluation of a toolkit to improve cardiovascular disease screening and treatment for people with type 2 diabetes: protocol for a cluster‐randomized pragmatic trial. Trials. 2010/04/27 2010; Vol. 11:44. [DOI] [PMC free article] [PubMed]
Zwarenstein 2007 {published data only}
- Zwarenstein M, Hux JE, Kelsall D, Paterson M, Grimshaw J, Davis D, et al. The Ontario printed educational message (OPEM) trial to narrow the evidence‐practice gap with respect to prescribing practices of general and family physicians: a cluster randomized controlled trial, targeting the care of individuals with diabetes and hypertension in Ontario, Canada. Implementation Science 2007; Vol. 2:37. [DOI] [PMC free article] [PubMed]
Additional references
Ancker 2007
- Ancker JS, Kaufman D. Rethinking health numeracy: a multidisciplinary literature review. Journal of the American Medical Informatics Association 2007; Vol. 14, issue 6:713‐21. [DOI] [PMC free article] [PubMed]
Arnold 2005
- Arnold SR, Straus SE. Interventions to improve antibiotic prescribing practices in ambulatory care. Cochrane Database of Systematic Reviews 2005, issue 4:CD003539. [DOI] [PMC free article] [PubMed]
Baker 2010
- Baker R, Camosso‐Stefinovic J, Gillies C, Shaw EJ, Cheater F, Flottorp S, et al. Tailored interventions to overcome identified barriers to change: effects on professional practice and health care outcomes. Cochrane Database of Systematic Reviews 2010, issue 3. [DOI] [PMC free article] [PubMed]
Bero 1998
- Bero L, Grilli R, Grimshaw J, Harvey E, Oxman A, Thomson MA. Closing the gap between research and practice: an overview of systematic reviews of interventions to promote the implementation of research findings. BMJ 1998;317(7156):465‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bull 2001
- Bull F, Kreuter M, Clark E, Scharff D. Understanding the effects of printed health education material which features lead to which outcomes. Journal of Health Communication 2001;6(3):265‐79. [DOI] [PubMed] [Google Scholar]
Burgers 2003a
- Burgers J, Grol R, Zaat J, Spies T, Bij A, Mokkink H. Characteristics of effective guidelines for general practice. British Journal of General Practice 2003;53(486):15‐9. [PMC free article] [PubMed] [Google Scholar]
Chastre 2003
- Chastre J. Antimicrobial treatment of hospital‐acquired pneumonia. Infectious disease clinics of North America 2003;17(4):727‐37. [PUBMED: 15008595] [DOI] [PubMed] [Google Scholar]
Chatfield 2001
- Chatfield C. Time‐series forecasting. Florida: Chapman & Hall/CRC Press, 2001. [Google Scholar]
Davis 2009
de Belvis 2009
Donner 2001
Flodgren 2011
- Flodgren G, Parmelli E, Doumit G, Gattellari M, O'Brien MA, Grimshaw J, et al. Local opinion leaders: effects on professional practice and health care outcomes. Cochrane Database of Systematic Reviews 2011, issue 8:CD000125. [DOI] [PMC free article] [PubMed]
Forsetlund 2009
- Forsetlund L, Bjorndal A, Rashidian A, Jamtvedt G, O'Brien MA, Wolf F, et al. Continuing education meetings and workshops: effects on professional practice and health care outcomes. Cochrane Database of Systematic Reviews 2009, issue 2:CD003030. [DOI] [PMC free article] [PubMed]
Foy 2002
- Foy R, McLennan G, Grimshaw J, Penney G, Campbell M, Grol R. Attributes of clinical recommendations that influence change in practice following audit and feedback. Journal of Clinical Epidemiology 2002;55:717‐22. [DOI] [PubMed] [Google Scholar]
Freemantle 1997
- Freemantle N, Harvey EL, Wolf F, Grimshaw JM, Grilli R, Bero LA. Printed educational materials: effects on professional practice and health care outcomes. Cochrane Database of Systematic Reviews 1997, Issue 2. [DOI: 10.1002/14651858.CD000172] [DOI] [PubMed] [Google Scholar]
French 2010
- French SD, Green S, Buchbinder R, Barnes H. Interventions for improving the appropriate use of imaging in people with musculoskeletal conditions. Cochrane Database of Systematic Reviews 2010, issue 1:CD006094. [DOI] [PMC free article] [PubMed]
Graham 2006
Grandage 2002
- Grandage KK, Slawson DC, Shaughnessy AF. When less is more: a practical approach to searching for evidence‐based answers. Journal of the Medical Library Association 2002; Vol. 90, issue 3:298‐304. [PMC free article] [PubMed]
Grilli 2002
Grimshaw 2003
- Grimshaw J, McAuley LM, Bero LA, Grilli R, Oxman AD, Ramsay C, et al. Systematic reviews of the effectiveness of quality improvement strategies and programmes. Quality and Safety in Health Care 2003; Vol. 12, issue 4:298‐303. [1475‐3898: (Print); MEDLINE: ] [DOI] [PMC free article] [PubMed]
Grimshaw 2004
- Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technology Assessment 2004;8(6):iii‐iv, 1‐72. [DOI] [PubMed] [Google Scholar]
Grimshaw 2006
- Grimshaw J, Eccles M, Thomas R, MacLennan G, Ramsay C, Fraser C, et al. Toward evidence‐based quality improvement. Evidence (and its limitations) of the effectiveness of guideline dissemination and implementation strategies 1966‐1998. Journal of General Internal Medicine 2006; Vol. 21 Suppl 2:S14‐20. [DOI] [PMC free article] [PubMed]
Grol 1998
- Grol R, Dalhuijsen J, Thomas S, Veld C, Rutten G, Mokkink H. Attributes of clinical guidelines that influence of guidelines in general practice: observational study. BMJ 1998;317:858‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grol 2001
Grol 2003
Grol 2007
- Grol RP, Bosch MC, Hulscher ME, Eccles MP, Wensing M. Planning and studying improvement in patient care: the use of theoretical perspectives. Milbank Quarterly 2007; Vol. 85, issue 1:93‐138. [DOI] [PMC free article] [PubMed]
Gross 2001
- Gross PA, Greenfield S, Cretin S, Ferguson J, Grimshaw J, Grol R, et al. Optimal methods for guideline implementation: conclusions from Leeds Castle meeting. Medical Care 2001; Vol. 39, issue 8 Suppl 2:II85‐92. [PubMed]
Hakkennes 2008
Haynes 2007
Higgins 2011
- Higgins J, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated March 2011]. The Cochrane Collaboration, 2011. http://www.cochrane‐handbook.org/.
Hoffman 2004
- Hoffman T, Worrall L. Designing effective written health education materials: considerations for health professionals. Disability and Rehabilitation 2004;26(19):1163‐73. [DOI] [PubMed] [Google Scholar]
Jamtvedt 2006
Kanouse 1995
- Kanouse D, Kallich J, Kahan J. Dissemination of effectiveness and outcomes research. Health Policy 1995;34(3):167‐92. [DOI] [PubMed] [Google Scholar]
Kreuter 1996
- Kreuter MW, Strecher VJ. Do tailored behaviour change messages enhance the effectiveness of health risk appraisals? results from a randomized controlled trial. Health Education Research 1996;11(1):97‐105. [DOI] [PubMed] [Google Scholar]
Lagarde 2012
Marriott 2000
- Marriott S, Palmer C, Lelliott P. Disseminating healthcare information: getting the message across. Quality in Health Care 2000;9(1):58‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
McGuire 1989
- McGuire WJ. Theoretical foundations of campaigns. In: Rice R, Atkins C editor(s). Public Communication Campaigns. California: Sage Publication, Inc., 1989. [Google Scholar]
NHS 1999
- NHS CforRandD. Getting evidence into practice. Effective Health Care 1999; Vol. 5:1–16.
O'Brien 2007
- O'Brien MA, Rogers S, Jamtvedt G, Oxman AD, Odgaard‐Jensen J, Kristoffersen DT, et al. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database of Systematic Reviews. 2007/10/19 2007, issue 4:CD000409. [DOI] [PMC free article] [PubMed]
Ramsay 2003
- Ramsay CR, Matowe L, Grilli R, Grimshaw JM, Thomas RE. Interrupted time series designs in health technology assessment: lessons from two systematic reviews of behavior change strategies. International Journal of Technology Assessment in Health Care 2003;19(4):613‐23. [DOI] [PubMed] [Google Scholar]
Revere 2001
- Revere D, Dunbar P. Review of computer‐generated outpatient health behavior interventions: clinical encounters in absentia. Journal of the American Medical Informatics Association 2001;8(1):62‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rogers 1995
- Rogers EM. Diffusion of innovations. New York: Free Press, 1995. [Google Scholar]
Rosenbaum 2010
- Rosenbaum ME. Improving the User Experience of Evidence: a Design Approach to Evidence‐informed Healthcare [PhD thesis]. Oslo: The Oslo School of Architecture and Design, 2010:170. [Google Scholar]
Schuster 2005
- Schuster MA, McGlynn EA, Brook RH. How good is the quality of health care in the United States? 1998. Milbank Quarterly. 2005/11/11 2005; Vol. 83, issue 4:843‐95. [DOI] [PMC free article] [PubMed]
Seitz 2011
Shojania 2010
- Shojania KG, Jennings A, Mayhew A, Ramsay C, Eccles M, Grimshaw J. Effect of point‐of‐care computer reminders on physician behaviour: a systematic review. CMAJ: Canadian Medical Association Journal. 2010/03/10 2010; Vol. 182, issue 5:E216‐25. [DOI] [PMC free article] [PubMed]
Stergiou‐Kita 2010
Tseng 1999
- Tseng S, Fogg B. Credibility and computing technology. Communications of the ACM 1999;42(5):39‐44. [Google Scholar]
Wang 2009
Wathen 2002
- Wathen CN, Burkell J. Believe it or not: factors influencing credibility on the web. Journal of the American Society for Information Science and Technology 2002;53(2):134‐44. [Google Scholar]
Weinmann 2007
Wilson 2010
- Wilson PM, Petticrew M, Calnan MW, Nazareth I. Disseminating research findings: what should researchers do? A systematic scoping review of conceptual frameworks. Implementation Science. 2010/11/26 2010; Vol. 5:91. [DOI] [PMC free article] [PubMed]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ. 2008/03/05 2008; Vol. 336, issue 7644:601‐5. [DOI] [PMC free article] [PubMed]
Woolf 1999
- Woolf SH, Grol R, Hutchinson A, Eccles M, Grimshaw J. Clinical guidelines: potential benefits, limitations, and harms of clinical guidelines. BMJ. 1999/02/19 1999; Vol. 318, issue 7182:527‐30. [DOI] [PMC free article] [PubMed]


