Abstract
Purpose
To assess the effect of age and test-retest reliability of the intensity response function of the full field Photopic Negative Response (PhNR) in normal healthy human subjects.
Methods
Full field electroretinograms (ERGs) were recorded from one eye of 45 subjects and 39 of these subjects were tested on two separate days with a Diagnosys Espion System (Lowell, MA, USA). The visual stimuli consisted of brief (<5ms) red flashes ranging from 0.000625 to 6.4 phot cd s/m2, delivered on a constant 7 cd/m2 blue background. PhNR amplitudes were measured at its trough from baseline (BT) and from the preceding b-wave peak (PT) and b-wave amplitude was measured at its peak from the preceding a-wave trough or baseline if the a-wave was not present. The intensity response data of all three ERG measures was fitted with a generalized Naka-Rushton function to derive the saturated amplitude (Vmax), semisaturation constant (K) and slope (n) parameters. Effect of age on the fit parameters was assessed with linear regression and test-retest reliability was assessed with the Wilcoxon Signed Rank test and Bland-Altman analysis. Holm’s correction was applied to account for multiple comparisons.
Results
Vmax of BT was significantly smaller than that of PT and b-wave and the Vmax of PT and b-wave were not significantly different from each other. The slope parameter n was smallest for BT and largest for b-wave and the difference between the slopes of all three measures were statistically significant. Small differences observed in the mean values of K for the different measures did not reach statistical significance. The Wilcoxon signed rank test indicated no significant differences between the two test visits for any of the Naka-Rushton parameters for the three ERG measures and the Bland-Altman plots indicated that the mean difference between test and retest measurements of the different fit parameters were close to zero and within 6% of the average of the test and retest values of the respective parameters for all three ERG measurements, indicating minimal bias. While the Coefficient of Reliability (COR, defined as 1.96 times the standard deviation of the test and retest difference) of each fit parameter was more or less comparable across the three ERG measurements, the %COR (COR normalized to the mean test and retest measures) was generally larger for BT compared to both PT and b-wave for each fit parameter. The Naka-Rushton fit parameters did not show statistically significant changes with age for any of the ERG measures when corrections were applied for multiple comparisons. However, the Vmax of BT demonstrated a weak correlation with age prior to correction for multiple comparisons and the effect of age on this parameter showed greater significance when the measure was expressed as a ratio of the Vmax of the b-wave from the same subject.
Conclusion
The Vmax of the BT amplitude measure of PhNR at the best was weakly correlated with age. None of the other parameters of the Naka-Rushton fit to the intensity response data of either the PhNR or the b-wave showed any systematic changes with age. The test-retest reliability of the fit parameters for PhNR BT amplitude measurements appear to be lower than those of the PhNR PT and b-wave amplitude measurements.
Keywords: Photopic Negative Response (PhNR), Aging, Intensity response function, Naka-Rushton, Electroretinogram (ERG)
INTRODUCTION
The electroretinogram (ERG) recorded to a full-field flash stimulus under photopic condition contains an initial negative deflection, the photopic a-wave that originates from cone photoreceptor and off-cone bipolar cell activity [1], followed by a positive deflection, the photopic b-wave that originates from the combined activity of on- and off-cone bipolar cells [2], and finally a slow negative potential, the Photopic Negative Response (PhNR) that originates from the spiking activity of primarily the retinal ganglion cells [3]. The PhNR is altered in myriad clinical conditions where it could be useful for evaluating retinal ganglion cell health [4–36]. However, most of the aforementioned clinical studies with two exceptions [26, 27] analyzed PhNRs at a few fixed flash intensities or restricted the analysis to qualitative descriptions of the PhNR intensity response relationship. A quantitative description of the PhNR intensity response data can be potentially useful in understanding the mechanisms and severity of retinal ganglion cell dysfunction in disease conditions and to this effect the Naka-Rushton function has been recently employed to fit this data and quantify the intensity response characteristics of the PhNR in normal subjects [26, 27, 37] and disease patients [26, 27]. Previously, the Naka-Rushton function has been used quite extensively to describe the intensity response relationship of other flash ERG components, especially the dark-adapted b-wave and the fit parameters of saturated amplitude (Vmax), semisaturation constant (K) and slope (n) have been used to explore the underlying mechanisms of different retinal degenerative conditions [38–53]. Naka and Rushton originally developed this function to characterize the s-potentials from color units in the fish [54]. Arden and co-workers [38] as well as Massof and colleagues [39] later applied the Naka-Rushton function to describe the stimulus intensity response relationship of ERG components in clinical populations. As shown in recent studies [26, 27, 37], the Naka-Rushton function seems to provide a reasonable description of the PhNR intensity response relationship. Binns and co-workers [37] were the first to fit the PhNR intensity response data with the Naka-Rushton function and examined the test-retest variability of the best fit parameters from young healthy subjects. Kremers and colleagues [27] employed the Naka-Rushton function to examine the behavior of the PhNR intensity response data for different test and background spectral combinations over a wide range of background intensities from glaucoma patients as well as control subjects and reported that the saturated amplitudes derived from Naka-Rushton fits were significantly reduced for glaucoma patients relative to controls. Using red test flashes on a steady blue background, Wang et al., [26] performed similar analysis to look at differences in the values of fit parameters between multiple sclerosis patients and normal subjects. The purpose of our study was to evaluate the effect of age and the test-retest reliability of the Naka-Rushton function fit parameters of the PhNR intensity-response data in normal healthy subjects over a wider age range than previously employed by Binns and co-workers. The PhNR amplitudes were measured at its trough from baseline (BT) or from the preceding b-wave peak (PT). We found that the Vmax of the PhNR BT measurements, at the best showed a weak correlation with age. None of the other fit parameters for either the PhNR or the b-wave weresignificantly altered by age. We also found that for both PhNR amplitude measurement techniques, the test-retest reliability of Vmax was better than that of K or n. Further, the test-retest reliability of all three fit parameters was better for PT relative to BT and comparable or better for b-wave relative to PT. The subject’s age did not have any significant effect on the absolute values of the test-retest difference for any of the fit parameters.
METHODS
A total of 45 healthy, visually normal healthy subjects in the age range of 24 to 74 years (median age 49 years, 22 males and 23 females) who had a clinical eye examination within the previous year participated in the study. The spherical equivalent of the subjects refractive error was within ± 6 Diopters. Written consent was obtained from all the participants and the study strictly adhered to the declaration of Helsinki and was approved by the Institutional Review Board at the State University of New York College of Optometry.
ERGs were recorded from one eye of 45 subjects and 39 of these subjects were tested on two separate days using DTL electrodes [55] from Diagnosys LLC (Lowell, Massachusetts, USA). The electrode was placed in the lower cul-de-sac and held in place by sticky pads on the skin of the outer and inner canthi after the topical application of 1% Proparacaine Hydrochloride (Akorn, Lake Forest, Illinois, USA). The active electrode was placed in the right eye and the electrode in the left eye served as reference. The pupil of the tested eye was dilated with 1% Tropicamide (Akorn, Lake Forest, Illinois, USA) prior to electrode placement. The eye with the reference electrode was covered with a light proof patch and the subject was requested to keep the eye lids open under the patch to minimize electrode movement. An ear clip electrode placed on the right earlobe served as ground.
An Espion System from Diagnosys LLC (Lowell, Massachusetts, USA) was used for visual stimulation and response acquisition. Visual stimuli consisted of brief (<5ms) red (660 nm peak λ and 20 nm half band width) LED flashes (0.000625 – 6.4 phot cd.s/m2) delivered on 7 phot cd/m2 constant blue (485 nm peak λ and 20 nm half band width) background delivered in an Espion Ganzfeld ColorDome after 10 minutes of adaptation to the background light. Test flashes were delivered in the order of increasing flash intensity with responses averaged in blocks of 25 trials with an interstimulus interval of at least 1 second. The subjects fixated on a red LED target at the center of the Ganzfeld and the subject’s fixation, eye movements and blinks were continuously monitored through an IR video camera inside the Ganzfeld and all traces contaminated by eye blinks and large eye movements were rejected. Signals were amplified, filtered (0–300 Hz), digitized and sampled at 1kHz.
PhNR amplitudes were measured at its trough after the positive i-wave, from baseline (BT) as well as from the preceding b-wave peak (PT). The time point beyond the i-wave, where the amplitude was most negative was chosen for the amplitude measurement, if the most negative amplitude extended over a range of time points then the middle of the range was chosen for amplitude measurement. For the lower flash intensity responses for which the PhNR was not very well developed, we used the same time point of amplitude measurement as with the first succeeding brighter flash intensity for which the PhNR trough was prominently displayed. We did not use one fixed time for amplitude measurements for all test intensities as the timing of the PhNR trough could vary as a function of not just the flash intensity but also with age [4]. Adopting the same strategy, the b-wave amplitudes were also measured at its peak from the preceding a-wave trough or baseline if the a-wave was not present.
BT, PT and b-wave amplitudes were then plotted as a function of flash intensity and a generalized Naka-Ruston equation [38, 39] of the form V(I) = (Vmax*In)/(In+Kn) was used to fit the intensity response data. In this function, I is the stimulus intensity, V is the amplitude at intensity I, Vmax is the saturated amplitude, K is the semisaturation constant or the intensity at which the amplitude is half of Vmax and n is the slope. Curve fitting was performed by minimizing the sum of the squared differences between the observed and predicted data and without constraining the slope to 1. SigmaPlot version 10 (Systat Software, Inc., San Jose, CA) was used for curve fitting and all subsequent statistical analysis.
The relative reliability of test-retest differences of the Naka-Rushton fit parameters of BT, PT and b-wave intensity response data as well as Vmax of BT/Vmax of b-wave was assessed with the Wilcoxon signed rank test at p<0.05 with correction for multiple comparisons using the Holm’s method [56]. The Shapiro-Wilk normality test was used to assess whether the Vmax values of BT, PT and b-wave were normally distributed. A simple linear regression analysis was used to study the effect of age of the different fit parameters, again with Holm’s correction for multiple comparisons. Bland-Altman analysis [57] was performed to assess the absolute test-retest reliability of the Naka-Rushton fit parameters for BT, PT and b-wave intensity response data. The mean test-retest difference and the Coefficient of Reliability (COR or 1.96 times the standard deviation of the test-retest difference) was calculated for each fit parameter for BT, PT and b-wave intensity response data. The limits of agreement (LOA) were calculated as the mean ± COR. Further, COR was normalized to the mean test and retest values for each parameter to compute the %COR that would allow comparison of COR across parameters of the BT, PT and b-wave ERG measures.
RESULTS
Figure 1A illustrates an example of the photopic ERG waveforms recorded to increasing flash intensities averaged from recordings on two separate occasions from a 63 year old female subject and Figures 1B, C and D illustrate the Naka-Rushton fits to the PhNR intensity response data for amplitude measurements from baseline (BT), from preceding b-wave peak (PT) and the b-wave itself. The amplitudes of BT, PT and b-wave all grew with flash intensity to reach saturation between 0.2 and 0.8 phot cd.s/m2 respectively. Beyond 1.6 phot cd.s/m2, as indicated for this subject in Figures 1B, C and D, the amplitudes of all three measures showed a tendency to reduce with further increase in flash intensity. Thus, the Naka-Rushton fits to all data were restricted to the flash intensity range of 0.000625 – 1.6 phot cd.s/m2 and within this intensity range the Naka-Rushton fits provided a reasonable description of the intensity response data of all three ERG measures. Table 1 shows the mean (± standard deviation) as well as the median (with low and high 95% confidence limits) of saturated amplitude (Vmax), semisaturation constant (K) and slope (n) parameters derived from the Naka-Rushton fits to the intensity responses of BT, PT and b-wave from all the subjects. On average, the values of Vmax were smallest for BT and a paired student’s t-test with Holm’s correction for multiple comparisons indicated that the difference between Vmax of BT and PT on the one hand and between BT and the b-wave on the other were highly significant (p<0.001), but the Vmax of PT and b-wave were not significantly different. The slope parameter n was smallest for BT and largest for b-wave and the difference between the slopes of all three measures were statistically significant (p<0.001). Small differences observed in the mean values of K for the different measures did not reach statistical significance.
Figure 1.

(Fig. A) Example of photopic ERG waveforms to increasing test flash intensities from a 63 year old female subject with illustrations of the a-wave, b-wave and PhNR measured from baseline (BT) and preceding b-wave peak (PT). (Figs. B, C, D) Naka-Rushton fits to BT, PT and b-wave intensity response data with corresponding values of saturated amplitude (Vmax), semisaturation constant (K) and slope (n) parameters
Table 1.
Mean and standard deviations (sd) as well as median and low and high 95% confidence limits of saturated amplitude (Vmax) in microvolts (μV), semisaturation constant (K) in phot cd.s/m2 and slope (n) of PhNR intensity response functions for measurements from baseline to trough (BT), b-wave peak to trough (PT) and b-wave intensity response function of all subjects along with the statistical significance of the differences between measurements for each parameter determined by Student t-test with Holm’s correction (HC) for multiple testing
| Naka-Rushton parameters | Vmax | K | n |
|---|---|---|---|
| Mean ± sd | |||
| BT | 27.7±5.11 | 0.2±0.13 | 0.99± 0.27 |
| PT | 77.91±18.5 | 0.197±0.1 | 1.35±0.32 |
| b-wave | 78.97±22.7 | .20±0.06 | 1.86±0.47 |
| Median (low/high_95%CI) | |||
| BT | 27.74 (25.7/29.93) | 0.19 (0.15/0.22) | 0.92 (0.87/0.97) |
| PT | 73.49 (69.24/83.42) | 0.17 (0.16/0.21) | 1.37 (1.26/1.45) |
| b-wave | 73.5 (65.01/80.91) | 0.2 (0.17/0.23) | 2.04 (1.78/2.11) |
| Student t-test p value (with HC) | |||
| BT vs PT | <0.001 | 1 | <0.001 |
| BT vs b-wave | <0.001 | 1 | <0.001 |
| PT vs b-wave | 1 | 1 | <0.001 |
Linear dependence of Naka-Rushton fit parameters of the BT, PT and b-wave intensity response functions on age was assessed by computing Pearson correlation coefficients. Figures 2 A–C illustrate the effect of age on the fit parameters for BT, Figures 2 D–F for PT and Figures 2 G–I for b-wave intensity response data. The correlation between age and Naka-Rushton fit parameters was quite low (r2 between 0.04 and 0.14) and was not statistically significant (p≥0.09) after application of Holm’s correction for multiple comparisons.
Figure 2.
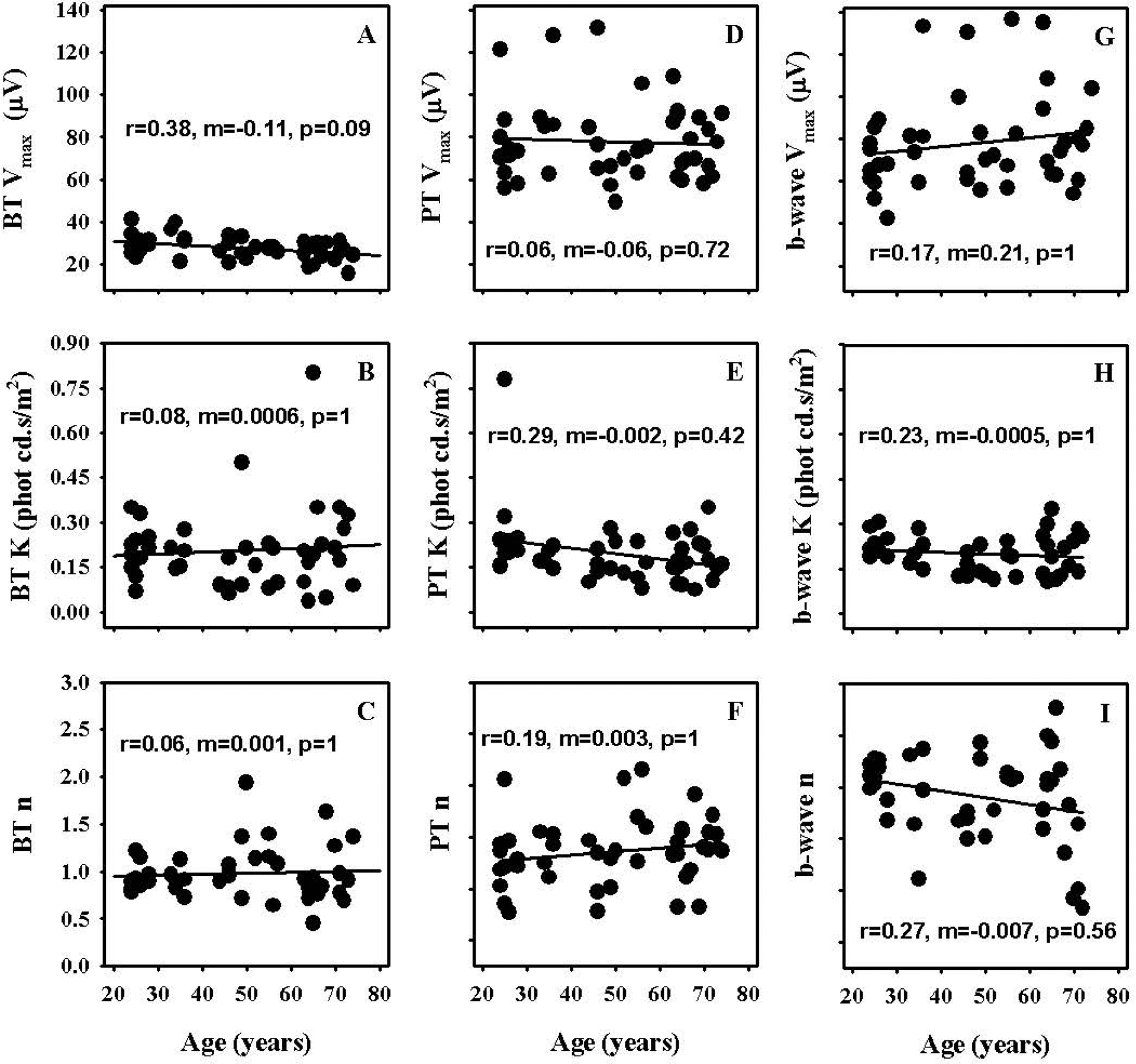
Effect of age on saturated amplitude (Vmax), semisaturation constant (K) and slope (n) parameters of Naka-Rushton fits to intensity response data of PhNR measured from baseline (BT – Figs. 2A, B, C) and preceding b-wave peak (PT – Figs. 2D, E, F) as well as b-wave (Figs. 2G, H, I)
Figure 3A which illustrates a comparison of the ERG waveforms to increasing flash intensities recorded on two different days for the subject whose average waveforms were shown in Figure 1A, captures the general finding that the ERG waveforms were quite repeatable from day to day, with a greater variability observed for the PhNR portion of the waveform. Figures 3B, C and D illustrate the Naka-Rushton fits to the intensity response data for BT, PT and b-wave respectively for the recordings from the two different days for the same subject. These figures again capture the general finding that the intensity response functions were more variable for the BT relative to PT and b-wave measurements.
Figure 3.

(Fig. A) Example of photopic ERG waveforms to increasing test flash intensities measured on two separate days from the same subject whose averaged waveforms from the two days are illustrated in Fig. 1A. (Figs. 3B, C and D) Naka-Rushton fits to BT, PT and b-wave intensity response data from the two days with corresponding values of saturated amplitude (Vmax), semisaturation constant (K) and slope (n) parameters
The mean values and standard deviations of the test and retest measurements of Vmax, K and n for BT, PT and b-wave are shown in Table 2. The test-retest values were not statistically significant for any of the parameters as determined by Wilcoxon signed rank test with correction for multiple comparisons.
Table 2.
Mean test and retest values and standard deviations (sd) of saturated amplitude (Vmax) in microvolts (μV), semisaturation constant (K) in phot cd.s/m2 and slope (n) of PhNR intensity response functions for measurements from baseline to trough (BT), b-wave peak to trough (PT) and b-wave intensity response functions along with the statistical significance of the differences between test and retest amplitudes determined by Wilcoxon match-pair signed rank test with Holm’s correction (HC) for multiple testing
| Naka-Rushton parameters | Vmax | K | n |
|---|---|---|---|
| BT | |||
| Mean test ± sd | 27±5 | 0.18±0.08 | 0.99±0.26 |
| Mean retest ± sd | 27±8 | 0.17±0.15 | 0.95±0.46 |
| p value (with HC) | 1 | 1 | 1 |
| PT | |||
| Mean test ± sd | 77±18 | 0.2±0.13 | 1.38±0.3 |
| Mean retest ± sd | 77±19 | 0.19±0.09 | 1.44±0.38 |
| p value (with HC) | 1 | 1 | 1 |
| b-wave | |||
| Mean test ± sd | 78±23 | 0.19±0.06 | 2.05±0.34 |
| Mean retest ± sd | 78±25 | 0.2±0.06 | 1.93±0.4 |
| p value (with HC) | 0.95 | 0.64 | 0.29 |
Figures 4A–I depict scatter plots of the test and retest amplitudes of the Naka-Rushton fit parameters for BT, PT and b-wave intensity response functions to provide an overview of how test and retest values of the fit parameters relate to each other across the overall range of values for each parameter. The dashed line indicates the one to one correspondence line. In general, for all parameters the data were almost equally distributed on either side of the dashed line but with a wide spread. The only exception was the slope parameter n of PT where the retest data on average appeared to be greater than the test values.
Figure 4.

Scatter plots of test and retest values of saturated amplitude (Vmax), semisaturation constant (K) and slope (n) parameters of Naka-Rushton fits to intensity response data of PhNR measured from baseline (BT – Figs. 4A, B, C) and preceding b-wave peak (PT – Figs. 4D, E, F) as well as b-wave (Figs. 4G, H, I). The dashed line indicates the one to one correspondence line
Figures 5A–I show the Bland-Altman plots for the different Naka-Rushton fit parameters for BT, PT and b-wave intensity response data where the difference between the test and retest values are plotted against the mean of the two tests. The dashed line in each plot indicates the mean test and retest difference and the solid lines indicate the limits of agreement (LOA) defined as mean ± 1.96 times the standard deviation of the test and retest differences. The mean test and retest differences of all parameters were close to zero. As indicated in Table 3, the mean test and retest values of all parameters were within ±6% of the mean value of all of their respective test and retest measures indicating minimal bias in the test and retest differences. Also, as indicated in Table 3, the coefficient of reliability (COR) defined as the 95% confidence interval (CI) or 1.96 times the standard deviation of the test retest differences for Vmax was slightly larger for PT and b-wave relative to BT in absolute terms (μV). However, when the COR was normalized to the mean value of the test and retest measures for each measurement technique (%COR), the value was larger for BT compared to both PT and b-wave. For K and n, the COR and %COR were largest for BT and smallest for b-wave.
Figure 5.
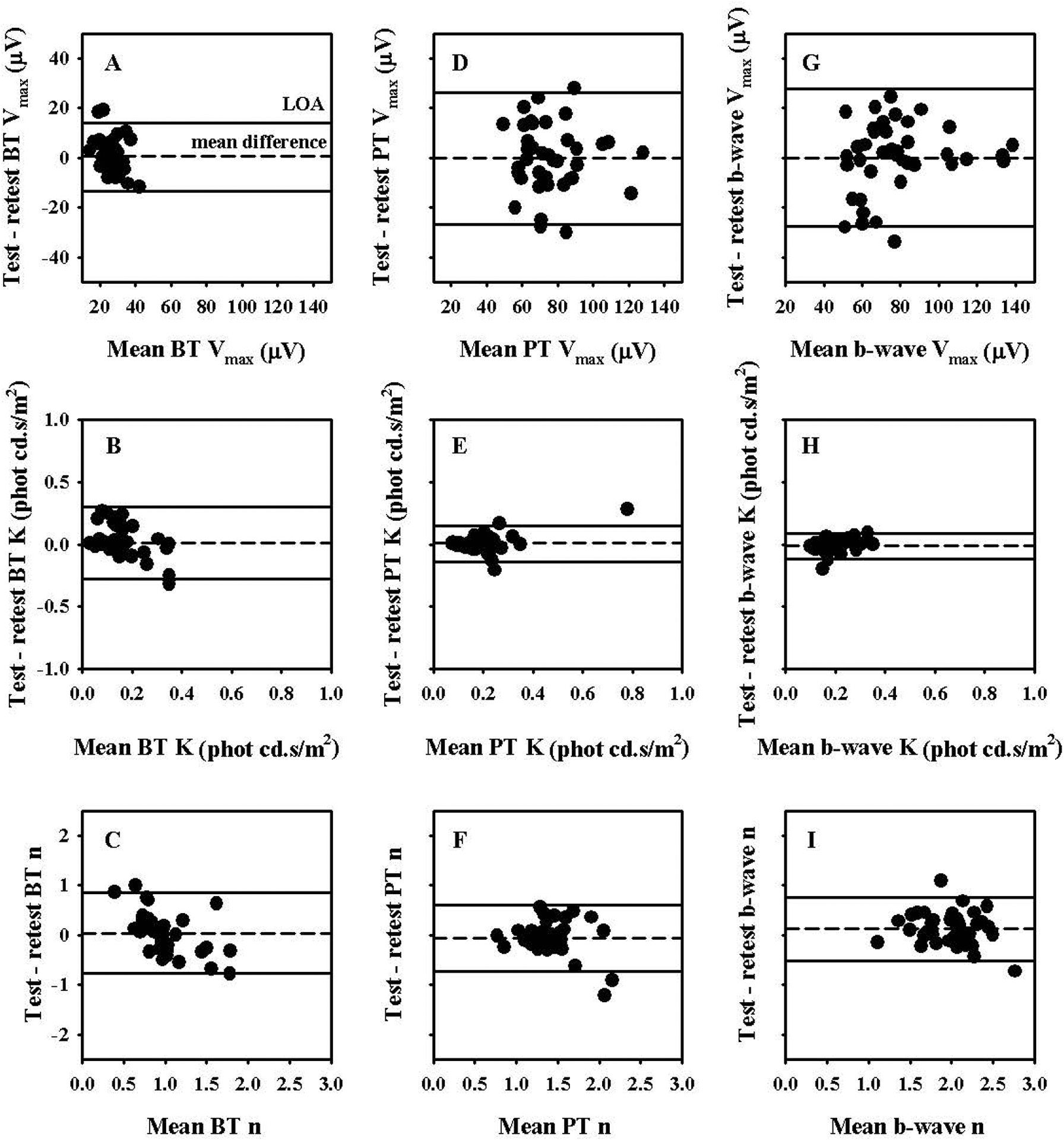
Bland Altman plots of test and retest measures of saturated amplitude (Vmax), semisaturation constant (K) and slope (n) parameters of Naka-Rushton fits to intensity response data of PhNR measured from baseline (BT – Figs. 5A, B, C) and preceding b-wave peak (PT – Figs. 5D, E, F) as well as b-wave (Figs. 5G, H, I). The dashed line represents the mean test-retest amplitude difference and the solid lines represent the 95% Limits of Agreement (LOA)
Table 3.
Mean difference (Mean diff) between test and retest measures, mean difference normalized to the mean of all test and retest values, Coefficient of Reliability (COR: 1.96 times the standard deviation of the mean test-retest difference) and COR normalized to the mean of all test and retest values (%COR) of saturated amplitude (Vmax) in microvolts (μV), semisaturation constant (K) in phot cd.s/m2 and slope (n) of PhNR intensity response functions for measurements from baseline to trough (BT), b-wave peak to trough (PT) and b-wave intensity response function
| Naka-Rushton parameters | Vmax | K | n |
|---|---|---|---|
| BT | |||
| Mean diff | 0.6 | −0.01 | −0.04 |
| Normalized mean diff | 2% | −6% | −4% |
| COR | 14 | 0.29 | 0.8 |
| %COR | 52% | 161% | 82% |
| PT | |||
| Mean diff | −0.25 | 0.003 | −0.06 |
| Normalized mean diff | −0.3% | 2% | −4% |
| COR | 26 | 0.15 | 0.67 |
| %COR | 34% | 75% | 48% |
| b-wave | |||
| Mean diff | −0.1 | −0.01 | 0.12 |
| Normalized mean diff | <−1% | −5% | 6% |
| COR | 28 | 0.1 | 0.64 |
| %COR | 36% | 50% | 32% |
Figures 6A–I illustrate the absolute value of the test and retest differences of different Naka-Rushton fit parameters of BT, PT and b-wave intensity response function plotted as a function of the subject’s age. The absolute differences of none of the parameters for any of the three ERG measures were significantly altered as a function age or the magnitude of the mean test and retest measures (results not shown for the latter)
Figure 6.
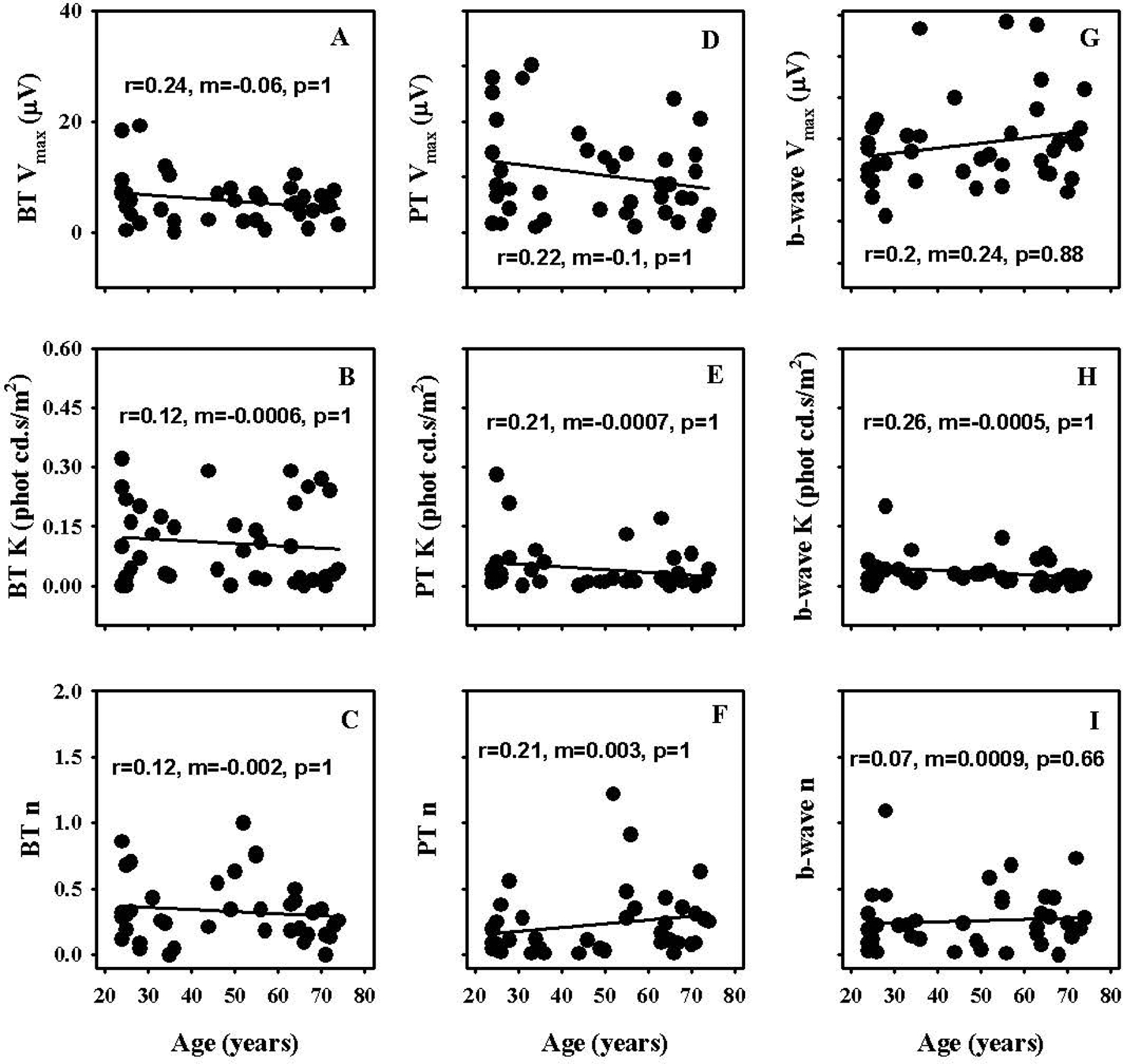
Effect of age on absolute values of test and retest differences of saturated amplitude (Vmax), semisaturation constant (K) and slope (n) parameters of Naka-Rushton fits to intensity response data of PhNR measured from baseline (BT – Figs. 6A, B, C) and preceding b-wave peak (PT – Figs. 6D, E, F) as well as b-wave (Figs. 6G, H, I)
Figure 7A shows the effect of age on the Vmax of BT expressed as a ratio of the Vmax of b-wave. The BT Vmax normalized in this fashion demonstrated reduction with age (m=−0.003, p=0.0032). The mean ± standard deviation of these ratios was 0.37 ± 0.1 and the median and upper and lower limits of the 95% confidence intervals were 0.36, 0.31 and 0.43 respectively. The mean of the test and retest values of these ratios (± standard deviation) were 0.37 (±0.12) and 0.36 (±0.13) respectively and the test and retest values were not significantly different as determined by Wilcoxon signed rank test. Figure 7B shows the Bland-Altman plot for the BT Vmax normalized to the b-wave Vmax. The mean of the test and retest differences of this measure was close to zero (0.008) and approximately 2% of the mean of all test and retest values. The COR and %COR values of the ratios were 0.9 and 78% respectively. Figures 7C and D show the absolute values of the test and retest differences of the ratios plotted as a function of the mean of the test and retest values of the ratios and age respectively. The absolute values of the test and retest differences of the ratios demonstrated significant increase (m=0.55, p<0.0001) with increase in the magnitude of the mean values of the test and retest measures but not with age.
Figure 7. (A).
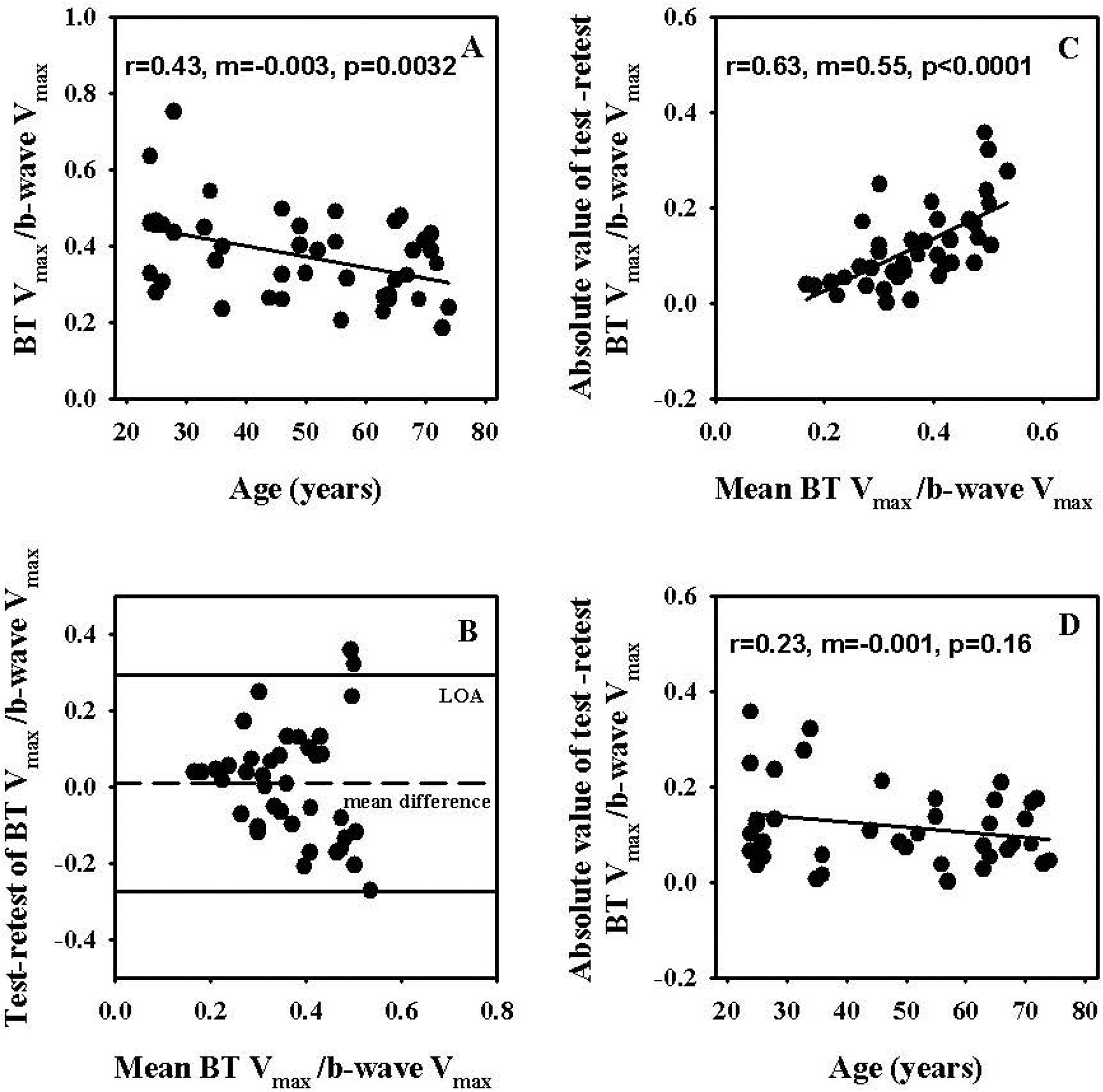
Effect of age on saturated amplitude (Vmax) of PhNR measured from baseline (BT) normalized to the Vmax of b-wave. (B) Bland-Altman plots of test and retest measures of saturated amplitude (Vmax) of PhNR measured from baseline (BT) normalized to the Vmax of b-wave. The dashed line represents the mean test-retest amplitude difference and the solid lines represent the 95% Limits of Agreement (LOA). (C) Effect of the means of test and retest measures of saturated amplitude (Vmax) of PhNR measured from baseline (BT) normalized to the Vmax of b-wave on absolute values of test and retest differences of these measures. (D) Effect of age on absolute values of test and retest differences of saturated amplitude (Vmax) of PhNR measured from baseline (BT) normalized to the Vmax of b-wave.
DISCUSSION
In the present study we recorded photopic flash ERG responses to test flashes in the intensity range of 0.000625 – 6.4 phot cd.s/m2. However, data only in the range of 0.000625 – 1.6 phot cd.s/m2 was used for the Naka-Rushton fit as BT, PT and b-waves in most subjects showed amplitude reductions at 3.2 and 6.4 phot cd.s/m2. The b-wave amplitude reduction at the higher flash intensities is well documented as the photopic hill phenomenon [e.g., 58, 59]. Our finding that similar reductions are observed with the PhNR for red flash at intensities greater than 1.6 phot cd.s/m2 on a 7 cd/m2 blue background reiterates the findings from other groups for recordings under similar stimulus conditions [26, 27, 37]. Ueno and co-workers [60] demonstrated that the photopic hill of the non-human primate ERG b-wave was preserved after elimination of inner-retinal responses in the ERG including the PhNR, and based on this and additional findings they concluded that the photopic hill phenomenon associated with the b-wave is likely due to a reduction of the ON-pathway response and a delay of the positive potential generated by the off pathway before the level of the inner retina (the term inner retina used here refers to retinal ganglion and amacrine cells). Given their findings, it is quite likely that the photopic hill like phenomena of the PhNR simply reflects the phenomena observed at the level of the b-wave and not necessarily induced at the level of the inner retina. Nevertheless, the fact that the photopic hill like phenomenon is seen at the level of the PhNR highlights the importance of restricting the flash intensities around 1.6 phot cd.s/m2 so that the PhNR amplitude can be maximized when recordings under these stimulus conditions.
Our finding that the Naka-Rushton function provides a reasonable fit to the intensity response data for both BT and PT amplitude measurement techniques is in agreement with previous findings of Binns and co-workers [37] who first employed this function to fit the BT and PT responses and with those of Wang and colleagues [26] as well as Kremers et al., [27] who measured BT amplitudes. While the absolute values of Vmax for PhNR and b-wave obtained with DTL electrodes were comparable between our study and that of Binns and co-workers, the amplitudes were generally larger in the recordings by Kremers et al. However, when the reported average values of Vmax of BT was considered as a ratio of the reported average Vmax of b-wave, the values were in good agreement between our study and those of Binns and co-workers as well as Kremers et al., which were all in the range of 31–35%. Wang and colleagues did not report the Vmax of the b-wave and thus we could not compute such ratios from their work. Our finding that the magnitudes of Vmax for PT and b-wave are comparable is also in agreement with Binns and co-workers. Also, the average K values for the BT, PT and b-wave were similar between our study (0.2, 0.2 and 0.2 phot cd.s/m2) and that of Binns and co-workers (0.2, 0.26 and 0.26 phot cd.s/m2). However, while we found that the average value for n increased from BT to PT to b-wave (0.99, 1.35 and 1.86 respectively) and that these differences were statistically significant, the values were quite similar for the three amplitude measurements in the study by Binns and co-workers (1.6, 1.9 and 1.7). These differences in the values for n could have been due to the wider range of test flashes (0.00625 – 1.6 vs 0.05 – 3.38 phot cd.s/m2) and the greater number of intensities sampled (9 vs 6) in our study, especially in the lower intensity range. The values for n and K of BT were comparable between our study and that of Wang and colleagues and not reported by Kremers et al., for the subjects whose responses were recorded with DTL fiber electrodes.
We found that none of the Naka-Rushton fit parameters were significantly affected by age for PT, BT or b-wave measurements after applying Holm’s correction for multiple comparisons. It may be noteworthy to mention here that Vmax for BT measurements alone demonstrated a weak but statistically significant change with age (r=0.38, m=−0.11, p=0.01) prior to Holm’s correction. Interestingly, we also found that the Vmax of BT when normalized to the Vmax of b-wave showed a statistically significant decline with increasing age. These findings taken together possibly suggest different rates of decline of the Vmax of BT and b-wave with increasing age and that normalization to an internal control, perhaps by reducing inter-subject variability, can better highlight the effect of age on the BT measure of PhNR. In our analysis of the PhNR amplitude data we used linear values. It has been reported that ERG measures like b-wave amplitudes are not normally distributed [61, 62] and consequently logarithmic values of amplitude measures might be appropriate to normalize the distribution in order to allow parametric statistics [62]. A Shapiro-Wilk normality test indicated that the PhNR Vmax of BT measures were normally distributed (W = 0.98601, p-value = 0.8715), whereas the Vmax of the b-wave (W = 0.87116, p-value = 0.0001864) and PT (W = 0.90029, p-value = 0.0009675) that is dependent on the b-wave were not. Thus, we re-examined the effect of age on the Vmax of PT and b-wave after converting them to logarithmic values, but still the measures did not show any significant change with age. Previous studies that used Naka-Rushton function to fit the PhNR [26, 27, 37] have not examined the effect of age and studies that examined the effect of age on BT and/or PT amplitudes for fixed high intensity test flashes have reported either no significant correlation with age [4, 11, 63] or a very weak correlation [7]. Since we found a weak effect of age on Vmax of BT before correction for multiple comparisons and as the Vmax of BT normalized to the Vmax of b-wave demonstrated significant reduction with age, and also because some studies have found a trend for single flash amplitudes to reduce with age, we would recommend erring on the side of caution and using appropriate age-matched control data to make comparisons while analyzing PhNR data from clinical cohorts.
Since previous studies have reported significant age effects on the photopic b-wave amplitude [62, 64], we were surprised on the finding in our study that the Vmax of the b-wave did not show statistically significant changes with age. This could be partly because we were estimating the saturated amplitudes of the photopic b-waves while the earlier studies measured amplitudes at a fixed flash intensity, most likely in the linear portion of the intensity response curve. However, we did find a slight tendency for reduction in K and n with increase in age, but that effect did not reach statistical significance. Differences in background intensity as well as the spectral characteristics of test flash and background stimuli could have contributed to the disparate findings between our study and previous ones. Based on the appearance of our data we used a linear fit similar to Weleber [64] while Birch and Anderson [62] used an exponential fit that best described their data. The higher limit of the age range of the subjects was similar for the three studies and is less likely to have contributed to the differences observed.
Wilcoxon signed rank test with correction for multiple testing indicated that the mean test and retest values of the Naka-Rushton function fit parameters of BT, PT and b-wave intensity response data were not significantly different and the Bland-Altman analysis indicated that the average test-retest difference was close to zero (within 6% of the mean of the test and retest values) for all parameters and ERG components, thereby indicating good test-retest reliability. Furthermore, the %COR values (percent Coefficient of Reliability or in other words 1.96 times the standard deviation of the test-retest difference of each fit parameter normalized to the mean of all test and retest values for that parameter) were smaller for PT than BT measurements, suggesting that the test retest reliability of the fit parameters for PT measurements were superior to the reliability of the fit parameters for BT measurements. Binns and co-workers [37] have previously shown similar results for test-retest reliability of Naka-Rushton fit parameters for BT and PT and similar results have been reported by others for PhNR amplitude analysis at fixed flash intensities [63, 65].
In terms of its clinical applicability, a lower test retest variability of the PT parameters could be taken to mean that a smaller proportional change may be needed with PT than BT parameters in order to obtain clinically significant changes. For example, according to Table 3, to observe a clinically meaningful change in Vmax, a 52% reduction is needed for BT, whereas only a 34% reduction is needed for PT. However, this argument that a smaller proportional change in PT is required of a meaningful clinical effect may be misleading for the following reason. The PT amplitude measure of the PhNR already includes the BT amplitude measure and it is only the BT component of the PT measure that is likely to be compromised in a disease like glaucoma. The remaining portion of the PT measure is a reflection of the ERG b-wave that originates from bipolar cell activity and is relatively uncompromised in glaucoma. Based on the average values of Vmax that appear in Table 2, the BT amplitude value is approximately 35% of the normal PT amplitude value. Thus, to see a clinically meaningful change in PT (34% as per Table 3) in a disease like glaucoma, nearly a 100% reduction in BT amplitude should occur. However, also according to Table 3, only a 52% change is required in the BT amplitude for a significant clinical effect with this measure. Thus, based on the latter argument one would actually expect the BT amplitude measure of PhNR to be more sensitive than the PT measure for detecting glaucomatous neural damage.
Earlier studies that examined PhNR amplitudes at fixed high intensity flashes have reported that the test retest repeatability of BT/PT amplitude ratios is better than that of BT amplitudes alone in control subjects [63, 65, 66]. It has also been shown that BT/b-wave measurements demonstrate a greater sensitivity in the detection of glaucomatous damage compared to BT measurements alone [67] and that its sensitivity to detect glaucomatous damage improves with increasing disease severity and magnitude of the b-wave amplitude [68]. Normalization of BT to b-wave for detection of glaucomatous damage makes sense intuitively as the b-wave which is generated by bipolar cell activity, in theory should not be altered in a disease like glaucoma where there is selective damage of retinal ganglion cells. Based on our findings that the ratio of Vmax of BT and b-wave is age dependent, we suggest that appropriate age norms be used if such ratios are computed in disease populations along with the caveat that the test retest reliability of this ratio measure in normal subjects is worse than that of Vmax of BT, PT or b-wave alone. In our study we did not assess the effect of age and the test retest reliability of Vmax of BT normalized to the Vmax of PT, as both BT and PT are likely to be affected in disease like glaucoma and consequently computing the ratios of their Vmax may not be very useful.
Compliance with ethical standards
Acknowledgements
The authors would like to acknowledge the assistance of Dr. Usha Govindarajulu for assistance with some of the statistical analysis included in this study.
Funding:
National Institutes of Health provided financial support in the form of an institutional training grant (National Eye Institute grant T35 EY020481) to State University of New York College of Optometry. The sponsor had no role in the design or conduct of this research.
Footnotes
Ethical approval:
“All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.”
Informed consent:
“Informed consent was obtained from all individual participants included in the study.”
Conflict of Interest:
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
REFERENCES
- 1.Bush RA, Sieving PA. (1994) A proximal retinal component in the primate photopic ERG a-wave. Invest Ophthalmol Vis Sci 35:635–645 [PubMed] [Google Scholar]
- 2.Sieving PA, Murayama K, Naarendorp F. (1994) Push-pull model of the primate photopic electroretinogram: a role for hyperpolarizing neurons in shaping the b-wave. Vis Neurosci 11:519–532 [DOI] [PubMed] [Google Scholar]
- 3.Viswanathan S, Frishman LJ, Robson JG, Harwerth RS, Smith EL 3rd. (1999) The photopic negative response of the macaque electroretinogram: reduction by experimental glaucoma. Invest Ophthalmol Vis Sci 40:1124–1136 [PubMed] [Google Scholar]
- 4.Viswanathan S, Frishman LJ, Robson JG, Walters JW. (2001) The photopic negative response of the flash electroretinogram in primary open angle glaucoma. Invest Ophthalmol Vis Sci 42:514–522 [PubMed] [Google Scholar]
- 5.Drasdo N, Aldebasi YH, Chiti Z, Mortlock KE, Morgan JE, North RV. (2001) The s-cone PHNR and pattern ERG in primary open angle glaucoma. Invest Ophthalmol Vis Sci 42:1266–1272 [PubMed] [Google Scholar]
- 6.Gotoh Y, Machida S, Tazawa Y. (2004) Selective loss of the photopic negative response in patients with optic nerve atrophy. Arch Ophthalmol. 122:341–346 [DOI] [PubMed] [Google Scholar]
- 7.Rangaswamy NV, Frishman LJ, Dorotheo EU, Schiffman JS, Bahrani HM, Tang RA. (2004) Photopic ERGs in patients with optic neuropathies: comparison with primate ERGs after pharmacologic blockade of inner retina. Invest Ophthalmol Vis Sci 45:3827–3837 [DOI] [PubMed] [Google Scholar]
- 8.Machida S, Gotoh Y, Tanaka M, Tazawa Y. (2004) Predominant loss of the photopic negative response in central retinal artery occlusion. Am J Ophthalmol 137:938–940 [DOI] [PubMed] [Google Scholar]
- 9.Ueno S, Kondo M, Piao CH, Ikenoya K, Miyake Y, Terasaki H.(2006) Selective amplitude reduction of the PhNR after macular hole surgery: ganglion cell damage related to ICG-assisted ILM peeling and gas tamponade. Invest Ophthalmol Vis Sci 47:3545–3549 [DOI] [PubMed] [Google Scholar]
- 10.Kizawa J, Machida S, Kobayashi T, Gotoh Y, Kurosaka D. Changes of oscillatory potentials and photopic negative response in patients with early diabetic retinopathy. (2006) Jpn J Ophthalmol. 50:367–373 [DOI] [PubMed] [Google Scholar]; Chen H, Wu D, Huang S, Yan H. (2006) The photopic negative response of the flash electroretinogram in retinal vein occlusion. Doc Ophthalmol 113:53–59 [DOI] [PubMed] [Google Scholar]
- 11.Miyata K, Nakamura M, Kondo M, Lin J, Ueno S, Miyake Y, Terasaki H. (2007) Reduction of oscillatory potentials and photopic negative response in patients with autosomal dominant optic atrophy with OPA1 mutations. Invest Ophthalmol Vis Sci 48:820–824 [DOI] [PubMed] [Google Scholar]
- 12.Chen H, Zhang M, Huang S, Wu D (2008) The photopic negative response of flash ERG in nonproliferative diabetic retinopathy. Doc Ophthalmol 117:129–135 [DOI] [PubMed] [Google Scholar]
- 13.Shinoda K, Yamada K, Matsumoto CS, Kimoto K, Nakatsuka K. (2008) Changes in retinal thickness are correlated with alterations of electroretinogram in eyes with central retinal artery occlusion. Graefes Arch Clin Exp Ophthalmol 246:949–954 [DOI] [PubMed] [Google Scholar]
- 14.Machida S, Gotoh Y, Toba Y, Ohtaki A, Kaneko M, Kurosaka D. (2008) Correlation between photopic negative response and retinal nerve fiber layer thickness and optic disc topography in glaucomatous eyes. Invest Ophthalmol Vis Sci 49:2201–2207 [DOI] [PubMed] [Google Scholar]
- 15.Sustar M, Cvenkel B, Brecelj J. (2009) The effect of broadband and monochromatic stimuli on the photopic negative response of the electroretinogram in normal subjects and in open-angle glaucoma patients. Doc Ophthalmol. 118:1671–1677 [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto CS, Shinoda K, Yamada K, Nakatsuka K. (2009) Photopic negative response reflects severity of ocular circulatory damage after central retinal artery occlusion. Ophthalmologica 223:362–369 [DOI] [PubMed] [Google Scholar]
- 17.North RV, Jones AL, Drasdo N, Wild JM, Morgan JE. (2010) Electrophysiological evidence of early functional damage in glaucoma and ocular hypertension. Invest Ophthalmol Vis Sci 51:1216–1222 [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto CS, Shinoda K, Nakatsuka K. (2011) High correlation of scotopic and photopic electroretinogram components with severity of central retinal artery occlusion. Clin Ophthalmol 5:115–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson DA, Feather S, Stanescu HC, Freudenthal B, Zdebik AA, Warth R, Ognjanovic M, Hulton SA, Wassmer E, van’t Hoff W, Russell-Eggitt I, Dobbie A, Sheridan E, Kleta R, Bockenhauer D. (2011) Altered electroretinograms in patients with KCNJ10 mutations and EAST syndrome. J Physiol 589:1681–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horn FK, Gottschalk K, Mardin CY, Pangeni G, Jünemann AG, Kremers J. (2011) On and off responses of the photopic fullfield ERG in normal subjects and glaucoma patients. Doc Ophthalmol 122:53–62 [DOI] [PubMed] [Google Scholar]
- 21.Machida S, Tamada K, Oikawa T, Gotoh Y, Nishimura T, Kaneko M, Kurosaka D. (2011) Comparison of photopic negative response of full-field and focal electroretinograms in detecting glaucomatous eyes. J Ophthalmol 2011. Pii: 564131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moon CH, Hwang SC, Kim BT, Ohn YH, Park TK. (2011) Visual prognostic value of optical coherence tomography and photopic negative response in chiasmal compression. Invest Ophthalmol Vis Sci 52:8527–8533 [DOI] [PubMed] [Google Scholar]
- 23.Gowrisankaran S, Anastasakis A, Fishman GA, Alexander KR. (2011) Structural and functional measures of inner retinal integrity following visual acuity improvement in a patient with hereditary motor and sensory neuropathy type VI. Ophthalmic Genet 32:188–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McFarlane M, Wright T, Stephens D, Nilsson J, Westall CA. (2012) Blue flash ERG PhNR changes associated with poor long-term glycemic control in adolescents with type 1 diabetes. Invest Ophthalmol Vis Sci 53:741–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pangeni G, Lämmer R, Tornow RP, Horn FK, Kremers J. (2012) On- and off-response ERGs elicited by sawtooth stimuli in normal subjects and glaucoma patients. Doc Ophthalmol 124:237–248 [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Cheng H, Hu YS, Tang RA, Frishman LJ. (2012) The photopic negative response of the flash electroretinogram in multiple sclerosis. Invest Ophthalmol Vis Sci 53:1315–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kremers J, Jertila M, Link B, Pangeni G, Horn FK. (2012) Spectral characteristics of the PhNR in the full-field flash electroretinogram of normals and glaucoma patients. Doc Ophthalmol 124:79–90 [DOI] [PubMed] [Google Scholar]
- 28.Niyadurupola N, Luu CD, Nguyen DQ, Geddes K, Tan GX, Wong CC, Tran T, Coote MA, Crowston JG. (2013) Intraocular pressure lowering is associated with an increase in the photopic negative response (PhNR) amplitude in glaucoma and ocular hypertensive eyes. Invest Ophthalmol Vis Sci 54:1913–1919 [DOI] [PubMed] [Google Scholar]
- 29.Preiser D, Lagrèze WA, Bach M, Poloschek CM. (2013) Photopic negative response versus pattern electroretinogram in early glaucoma. Invest Ophthalmol Vis Sci 54:1182–1191 [DOI] [PubMed] [Google Scholar]
- 30.Gardašević Topčić I, Šuštar M, Brecelj J, Hawlina M, Jaki Mekjavić P. (2014) Morphological and electrophysiological outcome in prospective intravitreal bevacizumab treatment of macular edema secondary to central retinal vein occlusion. Doc Ophthalmol 129:27–38 [DOI] [PubMed] [Google Scholar]
- 31.Machida S, Toba Y, Nishimura T, Ohzeki T, Murai K, Kurosaka D. (2014) Comparisons of cone electroretinograms after indocyanine green-, brilliant blue G-, or triamcinolone acetonide-assisted macular hole surgery. Graefes Arch Clin Exp Ophthalmol 252:1423–1433 [DOI] [PubMed] [Google Scholar]
- 32.Moss HE, Park JC, McAnany JJ. (2015) The Photopic Negative Response in Idiopathic Intracranial Hypertension. Invest Ophthalmol Vis Sci 56:3709–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abed E, Piccardi M, Rizzo D, Chiaretti A, Ambrosio L, Petroni S, Parrilla R, Dickmann A, Riccardi R, Falsini B. (2015) Functional Loss of the Inner Retina in Childhood Optic Gliomas Detected by Photopic Negative Response. Invest Ophthalmol Vis Sci 56:2469–2474 [DOI] [PubMed] [Google Scholar]
- 34.Noma H, Mimura T, Kuse M, Yasuda K, Shimura M. (2015) Photopic negative response in branch retinal vein occlusion with macular edema. Int Ophthalmol 35:19–26 [DOI] [PubMed] [Google Scholar]
- 35.Morny EK, Margrain TH, Binns AM, Votruba M. (2015) Electrophysiological ON and OFF Responses in Autosomal Dominant Optic Atrophy. Invest Ophthalmol Vis Sci 56:7629–7637 [DOI] [PubMed] [Google Scholar]
- 36.Kirkiewicz M, Lubiński W, Penkala K. (2016) Photopic negative response of full-field electroretinography in patients with different stages of glaucomatous optic neuropathy. Doc Ophthalmol. 132:57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Binns AM, Mortlock KE, North RV. (2011) The relationship between stimulus intensity and response amplitude for the photopic negative response of the flash electroretinogram. Doc Ophthalmol 122:39–52 [DOI] [PubMed] [Google Scholar]
- 38.Arden GB, Carter RM, Hogg CR, Powell DJ, Ernst WJ, Clover GM, Lyness AL, Quinlan MP. (1983) A modified ERG technique and the results obtained in X-linked retinitis pigmentosa. Br J Ophthalmol 67:419–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Massof RW, Wu L, Finkelstein D, Perry C, Starr SJ, Johnson MA.(1984) Properties of electroretinographic intensity-response functions in retinitis pigmentosa. Doc Ophthalmol 57:279–296 [DOI] [PubMed] [Google Scholar]
- 40.Birch DG, Herman WK, deFaller JM, Disbrow DT, Birch EE. (1987) The relationship between rod perimetric thresholds and full-field rod ERGs in retinitis pigmentosa. Invest Ophthalmol Vis Sci 28:954–965 [PubMed] [Google Scholar]
- 41.Johnson MA, Marcus S, Elman MJ, McPhee TJ. (1988) Neovascularization in central retinal vein occlusion: electroretinographic findings. Arch Ophthalmol 106:348–352. [DOI] [PubMed] [Google Scholar]
- 42.Peachey NS, Fishman GA, Derlacki DJ, Alexander KR. (1988) Rod and cone dysfunction in carriers of X-linked retinitis pigmentosa. Ophthalmology 95:677–685 [DOI] [PubMed] [Google Scholar]
- 43.Breton ME, Quinn GE, Keene SS, Dahmen JC, Brucker AJ. Electroretinogram parameters at presentation as predictors of rubeosis in central retinal vein occlusion patients. Ophthalmology 96:1343–1352 [DOI] [PubMed] [Google Scholar]
- 44.Birch DG, Anderson JL. (1990) Rod visual fields in cone-rod degeneration. Comparisons to retinitis pigmentosa. Invest Ophthalmol Vis Sci 31:2288–2299 [PubMed] [Google Scholar]
- 45.Breton ME, Montzka DP, Brucker AJ, Quinn GE. (1991) Electroretinogram interpretation in central retinal vein occlusion. Ophthalmology 98:1837–1844 [DOI] [PubMed] [Google Scholar]
- 46.Hood DC, Shady S, Birch DG. (1994) Understanding changes in the b-wave of the ERG caused by heterogeneous receptor damage. Invest Ophthalmol Vis Sci 35:2477–2488 [PubMed] [Google Scholar]
- 47.Velten IM, Horn FK, Korth M, Velten K. (2001) The b-wave of the dark adapted flash electroretinogram in patients with advanced asymmetrical glaucoma and normal subjects. Br J Ophthalmol. 85:403–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gabrieli CB, Regine F, Vingolo EM, Rispoli E, Fabbri A, Isidori A. Subjective visual halos after sildenafil (Viagra) administration: Electroretinographic evaluation. Ophthalmology. 108:877–81 [DOI] [PubMed] [Google Scholar]
- 49.Balacco Gabrieli C, Regine F, Vingolo EM, Rispoli E, Isidori A. Acute electroretinographic changes during sildenafil (Viagra) treatment for erectile dysfunction. Doc Ophthalmol. 107:111–114 [DOI] [PubMed] [Google Scholar]
- 50.Ziemssen F, Lüke M, Messias A, Beutel J, Tatar O, Zrenner E, Bartz-Schmidt KU; Tuebingen Bevacizumab Study Group. (2008) Safety monitoring in bevacizumab (Avastin) treatment: retinal function assessed by psychophysical (visual fields, colour vision) and electrophysiological (ERG/EOG) tests in two subgroups of patients. Int Ophthalmol 28:101–109 [DOI] [PubMed] [Google Scholar]
- 51.Stahl A, Feltgen N, Fuchs A, Bach M. (2009) Electrophysiological evaluation of retinal photoreceptor function after repeated bevacizumab injections. Doc Ophthalmol 118:81–88 [DOI] [PubMed] [Google Scholar]
- 52.Varghese SB, Reid JC, Hartmann EE, Keyser KT. (2011) The effects of nicotine on the human electroretinogram. Invest Ophthalmol Vis Sci 52:9445–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Constable PA, Gaigg SB, Bowler DM, Jägle H, Thompson DA. (2016) Full-field electroretinogram in autism spectrum disorder. Doc Ophthalmol 132:83–99 [DOI] [PubMed] [Google Scholar]
- 54.Naka KI, Rushton WA. (1966) S-potentials from colour units in the retina of fish (Cyprinidae). J Physiol 185:536–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dawson WW, Trick GL, Litzkow CA. (1979) Improved electrode for electroretinography. Invest Ophthalmol Vis Sci 18:988–991 [PubMed] [Google Scholar]
- 56.Holm S 1979. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics 6:65–70 [Google Scholar]
- 57.Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310 [PubMed] [Google Scholar]
- 58.Wali N, Leguire LE. (1992) The photopic hill: a new phenomenon of the light adapted electroretinogram. Doc Ophthalmol 80:335–345 [DOI] [PubMed] [Google Scholar]
- 59.Rufiange M, Dassa J, Dembinska O, Koenekoop RK, Little JM, Polomeno RC, Dumont M, Chemtob S, Lachapelle P. (2003) The photopic ERG luminance-response function (photopic hill): method of analysis and clinical application. Vision Res 43:1405–1412 [DOI] [PubMed] [Google Scholar]
- 60.Ueno S, Kondo M, Niwa Y, Terasaki H, Miyake Y. (2004) Luminance dependence of neural components that underlies the primate photopic electroretinogram. Invest Ophthalmol Vis Sci 45:1033–1040 [DOI] [PubMed] [Google Scholar]
- 61.McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, Bach M. ISCEV Standard for full-field clinical electroretinography (2015. update). Doc Ophthalmol 130:1–12 [DOI] [PubMed] [Google Scholar]
- 62.Birch DG, Anderson JL. (1992) Standardized Full-Field Electroretinography. Arch Ophthalmol 110:1571–1576. [DOI] [PubMed] [Google Scholar]
- 63.Tang J, Edwards T, Crowston JG, Sarossy M (2014) The Test-Retest Reliability of the Photopic Negative Response (PhNR). Transl Vis Sci Technol 3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weleber RG. (1981) The effect of age on human cone and rod ganzfeld electroretinograms. Invest Ophthalmol Vis Sci 20:392–399. [PubMed] [Google Scholar]
- 65.Mortlock KE, Binns AM, Aldebasi YH, North RV (2010) Inter-subject, inter-ocular and inter-session repeatability of the photopic negative response of the electroretinogram recorded using DTL and skin electrodes. Doc Ophthalmol 121:123–134 [DOI] [PubMed] [Google Scholar]
- 66.Kundra H, Park JC, McAnany JJ. (2016) Comparison of photopic negative response measurements in the time and time-frequency domains. Doc Ophthalmol 133:91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Preiser D, Lagrèze WA, Bach M, Poloschek CM. (2013) Photopic negative response versus pattern electroretinogram in early glaucoma. Invest Ophthalmol Vis Sci 54:1182–1191 [DOI] [PubMed] [Google Scholar]
- 68.Wu Z, Hadoux X, Fan Gaskin JC, Sarossy MG, Crowston JG (2016) Measuring the Photopic Negative Response: Viability of Skin Electrodes and Variability Across Disease Severities in Glaucoma. Transl Vis Sci Technol 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]


