Abstract
Gender identity is a core aspect of self-identity and is usually congruent with birth-assigned sex and own body sex-perception. The neuronal circuits underlying gender identity are unknown, but greater awareness of transgenderism has sparked interest in studying these circuits. We did this by comparing brain activation and connectivity in transgender individuals (for whom gender identity and birth-assigned sex are incongruent) with that in cisgender controls (for whom they are congruent) when performing a body self-identification task during functional magnetic resonance imaging. Thirty transgender and 30 cisgender participants viewed images of their own bodies and bodies morphed in sex toward or opposite to birth-assigned sex, rating each image to the degree they identified with it. While controls identified with images of themselves, transgender individuals identified with images morphed “opposite” to their birth-assigned sex. After covarying out the effect of self-similarity ratings, both groups activated similar self- and body-processing systems when viewing bodies that aligned with their gender identity rather than birth-assigned sex. Additionally, transgender participants had greater limbic involvement when viewing ambiguous, androgynous images of themselves morphed toward their gender identity. These results shed light on underlying self-processing networks specific to gender identity and uncover additional involvement of emotional processing in transgender individuals.
Keywords: body perception, functional magnetic resonance imaging, gender dysphoria, gender identity, gender incongruence, self-perception
Introduction
Gender identity is a person’s fundamental, inner sense of feeling male, female, or another gender, but how it is represented in the brain is not fully understood. In individuals whose gender identity aligns with their birth-assigned sex, that is, those who are “cisgender”, disentangling the neuronal circuits involved in gender identity from those involved in other aspects of self-identity, including own body recognition, is challenging. However, studying individuals whose gender identity does not align with their birth-assigned sex may provide valuable insights in this regard.
Gender dysphoria (GD) in the DSM-5 (American Psychiatric Association 2013), or gender incongruence in ICD-11 (World Health Organization, 1992), refers to a feeling of distress due to an incongruence between one’s experienced gender and their birth-assigned sex. Individuals with GD comprise a subset of those who identify as “transgender”. GD has been theorized to be a consequence of different cerebral sexual differentiation (Dörner 1988; Swaab and Hofman 1995), and many transgender persons report estrangement from, or at times even disgust with, their own bodies from an early age (Nieder et al. 2011). Since perception of one’s own body is tightly connected to perception of self and one’s gender, studying the neurobiology of transgender in relationship to cisgender groups when viewing own and others’ bodies may prove informative about the fundamental neurobiology of gender identity and may be advantageous over studying gender identity in either group alone.
The perception of one’s body is molded by a reciprocal interaction between one’s perception of physical appearance based on self-observation or the reactions of others (Cash and Pruzinsky 2004) and one’s body representation in the brain (Vocks et al. 2010). We recently published a series of studies suggesting that cortical thickness and both functional and structural connectivity in regions believed to process own-body perception differed between transgender and cisgender individuals, independent of sex and sexual orientation (Savic and Arver 2011, 2014; Feusner et al. 2016, 2017; Burke et al. 2017, 2018; Manzouri and Savic 2019). These self-referential regions include medial prefrontal cortical regions such as the dorsal and pregenual anterior cingulate cortex (dACC and pACC) (Northoff et al. 2006); the insular cortex (Craig 2010); the temporo-parietal junction at the confluence of the angular gyrus, supramarginal gyrus, and the posterior superior temporal gyrus (Blanke et al. 2005); and the extrastriate and fusiform body areas (EBA and FBA) (Vocks et al. 2010). The pACC draws particular interest for its role in self-conscious emotion (Sturm et al. 2013) and its functional connection with other regions such as the anterior insula in facilitating decisions about self-representation, making it an important component in a conceptual “self”-network (Murray et al. 2015).
Tentatively, differences in coordinated activation and connections between own-body and self-perception networks could explain the reported discomfort that individuals with GD feel with their own bodies. Own-body representation in the brains of individuals with GD may not incorporate the typical physical traits of their birth-assigned sex, as suggested by a report that transgender men with GD (who were assigned female at birth) showed diminished cerebral homunculus reactivity to the breast (Case et al. 2017). Alternatively, the representation may be opposite to one’s physical sex and unintegrated with the “self-network,” possibly leading to avoidance when viewing one’s own body—a core feature of GD (Cohen-Kettenis and Pfäfflin 2010; Bandini et al. 2013; Case et al. 2017). In the former example, these networks may be neuroanatomically and/or functionally different between groups. The latter example, on the other hand, includes the possibility that cisgender and transgender persons employ similar self- and own-body perception networks but that they are unintegrated with each other for transgender individuals. The whole concept of how own-body networks are related to gender identity among transgender and cisgender persons is novel and has not previously been tested.
We thus investigated whether and how the described differences in gender identity among treatment-naïve transgender individuals with GD and cisgender persons are related to the de facto neuronal processing underlying perception of the own body in relation to self. Undergoing functional magnetic resonance imaging (fMRI), participants viewed photographs of their own bodies incrementally morphed by different degrees to the images of other bodies whose sex was aligned or opposite to participants’ birth-assigned sex. Participants then had to assess the degree each image represented him/her. We hypothesized that transgender and cisgender individuals would activate “self-perception” networks (including the medial prefrontal cortex). We also anticipated they would demonstrate inter-regional connectivity involving the pACC, a self-network node, when the morphed image aligned closely with their identified gender. In keeping with this, we predicted less activity in these networks in the transgender compared to cisgender group when viewing their own unmorphed body as well as when viewing their body morphed toward bodies of the same birth-assigned sex (away from transgender individuals’ identified gender). Moreover, we anticipated stronger activation when participants view images over a longer rather than shorter time period, suggesting greater reflective (top-down) rather than reflexive (bottom-up) processing.
Materials and Methods
Participants
Transgender participants and cisgender controls were recruited by the Gender Team at analysis of variance (ANOVA), Center for Andrology, Sexual Medicine and Transgender Medicine at Karolinska University Hospital (Stockholm, Sweden), a center specializing in the evaluation and treatment of individuals with GD. All adults aged 18–45 who were diagnosed with Transsexualism based on ICD-10 diagnostic criteria (F64.0, World Health Organization, 1992), identified with the gender opposite to their birth-assigned sex, and sought gender affirming medical interventions at the center between January 2015 and June 2016 were approached to enter the study with age- and sex-matched controls. Participants were excluded for previous or current hormonal treatment, any known chromosomal or hormonal disorder, any concurrent psychiatric disorder (determined by the Mini International Neuropsychiatric Interview, Sheehan et al. 1998), neurological or other medical disorder including autism spectrum disorder, substance abuse, the use of psychoactive medications, or any contraindication to undergoing magnetic resonance imaging such as metal or electronic implants. All participants provided full informed consent in accordance with the Karolinska Institutet ethical committee (Application # Dnr 2011/281-31/4). All transgender participants reported an early (pre-pubescent) awareness of their transgender identity. Sexual orientation was assessed using the self-report Kinsey scale (from 0 or “exclusively heterosexual” to 6 or “exclusively homosexual”) (Kinsey et al. 2003) in relation to birth-assigned sex. While data about the cisgender control group alone were previously presented in Burke et al. 2019, data about the transgender group have not been previously presented.
Body Localizer Task
Participants first engaged in a body localizer task in the scanner, where they viewed images of other human bodies versus inanimate chairs. We used the body localizer for a separate analysis in order to ascertain whether the two study groups used similar networks for the general perception of others’ bodies, rather than to obtain regions of interest for other analyses. This is similar to what we performed in Burke et al. 2019, where the body localizer task is described in full.
Body Perception
Details of the Body Perception Task can be found in Burke et al. 2019. Each participant was photographed while standing identically before a wall dressed in a skin-colored, skin-tight, full-body unitard to provide an accurate representation of their body shape without the discomfort of being nude. Hands, feet, and head were cropped from the photos. FantaMorph Software, version 5.0 (Abrosoft http://www.fantamorph.com/) was used to morph each participants picture toward those of five different female and five different male participant targets at degree intervals of 20%. This led to a total of 11 morph conditions ranging between −100% (morphed completely opposite to birth-assigned sex) to +100% (morphed completely toward birth-assigned sex), with 0% referring to the unmorphed original image. A scrambled control image was created using a Fourier phase randomization procedure that preserved the luminance, contrast, color distribution, and special frequency spectrum of the original image while degrading the shape (Näsänen 1999) (see Fig. 1).
Figure 1.
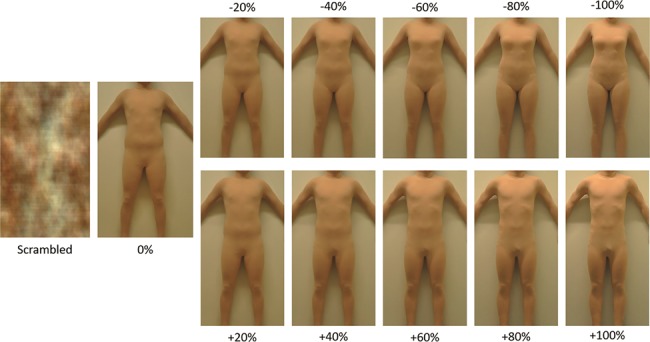
Examples of a scrambled image and a male’s body image morphed, from left to right, to 20, 40, 60, 80, and 100% to the same (denoted by positive morph degrees) and the opposite (denoted by negative morph degrees) sex. Note that “100%” photographs were unaltered images of another person.
Fifteen repetitions of each of the 11 body images plus the scrambled image were presented for two different viewing conditions (a short 0.5 s and long 2 s duration), totaling 360 trials (15 × 12 × 2). These images were randomized and presented over three fMRI runs using Presentation version 18.1. Each trial consisted of the image (presented for either 0.5 or 2 s) followed by a 1 s response screen with button press options, followed by a fixation cross with a jittered inter-trial interval. Participants were instructed to respond as quickly as possible to the question “To what degree is this picture you?” on a 4-point scale (1: 0–25% “me,” 2: 25–50% “me,” 3: 50–75% “me,” and 4: 75–100% “me”). Before the main runs, participants engaged in a practice session in the scanner to ensure task comprehension.
MR Data Acquisition
MRI data were acquired on a 3 Tesla MRI scanner (Discovery 3 T GE-MR750, General Electric, Milwaukee, Wisconsin). Functional MRI of both the body perception and body localizer tasks was performed with a gradient echo pulse sequence using a voxel size of 3.03 × 3.03 × 3.5 mm (time echo [TE] = 30 ms, time repetition [TR] = 2000 ms, field of view [FoV] = 23 cm, 41 bottom up interleaved axial slices, 3 mm thickness, 75° flip angle) and a 32-channel head coil. 3D T1-weighted spoiled gradient echo pulse sequence images were acquired with 1 mm3 isotropic voxel size (TE = 3.1 ms, TR = 7.9 ms, TI = 450 ms, FoV = 23 cm, 176 axial slices, 12° flip angle) using an 8-channel coil. Each fMRI run totaled 9.5 min with 280 volumes per run.
Data Analysis
Sample characteristics and behavioral data were analyzed using SPSS Statistics 21 (SPSS Inc., Chicago, IL). For behavioral ratings, we used a general linear mixed model with repeated-measures on image ratings, with two between-subject factors (group [transgender vs cisgender] and birth-assigned sex) and two within-subject factors (morph degree and viewing duration).
MRI analysis was performed using fMRI expert analysis tool (FEAT) version 5.0.8, part of FMRIB Software Library (FSL) http://www.fmrib.ox.ac.uk/fsl) (Jenkinson et al. 2012). Blood oxygen level-dependent sequences were motion-corrected (using the FMRIB linear image registration tool, MCFLIRT) and spatially smoothed (using FEAT) with a smoothing kernel of 5 mm. Portions of subject runs with notable movement greater than a maximum displacement of 1.5 mm at either the beginning or end were truncated to minimize the effect of movement. To summarize standardized FEAT procedures, functional images were first registered to the participant’s T1-weighted image (using the FMRIB non-linear image registration tool, FNIRT) after brain extraction using the FSL Brain Extraction Tool (BET) with a fractional intensity threshold of 0.3. Images were then registered to the MNI-152 brain for group analysis (using FNIRT). We used FMRIB’s Improved Linear Model (FILM) to apply the General Linear Model to first-level time-series data. We then used Fixed Effects Modeling to combine each participant’s three functional runs into a single statistical map. This was followed by using the FMRIB’s Local Analysis of Mixed Effects (FLAME 1) tool to create group maps for cross-subject comparisons (Beckmann et al. 2003; Woolrich et al. 2004, 2009). To provide family-wise error correction, these were thresholded using a value of Z > 2.3 to produce a set of contiguous voxels, or “clusters,” and each cluster’s estimated significance level (from Gaussian random field theory) was compared to the cluster probability threshold to exclude all clusters whose sizes did not fall in the top 5% of the distribution (P < 0.05) (see Worsley et al. 2002).
To determine significant effects between groups, we used an ANOVA with two factors: group (cisgender vs transgender) and birth-assigned sex (female vs male) were used for both the body perception task and the body localizer task. Since the focus of this paper was to test our hypotheses about differences between transgender and cisgender groups rather than sex differences, and since there were no significant interactions between the effects of group and birth-assigned sex, significant main effects of birth-assigned sex are provided in the supplement. For the body perception task, viewing duration was a “within-subjects” variable. Therefore, this was not included in the ANOVA, and instead, trials in the long (2 s) and short (0.5 s) viewing condition were analyzed and compared at the individual subject level.
Four primary contrasts were determined for comparison with the scrambled image baseline for both the long and short viewing conditions to explore our hypotheses: (1) the own body (0% morph) condition when viewing the unmorphed image of self; (2) the other body same as birth-assigned sex (+80% and +100% morph) condition, (3) the other body opposite to birth-assigned sex (−80% and −100% morph) condition, and (4) the androgynous morph (−40% and −60% morph) condition when viewing images of oneself morphed intermediately to another body whose sex is “opposite” to one’s birth-assigned sex, creating an androgynous image. Images morphed −20%, +20%, +40%, and +60% morphed images were modeled in the general linear model but not analyzed.
These contrasts were run both with behavioral ratings as two additional parametric covariates, modeled separately for responses to images morphed (1) toward and (2) opposite to birth-assigned sex. This allowed us to isolate the component of functional activity that was dependent and parametrically scaled with participants’ subjective assessment of self-similarity when they were viewing images in each of the two conditions separately. The regression variables were modeled at the individual level by de-meaning participants’ self-rating scores, such that significant results indicated regions where activation scaled with greater “me” rating. Conversely, by using these parametric covariates as regressors of non-interest, we sought to characterize participants’ functional responses to viewing bodies that were independent of their subjective assessment of the bodies’ similarity to self. An ANOVA with two factors (group and birth-assigned sex) was used at the whole brain level.
Functional Connectivity Analysis
Given the pACC’s role in self-processing (Sturm et al. 2013; Murray et al. 2015), we queried whether its task-dependent connectivity with other regions differed between groups, which would suggest distinct coordinated activation. We therefore carried out a psychophysiological interaction (PPI) analysis. A physiological seed was obtained from the intersection of reverse-inference map derived from Neurosynth.org (search term “self,” thresholded to Z ≥ 4) and the Harvard-Oxford Cortical Atlas mask for the anterior cingulate cortex (Desikan et al. 2006). This provided an unbiased pACC seed region of interest. The mean time course of this region of interest was obtained for each participant and an interaction was modeled between this and each task event, deconvolving with custom tools (see McLaren et al. 2012 regarding modeling of generalized PPI analysis). Once again, we compared connectivity during the four viewing categories of interest (see above) compared to connectivity when viewing the scrambled images at both long and short viewing conditions.
Results
Participant Characteristics
Sixty individuals, consisting of 30 transgender (mean age 25.4 ± 5.8 years) and 30 cisgender individuals (mean age 25.8 ± 3.5 years), participated in the study (Table 1). The transgender group comprised of 14 transgender men (assigned female at birth) and 16 transgender women (assigned male at birth), while the cisgender group comprised of 15 cisgender men and 15 cisgender women (previously described in Burke et al., 2019). There were no significant group differences in age, education, or handedness between transgender and cisgender groups (Table 1). All cisgender controls identified as heterosexual (Kinsey Score: cisgender women: 0.5 ± 0.6; cisgender men: 0.4 ± 0.6; n.s.), whereas transgender participants showed greater inter-individual variance in scores (transgender men: 3.0 ± 2.0; transgender women: 2.2 ± 2.2; n.s.), as reported in our earlier studies (e.g., Manzouri and Savic 2019).
Table 1.
Demography
| All transgender (n = 30) | Transgender men (n = 14) | Transgender women (n = 16) | All cisgender (n = 30) | Cisgender men (n = 15) | Cisgender women (n = 15) | Group T-value | P value | |
|---|---|---|---|---|---|---|---|---|
| Age | 25.4 ± 5.8 | 22.2 ± 5.8 | 28.2 ± 4.3 | 25.8 ± 3.5 | 26.4 ± 3.4 | 25.3 ± 3.7 | 0.35 | .73 |
| Education | 14.6 ± 2.5 | 13.9 ± 2.1 | 15.3 ± 2.7 | 15.5 ± 2.2 | 15.3 ± 1.7 | 15.7 ± 2.6 | 1.45 | .15 |
| Handedness | 82.6 ± 26.8 | 94.1 ± 9.3 | 82.6 ± 26.8 | 78.4 ± 48.2 | 78.4 ± 48.2 | 62.6 ± 71.2 | 1.31 | .20 |
| Sexual orientation | 2.6 ± 2.1 | 3.0 ± 2.0 | 2.2 ± 2.2 | 0.5 ± 0.6 | 0.4 ± 0.6 | 0.5 ± 0.6 | 5.11 | <0.0001 |
Sexual orientation was assessed using the self-report Kinsey scale (Kinsey et al. 2003). Scores range from 0 = “exclusively heterosexual” to 6 = “exclusively homosexual” in relation to one’s birth-assigned sex; Handedness was assessed according to Oldfield (1971): scores could range from −100 (exclusively left-handed) to +100 (exclusively right-handed)
Body Localizer Task
There were no significant group differences between transgender and cisgender groups in the body localizer task, suggesting the two study groups used similar networks for the general perception of bodies.
Body Perception Task: Behavioral Results
One cisgender man was excluded from the behavioral analysis on account of being mistakenly presented images of other individuals in the 0% morph condition only rather than his own unmorphed body. In the remaining participants, ANOVA showed no significant four-way interaction between factors, but there was one significant three-way interaction between morph degree, viewing duration, and group (F(10,46) = 3.535, P = 0.002). Exploring this further, there were related significant two-way interactions of morph degree and group (F(10,46) = 15.131, P < 0.001) and morph degree and viewing duration (F(10,46) = 5.081, P < 0.001), but there was no significant interaction between viewing duration and group (F(1,55) < 1, P = 0.36). Thus, we sought to characterize these interactions by investigating (1) differences by group at each image morph degree and (2) differences by viewing duration at each morph degree. Regarding differences by group, the transgender group had greater self-similarity ratings compared to the cisgender group for images morphed −60%, −80%, and −100% (opposite to birth-assigned sex), whereas the cisgender group had greater self-similarity ratings compared to the transgender group for all morph degrees greater than or equal to −20% for both the short and long viewing conditions (Fig. 2). Regarding differences by viewing duration, the long (2 s) viewing condition was associated with slightly greater self-similarity ratings compared to the short (0.5 s) viewing condition for images morphed between −20% and +40%, though individual pair-wise comparisons did not reach statistical significance.
Figure 2.
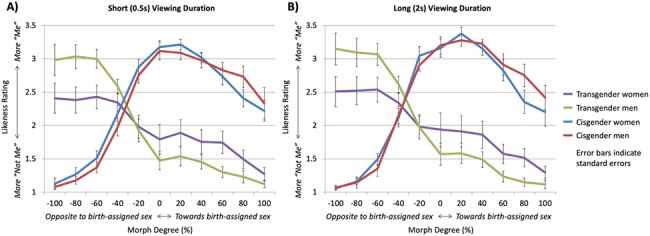
Participant ratings of self-similarity at each morph degree for the 2 s long (A) and 0.5 s short (B) viewing duration conditions. Participants were asked, “To what degree is this picture you?” on a scale from 1 to 4, with 1 representing least similarity to self and 4 representing most similarity to self. A morph degree of 0% represented the participants unmorphed own body. Positive and negative morph degrees represented morphing toward another body with the same and opposite sex, respectively, as the participant’s birth-assigned sex. Error bars represent standard errors of the mean. There were no significant differences in behavioral scores between transgender women and transgender men, nor were there significant differences between cisgender men and cisgender women.
Short 0.5 s Viewing Condition
There were no significant results for any of the activation or connectivity analyses for the short 0.5 s viewing condition and no significant differences found when comparing long and short viewing conditions. The following results are thus entirely from the long 2 s viewing condition.
Qualitative Comparison of Groups while Viewing Morphed Images
We first qualitatively compared within-group activation results when viewing bodies morphed toward and opposite to birth-assigned sex. Transgender and cisgender groups showed similar activation when viewing bodies morphed toward their gender identity, even though these morphs were opposite to birth-assigned sex for the transgender group and toward birth-assigned sex for the cisgender group. This similarity between cisgender and transgender participants is visualized in Figure 3, which compares the activation maps of transgender and cisgender participants viewing both other bodies same as birth-assigned sex (+80% and +100% morphs) and other bodies opposite to birth-assigned sex (−80% and −100% morphs) compared to the scrambled image baseline. Inspecting this further, when compared to the scrambled image baseline, there was considerable overlap between activation in transgender individuals viewing other bodies opposite to birth-assigned sex (−80% and −100% morphs) and activation in cisgender individuals viewing other bodies same as birth-assigned sex (+80% and +100% morphs) (Fig. 4).
Figure 3.
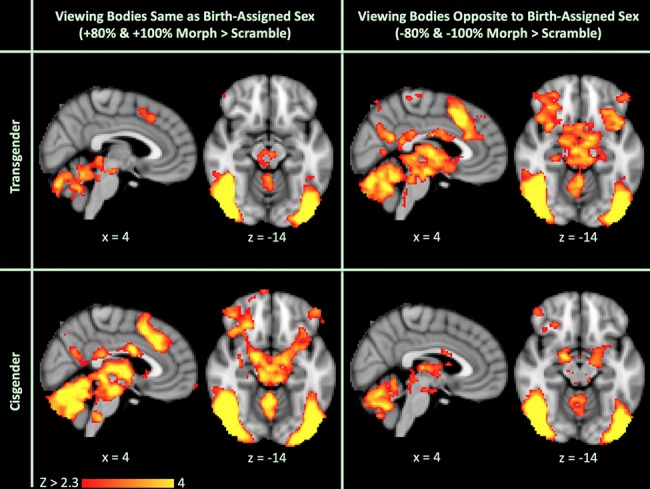
Comparison of activation maps in both transgender and cisgender groups when viewing other bodies same as birth-assigned sex (+80% and +100% morphs) vs. other bodies opposite to birth-assigned sex (+80% and +100% morphs) compared to scrambled images in the long viewing condition after covarying out self-similarity ratings. Here, activation in transgender participants viewing bodies opposite to their birth-assigned sex (and same as their gender identity) is similar to activation in cisgender participants viewing bodies same as their birth-assigned sex (also same as their gender identity).
Figure 4.
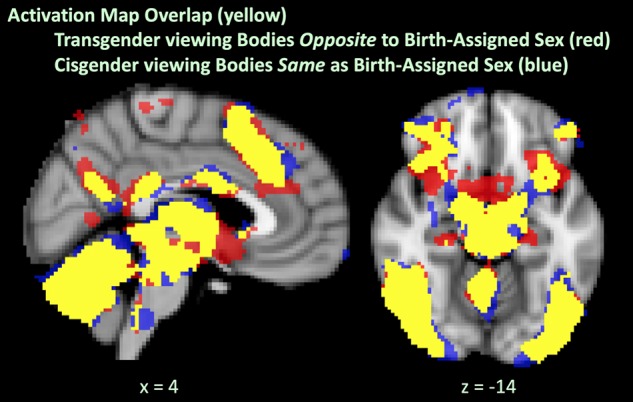
A further elaboration of Figure 3. We overlay (in yellow) activation maps to demonstrate the similarity between transgender participants viewing bodies opposite to birth-assigned sex (in red) and cisgender participants viewing bodies same as birth-assigned sex (in blue).
Own Body Perception (0% Morph)
One cisgender man was excluded from this analysis on account of being mistakenly presented images of other individuals rather than his own unmorphed body. In the remaining participants, when contrasting perception of the own body (0% morph) to the scrambled image baseline, there was a significant group effect, with cisgender participants showing greater activation in the left postcentral gyrus and left superior parietal lobule, and the right cerebellum (Fig. 5A, Table 2).
Figure 5.
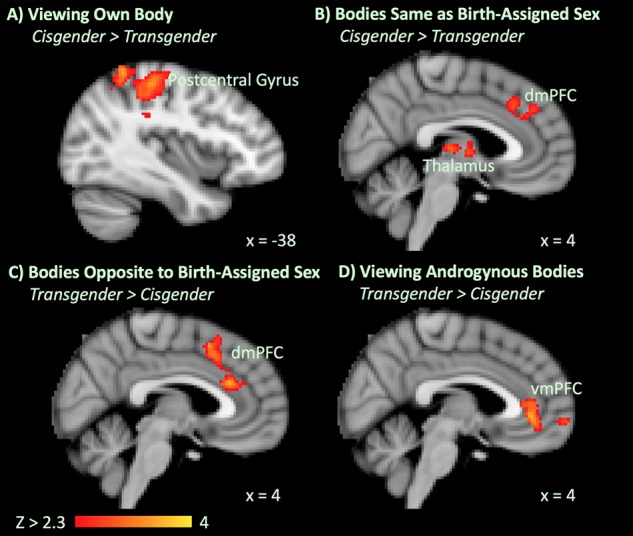
Significant ANOVA group effects when viewing bodies compared to scrambled images in the long viewing condition after covarying out self-similarity ratings. (A) Cisgender > transgender activation when viewing the own body (0% Morphs). (B) Cisgender > transgender activation when viewing bodies same as birth-assigned sex (+80% and +100% morphs). (C) Transgender > cisgender activation when viewing bodies opposite to birth-assigned sex (−80% and −100% morphs). (D) Transgender > cisgender activation when viewing androgynous bodies (−40% and −60% morphs).
Table 2.
Significant ANOVA group effects
| Region | Side | MNI coordinates | Z-max | Cluster size (mm3) |
|---|---|---|---|---|
| Own body (0% morph) condition: cisgender > transgender | ||||
| Cerebellum | R | (4, −72, −22) | 3.28 | 1463 |
| Postcentral gyrus | L | (−46, −28, 58) | 3.75 | 1462 |
| Superior parietal lobule | L | (−34, −50, 62) | 3.72 | |
| Bodies same as birth-assigned sex (+80% and +100% morph) condition: cisgender > transgender | ||||
| Postcentral gyrus | L | (−48, −30, 48) | 3.76 | 2429 |
| Superior parietal lobule | L | (−38, −52, 62) | 3.50 | |
| Supramarginal gyrus | L | (−50, −38, 46) | 3.47 | |
| Left thalamus | L | (−10, −16, 6) | 3.59 | 837 |
| Right thalamus | R | (14, −10, 12) | 3.39 | |
| Cerebellum | R | (28, −52, −26) | 3.20 | 655 |
| Superior lateral occipital cortex | R | (32, −66, 44) | 3.27 | 578 |
| dACC and paracingulate | L | (−6, 28, 36) | 3.29 | 526 |
| Bodies opposite to birth-assigned sex (−80% and −100% morph) condition: transgender > cisgender | ||||
| dACC and paracingulate | R | (10, 30, 24) | 3.84 | 1141 |
| Androgynous body (−40% and −60% morph) condition: transgender > cisgender | ||||
| pACC and paracingulate | L | (−4, 40, −2) | 3.53 | 882 |
| Parametric analysis (regions correlated to greater “me” rating when viewing morphs opposite to birth-assigned sex [−20% to −100% Morph]): transgender > cisgender | ||||
| Posterior cingulate gyrus | (0, −36, 46) | 3.76 | 8350 | |
| Posterior parahippocampus and hippocampus | L | (−24, −40, −12) | 3.64 | |
| Lateral occipital cortex | L | (−48, −68, 14) | 3.62 | |
| Temporal occipital fusiform cortex | L | (−26, −48, −12) | 3.57 | |
| Precuneous cortex | R | (2, −40, 56) | 3.53 | |
| Superior lateral occipital cortex | R | (58, −60, 20) | 3.41 | 1257 |
| Inferior lateral occipital cortex | R | (42, −64, 6) | 3.14 | |
| Frontal pole | L | (−18, 72, 4) | 3.26 | 843 |
| Frontal pole | R | (6, 58, −10) | 3.23 | |
| pACC | L | (−2, 44, 2) | 3.19 | |
| Frontal medial cortex | L | (−4, 54, −10) | 3.16 | |
| PPI analysis (regions functionally connected to pACC region of interest during the androgynous body [−40% and −60% morph] condition): transgender > cisgender | ||||
| Occipital pole | R | (24, −96, 2) | 3.46 | 1205 |
| Inferior lateral occipital cortex | R | (42, −76, −4) | 3.10 | |
| Occipital fusiform gyrus | R | (28, −82, −8) | 3.03 | |
| Lingual gyrus | R | (2, −72, −12) | 3.22 | 725 |
| Occipital pole | L | (−28, −96, 0) | 3.18 | |
| Occipital fusiform gyrus | L | (−18, −84, −14) | 3.05 | |
All findings were in the long 2 s viewing condition. All contrasts except the parametric analysis are in contrast with the scrambled image baseline
Perception of Other Bodies Same as Birth-Assigned Sex (+80% and +100% Morphs)
When contrasting perception of bodies same as birth-assigned sex (+80% and +100% morphs) to the scrambled image baseline, there was a significant group effect with cisgender controls showing significantly greater activation in the left postcentral, superior parietal, and supramarginal gyri, the bilateral thalami, the right cerebellum, the right superior lateral occipital cortex, and the dorsomedial prefrontal cortex (dmPFC, including dACC and paracingulate gyrus) (Fig. 5B, Table 2). Thus, as expected, cisgender controls appeared to engage own-body perception networks during this condition, which was not evident among transgender participants.
Perception of Other Bodies Opposite to Birth-Assigned Sex (−80% and −100% Morphs)
In contrast to the above findings, when contrasting perception of bodies opposite to birth-assigned sex (−80% and −100% morphs) to the scrambled image baseline, there was a significant group effect with transgender participants showing significantly greater activation in the dmPFC (including the dACC and paracingulate gyrus) (Fig. 5C, Table 2).
Perception of Androgynous Morphs (−40% and −60% Morphs)
When contrasting perception of androgynous morphs (−40% and −60% morphs) to the scrambled image baseline, there was a significant group effect with transgender participants showing significantly greater activation in the ventromedial prefrontal cortex (vmPFC, including the pACC and paracingulate gyrus) (Fig. 5D, Table 2).
Response-Dependent Activation for Images Morphed Toward, and Opposite to, Birth-Assigned Sex
Although the above results covaried out self-similarity rating, we also sought to understand the component of functional activity that was “dependent” and parametrically scaled with self-similarity rating. This was studied in an analysis with two parametric regressors of self-perception rating when participants were viewing any image morphed either (1) toward, or (2) opposite, to birth-assigned sex.
Qualitative comparisons of within-group parametric results when viewing bodies morphed toward and opposite to birth-assigned sex are discussed in the Supplemental Information. We found no significant effects (whether effects of group or birth-assigned sex) when viewing images morphed “toward” birth-assigned sex. By contrast, when viewing images morphed opposite to birth-assigned sex, there was a significant group effect with transgender participants showing a significantly greater association between greater self-perception (“me”) rating and activation in the dmPFC and the vmPFC (including the pACC), frontal poles, the posterior cingulate cortex (PCC) and the precuneus, the left parahippocampal gyrus, hippocampus, temporal occipital fusiform cortices, and the bilateral lateral occipital cortices (Fig. 6, Table 2).
Figure 6.
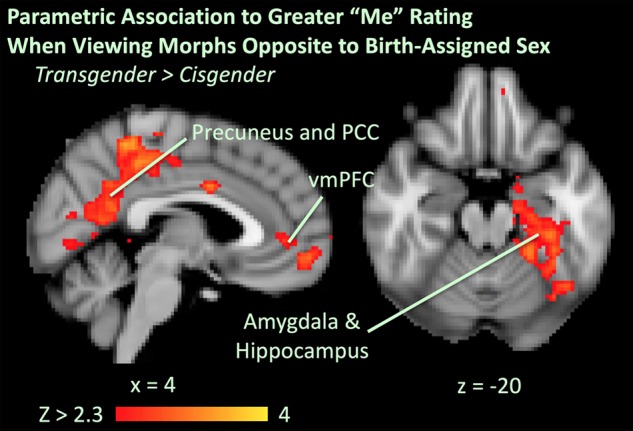
Significant ANOVA group effect of regions with stronger parametrical correlation to self-similarity scores in transgender > cisgender when viewing any image morphed opposite to birth-assigned sex (between −20 and −100%) during the long viewing condition.
Connectivity Analysis
We used a PPI analysis to query whether task-dependent connectivity between a pACC seed region and other brain regions differed between groups. There were no significant ANOVA effects in connectivity for any morph category of interest except when viewing androgynous morphs (−40% and −60% morphs). Here, there was a significant group effect with transgender participants showing greater connectivity than cisgender controls between the pACC and early visual cortex (bilateral lateral occipital cortex—adjacent to and partially overlapping with EBA—extending into the bilateral occipital fusiform cortices and cerebellum (Fig. 7, Table 2).
Figure 7.
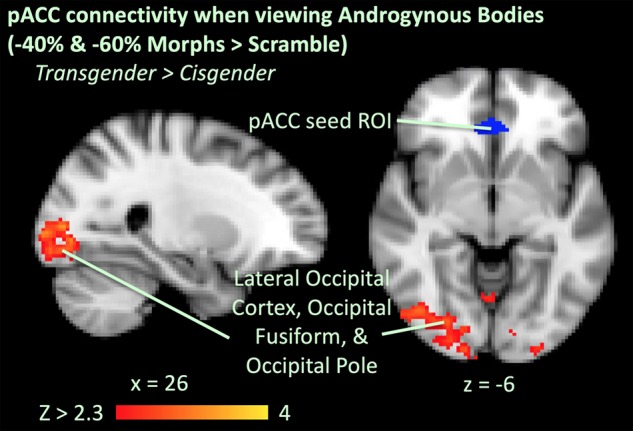
PPI analysis, showing ANOVA group effect of regions of greater functional connectivity with a pACC region of interest (ROI) (defined from neurosynth.org, in blue) in transgender > cisgender when viewing androgynous bodies (−40 and −60% morph) compared to scrambled images during the long viewing condition.
Discussion
This study used fMRI in transgender and cisgender persons to investigate the cerebral processing of self- and gender identity in the context of own-body perception. Participants were asked to rate images of themselves, as well as images of their bodies morphed to either masculine or feminine forms, as to the degree by which these images represented “me.” Whereas, both cisgender men and women rated images of their own bodies as more representative of “me,” with a strong bias away from images morphed to the opposite sex, both transgender men and women identified more closely with images morphed opposite their birth-assigned sex. In line with these behavioral results were the observed brain activation patterns in transgender and cisgender groups. Both groups activated self-referential and body processing regions (Hodzic et al. 2009; Kruse et al. 2016) when viewing images of bodies morphed toward their gender identity (whether that be opposite to birth-assigned sex for transgender individuals or toward birth-assigned sex for cisgender individuals). This activation of self-referential brain regions, occurring after covarying out participants’ individual responses, was not associated with participants’ subjective assessments of self-similarity. The findings suggest the presence of a distributed network connecting self-referential and visual body processing regions to assess congruence with self that exists in both cisgender and transgender individuals. Yet, the stimuli that activate this distributed network may differ, aligning with each group’s gender identity rather than their birth-assigned sex.
The implications of these findings are considerable. Transgender and cisgender groups differed when viewing other bodies same or opposite to their birth-assigned sex. Cisgender individuals activated the dmPFC and the thalamus more than transgender individuals when viewing bodies morphed toward their birth-assigned sex (Fig. 5A). Activation in these regions has been implicated in self-referential tasks (Johnson et al. 2002; Schmitz et al. 2004; Amodio and Frith 2006; D’Argembeau et al. 2007, 2014; van der Meer et al. 2010; Denny et al. 2012; Murray et al. 2015) and is consistent with prior literature showing involvement in such self-referential processes when viewing others who look similar to us (Platek et al. 2008; Tsakiris 2017). Yet, if the findings were simply a result of the degree by which individuals consciously identified with an image, we would not expect these findings to remain after covarying out subjective self-similarity scores. Engagement of the dmPFC and thalamus in cisgender individuals viewing bodies of the same sex and gender may thus suggest an unconscious process or an invariable response to the sex and gender of the stimuli.
Like cisgender individuals, treatment-naïve transgender individuals showed analogous responses in the dmPFC when viewing bodies that align with their gender identity (Fig. 5B), although these bodies did not align with the secondary sex characteristics of their own bodies. Like cisgender individuals, this activation would not be expected to remain after covarying out self-similarity scores if this were simply a function of conscious volitional identification with bodies aligned with their gender identity. Again, engagement of the dmPFC in transgender individuals viewing bodies of the same gender may suggest an unconscious process or an invariable response to the gender of the stimuli.
Our parametric analysis allowed us to distinguish the component of the neural signal that scaled with self-similarity scores from the component of the neural signal described above that was an invariable response to the stimuli. Here, we saw that transgender individuals had greater engagement of amygdala, hippocampus, dmPFC, vmPFC, precuneus, PCC, and visual cortex (Fig. 6) when deciding that an image morphed opposite to birth-assigned sex was more reflective of self. Involvement of the vmPFC (specifically pACC) in such self-referential decision making is consistent with literature suggesting a ventral-dorsal gradient in the prefrontal cortex where decisions about “self” engage more ventral regions than decisions about the “similar other” (Denny et al. 2012). Ventral prefrontal involvement, as well as involvement of limbic nodes such as the amygdala, may also suggest a role of emotional processing in transgender individuals, consistent with literature suggesting pACC’s role in processing emotional valence of self (Yu et al. 2011) and the amygdala’s role in emotional processing, motivation, and reward (Rolls 2007; Pessoa 2017). This emotional processing may relate to the emotional significance of body-self alignment in the transgender individuals in this study and relate to their motivation toward hormone and/or gender-affirming surgery in the clinic they were recruited. Also notable is recruitment of the precuneus and PCC, which has been reported to be involved in self–other decision making (Ochsner et al. 2004; Cavanna and Trimble 2006) and autobiographical memory retrieval (Fink et al. 1996; Summerfield et al. 2009), as well as recruitment of other regions associated with memory (hippocampus) and visual attention (occipital cortex).
Particularly interesting is the greater pACC activation in transgender individuals when viewing images morphed −40% and −60% (opposite to birth-assigned sex) after covarying out self-similarity ratings. These images, morphed approximately half-way between their own bodies and the bodies of others opposite to their birth-assigned sex, represent gender ambiguous, androgynous bodies. Consistent with the ventral-dorsal prefrontal hypothesis of self-processing discussed above (Denny et al. 2012), transgender individuals may engage greater self-referential processing when viewing these androgynous images, but in a way that is invariable and does not associate parametrically with subjective self-similarity ratings. Additionally, given the pACC’s involvement in limbic processing and emotional salience monitoring (Brown et al. 2011; Yu et al. 2011), particularly its associations with positive reward processing (Liu et al. 2011) and happy emotions (Vogt 2005), transgender individuals may also experience a positive emotional response or reward from these images, which may be dissimilar to their own bodies but closer to their “ideal self.” We further found greater task-dependent connectivity between the pACC and early (secondary) visual cortical regions in the transgender group compared to the cisgender group when viewing these ambiguous images. In transgender individuals, this connectivity may facilitate transfer of visual information about “selfness” to the pACC, whereas it may be suppressed in cisgender individuals when confronted with images which may be deemed androgynous and sex-discordant.
An interesting but unexpected finding is that individuals assigned male at birth—cisgender men and transgender women—had greater activation than individuals assigned female at birth—cisgender women and transgender men—in each of the morphed conditions regardless of whether the body was morphed toward or opposite to birth-assigned sex. This activation was observed in similar regions in each condition, including the right angular gyrus and left occipital pole (see supplemental material). By contrast, groups showed no differences when viewing the unmorphed self. These findings are consistent with greater engagement of visual processing and body space representation when viewing others (regardless of that body’s sex) (Seghier 2013; Spitoni et al. 2013) and may suggest a greater general saliency effect for the visual aspects of bodies in individuals assigned male at birth.
We acknowledge a general limitation in our study based on group sizes, which included 30 individuals in both cisgender and transgender groups. Another limitation of the present study is that we were unable to concurrently query participants’ emotional valence when they engaged in the task, which might have been useful to better clarify the involvement of limbic regions, such as the pACC seen here. The role of emotional valence and motivation in underlying group differences should be further explored.
Our findings occurred entirely in the long 2 s viewing condition, with no significant results for the short 0.5 s viewing condition. We had hypothesized that such a finding could suggest that participants were engaging in more “reflective” top-down processing rather than “reflexive” bottom-up processing. However, we caution that this interpretation is not conclusive. Though we did not see significant effects in the short viewing condition, we also did not see significant differences between the long and short conditions when they were compared directly. The absence of findings for the short viewing condition may thus be a result of lower power (given less TRs in the short condition), and there is no evidence to suggest that reflexive bottom-up processing is not occurring in the long viewing condition, especially when participant behavior was similar between the two conditions.
The behavioral results in this study, where transgender participants identified most with images morphed ≥60% opposite to birth-assigned sex regardless of viewing duration, were not entirely consistent with those observed in a previous behavioral study of a different group of transgender men using the body perception task paradigm. In that study, transgender participants identified most with images morphed 40% opposite to birth-assigned sex (Feusner et al. 2016). We speculate this may be due to differences in the experimental setting. This present task was conducted in the fMRI scanner, whereas the previous behavioral task was conducted in a clinical research setting in the presence of an investigator, raising concerns participants may have been more self-conscious about their responses. Furthermore, the present fMRI task consisted of more trials, allowing greater sensitivity to detect the behavioral response.
In previous work using a larger transgender study group, we saw distinct anatomical differences with greater cortical thickness than cisgender controls in regions of self-perception independent of sex and sexual orientation (Manzouri et al. 2017; Manzouri and Savic 2019). However, these anatomical differences reduced with sex-hormone treatment while concurrently increasing transgender individuals’ self-similarity ratings when viewing their own bodies (Kilpatrick et al. 2019). We thus hypothesize that hormonal treatment may allow transgender individuals to have greater congruence between their physical bodies and their internal body image representation, leading to greater convergence with the own body-self processing seen in cisgender people. We plan to test this hypothesis in the near future.
In conclusion, our observations add new insights to our understanding of the neurobiology of gender identity in general and GD in particular. Transgender and cisgender participants, regardless of sex, activate similar self-referential networks involving the dmPFC, but the stimuli activating these networks differ, aligning with gender identity and not birth-assigned sex. Moreover, this is not simply an effect of subjective assessments of self-similarity. Treatment-naïve transgender participants may also engage greater emotional processing when viewing ambiguous, androgynous images of themselves morphed toward their experienced gender identity and when making decisions as to the degree images corresponded to their sense of self. Our findings thus shed light on mechanisms of gender processing in the brain. They also set the stage for further exploration of how neural signatures of gender might change in transgender individuals with hormonal treatment and/or surgical interventions that allow for their bodies to finally align with their gender identity.
Supplementary Material
Funding
This work was supported by the Swedish Science Council (Dnr 200 7-3107 to I.S) and the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development (1R01HD087712 to J.F. and I.S.)
Notes
We would like to thank Barbara Nordhjem for her assistance, and Jeanette Mumford for providing custom tools for the PPI analysis. Conflicts of Interests: None declared.
Author Contributions
D.S.A.M. analyzed and wrote the manuscript. S.B. conducted the study and contributed to the writing. A.M. also conducted the study. C.D. recruited participants and contributed to the writing. J.F. designed the study, supervised analyses, and contributed to the writing. I.S. initiated, designed, and supervised the study and contributed to writing.
References
- American Psychiatric Association 2013. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Amodio DM, Frith CD. 2006. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 7(4):268–277. [DOI] [PubMed] [Google Scholar]
- Bandini E, Fisher AD, Castellini G, Sauro CL, Lelli L, Meriggiola MC, Casale H et al. 2013. Gender identity disorder and eating disorders: similarities and differences in terms of body uneasiness. J Sex Med. 10(4):1012–1023. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. 2003. General multilevel linear modeling for group analysis in FMRI. NeuroImage. 20(2):1052–1063. [DOI] [PubMed] [Google Scholar]
- Blanke O, Mohr C, Michel CM, Pascual-Leone A, Brugger P, Seeck M, Landis T, Thut G. 2005. Linking out-of-body experience and self processing to mental own-body imagery at the temporoparietal junction. J Neurosci Off J Soc Neurosci. 25(3):550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Gao X, Tisdelle L, Eickhoff SB, Liotti M. 2011. Naturalizing aesthetics: brain areas for aesthetic appraisal across sensory modalities. NeuroImage. 58(1):250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SM, Adnan Majid DS, Manzouri AH, Moody T, Feusner JD, Savic I. 2019. Sex differences in own and other body perception. Hum Brain Mapp. doi: 10.1002/hbm.24388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SM, Manzouri AH, Dhejne C, Bergström K, Arver S, Feusner JD, Savic-Berglund I. 2018. Testosterone effects on the brain in transgender men. Cereb Cortex. 28(5):1582–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SM, Manzouri AH, Savic I. 2017. Structural connections in the brain in relation to gender identity and sexual orientation. Sci Rep. 7(1):17954. doi: 10.1038/s41598-017-17352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case LK, Brang D, Landazuri R, Viswanathan P, Ramachandran VS. 2017. Altered white matter and sensory response to bodily sensation in female-to-male transgender individuals. Arch Sex Behav. 46(5):1223–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash TF, Pruzinsky T. 2004. Body image: a handbook of theory, research, and clinical practice. New York, NY: Guilford Press. [Google Scholar]
- Cavanna AE, Trimble MR. 2006. The Precuneus: a review of its functional anatomy and behavioural correlates. Brain J Neurol. 129(Pt 3):564–583. [DOI] [PubMed] [Google Scholar]
- Cohen-Kettenis PT, Pfäfflin F. 2010. The DSM diagnostic criteria for gender identity disorder in adolescents and adults. Arch Sex Behav. 39(2):499–513. [DOI] [PubMed] [Google Scholar]
- Craig AD., Bud 2010. The sentient self. Brain Struct Funct. 214(5–6):563–577. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Cassol H, Phillips C, Balteau E, Salmon E, Van der Linden M. 2014. Brains creating stories of selves: the neural basis of autobiographical reasoning. Soc Cogn Affect Neurosci. 9(5):646–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argembeau A, Ruby P, Collette F, Degueldre C, Balteau E, Luxen A, Maquet P, Salmon E. 2007. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. J Cogn Neurosci. 19(6):935–944. [DOI] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN. 2012. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. J Cogn Neurosci. 24(8):1742–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner Randy L. et al. 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into Gyral based regions of interest. NeuroImage. 31(3):968–980. [DOI] [PubMed] [Google Scholar]
- Dörner G. 1988. Neuroendocrine response to estrogen and brain differentiation in heterosexuals, homosexuals, and transsexuals. Arch Sex Behav. 17(1):57–75. [DOI] [PubMed] [Google Scholar]
- Feusner JD, Dervisic J, Kosidou K, Dhejne C, Bookheimer S, Savic I. 2016. Female-to-male transsexual individuals demonstrate different own body identification. Arch Sex Behav. 45(3):525–536. [DOI] [PubMed] [Google Scholar]
- Feusner JD, Lidström A, Moody TD, Dhejne C, Bookheimer SY, Savic I. 2017. Intrinsic network connectivity and own body perception in gender dysphoria. Brain Imaging Behav. 11(4):964–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GR, Markowitsch HJ, Reinkemeier M, Bruckbauer T, Kessler J, Heiss W-D. 1996. Cerebral representation of one’s own past: neural networks involved in autobiographical memory. J Neurosci Off J Soc Neurosci. 16(13):4275–4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodzic A, Kaas A, Muckli L, Stirn A, Singer W. 2009. Distinct cortical networks for the detection and identification of human body. NeuroImage. 45(4):1264–1271. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, Smith SM. 2012. FSL. NeuroImage. 62(2):782–790. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. 2002. Neural correlates of self-reflection. Brain J Neurol. 125(Pt 8):1808–1814. [DOI] [PubMed] [Google Scholar]
- Kilpatrick LA, Holmberg M, Manzouri AH, Savic I. 2019. Cross sex hormone treatment is linked with a reversal of cerebral patterns associated with gender dysphoria to the baseline of cisgender controls. Eur J Neurosci. 50(8):3269–3281. doi: 10.1111/ejn.14420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey AC, Pomeroy WR, Martin CE. 2003. Sexual behavior in the human male. 1948. Am J Public Health. 93(6):894–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse B, Bogler C, Haynes J-D, Schütz-Bosbach S. 2016. Am I seeing myself, my friend or a stranger? The role of personal familiarity in visual distinction of body identities in the human brain. Cortex. 83:86–100. doi: 10.1016/j.cortex.2016.07.010. [DOI] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. 2011. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 35(5):1219–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzouri AH, Kosidou K, Savic I. 2017. Anatomical and functional findings in female-to-male transsexuals: testing a new hypothesis. Cereb Cortex. 27(2):998–1010. [DOI] [PubMed] [Google Scholar]
- Manzouri A, Savic I. 2019. Possible neurobiological underpinnings of homosexuality and gender dysphoria. Cereb Cortex. 29(5):2084–2101. doi: 10.1093/cercor/bhy090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC. 2012. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. NeuroImage. 61(4):1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray RJ, Debbané M, Fox PT, Bzdok D, Eickhoff SB. 2015. Functional connectivity mapping of regions associated with self- and other-processing. Hum Brain Mapp. 36(4):1304–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näsänen R. 1999. Spatial frequency bandwidth used in the recognition of facial images. Vis Res. 39(23):3824–3833. [DOI] [PubMed] [Google Scholar]
- Nieder TO, Herff M, Cerwenka S, Preuss WF, Cohen-Kettenis PT, De Cuypere G, Hebold Haraldsen IR, Richter-Appelt H. 2011. Age of onset and sexual orientation in transsexual males and females. J Sex Med. 8(3):783–791. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, Greck M, Bermpohl F, Dobrowolny H, Panksepp J. 2006. Self-referential processing in our brain--a meta-analysis of imaging studies on the self. NeuroImage. 31(1):440–457. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, Mackey SC. 2004. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci. 16(10):1746–1772. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. 1971. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 9(1):97–113. [DOI] [PubMed] [Google Scholar]
- Pessoa L. 2017. A network model of the emotional brain. Trends Cogn Sci. 21(5):357–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platek SM, Krill AL, Kemp SM. 2008. The neural basis of facial resemblance. Neurosci Lett. 437(2):76–81. [DOI] [PubMed] [Google Scholar]
- Rolls ET. 2007. Reward- and punishment-related learning; emotion and motivation In: Memory, attention, and decision-making: A unifying computational neuroscience approach. 1st Edition. Oxford University Press; pp. 113–261. [Google Scholar]
- Savic I, Arver S. 2014. Sex differences in cortical thickness and their possible genetic and sex hormonal underpinnings. Cereb Cortex. 24(12):3246–3257. [DOI] [PubMed] [Google Scholar]
- Savic I, Arver S. 2011. Sex dimorphism of the brain in male-to-female transsexuals. Cereb Cortex. 21(11):2525–2533. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Kawahara-Baccus TN, Johnson SC. 2004. Metacognitive evaluation, self-relevance, and the right prefrontal cortex. NeuroImage. 22(2):941–947. [DOI] [PubMed] [Google Scholar]
- Seghier ML. 2013. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist. 19(1):43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 59(Suppl 20):22–33; quiz 34–57. [PubMed] [Google Scholar]
- Spitoni GF, Pireddu G, Cimmino RL, Galati G, Priori A, Lavidor M, Jacobson L, Pizzamiglio L. 2013. Right but not left angular gyrus modulates the metric component of the mental body representation: a tDCS study. Exp Brain Res. 228:63. doi: 10.1007/s00221-013-3538-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm VE, Sollberger M, Seeley WW, Rankin KP, Ascher EA, Rosen HJ, Miller BL, Levenson RW. 2013. Role of right pregenual anterior cingulate cortex in self-conscious emotional reactivity. Soc Cogn Affect Neurosci. 8(4):468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield JJ, Hassabis D, Maguire EA. 2009. Cortical midline involvement in autobiographical memory. NeuroImage. 44(3):1188–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaab DF, Hofman MA. 1995. Sexual differentiation of the human hypothalamus in relation to gender and sexual orientation. Trends Neurosci. 18(6):264–270. [PubMed] [Google Scholar]
- Tsakiris M. 2017. The multisensory basis of the self: from body to identity to others [formula: see text]. Q J Exp Psychol. 70(4):597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meer L, Costafreda S, Aleman A, David AS. 2010. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci Biobehav Rev. 34(6):935–946. [DOI] [PubMed] [Google Scholar]
- Vocks S, Busch M, Grönemeyer D, Schulte D, Herpertz S, Suchan B. 2010. Differential neuronal responses to the self and others in the extrastriate body area and the fusiform body area. Cogn Affect Behav Neurosci. 10(3):422–429. [DOI] [PubMed] [Google Scholar]
- Vogt BA. 2005. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 6(7):533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Beckmann CF, Nichols TE, Smith SM. 2009. Statistical analysis of fMRI data In: Filippi M. (eds) fMRI Techniques and Protocols Neuromethods. Totowa, NJ: Humana Press, 41 pp. 179–236. [Google Scholar]
- Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM. 2004. Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage. 21(4):1732–1747. [DOI] [PubMed] [Google Scholar]
- World Health Organization 1992. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. World Health Organization; https://www.who.int/health-topics/international-classification-of-diseases. [Google Scholar]
- Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, Evans AC. 2002. A general statistical analysis for fMRI data. NeuroImage. 15(1):1–15. [DOI] [PubMed] [Google Scholar]
- Yu C, Zhou Y, Liu Y, Jiang T, Dong H, Zhang Y, Walter M. 2011. Functional segregation of the human cingulate cortex is confirmed by functional connectivity based neuroanatomical parcellation. NeuroImage. 54(4):2571–2581. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


