Abstract
Dexterous object manipulation is a hallmark of human evolution and a critical skill for everyday activities. A previous work has used a grasping context that predominantly elicits memory-based control of digit forces by constraining where the object should be grasped. For this “constrained” grasping context, the primary motor cortex (M1) is involved in storage and retrieval of digit forces used in previous manipulations. In contrast, when choice of digit contact points is allowed (“unconstrained” grasping), behavioral studies revealed that forces are adjusted, on a trial-to-trial basis, as a function of digit position. This suggests a role of online feedback of digit position for force control. However, despite the ubiquitous nature of unconstrained hand–object interactions in activities of daily living, the underlying neural mechanisms are unknown. Using noninvasive brain stimulation, we found the role of primary motor cortex (M1) and somatosensory cortex (S1) to be sensitive to grasping context. In constrained grasping, M1 but not S1 is involved in storing and retrieving learned digit forces and position. In contrast, in unconstrained grasping, M1 and S1 are involved in modulating digit forces to position. Our findings suggest that the relative contribution of memory and online feedback modulates sensorimotor cortical interactions for dexterous manipulation.
Keywords: feedback, grasping, memory, sensorimotor control
Introduction
Dexterous object manipulation is a hallmark of human evolution (Napier 1956; Washburn 1960; Marzke 1997; Lemon 2008). Coadaptation of anatomical features and sensorimotor control mechanisms (Santello et al. 2013) have made dexterous manipulation a versatile means of interacting with the environment while inspiring (Santello et al. 2016) and challenging (Valero-Cuevas and Santello 2017) efforts to build dexterous robotic and prosthetic hands. The ability to skillfully use our hands depends on cortical mechanisms supporting several sensorimotor processes (Ehrsson et al. 2000; Castiello 2005; Lemon 2008; Davare et al. 2011), including integration of sensorimotor memory of previous hand–object interactions with online sensory feedback (Johansson and Cole 1992; Johansson and Flanagan 2009). Although the role of motor and parietal cortices in this sophisticated interplay has been extensively studied (Lemon et al. 1995; Chouinard et al. 2005; Davare et al. 2006, 2007; Jenmalm et al. 2006), this work has drawn an incomplete picture of these cortical mechanisms. Research over the past three decades has focused on the control of digit forces through a paradigm based on grasping objects at visually cued contacts (constrained grasping). These studies have shown that subjects use the same digit forces over consecutive trials by relying on a sensorimotor memory (Westling and Johansson 1984; Johansson and Westling 1988; Johansson and Cole 1992; Gordon et al. 1993; Dimitriou and Edin 2010). Upon lifting the object, online sensory feedback is used to assess the accuracy of the force plan and update the sensorimotor memory of digit forces for future manipulations if an error occurs, for example, object slip or tilt (for review, see Johansson and Flanagan, 2009). The “constrained grasping” paradigm, while providing significant insights into neural control of object manipulation, has neglected a critical component of sensorimotor control that is fundamental to natural hand–object interactions: choice of contact points.
When individuals can choose where to grasp an object (unconstrained grasping)—as it happens in many activities of daily living—the central nervous system is presented with unique challenges: As there are no visual cues constraining where to grasp an object, unconstrained grasping is characterized by greater trial-to-trial variability of digit position than constrained grasping; this occurs even after the object dynamics have been fully learned (Lukos et al. 2007, 2008, 2010; Fu et al. 2010, 2011; Mojtahedi et al. 2015). If control of digit forces in unconstrained grasping relied predominantly on sensorimotor memory, the same forces would be applied on each trial regardless of contact points. This behavior would lead to task failure. Remarkably, skilled manipulation can still be accurately performed because participants modulate digit forces as a function of digit position on a trial-to-trial basis (Fu et al. 2010, 2011; Mojtahedi et al. 2015). This evidence suggests that individuals do not rely primarily on memory of digit forces in unconstrained grasping. We have proposed that the predominant mechanism involves online feedback of digit position to change the force distribution every time an object is grasped at novel contact points (Fu et al. 2010, 2011).
The ability to modulate digit forces to position raises the question as to whether adding choice of digit placement to manipulation would elicit distinct interactions among cortical grasp regions. Allowing choice of contact points has revealed differences in brain activation (Marneweck et al. 2018) and corticospinal excitability (CSE) (Davare et al. 2019). However, the causal role of primary motor and somatosensory cortices (M1 and S1, respectively) for the control of digit forces and position remains to be established. We addressed this issue by combining brain stimulation and a dexterous manipulation paradigm. We hypothesized that, in unconstrained grasping, M1 and S1 are both involved in digit force-to-position modulation: S1 would relay somatosensory feedback about digit position to M1, while M1 would process this feedback to modulate digit forces. In contrast, in constrained grasping, M1 but not S1 is involved in the retrieval of digit forces used in previous manipulations (Chouinard 2006; Schabrun et al. 2008). Therefore, we predicted that a virtual lesion to M1 or S1 in unconstrained grasping should interfere with digit force-to-position modulation. In contrast, in constrained grasping, a virtual lesion to M1 should only impair retrieval of digit forces used in previous manipulations. This hypothesis is based on evidence implicating M1 with building, storing, and retrieving sensorimotor memories of grasp forces in constrained grasping (Chouinard et al. 2005; Jenmalm et al. 2006). Lastly, based on the previous work (Schabrun et al. 2008), we expected a virtual lesion to S1 to have no effect on digit forces.
Materials and Methods
Subjects
Seventy-eight naïve right-handed volunteers (22 ± 4.29 years [mean ± standard deviation {SD}]; 35 females) with normal or corrected-to-normal vision and no history of musculoskeletal disorders or neurological disease participated in this study. Subjects were screened for potential risks of adverse reactions to transcranial magnetic stimulation (TMS) using the Transcranial Magnetic Stimulation Adult Safety Screen (Keel et al. 2001) and gave their written informed consent according to the Declaration of Helsinki. All protocols were approved by the institutional review boards at Arizona State University and the University of Houston.
Grip Device
A custom-designed inverted T-shaped object instrumented with two six-dimensional force and torque transducers (Nano 25; ATI Industrial Automation, Garner, NC) (Fig. 1A) was used to record forces and torques exerted by the index fingertip and thumb. Graspable surfaces (sandpaper, grit #320) consisted of two long parallel PVC plates (140 × 22 mm), each mounted vertically on one transducer (Fig. 1A). The grip device measured grip and load force (normal and tangential/vertical to the graspable surface) and each digit’s center of pressure. The transducers’ location relative to the graspable surfaces was blocked from the subject’s view to prevent visual cues from biasing the choice of digit placement. A 400 g mass was placed in the right (relative to the subject) compartment at the base of the grip device and was hidden from view to prevent subjects from anticipating the object’s mass distribution. The added mass created an external torque in the frontal x–y plane of 255 N mm (Text, Fig. 1A). The object’s total mass was 790 g. Each end of the object’s base was placed on a lift switch. The release of either switch by upward movement of the object from the table signaled object lift onset. We used a wireless inertial measurement unit (IMU; Emerald, APDM) fastened to the top of the object to measure object tilt during the lifting phase.
Figure 1.

Grip device, experimental conditions, and experimental variables. (A) Schematic and free-body diagrams of the custom-built grip device for the unconstrained and constrained grasp conditions. (B) Experimental variables are shown for one representative trial of the manipulation task performed using an unconstrained grasp. From top to bottom, traces are the thumb and index finger center of pressure (CoP), load and grip forces, compensatory and external torque (Tcom, thick line, and Text, dotted horizontal line, respectively), and object roll (thin line). The sign of Tcom has been inverted for graphical purposes. At object contact, the index finger is placed higher than the thumb and exerts larger load force. Nearly identical grip force is exerted by each digit. This subject generates a Tcom that approaches Text at object lift onset, thus minimizing object roll (thin line; peak value <5°). Vertical dashed lines from left to right denote reach onset, contact, and object lift off.
Experimental Protocol
Subjects sat comfortably in a custom TMS chair (Rogue Research Inc.) with the right hand pronated and resting on a hand switch. Subjects were asked to reach and grasp the grip device placed on a table 15 cm in front of them using the thumb and index fingertip at a self-selected speed, lift the object vertically to a height of 5–10 cm above the table, hold the object for 2–3 s, replace the object on the table, and return their hand to the hand switch until the next trial. During each trial, subjects were asked to lift the object as straight as possible, that is, to prevent the object from rotating on the frontal plane due to the right-sided asymmetrical mass distribution (Fig. 1A). A successful performance required subjects to exert a compensatory torque (Tcom) of the same magnitude but in the opposite direction of Text in an anticipatory fashion, that is, at object lift onset (Fu et al. 2010). We chose to study this task over the classic task of lifting an object with a symmetrical center of mass because the task goal of object roll minimization introduces an element of dexterity in addition to those that have been extensively studied, for example, modulating normal force to load force to prevent object slip during lift and hold. Importantly, by combining our dexterity requirement with choice of contact points, we had earlier found that digit load force distribution is modulated to variable digit position prior to object lift onset on a trial-to-trial basis (see Introduction). It is important to note that this phenomenon of digit force-to-position modulation is also found when manipulating objects with a symmetrical mass distribution and there is no requirement for exerting a compensatory torque—in fact, in this scenario the covariation between digit load force distribution and position is even stronger than when manipulating objects with an asymmetrical mass distribution (see Fig. 8C in Fu et al. 2010). These observations led to the proposition that digit force-to-position modulation is a task- and object-independent phenomenon underlying skilled manipulation through unconstrained grasp contacts (Santello 2018).
Subjects were asked to perform our manipulation task by either allowing them to choose grasp contact locations (unconstrained grasping, uncon) or constraining contact locations by visually cueing grasp points on the object (constrained grasping, con) (top and bottom objects, respectively, in Fig. 1A). The functional roles of M1 and S1 underlying control of “con” and “uncon” grasping were investigated in two separate experiments using TMS.
For the uncon grasping condition, subjects were instructed that they could grasp anywhere along the vertical plates to perform the task. We asked subjects to perform a learning block of 10 uncon trials (Fig. 2). The con grasping condition in the TMS experiment was designed to address the question of whether the control of digit force and position differed following a virtual lesion to M1 (Fig. 2). To allow comparison with previous studies of con grasping, subjects were instructed to grip the object at fixed collinear locations indicated by a horizontal marker placed across the front of the object (vertical length: 20 mm; see Fig. 1A). For both uncon and con grasping conditions, a computer monitor placed behind the object presented two visual cues to the subject to guide each trial. The first “ready” cue signaled the beginning of a trial, and after a random delay (1–3 s), subjects were shown a “go” cue to initiate the reach. To allow subjects to learn the dynamics of the object, they were asked to perform 10 practice trials (“Learn” block) (Fig. 2). Following this block, subjects then performed two blocks (“Pre” block, “Post” block) of 15 trials each. TMS was delivered between the Pre and Post block (see below). Each block was separated by a rest time of 5 min (Fig. 2).
Figure 2.

Experimental protocols. We delivered spTMS and/or cTBS. The experimental groups (n = 4) performed our manipulation task in both con or uncon grasp conditions and received cTBS over M1 or S1. All control groups (n = 5), with the exception of No Move, were tested in the uncon grasp condition. All groups, with the exception of No Stim, received spTMS before and after cTBS. All groups, with the exception of No Move, performed 10 repetitions of the manipulation task during the Learn block and 15 repetitions each during the Pre- and Post-cTBS blocks.
TMS: Procedures
We delivered single-pulse TMS (spTMS) to the primary motor cortex (M1) using a Rapid2 stimulator (Magstim, 70-mm figure-of-eight coil). The TMS coil was held tangential to the scalp, perpendicular to the direction of the central sulcus, 45° from the midsagittal line, with the handle pointing backward to induce current in the posteroanterior direction (Mills et al. 1992). Suprathreshold TMS pulses delivered over contralateral (left) M1 representing the right first dorsal interosseous (FDI) muscle were used to estimate resting motor threshold (rMT) (Parikh et al. 2014; Davare et al. 2019). rMT was defined as the TMS intensity that induced 50 μV peak-to-peak motor evoked potentials (MEPs) in 5 of 10 trials in the FDI muscle. Active motor threshold (aMT) was estimated by stimulating M1 at the same site used for rMT while the subject maintained a static contraction using the thumb and index finger on the object at approximately 20% of maximum voluntary contraction, defined as the average of three trials. We defined aMT as the TMS intensity that induced 200 μV peak-to-peak MEPs in 5 of 10 trials in the FDI muscle (Parikh and Santello 2017). To assess CSE, we delivered spTMS with the intensity set at 120% of rMT over the identified FDI region.
We delivered continuous theta burst stimulation (cTBS) to M1 and S1 at an intensity of 80% of aMT (Parikh and Santello 2017) to transiently disrupt neural activity. Repetitive pulses were delivered in the form of three pulses at 50 Hz repeated every 200 ms for 40 s (600 pulses) (Huang et al. 2005; Parikh and Santello 2017). We assessed the CSE to verify the effects of cTBS over M1 and S1 on the excitability of corticospinal tract. Earlier studies have found a reduction in the size of MEP, a measure of CSE, following cTBS over M1 (Huang et al. 2005) and S1 (Jacobs et al. 2012).
For M1 cTBS, the TMS coil was positioned over the left cerebral hemisphere representing the right FDI muscle, as identified during rMT estimation. For S1 cTBS, we positioned the TMS coil over the postcentral gyrus posterior to the M1 FDI hotspot (Ni et al. 2009). To locate the stimulation site, we used high-resolution T1-weighted MRI scan (3T Philips Ingenia scanner) obtained from each subject and used it to reconstruct a three-dimensional brain to display the cortical surface (Brainsight software, Rogue Research Inc.) (Parikh and Santello 2017). The mean Montreal Neurological Institute coordinates of the stimulation sites for left S1 were −41.75 ± 10.37, −25.27 ± 15.21, and 57.11 ± 5.47 (x, y, z, mean ± SD; n = 20). For vertex stimulation (see below), the TMS coil was positioned over Cz, based on the 10–20 international system (Jasper 1958) with the TMS handle oriented posteriorly in alignment with the interhemispheric fissure (Legon et al. 2013). The coil position for S1 and vertex was confirmed by the delivery of single TMS pulses at 120% of rMT to ensure that there were no MEPs in the FDI muscle.
TMS Experiment: Experimental Groups
We delivered cTBS to four groups of subjects (Fig. 2). We stimulated M1 and S1 of subjects performing the con and uncon grasping condition (“M1 con”, “S1 con”, M1 uncon, and S1 uncon; n = 10 in each group). cTBS was delivered between the Pre and Post blocks. CSE was assessed using spTMS immediately before and 5 min after cTBS (Huang et al. 2005) (Fig. 2).
TMS Experiment: Control Groups
We performed five control experiments to assess the specificity of cTBS effects over M1 and S1 and the efficacy of the cTBS protocol: “Vertex” (n = 10), “Sham” (n = 10), “No cTBS” (n = 6), “No Stim” (n = 6), and “No Move” (n = 6). All control groups, with the exception of the No Move group, performed the manipulation task in the uncon grasping condition (Fig. 2). Unless otherwise stated, cTBS occurred between the Pre and Post blocks, and CSE was assessed over contralateral (left) M1 region immediately after the Pre block and before the Post block (Fig. 2).
In the Vertex group, cTBS was delivered over the vertex to assess specificity of cTBS-induced effects observed in the M1 and S1 groups (Kobayashi et al. 2004; Parikh and Santello 2017).
In the Sham group, cTBS was delivered using a second coil placed directly behind the TMS chair’s headrest with current directed away from the scalp while the coil over the contralateral (left) M1 remained in place. Thus, subjects heard the sound elicited by stimulation, but did not experience any somatosensory effect of stimulation on the scalp. This group was used to control for any somatosensory effects caused by the auditory cue of cTBS on the control of object manipulation (Duecker et al. 2013).
For the No cTBS group, No cTBS stimulation was used. CSE was assessed to study the influence of MEP-induced movements on object manipulation control. This served to quantify the potential effects of MEP-induced movements on manipulation performance. Muscle twitches caused by spTMS over M1 have been shown to affect grasping behavior in subsequent lifts (Nowak et al. 2005). Therefore, the results of this control condition were analyzed to ensure that any change in behavior found in the experimental groups was specifically due to a “virtual lesion” over the cortical area targeted by cTBS.
Subjects in the No Move group received cTBS over contralateral M1 and saw the same visual cues as those presented to all other groups. However, they were asked to remain at rest when seeing the go cue rather than performing the motor task. This control group was used to validate the effects of cTBS over M1 on MEP size using the protocol that has been previously reported in the literature (Huang et al. 2005).
Subjects in the No Stim group received neither spTMS nor cTBS (Fig. 2). This group was used to control for any somatosensory effects caused by spTMS and cTBS on the control of object manipulation (Duecker et al. 2013).
Behavioral Data Analysis
Data from force/torque sensors and the IMU gyroscope (range of ±2000°/s and noise density of 0.05 rad/s/√Hz) were sampled at 1000 and 128 Hz, respectively. Force, torque, and object roll data were used to compute the following variables (Mathworks): 1) “Digit forces”: Digit tangential force (Ftan) is the vertical force component parallel to the grip surface produced by each digit to lift the object (Fig. 1A,B). Digit load force data exerted by each digit were used to compute the difference between thumb and index finger load forces (Ftan1–Ftan2 = dLF). Digit normal force (Fn) is the force component normal to the grip surface produced by each digit (Fig. 1A,B). Digit grip force was defined as the average of the thumb and index finger normal forces ([Fn1 + Fn2]/2 = FGF). 2) “Digit center of pressure”: The center of pressure of the thumb and index fingertip (CoP1 and CoP1, respectively) was computed using the force and torque output of each sensor (Fu et al. 2010) (Fig. 1A,B). CoP data were then used to compute the vertical distance between the CoP on the thumb and finger side of the grip device (CoP1—CoP2 = dY). We computed the compensatory torque exerted on the object (Tcom, Fig. 1A,B) using the following equation:
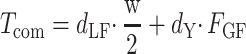 |
(1) |
where “w” denotes the width of the object. 3) “Peak object roll”: Our previous studies have demonstrated that Tcom is a valid predictor of manipulation performance, that is, object roll. Specifically, as subjects learn the appropriate Tcom required to minimize object roll, peak object roll negatively correlates with the magnitude of Tcom (25, 26, 31, 63). This was confirmed by a significant linear correlation between Tcom and peak object roll (Pearson correlation coefficient on data pooled across all experimental groups and subjects: 0.68; P < 0.001). We also note that the results of the analysis of peak object roll and Tcom were identical across all experimental and control groups. Therefore, as both variables capture two interrelated phenomena associated with learning dexterous manipulation, for the sake of brevity, we report only results of the analysis of Tcom.
All of the above variables were computed at the time of object lift onset to quantify anticipatory control of manipulation (Lukos et al. 2007; Fu et al. 2010). Object lift onset was defined as the time at which the first of the two object switches was released from the object switch plate and remained open for 50 ms.
TMS Data Analysis
Electromyography (EMG) signals were recorded from the right FDI muscle using bipolar surface electrodes (Delsys Bagnoli System) and digitized at 5 kHz (Power 1401 Cambridge Electronic Design). For MEP analysis, we removed trials in which EMG activity during the 150-ms window prior to the spTMS was larger than two SDs of the mean baseline activity (calculated as the mean of the rectified EMG signal during a short period of rest). This was done to ensure that recorded MEP values were not affected by baseline EMG activity at the time of TMS stimulation (Parikh et al. 2014). Peak-to-peak MEP amplitudes (mV) were measured and extracted using a custom-written Spike2 script and analyzed using MATLAB. We did not remove any outlier during rMT and aMT estimations. EMG signals were screened online and recorded during cTBS stimulation to verify that cTBS did not evoke MEPs.
Statistical Analysis
We assessed subjects’ ability to perform the manipulation task by comparing Tcom from the first trial with the average of the last five trials of each block (Learn, Pre, Post) within and across experimental groups (Fig. 2). Our previous work has shown that subjects quickly learn to generate the necessary Tcom (Fig. 3) within the first three trials so as to minimize object roll (Salimi et al. 2003; Fu et al. 2010). Analysis of the first trial of each block thus allowed the assessment of subjects’ performance without any previous experience (“learn1”), and recall of stored sensorimotor memory of grasp position and forces is acquired after learning the manipulation task (“pre1,” “post1”). Subsequently, averaging the last five trials of each block (“learn5,” “pre5,” “post5”) was performed to obtain a measure of stability of performance for each block.
Figure 3.

Compensatory torque: Experimental groups and control condition (Vertex). (A) Compensatory torque (Tcom) during the Learn, Pre, and Post blocks in the M1 con, M1 uncon, S1 con, S1 uncon, and Vertex groups. The horizontal dashed line denotes the external torque induced by the added mass at the bottom of the object (Text) that should be compensated for by Tcom. Shaded data denote trials used for plotting in B and analysis. (B) Tcom on the first trial and the average of the last five trials for each block. **P < 0.0125. Data are averages (±SE) of all subjects.
To assess learning-related changes in Tcom, we performed a 5×2 between-within repeated-measures (rm) ANOVA with “Group” (5 levels: M1 uncon, M1 con, S1 uncon, S1 con, Vertex) as the between-subject factor and “Block” (2 levels: learn1, learn5) as the within-subject factor. To assess learning-related changes in Tcom in control groups, we performed a 4×2 between-within repeated-measures (rm) ANOVA with Group (4 levels: Sham, no cTBS, No Stim, Vertex) as the between-subject factor and Block (2 levels, learn1, learn5) as the within-subject factor. We chose to include the Vertex group in this analysis to ensure that there were no differences across any of the control groups. This inclusion also served to validate that having included any control groups in the main analysis with the M1 con, M1 uncon, S1 con, and S1 uncon groups would have produced similar results.
To confirm that subjects’ performance remained stable during trials after learning and prior to cTBS in the experimental and control groups, we performed separate 5 × 3 between-within repeated measures ANOVA (rmANOVA) with Group as the between-subject factor and Block (3 levels: learn5, pre1, pre5) as the within-subject factor. To assess the effects of cTBS on Tcom in the experimental and control groups, we performed separate 5 × 3 between-within rmANOVA with Group as the between-subject factor and Block (three levels: pre5, post1, post5) as the within-subject factor. Post hoc t-tests and one-way ANOVAs were used to compare between- and within-group differences, respectively.
We performed separate one-way rmANOVA to assess the effects of cTBS on individual Tcom components (load force distribution, dy, and grip force). We chose to perform only within-group analyses for individual digit position and force data because subjects exhibit idiosyncratic patterns of digit force–position relations (Fu et al. 2010, 2011; Zhang et al. 2010), for example, some subjects may have chosen to vertically spread the thumb and index fingertip more than other subjects, thus resulting in different digit force distributions. These across-subject differences in digit force–position relations prior to cTBS could have confounded the effects of TMS. We note that a between-group statistical design is not an issue for the analysis of Tcom because all subjects are required to exert the same Tcom. Finally, we used one-sample t-tests on the percentage changes (post- vs. pre-cTBS or following rest for the No Move group) in MEP data. We applied Huynh–Feldt corrections when sphericity assumption was violated. We used Dunnett’s post hoc t-test to compare each experimental group with the control group (i.e., Vertex). We used Bonferroni t-test for post hoc comparisons between experimental groups. For within-subject factors, we performed post hoc comparisons using paired t-tests with appropriate Bonferroni corrections.
Our analysis examining the difference in Tcom of pre5 and post1 trials allowed us to quantify the immediate effect of cTBS to different neural sites. This revealed differential changes in Tcom components (Fig. 4A,B). To understand whether these effects persisted or changed during the 15 post-cTBS trials, we calculated the difference of each post-cTBS trial and pre5 data to create a time series of values. We notate these values with a ∆ to represent the difference; for example, the difference in dY pre- versus post-cTBS is denoted as ∆dY. To analyze the potential changes in these components as a function of Group over the time course of “post-cTBS trials” (1:15), ∆load force distribution, ∆grip force, and ∆dY were analyzed using a repeated-measures mixed-model framework. This approach allows us to account for between-subject variability that often accompanies behavioral responses to TMS, rendering a more robust estimate of post-TMS effects while considering all groups and subjects’ variability simultaneously. The rationale underlying our statistical analysis is that mixed-model approaches are more attuned to detect differences between groups, given that the between-subject and between-group variability for an intercept (the measure of first post-TMS trial effects), on any given variable (e.g., dY), is likely to differ post-TMS. Group entered the model as a between-subject categorical factor, and post-cTBS trials entered as a continuous covariate. Because we anticipated time-based effects due to the sequential nature of the task, we set the residual-covariance matrix to be scaled within subject and have an autoregressive (lag 1) structure. The model was also set to include random intercepts for individual subjects. Each model was fitted using restricted maximum likelihood and always started with a full structure with both the effects and interaction of Group and post-cTBS trial. This full model was compared to each of the simpler, nested models of just the effects using a likelihood ratio test to determine the appropriate model. All of the analyses were computed in the R environment using the lme4 (Pinheiro et al. 2017) and lmerTest (Kuznetsova et al. 2019) packages.
Figure 4.

Effect of cTBS on digit load force, grip force, and position across all experimental conditions. (A) From top to bottom, traces denote time course of the difference between the thumb and index finger load force, grip force averaged across the thumb and index finger, and vertical distance between the thumb and index finger center of pressure (dy) from contact (“0”) to object lift onset. Data are averages of the last five trials prior to cTBS (pre5) and first trial following cTBS (post1). dY data are plotted from the time at which they can be accurately estimated using force and torque sensors (Fu et al. 2010). Data from each experimental group are shown across columns. Shaded plots denote Tcom variables that were significantly affected by cTBS. (B) Data from pre5 and each post-cTBS trial are shown for each Tcom variable and experimental group. ** denotes P < 0.0125. Data are averages (±SE) of all subjects.
Results
For both constrained and unconstrained grasp contexts (con and uncon, respectively), the task consisted of grasping and lifting a sensorized object using the thumb and index fingertip. The task’s goal was to minimize object roll during lift. Participants achieved this goal by exerting a compensatory torque (Tcom) on the object prior to object lift to counteract the object’s external torque (Text) caused by its asymmetrical mass distribution (Fig. 1A; eq. 1, see Materials and Methods). As expected from our previous work, we found a significant negative correlation between Tcom and peak object roll (see Materials and Methods). Therefore, for brevity we focus on Tcom as the measure of anticipatory grasp control and manipulation performance.
Learning Dexterous Manipulation in Constrained and Unconstrained Grasping
The con and uncon grasp contexts differed only in terms of whether object contact locations were visually cued or could be chosen by the subject. During the Learn and pre-cTBS trial blocks (Fig. 2), subjects from all experimental groups learned to generate compensatory torque (Tcom) appropriate to minimize object roll. Learning of Tcom occurred within the first three trials, after which Tcom was consistently attained (Fig. 3A). Trial-to-trial modulation of digit load force distribution, grip force, and digit position (dY) measured at lift onset was similar to that described in previous work (Fu et al. 2010, 2011; Zhang et al. 2010). dY and load force distribution from each trial were normalized to generate z-scores and used for linear regression analysis (Fu et al. 2010).
As Tcom is learned within the first three trials (Fu et al. 2010, 2011), we used trials 4–10 of the Learn block and all trials in the Pre block (22 trials per subject) for the TMS experiment. As expected from our previous work, we found 1) higher dy variability in uncon than con grasping conditions (P = 0.02), and 2) the larger dY variability in uncon was compensated by trial-to-trial modulation of load force distribution (Fu et al. 2010, 2011; Davare et al. 2019). Specifically, we found significant negative correlations between load force distribution and dY only for the uncon grasping condition. We found significant negative correlations between load force distribution and dy in M1 uncon, S1 uncon, and Vertex conditions (r = −0.45, −0.67, and −0.46; all P < 0.0001), but not in M1 con and S1 con groups (r = 0.08 and −0.12, respectively; all P > 0.1).
To determine the extent to which the role of M1 and S1 is grasp context-dependent, we conducted TMS experiments (Fig. 2).
A Virtual Lesion of M1 and S1 Impairs Execution of Learned Manipulation in a Grasp Context-Specific Fashion
On the first trial of the Learn block, subjects were unaware of the object’s mass distribution, as the object is visually symmetrical and therefore exerted negligible Tcom (Fig. 3A; Learn 1, Fig. 3B). Consistent with the previous work (Fu et al. 2010), all subjects quickly learned to compensate for the object’s mass distribution and generated the necessary Tcom over the remaining trials of the Learn block (main effect of Block, F1,45 = 522.14, P < 0.0001, 0.92; Fig. 3B) similarly across experimental and control groups (no significant Group × Block interaction: F4,45 = 2.26, P = 0.08 or main effect of Group, F4,45 = 1.71, P = 0.16). Following learning, Tcom was stable for all subjects during the remaining Learn and Pre block trials across experimental and control groups (no significant Group × Block interaction, F7.85,88.32 = 0.8, P = 0.6; no main effect of Block: F1.96, 88.32 = 2.69, P = 0.08; or no main effect of Group: F4,45 = 0.69; P = 0.6; Fig. 3B). We delivered cTBS to the M1 con, M1 uncon, S1 con, S1 uncon, and Vertex groups immediately following the Pre block, but prior to the beginning of the Post block (Fig. 2). We selected the vertex as a neutral control site to assess the specificity of cTBS-induced effects on the control of our manipulation task following stimulation of M1 and S1.
0.92; Fig. 3B) similarly across experimental and control groups (no significant Group × Block interaction: F4,45 = 2.26, P = 0.08 or main effect of Group, F4,45 = 1.71, P = 0.16). Following learning, Tcom was stable for all subjects during the remaining Learn and Pre block trials across experimental and control groups (no significant Group × Block interaction, F7.85,88.32 = 0.8, P = 0.6; no main effect of Block: F1.96, 88.32 = 2.69, P = 0.08; or no main effect of Group: F4,45 = 0.69; P = 0.6; Fig. 3B). We delivered cTBS to the M1 con, M1 uncon, S1 con, S1 uncon, and Vertex groups immediately following the Pre block, but prior to the beginning of the Post block (Fig. 2). We selected the vertex as a neutral control site to assess the specificity of cTBS-induced effects on the control of our manipulation task following stimulation of M1 and S1.
Following cTBS over M1 and S1, but not vertex, subjects were unable to exert the previously learned Tcom (significant Group × Block interaction: F7.6, 85.53 = 4.36, P < 0.0001, 0.28; main effect of Block: F1.9, 85.53 = 33.55, P < 0.0001,
0.28; main effect of Block: F1.9, 85.53 = 33.55, P < 0.0001, 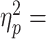 0.43; main effect of Group, F4,45 = 3.58, P = 0.013,
0.43; main effect of Group, F4,45 = 3.58, P = 0.013,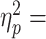 0.24; Fig. 3B). Specifically, on the first trial following cTBS (post1), subjects in all experimental groups exerted significantly smaller Tcom than those in the Vertex group (Dunnett’s t-test: all P-values ≤ 0.004, 1.62 < Cohen’s d < 2.82), although Tcom reduction was not significantly different across experimental groups (Bonferroni’s t-test: all P-values > 0.99).
0.24; Fig. 3B). Specifically, on the first trial following cTBS (post1), subjects in all experimental groups exerted significantly smaller Tcom than those in the Vertex group (Dunnett’s t-test: all P-values ≤ 0.004, 1.62 < Cohen’s d < 2.82), although Tcom reduction was not significantly different across experimental groups (Bonferroni’s t-test: all P-values > 0.99).
However, the persistence of the cTBS effect on Tcom during the Post block was dependent on whether subjects performed the manipulation task in the con or uncon condition and the cortical area targeted by cTBS. For the M1 uncon, S1 uncon, and S1 con groups, Tcom impairment was short-lived, returning to the same magnitude as Tcom exerted by the Vertex group at the end of the Post block (Dunnett’s t-test: all P-values > 0.43; Fig. 3B). In contrast, the drop in Tcom for the M1 con group persisted until the end of the Post block, as revealed by significantly smaller Tcom relative to the Vertex group (Dunnett’s t-test: P = 0.016, Cohen’s d = 1.12). There was no difference in Tcom at the end of the Post block between the Vertex group and M1 uncon, S1 uncon, and S1 con groups (Dunnett’s t-test: all P-values > 0.9).
Because Tcom results from the coordination of digit position (dy), grip force, and load force distribution (eq. 1, see Materials and Methods), we analyzed the extent to which virtual lesions affected each of these variables for each experimental group using the same subset of trials as those used for the above Tcom analysis. The similar reduction in Tcom observed on the first trial following cTBS could have been interpreted as a nonspecific effect on Tcom variables regardless of grasp type and cortical area targeted by TMS. However, the different time courses after cTBS (Fig. 3) indicate that digit position and forces were highly sensitive to the grasp context and cortical area being stimulated.
Constrained Grasping: Virtual Lesions of M1 and S1
The M1 and S1 con groups learned Tcom within the first few trials of the Learn block by exerting a greater load force on the index finger than the thumb [negative load force distribution; eq. 1, Materials and Methods; this behavior has also been described in previous studies (Fu et al. 2010, 2011; Zhang et al. 2010)]. Furthermore, participants consistently exerted the learned Tcom throughout the blocks of trials preceding cTBS.
Disruption of M1 Impairs Retrieval of Learned Grip and Load Forces
Following cTBS, M1 con participants were unable to retrieve and use the same digit forces used in previous trials, such retrieval being a key feature of con grasping (Johansson and Westling 1984, 1988). This effect of cTBS started ~ 200 ms after contact, leading to significantly smaller digit forces by the time the object was lifted (Fig. 4A, M1 con column) (main effect of Block: F2,18 = 14.30 (P < 0.0001, 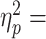 0.61), load force distribution, and 13.76 (P < 0.0001,
0.61), load force distribution, and 13.76 (P < 0.0001,  0.60) grip force. Load force distribution and grip force on the first Post block trial were significantly smaller than on the late Pre block trials (post1 vs. pre5: t9 = −7.081 (P < 0.0001, Cohen’s dz = 2.24) and 3.936 (P = 0.0017, Cohen’s dz = 1.24), respectively; Fig. 4A). Importantly, and in contrast to digit forces, dy was unaffected by cTBS (no main effect of Block, F2,18 = 0.045, P = 0.956;
0.60) grip force. Load force distribution and grip force on the first Post block trial were significantly smaller than on the late Pre block trials (post1 vs. pre5: t9 = −7.081 (P < 0.0001, Cohen’s dz = 2.24) and 3.936 (P = 0.0017, Cohen’s dz = 1.24), respectively; Fig. 4A). Importantly, and in contrast to digit forces, dy was unaffected by cTBS (no main effect of Block, F2,18 = 0.045, P = 0.956; 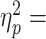 0.005; Fig. 4A,B). These results indicate that the effect of cTBS on Tcom in con grasping (Fig. 3) was due to selective disruption of the retrieval of learned digit forces while sparing the control of visually cued digit position.
0.005; Fig. 4A,B). These results indicate that the effect of cTBS on Tcom in con grasping (Fig. 3) was due to selective disruption of the retrieval of learned digit forces while sparing the control of visually cued digit position.
Disruption of S1 Does Not Impair the Modulation of Digit Forces or Position
Unlike the M1 con group, none of the Tcom components was affected by cTBS in the S1 con group (no main effect of Block; load force distribution, F1.3, 11.74 = 2.6, P = 0.13; grip force, F2,18 = 2.2, P = 0.14; and dY, F1.8, 16.5 = 3.5, P = 0.06; Fig. 4A, S1 con column). These results indicate that the effect of cTBS of S1 on con grasping was not due to a significant disruption of the retrieval of learned digit forces or the control of visually cued digit position. Therefore, the reduction in Tcom on the first post-cTBS trial (Fig. 3) was caused by a small, nonsignificant effect on dY and its multiplicative effect on grip force contribution to Tcom (eq. 1, see Materials and Methods).
Unconstrained Grasping: Virtual Lesions of M1 and S1
Consistent with our previous behavioral work (Fu et al. 2010, 2011; Zhang et al. 2010), M1 and S1 uncon groups learned to exert Tcom to counter the clockwise Text by exerting greater load force with the index finger and placing it higher than the thumb (negative load force distribution and dY respectively; eq. 1, Methods). Unlike con grasping, load force distribution and dy significantly covaried across the Learn and Pre block of trials.
Disruption of M1 Impairs Control of Learned Digit Position and Modulation of Load Force
cTBS to M1 impaired subjects’ ability to use similar digit positions learned in previous uncon trials (main effect of Block: F2,18 = 10.29, P = 0.001,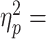 0.53). Specifically, on the first post-cTBS trial, the vertical distance between the thumb and index finger center of pressure (dY) significantly decreased relative to the late Pre block trials (post1 vs. pre5: t9 = −4.384, P = 0.002, Cohen’s dz = 1.39) (Fig. 4A, M1 uncon column). Note that the large change in dY caused by cTBS was not accompanied by a significant modulation of load force distribution or grip force (load force distribution: no main effect of Block, F2,18 = 5.27, P = 0.051; grip force: no main effect of Block, F2,18 = 0.16, P = 0.85). This is an important observation, given that the modulation of load force distribution to dY is a key feature of uncon grasping, which was found during Learn and Pre block trials. Thus, the effects of cTBS during uncon grasping were opposite to those found for con grasping: Virtual lesion to M1 impaired the control digit placement, but not digit forces. These results indicate that the lack of modulation of load force distribution to the cTBS-induced change in dY caused the drop in Tcom in the early trials of the uncon grasping condition (Fig. 3).
0.53). Specifically, on the first post-cTBS trial, the vertical distance between the thumb and index finger center of pressure (dY) significantly decreased relative to the late Pre block trials (post1 vs. pre5: t9 = −4.384, P = 0.002, Cohen’s dz = 1.39) (Fig. 4A, M1 uncon column). Note that the large change in dY caused by cTBS was not accompanied by a significant modulation of load force distribution or grip force (load force distribution: no main effect of Block, F2,18 = 5.27, P = 0.051; grip force: no main effect of Block, F2,18 = 0.16, P = 0.85). This is an important observation, given that the modulation of load force distribution to dY is a key feature of uncon grasping, which was found during Learn and Pre block trials. Thus, the effects of cTBS during uncon grasping were opposite to those found for con grasping: Virtual lesion to M1 impaired the control digit placement, but not digit forces. These results indicate that the lack of modulation of load force distribution to the cTBS-induced change in dY caused the drop in Tcom in the early trials of the uncon grasping condition (Fig. 3).
Disruption of S1 Impairs the Modulation of Load Force Distribution
cTBS over S1 affected only digit load force distribution, load force distribution being significantly reduced relative to Pre block trials (main effect of Block, F2,18 = 16.50, P < 0.0001,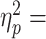 0.65; post1 vs. pre5, t9 = −4.187, P = 0.002, Cohen’s dz = 1.32; Fig. 4A, S1 uncon column). In contrast, grip force and dy were statistically indistinguishable from trials preceding cTBS (grip force: no main effect of Block, F2,18 = 0.867; P = 0.44 and dy: no main effect of Block, F2,18 = 2.34; P = 0.13; Fig. 4A). Therefore, the reduction in load force distribution was the primary cause of Tcom reduction on the first post-cTBS trial (Fig. 3).
0.65; post1 vs. pre5, t9 = −4.187, P = 0.002, Cohen’s dz = 1.32; Fig. 4A, S1 uncon column). In contrast, grip force and dy were statistically indistinguishable from trials preceding cTBS (grip force: no main effect of Block, F2,18 = 0.867; P = 0.44 and dy: no main effect of Block, F2,18 = 2.34; P = 0.13; Fig. 4A). Therefore, the reduction in load force distribution was the primary cause of Tcom reduction on the first post-cTBS trial (Fig. 3).
Persistence of cTBS Effects on Compensatory Torque Components Is Sensitive to Grasp Context and Cortical Area
Analysis of the first post-cTBS trial, as well as subsequently post-cTBS trials, revealed a differential effect on Tcom components depending on the cortical area being stimulated and grasp context (Fig. 4A,B). Therefore, we quantified how these changes in Tcom components persisted over post-cTBS trials. We used mixed models to examine changes in each Tcom component over post-cTBS trials (see Methods). For each Tcom variable, the model used the difference (∆) of the average of pre5 trials and each post-cTBS trial as the dependent variable.
The results for ∆load force distribution revealed a significant interaction between Group and post-cTBS trial (F1,3 = 3.25, P < 0.015). The interaction resulted from a significant difference in slopes between M1 uncon and S1 uncon groups (t24 = −2.41, P = 0.036; Fig. 5, top row). Follow-up examination revealed conditions with slopes different from 0 included M1 uncon (t24 = −2.76, P = 0.027) and S1 uncon (t24 = −2.91, P = 0.021). In contrast, the model examining ∆grip force only revealed an effect of Group (F1,3 = 8.67, P = 0.01). This Group effect is apparent in Figure 5 (middle row), as the mean level of the line for each group differs at a steady state across post-cTBS trials. More specifically, follow-up analysis of the intercept revealed only the M1 con group differed from zero (t12 = −2.02 P = 0.022). Therefore, M1 con was the only experimental condition with the strongest and more persistent effects of cTBS on grip force. The final model examining ∆dy also yielded a significant interaction (F1,3 = 6.95, P < 0.014; Fig. 5, bottom row). Follow-up revealed comparing individual condition slopes to 0 indicated differences for M1 uncon (t24 = 3.24, P = 0.024) and S1 con (t24 = 3.11, P = 0.033), with both exhibiting a positive trend post-cTBS.
Figure 5.

Effect of cTBS on digit placement, load, and grip force. Plots show predicted difference (∆) between the value of each Tcom variable averaged across the last five pre-cTBS trials and each post-cTBS trial. Predicted values were obtained by fitting a mixed model that predicted the variable (e.g., load force distribution) as a function of experimental group and post-cTBS trial. Each plot shows the predicted slope. ✭ and ✭✭ denote a slope significantly different than zero at P < 0.05 and 0.01, respectively.
These findings indicate that the effects of cTBS on digit forces and positions were highly sensitive to the grasp context and cortical area targeted by TMS. Specifically, the persistency of the effects of virtual lesion on Tcom for M1 con grasping throughout all post-cTBS trials (Fig. 3B) can be solely attributed to alteration of grip force, as indicated by the persistent and large nonzero intercept across post-cTBS trials (M1 con, Fig. 5). In contrast, the faster recovery of Tcom to pre-cTBS levels for M1 uncon grasping can be attributed to the re-establishment of a negative covariation between dy and load force distribution (M1 uncon, Fig. 5), despite large trial-to-trial fluctuations in dy (Fig. 4B). For the S1 con group, the quick recovery of Tcom to pre-cTBS level after the first post-cTBS trial was mediated only by adjustments in relative positioning of the thumb and index finger within the marked contact boundaries on the object (Fig. 5). Lastly, the rate at which Tcom recovered within the first 10 post-cTBS trials (Fig. 3B) following cTBS in the S1 uncon group was mostly driven by change in load force distribution (right column, Fig. 4).
cTBS Delivered to Control Groups Does Not Affect Compensatory Torque
We report a significant main effect of Block (F1,28 = 320.46, P < 0.0001, 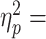 0.91), but no significant Block × Group interaction (F1,28 = 2.50, P = 0.13). Individual rmANOVAs for each group confirmed that the magnitude of Tcom significantly increased by the end of the Learn block (learn1 vs. learn5, Fig. 6) for all control groups (significant main effect of Block, all P < 0.05). After learning, Tcom remained invariant at the beginning of the Pre block (no main effect of Block: F2,56 = 0.267, P = 0.77; no significant Block × Group interaction: F2,56 = 0.572, P = 0.57). This comparison confirmed both that the rest period between the Learn and Pre blocks had no significant effect on the learned Tcom (learn5 vs. pre1) and that Tcom was stable throughout the Pre block (pre1 vs. pre5) (Fig. 6B). These results are identical to those reported for the experimental groups. We found no main effect of Block (F2,56 = 2.73, P = 0.08) nor significant Block × Group interaction (F2,56 = 0.42, P = 0.66). Lastly, between-group comparisons for all control groups revealed no differences during the Learn, Pre, or Post block trials (all P > 0.05, Fig. 6). Therefore, subjects in all control groups attained and maintained similar Tcom throughout the remainder of the Pre and Post blocks. Together, these analyses confirm that cTBS to Vertex, and/or the presence of spTMS between blocks, did not affect skilled object manipulation performance.
0.91), but no significant Block × Group interaction (F1,28 = 2.50, P = 0.13). Individual rmANOVAs for each group confirmed that the magnitude of Tcom significantly increased by the end of the Learn block (learn1 vs. learn5, Fig. 6) for all control groups (significant main effect of Block, all P < 0.05). After learning, Tcom remained invariant at the beginning of the Pre block (no main effect of Block: F2,56 = 0.267, P = 0.77; no significant Block × Group interaction: F2,56 = 0.572, P = 0.57). This comparison confirmed both that the rest period between the Learn and Pre blocks had no significant effect on the learned Tcom (learn5 vs. pre1) and that Tcom was stable throughout the Pre block (pre1 vs. pre5) (Fig. 6B). These results are identical to those reported for the experimental groups. We found no main effect of Block (F2,56 = 2.73, P = 0.08) nor significant Block × Group interaction (F2,56 = 0.42, P = 0.66). Lastly, between-group comparisons for all control groups revealed no differences during the Learn, Pre, or Post block trials (all P > 0.05, Fig. 6). Therefore, subjects in all control groups attained and maintained similar Tcom throughout the remainder of the Pre and Post blocks. Together, these analyses confirm that cTBS to Vertex, and/or the presence of spTMS between blocks, did not affect skilled object manipulation performance.
Figure 6.

Compensatory torque: Control groups. (A) Compensatory torque (Tcom) during Learn, Pre, and Post blocks in the Vertex, No Stim, Sham, and No cTBS groups. (B) Tcom on the first trial and the average of the last five trials for each block. Data are plotted in the same format as Figure 3. **P < 0.0125.
cTBS to M1 and S1 Does Not Reduce Corticospinal Excitability Following Exposure to Object Manipulation
We found no change in CSE after cTBS was delivered over M1 and S1 in the experimental groups (M1 con: t9 = −2.052, P = 0.07; M1 uncon: t9 = −2.314, P = 0.06; S1 con: t9 = −0.98, P = 0.35; and S1 uncon: t9 = −0.991, P = 0.35), nor M1 in the control groups (Sham: t9 = 2.2, P = 0.054; no cTBS: t9 = 0.68, P = 0.51; and Vertex: t9 = 1.88; P = 0.1; Fig. 7). These findings may seem surprising, as previous work reported a reduction in CSE following cTBS to M1 (Huang et al. 2005). Unlike our protocol, however, in this previous work, subjects did not perform a motor task prior to M1 cTBS. This is an important methodological difference, as a later study by the same group reported no reduction in CSE when subjects performed an isometric force contraction during cTBS stimulation (Huang et al. 2008). Therefore, the lack of CSE reduction following cTBS in our study, where subjects performed a series of object lifts prior to cTBS, is consistent with the follow-up study by Huang and colleagues (Huang et al. 2008). Nevertheless, to further validate our cTBS protocol, we performed an additional test on a No move group (n = 6) where we assessed the effects of cTBS over M1 on MEP size without having subjects perform our manipulation task (Fig. 2). In this group and consistent with previous work where subjects performed no motor tasks prior to M1 cTBS (Huang et al. 2005), we found a significant decrease in MEP amplitude (t5 = −7.172, P = 0.001; Cohen’s dz = 2.93; Fig. 7).
Figure 7.

Corticospinal excitability. Change in CSE was assessed as percentage change in the amplitude of MEPs by comparing pre- versus post-cTBS or following rest (No Move group). All groups except the No cTBS group received cTBS over M1, S1, or Vertex. **P < 0.0125. Data are averages (±SE) of all subjects.
Discussion
We found a differential involvement of M1 and S1 in unconstrained relative to constrained grasping. By using TMS, we confirmed previous work on constrained grasping showing that M1 (Chouinard et al. 2005; Jenmalm et al. 2006), but not S1 (Schabrun et al. 2008), mediates a memory-based control of manipulative forces. Importantly, for unconstrained grasping, we demonstrated that integrity of both M1 and S1 is critical as they have complementary roles in mediating digit force-to-position modulation. Together, our results suggest that control of dexterous manipulation relies on a flexible organization of the sensorimotor cortical network depending on whether contact points can be chosen or not.
Effects of cTBS on Grasp Control Variables Are Sensitive to Grasp Context
Our findings extend the role of M1 beyond storage and retrieval of sensorimotor memory of grasp forces (Chouinard et al. 2005; Nowak et al. 2005; Jenmalm et al. 2006) in important ways. When contact points were predictable, virtual lesions to M1, but not S1, prevented retrieval of learned digit forces. Importantly, the M1 con group’s inability to retrieve digit forces and restore pre-cTBS Tcom persisted for all post-cTBS trials. For the S1 con group, the small (nonsignificant) effect of cTBS on dY and Tcom reduction disappeared after the first post-cTBS trial due to small changes in digit centers of pressure. In contrast, when contact points were not as predictable as in constrained grasping, cTBS to M1 elicited two inter-related phenomena: Subjects could not implement digit placement similar to that before delivery of cTBS, and digit forces were not modulated as a function of the new digit placement. Furthermore, cTBS to S1 impaired digit force-to-position modulation. Thus, in both uncon groups, cTBS impaired the critical ability to modulate digit forces to position, but did so by selectively affecting different Tcom variables. Both uncon groups were able to restore digit force-to-position modulation and Tcom within the first five post-cTBS trials.
The different time courses of post-cTBS recovery in each Tcom variable further indicate differences in the roles of M1 and S1 according to the grasp context. The most striking difference was found in the timeline of post-cTBS effects across both con groups, that is, grip force and load force distribution were affected for 15 trials, whereas the small effect on dY lasted 1 trial, respectively. These findings confirm M1—but not S1—is involved in storing or retrieving memory of digit forces. As cTBS to S1 did not affect digit forces, the quick recovery of dY through small changes in digit position in the S1 con group could have been driven by visual feedback of object roll caused by sudden Tcom reduction on the first post-cTBS trial. These results suggest that the memory-based force control mechanism affected by cTBS cannot benefit from visual feedback of manipulation error to the same extent as digit placement, even when such errors continue to occur across multiple trials.
With regard to the uncon groups, cTBS to M1 and S1 again affected different Tcom variables, that is, dY and load force distribution, respectively. Importantly, both groups were able to restore pre-cTBS Tcom by re-establishing digit force-to-position modulation, but did so in different ways. Specifically, the M1 uncon group modulated both dY and load force distribution, whereas the S1 uncon group modulated only load force distribution. We speculate that this recovery in the uncon groups was mediated by visual feedback of object roll, as well as digit placement, which could be changed on a trial-to-trial basis. These differences in short- and long-term effects of cTBS, as well as the Tcom variables affected by the virtual lesion, underscore the complementary yet different roles of M1 and S1 in unconstrained grasping. Moreover, our unconstrained grasping findings indicate the role of M1 is not limited to a memory-based control process (Orban De Xivry et al. 2011; Hamel et al. 2017): It is also directly involved in using trial-by-trial sensory feedback of digit position to scale forces (Fig. 4A). Our focus on S1 was motivated by a long history of research on the role of S1 in the context of online feedback control (London and Miller 2012; Omrani et al. 2016; Wolpaw 2017). Recent evidence further supports this proposition by indicating that S1 is also involved in motor learning (Mathis et al. 2017). Together, these findings suggest that S1 plays a critical role in providing feedback information for both online control and learning.
S1 would be equally important for both grasp contexts in processing tactile afferent inputs triggered by contact and finger pad deformation during the loading phase, as well as monitoring for potential discrepancies between the temporal evolution of grasp events, that is, transition from contact to onset of loading phase, from loading phase to lift onset, from lift onset to object acceleration, and so forth (Johansson and Flanagan, 2009). We hypothesized that S1 is involved in 1) detecting the dY via somatosensory inputs and 2) communicating this feedback to M1 for modulating load force distribution as a function of dY. In constrained grasping, trial-to-trial variability in dY is significantly lower than in unconstrained grasping because contact points are constrained by visual cues on the object. Therefore, participants use the same grip and load forces, thus generating a consistent compensatory torque, on each trial. Although S1 still receives somatosensory inputs triggered by vertically spreading the fingertips (dY), this information is not functionally relevant nor used for the trial-to-trial modulation of load force distribution because there is no need to modulate load forces to dY. Nevertheless, S1 could still contribute to the positioning of the digits in constrained grasping. The small (nonstatistically significant) effect of cTBS in the S1 con condition in the first post-cTBS trial (Fig. 4) suggests that S1 may play a role in fingertip position control even when contact points are visually cued. We speculate that cTBS might have interfered with integration of visual and somatosensory afferent inputs driving fingertip position control in both grasp contexts by reducing the reliability or weight of somatosensory inputs. Further work is needed to understand this integration process.
Primacy of Contact Event for Somatosensory Feedback
We should note that S1’s role in processing sensory inputs associated with digit position is not obligatory. Conceptually, force planning could arise prior to contact, when the digits are visible. While such vision-based force planning is still possible, contact detection through sensory feedback has been shown to be a critical event for signaling the transition from the end of hand transport and onset of force application (Johansson and Flanagan 2009). We propose that feedback during object contact is also instrumental for estimating the relative position of the digits. Importantly, the S1 uncon results indicate that visual feedback of the hand trajectory and contact points—available throughout the task—could not compensate for the effect of cTBS on digit force-to-position modulation. The notion that object contact is the most relevant event for feedback processing in unconstrained grasping is supported by our recent study showing grasp context differences in CSE at contact, but not during the reach (Davare et al. 2019). In summary, our findings support the imperative role of somatosensory feedback of digit position for digit force modulation at object contact.
Grasp Cortical Network for Constrained and Unconstrained Grasping
The cortical network underlying grasp control, which has been defined primarily based on research on constrained grasping, includes premotor dorsal (PMd) and ventral (PMv), M1, S1, and posterior parietal regions (Grafton et al. 1998; Ehrsson et al. 2000; Ehrsson et al. 2001; Kuhtz-Buschbeck et al. 2001; Chouinard et al. 2005; Tunik et al. 2005; Davare et al. 2006; Jenmalm et al. 2006; Grol et al. 2007; Schabrun et al. 2008; Schettino et al. 2015; Parikh and Santello 2017). To the best of our knowledge, only one study examined the extent to which control of constrained and unconstrained grasping is mediated by different brain areas (Marneweck et al. 2018). This fMRI study found that the cerebellum, BA44, and PMv were differentially activated across the two grasp contexts. However, in contrast to our findings, no differences were found in M1 and S1 activity. A direct comparison between these studies is not possible due to methodological differences, that is, Tcom variables could not be measured in the fMRI scanner and subjects were instructed to deliberately vary digit position across trials in the unconstrained condition.
Revised Conceptual Framework of Neural Control of Manipulation
Our theory posits that following object contact, subjects use feedback of digit position to determine the similarity of contact points with those used in previous manipulations. For constrained grasping, sensing digit position is minimally important beyond ensuring force control that satisfies mechanical requirements, that is, normal-to-load force modulation (Johansson and Westling 1984; Johansson et al. 1992). In contrast, during unconstrained grasping force control would be predominantly driven by a mechanism that compares predicted and actual sensory feedback of digit position (Fig. 8). This mechanism is used to determine the relative contribution memory and online feedback depending on the extent to which predicted and actual contact points match. Our findings suggest that allowing or preventing choice of contact points influences the relative contribution of memory and online feedback and therefore modulates sensorimotor cortical interactions for the control of dexterous manipulation.
Figure 8.

Cortical sensorimotor mechanisms for neural control of dexterous manipulation. Prior to object contact, interactions between M1, sensory, as well as premotor and parietal cortical areas lead to hand shaping (Grol et al. 2007; Cavina-Pratesi et al. 2017) and positioning the digits at remembered locations used in previous manipulations. Somatosensory and visual inputs contribute to guiding the hand toward the planned contact points on the object. Following contact, the roles of M1 and S1 for the control of dexterous manipulation differ according to whether contact points are constrained or unconstrained (see text for more details).
Funding
National Science Foundation (NSF) (Collaborative Research Grant BCS-1455866 to M.S.); Core for Advanced MRI (CAMRI) at Baylor College of Medicine to P.J.P.
Notes
We thank Dr Patrick McGurrin for contributing to TMS data collection and analyses. We thank Nishant Rao at the University of Houston for assistance with data collection and MR technologist Lacey Berry, BS, RT(R)(MR) at Baylor College of Medicine for assistance with MR scanning. We thank Drs Qiushi Fu, Jamie Tyler, Jeffrey Kleim, Andrew Gordon, and Marco Davare for comments on the manuscript. The contents are solely the responsibility of the authors and do not necessarily represent the official views of NSF. Conflict of Interest: None declared.
Author Contributions
All authors contributed to the design of the study, data analysis and interpretation, and manuscript preparation. P.J.P performed experiments. All authors approved the final version of the manuscript.
References
- Castiello U. 2005. The neuroscience of grasping. Nat Rev Neurosci. 6:726–736. [DOI] [PubMed] [Google Scholar]
- Cavina-Pratesi C, Connolly JD, Monaco S, Figley TD, Milner AD, Schenk T, Culham JC. 2017. Human neuroimaging reveals the subcomponents of grasping, reaching and pointing actions. Cortex. 98:128–148. [DOI] [PubMed] [Google Scholar]
- Chouinard P a. 2006. Different roles of PMv and PMd during object lifting. J Neurosci.. 26:6397–6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard PA, Leonard G, Paus T. 2005. Role of the primary motor and dorsal premotor cortices in the anticipation of forces during object lifting. J Neurosci.. 25:2277–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare M, Andres M, Clerget E, Thonnard J-L, Olivier E. 2007. Temporal dissociation between hand shaping and grip force scaling in the anterior intraparietal area. J Neurosci. 27:3974–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare M, Andres M, Cosnard G, Thonnard J-L, Olivier E. 2006. Dissociating the role of ventral and dorsal premotor cortex in precision grasping. J Neurosci. 26:2260–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare M, Kraskov A, Rothwell JC, Lemon RN. 2011. Interactions between areas of the cortical grasping network. Curr Opin Neurobiol. 21:565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare M, Parikh PJ, Santello M. 2019. Sensorimotor uncertainty modulates corticospinal excitability during skilled object manipulation. J Neurophysiol 121(4): 1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriou M, Edin BB. 2010. Human muscle spindles act as forward sensory models. Curr Biol. 20:1763–1767. [DOI] [PubMed] [Google Scholar]
- Duecker F, de Graaf TA, Jacobs C, Sack AT. 2013. Time- and task-dependent non-neural effects of real and sham TMS. PLoS One. 8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren A, Forssberg H. 2001. Differential fronto-parietal activation depending on force used in a precision grip task: an fMRI study. J Neurophysiol. 86(6):2613–2623. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren A, Jonsson T, Westling G, Johansson RS, Forssberg H. 2000. Cortical activity in precision- versus power-grip tasks: an fMRI study. J Neurophysiol. 83:528–536. [DOI] [PubMed] [Google Scholar]
- Fu Q, Hasan Z, Santello M. 2011. Transfer of learned manipulation following changes in degrees of freedom. J Neurosci. 31:13576–13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Zhang W, Santello M. 2010. Anticipatory planning and control of grasp positions and forces for dexterous two-digit manipulation. J Neurosci. 30:9117–9126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A, Westling G, Cole KJ, Johansson RS. 1993. Memory representations underlying motor commands used during manipulation of common and novel objects. J Neurophysiol. 69:1789–1796. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Fagg A, Arbib M. 1998. Dorsal premotor cortex and conditional movement selection: a PET functional mapping study. J Neurophysiol. 79:1092–1097. [DOI] [PubMed] [Google Scholar]
- Grol MJ, Majdandzić J, Stephan KE, Verhagen L, Dijkerman HC, Bekkering H, Verstraten FAJ, Toni I. 2007. Parieto-frontal connectivity during visually guided grasping. J Neurosci. 27:11877–11887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel R, Trempe M, Bernier P-M. 2017. Disruption of M1 activity during performance plateau impairs consolidation of motor memories. J Neurosci.. 37(38):9197–9206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. 2005. Theta burst stimulation of the human motor cortex. Neuron. 45:201–206. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Rothwell JC, Edwards MJ, Chen RS. 2008. Effect of physiological activity on an NMDA-dependent form of cortical plasticity in human. Cereb Cortex. 18:563–570. [DOI] [PubMed] [Google Scholar]
- Jacobs MF, Zapallow CM, Tsang P, Lee KGH, Asmussen MJ, Nelson AJ. 2012. Current direction specificity of continuous theta-burst stimulation in modulating human motor cortex excitability when applied to somatosensory cortex. Neuroreport. 23:927–931. [DOI] [PubMed] [Google Scholar]
- Jasper H. 1958. The ten twenty system of the International Federation. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Suppl. 10:371–375. [PubMed] [Google Scholar]
- Jenmalm P, Schmitz C, Forssberg H, Ehrsson HH. 2006. Lighter or heavier than predicted: neural correlates of corrective mechanisms during erroneously programmed lifts. J Neurosci. 26:9015–9021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Cole KJ. 1992. Sensory-motor coordination during grasping and manipulative actions. Curr Opin Neurobiol. 2:815–823. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Flanagan JR. 2009. Coding and use of tactile signals from the fingertips in object manipulation tasks. Nat Rev Neurosci. 10:345–359. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Riso R, Hiiger C, Backstrom L. 1992. Somatosensory control of precision grip during unpredictable pulling loads. Exp Brain Res. 89:181–191. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G. 1984. Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp Brain Res. 56:550–564. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G. 1988. Programmed and triggered actions to rapid load changes during precision grip. Exp Brain Res. 71:72–86. [DOI] [PubMed] [Google Scholar]
- Keel J, Smith M, Wassermann E. 2001. A safety screening questionnaire for transcranial magnetic stimulation. Clin Neurophysiol. 112(4):720. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Hutchinson S, Théoret H, Schlaug G, Pascual-Leone A. 2004. Repetitive TMS of the motor cortex improves ipsilateral sequential simple finger movements. Neurology. 62:91–98. [DOI] [PubMed] [Google Scholar]
- Kuhtz-Buschbeck JP, Ehrsson HH, Forssberg H. 2001. Human brain activity in the control of fine static precision grip forces: an fMRI study. Eur J Neurosci. 14:382–390. [DOI] [PubMed] [Google Scholar]
- Kuznetsova A, Brockhoff PB, Christensen RHB. 2017. Tests in linear mixed effects models [R package lmerTest version 2.0-36]. Comprehensive R Archive Network (CRAN). [Google Scholar]
- Legon W, Dionne JK, Staines WR. 2013. Continuous theta burst stimulation of the supplementary motor area: effect upon perception and somatosensory and motor evoked potentials. Brain Stimul. 6:877–883. [DOI] [PubMed] [Google Scholar]
- Lemon RN. 2008. Descending pathways in motor control. Annu Rev Neurosci. 31:195–218. [DOI] [PubMed] [Google Scholar]
- Lemon RN, Johansson RS, Westling G. 1995. Corticospinal control during reach, grasp, and precision lift in man. J Neurosci. 15:6145–6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London BM, Miller LE. 2012. Responses of somatosensory area 2 neurons to actively and passively generated limb movements. J Neurophysiol. 109(6):1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukos JR, Ansuini C, Santello M. 2007. Choice of contact points during multidigit grasping: effect of predictability of object center of mass location. J Neurosci. 27:3894–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukos JR, Ansuini C, Santello M. 2008. Anticipatory control of grasping: independence of sensorimotor memories for kinematics and kinetics. J Neurosci. 28:12765–12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukos JR, Lee D, Poizner H, Santello M. 2010. Anticipatory modulation of digit placement for grasp control is affected by Parkinson’s disease. PLoS One. 5(2):e9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marneweck M, Barany DA, Santello M, Grafton ST. 2018. Neural representations of sensorimotor memory- and digit position-based load force adjustments before the onset of dexterous object manipulation. J Neurosci. 38:4724–4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzke MW. 1997. Precision grips, hand morphology, and tools. Am J Phys Anthropol. 102:91–110. [DOI] [PubMed] [Google Scholar]
- Mathis MW, Mathis A, Uchida N. 2017. Somatosensory cortex plays an essential role in forelimb motor adaptation in mice. Neuron. 93(6):1493:1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills K, Boniface S, Schubert M. 1992. Magnetic brain stimulation with a double coil: the importance of coil orientation. Electroencephalogr Clin Neurophysiol. 85:17–21. [DOI] [PubMed] [Google Scholar]
- Mojtahedi K, Fu Q, Santello M. 2015. Extraction of time and frequency features from grip force rates during dexterous manipulation. IEEE Trans Biomed Eng. 62:1363–1375. [DOI] [PubMed] [Google Scholar]
- Napier JR. 1956. The prehensile movements of the human hand. J Bone Joint Surg Br. 38-B:902–913. [DOI] [PubMed] [Google Scholar]
- Ni Z, Gunraj C, Nelson AJ, Yeh IJ, Castillo G, Hoque T, Chen R. 2009. Two phases of interhemispheric inhibition between motor related cortical areas and the primary motor cortex in human. Cereb Cortex. 19:1654–1665. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Voss M, Huang Y-Z, Wolpert DM, Rothwell JC. 2005. High-frequency repetitive transcranial magnetic stimulation over the hand area of the primary motor cortex disturbs predictive grip force scaling. Eur J Neurosci. 22:2392–2396. [DOI] [PubMed] [Google Scholar]
- Omrani M, Murnaghan CD, Pruszynski JA, Scott SH. 2016. Distributed task-specific processing of somatosensory feedback for voluntary motor control. Elife. 5:e13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban De Xivry JJ, Criscimagna-Hemminger SE, Shadmehr R. 2011. Contributions of the motor cortex to adaptive control of reaching depend on the perturbation schedule. Cereb Cortex. 21(7):1475–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh PJ, Davare M, McGurrin P, Santello M. 2014. Corticospinal excitability underlying digit force planning for grasping in humans. J Neurophysiol. 111:2560–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh PJ, Santello M. 2017. Role of human premotor dorsal region in learning a conditional visuomotor task. J Neurophysiol. doi. 10(11):445–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team . 2017. Nlme: linear and nonlinear mixed effects models. R package version. 3:1–131. [Google Scholar]
- Salimi I, Frazier W, Reilmann R, Gordon AM. 2003. Selective use of visual information signaling objects’ center of mass for anticipatory control of manipulative fingertip forces. Exp brain Res. 150:9–18. [DOI] [PubMed] [Google Scholar]
- Santello M. 2018. Dexterous manipulation: Understanding the continuum from hand kinematics to kinetics In: Corbetta D, Santello M, editors. Reach-to-grasp behavior. Brain, behavior, and modelling across the lifespan. 1st ed. Boca Raton (FL): CRC Press/Taylor & Francis. [Google Scholar]
- Santello M, Baud-Bovy G, Jörntell H. 2013. Neural bases of hand synergies. Front Comput Neurosci. 7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santello M, Bianchi M, Gabiccini M, Ricciardi E, Salvietti G, Prattichizzo D, Ernst M, Moscatelli A, JÃrntell H, AML K et al. 2016. Hand synergies: integration of robotics and neuroscience for understanding the control of biological and artificial hands. Phys Life Rev. 17:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabrun SM, Ridding MC, Miles TS. 2008. Role of the primary motor and sensory cortex in precision grasping: a transcranial magnetic stimulation study. Eur J Neurosci. 27:750–756. [DOI] [PubMed] [Google Scholar]
- Schettino LF, Adamovich SV, Bagce H, Yarossi M, Tunik E. 2015. Disruption of activity in the ventral premotor but not the anterior Intraparietal area interferes with on-line correction to a haptic perturbation during grasping. J Neurosci. 35:2112–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunik E, Frey SH, Grafton ST. 2005. Virtual lesions of the anterior intraparietal area disrupt goal-dependent on-line adjustments of grasp. Nat Neurosci. 8:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero-Cuevas FJ, Santello M. 2017. On neuromechanical approaches for the study of biological and robotic grasp and manipulation. J Neuroeng Rehabil. 14:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn SL. 1960. Tools and human evolution. Sci Am. 203:63–75. [PubMed] [Google Scholar]
- Westling G, Johansson RS. 1984. Factors influencing the force control during precision grip. Exp Brain Res. 53:277–284. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. 1980. Amplitude of responses to perturbation in primate sensorimotor cortex as a function of task. J Neurophysiol. 44(6):1139–1147. [DOI] [PubMed] [Google Scholar]
- Zhang W, Gordon AM, Fu Q, Santello M. 2010. Manipulation after object rotation reveals independent sensorimotor memory representations of digit positions and forces. J Neurophysiol. 103:2953–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]


