Abstract
Recent work suggests an important role for cortical–subcortical networks in seizure-related loss of consciousness. Temporal lobe seizures disrupt subcortical arousal systems, which may lead to depressed cortical function and loss of consciousness. Extracellular recordings show ictal neocortical slow waves at about 1 Hz, but it is not known whether these simply represent seizure propagation or alternatively deep sleep-like activity, which should include cortical neuronal Up and Down states. In this study, using in vivo whole-cell recordings in a rat model of focal limbic seizures, we directly examine the electrophysiological properties of cortical neurons during seizures and deep anesthesia. We found that during seizures, the membrane potential of frontal cortical secondary motor cortex layer 5 neurons fluctuates between Up and Down states, with decreased input resistance and increased firing rate in Up states when compared to Down states. Importantly, Up and Down states in seizures are not significantly different from those in deep anesthesia, in terms of membrane potential, oscillation frequency, firing rate, and input resistance. By demonstrating these fundamental similarities in cortical electrophysiology between deep anesthesia and seizures, our results support the idea that a state of decreased cortical arousal may contribute to mechanisms of loss of consciousness during seizures.
Keywords: arousal, consciousness, epilepsy, sleep, slow waves, whole-cell recordings
Introduction
Neocortical slow oscillations are a fundamental rhythm observed in states where the cortex is disconnected from the external world and consciousness is depressed. First characterized in intracellular recordings from anesthetized cats in a classic series of papers by Steriade et al. (1993a, 1993b, 1993c, 2001), the cellular hallmarks of these slow oscillations are termed Up and Down states. Up and Down states underlie the slow membrane potential oscillations of cortical and thalamic neurons at about 0.5–1.5 Hz, continuously alternating between depolarized, active Up states and hyperpolarized, almost completely silent Down states. Up states typically demonstrate increased action potential firing and increased synaptic activity evidenced by reduced membrane potential resistance compared to Down states (Contreras et al. 1996; Shu et al. 2003a). Cortical slow oscillations have been related to various physiological and pathological states of unconsciousness such as natural sleep, anesthesia, and coma and are generally considered to indicate a depressed cortical state (Steriade et al. 1993b, 1996, 2001; Young 2000; Englot et al. 2008). Interestingly, evidence for the association of neocortical slow oscillations with focal limbic seizures is also abundant, with several human intracranial EEG studies revealing ictal neocortical slowing in bilateral frontal and ipsilateral parietal association cortex (Lieb et al. 1991; Eisenschenk et al. 2001; Blumenfeld et al. 2004b; Englot et al. 2010). This has been further confirmed by animal studies using local field potential (LFP)/multiunit activity (MUA) recordings (Englot et al. 2008; Motelow et al. 2015). However, unlike states like coma or sleep with depressed neuronal activity, seizures are typically marked by intense neuronal firing and overexcitation of neuronal networks, which could extend beyond the mesial temporal lobe to influence activity in the neocortex (Lieb et al. 1991; Englot et al. 2008). Whether ictal neocortical slowing in focal limbic seizures produces cellular neurophysiological changes in cortical neurons consistent with seizure propagation or consistent with a sleep-like depressed cortical state requires direct investigation.
Temporal lobe epilepsy is characterized by recurrent focal seizures and impaired consciousness, despite the temporal lobe not being regarded as part of traditional consciousness networks (Rees et al. 2002; Motelow et al. 2015). It has been proposed that temporal lobe seizures may cause remote dysfunction in other regions (Van Paesschen et al. 2003; Li et al. 2019). The network inhibition hypothesis supports a shared mechanism between sleep- and seizure-related impairment of consciousness; sleep-promoting regions such as the hypothalamus and lateral septum are activated due to seizure propagation, which in turn inhibits subcortical arousal systems rendering sleep-like states in the brain (Englot et al. 2008; Motelow et al. 2015; Zhan et al. 2016; Feng et al. 2017; Andrews et al. 2019). Notably, the network inhibition hypothesis predicts that seizure activity in the hippocampus can depress subcortical arousal, giving rise to neocortical slow-wave activity and impaired consciousness (Englot et al. 2008, 2009; Motelow et al. 2015). Indeed, by using functional magnetic resonance imaging (fMRI) in a rat model of partial limbic seizures, sleep-promoting regions were found to be activated, while cortex and subcortical arousal system were inhibited (Motelow et al. 2015). Furthermore, Englot et al. (2008) found using a range of multimodal techniques that neocortical slow waves are related to reduced neuronal activity and cerebral metabolism in contrast with increased neuronal activity and metabolism during propagated seizures.
However, the techniques used in the previous work are indirect in understanding the intracellular properties of neurons during ictal neocortical slowing. Neuroimaging and extracellular recordings are inherently limited in the resolution they provide to examine the activity of individual cells. Whole-cell intracellular recordings are an excellent tool for investigating membrane potential changes in single neurons. Here, using in vivo whole-cell recordings in a lightly anesthetized ketamine–xylazine rat model of focal limbic seizures, we compare the states of single cortical layer 5 neurons during ictal neocortical slowing and during deep anesthesia. We find that these 2 states share similar properties in terms of membrane potential, oscillation frequency, neuronal firing rate, and input resistance. These findings are consistent with the concept that ictal neocortical slow waves represent a depressed cortical sleep-like state and might be involved in impaired consciousness during focal seizures with loss of awareness.
Materials and Methods
Animals
All procedures were performed in accordance with approved protocols of Yale University’s Institutional Animal Care and Use Committee. A total of 87 adult female Sprague Dawley rats (Charles River Laboratories) aged 6–10 weeks, weighing 160–260 g, were used in these experiments. Female rats were used because seizures have been shown less likely to secondarily generalize in female rats compared to males (Mejias-Aponte et al. 2002; Janszky et al. 2004). Sixty-five animals were used for whole-cell recordings measuring membrane potential and 12 for calculating input resistance with repeated current pulses at baseline and during seizures (repeated current pulses were not used in all experiments because they skew the Vm). Another 10 animals were used for simultaneous MUA and LFP recordings in secondary motor cortex (M2) and lateral orbitofrontal cortex (LO) as pilot experiments (data not shown) to confirm that M2 displays slow oscillations during seizures and deep anesthesia similar to prior recordings from LO (Englot et al. 2008, 2009; Motelow et al. 2015). M2 was chosen as the target region because it is close to LO, which is proven to have classic slow waves during seizures and because M2 and LO show similar fMRI decreases during focal limbic seizures (Motelow et al. 2015). Furthermore, M2 is easier to access from the brain surface than LO, allowing us to maximize the rate of successful patch clamp experiments.
Surgery and Electrode Implantation
Surgeries were performed under deep anesthesia (90 mg/kg ketamine [Henry Schein Animal Health]; 15 mg/kg xylazine [Amassed; Lloyd Laboratories]), following which animals were transitioned to light anesthesia (40 mg/kg ketamine; 7 mg/kg xylazine) (Motelow et al. 2015). Focal limbic seizures were induced in rats as described previously (Englot et al. 2008; Feng et al. 2017) and in a later section (see Seizure Induction). All stereotaxic coordinates are reported relative to bregma (Paxinos and Watson 1998). A single tungsten monopolar microelectrode (Catalog Number: UEWMGGSEDNNM; Company name: FHC) with an impedance of 3–4 MΩ for local field potential and multiunit activity recordings was implanted at an approach angle of 20° vertically in the right secondary motor cortex (M2) to reach the following final coordinates: anteroposterior (AP) 4.20 mm, mediolateral (ML) 2.0 mm, and superoinferior (SI) 2.0 mm. Twisted-pair bipolar electrodes (50–100 kΩ resistance; Plastics One, E363/2–2TW), with tips separated by 1 mm in the coronal plane and insulation shaved from the distal 0.3 mm, were implanted into the dorsal hippocampus (AP, −3.8; ML, ±2.5; SI, 2.7 mm) for stimulation and LFP recording. All stereotaxic coordinates relative to bregma were adjusted by multiplying target coordinates by the ratio of (bregma–lambda distance [mm])/8.7 mm (Paxinos and Watson 1998). In summary, whole-cell recordings (described below) were obtained in left M2, while neocortical LFP and MUA were recorded in right M2, and seizures were elicited by stimulating either side of the HC. We found as in prior work from the rat model (Englot et al. 2008, 2009; Motelow et al. 2015) that unilateral stimulation of either HC-produced bilateral hippocampal seizures; and neocortical slow wave activity was approximately equal in both hemispheres (data not shown).
LFP and MUA Electrophysiology
Local field potential and multiunit activity from M2, as well as LFP from hippocampi (HC), were acquired and amplified as previously described (Motelow et al. 2015; Zhan et al. 2016; Andrews et al. 2019). LFP and MUA recordings were amplified using a microelectrode AC amplifier (model 1800 A-M Systems) and broadband filtered from 0.1 Hz to 10 kHz (×1000 gain). A model 3363 Krohn–Hite filter was used to low-pass filter LFP at 0.1–100 Hz and high pass filter MUA at 400 Hz–10 kHz. A Micro1401 (CED) A/D converter was used with a sampling rate of 1000 Hz for M2 LFP, 25 kHz for M2 MUA, 1000 Hz for HC LFP, and 25 kHz for whole-cell recordings of M2 neurons (below). Continuous recordings were made using Spike2 v8.06 (CED) software.
Seizure Induction
Seizures were induced during a lightly anesthetized state as described previously (Englot et al. 2008; Motelow et al. 2015). Animals were defined as lightly anesthetized when the frequency of large, positively deflected slow-wave oscillations on frontal cortex LFPs decreased to less than 3 per 10 s of recordings, approaching awake cortical physiology, and yet animals remained unresponsive to somatosensory stimulation. Bipolar stimulating–recording electrodes were placed in the HC bilaterally to incorporate redundancy into the model and to allow for maximal opportunities for seizure induction. Either the right or left HC was stimulated, using a 2 s, 60 Hz biphasic square wave (1 ms per phase) stimulus train. HC stimulus current amplitude was titrated to produce focal hippocampal seizure activity based on polyspike discharges in HC without propagation to frontal cortex (Fig. 1). The current was titrated by steps of ~200 μA until a seizure was observed. Seizures longer than 10 s were stipulated for inclusion in analyses (range of duration for included seizures was 10–110 seconds, average 27 s).
Figure 1.

In vivo whole-cell recording from layer 5 secondary motor cortex (M2) pyramidal neuron during focal seizure shows slow oscillations, which resemble Up and Down states in deep anesthesia. (A, B) Example of whole-cell recording showing membrane potential (Vm) changes (red trace) in 5 epochs (B). Up and Down states were seen in Vm, local field potential, and multiunit activity recordings during deep anesthesia. Full action potentials in Vm recordings are shown in (A) while truncated in (B). During light anesthesia, large-amplitude, slow frequency oscillation was transformed into low-amplitude, fast frequency activity on Vm and LFP recordings and tonic MUA firing. Seizure was induced in the hippocampus by a 2 s, 60 Hz stimulus (Stim), with large-amplitude, fast polyspike activity seen in hippocampal LFP recording during seizure. Up and Down states are similar in Vm and LFP recordings during deep anesthesia, seizure, and postictal periods. Histology of the recorded neuron is shown in Figure 2A–C.
Whole-Cell Recordings
Whole-cell patch clamp recordings were obtained in vivo in layer 5 secondary motor cortex (M2) with slight modification of procedures described previously (Andrews et al. 2019). The M2 cortical layer 5 in the left hemisphere was targeted at the following coordinates: AP 4.20 mm, ML 2.0 mm, and SI 2.0 mm (coordinates adjusted for each animal relative to bregma as already described). A potassium gluconate-based electrode internal solution with 0.5% biocytin, the same as is used for in vitro whole-cell recordings, was used for micropipettes (Neske et al. 2015; Andrews et al. 2019). Borosilicate glass pipettes (1B150F-4, World Precision instruments) were fabricated using a multi-line program on a Sutter Instruments P-1000 micropipette puller, resulting in a final tip resistance of 4–6 MΩ. Recordings were made using a MultiClamp 700B (Axon) amplifier and digitized at 25 kHz using a Micro1401 (CED) and Spike2 v8.06 (CED) software. The neuron-searching step of acquiring whole-cell recordings was undertaken in voltage clamp mode, with current response visualized in real time both in Spike2 software and on an external oscilloscope for blind patching. The initial access resistance of most cells was between 20 and 50 MΩ. Recordings were conducted in current clamp mode soon after attaining whole-cell configuration, the bridge was balanced and access resistance rechecked periodically throughout recordings. The intracellular membrane potential (Vm) was corrected for junction potential, measured to be approximately 10 mV or 14 mV depending on the batch of internal solution, similar to values determined previously for our internal pipette solution (Cruikshank et al. 2007; Neske et al. 2015). Recordings were terminated if any obvious change in resting Vm, access resistance, or action potential shape was observed. For inclusion in analysis, we required that focal seizures were induced as described above, that included neurons had an RC charging curve compatible with transition to intracellular recordings and exhibited reproducible action potentials (either spontaneous or stimulated by current injection) and stable resting potential, and that recorded neurons were identified histologically by intracellular staining to be located in M2 layer 5 (Fig. 2).
Figure 2.

Histology of layer 5 secondary motor cortex (M2) pyramidal neurons recorded by in vivo whole-cell recordings. (A) Representative coronal sections from left hemisphere showing a recorded neuron (Red, Biocytin) located in M2 layer 5 (same neuron as in Fig. 1). Hoechst staining in blue was used to characterize cortex layer distribution. Photomicrographs from the same neuron at 200x and 400x magnification are shown in (B) and (C). (D) Schematic summary of the locations of neurons recorded from all experiments including whole-cell recordings with and without pulses (47 neurons from 28 rats). Of these, 42 neurons from 25 rats were located in M2 layer 5 (3 neurons were recovered from layers 2–3 and 2 from layer 6, not included in analyses shown here). Background drawing based on section in (A) (Paxinos and Watson 1998; Ueta et al. 2014). M1, primary motor cortex; LO, lateral orbitofrontal cortex; Cg1, cingulate cortex, area 1.
Histology
Animals were perfused, and brains were fixed with 4% paraformaldehyde and cut in 60 μm slices for histology. Recorded neurons were stained with the biocytin cy3-streptavidin construct as previously described (Motelow et al. 2015). To visualize the location of neurons relative to cortical laminae slices were co-stained with Hoechst (Sigma–Aldrich, dilution 1:10 000, final solution 0.12 g/mL) (Chen et al. 2015). Microscopy was performed with a Leica DM6B Microsystems microscope and the Leica LAS Microimage system. To confirm the locations of neurons, slices containing labeled neurons were compared to a standard anatomical atlas of the rat brain (Paxinos and Watson 1998).
Epoch Definitions
Analysis epochs were defined as follows: 1) Light anesthesia: up to 20 s of data from immediately before the seizure satisfying criteria described above for the light anesthesia state (see Seizure Induction). 2) Ictal: the first 20 s of HC seizure activity (or the entire period of seizure activity when duration <20 s and >10 s), based on large amplitude, polyspike activity in HC LFP recordings. 3) Postictal: up to 20 s of data starting at end of HC seizure until time when M2 LFP no longer shows Up and Down states. 4) Recovery: 20 s following the postictal period 5. Deep anesthesia: 20 s of clear Up and Down states at time closest to seizure but not overlapping any of other epochs above. A minimum of 10 s was required for all epochs used for analysis.
Up/Down State Detection–Segmentation
To identify Up and Down state characteristic of cortical slow-wave oscillations, we classified the membrane potential Vm data from the deep anesthesia, seizure, and postical epochs using a custom algorithm, loosely based on the methods of Seamari et al. (Seamari et al. 2007) implemented in MATLAB (MathWorks). First, we band-pass filtered the Vm data to remove low-frequency signal drift (<0.5 Hz) and high-frequency noise (>5000 Hz). Action potentials were removed and data interpolated by finding all local minima in the 25 kHz data (data points less than their immediate neighboring data points) and interpolating between the remaining points. This procedure effectively removed positive action potential and at the same time led to a 5–10-fold decrease in time resolution of the 25 kHz data, which on visual review led to no change in the voltage time course or membrane potential probability distribution function. Next, the signal was smoothed using a moving average with a window of 200 ms. The mean signal was then calculated for each analysis epoch (deep, seizure, or postictal). Up state peak data points were then defined by values greater than the mean for that epoch and greater than the preceding and following local neighboring points. Downstate trough data points similarly were defined by values below the mean for the entire epoch, as well as less than the preceding and following local data points. This procedure identified sequences of prominent data points occurring during Up and Down states. However, because the membrane potential tended to fluctuate more during Up than during Down states, including occasional very brief negative dips, we excluded any Down state trough data points if they occurred within 100 ms of an Up state peak data point. Segments of data representing Up states were next identified by stringing together consecutive Up state peak data points with no intervening Down state trough data points and then adding a small additional segment of 67 ms of data on either side to yield a complete Up state segment. Down state segments were identified by the analogous procedure using consecutive Down state trough data points encompassing no Up state peak data points. Data points during the transitions between Up and Down states were classified as Neither. Finally, all times obtained using the above Up and Down state and Neither classification were applied back to the original band-pass filtered, action potential-removed Vm data series for subsequent analysis (see examples of segmented, labeled Vm data in Fig. 3A–C, left traces). We found that this approach produced excellent results in reliably labeling up, down, and Neither data segments from the deep anesthesia, seizure, and postictal data epochs. Data from the light anesthesia and recovery epochs underwent band-pass filtering and action potential removal as above but did not undergo Up/Down state segmentation. The Vm data from all 5 epoch types was next used for the creation of probability density histograms and analysis of mean Vm under different conditions (Fig. 3).
Figure 3.

Membrane potential fluctuations during different epochs. (A–E) Side plot on the right shows the probability density histogram of a 10 second example trace of each state seen on the left. Red lines indicate the labeled Vm segments (left) and distribution (right) of Up states, and blue lines indicate the Vm segments and distribution of Down states, while green lines indicate the overall distribution of Vm throughout the 10-s period. Black lines in tracings on left indicate either action potentials or Vm segments labeled as Neither by the segmentation algorithm. Notice the bimodal distribution of membrane potential in deep anesthesia, seizure, and postictal epochs (A, B, C), versus the unimodal distribution in light anesthesia and recovery (D, E). (F) Group comparison between each epoch shows statistically significant differences in mean Vm between Up and Down states in deep anesthesia, seizure, and postictal periods. See text for additional statistical comparisons. Data are mean Vm and error bars are SEM. One-way ANOVA mixed-effects analysis, with post hoc Tukey test, ****P < 0.0001).
Probability Density Histograms and Mean Vm Analysis
To display the distribution of membrane potential values in Up and Down states during cortical slow-wave oscillations and during times without slow waves, we constructed probability density histograms using the “histcounts” function in MATLAB applied separately to Vm data segmented into Up states, Down states, or to the entire unsegmented data set (see Fig. 3A–E). Histogram values were normalized in each case to the total area or equivalently to the total number of Vm data points (time points) in each histogram. This procedure produced histograms representing the proportion of time neurons spent at different membrane potentials in each condition.
To evaluate whether Vm histograms showed a bimodal or unimodal distribution, we fit a mixture model of 2 Gaussian distributions to the data drawn from each cell, for each epoch. The mixture model was fit using the expectation–maximization algorithm (Dempster et al. 1977). We then assessed whether each fit could be plausibly described as bimodal (or whether the 2 Gaussians simply summed to give a single unimodal distribution) using the d statistic, calculated as 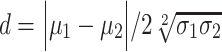 , where
, where  and
and  represent the mean and standard deviation, calculated via the EM algorithm, of each of the Gaussian distributions, respectively (Holzmann and Vollmer 2008), which sets a strict criterion for whether a distribution is unimodal or not. Using this approach, we assessed whether the data for each epoch type in each cell was uni- or bimodal. We then compared the proportion of cells with bimodal versus unimodal fits between different epoch types using a Chi-square test with significance threshold P < 0.05.
represent the mean and standard deviation, calculated via the EM algorithm, of each of the Gaussian distributions, respectively (Holzmann and Vollmer 2008), which sets a strict criterion for whether a distribution is unimodal or not. Using this approach, we assessed whether the data for each epoch type in each cell was uni- or bimodal. We then compared the proportion of cells with bimodal versus unimodal fits between different epoch types using a Chi-square test with significance threshold P < 0.05.
The calculation of mean Vm and statistical analysis for Up states and Down states in the deep anesthesia, seizure, and postictal epoch was performed on the band-pass filtered, action potential-removed, segmented data; Vm calculations for the light anesthesia and recovery epochs were performed on the band-pass filtered, action potential-removed data without Up/Down state segmentation (Fig. 3F).
Slow-Wave Oscillation Frequency Analysis
The mean oscillation frequency of cortical slow waves was calculated from autocorrelograms of the Vm data for the deep anesthesia and seizure epochs. We obtained band-pass filtered, action potential-removed Vm data as already described. Each data epoch was z-scored using its own mean and standard deviation. An autocorrelogram was then calculated from this time series, and the resulting values were smoothed by a moving average window of 0.35 s to remove spurious local peaks. Positive peaks were then determined using the “findpeaks” function in MATLAB, and the period of the signal was calculated as the time of the largest peak with offset ≤3 s (excluding the peak at centered at 0 s). We therefore generated a single value for the period for each epoch. These were averaged together for all epochs of a given type (deep anesthesia or seizure) across all neurons and seizures within a single animal. The reciprocal was then taken to calculate the slow-wave oscillation frequency.
Action Potential Firing Rate Analysis
To analyze action potential firing rates during Up and Down states, Vm data were segmented for the deep anesthesia and seizure epochs into Up and Down states using the same procedures described above. Segmentation of data time points into Up and Down states was applied back to the original Vm data, and action potentials were reliably identified as instances where voltage exceeded a threshold of −30 mV for at least 10 consecutive samples (400 μs). Spiking rates in Up and Down states were then calculated by dividing the number of action potentials by the time in each state. For the inclusion of an animal in the analysis, we required the mean firing rate in Up states across all epochs to lie between 0.05 Hz and 2.00 Hz. In addition, only cortical regular spiking neurons were included, and the rare intrinsic burst firing neurons were excluded due to their extreme high mean firing rates (Nowak et al. 2003).
Membrane Input Resistance Analysis
Input resistance was measured by delivering repeated hyperpolarizing square current pulses (80–140 pA, 50 ms duration, 10 Hz) and measuring the change in membrane potential for each step, as resistance (R) is related to change in voltage (ΔV) and current (ΔI) through Ohm’s law R = ΔV/ΔI. See Figure 4 for examples of membrane voltage changes in response to square current pulses. Because the current pulses changed the shape of the Vm waveform, we performed Up/Down state classification on the LFP data instead of Vm, but otherwise using the same procedures already described. The identified times of Up and Down states were then applied back to the original Vm data. We calculated ΔV for each pulse by calculating the mean Vm in the last 25 ms of baseline activity before the negative pulse and mean Vm in the last 25 ms of the 50 ms pulse (when Vm reached steady state) and then taking the difference. We only included pulses for which Vm data were available for both baseline and pulse within the same Up or Down state. Any pulses that overlapped with an action potential (detected as described in the preceding section) were excluded. We divided ΔV for each pulse by the current pulse amplitude to calculate input resistance. In addition, we attempted to analyze membrane potential RC time constants using 1 or 2 exponential fits as described previously (Amzica and Neckelmann 1999). However, we found relatively large variance in fits due to membrane potential fluctuations and synaptic noise, which made this approach insensitive to detect differences between states (data not shown).
Figure 4.

Decreased input resistance in Up states during seizures resembles deep anesthesia. (A, B) An example of voltage response to negative current pulses in M2 cortical pyramidal layer 5 neuron in deep anesthesia and seizure epochs. Top traces, Vm; Middle traces, expanded from top traces where time segments are indicated by horizontal colored lines, with Up states (red), Down states (blue); Bottom traces, 10 Hz 50 ms 100pA square negative (hyperpolarizing) current pulses. Action potentials are truncated at top. (C) Example of Up and Down states free of negative pulses in deep anesthesia epoch from the same cell. (D, E) Mean Vm response for pulses from example traces (A, B) in deep anesthesia and seizure epochs. Voltage deflection in Up states is smaller than Down states. To facilitate comparison in D, E, voltages at the end of baseline period (just before start of hyperpolarizing current pulse) have been aligned vertically to remove the offset from the mean Up and Down state traces. Voltages including the vertical displacement are shown in A, B. (F) Comparison of input resistance in Up and Down states during seizure and deep anesthesia epochs. The mean input resistance in Up states (red lines) was lower than in Down states (blue lines) (deep down vs. deep up; n = 12 neurons, ****P < 0.0001, paired, 2-tailed t-test; seizure down vs. seizure up; n = 12 neurons, ***P = 0.0005, paired 2-tailed t-test). There is no difference between Up states and Down states in seizures versus deep anesthesia. Seizure up versus deep up, n = 12 neurons, P = 0.86 (NS), paired 2-tailed t-test; seizure down versus deep down; n = 12 neurons, P = 0.64 (NS), paired 2-tailed t-test.
Statistical Analysis
Data analysis and figure preparation were performed using Spike2 (CED, v5.20a), GraphPad Prism8 (GraphPad, 8.0.1), and in-house software written on MATLAB (R2009a, MathWorks). We typically obtained data from 1 to 5 (median 2) seizures in each neuron and from 1 to 3 (median 1) neurons in each animal. Taking a conservative approach, prior to statistical analysis of mean Vm, slow-wave oscillation frequency, and spiking rates, we first pooled data by combining results across all neurons and seizures within each animal. This was done by concatenating and then averaging voltage, slow-wave periods, or action potential rate data across all neurons, seizures, epoch types (Deep Anesthesia, Light anesthesia, Seizure, Post-ictal or Recovery), and Up or Down states as applicable within each animal. Individual animals were thus the unit of statistical analysis for Vm, oscillation frequency, and spiking rate calculations.
Because mean Vm had the largest data set (fewest exclusion criteria), we analyzed data across all 5 epoch types. Thus, for analysis of differences in Vm between conditions and across animals (Fig. 3F), we performed a mixed-effect one-way ANOVA analysis with post hoc Tukey test, with a statistical significance threshold P < 0.05. A statistical analysis of bimodal versus unimodal Vm histogram fits for different epoch types was already described above. For analysis of slow-wave oscillation frequency, spiking rates, and input resistance (below), we focused our analyses on the most important comparisons, looking at differences between deep anesthesia and seizures or between Up and Down states as applicable. Therefore, analysis of differences in these data sets between deep anesthesia and seizures or between Up and Down states were by paired 2-tailed t-tests with significance threshold P < 0.05.
For the analysis of membrane input resistance data, because we had a lower total number of animals for analysis (n = 5), we used the slightly less conservative but valid approach of analyzing data using individual neurons as the units of statistical analysis (n = 12), and we first pooled data by averaging all resistance values within each neuron across all seizures, epoch types (Deep Anesthesia or Seizure) and Up or Down states. An analysis of differences in input resistance between Up and Down states and between deep anesthesia and seizures (Fig. 4E) was analyzed by paired 2-tailed t-test with a significance threshold P < 0.05.
Results
We found that cortical neurons exhibit Up and Down states during focal limbic seizures, very similar to cortical slow-wave oscillations seen in deep anesthesia. Previous studies have shown that focal limbic seizures are associated with 1–2 Hz ictal neocortical slow activity, demonstrated using extracellular multiunit activity and local field potential recordings (Englot et al. 2008, 2009; Motelow et al. 2015). Here, we aimed to determine if Up and Down states also exist at the single neuron level during focal seizures. To this end, in vivo whole-cell recordings were targeted to layer 5 pyramidal cells because of their known role in cortical slow waves in other conditions (Sanchez-Vives and McCormick 2000; Beltramo et al. 2013; Neske 2016). In focal limbic seizures induced by HC stimulation, prior LFP and MUA studies in the rat showed slow waves in several areas, most prominent in lateral orbital frontal cortex (Englot et al. 2008, 2009; Motelow et al. 2015). To facilitate access for in vivo whole-cell recordings, we first established with LFP and MUA recordings that the more dorsally located frontal secondary motor cortex (M2) exhibited the same slow-wave activity during focal limbic seizures as seen with simultaneous recordings from lateral orbital frontal cortex (n = 10, data not shown). Experiments targeting M2 for in vivo whole-cell recordings were performed in 77 animals, with successful recordings obtained from 28 animals (47 neurons histologically recovered). This involved a total of 472 micropipette passes targeting M2 layer 5 for an overall success rate of 9.5%. Of the 47 histologically recovered neurons, 42 neurons in 25 animals had locations confirmed in M2 layer 5 (Fig. 2) and were available for analysis, subject to other electrophysiological inclusion criteria (see Methods).
The membrane potential (Vm) was measured using whole-cell current clamp in left M2, while MUA and LFP were recorded simultaneously from right M2 (Fig. 1). Whole-cell recordings typically began in deep anesthesia, where we observed characteristic Up and Down state fluctuations in Vm with occasional action potential firing in the Up states (Fig. 1A,B, left traces). As the anesthesia lightened, large-amplitude slow waves in M2 LFP begin to vanish and transform into low-amplitude fast activity associated with MUA tonic firing (Fig. 1, Light anesthesia). A seizure was then triggered producing large-amplitude, fast polyspike activity in HC LFP. During the seizure, Up and Down states appeared again in M2 LFP and M2 MUA and in Vm recordings, resembling those seen under deep anesthesia (Fig. 1, Seizure and Deep Anesthesia traces). When seizure activity stopped in the HC LFP, cortical slow oscillations still persisted during the postictal period but eventually stopped in the recovery period (Fig. 1, Postictal and Recovery traces). These recordings suggest that during and following seizures, cortical neurons switch temporarily to a depressed state similar to deep anesthesia. We observed clear Up and Down state oscillations in Vm during all focal seizures recorded from histologically recovered cortical M2 layer 5 neurons (Fig. 2).
Up and Down State Vm Changes During Seizures Resemble Those in Deep Anesthesia
We next proceeded to probe the similarities between Up and Down states seen in deep anesthesia and seizures. First, we compared membrane voltage changes within different epochs including the seizure and deep anesthesia epoch (Fig. 3). The membrane potential probability density histogram or frequency plot during the deep anesthesia, seizure, and postictal epochs follows a classic bimodal distribution with 2 peaks corresponding to Up states (higher Vm peak) and Down states (lower Vm peak) (Fig. 3A–C). In contrast, the membrane potential frequency plot during light anesthesia and recovery is unimodal, with a single intermediate peak (Fig. 3D,E).
The proportion of cells with bimodal Vm distributions was not significantly different between the deep anesthesia, seizure, and postictal epochs, respectively, 15/20, 16/29, and 24/28 cells (Chi-square = 3.37, df = 2, P = 0.19). Similarly, light anesthesia and recovery epochs did not differ significantly in proportion of cells with bimodal Vm distributions at 3/28 and 2/9 cells, respectively (Chi-square = 0.58, df = 1, P = 0.58). Finally, pooling together data from deep anesthesia, seizure, and postictal epochs versus pooled data from light anesthesia and recovery epochs yielded a significant difference between these groupings (Chi-square = 27.49, df = 1, P < 0.0001), strongly suggesting that the chance of finding bimodal cells in seizure, deep anesthesia, and postictal is significantly different than in light anesthesia and recovery epochs.
Membrane potential comparison among 5 epochs for the group data based on 20 animals (27 neurons, 48 seizures) is shown in Figure 3F. Up states are significantly more depolarized than down states during deep anesthesia, seizure, and postictal epoch (mean Vm: deep up −63.86 mV vs. deep down −75.93 mV, n = 16 animals, P < 0.0001; seizure up −63.19 mV vs. seizure down −72.63 mV, n = 20 animals, P < 0.0001; postictal up −61.08 mV vs. postictal Down −71.65 mV, n = 15 animals, P < 0.0001; one-way ANOVA mixed-effect analysis, with post hoc pairwise Tukey tests, F = 40.75, P < 0.0001). No significant differences were found between Up state membrane potentials in deep anesthesia, seizure, and postictal epochs, and similarly down state membrane potentials were not significantly different when compared across these 3 conditions. Taken together, the changes in membrane potential between Up and Down states affect the neuron in the same pattern during seizure, deep anesthesia, and the postictal epochs. The mean Vm for the light anesthesia preictal epochs was −67.08 mV, which was intermediate between the Vm for Up and Down states as reported previously (Steriade et al. 2001; McGinley et al. 2015). The mean Vm for light anesthesia was significantly different from the Up and Down state mean Vm for seizure, postictal, and deep anesthesia epochs except for the deep anesthesia Up state (light vs. seizure up, P < 0.0001; light vs. seizure down, P < 0.0001; light vs. postictal up, P = 0.0020; light vs. postictal down, P = 0.0150; light vs. deep up, P = 0.1399; light vs. deep down, P < 0.0001). The mean Vm for recovery was −67.39 mV, not significantly different from the preictal light anesthesia Vm. Having thus characterized the Vm changes during the different epoch types and finding very similar Up and Down states in the seizure and deep anesthesia epochs, the remainder of our analyses focused on these 2 epochs.
Slow-Wave Frequency and Firing Rates During Seizures Resemble Deep Anesthesia
The mean frequency of the slow-wave oscillations during the seizure epochs was 0.8 Hz (±0.1 Hz SEM), while the frequency in deep anesthesia was 1.1 Hz (±0.2 Hz SEM), and these 2 values were not significantly different (P = 0.14, paired 2-tailed t-test, n = 9 animals). Action potential firing rates in Up states were much higher than firing rates in Down states in both deep anesthesia (deep up 0.44 Hz ± SEM 0.14 Hz vs. deep down 0.00 Hz ± SEM 0.00 Hz; P = 0.021, paired 2-tailed t-test, n = 7 animals) and during seizure (seizure up 0.74 Hz ± SEM 0.22 Hz vs. seizure down 0.00 Hz ± SEM 0.00 Hz; P = 0.0081, paired 2-tailed t-test, n = 10 animals). A comparison of firing rates in seizure Up states and deep anesthesia Up states showed no significant difference (P = 0.40, paired 2-tailed t-test, n = 7 animals) as is similarly the case in Down states, which had essentially zero firing in both seizure and deep anesthesia.
Input Resistance Changes in Up and Down States During Seizures Resemble Deep Anesthesia
Another hallmark of Up and Down states in cortical slow-wave oscillations is the decrease of input resistance during Up states (Shu et al. 2003a), likely reflecting heightened synaptic activity. 80–140 pA, 50 ms, hyperpolarizing, square current pulses at a rate of 10 Hz were applied in whole-cell current clamp mode to measure moment-to-moment input resistance. Figure 4A,B shows example traces from deep anesthesia and seizure epochs, respectively. Up and Down states are still visible on top of current pulses in the Vm channel. Mean response to pulses from the example traces were calculated and are shown in Figure 4D,E where we see a clear difference in the magnitude of deflection in response to the injection of current for Up versus Down states. This difference is explored further in Figure 4F by calculating mean input resistance values during Up states and Down states for deep anesthesia and seizure periods in each neuron. The mean input resistance was significantly higher in Down states than Up states (deep down 172.9 MΩ ± SEM 6.2 MΩ vs. deep up 152.6 MΩ ± SEM 6.3 MΩ; n = 12 neurons, P < 0.0001, paired 2-tailed t-test; seizure down 176.7 MΩ ± SEM 5.6 MΩ vs. seizure up 154.6 MΩ ± SEM 7.7 MΩ; n = 12 neurons, P = 0.0005, paired 2-tailed t-test). In comparing the mean input resistance in seizure to deep anesthesia, we found no significant difference between Up states (seizure up 154.6 MΩ ± SEM 7.7 MΩ vs. deep up 152.6 MΩ ± SEM 6.3 MΩ; n = 12 neurons, P = 0.86, paired 2-tailed t-test) or between Down states (seizure down 176.7 MΩ ± SEM 5.6 MΩ vs. deep down 172.9 MΩ ± SEM 6.2 MΩ; n = 12 neurons, P = 0.64, paired 2-tailed t-test) (Fig. 4F). The membrane resistance changes during Up and Down states in seizures are thus very similar to those during deep anesthesia.
Discussion
By using in vivo whole-cell recordings, we have demonstrated that the membrane potential of cortical neurons shows Up and Down states during focal limbic seizures. The Up and Down states seen during seizures have similar membrane potential fluctuations, oscillation frequency, firing rates, and input resistances as those seen in deep anesthesia. Our measurements are in agreement with the previous studies of Up and Down states and provide strong evidence of a depressed cortical state during seizures (Steriade et al. 1993b; Pare et al. 1998; Sanchez-Vives and McCormick 2000; Compte et al. 2003; Rigas and Castro-Alamancos 2009; Neske 2016).
The generation of ictal neocortical slow waves during temporal lobe seizures is an intriguing phenomenon for 2 reasons. First, temporal lobe seizures originate within limbic structures, such as the hippocampus, which are distant from the neocortex. Second, neocortical slow waves are generally associated with depressed mean cortical activity observed in sleep and anesthesia (Steriade et al. 1996; Englot et al. 2008; Motelow et al. 2015), whereas seizures are marked by overactivation in neuronal networks (Scharfman 2007). These contradictory observations have given rise to an important question—What is the actual state of the neocortex during focal seizures; is it overexcitation or deactivation? Multiple studies have tried to answer this question using different techniques. Electrophysiological methods, such as intracranial EEG, local field potential, and multiunit recordings have demonstrated ictal neocortical slow oscillations (Blumenfeld et al. 2004b; Englot et al. 2008, 2010; Motelow et al. 2015; Feng et al. 2017). Hemodynamic techniques, such as single photon emission computed tomography (SPECT), fMRI, or laser Doppler cerebral blood flow recordings, have revealed decreased hemodynamic responses and neuronal metabolism both in humans and animal models of temporal lobe seizures (Blumenfeld et al. 2004a;Englot et al. 2008; Motelow et al. 2015). Although those studies largely favor slow oscillations as a result of depressed cortical states, these techniques measured neuronal activity within a broad scope—lack of resolution risks masking unique activity within a small subtype of neurons by the large-scale activity observed across the ensemble. There is an intrinsic gap between hemodynamic measures and neuronal activity, and negative BOLD signals can paradoxically occur even with increased neuronal activity (Schridde et al. 2008; Mishra et al. 2011). In contrast to previous work, this study provides a direct observation of ictal slow waves at single neuron resolution. Layer 5 pyramidal neurons, which are essential for the generation of Up and Down states (Sanchez-Vives and McCormick 2000; Neske 2016), were recorded continuously during deep anesthesia and seizures. We found that cortical layer 5 neurons indeed present with Up and Down states during seizures, with these states closely resembling those seen during deep anesthesia.
How do seizures originating in the hippocampus affect remote structures such as the neocortex? The mechanism could fall into the following 2 categories: a direct mechanism in which seizures propagate to the neocortex or an indirect mechanism where seizures originating in the hippocampus interact with other structures that are functionally connected to the neocortex. Our results suggest a depressed state of cortical neurons, making direct seizure propagation unlikely. Furthermore, the similar electrophysiological properties of Up and Down states during deep anesthesia and seizures imply a shared mechanism of sleep and temporal lobe seizures. Because subcortical arousal systems and neurotransmitters such as acetylcholine play an important role modulating cortical activity and consciousness during sleep (Halasz et al. 2004; Scammell et al. 2017), it is possible that these systems are also involved in temporal lobe seizures. Recent work has provided further evidence in support of the network inhibition hypothesis, which is centered on a deficit in subcortical arousal systems leading to cortical deactivation during limbic seizures. Indeed, key subcortical arousal regions, such as the brainstem pedunculopontine tegmental nucleus (PPT), thalamic intralaminar central lateral nucleus (CL), and basal forebrain have been shown to exhibit a reduction in activity during temporal lobe seizures, using neuroimaging or electrophysiological tools in human or animal models, respectively (Blumenfeld et al. 2004a; Englot et al. 2008; Motelow et al. 2015; Andrews et al. 2019). Furthermore, the disconnection of limbic and subcortical structures prevented cortical slowing, and stimulation of the PPT or CL are able to decrease slow-wave activity and reverse seizure-related impairment of consciousness with potential implications for human therapy (Englot et al. 2009; Furman et al. 2015; Gummadavelli et al. 2015a, 2015b; Kundishora et al. 2017).
In addition to ictal effects, we found that slow oscillations persisted into the postictal period, as shown previously in other animal studies and patients with epilepsy (Blumenfeld et al. 2004b; Englot et al. 2010; Motelow et al. 2015). Prior work has shown that activity in the hippocampus, lateral septum, and the anterior hypothalamus is increased ictally but suppressed postically, suggesting different mechanisms of slow oscillations and loss of consciousness in the ictal and postictal periods (Motelow et al. 2015). However, cholinergic neurons in subcortical regions such as the PPT and basal forebrain showed decreased firing rates in the postictal period, simultaneously with cortical slow oscillations, which is similar to ictal findings, suggesting depressed subcortical arousal may still contribute in the postictal period (Englot et al. 2008; Motelow et al. 2015). Future studies are needed to investigate the exact network mechanism of loss of consciousness during the postictal period.
Up and Down states have been studied extensively in prior work with variability in results mainly caused by different experimental settings. Indeed, studies of Up and Down states have been done in species including anesthetized cat, rat, mouse, ferret, and guinea pig (Steriade et al. 1993b; Cowan and Wilson 1994; Stern et al. 1997; Sanchez-Vives and McCormick 2000; Shu et al. 2003a; Hasenstaub et al. 2005; Rigas and Castro-Alamancos 2009; Beltramo et al. 2013). Some studies have also been performed on naturally sleeping cats and in vitro slices (Steriade et al. 1996; Sanchez-Vives and McCormick 2000; Shu et al. 2003b). Anesthetics have included ketamine–xylazine, urethane, pentobarbital, and halothane (Steriade et al. 1993b; Amzica and Steriade 1998; Pare et al. 1998; Sanchez-Vives and McCormick 2000). Targeting of recordings has varied from motor and sensory cortex to association cortices and from layer 2 to layer 6, with substantial evidence indicating layer 5 is the initiation site of Up states followed by propagation to layer 6 and then layers 2/3 (Sanchez-Vives and McCormick 2000; Neske 2016). Intracellular recording techniques have also varied from sharp microelectrodes with large resistance (60–90 MΩ or larger) to whole-cell patch clamp recordings with lower resistance, which can cause a significant difference in membrane input resistance measurements (Li et al. 2004). In our study, the classic pattern of Up and Down states was found in membrane potential fluctuations in layer 5 cortical neurons during seizures and deep anesthesia largely similar to previous research (Neske 2016). We observed ~ 9 to 12 mV depolarization of the membrane potential in up compared with Down states during deep anesthesia and seizures. These numbers are in line with in vivo whole-cell recordings performed in anesthetized rats (Waters and Helmchen 2006; Rigas and Castro-Alamancos 2009) and in anesthetized cats (Contreras et al. 1996; Neske 2016) while slightly higher than in in vitro studies (Shu et al. 2003a). We also report a slow-wave oscillation frequency of about 1 Hz in seizures and deep anesthesia. In prior work, Stern et al. reported a weak periodicity with a peak frequency near 1 Hz in anesthetized rats, and Cowan et al. reported an occasional periodic frequency of 0.3–1.5 Hz (Cowan and Wilson 1994; Stern et al. 1997). A 0.5–2 Hz range has been suggested in human by the American Academy of Sleep Medicine. Interestingly, we report a relatively large absolute input resistance during Up and Down states and relatively small decrease in input resistance from Down states to Up states in both seizure and deep anesthesia. Estimates of input resistance changes in previous literature vary considerably, from 10% to 200–500% with the majority of studies reporting a decrease in Up states (Steriade et al. 1993b; Shu et al. 2003a; Rigas and Castro-Alamancos 2009) although one study reported a small increase due to anomalous rectification (Waters and Helmchen 2006).
The present work is limited in several ways but serves as groundwork for additional future studies. First, the use of “light anesthesia,” which was necessary for the stabilization of patch clamp technique, could alter neuronal activity during seizures. We used low dose of anesthetic agent to mitigate those potential confounding effects. Furthermore, prior studies on human or animal models without anesthesia revealed similar slow oscillations during ictal and postictal periods associated with behavioral impairment (Blumenfeld et al. 2004b; Englot et al. 2008, 2009, 2010; Gummadavelli et al. 2015b; Kundishora et al. 2017). In the present work, however, we did not measure the extent of behavioral impairment simultaneously with the electrical recordings. To allow behavior to be evaluated directly and to eliminate effects of anesthesia, future work could be done with whole-cell recordings in awake head-fixed animals, which, although considerably more challenging especially in rats, could be feasible. In our present study, we measured membrane potential, oscillation frequency, input resistance, and firing rate of cortical layer 5 pyramidal neurons—another important direction for future study would be to examine the changes in excitatory and inhibitory postsynaptic potentials in these neurons with voltage clamp recordings and channel blockers. In addition, it would be of interest to compare other neuronal groups, such as inhibitory cortical neurons or pyramidal neurons, in different cortical layers. Finally, future work is also needed to extend previous investigations of the potential contribution of subcortical cholinergic, serotonergic, or putative glutamatergic nuclei to cortical depression during seizures (Motelow et al. 2015; Zhan et al. 2016; Feng et al. 2017), by investigating other systems such as the noradrenergic neurons in the locus coeruleus and orexin neurons in the lateral hypothalamus, ideally in nonanesthetized behaving animals.
In summary, the present study demonstrates that single cortical neurons follow a pattern of Up and Down states during focal limbic seizures and display similar membrane potential fluctuations, oscillation frequency, firing rate, and input resistance changes to those seen in deep anesthesia. Further work is needed to fully understand the basis and significance of these oscillations. However, based on our work and previous studies, we can speculate that temporal lobe epilepsy and sleep might share similar mechanisms related to the loss of consciousness, in which subcortical–cortical interplay might have a major role. Our work serves as an initial step to understand cortical activity and its relevance to impaired consciousness and offers insight into how the remote effects of focal limbic seizures may lead to potential targets to improve cortical function and epilepsy treatment.
Funding
National Institutes of Health (grant numbers: R01 NS066974 and R01 NS096088 to H.B.); the Loughridge–Williams Foundation (to H.B.); the Betsy and Jonathan Blattmachr Family (to H.B.); China Scholarship Council Postgraduate Scholarship Program Award (grant number: CSC 201606370154 to Z.Y.).
Notes
We thank Joshua C. Brumberg for the helpful advice on histological methods. Conflict of Interest: None declared.
References
- Amzica F, Neckelmann D. 1999. Membrane capacitance of cortical neurons and glia during sleep oscillations and spike-wave seizures. J Neurophysiol. 82:2731–2746. [DOI] [PubMed] [Google Scholar]
- Amzica F, Steriade M. 1998. Electrophysiological correlates of sleep delta waves. Electroencephalogr Clin Neurophysiol. 107:69–83. [DOI] [PubMed] [Google Scholar]
- Andrews JP, Yue Z, Ryu JH, Neske G, DA MC, Blumenfeld H. 2019. Mechanisms of decreased cholinergic arousal in focal seizures: in vivo whole-cell recordings from the pedunculopontine tegmental nucleus. Exp Neurol. 314:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltramo R, D'Urso G, Dal Maschio M, Farisello P, Bovetti S, Clovis Y, Lassi G, Tucci V, De Pietri Tonelli D, Fellin T. 2013. Layer-specific excitatory circuits differentially control recurrent network dynamics in the neocortex. Nat Neurosci. 16:227–234. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, KA MN, Vanderhill SD, Paige AL, Chung R, Davis K, Norden AD, Stokking R, Studholme C, Novotny EJ Jr et al. 2004a. Positive and negative network correlations in temporal lobe epilepsy. Cereb Cortex. 14:892–902. [DOI] [PubMed] [Google Scholar]
- Blumenfeld H, Rivera M, KA MN, Davis K, Spencer DD, Spencer SS. 2004b. Ictal neocortical slowing in temporal lobe epilepsy. Neurology. 63:1015–1021. [DOI] [PubMed] [Google Scholar]
- Chen CC, Chu P, Brumberg JC. 2015. Experience-dependent regulation of tissue-type plasminogen activator in the mouse barrel cortex. Neurosci Lett. 599:152–157. [DOI] [PubMed] [Google Scholar]
- Compte A, Sanchez-Vives MV, DA MC, Wang XJ. 2003. Cellular and network mechanisms of slow oscillatory activity (<1 Hz) and wave propagations in a cortical network model. J Neurophysiol. 89:2707–2725. [DOI] [PubMed] [Google Scholar]
- Contreras D, Timofeev I, Steriade M. 1996. Mechanisms of long-lasting hyperpolarizations underlying slow sleep oscillations in cat corticothalamic networks. J Physiol. 494:251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan RL, Wilson CJ. 1994. Spontaneous firing patterns and axonal projections of single corticostriatal neurons in the rat medial agranular cortex. J Neurophysiol. 71:17–32. [DOI] [PubMed] [Google Scholar]
- Cruikshank SJ, Lewis TJ, Connors BW. 2007. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat Neurosci. 10:462–468. [DOI] [PubMed] [Google Scholar]
- Dempster AP, Laird NM, Rubin DB. 1977. Maximum likelihood from incomplete data via the EM algorithm. J R Stat Soc. 39:1–38. [Google Scholar]
- Eisenschenk S, Gilmore RL, Cibula JE, Roper SN. 2001. Lateralization of temporal lobe foci: depth versus subdural electrodes. Clin Neurophysiol. 112:836–844. [DOI] [PubMed] [Google Scholar]
- Englot DJ, Mishra AM, Mansuripur PK, Herman P, Hyder F, Blumenfeld H. 2008. Remote effects of focal hippocampal seizures on the rat neocortex. J Neurosci. 28:9066–9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englot DJ, Modi B, Mishra AM, DeSalvo M, Hyder F, Blumenfeld H. 2009. Cortical deactivation induced by subcortical network dysfunction in limbic seizures. J Neurosci. 29:13006–13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englot DJ, Yang L, Hamid H, Danielson N, Bai X, Marfeo A, Yu L, Gordon A, Purcaro MJ, Motelow JE et al. 2010. Impaired consciousness in temporal lobe seizures: role of cortical slow activity. Brain. 133:3764–3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Motelow JE, Ma C, Biche W, McCafferty C, Smith N, Liu M, Zhan Q, Jia R, Xiao B et al. 2017. Seizures and sleep in the thalamus: focal limbic seizures show divergent activity patterns in different thalamic nuclei. J Neurosci. 37:11441–11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman M, Zhan Q, McCafferty C, Lerner BA, Motelow JE, Meng J, Ma C, Buchanan GF, Witten IB, Deisseroth K et al. 2015. Optogenetic stimulation of cholinergic brainstem neurons during focal limbic seizures: effects on cortical physiology. Epilepsia. 56:e198–e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gummadavelli A, Kundishora AJ, Willie JT. 2015a. Improving level of consciousness in epilepsy with neurostimulation. Neurosurg Focus. 38(6):E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gummadavelli A, Motelow JE, Smith N, Zhan Q, Schiff ND, Blumenfeld H. 2015b. Thalamic stimulation to improve level of consciousness after seizures: evaluation of electrophysiology and behavior. Epilepsia. 56:114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halasz P, Terzano M, Parrino L, Bódizs R. 2004. The nature of arousal in sleep. J Sleep Res. 13:1–23. [DOI] [PubMed] [Google Scholar]
- Hasenstaub A, Shu Y, Haider B, Kraushaar U, Duque A, McCormick DA. 2005. Inhibitory postsynaptic potentials carry synchronized frequency information in active cortical networks. Neuron. 47:423–435. [DOI] [PubMed] [Google Scholar]
- Holzmann H, Vollmer S. 2008. A likelihood ratio test for bimodality in two-component mixtures with application to regional income distribution in the EU. Asta Adv Stat Anal. 92:57–69. [Google Scholar]
- Janszky J, Schulz R, Janszky I, Ebner A. 2004. Medial temporal lobe epilepsy: gender differences. J Neurol Neurosurg Psychiatry. 75:773–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundishora AJ, Gummadavelli A, Ma C, Liu M, McCafferty C, Schiff ND, Willie JT, Gross RE, Gerrard J, Blumenfeld H. 2017. Restoring conscious arousal during focal limbic seizures with deep brain stimulation. Cereb Cortex. 27:1964–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Hu C, Wang L, Liu D, Liao W, Xiao B, Chen H, Feng L. 2019. Disruption of functional connectivity among subcortical arousal system and cortical networks in temporal lobe epilepsy. Brain Imaging Behav. doi: 10.1007/s11682-018-0014-y. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Li WC, Soffe SR, Roberts A. 2004. A direct comparison of whole cell patch and sharp electrodes by simultaneous recording from single spinal neurons in frog tadpoles. J Neurophysiol. 92:380–386. [DOI] [PubMed] [Google Scholar]
- Lieb JP, Dasheiff RM, Engel J Jr. 1991. Role of the frontal lobes in the propagation of mesial temporal lobe seizures. Epilepsia. 32:822–837. [DOI] [PubMed] [Google Scholar]
- McGinley MJ, Vinck M, Reimer J, Batista-Brito R, Zagha E, Cadwell CR, Tolias AS, Cardin JA, McCormick DA. 2015. Waking state: rapid variations modulate neural and behavioral responses. Neuron. 87:1143–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejias-Aponte CA, Jimenez-Rivera CA, Segarra AC. 2002. Sex differences in models of temporal lobe epilepsy: role of testosterone. Brain Res. 944:210–218. [DOI] [PubMed] [Google Scholar]
- Mishra AM, Ellens DJ, Schridde U, Motelow JE, Purcaro MJ, DeSalvo MN, Enev M, Sanganahalli BG, Hyder F, Blumenfeld H. 2011. Where fMRI and electrophysiology agree to disagree: corticothalamic and striatal activity patterns in the WAG/Rij rat. J Neurosci. 31:15053–15064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motelow JE, Li W, Zhan Q, Mishra AM, Sachdev RN, Liu G, Gummadavelli A, Zayyad Z, Lee HS, Chu V et al. 2015. Decreased subcortical cholinergic arousal in focal seizures. Neuron. 85:561–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neske GT. 2016. The slow oscillation in cortical and thalamic networks: mechanisms and functions. Front Neural Circuit. 9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neske GT, Patrick SL, Connors BW. 2015. Contributions of diverse excitatory and inhibitory neurons to recurrent network activity in cerebral cortex. J Neurosci. 35:1089–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak LG, Azouz R, Sanchez-Vives MV, Destexhe A, Lang EJ. 2003. Electrophysiological classes of cat primary visual cortical neurons in vivo as revealed by quantitative analyses. J Neurophysiol. 89:1541–1566. [DOI] [PubMed] [Google Scholar]
- Pare D, Shink E, Gaudreau H et al. 1998. Impact of spontaneous synaptic activity on the resting properties of cat neocortical pyramidal neurons in vivo. J Neurophysiol. 79:1450–1460. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. 1998. The rat brain in stereotaxic coordinates. San Diego (CA): Academic Press. [Google Scholar]
- Rees G, Kreiman G, Koch C. 2002. Neural correlates of consciousness in humans. Nat Rev Neurosci. 3:261–270. [DOI] [PubMed] [Google Scholar]
- Rigas P, Castro-Alamancos MA. 2009. Impact of persistent cortical activity (up states) on intracortical and thalamocortical synaptic inputs. J Neurophysiol. 102:119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. 2000. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci. 3:1027–1034. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Arrigoni E, Lipton JO. 2017. Neural circuitry of wakefulness and sleep. Neuron. 93:747–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. 2007. The neurobiology of epilepsy. Curr Neurol Neurosci Rep. 7:348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schridde U, Khubchandani M, Motelow JE, Sanganahalli BG, Hyder F, Blumenfeld H. 2008. Negative BOLD with large increases in neuronal activity. Cereb Cortex. 18:1814–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamari Y, Narvaez JA, Vico FJ, Lobo D, Sanchez-Vives MV. 2007. Robust off- and online separation of intracellularly recorded up and down cortical states. PLoS One. 2:e888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, Badoual M, Bal T, McCormick DA. 2003a. Barrages of synaptic activity control the gain and sensitivity of cortical neurons. J Neurosci. 23:10388–10401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, McCormick DA. 2003b. Turning on and off recurrent balanced cortical activity. Nature. 423:288–293. [DOI] [PubMed] [Google Scholar]
- Steriade M, Amzica F, Contreras D. 1996. Synchronization of fast (30-40 Hz) spontaneous cortical rhythms during brain activation. J Neurosci. 16:392–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Contreras D, Curro Dossi R, Nuñez A. 1993a. The slow (<1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci. 13:3284–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. 1993b. Intracellular analysis of relations between the slow (<1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci. 13:3266–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nunez A, Amzica F. 1993c. A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 13:3252–3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Timofeev I, Grenier F. 2001. Natural waking and sleep states: a view from inside neocortical neurons. J Neurophysiol. 85:1969–1985. [DOI] [PubMed] [Google Scholar]
- Stern EA, Kincaid AE, Wilson CJ. 1997. Spontaneous subthreshold membrane potential fluctuations and action potential variability of rat corticostriatal and striatal neurons in vivo. J Neurophysiol. 77:1697–1715. [DOI] [PubMed] [Google Scholar]
- Ueta Y, Otsuka T, Morishima M, Ushimaru M, Kawaguchi Y. 2014. Multiple layer 5 pyramidal cell subtypes relay cortical feedback from secondary to primary motor areas in rats. Cereb Cortex. 24:2362–2376. [DOI] [PubMed] [Google Scholar]
- Van Paesschen W, Dupont P, Van Driel G, Van Billoen H, Maes A. 2003. SPECT perfusion changes during complex partial seizures in patients with hippocampal sclerosis. Brain. 126:1103–1111. [DOI] [PubMed] [Google Scholar]
- Waters J, Helmchen F. 2006. Background synaptic activity is sparse in neocortex. J Neurosci. 26:8267–8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young GB. 2000. The EEG in coma. J Clin Neurophysiol. 17:473–485. [DOI] [PubMed] [Google Scholar]
- Zhan Q, Buchanan GF, Motelow JE, Andrews J, Vitkovskiy P, Chen WC, Serout F, Gummadavelli A, Kundishora A, Furman M et al. 2016. Impaired serotonergic brainstem function during and after seizures. J Neurosci. 36:2711–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]


