Abstract
Contact lens wear is one of the primary risk factors for the development of ocular surface inflammatory events. The purpose of this review is to examine and summarize existing knowledge on the mechanisms of contact lens related ocular surface inflammation and the evidence for the effectiveness of current objective methods to measure ocular surface inflammation. Contact lens wear is postulated to trigger an inflammatory response on the ocular surface due to mechanical, chemical, hypoxic stress, or by the introduction of microbes and their toxins. Apart from the traditional signs of inflammation, such as swelling, oedema, redness and heat, on the ocular surface, other methods to measure ocular surface inflammation in sub-clinical levels include tear inflammatory mediator concentrations, conjunctival cell morphology, and corneal epithelial dendritic cell density and morphology. Tear inflammatory mediator concentrations are up- or down-regulated during contact lens wear, with or without the presence of associated inflammatory events. There is higher conjunctival cell metaplasia observed with contact lens wear, but changes in goblet cell density are inconclusive. Dendritic cell density is seen to increase soon after initiating soft contact lens wear. The long term effects of contact lens wear on dendritic cell migration in the cornea and conjunctiva, including the lid wiper area, require further investigation. Currently patient factors, such as age, smoking, systemic diseases and genetic profile are being studied. A better understanding of these mechanisms may facilitate the development of new management options and strategies to minimize ocular surface inflammation related to contact lens wear.
Keywords: contact lens wear, tear cytokine conjunctival cell morphology dendritic cells, ocular surface inflammation
1. Introduction
The use of contact lenses is one of the primary risk factors associated with corneal and ocular surface inflammatory events [1–4]. It has been reported that soft contact lens related corneal inflammatory and infiltrative events occur in 7–44% of wearers per year and are associated with significant morbidity and economic cost (> $175 million US dollars in 2010, USA) [1,5–7]. Contact lens induced adverse events can be inflammatory and/or infectious in nature [2,8]. Contact lens wear can induce hypoxic or mechanical stress on the ocular surface and may also act as a vehicle for microbial inoculation, leading to pathogenic events ranging from subtle epithelial injury and infiltration by pathogens, antigens and white blood cells to the most severe microbial keratitis (MK)[9].
It has been hypothesized that a contact lens on the eye induces an ocular surface inflammatory process. Efron has comprehensively shown how contact lens wear can lead to the five cardinal signs of inflammation (mild and severe) that are clinically seen on the ocular surface namely- rubor (redness), calor (heat), tumor (swelling), loss of function (Functio laesa) and dolor (pain), which is encompassed as discomfort [10], and this is consistent with the Merriam- Webster dictionary definition of inflammation. Even though the forms of inflammations are mild in successful contact lens wearers, cardinal signs, such as hyperaemia [11], increased ocular temperature when wearing contact lens [12,13], symptoms [14], and corneal oedema in subjects who wear low oxygen transmissibility contact lenses [15], can be more readily observed than in non-contact lens wearers. These milder forms of inflammation can be managed by altering the contact lens material, fitting, decreasing wearing time and instillation of artificial tears [13,15–17]. However, in severe cases, the contact lens induced inflammation can lead to adverse events that warrant discontinuation of lens wear [18].
A large proportion of soft contact lens wearers report ocular dryness and discomfort [19,20]. It has been stated that this, so-called, ectopic corneal pain could be due to subclinical inflammation with the presence of normal tear secretion and corneal sensitivity [21,22]. Contact lens wear has been shown to induce higher ocular temperature and conjunctival hyperemia, which supports the notion that soft contact lens wear induces ocular surface inflammation, along with other compromised ocular surface parameters, such as lower tear stability and higher ocular surface staining [13,23–25].
This manuscript aimed to review the findings of these non-invasive contemporary techniques for detecting inflammatory responses at the cellular and molecular levels, including a) Ocular inflammatory response related to contact lens wear in humans; b) Recent objective methods used to evaluate the inflammatory responses on the ocular surface; and c) Potential factors that may be related to the risk of ocular inflammatory events in contact lens wearers.
2. Ocular surface inflammation in contact lens wear
The proposed mechanism driving this contact lens related inflammatory response can be described in two main steps: First, the ocular surface releases pro-inflammatory molecules and proteins [2,26] in response to the presence of a contact lens. These proteins then modulate the ocular surface (i.e, migration of antigen presenting cells and changes in the morphology of conjunctival cells) and these changes further drive the inflammatory cascade, in a vicious cycle (Fig. 1) [27]. These inflammatory responses can be measured noninvasively as is discussed in this manuscript (Fig. 1).
Fig. 1.
Brief schematic diagram of ocular inflammation induced by contact lens wear and its measurements. TLRs: toll-like receptor; NK cells: natural killer cells. The blue boxes describe recent methods used to objectively measured lens induced inflammation. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
When a pathogen or foreign body is presented to the ocular surface, the innate immune system is activated, which leads to the secretion of certain inflammatory proteins by natural killer cells (NK cells) which in turn, can damage the ocular surface [28]. One family of the innate immune response proteins, the toll-like receptors (TLRs), can be activated by pathogen associated molecular patterns (PAMPs) on pathogens and endogenous ligands of intracellular components of dead cells, such as small nuclear ribonucleoprotein particles (snRNPs) [29]. In contact lens wear, TLRs are likely to be modulated by microbes and their products on the contact lenses and/or the damaged epithelial cells caused by contact lens wear [30]. After the activation of TLRs, Interleukin-1R associated kinase (IRAK) is activated and leads to activation of the Nuclear Factor kappa light chain enhancer of activated B cells (NF-kβ) pathway on the ocular surface. This leads to expression of multiple cytokines and chemokines, including interleukin (IL)-1α, IL-1β, IL-6, IL-8, IL-23, IL-17A, interferon (IFN)-γ and tumor necrosis factor (TNF)-α. Even though the activation of TLRs has been identified in dry eye [31], corneal inflammation and infection in animal studies [32,33], an appropriate animal model to study the role that TLRs in contact lens wear induced inflammation is warranted.
Damaged cells of the ocular surface can also release cytokines and chemokines and transform immature antigen presenting cells (APCs), which are dendritic cells and macrophages, into mature APCs [34]. APCs play a vital role in the activation of the immune system and the communication between B and T cells [35–37]. In the presence of certain up-regulating inflammatory mediators (IL-1, IL-6, IL-8, TNF- α and IFN-γ), infiltrating T cells, macrophages and HLA class II molecules on APCs increase in the epithelium on the ocular surface [38,39], which may up-regulate neuropeptides as inflammatory mediators [40,41]. Increased expression of inflammatory mediators was shown to correlate negatively with goblet cell density of the conjunctiva [42]. The released cytokines and chemokines may also lead to apoptosis of the ocular surface cells (Fig. 1). However, these relationships have not been fully investigated in contact lens wearers.
2.1. Inflammatory mediators on the ocular surface
More than 1500 proteins have been identified on the ocular surface and tear film [43,44]. Among them, at least 25 inflammatory mediators can be detected in tears in healthy subjects[45]. In this review, the known inflammatory mediators related to contact lens wear are briefly discussed in this section and the association with contact lens wear is further addressed in Section 3.1.2.
IL-6, IL-8 and TNF-α are the most studied inflammatory mediators during contact lens wear and are up-regulated in inflammation and/or infection by activation of inflammatory cells. Their role on the ocular surface is to defend against pathogens and promote epithelial wound healing [46–50]. Higher tear IL-6 and IL-8, but not TNF-α, concentrations are significantly associated with ocular surface integrity and goblet cell density [51,52]. TNF-α stimulates the acute phase reaction and the reaction with other cytokines (IL-1, IL-17 and IFN-γ) also leads to cell apoptosis on the ocular surface [53].
IL-10, IL-12 and matrix metalloproteinases-9 (MMP-9) have also been studied during contact lens wear. IL-10, which is suppressed by IL-12 [54], is a potent inhibitor or autoregulator of IL-8. The ratio of IL-8/ IL-10 may be an important marker of the overall ocular inflammatory status. IL- 12, released by APCs, is a potent regulator of T-helper type 1 (Th1) cells, whose role it is to terminate bacteria on the ocular surface; however, it also damages normal tissue [55]. MMP-9 is produced by stressed corneal and conjunctival epithelial cells [56]. An increase in MMP-9 could be related to immunological inflammation and by degrading collagen, it may contribute to poorer corneal barrier function and corneal epithelial desquamation. This may further affect wound healing on the ocular surface [57–59].
Mature IL-1β acts as a mediator of the inflammatory response for defending against infection, increasing cellular activity [53] and inducing other cytokines (IL-6, IL-8 and TNF α) and stimulating MMPs, particularly MMP-9 [60–63]. It has been shown that higher tear IL-1β concentration is associated with severity of dry eye disease and ocular surface staining [51,52]. In contrast, IL-1 receptor antagonist (IL-1Ra) inhibits the activity of the IL-1β by binding to the type 1 IL-1 receptor [64]. Therefore, the imbalance between IL-1β and IL-1Ra has been speculated as a reason for the excess presentation of IL-1β on the ocular surface in bacterial keratitis [65]. However, the balance between proand anti-inflammatory mediators has not been investigated in contact lens induced ocular surface inflammatory events.
During an inflammatory event such as bacterial or an autoimmune keratitis, IL-17, which is activated by memory T cells, is responsible for the induction of other cytokines, chemokines and MMPs by the epithelial cells of the ocular surface [65,66]. An increased concentration of IL-17 is also associated with corneal damage during systematic inflammatory disease [66] and linked to many immuno-pathological changes [53,65,67]. In support of this, elimination of IL-17 has been shown to attenuate the severity of dry eye [53].
Pain sensation is also one of the clinical signs of inflammation. Therefore, neuromediators, such as nerve growth factor, substance P and calcitonin gene related peptides, are also considered as inflammatory mediators [40,41,68]. Up-regulation of neurotransmitters have been reported in allergy, inflammation and injury on the ocular surface [68–70]. Differences in substance P and calcitonin gene related peptides were not found in healthy contact lens wearers [14], however further research is needed in those with ocular surface inflammation.
2.2. Conjunctival cells
Mucin has an important role in the stabilization of the tear film [71] and is also identified as a defense layer against bacteria. The mucin layer of the tear film functions to bind with foreign particles and bacteria which is then removed by the blinking action of the eyelids. For example, higher Pseudomonas aeruginosa adhesion to mucin was found in ocular pathogenic strains [72]. Mucin also contains molecules with antimicrobial properties such as IgA, lactoferrin and lysozyme to protect the ocular surface from infections [73].
Contact lens wear interferes with the structure of the mucin layer and induces inflammatory cells which may cause actual goblet cells loss [74–76]. The consequent decrease in mucin secretion can lead to keratinization [77] in which epithelial cells separate and show upturning of their edges. These features are thought to be morphologically characteristic of cells that are about to be shed from the ocular surface [77]. Therefore, the density of goblet cells and the morphology of human conjunctival epithelium have been evaluated in contact lens wear and this is further addressed in Section 3.2.
2.3. Antigen presenting cells (APCs) on the ocular surface
APCs are Y- or X-shaped forms with a small cell body [78,79]. As mentioned in Section 2, inflammatory mediators activate APCs to morph from an immature form (cell bodies only) into a mature form (long dendrites) [34–37,80]. Migration of epithelial dendritic cells into the central cornea is considered pathognomonic of activation of the immune response on the ocular surface [81,82]. Also, dendritic cell density and maturation in conjunctiva and eyelid may represent the status of ocular inflammation [83–85].
3. Evaluation of ocular surface inflammation at the cellular and molecular level
In this section, the methodology of sample collections and analyses of tear inflammatory mediators, conjunctival cell morphology, including goblet cell density and epithelial squamous metaplasia, and antigen presenting cells on the ocular surface and the effects of contact lens wear are discussed.
3.1. Inflammatory mediators
A broad range of inflammatory mediators, including those pro-inflammatory cytokines, chemokines, MMPs and neuromediators described in Section 2.1, can be detected in human tears and conjunctival cells. Inflammatory mediator concentrations have been shown to be directly associated with ocular surface staining, severity of dry eye (including contact lens related dry eye) and allergies. For example, there are significant increases in IL-1β, IL-1Ra, IL-6, IL-8, IL-10, IL-17A, TNF-α, MMP-9 and nerve growth factors and decrease in calcitonin gene related peptide in dry eye and associated with staining [52,56,57,66,86–94] and increased IL-1β, IL-2, IL-6, IL-10, IL-12, TNF-α, substance P and calcitonin gene related peptide in allergy [95,96]. Additional tear film biomarkers such as IgE, histamine, tryptase, prostaglandin, leukotriene B4, and eosinophilic cationic protein are also present in allergy, often related to degranulation of a mast cell or as a consequence of the release of inflammatory mediators from the degranulation of a mast cell [97]. Tear analysis of inflammatory mediators is more common than conjunctival cell analysis since it is relatively less invasive and requires fewer procedures in sample collection and extraction.
3.1.1. Tear analysis
A number of different techniques of tear collection have been reported in the literature. Possible methods used to collect tears include microcapillary tubes (Fig. 2A), Schirmer strips (Fig. 2B) and microsponges (Fig. 2C). Different types of tears [98], such as flush, reflex or basal tears can also be collected. The collection method and/or the type of tears collected can significantly affect tear protein concentrations [99–101]. For example, levels of albumin, transferrin and Immunoglobulin G are significantly higher in samples collected by Schirmer strips than by glass capillary [102]. It was postulated that reflex tears may have resulted from the discomfort produced by the strips or sponges in the eye [99,100]. Tear proteins may also interact with the collecting tube material (glass/plastic). Flush and reflex tears have been shown to have lower concentration of tear proteins and inflammatory mediators than normal tears, due to the dilution of the tears [103]. Thus the method and type of tear collection must be taken into account when comparing studies.
Fig. 2.
Possible methods of tear collections, using a microcapillary tube (A), a Schirmer strip (B) and a microsponge (C).
Concentrations of tear cytokines have been quantified using Enzyme Linked Immuno Sorbent Assay (ELISA), Cytometric Bead Assay (CBA), microarrays, membrane assay (MA), Polyacrylamide Gel Electrophoresis (PAGE), and multiplex bead and electrochemiluminescence-based assays [45,52,89,104–109]. Among these, ELISA is considered the current gold standard and remains the most commonly used method to measure tear cytokine concentrations. However, the small volume of tears that can be collected from human subjects limits the number of cytokines that can be evaluated from a single sample of up to 10–12 μl of basal tears. With the recent advent of multiplex techniques, multiple tear proteins can be measured from a single sample of volume as small as a few microliters (μl). Multiplex assays have, as a result, been increasingly used, but they are not without their limitations [45,110–113]. La Fránce et al. [114] showed that in different multiplex assays, the level of tear dilution and the sample preparation can affect the concentration of tear cytokines measured. Good within-kit/operator repeatability was recently reported using custom bead-based multiplex assays but significant differences across studies of similar patient populations were also found [115].
The relative ability of ELISA and multiplex assays to measure tear cytokines has not, so far, been assessed. However, preliminary data indicate coefficients of repeatability for measuring tear IL-6 concentration using both validated techniques at 234 pg/ml for bead-based multiplex analyses (Bio-Rad Laboratories, CA) and 5.3 pg/ml for ELISA (Affymetrix ebioscience, CA) (unpublished data Chao and Richdale). There are large differences in the level of IL-6 detected [multiplex: mean 406 ± 132 pg/ml; ELISA: mean 5 ± 11 pg/ml; n = 24] and no correlation between the concentrations determined by these two methods. Similar to tear collection methods, the use of different tear analysis methods makes it difficult to compare across studies. There are not enough published data to indicate which method may be superior.
3.1.2. Tear inflammatory mediators in contact lens wear
Both contact lens wear and contact lens associated inflammatory diseases have the ability to up- or down-regulate certain tear cytokines [2,116–118] (Table 1). For example, IL-6, 8, TNF-α and MMP-9 are increased in healthy contact lens wearers [2,108,119–122]. The influence of contact lenses on ocular surface inflammation was seen in a study where IL-6 levels could only be detected in contact lens wearers but not in non-lens wearers [108]. Furthermore, IL-6 was not detectable when contact lens wear was ceased for 6 days, and returned to the original values upon resuming lens wear [108]. However, the changes in tear IL-6 and TNF-α after short term daily soft contact lens wear could not be replicated when measured using multiplex assays [112]. This highlights the impact of analysis methods on the results of changes in tear film inflammatory mediator concentration after contact lens wear. Differences in tear nerve growth factor, substance P and calcitonin gene related peptides were not found between healthy contact lens and non-lens wearers [14,123]. In contrast, concentrations of IL-1β and IL-12(p70) decreased after short-term daily contact lens measured using multiplex assays [112].
Table 1.
Changes in key known tear inflammatory mediators in soft contact lens wear and contact lens related situations in human’s studies.
| Tear Protein | Healthy SCL Wear | Multipurpose Solution | Contact lens induced CIEs |
|---|---|---|---|
| IL-1β | ↓[112], ↑[124] | – | – |
| IL-6 | ↑[2,108,116]; ↓[124] ↑Trend[120] |
↑[125,126] | ↑[117,127] |
| IL-8 | ↑[2,116], =[112,124] ↑Trend[120] |
↑[126] | ↑[117,127] |
| IL-10 | – | – | ↑[127] |
| IL-12 | ↓[112] | – | – |
| IL-17A | – | ↑[125] | – |
| TNF- α | =[112] | ↑[125] | – |
| MMP-9 | ↑[128] ↑Trend[120] |
– | – |
| NGF | =[123] | – | – |
| Substance P | =[14] | – | – |
| CGRP | =[14] | – | – |
NGF: nerve growth factor, CGRP: calcitonin gene related peptide; SCL: soft contact lens; CIEs: corneal infiltrative events; ↑: increase; ↓: decrease; ___: not reported; =: No significant changes.
Contact lens discomfort is a major problem for contact lens wear [19,20,129]. However, there have been no reported association between absolute concentrations of cytokines and contact lens induced discomfort [112]. Interestingly, a higher concentration of tear nerve growth factor has been reported in symptomatic soft contact lens wearers [123]. The comparisons in tear neuropeptide and cytokine concentrations between symptomatic and asymptomatic contact lens wearers require further investigation.
Many tear proteins, including GM-CSF, IFN-γ, TNF-α, IL-2, IL-4, IL-5, IL-6, IL-13, IL-15 and IL-17, increased with the use of certain lens care products [125]. MMP-9 and its tissue inhibitor metalloproteinase (TIMP)-1 are up-regulated during overnight contact lens wear [128]. However, differences could not be demonstrated in 27 cytokine concentrations (IL-1β, IL-1Ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12(p70), IL-13, IL-15 and IL-17A, TNF-α, IFN-γ, GM-CSF, Exotaxin, FGF-b, G-CSF, IP-10, MCP-1, MIP-1α and β, PDGF-BB, RANTES and VEGF) between 30 days of extended, versus daily, silicone hydrogel soft contact lens wear [127].
A trend for increased levels of IL-6, IL-8 and GM-CSF in reflex tear has been suggested in subjects with contact lens induced red eye (CLARE) and contact lens induced peripheral ulcer (CLPU) [117]. Higher tear IL-6, IL-8 and IL-10 concentrations were found in a patient with CLARE using the aforementioned 27 cytokine multiplex assay [127]. However, these 27 cytokines mentioned above have only been studied when first initiating lens wear [112]. Also, the levels of these cytokines/inflammatory mediators, including neuromediators, need to be evaluated in contact lens-induced inflammatory events, due to their role in the normal ocular immune defense system.
3.2. Conjunctival cell morphology
Apart from tear analysis, conjunctival cells have been used to assess the ocular surface inflammatory responses. Abnormal cellular findings reported in ocular surface disease have been typically described as an increase/enlargement in the epithelial cell size and change to epithelial cell shape from round or oval to polygonal, with concurrent decrease in the size of the nuclei and/or a snake like appearance of the nucleus, and a decrease in goblet cell density [130–132]. Conjunctival epithelium morphology and goblet cell density have been evaluated in ocular inflammation associated with dry eye [31,133], after LASIK surgery [134,135], trachoma [136], chemical burn [137] and other ocular surface diseases [132,138–144].Lower goblet cell density and MUC5AC and higher squamous metaplasia were also found in atopic subjects with corneal ulcer [145]. Recently, impression cytology of a specific conjunctival region at the lid margin (often called the lid wiper) has been used to characterize ocular surface health [146,147]. Differences in cell morphology in the lid wiper area between contact lens and non-lens wearers have not been investigated.
3.2.1. Conjunctival cell collection and analysis
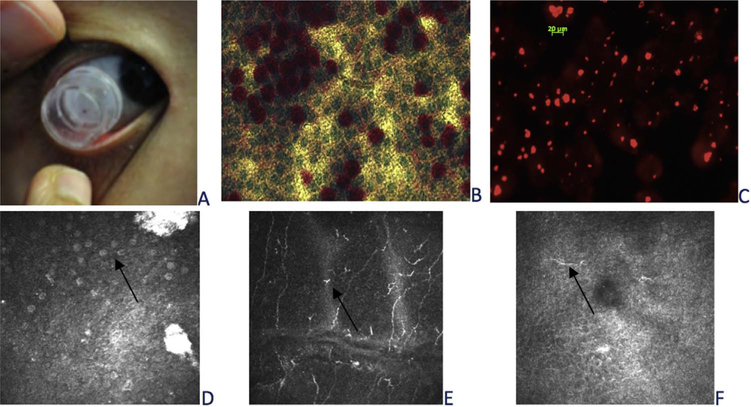
Conjunctival impression cytology is considered as a form of “conjunctival biopsy.” Impression cytology can be easily conducted on the ocular surface with filter inserts (Fig. 3A) or filter papers. Filters with different pore size, between 0.025–0.45 μm, and the presence of surfactant, affect the consistency of cells yielded and the details of the cell morphology [148,149]. Larger pore size filters can pick up more cells than smaller pore size filters without being affected by the presence of surfactant [148]. However, the details of cell morphology could be better observed with smaller pore size [148]. Periodic acid-Schiff staining (PAS) (Fig. 3B) is one of the most common methods to evaluate conjunctival impression cytology samples. In addition, immunolabelling staining of MUC5AC, a type of mucin secreted by conjunctival goblet cells, can be used for the assessment of mucin expression (Fig. 3C) [145]. The most common assessment of conjunctival cell homeostasis is conducted through measurement of goblet cell density and epithelial metaplasia [150]. Therefore, the selection of filters depends on the purpose of the observation and has to be considered when comparing studies.
Fig. 3.
Ocular surface cell morphology. Conjunctival impression cytology using filter insert (A); conjunctival cells stained with PAS (B) and MUC5AC (C). Presumed goblet cells (arrow) (C), epithelial dendritic cells (arrow) (D) on conjunctiva and presumed dendritic cells on cornea using in vivo confocal microscopy.
In vivo confocal microscopy has also been used to assess human conjunctival cells and image the superficial conjunctival epithelial layer with its hyper-refractive desquamating cells and presumed goblet cells (Fig. 3D). Impression cytology and confocal microscopy methods have shown good correlation in the measurement of goblet cell density [151,152]. Even though in vivo confocal microscopy allows less invasive conjunctival cell assessment, limitations of in vivo confocal microscopy include: a. Small area (400 μm2) scanned, which is likely to induce higher variability of density estimations; b. Significant inter-observer difference in counting assumed goblet cells density [153]; c. Less detailed cell morphology, such as cell nuclei and cytoplasm comparing with impression cytology sample (Fig. 3B and D).
3.2.2. Conjunctival cell morphology in contact lens wear
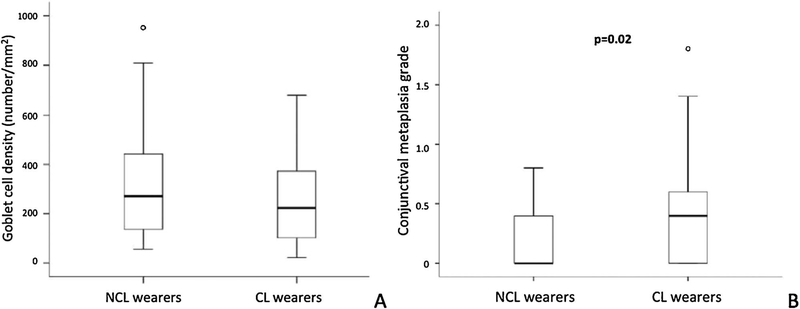
Reduced goblet cell density has been widely reported in contact lens wearers (reviewed in Doughty 2011 [154]), even though a small number of prospective studies have not been able to show this effect [138,155–158]. Significant differences in temporal bulbar conjunctival goblet cell density were not demonstrated using impression cytology between habitual contact lens wearers (11 years) and non-lens wearers (Fig. 4A) [159]. This is consistent with findings previously reported from a study using in vivo confocal microscopy [79]. There was also no difference in goblet cell density between hydrogel and silicone hydrogel in extended wear over a 6-month study period [155]. However, higher goblet cell density was found with 2-weekly reusable soft lens wear compared to daily disposable lens wear over a 6-month period [160]. Changes in goblet cells were associated with contact lens intolerance and/or contact lens papillary conjunctivitis, which could be attributed to possible lipid deposition from the irritated goblet cells [77,154,155,161,162]. These inconclusive findings suggest the use of a more functional measure, such as the number of MUC5AC positive goblet cells, as a more efficient method to characterize the ocular surface inflammation [163]. Future randomized controlled clinical trials are needed to further understand the effect of contact lens wear on conjunctival cell morphology.
Fig. 4.
Goblet cell density (A) and conjunctival epithelial metaplasia (B) between contact lens and non-contact lens wearer [159]. The contact lens wear group demonstrated a significantly higher conjunctival metaplasia grade than the non-lens wear group.
Contact lens wear has been shown to cause conjunctival metaplasia [138]. Epithelial metaplasia increases with duration of contact lens wear [155,157,164,165]. It is hypothesized that mechanical friction, contact lens induced dry eye or solution toxicity may be causing the conjunctival epithelial changes. In a study with 34 habitual contact lens and 37 non lens wearers, significantly greater conjunctival metaplasia measured using Nelson grading scheme was found [132] in the contact lens wear group than the non-lens wearers (Fig. 4B, p = 0.02)[159]. Higher density of superficial conjunctival epithelial cells was also observed in contact lens wearers compared to controls using in vivo confocal microscopy [79]. Higher epithelial cell density in contact lens wearers may be because of the delayed desquamation/keratinization caused by retardation of the cells, which could not have been observed using in vivo confocal microscopy. Therefore, the grading of metaplasia using impression cytology may be a better biomarker of tissue damage than goblet cell density for contact lens wearers.
On the basis of ocular surface inflammation (Fig. 1), it can be expected that there are changes in goblet cell density, MUC5AC expression and squamous metaplasia in contact lens induced corneal infiltrative events. Further investigation is required in this area.
3.3. Epithelial dendritic cells
Density of epithelial dendritic cells is higher in the periphery compared to the central cornea [166,167]. Epithelial dendritic cells have been found in increased numbers in the central cornea of dry eye [168], diabetic neuropathy [27], Thygeson’s disease [169], bacterial keratitis [65] and mechanical corneal injury patients [170]. Higher conjunctival dendritic cell density was also found in Langerhans cells histocytosis [171], atopic dermatitis [172], ocular cicatricial pemphigoid [84] using eye specimens and vernal keratoconjunctivitis using in vivo confocal microscopy [83,173].
3.3.1. Ocular surface epithelial dendritic cell analysis
Confocal microscopy allows in vivo visualization and quantification of the number of dendritic cells in the cornea (Fig. 3E), conjunctiva (Fig. 3F) and lid wiper. The maturity of dendritic cell visualized using in vivo confocal microscopy may also provide clues to the status of inflammation [78,80,83,173].
3.3.2. Ocular surface epithelial dendritic cells in contact lens wear
Dendritic cell recruitment to the central cornea has been shown to occur following as little as one week of contact lens wear in humans [167,174,175] and in animal models [55,176]. With cessation of contact lens wear, a corresponding decline in dendritic cell density is noted, with minimal detection of dendritic cells after 2 weeks of no lens wear in an animal model [55]. Corneal dendritic cell recruitment may also be higher in silicone hydrogel than hydrogel wearers, as well as higher in peroxide than multipurpose solution users [174]. A relationship between corneal dendritic cell density and corneal staining could not be demonstrated, in either contact or non-contact lens wearers [177]. Changes in corneal dendritic cell response over a period of contact lens wear beyond 1 month has not been investigated, nor has the time course of dendritic cell changes following discontinuation of contact lens wear in humans. Levels of dendritic cells have also not been measured during active contact lens-related inflammation.
Dendritic cell density at nasal bulbar conjunctiva assessed using in vivo confocal microscopy increased 1 week after hydrogel contact lens wear and returned to baseline levels at 24 weeks [76]. Another study confirmed that there was no difference between experienced contact lens wearers and non-lens wearers [78]. Higher dendritic cell density in lid wiper was found in contact lens wearers with dry eye compared to contact lens wearers without dry eye and non contact lens wearers [76], which could be contributed to the rubbing effects [76] between lid wiper caused by insufficient tear film. These findings confirmed that conjunctival dendritic cells play a role in innate immune system with higher density immediately after contact lens wear. The migration and maturation of conjunctival dendritic cells in contact lens-related inflammation require further investigation.
4. Factors affecting ocular surface inflammation
Contact lens wear related factors, such as duration of wear, lens power, replacement schedule and lens materials [1,178]; and behaviors and non-modifiable patient factors, such as age and sex, must be taken into consideration when investigating contact lens related ocular surface inflammation. For example, the peak prevalence for contact lens induced corneal inflammatory events occurs in 15–44 years old [178–181]. However, the effect of age and innate immune system in contact lens related corneal infiltrates event has not been investigated. Smoking [182], poor hygiene [182], exposure of contact lenses to tap water, sleeping, showering or swimming in contact lenses are established risk factors for the development of ocular inflammation and infection [183]. General health issues such as diabetes, herpes and autoimmune diseases can also affect the ocular surface. General history taking and use of instruments such as the Contact Lens Risk Survey [183] can facilitate a proactive management approach to contact lens related ocular surface inflammation.
It is the clinical experience of the authors that some contact lens wearers remain more susceptible to ocular inflammation than others. Recent research in the field of genetics and ocular microbiome may provide further insight into the possible causes of contact lens related infiltrative events. Genetic differences in single nucleotide polymorphisms (SNPs) of key inflammatory cytokines [118,184,185] and ocular microbiome [186] have been linked to susceptibility to and severity of corneal infiltrative events during contact lens wear. Further work is needed in this area including confirmation of the existence of a microbiome on the ocular surface. Future studies should focus on whole genome sequencing, which has been successfully used in other eye and vision fields [187–189], and the association with microbiomes on the ocular surface in contact lens wear.
5. Conclusion
Recent improvements in technology have significantly increased the ability to quantitatively and objectively assess the inflammatory status of the human ocular surface in vivo. This manuscript provided a review of contemporary techniques to measure the inflammatory responses on the ocular surface during contact lens wear, and highlighted the potential limitations of techniques and challenges comparing results across studies, suggesting that standardizing test methods and interpretations are required. This review also identified the following future research needs.
To evaluate the TLRs that lead to a cascade of expression of multiple cytokines and chemokines in contact lens wear.
To understand the mechanism by which the inflammatory mediators, such as IL-1, IL-6, IL-8, TNF- α and IFN-γ, infiltrating T cells, macrophages and HLA class II molecules on APCs lead to ocular surface cell apoptosis during contact lens wear.
To consider the balance of pro- and anti- inflammatory cytokine concentrations, such as IL-1β:IL-1Ra, which is seen in bacterial keratitis, in contact lens wear and other associated inflammatory events.
To investigate the diurnal changes in the concentration of inflammatory mediators in order to standardize the tear collection time.
To assess the role of neurotransmitters such as substance P and calcitonin gene related peptides, which are linked to pain sensations, on symptoms of dryness and discomfort in symptomatic contact lens wearers.
To evaluate the ocular surface cellular changes by investigating the MUC5AC positive goblet cells and imaging techniques for conjunctival metaplasia in contact lens wearers.
To better understand environmental and general health related variations in the concentrations and of ocular surface inflammatory mediators.
To evaluate potential genetic differences in the SNPs of key inflammatory cytokines and the ocular microbiome between asymptomatic and symptomatic contact lens wearers to better understand risk factors.
Further work is required to understand the mechanism of contact lens related inflammation and link risk factors to the ocular inflammatory response. The results of future studies will hopefully allow for characterization of individual susceptibility to contact lens induced ocular surface inflammation and support the development of improved treatment and management options for all contact lens wearers.
Acknowledgments
This review did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Conflict of interest
The authors have no proprietary or commercial interests in any concept or product discussed in this article.
References
- [1].Wagner H, Chalmers RL, Mitchell GL, Jansen ME, Kinoshita BT, Lam DY, McMahon TT, Richdale K, Sorbara L, Group CS, Risk factors for interruption to soft contact lens wear in children and young adults, Optom. Vis. Sci. 88 (2011) 973–980. [DOI] [PubMed] [Google Scholar]
- [2].Thakur A, Willcox MD, Contact lens wear alters the production of certain inflammatory mediators in tears, Exp. Eye. Res. 70 (2000) 255–259. [DOI] [PubMed] [Google Scholar]
- [3].Robertson DM, The effects of silicone hydrogel lens wear on the corneal epithelium and risk for microbial keratitis, Eye Contact Lens 39 (2013) 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sweeney DF, Jalbert I, Covey M, Sankaridurg PR, Vajdic C, Holden BA, Sharma S, Ramachandran L, Willcox MD, Rao GN, Clinical characterization of corneal infiltrative events observed with soft contact lens wear, Cornea 22 (2003) 435–442. [DOI] [PubMed] [Google Scholar]
- [5].Stapleton F, Keay L, Jalbert I, Cole N, The epidemiology of contact lens related infiltrates, Optom. Vis. Sci. 84 (2007) 257–272. [DOI] [PubMed] [Google Scholar]
- [6].Sankaridurg PR, Sweeney DF, Sharma S, Gora R, Naduvilath T, Ramachandran L, Holden BA, Rao GN, Adverse events with extended wear of disposable hydrogels: results for the first 13 months of lens wear, Ophthalmology 106 (1999) 1671–1680. [DOI] [PubMed] [Google Scholar]
- [7].Collier SA, Gronostaj MP, MacGurn AK, Cope JR, Awsumb KL, Yoder J, Beach MJ, Estimated burden of keratitis – United States, 2010, MMWR 63 (2014) 1027–1030. [PMC free article] [PubMed] [Google Scholar]
- [8].Thakur A, Willcox MD, Stapleton F, The proinflammatory cytokines and arachidonic acid metabolites in human overnight tears: homeostatic mechanisms, J. Clin. Immunol. 18 (1998) 61–70. [DOI] [PubMed] [Google Scholar]
- [9].Sweeney DF, Naduvilath TJ, Are inflammatory events a marker for an increased risk of microbial keratitis? Eye Contact Lens 33 (2007) 383–387. [DOI] [PubMed] [Google Scholar]
- [10].Efron N, Contact lens wear is intrinsically inflammatory, Clin. Exp. Optom. 100 (2017) 3–19. [DOI] [PubMed] [Google Scholar]
- [11].Young G, Chalmers R, Napier L, Kern J, Hunt C, Dumbleton K, Soft contact lens-related dryness with and without clinical signs, Optom. Vis. Sci. 89 (2012) 1125–1132. [DOI] [PubMed] [Google Scholar]
- [12].Martin DK, Fatt I, The presence of a contact lens induces a very small increase in the anterior corneal surface temperature, Acta. Ophthalmol. (Copenh.) 64 (1986) 512–518. [DOI] [PubMed] [Google Scholar]
- [13].Purslow C, Wolffsohn JS, Santodomingo-Rubido J, The effect of contact lens wear on dynamic ocular surface temperature, Contact Lens Anterior Eye 28 (2005) 29–36. [DOI] [PubMed] [Google Scholar]
- [14].Golebiowski B, Chao C, Stapleton F, Jalbert I, Corneal nerve morphology, sensitivity, and tear neuropeptides in contact lens wear, Optom. Vis. Sci 94 (2017) 534–542. [DOI] [PubMed] [Google Scholar]
- [15].Morgan PB, Brennan NA, Maldonado-Codina C, Quhill W, Rashid K, Efron N, Central and peripheral oxygen transmissibility thresholds to avoid corneal swelling during open eye soft contact lens wear, Biomed. Mater. Res. B. Appl. Biomater. 92 (2010) 361–365. [DOI] [PubMed] [Google Scholar]
- [16].Stapleton F, Tan J, Impact of contact lens material, design, and fitting on discomfort, Eye Contact Lens 43 (2017) 32–39. [DOI] [PubMed] [Google Scholar]
- [17].Covey M, Sweeney DF, Terry R, Sankaridurg PR, Holden BA, Hypoxic effects on the anterior eye of high-Dk soft contact lens wearers are negligible, Optom. Vis. Sci. 78 (2001) 95–99. [DOI] [PubMed] [Google Scholar]
- [18].Santodomingo-Rubido J, Wolffsohn JS, Gilmartin B, Adverse events and discontinuations during 18 months of silicone hydrogel contact lens wear, Eye Contact Lens 33 (2007) 288–292. [DOI] [PubMed] [Google Scholar]
- [19].Richdale K, Sinnott LT, Skadahl E, Nichols JJ, Frequency of and factors associated with contact lens dissatisfaction and discontinuation, Cornea 26 (2007) 168–174. [DOI] [PubMed] [Google Scholar]
- [20].Dumbleton K, Caffery B, Dogru M, Hickson-Curran S, Kern J, Kojima T, Morgan PB, Purslow C, Robertson DM, Nelson JD, T.I.W.o.C.L.D. members of the, The TFOS international workshop on contact lens discomfort: report of the subcommittee on epidemiology, Invest. Ophthalmol. Vis. Sci. 54 (2013) TFOS20–TFOS36. [DOI] [PubMed] [Google Scholar]
- [21].Belmonte C, Acosta MC, Gallar J, Neural basis of sensation in intact and injured corneas, Exp. Eye Res. 78 (2004) 513–525. [DOI] [PubMed] [Google Scholar]
- [22].Rosenthal P, Borsook D, The corneal pain system. Part I: the missing piece of the dry eye puzzle, Ocul. Surf. 10 (2012) 2–14. [DOI] [PubMed] [Google Scholar]
- [23].Muselier-Mathieu A, Bron AM, Mathieu B, Souchier M, Brignole-Baudouin F, Acar N, Bretillon L, Creuzot-Garcher C, Ocular surface assessment in soft contact lens wearers; the contribution of tear osmolarity among other tests, Acta Ophthalmol. 92 (2014) 364–369. [DOI] [PubMed] [Google Scholar]
- [24].McMonnies CW, Chapman-Davies A, Assessment of conjunctival hyperemia in contact lens wearers. Part II, Am. J. Optom. Physiol. Opt. 64 (1987) 251–255. [DOI] [PubMed] [Google Scholar]
- [25].McMonnies CW, Chapman-Davies A, Assessment of conjunctival hyperemia in contact lens wearers. Part I, Am. J. Optom. Physiol. Opt. 64 (1987) 246–250. [DOI] [PubMed] [Google Scholar]
- [26].Oppenheim JJ, Zachariae CO, Mukaida N, Matsushima K, Properties of the novel proinflammatory supergene intercrine cytokine family, Ann. Rev. immunol 9 (1991) 617–648. [DOI] [PubMed] [Google Scholar]
- [27].Tavakoli M, Boulton AJ, Efron N, Malik RA, Increased Langerhan cell density and corneal nerve damage in diabetic patients: role of immune mechanisms in human diabetic neuropathy, Contact Lens Anterior Eye 34 (2011) 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chen Y, Chauhan SK, Saban DR, Sadrai Z, Okanobo A, Dana R, Interferongamma-secreting NK cells promote induction of dry eye disease, J. Leckoc. Biol. 89 (2011) 965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Redfern RL, McDermott AM, Toll-like receptors in ocular surface disease, Exp. Eye Res. 90 (2010) 679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pearlman E, Johnson A, Adhikary G, Sun Y, Chinnery HR, Fox T, Kester M, McMenamin PG, Toll-like receptors at the ocular surface, Ocul. Surf. 6 (2008) 108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].The definition and, classification of dry eye disease: report of the definition and classification subcommittee of the international dry eye workshop, Ocul. Surf. 5 (2007) 75–92. [DOI] [PubMed] [Google Scholar]
- [32].Poltorak A, Ricciardi-Castagnoli P, Citterio S, Beutler B, Physical contact between lipopolysaccharide and toll-like receptor 4 revealed by genetic complementation, Proc. Natl. Acad. Sci. U. S. A. 97 (97) (2000) 2163–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sun Y, Hise AG, Kalsow CM, Pearlman E, Staphylococcus aureus-induced corneal inflammation is dependent on Toll-like receptor 2 and myeloid differentiation factor 88, Infect. Immun. 74 (2006) 5325–5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Barabino S, Chen Y, Chauhan S, Dana R, Ocular surface immunity: homeostatic mechanisms and their disruption in dry eye disease, Prog. Retin. Eye. Res. 31 (2012) 271–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hamrah P, Liu Y, Zhang Q, Dana MR, Alterations in corneal stromal dendritic cell phenotype and distribution in inflammation, Arch. Ophthalmol. 121 (2003) 1132–1140. [DOI] [PubMed] [Google Scholar]
- [36].Dana MR, Hamrah P, Role of immunity and inflammation in corneal and ocular surface disease associated with dry eye, Adv. Exp. Med. Biol. 506 (2002) 729–738. [DOI] [PubMed] [Google Scholar]
- [37].Hamrah P, Huq SO, Liu Y, Zhang Q, Dana MR, Corneal immunity is mediated by heterogeneous population of antigen-presenting cells, J. Leukoc. Biol. 74 (2003) 172–178. [DOI] [PubMed] [Google Scholar]
- [38].Pflugfelder SC, Jones D, Ji Z, Afonso A, Monroy D, Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren’s syndrome keratoconjunctivitis sicca, Curr. Eye Res. 19 (1999) 201–211. [DOI] [PubMed] [Google Scholar]
- [39].Barabino S, Montaldo E, Solignani F, Valente C, Mingari MC, Rolando M, Immune response in the conjunctival epithelium of patients with dry eye, Exp. Eye Res. 91 (2010) 524–529. [DOI] [PubMed] [Google Scholar]
- [40].Springer J, Geppetti P, Fischer A, Groneberg DA, Calcitonin gene-related peptide as inflammatory mediator, Pulm. Pharmacol. Ther. 16 (2003) 121–130. [DOI] [PubMed] [Google Scholar]
- [41].O’Connor TM, O’Connell J, O’Brien DI, Goode T, Bredin CP, Shanahan F, The role of substance P in inflammatory disease, J. Cell Physiol. 201 (2004) 167–180. [DOI] [PubMed] [Google Scholar]
- [42].Pisella PJ, Brignole F, Debbasch C, Lozato PA, Creuzot-Garcher C, Bara J, Saiag P, Warnet JM, Baudouin C, Flow cytometric analysis of conjunctival epithelium in ocular rosacea and keratoconjunctivitis sicca, Ophthalmology 107 (2000) 1841–1849. [DOI] [PubMed] [Google Scholar]
- [43].de Souza GA, Godoy LM, Mann M, Identification of 491 proteins in the tear fluid proteome reveals a large number of proteases and protease inhibitors, Genome. Biol. 7 (2006) R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhou L, Zhao SZ, Koh SK, Chen L, Vaz C, Tanavde V, Li XR, Beuerman RW, In-depth analysis of the human tear proteome, J. Proteomics 75 (2012) 3877–3885. [DOI] [PubMed] [Google Scholar]
- [45].Carreno E, Enriquez-de-Salamanca A, Teson M, Garcia-Vazquez C, Stern ME, Whitcup SM, Calonge M, Cytokine and chemokine levels in tears from healthy subjects, Acta Ophthalmol. 88 (2010) e250–e258. [DOI] [PubMed] [Google Scholar]
- [46].Arranz-Valsero I, Schulze U, Contreras-Ruiz L, Garcia-Posadas L, LopezGarcia A, Paulsen F, Diebold Y, Involvement of corneal epithelial cells in the Th17 response in an in vitro bacterial inflammation model, Mol. Vis. 19 (2013) 85–99. [PMC free article] [PubMed] [Google Scholar]
- [47].Duan F, Liao J, Huang Q, Nie Y, Wu K, HSV-1 miR-H6 inhibits HSV-1 replication and IL-6 expression in human corneal epithelial cells in vitro, Clin. Dev. Immunol. 2012 (2012) 192791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ebihara N, Matsuda A, Nakamura S, Matsuda H, Murakami A, Role of the IL-6 classic- and trans-signaling pathways in corneal sterile inflammation and wound healing, Invest. Ophthalmol. Vis. Sci. 52 (2011) 8549–8557. [DOI] [PubMed] [Google Scholar]
- [49].Cope AP, Londei M, Chu NR, Cohen SB, Elliott MJ, Brennan FM, Maini RN, Feldmann M, Chronic exposure to tumor necrosis factor (TNF) in vitro impairs the activation of T cells through the T cell receptor/CD3 complex; reversal in vivo by anti-TNF antibodies in patients with rheumatoid arthritis, J. Clin. Invest. 94 (1994) 749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Aggarwal BB, Signalling pathways of the TNF superfamily: a double-edged sword, Nat. Rev. Immunol. 3 (2003) 745–756. [DOI] [PubMed] [Google Scholar]
- [51].Calonge M, Enriquez-de-Salamanca A, Diebold Y, Gonzalez-Garcia MJ, Reinoso R, Herreras JM, Corell A, Dry eye disease as an inflammatory disorder, Ocul Immun Inflam 18 (2010) 244–253. [DOI] [PubMed] [Google Scholar]
- [52].Lam H, Bleiden L, de Paiva CS, Farley W, Stern ME, Pflugfelder SC, Tear cytokine profiles in dysfunctional tear syndrome, Am. J. Ophthalmol. 147 (2009) 198–205 e191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Benitez del Castillo JM, Lemp MA, Ocular Surface Disorder, JP Medical Ltd., London UK, 2002. [Google Scholar]
- [54].Liu TF, Jones BM, Impaired production of IL-12 in systemic lupus erythematosus. I. Excessive production of IL-10 suppresses production of IL-12 by monocytes, Cytokine 10 (1998) 140–147. [DOI] [PubMed] [Google Scholar]
- [55].Hazlett LD, McClellan SM, Hume EB, Dajcs JJ, O’Callaghan RJ, Willcox MD, Extended wear contact lens usage induces Langerhans cell migration into cornea, Exp. Eye. Res. 69 (1999) 575–577. [DOI] [PubMed] [Google Scholar]
- [56].Meloni M, De Servi B, Marasco D, Del Prete S, Molecular mechanism of ocular surface damage: application to an in vitro dry eye model on human corneal epithelium, Mol. Vis. 17 (2011) 113–126. [PMC free article] [PubMed] [Google Scholar]
- [57].Chotikavanich S, de Paiva CS, Li de Q, Chen JJ, Bian F, Farley WJ, Pflugfelder SC, Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome, Invest. Ophthalmol. Vis. Sci. 50 (2009) 3203–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sambursky R, Davitt WF 3rd, Friedberg M, Tauber S, Prospective, multicenter, clinical evaluation of point-of-care matrix metalloproteinase-9 test for confirming dry eye disease, Cornea 33 (2014) 812–818. [DOI] [PubMed] [Google Scholar]
- [59].Parks WC, Wilson CL, Lopez-Boado YS, Matrix metalloproteinases as modulators of inflammation and innate immunity, Nat. Rev. Immunol. 4 (2004) 617–629. [DOI] [PubMed] [Google Scholar]
- [60].Dinarello CA, Biologic basis for interleukin-1 in disease, Blood 87 (1996) 2095–2147. [PubMed] [Google Scholar]
- [61].Black RA, Kronheim SR, Sleath PR, Activation of interleukin-1 beta by a coinduced protease, FEBS Lett. 247 (1989) 386–390. [DOI] [PubMed] [Google Scholar]
- [62].van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA, Inflammasome activation and IL-1beta and IL-18 processing during infection, Trends Immunol. 32 (2011) 110–116. [DOI] [PubMed] [Google Scholar]
- [63].Narayanan S, Glasser A, Hu YS, McDermott AM, The effect of interleukin-1 on cytokine gene expression by human corneal epithelial cells, Exp. Eye Res. 80 (2005) 175–183. [DOI] [PubMed] [Google Scholar]
- [64].Arend WP, Interleukin-1 receptor antagonist, Adv. Immunol. 54 (1993) 167–227. [DOI] [PubMed] [Google Scholar]
- [65].Yamaguchi T, Calvacanti BM, Cruzat A, Qazi Y, Ishikawa S, Osuka A, Lederer J, Hamrah P, Correlation between human tear cytokine levels and cellular corneal changes in patients with bacterial keratitis by in vivo confocal microscopy, Invest. Ophthalmol. Vis. Sci. 55 (2014) 7457–7466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kang MH, Kim MK, Lee HJ, Lee HI, Wee WR, Lee JH, Interleukin-17 in various ocular surface inflammatory diseases, J. Korean Med. Sci. 26 (2011) 938–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Taylor PR, Roy S, Leal SM Jr, Sun Y, Howell SJ, Cobb BA, Li X, Pearlman E, Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORgammat and dectin-2, Nat. Immunol. 15 (2014) 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].McMahon SB, NGF as a mediator of inflammatory pain, Philos. Trans. R. Soc. Lond. Series B Biol. Sci. 351 (1996) 431–440. [DOI] [PubMed] [Google Scholar]
- [69].Chao C, Stapleton F, Zhou X, Chen S, Zhou S, Golebiowski B, Structural and functional changes in corneal innervation after laser in situ keratomileusis and their relationship with dry eye signs and symptoms, Graefe’s Arch. Clin. Exp. Ophthalmol. 253 (2015) 2029–2039. [DOI] [PubMed] [Google Scholar]
- [70].Fujishima H, Takeyama M, Takeuchi T, Saito I, Tsubota K, Elevated levels of substance P in tears of patients with allergic conjunctivitis and vernal keratoconjunctivitis, Clin. Exp. Allergy 27 (1997) 372–378. [PubMed] [Google Scholar]
- [71].Watanabe H, Significance of mucin on the ocular surface, Cornea 21 (2002) S17–22. [DOI] [PubMed] [Google Scholar]
- [72].Aristoteli LP, Bojarski B, Willcox MD, Isolation of conjunctival mucin and differential interaction with Pseudomonas aeruginosa strains of varied pathogenic potential, Exp. Eye Res. 77 (2003) 699–710. [DOI] [PubMed] [Google Scholar]
- [73].Argueso P, Gipson IK, Epithelial mucins of the ocular surface: structure, biosynthesis and function, Exp. Eye Res. 73 (2001) 281–289. [DOI] [PubMed] [Google Scholar]
- [74].Ueta M, Hamuro J, Yamamoto M, Kaseda K, Akira S, Kinoshita S, Spontaneous ocular surface inflammation and goblet cell disappearance in I kappa B zeta genedisrupted mice, Invest. Ophthalmol. Vis. Sci. 46 (2005) 579–588. [DOI] [PubMed] [Google Scholar]
- [75].Mann A, Tighe B, Contact lens interactions with the tear film, Exp. Eye Res. 117 (2013) 88–98. [DOI] [PubMed] [Google Scholar]
- [76].Alzahrani Y, Colorado L, Pritchard N, Efron N, Inflammatory cell upregulation of the lid wiper in contact lens dry eye, Optom. Vis. Sci. 93 (2016) 917–924. [DOI] [PubMed] [Google Scholar]
- [77].Doughty MJ, Contact lens wear and the development of squamous metaplasia of the surface cells of the conjunctiva, Eye Contact Lens 37 (2011) 274–281. [DOI] [PubMed] [Google Scholar]
- [78].Efron N, Al-Dossari M, Pritchard N, In vivo confocal microscopy of the bulbar conjunctiva, Clin. Exp. Ophthalmol. 37 (2009) 335–344. [DOI] [PubMed] [Google Scholar]
- [79].Efron N, Al-Dossari M, Pritchard N, Confocal microscopy of the bulbar conjunctiva in contact lens wear, Cornea 29 (2010) 43–52. [DOI] [PubMed] [Google Scholar]
- [80].Resch MD, Marsovszky L, Nemeth J, Bocskai M, Kovacs L, Balog A, Dry eye and corneal langerhans cells in systemic lupus erythematosus, J. Ophthalmol. 2015 (2015) 543835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Hazlett LD, McClellan SA, Rudner XL, Barrett RP, The role of Langerhans cells in Pseudomonas aeruginosa infection, Invest. Ophthalmol. Vis. Sci. 43 (2002) 189–197. [PubMed] [Google Scholar]
- [82].Suzuki T, Sano Y, Kinoshita S, Conjunctival inflammation induces Langerhans cell migration into the cornea, Curr. Eye Res. 21 (2000) 550–553. [PubMed] [Google Scholar]
- [83].Wei Q, Le Q, Hong J, Xiang J, Wei A, Xu J, In vivo confocal microscopy of meibomian glands and palpebral conjunctiva in vernal keratoconjunctivitis, Indian J. Ophthalmol. 63 (2015) 327–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Bodaghi B, Bertin V, Paques M, Toublanc M, Dezutter-Dambuyant C, HoangXuan T, Limbal conjunctival Langerhans cell density in ocular cicatricial pemphigoid: an indirect immunofluorescence study on Dispase-split conjunctiva, Curr. Eye Res. 16 (1997) 820–824. [DOI] [PubMed] [Google Scholar]
- [85].Bhan AK, Fujikawa LS, Foster CS, T-cell subsets and Langerhans cells in normal and diseased conjunctiva, Am. J. Ophthalmol. 94 (1982) 205–212. [DOI] [PubMed] [Google Scholar]
- [86].Massingale ML, Li X, Vallabhajosyula M, Chen D, Wei Y, Asbell PA, Analysis of inflammatory cytokines in the tears of dry eye patients, Cornea 28 (2009) 1023–1027. [DOI] [PubMed] [Google Scholar]
- [87].Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC, Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease, Invest. Ophthalmol. Vis. Sci. 42 (2001) 2283–2292. [PubMed] [Google Scholar]
- [88].Boehm N, Riechardt AI, Wiegand M, Pfeiffer N, Grus FH, Proinflammatory cytokine profiling of tears from dry eye patients by means of antibody microarrays, Invest. Ophthalmol. Vis. Sci. 52 (2011) 7725–7730. [DOI] [PubMed] [Google Scholar]
- [89].Yoon KC, Jeong IY, Park YG, Yang SY, Interleukin-6 and tumor necrosis factor-alpha levels in tears of patients with dry eye syndrome, Cornea 26 (2007) 431–437. [DOI] [PubMed] [Google Scholar]
- [90].Tishler M, Yaron I, Geyer O, Shirazi I, Naftaliev E, Yaron M, Elevated tear interleukin-6 levels in patients with Sjogren syndrome, Ophthalmology 105 (1998) 2327–2329. [DOI] [PubMed] [Google Scholar]
- [91].Enriquez-de-Salamanca A, Castellanos E, Stern ME, Fernandez I, Carreno E, Garcia-Vazquez C, Herreras JM, Calonge M, Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease, Mol. Vis. 16 (2010) 862–873. [PMC free article] [PubMed] [Google Scholar]
- [92].Chung JK, Kim MK, Wee WR, Prognostic factors for the clinical severity of keratoconjunctivitis sicca in patients with Sjogren’s syndrome, Br. J. Ophthalmol. 96 (2012) 240–245. [DOI] [PubMed] [Google Scholar]
- [93].Huang JF, Zhang Y, Rittenhouse KD, Pickering EH, McDowell MT, Evaluations of tear protein markers in dry eye disease: repeatability of measurement and correlation with disease, Invest. Ophthalmol. Vis. Sci. 53 (2012) 4556–4564. [DOI] [PubMed] [Google Scholar]
- [94].Lambiase A, Micera A, Sacchetti M, Cortes M, Mantelli F, Bonini S, Alterations of tear neuromediators in dry eye disease, Arch. Ophthalmol. 129 (2011) 981–986. [DOI] [PubMed] [Google Scholar]
- [95].Leonardi A, Curnow SJ, Zhan H, Calder VL, Multiple cytokines in human tear specimens in seasonal and chronic allergic eye disease and in conjunctival fibroblast cultures, Clin. Exp. Allergy 36 (2006) 777–784. [DOI] [PubMed] [Google Scholar]
- [96].Sacchetti M, Micera A, Lambiase A, Speranza S, Mantelli F, Petrachi G, Bonini S, Bonini S, Tear levels of neuropeptides increase after specific allergen challenge in allergic conjunctivitis, Mol. Vis. 17 (2011) 47–52. [PMC free article] [PubMed] [Google Scholar]
- [97].Kolanu S, Measurement of Clinical, Bio- and Neuro- Markers of Ocular Surface and Tear Film in Subjects with Itchy Eyes, PhD Thesis, School of Optometry and Vision Science, University of New South Wales, Sydney, Australia, 2016. [Google Scholar]
- [98].Duong K, Chao C, Willcox M, Richdale K, Changes in tear cytokine concentration following a short period of daily wear and extended wear: a pilot study, Optom Vis Sci Conference (2015) E-. [Google Scholar]
- [99].Cho P, The cotton thread test on Chinese eyes: effect of age and gender, J. Br Contact Lens Assoc. 17 (1994) 25–28. [Google Scholar]
- [100].Sakamoto R, Bennett ES, Henry VA, Paragina S, Narumi T, Izumi Y, Kamei Y, Nagatomi E, Miyanaga Y, Hamano H, et al. , The phenol red thread tear test: a cross-cultural study, Invest. Ophthalmol. Vis. Sci. 34 (1993) 3510–3514. [PubMed] [Google Scholar]
- [101].Markoulli M, Gokhale M, You J, Substance P in flush tears and schirmer strips of healthy participants, Optom. Vis. Sci. 94 (2017) 527–533. [DOI] [PubMed] [Google Scholar]
- [102].Stuchell RN, Feldman JJ, Farris RL, Mandel ID, The effect of collection technique on tear composition, Invest. Ophthalmol. Vis. Sci. e 25 (1984) 374–377. [PubMed] [Google Scholar]
- [103].Markoulli M, Matrix metalloproteinase-9 in contact lens-related corneal erosions, PhD thesis, school of optometry and vision science, University of New South Wales, Sydney, Australia: (2011). [Google Scholar]
- [104].Narayanan S, Corrales RM, Farley W, McDermott AM, Pflugfelder SC, Interleukin-1 receptor-1-deficient mice show attenuated production of ocular surface inflammatory cytokines in experimental dry eye, Cornea 27 (2008) 811–817. [DOI] [PubMed] [Google Scholar]
- [105].Narayanan S, Miller WL, McDermott AM, Conjunctival cytokine expression in symptomatic moderate dry eye subjects, Invest. Ophthalmol. Vis. Sci. 47 (2006) 2445–2450. [DOI] [PubMed] [Google Scholar]
- [106].Sonoda S, Uchino E, Nakao K, Sakamoto T, Inflammatory cytokine of basal and reflex tears analysed by multicytokine assay, Br. J. Ophthalmol. 90 (2006) 120–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Sack RA, Conradi L, Krumholz D, Beaton A, Sathe S, Morris C, Membrane array characterization of 80 chemokines, cytokines, and growth factors in openand closed-eye tears: angiogenin and other defense system constituents, Invest. Ophthalmol. Vis. Sci. 46 (2005) 1228–1238. [DOI] [PubMed] [Google Scholar]
- [108].Schultz CL, Kunert KS, Interleukin-6 levels in tears of contact lens wearers, J. Interferon Cytokine Res. 20 (2000) 309–310. [DOI] [PubMed] [Google Scholar]
- [109].Subbaraman L, Thangavelu M, McCanna D, Jones L, Tear film cytokine analyses using a novel electrochemiluminescent array technique, Invest. Ophthalmol. Vis. Sci. 54 (2014) E–abstract: 4325. [Google Scholar]
- [110].Chen R, Lowe L, Wilson JD, Crowther E, Tzeggai K, Bishop JE, Varro R, Simultaneous quantification of six human cytokines in a single sample using microparticle-based flow cytometric technology, Clin. Chem. 45 (1999) 1693–1694. [PubMed] [Google Scholar]
- [111].Hagan S, Tomlinson A, Tear fluid biomarker profiling: a review of multiplex bead analysis, Ocul. Surf. 11 (2013) 219–235. [DOI] [PubMed] [Google Scholar]
- [112].Willcox MD, Zhao Z, Naduvilath T, Lazon P de la Jara, Cytokine changes in tears and relationship to contact lens discomfort, Mol. Vis. 21 (2015) 293–305. [PMC free article] [PubMed] [Google Scholar]
- [113].Malvitte T, Montange A, Vejux C, Baudouin AM, Creuzot-Garcher C, Lizard G, Measurement of inflammatory cytokines by multicytokine assay in tears of patients with glaucoma topically treated with chronic drugs, Br. J. Ophthalmol. 91 (2007) 29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].LaFrance MW, Kehinde LE, Fullard RJ, Multiple cytokine analysis in human tears: an optimized procedure for cytometric bead-based assay, Curr. Eye Res. 33 (2008) 525–544. [DOI] [PubMed] [Google Scholar]
- [115].Chao C, Golebiowski B, Stapleton F, Richdale K, Changes in Tear Cytokine Concentrations Following Discontinuation of Soft Contact Lenses-a Pilot Study, Eye Contact Lens, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Poyraz C, Irkec M, Mocan MC, Elevated tear interleukin-6 and interleukin-8 levels associated with silicone hydrogel and conventional hydrogel contact lens wear, Eye Contact Lens 38 (2012) 146–149. [DOI] [PubMed] [Google Scholar]
- [117].Thakur A, Willcox MD, Cytokine and lipid inflammatory mediator profile of human tears during contact lens associated inflammatory diseases, Exp. Eye Res. 67 (1998) 9–19. [DOI] [PubMed] [Google Scholar]
- [118].Carnt NA, Willcox MD, Hau S, Garthwaite LL, Evans VE, Radford CF, Dart JK, Chakrabarti S, Stapleton F, Association of single nucleotide polymorphisms of interleukins-1beta, −6, and −12B with contact lens keratitis susceptibility and severity, Ophthalmology 119 (2012) 1320–1327. [DOI] [PubMed] [Google Scholar]
- [119].Gonzalez-Perez J, Villa-Collar C, Gonzalez-Meijome JM, Porta NG, Parafita MA, Long-term changes in corneal structure and tear inflammatory mediators after orthokeratology and LASIK, Invest. Ophthalmol. Vis. Sci. 53 (2012) 5301–5311. [DOI] [PubMed] [Google Scholar]
- [120].Gonzalez-Perez J, Villa-Collar C, Sobrino Moreiras T, Lema Gesto I, Gonzalez-Meijome JM, Rodriguez-Ares MT, Parafita M, Tear film inflammatory mediators during continuous wear of contact lenses and corneal refractive therapy, Br. J. Ophthalmol. 96 (2012) 1092–1098. [DOI] [PubMed] [Google Scholar]
- [121].Lema I, Duran JA, Ruiz C, Diez-Feijoo E, Acera A, Merayo J, Inflammatory response to contact lenses in patients with keratoconus compared with myopic subjects, Cornea 27 (2008) 758–763. [DOI] [PubMed] [Google Scholar]
- [122].Kallinikos P, Morgan P, Efron N, Assessment of stromal keratocytes and tear film inflammatory mediators during extended wear of contact lenses, Cornea 25 (2006) 1–10. [DOI] [PubMed] [Google Scholar]
- [123].Liu Q, McDermott AM, Miller WL, Elevated nerve growth factor in dry eye associated with established contact lens wear, Eye Contact Lens 35 (2009) 232–237. [DOI] [PubMed] [Google Scholar]
- [124].Yuksel Elgin C, Iskeleli G, Talaz S, Akyol S, Comparative analysis of tear film levels of inflammatory mediators in contact lens users, Curr. Eye Res. 41 (2016) 441–447. [DOI] [PubMed] [Google Scholar]
- [125].Kalsow CM, Reindel WT, Merchea MM, Bateman KM, Barr JT, Tear cytokine response to multipurpose solutions for contact lenses, Clin. Ophthalmol. 7 (2013) 1291–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Cole N, Garthwaite L, Chen R, Willcox MD, Effect of multipurpose solutions on cell morphology and cytokine production by corneal epithelial cells, Optom. Vis. Sci. 89 (2012) 1460–1467. [DOI] [PubMed] [Google Scholar]
- [127].Kehinde LE, Effects of Daily Versus 30-day Continuous Contact Lens Wear on Tear Cytokine Levels, Vision Science, PhD Thesis, The University of Alabama at Birmingham, Birmingham Alabama, 2009. [Google Scholar]
- [128].Markoulli M, Papas E, Cole N, Holden B, Effect of contact lens wear on the diurnal profile of matrix metalloproteinase 9 in tears, Optom. Vis. Sci. 90 (2013) 419–429. [DOI] [PubMed] [Google Scholar]
- [129].Nichols JJ, Jones L, Nelson JD, Stapleton F, Sullivan DA, Willcox MD, T.I.W.o.C.L.D. members of the, The TFOS international workshop on contact lens discomfort: introduction, Invest. Ophthalmol. Vis. Sci. 54 (2013) TFOS1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Adams GG, Dilly PN, Kirkness CM , Monitoring ocular disease by impression cytology, Eye 2 (1988) 506–516. [DOI] [PubMed] [Google Scholar]
- [131].Marner K, ‘Snake-like’ appearance of nuclear chromatin in conjunctival epithelial cells from patients with keratoconjunctivitis sicca, Acta Ophthalmol. (Copenh.) 58 (1980) 849–853. [DOI] [PubMed] [Google Scholar]
- [132].Nelson JD, Havener VR, Cameron JD, Cellulose acetate impressions of the ocular surface. Dry eye states, Arch. Ophthalmol. 101 (1983) 1869–1872. [DOI] [PubMed] [Google Scholar]
- [133].Tseng SC, Staging of conjunctival squamous metaplasia by impression cytology, Ophthalmology 92 (1985) 728–733. [DOI] [PubMed] [Google Scholar]
- [134].Rodriguez AE, Rodriguez-Prats JL, Hamdi IM, Galal A, Awadalla M, Alio JL, Comparison of goblet cell density after femtosecond laser and mechanical microkeratome in LASIK, Invest. Ophthalmol. Vis. Sci. 48 (2007) 2570–2575. [DOI] [PubMed] [Google Scholar]
- [135].Rodriguez-Prats JL, Hamdi IM, Rodriguez AE, Galal A, Alio JL, Effect of suction ring application during LASIK on goblet cell density, J. Refract. Surg. 23 (2007) 559–562. [DOI] [PubMed] [Google Scholar]
- [136].Blodi BA, Byrne KA, Tabbara KF, Goblet cell population among patients with inactive trachoma, Int. Ophthalmol. 12 (1988) 41–45. [DOI] [PubMed] [Google Scholar]
- [137].Shimazaki J, Yang HY, Tsubota K, Amniotic membrane transplantation for ocular surface reconstruction in patients with chemical and thermal burns, Ophthalmology 104 (1997) 2068–2076. [DOI] [PubMed] [Google Scholar]
- [138].Doughty MJ, Goblet cells of the normal human bulbar conjunctiva and their assessment by impression cytology sampling, Ocul. Surf. 10 (2012) 149–169. [DOI] [PubMed] [Google Scholar]
- [139].Egbert PR, Lauber S, Maurice DM, A simple conjunctival biopsy, Am. J. Ophthalmol. 84 (1977) 798–801. [DOI] [PubMed] [Google Scholar]
- [140].Lopin E, Deveney T, Asbell PA, Impression cytology: recent advances and applications in dry eye disease, Ocul. Surf. 7 (2009) 93–110. [DOI] [PubMed] [Google Scholar]
- [141].Wang Y, Ogawa Y, Dogru M, Kawai M, Tatematsu Y, Uchino M, Okada N, Igarashi A, Kujira A, Fujishima H, Okamoto S, Shimazaki J, Tsubota K, Ocular surface and tear functions after topical cyclosporine treatment in dry eye patients with chronic graft-versus-host disease, Bone Marrow Transplant. 41 (2008) 293–302. [DOI] [PubMed] [Google Scholar]
- [142].Tsifetaki N, Kitsos G, Paschides CA, Alamanos Y, Eftaxias V, Voulgari PV, Psilas K, Drosos AA, Oral pilocarpine for the treatment of ocular symptoms in patients with Sjogren’s syndrome: a randomised 12 week controlled study, Ann Rheu Dis 62 (2003) 1204–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Dursun D, Ertan A, Bilezikci B, Akova YA, Pelit A, Ocular surface changes in keratoconjunctivitis sicca with silicone punctum plug occlusion, Curr. Eye Res. 26 (2003) 263–269. [DOI] [PubMed] [Google Scholar]
- [144].Kinoshita S, Kiorpes TC, Friend J, Thoft RA, Goblet cell density in ocular surface disease. A better indicator than tear mucin, Arch. Ophthalmol. 101 (1983) 1284–1287. [DOI] [PubMed] [Google Scholar]
- [145].Dogru M, Asano-Kato N, Tanaka M, Igarashi A, Shimmura S, Shimazaki J, Okada N, Takano Y, Fukagawa K, Tsubota K, Fujishima H, Ocular surface and MUC5AC alterations in atopic patients with corneal shield ulcers, Curr. Eye Res. 30 (2005) 897–908. [DOI] [PubMed] [Google Scholar]
- [146].Efron N, Brennan NA, Morgan PB, Wilson T, Lid wiper epitheliopathy, Prog. Retin. Eye Res. 53 (2016) 140–174. [DOI] [PubMed] [Google Scholar]
- [147].Jalbert I, Madigan MC, Shao M, Ng J, Cheng J, Wong D, McMonnies C, Assessing the human lid margin epithelium using impression cytology, Acta Ophthalmol. 90 (2012) e547–552. [DOI] [PubMed] [Google Scholar]
- [148].Vadrevu VL, Fullard RJ, Enhancements to the conjunctival impression cytology technique and examples of applications in a clinico-biochemical study of dry eye, CLAO J. 20 (1994) 59–63. [PubMed] [Google Scholar]
- [149].Refaat RF, Allan D, Optimal surface for impression cytology, Br. J. Ophthalmol. 74 (1990) 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Singh R, Joseph A, Umapathy T, Tint NL, Dua HS, Impression cytology of the ocular surface, Br. J. Ophthalmol. 89 (2005) 1655–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Hong J, Zhu W, Zhuang H, Xu J, Sun X, Le Q, Li G, Wang Y, In vivo confocal microscopy of conjunctival goblet cells in patients with Sjögren’s syndrome dry eye, Br. J. Ophthalmol. 94 (2010) 1454–1458. [DOI] [PubMed] [Google Scholar]
- [152].Le QH, Wang WT, Hong JX, Sun XH, Zheng TY, Zhu WQ, Xu JJ, An in vivo confocal microscopy and impression cytology analysis of goblet cells in patients with chemical burns, Invest. Ophthalmol. Vis. Sci. 51 (2010) 1397–1400. [DOI] [PubMed] [Google Scholar]
- [153].Villani E, Beretta S, Galimberti D, Viola F, Ratiglia R, In vivo confocal microscopy of conjunctival roundish bright objects: young, older, and Sjogren subjects, Invest. Ophthalmol. Vis. Sci. 52 (2011) 4829–4832. [DOI] [PubMed] [Google Scholar]
- [154].Doughty MJ, Contact lens wear and the goblet cells of the human conjunctiva-A review, Contact Lens Anterior Eye 34 (2011) 157–163. [DOI] [PubMed] [Google Scholar]
- [155].Lievens CW, Connor CG, Murphy H, Comparing goblet cell densities in patients wearing disposable hydrogel contact lenses versus silicone hydrogel contact lenses in an extended-wear modality, Eye Contact Lens 29 (2003) 241–244. [DOI] [PubMed] [Google Scholar]
- [156].Anshu MM, Munshi V, Sathe Ganar A, Conjunctival impression cytology in contact lens wearers, Cytopathology 12 (2001) 314–320. [DOI] [PubMed] [Google Scholar]
- [157].Simon P, Jaison SG, Chopra SK, Jacob S, Conjunctival impression cytology in contact lens wearers, Indian J. Ophthalmol. 50 (2002) 301–306. [PubMed] [Google Scholar]
- [158].Connor CG, Campbell JB, Steel SA, Burke JH, The effects of daily wear contact lenses on goblet cell density, J. Am. Optom. Assoc. 65 (1994) 792–794. [PubMed] [Google Scholar]
- [159].Gokhale M, Contemporary Evaluation of Association Between Symtpoms of Ocular Comforts, Clinical Indicators and Biomarkers of Ocular Surface Health, PhD Thesis, School of Optometry and Vision Science, University of New South Wales, Sydney, Australia, 2014. [Google Scholar]
- [160].Connor C, Campbell J, Steel S, B. J., Sofy contact lens wear modality affects goblet cell density, Optom. Vis. Sci. (1995) E-abstract. [Google Scholar]
- [161].Adar S, Kanpolat A, Surucu S, Ucakhan OO, Conjunctival impression cytology in patients wearing contact lenses, Cornea 16 (1997) 289–294. [PubMed] [Google Scholar]
- [162].Hori Y, Argueso P, Spurr-Michaud S, Gipson IK, Mucins and contact lens wear, Cornea 25 (2006) 176–181. [DOI] [PubMed] [Google Scholar]
- [163].Chao C, Structural and Functional Changes in Corneal Innervation After Laser in Situ Keratomilesis and Their Relationship with Dry Eye Signs and Symptoms, PhD Thesis, Optometry and Vision Science, University of New South Wales, Sydney, Australia, 2015. [DOI] [PubMed] [Google Scholar]
- [164].Gurdal C, Aydin S, Kirimlioglu H, Toprak E, Sengor T, Effects of extended-wear soft contact lenses on the ocular surface and central corneal thickness, Ophthalmologica 217 (2003) 329–336. [DOI] [PubMed] [Google Scholar]
- [165].Tomatir DK, Erda N, Gurlu VP, Effects of different contact lens materials and contact lens-wearing periods on conjunctival cytology in asymptomatic contact lens wearers, Eye Contact Lens 34 (2008) 166–168. [DOI] [PubMed] [Google Scholar]
- [166].Zhivov A, Stave J, Vollmar B, Guthoff R, In vivo confocal microscopic evaluation of Langerhans cell density and distribution in the normal human corneal epithelium, Graefe’s Arch. Clin. Exp. Ophthalmol. 243 (2005) 1056–1061. [DOI] [PubMed] [Google Scholar]
- [167].Zhivov A, Stave J, Vollmar B, Guthoff R, In vivo confocal microscopic evaluation of langerhans cell density and distribution in the corneal epithelium of healthy volunteers and contact lens wearers, Cornea 26 (2007) 47–54. [DOI] [PubMed] [Google Scholar]
- [168].Marsovszky L, Resch MD, Nemeth J, Toldi G, Medgyesi E, Kovacs L, Balog A, In vivo confocal microscopic evaluation of corneal Langerhans cell density, and distribution and evaluation of dry eye in rheumatoid arthritis, Innate Immun. 19 (2013) 348–354. [DOI] [PubMed] [Google Scholar]
- [169].Kawamoto K, Chikama T, Takahashi N, Nishida T, In vivo observation of Langerhans cells by laser confocal microscopy in Thygeson’s superficial punctate keratitis, Mol. Vis. 15 (2009) 1456–1462. [PMC free article] [PubMed] [Google Scholar]
- [170].Resch MD, Imre L, Tapaszto B, Nemeth J, Confocal microscopic evidence of increased Langerhans cell activity after corneal metal foreign body removal, Eur. J. Ophthalmol. 18 (2008) 703–707. [DOI] [PubMed] [Google Scholar]
- [171].Kiratli H, Kocabeyoglu S, Saglam A, Soylemezoglu F, Langerhans cell histiocytosis of the caruncle, Clin. Exp. Ophthalmol. 3 (35) (2007) 661–663. [DOI] [PubMed] [Google Scholar]
- [172].Yoshida A, Imayama S, Sugai S, Kawano Y, Ishibashi T, Increased number of IgE positive Langerhans cells in the conjunctiva of patients with atopic dermatitis, Br. J. Ophthalmol. 81 (1997) 402–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Le Q, Hong J, Zhu W, Sun X, Xu J, In vivo laser scanning confocal microscopy of vernal keratoconjunctivitis, Clin. Exp. Ophthalmol. 39 (2011) 53–60. [DOI] [PubMed] [Google Scholar]
- [174].Sindt CW, Grout TK, Critser DB, Kern JR, Meadows DL, Dendritic immune cell densities in the central cornea associated with soft contact lens types and lens care solution types: a pilot study, Clin. Ophthalmol. 6 (2012) 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Alzahrani YA, In vivo assessment of inflammatory cells in contact lens wearers, Optom Vis. Sci. (2014) abstract-14024. [Google Scholar]
- [176].Sankaridurg PR, Rao GN, Rao HN, Sweeney DF, Holden BA, ATPase-positive dendritic cells in the limbal and corneal epithelium of guinea pigs after extended wear of hydrogel lenses, Cornea 19 (2000) 374–377. [DOI] [PubMed] [Google Scholar]
- [177].Sindt CW, Critser DB, Grout TK, Kern JR, Effects of fluorescein staining on laser in vivo confocal microscopy images of the cornea, J. Ophthalmol. 2012 (2012) 541974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Chalmers RL, Wagner H, Mitchell GL, Lam DY, Kinoshita BT, Jansen ME, Richdale K, Sorbara L, McMahon TT, Age and other risk factors for corneal infiltrative and inflammatory events in young soft contact lens wearers from the Contact Lens Assessment in Youth (CLAY) study, Invest. Ophthalmol. Vis. Sci. 52 (2011) 6690–6696. [DOI] [PubMed] [Google Scholar]
- [179].Chalmers RL, McNally JJ, Schein OD, Katz J, Tielsch JM, Alfonso E, Bullimore M, O’Day D, Shovlin J, Risk factors for corneal infiltrates with continuous wear of contact lenses, Optom. Vis. Sci. 84 (2007) 573–579. [DOI] [PubMed] [Google Scholar]
- [180].McNally JJ, Chalmers RL, McKenney CD, Robirds S, Risk factors for corneal infiltrative events with 30-night continuous wear of silicone hydrogel lenses, Eye Contact Lens 29 (2003) S153–156. [DOI] [PubMed] [Google Scholar]
- [181].Lim CH, Carnt NA, Farook M, Lam J, Tan DT, Mehta JS, Stapleton F, Risk factors for contact lens-related microbial keratitis in Singapore, Eye (Lond.) 30 (2016) 447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Stapleton F, Edwards K, Keay L, Naduvilath T, Dart JK, Brian G, Holden B, Risk factors for moderate and severe microbial keratitis in daily wear contact lens users, Ophthalmology 119 (2012) 1516–1521. [DOI] [PubMed] [Google Scholar]
- [183].Cope JR, Collier SA, Rao MM, Chalmers R, Mitchell GL, Richdale K, Wagner H, Kinoshita BT, Lam DY, Sorbara L, Zimmerman A, Yoder JS, Beach MJ, Contact lens wearer demographics and risk behaviors for contact lens-related eye infections–United States, MMWR 64 (2014) 865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Carnt NA, Willcox MD, Hau S, Keay L, Dart JK, Chakrabarti S, Stapleton F, Immune defense single nucleotide polymorphisms and recruitment strategies associated with contact lens keratitis, Ophthalmology 119 (2012) 1997–2002. [DOI] [PubMed] [Google Scholar]
- [185].Keijser S, Kurreeman FA, de Keizer RJ, Dogterom-Ballering H, van der Lelij A, Jager MJ, Nibbering PH, IL-10 promotor haplotypes associated with susceptibility to and severity of bacterial corneal ulcers, Exp. Eye Res. 88 (2009) 1124–1128. [DOI] [PubMed] [Google Scholar]
- [186].Willcox M, Sharma S, Naduvilath TJ, Sankaridurg PR, Gopinathan U, Holden BA, External ocular surface and lens microbiota in contact lens wearers with corneal infiltrates during extended wear of hydrogel lenses, Eye Contact Lens 37 (2011) 90–95. [DOI] [PubMed] [Google Scholar]
- [187].Hysi PG, Wojciechowski R, Rahi JS, Hammond CJ, Genome-wide association studies of refractive error and myopia, lessons learned, and implications for the future, Invest. Ophthalmol. Vis. Sci. 55 (2014) 3344–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Li X, Bykhovskaya Y, Haritunians T, Siscovick D, Aldave A, Szczotka-Flynn L, Iyengar SK, Rotter JI, Taylor KD, Rabinowitz YS, A genome-wide association study identifies a potential novel gene locus for keratoconus, one of the commonest causes for corneal transplantation in developed countries, Hum. Mol. Genet. 21 (2012) 421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA, Complement factor H variant increases the risk of age-related macular degeneration, Science 308 (2005) 419–421. [DOI] [PubMed] [Google Scholar]