Abstract
Recent studies indicate that a significant reorganization of cerebral networks may occur in patients with chronic pain, but how immediate pain experience influences the organization of large-scale functional networks is not yet well characterized. To investigate this question, we used functional magnetic resonance imaging in 106 participants experiencing both noxious and innocuous heat. Painful stimulation caused network-level reorganization of cerebral connectivity that differed substantially from organization during innocuous stimulation and standard resting-state networks. Noxious stimuli increased somatosensory network connectivity with (a) frontoparietal networks involved in context representation, (b) “ventral attention network” regions involved in motivated action selection, and (c) basal ganglia and brainstem regions. This resulted in reduced “small-worldness,” modularity (fewer networks), and global network efficiency and in the emergence of an integrated “pain supersystem” (PS) whose activity predicted individual differences in pain sensitivity across 5 participant cohorts. Network hubs were reorganized (“hub disruption”) so that more hubs were localized in PS, and there was a shift from “connector” hubs linking disparate networks to “provincial” hubs connecting regions within PS. Our findings suggest that pain reorganizes the network structure of large-scale brain systems. These changes may prioritize responses to painful events and provide nociceptive systems privileged access to central control of cognition and action during pain.
Keywords: functional network, hub disruption, inter-system connectivity, immediate pain, reorganization
Introduction
Pain is a conscious experience defined as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” (https://www.iasp-pain.org). Noxious stimuli engage multiple systems distributed across the brain, including the insula, anterior cingulate cortex (ACC), amygdala (sometimes grouped under the rubric of “salience network” (SN) or “ventral attention network”), and somatosensory areas S1, S2, and dorsal posterior insula (part of the “somatomotor network”), thalamus, and brainstem. The involvement of multiple functional systems suggests cooperation and integration in processing pain-related information, which may provide a substrate for generating the conscious experience of pain and prioritize access to action-planning systems (Bornhövd et al. 2002; Apkarian et al. 2005; Tracey and Mantyh 2007; Bastuji et al. 2008; Boly et al. 2008). Advances in functional brain imaging have allowed researchers to characterize patterns of functional activation and deactivation to painful stimuli across different types of evoked pain (Davis et al. 1997; Kwan et al. 2000; Bornhövd et al. 2002; Wager et al. 2013; Favilla et al. 2014; Krishnan et al. 2016), predict pain intensity from patterns of brain activity (Wager et al. 2013; Atlas et al. 2014; Favilla et al. 2014; Wiech 2016; Lindquist et al. 2017), and identify brain mediators of pain (Atlas et al. 2010, 2014; Woo et al. 2015). Though important, these findings chiefly concern patterns of brain activity and do not address the issue of how nociception and pain alter brain connectivity and modularity in functional brain networks.
The important issue of pain-related alterations in brain connectivity and network structure has recently been investigated in a series of important studies that have identified alterations in brain connectivity in both evoked experimental and chronic pain (Zaki et al. 2007; Napadow et al. 2010; Farmer et al. 2012; Jensen et al. 2012; Kong et al. 2013; Kucyi et al. 2014; Martucci et al. 2014; Kucyi and Davis 2015; Hemington et al. 2016; Kutch et al. 2017). For example, chronic pain is associated with abnormalities in brain functional connectivity (FC), that is, increased FC between medial prefrontal cortex (mPFC) and regions that receive nociceptive afferents, including ACC and insula (Cauda et al. 2009; Napadow et al. 2010; Baliki et al. 2014; Kucyi and Davis 2015). At the network level, chronic pain is associated with reduced task-related deactivation in the “default mode” network (DMN, which includes mPFC) (Baliki et al. 2008, 2011); reduced positive correlations among “default-mode” regions (Kornelsen et al. 2013); reduced negative correlations between “default mode” and other brain networks (Baliki et al. 2011), particularly the “SN” (which contains the anterior insula) (Hemington et al. 2016); and increased correlations between the anterior and posterior insula with “default mode”- and “ventral attention”-related areas (Napadow et al. 2010; Tagliazucchi et al. 2010; Loggia et al. 2013).
Some studies have begun to analyze connectivity patterns in pain-related networks from the vantage point of network topology, using concepts from graph theory (Sporns and Zwi 2004; Bullmore and Sporns 2009). Graph-theoretic analysis provides rich quantitative measures (Rubinov and Sporns 2010) that efficiently describe the segregation and integration of complex brain networks. Graph theoretic metrics serve as high-level topological features that can characterize complex alterations in neurodegenerative, neurological, and psychiatric disorders (Bullmore and Sporns 2009; Heuvel and Sporns 2013; Fornito et al. 2015; Yao et al. 2015; Sha et al. 2017; Zheng et al. 2018). Pain studies have begun to identify altered network organization in chronic pain patients compared with normative samples (Mano and Seymour 2015; Mansour et al. 2016; Mano et al. 2018). For example, Mano et al. found evidence for several global network changes in chronic low back pain patients, including hub disruption (reorganization of regions that are highly connected), reduced mean clustering coefficient (a measure of whether regions are tightly inter-connected into functional modules), and reduced betweenness-centrality (incidence of “connector nodes” that connect multiple networks). These changes were replicated across three separate patient samples (Mano et al. 2018). Together, previous pain-related connectivity studies point to increased cross-talk among networks and reduced functional specialization in chronic pain, and particularly greater connectivity between the DMN and SN, which are usually anticorrelated (e.g., increased DMN connectivity with putatively pain-related regions in the insula).
Other studies have shown that resting-state functional connectivity predicts later symptom improvements. For example, increased connectivity among predominantly left frontoparietal network regions has been reported to predict symptom improvements 3 months later in patients with urologic chronic pelvic pain (Kutch et al. 2017). Increased connectivity between lateral prefrontal cortex and other regions (Tétreault et al. 2016), and between mPFC and insula (Hashmi et al. 2012), has been found to predict the magnitude of subsequent placebo responses in chronic back pain patients.
In this article, we attempted to address two inter-related issues not addressed in previous studies. First, the vast majority of connectivity studies have identified changes related to chronic pain, and to our knowledge, there has been no systematic characterization of network topology in evoked experimental pain. Understanding the network topology of evoked pain would provide an important basis for comparing experimental and chronic pain. Second, previous studies of chronic pain have not separated connectivity related to pain experience itself from connectivity changes related to the “kinds of individuals” who experience pain. Pain patients differ from controls in many ways, including medication status, depression and anxiety, exercise and body weight, and socioeconomic status, among other variables. Thus, it is desirable to identify connectivity changes directly associated with nociception and pain experience [e.g., (Seminowicz and Davis 2007; Zaki et al. 2007)], which may be compared with the more complex set of changes associated with individuals with chronic pain conditions.
We analyzed functional magnetic resonance imaging (fMRI) data from 5 independent studies, with a total of 106 participants, to construct group-level functional networks for noxious (painfully hot) and innocuous (non-painful warmth) thermal stimuli and characterize the differences between them. Rather than focusing on time series connectivity, we estimated functional connectivity matrices and network topology measures based on “inter-individual differences” (He et al. 2007, 2008; Wager et al. 2007; Evans 2013; Palaniyappan et al. 2015; Yao et al. 2015). This (a) constrains connectivity estimates to brain responses to painful (or nonpainful) stimulation and (b) estimates networks such that “connected” regions are coactivated in the same individuals, making network estimates more relevant for individual differences in pain sensitivity. Other benefits of estimating connectivity in stimulus-evoked responses averaged over trials is that it enhances the signal-to-noise ratio for pain-related signals, reduces susceptibility to intrinsic neural dynamics and time series artifacts (Simony et al. 2016), and reduces extraneous sources of inter-individual variability relative to resting-state studies (Geerligs et al. 2015; Finn et al. 2017). Participants were selected to have matched numbers of noxious and innocuous thermal stimuli, to allow comparison of activity and individual differences between the two conditions. We then calculated graph theoretic measures, including small-world-ness, modules and hub nodes, and connectivity patterns, to examine stimulus intensity-dependent and pain-dependent alterations in network organization. Overall, the results provided a coherent picture of reduced network diversity and complexity (across multiple graph-theoretic measures) during pain, paralleled by increased integration in particular cortical and subcortical systems.
Materials and Methods
Participants
For network analysis, we used fMRI data from 119 healthy participants (before exclusion criterion were applied) from 5 published studies (Atlas et al. 2010, 2012, 2014; Woo et al. 2015; Lindquist et al. 2017). All studies were approved by the institutional review board of Columbia University and the University of Colorado Boulder. Participants were recruited from New York City and Boulder/Denver Metro Areas, and all participants provided written informed consent. Participants with psychiatric, physiological or pain disorders, neurological conditions, and MRI contraindications were excluded prior to enrollment. Preliminary eligibility of participants was determined through an online questionnaire, a pain safety screening form, and a fMRI safety screening form. Descriptive information about age, gender, and other features of each study are provided in Table 1. In all studies, participants received a series of contact-heat stimulus using a TSA-II Neurosensory Analyzer (Medoc Ltd) with a 16 mm Peltier thermode endplate (Study 7:32 mm) and rated the magnitude of pain they felt on a visual analog scale (VAS) after stimulus offset. The number of trials, stimulation sites, rating scales, intensities and durations of stimulus, and the specific psychological manipulation each study comprised varied across studies as shown in Table 1 (also see Supplementary Table 1 for details about the fMRI acquisition parameters). Notably, the psychological and physical manipulations that influenced pain (except for stimulus intensity) were irrelevant for our analyses, as our focus was on investigating the difference between noxious and innocuous stimuli in functional network organization, and all psychological manipulations were balanced across (orthogonal to) noxious and innocuous conditions.
Table 1.
Demographics and study information
| Study | Study name | Sample (included) |
Sex | Mean age | Duration (s) | Mean stimulation temperature (°C) |
Trial number | Rating scale |
|---|---|---|---|---|---|---|---|---|
| Study 1 | NSF (Atlas et al. 2014) | 26 (23) | 9F | 27.8 | 10 | 40.8, 43.5, 45.1, 47.0 | 35–48 | 0–10 VAS |
| Study 2 | BMRK3 (Woo et al. 2015) | 33 (33) | 22F | 27.9 | 12.5 | 44.3, 45.3, 46.3, 47.3, 48.3, 49.3 |
97 | 0–100 VAS for no pain and pain, respectively |
| Study 3 | EXP (Atlas et al. 2010) | 17 (15) | 9F | 25.5 | 10 | 41.2, 44.4, 47.2 | 61–64 | 0–10 VAS |
| Study 4 | ILCP (Lindquist et al. 2017) | 29 (24) | 16F | 20.4 | 10 | 44.7, 46.7 | 64 | 0–100 VAS |
| Study 5 | REMI (Atlas et al. 2012) | 14 (11) | 7F | 22 | 11 | 41.2, 47.1 | 75 | 0–8 VAS |
Image Preprocessing
Functional images were preprocessed using Statistical Parametric Mapping (SPM) software (http://www.fil.ion.ucl.ac.uk/spm/). Our goal was not to maintain perfect homogeneity in analyses across studies, but rather to establish broad generalizability across a range of studies with standard, but different, state-of-the-art methods. This has disadvantages in spatial precision (though we focus on pre-defined parcels, which mitigates this drawback) but has advantages in (a) establishing generalizability [e.g., (Kragel et al. 2018; Mano et al. 2018; Zunhammer et al. 2018)] and (b) maintaining consistency with published and quality-checked final analyses for each study. However, the normalization template and general linear model framework used were identical for all studies.
Briefly, structural T1-weighted images were coregistered to the average functional image for each subject with the mutual information-based coregistration method in SPM and were then normalized to Montreal Neurological Institute (MNI) space (avg152T1.nii). Functional images were corrected for slice acquisition timing and motion; warped to MNI space by applying motion parameters estimated from coregistered, high-resolution structural images; interpolated to 2 × 2 × 2 mm3 voxels; and smoothed with an 8-mm full width at half maximum (FWHM) Gaussian kernel.
Prior to processing, global outlier time points were identified by calculating the mean and standard deviation (SD) of intensity across voxels for each image. Mahalanobis distances were computed for the matrix of slice-wise mean and SD values (concatenated) by functional volumes, and values with significant χ2 value after multiple comparison correction (Bonferroni) were considered outliers. The outputs of this procedure were later included as nuisance covariates in the first-level models. The number of removed volumes in each study can be found in referenced publications but averages around 1% of functional volumes (Atlas et al. 2010, 2012, 2014; Woo et al. 2015; Lindquist et al. 2017).
Single-Trial Analyses
For studies except Study 3, magnitudes of single-trial responses were quantified by constructing a general linear model (GLM) design matrix with separate regressors for each trial. Boxcar regressors, convolved with the canonical hemodynamic response function (HRF), were constructed to model cue, pain, and rating periods in each study. One regressor was included for each trial, as well as several types of nuisance covariates (images with high artifact/outlier scores as defined above, head movement parameter estimates). Because trial estimates could be strongly affected by artifacts occurring during acquisition (e.g., sudden motion), trial-by-trial variance inflation factors (VIF, a measure of design-induced uncertainty due, in this case, to collinearity with nuisance regressors) were calculated, and any trials with VIFs that exceeded 2.5 were excluded. For Study 1, trials that exceeded 3 SDs above the mean were excluded, and a principal components-based denoising approach during preprocessing to minimize artifacts was employed. This step generated single-trial estimates that reflected the amplitude of the fitted HRF on each trial and referred to the magnitude of anticipatory and pain-period activity for each trial in each voxel.
For Study 3, single-trial analyses were based on fitting a set of three basis functions, rather than on the canonical HRF. This flexible strategy allowed the shape of the modeled HRF to vary across trials and voxels. This procedure differed from other studies because it maintained consistency with the procedures used in the original publication (Atlas et al. 2010) and provided an opportunity to examine predictive performance using a flexible basis set. The pain period basis set consisted of 3 curves shifted in time and was customized for thermal pain responses based on previous studies (Lindquist et al. 2009; Atlas et al. 2010). To estimate cue-evoked responses, the pain anticipation period was modeled using a boxcar epoch convolved with a canonical HRF. This epoch was truncated at 8 s to ensure that fitted anticipatory responses were not affected by noxious stimulus-evoked activity. As with the other studies, nuisance covariates and excluded trials with VIFs > 2.5 were included. Trials that were global outliers (those that exceeded 3 SDs above the mean) were also excluded. The fitted basis functions from the flexible single-trial approach were reconstructed to compute the area under the curve (AUC) for each trial and in each voxel. These trial-by-trial AUC values were used as estimates of trial-level anticipatory or pain-period activity.
Categorization Criteria of Nonpainful and Painful Stimulus
Previous studies have shown that the threshold for specific nociceptors is around 45°C (LaMotte and Campbell 1978) and have identified human pain thresholds in the range of 45–46 °C (Price et al. 1989). Here, we used 45.3 °C as a threshold for dividing thermal stimulation into innocuous (stimulation intensity <45.3 °C) and noxious (stimulation intensity > 45.3 °C) conditions. Participants with both of the two conditions were included. We excluded stimulation level of 49.3 °C in Study 2, as it included only four trials per participant. We also excluded subjects with missing heat ratings and trials delivered during drug administration and active placebo conditions. Finally, as our goal was to compare noxious and innocuous conditions across the same set of participants, for datasets with multiple stimulus intensities within the noxious or innocuous range, we randomly selected one stimulus level for analysis. A total of 106 participants remained in the final analysis. Detailed information regarding pain ratings and stimulation intensity of the included participants are shown in Supplementary Figure 1. Painfully hot stimuli caused significant increases in intensity ratings relative to nonpainful warmth and were in the clearly noxious stimulus range (46–49 °C), whereas warm stimuli were below the threshold for specific nociceptors.
Functional Network Construction
Figure 1 provides a flowchart of network construction. The single-trial images were generated by constructing a GLM over the entire time-course of the study for a given person, with one regressor per trial. The trial-level regressors modeled activity during each 10-s stimulation epoch, which were convolved with the HRF. To reduce differences in image scaling across studies, we rescaled the activity of all included trials for the selected stimulus intensity study-wise, by the study’s global average median absolute deviation (MAD). Then, trial-level maps were averaged at the same stimulation intensity within each subject to yield 1 pain-associated and 1 no pain-associated image for each participant. Average activity was calculated for each of 274 brain parcels as defined in a recent atlas (the “Brainnetome” atlas) (Fan et al. 2016) with 210 cortical, 36 subcortical, and 28 cerebellar regions spanning the brain (K = 274 in total). These averages were concatenated into a 274 × N matrix, where N is the number of subjects (i.e., 106). We then calculated a 274 × 274 matrix of Pearson correlations among regions (37 401 total connections). Prior to connectivity estimation, linear regression was applied within each study to remove the effects of white matter (WM), cerebral spinal fluid (CSF), and global grey matter (GM) signals, which were also rescaled and averaged across trials, from the regional activity estimates (i.e., the subjects × regions matrix for each of high-pain and low-pain conditions). Because the individual differences level is the level of primary interest here, regression at this level provides results that most closely related to the variables of interest. This is in addition to covariates included in the first-level models for head movement, spikes, and artifacts detected as outliers in global signal and root mean square successive deviations (dvars). We chose to regress out GM because previous studies have suggested that the removal of global GM signal may have little influence on community structure but can increase the signal-to-noise in task-related graph theoretic measures (Herrera et al. 2017). Graph metrics should thus be interpreted as inter-regional covariation around the whole-brain mean.
Figure 1.

Pipeline of network construction and analysis. (A) For each study, pain-related brain activity maps for each trial (from single-trial regression) were rescaled by dividing by the MAD across the entire study, avoiding artifacts related to differences in field strength and other analysis choices that impact the scale of activation maps. The rescaled images were then averaged across trials delivered at the same stimulation intensity within each subject, yielding an average map for each person for each of high- and low-pain intensity. Then, a brain template including 274 brain regions was used to extract the average in-region activity from each trial-averaged image, yielding an N-participants (N = 106) × 274 matrix of pain-related activity values. The global averages of GM, WM, and CSF signals were regressed out from the regional activity separately for each study. The functional connectivity between pairs of brain regions was then calculated by measuring the Pearson correlations across individuals, separately for high-pain and low-pain conditions. (B) The optimal module partitions were found based on a weighted network by clustering nodes that are densely connected, using correlation values as weights after retaining only edges with significant positive connectivity (q < 0.05, FDR corrected). These were then applied to both weighted and binary networks at multiple levels of network sparsity to investigate the differences in network-level graph metrics.
Module Detection
The optimal division of module structure is the nonoverlapping communities with maximization of intra-module edges and minimization of inter-module edges. Both negative connections and positive connections with weights near zero were excluded, because these links may represent spurious functional connections (Power et al. 2010; Rubinov and Sporns 2010). False discovery rate (FDR) correction with q = 0.05 was performed to remove the nonsignificant positive links by setting weights of links with P values above the threshold to 0 (Chen et al. 2008), and the resulting networks were used for module detection (Power et al. 2011). In this study, all nodes in the networks after thresholding were connected, and the link densities were close to 10% (9.23% and 9.97% for innocuous and noxious condition, respectively). The module detection algorithm is based on maximizing the modularity measurement Q for the network (Newman 2006), which is defined as:
 |
where lw is the sum of all weights in the network, wij is the connection weight between node i and j,  is the weighted degree of node i, and mi is the module to which node i belongs (
is the weighted degree of node i, and mi is the module to which node i belongs ( = 1 if mi = mj, and 0 otherwise).
= 1 if mi = mj, and 0 otherwise).
To examine whether the detected modules were stable across different link density, we varied the thresholds in the range of [q < 0.01 (FDR corrected), q < 0.05 (FDR corrected), P < 0.001, P < 0.01, P < 0.05]. Module detection algorithm was performed to the thresholded networks with weighted edges (positive only) to estimate the partitions of each condition under different link densities.
Network Properties
Prior to graph theoretic analysis, the connection matrix was thresholded by using a certain threshold. Nodes were considered to be “neighboring” if their edge survived from thresholding. To characterize the robustness of our analyses, network properties of both weighted and binary networks were calculated and compared. Following the traditional approach (Rubinov and Sporns 2010; Power et al. 2011; Power et al. 2013; Xu et al. 2016), negative links were excluded from our analysis because the biological meaning of negative functional connectivity remains unclear (Parente et al. 2018). However, in this case, we did not observe strong negative connectivity (e.g., strength >−0.6). For weighted network, we thresholded the network by preserving only positive links with P < 0.01 to remove the near zero links that may represent spurious functional connections (Power et al. 2010; Rubinov and Sporns 2010). For binary network, we performed analyses with varying network sparsity (retaining the strongest 10–25% of links in 5% increments) and setting suprathreshold links (edges) to 1 and the rest to 0. This range of sparsity was chosen because it allows for the creation of fully connected graphs that permit a reasonable estimation of the graph metrics during the bootstrap test (see Statistical analysis section). The results are identical whether one includes only positive correlations before binarization or not. In addition, since we focus on task-related responses that are less subject to spurious connectivity from time series artifacts than the more common time series connectivity approach, a higher density threshold is appropriate (e.g., started from 10% density).
Four common properties of the graph that reflect local and global organization as well as the architecture of the graph, including the clustering coefficient (a measure of graph segregation), characteristic path length (a measure of graph integration), small-world-ness (evaluates the network organization compare to a matched random graph), and modularity (Q, measures the decomposability of a graph into several sparsely interconnected communities), were extracted for both weighted and binary graphs. Though some of them (e.g., clustering coefficient and modularity) may represent some common information, each property contributes unique information to the whole picture of network organization. Notably, for binary graphs, we averaged the graph metrics across link densities to ensure that the differences between conditions were not due to the choice of link density (Lynall et al. 2010; Cohen and D’Esposito 2016; Kaplan et al. 2019).
The clustering coefficient of a node is defined as the average intensity of all triangles associated with each node for weighted network and the number of suprathreshold edges between neighbors of this node divided by all possible edges between its neighbors for binary network. The average cluster coefficient, C, averages this value across nodes. Characteristic path length, L, is the shortest path between pairs of nodes, averaged across all pairs. The higher L value indicates longer route, on average, from node to node, resulting in lower efficiency of information transfer along the graph. The modularity of binary network can be calculated through the equation for weighted network as mentioned above, in which degree (k) of node i is the number of edges that connected to this node. These network properties were calculated using the Brain Connectivity Toolbox (Rubinov and Sporns 2010).
Typically, a small-world network shows more clustering than a random graph but maintains a similar shortest path length (Watts and Strogatz 1998). In other words, a small-world network should meet the following criteria: γ = Creal/Crandom > 1 and λ = Lreal/Lrandom ≈ 1, where Creal and Lreal are averaged clustering coefficient and averaged characteristic path length of the real network, respectively; and Crandom and Lrandom are averaged clustering coefficient and averaged characteristic path length of a matched random network, respectively, generated by preserving the degree of each node but randomizing the nodes’ connections 100 times. Small-world-ness is defined as σ = γ/λ, and a network with σ > 1 indicates that the network is “small-world.”
Hub Region Detection
Degree centrality was utilized to measure the nodal importance, which is calculated as the sum of edges connected to a node. Here, nodal degree was calculated based on the weighted thresholded network that only preserved positive links with P values <0.01. Nodes with Z-scored degree value >1.5 were defined as hubs of the whole brain. Within-module degree (WD) and participation coefficient (PC) were also calculated as measures related to the role each hub node plays in the network (Guimerà and Amaral 2005a, 2005b). The WD value of node i is defined as 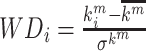 , where
, where  is the weighted degree of node i within its own module (m),
is the weighted degree of node i within its own module (m),  is the average of the degree within module m, and
is the average of the degree within module m, and  is the of degree of nodes in module m. The PC value of node i is defined as
is the of degree of nodes in module m. The PC value of node i is defined as 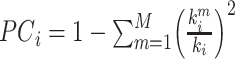 , where M is the number of modules, and ki is the total weighted degree of node i. Nodes with high WD and low PC values are “provincial” hubs connected to other nodes in the same module (cluster), whereas nodes with low WD but high PC values are “connector” hubs that link different modules together. Here, we defined provincial hubs as those with a z-score z (WD) ≥ 1.5 and z (PC) ≤ 0.3, which primarily connect to nodes within their own modules; and connector hubs as those with z (WD) < 1.5 and z (PC) > 0.6, which predominantly link different modules. Similar definition of provincial hub can also be found in (Cohen and D’Esposito 2016).
, where M is the number of modules, and ki is the total weighted degree of node i. Nodes with high WD and low PC values are “provincial” hubs connected to other nodes in the same module (cluster), whereas nodes with low WD but high PC values are “connector” hubs that link different modules together. Here, we defined provincial hubs as those with a z-score z (WD) ≥ 1.5 and z (PC) ≤ 0.3, which primarily connect to nodes within their own modules; and connector hubs as those with z (WD) < 1.5 and z (PC) > 0.6, which predominantly link different modules. Similar definition of provincial hub can also be found in (Cohen and D’Esposito 2016).
Assortativity coefficient, defined as correlation between the strength of nodes (degree) on opposite sides of a connection (Newman 2002), was utilized to investigate whether noxious stimuli influence the assortativity of the network structure. Nodes in an assortative network tend to link with other nodes with similar strength, for example, hub regions are more strongly clustered with other hub regions, making the network more robust to disruption (Newman 2002; Bassett et al. 2008).
Statistical Analysis
We performed statistical tests on the difference between noxious and innocuous stimulus on brain activity, functional connectivity, and network measures. For brain activity, we performed paired t-tests (P < 0.05, Bonferroni connected across parcels) on noxious versus innocuous stimulation. For between-group differences in functional connectivity, Steiger’s z test (Steiger 1980) for dependent correlations was performed, with FDR correction at q < 0.05. For network properties, the bias corrected, accelerated bootstrap tests were performed on painfully hot versus nonpainful warm paired (within-person) differences. This test is preferred because of the expected nonnormal distribution of differences in network measures. In each bootstrap iteration, participants were resampled with replacement, and paired noxious versus innocuous differences in each network property (e.g., C) was calculated. This procedure was repeated 5000 times, and two-tailed, uncorrected P values were calculated from the bootstrap confidence interval. FDR correction with q < 0.05 was used for correcting multiple comparisons across connectivity densities.
Contrasting Subjectively Painful versus Nonpainful Stimuli
We used Study 2 to investigate how changes in network organization varied according to subjective feelings of pain. In Study 2, participants experienced 6 levels of thermal stimuli and were asked to judge whether each individual stimulus was painful or not (Woo et al. 2015). They then rated warmth or pain on separate 100-point VAS scales, coded here as (1–100) or (101–200), respectively. For each participant, we grouped trials rated as nonpainful and those rated as painful and averaged these within condition. These averages were used to identify networks based on individual differences and to contrast explicitly painful versus nonpainful conditions.
In addition to the aforementioned analysis, normalized mutual information (NMI) (Kuncheva and Hadjitodorov 2004; Alexander-Bloch et al. 2012) was utilized to quantify the similarity of modular partitions between subjective evaluation and objective categorization of pain. NMI is a widely used measure to assess pairwise difference between modular partitions, defined as:
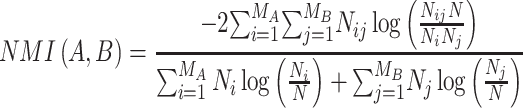 |
where A and B are the partitions of two networks;  is the number of modules in A; N is the number of nodes in the network;
is the number of modules in A; N is the number of nodes in the network; 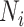 and
and 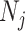 represent the number of nodes in module i of A and module j of B, respectively;
represent the number of nodes in module i of A and module j of B, respectively;  is the number of nodes that the two modules have in common. The NMI lies between 0 and 1, a value close to 1 implies that the two partitions are relatively similar.
is the number of nodes that the two modules have in common. The NMI lies between 0 and 1, a value close to 1 implies that the two partitions are relatively similar.
Results
Regional Activity for Nonpainful Warm versus Painfully Hot Stimuli
The comparison pattern of brain activation differences between innocuous and noxious stimulation is shown in Figure 2. Compared to innocuous stimuli, noxious stimuli significantly activated bilateral insular and opercular cortices, ACC, S2, ventral and caudal inferior frontal gyrus, medial superior frontal gyrus (SFG), premotor cortex, right anterior superior temporal gyrus (STG), some subcortical tissues (e.g., thalamus), and cerebellum. Significant deactivation induced by painfully hot stimuli was found in the left postcentral gyrus, ventromedial prefrontal cortex (vmPFC), left middle and inferior temporal gyrus, right STG, medial precuneus, and bilateral occipital cortices (paired t test, Bonferroni corrected, q < 0.05). This pattern is consistent with previous findings on evoked pain (Treede et al. 2000; Bushnell and Apkarian 2006; Kong et al. 2010; Mouraux et al. 2011; Wager et al. 2013; Favilla et al. 2014; Tanasescu et al. 2016; Lindquist et al. 2017).
Figure 2.

Parcellation of the brain and significant activation (yellow/orange) and deactivation (blue) for noxious versus innocuous stimulation. Red and blue colors indicate the significance of activity (paired t test P < 0.05, Bonferroni corrected).
Altered Network Properties Induced by Noxious Stimuli
Results from the 5000 paired-sample bootstrap test (see Materials and Methods) showed that noxious (vs. innocuous) stimulation significantly influenced the overall organization of functional networks. We found that increased stimulation intensity caused significant increases in both the brain-wide average shortest path length and clustering coefficient (P < 0.05, FDR corrected, see Fig. 3A,B), resulting in reduced small-world-ness (Fig. 3C). This indicates that brain regions are more locally clustered and less globally connected across local clusters (i.e., reduced global efficiency). These changes are coherent in pointing to more tightly clustered results “within” networks and reduced global connectivity “between” functional networks. We unpack these global changes in more detail below.
Figure 3.

Comparison of network properties between innocuous and noxious stimulations. The network metrics of both weighted and binary networks were extracted. Metrics of weighted network were extracted from networks that only preserved positive links with P < 0.01, whereas metrics of binary networks are the average of the graph metrics across link densities (from 10% to 25% in 5% increments). Bars show the mean value and SD from the bootstrap procedure. (A) Both networks show small-world-ness, but the small-world architecture is disrupted during pain. (B–D) Increased average clustering coefficient (C) and average characteristic path length (L), and decreased modularity (Q) under noxious heat stimulus. Asterisk indicates the P value exceeds the threshold (bootstrap test, P < 0.05).
Reorganized Modular Architecture during Noxious Stimuli
By applying the module detection algorithm, we found significantly lower modularity values (Q) during pain, suggesting pain integrates brain systems into fewer functional communities (i.e., clusters) (Fig. 3D). Eight distinct modules were identified in the innocuous condition when preserving only significant positive connections (connections with q < 0.05, FDR corrected) in the network, whose spatial distribution is shown in Figure 4A,B (different colors indicate different modules). These modules are consistent with intrinsic functional subnetworks identified in other studies, including networks heuristically termed the cognitive control network (CCN, in red), “default mode” network (DMN, in orange), sensorimotor network (SMN, in yellow), insular-opercular “ventral attention” or “salience” network (ION, in gold), a temporal system (TN, in light green), visual function (VN, in dark green), a subcortical network (SCN, in light blue), and a cerebellum system (CBN, in blue). These system partitions showed high coherence across multiple thresholds (Fig. 4C). In addition, CCN, ION, and SCN were positively activated under warm stimuli, whereas some regions of DMN and VN were deactivated (Fig. 4D).
Figure 4.

The detected functional systems of each condition. (A and B) Visualization of eight functional systems that were detected using network with significant positive connections (P < 0.05, FDR corrected) under innocuous stimuli. (C) The coherence modular structure under innocuous stimuli thresholded by using various P values [q < 0.01 (FDR corrected), q < 0.05 (FDR corrected), P < 0.005, P < 0.01, P < 0.05]. Brain regions belong to the same functional system across thresholds are shown in the same color. The partitions are coherent across thresholds but show a tendency to merge as the threshold P value increases. (D) Z-scored activities in each system during innocuous stimulation. (E and F) Functional systems detected under painfully hot stimuli. (G) The coherence modular structure under noxious stimuli thresholded by using various P values [q < 0.01 (FDR corrected), q < 0.05 (FDR corrected), P < 0.005, P < 0.01, P < 0.05]. Brain regions belong to the same functional system across thresholds are shown in the same color. (H) Z-scored activity in each system during noxious stimulation. (I) Comparison of module numbers of the two conditions (bootstrap test, P < 0.05). Bars show the mean value and SD from the bootstrap procedure.
By contrast, clustering during noxious stimulation resulted in four modules (Fig. 4E,F), which showed high coherence across multiple thresholds (Fig. 4G), and were significantly fewer than during innocuous stimulation (Fig. 4I, P < 0.05, bootstrap test). Specifically, an integrated “pain-related super-system” (PS)—so termed because it was the only module of the 4 to be significantly activated for painful events (Fig. 4H and Supplementary Fig. 2)—included most of the ION and SCN and components of the CCN (e.g., ACC) and SMN. Three other systems (OS) were also detected (Fig. 4F). OS1 included most of CCN and parts of DMN and was neither activated nor deactivated during painful versus innocuous stimulation (Fig. 4H). OS2 included most of DMN and several regions of TN, VN, and ION and was significantly deactivated during pain (Fig. 4G and Supplementary Fig. 2). OS3 included most of SMN, VN, and CBN and was neither activated nor de-activated during painful stimulation.
Analysis of individual differences in reported pain intensity during noxious stimulation also yielded significant relationships across individuals and studies. For analysis across the 5 studies, the average activity with PS was correlated with increased average pain intensity (r = 0.21, P < 0.05), and OS2 activity was negatively correlated with pain intensity (r = −0.19, P < 0.05) (see the black line in Supplementary Fig. 3). Together, multiple regression using the average of PS and OS2 to predict pain yielded a multiple correlation of r = 0.39 (P < 0.0001). These findings extend earlier work showing correlations between brain activity and perceived pain (Coghill et al. 1999; Koyama et al. 2005; Baliki et al. 2009; Wager et al. 2013; Atlas et al. 2014). Specifically, these findings extend earlier studies by demonstrating (a) prediction of individual differences by connectivity in a reproducible set of networks, (b) a much larger sample size, and (c) generalizability across multiple studies. No significant correlation was found between the pain ratings and activity in other subsystems. Interestingly, the alterations in network structure indicate that these communities likely could not be identified in resting-state studies.
Differences in FC with Painful Heat versus Nonpainful Warmth
Significant connectivity differences between innocuous versus noxious stimulation (q < 0.05, FDR corrected) are shown in Figure 5A. Red and blue connections indicate positive and negative changes during pain, respectively (see also Supplementary Table 2 for details). During noxious stimulation, connections within the PS became more positive. The increased connectivity integrated several components within the PS, including the CCN (e.g., mPFC, area 44 and 45, and ACC), ION, SMN (e.g., primary sensory cortex), and SCN. Other increased connections were found mainly between lateral SMN and VN within OS3. We also found most of the connections that connected the PS with OS were decreased, such as connections with hippocampus and temporal cortices.
Figure 5.

Altered connections and hub regions under noxious stimuli. (A) Visualization of the altered connections under noxious stimuli. The lines indicate more positive (red) and negative (blue) connectivity during noxious versus innocuous stimulation. Colors in the outer ring indicate the functional systems that each brain region belongs to. (B) Visualization of whole-brain hubs (z-scored degree > 1.5) under nonpainful warmth and painfully hot stimulation, respectively. The colors indicate the network membership as in (A). During pain, all whole-brain hubs are contained within the PS. (C) Differences in assortativity between innocuous and noxious stimuli. Noxious stimulation significantly increases the assortativity of the network (P < 0.01). (D) Brain regions with the highest regional activity (averaged across participants) also had a higher degree on average (r = 0.55, P < 0.001). Conversely, however, this implies that only 30% of the variance in degree is explained by average activity, and 70% is unexplained. The correlation under noxious stimuli (rpain = 0.64, Ppain < 0.001) is greater than the correlation in warm condition (rnopain = 0.47, Pnopain < 0.001). (E) The distribution of provincial hubs and connector hubs for each condition. The dashed lines indicate the threshold defining provincial hubs (z (WD) = 1.5, z (PC) = 0.3) and connector hub z (WD) = 1.5, z (PC) = 0.6). Provincial hubs are more frequent with painful stimulation, and connector hubs are more frequent with nonpainful stimulation.
These altered connectivity patterns further induced decreased shortest path length within each subsystem and increased shortest path length between different subsystems (Supplementary Fig. 4A), which may result in rises of local communication but reduction in global efficiency. In addition, the increased connectivity within the PS significantly improved its communication efficiency relative to OS2 and OS3 (P < 0.05, bootstrap test); the increase in efficiency compared to OS1 was marginally significant (P = 0.084, Supplementary Fig. 4B).
Changes in Distribution and Function of Hub Regions during Noxious Stimuli
Distributions of the whole-brain hub regions were also altered during noxious stimuli, partially paralleling reports of hub disruption in chronic pain (Mansour et al. 2016; Mano et al. 2018; Kaplan et al. 2019). As we can observe in Figure 5B, during warm stimulation, hubs were distributed broadly across multiple systems (i.e., CCN, ION, and SCN), including regions such as medial SFG, ventral middle frontal gyrus, STG, insula, basal ganglia, and other subcortical tissues (colors indicate functional systems in each condition). During painfully hot stimulation, whole-brain hubs were located mainly within PS, including bilateral insular-opercular cortices, mPFC, ACC, and many subcortical (e.g., striatal) regions, and preferred to link with other hubs rather than nonhub nodes (Fig. 5C). Similar findings were also reported in fibromyalgia (FM) patients with high pain intensity (Kaplan et al. 2019), which showed highly interconnected hubs (rich club) within the PS. We also found significant positive correlations (rinnocuous = 0.47, rnoxious = 0.64, Ps < 0.001) between regional activity and nodal degree (Z-scored across nodes) in both conditions (Fig. 5D).
The connector versus provincial roles of hub regions was also quite different for innocuous versus noxious stimulation. Hub regions in the innocuous condition were mostly connectors with high PCs but low WDs, promoting inter-system connectivity between modules. Hub regions during pain were mostly provincial, with low PCs but high WDs, connecting brain regions within the PS (Fig. 5E). The number of connector hubs in the noxious condition was significantly lower than in the innocuous condition (bootstrap test, P < 0.01), implying reduced information transfer across systems outside the PS during pain. This was consistent with findings of reduced global efficiency and the increased shortest path length between subsystems. Thus, in sum, pain results in significantly enhanced connections within a “supersystem” including networks associated with affect, attentional allocation to salient events and motivated action selection, cognitive control and/or representation of context and goal relevance, action initiation and valuation (e.g., striatum), and somatosensory perception. Conversely, pain causes reduced connectivity with OS involved in emotion, semantics, language, visuospatial processing, and long-term memory.
Altered Network Organization Related to Subjective Pain Experience
Analyses grouping trials into subjectively painful and subjectively nonpainful conditions, which were possible for one of the studies (N = 30), yielded similar results in many respects. Pain was associated with higher clustering coefficients and increased path length connecting nodes (i.e., reduced global efficiency), and the reduction in small-world-ness was marginally significant (Ps < 0.085, see Fig. 6A). However, modularity was significantly higher during painful than nonpainful trials (Fig. 6A). The modular structure of networks when grouping trials by subjective pain (see Fig. 6B) was similar to the structure identified when grouping trials based on objectively noxious stimuli (see Fig. 4F). The assortativity did not show significant change between the two conditions. The similarity in maps of module membership across the brain was highest for the pairs (painful, noxious) and (non-painful, innocuous) and significantly lower for pairs (non-painful, noxious) and (painful, innocuous) (P < 0.001, bootstrap test; Fig. 6C), suggesting similar reorganization mode induced by noxious stimuli and pain feeling, though discrepancies in module distribution were observed. The discrepancies, we speculated, may result from individual differences in pain threshold; for example, some of the participants may have experienced pain during low intensity, nominally innocuous stimulation (intensity <45.3 °C). In addition, paralleling analyses grouped by noxious versus innocuous stimulus intensity, there was a sharp reduction in the number of connector hubs during subjectively painful versus nonpainful trials, as indicated by reduced PCs of the top-ranked hubs (P < 0.01, bootstrap test, Fig. 6D). Thus, our observations of increased clustering and shortest path length, reduced small-world-ness, integration of multiple networks into a “pain-related super-system,” and a shift from connector hubs to provincial hubs were all confirmed to be related to subjective pain reports as well as objectively defined noxious stimulation.
Figure 6.

Comparison of network properties, modular organization, and PC values of subjective painful versus nonpainful. (A) Comparisons of average clustering coefficient (C), average characteristic path length (L), small-world-ness, and modularity (Q) between painful and nonpainful conditions. Asterisk indicates the P value exceeds the threshold (bootstrap test, P < 0.05), “~” indicates the difference is marginally significant. (B) Functional subsystems detected in subjective nonpainful and painful conditions. (C) Comparison of normalized mutual information (NMI) between module segmentations under noxious and innocuous stimuli and subjective painful and non-painful (bootstrap test, P < 0.01). (D) The role of hub regions played in each condition. The dashed line indicates the threshold (WD = 1.5, PC = 0.3) between provincial hub and connector hub. Nodes in orange and blue indicate hub regions under painful and non-painful condition, respectively.
More detailed analyses of changes across four levels of reported pain (non-painful [<100 on the VAS], 100–120, 120–150, and >150) helped explain the pattern of changes in global network measures we observed when defining conditions based on subjective pain (Supplementary Fig. 5). Clustering coefficient increased and small-world-ness decreased monotonically across four levels of reported pain. Minimum path length and modularity were nonmonotonic, highest for intermediate levels of pain and lower for nonpainful or very painful trials. This is likely because as pain increases, path length decreases within a “pain-related super-system” similar to the one we describe above (red in Fig. 6B), but increases between modules. Global path length averages over these changes. It is also likely that some aspects of modular organization are driven in part by thermosensory responses in the physiologically noxious (≥46.3 °C) versus innocuous (<45.3 °C) range, irrespective of self-reports. Graph measures more strongly related to noxious stimulus intensity (vs. subjective pain) should show smaller changes and/or different results from those based on objective stimulus intensity, as categories defined based on subjective pain mix noxious and innocuous stimuli. Our results show that global path length and modularity indices show the strongest differences, implying that they are relatively more stimulus-related than pain-related, but that other indices track noxious stimulus intensity and subjective pain in a similar fashion. This study was not designed to permit a full dissociation of stimulus intensity and subjective pain-related effects, as they are very strongly related in healthy individuals. A full characterization of potential differences should be undertaken in specialized studies and patient populations.
Discussion
The original concept of the “pain neuromatrix” (Melzack 2005) proposed that pain served to integrate neural systems related to somatosensation, affect and emotion, action, viscerosensation, and homeostatic regulation. Though the term was later co-opted as a shorthand for a set of brain regions typically activated by noxious stimulation (Jones 1998; Peyron et al. 2000; Bushnell and Apkarian 2006; Tracey and Mantyh 2007; Iannetti and Mouraux 2010), its intended spirit is perhaps better characterized in terms of patterns of connectivity across nociceptive and non-nociceptive circuits. The drive to understand pain in terms of functional connections and global network properties has led to the concept of the “dynamic pain connectome” (Kucyi and Davis 2015, 2017) and a number of recent papers characterize chronic pain in terms of associations with functional connectivity and network properties (Baliki et al. 2008, 2014; Cauda et al. 2009; Napadow et al. 2010; Jensen et al. 2012; Kong et al. 2013; Ichesco et al. 2014; Kucyi and Davis 2015; Hemington et al. 2016; Mansour et al. 2016; Bosma et al. 2018; Mano et al. 2018). This article extends this work by showing reliable patterns of pain-related changes in connectivity across studies. The eight functional systems we identified during innocuous stimulation were broadly consistent with modules reported in resting-state studies (Damoiseaux et al. 2006; Menon 2011; Power et al. 2011), suggesting that innocuous stimuli do not substantially alter the functional architecture observed at rest. However, pain resulted in substantial changes. Network analyses of stimulus-evoked responses based on both objectively noxious stimulus intensity and subjective pain reports revealed a coherent set of changes in network structure (summarize in Table 2), including (1) reduced small-world-ness, increased clustering coefficients, and a shift from “connector” hubs to “provincial” hubs, all changes that imply increased integration “within” large-scale functional networks and reduced connectivity across disparate networks and (2) an integration (increased coherence) of specific networks present at rest and during innocuous stimulation—namely, the CCN, ION, ‘somatomotor’ (SMN), and subcortical (mainly thalamic and striatal) network—into a highly interconnected “supersystem” (pain-related system, PS) with increased intra-system connectivity and reduced connectivity with OS.
Table 2.
Summary of the results
| Network metrics | Noxious versus innocuous | Painful versus non-painful | Interpretation | |
|---|---|---|---|---|
| Shortest path length | ↑ | ↑ | • Noxious stimulation results in fewer | |
| Clustering coefficient | ↑ | ↑ | networks with more efficient intra- | |
| Small-world-ness | ↓ | ↓ (~) | network connectivity and greater | |
| Modularity | ↓ | ↑ | resistance to disruption. Connectivity | |
| Assortativity | ↑ | ns | between networks is reduced. High pain experience was associated with similar changes. Modularity differences between analyses may reflect competing changes in within- and between-network connectivity (see below) |
|
| Modules | • Innocuous condition: 8 subsystems. • Noxious condition: 4 subsystems. (Components of the ION, SCN, CCN and SMN were reorganized to form the PS) |
• Non-painful condition: 6 subsystems (NMI (non-painful and innocuous) = 0.4287). • Painful condition: 4 subsystems (NMI (painful and noxious) = 0.4283). |
• There are fewer modules (networks) during painful stimulation and high-pain experience |
|
| Hubs | Whole brain | • Concentrated within the PS in noxious condition. | • Brain regions within the PS become more densely connected during painful stimulation | |
| • Distributed broadly across multiple systems in innocuous condition. | ||||
| Provincial | ↑ | ↑ | • Increased intra-module communication | |
| Connector | ↓ | ↓ | • Decreased inter-module communication | |
| Connections | Within PS | ↑ | ns | • Increased information transfer within the PS |
| Between systems | ↓ | • Disrupted communication between the PS and OS | ||
| (between PS and OS) | ns | |||
Notes. ns, non-significant; “~” indicates the alteration is marginally significant.
Coactivation of brain regions (e.g., regions in the “pain neuromatrix”) may result from common latent causes (e.g., variation in nociceptive input). In many cases, observations of stimulus-dependent connectivity may reflect such latent factors; we would argue that this provides a richer characterization of a functional brain system but does not definitively prove that the underlying architecture is stimulus-dependent. However, if regions are uncorrelated under one stimulus condition (implying that they are not driven by a common latent cause) but correlated under another condition, this provides stronger evidence for stimulus-dependent changes in network communication. We found that this was the case. Regions uncorrelated during nonpainful stimulation (and typically at rest as well) became correlated during painful stimulation. Moreover, connectivity changes that originated from activity changes induced by tasks are independent to the interregional “inherent connectivity” (Duff et al. 2018), though some of the “inherent connectivity” are likely always driven by task-induced activity that is unmodeled or whose timing and duration differ from the timing in the task model (Geuter et al. 2016). Indeed, the “communication-through-coherence” theory (Fries 2005) suggests that effective interactions occur when activated neuronal groups undergo coherent excitability fluctuations. However, they are informative in ways that activation magnitude alone is not, as they describe reorganization of the systems that are coactivated in response to painful stimulation. In addition, importantly, coactivation of a set of brain regions does not imply that these regions are all activated to the same degree for a given individual (i.e., that there would be coherent individual differences) or that activation would predict individual differences in behavior (activity in the PS we identified predicted increased pain sensitivity across individuals and studies, and OS2 activity predicted reduced pain sensitivity), as we observed here. Furthermore, the structure of which functional subnetworks are coherently activated in subsets of individuals, and how this relates to pain sensitivity, is largely uncharted territory. Thus, a main contribution of this article is to show that stimulus-dependent connectivity changes are an important, measurable property of pain-related systems that can provide a more complete description than measuring activity alone—and that connectivity is particularly for characterizing individual differences in pain sensitivity. We are largely agnostic about whether these connectivity changes reflect a common underlying factor such as stronger input to multiple regions or a change in network architecture; however, two facts point to a substantive change in network architecture. First is the fact that during painful stimulation regions outside typical pain-processing regions begin to inter-correlate. Second, many of these regions are not appreciably correlated at rest. These two pieces of evidence argue against a common-factor interpretation. Though our results were not designed to yield identical patterns to those that might be observed based on time series (within-person) functional connectivity, they were designed to identify differences that are maximally related to the processing of painful stimuli and useful for characterizing individual differences.
One broader interpretation of these findings is that the “supersystem” (PS) includes the systems required to perceive and respond to bodily threats at both short and long time-scales, from sensation to action initiation to cognitive planning and longer-term action policies. Because the type of pain we studied is immediate, evoked pain, systems related to long-term memory retrieval, and broader contextualization of pain relative to the self—for example, “default mode” DMN, including medial PFC and hippocampus—are suppressed and disconnected from the sensorimotor-action-planning “supersystem.” This interpretation recognizes that pain is a complex process involving many features beyond nociception (e.g., attention and emotion) (Melzack and Wall 1965; Seminowicz et al. 2004; Moriarty et al. 2011) and that DMN and hippocampal connectivity may play a different role in relation to chronic pain [e.g., (Baliki et al. 2008; Jensen et al. 2012; Loggia et al. 2013; Kucyi et al. 2014; Vachon-Presseau et al. 2016; López-Solà et al. 2017).
Increased Within-Module Connectivity Facilitates the Integration and Transmission of Pain-Related Information
The increases in connectivity within the PS coupled with reduced inter-module connectivity may serve to prioritize information processing-related pain and redirect attention and processing resources toward noxious stimuli. This may facilitate rapid responses to important exogenous stimuli. This idea is consistent with a previous study indicating that cerebral systems may reorganize during pain to enhance the processing efficiency of pain-related information, resulting in shorter times between nociceptive stimulus-evoked cerebral responses and behavioral reactions (Ploner et al. 2006; Tiemann et al. 2018).
More broadly, the pain-related integration of systems we observed may be important for integrating multiple cognitive and affective processes essential for overall pain experience and behavior, including encoding of spatial information and stimuli intensity (Greenspan et al. 1999; Bornhövd et al. 2002; Bingel et al. 2003, 2004; Arienzo et al. 2006), pain perception (Blomqvist et al. 2000; Garcia-Larrea 2012), transfer sensory information to motor system (Binkofski et al. 1999; Favilla et al. 2014) and magnitude estimation (Greenspan et al. 1999; Bornhövd et al. 2002), processing of emotional salience (Rainville et al. 1997; Johansen et al. 2001; Farrell et al. 2005; Zaki et al. 2012), mobilizing attentional resources toward stimuli (Davis et al. 1997), and pain-related decision-making (Wiech et al. 2010; Roy et al. 2014). The integration we observed spans systems implementing a collection of inter-related processes (Rainville 2002; Porro 2003).
Conversely, the disconnections we observed between systems may relate to the impairments in cognitive performance that are well known to accompany pain (Crombez et al. 1996; Eccleston and Crombez 1999; Seminowicz et al. 2004; Buhle and Wager 2010; Kucyi and Davis 2015). Kucyi and Davis found that enhanced DMN activity during pain was associated with mind-wandering away from pain and increased ability to maintain cognitive performance during pain (Kucyi and Davis 2015). More direct tests of the relationship between the connectivity patterns we identify here and cognitive performance during pain should be tested in future studies.
Decreased “Bridge” Nodes Reduce Connectivity Linking the PS and Other Subsystems
Provincial hubs are important for within-network communication, whereas connector hubs facilitate information flow between distinct brain networks (Heuvel and Sporns 2013). The significant shift from connector hubs during innocuous stimulation to provincial hubs during painful stimulation suggests that pain reduces inter-system communication. Unlike the PS, which was strongly activated during pain, the other three identified subsystems showed deactivation (OS2) or no activation (OS1, OS3). These systems are often thought to be related to descending pain-inhibitory systems [e.g., vmPFC, part of the DMN and OS2 here; (Bingel et al. 2006; Leknes et al. 2013)] and are often anticorrelated with pain, as we observed here (Kong et al. 2010; Wager et al. 2013). In addition to roles in descending inhibition, cortical DMN regions (part of OS2) are strongly connected to the hippocampus, forming a system that may be important for “contextualizing” pain, reducing pain experience in healthy participants and safe contexts (Woo et al. 2017), but increasing pain experience when contexts imply threat (Ploghaus et al. 1999). Thus, disconnection of PS and OS may imply reduced contextual influences on pain as noxious stimulus intensity increases. This possibility can be directly tested in future studies.
Comparisons between Evoked Pain and Chronic Pain Improved the Understanding of Chronic Pain beyond Nociception
An important question is how our findings on evoked pain relate to network-level changes in various chronic pain conditions. Integrating and comparing findings across studies, methods, and pain conditions is a complex but crucial endeavor as more studies produce network-level descriptions of pain. Comparing evoked and chronic pain can help identify whether brain changes with chronic pain are limited mainly to nociceptive pathways or extend beyond nociceptive systems to involve novel functional contributions of non-nociceptive central brain circuits [e.g., (Baliki et al. 2006; Mansour et al. 2016; Seminowicz and Moayedi 2017; Woo et al. 2017)]. When pain correlates are limited mainly to nociceptive pathways, it is more likely that changes are secondary to enhanced nociceptive input from the periphery, and peripheral treatments (e.g., surgery) are more likely to be helpful. Conversely, when changes in extra-nociceptive circuits support pain and functional impairment, peripheral pathology and peripheral treatments are less likely to be important. For this reason, it is important to understand which network-level correlates of chronic pain reflect nociceptive versus extra-nociceptive systems.
Our findings suggest that during evoked pain, nociceptive areas are increasingly integrated with extra-nociceptive areas important for attention, consciousness, memory, and other aspects of cognition and affect. These changes parallel recent findings of similar network-level changes with chronic pain. For example, Kaplan et al. (2019) identified a “rich club” of highly interconnected regions in FM that is similar to the “pain supersystem” (PS) we identified here. The strongest increases in global connectivity in FM versus controls were found in nociceptive target regions (e.g., mid- and anterior insula), and membership of nociceptive regions (e.g., posterior insula, S1) in the “rich club” was found only in patients with the highest clinical pain intensity. Other studies have focused on extra-nociceptive, global network measures associated with chronic pain [e.g., (Mansour et al. 2016; Mano et al. 2018)]. Nonetheless, these networks also share some features with evoked pain network topology identified in our study, including increased connectivity in S1 (Mano et al. 2018), lateral (putatively sensory) thalamus (Mansour et al. 2016), and posterior insula (Mansour et al. 2016).
Another common feature of chronic pain is increased connectivity between nociceptive and “default-mode” regions (Hemington et al. 2016), paralleling the increased integration between nociceptive and OS we found in this study. Multiple studies have found pain-related, between-network connectivity increases in DMN-ION (Baliki et al. 2014; Kim et al. 2019), DMN-pain [e.g., (Hashmi et al. 2013; López-Solà et al. 2017)], and VN-SMN (Shen et al. 2019). Thus, some brain network-level changes with chronic pain may be related to the pain itself, either as a predisposing factor or consequence (Baliki et al. 2014); others may be truly extra-nociceptive differences in the kinds of patients with chronic pain or correlates of functional, behavioral, and emotional changes beyond pain experience itself. For example, changes in frontostriatal systems may be associated with withdrawal and avoidance (Roy et al. 2014; Ren et al. 2016; Schwartz et al. 2017).
It is interesting when compared with the results of Lee et al. (2018), who showed decreased frequency assortativity in chronic pain patients. This difference suggests that there are qualitative differences between brain changes associated with evoked pain and changes in baseline connectivity that develop with chronic pain. Our results suggest that the spread of activity is limited to one system (albeit one that is substantially expanded relative to the resting state) and hubs regions were more inclined to connect with each other, whereas chronic pain patients may experience broader across-module connectivity that makes them vulnerable to explosive synchronization with strong input (Lee et al. 2018). However, changes in chronic pain are complex, and there are also similarities between our findings and module organization with chronic pain (Kaplan et al. 2019), for example, brain regions within the PS (e.g., insula, ACC and primary somatosensory) are more tensely connected with the increase of clinical pain.
Though the integration of findings across various types of evoked and chronic pain is an important goal, there are also inconsistencies that make integration challenging. These include differences in methods (e.g., hub centrality metrics and region definitions), findings, and emphasis in discussion of what appear inevitably to be complex patterns of effects. For example, thalamic hyperconnectivity is a common feature across disorders in Mansour et al. 2016, but subcortical regions were not tested in some other studies (e.g., Kaplan et al. 2019). Posterior insula changes are a central focus in the study of Kaplan et al. (2019), and some corroborating evidence is apparent in figures in the work of Mansour et al. (2016), but this is not discussed. These incompatibilities could be harmonized in future studies in meta-analyses that directly integrate and compare data across types of stimuli, brain measures, and pain conditions.
Implications for Understanding Pain Consciousness
Another important, seldom addressed question concerns what makes pain conscious. Pain is by definition a subjective, reportable experience, implying consciousness as a required element; but our understanding of what makes pain conscious is limited. Some researchers have described pain as an emergent, global property (Baliki and Apkarian 2015). While there are multiple theories of consciousness with different brain substrates (Boly et al. 2012; Bonhomme et al. 2012; Heine et al. 2012; Barttfeld et al. 2015; Morsella et al. 2016; Tononi et al. 2016), one prominent theory—the “global workspace” theory—posits that integration of activity in the lateral prefrontal cortex amplifies representations in other cortical areas that would otherwise be subliminal, rendering them conscious. Our findings that pain integrates somatosensory activity with frontoparietal systems (the CCN here) similar to those identified by the “global workspace” theory are consistent with this view. The conscious experience of pain may require connectivity between cortical targets of nociceptive afferents (generally located in SMN and ION here) and frontoparietal systems (CCN). We found that all of these systems are integrated during pain. These findings are also broadly consistent with previous work showing loss of consciousness is associated with impeded integration of frontoparietal system (Schrouff et al. 2011). Interestingly, findings of decreased modularity when aware of noxious stimuli is also consistent with the view that awareness is associated with reductions in modularity (Godwin et al. 2015).
Other relationships between functional connectivity and conscious experience are also relevant. For example, we found increased connectivity between thalamus and SMN (both part of PS). Previous studies have associated consciousness with thalamocortical connectivity (Alkire and Miller 2005; Boveroux et al. 2010) and suggested that decreased thalamocortical connectivity during propofol-induced loss of consciousness might block cortical arousal to external stimuli (Boveroux et al. 2010). Thus, the pattern of integration of cortical and subcortical systems we observed here may be related to what makes pain conscious. This hypothesis needs to be examined further in future studies.
Limitations
A number of limitations could productively be addressed by future work. First, our analyses focused on individual differences, and other types of network-level characterizations based on different types of data are possible. Our focus on stimulation-induced responses minimizes sources of variability unrelated to pain (Geerligs et al. 2015; Finn et al. 2017), but time series connectivity similarities and differences within and between stimulation periods could paint a complementary picture. In addition, our results reflect average network changes, but characteristics may vary across individuals in ways we have not captured here. Constructing a functional network at the individual-person level is still a challenge for single-trial analysis, and the short duration of each trial and the limited number of trials are limitations. Second, we utilized data from 5 independent datasets with varied thermal intensity and duration of stimulation. This is a strength in one sense, as it promotes generalizability across samples, but it may be less sensitive to individual differences within-study when stimulus and acquisition parameters are tightly controlled. In addition, the findings could be further generalized, for example, to different types of pain. Third, in this study, we attempted to provide a descriptive label for this “supersystem,” “pain-related,” as it is indeed empirically pain-related. However, caution in interpreting its function is warranted, and validating its profile of sensitivity and specificity is an empirical matter. We make no claims that “pain-related” means that the system measures “pain” to the exclusion of any other process. It is still unclear whether the reorganization of the PS is a pain-specific change or whether it can be evoked by other types of salient, intense, affective states (Liang et al. 2019). Recently, we identified distinct cerebral representations that are related to pain across multiple types (thermal, mechanical, and visceral) and are not shared with cognitive control and negative emotion tasks (Kragel et al. 2018). Such analyses require generalization and test of specificity across multiple studies and task conditions; the generalizability and specificity of the PS to pain could productively be further examined using similar approaches. In addition, we speculated the brain functional reorganization may result from the pain protective function; however, other interpretations are also possible. One possible hypothesis could be that the altered modular organization and hub topology may represent a suboptimal or pathological brain state, similar as in patients with chronic pain (Mansour et al. 2016; Lee et al. 2018; Kaplan et al. 2019). Currently, it is difficult for us to directly examine other views, but we have indicated increased efficiency and assortativity of the PS, suggesting the reorganized network structure do promote the information exchange. More specific exploration will be performed in the future studies. Finally, current community detection algorithms based on modularity maximization may have degeneracy problem especially for large-scale hierarchical networks (Good et al. 2010), and the partitions largely rely on the chosen of resolution parameter that determines the scale of detected communities (He et al. 2018). Though our findings of the communities are in good accordance with previous literatures (Bushnell and Apkarian 2006; Kong et al. 2010; Power et al. 2011; Wager et al. 2013), whether these results are sensitive to the chosen of algorithms and the resolution parameters still need further exploration. This is beyond the scope of this study, but will be investigated in our future work through varying resolution parameters and via different modular detection algorithms [e.g., local community methods (Clauset 2005; Bagrow 2008) and generative models (Rosvall and Bergstrom 2007; Clauset et al. 2008; Hofman and Wiggins 2008)].
Conclusion
In conclusion, our study showed that painful stimulation drives a reorganization of functional networks. We found that, when experiencing nociceptive stimuli, the brain complexity of modular organization across brain regions is reduced. This may enable the integration of pain processing across functional subsystem, as well as reduce communication (and access to behavioral output systems) with OS that have less correlation with pain. The subjective pain experience induced similar changes in network organization. These alterations enable a person to rapidly respond to painful stimuli and may bear on understanding the bases of consciousness, especially in the domain of pain.
Supplementary Material
Author Contributions
W.Z. and T.D.W. drafted the manuscript. W.Z. conducted data analysis. C.-W.W., L.Y.A., M.R., L.S., A.K., and M.J. contributed neuroimaging data. All authors provided feedback and revised the manuscript.
Funding
National Institutes of Health (grant R01 MH076136 to T.D.W.); National Key Basic Research and Development Program of China (2014CB744600, to B.H.); the National Natural Science Foundation of China (61210010, 61632014, to B.H.); the Program of Beijing Municipal Science and Technology Commission (grant Z171100000117005 to B.H.); the scholarship of China Scholarship Council (201606180117 to W.Z.); the Postdoctoral Funding of Zhejiang Province, China (514000-X81901, to W.Z.); and is supported in part by intramural funding from the National Center for Complementary and Integrative Health (to L.Y.A.).
Notes
Conflicts of Interest: The authors declare there is no conflict of interest in relation to this work.
References
- Alexander-Bloch A, Lambiotte R, Roberts B, Giedd J, Gogtay N, Bullmore E. 2012. The discovery of population differences in network community structure: new methods and applications to brain functional networks in schizophrenia. NeuroImage. 59:3889–3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkire MT, Miller J. 2005. General anesthesia and the neural correlates of consciousness. Prog Brain Res. 150:229–597. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. 2005. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 9:463–463. [DOI] [PubMed] [Google Scholar]
- Arienzo D, Babiloni C, Ferretti A, Caulo M, Del Gratta C, Tartaro A, Rossini P, Romani G. 2006. Somatotopy of anterior cingulate cortex (ACC) and supplementary motor area (SMA) for electric stimulation of the median and tibial nerves: an fMRI study. NeuroImage. 33:700–705. [DOI] [PubMed] [Google Scholar]
- Atlas LY, Bolger N, Lindquist MA, Wager TD. 2010. Brain mediators of predictive cue effects on perceived pain. J Neurosci. 30:12964–12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas LY, Lindquist MA, Bolger N, Wager TD. 2014. Brain mediators of the effects of noxious heat on PAIN. Pain. 155:1632–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas LY, Whittington RA, Lindquist MA, Wielgosz J, Sonty N, Wager TD. 2012. Dissociable influences of opiates and expectations on pain. J Neurosci. 32:8053–8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagrow JP. 2008. Evaluating local community methods in networks. J Stat Mech Theory Exp. 2008:P05001. [Google Scholar]
- Baliki MN, Apkarian AV. 2015. Nociception, pain, negative moods, and behavior selection. Neuron. 87:474–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Baria AT, Apkarian AV. 2011. The cortical rhythms of chronic back pain. J Neurosci. 31:13981–13990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian AV. 2006. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 26:12165–12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Apkarian AV. 2009. Parsing pain perception between nociceptive representation and magnitude estimation. J Neurophysiol. 101:875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Apkarian AV, Chialvo DR. 2008. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 28:1398–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Mansour AR, Baria AT, Apkarian AV. 2014. Functional reorganization of the default mode network across chronic pain conditions. PLoS One. 9:e106133–e106133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barttfeld P, Uhrig L, Sitt JD, Sigman M, Jarraya B, Dehaene S. 2015. Signature of consciousness in the dynamics of resting-state brain activity. Proc Natl Acad Sci. 112:887–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. 2008. Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci. 28:9239–9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastuji H, Perchet C, Legrain V, Montes C, Garcia-Larrea L. 2008. Laser evoked responses to painful stimulation persist during sleep and predict subsequent arousals. Pain. 137:589–599. [DOI] [PubMed] [Google Scholar]
- Bingel U, Lorenz J, Glauche V, Knab R, Gläscher J, Weiller C, Büchel C. 2004. Somatotopic organization of human somatosensory cortices for pain: a single trial fMRI study. NeuroImage. 23:224–232. [DOI] [PubMed] [Google Scholar]
- Bingel U, Lorenz J, Schoell E, Weiller C, Büchel C. 2006. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 120:8–15. [DOI] [PubMed] [Google Scholar]
- Bingel U, Quante M, Knab R, Bromm B, Weiller C, Büchel C. 2003. Single trial fMRI reveals significant contralateral bias in responses to laser pain within thalamus and somatosensory cortices. NeuroImage. 18:740–748. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buccino G, Posse S, Seitz RJ, Rizzolatti G, Freund HJ. 1999. A fronto-parietal circuit for object manipulation in man: evidence from an fMRI-study. Eur J Neurosci. 11:3276–3286. [DOI] [PubMed] [Google Scholar]
- Blomqvist A, Zhang E-T, Craig A. 2000. Cytoarchitectonic and immunohistochemical characterization of a specific pain and temperature relay, the posterior portion of the ventral medial nucleus, in the human thalamus. Brain. 123:601–619. [DOI] [PubMed] [Google Scholar]
- Boly M, Faymonville M-E, Schnakers C, Peigneux P, Lambermont B, Phillips C, Lancellotti P, Luxen A, Lamy M, Moonen G. 2008. Perception of pain in the minimally conscious state with PET activation: an observational study. Lancet Neurol. 7:1013–1020. [DOI] [PubMed] [Google Scholar]
- Boly M, Massimini M, Garrido MI, Gosseries O, Noirhomme Q, Laureys S, Soddu A. 2012. Brain connectivity in disorders of consciousness. Brain Connect. 2:1–10. [DOI] [PubMed] [Google Scholar]
- Bonhomme VLG, Boveroux P, Brichant JF, Laureys S, Boly M. 2012. Neural correlates of consciousness during general anesthesia using functional magnetic resonance imaging (fMRI). Arch Ital Biol. 150:155–163. [DOI] [PubMed] [Google Scholar]
- Bornhövd K, Quante M, Glauche V, Bromm B, Weiller C, Büchel C. 2002. Painful stimuli evoke different stimulus–response functions in the amygdala, prefrontal, insula and somatosensory cortex: a single-trial fMRI study. Brain. 125:1326–1336. [DOI] [PubMed] [Google Scholar]
- Bosma RL, Kim JA, Cheng JC, Rogachov A, Hemington KS, Osborne NR, Oh J, Davis KD. 2018. Dynamic pain connectome functional connectivity and oscillations reflect multiple sclerosis pain. Pain. 159:2267–2276. [DOI] [PubMed] [Google Scholar]
- Boveroux P, Vanhaudenhuyse A, Bruno M-A, Noirhomme Q, Lauwick S, Luxen A, Degueldre C, Plenevaux A, Schnakers C, Phillips C. 2010. Breakdown of within-and between-network resting state functional magnetic resonance imaging connectivity during propofol-induced loss of consciousness. Anesthesiology. 113:1038–1053. [DOI] [PubMed] [Google Scholar]
- Buhle J, Wager TD. 2010. Does meditation training lead to enduring changes in the anticipation and experience of pain? Pain. 150:382–383. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. 2009. Complex brain networks: graph theoretical analysis of structural and functional systems. Nature Rev Neurosci. 10:186. [DOI] [PubMed] [Google Scholar]
- Bushnell M, Apkarian A. 2006. Representation of pain in the brain In: Wall and Melzack’s textbook of pain. 5th ed. London: Elsevier. [Google Scholar]
- Cauda F, Sacco K, Duca S, Cocito D, D'Agata F, Geminiani GC, Canavero S. 2009. Altered resting state in diabetic neuropathic pain. PLoS One. 4:e4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ, He Y, Rosa-Neto P, Germann J, Evans AC. 2008. Revealing modular architecture of human brain structural networks by using cortical thickness from MRI. Cereb Cortex. 18:2374–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauset A. 2005. Finding local community structure in networks. Phys Rev E. 72:026132. [DOI] [PubMed] [Google Scholar]
- Clauset A, Moore C, Newman MEJ. 2008. Hierarchical structure and the prediction of missing links in networks. Nature. 453:98. [DOI] [PubMed] [Google Scholar]
- Coghill RC, Sang CN, Maisog JM, Iadarola MJ. 1999. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol. 82:1934–1943. [DOI] [PubMed] [Google Scholar]
- Cohen JR, D'Esposito M. 2016. The segregation and integration of distinct brain networks and their relationship to cognition. J Neurosci. 36:12083–12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombez G, Eccleston C, Baeyens F, Eelen P. 1996. The disruptive nature of pain: an experimental investigation. Behav Res Ther. 34:911–918. [DOI] [PubMed] [Google Scholar]
- Damoiseaux J, Rombouts S, Barkhof F, Scheltens P, Stam C, Smith SM, Beckmann C. 2006. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci. 103:13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KD, Taylor SJ, Crawley AP, Wood ML, Mikulis DJ. 1997. Functional MRI of pain-and attention-related activations in the human cingulate cortex. J Neurophysiol. 77:3370–3380. [DOI] [PubMed] [Google Scholar]
- Duff EP, Makin T, Cottaar M, Smith SM, Woolrich MW. 2018. Disambiguating brain functional connectivity. NeuroImage. 173:540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston C, Crombez G. 1999. Pain demands attention: a cognitive–affective model of the interruptive function of pain. Psychol Bull. 125:356. [DOI] [PubMed] [Google Scholar]
- Evans AC. 2013. Networks of anatomical covariance. NeuroImage. 80:489–504. [DOI] [PubMed] [Google Scholar]
- Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, Yang Z, Chu C, Xie S, Laird AR. 2016. The human brainnetome atlas: a new brain atlas based on connectional architecture. Cereb Cortex. 26:3508–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer MA, Baliki MN, Apkarian AV. 2012. A dynamic network perspective of chronic pain. Neurosci Lett. 520:197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MJ, Laird AR, Egan GF. 2005. Brain activity associated with painfully hot stimuli applied to the upper limb: a meta-analysis. Hum Brain Mapp. 25:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favilla S, Huber A, Pagnoni G, Lui F, Facchin P, Cocchi M, Baraldi P, Porro CA. 2014. Ranking brain areas encoding the perceived level of pain from fMRI data. NeuroImage. 90:153–162. [DOI] [PubMed] [Google Scholar]
- Finn ES, Scheinost D, Finn DM, Shen X, Papademetris X, Constable RT. 2017. Can brain state be manipulated to emphasize individual differences in functional connectivity? NeuroImage. 160:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Zalesky A, Breakspear M. 2015. The connectomics of brain disorders. Nat Rev Neurosci. 16:159. [DOI] [PubMed] [Google Scholar]
- Fries P. 2005. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends in cognitive sciences. 9:474–480. [DOI] [PubMed] [Google Scholar]
- Garcia-Larrea L. 2012. The posterior insular-opercular region and the search of a primary cortex for pain. Clin Neurophysiol. 42:299–313. [DOI] [PubMed] [Google Scholar]
- Geerligs L, Rubinov M, Henson RN. 2015. State and trait components of functional connectivity: individual differences vary with mental state. J Neurosci. 35:13949–13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuter S, Lindquist MA, Wager TD. 2016. Fundamentals of Functional Neuroimaging In: Berntson GG, Cacioppo JT, Tassinary LG, editors. Handbook of psychophysiology. 4th ed. Cambridge: Cambridge University Press, pp. 41–73. [Google Scholar]
- Godwin D, Barry RL, Marois R. 2015. Breakdown of the brain’s functional network modularity with awareness. Proc Natl Acad Sci. 112:3799–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good BH, Montjoye YA, Clauset A. 2010. Performance of modularity maximization in practical contexts. Phys Rev E Stat Nonlinear Soft Matter Phys. 81:046106. [DOI] [PubMed] [Google Scholar]
- Greenspan JD, Lee RR, Lenz FA. 1999. Pain sensitivity alterations as a function of lesion location in the parasylvian cortex. Pain. 81:273–282. [DOI] [PubMed] [Google Scholar]
- Guimerà R, Amaral LAN. 2005a. Cartography of complex networks: modules and universal roles. J Stat Mech. nihpa35573–nihpa35573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimerà R, Amaral LAN. 2005b. Functional cartography of complex metabolic networks. Nature. 433:895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmi JA, Baliki MN, Huang L, Baria AT, Torbey S, Hermann KM, Schnitzer TJ, Apkarian AV. 2013. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain. 136:2751–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashmi JA, Baria AT, Baliki MN, Huang L, Schnitzer TJ, Apkarian AV. 2012. Brain networks predicting placebo analgesia in a clinical trial for chronic back pain. Pain. 153:2393–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Chen Z, Evans A. 2008. Structural insights into aberrant topological patterns of large-scale cortical networks in Alzheimer's disease. J Neurosci. 28:4756–4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Chen ZJ, Evans AC. 2007. Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb Cortex. 17:2407–2419. [DOI] [PubMed] [Google Scholar]
- He Y, Lim S, Fortunato S, Sporns O, Zhang L, Qiu J, Xie P, Zuo X-N. 2018. Reconfiguration of cortical networks in MDD uncovered by multiscale community detection with fMRI. [DOI] [PMC free article] [PubMed]
- Heine L, Soddu A, Gómez F, Vanhaudenhuyse A, Tshibanda L, Thonnard M, Charland-Verville V, Kirsch M, Laureys S, Demertzi A. 2012. Resting state networks and consciousness. Front Psychol. 3:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemington KS, Wu Q, Kucyi A, Inman RD, Davis KD. 2016. Abnormal cross-network functional connectivity in chronic pain and its association with clinical symptoms. Brain Struct Funct. 221:4203–4219. [DOI] [PubMed] [Google Scholar]
- Herrera LC, Coan A, Marenco L, Cesar CL, Castellano G. 2017. Impact of gray matter signal regression in resting state and language task functional networks. bioRxiv. 094078. doi: https://doi.org/10.1101/094078. [Google Scholar]
- Hofman JM, Wiggins CH. 2008. Bayesian approach to network modularity. Phys Rev Lett. 100:258701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannetti GD, Mouraux A. 2010. From the neuromatrix to the pain matrix (and back). Exp Brain Res. 205:1–12. [DOI] [PubMed] [Google Scholar]
- Ichesco E, Schmidt-Wilcke T, Bhavsar R, Clauw DJ, Peltier SJ, Kim J, Napadow V, Hampson JP, Kairys AE, Williams DA. 2014. Altered resting state connectivity of the insular cortex in individuals with fibromyalgia. J Pain. 15:815–826. e811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KB, Loitoile R, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, Williams SC, Choy E, Mainguy Y. 2012. Patients with fibromyalgia display less functional connectivity in the brain’s pain inhibitory network. Mol Pain. 8:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Fields HL, Manning BH. 2001. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci. 98:8077–8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. 1998. The pain matrix and neuropathic pain. Brain J Neurol. 121:783–784. [DOI] [PubMed] [Google Scholar]
- Kaplan CM, Schrepf A, Vatansever D, Larkin TE, Mawla I, Ichesco E, Kochlefl L, Harte SE, Clauw DJ, Mashour GA et al. 2019. Functional and neurochemical disruptions of brain hub topology in chronic pain. Pain. 160:973–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Mawla I, Kong J, Lee J, Gerber J, Ortiz A, Kim H, Chan ST, Loggia ML, Wasan AD et al. 2019. Somatotopically specific primary somatosensory connectivity to salience and default mode networks encodes clinical pain. Pain. 160(7):1594–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Jensen K, Loiotile R, Cheetham A, Wey H-Y, Tan Y, Rosen B, Smoller JW, Kaptchuk TJ, Gollub RL. 2013. Functional connectivity of the frontoparietal network predicts cognitive modulation of pain. Pain. 154:459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Loggia ML, Zyloney C, Tu P, LaViolette P, Gollub RL. 2010. Exploring the brain in pain: activations, deactivations and their relation. Pain. 148:257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornelsen J, Sboto-Frankenstein U, McIver T, Gervai P, Wacnik P, Berrington N, Tomanek B. 2013. Default mode network functional connectivity altered in failed back surgery syndrome. J Pain. 14:483–491. [DOI] [PubMed] [Google Scholar]
- Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. 2005. The subjective experience of pain: where expectations become reality. Proc Natl Acad Sci USA. 102:12950–12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragel PA, Kano M, Van Oudenhove L, Ly HG, Dupont P, Rubio A, Delon-Martin C, Bonaz BL, Manuck SB, Gianaros PJ. 2018. Generalizable representations of pain, cognitive control, and negative emotion in medial frontal cortex. Nat Neurosci. 21:283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Woo C-W, Chang LJ, Ruzic L, Gu X, López-Solà M, Jackson PL, Pujol J, Fan J, Wager TD. 2016. Somatic and vicarious pain are represented by dissociable multivariate brain patterns. elife. 5:e15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A, Davis KD. 2015. The dynamic pain connectome. Trends Neurosci. 38:86–95. [DOI] [PubMed] [Google Scholar]
- Kucyi A, Davis KD. 2017. The neural code for pain: from single-cell electrophysiology to the dynamic pain connectome. Neuroscientist. 23:397–414. [DOI] [PubMed] [Google Scholar]
- Kucyi A, Moayedi M, Weissman-Fogel I, Goldberg MB, Freeman BV, Tenenbaum HC, Davis KD. 2014. Enhanced medial prefrontal-default mode network functional connectivity in chronic pain and its association with pain rumination. J Neurosci. 34:3969–3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuncheva LI, Hadjitodorov ST, editors. 2004. Using diversity in cluster ensembles In: IEEE International Conference on Systems, Man and Cybernetics (IEEE Cat. No.04CH37583); 10–13 Oct. 2004. Vol 1212, pp. 1214–1219. [Google Scholar]
- Kutch JJ, Labus JS, Harris RE, Martucci KT, Farmer MA, Fenske S, Fling C, Ichesco E, Peltier S, Petre B. 2017. Resting-state functional connectivity predicts longitudinal pain symptom change in urologic chronic pelvic pain syndrome: a MAPP network study. Pain. 158:1069–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan CL, Crawley AP, Mikulis DJ, Davis KD. 2000. An fMRI study of the anterior cingulate cortex and surrounding medial wall activations evoked by noxious cutaneous heat and cold stimuli. Pain. 85:359–374. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Campbell JN. 1978. Comparison of responses of warm and nociceptive C-fiber afferents in monkey with human judgments of thermal pain. J Neurophysiol. 41:509–528. [DOI] [PubMed] [Google Scholar]
- Lee U, Kim M, Lee K, Kaplan CM, Clauw DJ, Kim S, Mashour GA, Harris RE. 2018. Functional brain network mechanism of hypersensitivity in chronic pain. Sci Rep. 8(1):243. doi: 10.1038/s41598-017-18657-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leknes S, Berna C, Lee MC, Snyder GD, Biele G, Tracey I. 2013. The importance of context: when relative relief renders pain pleasant. Pain. 154:402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M, Su Q, Mouraux A, Iannetti GD. 2019. Spatial patterns of brain activity preferentially reflecting transient pain and stimulus intensity. Cereb Cortex. 29:2211–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist MA, Krishnan A, López-Solà M, Jepma M, Woo C-W, Koban L, Roy M, Atlas LY, Schmidt L, Chang LJ. 2017. Group-regularized individual prediction: theory and application to pain. NeuroImage. 145:274–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist MA, Loh JM, Atlas LY, Wager TD. 2009. Modeling the hemodynamic response function in fMRI: efficiency, bias and mis-modeling. NeuroImage. 45:S187–S198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loggia ML, Kim J, Gollub RL, Vangel MG, Kirsch I, Kong J, Wasan AD, Napadow V. 2013. Default mode network connectivity encodes clinical pain: an arterial spin labeling study. Pain. 154:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Solà M, Woo C-W, Pujol J, Deus J, Harrison BJ, Monfort J, Wager TD. 2017. Towards a neurophysiological signature for fibromyalgia. Pain. 158:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynall M-E, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U, Bullmore E. 2010. Functional Connectivity and Brain Networks in Schizophrenia. 30:9477–9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano H, Kotecha G, Leibnitz K, Matsubara T, Nakae A, Shenker N, Shibata M, Voon V, Yoshida W, Lee M. 2018. Classification and characterisation of brain network changes in chronic back pain: a multicenter study. Wellcome Open Res. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano H, Seymour B. 2015. Pain: a distributed brain information network? PLoS Biol. 13:e1002037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Baria AT, Tetreault P, Vachon-Presseau E, Chang P-C, Huang L, Apkarian AV, Baliki MN. 2016. Global disruption of degree rank order: a hallmark of chronic pain. Sci Rep. 6:34853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martucci KT, Ng P, Mackey S. 2014. Neuroimaging chronic pain: what have we learned and where are we going? Future Neurol. 9:615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R. 2005. Evolution of the neuromatrix theory of pain. The Prithvi Raj lecture: presented at the third world congress of world Institute of Pain, Barcelona 2004. Pain Pract. 5:85–94. [DOI] [PubMed] [Google Scholar]
- Melzack R, Wall PD. 1965. Pain mechanisms: a new theory. Science. 150:971–979. [DOI] [PubMed] [Google Scholar]
- Menon V. 2011. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 15:483–506. [DOI] [PubMed] [Google Scholar]
- Moriarty O, McGuire BE, Finn DP. 2011. The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol. 93:385–404. [DOI] [PubMed] [Google Scholar]
- Morsella E, Godwin CA, Jantz TK, Krieger SC, Gazzaley A. 2016. Homing in on consciousness in the nervous system: an action-based synthesis. Behav Brain Sci. 39: e168. [DOI] [PubMed] [Google Scholar]
- Mouraux A, Diukova A, Lee MC, Wise RG, Iannetti GD. 2011. A multisensory investigation of the functional significance of the “pain matrix”. NeuroImage. 54:2237–2249. [DOI] [PubMed] [Google Scholar]
- Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. 2010. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 62:2545–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman MEJ. 2002. Assortative mixing in networks. Phys Rev Lett. 89(2):208701. [DOI] [PubMed] [Google Scholar]
- Newman ME. 2006. Modularity and community structure in networks. Proc Natl Acad Sci. 103:8577–8582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyappan L, Park B, Balain V, Dangi R, Liddle P. 2015. Abnormalities in structural covariance of cortical gyrification in schizophrenia. Brain Struct Funct. 220:2059–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parente F, Frascarelli M, Mirigliani A, Di Fabio F, Biondi M, Colosimo A. 2018. Negative functional brain networks. Brain Imaging Behav. 12:467–476. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L. 2000. Functional imaging of brain responses to pain. A review and meta-analysis (2000). Clin Neurophysiol. 30:263–288. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JNP. 1999. Dissociating pain from its anticipation in the human brain. Science. 284:1979–1981. [DOI] [PubMed] [Google Scholar]
- Ploner M, Gross J, Timmermann L, Schnitzler A. 2006. Pain processing is faster than tactile processing in the human brain. J Neurosci. 26:10879–10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porro CA. 2003. Functional imaging and pain: behavior, perception, and modulation. Neuroscientist. 9:354–369. [DOI] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL. 2011. Functional network organization of the human brain. Neuron. 72:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Fair DA, Schlaggar BL, Petersen SE. 2010. The development of human functional brain networks. Neuron. 67:735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Schlaggar BL, Lessov-Schlaggar CN, SEJN P. 2013. Evidence for hubs in human functional brain networks. Neuron. 79:798–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD, McHaffie JG, Larson MA. 1989. Spatial summation of heat-induced pain: influence of stimulus area and spatial separation of stimuli on perceived pain sensation intensity and unpleasantness. J Neurophysiol. 62:1270–1279. [DOI] [PubMed] [Google Scholar]
- Rainville P. 2002. Brain mechanisms of pain affect and pain modulation. Curr Opin Neurobiol. 12:195–204. [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. 1997. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 277:968–971. [DOI] [PubMed] [Google Scholar]
- Ren W, Centeno MV, Berger S, Wu Y, Na X, Liu X, Kondapalli J, Apkarian AV, Martina M, Surmeier DJ. 2016. The indirect pathway of the nucleus accumbens shell amplifies neuropathic pain. Nat Neurosci. 19:220–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosvall M, Bergstrom CT. 2007. An information-theoretic framework for resolving community structure in complex networks. PNAS. 104:7327–7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Daw N, Jepma M, Wimmer GE, Wager TD. 2014. Representation of aversive prediction errors in the human periaqueductal gray. Nat Neurosci. 17:1607–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. 2010. Complex network measures of brain connectivity: uses and interpretations. NeuroImage. 52:1059–1069. [DOI] [PubMed] [Google Scholar]
- Schrouff J, Perlbarg V, Boly M, Marrelec G, Boveroux P, Vanhaudenhuyse A, Bruno M-A, Laureys S, Phillips C, Pélégrini-Issac M. 2011. Brain functional integration decreases during propofol-induced loss of consciousness. NeuroImage. 57:198–205. [DOI] [PubMed] [Google Scholar]
- Schwartz N, Miller C, Fields HL. 2017. Cortico-accumbens regulation of approach-avoidance behavior is modified by experience and chronic pain. Cell Rep. 19:1522–1531. [DOI] [PubMed] [Google Scholar]
- Seminowicz D, Mikulis D, Davis K. 2004. Cognitive modulation of pain-related brain responses depends on behavioral strategy. Pain. 112:48–58. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Davis KD. 2007. Pain enhances functional connectivity of a brain network evoked by performance of a cognitive task. J Neurophysiol. 97:3651–3659. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Moayedi M. 2017. The dorsolateral prefrontal cortex in acute and chronic pain. J Pain: Off J Am Pain Soc. 18:1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha Z, Xia M, Lin Q, Cao M, Tang Y, Xu K, Song H, Wang Z, Wang F, Fox PT. 2017. Meta-Connectomic analysis reveals commonly disrupted functional architectures in network modules and connectors across brain disorders. Cereb Cortex. 1–16. [DOI] [PubMed] [Google Scholar]
- Shen W, Tu Y, Gollub RL, Ortiz A, Napadow V, Yu S, Wilson G, Park J, Lang C, Jung M et al. 2019. Visual network alterations in brain functional connectivity in chronic low back pain: a resting state functional connectivity and machine learning study. NeuroImage: Clin. 22:101775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simony E, Honey CJ, Chen J, Lositsky O, Yeshurun Y, Wiesel A, Hasson U. 2016. Dynamic reconfiguration of the default mode network during narrative comprehension. Nat Commun. 7:12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O, Zwi JD. 2004. The small world of the cerebral cortex. Neuroinformatics. 2:145–162. [DOI] [PubMed] [Google Scholar]
- Steiger JH. 1980. Tests for comparing elements of a correlation matrix. Psychol Bull. 87:245. [Google Scholar]
- Tagliazucchi E, Balenzuela P, Fraiman D, Chialvo DR. 2010. Brain resting state is disrupted in chronic back pain patients. Neurosci Lett. 485:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanasescu R, Cottam WJ, Condon L, Tench CR, Auer DP. 2016. Functional reorganisation in chronic pain and neural correlates of pain sensitisation: a coordinate based meta-analysis of 266 cutaneous pain fMRI studies. Neurosci Biobehav Rev. 68:120–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tétreault P, Mansour A, Vachon-Presseau E, Schnitzer TJ, Apkarian AV, Baliki MN. 2016. Brain connectivity predicts placebo response across chronic pain clinical trials. PLoS Biol. 14:e1002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemann L, Hohn VD, Dinh ST, May ES, Nickel MM, Gross J, Ploner M. 2018. Distinct patterns of brain activity mediate perceptual and motor and autonomic responses to noxious stimuli. Nat Commun. 9:4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Boly M, Massimini M, Koch C. 2016. Integrated information theory: from consciousness to its physical substrate. Nat Rev Neurosci. 17:450. [DOI] [PubMed] [Google Scholar]
- Tracey I, Mantyh PW. 2007. The cerebral signature for pain perception and its modulation. Neuron. 55:377–391. [DOI] [PubMed] [Google Scholar]
- Treede R-D, Apkarian AV, Bromm B, Greenspan JD, Lenz FA. 2000. Cortical representation of pain: functional characterization of nociceptive areas near the lateral sulcus. Pain. 87:113–119. [DOI] [PubMed] [Google Scholar]
- Vachon-Presseau E, Tétreault P, Petre B, Huang L, Berger SE, Torbey S, Baria AT, Mansour AR, Hashmi JA, Griffith JW. 2016. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain. 139:1958–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuvel MP, Sporns O. 2013. Network hubs in the human brain. Trends Cogn Sci. 17:683–696. [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Lindquist MA, Roy M, Woo C-W, Kross E. 2013. An fMRI-based neurologic signature of physical pain. N Engl J Med. 368:1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Scott DJ, Zubieta J-K. 2007. Placebo effects on human μ-opioid activity during pain. Proc Natl Acad Sci. 104:11056–11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH. 1998. Collective dynamics of'small-world'networks. Nature. 393:440. [DOI] [PubMed] [Google Scholar]
- Wiech K. 2016. Deconstructing the sensation of pain: the influence of cognitive processes on pain perception. Science. 354:584–587. [DOI] [PubMed] [Google Scholar]
- Wiech K, Lin C-s, Brodersen KH, Bingel U, Ploner M, Tracey I. 2010. Anterior insula integrates information about salience into perceptual decisions about pain. J Neurosci. 30:16324–16331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C-W, Roy M, Buhle JT, Wager TD. 2015. Distinct brain systems mediate the effects of nociceptive input and self-regulation on pain. PLoS Biol. 13:e1002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C-W, Schmidt L, Krishnan A, Jepma M, Roy M, Lindquist MA, Atlas LY, Wager TD. 2017. Quantifying cerebral contributions to pain beyond nociception. Nat Commun. 8:14211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Lin Q, Han Z, He Y, YJN B. 2016. Intrinsic functional network architecture of human semantic processing: modules and hubs. NeuroImage. 132:542–555. [DOI] [PubMed] [Google Scholar]
- Yao Z, Hu B, Zheng J, Zheng W, Chen X, Gao X, Xie Y, Fang L, Initiative ADN. 2015. A FDG-PET study of metabolic networks in apolipoprotein E ε4 allele carriers. PLoS One. 10:e0132300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Davis JI, Ochsner KN. 2012. Overlapping activity in anterior insula during interoception and emotional experience. NeuroImage. 62:493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Ochsner KN, Hanelin J, Wager TD, Mackey SC. 2007. Different circuits for different pain: patterns of functional connectivity reveal distinct networks for processing pain in self and others. Soc Neurosci. 2:276–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Yao Z, Xie Y, Fan J, Hu B. 2018. Identification of Alzheimer's disease and mild cognitive impairment using networks constructed based on multiple morphological brain features. Biol Psychiatry Cogn Neurosci Neuroimaging. 3:887–897. [DOI] [PubMed] [Google Scholar]
- Zunhammer M, Bingel U, Wager TD. 2018. Placebo effects on the neurologic pain signature: a meta-analysis of individual participant functional magnetic resonance imaging data. JAMA Neurol. 75(11):1321–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


