Abstract
Background:
Paralysis of the diaphragm in newborn infants can lead to recurrent infections and life-threatening respiratory insufficiency. The clinical diagnosis of unilateral diaphragmatic paralysis has been reported in infants with laboratory evidence of congenital Zika virus infection and/or the congenital Zika syndrome (CZS) phenotype but no evaluation of phrenic nerve function has been described. All reported infants have had accompanying arthrogryposis. High infant mortality is reported.
Methods:
The causal mechanism of congenital diaphragmatic paralysis was evaluated in three infants with arthrogryposis as a manifestation of CZS (two of the three infants had laboratory evidence of ZIKV infection shortly after birth; the remaining infant had negative serology for ZIKV when first tested at 7 months of age). Electromyography and phrenic nerve compound muscle action potential (CMAP) were performed in all infants with diaphragmatic paralysis demonstrated on imaging studies.
Results:
All infants had evidence of moderate chronic involvement of peripheral motor neurons. Phrenic nerve CMAP was reduced on the side of the diaphragmatic paralysis in two infants and reduced bilaterally in the remaining infant who had primarily anterior involvement of the diaphragm. All three infants had multiple medical complications and one infant died at 18 months of age.
Conclusion:
Evaluation of three infants with CZS and diaphragmatic paralysis demonstrated phrenic nerve dysfunction. In these and other affected infants, arthrogryposis appears to be a constant co-occurring condition and health problems are significant; both conditions are likely due to involvement of the peripheral nervous system in some infants with CZS.
Keywords: arthrogryposis, congenital infection, diaphragmatic paralysis, phrenic nerve, Zika virus
1 |. INTRODUCTION
Since the first reports in September 2015 of newborns with microcephaly in Brazil, much has been learned about the causative agent, Zika virus (ZIKV), and the variability of the clinical phenotype in infants who are affected in utero (Schuler-Faccini et al., 2016). Arthrogryposis (multiple joint contractures) has been a low-frequency finding in infants with confirmed or suspected ZIKV infection (Sarno et al., 2016). Evaluations of affected infants suggest long-term involvement of central and peripheral motor neurons (van der Linden et al., 2016).
Paralysis of the diaphragm in newborn infants can lead to recurrent infections and life-threatening respiratory insufficiency (Kokatnur & Rudrappa, 2018). There are four reports of infants with laboratory evidence of congenital ZIKV infection and a clinical diagnosis of unilateral diaphragmatic paralysis (Melo et al., 2016; Meneses et al., 2017; Rajapakse et al., 2018; Souza et al., 2016); however, the mechanism of the diaphragmatic paralysis was not described in any of these infants. All affected infants had arthrogryposis with other features of CZS. Most of these infants died in the first year of life. We report the evaluation of three additional infants with arthrogryposis and diaphragmatic paralysis associated with congenital Zika syndrome (CZS) (Moore et al., 2016).
2 |. METHODS
Three infants with CZS were evaluated in Recife, Pernambuco, Brazil by a pediatric neurologist (VvdL) and colleagues. Each infant received a comprehensive evaluation including laboratory studies, clinical examination, neuroimaging, and neuro-logic diagnostic procedures. Microcephaly was defined as a head circumference (HC) exceeding two SD below the mean for gestational age and sex, according to the Fetal International and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st) (https://intergrowth21.tghn.org/) and for infants, the World Health Organization Child Growth Standards (www.who.int/childgrowth/en/). Testing for congenital ZIKV infection included conventional reverse transcription-polymerase chain reaction (RT-PCR) for detection of ZIKV RNA and ZIKV-specific immunoglobulin M (IgM) capture enzyme-linked immunosorbent testing. Testing for disease-specific IgM was done for five congenital infections that have been associated with congenital microcephaly—toxoplasmosis, cytomegalovirus, rubella, syphilis, and human immunodeficiency virus. Testing for two additional viruses (dengue and chikungunya) that can cause rash and fever in pregnancy, and rarely, congenital infection, was performed using conventional RT-PCR for the detection of dengue virus RNA and real-time RT-PCR for the detection of chikungunya virus RNA as well as disease-specific IgM for both. Congenital ZIKV infection was inferred if testing for ZIKV RNA or ZIKV-specific IgM was positive and testing for other congenital infections was negative. Imaging included a noncontrast computed tomography (CT) scan with or without noncontrast magnetic resonance imaging (MRI) of the brain, X-ray of the chest, and X-ray of the hips. Bilateral phrenic nerve conduction studies (NCS) and ultrasound-guided needle electromyography (EMG) of the diaphragm were also performed. Additional evaluation included an MRI of the spinal cord (one infant), CT scan of the chest (one infant), and ultrasound of the hips (one infant). The infants also had orthopedic evaluation. Comprehensive neurologic examinations with assessment of developmental milestones were performed during clinic visits. All infants underwent a sleep electroencephalogram (EEG) and one also had a video EEG.
The reporting of case histories was approved by the Osvaldo Cruz University Hospital (CAAE5283316.8.0000.5192), Recife, Pernambuco, Brazil and Association for Assistance of Disabled Children ethics committee (CAAE: 68400017.9.0000.0085). Parental informed consent was obtained for photography and inclusion of medical findings in scientific reports and presentations for each of the three infants. Based on the study methods, this work was deemed human subjects research without CDC engagement upon review by the human subjects contact for the National Center on Birth Defects and Developmental Disabilities.
3 |. RESULTS
All three infants (one female, two males) were term births (Table S1). The mothers of infant 1 and infant 2 reported a rash illness at 4 and 2 months’ gestation, respectively. Birth HC was more than 2 SD below the mean for infants 1 and 3 and slightly below the mean for infant 2. HC measurements for infants 2 and 3 were more than 3 SD and 5 SD below the mean at 19 months, respectively; infant 1 had a HC that measured 5 SD below the mean at 7 months. The cranial phenotype at birth varied as follows: normocephaly in infant 2, microcephaly in infant 1, and findings consistent with the fetal brain disruption sequence (Russell, Weaver, Bull, & Weinbaum, 1984) in infant 3.
Arthrogryposis was present in both upper and lower extremities in each infant (Figure S1). Diaphragmatic paralysis was unilateral and right-sided in two infants (infants 1 and 2) and bilateral in infant 3 although the anterior segments were more severely affected in this infant. Pneumonia was diagnosed in all three infants and was a presenting feature of the diaphragmatic paralysis in two infants. Of the two latter infants, one died at 18 months due to respiratory failure and the other infant had pneumonia twice and required noninvasive intermittent ventilation. Two infants required tube feedings and one had a gastrostomy placed. Infants 2 and 3 had Zika-specific immuno-globulin M (IgM) detected in cerebrospinal fluid (CSF) by 1 month of age. IgM results were negative at 7 months in CSF for infant 1 who was not tested in the first month of life; however, her phenotype was consistent with CZS and her mother was symptomatic. None of the three infants had ZIKV RNA detected.
Developmental milestones including visual tracking, interaction with the environment, social smile, head control, and sitting without support were not attained by infants 1 and 3 by age of last evaluation (i.e., 7 months in infant 1 and 19 months in infant 3) (Table S2). Infant 2 displayed visual tracking and social smile at 12 months but had not attained other tested developmental achievements at 19 months. Persistent primitive reflexes, nystagmus, and dysphagia were present in all three infants at time of last follow-up. Two of the three infants had epilepsy as deter-mined by clinical and EEG findings. Infant 1 had no report of seizure, but had an abnormal EEG with almost continuous focal discharges.
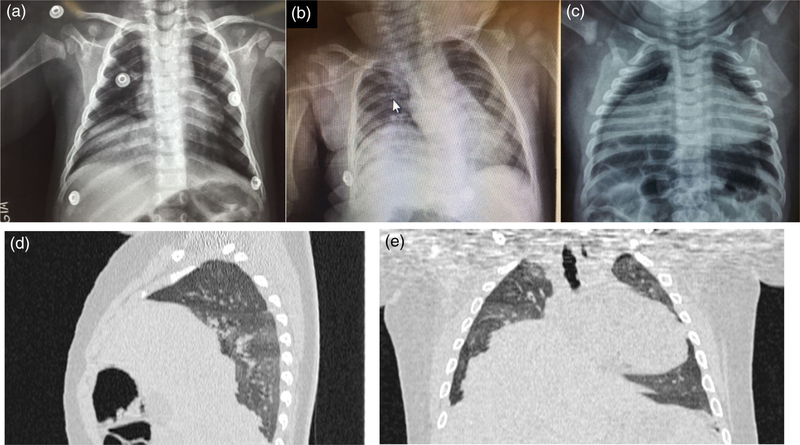
All infants had brain imaging (either CT or MRI) consistent with severe effects of congenital ZIKV infection including cortical loss, ventriculomegaly, and intracranial calcifications (Soares de Oliveira-Szejnfeld et al., 2016) (Table 1). Brainstem hypoplasia was noted in infants 2 and 3, and cerebellar hypoplasia with Dandy–Walker malformation was noted in infant 3 (Figure S2). Additionally, infant 2 had an MRI of the spinal cord which showed a thin cord in the thoracic region predominantly in the ventral aspect, reducing the ventral roots. Unilateral diaphragmatic paralysis was documented by chest X-ray in infants 1 and 2 and bilateral in infant 3 (Figure 1). NCS of the phrenic nerve showed reduced amplitudes of the compound muscle action potential on the right in infants 1 and 2 and bilaterally in infant 3. Ultrasound-guided needle EMG showed signs of chronic denervation of the diaphragm consistent with the pattern of the NCS. These findings are consistent with chronic involvement of peripheral motor neurons innervating the diaphragm. Chest CT scan indicated that the diaphragmatic paralysis was not uniform in the infant with bilateral involvement, with the anterior area appearing to be more affected than the posterior (Figure 1).
TABLE 1.
Imaging and neurologic studies findings in three infants with congenital Zika syndrome, arthrogryposis, and diaphragmatic paralysis—Recife, Brazil
| Evaluation | Infant 1 (7 months) | Infant 2 (19 months) | Infant 3 (19 months) |
|---|---|---|---|
| Brain imaging by CT scan/MRI | Cortical loss, moderate ventriculomegaly, intracranial calcifications | Cortical loss, mild ventriculomegaly, intracranial calcifications, brainstem hypoplasia; cortical dysplasia by MRI | Cortical loss, severe ventriculomegaly, intracranial calcifications, brainstem and cerebellar hypoplasia, Dandy–Walker malformation |
| Chest X-ray (CXR)/diaphragm ultrasound (USG)/CT scan | Paralysis of the right diaphragm by CXR and USG | Paralysis of the right diaphragm by CXR and USG | Paralysis of the diaphragm, bilateral; anterior more affected by CXR, USG, and CT scan |
| Spinal cord MRI | Not done | Thin cord thoracic region, ventral predominance | Not done |
| Electromyography | Moderate chronic involvement of peripheral motor neurons | Moderate chronic involvement of peripheral motor neurons | Moderate chronic involvement of peripheral motor neurons |
| Nerve conduction study—phrenic nerve compound muscle action potential | Reduced on right | Reduced on right | Reduced bilaterally |
| Hip X-ray/ultrasound | Bilateral hip dislocation | Bilateral hip dislocation | Bilateral hip dislocation |
| Electroencephalogram | Focal discharge, almost continuous, temporal bilateral | Focal discharge, frontal bilateral | Focal discharge, frontal bilateral |
FIGURE 1.
Chest imaging of three infants with congenital Zika syndrome, arthrogryposis and diaphragmatic paralysis, Brazil. (a) Infant 1 at age 7 months, chest X-ray shows elevation of the right hemidiaphragm. (b) Infant 2 at age 11 months, chest X-ray shows elevation of the right hemidiaphragm. (c) Infant 3 at age 8 months, chest X-ray shows elevation of the right and left hemidiaphragms. (d, e) Infant 3 at 11 months, chest CT shows heterogeneous diaphragmatic paralysis, with greater involvement of the right side and anterior area. CT, computed tomography
4 |. DISCUSSION
This report adds to our understanding of the causal mechanism of diaphragmatic paralysis in infants with arthrogryposis as a manifestation of CZS. All mammals have movement of the diaphragm in utero preparing the newborn to breathe effectively upon delivery (Greer, 2012). In experimental animals, control of this process has been shown to be the result of medullary centers which generate rhythmic bursts as well as interaction between the phrenic motor neuron and the muscles of the diaphragm (Greer, 2012). The phrenic nerve arises from the neck primarily from C3–C5 and receives innervation from both the cervical and brachial plexus (Kokatnur & Rudrappa, 2018).
Diaphragmatic paralysis in the neonate can be the result of phrenic nerve palsy in conjunction with brachial plexus palsy as a peripartum event (Bowerson, Nelson, & Yang, 2010); but, injury to the nerve during thoracic surgery is the most common cause of phrenic nerve palsy in infants (Kokatnur & Rudrappa, 2018). Diaphragmatic paralysis occurring secondary to an intrauterine event has rarely been documented and can be difficult to discern from a peripartum event; however, prenatal studies have documented onset before birth (Alamo, Gudinchet, & Meuli, 2015).
In published reports of diaphragmatic issues and infections, distinguishing between diaphragmatic eventration (i.e., aplasia or atrophy of central muscle fibers) and paralysis is challenging. The term “eventration” has been used to designate any elevation of the diaphragm whether due to aplasia or paralysis (Christensen, 1959; Tiryaki, Livanelioglu, & Atayurt, 2006). Two infants with diaphragmatic eventration reported in the 1970s had congenital cytomegalovirus (CMV) (Becroft, 1979; Wayne, Burrington, Myers, Cotton, & Block, 1973). In a 2010 report, congenital CMV was linked to diaphragmatic dysfunction in three infants (Izumi et al., 2010); these infants did not have phrenic NCS or EMG so distinction between eventration and paralysis of the diaphragm is unclear. Other congenital infections have not been implicated as causes of either diaphragmatic paralysis or eventration. Of note, infectious causes of acquired diaphragmatic paralysis include viruses within the same genus as ZIKV (i.e., Flavivirus) including dengue virus (Ratnayake, Shivanthan, & Wijesiriwardena, 2012) and West Nile virus (Rudrappa, Kokatnur, & Chernyshev, 2018), but also other infectious agents as the Borrelia burgdorferi bacterium (Reddy, McCannon, & Venna, 2015). This is the fifth report of associated diaphragmatic paralysis perhaps consistent with ZIKV’s ability to affect both the central and peripheral nervous systems.
The infants reported in the literature to date with arthrogryposis, diaphragmatic paralysis, and congenital ZIKV infection have been severely affected and fit the pattern of CZS. In addition, the phenotype has been quite similar. Congenital contractures have affected both the upper and lower extremities, and with the exception of infant 3 in this report, all have had unilateral and when described, right-sided paralysis of the diaphragm. Previous evaluation of infants with CZS and arthrogryposis but without diaphragmatic paralysis have shown involvement of both central and peripheral motor neurons by EMG (van der Linden et al., 2016), thinning of the spinal cord and reduction in the ventral roots on MRI of the spine (Aragao et al., 2017), and severe neuronal loss in the spinal cord with architectural distortion and microcalcifications by histopathological examination (Ramalho et al., 2017).
The co-occurrence of phrenic nerve paralysis and upper limb arthrogryposis likely is related to the close proximity and limited shared function of nerve roots in the cervical and brachial plexuses—C3–5 for the phrenic nerve and C5-TI for innervation of the upper limb. However, paralysis of the diaphragm is not a reported co-occurring condition with arthrogryposis in general; rare reports of arthrogryposis with other congenital infections have not included paralysis of the diaphragm as a co-occurring defect (Hall & Reed, 1982; Konstantinidou et al., 2007). Why these two manifestations co-occur in this particular congenital infection is unknown and why the effect appears to be predominantly right-sided also is not readily apparent. In the lower extremities, there is potential, yet unproven, peripheral nervous system involvement in the occurrence of neurogenic bladder with CZS (Costa Monteiro et al., 2019). Although neurogenic bladder has not been specifically linked to arthrogryposis in CZS, neurogenic bladder in children with the generic diagnosis of arthrogryposis multiplex congenita has been reported (Arantes de Araujo, Ferraz de Arruda Musegante, de Oliveira Damasceno, Barroso, & Badaro, 2013) and sacral nerve roots innervate both the bladder and lower extremities. In addition, ZIKV has been demonstrated to persist in multiple tissues including those of the peripheral nervous system in infected rhesus macaques (Hirsch et al., 2017).
This report is subject to at least two limitations. Despite negative ZIKV testing in her CSF, infant 1 is presumed to have CZS based on her clinical findings and maternal symptoms. ZIKV IgM has been shown to wane after the first few months of life in multiple studies (de Araujo et al., 2018; Krow-Lucal et al., 2018). In addition, these findings might not be generalizable to all similarly affected infants.
Clinical presentations in infants with isolated diaphragmatic paralysis vary widely from asymptomatic to significant respiratory distress, recurrent infection, ventilator dependence, and in some cases, death (Bowerson et al., 2010; Commare, Kurstjens, & Barois, 1994; Kokatnur & Rudrappa, 2018). The infants in this report had numerous medical complications and infant 1 died at age 18 months. As demonstrated in infant 3, the diaphragmatic paralysis can be bilateral and, if severe, affected infants might die shortly after birth and diaphragmatic paralysis could remain undetected.
5 |. CONCLUSION
This report supports the observation that arthrogryposis and diaphragmatic paralysis are associated with congenital ZIKV infection. In these infants, phrenic nerve dysfunction appears to be the causal mechanism for the diaphragmatic paralysis, providing additional evidence of peripheral nervous system involvement with congenital ZIKV infection. Recognizing this phenotype could benefit clinical management of infants with CZS (Adebanjo et al., 2017) and provide insight into the causes of early mortality due congenital Zika infection (Franca et al., 2016; Panchaud, Stojanov, Ammerdorffer, Vouga, & Baud, 2016).
Supplementary Material
ACKNOWLEDGMENTS
We appreciate the willingness of the families to have their child’s clinical description included in this report.
Footnotes
CONFLICT OF INTEREST
The authors have no potential conflict of interest or financial disclosures. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
DATA AVAILABILITY STATEMENT
Research data are not shared due to privacy or ethical restrictions.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- Adebanjo T, Godfred-Cato S, Viens L, Fischer M, Staples JE, Kuhnert-Tallman W, … Contributors. (2017). Update: Interim guidance for the diagnosis, evaluation, and management of infants with possible congenital Zika virus infection - United States, October 2017. MMWR. Morbidity and Mortality Weekly Report, 66 (41), 1089–1099. 10.15585/mmwr.mm6641a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alamo L, Gudinchet F, & Meuli R (2015). Imaging findings in fetal diaphragmatic abnormalities. Pediatric Radiology, 45(13), 1887–1900. 10.1007/s00247-015-3418-5 [DOI] [PubMed] [Google Scholar]
- Aragao M, Brainer-Lima AM, Holanda AC, van der Linden V, Vasco Aragao L, Silva Junior MLM, … Valenca MM (2017). Spectrum of spinal cord, spinal root, and brain mri abnormalities in congenital Zika syndrome with and without arthrogryposis. AJNR. American Journal of Neuroradiology, 38(5), 1045–1053. 10.3174/ajnr.A5125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arantes de Araujo L, Ferraz de Arruda Musegante A, de Oliveira Damasceno E, Barroso U Jr., & Badaro R. (2013). Investigation into neurogenic bladder in arthrogryposis multiplex congenita. Journal of Pediatric Urology, 9(6 Pt A), 895–899. 10.1016/j.jpurol.2012.12.011 [DOI] [PubMed] [Google Scholar]
- Becroft DM (1979). Prenatal cytomegalovirus infection and muscular deficiency (eventration) of the diaphragm. The Journal of Pedi-atrics, 94(1), 74–75. [DOI] [PubMed] [Google Scholar]
- Bowerson M, Nelson VS, & Yang LJ (2010). Diaphragmatic paralysis associated with neonatal brachial plexus palsy. Pediatric Neurology, 42(3), 234–236. 10.1016/j.pediatrneurol.2009.11.005 [DOI] [PubMed] [Google Scholar]
- Christensen P. (1959). Eventration of the diaphragm. Thorax, 14, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commare MC, Kurstjens SP, & Barois A. (1994). Diaphragmatic paralysis in children: A review of 11 cases. Pediatric Pulmonology, 18(3), 187–193. [DOI] [PubMed] [Google Scholar]
- Costa Monteiro LM, Cruz GNO, Fontes JM, de Araujo GF, Ventura T, Monteiro AC, & Moreira MEL (2019). Neurogenic bladder in the settings of congenital Zika syndrome: A confirmed and unknown condition for urologists. Journal of Pediatric Urology, pii:S1477–5131(19)30090–7. 10.1016/j.jpurol.2019.04.018 [DOI] [PubMed] [Google Scholar]
- de Araujo TVB, Ximenes RAA, Miranda-Filho DB, Souza WV, Montarroyos UR, de Melo APL, … State Health Department of Pernambuco. (2018). Association between microcephaly, Zika virus infection, and other risk factors in Brazil: Final report of a case-control study. The Lancet Infectious Diseases, 18(3), 328–336. 10.1016/S1473-3099(17)30727-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franca GV, Schuler-Faccini L, Oliveira WK, Henriques CM, Carmo EH, Pedi VD, … Victora CG (2016). Congenital Zika virus syndrome in Brazil: A case series of the first 1501 livebirths with complete investigation. Lancet, 388(10047), 891–897. 10.1016/S0140-6736(16)30902-3 [DOI] [PubMed] [Google Scholar]
- Greer JJ (2012). Control of breathing activity in the fetus and new-born. Comprehensive Physiology, 2(3), 1873–1888. 10.1002/cphy.c110006 [DOI] [PubMed] [Google Scholar]
- Hall JG, & Reed SD (1982). Teratogens associated with congenital contractures in humans and in animals. Teratology, 25(2), 173–191. 10.1002/tera.1420250207 [DOI] [PubMed] [Google Scholar]
- Hirsch AJ, Smith JL, Haese NN, Broeckel RM, Parkins CJ, Kreklywich C, … Streblow DN (2017). Zika virus infection of rhesus macaques leads to viral persistence in multiple tissues. PLoS Pathogens, 13(3), e1006219. 10.1371/journal.ppat.1006219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi K, Hokuto I, Yamaguchi S, Uezono A, Ikeda K, Rice L, … Craven DI (2010). Diaphragm dysfunction with congenital cytomegalovirus infection. Journal of Perinatology, 30(10), 691–694. 10.1038/jp.2010.65 [DOI] [PubMed] [Google Scholar]
- Kokatnur L, & Rudrappa M. (2018). Diaphragmatic palsy. Diseases, 6(1), pii:E16. 10.3390/diseases6010016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinidou A, Anninos H, Spanakis N, Kotsiakis X, Syridou G, Tsakris A, & Patsouris E. (2007). Transplacental infection of Coxsackievirus B3 pathological findings in the fetus. Journal of Medical Virology, 79(6), 754–757. 10.1002/jmv.20887 [DOI] [PubMed] [Google Scholar]
- Krow-Lucal ER, de Andrade MR, Cananea JNA, Moore CA, Leite PL, Biggerstaff BJ, … Group PMW (2018). Association and birth prevalence of microcephaly attributable to Zika virus infection among infants in Paraíba, Brazil, in 2015–16: A case-control study. The Lancet Child & Adolescent Health, 2, 205–213. [DOI] [PubMed] [Google Scholar]
- Melo AS, Aguiar RS, Amorim MM, Arruda MB, Melo FO, Ribeiro ST, … Tanuri A. (2016). Congenital Zika virus infection: Beyond neonatal microcephaly. JAMA Neurology, 73(12), 1407–1416. 10.1001/jamaneurol.2016.3720 [DOI] [PubMed] [Google Scholar]
- Meneses JDA, Ishigami AC, de Mello LM, de Albuquerque LL, de Brito CAA, Cordeiro MT, & Pena LJ (2017). Lessons learned at the epicenter of Brazil’s congenital Zika epidemic: Evidence from 87 confirmed cases. Clinical Infectious Dis-eases, 64(10), 1302–1308. 10.1093/cid/cix166 [DOI] [PubMed] [Google Scholar]
- Moore CA, Staples JE, Dobyns WB, Pessoa A, Ventura CV, Fonseca EB, … Rasmussen SA (2016). Characterizing the pattern of anomalies in congenital Zika syndrome for pediatric clinicians. JAMA Pediatrics, 171(3), 288–295. 10.1001/jamapediatrics.2016.3982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchaud A, Stojanov M, Ammerdorffer A, Vouga M, & Baud D. (2016). Emerging role of Zika virus in adverse fetal and neonatal outcomes. Clinical Microbiology Reviews, 29(3), 659–694. 10.1128/CMR.00014-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajapakse NS, Ellsworth K, Liesman RM, Ho ML, Henry N, Theel ES, … Meneses J. (2018). Unilateral phrenic nerve palsy in infants with congenital Zika syndrome. Emerging Infectious Diseases, 24(8), 1422–1427. 10.3201/eid2408.180057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalho FS, Yamamoto AY, da Silva LL, Figueiredo LTM, Rocha LB, Neder L, … Mussi-Pinhata MM (2017). Congenital Zika virus infection induces severe spinal cord injury. Clinical Infectious Diseases, 65(4), 687–690. 10.1093/cid/cix374 [DOI] [PubMed] [Google Scholar]
- Ratnayake EC, Shivanthan C, & Wijesiriwardena BC (2012). Diaphragmatic paralysis: A rare consequence of dengue fever. BMC Infectious Diseases, 12, 46 10.1186/1471-2334-12-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy KP, McCannon JB, & Venna N. (2015). Diaphragm paralysis in Lyme disease: Late occurrence in the course of treatment and long-term recovery. Annals of the American Thoracic Society, 12(4), 618–620. 10.1513/AnnalsATS.201501-070LE [DOI] [PubMed] [Google Scholar]
- Rudrappa M, Kokatnur L, & Chernyshev O. (2018). Neurological respiratory failure. Diseases, 6(1), pii:E7. 10.3390/diseases6010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell LJ, Weaver DD, Bull MJ, & Weinbaum M. (1984). In utero brain destruction resulting in collapse of the fetal skull, microcephaly, scalp rugae, and neurologic impairment: The fetal brain disruption sequence. American Journal of Medical Genetics, 17(2), 509–521. 10.1002/ajmg.1320170213 [DOI] [PubMed] [Google Scholar]
- Sarno M, Aquino M, Pimentel K, Cabral R, Costa G, Bastos F, & Brites C. (2016). Progressive lesions of central nervous system in microcephalic fetuses with suspected congenital Zika virus syndrome. Ultrasound in Obstetrics & Gynecology, 50 (2), 717–722. 10.1002/uog.17303 [DOI] [PubMed] [Google Scholar]
- Schuler-Faccini L, Ribeiro EM, Feitosa IM, Horovitz DD, Cavalcanti DP, Pessoa A, … Brazilian Medical Genetics Society-Zika Embryopathy Task Force. (2016). Possible association between Zika virus infection and microcephaly — Brazil, 2015. MMWR. Morbidity and Mortality Weekly Report, 65(3), 59–62. [DOI] [PubMed] [Google Scholar]
- Soares de Oliveira-Szejnfeld P, Levine D, Melo AS, Amorim MM, Batista AG, Chimelli L, … Tovar-Moll F. (2016). Congenital brain abnormalities and Zika virus: What the radiologist can expect to see prenatally and postnatally. Radiology, 281(1), 203–218. 10.1148/radiol.2016161584 [DOI] [PubMed] [Google Scholar]
- Souza ASR, Cordeiro MT, Meneses JDA, Honorato E, Junior EA, Castanha PMDS, … Dhalia R. (2016). Clinical and laboratory diagnosis of congenital Zika virus syndrome and diaphragmatic unilateral palsy: Case report. Revista Brasileira de Saúde Materno Infantil, 16(4), 467–473. [Google Scholar]
- Tiryaki T, Livanelioglu Z, & Atayurt H. (2006). Eventration of the diaphragm. Asian Journal of Surgery, 29(1), 8–10. 10.1016/S1015-9584(09)60285-2 [DOI] [PubMed] [Google Scholar]
- van der Linden V, Filho EL, Lins OG, van der Linden A, Aragao Mde F, Brainer-Lima AM, … Ramos RC (2016). Congenital Zika syndrome with arthrogryposis: Retrospective case series study. BMJ, 354, i3899. 10.1136/bmj.i3899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne ER, Burrington JD, Myers DN, Cotton E, & Block W. (1973). Letter: Bilateral eventration of the diaphragm in a neonate with congenital cytomegalic inclusion disease. The Journal of Pediatrics, 83(1), 164–165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.