Abstract
Objective:
Durability of transcatheter aortic valves (TAV) remains their greatest disadvantage given that fixed tissue leaflets are not immune to structural degeneration from calcification and thrombosis. Therefore, a second intervention is necessary especially that TAV in low-risk patients showed non-inferior outcomes compared with surgery. This study aims at assessing hemodynamic and turbulent properties of the flow downstream different TAV-in-TAV configurations to offer basic hemodynamic guidance for future interventions when currently implanted valves structurally degrade.
Methods:
Six TAV-in-TAV configurations were chosen: (1)23mm Evolut in 26mm Evolut; (2)23mm Evolut in 23mm SAPIEN3; (3)26mm Evolut in 26mm Evolut; (4)26mm Evolut in 23mm SAPIEN3; (5)23mm SAPIEN3 in 26mm Evolut and (6)23mm SAPIEN3 in 23mm SAPIEN3. Their hemodynamic performance was assessed in a pulse duplicator for 100 cycles. High-speed imaging and particle image velocimetry were performed to assess turbulence. Effective orifice areas (EOA), pinwheeling indices (PI) and Reynolds Shear Stresses (RSS)were evaluated.
Results:
The largest EOA was obtained with 23mm SAPIEN in 26mm Evolut (2.07±0.06cm2) and the smallest with 23mm Evolut in 23mm SAPIEN (1.50±0.04cm2)(p<0.001); the highest PI was obtained with SAPIEN-in-SAPIEN(26.5±2.00%) and the lowest with 26mm Evolut-in-26mm Evolut (7.5±1.6%)(p<0.01); at peak systole the least detrimental RSS range was obtained with 23mm Evolut-in-26mm Evolut (up to around 340Pa) and the highest with 23mm Evolut-in-SAPIEN (around 900Pa)(p<0.01).
Conclusions:
This study shows that best hemodynamic parameters are TAV (implanted and to be implanted) specific. In addition, it shows that RSS levels, which are indicative of turbulence levels – associated with blood damage - are 2-3 folds higher post-TAV-in-TAV.
Keywords: TAVR, leaflet thrombosis, hemolysis, TAV-in-TAV, Turbulence
Graphical Abstract

Hemodynamic parameters and turbulent stresses results after implantation of a second transcatheter aortic valve in a failed or degenerated transcatheter aortic valve. The selection of the appropriate valve type depends strongly on the valve that is already in place and the interaction between both implanted and already existing valve. While the effective orifice areas were close between every arrangement, regurgitant fractions differed and so did the pinhweeling indices. The Reynolds shear stresses, which reflect the turbulence levels induced and correlate with blood damage in literature, were also a function of the valve combinations. Further clinical studies are necessary to develop selection guidelines based on patient-specific considerations. Results were presented ± standard deviations. TAV denotes transcatheter aortic valve and RSS denotes Reynolds shear stresses (Pa).
Introduction
Transcatheter aortic valve (TAV) replacement (TAVR) therapy became the standard of care for high and intermediate risk patients and recently for low-risk patients with the recent expansion of indication by the FDA (1-3). Despite the superior or non-inferior hemodynamic performance of TAVR compared to surgical aortic valve replacement (4,5), durability remains the greatest disadvantage given the degeneration of the biological leaflets including mainly calcification and thrombosis (6,7).
A recent clinical study by Yanagisawa et al(8) examining leaflet thrombosis formation after TAVR showed that early leaflet thrombosis occurred in 9.3% of 485 patients causing a higher mean pressure gradient at discharge compared to patients who did not show evidence of leaflet thrombosis(8). In addition, leaflet thrombosis occurred again within a three-year period. A recent publication by Chakravarty et al also suggested that the risk of leaflet thrombosis after TAVR is significant and higher than surgical bioprosthetic valve and therefore may result in an earlier risk of structural valve degeneration(9).
This relatively rapid degeneration risk of TAVs and the potential expansion of TAV application for low-risk patients in the future will probably lead them to undergo another TAVR procedure after degeneration of the originally implanted valve. TAV-in-TAV procedures are mostly currently performed to mitigate sub-optimal outcomes such as aortic regurgitation (10,11). Whether TAV-in-TAV as the next step after degeneration of the implanted TAV would yield optimal or favorable hemodynamic performance remains unknown and requires further investigation.
The implantation of a TAV modifies the hemodynamic characteristics downstream of the valve along with the sinus flow (12-15). Particularly, it was shown that the resulting non-physiological turbulent flow is TAV type and design dependent(12) and the turbulent stresses induced can be correlated to the initiation of platelet activation and hemolysis – both phenomena identified clinically(9,16).
Based on these, it may be hypothesized that TAV-in-TAV will produce a further increase in turbulent stresses and knowledge of which TAV in TAV combination produces the least increase in these stresses may be of importance in the context of the durability.
The objective of this study is to assess the hemodynamic properties of the flow downstream different TAV-in-TAV configurations in order to not only evaluate feasibility of this alternative for future therapies, but also provide guidance with respect to how hemodynamic performance and turbulence vary between these many possible combinations.
Methods
Valve Selection and Hemodynamic Assessment
To evaluate post-TAVR hemodynamics and turbulence, a combination of six TAVs were chosen to be deployed as shown in Figure 1: (1a) 23mm Evolut-in-26mm Evolut; (1b) 23mm Evolut-in-23mm SAPIEN 3; (1c) 26mm Evolut-in-26mm Evolut; (1d) 26mm Evolut-in-23mm SAPIEN 3; (1e) 23mm SAPIEN3-in-26mm Evolut and (1f) 23mm SAPIEN 3-in-23mm SAPIEN 3. The hemodynamic performance of the abovementioned TAV combinations was assessed in a pulse duplicator left heart simulator flow loop, as previously described(17-19), under physiological conditions of 120/80mmHg pressures, 4L/min cardiac output and 60 beats per minute heart rate. A hundred consecutive cardiac cycles of aortic pressure, pressure gradient, and flow rate data were recorded at a sampling rate of 100 Hz. The working fluid in this study was a blood analogue mixture of water and glycerin of density 1060 kg/m3 and a kinematic viscosity of 3.5×10−6 m2/s.
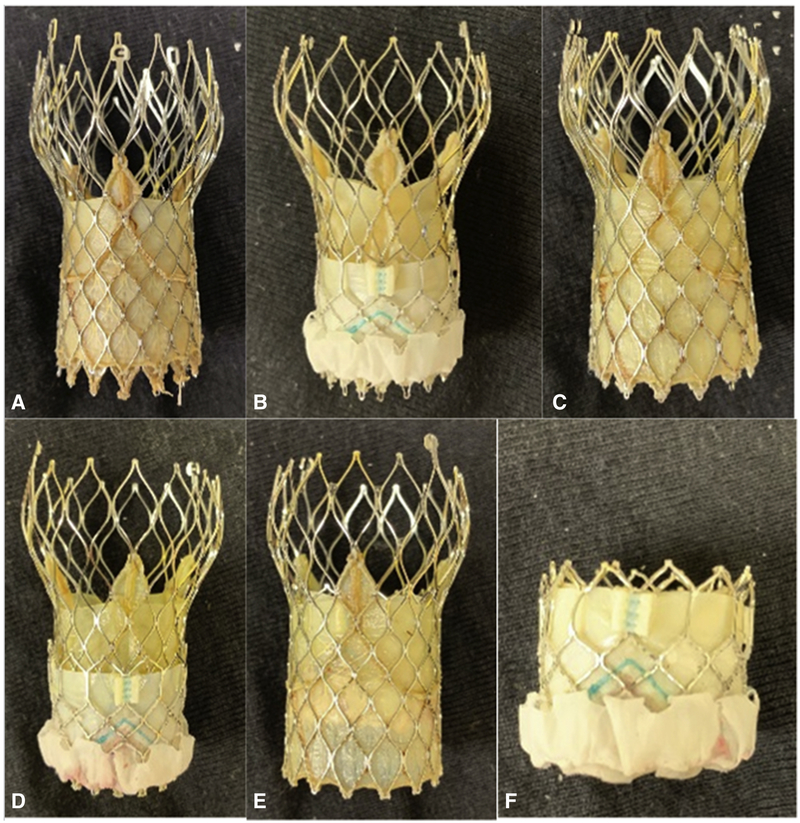
Figure 1:
Image showing the different TAV-in-TAV configurations that were tested in-vitro for hemodynamic performance and turbulence evaluation. The configurations were as follows: (a) Evolut 23-in-Evolut 26; (b) Evolut 23-in-SAPIEN 3 23; (c) Evolut 26-in-Eolut 26; (d) Evolut 26-in-SAPIEN 3 23; (e) SAPIEN 3 23-in-Evolut 26 and (d) SAPIEN 3 23-in-SAPIEN 3 23.
The mean transvalvular pressure gradient (PG) is defined as the average of positive pressure difference between the ventricular and aortic pressure curves during forward flow. The effective orifice area (EOA) is an important parameter to evaluate valve orifice opening. It is an indication of energy and a measure of how well a valve utilizes its orifice area(20). The larger the orifice area the more efficient the valve is and the less it impedes the flow. EOA was computed using the Gorlin’s equation:
| (1) |
Where Q represents the root mean square aortic valve flow over the same averaging interval of the PG.
Regurgitant fraction (RF) is the percentage of total fluid volume through the valve per beat owing to the retrograde flow from the overall forward flow(20). The larger the RF, the more the indication that the valve is regurging. RF is computed based on the equation below:
| (2) |
The pinwheeling index (PI) is an indication with implications on leaflet durability and resilience (21-23). Based on ISO heart valve guidelines(17), localized leaflet material bending, or pinwheeling, should be avoided due to correlation with premature tissue degradation. The larger the pinwheeling index, the more severe the twisting that exists in the leaflets post-deployment. It is computed from still frames obtained from high-speed imaging during diastole as follows and in accordance with previous publications(13,24):
| (3) |
Where Lactual represents the deflected free edge of the leaflet and Lideal represents the unconstrained ideal configuration of the leaflet free edge.
Particle Image Velocimetry (PIV)
Particle image velocimetry (PIV) is a high-fidelity fluid mechanic technique utilized to visualize the flow, obtain instantaneous velocity measurements and flow properties. For PIV, the flow was seeded with fluorescent PMMA-Rhodamine B particles with average diameter of ~10 μm. For all cases, the velocity field within the distal flow region was measured using high spatial and temporal resolution. Briefly, this involved illuminating the flow region using a laser sheet created by pulsed Nd:YLF single cavity diode pumped solid state laser coupled with external spherical and cylindrical lenses; while acquiring high-speed images of the fluorescent particles within the region. Time-resolved PIV images were acquired with a resulting spatial and temporal resolutions of 0.0532mm/pixel and 500Hz respectively. Phase locked measurements were recorded for 3 phases of the cardiac cycle (acceleration, peak and deceleration) repetitively over 250 cycles each with a spatial resolution of 0.0532mm/pixel. Refraction was corrected using a calibration in DaVis particle image velocimetry software (DaVis 7.2, LaVision Germany). Velocity vectors were calculated using adaptive cross-correlation algorithms. Further details of PIV measurements can be found in Hatoum et al(12,19,25,26).
Vorticity Dynamics
Using the velocity measurements from PIV, vorticity dynamics were also evaluated downstream of the valve. Vorticity is the curl of the velocity field and therefore captures the local rotational components of the blood flow shearing as well as visualizing turbulent eddies. Regions of high vorticity along the axis perpendicular to the plane indicate both shear and rotation of the fluid particles. High levels of fluctuating vorticity indicate elevated flow turbulence. Vorticity was computed using the following equation:
| (4) |
Where ωz is the vorticity component with units of s−1; U and V are the x and y components of the velocity vector with units of m/s. The x and y directions are axial and lateral respectively with the z direction being out of measurement plane.
Reynolds shear stress (RSS)
Reynolds shear stress has been widely correlated to platelet activation(27,28). It is a statistical quantity that measures the shear stress between fluid layers when fluid particles decelerate or accelerate while changing direction(29).
| (5) |
Where ρ is the blood density and u′ and v′ are the instantaneous velocity fluctuations in the x and y directions respectively.
Statistics
Statistical analysis in this study was performed using JMP Pro version 13.0.0 (SAS Institute Inc, Cary, NC). All data are presented as mean ± standard deviation. For data that were found to follow the normal distribution (based on Kolmogorov-Smirnov test with p-value > 0.05), ANOVA followed by the t-test between each pair were performed. For the data that do not follow the normal distribution, the non-parametric Kruskal–Wallis test was performed followed by Man-Whitney test for the direct comparisons between each pair. The data are now graphically represented using the box and whisker plot format. P-value < 0.05 was considered statistically significant. Analyses were performed over 100 replicates for pressure gradient, effective orifice area and regurgitant fractions and 6 replicates for the pinwheeling index.
Results
Hemodynamic Parameters
Supplementary Table 1 shows the hemodynamic parameters obtained post TAV-in-TAV. Supplementary Figure 1 shows the graphical representation (box and whisker plot) of the instantaneous results obtained for each group of TAV-in-TAV.
The pressure gradients from lowest to highest were obtained as follows with (1) 23mm SAPIEN in 26mm Evolut (6.35±0.29mmHg), (2) 23mm Evolut in 26mm Evolut (6.58±0.30mmHg), (3) 26mm Evolut in 23mm SAPIEN (6.69±0.27mmHg), (4) 26mm Evolut in 26mm Evolut (8.93±0.45mmHg), (5) 23mm SAPIEN in 23mm SAPIEN (9.86±0.51mmHg) and (6) 23mm Evolut in 23mm SAPIEN (9.95±0.39mmHg). The differences between all these groups are significant (p<0.05) as shown in Supplementary Table 1.
The EOAs from largest to lowest were obtained as follows with (1) 23mm SAPIEN in 26mm Evolut (2.07±0.06cm2), (2) 23mm Evolut in 26mm Evolut (2.04±0.07cm2), (3) 26mm Evolut in 23mm SAPIEN (1.89±0.06cm2), (4) 26mm Evolut in 26mm Evolut (1.88±0.04cm2), (5) 23mm SAPIEN in 23mm SAPIEN (1.66±0.06cm2) and (6) 23mm Evolut in 23mm SAPIEN (1.50±0.04cm2). The differences between all these groups are significant (p<0.0001) except for 26mm Evolut in 26mm Evolut and 26mm Evolut in 23mm SAPIEN (p=0.16), 23mm SAPIEN in 26mm Evolut and 26mm Evolut in 23mm SAPIEN (p = 0.006) and 23mm SAPIEN in 26mm Evolut and 23mm Evolut in 26mm Evolut (p = 0.1498) as shown in Supplementary Table 1. The RFs from largest to lowest were obtained as follows with (1) 26mm Evolut in 26mm Evolut (22.07±0.54%), (2) 23mm SAPIEN in 26mm Evolut (10.21±0.81%), (3) 26mm Evolut in 23mm SAPIEN (9.40±0.99%), (4) 23mm SAPIEN in 23mm SAPIEN (9.37±0.93%), (5) 23mm Evolut in 23mm SAPIEN (8.74±0.95%) and (6) 23mm Evolut in 26mm Evolut (8.18±1.01%). The differences between all these groups are significant (p<0.01) except for 26mm Evolut in 23mm SAPIEN and 23mm SAPIEN in 23mm SAPIEN (p=0.8871) as shown in Supplementary Table 1.
Pinwheeling (PI)
Video 1 shows the opening and closing of the valve leaflets for the different valve cases and Figure 2 shows still frames of the leaflets closed at diastole. From Supplementary Table 1, the highest PI was obtained with SAPIEN-in-SAPIEN (26.5±2.00%) followed by 23mm Evolut-in-SAPIEN (24.5±1.10%), then by 26mm Evolut-in-SAPIEN (18.2±5.1%), then by SAPIEN-in-Evolut (13.0±4.1%), then 23mm Evolut-in-26mm Evolut (11.3±2.6%) and the smallest PI was with 26mm Evolut-in-26mm Evolut(7.5±1.6%). Supplementary Table 1 shows how significant the differences in PI between each pair of TAV-in-TAV are.
Figure 2:
En-face imaging of the valves at different phases in the cardiac cycle while deployed in the left heart simulator in-vitro setup. The views correspond to the following TAV-in-TAV arrangements: (a) Evolut 23-in-Evolut 26; (b) Evolut 23-in-SAPIEN 23; (c) Evolut 26-in-Evolut 26; (d) Evolut 26-in-SAPIEN 23; (e) SAPIEN 23-in-Evolut 26 and (f) SAPIEN 23-in-SAPIEN 23.
Flow Velocity Field
Video 2 shows the main flow streaks of the different valves combinations. Figure 3 shows the phase averaged velocity vectors and vorticity contours at different phases (acceleration, peak and deceleration) throughout the cardiac cycle. The dark layers of red and blue vorticity contours represent the shear layers corresponding to the jet boundaries and the distance between them represents the width of the jet.
Figure 3:
Main jet hemodynamics shown in-vitro using Particle Image Velocimetry for every TAV-in-TAV arrangement. Phase averaged velocity vectors and vorticity contours at different phases in the cardiac cycle are shown.
As noted from the figure, the largest distance between shear layers corresponds to 23mm Evolut-in-26mm Evolut (15.57±0.32mm) and the smallest distance corresponds to 23mm Evolut-in-SAPIEN (10.17±0.35mm). For the remaining cases, going from highest to lowest, the distances between shear layers were as follows: 12.47±0.35, 12.17±0.21, 12.07±0.25 and 10.97±0.12mm for 26mm Evolut-in-26mm Evolut, 26mm Evolut-in-SAPIEN, SAPIEN-in-26mm Evolut and SAPIEN-in-SAPIEN respectively.
Moreover, specifically noticeable at peak, diffusion is more obvious with the cases where Evoluts are implanted compared with the cases where SAPIEN is the implanted valve (first 4 rows of Figure 3 compared to the last 2 rows). This turbulent flow dies out quickly after peak systole is achieved and the deceleration part starts.
The smallest velocity was obtained with SAPIEN-in-26mm Evolut (reaching 2.0±0.03m/s), 26mm Evolut-in-26mm Evolut (reaching a maximum of 2.05±0.03m/s) followed by then by 23mm Evolut-in-26mm Evolut(reaching 2.08±0.06m/s) then by SAPIEN-in-SAPIEN(reaching 2.36±0.03m/s), then 26mm Evolut-in-SAPIEN(reaching 2.42±0.01m/s) and the highest range of velocities was obtained with 23mm Evolut-in-SAPIEN(reaching 2.63±0.11m/s). The difference between the highest and the lowest velocities was significant (p<0.01).
Reynolds Shear Stress (RSS)
Figure 4 shows the principal RSS contours at different phases in the cardiac cycle for every valve combination. It is quite remarkable that the highest fluctuations of RSS along with their highest magnitudes are shown wherever the Evolut valve is present in the arrangement, particularly as an implanted valve. To quantify them, Figure 5 shows the probability distribution of RSS in acceleration, peak and deceleration.
Figure 4:
Principal Reynolds shear stress (RSS) at different phases in the cardiac cycle for the different TAV-in-TAV arrangement. The higher the RSS the more turbulent the flow downstream of the valves is.
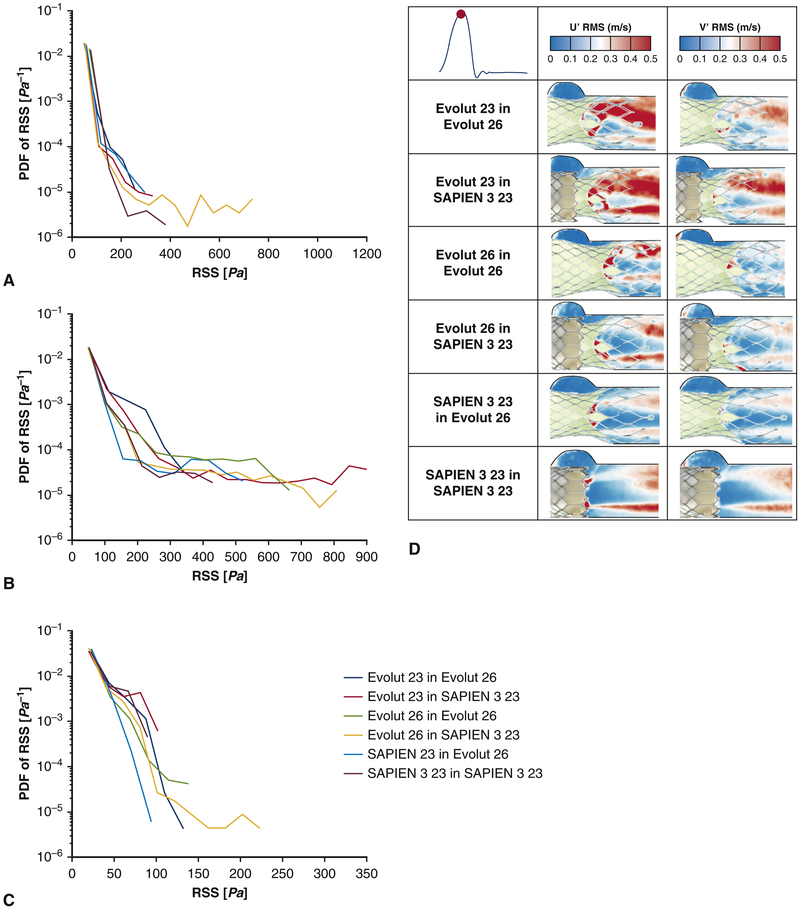
Figure 5:
Probability density function in semi-log scale of varying principal Reynolds Shear Stress (RSS) distribution values during (a) acceleration, (b) peak systole and (c) deceleration for the different TAV-in-TAV configurations. The larger the range of RSS and the higher the probability for elevated RSS values, the higher the turbulence induced is. (d) Root mean square velocity fluctuations U' and V' for the different valve models at peak systole. The larger the fluctuations, the more the flow is governed by instabilities.
At peak systole in Figure 5b, the smallest range of RSS over the main flow region is obtained with 23mm Evolut-in-26mm Evolut(up to around 340 Pa) however this region is characterized by the highest probabilities of having RSS between around 120 and 300Pa as displayed on the contour plots of Figure 4. The largest range of RSS over the same region is obtained with 23mm Evolut-in-SAPIEN(reaching around 900Pa). 26mm Evolut-in-SAPIEN shows the second largest RSS range (reaching around 800Pa), followed by 26mm Evolut-in-26mm Evolut(reaching around 660Pa), then by SAPIEN-in-26mm Evolut(reaching around 530Pa) then by SAPIEN in SAPIEN reaching around 420Pa.
This order is not maintained during acceleration and deceleration as shown in Figures 5a and 5c. 26mm Evolut-in-SAPIEN displays the largest RSS ranges during acceleration and deceleration (reaching around 750 and 220Pa respectively).
Turbulent Velocity Fluctuations
Figure 5d shows the root mean square (rms) velocity fluctuations U' and V' for the different valve models at peak systole and Figure 6 shows a plot of the vertical component of the velocity fluctuation (V’) versus the horizontal component (U’). This plot will help not only quantify the fluctuations but also help determine based on the concentration and dispersion of the points an estimation of turbulence ranking and type (isotropic or anisotropic) based on how U’rms and V’rms are correlated. If the points are all close together then the turbulence is close to being isotropic and homogeneous. More dispersed points mean strong anisotropic turbulence.
Figure 6:
Plots showing the fluctuations of velocity at peak systole in the y plotted versus the fluctuations in the x direction for (a) Evolut 23-in-Evolut 26; (b) Evolut 23-in-SAPIEN 23; (c) Evolut 26-in-Evolut 26; (d) Evolut 26-in-SAPIEN 23; (e) SAPIEN 23-in-Evolut 26 and (f) SAPIEN 23-in-SAPIEN 23. The more spread-out the plots are, the more they inform about turbulence nature downstream of the valves.
Fluctuations in both U and V are obviously more concentrated with the 23mm Evolut implanted in 26mm Evolut (~0.65m/s and ~0.48m/s respectively). When the 23mm Evolut is implanted in SAPIEN, there is more fluctuation in U’ than in V’ (1.3m/s and 0.58m/s respectively). For the case of SAPIEN in SAPIEN and SAPIEN in Evolut, the outlying fluctuating points are higher with the SAPIEN in 26mm Evolut as shown in Figure 6e and 6f. Similarly, more concentrated fluctuation plots are obtained with 26mm Evolut-in-SAPIEN compared to 26mm Evolut-in-26mm Evolut.
Discussion
In this study, an evaluation of the hemodynamic behavior and turbulence induced downstream 6 different TAV-in-TAV arrangements were assessed and related to the potential of blood damage. While all these combinations may be realizable in-vitro, several factors may come to play in-vivo that would make some of them implementable and others not.
Hemodynamic Performance
The effective orifice area is a measure of energy loss, thus large EOAs signify a smaller energy loss(20). Compared to pressure gradient, EOA is a measure that is less dependent on flow differences(30). As shown in the results, the effective orifice areas were highest with the 26mm Evolut being the original “host” valve while the smaller EOAs are found with the TAV-in-TAV cases being with 23mm SAPIEN as the original implanted valve. The reason may be because of the nitinol frame of the Evolut with self-expandable properties that allow the originally implanted TAV to further expand once another gets deployed in. This result is also reflected in smaller magnitudes of main flow velocities for these three cases as highlighted in the results of Figure 3.
The implantation of 23mm SAPIEN in another one of the same size yielded an EOA lower than that obtained with an implanted 26mm Evolut in SAPIEN 3. This may be due to the smaller thickness of the Evolut stent in addition to the exclusion of the skirt as is in the case of a SAPIEN 3. The smallest EOA was found with 23mm Evolut implanted in 23mm SAPIEN. This may be due to the probable inaccurate valve sizing choice as a fit in a 23mm SAPIEN. While the 23mm Evolut was fully expanded, there was more room left which was limited by the smaller size of 23mm Evolut.
The overall results from this study indicate that the possibility of patient-prosthesis mismatch (PPM) that is defined as an effective orifice area that is too small in relation to body size(14), may not be high but needs to be considered in certain combination of TAV-in-TAV cases. It is particularly true that the indexed effective orifice area (EOAI) of the initial implantation of either SAPIEN or Evolut in clinical setting may be smaller than that of this experimental study because significant calcification of native aortic valves may narrow the actual EOAI.
The abovementioned observations came in accordance with the vorticity contour profiles. Based on the distances between the shear layers – which are the size of the jet, an indication of the size of the vena contracta, also represented by EOA(18) – as reported in the results, the higher distances were found with the TAV-in-TAVs that have the 26mm Evolut as originally implanted.
From a regurgitation perspective, the TAV-in-TAV arrangements with SAPIEN being the originally implanted valve yield in most cases lower regurgitation fractions. The skirt that envelops the bottom of the SAPIEN valve plays a role in decreasing regurgitation compared to Evolut. However for both valves, regurgitation levels were mild(31).
These results demonstrate that the effectiveness and goodness of hemodynamic performance is valve-specific whether implanted or to be implanted.
It is important to note that while no sizing or selection guideline is yet available for the most optimal TAV-in-TAV combination, some of the cases studied here may not be achievable or viable in-vivo. In valve-in-valve procedures, where a TAV is implanted in a surgical aortic valve (SAV), usually the TAV diameter is larger than the true internal diameter of the SAV. In addition, usually the self-expandable TAVs necessitate a smaller annulus compared to a balloon-expandable TAV of the same size. Stemming from these concepts, the viability of implanting a 23mm Evolut in a 26mm Evolut or a 23mm SAPIEN remains unknown and requires further clinical investigation. In this study, these test cases were included for completion and for an overall presentation of the highest number of possible combinations.
Pinwheeling and durability
It was shown that when another TAV is implanted in SAPIEN, pinwheeling is highest. This is due to the large radial force exerted by the “rigid” SAPIEN on the implanted TAV. In literature, elevated PIs are often associated with low durability, and therefore accelerated failure of valve leaflets(21).
Assessment of the Induced Turbulence and Incidence of Blood Damage
The importance of turbulence assessment stems from its relationship with blood damage (32-34). The implantation of a medical device leads to non-physiological blood flow characterized by non-physiological turbulence levels(35,36). Smith et al. reported that red blood cell and platelet damage, which eventually lead to thrombosis, are caused by mechanical stresses of turbulence(37). Kawase and Moo-Young(38) studied cell damage in bioreactors and highlighted the impact of turbulent flow field on cell survival. These studies concluded that if the small scale turbulent eddies are equal in size or smaller than the blood cells, they can transfer energy to these cells, which can cause blood damage. In spite of the different previous in-vitro studies that investigated the risk of blood damage, thresholds are not well-established, and indeed the characterization of turbulent stress is still controversial(36). The level of turbulence generated downstream any valve could be determined by the type of the valve (12,17,19,36), and clinical studies highlighted different levels of blood damage (expressed in thrombus formation and hemolysis) based on valve type (8,39).
Compared to a recent study performed under the same conditions as this one, only using a SAPIEN 3 and an Evolut (12), this study shows that the magnitudes of RSS were increased by 2 and 3-folds. The peak RSS values obtained with one Evolut TAV were 337.22±7.05 Pa and with SAPIEN 157.91±1.80Pa. Thus, TAV-in-TAV arrangement may lead to higher turbulence than that obtained with only one TAV in place. Despite the absence of an official anti-coagulation regimen, thrombosis whether clinical or subclinical was detected after TAVR(9) and so was hemolysis(39).
As discussed for EOA results, implanting a second TAV in 26mm Evolut yielded lower RSS than the cases where TAVs were implanted in SAPIEN. The compromised EOA and higher velocities obtained with TAV-in-SAPIEN cases may have played a role in increasing turbulence for these cases. Having said that, SAPIEN-in-SAPIEN yielded lower magnitudes of RSS nevertheless showing that despite the lower EOA, the short profile (mesh-free) of the SAPIEN valve may have played a positive role in helping with minimizing turbulent diffusivity(12). Studies have shown that the presence of grids enhances turbulence and promotes rapid axial diffusion and velocity fluctuations(40), highlighting the advantage of the SAPIEN short profile. The leaflet flutter that was observed in Video 1 with the 23mm and 26mm Evolut implanted in SAPIEN also indicates enhanced turbulence for these cases. Leaflet flutter also occurs with Evolut-in-Evolut only it is not as significant as that obtained with Evolut-in-SAPIEN. A probable cause may be due to the supra-annular design of the Evolut leaflets, that when implanted in an intra-annular leaflet design valve are not well supported all around compared to a supra-annular design where they can rest against the originally implanted Evolut leaflets.
While this study controlled the receiving TAV to be either a 26mm Evolut or a 23mm SAPIEN 3, it is important to comment on the applicability of our results to receiving TAVs of other sizes. As our study showed that implanting the specified TAVs in a 26mm Evolut or a 23mm SAPIEN 3 does not necessarily compromise the effective orifice areas generated, and may actually provide less regurgitation as is currently the case clinically, and may lead to increased turbulence. We expect the same conclusions to be largely applicable for all receiving TAV sizes. However, receiving TAVs of smaller sizes may need to be further explored for patient prosthesis mismatch (particularly for TAV in a ViV scenario). For receiving TAVs with larger orifices, we expect less hemodynamic concerns with respect to gradients. With respect to turbulence, similar flow velocities in a larger orifice will yield a dynamically similar and proportional modifications in turbulent stresses.
Further in-vivo and clinical studies are necessary to generate recommendations based on patient outcomes as the selection process depends on many more considerations. Examples of these considerations, particularly anatomical, are important when choosing both the original valve type as well as the best choice of valve for TAV in TAV. These include, among others, the risk of acute coronary obstruction for instance, as well as a potential need for subsequent coronary access, especially in low risk younger patients who may not initially have but then subsequently develop coronary obstructive disease.
Limitations
This study was performed using the original Evolut valve. Further studies are needed to evaluate the impact and the differences induced with the newer generations (R and PRO). In addition, the study imposed a certain heart rate of 60 beats per minute. Further evaluation is required to test several different heart rates and assess the resulting variations.
Conclusion
In this study, the hemodynamic parameters along with the turbulence induced downstream of the TAV-in-TAV arrangement were assessed. Given the relatively short durability of TAVs and the anticipated approval of TAVR as appropriate therapy for low-risk patients, TAV-in-TAV seems to be the plausible future therapy. As summarized in Figure 7 (graphical abstract), this study shows that hemodynamic parameters are valve (implanted and to be implanted) specific. In addition, it shows that RSS levels, which are indicative of turbulence levels are 2-3 folds higher, compared to a traditional TAVR. Further clinical studies are necessary to develop selection guidelines based on patient-specific considerations.
Central figure.

Importance of TAV-in-TAV choice to produce lowest turbulent stresses and best hemodynamics
Supplementary Material
Supplementary Table 1: Average hemodynamic parameters of the different TAV-in-TAV configurations. Values reported are ± standard deviation. P-values showing the significance in differences between each pair of the groups. P-values lower than 0.05 were considered significant.
Supplementary Figure 1: Box and whisker graphical representation of the instantaneous results obtained for each group with (a) pressure gradients results, (b) effective orifice area results, (c) regurgitation fractions results, and (d) pinwheeling index results.
Video 1: En-face imaging views of the valve arrangement throughout the cardiac cycle.
Video 2: Main flow streak visualization of the different valves.
Central Message.
Appropriate valve type selection depends strongly on host valve already in place and interaction between both host and guest valves.
Perspective statement.
This study aims at assessing hemodynamic and turbulent properties of the flow downstream different TAV-in-TAV configurations in order to evaluate options for future interventions when current TAVs undergo structural valve failure. The best hemodynamic parameters are TAV (implanted and to be implanted) specific and more turbulence is obtained overall compared to a traditional TAVR
Acknowledgments
Sources of Funding
The research done was partly supported by National Institutes of Health (NIH) under Award Number R01HL119824 and the American Heart Association (AHA) under Award Number 19POST34380804.
Abbreviation List
- TAVR
Transcatheter aortic valve replacement
- EOA
Effective Orifice Area
- PI
Pin-wheeling Index
- RSS
Reynolds Shear Stress
Footnotes
Disclosures
Dr. Dasi reports having patent applications filed on novel polymeric valves, vortex generators and superhydrophobic/omniphobic surfaces. Dr. Crestanello reports having grants from Medtronic, Boston Scientific, and St. Jude in addition to being part of the Medtronic advisory board. No other conflicts were reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Waksman R, Rogers T, Torguson R et al. Transcatheter aortic valve replacement in low-risk patients with symptomatic severe aortic stenosis. Journal of the American College of Cardiology 2018;72:2095–2105. [DOI] [PubMed] [Google Scholar]
- 2.Waksman R, Corso PJ, Torguson R et al. Transcatheter Aortic Valve Replacement in Low-Risk Patients: One-Year Results from the LRT Trial. JACC: Cardiovascular Interventions 2019. [DOI] [PubMed] [Google Scholar]
- 3.FDA. FDA expands indication for several transcatheter heart valves to patients at low risk for death or major complications associated with open-heart surgery. 2019.
- 4.Mack MJ, Leon MB, Thourani VH et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. New England Journal of Medicine 2019. [DOI] [PubMed] [Google Scholar]
- 5.Søndergaard L, Ihlemann N, Capodanno D et al. Durability of transcatheter and surgical bioprosthetic aortic valves in patients at lower surgical risk. Journal of the American College of Cardiology 2019;73:546–553. [DOI] [PubMed] [Google Scholar]
- 6.Sellers SL, Turner CT, Sathananthan J et al. Transcatheter aortic heart valves: histological analysis providing insight to leaflet thickening and structural valve degeneration. JACC: Cardiovascular Imaging 2019;12:135–145. [DOI] [PubMed] [Google Scholar]
- 7.Blackman DJ, Saraf S, MacCarthy PA et al. Long-Term Durability of Transcatheter Aortic Valve Prostheses. Journal of the American College of Cardiology 2019;73:537–545. [DOI] [PubMed] [Google Scholar]
- 8.Yanagisawa R, Tanaka M, Yashima F et al. Early and Late Leaflet Thrombosis After Transcatheter Aortic Valve Replacement: A Multicenter Initiative From the OCEAN-TAVI Registry. Circulation: Cardiovascular Interventions 2019;12:e007349. [DOI] [PubMed] [Google Scholar]
- 9.Chakravarty T, Søndergaard L, Friedman J et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. The Lancet 2017;389:2383–2392. [DOI] [PubMed] [Google Scholar]
- 10.Nijhoff F, Agostoni P, Amrane H et al. Transcatheter aortic valve implantation in patients with severe aortic valve stenosis and large aortic annulus, using the self-expanding 31-mm Medtronic CoreValve prosthesis: First clinical experience. The Journal of thoracic and cardiovascular surgery 2014;148:492–499. e1. [DOI] [PubMed] [Google Scholar]
- 11.Unbehaun A, Pasic M, Buz S et al. Transapical aortic valve implantation in patients with poor left ventricular function and cardiogenic shock. The Journal of thoracic and cardiovascular surgery 2014;148:2877–2882. e1. [DOI] [PubMed] [Google Scholar]
- 12.Hatoum H, Yousefi A, Lilly S, Maureira P, Crestanello J, Dasi LP. An in vitro evaluation of turbulence after transcatheter aortic valve implantation. The Journal of thoracic and cardiovascular surgery 2018;156:1837–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatoum H, Dollery J, Lilly SM, Crestanello J, Dasi LP. Impact of patient-specific morphologies on sinus flow stasis in transcatheter aortic valve replacement: an in vitro study. The Journal of thoracic and cardiovascular surgery 2019;157:540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dasi LP, Hatoum H, Kheradvar A et al. On the mechanics of transcatheter aortic valve replacement. Annals of biomedical engineering 2017;45:310–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatoum H, Moore BL, Maureira P, Dollery J, Crestanello JA, Dasi LP. Aortic sinus flow stasis likely in valve-in-valve transcatheter aortic valve implantation. The Journal of thoracic and cardiovascular surgery 2017;154:32–43. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eleid MF, Cabalka AK, Malouf JF, Sanon S, Hagler DJ, Rihal CS. Techniques and outcomes for the treatment of paravalvular leak. Circulation: Cardiovascular Interventions 2015;8:e001945. [DOI] [PubMed] [Google Scholar]
- 17.Hatoum H, Dollery J, Lilly SM, Crestanello J, Dasi LP. Impact of patient-specific morphologies on sinus flow stasis in transcatheter aortic valve replacement: An in vitro study. The Journal of thoracic and cardiovascular surgery 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatoum H, Dollery J, Lilly SM, Crestanello JA, Dasi LP. Implantation Depth and Rotational Orientation Effect on Valve-in-Valve Hemodynamics and Sinus Flow. The Annals of thoracic surgery 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatoum H, Maureira P, Dasi LP. A turbulence in vitro assessment of On-X and St Jude Medical prostheses. The Journal of thoracic and cardiovascular surgery 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoganathan AP, He Z, Casey Jones S. Fluid mechanics of heart valves. Annu Rev Biomed Eng 2004;6:331–362. [DOI] [PubMed] [Google Scholar]
- 21.Martin C, Sun W. Simulation of long-term fatigue damage in bioprosthetic heart valves: effects of leaflet and stent elastic properties. Biomechanics and modeling in mechanobiology 2014;13:759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doose C, Kütting M, Egron S et al. Valve-in-valve outcome: design impact of a pre-existing bioprosthesis on the hydrodynamics of an Edwards Sapien XT valve. European Journal of Cardio-Thoracic Surgery 2016:ezw317. [DOI] [PubMed] [Google Scholar]
- 23.Gunning PS, Saikrishnan N, Yoganathan AP, McNamara LM. Total ellipse of the heart valve: the impact of eccentric stent distortion on the regional dynamic deformation of pericardial tissue leaflets of a transcatheter aortic valve replacement. Journal of The Royal Society Interface 2015;12:20150737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Midha PA, Raghav V, Condado JF et al. Valve type, size, and deployment location affect hemodynamics in an in vitro valve-in-valve model. JACC: Cardiovascular Interventions 2016;9:1618–1628. [DOI] [PubMed] [Google Scholar]
- 25.Hatoum H, Dollery J, Lilly SM, Crestanello JA, Dasi LP. Sinus hemodynamics variation with tilted transcatheter aortic valve deployments. Annals of biomedical engineering 2019;47:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hatoum H, Moore BL, Dasi LP. On the significance of systolic flow waveform on aortic valve energy loss. Annals of biomedical engineering 2018;46:2102–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giersiepen M, Wurzinger L, Opitz R, Reul H. Estimation of shear stress-related blood damage in heart valve prostheses--in vitro comparison of 25 aortic valves. The International journal of artificial organs 1990;13:300–306. [PubMed] [Google Scholar]
- 28.Nygaard H, Giersiepen M, Hasenkam J et al. Two-dimensional color-mapping of turbulent shear stress distribution downstream of two aortic bioprosthetic valves in vitro. Journal of biomechanics 1992;25:437–440. [DOI] [PubMed] [Google Scholar]
- 29.Gunning PS, Saikrishnan N, McNamara LM, Yoganathan AP. An in vitro evaluation of the impact of eccentric deployment on transcatheter aortic valve hemodynamics. Annals of biomedical engineering 2014;42:1195–1206. [DOI] [PubMed] [Google Scholar]
- 30.Bech-Hanssen O, Caidahl K, Wallentin I, Ask P, Wranne B. Assessment of effective orifice area of prosthetic aortic valves with Doppler echocardiography: an in vivo and in vitro study. The Journal of thoracic and cardiovascular surgery 2001;122:287–295. [DOI] [PubMed] [Google Scholar]
- 31.Nishimura RA, Otto CM, Bonow RO et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology 2014;63:e57–e185. [DOI] [PubMed] [Google Scholar]
- 32.Yen J-H, Chen S-F, Chern M-K, Lu P-C. The effect of turbulent viscous shear stress on red blood cell hemolysis. Journal of Artificial Organs 2014;17:178–185. [DOI] [PubMed] [Google Scholar]
- 33.Morshed KN, Bark D Jr, Forleo M, Dasi LP. Theory to predict shear stress on cells in turbulent blood flow. PloS one 2014;9:e105357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kameneva MV, Burgreen GW, Kono K, Repko B, Antaki JF, Umezu M. EFFECTS OF TURBULENT STRESSES ON MECHANICAL HEMOLYSIS: EXPERIMENTAL AND COMPUTATIONAL ANALYSIS. ASAIO journal (American Society for Artificial Internal Organs: 1992) 2004;50:418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sotiropoulos F, Le TB, Gilmanov A. Fluid mechanics of heart valves and their replacements. Annual Review of Fluid Mechanics 2016;48:259–283. [Google Scholar]
- 36.Hatoum H, Dasi LP. Reduction of pressure gradient and turbulence using vortex generators in prosthetic heart valves. Annals of biomedical engineering 2019;47:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith RL, Blick EF, Coalson J, Stein PD. Thrombus production by turbulence. Journal of applied physiology 1972;32:261–264. [DOI] [PubMed] [Google Scholar]
- 38.Kawase Y, Moo-Young M. Mathematical models for design of bioreactors: Applications of: Kolmogoroff's theory of isotropic turbulence. The Chemical Engineering Journal 1990;43:B19–B41. [Google Scholar]
- 39.Laflamme J, Puri R, Urena M et al. Incidence and risk factors of hemolysis after transcatheter aortic valve implantation with a balloon-expandable valve. The American journal of cardiology 2015;115:1574–1579. [DOI] [PubMed] [Google Scholar]
- 40.Yang SK, Chung MK. Turbulent flow through mixing spacer grids in rod bundles. 1995 national heat transfer conference: Proceedings Volume 14: Thermal hydraulics of advanced nuclear reactors, containment thermal-hydraulics; HTD Volume 316, 1995. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Average hemodynamic parameters of the different TAV-in-TAV configurations. Values reported are ± standard deviation. P-values showing the significance in differences between each pair of the groups. P-values lower than 0.05 were considered significant.
Supplementary Figure 1: Box and whisker graphical representation of the instantaneous results obtained for each group with (a) pressure gradients results, (b) effective orifice area results, (c) regurgitation fractions results, and (d) pinwheeling index results.
Video 1: En-face imaging views of the valve arrangement throughout the cardiac cycle.
Video 2: Main flow streak visualization of the different valves.