Abstract
Background
Breast cancer is the most common cancer types among women. Recent researches have focused on determining the efficiency of alternative molecules and miRNAs in breast cancer treatment. The aim of this study was to determine the effect of usnic acid response-miR-185-5p on proliferation in the breast cancer cell and to determine its relationship with apoptosis pathway.
Methods
The cell proliferation and cell apoptosis rate were significantly increased following the ectopic expression of miR-185-5p in BT-474 cells. Furthermore, the results of cell cycle assay performed by flow cytometry revealed that the transfection with miR-185-5p induced G1/S phase arrest. The apoptosis-related genes expression analysis was performed by qRT-PCR and the direct target of miR-185-5p in BT-474 cells was identified by western blot and luciferase reporter assay.
Results
Our data showed that miR-185-5p can cause significant changes in apoptosis-related genes expression levels, suggesting that cell proliferation was suppressed by miR-185-5p via inducing apoptosis in breast cancer cells. According to western blot results, miR-185-5p lead to decrease BCL2 protein level in BT-474 cells and direct target of miR-185-5p was identified as BCL by luciferase reporter assay.
Conclusion
This study revealed that miR-185-5p may be an effective agent in the treatment of breast cancer.
Keywords: miR-185-5p, Usnic acid, Breast cancer, Apoptosis, BCL2
Background
Breast cancer is the most common cancer among women and morbidity of this disease increases every year in the world. Breast cancer is a heterogeneous disease with different histological and molecular characteristics according to the data obtained from gene expression analysis. The surgery, chemotherapy, radiation and the combination of these therapeutic approaches are used in cancer patients for cancer management and therapy stage. Each of the therapy method has some advantages and disadvantages. Chemotherapies have been determined to be effective in the early stages of breast cancer by interfering with DNA synthesis and tumor replication. Besides, chemotherapies play a role as adjuvant therapy to reduce micrometastases and cancer recurrence or to reduce tumor as a neoadjuvant [1]. The endocrine, chemotherapeutic and biological therapies which include Trastuzumab, Pertuzumab, Lapatinib, and Neratinib are used to produce a positive effect and a reduced death rate for breast cancer patients [2]. But, the traditional non-surgical therapies are associated with significant toxicity and some individuals due to drug resistance [2, 3]. The novel molecules derived from biological origin such as lichens can kill the cancer cells by the induction of apoptosis with the minimum side effect on non-cancerous cells. Induction of apoptosis is one of the most important steps of cytotoxic anticancer agents. Novel drug molecules induce apoptosis pathways that are blocked in cancer cells by manipulating different mechanisms in cancer cells [4, 5]. Therefore, the development of novel molecular targeted therapies is required for many patients as different therapeutic options [2].
Natural compounds derived from plants, fungi, and lichens have long been a source of anticancer compounds for cancer treatments for many years. The valuable compounds obtained from biological organisms are generally low in cost, plentiful in nature, and cause little toxicity or side effects in applications. Usnic acid is well-known as medicinal lichen secondary metabolite and is a potentially interesting candidate to use in cancer treatment [6]. The activity of usnic acid has been investigated in various pathways including cytotoxicity, regulation of the cell cycle, stimulation of cell death, modulating mechanisms of immune activity, angiogenesis and energy metabolism [7]. Usnic acid can inhibit tumor cell growth, metastasis, and proliferation, and induce the apoptosis of different cancer cells through a signaling cascade of death receptor-mediated extrinsic and mitochondria-mediated intrinsic caspase pathways [8, 9]. Besides, usnic acid has a high anti-proliferative effect against cancer cells, whereas it has little effect on normal epithelial cells [6, 10]. This indicates that usnic acid may be a very important therapeutic molecule in cancer treatment.
Recent advances promise hope for cancer treatment by regulating the gene expression of non-coding small RNAs. These developments aim to provide new therapeutic approaches for cancer treatment and to expand a new method to inhibit cancer by miRNA [11, 12]. MicroRNAs (miRNAs) are endogenous, highly conserved, and small noncoding RNAs of about 22 nucleotides in length that regulate the gene expression at the posttranscriptional level [13]. Since avoiding from apoptosis is a critical characteristics of malignant tumors, miRNAs are well known to have key roles in apoptosis regulation [14, 15]. miRNAs as candidate therapeutic molecules suppress tumor formation by regulating apoptosis and this needs to be addressed [2]. Given that most chemotherapeutic drugs kill cancer cells through apoptosis and miRNAs are also involved in the regulation of apoptosis, it is obvious that miRNAs can also be an effective target for cancer therapies [2]. The number of miRNA profiling studies have rapidly increased in recent decades. Many studies have demonstrated that miRNAs play key roles in the occurrence and progression of malignancies including breast cancer [16, 17]. Moreover, several studies have revealed that the dysregulation of miRNA expression is involved in cancer, suggesting their function as either oncogene (oncomir’s) or tumor suppressor in a tissue-specific manner [18]. Pre-clinic and clinic studies include the evaluation of miRNAs as therapeutic agents in recent years. Numerous preclinical studies have revealed miR-34a as a tumor suppressor is against various types of cancers through targeting of many oncogenic pathways [19]. Phase I clinical study on the safety, pharmacokinetics, and effectiveness of a liposome-encapsulated synthetic miR-34a mimic, namely MRX34, was conducted in patients with advanced solid tumors [20, 21].
Unlike chemically synthesized drugs, the effect of usnic acid on breast cancer treatment was investigated at the molecular (miRNA) level in the previous studies [22]. Kılıç et al. investigated the anti-proliferative effect of usnic acid, an alternative drug candidate molecule, on different breast cancer cell lines (MDA-MB-231, BT-474 and MCF-7). The inhibitory effect of usnic acid on cell growth was determined in breast cancer cell lines and various miRNAs that respond to usnic acid in three different breast cancer cells were identified by miRNA microarray analysis. With bioinformatics and validation studies, the most effective miRNA associated with BT-474 cell line treated with usnic acid was determined as miR-185-5p in our previous study [21]. According to Kılıç et al. studies, the miRNA targets of usnic acid were mostly found to play role in Hedgehog signaling pathway, Transforming growth factor beta (TGF-Beta), Mitogen-activated protein kinase (MAPK) and apoptosis pathways [21]. In the light of these data, this is the first study which give comprehensive information about the changes in the expression level of miRNAs following the application of usnic acid to breast cancer cells, apoptosis pathways targeted by these miRNAs and how these events can be exploited in breast cancer cells.
In the current study, we investigated the effects of overexpression of miR-185-5p on proliferation and apoptosis in a breast cancer cell in vitro. This is the first study that the efficiency of miR-185-5p on cell apoptosis was investigated in BT-474 breast cancer cell line.
Materials and methods
Cell culture
Human breast cancer BT-474 cell line and human breast epithelial MCF-12A cell line obtained from ATCC. BT-474 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma, USA) with high glucose containing 10% Fetal Bovine Serum (FBS) (Biological Industries, Israel) and 1% penicillin/streptomycin (Biowest, USA) at incubated 37 °C, 5% CO2. MCF-12A cells were maintained in DMEM Ham’s F12 (Sigma, USA) including 10% FBS, 1% penicillin/streptomycin, l-glutamine, 10 μg/ml insulin, 20 ng/ml Epidermal Growth Factor (EGF) (Goquick) and 0.5 mg/ml hydrocortisone (PubChem) at 37 °C with 5% CO2.
Cell viability and proliferation analysis
Cell viability of BT-474 and MCF-12A was determined by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay (Sigma) according to the manufacturer’s protocol. 1 × 105 cells per well were seeded in 96 well-plate and after 24 h, cells transfected with miRNA mimics and scramble in different concentrations of 0, 10, 15, 25 and 50 nM. After 24, 48, and 72 h incubation time, 10 µl MTT (5 mg/ml) was added to each well and the plates were incubated at 37 °C for 4 h. After 4 h incubation, dimethyl sulfoxide (DMSO) of 100 µl was added to each sample and the absorbance was measured at 570 nm using microplate reader (PerkinElmer 1420 Multilabel Counter, USA). Each experiment was performed three times.
miRNA transfection
miRNA mimics and scramble transfection were carried out by HiPerfect Transfection Reagent (Qiagen, Germany) according to the manufacturer’s protocol. miRNA mimics and scramble were separately transfected to BT-474 and MCF-12A cells in the final concentration of 25 nM. These transfected cells were incubated for 48 h and then cells were collected for further investigations.
The validation of transfection
miR-185-5p (5′UGGAGAGAAAGGCAGUUCCUGA3′) transfection into cells was validated by using qRT-PCR. The thermal cycling parameters used for amplification were as follows: initial activation at 95 °C for 15 min, followed by 40 cycles at 94 °C for 15 s, 55 °C for 30 s, and 70 °C for 30 s. The relative expression level of miR-185-5p was calculated by using 2−ΔΔCt formula, normalizing with RNU-6 (5′CGCAAGGATGACACGCAAATTC3′) housekeeping gene.
Cell-cycle analysis
BT-474 cells (5 × 105)/well were seeded into six-well plates. After 48 h transfection, harvested cells were washed with cold PBS and fixed with 70% ethanol at − 20 °C for at least 2 h. Propidium iodide (PI)/RNase staining buffer (BD Biosciences, USA) was used to analyze cell cycle. 1 × 106 cells were resuspended in 500 µl PI/RNase staining buffer and the cells were incubated 15 min at room temperature and analyzed by flow cytometry at 488 nm (BD Accuri Plus, US). The cells used in the control and experimental group were synchronized to perform cell cycle assay.
Apoptosis detection assay
Cell apoptosis assay was performed Annexin V-FITC/PI apoptosis assay kit (Invitrogen, USA). Briefly, BT-474 and MCF-12A cells (5 × 105)/well were seeded into six-well plates. After 48 h miRNA transfection process, treated cells were harvested and washed twice with cold PBS. The treated cells were resuspended with 1 µl YO-PRO and 1 µl PI and incubated in ice for 30 min. Cell apoptosis rate was measured with flow cytometry (BD Accuri Plus, US).
Quantification of mRNA with reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA extraction from transfected and non-transfected cells was performed using Genezol Reagent (Geneaid, Taiwan), in accordance with the manufacturer’s protocol. NG dART RT kit (EURx, Poland) was used to reverse transcription according to the manufacturer’s instructions. The reverse transcription reaction conditions were 10 min at 25 °C, 30 min at 48 °C, and a final step of 5 min at 95 °C. The level of gene expression was determined by Human Apoptosis Primer Library (Real Time Primers, US), containing 88 apoptosis-related genes and 8 housekeeping genes. All qRT-PCR experiments were repeated three times.
Western blot analysis
Cultured cells in the six-well plate were harvested and washed with cold phosphate-buffered saline (PBS). The protein isolation from cell pellets was carried out using lysis buffer containing 7 M Urea, 2 M Thiourea, 4% Chaps, 1% dichlorodiphenyltrichloroethane (DDT), 2% Ampholyte and supplemented with protease inhibitor. The protein amounts for each sample were quantified using BCA™ Protein Assay Kit (Merck, Germany) and an equal amount of protein from each sample was separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After the separation process, proteins were transferred onto a nitrocellulose membrane (Thermo Fisher Scientific, USA), followed by blocking for 1 h with 5% nonfat milk. The membrane was incubated at 4 °C overnight with diluted anti-BCL2 primary antibodies (Santa Cruz Biotechnology, USA) and anti-β-Actin primary antibodies (FineTest, China) as a control. Subsequently, the membranes were washed with Tris-Buffered Saline Tween 20 (TBST) buffer for 3 times. After washing, the membranes were incubated with horseradish peroxide (HRP) conjugated secondary antibodies (Abcam, US) at room temperature for 1.5 h. Finally, the chemiluminescence kit (Thermo Fisher Scientific, USA) was used to visualize immunoreactive bands and evaluated with ImageJ program.
Luciferase reporter assay
BT-474 cells (3 × 104 cells/well) were seeded into a 96-well plate and cultured for 24 h. Subsequently, the cells were co-transfected with miR-185-5p (Qiagen, USA) and luciferase reporter plasmid containing 3′UTR of BCL2 (OriGene, USA) and transfected with luciferase reporter plasmid as a control group by using Lipofectamine 2000 (Thermo Fisher Scientific, USA) according to manufacturer’s protocol. After 24 h transfection, luciferase assay was performed using Luciferase Assay Systems (Promega, USA) and luciferase activity was measured by a luminometer (PerkinElmer 1420 Multilabel Counter, USA).
miRNA target prediction
miRSystem (http://mirsystem.cgm.ntu.edu.tw/), miRDB (http://mirdb.org/), and DIANA (http://diana.imis.athena-innovation.gr/DianaTools/index.php) bioinformatics tools were used to predict in silico miRNA targets and 3′UTR BCL2 site binding with seed region was determined by miRmap (https://mirmap.ezlab.org/) bioinformatics tool.
Statistical analysis
Data are presented as the means ± (SD) from at three separate experiments of the apoptosis, cell cycle assay, western blot, and luciferase assay analysis. Differences between experiment and control group were defined with two-tailed Student’s t-tests and one-way ANOVA. p values ˂ 0.05 were considered significant. For qRT-PCR analysis, GAPDH was used as housekeeping gene and qRT-PCR data was analyzed according to the ΔΔCT method and SPSS16.0 software. Graphpad Prism 8.0 software was used to represent data on graph.
Results
miR-185-5p suppresses proliferation of BT-474 cells in a dose-dependent manner
To investigate the effect of miR-185-5p on the cell proliferation of BT-474 and MCF-12A, the miR-185-5p transfection was performed in the different concentrations (0, 10, 15, 25 and 50 nM) for 24, 48 and 72 h and then MTT assay was carried out. Subsequently, the most effective concentration of miR-185-5p for BT-474 cells was determined as 25 nM at 48 h (Fig. 1a). Unlike, we observed that there was no significant reduction of cellular viability in MCF-12A under the different concentrations (Fig. 1b). 25 nM miR-185-5p was able to inhibit BT-474 cell viability by about 15% compared with the control group and scramble (p < 0.0001) (Fig. 2a). On the other hand, the ectopic expression of 25 nM miR-185-5p in the MCF-12A cells resulted in a reduction of cell viability by 3.5% compared with the control group (Fig. 2b). As a result of this data, we indicated that miR-185-5p has an anti-proliferative effect on BT-474 breast cancer cell viability.
Fig. 1.
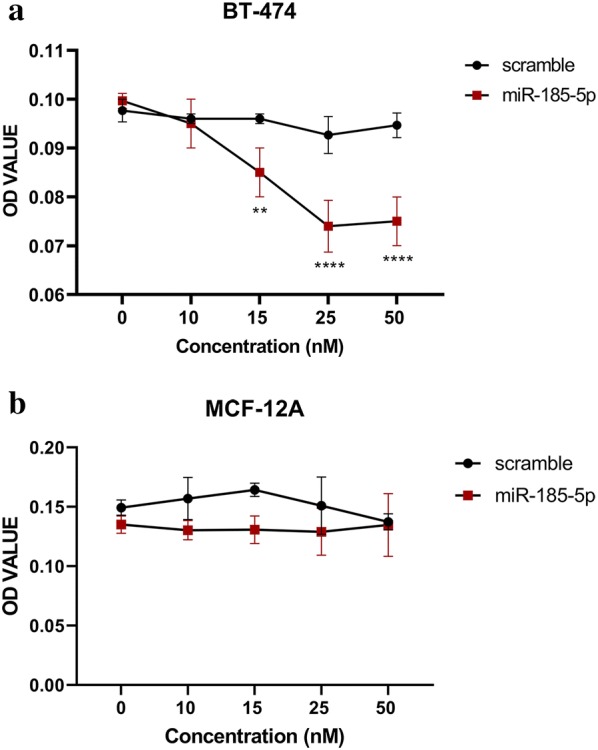
a The effect of miR-185-5p on BT-474 cell viability under different concentrations. The most significant inhibition of cell proliferation observed at 25 nM (**p < 0.01, ****p < 0.0001). b The effect of miR-185-5p on MCF-12A cell viability under different concentrations. Even under the different concentrations of miR-185-5p, no reduction in MCF-12A cell proliferation
Fig. 2.
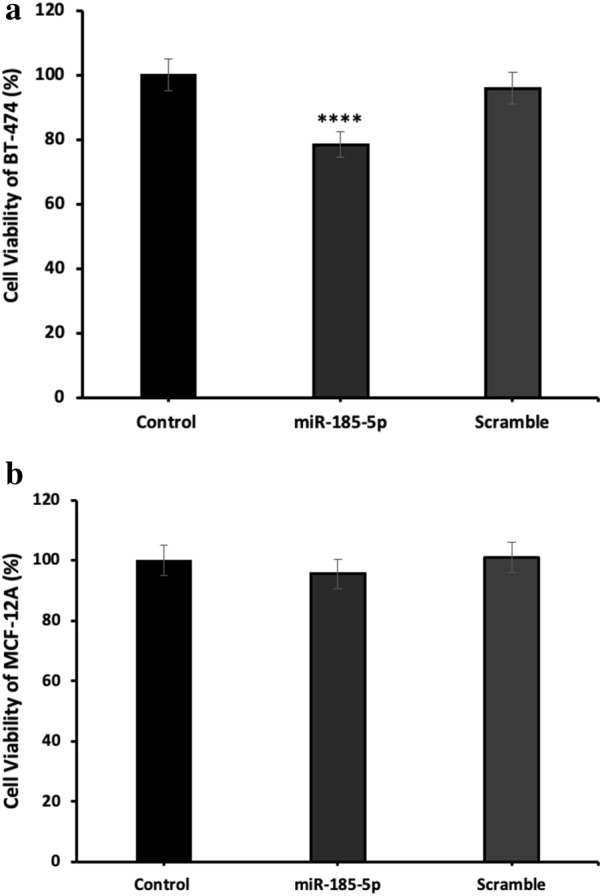
a miR-185-5p inhibits the BT-474 cell proliferation. The cell viability analysis performed using MTT assay by the transfection of 25 nM miR-185-5p and scramble in BT-474 cells (****p < 0.0001). b The overexpression of miR-185-5p in MCF-12A do not affect the cell viability significantly. MTT assay performed for the determination of the cell viability after 25 nM miR-185-5p and scramble transfection into MCF-12A cell line (Control: DMEM)
Transfection efficiency of miR-185-5p in BT-474
To validate the transfection efficiency of miR-185-5p in BT-474, the qRT-PCR assay was performed. The qRT-PCR data showed that miR-185-5p expression was up-regulated in breast cancer cells with transfection of miR-185-5p mimic, while there was no prominent increase in miR-185-5p levels in control group, suggesting that the ectopic expression of miR-185-5p was successfully carried out as seen Fig. 3 (p < 0.05).
Fig. 3.
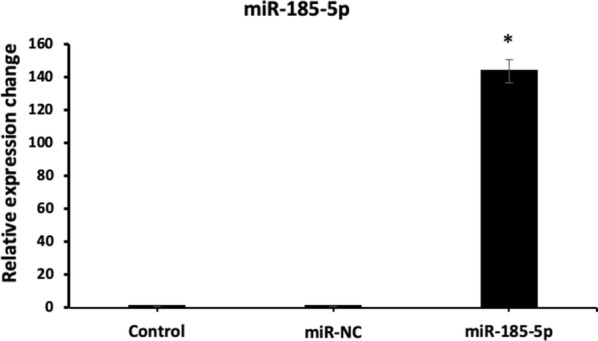
The transfection efficiency results of miR-185-5p in BT-474 cells (*p < 0.05) (Control: The level of expression given as control is proportional to the number 1, miR-NC: Scramble)
miR-185-5p induces apoptosis in breast cancer cells
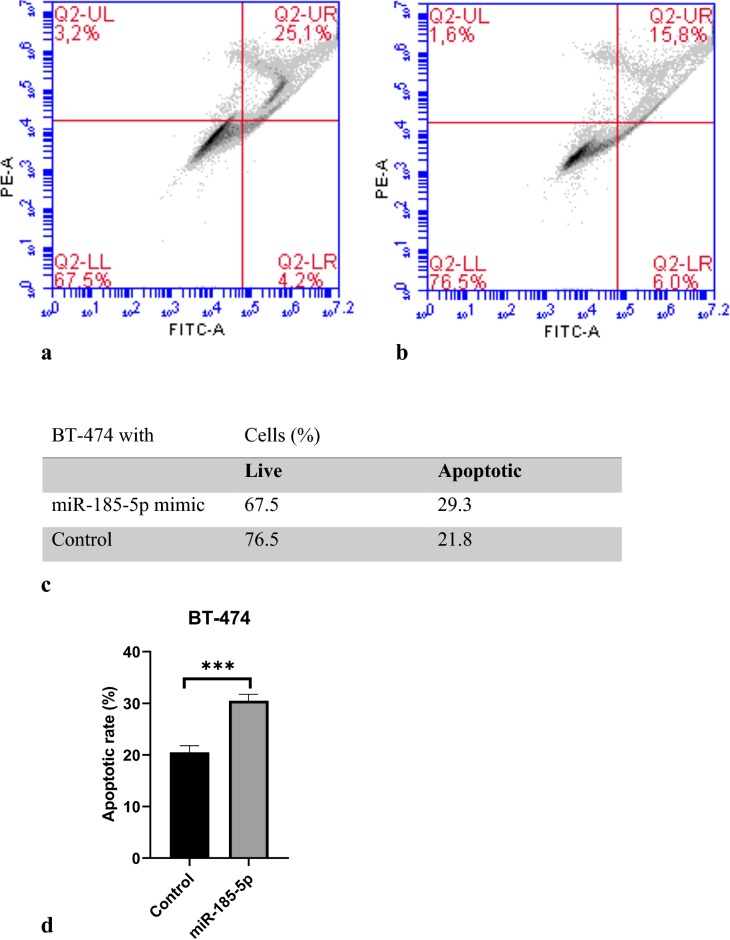
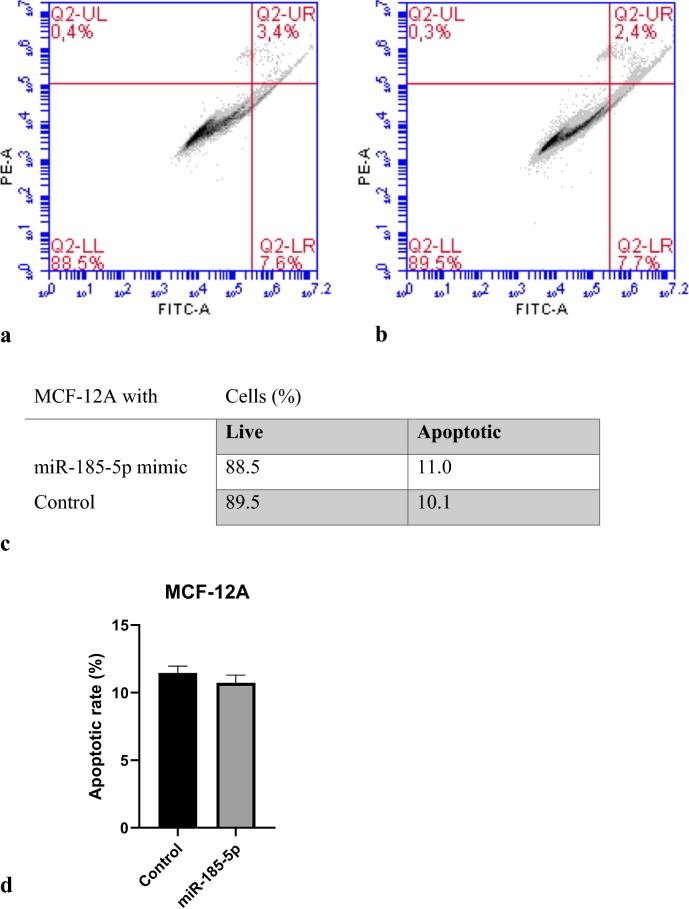
To explore the mechanism by which cell viability was suppressed, the apoptosis ratio of BT-474 and MCF-12A cells transfected with miR-185-5p was measured by flow cytometry. Our data revealed that the BT-474 cell apoptosis was significantly increased with the overexpression of miR-185-5p compared to the control group. Our results suggested that miR-185-5p may be as a tumor suppressor for breast cancer (Fig. 4). In addition, the apoptosis ratio of BT-474 cells in terms of cell percentage was determined as 9% as shown in Fig. 4 (p < 0.001). In contrast, by observing the data, there was not significantly increase in the apoptosis of miR-185-5p transfected MCF-12A cells (Fig. 5). Together, these results indicated that miR-185-5p could induce the apoptosis in BT-474 breast cancer cell compared with the MCF-12A non-cancerous epithelial breast cell.
Fig. 4.
miR-185-5p effect on BT-474 cell apoptosis. a Flow cytometric analysis revealed miR-185-5p transfected apoptotic BT-474 cells ratio. b Non-transfected apoptotic BT-474 cells ratio. c Percentages of apoptotic and live cells. d Apoptotic rate of BT-474 following miRNA-185-5p transfection on graph (***p < 0.001) (Control: DMEM)
Fig. 5.
miR-185-5p effect on MCF-12A cell apoptosis. a Vybrant Apoptosis assay showed any significant effect of ectopic expression of miR-185-5p on cell apoptosis. b In control group, similar results observed with (a). c Percentages of apoptotic and live cells. d Apoptotic rate of MCF-12A following miRNA-185-5p transfection on graph (Control: DMEM)
miR-185-5p regulates the cell cycle in BT-474 cells
In order to further support the inhibitory effect of miR-185-5p on breast cancer cell proliferation, cell cycle analysis was performed by flow cytometry. The flow cytometric analysis results suggested that the proportion of cells stagnated in the G0/G1 phase was higher in the BT-474 cells transfected with miR-185-5p than the control group. Besides, we found that the number of cells transfected with the miR-185-5p in the S and G2/M phases was less than the control group, indicating that the miR-185-5p could block the mitosis in the breast cancer cells (Fig. 6) (p < 0.05). These results demonstrated that the inhibition of breast cancer cell viability via miR-185-5p can be mediated by regulating the cell cycle.
Fig. 6.
miR-185-5p regulates cell-cycle. a Cell- cycle analysis graph of the miR-185-5p transfected BT-474 cells. b Control group cell cycle analysis graph. c Percentages of G1, S, and G2/M phase cells. d Comparison of the miR-185-5p transfected and non-transfected cells cell cycle analysis results on graph (*p < 0.05) (Control: DMEM)
miR-185-5p regulates the expression of the apoptosis-related genes
In order to explore the apoptotic gene expression pattern after the transfection of miRNA-185-5p in BT-474 cells, we performed real-time PCR using Human Apoptosis Primer Library assay that profiles the expression of 88 apoptosis-related genes. However, we obtained reasonable results for 69 genes by qRT-PCR (Additional file 1). According to our results, all Caspase gene family members exhibited an expression increase after transfection of miRNA-185-5p in BT-474 cell line and also the maximum increase rate was observed in Caspase 3 gene (p < 0.01) (Fig. 7).
Fig. 7.
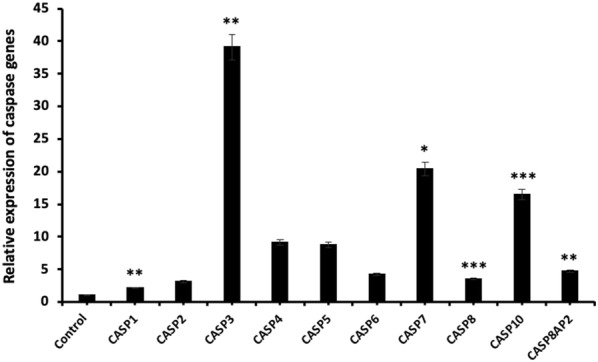
Relative expression of caspase genes after miR-185-5p transfection in BT-474 cell line (*p < 0.05, **p < 0.01, ***p < 0.001)
One of the other primer group in Human Apoptosis Primer Library is BCL2-related gene family and we observed that the BCL2 (p < 0.001) and BAG4 (p < 0.05) mRNA levels were significantly decreased with miR-185-5p transfection. These results also demonstrated that BOK gene expression was mostly increased compared with the control group. On the other hand, there was no significant change in BCL2A1 and BAG1 mRNA levels (Fig. 8).
Fig. 8.

Relative expression of BCL2 genes after miR-185-5p transfection in BT-474 cell line (*p < 0.05, **p < 0.01, ***p < 0.001)
We also investigated the relative expression of Kinase and TRAF gene family and following qRT-PCR results, the highest increase was observed in the mRNA expression levels of CHECK2 (p < 0.01) and TRAF6 (p < 0.05) (Figs. 9 and 10, respectively). In addition, the mRNA expression of the other genes was up-regulated after miR-185-5p transfection (Figs. 9, 10) (p < 0.05, p < 0.01, p < 0.001).
Fig. 9.

Relative expression of Kinase genes after miR-185-5p transfection in BT-474 cell line (*p < 0.05, **p < 0.01)
Fig. 10.
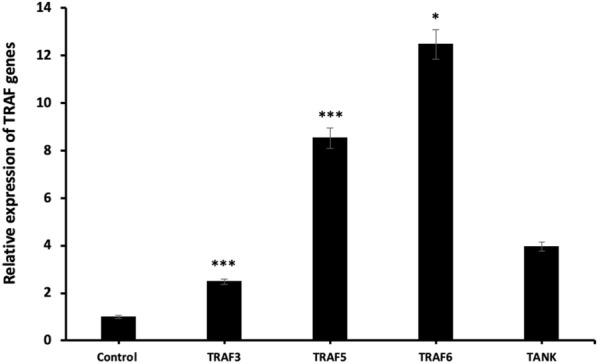
Relative expression of TRAF genes following miR-185-5p transfection in BT-474 cell line (*p < 0.05, ***p < 0.001)
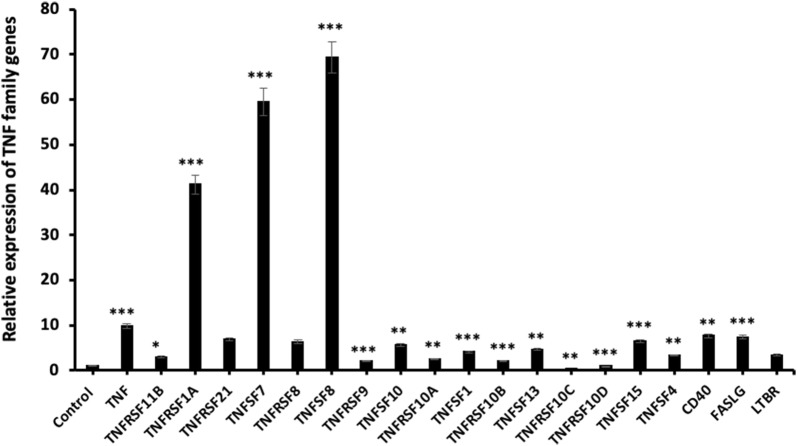
We also performed qRT-PCR to determine the relative expression of TNF family genes and this study revealed a substantial change in mRNA expression levels for almost all genes. TNFRSF10C (p < 0.01) and TNFRSF10D (p < 0.001) exhibited a significant increase in mRNA expression in the BT-474 cells. On the other side, the relative mRNA expression levels of the other genes were significantly increased and also TNFSF8 (p < 0.001) was highly up-regulated in comparison with the other genes. The qRT-PCR results indicated that TNFSF7 (p < 0.001) and TNFRSF1A (p < 0.001) genes mRNA levels were increased with the transfection of miR-185-5p in BT-474 cells (Fig. 11).
Fig. 11.
Relative expression of TNF genes with miR-185-5p transfection in BT-474 cells (*p < 0.05, ** p < 0.01, ***p < 0.001)
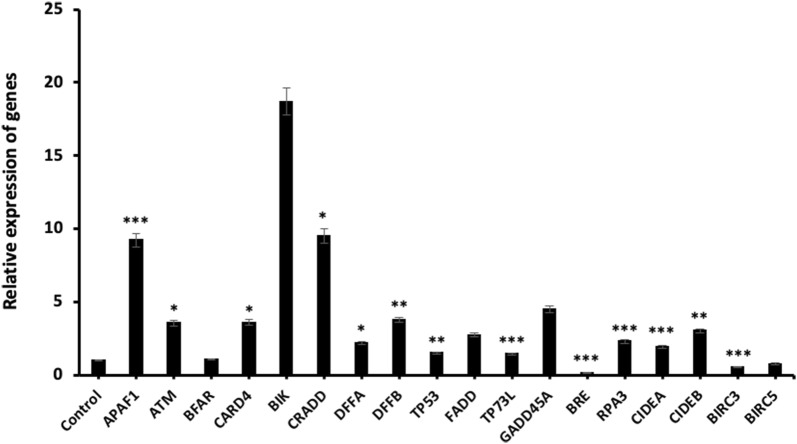
Finally, we found that the other apoptosis-related genes groups also showed a considerable change in terms of mRNA expression levels and the highest mRNA expression increase rate was observed in BIK gene. According to our results, BRE (p < 0.001) gene expression level was decreased in miR-185-5p transfected BT-474 cells and also BRC3 and BRC5 genes were slightly decreased at the mRNA level. On the other hand, there was no significant change in the BFAR gene expression (Fig. 12). In brief, all these qRT-PCR data suggested that miR-185-5p mediated the mRNA levels of many different genes associated with apoptosis.
Fig. 12.
The expression analysis of apoptosis related-genes at mRNA level (*p < 0.05, **p < 0.01, ***p < 0.001)
miR-185-5p targets BCL2 in BT-474 breast cancer cells
To find out the target of miR-185-5p in breast cancer cells, firstly we identified the potential target genes of miR-185-5p by using three different miRNA target prediction databases (miRDB, DIANA, and miRSystem). BCL2, which is common in three bioinformatics tools, is selected for target analysis. Also, the potential binding site between miR-185-5p and BCL2 was predicted by miRmap database as shown in Fig. 13a. To experimentally confirm the targeting of BCL2 by miR-185-5p, BCL2 protein expression level was shown by western blot. The results indicated that the BCL2 expression was significantly decreased after miR-185-5p transfection compared to the control group (p < 0.01) (Fig. 13b–c).
Fig. 13.
The target identification of miR-185-5p. a The predicted binding site between miR-185-5p and 3′UTR BCL2. b Western blot band results. c Graph of relative BCL2 protein level change (n = 3, **p < 0.01). d Relative luciferase activity results on graph (****p < 0.0001) (Control: DMEM)
Luciferase reporter assay
To further demonstrate whether BCL2 is a direct target of miR-185-5p, luciferase assay was performed therefore the luciferase plasmid inserted with 3′UTR of BCL2 and miR-185-5p mimic were co-transfected to BT-474 cells. These obtained data indicated that the relative luciferase activity significantly decreased with the miR-185-5p transfection compared with the control group as seen Fig. 13d (p < 0.0001). By observing the western blot and luciferase assay analysis, it was determined that miR-185-5p targeted the 3′UTR of BCL2 in the breast cancer cell.
Discussion
Although breast cancer can be diagnosed by improved diagnostic techniques and cured with different therapeutic approaches, this cancer is still the second leading cause of cancer-related death in woman worldwide [23, 24]. Some complications such as high recurrence rate and the formation of resistance to chemotherapy and endocrine therapy obstruct to combat breast cancer [25, 26].
Investigating the underlying molecular mechanisms of breast cancer at great length and the development of new therapeutic approaches are needed to provide better treatment for breast cancer patients. One of the well-known naturally occurring compounds is usnic acid which is a type of most commonly studied lichen secondary metabolite and it exerts an anti-proliferative effect on many cancer cell lines [6, 27] such as glioma cells (PRCC and U87MG) [28], human gastric cells (BGC823, SGC7901 and AGS) [29, 30], human lung carcinoma (A549) [8], prostate cancer cell (CWR22Rv-1) [30], human colon adenocarcinoma cell (HT-29) [30], and breast cancer cells (MCF-7, BT-474 and MDA-MB-231) [22]. Besides anti-proliferative properties, usnic acid can also inhibit tumor progression via anti-angiogenesis and anti-invasion activity through manipulating different signaling pathways in different cancer cells, including lung cancer cells (A549, H1650 and H1975) [31] and breast cancer cells (Bcap-37) [32].
Accompanied by the discovery of miRNAs and their investigations at the molecular level in cancer biology, it was observed that miRNAs have been deregulated in many human cancer cells [33–35]. Moreover, several studies have demonstrated that the miRNAs can act as a key regulator for oncogenes or tumor suppressor genes in cancer development and progression by mediating vital cellular processes including cell proliferation, differentiation, and apoptosis [36]. Due to the main cellular role of miRNAs in cancer progression, miRNAs gained attention as a novel therapeutic approach for cancer treatment. Indeed, many studies revealed that tumor development can be managed by miRNAs by inhibition of pro-oncogenic miRNAs or re-introduction of tumor suppressor miRNAs into breast cancer cells [37]. Han et al. [38] showed that the miR-1307-3p stimulates breast cancer development by targeting SMYD4, while downregulation of miR-1307-3p could inhibit the cell proliferation in breast cancer cells. Zou et al. [39] demonstrated that overexpression of the miRNA-375 could inhibit the cell viability, invasion and migration by targeting PAX6. Moreover, Ji et al. [40] indicated that the miR-3196 was significantly downregulated in breast cancer tissues compared with adjacent normal tissues and ectopic expression of miR-3196 in breast cancer cells could suppress cell proliferation via inducing apoptosis by targeting ERBB3. Tang et al. investigated the effect of miR-185 on tumor progression in various breast cancer cell lines. As a result of this study, it was demonstrated that miR-185 expression decreased in BT-474 breast cancer cell [41]. Ostadrahimi et al. [42] revealed that the miR-185-5p can trigger apoptosis in 12.82% and 9.2% of DU145 and PC3 prostate cancer cell population, respectively. Our previous study demonstrated that miR-185-5p, the most promising candidate usnic acid response miRNA, was remarkably up-regulated through usnic acid treatment to BT-474 breast cancer cell [22]. In this study, we tested that transfection of miR-185-5p in BT-474 breast cancer cell might suppress tumorigenicity by inhibiting cell proliferation and promoting apoptosis. In addition, we focused on the function of the miR-185-5p in breast cancer cells thus the underlying molecular mechanisms mediated by miR-185-5p on cell apoptosis was investigated in BT-474 cells.
To elucidate the role of miR-185-5p on cell proliferation and apoptosis, MTT assay and flow cytometry analysis were conducted in BT-474 cells and the results showed that the miR-185-5p decreased cell viability. Our cell viability and apoptosis assay results revealed that there is no significant effect of miR-185-5p on cell viability of normal breast cells (MCF-12A), suggesting that miR-185-5p can inhibit breast cancer cell proliferation without detriment to normal breast cells. Hao et al. [43] showed that cell apoptosis rate was increased under the miR-374c-5p transfection by about 12% and 14% in BT-549 and MDA-MB-231 breast cancer cell lines, respectively. In other study, Cui et al. [43] demonstrated that tumor progression was suppressed by miR-216a, stimulating cell apoptosis by 27% in MCF-7 breast cancer cell line. Qin et al. also reported that miR-99a-5p increased apoptosis rate 15% in MCF-7 and MDA-MB-231 breast cancer cells. Our data showed that the miR-185-5p obviously induced cell apoptosis in the 9% ratio, whereas there was no significant change in apoptosis ratio in MCF-12A normal breast cancer cells.
Apoptosis is a programmed cell death process that provides homeostasis in tissues and mediated by extrinsic and intrinsic pathway. Several in vitro studies have documented a role for miRNAs in apoptosis of human cancer cells [44, 45]. In the literature, there are many studies performed with flow cytometry and western blot assay to determine the apoptosis rate of miRNAs. Therefore, there are few studies that determine the mRNA level in relation to apoptosis of miRNAs. In our study, apoptosis data obtained after flow cytometry assay was analyzed by qRT-PCR technology in mRNA level. Liu et al. [44] reported western blot assay and real-time PCR were used to measure apoptosis related factors Bax and BCL-2 expression level. Liu et al. studies have shown that Bax expression level significantly decreased, but BCL-2 expression significantly increased compared with the control. In another studies, Li et al. showed that miR-886-5p negatively regulates Bax up-regulation of miR-886-5p in cancer cell line. This study revealed the role of miR-886 in apoptosis in cervical squamous cell carcinoma by using qRT-PCR and western blot assays [46]. The gene expression levels of our study showed similar levels with the literature. BCL2 is a well-known regulator gene which inhibits apoptosis by contributing the intrinsic apoptosis pathway [47, 48]. In more recent studies, it has been reported that tumorigenesis could be abolished by inducing cell apoptosis via miRNA-mediated BCL2 downregulation in different cancer cells. Yu et al. [49] demonstrated that apoptosis can be induced via directly targeting the 3′UTR of BCL2 by miRNA-136 in human gastric cancer cells. Zhu et al. [50] elucidated that miR-365 targeted the BCL2 thus and could suppress cell growth by promoting apoptosis in melanoma cells. Li et al. [51] revealed that miR-34c involved in the regulation of cell apoptosis by targeting the 3′UTR of BCL2, which led to inhibition of the cell viability in M4e laryngeal carcinoma cells. Pekarsky et al. indicated that the expression of miR-15/16 do not observe in MEG-01 leukemic cells, whereas the ectopic expression of miR-15/16 promoted cell apoptosis via direct targeting of BCL2 in leukemic cells [52]. In this study, we also demonstrated that miR-185-5p targeted the BCL2 in BT-474 cells, therefore, miR-185-5p significantly increased the cell apoptosis, suggesting that miR-185-5p can act as a tumor suppressor in breast cancer cells. This is the first study based on the determination of the expression level of all genes in the apoptosis pathway by miR-185-5p transfection.
The possible candidate gene of miR-185-5p was identified by bioinformatic miRNA target prediction tools, which showed that the 3′UTR of BCL2 has a binding site with miR-185-5p seed region. Luciferase reporter assay results showed that the increased expression of miR-185-5p in BT-474 cells inhibited the luciferase activity of reporter plasmid with 3′UTR of BCL2, confirming that the miR-185-5p could negatively regulate the BCL2 expression in breast cancer cells. To the best our knowledge, our results firstly uncovered that BCL2 is a target of miR-185-5p in BT-474 cells. Li et al. [53] suggested that the miR-148a directly targeted BCL2 by binding with 3′UTR sequence of BCL2 mRNA in MCF-7 breast cancer cell line.
Conclusion
In conclusion, we investigated the potential underlying mechanisms of miR-185-5p on apoptosis in BT-474 cells. Our findings showed that the up-regulation of miR-185-5p could block the cell growth by stimulating to apoptosis through directly targeting the 3′UTR of BCL2. It was suggested that usnic acid-induced BCL2 downregulation might be regulated by miR-185-5p in BT-474 breast cancer cell line. Therefore miR-185-5p could be considered as a tumor suppressor in BT-474 cells and might serve as a potential therapeutic candidate in breast cancer treatment. Further studies involving in vivo and clinical studies need to confirm the therapeutic effect of miR-185-5p on breast cancer.
Supplementary information
Additional file 1. The relative gene expression analysis of apoptosis related genes.
Acknowledgements
Authors would like to thank Dr Bala Gür Dedeoğlu for her help in obtaining cell lines.
Abbreviations
- 3′ UTR
3′ untranslated region
- APAF1
Apoptotic protease activating factor
- ATCC
American Type culture collection
- ATM
Ataxia telangiectasia mutated
- BAG1
BCL2-associated athanogene
- BAG3
BCL2-associated athanogene 3
- BAG4
BCL2-associated athanogene 4
- BAK1
BCL2-antagonist/killer 1
- BAX
BCL2-associated X protein
- BCL10
B-cell CLL/lymphoma 10
- BCL2
B-cell CLL/lymphoma 2
- BCL2A1
BCL2-related protein A1
- BCL2L11
BCL2-like 11 (apoptosis facilitator)
- BCL2L2
BCL2-like 2
- BFAR
Bifunctional apoptosis regulator
- BIK
BCL2-interacting killer (apoptosis-inducing)
- BIRC3
Baculoviral IAP repeat-containing 3
- BIRC5
Baculoviral IAP repeat-containing 5 (survivin)
- BOK
BCL2-related ovarian killer
- BRE
Brain and reproductive organ-expressed
- CARD4
Caspase recruitment domain family, member 4
- CASP1
Caspase 1, apoptosis-related cysteine protease
- CASP10
Caspase 10, apoptosis-related cysteine protease
- CASP2
Caspase 2, apoptosis-related cysteine protease
- CASP3
Caspase 3, apoptosis-related cysteine protease
- CASP4
Caspase 4, apoptosis-related cysteine peptidase
- CASP5
Caspase 5, apoptosis-related cysteine protease
- CASP6
Caspase 6, apoptosis-related cysteine protease
- CASP7
Caspase 7, apoptosis-related cysteine protease
- CASP8
Caspase 8, apoptosis-related cysteine protease
- CASP8AP2
CASP8 associated protein 2
- CD40
CD40 antigen (TNF receptor superfamily member 5)
- CHEK1
CHK1 checkpoint homolog (S. pombe)
- CHEK2
CHK2 checkpoint homolog (S. pombe)
- CIDEA
Cell death-inducing DFFA-like effector a
- CIDEB
Cell death-inducing DFFA-like effector b
- CRADD
CASP2 and RIPK1 domain containing adaptor
- DAPK2
Death-associated protein kinase 2
- DDT
Dichlorodiphenyltrichloroethane
- DFFA
DNA fragmentation factor, 45 kDa, alpha polypeptide
- DFFB
DNA fragmentation factor, 40 kDa, beta polypeptide
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
Dimethyl sulfoxide
- DNA
Deoxyribonucleic acid
- EGF
Epidermal Growth Factor
- FADD
Fas (TNFRSF6)-associated via death domain
- FASLG
Fas ligand (TNF superfamily, member 6)
- FBS
Fetal Bovine Serum
- G1 Phase
Gap1 Phase
- G2 Phase
Gap2 phase
- GADD45A
Growth arrest and DNA-damage-inducible, alpha
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- HRK
Harakiri, BCL2 interacting protein
- HRP
Horseradish peroxide
- LTBR
Lymphotoxin beta receptor (TNFR superfamily, member 3)
- M Phase
Mitotic Phase
- MAPK
Mitogen-activated protein kinase
- MCL1
Myeloid cell leukemia sequence 1 (BCL2-related)
- miR-185-5p
microRNA-185-5p
- miRNA
microRNA
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NM
Nano molar
- PBS
Phosphate buffered saline
- PI
Propidium iodide
- qRT-PCR
Quantitative reverse transcription polymerase chain reaction
- RIPK2
Receptor-interacting serine-threonine kinase 2
- RPA3
Replication protein A3, 14 kDa
- S Phase
Synthesis phase
- SDS-PAGE
Sodium dodecyl sulfate-polyacrylamide
- SEM
Standard error of the mean
- TANK
TRAF family member-associated NFKB activator
- TBST
Tris-Buffered Saline Tween
- TGF BETA
Transforming growth factor beta
- TNF
Tumor necrosis factor (TNF superfamily, member 2)
- TNFRSF10A
Tumor necrosis factor receptor superfamily, member 10a
- TNFRSF10B
Tumor necrosis factor receptor superfamily, member 10b
- TNFRSF10C
Tumor necrosis factor receptor superfamily, member 10c
- TNFRSF10D
Tumor necrosis factor receptor superfamily, member 10d
- TNFRSF11B
Tumor necrosis factor receptor superfamily, member 11b
- TNFRSF1A
Tumor necrosis factor receptor superfamily, member 1A
- TNFRSF21
Tumor necrosis factor receptor superfamily, member 21
- TNFRSF8
Tumor necrosis factor receptor superfamily, member 8
- TNFRSF9
Tumor necrosis factor receptor superfamily, member 9
- TNFSF10
Tumor necrosis factor (ligand) superfamily, member 10
- TNFSF13
Tumor necrosis factor (ligand) superfamily, member 12
- TNFSF15
Tumor necrosis factor (ligand) superfamily, member 15
- TNFSF4
Tumor necrosis factor (ligand) superfamily, member 4
- TNFSF7
Tumor necrosis factor (ligand) superfamily, member 7
- TNFSF8
Tumor necrosis factor (ligand) superfamily, member 8
- TP53
Tumor protein p53 (Li-Fraumeni syndrome)
- TP73L
Tumor protein p73-like
- TRAF3
TNF receptor-associated factor 3
- TRAF5
TNF receptor-associated factor 5
- TRAF6
TNF receptor-associated factor 6
Authors’ contribution
ED set up and carried out experiments. VT performed MTT assays. ED and DC-D executed data analysis and wrote the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Ankara University Management of Scientific Research Projects, Turkey (Project no. 15B0415001)
Availability of data and materials
The datasets used and/or analyzed in the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no conflicts of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s40659-020-00285-4.
References
- 1.Gates R, Regina M. Oncology nursing secrets. 3. Amsterdam: Elsevier; 2008. [Google Scholar]
- 2.Gong Y, He T, Yang L, Yang G, Chen Y, Zhang X. The role of miR-100 in regulating apoptosis of breast cancer cells. Sci Rep. 2015;5:1–13. doi: 10.1038/srep11650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang S, Shan C, Kong G, Du Y, Ye L, Zhang X. MicroRNA-520e suppresses growth of hepatoma cells by targeting the NF-B-inducing kinase (NIK) Oncogene. 2012;31(31):3607–3620. doi: 10.1038/onc.2011.523. [DOI] [PubMed] [Google Scholar]
- 4.Rogers Seton S. Chemotherapy: preventing competitive release. Nat Rev Cancer. 2016;16(4):199. doi: 10.1038/nrc.2016.28. [DOI] [PubMed] [Google Scholar]
- 5.Plantamura I, Cosentino G, Cataldo A. MicroRNAs and DNA-damaging drugs in breast cancer: strength in numbers. Front Oncol. 2018;8(September):1–10. doi: 10.3389/fonc.2018.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinçsoy AB, Duman DC. Changes in apoptosis-related gene expression profiles in cancer cell lines exposed to usnic acid lichen secondary metabolite. Turk J Biol. 2017;41:484–493. [Google Scholar]
- 7.Kim H, Kim KK, Hur J-S. Anticancer activity of lichen metabolites and their mechanisms at the molecular level. In: Upreti DK, Divakar PK, Shukla V, Bajpai R, editors. Recent advances in lichenology. New Delhi: Springer India; 2015. pp. 201–208. [Google Scholar]
- 8.Singh N, Nambiar D, Kale R, Singh R. Usnic acid inhibits growth and induces cell cycle arrest and apoptosis in human lung carcinoma A549 cells. Nutr Cancer. 2013;65:36–43. doi: 10.1080/01635581.2013.785007. [DOI] [PubMed] [Google Scholar]
- 9.Bačkorová M, Jendželovský R, Kello M, Bačkor M, Mikeš J, Fedoročko P. Lichen secondary metabolites are responsible for induction of apoptosis in HT-29 and A2780 human cancer cell lines. Toxicol In Vitro. 2012;26(3):462–468. doi: 10.1016/j.tiv.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Yurdacan B, Egeli U, Eskiler GG, Eryilmaz IE, Cecener G, Tunca B. The role of usnic acid-induced apoptosis and autophagy in hepatocellular carcinoma. Hum Exp Toxicol. 2019;38(2):201–215. doi: 10.1177/0960327118792052. [DOI] [PubMed] [Google Scholar]
- 11.Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: rationale, strategies and challenges. Nat Rev Drug Discov. 2010;9(10):775–789. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Rooij E, Kauppinen S. Development of microRNA therapeutics is coming of age. EMBO Mol Med. 2014;6(7):851–864. doi: 10.15252/emmm.201100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ambros V. MicroRNAs: tiny regulators with great potential. Signal Pathways Liver Dis. 2001;107:823–826. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- 14.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6(11):857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 15.Lima RT, Busacca S, Almeida GM, Gaudino G, Fennell DA, Vasconcelos MH. MicroRNA regulation of core apoptosis pathways in cancer. Eur J Cancer. 2011;47(2):163–174. doi: 10.1016/j.ejca.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Lou W, Liu J, Ding B, Xu L, Fan W. Identification of chemoresistance-associated miRNAs in breast cancer. Cancer Manage Res. 2018;10:4747–4757. doi: 10.2147/CMAR.S172722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi X, Zhang H, Wang M, Xu X, Zhao Y, He R, et al. LncRNA AFAP1-AS1 promotes growth and metastasis of cholangiocarcinoma cells. Oncotarget. 2017;8(35):58394–58404. doi: 10.18632/oncotarget.16880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang B, Pan X, Cobb GP, Anderson TA. MicroRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 19.Bader AG. MiR-34—a microRNA replacement therapy is headed to the clinic. Front Genet. 2012;3:1–9. doi: 10.3389/fgene.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beg MS, Brenner AJ, Sachdev J, Borad M, Kang YK, Stoudemire J, et al. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Invest New Drugs. 2017;35(2):180–188. doi: 10.1007/s10637-016-0407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu AM, Jian C, Yu AH, Tu MJ. RNA therapy: are we using the right molecules? Pharmacol Ther. 2019;196:91–104. doi: 10.1016/j.pharmthera.2018.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kilic N, Islakoglu YO, Buyuk A, Gur-Dedeoglu B, Cansaran-Duman D. Determination of usnic acid responsive miRNAs in breast cancer cell lines. Anticancer Agents Med Chem. 2018;18:1463–1472. doi: 10.2174/1871520618666181112120142. [DOI] [PubMed] [Google Scholar]
- 23.Hu X, Wang J, He W, Zhao P, Ye C. MicroRNA-433 targets AKT3 and inhibits cell proliferation and viability in breast cancer. Oncol Lett. 2018;15:3998–4004. doi: 10.3892/ol.2018.7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keklikoglou I, Koerner C, Schmidt C, Zhang JD, Heckmann D, Shavinskaya A, et al. MicroRNA-520/373 family functions as a tumor suppressor in estrogen receptor negative breast cancer by targeting NF-jB and TGF-b signaling pathways. Oncogene. 2012;31:4150–4163. doi: 10.1038/onc.2011.571. [DOI] [PubMed] [Google Scholar]
- 25.Yin C, Zhang G, Sun R, Pan X, Wang X, Li H, et al. miR-185-5p inhibits F-actin polymerization and reverses epithelial mesenchymal transition of human breast cancer cells by modulating RAGE. Mol Med Rep. 2018;18:2621–2630. doi: 10.3892/mmr.2018.9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagini S. Breast cancer: current molecular therapeutic targets and new players. Anticancer Agents Med Chem. 2017;17(2):152–163. doi: 10.2174/1871520616666160502122724. [DOI] [PubMed] [Google Scholar]
- 27.Koparal T. Anti-angiogenic and antiproliferative properties of the lichen substances (−) -usnic acid and vulpinic acid. Zeitschrift für Naturforsch C. 2015;70(5–6):159–164. doi: 10.1515/znc-2014-4178. [DOI] [PubMed] [Google Scholar]
- 28.Emsen B, Turkez H, Joughi AT, Kaya A. The anti-cancer efficacies of diffractaic, lobaric, and usnic acid: in vitro inhibition of glioma. J Cancer Res Ther. 2016;14:941–951. doi: 10.4103/0973-1482.177218. [DOI] [PubMed] [Google Scholar]
- 29.Geng X, Zhang X, Zhou B, Zhang C, Tu J, Chen X, et al. Usnic acid induces cycle arrest, apoptosis, and autophagy in gastric cancer cells in vitro and in vivo. Med Sci Monit. 2018;24:556–566. doi: 10.12659/MSM.908568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen TT, Yoon S, Yang Y, Lee H, Oh S, Jeong M, et al. Lichen secondary metabolites in Flavocetraria cucullata exhibit anti-cancer effects on human cancer cells through the induction of apoptosis and suppression of tumorigenic potentials. PLoS ONE. 2014;9(10):1–14. doi: 10.1371/journal.pone.0111575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, Nguyen TT, Jeong M, Cri F, Yu YH. Inhibitory activity of (+) -usnic acid against non-small cell lung cancer cell motility. PLoS ONE. 2016 doi: 10.1371/journal.pone.0146575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song Y, Dai F, Zhai D, Dong Y, Zhang J, Lu B, et al. Usnic acid inhibits breast tumor angiogenesis and growth by suppressing VEGFR2-mediated AKT and ERK1/2 signaling pathways. Angiogenesis. 2012;15(3):421–432. doi: 10.1007/s10456-012-9270-4. [DOI] [PubMed] [Google Scholar]
- 33.Cullen BR. MicroRNAs as mediators of viral evasion of the immune system. Nat Immunol. 2013;14(3):205–210. doi: 10.1038/ni.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang W, Luo Y. MicroRNAs in breast cancer: oncogene and tumor suppressors with clinical potential. J Zhejiang Univ B. 2015;16(1):18–31. doi: 10.1631/jzus.B1400184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torun V, Değerli E, Cansaran-Duman D. A promising role of lichens for early detection and their secondary metabolites on treatment of cancer disease after exposure to carcinogenic heavy metals. In: Phytoremediation. 2019. p. 203–14.
- 36.Munshi A, Mohan V, Yr A. Non-coding RNAs: a dynamic and complex network of gene regulation. J Pharmacogenomics Pharmacoproteomics. 2016;7(1):1–11. [Google Scholar]
- 37.Jansson M, Lund A. MicroRNA and cancer. Mol Oncol. 2012;6:590–610. doi: 10.1016/j.molonc.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han S, Zou H, Lee J, Han J, Kim HC, Cheol JJ, et al. miR-1307-3p stimulates breast cancer development and progression by targeting SMYD4. J Cancer. 2019;10:441–448. doi: 10.7150/jca.30041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zou Q, Yi W, Huang J, Fu F, Chen G, Zhong D. MicroRNA-375 targets PAX6 and inhibits the viability, migration and invasion of human breast cancer MCF-7 cells. Exp Ther Med. 2017;14:1198–1204. doi: 10.3892/etm.2017.4593. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Ji Z, Han S, Xing Y. Overexpression of miR-3196 suppresses cell proliferation and induces cell apoptosis through targeting ERBB3 in breast cancer. Eur Rev Med Pharmacol Sci. 2018;22:8383–8390. doi: 10.26355/eurrev_201812_16536. [DOI] [PubMed] [Google Scholar]
- 41.Tang H, Liu P, Yang L, Xie X, Ye F, Wu M, et al. miR-185 suppresses tumor proliferation by directly targeting E2F6 and DNMT1 and indirectly upregulating BRCA1 in triple-negative breast cancer. Mol Cancer Ther. 2014;13(12):3185–3197. doi: 10.1158/1535-7163.MCT-14-0243. [DOI] [PubMed] [Google Scholar]
- 42.Ostadrahimi S, Fayaz S, Parvizhamidi M, Esfahani PF, Valugerdi MA, Hassan M, et al. miR-1266-5p and miR-185-5p promote cell apoptosis in human prostate cancer cell lines. Asian Pac J Cancer Prev. 2018;19(8):2305–2311. doi: 10.22034/APJCP.2018.19.8.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hao S, Tian W, Chen Y, Wang L, Jiang Y, Gao B, et al. MicroRNA-374c-5p inhibits the development of breast cancer through TATA-box binding protein associated factor 7-mediated transcriptional regulation of DEP domain containing 1. J Cell Biochem. 2019;120(9):15360–15368. doi: 10.1002/jcb.28803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu X, Tong Z, Chen K, Hu X, Jin H, Hou M. The role of miRNA-132 against apoptosis and oxidative stress in heart failure. Biomed Res Int. 2018 doi: 10.1155/2018/3452748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Du H, Xu Q, Xiao S, Wu Z, Gong J, Liu C, et al. MicroRNA-424-5p acts as a potential biomarker and inhibits proliferation and invasion in hepatocellular carcinoma by targeting TRIM29. Life Sci. 2019;1(224):1–11. doi: 10.1016/j.lfs.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 46.Li JH, Xiao X, Zhang YN, Wang YM, Feng LM, Wu YM, et al. MicroRNA miR-886-5p inhibits apoptosis by down-regulating Bax expression in human cervical carcinoma cells. Gynecol Oncol. 2011;120(1):145–151. doi: 10.1016/j.ygyno.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 47.Cui J, Placzek WJ. Post-transcriptional regulation of anti-apoptotic BCL2 family members. Int J Mol Sci. 2018 doi: 10.3390/ijms19010308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martínez-Arribas F, Alvarez T, Del Val G, Martín-Garabato E, Núñez-Villar MJ, Lucas R, et al. Bcl-2 expression in breast cancer: a comparative study at the mRNA and protein level. Anticancer Res. 2007;27:219–222. [PubMed] [Google Scholar]
- 49.Yu L, Zhou G-Q, Li D-C. MiR-136 triggers apoptosis in human gastric cancer cells by targeting AEG-1 and BCL2. Eur Rev Med Pharmacol Sci. 2018;22(21):7251–7256. doi: 10.26355/eurrev_201811_16259. [DOI] [PubMed] [Google Scholar]
- 50.Zhu Y, Wen X, Zhao P. MicroRNA-365 inhibits cell growth and promotes apoptosis in melanoma by targeting BCL2 and cyclin D1 (CCND1) Med Sci Monit. 2018;24:3679–3692. doi: 10.12659/MSM.909633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li R, Zhang H, Zheng X. MiR-34c induces apoptosis and inhibits the viability of M4e cells by targeting BCL2. Oncol Lett. 2018;15(3):3357–3361. doi: 10.3892/ol.2017.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pekarsky Y, Balatti V, Croce CM. BCL2 and miR-15/16: from gene discovery to treatment. Cell Death Differ. 2018;25(1):21–26. doi: 10.1038/cdd.2017.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Q, Ren P, Shi P, Chen Y, Xiang F, Zhang L, et al. MicroRNA-148a promotes apoptosis and suppresses growth of breast cancer cells by targeting B-cell lymphoma 2. Anticancer Drugs. 2017;28(6):588–595. doi: 10.1097/CAD.0000000000000498. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. The relative gene expression analysis of apoptosis related genes.
Data Availability Statement
The datasets used and/or analyzed in the current study are available from the corresponding author on reasonable request.