Abstract
Background
An increasing rate of antibiotic resistance among Gram-negative bacterial pathogens has created an urgent need to discover novel therapeutic agents to combat infectious diseases. Use of bacteriocins as therapeutic agents has immense potential due to their high potency and mode of action different from that of conventional antibiotics.
Results
In this study, a novel bacteriocin E20c of molecular weight 6.5 kDa was purified and characterized from the probiotic strain of Enterococcus hirae. E20c had bactericidal activities against several multidrug resistant (MDR) Gram-negative bacterial pathogens. Flow cytometry and scanning electron microscopy studies showed that it killed the Salmonella enterica cells by forming ion-permeable channels in the cell membrane leading to enhanced cell membrane permeability. Further, checkerboard titrations showed that E20c had synergistic interaction with antibiotics such as ampicillin, penicillin, ceftriaxone, and ciprofloxacin against a ciprofloxacin- and penicillin-resistant strain of S. enterica.
Conclusion
Thus, this study shows the broad spectrum antimicrobial activity of novel enterocin E20c against various MDR pathogens. Further, it highlights the importance of bacteriocins in lowering the minimum inhibitory concentrations of conventional antibiotics when used in combination.
Keywords: Bacteriocin, Lactic acid bacteria, Antimicrobial agents, Synergistic effect, Multi-drug resistant pathogens
Background
The crisis of antibiotic resistance has been acknowledged as a global health emergency by WHO [1]. Concomitant to increasing rates of antibiotic resistance, the discovery of novel antimicrobials has stalled. According to a recent report, most of the new antimicrobials currently in clinical trials are the derivatives of the existing antibiotics [2]. Thus, their modes of action are similar to the parent drug that offers a short term solution to the problem of antibiotic resistance. Hence, novel therapeutic options for treating MDR infections are urgently required. Bacteriocins are considered as the next wave of protein antibiotics owing to their unique modes of action such as disruption of cell membrane integrity, inhibition of protein production, DNA replication and septum formation [3]. The applications of bacteriocins are being explored in different areas, such as healthcare, food preservation, and veterinary medicines [4]. Bacteriocins such as nisin and pediocin purified from lactic acid bacteria (LAB) have evoked commercial interest. Nisin has shown therapeutic effects for the treatment of mastitis in both human [5] and animal studies [6]. Apart from their use as standalone therapeutics, bacteriocin-antibiotic combinations have tremendous value in terms of decreasing the minimum inhibitory concentration (MIC) of antibiotics. For example, thuricin CD reduced the MIC of various antibiotics against Clostridium spp. [7]; and nisin showed synergistic interaction with various antibiotics against methicillin-resistant Staphylococcus aureus and vancomycin resistant enterococci [8]. This strategy will, in turn, reduce the probability of development of antibiotic resistance among pathogens [9].
Salmonella-related enteric infection is a significant health problem worldwide that poses a substantial economic burden to both developed and under-developed countries [10]. Every year, 11–20 million people become sick, and 128,000 to 161,000 people die due to typhoid fever [11]. The antibiotics commonly used for the treatment of typhoid fever belong to the classes ß-lactam and fluoroquinolone. The fast-emerging resistance to quinolones among Salmonella spp. has necessitated the use of other antimicrobials such as ceftriaxone, a third generation cephalosporin and azithromycin [12]. Recently sporadic cases of ceftriaxone- or azithromycin-resistant S. typhi have also been reported. Therefore, Salmonella has been classified as a high priority pathogen for the development of novel antimicrobials [13, 14]. A bacteriocin-antibiotic combination is an innovative approach to lower the dose of currently used antibiotics thereby decreasing the selective pressure that leads to the emergence of resistant bacterial strains. Thus, in this study we have purified and characterized a low molecular weight enterocin, E20c from a probiotic strain of Enterococcus hirae 20c that was previously isolated from healthy human vaginal swab samples [15]. Further, we studied the mechanism of antimicrobial activity of E20c and the interaction of E20c with conventionally used antibiotics against S. enterica.
Results
Purification and characterization of E20c
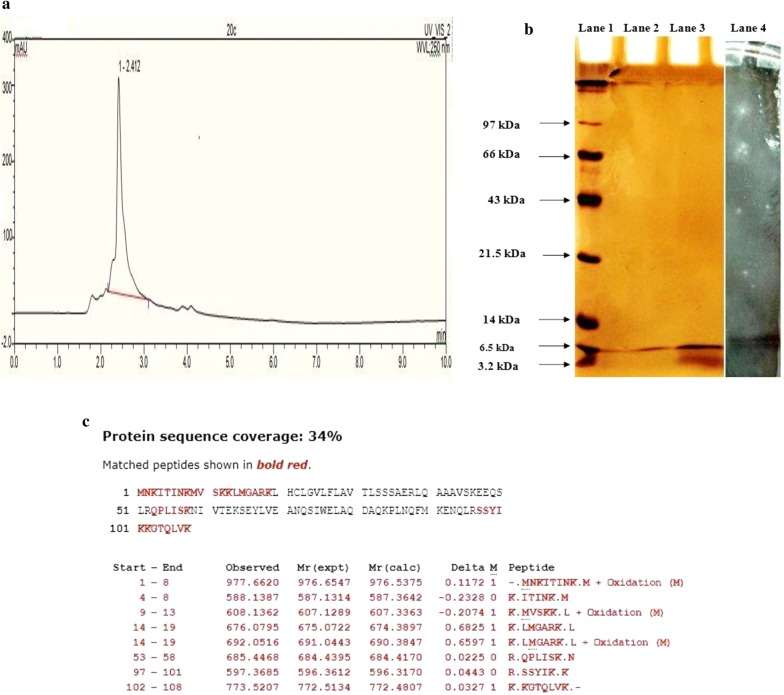
E20c was purified from the cell free culture supernatant (CS) of E. hirae 20c by ammonium sulphate precipitation followed by cation-exchange chromatography. Cation-exchange chromatography resulted in a 2-fold increase in the specific activity of E20c (Table 1) with the final yield percentage of 1.62. The active fractions were concentrated by lyophilization and subjected to U-HPLC and SDS PAGE analysis to check the purity of the protein. A single peak was observed in the U-HPLC chromatogram (Fig. 1a). The resolution of the concentrated active fractions on SDS-PAGE yielded a single band with a molecular weight of approximately 6.5 kDa (Fig. 1b; Lane 2). Simulataneously, a lane of agar gel was cut and subjected to agar gel overlay assay. A clear zone corresponding to 6.5 kDa band was obtained against the indicator strain S. enterica (Fig. 1b). Further, to characterize the protein band, it was cut, trypsin-digested and subjected to matrix-assisted laser desorption-ionization time-of-flight (MALDI TOF/TOF) mass spectrometry (MS) for protein identification. Peptide mass fingerprinting (Pmf) analysis of the fragments obtained was performed by Matrix Science Mascot UK software, and significant (p < 0.05) result was obtained. The analysis revealed eight peptides that shared similarity with 34% of the hypothetical protein of E. faecalis with significant (p < 0.05) coverage score of 102 (Fig. 1c).
Table 1.
Purification of enterocin from E. hirae 20c
| Purification step | Total volume (ml) | Activity (AU/ml) | Total activitya (AU) | Total protein (mg) | Specific activityb (AU/mg) | Increase in specific activity | Yield (%) |
|---|---|---|---|---|---|---|---|
| CS | 1000 | 4266 | 4.2 × 106 | 250 | 16800 | 1 | 100 |
| Ammonium sulphate ppt. | 50 | 17066 | 8.5 × 105 | 21 | 40476.2 | 2.41 | 20.24 |
| Cation-exchange fraction | 1 | 68266 | 6.8 × 104 | 2 | 34000 | 2.02 | 1.62 |
aTotal activity = activity × total volume of sample used at each purification step
bSpecific activity = total activity/total protein
Fig. 1.
Purification and characterization of E20c. a U-HPLC chromatogram of the purified E20c. b SDS-PAGE of purified E20c. Lane 1: molecular weight marker. Lane 2: cation-exchange fraction eluted with 0.4 mM NaCl-containing sodium acetate buffer. Lane 3: cation-exchange fraction eluted with 0.2 mM NaCl-containing sodium acetate buffer c PMF analysis of E20c. The PMF followed by MASCOT search showed the matched amino acid residues (in bold red) of the peptide fragments with the hypothetical protein of E. faecalis following NCBI BLASTsearch
Antimicrobial activity of purified enterocins
Purified E20c had antimicrobial activities against various Gram-positive and Gram-negative pathogens viz. S. enterica, Shigella flexneri, Escherichia coli, Streptococcus pyogenes and Listeria monocytogenes. On the other hand, it did not inhibit any of the tested commensal lactobacilli spp. isolated from the stool samples of healthy children, and other tested pathogenic bacteria viz. Vibrio cholerae, Mycobacterium smegmatis, S. aureus, and Pseudomonas aeruginosa (Table 2).
Table 2.
Antimicrobial activity of E20c against various indicator bacterial strains
| Indicator Bacteria | Zone of inhibition (mm) |
|---|---|
| E20c | |
| S. enterica Microbial Type Culture Collection (MTCC) 733 | 16 ± 0.1 |
| Es. coli MTCC 119 | 13 ± 0.12 |
| Sh. flexneri MTCC 1457 | 15 ± 0.2 |
| Lis. monocytogenes MTCC 657 | 14 ± 0.03 |
| V. cholerae MTCC 3906 | –a |
| M. smegmatis MTCC 6 | – |
| St. pyogenes MTCC 1927 | 13 ± 0.11 |
| Staph. aureus MTCC 96 | – |
| P. aeruginosa MTCC 741 | – |
| L. plantarum L14 | – |
| L. fermentum L32 | – |
| L. pentosus S45 | – |
| L. fermentum L13 | – |
| L. plantarum L12 | – |
| L. fermentum L18 | – |
| L. casei S49 | – |
Zones of inhibition (mm) of E20c against various indicator strains were determined by using agar gel diffusion assay. The results are the mean ± SD of three independent experiments
–a No zone of inhibition
Physico-chemical characterization of purified E20c
The thermostability of E20c was determined at different temperature treatments for different time periods. Results (Table 3) showed that the antimicrobial activity of E20c remained stable till 100 °C treatment for 60 min. However, at the autoclaving temperature, its activity was completely abrogated. Further, the purified E20c remained active at pH ranging from 2 to 8; maximum antimicrobial activity was observed at pH 4 and 6.
Table 3.
Physico-chemical characterization of E20c
| Physico-chemical parameter | Treatment | Zone of inhibition (mm) |
|---|---|---|
| Untreated control | 16 ± 0.11 | |
| Temperature | 60 °C (30 min) | 16 ± 0.12 |
| 60 °C (60 min) | 14 ± 0.10 | |
| 80 °C (30 min) | 14 ± 0.22 | |
| 80 °C (60 min) | 14 ± 0.08 | |
| 100 °C (15 min) | 14 ± 0.14 | |
| 100 °C (30 min) | 13 ± 0.15 | |
| 100 °C (60 min) | 13 ± 0.15 | |
| 121 °C (15 min) | –* | |
| pH | 2 | 10 ± 0.17 |
| 4 | 16 ± 0.11 | |
| 6 | 16 ± 0.04 | |
| 8 | 13 ± 0.24 | |
| 10 | – | |
| Enzymes (1 mg/ml) | Proteinase K | – |
| Pepsin | – | |
| Trypsin | – | |
| Lipase | 16 ± 0.12 |
Zone of inhibition (mm) of E20c was determined by using agar gel diffusion assay against S. enterica. The results are the mean ± SD of three independent experiments preformed in triplicates
–, No zone of inhibition observed
The susceptibility of E20c to various enzymes was also determined. Results showed that treatment of E20c with proteinase K, pepsin and trypsin resulted in complete abrogation of the antimicrobial activity. On the other hand, lipase treatment of E20c had no effect on the antimicrobial activity of E20c (Table 3).
Hemolytic activity
The toxicity of E20c was determined by testing its hemolytic activity against human red blood cells (RBCs). E20c at the highest concentration of 5 µg/ml did not cause any significant (p < 0.001) hemolysis of RBCs as compared to the phosphate buffer saline (PBS)-treated negative contol. On the other hand, treatment of RBCs with 1% Triton X-100 resulted in 98% hemolysis (Fig. 2).
Fig. 2.

Hemolytic activity of E20c. Enterocin at different concentrations was incubated with human RBCs in PBS for 1 h. RBCs suspended in PBS were used as untreated control. Error bars represent ± SD of three independent experiments performed in triplicates. All groups were compared to the untreated control and asterisks show significant difference (p < 0.001) from untreated control
Minimum inhibitory concentration (MIC) and time-kill assay
The MIC of E20c for S. enterica was determined by broth microdilution assay that showed that E20c inhibited the visible growth of S. enterica, and the MIC was calculated as 0.5 µg/ml. Further, to determine the bactericidal mode of antimicrobial activity of E20c, time-kill assay of enterocin at 2× MIC i.e. 1 µg/ml concentration was performed against S. enterica. Results showed that the treatment of S. enterica cells with E20c resulted in 2.7 and 5.0 log10 reduction in CFU/ml at15 and 60 min, respectively (Fig. 3).
Fig. 3.

Time-kill assay of E20c against S. enterica. The S. enterica cells were treated with 2× MIC of E20c i.e. 1 µg/ml and plated on nutrient agar plates at different time points. Error bars are representative of ± SD of the three independent experiments performed in triplicates
Effect of E20c on the cell membrane permeability
The membrane damaging effect of E20c on S. enterica cells was determined by flow cytometry after staining with DNA intercalating dye, propidium iodide (PI) that stains only the cells having damaged membranes. Treatment of S. enterica cells with E20c resulted in increase in the PI fluorescence of cells from 17% at 0 h to 63% after 15 min and to 84% at 30 min (Fig. 4a). The membrane damaging effect of E20c on S. enterica cells was simultaneously visualized by confocal microscopy. The images (Fig. 4b) of E20c-treated S. enterica cells stained with PI showed an exponential increase in the number of PI-fluorescent cells with increase in the treatment time as compared to the untreated cells.
Fig. 4.
a Histograms showing fluorescence of PI-stained enterocin-treated/untreated S. enterica cells b confocal microscopic images depicting PI fluorescence of enterocin-treated/untreated S. enterica cells. Lane 1 shows fluorescence of untreated S. enterica cells. Lane 2 and Lane 3 show fluorescence after 15 and 30 min of enterocin treatments, respectively. S. enterica cells in the mid-log phase were suspended in PBS and treated with 1 µg/ml of E20c for 15 and 30 min. Thereafter, the cells were stained with PI and the fluorescence was determined in flow cytometer. Data is represented as histograms with counted bacterial events displayed on y axis and increase in fluorescence on x axis
Scanning electron microscopy (SEM) studies
Further, the effect of E20c on the cell surface of S. enterica cells was visualized by electron microscopy. SEM images showed that the cell surface morphology of untreated S. enterica cells was smooth and continuous with good structural integrity (Fig. 5a). On the other hand, E20c treatment of S. enterica cells resulted in the shrinkage of their volume and appearance of surface indentations probably due to the loss of cell turgidity or cell membrane damage caused by the action of E20c.
Fig. 5.

Scanning electron microscopy images of E20c-treated/untreated S. enterica cells at 15000X magnification a untreated S. enterica cells, bS. enterica cells treated with 1 µg/ml of E20c for 60 min
Efflux of potassium ions
Disruption of the integrity of the cell membrane results in efflux of small ions. Therefore, we evaluated the effect of different doses of E20c on the efflux of potassium ions from S. enterica cells. As shown in Fig. 6, treatment of S. enterica cells with E20c resulted in significant (p < 0.05) increase in extracellular concentration of potassium ions at all time points in a dose-dependent manner. Efflux of potassium ions was observed within 2 min of the addition of E20c that peaked to 16.3 and 8.4 parts per million (ppm) at concentrations 1.0 and 0.5 µg/ml, respectively at 6 min, after which the effect plateaued (Fig. 6).
Fig. 6.
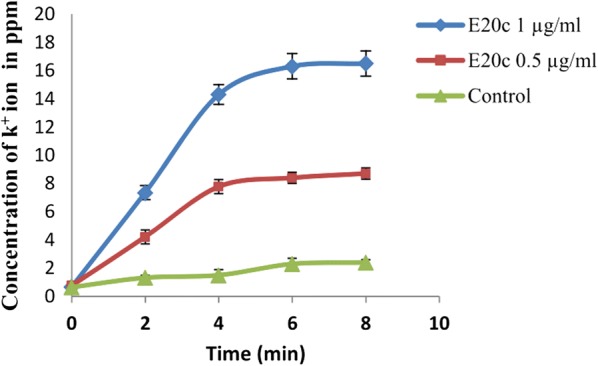
Efflux of potassium ions from S. enterica cells in response to the treatment of different concentrations of E20c. S. enterica cells without E20c was used as controls. Error bars are representative of ± SD of the three independent experiments performed in triplicates
Checkerboard titrations
The antibiotic susceptibility profile of S. enterica MTCC 733 used in the study was determined by standard Kirby Bauer method that showed that it was resistant to number of antibiotics in different classes (Additional file 1, Table S1). Fast emerging antibiotic resistance among S. enterica strains is a serious problem that warrants novel alternatives. One of the alternatives could be to use bacteriocins as adjuncts to lower the therapeutic doses of antibiotics. Thus, interaction of E20c with conventional antibiotics and its effect on lowering the MIC of antibiotics for S. enterica was studied by using checkerboard titrations. Interestingly, as shown in Table 4, the MIC of E20c for S. enterica was lower than the MIC of all the tested antibiotics. Further, checkerboard titrations revealed that E20c had synergistic interaction with ciprofloxacin and the tested ß-lactams viz. ampicillin, penicillin and ceftriaxone as the fractional inhibitory concentration index (FICI) obtained in combination with E20c in all the cases was less than 0.5. E20c lowered the MIC of ciprofloxacin by 12.8 times and that of the three tested ß-lactams in the range 6.4–13.1 (Table 4). However, in combination with carbenicillin, E20c showed indifference.
Table 4.
MIC and FICI of E20c alone and in combination with antibiotics against S. enterica
| Antibiotic | MIC (µg/ml) | FIC of antibiotic | FIC of enterocin 20c | FICI antibiotic + E20c | Fold decrease in MIC of antibiotic | ||
|---|---|---|---|---|---|---|---|
| Antibiotic | E20c | Antibiotic/E20c | |||||
| Ampicillin | 12.5 | 0.5 | 1.81/0.07 | 0.14 | 0.14 | 0.28 | 6.9 |
| Penicillin | 25 | 0.5 | 1.9/0.156 | 0.08 | 0.32 | 0.39 | 13.1 |
| Ceftriaxone | 43.5 | 0.5 | 6.7/0.07 | 0.15 | 0.14 | 0.29 | 6.4 |
| Ciprofloxacin | 400 | 0.5 | 31.2/0.15 | 0.078 | 0.31 | 0.38 | 12.8 |
| Carbenicillin | 21.7 | 0.5 | 6.5/0.15 | 0.29 | 0.31 | 0.60 | 3.3 |
FICI = FIC of antibiotic + FIC of E20c = (MIC of antibiotic in combination/MIC of antibiotic alone) + (MIC of E20c in combination/MIC of E20c alone). The interaction was interpreted as follows: synergy, FICI ≤ 0.5; indifference, 0.5 < FICI < 2; and antagonism, FICI > 2. The values are mean ± SD of experiments performed in triplicate
Discussion
In this study, enterocin E20c was purified from the CS of probiotic strain E. hirae 20c. E. hirae 20c was selected for the purification of E20c as its CS inhibited the growth of several Gram-negative bacterial pathogens, and the strain possessed good probiotic properties with no known virulence factors or genes [15]. The purified E20c was obtained as a single peak with the molecular weight of 6.5 kDa. The peak was further characterized by MALDI TOF/TOF MS and Pmf analysis. MASCOT search of the peptides obtained by trypsin digestion did not matched with any of the known bacteriocin; but it shared 34% homology with a hypothetical protein of E. faecalis. The sequence of the hypothetical protein was searched by Phobius software [16] to determine the presence of signal peptide that indicates the secretory nature of the protein. Results showed that the hypothetical protein possessed 41 amino acid long signal sequence (data not shown) that indicated its secretory nature. One of the peptide fragment of E20c matched with the signal peptide sequence of the hypothetical protein thereby indicating the secretory nature of E20c. The physico-chemical characterization of E20c was done that showed that it was both heat stable and stable at pH ranging between 2 and 8. The antimicrobial spectrum of E20c showed that it was active against both Gram-positive and Gram-negative pathogens but not against commensal lactobacilli isolated from the stool samples of healthy individuals. The antibiotic susceptibility profiles (Additional file 1) of S. enterica, Sh. flexneri and Es. coli strains used in the current study revealed that all the three pathogens could be MDR strains as they were resistant to at least one agent in more than 3 different antibiotic classes. Thus, enterocin 20c inhibited the growth of MDR Gram-negative human gut pathogens but not that of commensal gut lactobacilli spp.
Further, literature search revealed that only two enterocins, i.e., hiracin JM79 and LD3 have been earlier reported from E. hirae. Hiracin JM79 of molecular weight 8.157 kDa had antimicrobial activities against Gram-positive bacteria only, including various species of Lactobacillus, Enterococcus, and Pediococcus [17]. Whereas, enterocin LD3 (6 kDa) inhibited Gram-positive bacteria such as Enterococcus, Lactococcus spp. including lactobacilli, and Gram-negative bacteria, including S. typhi [18]. E20c, in contrast, had no activity against human commensal lactobacilli. Commensal gut lactobacilli are beneficial to human health owing to the number of important functional properties; therefore, their inhibition may lead to gut dysbiosis [19].
Further, time-kill studies were performed to determine the mode of action of E20c against S. enterica. Antimicrobial agents are categorized as bactericidal if they result in > 3 log decrease in CFU/ml [20]. Treatment of S. enterica cells with E20c at a dose of 1 µg/ml, resulted in a time-dependent decrease in the viable cell counts with 5 log10 CFU change within 60 min. This indicated the bactericidal mode of action of the enterocin. Similar results were obtained for bacteriocin BM1157 which caused more than 3 log10 decrease in CFU of Lis. monocytogenes after 30 min treatment [21]. In another study, pentocin JL-1 treatment resulted in 4.5 log10 decrease in CFUs of Staph. aureus cells within 60 min [22]. As bacteriocins are known to kill the target cell by permeabilizing their cell membrane due to their cationic nature, the effect of E20c on the cell membrane permeability of S. enterica cells was determined by flow cytometry and confocal microscopy. E20c treatment of S. enterica cells resulted in membrane destabilization that led to enhanced uptake of PI within the cells as shown by increase in their fluroscence with time. These results are similar to that reported for other bacteriocins such as nisin [23] and lactacin F [24]. Further, the SEM images of E20c-treated S. enterica clearly showed cell surface damaging effects such as cell shrinkage and surface indentations due to the loss of cell turgidity or membrane damage. Disruption of cell membrane integrity is associated with efflux of small molecules such as potassium ions and ATP, and large molecules such as proteins depending on the size of the pore [25]. Our results showed that E20c disrupted the cell membrane integrity of S. enterica cells resulting in efflux of potassium ions in a dose-dependent manner. Other enterocins such as enterocin P [26] and enterocin CRL35 [27] are also known to induce the efflux of potassium ions from the target bacterial cell.
Conventional antibiotics such as ampicillin and ciprofloxacin are commonly used for the treatment of S. enterica infections. However, resistance to these antibiotics limits the therapeutic options. The antibiotic susceptibility profile of S. enterica used in this study indicated that the strain was resistant to a number of antibiotics in the classes β-lactam, fluoroquinolone, tetracycline and aminoglycoside (Additional file 1; [28]). MIC of penicillin for S. enterica strain used in the current study was 25 µg/ml and that of ciprofloxacin was 400 µg/ml. Next, we determined the ability of E20c to lower the MIC of resistant antibiotics. Checkerboard titrations was revealed synergistic interaction between E20c and conventional antibiotics such as ampicillin, penicillin, ceftriaxone and ciprofloxacin. Treatment of S. enterica cells with conventional antibiotics in the presence of E20c lowered the MIC of antibiotics in the range 6.4 (ceftriaxone) to 13.1 (penicillin). However, no synergism was observed with carbenicillin. Few other studies have reported the synergistic effect of purified bacteriocins with antibiotics. For example, Singh et al. [29] showed the synergistic effect of nisin in combination with ampicillin, ceftriaxone, and cefotaxime against the clinical strains of S. enterica serovar Typhi. Enterocin CRL35 at a very low dose of 4 ng/ml showed synergy with tetracycline, erythromycin, and chloramphenicol but not with ciprofloxacin and ampicillin against Lis. innocua [30]. In another study, enterocins DD28 and DD93 at the dose of 50 µg/ml synergized with erythromycin and kanamycin against methicillin-resistant Staph. aureus [31].
The S. enterica strain used in the present study showed resistance to antibiotics penicillin, methicillin and ciprofloxacin. ß-lactam antibiotics are known to enter Gram-negative bacterial cells through porins; whereas, fluoroquinolones enter through both porins and lipid-mediated pathways [32]. Therefore, one of the mechanism of ß-lactam and ciprofloxacin resistance used by Gram-negative bacteria includes loss or severe reduction in the numbers of porins, or mutation leading to reduced permeability of porins [33]. Thus, the mechanism by which E20c synergized with these antibiotics may be explained by their ability to enhance the permeability of the outer membrane to the antibiotics thereby reversing their resistance and reducing their MIC. Another study that evaluated the combinations of nisin-cefotaxime and nisin-ceftriaxone against S. enterica showed that the combination of bacteriocin-β-lactams significantly increased (p < 0.05) the cell membrane permeability of the treated cells to hydrophobic fluorescent probe, 1-N-phenylnaphthylamine [29]. Further, they also observed dose- and time-dependent inhibition of DNA, RNA and protein synthesis in the presence of the tested combinations.
Conclusion
E20c appears to be a novel low molecular weight enterocin having bactericidal activity against several Gram-negative bacteria due to its cell membrane-permeabilising action. It is a promising candidate for standalone and adjunct therapy against S. enterica infections. With fast emerging problem of multidrug resistance in Salmonella-related infections, enterocin E20c can serve as an effective therapeutic option that should be further evaluated in animal models.
Methods
Bacterial strains
E. hirae 20c used for the purification of enterocin was obtained from the vaginal microflora of healthy women as reported previously [15]. Commensal Lactobacillus spp. used as indicator strains were isolated from the stool samples of healthy children and the method for the isolation and characterization were reported in the previous study [34]. All the LAB strains were cultured in De-Man Rogosa and Sharpe (MRS) media at 37 °C in anaerobic jars. All the chemicals and bacterial growth media used in this study, except where mentioned, were purchased from HiMedia Laboratories Pvt. Ltd. (Mumbai, India).
All the indicator pathogenic strains used in the study were procured from MTCC, Institute of Microbial Technology, Chandigarh, India. The pathogenic indicator strains, except M. smegmatis were propagated in Brain heart infusion (BHI) broth at 37 °C under aerobic stationary conditions. M. smegmatis was cultured in the 7H9 broth supplemented with Middlebrook OADC Growth Supplement containing bovine serum albumin fraction V and Tween 80 under aerobic conditions at 37 °C.
Agar gel diffusion assay
The antimicrobial activities of E20c against various indicator pathogenic bacterial strains was determined by modified agar gel diffusion assay [35].
Purification and characterization of E20c
E20c was purified from the CS of E. hirae 20c by using ammonium sulphate precipitation followed by cation-exchange chromatography. Briefly, 1 litre of MRS broth was inoculated with 2% of overnight grown culture of E. hirae 20c and incubated at 37 °C for 16 h in anaerobic jars. To prepare the CS, culture was centrifuged at 10,000 rpm at 4 °C. The proteins in the CS were precipitated by adding ammonium sulphate till 60% saturation (w/v) at 4 °C. The protein precipitates were separated by centrifugation (10,000 rpm at 4 °C) and dissolved in sodium acetate buffer (20 mM; pH 4.5). The dissolved precipitates were further desalted by passing through Biogel PD-10 column (GE HealthCare, USA) equilibrated with sodium acetate buffer. The active fractions obtained from Biogel PD-10 column were pooled and applied on SP-Sepharose Fast Flow cation-exchange column (50 × 10 mm; GE Healthcare) and eluted with a linear salt gradient of 0.1 to 1 M NaCl in sodium acetate buffer. The fractions were tested for the antimicrobial activity against various indicator pathogens by agar gel diffusion assay. The active fractions were lyophilized and dissolved in MilliQ water. At every purification step, protein concentration was determined by using Bradford’s method [36].
Denaturing gel electrophoresis was carried out under reducing conditions to determine the molecular weight of the purified E20c [37]. Protein separation was carried out in 15% (w/v) polyacrylamide separating gel and 6% stacking gel. After electrophoresis, one lane of the gel was cut and stained with silver nitrate (SRL, India) whereas, the other lane of the gel was subjected to agar gel overlay assay against S. enterica in BHI soft agar [38].
MALDI TOF/TOF MS
The silver-stained gel bands were cut, destained, trypsinized, extracted and subjected to MS by using MALDI-TOF/TOF-Proteomics Analyzer (UltrafleXtremeTM mass spectrometer; Bruker Daltonics Inc. Germany). A combined MS and LIFT-MS/MS were performed using BioTools 3.0 software (Bruker Daltonics Inc. Germany). The TOF spectra were recorded in positive ion reflector mode with a mass range from 700 to 3500 Da. Five hundred shots were accumulated for each spectra. Two most abundant peptide ions were then subjected to fragmentation analysis to determine the peptide sequence. Database search was performed using MASCOT search engine (Version 2.1) and NCBInr protein databases. The parameters used for search were as follows: taxonomy, Firmicutes; enzyme, trypsin; the fixed modification, carbamidomethyl (C); the variable modification, Glu- > pyro-Glu (N-term Q) and oxidation (M); parent ion mass tolerance at 50 ppm and MS/MS mass tolerance of 0.7 Da; one missed cleavage allowed. The identified proteins among the top hits on the search report with individual ions scores > 44 indicated identity or extensive homology (p < 0.05).
Physico-chemical characterization of purified E20c
Thermostability of purified E20c, was determined by subjecting it at a concentration of 1 µg/ml to different temperatures for different time periods. For determining the pH sensitivity, the pH of E20c was set to different values between 2 and 10 and incubated at 37 °C for 1 h. The pH was reset at 6.5 and the antimicrobial activity was determined by agar well diffusion assay. Further, the effect of various proteolytic and lipolytic enzymes on E20c was determined. E20c was treated with enzymes proteinase K, trypsin, pepsin, and lipase (Sigma Aldrich, India) at the concentration of 1 mg/ml for 1 h at 37 °C, followed by heat inactivation at 60 °C for 10 min. The residual antimicrobial activity was determined by agar gel diffusion assay.
Safety evaluation
Hemolytic activity of E20c was evaluated by hemoglobin release assay [39] against human RBCs. The defibrinated human blood was centrifuged at 1200 rpm for 15 min at 37 °C and RBC-containing pellet was suspended in 10 ml PBS (pH 7.2). RBC suspensions (500 µl) were incubated with 100 µl of different concentrations of E20c at 37 °C for 1 h. Thereafter, the suspensions were centrifuged at 3000 rpm for 5 min and the hemoglobin release in the supernatant was monitored by taking OD415. TritonX-100 (1%) treated and PBS-treated RBCs were used as positive and negative control, respectively. The percentage of RBC lysis was calculated by using the equation: (AT–AC)/(AX–AC) × 100.
Where AT is OD415 of E20c-treated RBCs, AC is OD415 of PBS treated-RBCs and AX is OD415 of 1% tritonX-100-treated RBCs.
Determination of MIC and time-kill studies
MIC of E20c and conventional antibiotics against S. enterica was determined by broth dilution method [40]. Time-kill assay was performed as per the protocol by Joshi et al. [41] with slight modifications. E20c at the concentration of 2× MIC was added to 1 ml of BHI and inoculated with 107 CFU/ml of S. enterica cells. The cells were incubated at 37 °C and 100 µl of the culture at different time points were serially-diluted and plated on nutrient agar plates for viable cell counting.
Effect of E20c on the cell membrane permeability
The effect of E20c on cell membrane integrity was studied by using a flow cytometric method [42] and confocal microscopy with slight modifications. S. enterica cells were suspended in PBS at a concentration of 1 × 106 CFU/ml. E20c (1 µg/ml) was added to the cell suspension and incubated at 37 °C for 15 and 30 min. The cell suspension was centrifuged (10,000 rpm; 5 min) to obtain cell pellet that was treated with PI (1 µg/ml) and incubated for 15 min at 4 °C under dark. Fluorescence of S. enterica cells was monitored by running the cells through flow cytometer (Accuri C6 Flow Cytometer) in FL2 channel. Data was analyzed by using C Flow Plus software (Becton–Dickinson, San Jose, CA, USA). Simultaneously, 10 µl of PI-stained cell suspension was placed on the glass slides, fixed with 5 µl Flourmount solution (Sigma) and viewed under a confocal microscope (Nikon, A1R).
Scanning electron microscopy studies
SEM was performed to view the morphological changes induced in the E20c-treated S. enterica cells. Overnight grown culture of S. enterica at OD595 of 0.3 was treated with E20c (1 µg/ml) for 60 min at ambient temperature. After 60 min, the cells were centrifuged at 10,000 rpm for 5 min and re-suspended in PBS. Untreated S. enterica cells were used as control. The cell suspensions were placed on the glass coverslips and dehydrated according to the method by Kalab et al. [43]. Silver sputtering was done and the stubs were examined under SEM-EVO LS-10 (Carl Zeiss, Germany).
Efflux of potassium ions
Destabilization of the cell membrane results in efflux of small ions. Therefore, we evaluated the effect of different doses of E20c treatment of S. enterica on the extracellular potassium ion concentration [44]. The bacterial cells grown till mid-log phase were centrifuged (10,000 rpm; 5 min) to obtain cell pellet, washed twice and suspended in 2.5 mM sodium HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) buffer (pH 7.0) to obtain an OD595 of 1.0. Purified E20c was added to the cell pellets of S. enterica in two separate tubes to obtain final concentrations of 1 µg/ml and 0.5 µg/ml. Samples (1 ml) were taken at intervals of 2, 4, 6, and 8 min and immediately chilled on ice. S. enterica cells without E20c was used as controls. The samples were filter sterlised (0.2 μ) to separate the cells and the potassium ion concentration in the supernatants was determined by flame photometry (Systronics 128, Gujarat, India). The experiment was performed thrice in triplicates.
Checkerboard titrations
Interaction of E20c with conventional antibiotics was determined by using checkerboard titration method [45]. Each antimicrobial was used at tenfold higher concentration than its MIC and diluted to test concentrations higher, equal and lower than MIC. Two-fold serial dilutions of the combination of antibiotics with E20c were made in 100 µl Mueller–Hinton broth in 96-well microtitre plate. Overnight grown culture of S. enterica was diluted using sterile BHI broth to obtain OD595 of 0.1 (108 log10 CFU/ml). Five microlitres (5 × 105 log10 CFU) of the culture suspension was added to each well and the plate was incubated at 37 °C for 24 h and observed for visual turbidity. The FICI was calculated as follows: FICI = FIC of antibiotic + FIC of E20c, where FIC of antibiotic is the MIC of antibiotic in the combination/MIC of antibiotic alone, and FIC of E20c is the MIC of E20c in the combination/MIC of E20 alone. FICI ≤ 0.5 indicate synergy; 0.5 < ΣFIC < 2 indicate Indifference; and ΣFIC > 2 indicate antagonism.
Supplementary information
Additional file 1: Table S1: Antibiotic susceptibility profile of Salmonella enterica MTCC 733. Table S2: Antibiotic susceptibility profile of E. coli MTCC119. Table S3: Antibiotic susceptibility profile of Shigella flexneri MTCC1457.
Abbreviations
- CS
Culture supernatant
- LAB
Lactic acid bacteria
- MRS
De-Man Rogosa and Sharpe
- MIC
Minimum inhibitory concentration
- FIC
Fractional inhibitory concentration
- RBC
Red blood cells
- WHO
World health organization
- BHI
Brain heart infusion media
- CFU
Colony forming units
- PBS
Phosphate buffered saline
- MS
Mass spectrometry
- PMF
Peptide mass fingerprinting
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
Authors’ contributions
SK conceived the idea and supervised the experiments. PS and SK designed the experiments and PS performed all of the experiments except potassium efflux studies. MR designed and executed potassium efflux studies and flame photometry. PS and SK discussed the results and contributed to the final manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by research grants (Grant Number: 42-478/2013 SR) sponsored by University Grants Commission (UGC), New Delhi, India and Promotion of University Research and Scientific Excellence (PURSE) by Department of Science and Technology, India. Preeti Sharma is thankful to University of Potential for Excellence scheme of UGC for the fellowship. Muzamil Rashid is thankful to Rashtriya Uchchattar Shiksha Abhiyan (RUSA), Component 2.0 scheme of Ministry of Human Resource Development, Government of India for Junior Research Fellowship.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Ethics approval and consent to participate
For the isolation of RBCs, the blood of healthy volunteers above the age of 18 yrs was drawn after taking their written informed consent. The protocol was approved by the Institutional Human Ethics Committee, Guru Nanak Dev University and performed in accordance with the guidelines of the Ethics Committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12934-020-01352-x.
References
- 1.Toner E, Adalja A, Gronvall GK, Cicero A, Inglesby TV. Antimicrobial resistance is a global health emergency. Health Secur. 2015;13(3):153–155. doi: 10.1089/hs.2014.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pewtrusts. https://www.pewtrusts.org//media/assets/2018/09/antibiotics_currently_in_global_clinical_development_sept2018.pdf.
- 3.Cavera VL, Arthur TD, Kashtanov D, Chikindas ML. Bacteriocins and their position in the next wave of conventional antibiotics. Int J Antimicrob Agents. 2015;46(5):494–501. doi: 10.1016/j.ijantimicag.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Chikindas ML, Weeks R, Drider D, Chistyakov VA, Dicks LM. Functions and emerging applications of bacteriocins. Curr Opin Biotechnol. 2018;49:23–28. doi: 10.1016/j.copbio.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernández L, Delgado S, Herrero H, Maldonado A, Rodríguez JM. The bacteriocin nisin, an effective agent for the treatment of Staphylococcal mastitis during lactation. J Hum Lact. 2008;24:311–316. doi: 10.1177/0890334408317435. [DOI] [PubMed] [Google Scholar]
- 6.Wu J, Hu S, Cao L. Therapeutic effect of nisin Z on subclinical mastitis in lactating cows. Antimicrob Agents Chemother. 2007;51(9):3131–3135. doi: 10.1128/AAC.00629-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathur H, O’Connor PM, Hill C, Cotter PD, Ross RP. Analysis of anti-Clostridium difficile activity of thuricin CD, vancomycin, metronidazole, ramoplanin, and actagardine, both singly and in paired combinations. Antimicrob Agents Chemother. 2013;57(6):2882–2886. doi: 10.1128/AAC.00261-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brumfitt W, Salton MR, Hamilton-Miller JM. Nisin, alone and combined with peptidoglycan-modulating antibiotics: activity against methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. J Antimicrob Chemother. 2002;50:731–734. doi: 10.1093/jac/dkf190. [DOI] [PubMed] [Google Scholar]
- 9.Mathur H, Field D, Rea MC, Cotter PD, Hill C, Ross RP. Bacteriocin-antimicrobial synergy: a medical and food perspective. Front Microbiol. 2017;8:1205. doi: 10.3389/fmicb.2017.01205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346–353. [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. (2018). http://www.who.int/mediacentre/factsheets/typhoid/en/.
- 12.Crump JA, Sjölund-Karlsson M, Gordon MA, Parry CM. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. Clin Microbiol Rev. 2015;28(4):901–937. doi: 10.1128/CMR.00002-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karkey A, Thwaites GE, Baker S. The evolution of antimicrobial resistance in Salmonella Typhi. Curr Opin Gastroenterol. 2018;34(1):25–30. doi: 10.1097/MOG.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 14.WHO . Global priority list of antibiotic-resistance bacteria to guide research, discovery, and development of new antibiotics. Geneva: WHO; 2017. [Google Scholar]
- 15.Sharma P, Kaur S, Kaur R, Kaur M, Kaur S. Proteinaceous secretory metabolites of probiotic human commensal Enterococcus hirae 20c, E. faecium 12a and L12b as antiproliferative agents against cancer cell lines. Front Microbiol. 2018 doi: 10.3389/fmicb.2018.00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Käll L, Krogh A, Sonnhammer EL. A combined transmembrane topology and signal peptide prediction method. J Mol Biol. 2004;338(5):1027–1036. doi: 10.1016/j.jmb.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez J, Diep DB, Herranz C, Nes IF, Cintas LM, Hernández PE. Amino acid and nucleotide sequence, adjacent genes, and heterologous expression of hiracin JM79, a sec-dependent bacteriocin produced by Enterococcus hirae DCH5, isolated from Mallard ducks (Anas platyrhynchos) FEMS Microbiol Lett. 2007;270(2):227–236. doi: 10.1111/j.1574-6968.2007.00673.x. [DOI] [PubMed] [Google Scholar]
- 18.Gupta A, Tiwari SK, Netrebov V, Chikindas ML. Biochemical properties and mechanism of action of enterocin LD3 purified from Enterococcus hirae LD3. Probiotics Antimicrob Proteins. 2016;8:161–169. doi: 10.1007/s12602-016-9217-y. [DOI] [PubMed] [Google Scholar]
- 19.Francino MP. Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Front Microbiol. 2016;6:1543. doi: 10.3389/fmicb.2015.01543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalia V, Miglani R, Purnapatre KP, Mathur T, Singhal S, Khan S, Voleti SR, Upadhyay DJ, Saini KS, Rattan A, Raj VS. Mode of action of ranbezolid against staphylococci and structural modeling studies of its interaction with ribosomes. Antimicrob Agents Chemother. 2009;53:1427–1433. doi: 10.1128/AAC.00887-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yi L, Luo L, Lü X. Heterologous expression of two novel bacteriocins produced by Lactobacillus crustorum MN047 and application of BM1157 in control of Listeria monocytogenes. Food Control. 2018;86:374–382. doi: 10.1016/j.foodcont.2017.11.042. [DOI] [Google Scholar]
- 22.Jiang H, Zou J, Cheng H, Fang J, Huang G. Purification, characterization, and mode of action of Pentocin JL-1, a novel bacteriocin isolated from Lactobacillus pentosus, against drug-resistant Staphylococcus aureus. Biomed Res Int. 2017;2017:1. doi: 10.1155/2017/7657190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weeks ME, von Caron GN, James DC, Smales CM, Robinson GK. Monitoring changes in nisin susceptibility of Listeria monocytogenes Scott A as an indicator of growth phase using FACS. J Microbiol Meth. 2006;66(1):43–55. doi: 10.1016/j.mimet.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Dal Peraro M, Van Der Goot FG. Pore-forming toxins: ancient, but never really out of fashion. Nat Rev Microbiol. 2016;14(2):77–92. doi: 10.1038/nrmicro.2015.3. [DOI] [PubMed] [Google Scholar]
- 25.Herranz C, Cintas LM, Hernández PE, Moll GN, Driessen AJ. Enterocin P causes potassium ion efflux from Enterococcus faecium T136 cells. Antimicrob Agents Chemother. 2001;45(3):901–904. doi: 10.1128/AAC.45.3.901-904.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minahk CJ, Farías ME, Sesma F, Morero RD. Effect of enterocin CRL35 on Listeria monocytogenes cell membrane. FEMS Microbiol Lett. 2000;192(1):79–83. doi: 10.1111/j.1574-6968.2000.tb09362.x. [DOI] [PubMed] [Google Scholar]
- 27.Dalmau M, Maier E, Mulet N, Vinas M, Benz R. Bacterial membrane injuries induced by lactacin F and nisin. Int Microbiol. 2002;5:73–80. doi: 10.1007/s10123-002-0063-2. [DOI] [PubMed] [Google Scholar]
- 28.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 29.Singh AP, Preet S, Rishi P. Nisin/β-lactam adjunct therapy against Salmonella enterica serovar Typhimurium: a mechanistic approach. J Antimicrob Chemother. 2014;69(7):1877–1887. doi: 10.1093/jac/dku049. [DOI] [PubMed] [Google Scholar]
- 30.Minahk CJ, Dupuy F, Morero RD. Enhancement of antibiotic activity by sub-lethal concentrations of enterocin CRL35. J Antimicrob Chemother. 2004;53(2):240–246. doi: 10.1093/jac/dkh079. [DOI] [PubMed] [Google Scholar]
- 31.Al Atya AK, Belguesmia Y, Chataigne G, Ravallec R, Vachée A, Szunerits S, et al. Anti-MRSA activities of enterocins DD28 and DD93 and evidences on their role in the inhibition of biofilm formation. Front Microbiol. 2016;7:817. doi: 10.3389/fmicb.2016.00817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nikaido H, Rosenberg EY, Foulds J. Porin channels in Escherichia coli: studies with beta-lactams in intact cells. J Bacteriol. 1983;153(1):232–240. doi: 10.1128/JB.153.1.232-240.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delcour AH. Outer membrane permeability and antibiotic resistance. Biochim Biophys Acta Proteins Proteom. 2009;1794:808–816. doi: 10.1016/j.bbapap.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaur S, Kaur S, Sharma P, Singh J, Kalia N. Anti-biofilm properties of the faecal probiotic lactobacilli against Vibrio spp. Front Cell Infect Microbiol. 2018;8:120. doi: 10.3389/fcimb.2018.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaur S, Sharma P. Protease-sensitive inhibitory activity of cell-free supernatant of Lactobacillus crispatus 156 synergizes with ciprofloxacin, moxifloxacin and streptomycin against Pseudomonas aeruginosa: an in vitro study. Probiotics Antimicrob Proteins. 2015;7(2):172–180. doi: 10.1007/s12602-015-9188-4. [DOI] [PubMed] [Google Scholar]
- 36.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 37.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 38.Dezwaan DC, Mequio MJ, Littell JS, Allen JP, Rossbach S, Pybus V. Purification and characterization of enterocin 62-6, a two-peptide bacteriocin produced by a vaginal strain of Enterococcus faecium: potential significance in bacterial vaginosis. Microb Ecol. 2007;19:241–250. doi: 10.1080/08910600701538240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paiva AD, de Oliveira MD, de Paula SO, Baracat-Pereira MC, Breukink E, Mantovani HC. Toxicity of bovicin HC5 against mammalian cell lines and the role of cholesterol in bacteriocin activity. Microbiology. 2012;158(11):2851–2858. doi: 10.1099/mic.0.062190-0. [DOI] [PubMed] [Google Scholar]
- 40.Ruíz FO, Gerbaldo G, García MJ, Giordano W, Pascual L, Barberis IL. Synergistic effect between two bacteriocin-like inhibitory substances produced by lactobacilli strains with inhibitory activity for Streptococcus agalactiae. Curr Microbiol. 2012;64(4):349–356. doi: 10.1007/s00284-011-0077-0. [DOI] [PubMed] [Google Scholar]
- 41.Joshi S, Bisht GS, Rawat DS, Kumar A, Kumar R, Maiti S, et al. Interaction studies of novel cell selective antimicrobial peptides with model membranes and E. coli ATCC 11775. Biochim Biophys Acta Biomembr. 2010;1798:1864–1875. doi: 10.1016/j.bbamem.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 42.Chopra L, Singh G, Jena KK, Sahoo DK. Sonorensin: a new bacteriocin with potential of an anti-biofilm agent and a food biopreservative. Sci Rep. 2015;5:13412. doi: 10.1038/srep13412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalab M, Yang AF, Chabot D. Conventional scanning electron microscopy of bacteria. Infocus Magazine. 2008;10:42–61. doi: 10.22443/rms.inf.1.33. [DOI] [Google Scholar]
- 44.McAuliffe O, Ryan MP, Ross RP, Hill C, Breeuwer P, Abee T. Lacticin 3147, a broad-spectrum bacteriocin which selectively dissipates the membrane potential. Appl Environ Microbiol. 1998;64(2):439–445. doi: 10.1128/AEM.64.2.439-445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petersen PJ, Labthavikul P, Jones CH, Bradford PA. In vitro antibacterial activities of tigecycline in combination with other antimicrobial agents determined by chequerboard and time-kill kinetic analysis. J Antimicrob Chemother. 2006;57(3):573–576. doi: 10.1093/jac/dki477. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1: Antibiotic susceptibility profile of Salmonella enterica MTCC 733. Table S2: Antibiotic susceptibility profile of E. coli MTCC119. Table S3: Antibiotic susceptibility profile of Shigella flexneri MTCC1457.
Data Availability Statement
All data generated or analysed during this study are included in this published article.