Abstract
Background:
There is a growing base of literature describing BK nephropathy (BKVN) in patients outside of the setting of kidney transplant. Previous systematic reviews of the literature have been limited by methodology or by the scope of patients included.
Study Design and Methods:
Systematic Review (Prospero # CRD42018088524).
Setting & Population:
Patients without kidney transplant who had biopsy-proven BKVN.
Selection Criteria for Studies:
Full-text articles that describe native BKVN patient cases.
Analytical Approach:
Descriptive synthesis.
Results:
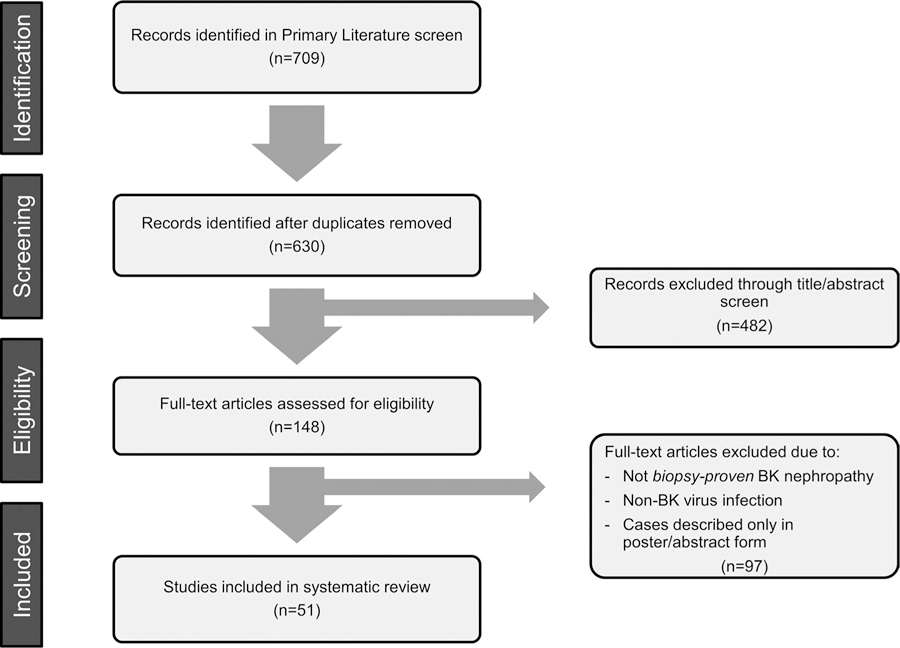
The search identified 630 unique articles of which 51 were included in the final review. Sixty-five cases (including two new cases presented in this review) were identified, all but one occurred in the setting of known immunosuppression.
Limitations:
The primary limitation was the exclusion of studies that did not fulfill the stringent review criteria. We excluded reports with only a clinical diagnosis of BKVN, such as those with viruria and/or viremia without biopsy.
Conclusions:
As of May 2018, there are 65 reported cases of BKVN in native kidneys. This represents the most comprehensive description of biopsy-proven BKVN in native kidneys to date. Evaluation for BK nephropathy should be considered in immunocompromised patients who exhibit unexplained renal failure.
Keywords: BK Nephropathy, BK Virus, NRSOT, transplantation, Tubulointerstitial nephritis
1 |. INTRODUCTION
BK virus, a member of Polyomaviridae family, was first discovered in 1971 in the urine of a kidney transplant recipient.1 BK is a non-enveloped, circular, and double-stranded DNA virus.2 There are four subtypes of BK virus, I-IV, with subtype I being the most prevalent and consisting for four subgroups (Ia, Ib-1, Ib-2, and Ic).3,4 Primary BK virus is typically acquired in childhood with seroprevalence rates in adults reaching 80%.5,6 After primary infection, the virus establishes latency in the renourinary epithelium and is usually asymptomatic in immunocompetent hosts.7 The clinical spectrum of BK virus infection in immunocompromised patients includes nephropathy, ureteral stricture, and hemorrhagic cystitis.8 Less commonly, systemic vasculitis, pneumonia, pneumonitis, retinitis, and hepatitis have been described.9–13 BK nephropathy (BKVN) are most common after kidney transplantation. Routine screening after kidney transplant leads to the diagnosis of BK viremia in 10%-20% of patients and BKVN in 2%-12% of patients.14–17 The best-studied intervention is the careful reduction of immunosuppression, and no there are no definitively effective antiviral prophylaxis or treatments.18
BKVN is rare in non-renal transplant recipients, but there is a growing literature of case reports and case series describing BKVN outside of the setting of kidney transplantation.19 The largest description to date of BKVN in non-kidney transplant recipients was published in 2016 and described 39 patients. However, previous publications on this topic have been limited by search methodology, inclusion of patients who have not undergone kidney biopsies, inclusion of patients with BK cystitis, and/or by limiting the scope of patient populations reviewed. In this study, we aimed to provide the most comprehensive description of this entity to date by presenting two cases and performing a systematic review of the literature of biopsy-proven BKVN in native kidney.
1.1 |. Case 1
A 65-year-old Caucasian man with ischemic cardiomyopathy, hypertension, and hyperlipidemia underwent an orthotopic heart transplant. His maintenance immunosuppression was tacrolimus, mycophenolate mofetil (MMF), and prednisone. His pre-transplant serum creatinine (SCr) was 1.5-1.7 mg/dL (estimated glomerular filtration rate 42-49 mL/min/1.73 m2 by the Chronic Kidney Disease Epidemiology (CKD-EPI) Collaboration equation) without hematuria or proteinuria on urinalysis, and his kidneys measured 10.0 and 10.9 cm in maximal longitudinal dimension on kidney ultrasound. His SCr remained stable after transplant. He developed post-transplant diabetes mellitus which resolved within 1 year after transplant. He had stable cardiac allograft function with an ejection fraction of 65% and no episodes of rejection.
Three years post-transplant, he developed acute kidney injury (AKI) with a SCr rising to 3.8 mg/dL over 5 months. His physical exam at that time was only remarkable for trace lower extremity edema, without evidence of intravascular volume overload. Tacrolimus trough levels were 5-7 µg/L. His urinalysis was negative for blood and albumin, urine microscopy showed no red blood cells (RBCs) or white blood cells per high powered field (hpf), and a random urine protein:creatinine ratio (UPCR) was 0.2 g/g. An ultrasound showed both kidneys to be 10.5 cm without evidence of obstruction.
He then underwent a kidney biopsy. The light microscopy showed acute tubular injury and tubulointerstitial nephritis with moderate interstitial fibrosis. There were focal tubular epithelial cells with glassy nuclear inclusions. Immunohistochemical staining for polyomavirus using SV40 and Pab597 antibodies was positive in focal tubular epithelial cell nuclei. A serum BK polymerase chain reaction (PCR) was positive (296 075 copies/mL - linear range of assay 325 to 100 million copies/mL).
His MMF was discontinued with improvement in his serum BK viral load to 6500 copies/mL, and his SCr remained stable at 3.5 mg/dL for 2 years, at which time his SCr began to rise again. Repeat serum BK PCR testing was not performed. He progressed to endstage kidney disease (ESKD). Six weeks after beginning hemodialysis, he developed a malignant pleural effusion and was subsequently found to have metastatic pancreatic cancer. He transitioned to hospice care and expired.
1.2 |. Case 2
A 34-year-old African American man with Hodgkin’s lymphoma had been treated 7 years prior to his recent presentation with cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisone. He was in remission when he presented to the hospital with diarrhea, weakness, and volume depletion. He was diagnosed with recurrence of his lymphoma as well as Staphylococcus aureus bacteremia that was treated with intravenous vancomycin. Laboratory testing was notable for AKI with a SCr of 5.8 mg/dL (increased from baseline 1.2 mg/dL) which did not improve with intravenous fluids. A urinalysis showed 500+ mg/dl albumin and small blood with 1-2 RBCs/hpf on microscopy. UPCR was 13.0 g/g.
He underwent a kidney biopsy which was notable for focal segmental glomerulosclerosis, nuclear inclusions in focal tubular epithelial cell nuclei, and immunohistochemistry for SV40 (clone Pab416) showed positive staining of focal tubular epithelial cell nuclei. These findings fulfill Banff 2018 criteria for BK nephropathy.20 At the time of this biopsy, his serum BK was negative by qualitative PCR (lower limit of detection not reported).
He required intermittent hemodialysis for two weeks with subsequent recovery of his kidney function (SCr 1.2 mg/dL). Because his Reed-Sternberg cells expressed CD20, he was treated with rituximab. One month after discontinuation of hemodialysis, his SCr worsened again, peaking at 6.5 mg/dL, along with transaminitis (aspartate aminotransferase 134, alanine aminotransferase 61 IU/L). Urine and serum BK PCR were undetectable, urinalysis showed moderate blood with 500+ mg/dL albumin with 1-2 RBCs/hpf on microscopy and UPCR was 3.3 g/g. A second kidney biopsy showed tubular epithelial cells with widespread degenerative and regenerative changes, frequent epithelial mitoses and intranuclear inclusions. Immunostaining for SV40 was positive in focal tubular epithelial cell nuclei.
He was subsequently diagnosed with hemophagocytic lymphohistiocytosis due to his lymphoma and died of multi-organ system failure.
1.3 |. Systematic review methods
We searched MEDLINE and EMBASE from inception to May 2018 using predefined keywords and/or medical subject headings (MeSH) to discover biopsy-proven cases of native kidney BKVN (Prospero # CRD42018088524). The detailed search strategy can be found in the Supplement (Tables S1 and S2). Two authors independently screened the titles and abstracts of identified references, and those meeting the inclusion criteria were retrieved for dual independent review of full-text. Searches were performed for relevant primary studies that were restricted to English but not by date. References of all papers meeting inclusion criteria were manually reviewed for identification of additional cases.
Primary studies were included for full-text articles that describe native BKVN patient cases. Exclusion criteria were (a) patients who underwent kidney transplant; (b) non-BK virus infection (eg JC virus); (c) BK viremia/viruria without immunohistochemical biopsy-proven BKVN; (d) non-human or in vitro models; and (e) cases described only in posters and abstract form. Previously published systematic reviews were only used to identify the relevant primary literature and were not included in this systematic review.
2 |. RESULTS
The search identified 630 unique articles of which 51 were included in the final review. Sixty-five cases (including our two patients) met our search criteria (Figure 1). The relevant medical histories for these patients include solid organ (heart, lung, liver, pancreas) transplant (n = 21), hematopoietic cell transplant (HCT, n = 19), human immunodeficiency virus (HIV) infection (n = 10), hematologic malignancy (n = 10), autoimmune disease (n = 1), primary immunodeficiency (n = 1), prostate cancer (n = 1), and pulmonary tuberculosis and diabetes (n = 1). One patient had no identified immunodeficiency (Table 1; Figure 2).
FIGURE 1.
Article screening process for systematic review
TABLE 1.
Individual cases of native kidney BK virus nephropathy included in our case series and systematic review of the literature
| Reference | Age/ gender | Relevant medical history | Time to diagnosisa | Therapy attempted | Therapy effect | Dialysis | Outcome |
|---|---|---|---|---|---|---|---|
| HCT | |||||||
| Aksenova43 | 10 F | Allo-HCT | 13 mo | Add IVIG, leflunomide, cidofovir, and ciprofloxacin. | Leflunomide/Cipro/IVIG simultaneously had no effect, but addition of cidofovir resulted din resolution of viremia and improved Cr | CKD | |
| Bruno44 | Nr | Allo-HCT | NR | Not reported | Not reported | N/A | Death |
| Burbach45 | 45 F | Allo-HCT | 46 mo | Reduced immunosuppression. | Viremia remained elevated. | Yes | Death |
| Lekakis46 | 51 M | Allo-HCT | 5 mo | Add leflunomide, cidofovir, and ciprofloxacin. | Viruria improved, Viremia not reported | Yes | Death |
| Maximova47 | 15 F | Allo-HCT | 3 mo | Stopped immunosuppression | Viremia improved. Persistent viremia. | No | Death |
| O’Donnell48 | 41 F | Matched-unrelated donor HCT | 30 mo | Add leflunomide. | Viremia not changed. | Yes | ESRD |
| Papanicolaou49 | 58 M | Allo-HCT | 16 mo | Add brincidofovir | Viremia improved. Persistent viremia. Cr stable. | No | Death |
| Shapiro50 | 14 M | Unrelated cord blood transplant | 1 mo | Reduced immunosuppression. Add cidofovir. | Received too few doses. Died of multiorgan failure shortly after 2nd dose of cidofovir. | No | Death |
| Sharma51 | 17 M | CML, haploidentical BMT complicated by GVHD | 2 y | Add leflunomide and IVIG. | Viremia improved. Persistent viremia. Cr worsened. | No | Death |
| Sharma51 | 16 M | AML, Allo-HCT | 4 mo | Diagnosed on autopsy | N/A | Death | |
| Sharma51 | 58 M | CLL, Allo-HCT complicated by GVHD | 24 mo | Add cidofovir. Add sirolimus due to GVHD. | Viremia improved. Persistent viremia. Cr stable. | No | CKD |
| Stracke52 | 28 F | Haploidentical T-cell depleted peripheral blood SCT | 2 y | Add cidofovir | Viremia not improved. Cr not improved | Yes | ESRD |
| Van der Bij53 | 57 M | Allo-HCT with leukemic invasion | 21 mo | Add leflunomide. | Cr worsened to ESRD, started on HD. | Yes | ESRD |
| Verghese54 | 10 M | unrelated cord blood transplant | 3.5 y | Add cidofovir and ciprofloxacin. | No effect to cidofovir, resolution of viremia to cipro. Cr improved | No | CKD |
| Verghese54 | 13 M | Allo-HCT | 2 y | Add cidofovir. | Viremia unchanged. Cr worsening. | No | Death |
| Vigil42 | 30 M | Allo-HCT | Approx. 4 y | Add ciprofloxacin. | Viremia worsened. | No | Death |
| Limaye55 | 41 M | Autologous Peripheral blood cell transfusion | 6.2 y | Add cidofovir. | Viremia resolved. | Yes | ESRD |
| O’Donnell48 | 36 F | Auto-HCT | 9 mo | Add leflunomide. | Viremia not changed. | Yes | Death |
| Sanchez-pinto56 | 10 F | Auto-HCT | 5 mo | Add cidofovir and ciprofloxacin. | Viremia improved. Persistent viremia. Cr worsened. | Yes | Death |
| Hematologic malignancy | |||||||
| Filler57 | 10 M | ALL | 3 y | Add IVIG | Viremia improved. Persistent viremia | No | CKD |
| Inaba58 | 5 F | ALL | 21 mo | Reduce chemotherapy dose. Add IVIG, cidofovir, leflunomide | Viremia improved. Persistent viremia. Cr stable. | No | Unclear |
| Collett59 | 56 M | CLL | NR | Add IVIG and ciprofloxacin. | Viremia did not respond. | No | CKD |
| Mccrory60 | 73 M | CLL | 1 y | Stopped IVIG, started leflunomide | Viremia improved. Persistent viremia. | No | CKD |
| Sangala61 | 72 M | CLL | 19 mo | Short course of methylprednisolone, added IVIG and ciprofloxacin. | Cr worsened to ESRD requiring regular HD | Yes | Unclear |
| Sharma51 | 66 M | CLL | 10 y | Add IVIG and cidofovir | Cr worsened to ESRD | Yes | ESRD |
| Sharma51 | 73 M | CLL | 1 y | Add IVIG and cidofovir | Cr remained elevated. | No | CKD |
| De Silva62 | 6 mM | Cartilage-hair hypoplasia and Hodgkin’s disease | 26 mo | Not reported | No | Death | |
| Sharma51 | 53 M | Non-Hodgkin Lymphoma | 4 y | Add cidofovir | Viremia improved. Persistent viremia. | No | Death |
| Shah (this paper) | 34 M | Non-Hodgkin lymphoma | No specific therapy | Yes | Death | ||
| HIV | |||||||
| Bratt63 | 26 M | HIV: CD4 20 × 106/L | N/A | Diagnosed on autopsy | No | Death | |
| Crum-Cianflone64 | 38 M | HIV: CD4 < 50*106/L but at time of renal failure was 0*106/L | N/A | No specific therapy | Required hemodialysis. Died 1 y later du to CNS lymphoma. | Yes | ESRD |
| Cubukcu-Dimopulo65 | 14 M | HIV: CD4 = 0 | N/A | Diagnosed on autopsy | No | Death | |
| Jung66 | 49 M | HIV: CD4 4 *106/L | VL 96800 copies/mL | N/A | No specific therapy | Worsened viremia. Cr worsened. Died of nonrenal reasons. | No | CKD |
| Manabe67 | 32 M | HIV: CD4 3 *106/ L | VL 4400 copies/mL | N/A | Diagnosed on autopsy | No | CKD | |
| Mouratoff68 | 49 M | HIV: CD4 3 × 106/L | N/A | No specific therapy | Renal function improved with antiretrovirals for HIV, but worsened within 2 mo of biopsy. | No | CKD |
| Nebuloni69 | 31 M | HIV: CD4 0.01 × 109/L | N/A | No specific therapy | 2 mo later, no clinical worsening. | No | CKD |
| Smith70 | 36 M | HIV: CD4 < 50 × 106/L | N/A | No specific therapy | Left hospital abruptly and died. | Yes | ESRD |
| Sukov71 | 43 M | HIV: CD4 2.0 × 109/L | N/A | No specific therapy | Became HD-dependent. No comment on viremia. | Yes | ESRD |
| Vallbracht10 | 27 M | HIV: n/a (autopsy) | N/A | Diagnosed on autopsy | No | Death | |
| Solid organ transplant | |||||||
| Ali72 | 12 M | Heart transplant | 21 mo | Reduce immunosuppression, add leflunomide and cidofovir | Effect unclear | No | CKD |
| Barber73 | 26 M | Heart transplant | 18 mo | Reduce immunosuppression, add cidofovir | Viremia resolved with cidofovir 1 mg/kg | Yes | ESRD |
| Butts74 | 9 F | Heart transplant | 8 y | Reduce immunosuppression, add leflunomide | Viremia reduced. Stable viremia. | No | CKD |
| Joseph75 | 60 M | Heart transplant | 3 y | Reduce immunosuppression, add ciprofloxacin and IVIG | Viremia N/A. Cr worsened. | Yes | ESRD |
| Joseph75 | 43 M | Heart transplant | 2 y | Reduce immunosuppression, add ciprofloxacin | Stable GFR. Viremia reduced. Stable viremia. | No | CKD |
| Limaye55 | 59 M | Heart transplant | 6.8 y | Diagnosed on autopsy | Refused | Death | |
| Lorica76 | 14 M | Heart transplant | 6 mo | Reduce immunosuppression, add IVIG, cidofovir, and ciprofloxacin | Viremia reduced. Stable viremia. | Yes | Death |
| Maddirala77 | 54 M | Heart transplant | 3.5 y | Reduce immunosuppression | Viremia N/A. Cr not improving. | Yes | Death |
| Menahem78 | 59 F | Heart transplant | 16 mo | Reduce immunosuppression, add cidofovir | Viruria remained positive. GFR had no improvement | Yes | ESRD |
| Pereira79 | 5 F | Heart transplant | 2 y | Reduce immunosuppression, add IVIG and cidofovir | Viral loads decreased at cidofovir 1 mg/kg but remained positive. Died of BKV rhombo-encephalomyelitis | Yes | Death |
| Sahney80 | 8 M | Heart transplant | 10 mo | Reduce immunosuppression, add IVIG and cidofovir | Viremia reduced. Stable viremia. | Yes | ESRD |
| Schmid81 | 57 M | Heart transplant | 29 mo | Reduce immunosuppression, add cidofovir | Cr improved. Viremia improved. Persistent viremia. | Yes | ESRD |
| Shah (this paper) | 70 M | Heart transplant | Reduce immunosuppression | Cr worsened. Viremia improved. Persistent viremia. | Yes | Death | |
| Dufek82 | 9 M | Lung transplant | 2 y | Reduce immunosuppression, add cidofovir | Viremia persisted | Yes | Death |
| Egli83 | 72 F | Lung transplant | 5 y | Reduce immunosuppression, add leflunomide | Viremia resolved. Cr improved. | No | CKD |
| Kuppachi84 | 63 M | Lung transplant | CKD at 2 y, biopsy at 4 y | Reduce immunosuppression, add cidofovir and leflunomide | Cr stabilized and improved. Persistent viremia | No | CKD |
| Schwarz85 | 40 M | Lung transplant | 15 mo | Add cidofovir and leflunomide | Resolution of viremia but relapsed after therapy discontinued | Yes | ESRD |
| Sharma51 | 30 M | Lung transplant | 2 y | Reduced immunosuppression | Cr worsened. Viremia improved. Viremia persistent. | No | CKD |
| Vigil42 | 70 M | Lung transplant | 2 y | Reduce immunosuppression, add IVIG and leflunomide | Viremia improved. Viremia persistent. | No | CKD |
| Zeng86 | 59 M | Liver transplant | 7 y | Not reported | Not reported | None reported | Not reported |
| Haririan87 | 54 M | Pancreas transplant | 9 mo | Not reported | Not reported | No | CKD |
| Other | |||||||
| Go88 | 79 M | Prostate cancer | N/A | Diagnosed on nephrectomy | Not reported | No | CKD |
| Krystel-Whittemore89 | 55 M | Rheumatoid Arthritis | 6 y | Reduce immunosuppression, add IVIG, ciprofloxacin, leflunomide | Viremia improved. Persistent viremia. | Temporary HD | CKD |
| Park90 | 34 M | None | N/A | Not reported | Not reported | No | CKD |
| Rosen91 | 6 M | Hyper-IGM | 5 y | Not reported | Not reported | Yes | Death |
| Sharma51 | 66 M | Pulmonary tuberculosis | 9 mo | Not reported | Not reported | Yes | Death |
ALL, acute lymphoblastic leukemia; CKD, chronic kidney disease; CLL, chronic lymphocytic leukemia; ESRD, end stage renal disease; F, female; HCT, hematopoietic stem cell transplantation; HCT, hematopoietic cell transplant; HD, hemodialysis; HIV, human immunodeficiency virus; IVIG, intravenous IgG; M, male.
Time to diagnosis is defined as the time from a known immunologic inult to diagnosis of BKVN. In practice, this refers to the time from transplant/diagnosis of illness to diagnosis of BKVN. HIV was not included in this because the time to development and diagnosis of BKVN is not a meaningful metric when the underlying HIV can be well-controlled on antiretroviral therapy, yielding long time frames or highly variable times.
FIGURE 2.
This diagram represents a breakdown of the primary medical issue faced by the native-kidney
2.1 |. Solid organ transplantation
Twelve cases of kidney biopsy-proven BKVN in heart transplant recipients were already reported in the literature and our case increased the total to 13. Ten of 13 cases occurred in males, with a mean age of 36.6 years and 38.5% of cases occurring in children. Of these cases, 9 (69%) required renal replacement therapy and mortality at time of publication was 38.5%.
Six cases of native kidney biopsy-proven BKVN were identified in patients with lung transplants (one pediatric case, five adults). The mean age at diagnosis was 47.3 years. Of these cases, two (33.3%) required renal replacement therapy and mortality at time of publication was 16.7%.
A single case of native kidney biopsy-proven BKVN was described in a liver transplant recipient whose immunosuppressive regimen consisted of tacrolimus (trough 10-12 µg/L), sirolimus (trough 8-12 µg/L), MMF, and prednisone. A single case of native kidney biopsy-proven BKVN has been noted in a pancreas transplant recipient whose immunosuppression regimen included tacrolimus (trough 12-15 ng/dL), MMF, and prednisone.
The average time from transplant to diagnosis of BKVN for the collective solid organ transplant population is 2.88 years.
2.2 |. Hematopoietic cell transplantation
A total of 19 cases of native kidney biopsy-proven BKVN were found in the literature for patients who had previously undergone HCT. The majority of cases (16/19) occurred in patients after allogeneic HCT. We excluded three cases (Iwamoto et al,21 Hoefele et al,22 Gagneux-Brunon et al23) which have previously been included in other native BKVN literature reviews because they did not perform immunohistochemistry to confirm the diagnosis. The mean age at diagnosis was 30.6 years with 42.1% of cases occurring in the pediatric population. In cases with demographic information, 58% were males. Half of these patients (8/16) required renal replacement therapy and 63.2% died during follow-up. A number of cases in the original search described BK virus-induced hemorrhagic cystitis, but these studies were excluded from our systematic review as these patients did not have BKVN.
The average time from transplant to diagnosis of BKVN for the hematopoietic stem cell transplant population is 1.91 years.
2.3 |. Hematologic malignancy
Including our second case, 10 cases of native kidney biopsy-proven BKVN in the setting of hematologic malignancy have been reported. Nine of 10 patients were males. The mean age was 44.8 years with 30% of cases occurring in children. Of these cases, 3 (30%) required renal replacement therapy and three patients died. The underlying pathology varied and outcome data is minimal in this cohort of patients. The average time from diagnosis of hematologic malignancy to diagnosis of BKVN is 3.06 years.
2.4 |. Human immunodeficiency virus
A total of 10 cases of native kidney biopsy-proven BKVN were reported in patients with HIV infection. All reported cases occurred in males, and the mean age was 34.5 years, with 10% of cases occurring in a pediatric population. Of these cases, 3 (30%) required renal replacement therapy and mortality at time of publication was 30%. The median CD4 count at diagnosis was 4 × 106/L (Interquartile Range (IQR): 1.5 × 106-35 × 106).
2.5 |. Other
Individual cases of native biopsy-proven BKVN were reported in the setting of rheumatoid arthritis, Hyper IgM immunodeficiency syndrome (with BK virus infection diffusely in both kidneys and the urothelium on autopsy), pulmonary tuberculosis and diabetes mellitus, and prostate cancer. A final case reported a 34-year-old male with no relevant medical history in whom long-term follow-up and workup for immunocompromised states were not reported.
3 |. DISCUSSION
BK virus infection is a well-described complication of kidney transplantation. BK viremia is associated with impaired graft function 24 and prevalence is estimated at 10%-20% while BKVN occurs in approximately 2%-12% of kidney transplant recipients and is associated with allograft loss.14,17 There is limited literature for non-renal transplant patients diagnosed with BKVN. Here, we have presented two cases from our institution and performed a systematic review of the literature describing published cases of native kidney BKVN. Our systematic review is comprised of 65 native BKVN cases in diverse populations including solid organ transplant recipients, HCT recipients and those with hematologic malignancy. Of these 65 cases, 14 (21.5%) resulted in chronic requirement for dialysis and 26 (40%) of patients died during follow-up. This study represents the most comprehensive description of BK VN in the native kidney to date.
Our systematic review suggests that BKVN should be included in the differential diagnosis for immunosuppressed or immunocompromised patients with native kidney dysfunction, and evaluation for BK nephropathy in these patients should be considered. Of note, BKV serum PCR screening is limited by lack of a universal standard assay, and the majority of BKV PCR assay primers are based on the genotype I sequence. However, if the patient’s clinical course or biopsy-findings are inconsistent with BKV serum PCR results, sample re-analysis at a lab that utilizes different BKV genotype sequences is recommended.19,25 The utility of screening immunosuppressed/immunocompromised patients with renal insufficiency for BK viremia, and the predictive of BK viremia for finding BK nephropathy on kidney biopsy in this patient population, remain to be studied.
Twelve of 65 (18.5%) patients identified in our review were children. Prior studies in pediatric kidney transplant have demonstrated an increased risk with primary infection but serostatus was unavailable in the two cases we presented.26,27
While effective anti-viral therapy is not currently available, the experience in kidney transplant recipients suggests that careful reduction of immunosuppression, when possible, can be effective in treating BKVN. In immunocompetent patients or those in whom immunosuppression reduction is not possible, alternative therapies can be considered. There is an extensive literature in the renal transplant population detailing use of mammalian target of rapamycin (mTOR) inhibitors, leflunomide, flouroquinolones, cidofovir, and intravenous immunoglobulin (IVIg) for the treatment of BKVN, but none have been shown to be particularly effective.28,29 Further research is ongoing, with newer agents such as brincidofovir, virus-specific T lymphocytes, and IVIg being currently studied.30–32
Noting the limitation of publication bias, a number of different treatment strategies were reported in the cases we review. Monotherapy with cidofovir resulted in undetectable viral loads in 3/12 patients reviewed, ciprofloxacin resulted in undetectable viral loads in 1/3 patients reviewed, and leflunomide in 1/6. Prospective trials are needed to determine efficacy of these agents used alone or in concert with others to better understand how to treat this entity.
Several authors have observed that BKVN is more common among heart transplant recipients than other solid organ transplant populations.33–35 While the reason for this is unknown, this disparity persists in our systematic review. It has been hypothesized that this may be related to their cumulative immunosuppressive burden or due to the effect of cardiac allograft dysfunction on renal perfusion, in accordance with a two-hit hypothesis.36–39 Conversely, only one patients with BKVN after liver transplant has been described, in a patient on fourdrug immunosuppression. Previous literature has described a lower prevalence of BK in liver transplant recipients compared to heart or kidney transplant recipients, which could reflect the lower cumulative immunosuppression exposure that liver transplant patients.40
It should also be noted that there is a large base of literature on the incidence of viremia and viruria in patients with systemic lupus erythematosus and lupus nephritis, but our systematic review did not find any cases of biopsy-proven BKVN in this population.41 Animal studies have shown that infection with BK can virus can lead to the formation of anti-dsDNA antibodies, resulting in speculation that the BK virus may have a pathogenic role in the development of systemic lupus.41
While our study is purely descriptive, we submit that the methodology and criteria for our systematic review represents the most comprehensive description of BKVN outside of the kidney transplant setting to date. These results show that our search methodology was more sensitive than prior reviews. Compared to the largest prior review, published in 2016 and which found 39 patients,42 we have added 26 cases to the literature. Taking into account our 2 cases, only 3 cases in our review were published in 2016 or after. This indicates that the majority of the cases we have added were found due to our search methodology, the strength of which include our robust review of the primary and secondary literature as well as the rigorous inclusion and exclusion criteria applied to studies identified by our search.
Our series is also more precise and specific than previous reviews, as we only included patients with biopsy-proven BKVN. This stringent inclusion criterion ensured high-quality data and minimized crossover with other BK complications such as hemorrhagic cystitis or asymptomatic viruria/viremia without biopsy-proven BKVN. A number of non-biopsy proven cases have been included in prior reviews based on elevated SCr in the setting of BK hemorrhagic cystitis, high levels of viruria, and high levels of viremia.
The primary limitation of our study in assessing the true burden of BKVN outside of the kidney transplant setting is the exclusion of studies that did not fulfill the stringent review criteria. We excluded reports with only a clinical diagnosis of BKVN, such as those with viruria and/or viremia without biopsy. As a kidney biopsy are not always pursued in immunocompromised patients with renal insufficiency, the total number of native-kidney BKVN patients may be greater than what is encompassed in our systematic review. As such, some patients that were excluded from our search may have had BKVN. The lack of a PCR standard for detecting BK viremia may have lead to discordant results between biopsy findings and blood testing. Finally, the retrospective nature of our review precludes us from establishing the incidence and prevalence of the disease.
4 |. CONCLUSION
This case series and systematic review of the literature provides the most comprehensive description of BKVN outside of the kidney transplant setting to date. While the incidence of BKVN in these patient populations remains to be determined evaluation for BKVN should be considered in immunocompromised patients with unexplained renal insufficiency.
Supplementary Material
Acknowledgments
Funding information
Benjamin Laskin is supported by K23 DK101600.
Footnotes
CONFLICT OF INTEREST
The authors declared that they have no relevant financial interests.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Gardner SD, Field AM, Coleman DV, Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971;297(7712):1253–1257. [DOI] [PubMed] [Google Scholar]
- 2.Bennett SM, Broekema NM, Imperiale MJ. BK polyomavirus: emerging pathogen. Microbes Infect. 2012;14(9):672–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng H-Y, Nishimoto Y, Chen Q, et al. Relationships between BK virus lineages and human populations. Microbes Infect. 2007;9(2):204–213. [DOI] [PubMed] [Google Scholar]
- 4.Zhong S, Randhawa PS, Ikegaya H, et al. Distribution patterns of BK polyomavirus (BKV) subtypes and subgroups in American, European and Asian populations suggest co-migration of BKV and the human race. J Gen Virol. 2009;90(Pt 1):144–152. [DOI] [PubMed] [Google Scholar]
- 5.Gossai A, Waterboer T, Nelson HH, et al. Seroepidemiology of Human Polyomaviruses in a US Population. Am J Epidemiol. 2016;183(1):61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egli A, Infanti L, Dumoulin A, et al. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis. 2009;199(6):837–846. [DOI] [PubMed] [Google Scholar]
- 7.Sawinski D, Goral S. BK virus infection: an update on diagnosis and treatment. Nephrol Dial Transplant. 2015;30(2):209–217. [DOI] [PubMed] [Google Scholar]
- 8.Rajpoot DK, Gomez A, Tsang W, Shanberg A. Ureteric and urethral stenosis: a complication of BK virus infection in a pediatric renal transplant patient. Pediatr Transplant. 2007;11(4):433–435. [DOI] [PubMed] [Google Scholar]
- 9.Mantyjarvi RA, Meurman OH, Vihma L, Berglund B. A human papovavirus (B.K.), biological properties and seroepidemiology. Ann Clin Res. 1973;5(5):283–287. [PubMed] [Google Scholar]
- 10.Vallbracht A, Lohler J, Gossmann J, et al. Disseminated BK type polyomavirus infection in an AIDS patient associated with central nervous system disease. Am J Pathol. 1993;143(1):29–39. [PMC free article] [PubMed] [Google Scholar]
- 11.Hedquist BG, Bratt G, Hammarin A-L, et al. Identification of BK virus in a patient with acquired immune deficiency syndrome and bilateral atypical retinitis. Ophthalmology. 1999;106(1):129–132. [DOI] [PubMed] [Google Scholar]
- 12.O’Reilly RJ, Lee FK, Grossbard E, et al. Papovavirus excretion following marrow transplantation: incidence and association with hepatic dysfunction. Transplant Proc. 1981;13(1 Pt 1):262–266. [PubMed] [Google Scholar]
- 13.Petrogiannis-Haliotis T, Sakoulas G, Kirby J, et al. BK-related polyomavirus vasculopathy in a renal-transplant recipient. N Engl J Med. 2001;345(17):1250–1255. [DOI] [PubMed] [Google Scholar]
- 14.Brennan DC, Agha I, Bohl DL, et al. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transplant. 2005;5(3):582–594. [DOI] [PubMed] [Google Scholar]
- 15.Yooprasert P, Rotjanapan P. BK virus-associated nephropathy: current situation in a resource-limited country. Transplant Proc. 2018;50(1):130–136. [DOI] [PubMed] [Google Scholar]
- 16.Ramos E, Drachenberg CB, Wali R, Hirsch HH. The decade of polyomavirus BK-associated nephropathy: state of affairs. Transplantation. 2009;87(5):621–630. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch HH, Randhawa P. BK polyomavirus in solid organ transplantation. Am J Transplant. 2013;13(Suppl 4):179–188. [DOI] [PubMed] [Google Scholar]
- 18.Scadden JR, Sharif A, Skordilis K, Borrows R. Polyoma virus nephropathy in kidney transplantation. World J Transplant. 2017;7(6):329–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirsch HH, Babel N, Comoli P, et al. European perspective on human polyomavirus infection, replication and disease in solid organ transplantation. Clin Microbiol Infect. 2014;20(Suppl 7):74–88. [DOI] [PubMed] [Google Scholar]
- 20.Nickeleit V, Singh HK, Randhawa P, et al. The Banff Working Group classification of definitive polyomavirus nephropathy: morphologic definitions and clinical correlations. J Am Soc Nephrol. 2018;29(2):680–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwamoto S, Azuma E, Hori H, et al. BK virus-associated fatal renal failure following late-onset hemorrhagic cystitis in an unrelated bone marrow transplantation. Pediatr Hematol Oncol. 2002;19(4):255–261. [DOI] [PubMed] [Google Scholar]
- 22.Hoefele J, Russmann D, Klein B, Weber LT, Fuhrer M. BK virus induced nephritis in a boy with acute myeloid leukaemia undergoing bone marrow transplantation. NDT Plus. 2008;1(5):336–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gagneux-Brunon A, Pillet S, Laurent B, et al. A case of BK virus nephropathy in a stem cell transplant recipient: a rare or under-recognized cause for Acute Kidney injury. Med Mal Infect. 2015;45(8):331–334. [DOI] [PubMed] [Google Scholar]
- 24.Korth J, Widera M, Dolff S, et al. Impact of low-level BK polyomavirus viremia on intermediate-term renal allograft function. Transpl Infect Dis. 2018;20(1):e12817. [DOI] [PubMed] [Google Scholar]
- 25.Trofe-Clark J, Sparkes T, Gentile C, Van Deerlin V, Sawinski D, Bloom RD. BK virus genotype variance and discordant BK viremia PCR assay results. Am J Transplant. 2013;13(4):1112–1113. [DOI] [PubMed] [Google Scholar]
- 26.Bohl DL, Storch GA, Ryschkewitsch C, et al. Donor origin of BK virus in renal transplantation and role of HLA C7 in susceptibility to sustained BK viremia. Am J Transplant. 2005;5(9):2213–2221. [DOI] [PubMed] [Google Scholar]
- 27.Andrews CA, Shah KV, Daniel RW, Hirsch MS, Rubin RH. A serological investigation of BK virus and JC virus infections in recipients of renal allografts. J Infect Dis. 1988;158(1):176–181. [DOI] [PubMed] [Google Scholar]
- 28.Santeusanio AD, Lukens BE, Eun J. Antiviral treatment of BK virus viremia after kidney transplantation. Am J Health Syst Pharm. 2017;74(24):2037–2045. [DOI] [PubMed] [Google Scholar]
- 29.Johnston O, Jaswal D, Gill JS, Doucette S, Fergusson DA, Knoll GA. Treatment of polyomavirus infection in kidney transplant recipients: a systematic review. Transplantation. 2010;89(9):1057–1070. [DOI] [PubMed] [Google Scholar]
- 30.Tylden GD, Hirsch HH, Rinaldo CH. Brincidofovir (CMX001) inhibits BK polyomavirus replication in primary human urothelial cells. Antimicrob Agents Chemother. 2015;59(6):3306–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tzannou I, Papadopoulou A, Naik S, et al. Off-the-shelf virus-specific T cells to treat BK virus, human herpesvirus 6, cytomegalovirus, epstein-barr virus, and adenovirus infections after allogeneic hematopoietic stem-cell transplantation. J Clin Oncol. 2017;35(31):3547–3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vu D, Shah T, Ansari J, Naraghi R, Min D. Efficacy of intravenous immunoglobulin in the treatment of persistent BK viremia and BK virus nephropathy in renal transplant recipients. Transplant Proc. 2015;47(2):394–398. [DOI] [PubMed] [Google Scholar]
- 33.Munoz P, Fogeda M, Bouza E, Verde E, Palomo J, Banares R. Prevalence of BK virus replication among recipients of solid organ transplants. Clin Infect Dis. 2005;41(12):1720–1725. [DOI] [PubMed] [Google Scholar]
- 34.Viswesh V, Yost SE, Kaplan B. The prevalence and implications of BK virus replication in non-renal solid organ transplant recipients: a systematic review. Transplant Rev (Orlando). 2015;29(3):175–180. [DOI] [PubMed] [Google Scholar]
- 35.Loeches B, Valerio M, Pérez M, et al. BK virus in liver transplant recipients: a prospective study. Transplant Proc. 2009;41(3):1033–1037. [DOI] [PubMed] [Google Scholar]
- 36.Atencio IA, Shadan FF, Zhou XJ, Vaziri ND, Villarreal LP. Adult mouse kidneys become permissive to acute polyomavirus infection and reactivate persistent infections in response to cellular damage and regeneration. J Virol. 1993;67(3):1424–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pendse SS, Vadivel N, Ramos E, et al. BK viral reactivation in cardiac transplant patients: evidence for a double-hit hypothesis. J Heart Lung Transplant. 2006;25(7):814–819. [DOI] [PubMed] [Google Scholar]
- 38.Agha I, Brennan DC. BK virus and immunosuppressive agents. Adv Exp Med Biol. 2006;577:174–184. [DOI] [PubMed] [Google Scholar]
- 39.Nickeleit V, Hirsch HH, Binet IF, et al. Polyomavirus infection of renal allograft recipients: from latent infection to manifest disease. J Am Soc Nephrol. 1999;10(5):1080–1089. [DOI] [PubMed] [Google Scholar]
- 40.Umbro I, Tinti F, Muiesan P, Mitterhofer AP. Different behaviour of BK-virus infection in liver transplant recipients. World J Gastroenterol. 2016;22(4):1532–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colla L, Mesiano P, Morellini V, et al. Human polyomavirus BK in patients with lupus nephritis: clinical and histological correlations. Lupus. 2007;16(11):881–886. [DOI] [PubMed] [Google Scholar]
- 42.Vigil D, Konstantinov NK, Barry M, et al. BK nephropathy in the native kidneys of patients with organ transplants: clinical spectrum of BK infection. World J Transplant. 2016;6(3):472–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aksenova M, Tsetlina V, Gutovskaya E, Mitrofanova A, Balashov D, Maschan A. BK virus nephropathy in a pediatric patient after hematopoietic stem cell transplantation. Pediatr Transplant. 2015;19(1):E29–32. [DOI] [PubMed] [Google Scholar]
- 44.Bruno B, Zager RA, Boeckh MJ, et al. Adenovirus nephritis in hematopoietic stem-cell transplantation. Transplantation. 2004;77(7):1049–1057. [DOI] [PubMed] [Google Scholar]
- 45.Burbach M, Birsen R, Denis B, et al. A case of BK virus nephropathy without hemorrhagic cystitis after hematopoietic stem cell transplantation. Ann Hematol. 2016;95:1567–1568. [DOI] [PubMed] [Google Scholar]
- 46.Lekakis LJ, Macrinici V, Baraboutis IG, Mitchell B, Howard DS. BK virus nephropathy after allogeneic stem cell transplantation: a case report and literature review. Am J Hematol. 2009;84(4):243–246. [DOI] [PubMed] [Google Scholar]
- 47.Maximova N, Pizzol A, Sonzogni A, Gregori M, Granzotto M, Tamaro P. Polyclonal gammopathy after BKV infection in HSCT recipient: a novel trigger for plasma cells replication? Virol J. 2015;12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Donnell PH, Swanson K, Josephson MA, et al. BK virus infection is associated with hematuria and renal impairment in recipients of allogeneic hematopoetic stem cell transplants. Biol Blood Marrow Transplant. 2009;15(9):1038–1048.e1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Papanicolaou GA, Lee YJ, Young JW, et al. Brincidofovir for polyomavirus-associated nephropathy after allogeneic hematopoietic stem cell transplantation. Am J Kidney Dis. 2015;65(5):780–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shapiro S, Robin M, Esperou H, et al. Polyomavirus nephropathy in the native kidneys of an unrelated cord blood transplant recipient followed by a disseminated polyomavirus infection. Transplantation. 2006;82:292–293. [DOI] [PubMed] [Google Scholar]
- 51.Sharma SG, Nickeleit V, Herlitz LC, et al. BK polyoma virus nephropathy in the native kidney. Nephrol Dial Transplant. 2013;28(3):620–631. [DOI] [PubMed] [Google Scholar]
- 52.Stracke S, Helmchen U, von Muller L, Bunjes D, Keller F. Polyoma virus-associated interstitial nephritis in a patient with acute myeloic leukaemia and peripheral blood stem cell transplantation. Nephrol Dial Transplant. 2003;18(11):2431–2433. [DOI] [PubMed] [Google Scholar]
- 53.van der Bij A, Betjes M, Weening J, et al. BK virus nephropathy in an immunodeficient patient with chronic lymphocytic leukemia. J Clin Virol. 2009;45(4):341–344. [DOI] [PubMed] [Google Scholar]
- 54.Verghese PS, Finn LS, Englund JA, Sanders JE, Hingorani SR. BK nephropathy in pediatric hematopoietic stem cell transplant recipients. Pediatr Transplant. 2009;13(7):913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Limaye AP, Smith KD, Cook L, et al. Polyomavirus nephropathy in native kidneys of non-renal transplant recipients. Am J Transplant. 2005;5(3):614–620. [DOI] [PubMed] [Google Scholar]
- 56.Sanchez-Pinto LN, Laskin BL, Jodele S, Hummel TR, Yin HJ, Goebel J. BK virus nephropathy in a pediatric autologous stem-cell transplant recipient. Pediatr Blood Cancer. 2011;56(3):495–497. [DOI] [PubMed] [Google Scholar]
- 57.Filler G, Licht C, Haig A. Native kidney BK virus nephropathy associated with acute lymphocytic leukemia. Pediatr Nephrol. 2013;28(6):979–981. [DOI] [PubMed] [Google Scholar]
- 58.Inaba H, Jones DP, Gaber LW, et al. BK virus-induced tubulointerstitial nephritis in a child with acute lymphoblastic leukemia. J Pediatr. 2007;151(2):215–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collett J, Fuller S, P’Ng CH, Gangadharan KM, January QP. A man with chronic lymphocytic leukemia and declining kidney function. Native kidney BK nephropathy and chronic lymphocytic leukemia/small lymphocytic lymphoma infiltration with chronic tubulointerstitial damage resulting in worsening kidney function. Am J Kidney Dis. 2016;67(1):A18–A21. [DOI] [PubMed] [Google Scholar]
- 60.McCrory R, Gray M, Leonard N, Smyth J, Woodman A. Native kidney BK virus nephropathy associated with chronic lymphocytic leukaemia. Nephrol Dial Transplant. 2012;27(3):1269–1271. [DOI] [PubMed] [Google Scholar]
- 61.Sangala N, Dewdney A, Marley N, Cranfield T, Venkat-Raman G. Progressive renal failure due to renal infiltration by BK polyomavirus and leukaemic cells: which is the culprit? NDT Plus. 2011;4(1):46–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Silva LM, Bale P, de Courcy J, Brown D, Knowles W. Renal failure due to BK virus infection in an immunodeficient child. J Med Virol. 1995;45(2):192–196. [DOI] [PubMed] [Google Scholar]
- 63.Bratt G, Hammarin AL, Grandien M, et al. BK virus as the cause of meningoencephalitis, retinitis and nephritis in a patient with AIDS. Aids. 1999;13(9):1071–1075. [DOI] [PubMed] [Google Scholar]
- 64.Crum-Cianflone N, Quigley M, Utz G, Hale B. BK virus-associated renal failure among HIV patients. AIDS. 2007;21:1501–1502. [DOI] [PubMed] [Google Scholar]
- 65.Cubukcu-Dimopulo O, Greco A, Kumar A, Karluk D, Mittal K, Jagirdar J. BK virus infection in AIDS. Am J Surg Pathol. 2000;24(1):145–149. [DOI] [PubMed] [Google Scholar]
- 66.Jung SW, Sung JY, Park SJ, Jeong KH. BK virus-associated nephropathy with hydronephrosis in a patient with AIDS: a case report and literature review. Clin Nephrol. 2016;85(3):173–178. [DOI] [PubMed] [Google Scholar]
- 67.Manabe M, Yoshii Y, Mukai S, et al. BK virus-associated nephropathy in an HIV-positive patient with gingival plasmablastic lymphoma. Int J Hematol. 2010;92(1):208–210. [DOI] [PubMed] [Google Scholar]
- 68.Mouratoff JG, Tokumoto J, Olson JL, Chertow GM. Acute renal failure with interstitial nephritis in a patient with AIDS. Am J Kidney Dis. 2000;35(3):557–561. [DOI] [PubMed] [Google Scholar]
- 69.Nebuloni M, Tosoni A, Boldorini R, et al. BK virus renal infection in a patient with the acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1999;123(9):807–811. [DOI] [PubMed] [Google Scholar]
- 70.Smith RD, Galla JH, Skahan K, et al. Tubulointerstitial nephritis due to a mutant polyomavirus BK virus strain, BKV(Cin), causing endstage renal disease. J Clin Microbiol. 1998;36(6):1660–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sukov WR, Lewin M, Sethi S, Rakowski TA, Lager DJ. BK virusassociated nephropathy in a patient with AIDS. Am J Kidney Dis. 2008;51(4):e15–18. [DOI] [PubMed] [Google Scholar]
- 72.Ali FN, Meehan SM, Pahl E, Cohn RA. Native BK viral nephropathy in a pediatric heart transplant recipient. Pediatr Transplant. 2010;14(4):E38–E41. [DOI] [PubMed] [Google Scholar]
- 73.Barber CE, Hewlett TJ, Geldenhuys L, Kiberd BA, Acott PD, Hatchette TF. BK virus nephropathy in a heart transplant recipient: case report and review of the literature. Transpl Infect Dis. 2006;8(2):113–121. [DOI] [PubMed] [Google Scholar]
- 74.Butts RJ, Uber WE, Savage AJ. Treatment of BK viremia in a pediatric heart transplant recipient. J Heart Lung Transplant. 2012;31:552–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Joseph A, Pilichowska M, Boucher H, Kiernan M, DeNofrio D, Inker LA. BK virus nephropathy in heart transplant recipients. Am J Kidney Dis. 2015;65(6):949–955. [DOI] [PubMed] [Google Scholar]
- 76.Lorica C, Bueno TG, Garcia-Buitrago MT, Rusconi P, Gonzalez IA. BK virus nephropathy in a pediatric heart transplant recipient with post-transplant lymphoproliferative disorder: a case report and review of literature. Pediatr Transplant. 2013;17(2):E55–E61. [DOI] [PubMed] [Google Scholar]
- 77.Maddirala S, Pitha JV, Cowley BD Jr, Haragsim L. End-stage renal disease due to polyomavirus in a cardiac transplant patient. Nat Clin Pract Nephrol. 2007;3(7):393–396. [DOI] [PubMed] [Google Scholar]
- 78.Menahem SA, McDougall KM, Thomson NM, Dowling JP. Native kidney BK nephropathy post cardiac transplantation. Transplantation. 2005;79:259–260. [DOI] [PubMed] [Google Scholar]
- 79.Pereira T, Rojas CP, Garcia-Buitrago MT, et al. A child with BK virus infection: inadequacy of current therapeutic strategies. Pediatr Transplant. 2012;16(7):E269–E274. [DOI] [PubMed] [Google Scholar]
- 80.Sahney S, Yorgin P, Zuppan C, Cutler D, Kambham N, Chinnock R. BK virus nephropathy in the native kidneys of a pediatric heart transplant recipient. Pediatr Transplant. 2010;14(3):E11–15. [DOI] [PubMed] [Google Scholar]
- 81.Schmid H, Burg M, Kretzler M, Banas B, Grone HJ, Kliem V. BK virus associated nephropathy in native kidneys of a heart allograft recipient. Am J Transplant. 2005;5(6):1562–1568. [DOI] [PubMed] [Google Scholar]
- 82.Dufek S, Haitel A, Muller-Sacherer T, Aufricht C. Duct Bellini carcinoma in association with BK virus nephropathy after lung transplantation. J Heart Lung Transplant. 2013;32(3):378–379. [DOI] [PubMed] [Google Scholar]
- 83.Egli A, Helmersen DS, Taub K, Hirsch HH, Johnson A. Renal failure five years after lung transplantation due to polyomavirus BK-associated nephropathy. Am J Transplant. 2010;10(10):2324–2330. [DOI] [PubMed] [Google Scholar]
- 84.Kuppachi S, Kaur D, Holanda DG, Thomas CP. BK polyoma virus infection and renal disease in non-renal solid organ transplantation. Clin Kidney J. 2016;9(2):310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schwarz A, Mengel M, Haller H, Niedermeyer J. Polyoma virus nephropathy in native kidneys after lung transplantation. Am J Transplant. 2005;5(10):2582–2585. [DOI] [PubMed] [Google Scholar]
- 86.Zeng Y, Magil A, Hussaini T, et al. First confirmed case of native polyomavirus BK nephropathy in a liver transplant recipient seven years post-transplant. Ann Hepatol. 2015;14(1):137–140. [PubMed] [Google Scholar]
- 87.Haririan A, Ramos ER, Drachenberg CB, Weir MR, Klassen DK. Polyomavirus nephropathy in native kidneys of a solitary pancreas transplant recipient. Transplantation. 2002;73(8):1350–1353. [DOI] [PubMed] [Google Scholar]
- 88.Go S, Conlin M, Hooper JE, Troxell ML. Polyoma virus nephropathy-related mass lesion in an apparently immunocompetent patient. Int Urol Nephrol. 2012;44(5):1585–1588. [DOI] [PubMed] [Google Scholar]
- 89.Krystel-Whittemore M, McCarthy ET, Damjanov I, Fields TA. Polyomavirus nephropathy of the native kidney in a patient with rheumatoid arthritis and pulmonary fibrosis. BMJ Case Rep. 2015;2015 https://casereports.bmj.com/content/2015/bcr-2015-211564.long [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Park S, Kim YW, Lee YJ, et al. Polyomavirus nephropathy in native kidneys of an immunocompetent individual. Am J Case Rep. 2017;18:498–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rosen S, Harmon W, Krensky AM, et al. Tubulo-interstitial nephritis associated with polyomavirus (BK type) infection. N Engl J Med. 1983;308(20):1192–1196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.