Abstract
BACKGROUND:
Our knowledge of central sensitization (CS) in chronic low back pain (CLBP) is limited. 2011 fibromyalgia criteria and severity scales (2011 FM survey) has been used to determine FM positive as a surrogate of CS. The major features of CS including widespread hyperalgesia and dysfunction of the descending inhibitory pathways can be identified by pressure pain threshold (PPT) and conditioned pain modulation (CPM) tests. The purpose of the study was to examine neurophysiological characteristics and psychosocial symptoms in a subgroup of FM positive CLBP compared to FM negative CLBP patients.
METHODS:
46 participants with CLBP and 22 healthy controls completed outcome measures of the 2011 FM survey, PPT and CPM tests, and psychosocial questionnaires. Differences between FM positive and FM negative CLBP participants on these measures and correlations were analyzed.
RESULTS:
The 2011 FM survey identified 22 (48%) participants with CLBP as FM positive. FM positive CLBP participants showed lower PPT values of the thumbnail (p=0.011) and lower back (p=0.003), lower CPM values of the thumbnail (p=0.002), and more severe pain catastrophizing, anxiety and depression symptoms (p<0.05) than FM negative CLBP participants. The 2011 FM scores were significantly correlated with the PPT and CPM values of the thumbnail and with psychosocial symptoms (p<0.001).
DISCUSSION:
Our findings suggest a subgroup of CLBP patients exhibiting with signs and symptoms of CS. Associations between subjective and objective CS measures indicate that the 2011 FM survey can be utilized to identify the presence of CS in CLBP in clinical practice.
Keywords: Central sensitization, chronic low back pain, quantitative sensory testing, psychosocial symptoms
Introduction
Chronic low back pain (CLBP) is a significant major health problem in the world[1–7] and 85–90% of patients with CLBP are classified as non-specific CLBP, indicating no specific underlying peripheral or mechanical cause can be found [8, 9]. Low back imaging findings are poorly correlated with the severity of pain and function, often leading to untargeted treatments with unsatisfactory outcomes in this population [8–13]. Hence, the CLBP population is quite heterogeneous and therefore includes several subgroups presenting with varying and/or co-existing underlying mechanisms [14, 15].
A certain proportion of patients with CLBP presents with multiple symptoms such as widespread hyperalgesia, disproportionate pain intensity to their injury, and somatic and psychosocial symptoms [16–19]. These symptoms are also present in other chronic pain conditions such as fibromyalgia and osteoarthritis, suggesting a common underlying pathophysiologic mechanism of central sensitization (CS) [20–26]. Accumulating evidence indicates CS, defined as augmented central pain processing, is a common cause of persistent pain [15, 16, 20, 21, 24, 26]. However, our knowledge about CS in the CLBP population is limited.
CS can be induced by chronic peripheral noxious input, causing hyperexcitability in the central nervous system [16, 20, 21, 25, 26]. Chronic noxious input can cause neuro-chemical abnormalities, i.e. an imbalance in the excitatory and inhibitory central neurotransmitters and alter gene regulations in the central nervous system, leading to the central hyperexcitability [15, 21, 22, 26]. The central hypersensitivity eventually can spread and expand to multiple brain regions such as the somatosensory cortices, insular cortex, anterior and midcingulate cortex, prefrontal cortex and the limbic system [20–22, 25, 27–29]. Therefore, patients with CS can present with various symptoms such as widespread hyperalgesia, fear avoidance behavior, pain catastrophizing thoughts, anxiety, and depression [15, 16, 20–22, 26, 30, 31]. Furthermore, the central hyperexcitability also causes disinhibition in the descending inhibitory pathways (i.e., dysfunction of the descending analgesic system), resulting in enhanced pain perception within the spinal cord [16, 20, 21, 26, 32]. In addition to these top-down mechanisms, bottom-up mechanisms also contribute to the presence of CS [25, 33]. Accumulating evidence suggests that the psychosocial symptoms, such as fear avoidance belief and pain catastrophizing, can facilitate the degree of central excitability through an increase in the neuronal activation of the prefrontal cortex and the limbic system [25, 33].
Patients with various underlying mechanisms leading to pain are mixed together in the CLBP population. A large amount of neuroscience literature has suggested that CS is present in a subgroup of chronic pain conditions [15–17, 20, 24, 29]. Clinically, some patients with CLBP present with widespread hyperalgesia and/or dysfunction of the descending inhibitory pathways determined by quantitative sensory testing (QST) [19, 34–40]. These clinical presentations are strong indicators of central nervous contribution to their chronic pain symptoms [19, 34–40]. A certain portion of the patients with CLBP also present with psychosocial symptoms [16–19]; these symptoms are considered to be related to CS [15, 16, 20–22, 26, 30, 31].
The 2011 Fibromyalgia (FM) Criteria and Severity Scales (2011 FM survey) used in the present study, is a validated self-reported questionnaire utilized to discriminate those with FM syndrome (FM positive) from those without (FM negative) [41–44]. FM is the prototypical state of CS [15, 22, 27, 28, 45, 46], but numerous imaging and clinical studies have determined that the neurophysiological alterations, i.e. CS, seen in FM are also present in a subgroup of other chronic pain conditions, including CLBP [34–37, 47–51]. The 2011 FM survey conceptualizes FM syndrome as a continuum of CS and thus aims to identify patients with CS across different chronic pain conditions [15, 42, 43]. One of the advantages of using the 2011 FM survey to identify a CS subgroup is that this survey substantially examines the degree of widespread hyperalgesia which is a key feature of CS [42, 43]. The FM scores have also been used as a surrogate of CS in the research studies to identify the CS subgroup, using its cut-off scores in knee osteoarthritis [52], rheumatoid arthritis [53], and spine pain [54].
Despite of the all aforementioned evidence, our knowledge of clinical characteristics in the subgroup of CS patients within the CLBP population has not been comprehensively studied. A majority of previous studies examined some aspects of the CS symptoms between CLBP patients and healthy individuals but lacked to compare the CS symptoms among CLBP patients and define CS in a subgroup within the population [34, 35, 38, 55, 56]. Furthermore, these studies did not comprehensively incorporate CS measures such as a battery of subjective and objective outcomes. Thus, the aim of this study was to examine potential neurophysiological characteristics, and psychosocial symptoms relating to CS in FM positive CLBP participants as compared to FM negative CLBP participants. We hypothesized that FM positive CLBP participants would display widespread hyperalgesia, dysfunction of the descending inhibitory pathways, pain catastrophizing, fear avoidance beliefs, depression and anxiety as compared to those with FM negative CLBP. Furthermore, as a secondary aim, we examined whether the 2011 FM scores are associated with QST values and psychosocial symptoms. The results of this study would provide a greater understanding of the presentation of CS in CLBP and help clinicians to classify CS in the patients with CLBP, potentially resulting in targeted interventions and better outcomes.
Methods
This cross-sectional study was conducted at the University of Kansas Medical Center (KUMC) from September 2017 to November 2018. The study protocol was approved by the Institutional Review Board of the University of Kansas Medical Center (KUMC IRB # STUDY00141062).
Participants
Participants were recruited through placement of flyers at various KUMC and community locations, online questionnaires, Frontiers and Pioneers Participant Registries associated with the University of Kansas Hospital, referrals from clinicians, and social media methods. We included those who had 1) low back pain more than 3 months, 2) low back pain severity greater than or equal to 3 on the 0 to 10 numeric rating pain scale within the last 7 days, 3) low back pain as a chief complaint, 4) age between 21 and 70 years, and 5) ability to read and understand English. We excluded those who had 1) numbness or decrease in sensation in the areas of the pain sensitivity tests as described below in the pressure pain threshold and conditioned pain modulation sections, 2) significant spinal pathologies such as fracture or tumor, 3) cervical, thoracic or peripheral joint pain as their chief complaint, 4) severe cardiovascular and neurological diseases, 5) cancer, 6) history of any spinal surgery, 7) resting blood pressure greater than 160/90 mmHg [57–59], 8) current use of blood thinning medications, 9) uncontrolled diabetes, and 10) current or prior participation in a similar study utilizing QST. We also included healthy controls (HC) as QST values do not have an established cut off point and can only be used to compare values between groups [19, 60–62]. In the present study, QST values of HC served as normative values to clarify the degree of pain sensitivity and functionality of the descending inhibitory pathways in the FM positive and negative CLBP participants.
All participants who were qualified and willing to participate in the study signed the informed consent form that had been approved by the Institutional Review Board prior to the enrollment.
Procedure and Outcome Measures
The participants completed a demographic questionnaire, pain assessments, CS assessments, and a number of self-reported questionnaires. Demographic information included age, gender, body mass index (BMI) and race. Pain assessments consisted of low back pain intensity as measured by the Numeric Rating Scale (NRS) and the duration of pain in months. CS assessments included the 2011 FM survey, pressure pain threshold (PPT) test, and conditioned pain modulation (CPM) test. The self-report questionnaires included Oswestry Disability Index (ODI), Pain Catastrophizing Scale (PCS), Fear Avoidance Belief Questionnaire (FABQ), Beck Anxiety Inventory (BAI), and Beck Depression Inventory (BDI).
Self-reported Questionnaires
Physical Function
Physical function was assessed with the ODI, which consists of questions related to daily functions and the degree to which back pain interferes with those functions [63, 64]. The ODI includes one item on pain and nine items on activities of daily living (personal care, lifting, walking, sitting, standing, sleeping, sex life, social life, and traveling). Total score possible is 50; higher scores indicate greater disability. Percent disability can be calculated by dividing the subject’s scores by the total score of 50 and multiplying by 100.
Fear Avoidance Belief and Pain Catastrophizing.
Fear avoidance behavior and pain catastrophizing are the two major traits associated with the development and maintenance of CLBP [19, 65, 66]. The FABQ categorizes these traits using items related to fear about physical and work activities, and items that do not fall under either the physical or work scale. Each item is scored from 0 to 6 with a higher number indicating increased fear of activity or work [67]. The PCS is a validated 13-item scale with questions related to catastrophizing behavior [68]. The total score is 52 with higher scores indicating greater catastrophic thoughts.
Anxiety and Depression.
Anxiety and depression were assessed with BAI and BDI respectively. The BAI is a self-report questionnaire that measures the severity of anxiety [69]. It contains 21 multiple-choice questions; each answer is scored on 0 (not at all) to 3 (severely) scale. Higher scores indicate more severe anxiety symptoms. The BDI is a 21-question multiple-choice self-report questionnaire with each answer being scored on a scale value of 0 to 3 [70]. Higher scores indicate more severe depressive symptoms.
The 2011 FM Survey.
The 2011 FM survey consists of an assessment of the Widespread Pain Index (WPI) and Symptom Severity (SS) [42, 43]. The WPI assesses whether or not patients experience pain or tenderness in the 19 specific body areas (score 0–19). Symptom severity was evaluated using the SS scale (score 0–12). As per the FM survey criteria, patients were classified FM positive if their scores were ≥ 7 for the WPI and ≥ 5 for the SS or if WPI scores were 3–6 and SS scores were ≥ 9. The 2011 FM survey has been shown to be a valid measure for identifying those with FM positive, i.e. fibromyalgia syndrome, in chronic pain conditions beyond the FM cohort in research settings [42, 43, 52–54]. In this study, we used the cut-off scores to divide the CLBP participants into two groups: FM positive and FM negative [41]. We also used the 2011 FM scores as a continuous variable to measure CS severity [42, 43, 52–54].
Quantitative Sensory Testing measures
Pressure Pain Threshold.
Pressure pain threshold (PPT) was measured using a pressure algometer (Wagner Instrument. USA) to two anatomical regions: 1) the thumbnail of the participant’s dominant hand (considered a remote site) and 2) the midline of the lower back, in the interspace between the fifth lumbar vertebra (L5) and first sacral vertebra (S1) (considered a local site)[38, 55, 56]. The pressure algometer has a one-cm2 circular rubber-tipped probe that was placed perpendicular to each body site at a rate of 0.5kg/sec [58, 71]. Participants were instructed to indicate when the pressure sensation changed to a painful sensation [34–36, 72, 73]. At the moment the participant indicated transition of pressure to pain, the algometer was immediately released and the amount of force (Kg/cm2) causing the perception of pain was recorded as a PPT value [34–36, 72]. Prior to the actual tests, familiarization with the PPT test was performed by its application on the participant’s dominant arm until the participant became familiar about the test procedure. Three PPT measurements on each body site with a 30-second rest interval were conducted and averaged for data analyses. The PPT test has been indicated in the literature as having excellent reliability [74, 75], and the most reproducible method for identifying individuals with CS [76].
Conditioned Pain Modulation.
Conditioned Pain Modulation (CPM) is a commonly used measurement to evaluate the functionality of the descending inhibitory pathways [15, 38–40, 77–79]. The CPM test assesses whether the pain sensitivity of the primary body site of interest changes with a conditioning painful stimulus applied to a remote site of the body. In normal response to a conditioning painful stimulus, a normal neurophysiological response causes release of endogenous opioids and activation of the descending analgesic system, leading to decreased pain sensitivity (increased pain threshold) [78]. In this study, the conditioning painful stimulus was created by applying intense pressure to the non-dominant upper arm with a 12-cm wide pressure cuff to temporarily induce ischemic pain [39, 40, 57, 58, 77, 79–81]. The cuff was inflated to 260 mmHg and the participant was instructed to lift a dumbbell weight (females lifted 1 kg and males lifted 2 kg) by extending the non-dominate wrist until the participant either completed 45 wrist lifts or reported pain intensity of the arm 7 or greater on 0 to 10 scale [39, 57, 58, 77, 79]. As soon as the participant finished the lifting task, the PPT on the dominant side thumbnail and the lower back region was re-assessed 3 times. The PPT values with the conditioning painful stimulus were averaged, and the differences between the PPT values with and without the painful stimulus (CPM values) were used for the analyses, as shown below [38, 39, 71, 77–79]. The CPM test is a successful QST method to discriminate individuals with CS, and has been utilized in multiple previous studies [15, 16, 38, 40, 58, 77–79, 82].
CPM values were calculated by subtracting the PPT values with the conditioning painful stimulus from PPT values without the conditioning painful stimulus.
Statistical Analysis
The data were analyzed using IBM SPSS Statistics Version 25 (SPSS Inc, Chicago, Illinois). Normality assumption of outcome variables was tested using the Shapiro-Wilk test. For the participant characteristics, mean and standard deviation were calculated for continuous variables, and percentages or binary labels (i.e., yes/no) were given for the categorical variables. The differences between groups on these participant characteristics were analyzed by independent two sample t test, chi-square test or one-way analysis of variance (ANOVA).
First, the differences in PPT and CPM values between FM positive CLBP participants, FM negative CLBP participants, and healthy controls were analyzed using one-way ANOVA. Family wise error rate was controlled with Tukey correction. Non-parametric tests were performed when data were not normally distributed. If a significant difference was observed, a post-hoc analysis was performed. Secondly, self-reported questionnaires between the FM positive CLBP and FM negative CLBP groups were analyzed with independent two sample t tests or Mann-Whitney U-tests, when data were not normally distributed. Thirdly, Pearson’s correlation coefficient r was estimated and tested to examine associations between 2011 FM scores (as a continuous variable), PPT and CPM values, and self-reported outcomes related to psychosocial symptoms. The interpretation of the correlation coefficient was as follows: a correlation coefficient between 0 and 0.19 = a very weak correlation, between 0.20–0.39 = weak, between 0.40–0.59 = moderate, between 0.60–0.79 = strong, and between 0.80–1.00 = very strong [83]. The significance level was set at .05.
Results
A total of 46 participants with CLBP and 22 healthy controls were enrolled. Of the 46 participants with CLBP, 22 (48%) were identified as having FM syndrome (FM positive) based on the cut-off scores on the 2011 FM survey (WPI ≥ 7 and SS ≥ 5 or WPI between 3 and 6 and SS ≥ 9).
Participant characteristics are described in Table 1. FM positive CLBP participants had greater low back pain intensity (p < 0.01; although the difference is not clinically significant [84]) and more widespread pain locations (p < 0.001) than FM negative CLBP participants. Eight participants were taking opioid medications on either as needed or regular basis and all of them were identified as FM positive (p < 0.001). With regards to age, gender, body mass index (BMI), and duration of pain, there were no significant differences between the 2 groups (Table1).
Table1:
Participant characteristics
| HC (n = 22) Mean ± SD | FM negative (n = 24) Mean ± SD | FM positive (n = 22) Mean ± SD | P value (t test)! | P value (ANOVA) | |
|---|---|---|---|---|---|
| Age (years) | 41.15 ± 8.830 | 42.38 ± 12.37 | 43.95 ± 14.00 | 0.687 | 0.788 |
| Gender (% Female) | 68% | 63% | 77% | 0.277 | 0.552 |
| BMI | 28.35 ± 8.10 | 28.76 ± 6.20 | 31.34 ± 6.13 | 0.163 | 0.296 |
| Race | 0.576 | 0.316 | |||
| White | 68.00% | 66.70% | 72.70% | ||
| Black | 5% | 16.70% | 13.70% | ||
| Asian | 18.00% | 4.20% | 0.00% | ||
| More than one race | 9.00% | 0.00% | 4.55% | ||
| Other | 0.00% | 0.00% | 4.55% | ||
| Pain average (NRS) | ----------- | 4.13 ± 1.12 | 5.27 ± 1.49 | < 0.01* | |
| Pain duration (months) | ----------- | 75.04 ± 104.87 | 135.09 ± 171.12 | 0.155 | |
| number of pain locations | ----------- | 4.50 ± 1.79 | 9.77 ± 3.84 | < 0.001* | |
| Opioid use (%Yes) | ----------- | 0% | 36% | < 0.001* |
FM: Fibromyalgia
HC: Healthy controls
BMI: Body mass index, NRS: Numeric rating scale
Comparisons of FM negative CLBP participants and FM negative CLBP participants
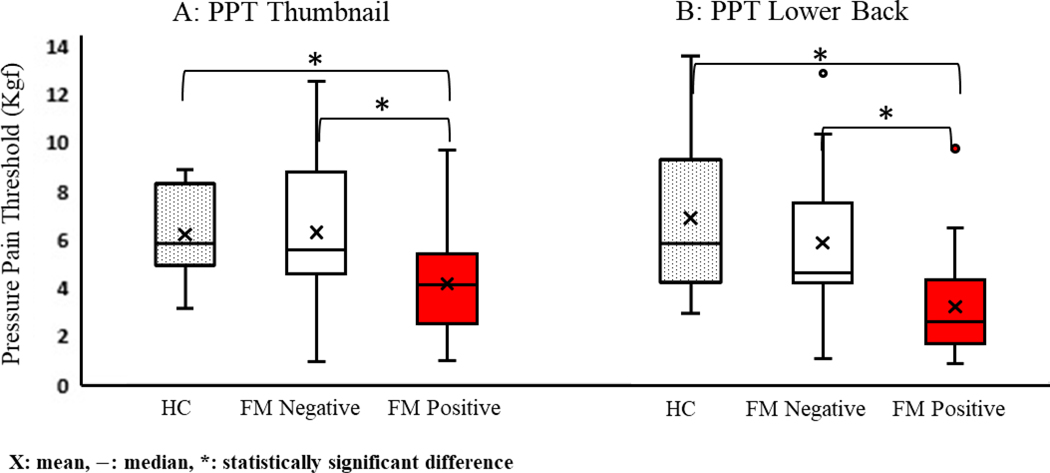
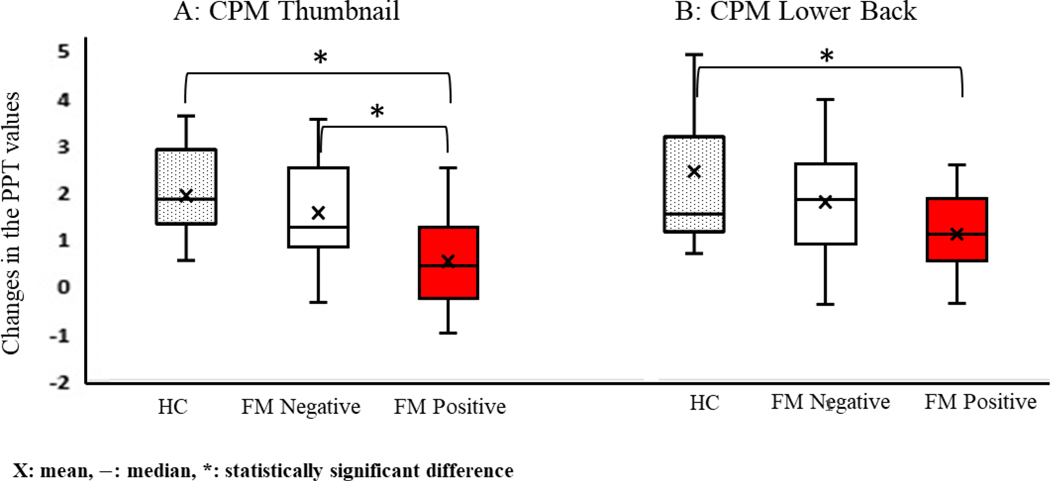
The results of PPT and CPM tests are shown in Table 2, Figure 1 and 2. Statistically significant differences between the FM positive CLBP, FM negative CLBP, and HC controls were found in all 4 values; PPT on the thumbnail, PPT on the lower back, CPM on the thumbnail, and CPM on the lower back (all p < 0.01). The subsequent post-hoc analyses revealed that PPT values of the thumbnail, PPT values of the lower back, and CPM values of the thumbnail were significantly lower in FM positive CLBP than FM negative CLBP (p = 0.011, p = 0.003, and p = 0.002 respectively), while CPM values of the lower back between FM positive CLBP and FM negative CLBP were not statistically different (p = 0.141). All of these values were also significantly lower in FM positive CLBP than healthy controls (p < 0.01).
Table2:
PPT and CPM values
| HC (n = 22) | FM negative (n = 24) | FM positive (n = 22) | ||
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | P value | |
| PPT Thumbnail (Kg/cm2) | 6.21 ± 1.83 | 6.30 ± 3.16 | 4.17 ± 2.12 | < 0.01* |
| PPT lower back (Kg/cm2) | 6.82 ± 3.10 | 5.80 ± 3.46 | 3.14 ± 2.04 | < 0.001* |
| CPM thumbnail (Kg/cm2) | 1.79 ± 0.84 | 1.51 ± 1.13 | 0.49 ± 0.94 | < 0.001* |
| CPM lower back (Kg/cm2) | 2.42 ± 1.59 | 1.72 ± 1.11 | 1.05 ± 0.73 | < 0.001* |
FM: Fibromyalgia
PPT: Pressure pain threshold values
CPM: Conditioned pain modulation values
Figure 1.
PPT values on the thumbnail (A) and lower back (B): PPT values between FM positive CLBP, FM negative CLBP and healthy controls
Figure 2:
CPM values on the thumbnail (A) and lower back (B): CPM values between FM positive CLBP, FM negative CLBP and healthy controls
The differences between FM positive CLBP and FM negative CLBP groups regarding the degree of CS and function, and psychosocial symptoms are shown in Table 3. FM positive CLBP showed poorer function (p = 0.002) and more severe pain catastrophizing thoughts (p =0.033), and more anxiety (p < 0.001) and depression (p < 0.01) symptoms compared to the FM negative CLBP scores. For the degree of fear avoidance belief, both groups were not significantly different.
Table 3:
Degree of CS, function, and psychosocial symptoms of FM positive CLBP and FM negative CLBP scores
| FM negative (n = 24) | FM positive (n = 22) | ||
|---|---|---|---|
| Mean ± SD | Mean ± SD | P value | |
| 2011FM survey | 8.25 ± 2.63 | 17.14 ± 4.04 | < 0.001* |
| ODI (%) | 19.24 ± 10.40 | 30.24 ± 12.74 | < 0.01* |
| PCS | 12.33 ± 11.43 | 20.73 ± 14.39 | 0.033* |
| FABQp | 11.58 ± 4.84 | 13.52 ± 7.16 | 0.301 |
| FABQw | 10.33 ± 10.74 | 15.50 ± 12.86 | 0.145 |
| BAI | 7.50 ± 6.14 | 21.59 ± 13.61 | < 0.001* |
| BDI | 8.33 ± 5.56 | 15.27 ± 8.81 | < 0.01* |
ODI: Oswestry Disability Index, PCS: Pain Catastrophizing scale, FABQp: Fear avoidance belief questionnaire, physical activity subscale, FABQw: Fear avoidance belief questionnaire, work subscale, BAI: Beck anxiety inventory, BDI: Beck depression index
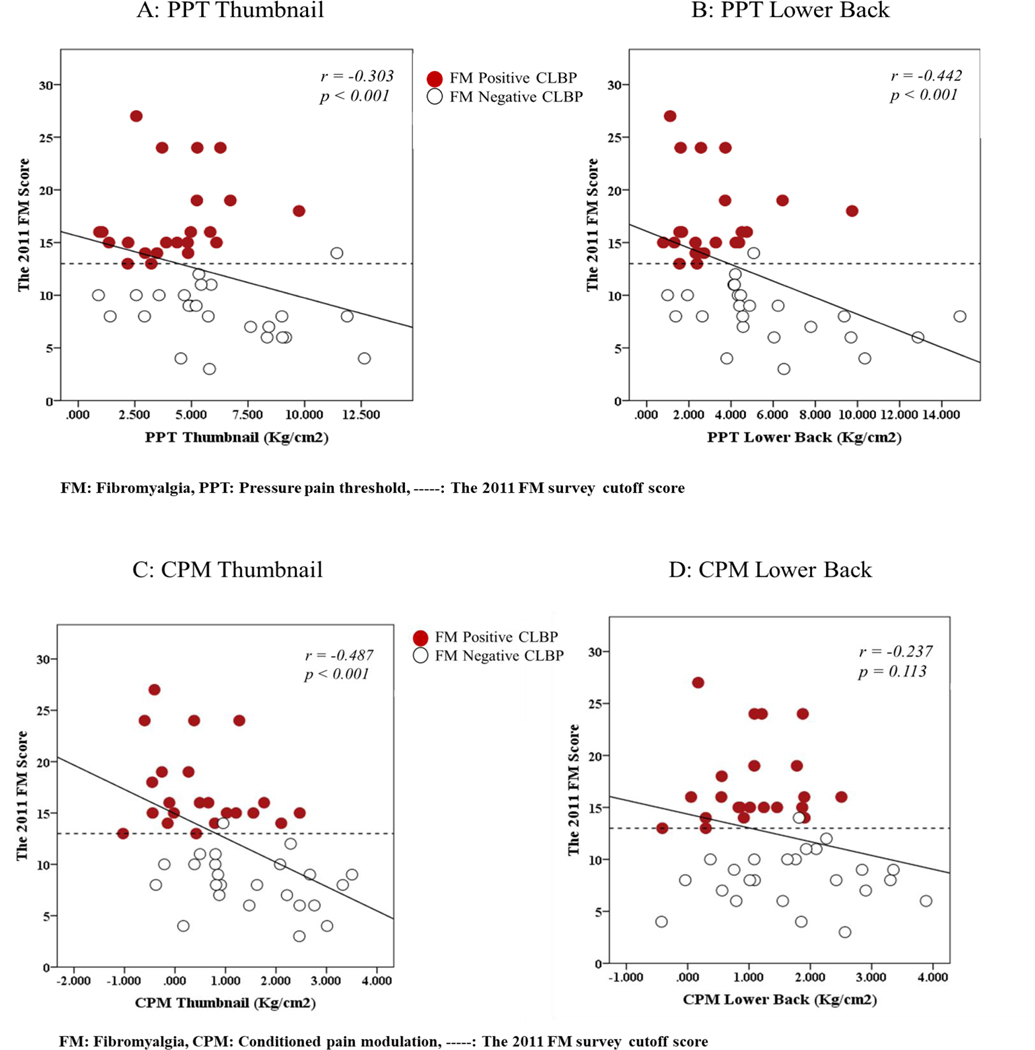
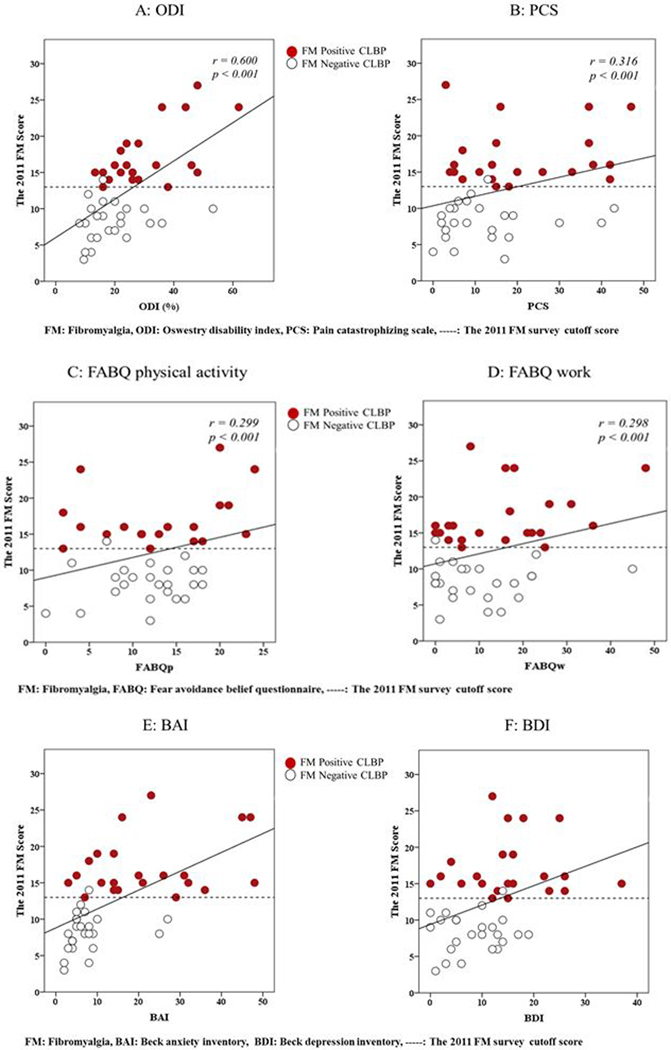
The 2011 FM scores were significantly correlated with BMI (r = 0.315; p= 0.033) and average pain intensity (r = 0.403; p < 0.01) but not with duration of pain (r = 0.243, p = 0.103). The correlation analysis results between the 2011 FM scores and QST are shown in Figure 3 and between the 2011 FM scores and self-reported questionnaires are shown in Figure 4. All variables were significantly correlated to 2011 FM scores except CPM values on lower back (r = −0.237 p = 0.113). The FM scores were also significantly correlated with all self-reported questionnaires with the correlation coefficients ranging from r=0.298 to 0.600. Among those significant correlations, high correlation coefficients (with moderate to strong strength) were found with ODI and BAI (Figure 4).
Figure 3:
Pearson’s r Correlations Between the 2011 FM scores and QST: Correlation figures of each QST value for the 2011 FM scores
Figure 4:
Pearson’s r Correlations Between the 2011 FM scores and Self-reported Measures: Correlation figures of each psychosocial symptom for the 2011 FM scores
Discussion
To the best of our knowledge, this was the first study to use the 2011 FM survey to identify a subgroup of individuals with CLBP patients who appeared to have symptoms and QST findings indicative of CS. Both the PPT and CPM tests suggested significant widespread hyperalgesia and dysfunction of the descending inhibitory pathways in FM positive CLBP participants when compared to the FM negative CLBP participants: these are key features of CS [15, 20, 21, 24, 25]. Secondly, FM positive CLBP participants showed significantly poorer function and more severe symptoms of pain catastrophizing thoughts, anxiety, and depression, compared to their counterparts with FM negative CLBP scores. Thirdly, the FM scores were significantly correlated with QST values, and psychosocial outcome measures. These findings strongly suggest a subgroup of patients within the CLBP population that prominently presents with an underlying pain mechanism of CS.
Central Sensitization in a Subgroup of Chronic Low Back Pain
We showed a decrease in the PPT values at the local (in the lumbar spine) and remote (in dominant thumbnail) sites in FM positive CLBP participants when compared to FM negative CLBP participants and healthy controls whose age, gender and BMI were not statistically different from the CLBP participants. These results suggest both primary (lower back region) and secondary (thumbnail) hyperalgesia in this subgroup. We also demonstrated less CPM values in FM positive CLBP participants compared to FM negative CLBP participants, suggesting dysfunction in the descending inhibitory mechanisms. Numerous previous studies have shown significant widespread hyperalgesia and dysfunction of the descending inhibitory pathways in several chronic pain conditions including CLBP [34, 35, 38, 55], FM [85–87], osteoarthritis [40], and chronic tension-type headache [81]. However previous studies compared the patient population with healthy controls only, whereas in the present study we compared CS symptoms within the CLBP patients using the FM survey cutoff scores.
The PPT values of the lower back in response to the conditioning painful stimulus did not differ significantly between the FM positive and FM negative CLBP subgroups (p = 0.141). This may be due to the relatively small sample size in the present study or difference in CPM test paradigms compared to a previous study that included a larger sample size and tested conditioned pain inhibition in response to a cold pressor [38]. Another possibility is that the FM positive CLBP participants are likely to have an on-going peripheral source of pain (peripheral sensitization) that may be overriding their descending endogenous inhibition and thus influencing their pain sensitivity of the lower back in response to the conditioning painful stimulus [20–22, 26]. Therefore, the lower back region as a local site may not be sensitive enough to test descending inhibitory pathways in CLBP. This warrants future studies.
One of the strengths of the present study is that we included both the 2011 FM survey and QST to comprehensively identify the presence of CS in CLBP while a majority of the previous studies included the 2011 FM survey or QST alone as the main CS related outcome measure(s) [34, 35, 38, 53–55]. The most similar study was conducted by Neville et al., in which both the 2011 FM survey and QST were included to examine the associations between the 2011 FM scores and results of QST in patients with knee osteoarthritis [88]. However, they did not use the FM scores as a dichotomous variable to categorize FM positive subgroup but rather used the FM scores as a continuous variable to examine associations with QST. In the present study, we used FM scores as a dichotomous variable to identify the presence of CS and as a continuous variable to examine association between CS, QST and other self-reported measures. Addition of QST tests validated the FM scores and provided valid and rigorous evidence regarding the clinical presence of CS within the CLBP population.
Psychosocial Symptoms
Our results show that clinical presentations of the subgroups are clearly different, based on all self-reported measures used in the present study. FM positive CLBP participants had poorer function and more severe pain catastrophizing thoughts, and greater anxiety and depression symptoms as compared to FM negative CLBP participants. In fact, the FM negative subgroup presented with normal to minimal levels of symptom while the FM positive subgroup presented with a moderate level of symptoms for most of the clinical outcomes. These findings are consistent with previous studies [54, 88, 89] and the current neuroscience literature [16, 17, 20, 24, 29]. For example, Huysmans et al. found associations between CS and function, pain catastrophizing, and fear avoidance behavior in CLBP [52, 89]. Theoretically, neuroplastic changes originated from the nociceptive pathways eventually diffuse and expand to various brain regions, including the insular cortex, cingulate cortex, prefrontal cortex, and the limbic system [15, 16, 20–22, 26, 30, 31], and can attribute to these psychosocial symptoms [15, 16, 20–22, 26, 30, 31]. Furthermore, these symptoms, especially pain catastrophizing thoughts, fear avoidance belief, anxiety, and depression, can also enhance forebrain activity[16, 90–92], leading to enhancement of central excitability (i.e., cognitive-emotional sensitization) [16, 90–92]. However, the cause and effect relationship between these symptoms and development, augmentation, or maintenance of CS has not been established yet; the relationship might be bidirectional [15, 16, 19, 20, 54, 90–93].
Interestingly, the present study did not show a significant difference between the CLBP subgroups with fear avoidance behavior, even though some scientific theories suggest fear avoidance belief is one of the main factors contributing to the chronicity of pain and potentially to CS [17, 20, 25, 65, 94]. This might be explained by the fact that neuropathological mechanisms of CS are multifactorial involving genetic, neurological and biopsychosocial parameters and the degree of each parameter’s contribution toward CS has not been fully investigated in the CLBP population [15–17, 20, 21, 26].
Associations between the 2011 FM scores and clinical outcomes
The clinical significance of the FM scores was further examined with the correlations with QST and self-reported questionnaires. The present study showed significant associations between the FM scores and the QST values and between the FM scores and self-reported questionnaires. The strength of the correlation was moderate with PPT of the lower back but weak with PPT values of the thumbnail, suggesting the greater degree of CS causing more primary hyperalgesia, especially in local peripheral tissues. The strength of correlation was moderate with CPM values of thumbnail but weak with CPM values of the lower back, suggesting dysfunction of the descending inhibition and the presence of CS.
These two QST values are strong indicators of the presence of CS, and the present study suggested the FM scores are associated with the degree of local and widespread hyperalgesia, and functionality of the descending inhibitory pathways. These results add a convergent validity for the 2011 FM scores in identifying a subgroup with CS in the CLBP population and consideration of use of the FM survey in clinical practice. Neville et al. also demonstrated significant but weak associations between the FM scores and PPT values of the thumbnail in patients with knee osteoarthritis [88]. However, they did not find significant associations between the FM scores and CPM values using a thumb pressure as their conditioning painful stimulus whereas we used a cuff pressure on the upper arm to induce an ischemic pain. Various CPM paradigms might influence the results across the studies. Further studies are needed to establish a reliable and valid CPM paradigm which could be consistently used to examine the integrity of the descending inhibitory pathways.
The FM scores were also significantly associated with all self-reported questionnaires related to function, pain catastrophizing thoughts, fear avoidance behavior, anxiety and depression. The strength of the correlation coefficients ranged between weak to strong; FM scores were strongly correlated with function, moderately correlated with anxiety but weakly correlated with pain catastrophizing thoughts and fear avoidance beliefs. These findings are also consistent with previous studies where FM scores were significantly associated with function, pain catastrophizing thoughts, anxiety, and depression in patients with spine pain[54] and knee osteoarthritis [52]. Taken together, these results support that the 2011 FM survey may be utilized as a simple clinical tool to identify a subgroup of CLBP patients with CS and the degree severity of clinical symptoms related to CS.
Clinical Application
CLBP is a heterogeneous population with various causes of pain, even though the general diagnosis is often given as non-specific CLBP [8, 9, 15, 23–25]. Emerging evidence suggests that CS is one of the major underlying mechanisms for a certain subset of CLBP patients [15–17, 20, 21, 24]. Our study provides further evidence and adds to the current knowledge about CS in CLBP. This can, in part, explain the high failure rate of current interventions for CLBP that only target the peripheral issues[95, 96] and the current high prevalence of CLBP [97]. Presence and assessment of CS with simple and valid tools should be considered in clinical practice to improve clinical examinations and to determine interventions targeting the central nervous system for the subgroup that presents with CS symptoms.
To identify the presence of CS in CLBP patients in clinical settings, the 2011 FM survey may be helpful as the present study demonstrated significant associations with the FM scores and the QST values that are valid, reliable and objective measures of CS. Psychosocial symptoms such as pain catastrophizing thoughts, anxiety and depression should also be considered as they are also associated with CS. Classifying the subgroup of CS in CLBP patients could lead to targeted and mechanistic-based interventions, which would likely improve outcomes for these patients. Targeted interventions for CS such as centrally acting analgesics such as serotonin-norepinephrine reuptake inhibitors [98], cognitive behavioral therapy [99], and aerobic exercises[100–102] have shown some promising results.
Limitations
Our study was limited to a cross-section design and thus, we cannot comment whether CS is a substrate or a consequence of low back pain. The study design did not separate the involvement of or the contribution of local back tissue pathologies toward CS. These patients may have peripheral sensitization, adding to their widespread pain, alerting pain threshold, and causing manifestations of psychosocial symptoms. Secondly, our sample size might be relatively small even though our study was powered for PPT values in the thumbnail (a statistical power of 0.80, α = 0.05), which is a key outcome measure of CS. However, we considered clinical significances and correlation strengths to guide readers about the magnitude of CS to account for our small sample size. A future study with a larger sample size should be considered to validate the present findings. Thirdly, even though the FM survey and QST are valid and reliable outcome measures for identifying the presence of CS, advance neuroimaging methods such as proton magnetic resonance spectroscopy that can measure metabolic concentration of some key biomarkers of CS (e,g,, glutamate and gamma-aminobutyric acid (GABA) [47, 103]) or functional magnetic resonance imaging (fMRI) that can measure neural activity in brain regions [34] might provide us with stronger evidence for presence of CS. Lastly, approximately 70% of the participants were female in the present study and therefore the results may be different if we had an even number of female and male participants. However, the results may still be acceptable given that chronic pain including CLBP is more common in females than males [104, 105].
Conclusion
The results of the present study identified 48% of CLBP participants with CS using the 2011 FM survey. PPT and CPM tests showed significant widespread hyperalgesia and dysfunction of the descending inhibitory pathways in FM positive CLBP participants compared to the FM negative CLBP participants. Furthermore, our results demonstrated the FM positive CLBP participants showed significantly poorer functional level and more severe pain catastrophizing thoughts, anxiety, and depression than FM negative CLBP participants. These findings strongly confirm that there is a subgroup of patients who present with CS as the predominant underlying pain mechanism in the CLBP population. The results suggest that CLBP patients should be assessed based on their underlying causes rather than diagnosis in clinical practice. Additionally, the 2011 FM scores were significantly associated with the PPT and CPM values, function, and psychosocial symptoms, suggesting that the 2011 FM survey could be used clinically for identifying the presence of CS in the CLBP population.
Acknowledgement
The authors would like to acknowledge Andrew Doyle, Garrett Holle, Ellen Marko, Jacquelyne Vaughan, and Elizabeth Meany for their assistances with the participant recruitment and data collection. This work was partially supported by the T32 Program (T32HD057850; PI: Randolph Nudo) to Andrew D and J Vaughan and by the Department of Physical Therapy and Rehabilitation Science to NK Sharma.
Conflict of interest disclosure and source of funding
The authors have no conflict of interest to declare. This work was partially supported by the T32 Program (T32HD057850; PI: Randolph Nudo) to Andrew D and J Vaughan and by the Department of Physical Therapy and Rehabilitation Science to NK Sharma. A Nicol has received research funding from National Institute of General Medical Sciences of the National Institutes of Health (NIH #K23GM123320) and served as a consultant for Heron Therapeutics and on an advisory board of Sandoz International.
Reference
- 1.Dillingham T, Evaluation and management of low back pain: an overview. SPINE-PHILADELPHIA-HANLEY AND BELFUS-, 1995. 9: p. 559–596. [Google Scholar]
- 2.Stubbs B, Schofield P, and Patchay S, Mobility Limitations and Fall-Related Factors Contribute to the Reduced Health-Related Quality of Life in Older Adults With Chronic Musculoskeletal Pain. Pain Practice, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson JH, Chronic back pain: searching for causes and cures. J Rheumatol, 2004. 31(12): p. 2323–5. [PubMed] [Google Scholar]
- 4.Gore M, et al. , The burden of chronic low back pain: clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine (Phila Pa 1976), 2012. 37(11): p. E668–77. [DOI] [PubMed] [Google Scholar]
- 5.Cottingham JT and Maitland J, A three-paradigm treatment model using soft tissue mobilization and guided movement-awareness techniques for a patient with chronic low back pain: a case study. J Orthop Sports Phys Ther, 1997. 26(3): p. 155–67. [DOI] [PubMed] [Google Scholar]
- 6.Hoy D, et al. , A systematic review of the global prevalence of low back pain. Arthritis Rheum, 2012. 64(6): p. 2028–37. [DOI] [PubMed] [Google Scholar]
- 7.Becker A, et al. , Low back pain in primary care: costs of care and prediction of future health care utilization. Spine, 2010. 35(18): p. 1714–1720. [DOI] [PubMed] [Google Scholar]
- 8.Airaksinen O, et al. , Chapter 4. European guidelines for the management of chronic nonspecific low back pain. Eur Spine J, 2006. 15 Suppl 2: p. S192–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krismer M and Van Tulder M, Low back pain (non-specific). Best Practice & Research Clinical Rheumatology, 2007. 21(1): p. 77–91. [DOI] [PubMed] [Google Scholar]
- 10.Jensen MC, et al. , Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med, 1994. 331(2): p. 69–73. [DOI] [PubMed] [Google Scholar]
- 11.Carragee E, et al. , Are first-time episodes of serious LBP associated with new MRI findings? Spine J, 2006. 6(6): p. 624–35. [DOI] [PubMed] [Google Scholar]
- 12.Borenstein DG, et al. , The value of magnetic resonance imaging of the lumbar spine to predict low-back pain in asymptomatic subjects : a seven-year follow-up study. J Bone Joint Surg Am, 2001. 83-a(9): p. 1306–11. [DOI] [PubMed] [Google Scholar]
- 13.Beattie KA, et al. , Abnormalities identified in the knees of asymptomatic volunteers using peripheral magnetic resonance imaging. Osteoarthritis Cartilage, 2005. 13(3): p. 181–6. [DOI] [PubMed] [Google Scholar]
- 14.Max MB, Is mechanism-based pain treatment attainable? Clinical trial issues. The Journal of Pain, 2000. 1(3): p. 2–9. [DOI] [PubMed] [Google Scholar]
- 15.Clauw DJ, Diagnosing and treating chronic musculoskeletal pain based on the underlying mechanism(s). Best Pract Res Clin Rheumatol, 2015. 29(1): p. 6–19. [DOI] [PubMed] [Google Scholar]
- 16.Roussel NA, et al. , Central sensitization and altered central pain processing in chronic low back pain: fact or myth? Clin J Pain, 2013. 29(7): p. 625–38. [DOI] [PubMed] [Google Scholar]
- 17.Nijs J, et al. , Low back pain: guidelines for the clinical classification of predominant neuropathic, nociceptive, or central sensitization pain. Pain Physician, 2015. 18(3): p. E333–46. [PubMed] [Google Scholar]
- 18.Neziri AY, et al. , Ranking of parameters of pain hypersensitivity according to their discriminative ability in chronic low back pain. Pain, 2012. 153(10): p. 2083–91. [DOI] [PubMed] [Google Scholar]
- 19.George SZ, et al. , Sex and pain-related psychological variables are associated with thermal pain sensitivity for patients with chronic low back pain. J Pain, 2007. 8(1): p. 2–10. [DOI] [PubMed] [Google Scholar]
- 20.Woolf CJ, Central sensitization: implications for the diagnosis and treatment of pain. Pain, 2011. 152(3 Suppl): p. S2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woolf CJ, Central sensitization: uncovering the relation between pain and plasticity. Anesthesiology, 2007. 106(4): p. 864–7. [DOI] [PubMed] [Google Scholar]
- 22.Woolf CJ, Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med, 2004. 140(6): p. 441–51. [DOI] [PubMed] [Google Scholar]
- 23.Nijs J, Van Houdenhove B, and Oostendorp RA, Recognition of central sensitization in patients with musculoskeletal pain: Application of pain neurophysiology in manual therapy practice. Man Ther, 2010. 15(2): p. 135–41. [DOI] [PubMed] [Google Scholar]
- 24.Nijs J, et al. , Applying modern pain neuroscience in clinical practice: criteria for the classification of central sensitization pain. Pain Physician, 2014. 17(5): p. 447–57. [PubMed] [Google Scholar]
- 25.Nijs J, et al. , How to explain central sensitization to patients with ‘unexplained’ chronic musculoskeletal pain: practice guidelines. Man Ther, 2011. 16(5): p. 413–8. [DOI] [PubMed] [Google Scholar]
- 26.Latremoliere A and Woolf CJ, Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain, 2009. 10(9): p. 895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clauw DJ, Fibromyalgia and related conditions. Mayo Clinic Proceedings, 2015. 90(5): p. 680–692. [DOI] [PubMed] [Google Scholar]
- 28.Clauw DJ, Fibromyalgia: a clinical review. JAMA: Journal of the American Medical Association, 2014. 311(15): p. 1547–1555. [DOI] [PubMed] [Google Scholar]
- 29.Nijs J, Van Houdenhove B, and Oostendorp RA, Recognition of central sensitization in patients with musculoskeletal pain: application of pain neurophysiology in manual therapy practice. Manual therapy, 2010. 15(2): p. 135–141. [DOI] [PubMed] [Google Scholar]
- 30.Langevin HM and Sherman KJ, Pathophysiological model for chronic low back pain integrating connective tissue and nervous system mechanisms. Med Hypotheses, 2007. 68(1): p. 74–80. [DOI] [PubMed] [Google Scholar]
- 31.O’Sullivan P, Diagnosis and classification of chronic low back pain disorders: maladaptive movement and motor control impairments as underlying mechanism. Man Ther, 2005. 10(4): p. 242–55. [DOI] [PubMed] [Google Scholar]
- 32.Purves D AG, Fitzpatrick D, Hall W, Lamantia AS, White L, Neuroscience, Fifth edition Sinauer Associates, 2012. [Google Scholar]
- 33.Samad TA, et al. , Interleukin-1β-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature, 2001. 410(6827): p. 471. [DOI] [PubMed] [Google Scholar]
- 34.Giesecke T, et al. , Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum, 2004. 50(2): p. 613–23. [DOI] [PubMed] [Google Scholar]
- 35.Giesbrecht RJ and Battie MC, A comparison of pressure pain detection thresholds in people with chronic low back pain and volunteers without pain. Phys Ther, 2005. 85(10): p. 1085–92. [PubMed] [Google Scholar]
- 36.Clauw DJ, et al. , Pain sensitivity as a correlate of clinical status in individuals with chronic low back pain. Spine (Phila Pa 1976), 1999. 24(19): p. 2035–41. [DOI] [PubMed] [Google Scholar]
- 37.Baliki MN, et al. , Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. Journal of Neuroscience, 2006. 26(47): p. 12165–12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Correa JB, et al. , Central sensitization and changes in conditioned pain modulation in people with chronic nonspecific low back pain: a case-control study. Exp Brain Res, 2015. 233(8): p. 2391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edwards RR, et al. , Individual differences in diffuse noxious inhibitory controls (DNIC): association with clinical variables. Pain, 2003. 106(3): p. 427–37. [DOI] [PubMed] [Google Scholar]
- 40.Arendt-Nielsen L, et al. , Sensitization in patients with painful knee osteoarthritis. Pain, 2010. 149(3): p. 573–81. [DOI] [PubMed] [Google Scholar]
- 41.Fatemi G, et al. , Deconstructing chronic low back pain in the older adult--Step by step evidence and expert-based recommendations for evaluation and treatment part III: Fibromyalgia syndrome. Pain Med, 2015. 16(9): p. 1709–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolfe F, et al. , Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol, 2011. 38(6): p. 1113–22. [DOI] [PubMed] [Google Scholar]
- 43.Wolfe F, et al. , 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum, 2016. 46(3): p. 319–329. [DOI] [PubMed] [Google Scholar]
- 44.Harte SE, et al. , The 2011 fibromyalgia (FM) survey criteria are a surrogate measure of pain centralization. Arthritis & Rheumatology, 2015. 67: p. 2764–2765. [Google Scholar]
- 45.Hudson JI and Pope HG, The concept of affective spectrum disorder: relationship to fibromyalgia and other syndromes of chronic fatigue and chronic muscle pain. Baillière’s clinical rheumatology, 1994. 8(4): p. 839–856. [DOI] [PubMed] [Google Scholar]
- 46.Clauw DJ, et al. , The relationship between fibromyalgia and interstitial cystitis. Journal of psychiatric research, 1997. 31(1): p. 125–131. [DOI] [PubMed] [Google Scholar]
- 47.Sharma NK, et al. , Primary somatosensory cortex in chronic low back pain–a 1H-MRS study. Journal of pain research, 2011. 4: p. 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma NK, et al. , Neurochemical analysis of primary motor cortex in chronic low back pain. Brain Sci, 2012. 2(3): p. 319–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cagnie B, et al. , Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI. Semin Arthritis Rheum, 2014. 44(1): p. 68–75. [DOI] [PubMed] [Google Scholar]
- 50.Kong J, et al. , S1 is associated with chronic low back pain: a functional and structural MRI study. Mol Pain, 2013. 9: p. 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grachev ID, Fredrickson BE, and Apkarian AV, Abnormal brain chemistry in chronic back pain: an in vivo proton magnetic resonance spectroscopy study. Pain, 2000. 89(1): p. 7–18. [DOI] [PubMed] [Google Scholar]
- 52.Neville SJ, et al. , Association between the 2011 Fibromyalgia Survey Criteria and Multisite Pain Sensitivity in Knee Osteoarthritis. Clin J Pain, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gist AC, et al. , Fibromyalgia remains a significant burden in rheumatoid arthritis patients in Australia. International journal of rheumatic diseases, 2018. 21(3): p. 639–646. [DOI] [PubMed] [Google Scholar]
- 54.Brummett CM, et al. , Prevalence of the fibromyalgia phenotype in patients with spine pain presenting to a tertiary care pain clinic and the potential treatment implications. Arthritis & Rheumatism, 2013. 65(12): p. 3285–3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Neill S, et al. , Generalized deep-tissue hyperalgesia in patients with chronic low-back pain. Eur J Pain, 2007. 11(4): p. 415–20. [DOI] [PubMed] [Google Scholar]
- 56.Laursen BS, et al. , Health related quality of life and quantitative pain measurement in females with chronic non-malignant pain. Eur J Pain, 2005. 9(3): p. 267–75. [DOI] [PubMed] [Google Scholar]
- 57.Tuveson B, Leffler A-S, and Hansson P, Influence of heterotopic noxious conditioning stimulation on spontaneous pain and dynamic mechanical allodynia in central post-stroke pain patients. Pain, 2009. 143(1): p. 84–91. [DOI] [PubMed] [Google Scholar]
- 58.Leffler AS, Hansson P, and Kosek E, Somatosensory perception in a remote pain-free area and function of diffuse noxious inhibitory controls (DNIC) in patients suffering from long-term trapezius myalgia. Eur J Pain, 2002. 6(2): p. 149–59. [DOI] [PubMed] [Google Scholar]
- 59.Wolf-Maier K, et al. , Hypertension treatment and control in five European countries, Canada, and the United States. Hypertension, 2004. 43(1): p. 10–7. [DOI] [PubMed] [Google Scholar]
- 60.Katz NP, Paillard FC, and Edwards RR, Review of the performance of quantitative sensory testing methods to detect hyperalgesia in chronic pain patients on long-term opioids. Anesthesiology, 2015. 122(3): p. 677–85. [DOI] [PubMed] [Google Scholar]
- 61.Hubscher M, et al. , Relationship between quantitative sensory testing and pain or disability in people with spinal pain-a systematic review and meta-analysis. Pain, 2013. 154(9): p. 1497–504. [DOI] [PubMed] [Google Scholar]
- 62.de Kruijf M, et al. , Determinants for Quantitative Sensory Testing and the Association with Chronic Musculoskeletal Pain in the General Elderly Population. Pain Pract, 2016. 16(7): p. 831–41. [DOI] [PubMed] [Google Scholar]
- 63.Fairbank JC and Pynsent PB, The Oswestry Disability Index. Spine (Phila Pa 1976), 2000. 25(22): p. 2940–52; discussion 2952. [DOI] [PubMed] [Google Scholar]
- 64.Fairbank JC, et al. , The Oswestry low back pain disability questionnaire. Physiotherapy, 1980. 66(8): p. 271–3. [PubMed] [Google Scholar]
- 65.Vlaeyen JW and Linton SJ, Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain, 2000. 85(3): p. 317–332. [DOI] [PubMed] [Google Scholar]
- 66.Lethem J, et al. , Outline of a fear-avoidance model of exaggerated pain perception—I. Behaviour research and therapy, 1983. 21(4): p. 401–408. [DOI] [PubMed] [Google Scholar]
- 67.Waddell G, et al. , A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain, 1993. 52(2): p. 157–68. [DOI] [PubMed] [Google Scholar]
- 68.Sullivan MJ, Bishop SR, and Pivik J, The pain catastrophizing scale: development and validation. Psychological assessment, 1995. 7(4): p. 524. [Google Scholar]
- 69.Steer RA and Beck AT, Beck Anxiety Inventory. 1997. [Google Scholar]
- 70.Beck AT and Steer RA, Internal consistencies of the original and revised Beck Depression Inventory. Journal of clinical psychology, 1984. 40(6): p. 1365–1367. [DOI] [PubMed] [Google Scholar]
- 71.Pud D, Granovsky Y, and Yarnitsky D, The methodology of experimentally induced diffuse noxious inhibitory control (DNIC)-like effect in humans. Pain, 2009. 144(1–2): p. 16–9. [DOI] [PubMed] [Google Scholar]
- 72.Lindbäck Y, et al. , Association between pain sensitivity in the hand and outcomes after surgery in patients with lumbar disc herniation or spinal stenosis. European Spine Journal, 2017: p. 1–8. [DOI] [PubMed] [Google Scholar]
- 73.De Groef A, et al. , Unraveling Self-Reported Signs of Central Sensitization in Breast Cancer Survivors with Upper Limb Pain: Prevalence Rate and Contributing Factors. Pain Physician, 2018. 21(3): p. E247–e256. [PubMed] [Google Scholar]
- 74.Nussbaum EL and Downes L, Reliability of clinical pressure-pain algometric measurements obtained on consecutive days. Physical therapy, 1998. 78(2): p. 160–169. [DOI] [PubMed] [Google Scholar]
- 75.Fischer AA, Pressure algometry over normal muscles. Standard values, validity and reproducibility of pressure threshold. Pain, 1987. 30(1): p. 115–126. [DOI] [PubMed] [Google Scholar]
- 76.Greenspan JD, et al. , Pain sensitivity risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case control study. The Journal of Pain, 2011. 12(11): p. T61–T74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tuveson B, Leffler AS, and Hansson P, Time dependent differences in pain sensitivity during unilateral ischemic pain provocation in healthy volunteers. Eur J Pain, 2006. 10(3): p. 225–32. [DOI] [PubMed] [Google Scholar]
- 78.Lewis GN, Rice DA, and McNair PJ, Conditioned pain modulation in populations with chronic pain: a systematic review and meta-analysis. J Pain, 2012. 13(10): p. 936–44. [DOI] [PubMed] [Google Scholar]
- 79.Kosek E and Ordeberg G, Lack of pressure pain modulation by heterotopic noxious conditioning stimulation in patients with painful osteoarthritis before, but not following, surgical pain relief. Pain, 2000. 88(1): p. 69–78. [DOI] [PubMed] [Google Scholar]
- 80.Krishnan S, et al. , Comparison of pain models to detect opioid-induced hyperalgesia. J Pain Res, 2012. 5: p. 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cathcart S, et al. , Noxious inhibition of temporal summation is impaired in chronic tension-type headache. Headache, 2010. 50(3): p. 403–12. [DOI] [PubMed] [Google Scholar]
- 82.Le Bars D, Dickenson AH, and Besson J-M, Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain, 1979. 6(3): p. 283–304. [DOI] [PubMed] [Google Scholar]
- 83.Evans JD, Straightforward statistics for the behavioral sciences. 1996: Brooks/Cole. [Google Scholar]
- 84.Ostelo RW, et al. , Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine, 2008. 33(1): p. 90–94. [DOI] [PubMed] [Google Scholar]
- 85.Schoen CJ, et al. , A novel paradigm to evaluate conditioned pain modulation in fibromyalgia. J Pain Res, 2016. 9: p. 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Julien N, et al. , Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain, 2005. 114(1–2): p. 295–302. [DOI] [PubMed] [Google Scholar]
- 87.Clauw DJ, Fibromyalgia: a clinical review. Jama, 2014. 311(15): p. 1547–1555. [DOI] [PubMed] [Google Scholar]
- 88.Neville SJ, et al. , Association Between the 2011 Fibromyalgia Survey Criteria and Multisite Pain Sensitivity in Knee Osteoarthritis. Clin J Pain, 2018. 34(10): p. 909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huysmans E, et al. , Association Between Symptoms of Central Sensitization and Cognitive Behavioral Factors in People With Chronic Nonspecific Low Back Pain: A Cross-sectional Study. J Manipulative Physiol Ther, 2018. 41(2): p. 92–101. [DOI] [PubMed] [Google Scholar]
- 90.Nijs J, et al. , Dysfunctional endogenous analgesia during exercise in patients with chronic pain: to exercise or not to exercise? Pain Physician, 2012. 15(3 Suppl): p. Es205–13. [PubMed] [Google Scholar]
- 91.Seminowicz DA and Davis KD, Cortical responses to pain in healthy individuals depends on pain catastrophizing. Pain, 2006. 120(3): p. 297–306. [DOI] [PubMed] [Google Scholar]
- 92.Brosschot JF, Cognitive-emotional sensitization and somatic health complaints. Scand J Psychol, 2002. 43(2): p. 113–21. [DOI] [PubMed] [Google Scholar]
- 93.O’Sullivan P, et al. , Sensory characteristics of chronic non-specific low back pain: a subgroup investigation. Man Ther, 2014. 19(4): p. 311–8. [DOI] [PubMed] [Google Scholar]
- 94.Leeuw M, et al. , The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. Journal of behavioral medicine, 2007. 30(1): p. 77–94. [DOI] [PubMed] [Google Scholar]
- 95.Cohen SP, et al. , Lumbar zygapophysial (facet) joint radiofrequency denervation success as a function of pain relief during diagnostic medial branch blocks: a multicenter analysis. The Spine Journal, 2008. 8(3): p. 498–504. [DOI] [PubMed] [Google Scholar]
- 96.Cohen SP, et al. , Factors predicting success and failure for cervical facet radiofrequency denervation: a multi-center analysis. Regional anesthesia and pain medicine, 2007. 32(6): p. 495–503. [DOI] [PubMed] [Google Scholar]
- 97.Freburger JK, et al. , The rising prevalence of chronic low back pain. Arch Intern Med, 2009. 169(3): p. 251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Skljarevski V, et al. , Efficacy and safety of duloxetine in patients with chronic low back pain. Spine, 2010. 35(13): p. E578–E585. [DOI] [PubMed] [Google Scholar]
- 99.Glombiewski JA, et al. , Psychological treatments for fibromyalgia: a meta-analysis. PAIN®, 2010. 151(2): p. 280–295. [DOI] [PubMed] [Google Scholar]
- 100.Whiteside A, Hansen S, and Chaudhuri A, Exercise lowers pain threshold in chronic fatigue syndrome. Pain, 2004. 109(3): p. 497–499. [DOI] [PubMed] [Google Scholar]
- 101.Sharma NK, et al. , Aerobic exercise alters analgesia and neurotrophin-3 synthesis in an animal model of chronic widespread pain. Physical therapy, 2010. 90(5): p. 714–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meeus M, et al. , Reduced pressure pain thresholds in response to exercise in chronic fatigue syndrome but not in chronic low back pain: an experimental study. J Rehabil Med, 2010. 42(9): p. 884–90. [DOI] [PubMed] [Google Scholar]
- 103.Zhao X, et al. , Neurochemical changes in patients with chronic low back pain detected by proton magnetic resonance spectroscopy: A systematic review. Neuroimage Clin, 2017. 13: p. 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Martel MO, Wasan AD, and Edwards RR, Sex differences in the stability of conditioned pain modulation (CPM) among patients with chronic pain. Pain Medicine, 2013. 14(11): p. 1757–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Boyan BD, et al. , Addressing the gaps: sex differences in osteoarthritis of the knee. Biology of sex differences, 2013. 4(1): p. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]