Abstract
Aims:
The study aims to determine the modulatory roles of ovarian hormones, estrogen (E2) and progesterone(P), on the expression of endothelin A (ETA) and B (ETB) receptors in lung, liver and kidney tissues.
Main methods:
Female Sprague–Dawley rats were subjected to bilateral ovariectomy and divided into four groups ovariectomized (OVX), OVX + E2, OVX + P, and OVX + E2 + P. A separate group of rats underwent sham surgery and served as a control. Three weeks after OVX or sham surgery, tissues from lungs, liver, renal cortex, and inner medulla were collected, snap-frozen, and kept at −80 °C for assessment of ETA and ETB receptor expression using real-time PCR.
Key findings:
E2-treated OVX animals had significantly lower expression of ETA receptors in the lungs, compared to OVX rats. Pulmonary ETB receptor mRNA was not measurably affected by any of the interventions. Hepatic ETA and ETB were significantly increased in OVX + E2 + P rats, compared to sham rats. Renal inner medullary ETA and ETB receptor expressions were significantly elevated in OVX compared to sham, an effect that was prevented by co-supplementation of OVX with E2 and P. Additionally, both ETA and ETB receptor expression in the renal cortex were significantly attenuated by ovariectomy, and this reduction was not evident in OVX + E2 rats.
Significance:
These data suggest that ovarian hormones regulate ET receptor expression and may contribute to sex differences in cardiovascular and renal health.
Keywords: Endothelin, ETA receptors, ETB receptors, Estrogen, Progesterone
1. Introduction
Premenopausal women are largely protected from hypertension and kidney-related diseases compared with men of similar age [15,25,28,31]. This phenomenon is gradually lost after menopause, pointing to a pivotal role of ovarian hormones in the maintenance of cardiovascular and renal health in women [11,34,37]. Endothelin-1 (ET-1), a potent vasoactive peptide found in cardiovascular and renal tissues, has been implicated in sex-differences in blood pressure control and kidney function [17–19,38]. The actions of ET-1 are mediated through activation of the G-protein-coupled receptors, ETA and ETB, which are found in a variety of cells in the cardiovascular and renal systems [38]. The activation of ETA receptors by ET-1 results in strong and sustained vasoconstriction and promotes oxidative stress [3,6], inflammation, and proteinuria [30]. On the other hand, the activation of ETB receptors by ET-1 causes vasorelaxation [12] and inhibits sodium reabsorption in the renal collecting ducts [20]. Additionally, ETB receptors in kidney and lung may serve to clear ET from circulation by receptor-mediated uptake and subsequent lysosomal degradation [7]. Therefore, ET-1 appears to have paradoxical effects on blood pressure regulation and renal function by acting either on ETA or ETB receptors.
Sex differences in ET-1 signaling system have been observed in renal [14,17,19], vascular [33], pulmonary [8], as well as hepatic tissues [13]. Despite the important role for the ET-1 system in mediating sex-differences in blood pressure and renal diseases, the roles of female sex steroids, estrogen (E2) and progesterone (P), in regulating this critically important system are unclear. Similar to E2, ET receptor antagonists have vasculoprotective effects in ovariectomized rats [16]. E2 suppresses renal ET-1 overproduction and the consequent renal damage in acute kidney injury [35]. In the study reported by Lamping and Nuno, P alleviates the vasoconstrictor effect of ET-1, but is less effective than E2 [23]. Additionally, Zhang et al. showed that P treatment in OVX mice enhances renal ETA receptor mRNA expression [40]. In the present study, we hypothesized that ovarian hormones provide cardiovascular and renal benefit that may be related to increased ETB and decreased ETA activity. To test this hypothesis, we studied the effect of ovarian hormonal depletion (by ovariectomy) and replacement (by E2 and/or P supplementation in OVX rats) on ETA and ETB receptor mRNA expression in lung, liver, renal cortex and inner medulla.
2. Materials and methods
2.1. Animals
Studies were performed on female (13–15 weeks old) Sprague Dawley rats, purchased from Harlan Laboratories (Indianapolis, IN). Rats were maintained on standard rodent diet (NIH-31 Open Formula, 7017 (0.3% Na), Teklad Diets, Madison, WI) with free access to water. During the entire experimental period, animals were housed in a temperature and humidity-controlled room with a 12:12-h light–dark cycle. All experimental procedures executed in this study were in accordance with National Institutes of Health guidelines for use and care animals and were approved by the Institutional Animal Care and Use Committee at University of Alabama at Birmingham.
2.2. Ovariectomy and hormonal replacement
Under isoflurane (2%) anesthesia, bilateral ovariectomy was performed through dorsal bilateral incisions. Ovaries were exposed, tied off and removed. The muscle layer was sutured and the incisions were closed with wound clips. An additional group of rats underwent sham operation (dorsal bilateral incisions and ovarian exposure without removal of ovaries) and served as a control group. At the time performing ovariectomy, OVX rats were subcutaneously implanted with controlled-release pellets (21-day release, Innovative Research America, Sarasota, Fl., USA) containing: (1) 17β-estradiol (0.35 mg/pellet, OVX + E2), (2) progesterone (25 mg/pellet, OVX + P), or (3) both (OVX + E2 + P). A separate group of OVX rats were implanted with cebo pellets.
2.3. Tissue collection and real time PCR
On day 21 after OVX or sham surgery, rats (16–18 weeks old) were anesthetized (isoflurane) and tissues from lung, liver and different parts of the kidney were harvested, snap frozen in liquid nitrogen and stored at −80 °C until molecular assays were performed.
RNA from different tissues was isolated using Purelink Mini RNA traction kit (Ambion) according to manufacturer’s instructions. Then, the isolated RNA was reverse transcribed using QuantiTect Reverse Transcription kit (Qiagen). Finally, the resulting cDNA was used quantify mRNA by real time PCR (CFX96 Real-Time System, BIORAD) using TaqMan primer gene expression assays with ETA receptor (catalog no. Rn00561137_m1), ETB receptor (catalog no. Rn00569139_m1), and GAPDH (catalog no. Rn01775763_g1) primers. ET receptor gene expression was quantified relative to GAPDH using 2−ΔΔCt method [2].
2.4. Estradiol and progesterone assay
The levels of serum estradiol and progesterone were measured the ELISA (CALBIOTEC® Mouse/Rat Estradiol ELISA, Spring Valley, CA) and (ALPCO® Progesterone rat/mouse ELISA, Salem, NH), following the manufacturer instructions.
2.5. Statistics
Values are presented as means ± SEM. Statistical comparison tween several groups was done by one-way ANOVA followed by post hoc analysis with t-test and Bonferroni correction. P values <0.05 were considered significant.
3. Results
Figs. 1–3 illustrate the effects of ovariectomy and supplementation of OVX rats with different hormone replacement regimens (E2 and each one alone or combined together) on the mRNA expression of ET and ETB receptors in lung, liver and kidney tissues of female Sprague Dawley rats. Compared with sham values, pulmonary ETA mRNA levels were not affected by ovariectomy or supplementation of OVX rats with P alone or combined with E2. However, hormone replacement of OVX rats with E2 significantly attenuated ETA receptor expression in lung, compared to OVX or sham values (Fig. 1A). On the other hand, pression of ETB receptor mRNA in lungs were not significantly different among sham, OVX, OVX + E2, OVX + E2 + P, OVX + P groups of rats, (Fig. 1B). The expression of ETA and ETB receptor mRNA appeared to be enhanced in liver tissues obtained from OVX rats, however this increase did not reach statistical significance (Fig. 2A & B). Compared to sham rats, OVX rats co-treated with E2 and P showed significant increases in the expression of hepatic ETA and ETB receptor mRNA. The increase in ETB receptor mRNA levels was also evident in rats treated with each hormone individually, compared to sham values, as demonstrated in Fig. 2A & B.
Fig. 1.
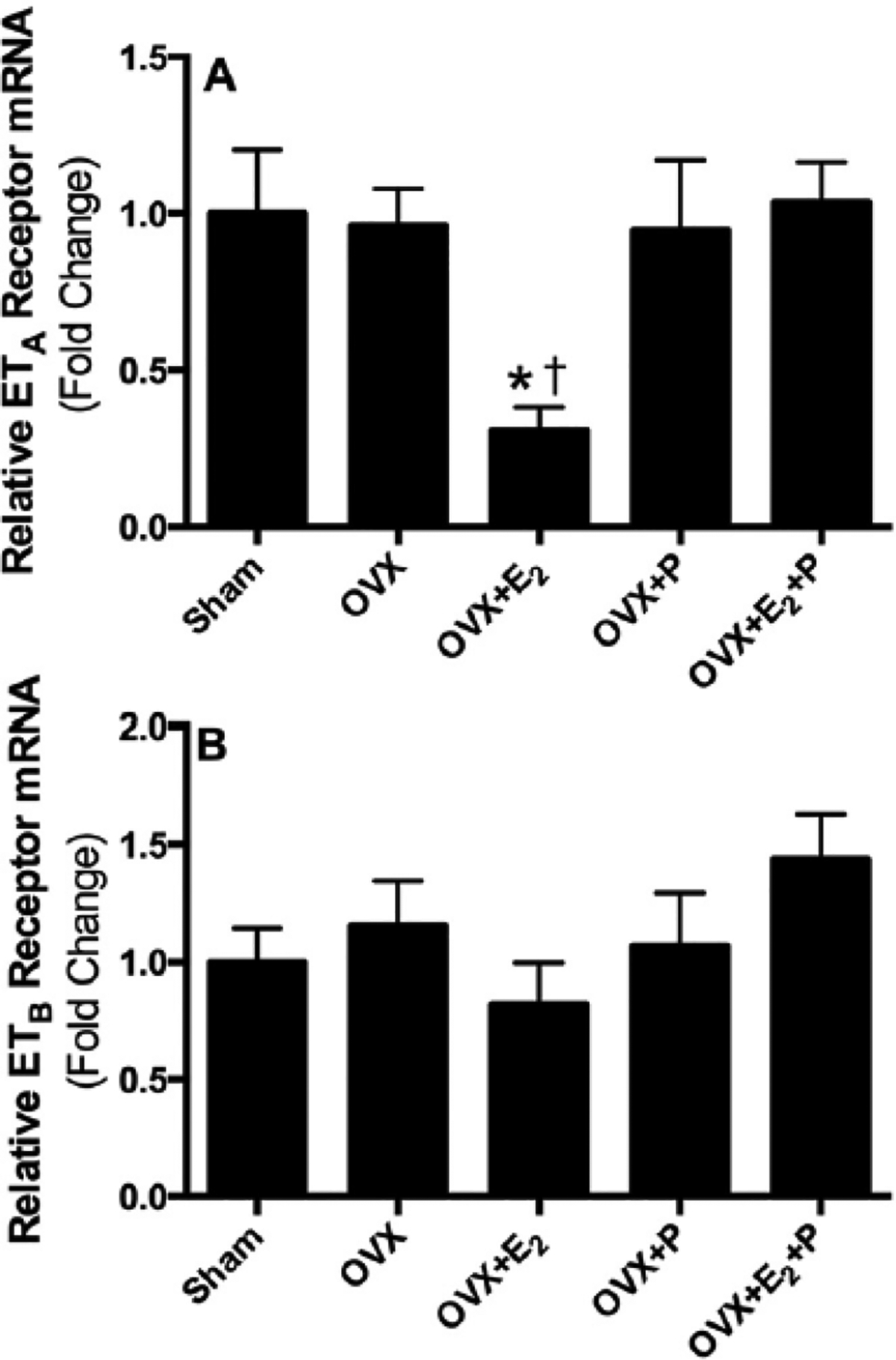
Relative mRNA expression of ETA (A) and ETB (B) receptors in lung tissues obtained from sham, ovariectomized (OVX) rats implanted with subcutaneous pellets containing estrogen (OVX + E2), progesterone (OVX + P), their combination (OVX + E2 + P) or placebo pellets (OVX). Values represent fold change from sham levels. Data are means ± SE of 4–6 samples in each group. *P < 0.05 vs. sham. †P 0.05 vs. OVX.
Fig. 3.
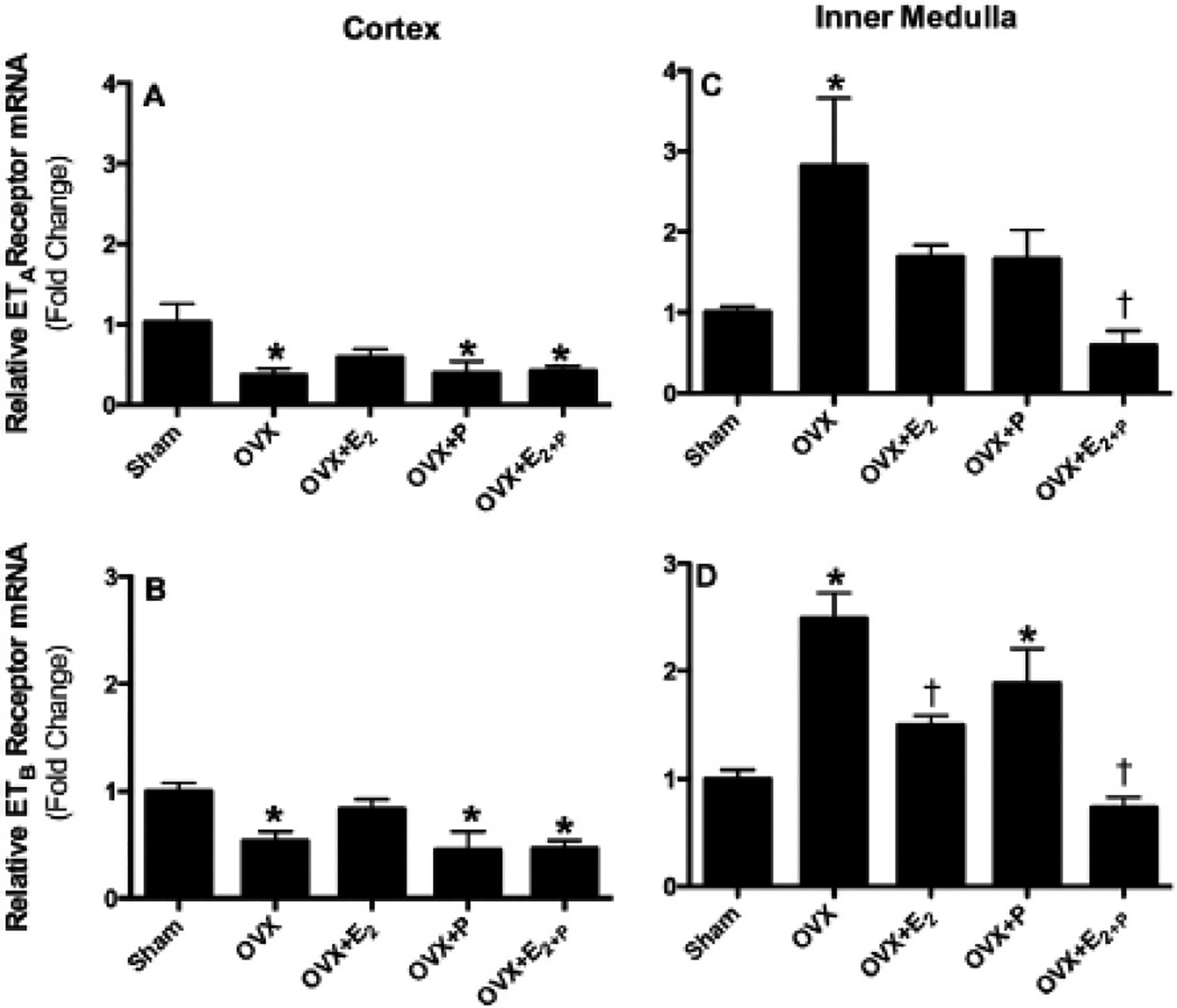
Relative mRNA expression of ETA and ETB receptors in renal cortex (A & B) and inner medulla (C & D) obtained from sham, ovariectomized (OVX) rats implanted with subcutaneous pellets containing estrogen (OVX + E2), progesterone (OVX + P), their combination (OVX + E2 + P) or placebo pellets (OVX). Values represent fold change from sham levels. Data are means ± SE of 4–7 samples in each group. *P < 0.05 vs. sham. †P 0.05 vs. OVX.
Fig. 2.
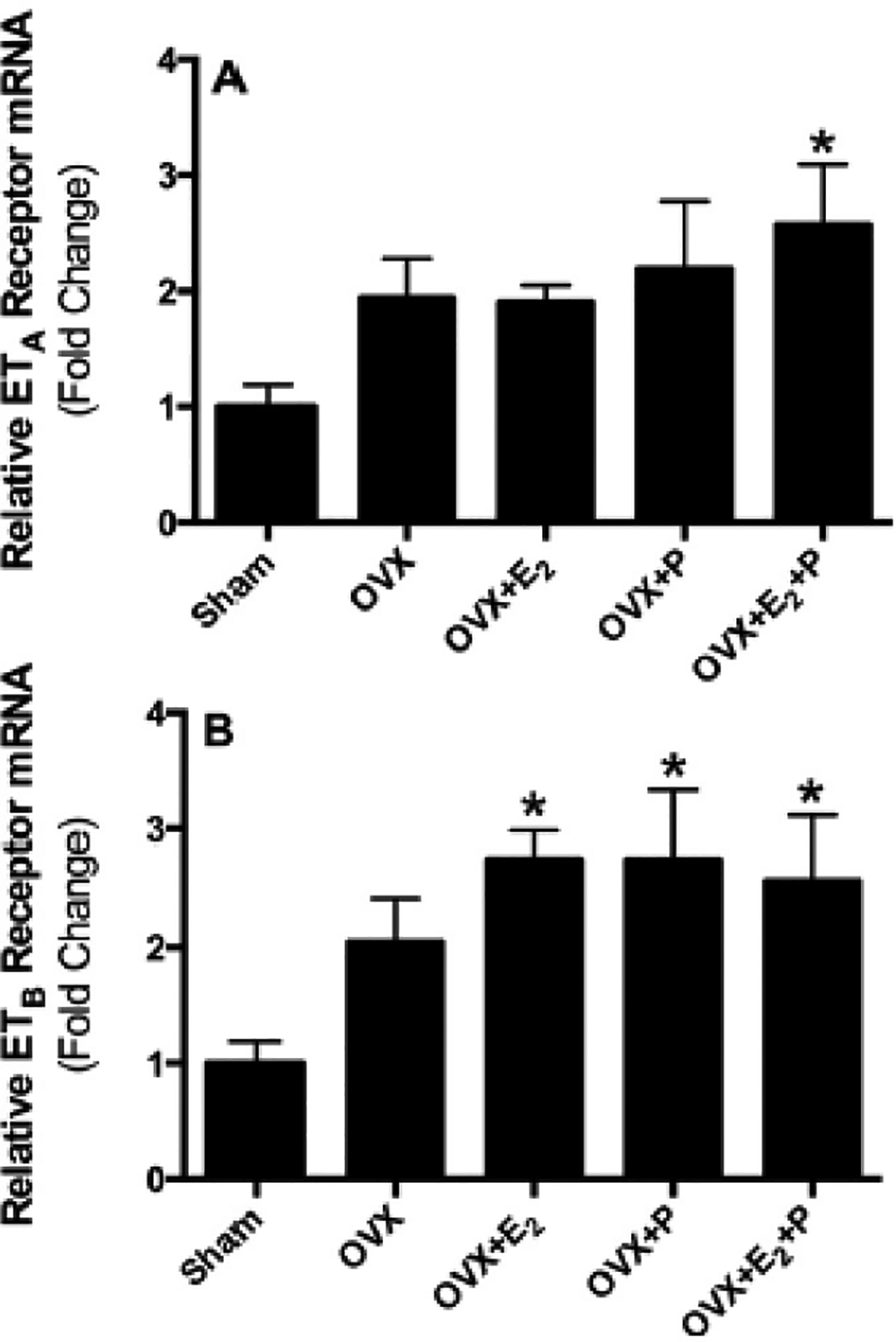
Relative mRNA expression of ETA (A) and ETB (B) receptors in liver tissues obtained from sham, ovariectomized (OVX) rats implanted with subcutaneous pellets containing estrogen (OVX + E2), progesterone (OVX + P), their combination (OVX + E2 + P) or placebo pellets (OVX). Values represent fold change from sham levels. Data are means ± SE of 5–7 samples in each group. *P < 0.05 vs. sham.
Ovariectomy significantly decreased the expression of ETA as well as ETB receptor mRNA within the renal cortex (Fig. 3A & B). This reduced expression of cortical ETA and ETB receptors caused by ovariectomy was not observed when OVX rats were supplemented with E2 alone. In contrast, supplementation of OVX rats with P alone or combined with E2 did not restore the OVX-induced changes in cortical ET-1 receptor expression (Fig. 3A & B). Within renal inner medullary tissue, the expression of ETA receptor mRNA was significantly enhanced in OVX compared to sham rats (Fig. 3C). Similar increases in the expression of inner medullary ETB mRNA were observed also in OVX rats, compared to sham rats (Fig. 3D). These elevations in the expression of both receptors were slightly decreased in OVX rats supplemented with E2 or P but differences were not statistically significant except that ETB receptor mRNA expression was significantly decreased in OVX + E2 rats (Fig. 3D). Interestingly, the elevated expression of cortical ETA and ETB receptor mRNA induced by ovariectomy was significantly reduced to sham levels by co-supplementation of OVX rats with E2 and P (Fig. 3C & D).
Similar serum E2 levels were observed in OVX and OVX + P rats(2.2 ± 0.2 and 2.3 ± 0.2 pg/ml) that were significantly lower than those of sham rats (13.7 ± 5.3 pg/ml). Circulating E2 levels in OVX + E2 and OVX + E2 + P rats were increased to levels significantly higher than sham levels (58.5 ± 6.2 and 42.5 ± 4.9 pg/ml, respectively). Assessment of serum levels of progesterone showed that ovariectomy significantly reduced the level of serum progesterone compared to sham rats (42.9 ± 3.9 vs. 92.2 ± 5.5 ng/ml, respectively), and this was restored to sham levels in OVX + E2 + P rats (82.5 ± 8.2 ng/ml). Surprisingly, serum progesterone concentrations in OVX + P rats(42.4 ± 8.4 ng/ml) were similar to those of OVX rats and lower than those in OVX + E2 + P rats (91.9 ± 8.1 ng/ml).
4. Discussion
The main finding of the present study is that ET-1 receptor expression is modulated by the ovarian hormonal status, particularly in the kidney, and this finding may contribute to the cardiovascular and renal protection demonstrated in premenopausal females that is lost after menopause. Our data further suggest a regulatory role for sex-hormonal replacement on ET-1 receptors profile.
As a potent endothelium-derived vasoactive peptide, ET-1 plays a pivotal role in regulating vascular tone and appears to play an important role in the development of pulmonary hypertension [4], liver cirrhosis and portal hypertension [29]. In addition, administration of selective ETA or mixed ETA/B antagonists reduces pulmonary [4] and portal pressure [32]. In the present study, E2 supplementation to OVX rats had an attenuating effect on pulmonary ETA receptor mRNA expression. This observation is in line with previous data suggesting that raloxifene, having estrogen agonistic actions in the cardiovascular system [1,24], effectively prevents the development pulmonary hypertension in OVX rats, at least in part, by suppressing ET-1 overproduction [27]. These two findings demonstrate an important interaction between E2 and the ET-1 system in the lung, suggesting a protective effect of E2 on ET-1-induced control of pulmonary vascular tone. On the other hand, the expression of pulmonary ETB receptors that function mainly as clearance receptors for circulating ET-1 was not significantly affected by ovariectomy or hormonal replacement to OVX rats. Additionally, our results show that neither hepatic ETA nor ETB receptor mRNA is significantly changed in response to ovarian hormone depletion. However, replacement of OVX rats with the dual hormonal replacement regimen showed significant increases in the hepatic ETA and ETB receptor mRNA expression levels compared to sham levels. Individual supplementation of E2 or P also caused significant increases only in hepatic ETB receptor mRNA levels, compared to sham rats. Thus, recent observations by Ho et al. [13] demonstrating that E2 attenuates intrahepatic vasoconstriction to ET-1 may be linked to E2-induced enhancement of ETB receptor expression in the liver. However, we used whole liver extracts and did not specifically examine intrahepatic vascular tissue.
Within renal tissues, our study revealed a high potential for ovarian hormones to regulate ETA and ETB receptor mRNA expression in both the cortex and inner medulla. Overall expression of both ETA and ETB receptor levels in the cortex typically represent vascular structures, while ETB receptor expression in renal medulla is heavily weighted towards tubular structures due to high levels of expression in the inner medullary-collecting duct (IMCD). Taking into consideration that the kidney is very sensitive to the vasoconstrictor properties of ET-1 [5] and plays a critical role in water and Na+ homeostasis, changes in renal ET-1 receptor expression may have considerable effects on sex differences in renovascular resistance as well as water and electrolyte balance. Our findings that OVX reduced cortical ETA and ETB expression that was not evident with E2 replacement suggests that E2 normally functions to maintain renal cortical expression of both receptor sub-types, most likely in vascular structures. Further studies will be needed to determine to what extent the effects of E2 on ETB receptor expression are endothelial versus epithelial.
It is well established that the inner medullary ETB receptors are major contributors to the maintenance of water and Na+ balance [20], however the role of inner medullary ETA receptors is not clear. It is generally believed that inner medullary ETA receptors oppose ETB effects and are anti-natriuretic by virtue of their vasoconstrictor properties [14,26]. In contrast, some studies suggest that the ETA receptor may con-tribute to the natriuretic response to ET-1 [9] and that blockade of ETA receptors leads to fluid retention through effects on tubular reabsorption [21]. Sex-differences in renal medullary ETA and ETB receptor function have been previously reported [14,17–19,26,36]. Interestingly, our lab has demonstrated increased ETA receptor binding and intracellular Ca2+ signaling in IMCD obtained from male, compared to female rats [14]. In line with the former study, our current data show that inner medullary ETA receptor expression was enhanced by ovariectomy and restored back to sham-levels by supplementation of E2 and P to OVX rats. Our data, together with the previously published sex-difference in ETA receptor expression in IMCD [14], highlight a central role for ovarian hormones in attenuating inner medullary ETA receptor expression in females, that may be linked to decreased incidence of salt-sensitive hypertension premenopausal females vs. males and postmenopausal females.
Our results also showed that inner medullary ETB receptor mRNA expression was increased in response to depletion of endogenous ovarian hormones and normalized by E2 and P treatment to OVX rats. Thus it is apparent that the E2 and P work co-operatively to modulate inner medullary ETA and ETB receptor expression, because individual supplementation of each hormone was less effective in restoring receptor expression. This is consistent with previous studies reporting synergism between of E2 and P on renovascular tone control [10]. Overall, this work provides evidence that the ET-1 system is highly influenced by the sex-hormonal status in females. These effects are not general to all tissues since we observed that renal cortex and lung tissues are restored by E2 replacement alone, whereas, inner medullary tissues are more sensitive to the combined effect of E2 and P.
Collectively, our data pinpoint that ovariectomy affected renal and hepatic ET receptor mRNA expression, however, changes in the hepatic ET receptors with OVX did not reach statistical significance levels. On the other hand, ovariectomy did not affect pulmonary ET receptor mRNA expression. This might be related to many factors including the use of whole lung extracts that did not specifically examine pulmonary vascular tissue. In addition, although our results did not show effects on receptor expression in response to ovariectomy, changes might be detected at the receptor protein level.
It is imperative to comment on the apparently higher levels of circulating E2 in estrogen-replaced OVX rats compared to sham rats. Representation of the different stages of the estrus cycle, and not only the proestrus stage, in the sham group is very likely to contribute to relatively low E2 levels in sham rats because the level of E2 in the diestrus rats is as low as in OVX rats. Additionally, other factors that might be responsible for the comparatively higher E2 levels in OVX + E2 and OVX + E2 + P rats include continuous supplementation of the hormone as sustained release pellets which differs from the natural rhythmic fluctuations in the level of ovarian hormones in sham rats. On the other hand, the unexpected levels of serum progesterone in OVX + P and OVX + E2 rats might be related to multiple causes. One conceivable candidate is cross-reactivity of the antibodies provided in the commercial assay with a number of related steroid compounds already existing in serum samples [22]. Interestingly, however, the combination of E2 with P resulted in restored P concentrations. Going forward, it will be important to determine the contribution of free progesterone versus progesterone associated with steroid binding proteins, which was not done in the current study, to get a clearer explanation for serum progesterone measurements.
Finally, our findings suggest that the ability of ovarian hormones to modulate the expression of both ETA and ETB receptors within the renal cortex and inner medulla are in the same pattern, thus maintaining the normal balance between the usually contrasting effects of ETA and ETB activation within the kidney. We postulate that the differences in cortical ETA and inner medullary ETB receptor expression observed in females with different hormonal status might be a compensatory mechanism to changes in cortical ETB receptor and inner medullary ETA receptor expression, respectively, rather that a direct effect of ovarian hormones on receptor expression. This idea will require further investigation. Taken together, these changes in renal ET-1 receptor expression could be related, at least in part, to the normal physiologic responses to keep the balance between the opposing effects of ETA (vasoconstrictor and anti-natriuretic) and ETB (vasodilator and natriuretic) receptor function.
5. Limitations of the study
In the current study, we measured the level mRNA expression and not the receptor protein levels. Although, the expression of mRNA has been often interpreted as the concentration of the corresponding protein assuming that the transcript abundances are the main determinant of the protein abundances, however, protein abundances reflect a dynamic balance among a series of linked processes including, but not limited to transcription. Processing of mRNA to translation, localization, modification, and programmed destruction of the receptor protein itself have important effects on the level of receptor protein [39]. So our results are limited to the effect of ovarian hormones on ETA and ETB mRNA expression and do not extend to interpret ETA and ETB receptor abundances, which could be assessed in future studies using other techniques as binding studies.
6. Conclusion
This preliminary study points to a potential modulatory role of ovarian hormones on ETA and ETB receptor expression that may have a considerable impact on cardiovascular and renal health in the female population. Future studies are needed to clarify the effects of the ovarian hormones on vascular versus tubular ET-1 receptors expression, signaling and function.
Acknowledgments
This study was supported by a grant from the National Heart, Lung, and Blood Institute (P01 HL095499 to DM Pollock) and a postdoctoral grant from the American Heart Association (15POST25090329 to EY Gohar).
Footnotes
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- [1].Black LJ, Sato M, Rowley ER, Magee DE, Bekele A, Williams DC, et al. , Raloxifene (LY139481 HCI) prevents bone loss and reduces serum cholesterol without causing uterine hypertrophy in ovariectomized rats, J. Clin. Invest 93 (1994) 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Boesen EI, Sasser JM, Saleh MA, Potter WA, Woods M, Warner TD, et al. , Inter-leukin-1beta, but not interleukin-6, enhances renal and systemic endothelin production in vivo, Am. J. Physiol. Ren. Physiol 295 (2008) F446–F453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chen DD, Dong YG, Yuan H, Chen AF, Endothelin 1 activation of endothelin a receptor/NADPH oxidase pathway and diminished antioxidants critically contribute to endothelial progenitor cell reduction and dysfunction in salt-sensitive hypertension, Hypertension 59 (2012) 1037–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chester AH, Yacoub MH, The role of endothelin-1 in pulmonary arterial hypertension, Glob. Cardiol. Sci. Pract 2014 (2014) 62–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Clozel M, Clozel JP, Effects of endothelin on regional blood flows in squirrel mon-keys, J. Pharmacol. Exp. Ther 250 (1989) 1125–1131. [PubMed] [Google Scholar]
- [6].Elmarakby AA, Loomis ED, Pollock JS, Pollock DM, NADPH oxidase inhibition attenuates oxidative stress but not hypertension produced by chronic ET-1, Hypertension 45 (2005) 283–287. [DOI] [PubMed] [Google Scholar]
- [7].Frommer KW, Muller-Ladner U, Expression and function of ETA and ETB receptors in SSc, Rheumatology 47 (Suppl. 5) (2008) v27–v28. [DOI] [PubMed] [Google Scholar]
- [8].Gabler NB, French B, Strom BL, Liu Z, Palevsky HI, Taichman DB, et al. , Race and sex differences in response to endothelin receptor antagonists for pulmonary arterial hypertension, Chest 141 (2012) 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ge Y, Bagnall A, Stricklett PK, Webb D, Kotelevtsev Y, Kohan DE, Combined knockout of collecting duct endothelin A and B receptors causes hypertension and sodium retention, Am. J. Physiol. Ren. Physiol 295 (2008) F1635–F1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gohar EY, El-gowilly SM, El-Gowelli HM, El-Demellawy MA, El-Mas MM, PI3K/Akt-independent NOS/HO activation accounts for the facilitatory effect of nicotine on acetylcholine renal vasodilations: modulation by ovarian hormones, PLoS One 9 (2014), e95079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gouva L, Tsatsoulis A, The role of estrogens in cardiovascular disease in the aftermath of clinical trials, Hormones 3 (2004) 171–183. [DOI] [PubMed] [Google Scholar]
- [12].Hirata Y, Emori T, Eguchi S, Kanno K, Imai T, Ohta K, et al. , Endothelin receptor subtype B mediates synthesis of nitric oxide by cultured bovine endothelial cells, J. Clin. Invest 91 (1993) 1367–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ho HL, Lee FY, Hsu SJ, Wang SS, Hsin IF, Huang HC, et al. , The ability of 17 beta-estradiol to attenuate intrahepatic vasoconstriction to endothelin-1 in female rats is lost in cirrhosis, Ann. Hepatol 14 (2015) 404–413. [PubMed] [Google Scholar]
- [14].Jin C, Speed JS, Hyndman KA, O’Connor PM, Pollock DM, Sex differences in ET-1 receptor expression and Ca2+ signaling in the IMCD, Am. J. Physiol. Ren. Physiol 305 (2013) F1099–F1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kim JM, Kim TH, Lee HH, Lee SH, Wang T, Postmenopausal hypertension and sodium sensitivity, J. Menopausal Med 20 (2014) 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kitada K, Yui N, Mori T, Ohkita M, Matsumura Y, Vasoprotective effects of an endothelin receptor antagonist in ovariectomized female rats, Life Sci. 118 (2014) 379–385. [DOI] [PubMed] [Google Scholar]
- [17].Kittikulsuth W, Looney SW, Pollock DM, Endothelin ETB receptors contribute to sex differences in blood pressure elevation in angiotensin II hypertensive rats on a high-salt diet, Clin. Exp. Pharmacol. Physiol 40 (2013) 362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kittikulsuth W, Pollock JS, Pollock DM, Sex differences in renal medullary endothelin receptor function in angiotensin II hypertensive rats, Hypertension 58 (2011) 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kittikulsuth W, Sullivan JC, Pollock DM, ET-1 actions in the kidney: evidence for sex differences, Br. J. Pharmacol 168 (2013) 318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kohan DE, Inscho EW, Wesson D, Pollock DM, Physiology of endothelin and the kidney, Compr. Physiol 1 (2011) 883–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kohan DE, Rossi NF, Inscho EW, Pollock DM, Regulation of blood pressure and salt homeostasis by endothelin, Physiol. Rev 91 (2011) 1–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Krasowski MD, Drees D, Morris CS, Maakestad J, Blau JL, Ekins S, Cross-reactivity of steroid hormone immunoassays: clinical significance and two-dimensional molecular similarity prediction, BMC Clin. Pathol 14 (2014) 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lamping KG, Nuno DW, Effects of 17 beta-estradiol on coronary microvascular responses to endothelin-1, Am. J. Physiol. Heart Circ. Physiol 271 (1996) H1117–H1124. [DOI] [PubMed] [Google Scholar]
- [24].Leung FP, Tsang SY, Wong CM, Yung LM, Chan YC, Leung HS, et al. , Raloxifene, tamoxifen and vascular tone, Clin. Exp. Pharmacol. Physiol 34 (2007) 809–813. [DOI] [PubMed] [Google Scholar]
- [25].Mendelsohn ME, Karas RH, The protective effects of estrogen on the cardiovascular system, N. Engl. J. Med 340 (1999) 1801–1811. [DOI] [PubMed] [Google Scholar]
- [26].Nakano D, Pollock DM, Contribution of endothelin A receptors in endothelin 1dependent natriuresis in female rats, Hypertension 53 (2009) 324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nishida M, Hasegawa Y, Tanida I, Nakagawa E, Inaji H, Ohkita M, et al. , Preventive effects of raloxifene, a selective estrogen receptor modulator, on monocrotaline induced pulmonary hypertension in intact and ovariectomized female rats, Eur. J. Pharmacol 614 (2009) 70–76. [DOI] [PubMed] [Google Scholar]
- [28].Okada K, Yanai M, Takeuchi K, Matsuyama K, Nitta K, Hayashi K, et al. , Sex differences in the prevalence, progression, and improvement of chronic kidney disease, Kidney Blood Press. Res 39 (2014) 279–288. [DOI] [PubMed] [Google Scholar]
- [29].Rockey DC, Vasoactive agents in intrahepatic portal hypertension and fibrogenesis: implications for therapy, Gastroenterology 118 (2000) 1261–1265. [DOI] [PubMed] [Google Scholar]
- [30].Saleh MA, Boesen EI, Pollock JS, Savin VJ, Pollock DM, Endothelin-1 increases glomerular permeability and inflammation independent of blood pressure in the rat, Hypertension 56 (2010) 942–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sandberg K, Ji H, Sex differences in primary hypertension, Biol. Sex Differ 3 (2012)7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sogni P, Moreau R, Gomola A, Gadano A, Cailmail S, Calmus Y, et al. , Beneficial hemodynamic effects of bosentan, a mixed ETA and ETB receptor antagonist, in portal hypertensive rats, Hepatology 28 (1998) 655–659. [DOI] [PubMed] [Google Scholar]
- [33].Stauffer BL, Westby CM, Greiner JJ, Van Guilder GP, Desouza CA, Sex differences in endothelin-1-mediated vasoconstrictor tone in middle-aged and older adults, Am. J. Physiol. Regul. Integr. Comp. Physiol 298 (2010) R261–R265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Suzuki H, Kondo K, Chronic kidney disease in postmenopausal women, Hypertens. Res 35 (2012) 142–147. [DOI] [PubMed] [Google Scholar]
- [35].Takaoka M, Yuba M, Fujii T, Ohkita M, Matsumura Y, Oestrogen protects against ischaemic acute renal failure in rats by suppressing renal endothelin-1 overproduction, Clin. Sci. (Lond.) 103 (Suppl. 48) (2002) 434s–437s. [DOI] [PubMed] [Google Scholar]
- [36].Taylor TA, Gariepy CE, Pollock DM, Pollock JS, Gender differences in ET and NOS systems in ETB receptor-deficient rats: effect of a high salt diet, Hypertension 41 (2003) 657–662. [DOI] [PubMed] [Google Scholar]
- [37].Teede HJ, Vincent A, Hormone therapy — where are we now? Aust. Fam. Physician 40 (2011) 280–285. [PubMed] [Google Scholar]
- [38].Tostes RC, Fortes ZB, Callera GE, Montezano AC, Touyz RM, Webb RC, et al. , Endothelin, sex and hypertension, Clin. Sci. (Lond.) 114 (2008) 85–97. [DOI] [PubMed] [Google Scholar]
- [39].Vogel C, Marcotte EM, Insights into the regulation of protein abundance from proteomic and transcriptomic analyses, Nat. Rev. Genet 13 (2012) 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhang Y, Knutsen GR, Brown MD, Ruest LB, Control of endothelin-a receptor expression by progesterone is enhanced by synergy with Gata2, Mol. Endocrinol 27 (2013) 892–908. [DOI] [PMC free article] [PubMed] [Google Scholar]


