Abstract
Pancreatic ductal adenocarcinoma (PDAC) is a fatal disease with a low survival rate (9%). Epidemiologic studies show that healthy dietary patterns enriched of fruits and vegetables lower the risk of PDAC. We previously showed that supplementing black raspberries (BRBs) to patients with colorectal cancer increased tumor-infiltrating NK cells and their cytotoxicity. We aimed to determine whether BRBs combat PDAC by modulating cancer immunity. NOD.SCID mice lacking T and B cells were injected with human Panc-1-Luc cells orthotopically, and immunocompetent KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice were fed BRBs. Peripheral blood mononuclear cells (PBMCs) from PDAC patients were treated with butyrate, a microbial metabolite of BRBs. The absence of T and B cells did not dampen BRBs’ anti-tumor effects in the NOD.SCID mice. In the KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice, BRBs significantly prolonged survival (189 days versus 154 days). In both models, BRBs decreased tumor-infiltrating CD11b+ cells and the expression of IL-1β, sEH, and Ki67. BRBs also increased tumor-infiltrating NKp46+ cells and the expression of CD107a, a functional marker of cytolytic NK and CD8+ T cells. In KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice, tumor infiltration of CD8+ T cells was increased by BRBs. Further using the PBMCs from PDAC patients, we show that butyrate decreased the population of myeloid-derived suppressor cells (MDSCs). Butyrate also reversed CD11b+ cell-mediated suppression on CD8+ T cells. Interestingly, there is a negative association between MDSC changes and patients’ survival, suggesting that the more decrease in MDSC population induced by butyrate treatment, the longer the patient had survived. Our study suggests the immune-modulating potentials of BRBs in PDAC.
Keywords: Pancreatic ductal adenocarcinoma, black raspberries, butyrate, orthotopic xenograft, KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre, cancer immunity, PDAC patients
Introduction
Pancreatic cancer is the 12th most common cancer in men and the 11th in women worldwide (1). In the United States, 56,770 new cases and 45,750 deaths were estimated for 2019 (2). Most (93%) cases are pancreatic ductal adenocarcinoma (PDAC), which originates in exocrine cells. For all stages combined, the estimated 5-year survival rate of diagnosed PDAC patients is low (9%), making PDAC the 4th leading cause of cancer death in the U.S. (2). Many risk factors for PDAC have been reported, including smoking, type 2 diabetes, obesity, inadequate physical exercise, and chronic pancreatitis. Constitutive activation of the Kras gene is an initiating event and the strongest contributor to PDAC development in humans: more than 90% of human PDAC cases has activated Kras. In addition, inactivation of the Trp53 tumor suppressor gene at a later stage of PDAC development has been observed in 50%–75% of human cases (3,4). Currently, there is no reliable method for early detection, and PDAC has usually metastasized beyond the pancreas before symptoms become noticeable (5). Surgical intervention is not suitable for patients with metastatic PDAC. Thus, there is an unmet need for new approaches to preventing and treating the disease.
Desmoplastic stroma infiltrated with immune cells is characteristic of PDAC and its precursor lesion, pancreatic intraepithelial neoplasia (PanIN). Myeloid cells are a predominant population in desmoplastic stroma, and most of these immune cells belong to immune-suppressive subsets, such as tumor-associated macrophages, or multiple subsets of immature myeloid cells/myeloid-derived suppressor cells (MDSCs). In contrast, CD8+ T and natural killer (NK) cells are rare. In a genetically engineered mouse model of PDAC, depleting CD11b+ cells, which contained mainly myeloid cells, prevented initiation of KrasG12D-driven PDAC (6). In pre-established tumors, myeloid cell (CD11b+) depletion arrested tumor growth and induced tumor regression through increased infiltration and activation of CD8+ T cells (7).
Epidemiologic studies show that healthy dietary patterns enriched of fruits, vegetables, vitamins and fiber lower the risk of PDAC (8). Natural dietary compounds with low toxicity have gained attention as chemopreventive agents against PDAC (9,10). These compounds decrease Kras activation and its downstream extracellular signal-regulated kinases (ERK or p42/44), as well as induce apoptosis (11). Evidence that natural compounds can modulate pancreatic cancer immunity is also beginning to emerge. For example, curcumin, curcuminoids, and ω−3 fatty acids potentiate the cytotoxicity of NK cells against cultured PDAC cells (12). Black raspberries (BRBs), which contain high levels of multiple chemopreventive components such as anthocyanins, ellagitannins, and dietary fiber (13), have been shown to inhibit cell transformation, cell proliferation, tumor-specific gene expression, inflammation, and angiogenesis, and to promote apoptosis and differentiation (9,14). We previously reported that BRBs were beneficial to patients with colorectal cancer (15,16) and patients with familial adenomatous polyposis (17). In addition, 5% BRBs in the diet inhibited the tumorigenesis of colorectal cancer (18–22) and esophageal cancer in rodent models (13,22–24). We recently showed that BRBs protectively modulated immune cells in esophageal cancer in rats (23) and BRBs enhanced cytotoxicity of NK cells in colorectal cancer in mice and humans (20). Furthermore, studies have shown that feeding berries and their components (phenolic extracts and fiber extracts) increased production of short-chain fatty acids, such as butyrate, in rat cecum (25,26). Human volunteers fed dietary fiber had higher levels of fecal butyrate (27). Butyrate has been shown to modulate T regulatory cells (28,29) and neutrophils (30). Our current study aimed to investigate whether BRBs and butyrate could modulate immune cells to combat PDAC.
Methods
Animals and cell lines
All protocols were carried out in accordance with the institutional guidelines for animal care dictated by the Medical College of Wisconsin Animal Care and Use Committee (AUA3067). Eight-week-old breeding pairs, including KrasLSL.G12D/+, Trp53LSL.R172H/+, and Pdx-1-Cre mouse strains, were obtained from the National Cancer Institute Mouse Repository. To produce transgenic KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice, we first generated double transgenic KrasLSL.G12D/+-Trp53LSL.R172H/+ mice and then mated them with heterozygous Pdx-1-Cre transgenic mice. Eight-week-old breeding pairs of NOD.SCID (NOD.CB17-Prkdcscid/J) mouse strain were purchased from the Jackson Laboratory (Bar Harbor, ME).
Panc-1 human pancreatic cancer cells (CRL-1469) were purchased from the American Type Culture Collection (ATCC, Manassas, VA) in November 2013, and then cultured as recommended and cryopreserved in liquid nitrogen. Panc-1 cells were sent to the Vector Core Laboratory at the University of Michigan, which generated Panc-1-Luc cell line by transfecting Panc-1 cells with the virus Lenti-Luc-VSVG. Clones positive for luciferase activity were generated for in vivo studies. The cells were not re-authenticated, as they were passaged for fewer than 6 months after resuscitation.
Diets
The control diet was the synthetic diet from the American Institute of Nutrition (AIN-76A; Dyets Inc., Bethlehem, PA). BRB powder was purchased from Berri Products LLC and stored at 4°C (16,18–21,31). Some potential chemopreventive agents in BRBs were measured and shown in Supplementary Table 1. The activities of these compounds have been summarized in our reviews (13,14). The content of sugar and starch in the BRB diet was adjusted to create an isocaloric diet. The composition of both the AIN-76A and BRB diets is shown in Supplementary Tables 2 and 3. 5% BRBs was used because our early study showed that 10% BRBs was not more effective than 5% BRBs in suppressing esophageal tumor in rats (32).
Animal experiments
Four-week old KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice were fed either the control AIN-76A diet (n=23) or the control diet supplemented with 5% BRBs (n=27). Pancreatic tumor tissues from these mice were collected, fixed in formalin, and embedded in paraffin (FFPE). The tissue sections were stained with hematoxylin and eosin (H&E) and examined histopathologically by our gastrointestinal pathologists.
We injected 0.5 × 106 Panc-1-Luc cells orthotopically into the pancreatic tail of 5-week-old NOD.SCID mice. Four weeks after the injection, we gave the mice either a control diet (n=8) or the control diet supplemented with 5% BRBs (n=19). After 6 weeks of BRB intervention, the mice were euthanized by CO2 asphyxiation, and pancreatic tumor tissues were collected. The gross volume of the orthotopic tumors was calculated using the following formula:
Bioluminescent imaging
For in vivo imaging, the substrate for luciferase, d-luciferin (150 mg/kg in PBS), was injected intraperitoneally into the mice that had been inoculated with Panc-1-Luc cells. The animals were anesthetized with isoflurane (2%–4%). Fifteen minutes after the substrate was administered, the anesthetized mice were placed onto a warm stage inside a light-tight camera box, where they continued to receive isoflurane (1%–2%). The acquisition time for photon emission measurements was normalized to 120 seconds. An IVIS camera system was used to visualize the tumors, and photon measurements taken around the circumference of each tumor were quantified as total number of photons per second, using Living Image software (Xenogen, Corp, Alameda, CA). All mice were imaged twice: before they received the experimental diet (baseline) and 6 weeks after the dietary intervention (end of study).
Immunohistochemistry
Paraffin-embedded pancreatic tumor blocks were cut into 4 μm sections and placed on glass slides. The slides were placed in an oven at 60°C for 1 hr, cooled, deparaffinized, and rehydrated through xylenes and graded ethanol solutions to water. To block endogenous peroxidase, we treated all slides for 5 min with a 3% H2O2 solution in water. Using a Dako Autostainer, we stained the slides for 1 hr at room temperature with primary antibodies against NKp46 (1:1000, #bs-10027R), CD8 (1:500, #bs-0648R), IL-12 (1:200, #bs-0767R) from Bioss (Woburn, Massachusetts), antibodies against CD11b (1:40000, #ab133357), IL-1β (1:400, #ab2105), CD107a (1:500, #ab25245) from Abcam (Cambridge, MA), antibodies against sEH (1:2500, #sc-25797) from Santa Cruz Biotechnology (Dallas, TX), antibodies against Ki67 (1:500, #M7249) from Dako (Carpinteria, CA), or antibodies against cleaved caspase-3 (1:200, #9661) from Cell Signaling Technology (Danvers, MA). The stained slides were photographed at 20 × magnification, and the staining area was quantified, as previously described (18–20,30).
Immunoblotting analysis
Protein lysates of pancreatic tumors were used for immunoblotting. Antibodies for phospho-c-Raf (p-c-Raf, #9421P), phospho-mitogen activated protein kinase kinase (MEK1/2) (p-MEK, #9154), phospho-p42/44 (p-ERK, #4370), phospho-signal transducer and activator of transcription 3 (STAT3) (p-STAT3, #9145), cyclin D3 (#2936), cyclin-dependent kinase 4 (CDK4) (#2906), caspase-3 (#9662), cleaved caspase-3 (#9661), phospho-B-cell lymphoma 2 (Bcl-2)-associated death promoter (BAD) (p-BAD, #5284P), and Bcl-2 (#2870P) were purchased from Cell Signaling Technology (Danvers, MA) and used to identify their respective proteins. The antibodies against β-actin (#691001) were purchased from MP Biomedical (Santa Ana, CA). Immunoblots were quantified by densitometry, using ImageJ software, and levels of the proteins of interest were normalized to the levels of β-actin.
PDAC patients’ samples and butyrate treatment
Peripheral blood mononuclear cells (PBMCs) samples of PDAC patients were requested and approved by Institutional Review Board and were obtained from Tissue Bank at the Medical College of Wisconsin. PBMCs were cultured in RPMI1640 containing 20% FBS and were treated with 5 mM butyrate for 16 hrs. The cells were collected for flow cytometric analysis to detect the levels of NK cells, CD8+ cells, and MDSCs. The gating strategies for different immune cells were used as followings: NK cells: CD45+CD3-CD56+; CD8+ T cells: CD45+CD3+CD8+; MDSCs: CD11b+CD33+HLA-DR-. Percentage change of MDSCs induced by butyrate treatment is defined as follows:
T cell suppression assay
Subgroups of PDAC patients’ PBMCs were used to isolate CD11b+ cells according to manufacturer’s instructions (CD11b MicroBeads, human and mouse, #130–049-601, Miltenyi Biotec, San Diego,CA). CD11b+ cells were treated with either PBS or 5 mM butyrate for 16 hrs. Whole blood of healthy donors were obtained from Froedtert Hospital and were used to isolate CD8+ cells according to manufacturer’s instructions (CD8 MicroBeads, human, #130–097-057, Miltenyi Biotec, San Diego,CA). After pre-treatment with butyrate, CD11b+ cells were co-cultured with CD8+ cells at a ratio of 1:1 for 72 hrs in the presence of human CD3/CD28 beads (Dynabeads™ Human T-Activator CD3/CD28) according to previous publication (33). The cells were collected for flow cytometric analysis to detect the levels of interferon-gamma (IFNγ) and CD107a in CD8+ cells. Percentage change is defined as follows:
Flow cytometry
Splenocytes were stained with surface marker antibodies (BD Biosciences, Franklin Lakes, NJ), and were then fixed and permeabilized by Cytofix/Cytoperm for intracellular cytokine staining. The samples were analyzed on a LSRII flow cytometer (BD Biosciences, Franklin Lakes, NJ), and FlowJo (Tree Star, Ashland, OR) was used to analyze the results. The data were presented as the percentage of positive cells. Examples of gating strategy were shown in Supplementary Figure 1.
Statistical analysis
Using Kaplan-Meier analysis, we determined differences in median survival rates between KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice fed with the control diet and those fed with the 5% BRB diet. Using SigmaPlot (Systat Software, San Jose, CA), we performed an unpaired, two-tailed t-test to analyze protein expression levels, cell proliferation, mean photon emissions, orthotopic pancreatic tumor size, and percentages of immune cells in animal studies, and applied a paired, two-tailed t-test to analyze levels of immune cells in PDAC patients’ samples. Pearson correlation and linear regression tests were performed using GraphPad Prism to determine the association between percentage change of MDSC in the PDAC patients and their overall survival (OS). A p value less than 0.05 was considered statistically significant.
Results
BRBs decrease the size of orthotopic pancreatic tumors in immune-deficient NOD.SCID mice
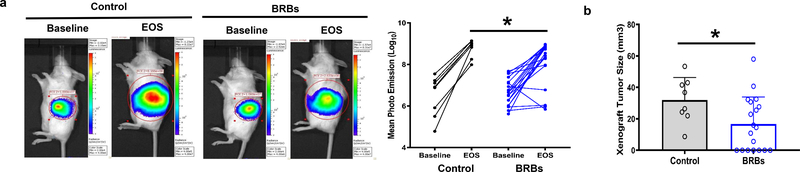
We first used immune-deficient NOD.SCID mice that lack functional B and T cells to investigate if BRBs are protective in these mice. To induce pancreatic tumors, we injected 0.5 × 106 Panc-1-Luc cells orthotopically into the pancreatic tail of 5-week-old NOD.SCID mice. Four weeks after the injection, we performed bioluminescent imaging to confirm that pancreatic tumors had developed (Figure 1a, baseline). The mice were then fed either the control diet or the BRB diet for 6 weeks. At the end of study (EOS), we performed bioluminescent imaging again. Mean photon emissions were significantly decreased by BRBs (Figure 1a). Afterwards, the mice were sacrificed, their orthotopic pancreatic tumors were collected. Gross tumor volumes of the orthotopic pancreatic tumors were significantly smaller in the NOD.SCID mice fed the BRB diet than in those fed the control diet (Figure 1b). Our results suggest that BRBs are protective in mice that are lack of functional B and T cells, suggesting that at least some of other immune cells, such as NK cells or MDSCs, might mediate the anti-tumor effects of BRB.
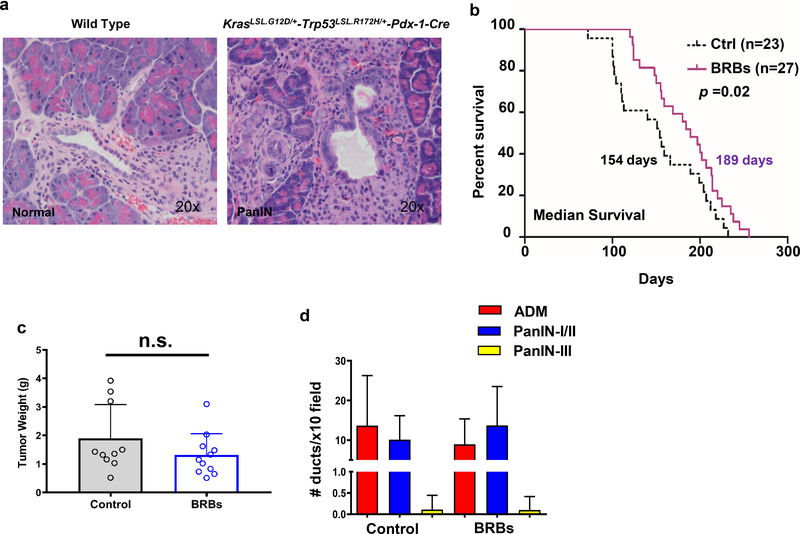
Figure 1: BRBs inhibited tumor growth and modulated tumor-infiltrating immune cells in an orthotopic immune-deficient pancreatic cancer model.
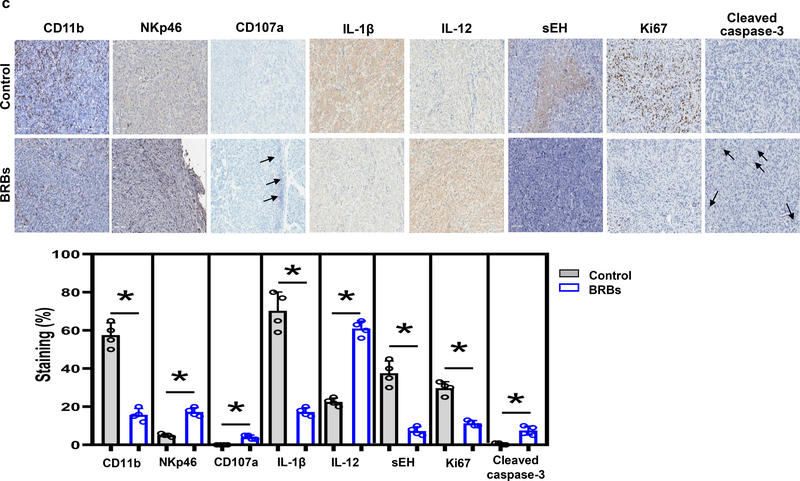
(a) The immune-deficient pancreatic cancer model was established by injecting Panc-1-Luc cells into the pancreas of NOD.SCID mice. Representative images and quantification of photon emission from in vivo bioluminescent imaging are shown. EOS: End of study. (b) BRBs decreased the gross volume of the orthotopic tumors. Control: n=8; BRBs: n=19. (c) Immunohistochemistry and quantification of CD11b, NKp46, CD107a, IL-1β, IL-12, sEH, Ki67, and cleaved caspase-3. Control: n=4; BRBs: n=4. Scale bar: 100μm. * p <0.05.
BRBs alter CD11b+ and NKp46+ cells and cytokine production in orthotopic pancreatic tumors of NOD.SCID mice
We then investigated if BRBs altered immune cells in the NOD.SCID mice. In the pancreatic orthotopic tumors, levels of tumor-infiltrating CD11b+ cells were significantly decreased by BRBs, while tumor-infiltrating NKp46+ cells were significantly increased, as were levels of CD107a, a functional marker of cytolytic cells. Expression of the pro-inflammatory cytokine IL-1β, inflammatory marker soluble epoxide hydrolase (sEH), and proliferative marker Ki67 was decreased, and levels of IL-12 and cleaved caspase-3 were increased (Figure 1c). Using flow cytometric analysis, we also analyzed the population and function of NKp46+ cells in the spleen of these NOD.SCID mice. We observed comparable numbers and function (CD107a) of NKp46+ cells, as well as the production of cytokine IFNγ of NKp46+ cells between mice fed the control and the BRB diet (Supplementary Figure 2). These results suggest that BRBs may modulate tumor-infiltrating immune cells, which contribute to suppress tumor development of pancreatic orthotopic tumors in the NOD.SCID mice. Of note, levels of MDSCs in the spleen of NOD.SCID mice were very low with no differences between the control and BRB groups (data not shown).
BRBs correct the dysregulated Raf/MEK/ERK pathway in orthotopic pancreatic tumors of NOD.SCID mice
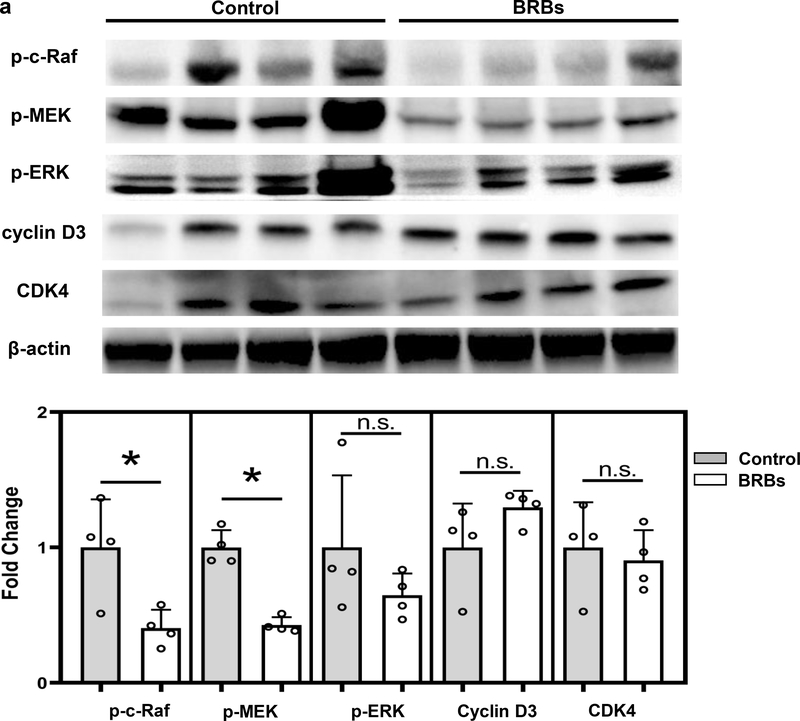
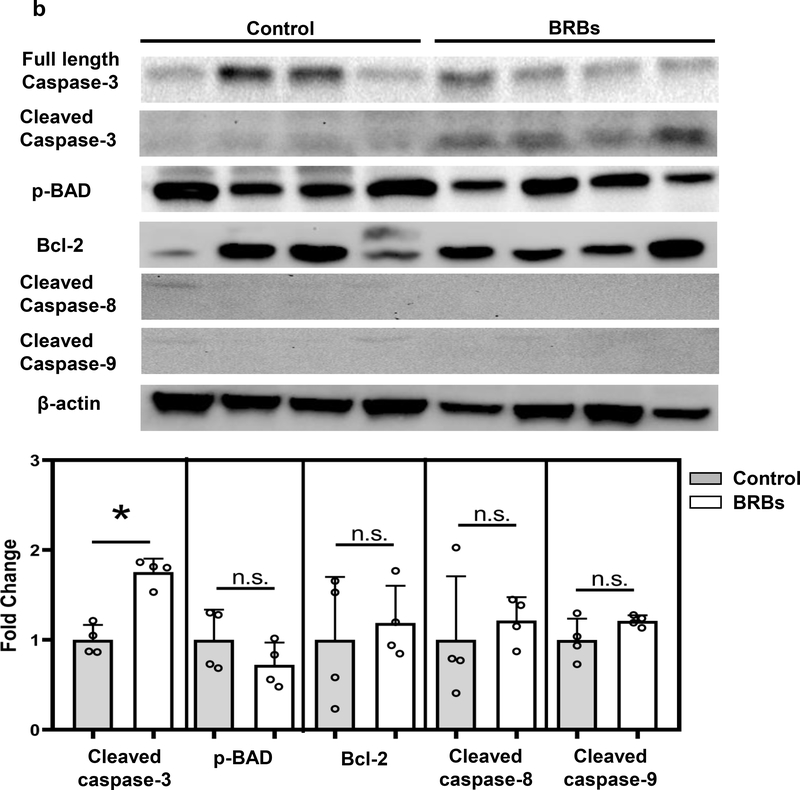
Because Panc-1 cells, a human pancreatic cancer cell line, carry a mutation that constitutively activates Kras, we determined whether BRBs could suppress pathways downstream of Kras in Panc-1-Luc tumors. BRBs decreased the activation of c-Raf and MAPKK (or MEK), but not ERK activation, and they had no significant effect on cyclin D3 and CDK4 expression (Figure 2a). BRBs increased the protein expression of cleaved capase-3 in the orthotopic tumors (Figure 2b). However, BRB-induced apoptosis may not involve the intrinsic mitochondrial pathways (caspase-8, caspase-9, Bcl-2, or BAD) (Figure 2b). These data suggest that in addition to the immune-modulating effects, BRBs may also directly act on tumor cells to induce cell apoptosis.
Figure 2: BRBs suppressed Raf/MEK activation and promoted apoptosis in Panc-1-Luc orthotopic tumors of NOD.SCID mice.
(a) Immunoblots of p-c-Raf, p-MEK, p-ERK, cyclin D3, and CDK4. Quantified results showed that protein expression of p-c-Raf and p-MEK was significantly lower in pancreatic tumors from BRB-treated mice than in those from mice on a control diet. (b) Immunoblots of full-length caspase-3, cleaved caspase-3, p-BAD, Bcl-2, cleaved caspase-8, and cleaved caspase-9. Quantified results showed that protein expression of cleaved caspase-3 was significantly higher in pancreatic tumors from BRB-fed mice than in those from control mice. Control: n=4; BRBs: n=4. * p <0.05; n.s.: not significant.
BRBs prolong median survival of immune-competent KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice that spontaneously develop PDAC
To stage PDAC before BRB treatment began, we euthanized an independent cohort (n=5) of 4-week-old KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice and collected their pancreatic tissues for H&E staining. In contrast to the normal pancreatic ductal structure seen in the age-matched wild-type mice (n=3) (Figure 3a, left panel), histopathological diagnosis indicated that 100% of these mutant mice developed PanIN (Figure 3a, right panel), a precursor to PDAC (4). The pancreatic ducts of the KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice showed mucinous epithelial lesions with cytological abnormalities such as nuclear crowding, enlarged nuclei, pseudo-stratification, and hyperchromatin.
Figure 3: BRBs prolonged median survival of KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice and modulated tumor-infiltrating immune cells.
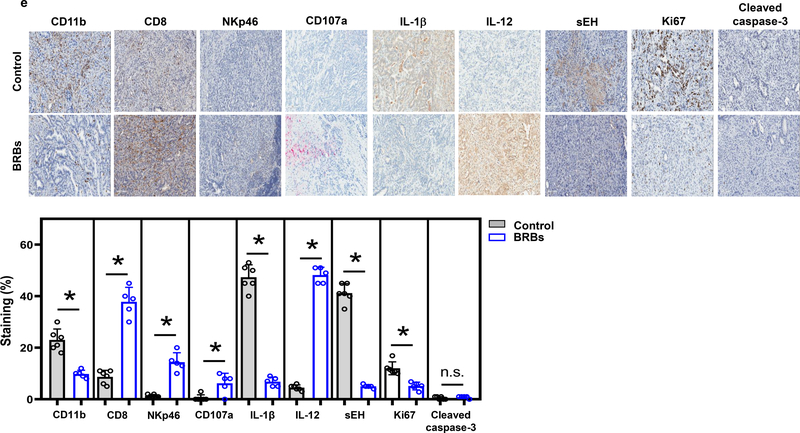
(a) Representative images of normal pancreas from a 4-week-old wild-type mouse (20x) and a PanIN lesion from a 4-week-old KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mouse (20x). (b) Kaplan-Meier survival analysis showing significantly (p =0.02) prolonged median survival in the BRB-treated mice (n=27) compared with the control mice (n=23). (c) Tumor weight of the KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice fed either the control diet or 5% BRBs. (d) Number of precancerous lesions per histopathologic section of the pancreas. (e) Immunohistochemistry and quantification of CD11b, CD8, NKp46, CD107a, IL-1β, IL-12, sEH, Ki67, and cleaved caspase-3. Control: n=6; BRBs: n=5. Scale bar: 100μm. * p <0.05; n.s.: not significant.
Previous studies have shown that combined point mutations in the Kras and Trp53 genes accelerated the development of PDAC and shortened median survival to approximately 5 months (34). Similarly in our study, the median survival for the KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice that received the control diet was only 154 days (Figure 3b). In contrast, BRBs prolonged their median survival to 189 days (Figure 3b), though they did not significantly change the weight of the pancreatic tumors (Figure 3c). Pathological analysis showed that BRBs did not change the numbers of precancerous lesions, including acinar-ductal metaplasia (ADM), PanIN-I/II, and PanIN-III (Figure 3d).
BRBs alter CD11b+, CD8+, and NKp46+ cells and cytokine production in pancreatic tumors of immune-competent KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice
We then asked whether BRBs change levels of immune cells in KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice. By staining the pancreatic tumors with antibodies against CD11b, CD8, NKp46, and CD4, we found that BRBs decreased levels of CD11b+ cells and increased levels of CD8+ T and NKp46+ cells in the pancreatic tumors. BRBs also significantly increased expression of CD107a in the pancreatic tumors (Figure 3e). No changes were observed in CD4+ T cells between the mice that received the control diet and the BRB-treated mice (data not shown). Levels of the pro-inflammatory cytokine IL-1β, inflammatory marker sEH, and proliferative maker Ki67 in the pancreatic tumors were decreased by BRBs. Further, expression of IL-12 was increased by BRBs, while there was no change in cleaved caspase-3 in the pancreatic tumors. We also investigated immune cells, including CD8+ T cells, NKp46+ cells, monocytic (M)-MDSCs, and polymorphonuclear (PMN)-MDSCs, in the spleen of these mice. The number of CD8+ T cells did not change, nor did their function (CD107a) or IFNγ production (Supplementary Figure 3a) in either the control or BRB group. Although the number and CD107a expression of NKp46+ cells in the spleen were comparable between the control and BRB group, levels of IFNγ in NKp46+ cells were significantly increased in the BRB-treated KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice (Supplementary Figure 3b), which may suggest enhanced NKp46+ cell cytolytic activities. In addition, the BRB-treated mice had a significantly decreased population of M-MDSCs (Supplementary Figure 3c). However, BRBs had no effect on levels of IL4 receptor (IL4R) or inducible nitric oxide synthase (iNOS) (Supplementary Figure 3c), both of which were previously shown to contribute to M-MDSCs’ immune-suppressing activities (35). No significant change in PMN-MDSCs was detected between the control and BRB groups (Supplementary Figure 3c). Collectively, our results suggest that BRBs may enhance tumor-infiltrating NKp46+ cells and CD8+ T cells and their functions while decrease tumor-infiltrating CD11b+ cells, thereby prolonging the survival of the KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice.
BRBs suppress Raf/MEK/ERK activation and inhibit cell cycle regulators in pancreatic tumors of KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice
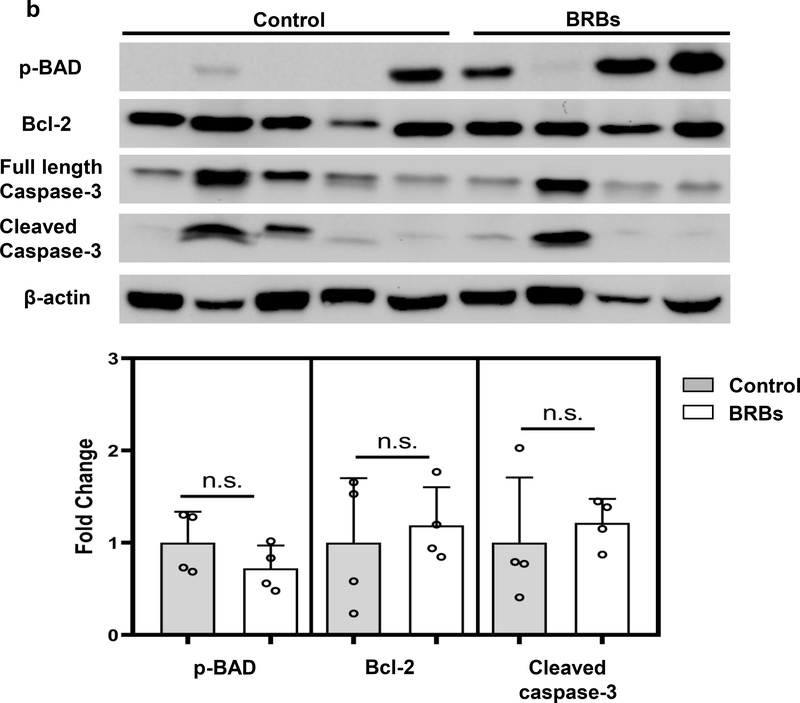
Because constitutive activation of Kras is the strongest contributor to the development of PDAC, we investigated potential effects of BRBs on pathways downstream of Kras. Immunoblotting analysis showed that BRBs suppressed the activation of c-Raf, MEK, ERK, and STAT3 (Figure 4a), which have been reported to be activated when Kras is activated and might be potential therapeutic targets of treating pancreatic cancer (36). In addition, BRBs significantly decreased the protein expression of cyclin D3 and CDK4 (Figure 4a), which are elevated in tumors from PDAC patients (37–39). Our observation of decreased expression of cyclin D3 and CDK4 was consistent with the decreased proliferative Ki67 nuclear staining in the pancreatic tumors from the BRB-fed mice (Figure 3e). However, there were no significant changes in apoptotic proteins, such as Bcl-2, BAD, or caspase-3 pathways (Figure 4b), which is consistent with the lack of immunohistochemical staining of cleaved caspase-3 in these mice (Figure 3e).
Figure 4: BRBs significantly inhibited Raf/MEK/ERK activation and cell cycle regulators in pancreatic tumors from KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice.
(a) Immunoblots of p-c-Raf, p-MEK, p-ERK, p-STAT3, cyclin D3, and CDK4. Quantified results showed a significant decrease in the protein expression of p-c-Raf, p-MEK, p-ERK, p-STAT3, cyclin D3, and CDK4 in the pancreatic tumors from the BRB-treated mice (n=5) in comparison with the control mice (n=5). (b) Immunoblots of p-BAD, Bcl-2, full-length caspase-3, and cleaved caspase-3. Quantified results showed no significant changes in the protein expression of p-BAD, Bcl-2, or cleaved caspase-3. Control: n=5; BRBs: n=4. * p <0.05; n.s.: not significant.
Butyrate, one of the BRB metabolites, reversed the CD11b+ cell-mediated T cell suppression on the PBMCs from PDAC patients
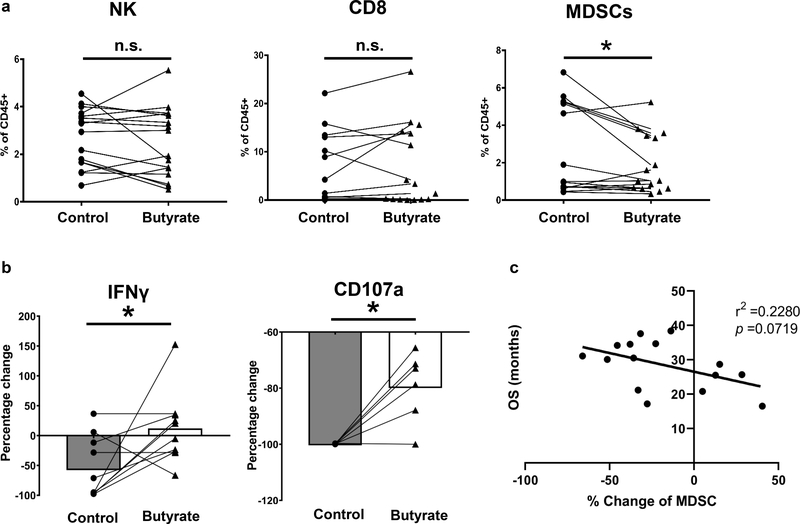
BRBs contain abundant dietary fibers, which can be fermented by gut bacteria into short-chain fatty acids, such as butyrate. We first determined the effects of butyrate on immune cells in the PBMCs from PDAC patients. Sixteen PBMC samples were used and butyrate treatment significantly decreased the levels of MDSCs, but not NK cells or CD8+ T cells (Figure 5a). Then we isolated CD11b+ cells from these PBMCs, pre-treated CD11b+ cells with butyrate, and co-cultured them at a ratio of 1:1 with CD3/CD28-activated CD8+ T cells from healthy donors. PDAC patients-derived CD11b+ cells induced a substantial decrease in CD107a expression and IFNγ release. Intriguingly, butyrate significantly restored these functions of CD8+ T cells (Figure 5b). These results suggest that butyrate dampened the immune-suppressive function of CD11b+ cells thereby enhancing function of CD8+ T cells. In addition, we analyzed the potential relationship between the percentage change of MDSC in these PDAC patients and their OS. Interestingly, we observed a negative association (p =0.0719) between MDSC changes and patients’ survival (Figure 5c), suggesting that the more decrease in MDSC population induced by butyrate treatment, the longer the patient had survived.
Figure 5: A BRB metabolite, butyrate, reversed CD11b+ cell-mediated T cell suppression in the PBMCs from PDAC patients.
(a) Percentage of NK cells (CD45+CD3-CD56+), CD8+ T cells (CD45+CD3+CD8+), and MDSCs (CD11b+CD33+HLA-DR-) in PBMCs of PDAC patients (n=16) in the absence or presence of butyrate. (b) CD11b+ cell-mediated T cell suppression assay. CD11b+ cells were isolated from PBMCs of PDAC patients, pre-treated with or without 5 mM butyrate for 16 hrs, and co-cultured at 1:1 ratio with CD3/CD28-activated CD8+ T cells from healthy donors. Butyrate increased the IFNγ release and CD107a production by CD8+ T cells. (c) Negative association between MDSC changes in the PDAC patients and their OS. * p <0.05; n.s.: not significant.
Discussion
Our current study investigated BRBs’ immune-modulating activities in the context of PDAC. In both immune-deficient NOD.SCID mice and immune-competent KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice that spontaneously develop PDAC, BRBs were protective against PDAC. BRBs decreased levels of tumor-infiltrating CD11b+ cells and increased infiltration of NKp46+ cells into the pancreatic tumors, where we also observed decreased expression of IL-1β and increased expression of IL-12. Moreover, BRBs corrected the dysregulated Raf/MEK/ERK pathway downstream of Kras in the pancreatic tumors from both models. In KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice, BRBs also increased tumor-infiltrating CD8+ T cells. Importantly, butyrate, a BRB metabolite from gut bacterial fermentation, decreased the levels of MDSCs in the PBMCs from PDAC patients, and butyrate reversed CD11b+ cell-mediated suppression of CD8+ T cell.
Major phytochemical compounds found in BRBs are polyphenols and their absorptions in the intestine after oral administration are low (16). These phytochemicals are metabolized by gut bacteria and transformed into different compounds that exert anti-cancer activities (16). It is well documented that butyrate is one of them. Butyrate can be detected in feces and plasma from the KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice fed BRBs but not in the pancreas (data not shown). The systematic circulation of gut bacteria metabolites such as butyrate modulates the host immunity throughout the whole body and thereby can prevent cancers in multiple organs. Indeed, this argument is supported by our data shown in Supplementary Figure 3 that IFNγ in NKp46+ cells were significantly increased in spleen from BRB-treated KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice and spleens of BRB-treated mice had a significantly decreased population of M-MDSCs. The systematic immune surveillance then influences the tumor microenvironment in the pancreas.
Treatment-induced changes in the immune system could affect outcome of pancreatic cancer. Increased circulating T cell and NK cell subsets significantly associated with treatment outcomes in a randomized phase II trial of the oncolytic virus pelareorep (Reolysin) in metastatic PDAC (40). Gemcitabine administration after resection of murine pancreatic tumors inhibited the accumulation of MDSCs, activated NK cell-mediated anti-tumor responses, and inhibited local tumor recurrence (41). These findings are consistent with observations from patients with PDAC (41). Thus, targeting immunosuppressive myeloid cells in combination with chemotherapy could be more effective than chemotherapy alone because chemotherapy-derived inflammatory responses could accelerate the infiltration of immunosuppressive myeloid cells in the tumor microenvironment of human pancreatic cancer (42).
In light of immune-modulating effects of BRBs, we obtained PBMC samples from PDAC patients and treated the samples with butyrate, which is a microbial metabolite of BRBs that has been shown to regulate T regulatory cells (28,29) and neutrophils (30). Though we didn’t observe any changes in NK cells and CD8+ T cells, we found significantly decreased levels of MDSCs. Furthermore, we isolated CD11b+ cells from PDAC patients’ PBMCs and pre-treated them with butyrate. CD8+ T cells were isolated from healthy donors and activated by CD3/CD28 antibodies. CD11b+ cells induced substantial decrease in the function of CD8+ T cells, suggesting an immune-suppressive role of isolated CD11b+ cells. Importantly, butyrate significantly restored the function of CD8+ T cells suppressed by CD11b+ cells. Further investigation is needed to determine if berries or their metabolites could suppress CD11b+ cells thereby enhancing CD8+ T cells in the tumors of PDAC patients.
Spleen is an important immune organ in the fight against cancer. One study reported increased immunosuppressive MDSCs in splenocytes from PDAC patients compared with patients with benign cysts (43). Similarly, another study observed increased splenic MDSCs in PDAC patients compared to patients with chronic pancreatitis (44). However, no differences were found for splenic CD4+ or splenic CD8+ T cells (44). In the current study, we performed flow cytometric analysis to measure the levels of CD8+ T, NKp46+ cells, and MDSCs in the spleen from the two mouse models of PDAC. BRBs only significantly increased the secretion of IFNγ by NKp46+ cells in the spleen of KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice.
MDSCs are a group of heterogeneous cells that play important roles in the immune response in diseases. Although numerous studies have investigated the characteristics of MDSCs in cancer, they should be interpreted carefully due to the complexity of these cells. The disease stages, specimen types, animal models, and surface markers chosen could all influence the conclusions (35). In the current study, M-MDSCs and PMN-MDSCs were defined according to a review (35). There were significantly decreased levels of M-MDSCs in the spleen of the BRB-fed KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice.
We also performed IHC staining to detect the expression of CD8, NKp46, and CD107a in the pancreatic tumor microenvironment. CD107a expression can be used as a functional marker of cytotoxic CD8+ T (45) and NK cells (46). Therefore, levels of CD107a expression represent anti-tumor activities of those cells. Our data showed that BRBs significantly increased the staining of CD8, NKp46, and CD107a in the pancreatic tumors, suggesting that BRBs boost both the numbers and cytotoxic activities of tumor-infiltrating CD8+ T cells and NKp46+ cells. In addition, we used IHC staining to determine levels of tumor-infiltrating CD11b+ cells and found significantly decreased CD11b+ staining in the BRB-treated pancreatic tumors from both mouse models. Collectively, our results suggest that BRBs have the potential to modulate PDAC immunity by decreasing tumor-infiltrating CD11b+ cells and increasing CD8+ T cells and NKp46+ cells in the pancreatic tumor microenvironment.
It should be noted that levels of immune cells in the spleen may not represent the cells’ functions and activities in the pancreatic tumor microenvironment, because spleen is not the target organ in PDAC. Tumor-infiltrating immune cells in the tumor microenvironment play more important roles in tumor immune surveillance. Direct measurement of immune cells in the pancreatic tumors would provide a better understanding of how BRBs affect PDAC immunity in the tumor microenvironment. Thus, further immune cell profiling inside pancreatic tumors is needed. In addition, studies have reported that BRBs decreased immune-suppressing cells in cancer, such as neutrophils (19) and T regulatory cells (47), thereby boosting anti-tumor immunities. Further investigation is much needed to determine whether both mechanisms–directly increase immune responses of NK cells and CD8+ T cells and indirectly inhibit immune-suppressing cells–contribute to BRBs’ anti-tumor effects.
Combining the Kras and Trp53 mutations in mice promotes PDAC and shortens median survival to 5 months (34). Pathologically, these KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice recapitulate human pancreatic cancer. We found that median survival was prolonged (by 35 days, to 189 days) when these mice received BRBs rather than the control diet (median survival 154 days). Based on good tolerance of BRB powder in our previous human clinical trials (15,17), we speculate that PDAC patients would tolerate BRB powder. Therefore, this prolonged survival of the BRB-fed KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice may have clinical significance.
To block PDAC carcinogenesis, it is necessary to target the Kras mutation or its effector pathways (48). Furthermore, it has been reported that cell cycle progression is induced by Ras-ERK activation (49). Cell cycle regulators, including cyclin D (37), cyclin B (38), and cyclin E (39), are overexpressed in human PDAC. We showed that BRBs suppressed Raf/MEK/ERK activation and decreased Ki67 expression in pancreatic tumors from both NOD.SCID and KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice. BRBs also decreased the expression of cyclin D3 and CDK4 in pancreatic tumors from the KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice but not the NOD.SCID mice, suggesting that BRBs may regulate different cell cycle regulators in NOD.SCID mice.
In conclusion, our results indicate that BRBs suppressed PDAC in both immune-deficient NOD.SCID mice lacking B and T cells and immune-competent KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice that spontaneously develop PDAC. BRBs have the potential to be immune-modulators that decreased tumor-infiltrating CD11b+ cells and increased infiltration of NKp46+ cells in the microenvironment of pancreatic tumors in both models. BRBs also increased tumor-infiltrating CD8+ T cells in the pancreatic tumors from the KrasLSL.G12D/+-Trp53LSL.R172H/+-Pdx-1-Cre mice. Importantly, BRBs increased the expression of CD107a in the pancreatic tumor microenvironment. A BRB metabolite, butyrate, reversed CD11b+ cell-mediated suppression of CD8+ T cell in the PBMCs from PDAC patients. Our results suggest that BRBs may have clinical potential for delaying the progression of PDAC by modulating immune cells.
Supplementary Material
Acknowledgements
We thank Chad Skaer, Hzin-Tsu Wang, and Chieh-Ti Kuo for their assistance in this study.
Funding: This work was supported by NIH grants CA148818 (to L.-S. Wang), and CA185301, AI129582 and NS106170 (to J. Yu).
List of abbreviations
- ADM
acinar-ductal metaplasia
- BAD
Bcl-2-associated death promoter
- Bcl-2
B-cell lymphoma 2
- BRBs
black raspberries
- CDK4
cyclin dependent kinase 4
- ERK (or p42/44)
extracellular signal-regulated kinases
- IFNγ
interferon-gamma
- MDSCs
myeloid-derived suppressor cells
- M-MDSCs
monocytic MDSCs
- MAPKK (or MEK)
mitogen activated protein kinase kinase
- NK
natural killer
- OS
overall survival
- PanIN
pancreatic intraepithelial neoplasia
- PBMCs
peripheral blood mononuclear cells
- PDAC
pancreatic ductal adenocarcinomas
- PMN-MDSCs
polymorphonuclear MDSCs
- STAT3
signal transducer and activator of transcription 3
References
- 1.Bernard W Stewart CPW. World Cancer Report 2014. 2014. [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019. [DOI] [PubMed] [Google Scholar]
- 3.Bryant KL, Mancias JD, Kimmelman AC, Der CJ. KRAS: feeding pancreatic cancer proliferation. Trends Biochem Sci 2014;39(2):91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer 2002;2(12):897–909. [DOI] [PubMed] [Google Scholar]
- 5.Teague A, Lim KH, Wang-Gillam A. Advanced pancreatic adenocarcinoma: a review of current treatment strategies and developing therapies. Ther Adv Med Oncol 2015;7(2):68–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Velez-Delgado A, Mathew E, Li D, Mendez FM, Flannagan K, et al. Myeloid cells are required for PD-1/PD-L1 checkpoint activation and the establishment of an immunosuppressive environment in pancreatic cancer. Gut 2017;66(1):124–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porembka MR, Mitchem JB, Belt BA, Hsieh CS, Lee HM, Herndon J, et al. Pancreatic adenocarcinoma induces bone marrow mobilization of myeloid-derived suppressor cells which promote primary tumor growth. Cancer Immunol Immunother 2012;61(9):1373–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng J, Guinter MA, Merchant AT, Wirth MD, Zhang J, Stolzenberg-Solomon RZ, et al. Dietary patterns and risk of pancreatic cancer: a systematic review. Nutr Rev 2017;75(11):883–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan P, Skaer C, Yu J, Zhao H, Ren H, Oshima K, et al. Berries and other natural products in the pancreatic cancer chemoprevention in human clinical trials. J Berry Res 2017;7(3):147–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amin AR, Kucuk O, Khuri FR, Shin DM. Perspectives for cancer prevention with natural compounds. J Clin Oncol 2009;27(16):2712–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montrose DC, Horelik NA, Madigan JP, Stoner GD, Wang LS, Bruno RS, et al. Anti-inflammatory effects of freeze-dried black raspberry powder in ulcerative colitis. Carcinogenesis 2011;32(3):343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halder RC, Almasi A, Sagong B, Leung J, Jewett A, Fiala M. Curcuminoids and omega-3 fatty acids with anti-oxidants potentiate cytotoxicity of natural killer cells against pancreatic ductal adenocarcinoma cells and inhibit interferon gamma production. Front Physiol 2015;6:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoner GD. Foodstuffs for preventing cancer: the preclinical and clinical development of berries. Cancer Prev Res (Phila) 2009;2(3):187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stoner GD, Wang LS, Casto BC. Laboratory and clinical studies of cancer chemoprevention by antioxidants in berries. Carcinogenesis 2008;29(9):1665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang LS, Arnold M, Huang YW, Sardo C, Seguin C, Martin E, et al. Modulation of genetic and epigenetic biomarkers of colorectal cancer in humans by black raspberries: a phase I pilot study. Clin Cancer Res 2011;17(3):598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan P, Skaer CW, Stirdivant SM, Young MR, Stoner GD, Lechner JF, et al. Beneficial Regulation of Metabolic Profiles by Black Raspberries in Human Colorectal Cancer Patients. Cancer Prev Res (Phila) 2015;8(8):743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang LS, Burke CA, Hasson H, Kuo CT, Molmenti CL, Seguin C, et al. A phase Ib study of the effects of black raspberries on rectal polyps in patients with familial adenomatous polyposis. Cancer Prev Res (Phila) 2014;7(7):666–74. [DOI] [PubMed] [Google Scholar]
- 18.Pan P, Skaer CW, Wang HT, Stirdivant SM, Young MR, Oshima K, et al. Black raspberries suppress colonic adenoma development in ApcMin/+ mice: relation to metabolite profiles. Carcinogenesis 2015;36(10):1245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan P, WS C, Wang HT, Oshima K, Huang YW, Yu J, et al. Loss of free fatty acid receptor 2 enhances colonic adenoma development and reduces the chemopreventive effects of black raspberries in ApcMin/+ mice. Carcinogenesis 2017;38(1):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan P, Kang S, Wang Y, Liu K, Oshima K, Huang Y-W, et al. Black Raspberries Enhance Natural Killer Cell Infiltration into the Colon and Suppress the Progression of Colorectal Cancer. Frontiers in Immunology 2017;8(997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan P, Skaer CW, Wang HT, Kreiser MA, Stirdivant SM, Oshima K, et al. Systemic Metabolite Changes in Wild-type C57BL/6 Mice Fed Black Raspberries. Nutr Cancer 2017;69(2):299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan P, Huang YW, Oshima K, Yearsley M, Zhang J, Yu J, et al. An immunological perspective for preventing cancer with berries. J Berry Res 2018;8(3):163–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peiffer DS, Wang LS, Zimmerman NP, Ransom BW, Carmella SG, Kuo CT, et al. Dietary Consumption of Black Raspberries or Their Anthocyanin Constituents Alters Innate Immune Cell Trafficking in Esophageal Cancer. Cancer Immunol Res 2016;4(1):72–82. [DOI] [PubMed] [Google Scholar]
- 24.Pan P, Dombkowski AA, Wang LS, Stoner GD. A nutrigenetic approach for investigating the chemopreventive effects of black raspberries during the development of preneoplastic esophagi in rats. J Berry Res 2018;8(4):263–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fotschki B, Juskiewicz J, Sojka M, Jurgonski A, Zdunczyk Z. Ellagitannins and Flavan-3-ols from Raspberry Pomace Modulate Caecal Fermentation Processes and Plasma Lipid Parameters in Rats. Molecules 2015;20(12):22848–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kosmala M, Jurgonski A, Juskiewicz J, Karlinska E, Macierzynski J, Roj E, et al. Chemical Composition of Blackberry Press Cake, Polyphenolic Extract, and Defatted Seeds, and Their Effects on Cecal Fermentation, Bacterial Metabolites, and Blood Lipid Profile in Rats. J Agric Food Chem 2017;65(27):5470–79. [DOI] [PubMed] [Google Scholar]
- 27.Vuholm S, Nielsen DS, Iversen KN, Suhr J, Westermann P, Krych L, et al. Whole-Grain Rye and Wheat Affect Some Markers of Gut Health without Altering the Fecal Microbiota in Healthy Overweight Adults: A 6-Week Randomized Trial. J Nutr 2017;147(11):2067–75. [DOI] [PubMed] [Google Scholar]
- 28.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013;504(7480):446–50. [DOI] [PubMed] [Google Scholar]
- 29.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013;341(6145):569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan P, Oshima K, Huang YW, Agle KA, Drobyski WR, Chen X, et al. Loss of FFAR2 promotes colon cancer by epigenetic dysregulation of inflammation suppressors. International journal of cancer 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan P, Lam V, Salzman N, Huang YW, Yu J, Zhang J, et al. Black Raspberries and Their Anthocyanin and Fiber Fractions Alter the Composition and Diversity of Gut Microbiota in F-344 Rats. Nutr Cancer 2017:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kresty LA, Morse MA, Morgan C, Carlton PS, Lu J, Gupta A, et al. Chemoprevention of esophageal tumorigenesis by dietary administration of lyophilized black raspberries. Cancer Res 2001;61(16):6112–9. [PubMed] [Google Scholar]
- 33.Bayne LJ, Vonderheide RH. A myeloid-derived suppressor cell-mediated T-cell suppression assay for functional evaluation of immune cells in tumor-bearing mice. Cold Spring Harb Protoc 2013;2013(9):849–53. [DOI] [PubMed] [Google Scholar]
- 34.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005;7(5):469–83. [DOI] [PubMed] [Google Scholar]
- 35.Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun 2016;7:12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He W, Wu J, Shi J, Huo YM, Dai W, Geng J, et al. IL22RA1/STAT3 Signaling Promotes Stemness and Tumorigenicity in Pancreatic Cancer. Cancer Res 2018;78(12):3293–305. [DOI] [PubMed] [Google Scholar]
- 37.Bachmann K, Neumann A, Hinsch A, Nentwich MF, El Gammal AT, Vashist Y, et al. Cyclin D1 is a strong prognostic factor for survival in pancreatic cancer: analysis of CD G870A polymorphism, FISH and immunohistochemistry. Journal of surgical oncology 2015;111(3):316–23. [DOI] [PubMed] [Google Scholar]
- 38.Zhou L, Li J, Zhao YP, Cui QC, Zhou WX, Guo JC, et al. The prognostic value of Cyclin B1 in pancreatic cancer. Med Oncol 2014;31(9):107. [DOI] [PubMed] [Google Scholar]
- 39.Deng J, He M, Chen L, Chen C, Zheng J, Cai Z. The loss of miR-26a-mediated post-transcriptional regulation of cyclin E2 in pancreatic cancer cell proliferation and decreased patient survival. PLoS One 2013;8(10):e76450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noonan AM, Farren MR, Geyer SM, Huang Y, Tahiri S, Ahn D, et al. Randomized Phase 2 Trial of the Oncolytic Virus Pelareorep (Reolysin) in Upfront Treatment of Metastatic Pancreatic Adenocarcinoma. Mol Ther 2016;24(6):1150–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gurlevik E, Fleischmann-Mundt B, Brooks J, Demir IE, Steiger K, Ribback S, et al. Administration of Gemcitabine After Pancreatic Tumor Resection in Mice Induces an Antitumor Immune Response Mediated by Natural Killer Cells. Gastroenterology 2016;151(2):338–50 e7. [DOI] [PubMed] [Google Scholar]
- 42.Takeuchi S, Baghdadi M, Tsuchikawa T, Wada H, Nakamura T, Abe H, et al. Chemotherapy-Derived Inflammatory Responses Accelerate the Formation of Immunosuppressive Myeloid Cells in the Tissue Microenvironment of Human Pancreatic Cancer. Cancer Res 2015;75(13):2629–40. [DOI] [PubMed] [Google Scholar]
- 43.Jordan KR, Kapoor P, Spongberg E, Tobin RP, Gao D, Borges VF, et al. Immunosuppressive myeloid-derived suppressor cells are increased in splenocytes from cancer patients. Cancer Immunol Immunother 2017;66(4):503–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Basso D, Fogar P, Falconi M, Fadi E, Sperti C, Frasson C, et al. Pancreatic tumors and immature immunosuppressive myeloid cells in blood and spleen: role of inhibitory co-stimulatory molecules PDL1 and CTLA4. An in vivo and in vitro study. PLoS One 2013;8(1):e54824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods 2003;281(1–2):65–78. [DOI] [PubMed] [Google Scholar]
- 46.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods 2004;294(1–2):15–22. [DOI] [PubMed] [Google Scholar]
- 47.Duncan FJ, Martin JR, Wulff BC, Stoner GD, Tober KL, Oberyszyn TM, et al. Topical treatment with black raspberry extract reduces cutaneous UVB-induced carcinogenesis and inflammation. Cancer Prev Res (Phila) 2009;2(7):665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Collins MA, Bednar F, Zhang Y, Brisset JC, Galban S, Galban CJ, et al. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. The Journal of clinical investigation 2012;122(2):639–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta 2007;1773(8):1263–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.