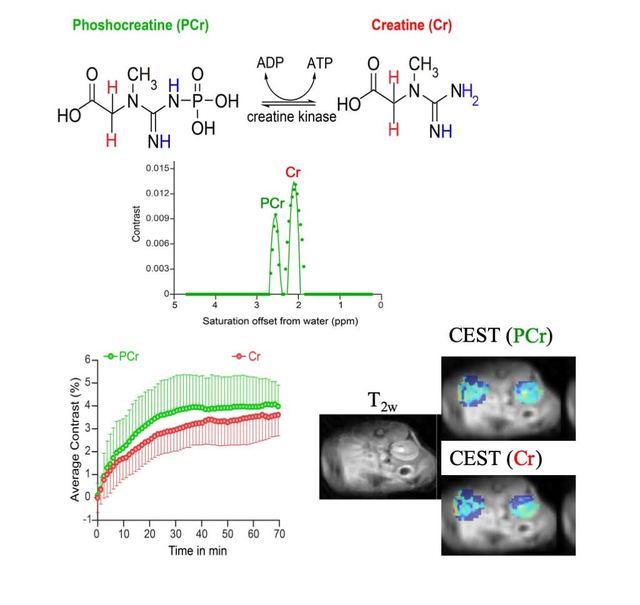
Abstract
Creatine is an important metabolite involved in muscle contraction. Administration of exogenous creatine (Cr) or phosphocreatine (PCr) has been used for improving exercise performance and protecting the heart during surgery including during valve replacements, coronary artery bypass grafting and repair of congenital heart defects. In this work we investigate whether it is possible to use chemical exchange saturation transfer (CEST) MRI to monitor uptake and clearance of exogenous creatine and phosphocreatine following supplementation. We were furthermore interested in determining the limiting conditions for distinguishing between creatine (1.9 ppm) and phosphocreatine (2.6 ppm) signals at ultra-high fields (21 T) and determine their concentrations could be reliably determined using Bloch equation fits of the experimental CEST spectra. We have tested these items by performing CEST MRI of hind limb muscle and kidneys at 11.7 T and 21.1 T both before and after intravenous administration of PCr. We observed up to 4% increase in contrast in the kidneys at 2.6 ppm which peaked ~ 30 minutes after administration and a relative ratio of 1.3 in PCr:Cr signal. Overall, these results demonstrate the feasibility of independent monitoring of PCr and Cr concentration changes using CEST MRI.
Keywords: CEST imaging, creatine, magnetic resonance imaging, molecular imaging
Graphical Abstract
Introduction
Creatine (Cr) is an important metabolite found mostly in muscle and involved in muscle contraction. Since the discovery of phosphocreatine (PCr) and of the creatine kinase (CK) reaction in 1927 and 1934 respectively, research efforts have focused on its involvement in energy metabolism (1). In fast twitch skeletal muscles, PCr stores phosphate for regeneration of adenosine triphosphate (ATP) from adenosine diphosphate (ADP) during high intensity, short duration activity and is converted to creatine (Cr) during this reaction. Because of this, Cr is regularly taken by athletes around the country as a nutritional supplement for performance enhancement, indeed it has been reported that all the National Football League teams have between 33 to 90% of their players using Cr supplementation (2–4). Besides this, some researchers have observed that PCr supplementation can be protective following heart surgery including for surgical valve replacements, coronary artery bypass grafting and repair of congenital heart defects (5,6). One of the challenges of supplementation is that there are large differences in dosage and duration between supplementation protocols, while methods which can assess Cr and PCr content in various organs of interest which would be helpful to evaluate the benefits are lacking.
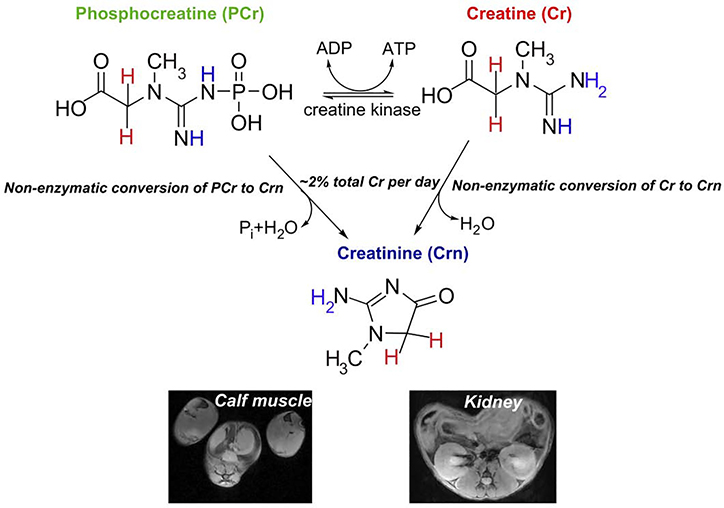
Molecular imaging enables a detailed understanding of biological function through detection of the distribution of important compounds in biological pathways (7), and can be accomplished using strategies which detect unaltered, natural compounds and their metabolites. MRI and MRSI are uniquely valuable tools within the field of molecular imaging for visualizing the spatial distribution of metabolites in soft tissue. Cr, PCr and creatinine (Crn) are the three compounds found in creatine metabolism, and possess several nuclei detectable via NMR (Fig 1). While 1H, 31P and 13C based MRSI have all been used for monitoring their presence in tissue (8–14), unfortunately MRSI presents disadvantages for widespread application. In the case of 31P MRSI the image resolution has limitations based on sensitivity, only PCr can be measured and 31P MRSI requires specialized hardware and software. For 1H MRSI, only total creatine is quantified instead of the three individual compounds due to limited spectral resolution. Based on this there is a need for new noninvasive methods to quantify creatine metabolism in tissue.
Figure 1.
Important molecules involved in creatine metabolism including CEST detectable protons (blue) and MRS detectable protons (red). Creatine has 3 equivalent labile protons, phosphocreatine has 1 equivalent labile proton (1 nonequivalent) and creatinine has 2 equivalent labile protons. (Lower panel) representative axial MRI of calf muscle and kidneys, two locations for detecting these metabolites.
Chemical exchange saturation transfer (CEST) contrast represents an attractive method for amplified detection of important compounds in biological pathways using the MRI saturation signatures of exchangeable protons (15–22). Indeed, a number of molecules have been reported as elegant examples of diamagnetic CEST (diaCEST) agents including urea (23,24), glucose (25,26), glutamate (27,28), Cr (29–31), glycosaminoglycans (32,33), barbituric acid (34,35), triiodobenzenes (36–39), thymidine analogues (40) salicylates (41), anthranillates (42), imidazoles (43) and free base porphyrins (44). In addition, several peptides and proteins have been optimized as CEST reporter genes including lysine rich protein and protamine (45–48). Herein, we show that it is possible to specifically follow the uptake and clearance of Cr and PCr separately via CEST MRI and demonstrate these concentrations can be modeled using multiple pool Bloch equations.
Methods
Phantom Preparation and CEST Experiments
All samples were dissolved in 0.01 M phosphate-buffered saline (PBS) at 25 mM concentrations and titrated to the pH values 6.2, 6.5, 6.8 and 7.1 using high concentration HCl/NaOH. The solutions were placed into 5 mm NMR tubes and assembled in a holder for CEST MR imaging. The samples were kept at 37 °C during imaging. 11.7 T phantom data were acquired on a Bruker vertical MR scanner (Bruker Avance, Ettlingen, Germany) using a 25mm transmit/receive coil and the RARE sequence (RARE Factor = 32). CW saturation pulse length = 3 sec, saturation field strength (B1) = 1.0 μT to 6 μT. The CEST Z-spectra were acquired by incrementing the saturation frequency every 0.12 ppm from −5.5 to 5.5 ppm. WASSR images were also acquired using a 3 sec CW saturation pulse with B1 = 0.5 μT, saturation frequency incremented from −1.5 ppm to 1.5 ppm. 21.1 T phantom CEST experiments were performed on 25 mM solutions in 0.01 M PBS as well. These CEST images were recorded on a 21.1 T vertical MR scanner (49) equipped with a Bruker Advance III console (Bruker Corp. Ettlingen, Germany). A home-built 35 mm transmit/receive coil was used for all experiments. CEST images were acquired using a RARE (RARE Factor = 12; effective TE = 3.58 ms) sequence with CW saturation pulse length of 3 seconds and B1 from 1.0 μT to 6 μT. The CEST Z-spectra were acquired for 85 offsets between −5 and +5 ppm and one M0 offset. Other parameters were kept same between 11.7 T and 21.1 T and were: TR = 6 s, effective TE = 4.5 ms, matrix size = 64×64 and slice thickness of 1.5 mm.
Animal Imaging
All animal experiments were performed under protocols approved by the Johns Hopkins University Animal Care and Use Committee and the Florida State University Animal Care and Use Committee. C57Bl/6 mice weighing 30–35 gr were used for all experiments. The 11.7 T Bruker Biospec at John Hopkins and the 21.1 T scanner at the National High Magnetic Field laboratory (NHMFL) were used to collect the images in this study through Paravision 6.0.1. On the 11.7 T scanner, a 70 mm transmit coil and an 8 channel mouse body phase array coil were used for collecting MRI data. On the 21.1 T scanner, the 35 mm transmit/receive volume coil was used for collecting MRI data. After confirming accurate placement of the mice in the coil, shimming was performed using a localized voxel placed over the left and right kidneys. The RARE sequence was used for all images. In vivo B0 inhomogeneity maps were generated from the WASSR experiment performed before the injection for each mouse. WASSR Z-spectra for ROI’s drawn over both the kidneys were extracted and interpolated in Matlab using cubic spline interpolation. The water shift was measured pixel by pixel as described previously (50,51) to obtain the ΔB0 maps over the kidney. For muscle and kidney CEST images collected without administering PCr, a 3 sec CW saturation pulse was used and CEST images were acquired with saturation B1 = 1, 1.5, 2, and 2.5 μT. Z-spectra were collected at 81 saturation frequencies between −5 and +5 ppm and one additional at +40 ppm for determining M0. Other experimental parameters include: TR = 10 sec, effective TE = 27.9 ms, matrix = 48×32, slice thickness = 1.5 mm. For the PCr administered experiments at 21.1 T, a micro cannula was inserted in the lateral tail vein of the mouse and secured for insertion into the magnet in order to inject 150 μl of 325 mM PCr at neutral pH during the MRI experiments. Prior to start of the CEST experiment, shimming was performed and a 1-mm thick axial slice was placed to bisect the center of the major calyces on both kidneys. CEST images were collected at 9 frequencies in this study (1.6, 1.8, 1.9, 2.1, 2.2, 2.4, 2.6, 2.7, 2.9 ppm) repetitively (each frequency was collected 85 times for a total of 9*85 = 765 images). Each offset was acquired in one-shot using repetition time (TR) = 8 s. The saturation preparation consisted of 15 pulses with block shape (bp) that were 200 ms long resulting in a 3 s saturation pulse train at B1 = 2.5 μT. CEST images acquired using this oversampling approach allows for correction of motion artifacts during the 1h 40 min scan through data temporal averaging (smoothing) as described previously (52). The injection of PCr occurred at 8 min following start of the acquisition to allow robust acquisition of pre-injection images.
Data Processing
In vitro and in vivo CEST MRI data were processed using custom written Matlab code. In vitro mean Z-spectra and MTRasym were calculated by drawing an ROI over each phantom tube. QUESP and Bloch simulations were used to fit the data and extracting the exchange rates at each pH value as described previously (53). In vivo muscle mean Z-spectra were produced and the baseline Z-spectrum without the PCr and Cr CEST peaks was calculated using spline interpolation and subtracted from the mean Z-spectra to calculate the difference (contrast) spectra for n= 3 mice on the 11.7 T scanner and n= 3 mice on the 21.1 T scanner. For calculating this difference spectra, one additional half width of the CEST peak from the lowest point on either side of these peaks to define the range of the peak. The mean Z-spectrum over an ROI covering the muscle was fit using 4 pool Bloch simulations (PCr, Cr, MT, water pools) to simulate the MT effect for calculating the water T1 and T2 values with parameters listed in Table 2, with errors estimated through Monte Carlo error estimation using 1000 iterations. Using these values, the muscle contrast spectra were fit to determine the in vivo PCr and Cr concentrations without administration of PCr. The in vivo kidney data for ROIs which exclude the collecting system were processed in a similar manner. For analyzing the 21.1 T Z-spectra without administering PCr, the mean Z-spectra were extracted and difference spectra calculated. The difference spectra were calculated in a similar manner as muscle, by allowing a half width space on each side of the CEST peaks and fitting these difference spectra using 4 pool Bloch simulations to calculate the Cr and PCr concentrations in kidney (Table 2). For the 21.1 T dynamic CEST contrast results, the mean Z-spectra of both pre and post contrast were extracted. The mean pre-injection z-spectra were subtracted from all post-injection images. For this dynamic data set, the ‘smooth’ function in Matlab was used to apply a moving time average to remove the motion induced fluctuations in the dynamic CEST contrast data as described previously (52). The smoothening factor used in the averaging process was ~10 neighboring images, with this choice based on the size of the data and the range of fluctuations. After smoothening ~2–3 images were averaged to generate the CEST contrast maps. Maximum contrast maps were generated by calculating the pixel-by-pixel ST (1-Mz/M0) on two ROIs, one over each kidney, overlaid on the corresponding T2W image. CEST dynamic contrast build up curve at two offsets correspond to Cr and PCr was obtained on the 21.1 T scanner by taking the average of the CEST build up curves for n = 6 mice.
Table 2:
Bloch simulation parameters for muscle Cr and PCr concentration calculations on 11.7 and 21.1 T scanners
| Muscle | 11.7 T scanner | |||
|---|---|---|---|---|
| ParameteR | Water | MT pool (A) | Cr pool (B) | PCr pool (C) |
| Δω (ppm) | 0 | 0 | 1.9 | 2.6 |
| R1 (s−1) | 1/(2±0.7) | 1 | 1 | 1 |
| R2 (s−1) | 1/(0.033±0.001) | 1/15×10−6 | 1/(0.5±0.1) | 1/(0.5±0.1) |
| Mol (mM) pH 6.5 pH 6.8 pH 7.1 |
- | 11.7 |
14.2 9.6 10.8 |
41.5 30.7 19.5 |
| kex (/sec) pH 6.5 pH 6.8 pH 7.1 |
- | 21 |
143±15 292±50 585±150 |
55±3 78±6 158±15 |
| Muscle | 21. 1 T scanner | |||
| Parameter | Water | MT pool (A) | Cr pool (B) | PCr pool (C) |
| Δω (ppm) | 0 | 0 | 1.9 | 2.6 |
| R1 (s−1) | 1/(2.33±0.06) | 1 | 1 | 1 |
| R2 (s−1) | 1/(0.015±0.0004) | 1/15×10−61 | 1/(0.3±0.1) | 1/(0.3±0.1) |
| Mol (mM) pH 6.5 pH 6.8 pH 7.1 |
- | 11.7 |
17.5 11 10.7 |
37.7 28 15.8 |
| kex (/sec) pH 6.5 pH 6.8 pH 7.1 |
- | 21 |
143±15 292±50 585±150 |
55±3 78±6 158±15 |
(R1, R2 values of MT, Cr, PCr without error estimates are similar to those found in reference 15)
Results
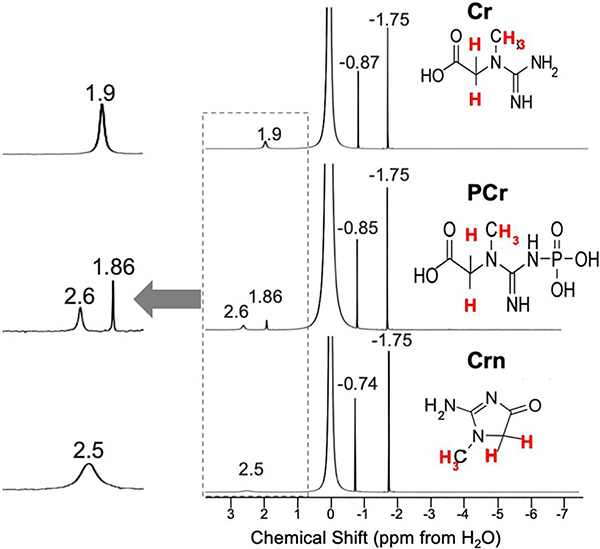
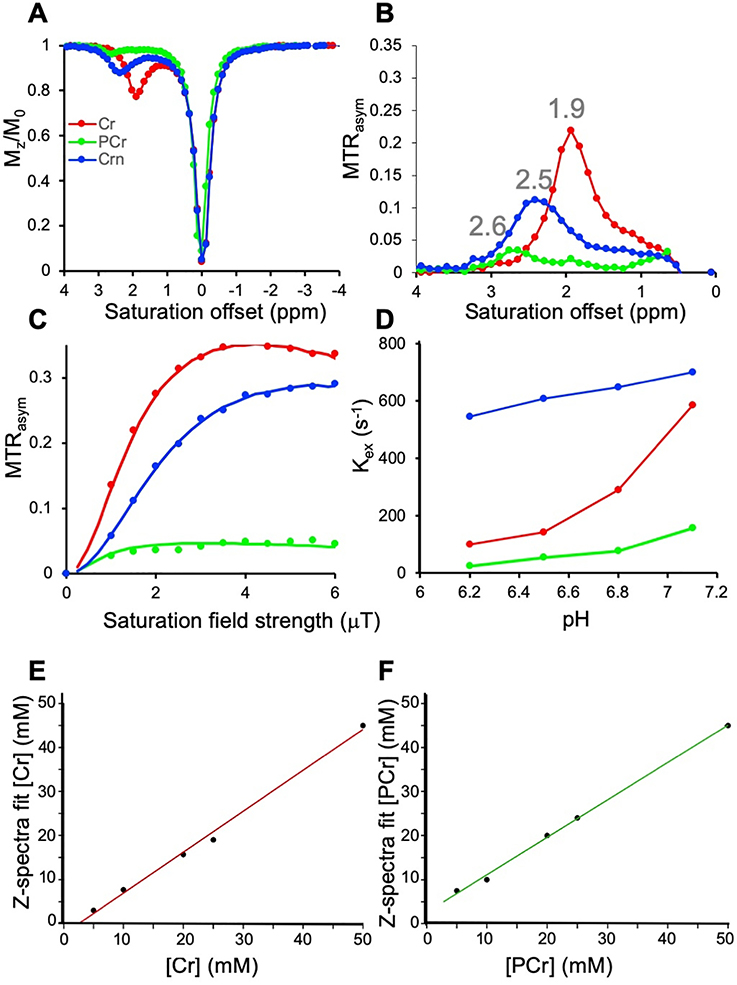
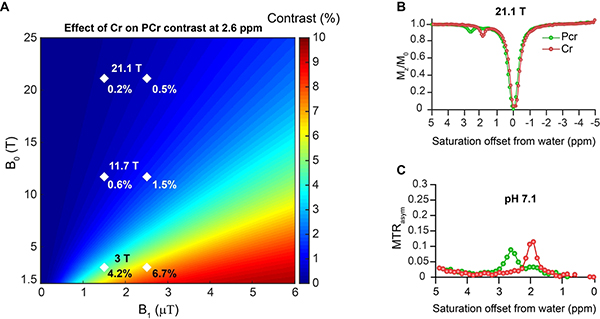
We were interested first in comparing how 1H NMR and 1H CEST would perform for detecting these metabolites. As is seen in Fig. 2, Table 1, there are some difficulties discriminating the three metabolites, as the 1H NMR spectra for all three consists of two peaks with the CH3 resonating at −1.75 ppm from H2O for all three. As is shown, there are small differences for the CH2 which for Cr is −0.87 ppm while PCr resonates at - 0.85 ppm and Crn a little removed at 0.74 ppm. Because of this, for MRS studies total creatine is quantified instead (13). This contrasts with the CEST Z-spectra of Cr, PCr and Crn (Fig. 3A,B) which displays peaks at 1.9 ppm, 2.6 ppm and 2.5 ppm respectively. Although Crn is not so well separated, this is a very low concentration species, as ~2% of total Cr in muscle is converted to Crn per day (1) and as a result Crn is not expected to contribute to signal in tissue. There are also peak intensity differences observed. Cr possesses 3 labile protons per molecule, PCr possesses 2 NH and CrN possesses 2 NH and there are also exchange rate (kex) differences for these labile protons which impact this sensitvity. As is shown in Fig 3A,B, at 11.7 T the most sensitive compound is Cr using moderate saturation pulses (B1 = 1.5 μT ) as is expected based on the factor of 1.5 larger labile proton content. We performed QUESP experiments to measure these rates (Fig 3C,D) which revealed the fastest exchanging protons were found on Crn, followed by Cr and PCr with the kex’s being pH dependent. Also as is shown in PBS in Fig 3C, increasing the B1 from 1 to 4 μT results in larger signal enhancements for PCr and Cr. Unfortunately, use of stronger saturation pulses results in overlap of the 1.9 ppm and 2.6 ppm peaks for these two metabolites. We performed a second round of phantom experiments with the phantom consisting of tubes with 5 different concentrations, 50, 25, 20, 10, 5 mM of Cr and PCr. The resulting difference spectra were fit to determine the concentrations as described in the methods section with the results shown in Fig 3E,F. We observed a linear correlation between the spectral fit determined concentration and measured concentrations with rmsd < 1 mM for both Cr and PCr indicating the suitability of our fitting methods over this range in concentrations. In order to determine what saturation conditions are ideal for sensitively detecting Cr and PCr and minimizing overlap, we performed a series of Bloch simulations (Fig. 4A). As is shown, minimal overlap can be achieved at 11.7 T using 1.5 μT saturation pulse trains, representing a good balance of sensitivity and resolution. This results in an enhancement in detection of ~ 200 for Cr and ~165 for PCr at neutral pH as calculated previously (54). Based on the same simulations, at 21.1 T we can achieve larger enhancements of ~350 and 200 for Cr, PCr while retaining good spectral resolution by using 2.5 μT saturation pulse trains as can be observed from the Z-spectra and asymmetry spectra at 21.1 T shown in Fig 4B,C and as quantified in Fig 4A which indicates a 0.5% increase in PCr contrast due to the 1.9 ppm Cr peak. We also Based on our results which show that CEST detection of Cr and PCr results in enhanced resolution over 1H NMR and also enhanced detection, we moved to in vivo studies.
Figure 2.
1H NMR spectra for Cr, PCr, Crn. Conditions: pH ~ 6.5, 10%/90% D2O/H2O at T = 20°C
Table 1:
Chemical shifts of labile protons for the compounds in this study
| Compound | chemical shift (ppm) | chemical shift (ppm) |
|---|---|---|
| Creatine | NH2: 1.9 ppm | NH: 1.9 ppm |
| Phosphocreatine | NH: 1.86 ppm | NH: 2.6 pm |
| Creatinine | NH2: 2.5 ppm |
Figure 3.
In vitro phantom results. A) CEST Z-spectra and B) MTRasym of Cr, PCr and Crn using B1 = 1.5 μT; C) QUESP plots of Cr, PCr and Crn at pH 7.1 obtained with B1 = 1, 1.5, 2, 2.5, 3, 3.5, 4, 4.5, 5, 5.5 and 6 μT respectively; D) Exchange rate vs pH of Cr, PCr and Crn measured using Bloch simulation fitting the QUESP dataset; E) Correlation between spectral fit concentration and measured concentrations for [Cr] = 50, 25, 20, 10, 5 mM; F) Correlation between spectral fit concentration and measured concentrations for [PCr] = 50, 25, 20, 10, 5 mM; Conditions: 20 mM Cr, PCr, and Crn phantoms, T= 37 °C and pH values 6.2, 6.5, 6.8 and 7.1 were used for CEST experiments.
Figure 4.
Bloch simulations of Cr, PCr contrast and in vitro CEST experiments on phantoms at 21.1 T. A) Bloch simulations of increase in PCr contrast due to presence of the 1.9 ppm Cr peak at different B0 and B1 fields. Diamonds are shown at the critical values of B1 = 1.5 μT and 2.5 μT to indicate that the PCr CEST contrast at 2.6 ppm is increased by 4.2 %, 0.6% and 0.2% by overlap with 1.9 ppm Cr peak for B1 = 1.5 μT and to 6.7 %, 1.5% and 0.5% for B1 = 2.5 μT on 3, 11.7 and 21 T MRI scanners respectively. T1 and T2 relaxations were kept constant for simulations as mentioned in Table 2; B) 21.1 T CEST z-spectra and C) 21.1 T MTRasym spectra of Cr, PCr samples at pH 7.1, T=37 °C using 2.5 μT RF saturation.
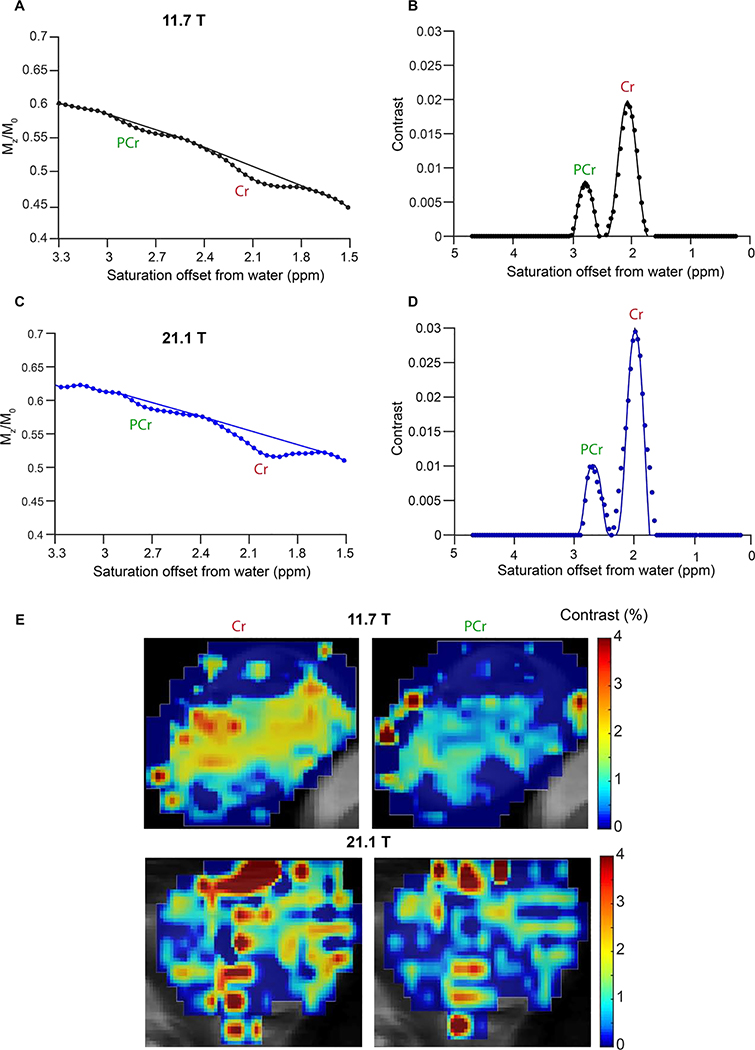
Next, we moved to determining whether we could quantify the amount of these compounds in tissue using CEST imaging in healthy C57Bl/6 mice. Based on in vivo monitoring of [4-13C] creatine injected mice, muscle possesses ~22.5 mM PCr and ~7.5 mM Cr, fairly large concentrations of these metabolites which should render these detectable by CEST imaging (12). Fig. 5 shows CEST MRI Z-spectra acquired on calf muscle at both 11.7 T (Fig. 5A) and 21.1 T (Fig. 5C), revealing two separate peaks for Cr and PCr at both field strengths. In order to quantify the concentrations, we fit the baseline around these two peaks to a 3rd order polynomial and subtracted this from the Z-spectra similar to the three point analysis performed by Tao and colleagues (55). Fig. 5B, D displays the resulting processed experimental data with Cr displaying a stronger signal than PCr at both 11.7 and 21.1 T despite the 3 fold lower concentration in tissue from previous studies(12). As is also seen, at 21.1 T there was enhanced resolution of the two peaks as expected. Based on fitting this data to numerical solutions to five pool Bloch equations using the kex determined in Fig. 3, we estimated the concentrations of Cr and PCr to be 10.8 mM, 19.5 mM (11.7T) and 10.7 mM, 15.8 mM (21.1T) with all parameters listed in Table 2 which is in excellent accord with the literature (12). As is also seen in Fig. 5, we were able to achieve a good fit (rmsd < 0.002) of the experimental muscle data at both 11.7T and 21.1T. Finally, we can also produce pixelwise contrast/difference maps at both PCr and Cr (Fig. 5E,F) frequencies revealing relatively homogeneous distribution of both through muscle tissue. If the muscle pH were to drop from 7.1 to 6.5, an error in concentration would be observed (Table 2) with measurements ranging from 9.6 to 17 mM for Cr and from 16 to 40 mM for PCr, however based on the literature we don’t expect muscle pH to go below 7.0 during exercise (56). These results show excellent agreement between prior [413C] creatine measurements (12) and CEST MRI, and excellent detection of Cr and PCr prior to supplementation.
Figure 5.
Comparison between 11.7 T and 21.1 T muscle CEST contrast of Cr and PCr. A) Z-spectrum from an ROI drawn over the muscle of a mouse, CEST data was acquired at B1= 1.5 μT on 11.7 T scanner. A polynomial data generated by removing the CEST contrast was fit to separate the CEST contrast of Cr and PCr; B) The difference spectrum displayed between 0 and 5 ppm of saturation offsets. This difference spectrum was obtained by subtracting from the experimental Z-spectrum a 3rd order polynomial fit to the experimental spectrum minus the PCr and Cr peaks. to determine the Cr and PCr concentrations in mouse muscle which were 10.8 mM and 19.5 mM respectively; C) Z-spectrum from an ROI drawn over the muscle of a mouse with CEST data acquired at B1=1.5 μT on 21.1 T scanner; D) Difference spectrum obtained by subtracting from the experimental Z-spectrum a 3rd order polynomial fit to the experimental spectrum minus the PCr and Cr peaks. The concentrations of Cr and PCr in muscle were found to be 10.7 and 15.8 mM respectively based on Bloch simulation fittings of the 21.1 T data; E) 11.7 and 21.1 T CEST contrast maps at Cr and PCr frequencies for a single calf muscle.
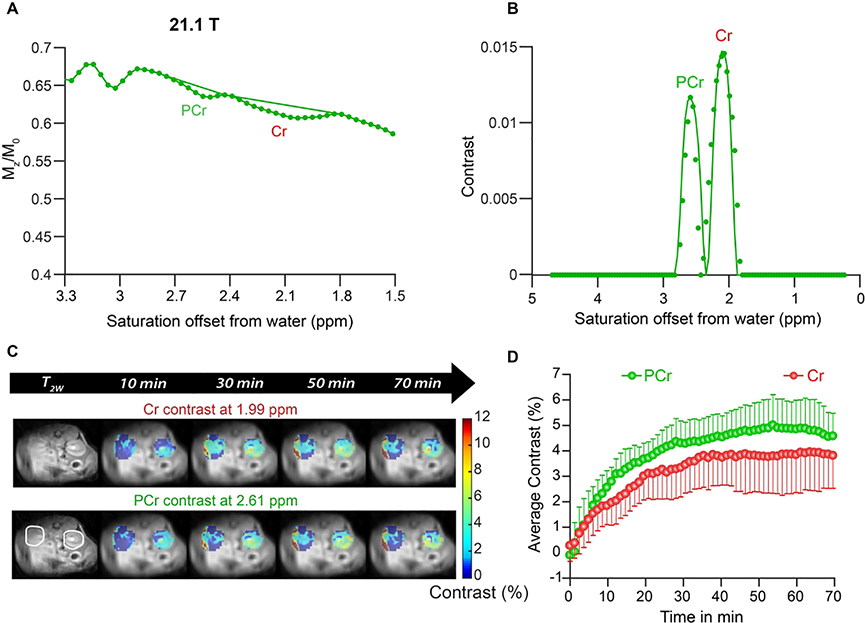
Finally, we tested how well we could detect creatine signal in the kidney and changes in these signals after PCr supplementation (Fig. 6). As is shown, the kidney also displays pronounced signals at PCr and Cr resonances, although these are broader than found in calf muscle (Fig. 6A,B) which may be due to different metabolites such as glutamine, alanine and ammonia contributing to these resonances (23,57). Again the fits of these two peaks using the parameters in Table 2 displayed excellent agreement with the experiments. We injected 325 mM PCr in saline into the tail vein of mice and monitored the PCr and Cr signals with time (Fig. 6C,D) by subtracting the pre-injection images at these offsets to remove the potential impact of additional metabolites. Peak contrast of ~4.6% was achieved earlier for PCr (~30 min) than for Cr (~3.8% at 40 min) in the medulla as is shown. Based on comparing with pre-injection and using the parameters in Table 3, this represents an increase in PCr of ~187 mM and increase of in Cr of ~23 mM.
Figure 6.
In vivo kidney CEST experiments on 21.1 T scanner on mice. A) Z-spectrum and 3rd order polynomial prior to PCr injection for an ROI drawn over the cortex of the kidney which avoided the collecting system. Cr and PCr contrast can be seen clearly at 1.99 and 2.61 ppm respectively; B) The difference spectrum calculated by subtracting the experimental Z-spectrum and the polynomial with experimental data (symbols) and fit (solid lines) to obtain the in vivo Cr and PCr concentrations= 6 mM and 22.5 mM respectively; C) In vivo kidney dynamic CEST contrast images overlaid on high resolution T2W images at 21.1 T after administering 325 mM PCr. ST = 1-Mz/M0 at 1.9 and 2.6 ppm corresponds to Cr and PCr frequencies; D) Average CEST contrast of Cr and PCr after administering PCr to healthy mice [n=6] and acquiring in vivo CEST kidney data at 21.1 T. Both Cr and PCr uptake peaked between 20 and 40 min.
Table 3:
Bloch simulation parameters for kidney Cr and PCr concentration calculations on 21.T scanner
| Kidney cortex | 21.1 T scanner | |||
|---|---|---|---|---|
| Parameter | Water | MT pool (A) | Cr pool (B) | PCr pool (C) |
| Δω (ppm) | 0 | 0 | 1.9 | 2.6 |
| R1 (s−1) | 1/3.1 | 1 | 1 | 1 |
| R2 (s−1) | 1/0.028 | 1/15×10−6 | 1/0.5 | 1/0.5 |
| Mol (mM) | - | 11.7 | 8.9 | 47.9 |
| kex (/sec) | - | 21 | 143 | 55 |
Discussion
In this study, we have tested the field dependence of CEST MRI detection of PCr and Cr both from Bloch simulations and experiments up to 21.1 T. We have shown that it is possible to obtain the Cr and PCr concentrations directly from fitting the CEST Z-spectra using numerical solutions to the Bloch equations at multiple magnetic field strengths. This approach is different from that of previous studies by Balaban, Reddy, Vandsburger, Xu, Sun, Jin and colleagues who have been interested in detecting endogenous Cr and PCr concentrations at lower magnetic field strengths (23,29–31,58–61). While monitoring signal intensity or performing lorentzian line-shape fitting could also be used to quantify concentration, as we show use of Bloch simulation fits results in numbers which are in good accord with literature values in muscle tissue and furthermore has the advantage that these allow testing how the concentration measurements are impacted by changes in tissue pH. Our approach for isolating these peaks is similar to the three point analysis method used by Jin and colleagues (55), however it diverges in that we use more frequency points. Muscle pH has been measured to drop from pH 7.3 to 6.9 in the cases of hemorrhage, hypoxia, hypothermia and exercise and in the extreme case of limb ischemia when blood flow was clamped for 2 hrs the muscle pH dropped to pH 6.55 (56,62). Indeed, using 4 pool Bloch equation fits we found that if there was a significant hemorrhage or ischemia in the imaged region, the PCr concentration measurements would be substantially altered but Cr measurements would be not so impacted. Cr concentration measurements are less effected by pH compared to that of PCr because of the impact changes in kex have on contrast for kex ~ 500 s−1 compared to when kex ~ 100 s−1 which also results in larger errors for the fast kex measurements similar to what we have found previously (53) and is seen in Table 2. Based on this, our approach should result in a reasonable range of concentration errors of < 10% for Cr and <20% for PCr as long as there is not prolonged limb ischemia.
We have also investigated use of natural PCr, a nutritional supplement, as a CEST agent and that there is specific enhancement at both Cr and PCr frequencies after intravenous administration. We compared post-injection images to those acquired over 8 minutes pre-injection to deconvolute the influence of PCr supplementation from other factors, although it is possible that hydration status due to injection of fluid may have some impact. The impact of hydration between post-injection and pre-injection is expected to be minimal based on the volume injected although this wasn’t explicitly evaluated. Our methodology of collecting CEST spectra of the regions of interest and determining concentrations using Bloch equation fits is applicable to monitor a wide variety of creatine or phosphocreatine supplementation protocols in use. Based on our experiments, this CEST imaging based approach presents two main advantages over MRS: 1) CEST doesn’t require isotopic enrichment of the supplement for discriminating between Cr and PCr in the spectra; 2) saturation transfer results in significant amplification factors (160 and higher) as shown experimentally at 11.7 T and 21.1 T to improve detection. Other investigators have reported ~5–500% changes in total creatine tissue concentrations through supplementation with the amount varying depending on dosage, frequency and tissue type (63) and we expect that changes in tissue Cr and PCr concentrations on that order could be spatially visualized and accurately quantified using our methods without need for invasive biopsy. As we show, the amplification of the signal is dependent on the magnetic field used for imaging, with higher fields allowing larger amplification factors while maintaining Cr and PCr peak resolution. We believe this approach should allow improvements in creatine supplementation protocols through direct monitoring of metabolite concentrations and quantification of creatine metabolism in response to muscle usage.
Conclusion
In conclusion, we have demonstrated that CEST detection of Cr and PCr is fairly robust over a wide range of experimental conditions and magnetic field strengths and that Bloch simulations allow robust quantification of these important compounds in phosphate storage. Improvements were seen using ultra high field magnets in terms of contrast (~33 %, 20% increase in Cr, PCr contrast respectively) and s in spectral resolution.
Highlights.
Natural creatine (Cr) and phosphocreatine (PCr) can be detected via Chemical Exchange Saturation Transfer (CEST) MRI with signal enhancement factors of 160 and higher.
Ultra high field up to 21.1 T show increased CEST contrast for Cr and PCr.
The Cr and PCr signals can be distinguished in living subjects and their concentrations determined from fitting CEST Z-spectra using numerical solutions to the Bloch equations.
Intravenous PCr supplementation increased the level of PCr and Cr in kidneys with peak contrast for PCr occurring at 30 minutes after injection.
Acknowledgements
This work was supported in part by P41EB024495. The National High Magnetic Field Laboratory is supported by the National Science Foundation through NSF/DMR1157490/1644779 and the State of Florida.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wyss M, Kaddurah-Daouk R. Creatine and Creatinine Metabolism. Physiological Reviews 2000;80(3):1107–1213. [DOI] [PubMed] [Google Scholar]
- 2.Kreider RB, Kalman DS, Antonio J, Ziegenfuss TN, Wildman R, Collins R, Candow DG, Kleiner SM, Almada AL, Lopez HL. International Society of Sports Nutrition position stand: safety and efficacy of creatine supplementation in exercise, sport, and medicine. Journal of the International Society of Sports Nutrition 2017;14(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tokish JM, Kocher MS, Hawkins RJ. Ergogenic aids: A review of basic science, performance, side effects, and status in sports. Am J Sports Med 2004;32(6):1543–1553. [DOI] [PubMed] [Google Scholar]
- 4.Olson D, Sikka RS, Labounty A, Christensen T. Injuries in Professional Football: Current Concepts. Curr Sport Med Rep 2013;12(6):381–390. [DOI] [PubMed] [Google Scholar]
- 5.Cisowski M, Bochenek A, Kucewicz E, Wnuk-Wojnar AM, Morawski W, Skalski J, Grzybek H. The use of exogenous creatine phosphate for myocardial protection in patients undergoing coronary artery bypass surgery. The Journal of cardiovascular surgery 1996;37(6 Suppl 1):75–80. [PubMed] [Google Scholar]
- 6.Guo-han C, Jian-hua G, Xuan H, Jinyi W, Rong L, Zhong-min L. Role of creatine phosphate as a myoprotective agent during coronary artery bypass graft in elderly patients. Coronary artery disease 2013;24(1):48–53. [DOI] [PubMed] [Google Scholar]
- 7.Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev 2003;17(5):545–580. [DOI] [PubMed] [Google Scholar]
- 8.Bottomley PA, Weiss RG. Non-invasive magnetic-resonance detection of creatine depletion in non-viable infarcted myocardium. Lancet (London, England) 1998;351(9104):714–718. [DOI] [PubMed] [Google Scholar]
- 9.Beer M, Seyfarth T, Sandstede J, Landschutz W, Lipke C, Kostler H, von Kienlin M, Harre K, Hahn D, Neubauer S. Absolute concentrations of high-energy phosphate metabolites in normal, hypertrophied, and failing human myocardium measured noninvasively with (31)P-SLOOP magnetic resonance spectroscopy. Journal of the American College of Cardiology 2002;40(7):1267–1274. [DOI] [PubMed] [Google Scholar]
- 10.Neubauer S, Beer M, Landschutz W, Sandstede J, Seyfarth T, Lipke C, Kostler H, Pabst TW, Meininger M, von Kienlin M, Horn M, Harre K, Hahn D. Absolute quantification of high energy phosphate metabolites in normal, hypertrophied and failing human myocardium. Magma (New York, NY) 2000;11(1–2):73–74. [DOI] [PubMed] [Google Scholar]
- 11.Nakae I, Mitsunami K, Omura T, Yabe T, Tsutamoto T, Matsuo S, Takahashi M, Morikawa S, Inubushi T, Nakamura Y, Kinoshita M, Horie M. Proton magnetic resonance spectroscopy can detect creatine depletion associated with the progression of heart failure in cardiomyopathy. Journal of the American College of Cardiology 2003;42(9):1587–1593. [DOI] [PubMed] [Google Scholar]
- 12.in ‘t Zandt HJ, de Groof AJ, Renema WK, Oerlemans FT, Klomp DW, Wieringa B, Heerschap A. Presence of (phospho)creatine in developing and adult skeletal muscle of mice without mitochondrial and cytosolic muscle creatine kinase isoforms. The Journal of physiology 2003;548(Pt 3):847–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Öz G, Alger JR, Barker PB, Bartha R, Bizzi A, Boesch C, Bolan PJ, Brindle KM, Cudalbu C, Dinçer A, Dydak U, Emir UE, Frahm J, González RG, Gruber S, Gruetter R, Gupta RK, Heerschap A, Henning A, Hetherington HP, Howe FA, Hüppi PS, Hurd RE, Kantarci K, Klomp DWJ, Kreis R, Kruiskamp MJ, Leach MO, Lin AP, Luijten PR, Marjańska M, Maudsley AA, Meyerhoff DJ, Mountford CE, Nelson SJ, Pamir MN, Pan JW, Peet AC, Poptani H, Posse S, Pouwels PJW, Ratai E-M, Ross BD, Scheenen TWJ, Schuster C, Smith ICP, Soher BJ, Tkáč I, Vigneron DB, Kauppinen RA. Clinical Proton MR Spectroscopy in Central Nervous System Disorders. Radiology 2014;270(3):658–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalia V, Leung DG, Sneag DB, Del Grande F, Carrino JA. Advanced MRI Techniques for Muscle Imaging. Semin Musculoskelet Radiol 2017;21(4):459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMahon MT, Gilad AA, Bulte JWM, van Zijl PCM. Chemical Exchange Saturation Transfer Imaging: Advances and Applications. Singapore: Pan Stanford Publishing; 2017. 479 p. [Google Scholar]
- 16.Liu G, Song X, Chan KW, McMahon MT. Nuts and bolts of chemical exchange saturation transfer MRI. NMR in Biomedicine 2013;26(7):810–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherry AD, Woods M. Chemical exchange saturation transfer contrast agents for magnetic resonance imaging. Annu Rev Biomed Eng 2008;10:391–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vinogradov E, Sherry AD, Lenkinski RE. CEST: From basic principles to applications, challenges and opportunities. Journal of Magnetic Resonance 2013;229:155–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaiss M, Bachert P. Chemical exchange saturation transfer (CEST) and MRZ-spectroscopyin vivo: a review of theoretical approaches and methods. Physics in Medicine and Biology 2013;58(22):R221–R269. [DOI] [PubMed] [Google Scholar]
- 20.Terreno E, Castelli DD, Aime S. Encoding the frequency dependence in MRI contrast media: the emerging class of CEST agents. Contrast Media & Molecular Imaging 2010;5(2):78–98. [DOI] [PubMed] [Google Scholar]
- 21.Jones KM, Pollard AC, Pagel MD. Clinical applications of chemical exchange saturation transfer (CEST) MRI. Journal of Magnetic Resonance Imaging 2018;47(1):11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward KM, Aletras AH, Balaban RS. A New Class of Contrast Agents for MRI Based on Proton Chemical Exchange Dependent Saturation Transfer (CEST). Journal of Magnetic Resonance 2000;143(1):79–87. [DOI] [PubMed] [Google Scholar]
- 23.Guivel-Scharen V, Sinnwell T, Wolff SD, Balaban RS. Detection of Proton Chemical Exchange between Metabolites and Water in Biological Tissues. Journal of Magnetic Resonance 1998;133(1):36–45. [DOI] [PubMed] [Google Scholar]
- 24.Shin SH, Wendland MF, Zhang B, Tran A, Tang A, Vandsburger MH. Noninvasive imaging of renal urea handling by CEST-MRI. Magnetic Resonance in Medicine 2019;0(0). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan KWY, McMahon MT, Kato Y, Liu G, Bulte JWM, Bhujwalla ZM, Artemov D, van Zijl PCM. Natural D-glucose as a biodegradable MRI contrast agent for detecting cancer. Magnetic Resonance in Medicine 2012;68(6):1764–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker-Samuel S, Ramasawmy R, Torrealdea F, Rega M, Rajkumar V, Johnson SP, Richardson S, Gonçalves M, Parkes HG, Årstad E, Thomas DL, Pedley RB, Lythgoe MF, Golay X. In vivo imaging of glucose uptake and metabolism in tumors. Nature Medicine 2013;19:1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin T, Kim S-G. Advantages of chemical exchange-sensitive spin-lock (CESL) over chemical exchange saturation transfer (CEST) for hydroxyl– and amine–water proton exchange studies. NMR in Biomedicine 2014;27(11):1313–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kogan F, Singh A, Debrosse C, Haris M, Cai K, Nanga RP, Elliott M, Hariharan H, Reddy R. Imaging of glutamate in the spinal cord using GluCEST. Neuroimage 2013;77:262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kogan F, Haris M, Singh A, Cai K, Debrosse C, Nanga RP, Hariharan H, Reddy R. Method for high-resolution imaging of creatine in vivo using chemical exchange saturation transfer. Magn Reson Med 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pumphrey A, Yang Z, Ye S, Powell DK, Thalman S, Watt DS, Abdel-Latif A, Unrine J, Thompson K, Fornwalt B, Ferrauto G, Vandsburger M. Advanced cardiac chemical exchange saturation transfer (cardioCEST) MRI for in vivo cell tracking and metabolic imaging. NMR in Biomedicine 2016;29(1):74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L, Barker PB, Weiss RG, van Zijl PCM, Xu J. Creatine and phosphocreatine mapping of mouse skeletal muscle by a polynomial and Lorentzian line-shape fitting CEST method. Magnetic Resonance in Medicine 2019;81(1):69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ling W, Regatte RR, Navon G, Jerschow A. Assessment of glycosaminoglycan concentration <em>in vivo</em> by chemical exchange-dependent saturation transfer (gagCEST). Proceedings of the National Academy of Sciences 2008;105(7):2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh A, Haris M, Cai K, Kassey VB, Kogan F, Reddy D, Hariharan H, Reddy R. Chemical exchange saturation transfer magnetic resonance imaging of human knee cartilage at 3 T and 7 T. Magnetic Resonance in Medicine 2012;68(2):588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan KW, Yu T, Qiao Y, Liu Q, Yang M, Patel H, Liu G, Kinzler KW, Vogelstein B, Bulte JW, van Zijl PC, Hanes J, Zhou S, McMahon MT. A diaCEST MRI approach for monitoring liposomal accumulation in tumors. Journal of controlled release : official journal of the Controlled Release Society 2014;180:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu T, Chan KW, Anonuevo A, Song X, Schuster BS, Chattopadhyay S, Xu Q, Oskolkov N, Patel H, Ensign LM, van Zjil PC, McMahon MT, Hanes J. Liposome-based mucus-penetrating particles (MPP) for mucosal theranostics: demonstration of diamagnetic chemical exchange saturation transfer (diaCEST) magnetic resonance imaging (MRI). Nanomedicine : nanotechnology, biology, and medicine 2015;11(2):401–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Longo DL, Dastrù W, Digilio G, Keupp J, Langereis S, Lanzardo S, Prestigio S, Steinbach O, Terreno E, Uggeri F. Iopamidol as a responsive MRI‐chemical exchange saturation transfer contrast agent for pH mapping of kidneys: In vivo studies in mice at 7 T. Magnetic resonance in medicine 2011;65(1):202–211. [DOI] [PubMed] [Google Scholar]
- 37.Chen LQ, Howison CM, Jeffery JJ, Robey IF, Kuo PH, Pagel MD. Evaluations of extracellular pH within in vivo tumors using acidoCEST MRI. Magnetic resonance in medicine 2014;72(5):1408–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun PZ, Longo DL, Hu W, Xiao G, Wu RH. Quantification of iopamidol multisite chemical exchange properties for ratiometric chemical exchange saturation transfer (CEST) imaging of pH. Physics in Medicine and Biology 2014;59(16):4493–4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu RH, Longo DL, Aime S, Sun PZ. Quantitative description of radiofrequency (RF) power-based ratiometric chemical exchange saturation transfer (CEST) pH imaging. Nmr in Biomedicine 2015;28(5):555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bar-Shir A, Liu GS, Liang YJ, Yadav NN, McMahon MT, Walczak P, Nimmagadda S, Pomper MG, Tallman KA, Greenberg MM, van Zijl PCM, Bulte JWM, Gilad AA. Transforming Thymidine into a Magnetic Resonance Imaging Probe for Monitoring Gene Expression. J Am Chem Soc 2013;135(4):1617–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang X, Song X, Li Y, Liu G, Ray Banerjee S, Pomper MG, McMahon MT. Salicylic Acid and Analogues as diaCEST MRI Contrast Agents with Highly Shifted Exchangeable Proton Frequencies. Angewandte Chemie International Edition 2013;52(31):8116–8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song X, Yang X, Ray Banerjee S, Pomper MG, McMahon MT. Anthranilic acid analogs as diamagnetic CEST MRI contrast agents that feature an intramolecular-bond shifted hydrogen. Contrast Media & Molecular Imaging 2015;10(1):74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang X, Song X, Ray Banerjee S, Li Y, Byun Y, Liu G, Bhujwalla ZM, Pomper MG, McMahon MT. Developing imidazoles as CEST MRI pH sensors. Contrast Media & Molecular Imaging 2016;11(4):304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X, Yuan Y, Li S, Zeng Q, Guo Q, Liu N, Yang M, Yang Y, Liu M, McMahon MT, Zhou X. Free-base porphyrins as CEST MRI contrast agents with highly upfield shifted labile protons. Magnetic Resonance in Medicine 2019;82(2):577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bar-Shir A, Liu G, Chan KWY, Oskolkov N, Song X, Yadav NN, Walczak P, McMahon MT, van Zijl PCM, Bulte JWM, Gilad AA. Human Protamine-1 as an MRI Reporter Gene Based on Chemical Exchange. ACS Chemical Biology 2014;9(1):134–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gilad AA, McMahon MT, Walczak P, Winnard PT, Raman V, van Laarhoven HWM, Skoglund CM, Bulte JWM, van Zijl PCM. Artificial reporter gene providing MRI contrast based on proton exchange. Nature Biotechnology 2007;25(2):217–219. [DOI] [PubMed] [Google Scholar]
- 47.McMahon MT, Gilad AA, DeLiso MA, Cromer Berman SM, Bulte JWM, van Zijl PCM. New “multicolor” polypeptide diamagnetic chemical exchange saturation transfer (DIACEST) contrast agents for MRI. Magnetic Resonance in Medicine 2008;60(4):803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farrar CT, Buhrman JS, Liu G, Kleijn A, Lamfers MLM, McMahon MT, Gilad AA, Fulci G. Establishing the Lysine-rich Protein CEST Reporter Gene as a CEST MR Imaging Detector for Oncolytic Virotherapy. Radiology 2015;275(3):746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fu R, Brey WW, Shetty K, Gor’kov P, Saha S, Long JR, Grant SC, Chekmenev EY, Hu J, Gan Z, Sharma M, Zhang F, Logan TM, Brüschweller R, Edison A, Blue A, Dixon IR, Markiewicz WD, Cross TA. Ultra-wide bore 900MHz high-resolution NMR at the National High Magnetic Field Laboratory. Journal of Magnetic Resonance 2005;177(1):1–8. [DOI] [PubMed] [Google Scholar]
- 50.Liu G, Gilad AA, Bulte JW, van Zijl PC, McMahon MT. High-throughput screening of chemical exchange saturation transfer MR contrast agents. Contrast Media Mol Imaging 2010;5(3):162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim M, Gillen J, Landman B, Zhou J, van Zijl P. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magnetic Resonance in Medicine 2009;61(6):1441–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pavuluri K, Manoli I, Pass A, Li Y, Vernon HJ, Venditti CP, McMahon MT. Noninvasive monitoring of chronic kidney disease using pH and perfusion imaging. Science Advances 2019;5(8):eaaw8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McMahon MT, Gilad AA, Zhou J, Sun PZ, Bulte JW, van Zijl PC. Quantifying exchange rates in chemical exchange saturation transfer agents using the saturation time and saturation power dependencies of the magnetization transfer effect on the magnetic resonance imaging signal (QUEST and QUESP): Ph calibration for poly-L-lysine and a starburst dendrimer. Magn Reson Med 2006;55(4):836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goffeney N, Bulte JW, Duyn J, Bryant LH Jr., van Zijl PC. Sensitive NMR detection of cationic-polymer-based gene delivery systems using saturation transfer via proton exchange. J Am Chem Soc 2001;123(35):8628–8629. [DOI] [PubMed] [Google Scholar]
- 55.Jin T, Wang P, Zong X, Kim S-G. MR imaging of the amide-proton transfer effect and the pH-insensitive nuclear overhauser effect at 9.4 T. Magnetic Resonance in Medicine 2013;69(3):760–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Street D, Bangsbo J, Juel C. Interstitial pH in human skeletal muscle during and after dynamic graded exercise. The Journal of physiology 2001;537(Pt 3):993–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stabinska J, Neudecker P, Ljimani A, Wittsack HJ, Lanzman RS, Muller-Lutz A. Proton exchange in aqueous urea solutions measured by water-exchange (WEX) NMR spectroscopy and chemical exchange saturation transfer (CEST) imaging in vitro. Magn Reson Med 2019;82(3):935–947. [DOI] [PubMed] [Google Scholar]
- 58.Haris M, Singh A, Cai K, Kogan F, McGarvey J, Debrosse C, Zsido GA, Witschey WR, Koomalsingh K, Pilla JJ, Chirinos JA, Ferrari VA, Gorman JH, Hariharan H, Gorman RC, Reddy R. A technique for in vivo mapping of myocardial creatine kinase metabolism. Nat Med 2014;20(2):209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DeBrosse C, Nanga RPR, Wilson N, D’Aquilla K, Elliott M, Hariharan H, Yan F, Wade K, Nguyen S, Worsley D, Parris-Skeete C, McCormick E, Xiao R, Cunningham ZZ, Fishbein L, Nathanson KL, Lynch DR, Stallings VA, Yudkoff M, Falk MJ, Reddy R, McCormack SE. Muscle oxidative phosphorylation quantitation using creatine chemical exchange saturation transfer (CrCEST) MRI in mitochondrial disorders. JCI insight 2016;1(18):e88207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun PZ, Wang Y, Xiao G, Wu RH. Simultaneous experimental determination of labile proton fraction ratio and exchange rate with irradiation radio frequency power-dependent quantitative CEST MRI analysis. Contrast Media & Molecular Imaging 2013;8(3):246–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chung JJ, Jin T, Lee JH, Kim SG. Chemical exchange saturation transfer imaging of phosphocreatine in the muscle. Magnetic Resonance in Medicine 2019;81(6):3476–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Filler RM, Das JB, Haase GM, Donahoe PK. Muscle surface pH as a monitor of tissue perfusion and acid-base status. Journal of Pediatric Surgery 1971;6(5):535–542. [DOI] [PubMed] [Google Scholar]
- 63.Ipsiroglu OS, Stromberger C, Ilas J, Hoger H, Muhl A, Stockler-Ipsiroglu S. Changes of tissue creatine concentrations upon oral supplementation of creatine-monohydrate in various animal species. Life sciences 2001;69(15):1805–1815. [DOI] [PubMed] [Google Scholar]