Abstract
Management of symptom-related distress is an important area of pediatric oncology nursing. Participants who attended the Children’s Oncology Group (COG) State of the Science Symposium on symptom distress completed an anonymous survey. The purpose was to explore participant perceptions of symptom distress experienced by children receiving cancer treatment on clinical trials, determine how symptom distress is currently assessed at COG institutions, and to identify what interventions are used to reduce symptom distress for these children. Among the 90 symposium attendees, 72% completed the survey, the majority (92%) of whom were nurses. The five most distressing symptoms in children with cancer enrolled on clinical trials identified by survey respondents were nausea/vomiting, fatigue, pain, anxiety, and sleep disturbances. Results from our survey also suggest that symptom distress may differ by disease type. For example, symptoms associated with leukemia/lymphoma included steroid side effects, procedural pain, and neuropathy. The majority of respondents (90%) also reported that symptoms go unrecognized by health care providers. The most commonly described unrecognized symptoms were behavioral (i.e., sadness, anxiety, fear, depression, and emotional needs; 45%) and fatigue (19%). Key focus areas reported by respondents included informal and inconsistent symptom assessment, the need for uniform measurement tools, and improved documentation of symptom-related distress. Management of symptom-related distress is an important aspect of pediatric oncology nursing. Further exploration of symptom distress experienced by children with specific types of cancers, and the development of standardized symptom assessment processes, will provide a foundation for developing future interventions aimed at alleviating symptom-related distress.
Keywords: symptom science, symptom distress, Children’s Oncology Group, survey
Introduction
Aggressive clinical trial regimens with curative intent are associated with symptom-related distress. Symptom distress is an individual’s perception and response to symptoms that cause discomfort. Children undergoing treatment for cancer may experience side effects on a daily basis and often live with unrelieved symptoms (Hockenberry & Hooke, 2007). In an effort to provide the most effective therapy, less focus may be placed on symptom-related distress. If symptom management is inadequate, this can lead to unnecessary suffering (Kestler & LoBiondo-Wood, 2012).
An understanding of symptom distress experienced by children with cancer enrolled on clinical trials is foundational to the work of the Children’s Oncology Group (COG) Nursing Discipline, which has focused its research agenda on key knowledge gaps that include understanding illness-related distress (Landier, Leonard, & Ruccione, 2013). This focus is aligned with the organizing framework for the COG Nursing Discipline, which aims to understand how children and their families sustain or regain optimal health in the context of serious illness, and is based on “doing well” concepts, such as resilience, quality of life, courageous coping, and hope (Kelly, Hooke, Ruccione, Landier, & Haase, 2014).
A State of the Science (SOS) Symposium was held the Fall of 2018 to explore major symptoms that cause distress during childhood cancer treatment. This article presents the results of a survey completed by participants during the SOS Symposium. The purpose of the survey was to explore participant perceptions of symptom distress experienced by children receiving cancer treatment on clinical trials, to determine how symptom distress is currently assessed at COG institutions, and to identify what interventions are used to reduce symptom distress for these children.
Method
Sample
Attendees at the COG Nursing State of the Science Symposium were invited to voluntarily participate in an open-ended survey during the symposium.
Instrument
The survey was developed by a panel of pediatric oncology nurses with expertise in symptom distress. The questions were evaluated by the State of the Science Symposium Steering Committee. Survey responses were anonymous and consisted of free-text responses to questions examining symptom distress experienced by pediatric oncology patients. Questions included the following: (1) From your perspective, list the five most distressing symptoms experienced by children with cancer receiving treatment on clinical trials. (2) Are there unique symptoms that are related to the child’s diagnosis and its related treatment? List the top three symptoms uniquely related to each diagnosis. (3) Do you think there are symptoms that go unrecognized by health care providers? If yes, please list them. (4) How is symptom distress assessed in your clinical setting? (5) What are the most common interventions used for symptom distress in your clinical setting? For this question, participants were asked to list and rate the effectiveness of the interventions used in their clinical setting to address symptom distress on a scale of 0 to 7 (0 being not effective, and 7 being extremely effective). Participant perceptions of the most important areas for symptom science research were also explored using open-ended questions. Surveys were returned to a collection box during the Symposium.
Data Analysis
We used a mixed methods approach for this analysis. For the initial qualitative portion of the analysis, the data were organized, evaluated, and prepared for review using the process outlined by Creswell and Creswell (2018). Responses to each question were analyzed separately. Extraction of themes from the completed surveys and coding of categories was conducted per the process described by Rossman and Rallis (2017). Responses were coded by bracketing chunks of text into broad categories and then labeling the categories, using words described by participants. All identified topics were entered into the data spreadsheet, and similar responses were clustered together. Categories were subsequently created by analyzing and summarizing clustered responses. Survey responses were coded by three independent members of the State of the Science Symposium Committee and categorized into themes. For the quantitative portion of the analysis, descriptive statistics were calculated using IBM SPSS Statistics, version 24.0. Findings from both the qualitative and quantitative data strands were then merged in the final analyses (Creswell & Creswell, 2018).
Results
Sixty-five of the 90 attendees completed the entire survey (response rate = 72%). The large majority (92%) of respondents were nurses (registered nurse = 24; advanced practice registered nurse = 36). The remaining 8% of participants identified themselves as physician (1), parent/caregiver (3), and pharmacist (1). Demographic data regarding the participants (other than role) were not collected. The majority of respondents identified themselves as experts in all childhood cancer disease groups.
Symptom-Related Distress
Respondents described the five most distressing symptoms experienced by children with cancer receiving treatment on clinical trials. Figure 1 shows the most frequently reported symptoms causing distress. All respondents identified nausea and vomiting as one of the most distressing symptoms. The large majority (91%) reported fatigue and pain as distressing symptoms. Almost half (46%) of respondents specifically reported anxiety as a distressing symptom. Additional distressing symptoms identified included depression, fear, and mood changes, accounting for 40% of the responses. Sadness was also identified as a distinct symptom causing distress, described by 27% of respondents. Additional distressing symptoms included problems with nutrition, neuropathy, alopecia, constipation, and fever.
Figure 1.
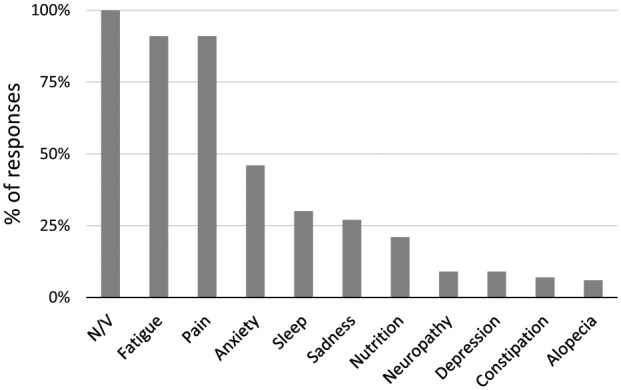
Most distressing symptoms. Most frequently reported symptoms elicited from respondents who were asked to list the five most distressing symptoms in children undergoing cancer therapy on clinical trials.
Respondents were also asked to identify specific symptoms that cause distress in patients receiving treatment for leukemia/lymphoma, solid tumors, or central nervous system (CNS) tumors. Table 1 depicts the three most commonly reported symptoms causing distress for each cancer group. The most commonly reported symptoms for leukemia and lymphoma were fatigue (59%), nausea/vomiting (53%), and pain (40%). Distressing symptoms reported uniquely for the leukemia and lymphoma disease group were constipation and procedural pain. The most common symptoms reported for children with solid tumors were pain (85%), nausea/vomiting (76%), and fatigue (32%). Distressing symptoms reported uniquely for solid tumors included delayed nausea/vomiting, hearing loss, and radiation-associated symptoms. Common symptoms in children with CNS tumors included problems with cognitive function (58%), nausea/vomiting (52%), and fatigue (44%). Distinct symptoms related to CNS tumors included headaches and problems with cognitive functioning.
Table 1.
Most Frequently Reported Disease-Specific Distressing Symptoms.
| Symptom | Leukemia (N = 58) | Solid tumor (N = 62) | CNS (N = 62) |
|---|---|---|---|
| Fatigue | 59% | 32% | 44% |
| Nausea/vomiting | 53% | 76% | 52% |
| Pain | 40% | 85% | 21% |
| Anxiety | 21% | 10% | 19% |
| Steroids | 17% | 0% | 2% |
| Procedural pain | 14% | 0% | 0% |
| Constipation | 12% | 0% | 0% |
| Neuropathy | 10% | 6% | 0% |
| Nutrition | 9% | 8% | 10% |
| Sleep | 7% | 2% | 13% |
| Blood counts | 5% | 0% | 0% |
| Other | 5% | 11% | 8% |
| Alopecia | 2% | 2% | 2% |
| Headache | 0% | 0% | 19% |
| Physical function | 0% | 3% | 19% |
| Depression/fear | 0% | 8% | 13% |
| Cognitive function | 0% | 0% | 58% |
| Sadness | 0% | 11% | 2% |
Note. CNS = central nervous system.
Unrecognized Symptoms
Unrecognized distressing symptoms were reported by 90% of respondents. Figure 2 shows the reported unrecognized distressing symptoms. The most commonly described unrecognized symptoms were behavioral (i.e., sadness, anxiety, fear, depression, and emotional needs; 45%) and fatigue (19%). Of note, sadness and anxiety each accounted for 10% of the reported unrecognized symptoms. Examples of unrecognized “other” symptoms included nutritional deficits, hearing loss, and physical function.
Figure 2.
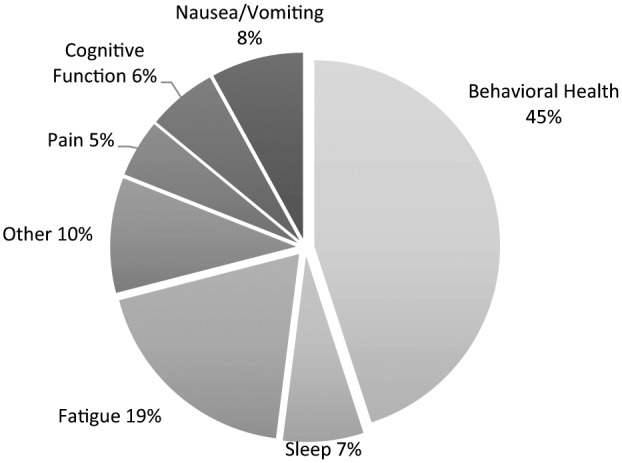
Unrecognized symptoms. Most commonly reported unrecognized distressing symptoms.
Symptom Distress Assessment
Participants described how symptom distress is assessed in their clinical setting. There were 95 responses from 60 participants. The most commonly reportedly mechanisms for assessment were history and physical (n = 29) followed by use of pain severity scales (n = 13). Common themes surrounding assessment included inconsistency and informal assessment in the clinical setting. Sixteen percent (n = 10) of respondents reported using some variation of a multidisciplinary, psychosocial, or palliative care approach to symptom assessment.
Symptom Distress Intervention and Effectiveness
Common symptoms that cause distress and interventions used in the clinical setting to address distressing symptoms were rated on an 8-point Likert-type scale (0 = not an effective intervention; 7 = extremely effective intervention). Table 2 shows the most commonly reported distressing symptoms with corresponding interventions, and median ratings for intervention effectiveness. Pain (including headaches, procedural pain, and neuropathic pain) was the most commonly reported symptom causing distress requiring intervention. The most highly rated interventions for pain were medications, management by pain/palliative care team, sleep/rest, and a combination of interventions, all of which received a median effectiveness rating of 6. The second most common symptom causing distress was nausea and vomiting; the most highly rated intervention for nausea and vomiting was medication (median rating = 5). Figure 3 summarizes the most common interventions and median ratings of intervention effectiveness. Engagement of the pain/palliative care team was the most effective intervention reported across symptoms, while sleep and rest were rated the least effective. “Other” reported interventions included; education, nutritional support, limiting screen time, and support/talking.
Table 2.
Most Commonly Reported Symptoms, Related Interventions, and Rating of Intervention Effectiveness (N = 65 Respondents).
| Symptom | Intervention | N | Median rating |
|---|---|---|---|
| Pain (N = 75) | Medication | 37 | 6.0 |
| Combination | 15 | 6.0 | |
| Palliative/pain team | 5 | 6.0 | |
| Sleep/rest | 1 | 6.0 | |
| Formal scale assessment | 4 | 5.5 | |
| Physical activity | 3 | 5.5 | |
| Complementary medicine | 2 | 3.5 | |
| Other | 6 | 3.0 | |
| Psychosocial services | 1 | None given | |
| Child life | 1 | None given | |
| N/V (N = 57) | Medication | 41 | 5.0 |
| Combination | 10 | 4.0 | |
| Complementary medicine | 5 | 3.0 | |
| Formal scale assessment | 1 | 3.0 | |
| Fatigue (N = 29) | Combination | 6 | 5.0 |
| Child life | 2 | 4.5 | |
| Physical activity | 7 | 4.0 | |
| Medication | 2 | 3.0 | |
| Sleep/rest | 5 | 2.0 | |
| Other | 2 | 1.0 | |
| Nothing | 3 | 0 | |
| Complementary medicine | 1 | None given | |
| Formal scale assessment | 1 | None given | |
| Anxiety (N = 19) | Complementary medicine | 1 | 6.0 |
| Child life | 5 | 4.0 | |
| Combination | 5 | 4.0 | |
| Psychosocial services | 4 | 4.0 | |
| Medication | 3 | 3.0 | |
| Other | 1 | 2.0 | |
| Sadness/depression (N = 18) | Other | 1 | 5.0 |
| Combination | 7 | 4.0 | |
| Psychosocial services | 7 | 3.0 | |
| Medication | 2 | 3.0 | |
| Nothing | 1 | 0 | |
| Nutritional (N = 15) | Nutrition | 5 | 6.0 |
| Combination | 5 | 4.5 | |
| Other | 1 | 4.0 | |
| Medication | 4 | 3.5 | |
| Sleep disturbance (N = 9) | Child life | 1 | 5.0 |
| Combination | 1 | 5.0 | |
| Medication | 3 | 2.0 | |
| Other | 3 | 1.0 | |
| Nothing | 1 | 0 | |
| Other symptoms (N = 9) | Combination | 2 | 5.0 |
| Physical activity | 1 | 5.0 | |
| Psychosocial services | 2 | 3.0 | |
| Nothing | 2 | 0 | |
| Child life | 1 | None given | |
| Formal scale assessment | 1 | None given | |
| GI (N = 8) | Medication | 5 | 5.0 |
| Combination | 3 | 2.0 |
Note. N/V = nausea/vomiting, GI = gastrointestinal. Rating on an 8-point Likert-type scale (0 = Least effective, 7 = Most effective).
Figure 3.
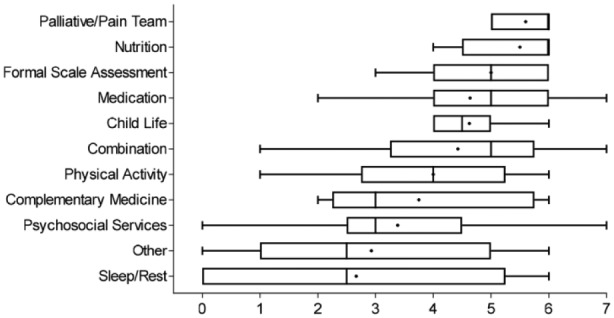
Boxplot of effectiveness ratings of common symptom interventions.
Note. Rating on an 8-point Likert-type scale (0 = Least effective, 7 = most effective). Line in center of each box is median. Mean rating is displayed by the dot in the box. The 25th percentile is the left side of each box. The 75th percentile is right side of box.
Symptom Focus Areas
Participants identified the most important areas for future research. A total of 81 responses were submitted and initial coding identified 22 separate themes. Analysis of responses revealed 4 primary and 18 secondary themes (Lincoln & Guba, 1985; Merriam, 1998).
Three interrelated themes constituted the primary responses from participants (43.5% of the total): (1) the need for consistent measurement tools across studies (20%), (2) improved assessment and documentation of identified symptoms using a common lexicon (13.5%), and (3) development of evidence-based interventions to manage identified symptoms (10%). Participants consistently noted that these three areas were closely related and that without standardized assessment tools, proper identification of symptoms and symptom clusters is not possible.
Symptom distress caused by fatigue was identified by participants (11%) as an area for continued research. Participants noted that while this symptom has been studied extensively in both the pediatric and adult oncology populations, assessment is still difficult and evidence-based management strategies are still lacking.
Discussion
From the perspective of the survey respondents, the five most distressing symptoms in children with cancer enrolled on clinical trials are nausea/vomiting, fatigue, pain, anxiety, and sleep disturbances. This is consistent with previous work examining symptom clusters (Baggott et al., 2011; Hockenberry & Hooke, 2007; Rodgers, Krance, Street, & Hockenberry, 2014). Pain, nausea/vomiting, and fatigue were the three most frequently reported distressing symptoms across disease groups. These findings are consistent with a previous study of more than 100 children and families describing the most commonly experienced symptoms as fatigue, pain, and nausea (Hedstrom, Haglund, Skolin, & von Essen, 2003).
Results from our survey suggest that symptom distress may differ by disease type. Symptoms associated with leukemia/lymphoma included procedural pain and constipation. Symptoms associated with solid tumors included delayed nausea/vomiting, hearing loss, and radiation-associated symptoms. Symptoms associated with CNS tumors included headaches and problems with cognitive function. These findings suggest that identification of symptoms profiles related to specific disease and treatment groups may be important in the development of targeted interventions and anticipatory guidance for different types of pediatric cancers, in order to prevent or minimize symptom-related distress.
Respondents from our study reported that symptoms often go unrecognized. The most underidentified symptoms reported were fatigue and behavioral symptoms (sadness, anxiety, fear, depression, emotional needs, and sleep disturbances). Fatigue is well documented in the literature as a frequent, distressing, and bothersome symptom in cancer patients (Rodgers, Hooke, Ward, & Linder, 2016; Tomlinson et al., 2019). A recent study of 104 children identified fatigue as negatively affecting health-related quality of life (Rosenberg et al., 2016), yet according to our survey respondents, fatigue is often unrecognized and underdiagnosed. Recent guidelines, endorsed by four national organizations, advocate for implementation of a more formal approach to assessment and treatment of cancer-related fatigue (Berger et al., 2015). Results of our study add to the evidence suggesting that adaptation of these guidelines for use in pediatric oncology is important to prevent continued unrecognized and undertreated fatigue.
Results from our study indicate that distressing symptoms are most routinely assessed by history and physical examination, or through use of formal pain scales. This is concerning, since formal pain scales do not evaluate myriad other symptoms children with cancer experience, and history and physical examinations are often brief and focused and may miss symptoms such as anxiety, sadness, and other emotional concerns. A common theme identified by our study was that symptoms are informally and inconsistently addressed. Involvement of multidisciplinary team members and/or the palliative care team in symptom assessment could potentially address this; however, only about 15% of our respondents indicated use of these teams. Interestingly, when asked to rate the effectiveness of interventions used to treat distressing symptoms, pain/palliative care teams were identified as most effective, regardless of the symptom requiring intervention. Palliative care has emerged in recent years as a valuable component of pediatric oncology care, with value not only at the end of life but throughout the course of therapy (Waldman & Wolfe, 2013). Palliative care offers multimodal approaches for managing a wide range of distressing symptoms. This holistic approach could be a reason for the reported effectiveness in our survey.
Additional common themes identified from our survey were the need for consistent evidence-based assessment and interventions for children with cancer suffering from treatment-related symptoms. Respondents reported that a standardized, multidisciplinary approach to assessment and intervention could lead to improved awareness and ultimately less symptom-related distress in these children. An understanding of factors that may predict symptoms, such as the disease-specific symptoms identified through this study, may contribute to prevention-based strategies in the future.
Limitations
The authors acknowledge some limitations of our survey that should be considered. First, the survey was conducted at a COG SOS symposium, the sample size was small, and participants were primarily nurses. These factors may have introduced bias and skewed the overall response. Surveying a group with more multidisciplinary representation, as well as the patient and parent perspectives, may have resulted in different outcomes. However, a strength of our study was that it drew from a group of nurses with expertise in pediatric oncology who were familiar with distressing symptoms experienced by this population. Thus, despite the limitations, our results provide important baseline information with regard to symptom distress assessment and interventions in pediatric oncology and identify areas on which to focus further research regarding symptom-related distress.
Conclusion
Management of symptom-related distress, both physiologic and emotional, is a key area of pediatric oncology nursing practice and has been a major focus area for nursing discipline within the Children’s Oncology Group over the past decade. Symptom science is also an area of high priority for the National Institute for Nursing Research and the National Cancer Advisory Board (Landier et al., 2013).
Attention must be given to the symptom experiences that occur during treatment in order to decrease distress for all children with cancer. Further research to develop unique symptom profiles for specific diseases, and the development of standardized symptom assessment processes, will provide a foundation for developing future interventions aimed at alleviating symptom-related distress experienced by children and adolescents undergoing cancer therapy.
Author Biographies
Micah A. Skeens, PhD, RN, CPNP, is a nurse scientist at Nationwide Children’s Hospital. Dr. Skeens has been an active member of the Children’s Oncology Group for 15 years, and currently serves as the Chair of the Nursing Clinical Trials Subcommittee. Her research focuses on adherence, supportive care and symptom experiences of children with cancer and those undergoing hematopoietic stem cell transplant.
Patsy Cullen, PhD, CPNP-PC, is assistant dean for Graduate Studies in the Loretto Heights School of Nursing at Regis University in Denver, Colorado. Dr. Cullen has been an investigator in the Children’s Oncology Group since 1985 and has served on the Nursing Discipline Committee and numerous CNS tumor trials. Her research focuses on neurocognitive late effects of treatment.
Joe Stanek, MS, is the biostatistician for the Division of Hematology, Oncology, and Bone Marrow Transplant at Nationwide Children’s Hospital. He aids investigators in the design and analysis of research projects ranging from prospective clinical trials to lab-based animal studies which have resulted in over 100 published abstracts and journal articles.
Marilyn Hockenberry, PhD, RN, CPNP, CPHON, FAAN, is a pediatric oncology nurse who has practiced as a nurse practitioner, educator and researcher for many years. Her research is focused on symptom experiences during childhood cancer treatment.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by National Cancer Institute R13CA232442 (PIs: Rodgers and Hockenberry) and National Clinical Trials Network (NCTN) Group Operations Centers Grant U10CA180886 (PI: Adamson).
ORCID iD: Micah A. Skeens  https://orcid.org/0000-0001-6786-8128
https://orcid.org/0000-0001-6786-8128
References
- Baggott C. R., Dodd M., Kennedy C., Marina N., Matthay K. K., Cooper B., Miaskowski C. (2011). An evaluation of the factors that affect the health-related quality of life of children following myelosuppressive chemotherapy. Supportive Care in Cancer, 19, 353-361. doi: 10.1007/s00520-010-0824-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A. M., Mooney K., Alvarez-Perez A., Breitbart W. S., Carpenter K. M., Cella D., . . . Smith C. (2015). Cancer-related fatigue, Version 2.2015. Journal of the National Comprehensive Cancer Network, 13, 1012-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell J. W., Creswell J. D. (2018). Research design: Qualitative, quantitative, and mixed methods approaches (5th ed.). Thousand Oaks, CA: Sage. [Google Scholar]
- Hedstrom M., Haglund K., Skolin I., von Essen L. (2003). Distressing events for children and adolescents with cancer: Child, parent, and nurse perceptions. Journal of Pediatric Oncology Nursing, 20, 120-132. doi: 10.1053/jpon.2003.76 [DOI] [PubMed] [Google Scholar]
- Hockenberry M., Hooke M. C. (2007). Symptom clusters in children with cancer. Seminars in Oncology Nursing, 23, 152-157. doi: 10.1016/j.soncn.2007.01.001 [DOI] [PubMed] [Google Scholar]
- Kelly K. P., Hooke M. C., Ruccione K., Landier W., Haase J. (2014). Children’s Oncology Group nursing research framework. Seminars in Oncology Nursing, 30, 17-25. doi: 10.1016/j.soncn.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestler S. A., LoBiondo-Wood G. (2012). Review of symptom experiences in children and adolescents with cancer. Cancer Nursing, 35(2), E31-E49. doi: 10.1097/NCC.0b013e3182207a2a [DOI] [PubMed] [Google Scholar]
- Landier W., Leonard M., Ruccione K. S. (2013). Children’s Oncology Group’s 2013 blueprint for research: Nursing discipline. Pediatric Blood & Cancer, 60, 1031-1036. doi: 10.1002/pbc.24415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln Y. S., Guba E. G. (1985). Naturalistic inquiry. Beverly Hills, CA: Sage. [Google Scholar]
- Merriam S. B. (1998). Qualitative research and case study applications in education. San Francisco, CA: Jossey-Bass. [Google Scholar]
- Rodgers C. C., Hooke M. C., Ward J., Linder L. A. (2016). Symptom clusters in children and adolescents with cancer. Seminars in Oncology Nursing, 32, 394-404. doi: 10.1016/j.soncn.2016.08.005 [DOI] [PubMed] [Google Scholar]
- Rodgers C. C., Krance R., Street R. L., Hockenberry M. J. (2014). Symptom prevalence and physiologic biomarkers among adolescents using a mobile phone intervention following hematopoietic stem cell transplantation. Oncology Nursing Forum, 41, 229-236. doi: 10.1188/14.Onf.229-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A. R., Orellana L., Ullrich C., Kang T., Geyer J. R., Feudtner C., . . . Wolfe J. (2016). Quality of life in children with advanced cancer: A report from the PediQUEST study. Journal of Pain and Symptom Management, 52, 243-253. doi: 10.1016/j.jpainsymman.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman G. B., Rallis S. F. (2017). An introduction to quality research: Learning in the field (4th ed.). Los Angeles, CA: Sage. [Google Scholar]
- Tomlinson D., Baggott C., Dix D., Gibson P., Hyslop S., Johnston D. L., . . . Sung L. (2019). Severely bothersome fatigue in children and adolescents with cancer and hematopoietic stem cell transplant recipients. Supportive Care in Cancer, 27, 2665-2671. doi: 10.1007/s00520-018-4555-9 [DOI] [PubMed] [Google Scholar]
- Waldman E., Wolfe J. (2013). Palliative care for children with cancer. Nature Reviews Clinical Oncology, 10, 100-107. doi: 10.1038/nrclinonc.2012.238 [DOI] [PubMed] [Google Scholar]


