Abstract
Background
Protocadherin 8 (PCDH8) functions as a tumor-suppressor gene in many types of cancer. This study aimed to investigate the role of PCDH8 in esophageal squamous cell carcinoma (ESCC).
Material/Methods
Cell proliferation, apoptosis, transwell assay, tube formation assays, and tumor xenograft experiment were performed to explore the role of PCDH8 in the progression of ESCC.
Results
PCDH8 was found to be downregulated in ESCC cells. Ectopic expression of PCDH8 blocked proliferation, invasion, and migration and induced apoptosis in ESCC cells. Furthermore, vascular endothelial growth factor A (VEGFA) secretion and the AKT signaling pathway were also inhibited when PCDH8 was upregulated. PCDH8 overexpression suppressed epithelial-mesenchymal transition (EMT) and pro-angiogenic activity of ESCC cells. In a mouse model of ESCC xenograft tumors, PCDH8 overexpression remarkably restrained tumor cell growth, with the tumor inhibition rate of 75.2%. PCDH8 was the target of miR-200c and had a negative correlation with miR-200c.
Conclusions
PCDH8 exerts a tumor-suppressive effect against ESCC cells. However, further studies are required to elucidate the exact molecular mechanism underlying the antitumor activity of PCDH8 in ESCC.
MeSH Keywords: Cadherins; Esophageal Neoplasms; Neoplasm Metastasis; RNA, Small Untranslated
Background
Esophageal cancer is one of the most common cancers in the world and had relatively high morbidity and mortality rates [1,2]. In China, esophageal cancer is the sixth most common cancer and the fourth leading cause of death from cancer [2]. Esophageal squamous cell carcinoma (ESCC) accounts for more than 90% of all esophageal cancer cases [3]. Despite advances in early detection and treatment, the prognosis of esophageal cancer is far from satisfactory [4]. Unfortunately, at the time of initial esophageal cancer diagnosis, approximately 50% of patients have metastatic disease [5]. Metastasis remains the leading cause of death from esophageal cancer, but limited progress has been made in understanding the epidemiology of cancer metastasis.
Protocadherins (PCDHs) are a large group of calcium-dependent transmembrane cell adhesion and signaling proteins that belong to the cadherin family [6]. PCDHs have a variety of molecular functions, such as cell adhesion and colony formation, and a primary function in cell signaling [6–11]. PCDH8 is one of the non-clustered PCDHs and belongs to the δ2 subgroup [9]. PCDH8 functions as a tumor-suppressor gene in many types of cancer, including ovarian cancer [12], glioma [13], liver cancer [14], and nasopharyngeal carcinoma [15]. Increasing evidence shows that promoter hypermethylation of PCDH8 is the key event leading to the transcriptional silencing or reduction. However, a recent study by Lin et al. [16] showed that PCDH8 exhibited oncogenic activity in gastric cancer, as its overexpression promoted cancer cell invasion and metastasis by upregulating laminin subunit γ2. The role of PCDH8 in ESCC remains largely unknown. Here, we demonstrated that PCDH8 has tumor-suppressor potential in ESCC, and its overexpression induced ESCC cell apoptosis and inhibited growth, metastasis, invasion, and angiogenesis of ESCC cells. Therefore, PCDH8 may serve as a potential therapeutic target for ESCC.
Material and Methods
Cell lines and ESCC tissues
Human ESCC cell lines Eca-109, TE-1, and KYSE-150, human normal esophageal epithelial cell line HET-1A, and human umbilical vein endothelial cells (HUVEC) were obtained from the Cell Bank of the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). The Eca-109, TE-1, KYSE-150, and HET-1A cells were cultured in Roswell Park Memorial Institute 1640 medium containing 10% FBS (Gibco, CA, USA), 1% penicillin-streptomycin (Invitrogen, CA, USA), and 1% L-glutamine at 37°C in a 5% CO2 humidified incubator. HUVEC cells were cultured in DMEM supplemented with 10% FBS (Gibco, CA, USA) and 1% penicillin-streptomycin, and 1% L-glutamine at 37°C with 5% CO2. Furthermore, 20 pairs of ESCC and adjacent non-cancerous tissues were collected from 20 ESCC patients and then immediately frozen in liquid nitrogen. This study was approved by the Ethics Committee of Taizhou People’s Hospital.
Dual luciferase assay
The 3′ untranslated regions (UTR) of PCDH8 containing wild-type (WT) or mutant (MT) miR-200c binding sites were cloned between the xhoI and NotI sites of the psicheck2 Dual luciferase vector (Promega, WI, USA). A total of 3×104 293T cells were seeded on 24-well culture plates 24 h prior to transfection, and then co-transfected with 0.5 μg of wild-type or mutated psicheck2-PCDH8 vectors and 20 μM of miR-200c mimics or scrambled control (Riobio, Guangzhou, China). After 48 h of incubation, the luciferase activities were measured using the Dual Luciferase Reporter Assay System (Promega, WI, USA) according to the manufacturer’s instructions.
Quantitative real-time PCR (qPCR)
Total RNA was isolated from cells using TRIzol® reagent (Invitrogen, CA, USA). The quantity and quality of total RNA was determined using an ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) and 1 μg was reverse-transcribed into complementary DNA using the RevertAid Reverse Transcriptase (Thermo Fisher Scientific, MA, USA). The qPCR was performed using a SYBR Premix Ex Taq II Kit (TaKaRa, Dalian, China) with the following primers:
-
PCDH8 (forward, 5′-AAGACTTCCTTAGCCTTTCGG-3′;
reverse, 5′-TGCTGTATCGGACTGTTTTGC-3′);
-
ACTB (forward, 5′-GGCACTCTTCCAGCCTTCC-3′;
reverse, 5′-GAGCCGCCGATCCACAC-3′).
The expression levels of miR-200c was detected as previously described [17]. The relative gene expression was calculated using the 2−ΔΔCt method.
Western blot analysis
Cells were washed with ice-cold PBS and lysed on ice in RIPA lysis buffer (Sigma, MO, USA). Protein concentration was measured using the Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific, MA, USA). Equal amounts of proteins were boiled at 100°C for 10 min in sodium dodecyl sulfate (SDS) loading buffer and then separated by SDS polyacrylamide gel electrophoresis (PAGE), followed by transfer to polyvinylidene fluoride (PVDF) membranes (Merck Millipore, MA, USA). The membranes were blocked with 5% non-fat milk in Tris-buffered saline (TBS) plus 0.1% Tween-20 (TBST) for 1 h and then incubated with primary antibodies against PCDH8 (1: 200 dilution, Abcam, MA, USA), Bcl2 (1: 1000 dilution, Abcam, MA, USA), Bax (1: 1000 dilution, ProteinTech Group Inc., IL, USA), cleaved caspase 3 (1: 1000 dilution, CST, MA, USA), phosphorylated-(p-) AKT (1: 1000 dilution, CST, MA, USA), p-MTOR (1: 1000 dilution, CST, MA, USA), Vimentin (1: 1000 dilution, CST, MA, USA), E-cadherin (1: 1000 dilution, CST, MA, USA), N-cadherin (1: 1000 dilution, CST, MA, USA), and Tubulin (1: 1000 dilution, Sungene Biotech, Tianjin, China) in blocking buffer overnight at 4°C. After being washed 3 times with TBST, the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG or rabbit anti-mouse IgG (1: 3000 dilution, Jackson ImmunoResearch, PA, USA) at room temperature for 1 h. Finally, the protein bands were visualized using an enhanced chemiluminescence reagent (Thermo Fisher Scientific, MA, USA).
Construction of expression plasmid
The full-length PCDH8 cloned from human cDNA was cloned into the using RT-PCR and were then religated into the eukaryotic expression vector pcDNA3.1 (Invitrogen, CA, USA). Lentiviral particles were transfected into KYSE-150 cells using Lipofectamine 2000 (Invitrogen, CA, USA) according to the manufacturer’s instructions.
Apoptosis assay
The stable overexpression PCDH8 cells and its negative control cells were harvested after 72 h of transfection and then washed twice with PBS. The cells were resuspended and incubated with Annexin V-FITC and propidium iodide (PI) at room temperature for 20 min in the dark. Cell apoptosis was analyzed using a flow cytometer (FACSAria Cell Sorter, BD Biosciences, NJ, USA).
Cell proliferation assay
The effect of PCDH8 on the proliferation of ESCC cells was evaluated using a Cell Counting Kit-8 (CCK-8; Dojindo, Kumamoto, Japan). KYSE-150 cells were seeded in 96-well plates at a density of 3×103/well in triplicate. After 1, 2, 3, 4, and 5 days of culture at 37°C with 5% CO2, 10 μL of CCK-8 reagent was added to each well and cells were incubated at 37°C for 2 h. Absorbance at 450 nm was measured using a Multiskan MK3 microplate reader (Thermo Fisher Scientific, MA, USA).
Transwell assay
For the invasion assay, KYSE-150 cells (4×104/well) were trypsinized and seeded into the upper chamber of transwell inserts (Corning, Cambridge, USA) precoated with Matrigel Basement Membrane Matrix (BD Bioscience, CA, USA). The bottom chamber was filled with 600 μL completed RPMI-1640 supplemented with 10% FBS as a chemoattractant. After 24 h of incubation, the nonmigrating cells in the upper chamber were removed with a cotton swab and the invasive cells on the lower surface of the membrane were fixed with 4% paraformaldehyde for 15 min and then stained with 0.1% crystal violet for 20 min. After taking images of the invasive cells, cells were incubated in cell lysis buffer at 37°C for 20 min, and then absorbance at 570 nm was measured using a Multiskan MK3 microplate reader (Thermo Fisher Scientific, MA, USA). For the transwell migration assay, transfected KYSE-150 cells were collected and seeded into the upper chamber of transwell inserts (Corning, Cambridge, USA) containing the noncoated membrane. The bottom chamber was filled with 1% FBS (600 μL). After 24 h of incubation, the cells on the upper surface of the membrane were wiped away with a cotton swab and the cells on the lower surface of membrane were fixed and stained with 0.1% crystal violet for 20 min.
Wound healing assay
KYSE-150 cells (3×105/well) were inoculated onto 6-well plates and cultivated at 37°C. After growing to 90% confluency, a pipette tip was used to scratch a wound. Detached and damaged cells were carefully removed by washing with PBS 3 times. The cells were then cultured in serum-free culture medium for 24 h at 37°C. The widths of scratch wounds were examined under a light microscope.
Enzyme-linked immunosorbent assay (ELISA)
ELISA was carried out to determine the protein level of vascular endothelial growth factor A (VEGFA) secreted by KYSE-150 cells. Briefly, 2×104 transfected cells were seeded onto 6-well plates and cultured in RPMI 1640 medium. After 72 h of incubation, the cell-free supernatant was collected by centrifugation twice at 3000×g at 4°C for 15 min to completely remove the cells and cell debris. VEGFA in the culture supernatants was examined using a specific VEGFA ELISA kit (Beijing 4A Biotech, China) according to the manufacturer’s protocol.
HUVEC tube formation assay
An in vitro endothelial cell tube formation assay was performed to evaluate the modulation of angiogenesis by PCDH8. HUVECs (2×104 cells/well) were seeded onto a 96-well plate precoated with 100 μL Matrigel Basement Membrane Matrix (BD Biosciences, NJ, USA) and cultured in 20 μL DMEM at 37°C for 6 h. Images were taken using a bright-field microscope at a magnification of 100×.
Tumor xenograft experiments
Female BALB/c nude mice (6 weeks old) were purchased from the Shanghai Lab Animal Research Center (Shanghai, China) and maintained in specific pathogen-free conditions. The mice were subcutaneously injected with 5×107 cells/mL of KYSE-150 cells stably transfected with pcDNA-PCDH8 or empty vector. Tumor growth was evaluated every 3–5 days, and tumor volumes were calculated as 0.5×length×width2. The mice were killed and the tumor masses were removed and weighed 30 days after injection.
Statistical analyses
All statistical analyses were performed using SPSS 22.0 software (SPSS Inc., IL, USA). Data are presented as mean±standard deviation. Comparisons between groups were carried out using the t test (two-sided). The criterion of statistical significance was P<0.05.
Results
PCDH8 induced apoptosis and inhibited the proliferation of ESCC cells
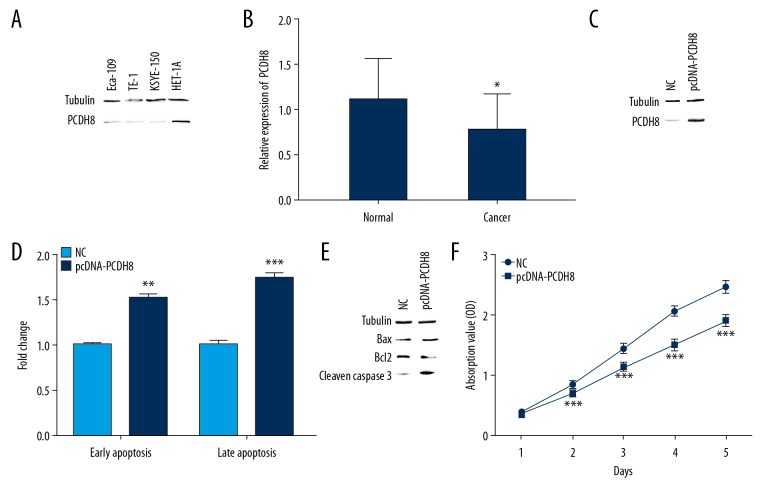
We first detected the expression levels of PCDH8 in cancer cell lines and tissues. PCDH8 was downregulated in ESCC cells compared to HET-1A cells (Figure 1A). The expression levels of PCDH8 in ESCC tissues were also lower than those in adjacent non-cancerous tissues (Figure 1B). To further investigate the function of PCDH8 in ESCC, we established stable PCDH8-overexpressing KYSE-150 cells by transfecting ESCC cells with an expression vector encoding the full-length PCDH8. Western blot analysis revealed that PCDH8 was substantially upregulated after transfected with pcDNA-PCDH8 in comparison with the vector control (Figure 1C). PCDH8 overexpression induced apoptosis of ESCC cells (Figure 1D). The rate of early apoptosis in cells transfected with pcDNA-PCDH8 was 1.533-fold higher than those transfected with empty vector (P=0.001). Cells transfected with pcDNA-PCDH8 also showed a similar change in the rate of late apoptosis (1.74-fold) compared with control (P<0.001). The levels of Bax and cleaved caspase-3 were elevated but Bcl2 levels were decreased in pcDNA-PCDH8-transfected cells compared with control cells (Figure 1E). In addition, PCDH8 showed a statistically significant proliferation-inhibitory effect on ESCC cells when evaluated by the CCK8 assay (Figure 1F).
Figure 1.
PCDH8 induced apoptosis and inhibited proliferation in KYSE-150 cells. (A, B) PCDH8 was downregulated in ESCC cell lines and tissues. (C) Stable overexpression of PCDH8 in KYSE-150 cell was confirmed by Western blot. (D) PCDH8 overexpression induced apoptosis. Histogram showed 1.53- and 1.74-fold increase in early and late apoptotic cell populations, respectively, upon pcDNA-PCDH8 transfection (E) PCDH8 overexpression upregulated the levels of Bax and cleaved caspase-3 but downregulated Bcl2 level. (F) Relative cell proliferation was measured using CCK-8 assay. PCDH8 overexpression significantly inhibited cell proliferation. * P<0.05; ** P<0.01; *** P<0.001.
PCDH8 inhibited ESCC invasion and migration
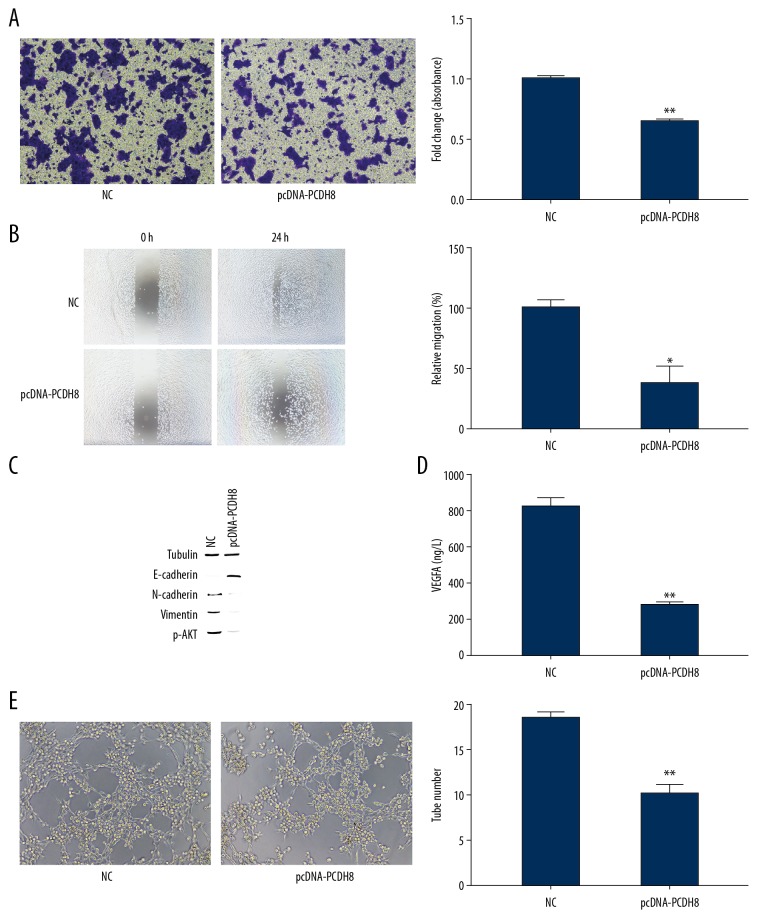
The effect of PCDH8 overexpression on invasion and migration of ESCC cells was evaluated using transwell assay. PCDH8 overexpression dramatically decreased the invasive ability of KYSE-150 cells (P<0.01, Figure 2A, 2B). We also found significant decreased migration in pcDNA-PCDH8-transfected cells compared with control (P<0.05). As a feature of aggressive tumors, epithelial–mesenchymal transition (EMT) accompanied by reduced E-cadherin and elevated N-cadherin expression gives cancer cells enhanced cell motility and invasive properties [18–20]. We further evaluated the effect of PCDH8 overexpression on EMT of ESCC cells. Western blot analysis showed that E-cadherin was upregulated whereas N-cadherin and Vimentin were downregulated when PCDH8 was overexpressed (P<0.05, Figure 2C). These results indicate that PCDH8 overexpression suppresses the invasion and migration of ESCC cells through inhibition of EMT.
Figure 2.
PCDH8 inhibited the invasion and migration of KYSE-150 cells. (A, B) Invasion and migration abilities of KYSE-150 cells transfected with pcDNA-PCDH8 or NC were measured by Transwell assays. PCDH8 overexpression significantly decreased the migratory and invasive ability of ESCC cells. (C) PCDH8 overexpression upregulated the levels of E-cadherin and p-AKT but downregulated the levels of N-cadherin and vimentin. (D) ELISA was performed to determine the protein level of VEGFA secreted by KYSE-150 cell. PCDH8 overexpression remarkably inhibited VEGFA expression. (E) Tube formation was significantly decreased when PCDH8 was overexpressed. Representative images of HUVEC tube formation were captured at 6 hours after cell seeding. * P<0.05, ** P<0.01.
PCDH8 suppresses angiogenic activity in ESCC cells
Angiogenesis plays a critical role in tumor growth and metastasis. Cancer cells secrete angiogenic factors that promote tube formation of endothelial cells [21,22]. Subsequently, we examined the level of secreted VEGFA from KYSE-150 cells using quantitative ELISA. The secretion of KYSE-150 cell transfected with pcDNA-PCDH8 was reduced by 65.2% (P=0.001, Figure 2D). Furthermore, the level of p-AKT was significantly decreased when PCDH8 was overexpressed (P=0.001, Figure 2C). Therefore, PCDH8 appears to inhibit VEGFA expression by suppressing the AKT signaling pathway. HUVEC tube formation assay revealed that conditioned media from PCDH8-overexpression KYSE-150 cells remarkably inhibited tube-like structure formation (P=0.002, Figure 2E). These results indicate that PCDH8 overexpression contributes to reduced angiogenic activity of ESCC cells by decreasing VEGFA production.
PCDH8 modulates ESCC tumor growth in vivo
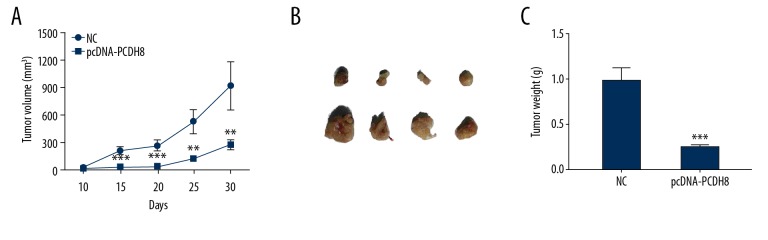
To further investigate the tumor-suppressor function of PCDH8 in ESCC in vivo, KYSE-150 cells with PCDH8 overexpression were injected into nude mice, and tumor size and body weight were recorded. Mice injected with KYSE-150 cells with empty vector showed tumor development as early as 10 days after injection of cancer cells, and average tumor volume was 928.0±251.2 mm3 at day 30 (Figure 3). On the contrary, tumors with PCDH8 overexpression were obviously smaller, with average volume of 277.0±45.4 mm3 after growth over the same time period. The tumor weight after treatment also exhibited a similar pattern and was lighter in mice with PCDH8 overexpression compared with control (P<0.001). This suggests that PCDH8 overexpression inhibits ESCC cell growth in vivo. These data further support the role of PCDH8 as a tumor-suppressor in ESCC.
Figure 3.
PCDH8 inhibited tumor growth in an ESCC xenograft nude mouse model. The mice were directly injected with pcDNA-PCDH8 to form xenograft tumors. The tumor growth was significantly suppressed after PCDH8 overexpression. (A) Growth curves of tumors. (B) Actual tumor size after harvest on day 30. (C) Quantitative analysis of tumor weight. * P<0.05; ** P<0.01; *** P<0.001.
PCDH8 is targeted by miR-200c in ESCC cell
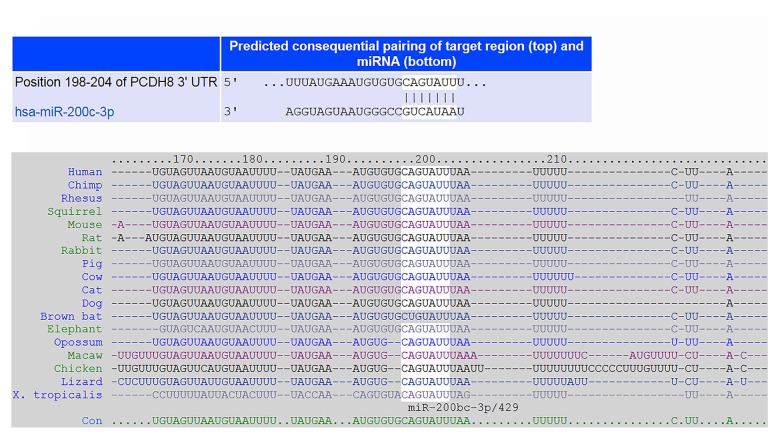
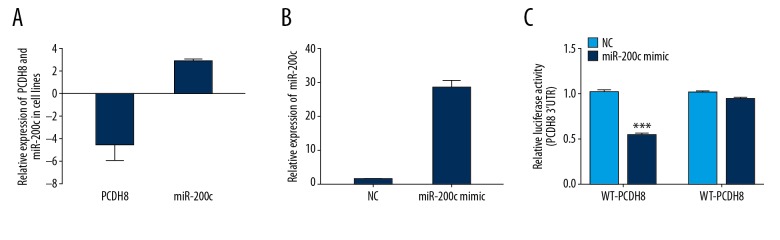
In general, miRNAs are critical negative regulators of target mRNA stability and translation through posttranscriptional regulation. We used 2 independent algorithms, TargetScan and PicTar, to identify the miRNA that regulates PCDH8 expression. As shown in Figure 4, PCDH8 appears to be the potential target of miR-200c. We further evaluated the expression levels of PCDH8 and miR-200c in ESCC cell line KYSE-150 and non-malignant Het-1A cell line. KYSE-150 cells had remarkably increased miR-200c expression and reduced PCDH8 expression compared with Het-1A cells (P<0.05).
Figure 4.
Identification of the binding site for miR-200c in the 3′ UTR of PCDH8. (A) Putative binding site between miR-200c and PCDH8 was predicted by TargetScan. (B) Seed sequence of miR-200c was strictly conserved across species.
To assess the interaction between miR-200c and the 3′UTR of PCDH8, luciferase reporter assays were performed. Luciferase activity was significantly decreased in cells co-transfected with wild-type 3′UTR of PCDH8 and miR-200c mimic (P<0.001, Figure 5), whereas mutant 3′UTR of PCDH8 reporter did not exhibit any difference in relative luciferase activity between cells transfected with mock and miR-200c mimic. These results imply that miR-200c can directly bind to the 3′UTR of PCDH8 and further repress its expression.
Figure 5.
miR-200c directly targeted PCDH8. (A) Expression of miR-200c and PCDH8 in KYSE-150 and HET-1A cells was examined. Fold expression in KYSE-150 cells was determined relative to expression in HET-1A cells. (B) Transfection efficiency of miR-200c mimics was confirmed by qPCR. (C) Dual luciferase assay revealed that miR-200c obviously reduced the luciferase activity of WT-PCDH8 but not MUT-PCDH8. *** P<0.001.
Discussion
Although improved therapeutic strategies and early diagnoses have decreased cancer-related deaths, cancer metastasis remains a major cause of cancer morbidity and mortality. There is still a lack of reliable and accurate predictive methods and there are only limited effective therapeutic options for cancer metastasis. Metastatic cancer is still a major clinical challenge, accounting for about 90% of cancer deaths [23]. Therefore, it is urgent to clarify the molecular mechanism underlying metastasis of ESCC.
PCDHs are involved in pathogenesis of many human diseases, including cancer [6]. It has been demonstrated that PCDHs exhibit tumor-suppressing and tumor-promoting functions [12,15,16,24–26]. PCDH8 is usually downregulated in many types of cancer, including ovarian, breast, and lung cancers, due to mutation and epigenetic alteration [12,14,25]. Inactivating PCDH8 may be a key step in the acquisition of malignant phenotype due to its high frequency in cancer [25]. Low expression and hypermethylation of PCDH8 are associated with poor prognosis of cancer patients [12,14,27,28]. However, there was no association between PCDH8 expression and survival time of ESCC patients in the GEPIA cohort [29], which may be due to the small sample size. PCDH8 inhibits the proliferation, migration, and invasion of cancer cells, such as breast cancer [25], nasopharyngeal carcinoma [15], glioma [13], and ovarian cancer [12]. However, Lin et al. reported that PCDH8 promoted gastric cancer cell invasion and migration and its overexpression was an indicator of poor prognosis [16]. In addition, activation of the AKT signaling pathway is involved in mediating cell survival, growth, proliferation, migration, and angiogenesis [30]. Zong et al. [13] found that PCDH8 suppressed glioma cell proliferation by downregulating the AKT/GSK3β/β-catenin signaling pathway. Subsequently, we investigated the effect of PCDH8 overexpression on angiogenesis. We found that VEGFA secretion and the AKT signaling pathway were inhibited by PCDH8, thus suppressing tumor-induced angiogenesis. Therefore, PCDH8 overexpression appears to affect at least 1 signaling pathway, which suppresses the expression and secretion of angiogenic factors and ultimately inhibits angiogenesis.
The EMT is essential for cancer metastasis by conferring an invasive and metastatic phenotype on cancer cells [18]. EMT-activated cancer cells have the ability to invade the surrounding local tissue and migrate to other organs. Disseminated cancer cells undergo mesenchymal-to-epithelial transition (MET) and revert to the epithelial phenotype to establish metastatic tumors when they arrive at a new location [31]. The miR-200 family, including miR-200a, miR-200b, miR-200c, miR-141, and miR-429, function as major regulators of EMT through a cluster of gene expression, such as E-cadherin, N-cadherin, and vimentin, by targeting zinc finger E-box-binding homeobox (ZEB) transcription factors [32]. miR-200c is not only an important regulator of EMT, but also is associated with resistance to anticancer drugs and poor prognosis of cancer patients [17,33,34]. Previous studies have demonstrated that miR-200c plays different roles in different types of cancer [17,33–35]. For example, miR-200c is overexpressed in esophageal squamous cancer (ESCC) but is downregulated in esophageal adenocarcinoma [34,35]. miR-200c appears to play an oncogenic role in ESCC, in which its overexpression contributes to chemotherapy resistance and poor prognosis through activation of the AKT signaling pathway [17,34]. In the present study, we found that PCDH8 was a target gene of miR-200c. PCDH8 overexpression inhibited ESCC growth, invasion, metastasis, and angiogenesis and induced apoptosis, which is consistent with previous studies conducted in other types of cancer [12,25]. A study by Li et al. [36] found that PCDH8 is the target gene of miR-429, an important member of the miR-200 family. miR-429 functions as a tumor suppressor in most types of cancer, including ESCC [37,38]. Both miR-200c and miR-429 are important modulators of EMT. It is interesting that miR-200c is upregulated but miR-429 is downregulated in ESCC [17,34,38]. PCDH8 overexpression suppresses EMT progression of ESCC cells through upregulation of E-cadherin and downregulation of N-cadherin and Vimentin. Therefore, the miR-200c can regulate EMT in ESCC cells, partly through downregulation of PCDH8.
Conclusions
In summary, our study demonstrates that PCDH8 is the target gene of miR-200c and is a key negative regulator of metastasis in ESCC. PCDH8 can downregulate VEGFA and inhibit the AKT signaling pathway in ESCC, consequently resulting in reduced proliferative, invasive, angiogenic, and metastatic abilities of cancer cells. However, further studies are required to elucidate the exact molecular mechanism underlying the antitumor activity of PCDH8 in ESCC.
Footnotes
Conflict of interest
None.
Source of support: This study was supported by the Scientific Research Project Foundation of Health Department, Jiangsu, China (grant No. H2017075), the Foundation of Jiangsu Provincial Medical Innovation Team (grant No. CXTDA2017042), and the Innovation Team Project Foundation of Taizhou People’s Hospital (grant No. CXTDB201904)
References
- 1.Sadiq A, Mansour KA. Esophageal cancer: Recent advances. Thorac Cancer. 2011;2(3):75–83. doi: 10.1111/j.1759-7714.2011.00054.x. [DOI] [PubMed] [Google Scholar]
- 2.Zheng RS, Sun KX, Zhang SW, et al. [Report of cancer epidemiology in China, 2015]. Zhonghua Zhong Liu Za Zhi. 2019;41(1):19–28. doi: 10.3760/cma.j.issn.0253-3766.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Zhang X, Xu Y, He C, et al. Elevated expression of CCAT2 is associated with poor prognosis in esophageal squamous cell carcinoma. J Surg Oncol. 2015;111(7):834–39. doi: 10.1002/jso.23888. [DOI] [PubMed] [Google Scholar]
- 4.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–75. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu SG, Zhang WW, He ZY, et al. Sites of metastasis and overall survival in esophageal cancer: A population-based study. Cancer Manag Res. 2017;9:781–88. doi: 10.2147/CMAR.S150350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El Hajj N, Dittrich M, Haaf T. Epigenetic dysregulation of protocadherins in human disease. Semin Cell Dev Biol. 2017;69:172–82. doi: 10.1016/j.semcdb.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Schalm SS, Ballif BA, Buchanan SM, et al. Phosphorylation of protocadherin proteins by the receptor tyrosine kinase Ret. Proc Natl Acad Sci USA. 2010;107(31):13894–99. doi: 10.1073/pnas.1007182107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang W, Xue X, Shan L, et al. Clinical significance of PCDH10 promoter methylation in diffuse large B-cell lymphoma. BMC Cancer. 2017;17(1):815. doi: 10.1186/s12885-017-3810-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SY, Yasuda S, Tanaka H, et al. Non-clustered protocadherin. Cell Adh Migr. 2011;5(2):97–105. doi: 10.4161/cam.5.2.14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shao Z, Noh H, Bin Kim W, et al. Dysregulated protocadherin-pathway activity as an intrinsic defect in induced pluripotent stem cell-derived cortical interneurons from subjects with schizophrenia. Nat Neurosci. 2019;22(2):229–42. doi: 10.1038/s41593-018-0313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shishodia G, Koul S, Koul HK. Protocadherin 7 is overexpressed in castration resistant prostate cancer and promotes aberrant MEK and AKT signaling. Prostate. 2019;79(15):1739–51. doi: 10.1002/pros.23898. [DOI] [PubMed] [Google Scholar]
- 12.Cao Y, Yu Y, Chen X, et al. Low expression of Protocadherin-8 promotes the progression of ovarian cancer. Int J Gynecol Cancer. 2018;28(2):346–54. doi: 10.1097/IGC.0000000000001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zong Z, Pang H, Yu R, Jiao Y. PCDH8 inhibits glioma cell proliferation by negatively regulating the AKT/GSK3beta/beta-catenin signaling pathway. Oncol Lett. 2017;14(3):3357–62. doi: 10.3892/ol.2017.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang C, Peng Y, Yang F, et al. PCDH8 is frequently inactivated by promoter hypermethylation in liver cancer: Diagnostic and clinical significance. J Cancer. 2016;7(4):446–52. doi: 10.7150/jca.13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He D, Zeng Q, Ren G, et al. Protocadherin8 is a functional tumor suppressor frequently inactivated by promoter methylation in nasopharyngeal carcinoma. Eur J Cancer Prev. 2012;21(6):569–75. doi: 10.1097/CEJ.0b013e328350b097. [DOI] [PubMed] [Google Scholar]
- 16.Lin Y, Ge X, Zhang X, et al. Protocadherin-8 promotes invasion and metastasis via laminin subunit gamma2 in gastric cancer. Cancer Sci. 2018;109(3):732–40. doi: 10.1111/cas.13502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu H, Duan B, Jiang L, et al. Serum miR-200c and clinical outcome of patients with advanced esophageal squamous cancer receiving platinum-based chemotherapy. Am J Transl Res. 2013;6(1):71–77. [PMC free article] [PubMed] [Google Scholar]
- 18.Heerboth S, Housman G, Leary M, et al. EMT and tumor metastasis. Clin Transl Med. 2015;4:6. doi: 10.1186/s40169-015-0048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66(17):8319–26. doi: 10.1158/0008-5472.CAN-06-0410. [DOI] [PubMed] [Google Scholar]
- 20.Kang Y, Massague J. Epithelial-mesenchymal transitions: Twist in development and metastasis. Cell. 2004;118(3):277–79. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Bao S, Wu Q, Sathornsumetee S, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66(16):7843–48. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 22.Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: Beyond discovery and development. Cell. 2019;176(6):1248–64. doi: 10.1016/j.cell.2019.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–64. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 24.He Y, Wang Z, Liu C, et al. Protocadherin 17 is a tumor suppressor and is frequently methylated in nasopharyngeal carcinoma. Cancer Manag Res. 2019;11:1601–13. doi: 10.2147/CMAR.S191102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Yu JS, Koujak S, Nagase S, et al. PCDH8, the human homolog of PAPC, is a candidate tumor suppressor of breast cancer. Oncogene. 2008;27(34):4657–65. doi: 10.1038/onc.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukai S, Oue N, Oshima T, et al. Overexpression of PCDHB9 promotes peritoneal metastasis and correlates with poor prognosis in patients with gastric cancer. J Pathol. 2017;243(1):100–10. doi: 10.1002/path.4931. [DOI] [PubMed] [Google Scholar]
- 27.Lin YL, Ma JH, Luo XL, et al. Clinical significance of protocadherin-8 (PCDH8) promoter methylation in bladder cancer. J Int Med Res. 2013;41(1):48–54. doi: 10.1177/0300060513475571. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Liu C, Wang Z, Hu G. Expression of protocadherin8: Function as a tumor suppressor in hypopharyngeal carcinoma. Cancer Biomark. 2018;22(3):495–502. doi: 10.3233/CBM-171137. [DOI] [PubMed] [Google Scholar]
- 29.Tang Z, Li C, Kang B, et al. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abid MR, Guo S, Minami T, et al. Vascular endothelial growth factor activates PI3K/Akt/forkhead signaling in endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24(2):294–300. doi: 10.1161/01.ATV.0000110502.10593.06. [DOI] [PubMed] [Google Scholar]
- 31.Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27(20):2192–206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill L, Browne G, Tulchinsky E. ZEB/miR-200 feedback loop: At the crossroads of signal transduction in cancer. Int J Cancer. 2013;132(4):745–54. doi: 10.1002/ijc.27708. [DOI] [PubMed] [Google Scholar]
- 33.Mutlu M, Raza U, Saatci O, et al. miR-200c: A versatile watchdog in cancer progression, EMT, and drug resistance. J Mol Med (Berl) 2016;94(6):629–44. doi: 10.1007/s00109-016-1420-5. [DOI] [PubMed] [Google Scholar]
- 34.Hamano R, Miyata H, Yamasaki M, et al. Overexpression of miR-200c induces chemoresistance in esophageal cancers mediated through activation of the Akt signaling pathway. Clin Cancer Res. 2011;17(9):3029–38. doi: 10.1158/1078-0432.CCR-10-2532. [DOI] [PubMed] [Google Scholar]
- 35.Smith CM, Watson DI, Leong MP, et al. miR-200 family expression is downregulated upon neoplastic progression of Barrett’s esophagus. World J Gastroenterol. 2011;17(8):1036–44. doi: 10.3748/wjg.v17.i8.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Z, Gou J, Jia J, Zhao X. MicroRNA-429 functions as a regulator of epithelial-mesenchymal transition by targeting Pcdh8 during murine embryo implantation. Hum Reprod. 2015;30(3):507–18. doi: 10.1093/humrep/dev001. [DOI] [PubMed] [Google Scholar]
- 37.Sheng N, Zhang L, Yang S. MicroRNA-429 decreases the invasion ability of gastric cancer cell line BGC-823 by downregulating the expression of heparanase. Exp Ther Med. 2018;15(2):1927–33. doi: 10.3892/etm.2017.5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Li M, Zang W, et al. MiR-429 up-regulation induces apoptosis and suppresses invasion by targeting Bcl-2 and SP-1 in esophageal carcinoma. Cell Oncol (Dordr) 2013;36(5):385–94. doi: 10.1007/s13402-013-0144-6. [DOI] [PubMed] [Google Scholar]