Abstract
Excessive vascular smooth muscle cell (VSMC) proliferation contributes to the development of atherosclerosis and restenosis. Furthermore, apoptosis of VSMCs accelerates plaque rupture in the atherosclerotic vessels. Therefore, a strategy that regulates both VSMC proliferation and apoptosis is essential for the development of novel pharmacological tools for the treatment of atherosclerosis. Despite mounting evidence supporting the benefits of melatonin in diverse metabolic diseases, the role of melatonin in VSMC growth remains largely unknown. The present study revealed that melatonin inhibited both proliferation and apoptosis of primary cultured rat VSMCs. Melatonin induced mitochondrial energetic stress in VSMCs and subsequent induction of Sestrin2 via C/EBPβ. Melatonin-induced Sestrin2 suppressed mTORC1 activity in VSMCs, contributing to suppression of VSMC proliferation. Additionally, melatonin-induced upregulation of Sestrin2 blocked apoptosis by preventing excessive ROS generation. The results demonstrated that melatonin controlled VSMC proliferation and apoptosis via Sestrin2-mediated inhibition of mTORC1 and ROS scavenging. Therefore, melatonin should be considered as a lead compound for therapies aimed at preventing vessel lumen constriction during the course of atherosclerosis and restenosis.
Keywords: vascular smooth muscle cells, melatonin, Sestrin2, proliferation, apoptosis
Introduction
Vascular smooth muscle cells (VSMCs) are the predominant cells in arteries (1). Excessive VSMC proliferation and neo-intima formation are critical processes in the development of atherosclerosis and restenosis after percutaneous balloon angioplasty (2). In addition to VSMC growth, proper regulation of apoptosis of VSMCs within vessel walls is also important for preventing plaque rupture in atherosclerotic vessels (3). For these reasons, many studies have focused on the regulation of VSMC growth and apoptosis by mechanistic target of rapamycin complex 1 (mTORC1) (4,5). It is well established that mTORC1 contributes to vascular pathologies such as intimal hyperplasia; accordingly, the use of the mTORC1 inhibitor rapamycin in drug-eluting stents has achieved a profound reduction in the incidence of restenosis (5).
Sestrin2 is a stress-inducible protein that regulates cell growth and survival, and can participate in cellular responses to stress conditions (6). Sestrin2 suppresses mTORC1 signaling via activation of AMPK-activated protein kinase (AMPK) or inhibition of Rag GTPases (7,8). Through these functions, Sestrin2 attenuates oxidative stress, fat accumulation, and insulin resistance, thereby attenuating various age- and obesity-related metabolic diseases (6). Sestrin2 is also induced by a range of mitochondrial dysfunctions and promotes mitochondrial biogenesis and mitohormesis (9). In the liver, Sestrin2 expression is induced upon chronic hypernutrition and acts as an important suppressor of hepatosteatosis by inhibiting mTORC1(10). Despite a great deal of evidence supporting the idea that Sestrin2 inhibits mTORC1 in the context of metabolic pathologies, the effect of Sestrin2/mTORC1 on VSMC growth has not been previously studied.
Melatonin was first described as an endogenous hormone that regulates circadian and seasonal rhythms. Several lines of evidence have revealed that melatonin has many other functions, including roles as an antioxidant, immune enhancer, promoter of mitochondrial homeostasis, and tumor suppressor (11). Multiple mechanisms, including cell cycle arrest and epithelial-to-mesenchymal transition, are thought to mediate the biologic effects of melatonin on growth and invasion of cancer (12). In addition, melatonin blocks TGF-β1-induced fibroblast proliferation, which leads to alleviated pulmonary fibrogenesis (13). However, it remains unclear whether melatonin regulates VSMC growth and proliferation. In this study, we investigated whether melatonin modulates VSMC growth and, if so, whether Sestrin2/mTORC1 is implicated in this effect.
Materials and methods
Cell culture
Rat aortic smooth muscle cells were isolated from 4-week-old male Sprague-Dawley rats (90 to 100 g). Cervical dislocation euthanasia was performed by well-trained individuals. Trimmed aortas was washed with sterilized cold phosphate-buffered saline (PBS) and sliced into pieces measuring 1-3 mm2. The pieces of aorta were attached to dishes and cultured in low-glucose Dulbecco's modified Eagle's medium (Hyclone) supplemented with 20% fetal bovine serum (FBS; Hyclone) for 2 weeks at 37˚C in 5% CO2. The medium was replaced every day. Cells were maintained in low-glucose Dulbecco's modified Eagle's medium supplemented with 20% FBS. Cells at passage 4-9 were used in all experiments. All protocols for animal use and euthanasia were reviewed and approved by the Animal Care and Use Committee of Kyunpook National University School of Medicine and conducted according to our institutional guideline (KNU-2011-0096-1).
Western blot analysis
Cells were lysed in lysis buffer (20 mM Tris-HCl [pH 7.4], 5 mM EDTA [pH 8.0], 10 mM Na4P2O7, 100 mM NaF, 2 mM Na3VO4, 1% NP-40) containing aprotinin, leupeptin, PMSF, and phosphatase inhibitors cocktail 3 (Sigma). Protein samples were separated on 10% SDS-PAGE gels and transferred to PVDF membranes. Membranes were blocked with 5% skim milk in Tris-buffered saline containing 0.1% Tween 20 (TBST) and incubated with primary antibody overnight at 4˚C. Primary antibodies against the following proteins were obtained from the indicated suppliers: C/EBPβ, Sestrin2, phospho-p70S6K (T389), p70S6K, p-Rb (S807/811) were from Cell Signaling Technology; Rb was from Santa Cruz Biotechnology; and β-actin (1:5,000) from Sigma.-Membranes were washed three times with TBST and incubated with HRP-conjugated anti-mouse (Santa Cruz Biotechnology) or anti-rabbit secondary antibody (Cell Signaling Technology). HRP was detected using the ECL reagent (BioNote).
Small interfering RNA (siRNA) transfection
For gene silencing, cells were transfected with 50 nM scrambled siRNA, siSestrin2, or siCEBP/β (Bioneer,) using Lipofectamine RNAiMAX (Invitrogen).
Transfection of expression vectors
For overexpression of Sestrin2, cells were transfected with a FLAG-Sestrin2 expressing vector (a gift from Dr. Jun Hee Lee, University of Michigan) or a control vector using TransIT-LT1 Transfection Reagent (Mirus Bio) according to the manufacturer's instructions.
Cell counting
Primary VSMCs transfected with scrambled small interfering RNA (siRNA) or siRNA targeting Sestrin2 (siSestrin2) for 24 h were serum-starved for 24 h and incubated with 10% FBS with or without 2 mM melatonin (Sigma). Cells were trypsinized, stained with Trypan blue solution, and counted with a hemocytometer.
Cell viability assay
Cells were seeded at 3-5x103 cells per well in 96-well plates. The cells were serum-starved for 24 h and incubated with 10% FBS with or without 2 mM melatonin for 3 days, and cell viability was measured using a CCK8 Solution Reagent (CK04; Dojindo). The absorbance of each well at 495 nm was measured on a VERSA MAX ELISA reader (Molecular Devices). The proportion of viable cells in each treatment group was normalized against that of control wells.
Flow cytometric analysis
Cell cycle distribution of VSMCs after treatment with 10% FBS with or without 2 mM melatonin was detected by flow cytometry. VSMCs were collected and fixed in 70% ethanol at 4˚C for 30 min. Before analysis, cells were centrifuged at 2000 rpm for 5 min to remove ethanol. Then, cells were suspended in 500 µl PBS containing propidium iodide (33 µg/ml) and Ribonuclease A (1 mg/ml) in darkness at 37˚C for 30 min. Fluorescence emitted by PI-DNA complexes was measured on an Epics XL flow cytometer (BD Bioscience).
Measurement of oxygen consumption rate
Oxygen consumption rate (OCR) was measured in 24-well plates using a Seahorse XF-24 analyzer (Seahorse Bioscience). VSMCs were serum-starved for 24 h and incubated with 10% FBS with or without melatonin (2 mM). On the day before the experiment, the sensor cartridge was placed in calibration buffer supplied by Seahorse Bioscience and incubated at 37˚C in a non-CO2 incubator for 1 h. Oligomycin (Sigma), carbonyl cyanide 3-chlorophenylhydrazone (CCCP, Sigma), and rotenone (Sigma) were added at the indicated times during OCR measurement.
Reverse transcription-quantitative PCR
Total RNA was prepared using QIAzol lysis reagent (Qiagen), and complementary DNA (cDNA) was synthesized from total RNA using the RevertAid First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.). The resultant cDNA was amplified on a 7500 Fast Real-Time PCR System (Applied Biosystems). Relative expression levels were calculated using the ∆∆Cq method (14); the levels of each mRNA were normalized against the corresponding level of 36B4 mRNA primer, and the sequences were as follows: CEBPβ forward, ACGAGCGGCTGCAGAAGA, reverse, GGCAGCTGCTTGAACAAGTTC; SESN2 forward, ACCTTTCGTGCCCAGGATTAT, reverse, GGGTAGAGCCGCTGGATCA; 36B4 forward, TTCCCACTGGCTGAAAAGGT, and reverse, GCCGCAGCCGCAAA.
MitoSox
Mitochondrial reactive oxygen species (ROS) generation was assessed using MitoSOX Red Mitochondrial Superoxide indicator (Invitrogen). VSMCs transfected with 50 nM scrambled siRNA or siSestrin2 for 24 h on a confocal dish were serum-starved for 24 h and incubated with 10% FBS with or without 2 mM melatonin. Cells were treated with 5 µM MitoSOX reagent working solution and incubated for 10 min at 37˚C in the dark. The cells were then washed gently three times with warm HBSS buffer. Finally, cells were counterstained with NucBlue Live Cell Stain ReadyProbes (Invitrogen) and mounted in warm buffer for imaging. MitoSOX fluorescence intensity were quantified using Image J software.
Immunocytochemistry
Cells were pretreated with or without 2 mM melatonin for 24 h and then treated with 1 mM H2O2 for 6 h. Cells were fixed with 4% paraformaldehyde (Biosesang) and washed with PBS. Cells were permeabilized with 0.1% Triton X-100 for 15 min and washed with PBS. Following 1 h of blocking in 5% normal goat serum (Vector Laboratories) in PBS, cells were incubated with primary anti-cleaved caspase-3 (1:400; Cell Signaling Technology) antibody overnight at 4˚C. After washing with PBS, the cells were incubated with Alexa Fluor® 568 goat anti-rabbit (1:100; Thermo Fisher Scientific Inc.) secondary antibodies for 2 h at room temperature. Nuclei were stained with DAPI (Vector Laboratories). Immnofluorescence intensity of cleaved caspase-3 was quantified using Image J software.
Statistical analysis
All values are presented as means ± SEM. ANOVA was used for comparisons between multiple groups, followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Melatonin inhibits VSMC proliferation
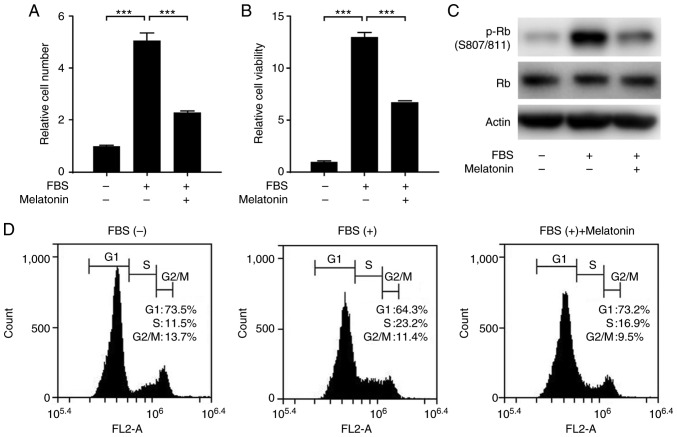
We first examined the effect of melatonin on FBS-stimulated proliferation of VSMCs. Treatment of VSMCs with FBS significantly increased the proliferation and viability of VSMCs, but this effect was blocked by melatonin (Fig. 1A and B). Next, we explored whether melatonin inhibits cell cycle progression in VSMCs. We found that melatonin reduced the level of phosphorylated retinoblastoma protein (p-Rb) (Fig. 1C). Flow cytometric analysis of cell cycles showed that melatonin attenuated serum-stimulated progression from G1 to S phase. In the melatonin-treated samples, the cells accumulated in G1 phase (73.2% in melatonin-treated cells vs. 64.3% in control cells) with a concomitant decrease in the percentage of cells in S phase (16.9% in melatonin-treated cells vs. 23.2% in control cells). Thus, melatonin arrests cells at the G1 cell cycle phase, blocking proliferation (Fig. 1D).
Figure 1.
Effects of melatonin on VSMC proliferation. Primary rat VSMCs were serum-starved for 24 h, and then treated with 10% FBS with or without 2 mM melatonin for 24 h. Relative (A) cell number and (B) cell viability showing the effect of melatonin in FBS-stimulated VSMCs. Data are presented as the mean ± SEM (n=3). ***P<0.001. Quiescent cells were treated with 10% FBS with or without 2 mM melatonin. (C) Phosphorylated Rb levels in primary rat VSMCs. (D) Representative flow cytometric data derived from analysis of cell cycle progression in VSMCs. VSMC, vascular smooth muscle cell; p-, phosphorylated; Rb, retinoblastoma protein.
Melatonin induces mitochondrial energetic stress and Sestrin2 expression in VSMCs
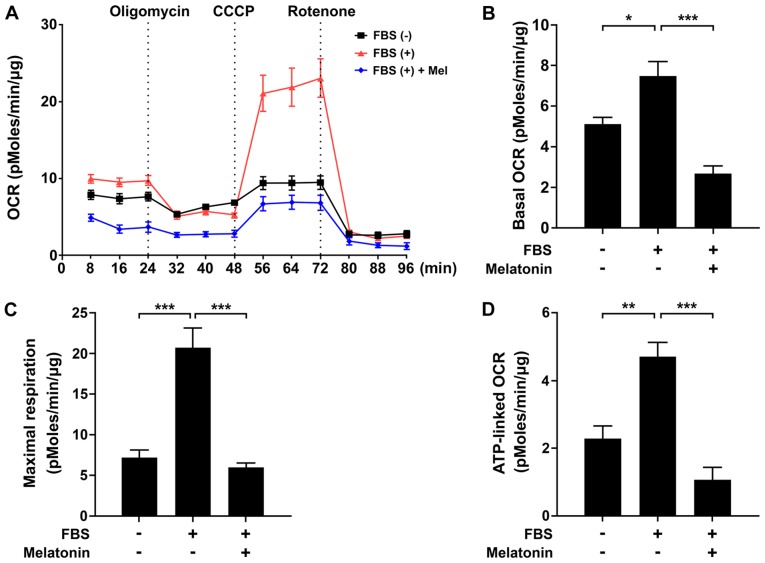
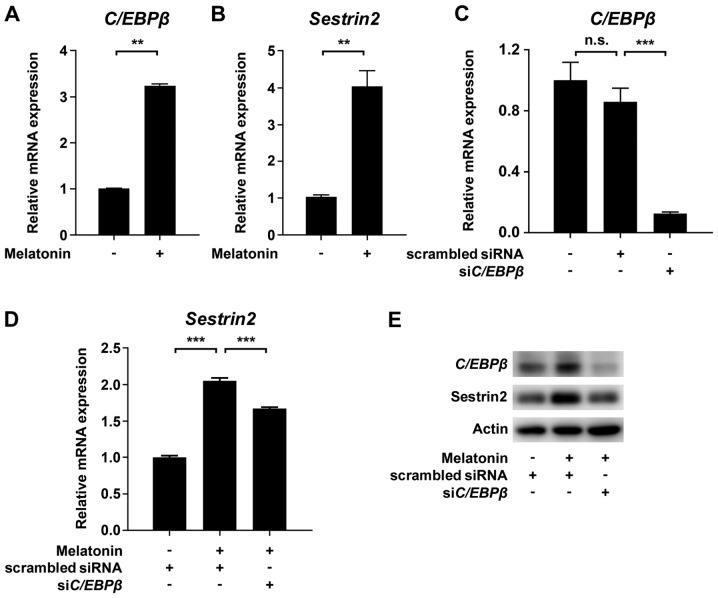
To determine the mechanism by which melatonin inhibits VSMC proliferation, we evaluated the effect of melatonin on mitochondrial function using an XF analyzer. We measured basal OCR over time, and then observed the effects of the mitochondrial inhibitors oligomycin, CCCP, and rotenone. Treatment of VSMCs with melatonin caused a significant decrease in the major parameters of mitochondrial function, including basal OCR and maximal respiration, as well as ATP-linked respiration (Fig. 2A-D). After demonstrating that melatonin induced mitochondrial dysfunction, we evaluated CCAAT/enhancer binding protein β (C/EBPβ) and Sestrin2, which promote the response to mitochondrial stress (15,16). As shown in Fig. 3A and B, treatment of VSMCs with melatonin increased C/EBPβ and Sestrin2 protein levels. Furthermore, siRNA-mediated knockdown of C/EBPβ inhibited melatonin-induced upregulation of Sestrin2 mRNA and protein level (Fig. 3C-E). Taken together, these data demonstrate that melatonin-induced mitochondrial energetic stress results in upregulation of Sestrin2 via C/EBPβ.
Figure 2.
Effects of melatonin on mitochondrial oxygen consumption rate and ATP production in VSMCs. (A) Mitochondrial oxygen consumption rate in FBS-stimulated primary rat VSMCs treated with or without melatonin for 24 h, following exposure to oligomycin (1 µM), CCCP (2 µM) and rotenone (1 µM). (B) Rates of basal respiration, (C) maximal respiratory capacity and (D) ATP-linked respiration in VSMCs shown in (A). Data were normalized against protein content and are presented as the mean ± SEM (n=4). *P<0.05; **P<0.01; ***P<0.001. VSMC, vascular smooth muscle cell; OCR, oxygen consumption rate; CCCP, carbonyl cyanide 3-chlorophenylhydrazone.
Figure 3.
Effects of melatonin on Sestrin2 and C/EBPβ in VSMCs. Levels of (A) C/EBPβ and (B) Sestrin2 mRNAs in primary rat VSMCs treated with or without 2 mM melatonin. (C-E) Primary rat VSMCs transfected with scrambled siRNA or siRNA targeting C/EBPβ (siC/EBPβ) for 24 h were serum-starved for 24 h, and then incubated with 10% FBS with or without 2 mM melatonin. Levels of (C) C/EBPβ and (D) Sestrin2 mRNA and (E) C/EBPβ and Sestrin2 protein in VSMCs treated with or without 2 mM melatonin. Data were normalized to the 36B4 mRNA level, and expressed as the mean ± SEM of three independent experiments. **P<0.01; ***P<0.001. n.s., not significant; VSMC, vascular smooth muscle cell; siRNA, small interfering RNA.
Melatonin-induced Sestrin2 prevents VSMC apoptosis
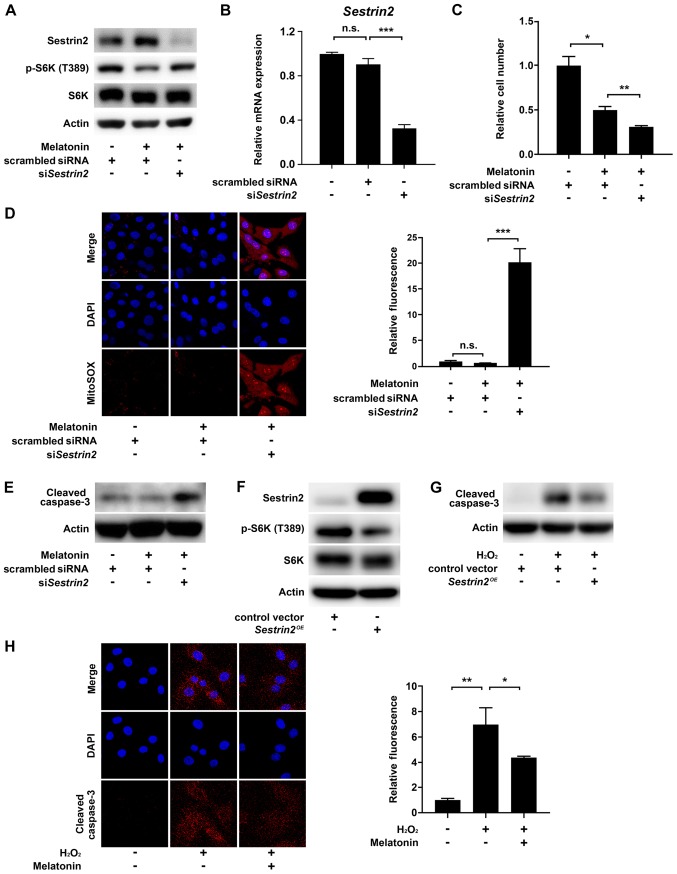
Next, we sought to confirm that Sestrin2 negatively regulates mTORC1 activity in VSMCs, consistent with previous results (7,8,17). As mTORC1 controls cell growth via direct phosphorylation of Thr389 in the hydrophobic motif site of p70S6 kinase 1 (S6K1), we determined mTORC1 activity by measuring the levels of S6K (T389) phosphorylation (18). The results showed that melatonin increased Sestrin2 but decreased the levels of S6K (T389) phosphorylation; this effect was abolished by siRNA-mediated knockdown of Sestrin2 (siSESN2) in VSMCs (Fig. 4A). Successful knockdown of Sestrin2 was confirmed by quantitative PCR (Fig. 4B). Given that melatonin-induced Sestrin2 suppressed mTORC1 activity, we next asked whether inhibition of Sestrin2 would restore melatonin-induced suppression of VSMC proliferation. Unexpectedly, siSESN2 further decreased the number of VSMCs beyond the reduction induced by melatonin treatment (Fig. 4C). Because Sestrin2 functions as a ROS scavenger (19), we investigated whether the siSESN2-induced decrease in cell number was caused by cell death triggered by intracellular ROS production. Indeed, siSESN2 significantly increased mitochondria-derived superoxide production (stained by MitoSOX) and the level of cleaved caspase 3 in melatonin-treated VSMCs (Fig. 4D and E). Moreover, Sestrin2 overexpression decreased mTORC1 activity in VSMCs and the level of cleaved caspase 3 in H2O2-treated VSMCs, further confirming the anti-proliferative and -apoptotic function of Sestrin2 (Fig. 4F and G). Finally, immunofluorescence using a cleaved caspase 3-specific antibody showed that melatonin reduced H2O2-induced apoptosis of VSMCs (Fig. 4H). Collectively, these data indicated that melatonin-induced Sestrin2 not only decreases VSMC proliferation, but also blocks apoptosis of VSMCs by preventing excessive ROS generation.
Figure 4.
Effects of si-Sestrin2 on ROS generation and apoptosis in melatonin-treated VSMCs. Primary rat VSMCs transfected with scrambled siRNA or siSestrin2 for 24 h were serum-starved for 24 h, and then incubated with 10% FBS with or without 2 mM melatonin. Levels of (A) phosphorylated S6K (T389) and (B) Sesn2 mRNA in VSMCs. (C) Relative VSMC number. Data are presented as the mean ± SEM. (D) Representative fluorescence micrographs showing intracellular superoxide production in VSMCs. Bar graph shows quantitation of MitoSOX fluorescence intensity (red) with nuclear counterstaining with DAPI (blue), expressed as the mean ± SEM. (E) Level of cleaved caspase 3 protein in VSMCs. (F and G) Primary rat VSMCs transfected with the Sestrin2-expression vector for 24 h were serum-starved for 24 h, and then incubated with or without 1 mM H2O2 for 6 h. (F) Phosphorylated S6K (T389) and (G) cleaved caspase 3 protein levels in VSMCs. (H) Primary rat VSMCs were pretreated with 2 mM melatonin for 24 h and then treated with or without 1 mM H2O2 for 6 h. Immunofluorescence images of cleaved caspase-3 (red) with nuclear counterstaining with DAPI (blue). Bar graphs show quantitation of cleaved caspase-3 immunofluorescence intensity, expressed as the mean ± SEM. Magnification, x100. *P<0.05; **P<0.01; ***P<0.001. n.s., not significant; VSMC, vascular smooth muscle cell; si/siRNA, small interfering RNA; ROS, reactive oxygen species.
Discussion
The results of this study demonstrate that melatonin induces mitochondrial energetic stress in VSMCs, leading to induction of Sestrin2 via C/EBPβ. Melatonin-induced upregulation of Sestrin2 suppressed mTORC1 activity in VSMCs, contributing to suppression of VSMC proliferation. Furthermore, melatonin decreased VSMC apoptosis through inhibition of ROS accumulation by Sestrin2.
Although some previous studies reported that melatonin improves mitochondrial respiratory function and enhances ATP production, more recent studies showed that a supra-physiologic dose of melatonin can be harmful to the kidney and other tissues (11). Moreover, melatonin induces mitochondrial depolarization and decreases oxidative phosphorylation by inhibiting complex IV in some cancer cells (20,21). In accordance with these findings, we also showed that melatonin increased mitochondrial energetic stress which was evident by decrease in basal OCR and maximal respiration, as well as ATP-linked respiration. Consequently, mitochondrial energetic stress by melatonin was responsible for induction of Sestrin2 in VSMCs.
Sestrin2 is an important regulator of mitohormesis in diverse tissues, including skeletal/cardiac muscle and brown adipose tissue (9). Previous study demonstrated that inhibitors of mitochondrial respiratory chain complex I and III induce transcription of Sestrin2(22). Here, we showed that melatonin-induced Sestrin2 upregulation is mediated by C/EBPβ, which is activated as an aspect of mitochondrial retrograde signaling in response to mitochondrial stress (23). Consistent with previous studies reporting Sestrin2-mediated inhibition of mTORC1 in various metabolic diseases, we also found that melatonin plays a critical role in limiting VSMC proliferation by the Sestrin2/mTORC1 axis.
It should be noted that knockdown of Sestrin2 promoted apoptosis in melatonin-treated VSMCs. Several lines of evidence show that apoptosis of VSMCs in atherosclerosis is sufficient to induce features of plaque vulnerability by thinning the fibrous cap and enlarging the necrotic core, ultimately resulting in plaque rupture (24). Accordingly, decreasing VSMC apoptosis could retard plaque size and instability, and thus has therapeutic potential (25). Sestrin2 promotes detoxification of ROS in two ways: By recycling peroxiredoxin as a part of an oxidoreductase enzyme, and by activating an antioxidant transcriptional program by stabilizing Nrf2(6). In line with previous findings, we also observed that melatonin-induced Sestrin2 prevents apoptosis of VSMCs by limiting excessive ROS accumulation. Therefore, melatonin represents a possible means to decrease both VSMC proliferation and apoptosis. Although it will be necessary to perform animal experiments to determine if these findings are valid in vivo, collectively, our results support previous studies showing that melatonin has anti-atherosclerotic effects in preclinical models (26,27).
In summary, we showed here that melatonin suppresses VSMC proliferation and apoptosis via Sestrin2-mediated inhibition of mTORC1 and ROS scavenging, respectively. Given its ability to control proliferation and apoptosis, melatonin represents a potential lead compound for prevention of vessel lumen constriction during the course of atherosclerosis and restenosis. Further studies should investigate how melatonin causes mitochondrial stress.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Research Foundation of Korea (NRF) grants funded by the Ministry of Science and ICT (grant nos. NRF 2017M3A9G7073086 and NRF-2018R1A2A1A05077703), an NRF grant funded by the Ministry of Education (grant no. NRF-2017R1A6A3A04010231), and grants from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute funded by the Ministry of Health and Welfare, Republic of Korea (grant nos. HI16C1501 and HI15C0001).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
SHL and JKB conceptualized the study, designed the research and performed the experiments. MP performed the experiments. SWK, SWL, JGK, and IKL analyzed and interpreted the data. YKC and KGP designed the experiments, analyzed and interpreted the data, wrote and edited the manuscript, and supervised the project. All authors reviewed the data and provided feedback on the manuscript.
Ethics approval and consent to participate
The present study was approved by The Animal Care and Use Committee of Kyunpook National University School of Medicine (approval no. KNU-2011-0096-1).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sehgel NL, Vatner SF, Meininger GA. ‘Smooth muscle cell stiffness syndrome’-Revisiting the structural basis of arterial stiffness. Front Physiol. 2015;6(335) doi: 10.3389/fphys.2015.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orford JL, Selwyn AP, Ganz P, Popma JJ, Rogers C. The comparative pathobiology of atherosclerosis and restenosis. Am J Cardiol. 2000;86:6H–11H. doi: 10.1016/s0002-9149(00)01094-8. [DOI] [PubMed] [Google Scholar]
- 3.Bennett MR, Sinha S, Owens GK. Vascular smooth muscle cells in atherosclerosis. Circ Res. 2016;118:692–702. doi: 10.1161/CIRCRESAHA.115.306361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosner D, McCarthy N, Bennett M. Rapamycin inhibits human in stent restenosis vascular smooth muscle cells independently of pRB phosphorylation and p53. Cardiovasc Res. 2005;66:601–610. doi: 10.1016/j.cardiores.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Martin KA, Rzucidlo EM, Merenick BL, Fingar DC, Brown DJ, Wagner RJ, Powell RJ. The mTOR/p70 S6K1 pathway regulates vascular smooth muscle cell differentiation. Am J Physiol Cell Physiol. 2004;286:C507–C517. doi: 10.1152/ajpcell.00201.2003. [DOI] [PubMed] [Google Scholar]
- 6.Lee JH, Budanov AV, Karin M. Sestrins orchestrate cellular metabolism to attenuate aging. Cell Metab. 2013;18:792–801. doi: 10.1016/j.cmet.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng M, Yin N, Li MO. Sestrins function as guanine nucleotide dissociation inhibitors for Rag GTPases to control mTORC1 signaling. Cell. 2014;159:122–133. doi: 10.1016/j.cell.2014.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ro SH, Semple I, Ho A, Park HW, Lee JH. Sestrin2, a regulator of thermogenesis and Mitohormesis in brown adipose tissue. Front Endocrinol (Lausanne) 2015;6(114) doi: 10.3389/fendo.2015.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JH, Budanov AV, Talukdar S, Park EJ, Park HL, Park HW, Bandyopadhyay G, Li N, Aghajan M, Jang I, et al. Maintenance of metabolic homeostasis by Sestrin2 and Sestrin3. Cell Metab. 2012;16:311–321. doi: 10.1016/j.cmet.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luchetti F, Canonico B, Betti M, Arcangeletti M, Pilolli F, Piroddi M, Canesi L, Papa S, Galli F. Melatonin signaling and cell protection function. FASEB J. 2010;24:3603–3624. doi: 10.1096/fj.10-154450. [DOI] [PubMed] [Google Scholar]
- 12.Favero G, Moretti E, Bonomini F, Reiter RJ, Rodella LF, Rezzani R. Promising antineoplastic actions of melatonin. Front Pharmacol. 2018;9(1086) doi: 10.3389/fphar.2018.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao X, Sun J, Su W, Shan H, Zhang B, Wang Y, Shabanova A, Shan H, Liang H. Melatonin protects against lung fibrosis by regulating the Hippo/YAP pathway. Int J Mol Sci. 2018;19(pii: E1118) doi: 10.3390/ijms19041118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Seo K, Ki SH, Shin SM. Sestrin2-AMPK activation protects mitochondrial function against glucose deprivation-induced cytotoxicity. Cell Signal. 2015;27:1533–1543. doi: 10.1016/j.cellsig.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Q, Wang J, Levichkin IV, Stasinopoulos S, Ryan MT, Hoogenraad NJ. A mitochondrial specific stress response in mammalian cells. EMBO J. 2002;21:4411–4419. doi: 10.1093/emboj/cdf445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parmigiani A, Nourbakhsh A, Ding B, Wang W, Kim YC, Akopiants K, Guan KL, Karin M, Budanov AV. Sestrins inhibit mTORC1 kinase activation through the GATOR complex. Cell Rep. 2014;9:1281–1291. doi: 10.1016/j.celrep.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saxton RA, Sabatini DM. mTOR Signaling in Growth, metabolism and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science. 2004;304:596–600. doi: 10.1126/science.1095569. [DOI] [PubMed] [Google Scholar]
- 20.Loureiro R, Magalhaes-Novais S, Mesquita KA, Baldeiras I, Sousa IS, Tavares LC, Barbosa IA, Oliveira PJ, Vega-Naredo I. Melatonin antiproliferative effects require active mitochondrial function in embryonal carcinoma cells. Oncotarget. 2015;6:17081–17096. doi: 10.18632/oncotarget.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarti P, Magnifico MC, Altieri F, Mastronicola D, Arese M. New evidence for cross talk between melatonin and mitochondria mediated by a circadian-compatible interaction with nitric oxide. Int J Mol Sci. 2013;14:11259–11276. doi: 10.3390/ijms140611259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garaeva AA, Kovaleva IE, Chumakov PM, Evstafieva AG. Mitochondrial dysfunction induces SESN2 gene expression through activating transcription Factor 4. Cell Cycle. 2016;15:64–71. doi: 10.1080/15384101.2015.1120929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biswas G, Guha M, Avadhani NG. Mitochondria-to-nucleus stress signaling in mammalian cells: Nature of nuclear gene targets, transcription regulation, and induced resistance to apoptosis. Gene. 2005;354:132–139. doi: 10.1016/j.gene.2005.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD, Bennett MR. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006;12:1075–1080. doi: 10.1038/nm1459. [DOI] [PubMed] [Google Scholar]
- 25.Lyon CA, Johnson JL, Williams H, Sala-Newby GB, George SJ. Soluble N-cadherin overexpression reduces features of atherosclerotic plaque instability. Arterioscler Thromb Vasc Biol. 2009;29:195–201. doi: 10.1161/ATVBAHA.108.178087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng X, Wan Y, Xu Y, Zhou Q, Wang Y, Zhu H. Melatonin alleviates myosin light chain kinase expression and activity via the mitogen-activated protein kinase pathway during atherosclerosis in rabbits. Mol Med Rep. 2015;11:99–104. doi: 10.3892/mmr.2014.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu ZP, Fang XL, Fang N, Wang XB, Qian HY, Cao Z, Cheng Y, Wang BN, Wang Y. Melatonin ameliorates vascular endothelial dysfunction, inflammation, and atherosclerosis by suppressing the TLR4/NF-κB system in high-fat-fed rabbits. J Pineal Res. 2013;55:388–398. doi: 10.1111/jpi.12085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.