Abstract
Nicotinic acetylcholine receptors (nAChRs) are the primary target for nicotine, the addictive component in tobacco products. These pentameric receptors are made up of various subunits which contribute to the diverse functions of nAChR subtypes. The β3 subunit of the nAChR has been understudied in nicotine dependence, even though it is expressed in brain regions important for drug reward. Therefore, we assessed nicotine dependence behaviors in β3 wildtype (WT) and knockout (KO) male and female mice. We evaluated nicotine reward in the conditioned place preference (CPP) test and then measured nicotine withdrawal signs after chronic exposure to the drug.. For the withdrawal studies, mice were continuously infused with 24 mg/kg/day of nicotine using surgically implanted osmotic mini-pumps for 14 days. Mini-pumps were removed at day 15, and withdrawal signs (somatic signs, hyperalgesia, anhedonia-like measure using the sucrose preference test and anxiety-like behaviors using the light dark boxes) were collected at 24 hours intervals for three days following spontaneous withdrawal of nicotine. Nicotine-induced CPP did not differ between β3 KO and WT mice. β3 KO mice displayed similar somatic symptoms and hyperalgesia compared to WT mice but showed significant absence in affective (anhedonia and anxiety-like behaviors) withdrawal signs in nicotine- dependent mice. These observations suggest that the β3 nicotinic subunits does not seem to influence nicotine reward but plays an important role in affective nicotine withdrawal signs. Given the health burden of tobacco use disorder and the modest effect of smoking cessation aids, it is important to understand underlying factor contributing to nicotine dependence. The results of this study will further our knowledge of the role of the β3 nAChR subunit in nicotine reward and withdrawal behaviors in hopes of finding new molecular targets for smoking cessation aids.
Introduction
Tobacco use disorder is associated with allelic variation in CHRNB3,(Bierut, 2007; Chen et al., 2012; Saccone et al., 2007; Zeiger et al., 2008), a gene that encodes for the β3 subunit of the nicotinic acetylcholine receptor (nAChR)(Thorgeirsson et al., 2010). The β3 subunit is not widely distributed in the brain, but is expressed in brain regions important in nicotine reward and withdrawal such as the ventral tegmental area, substantia nigra, nucleus accumbens and medial habenula (Deneris et al., 1989; Grady et al., 2009, 2007). In addition, nicotinic receptors containing the β3 subunit have been implicated in dopamine release from terminals in the mouse striatum (Cui et al., 2003; Sharon R. Grady et al., 2007). Further, research has shown that these brains regions are important for nicotine reward and withdrawal (Picciotto et al., 1998; Salas et al 2004; Tapper et al., 2004; Zhao-shea et al., 2015; Zhao-Shea et al 2013).
Nevertheless, surprisingly little is known about the role of β3-containing nAChRs in nicotine reward and withdrawal. Genetically modified mice may serve as important tools to understand the impact of the β3 subunit on nicotine reward and withdrawal behaviors. A recent study reported that oral nicotine consumption in the two-bottle choice paradigm was decreased in β3 knockout (KO) mice (Kamens et al., 2015) suggesting that the β3 subunit may partially mediate oral nicotine intake.
To extend these findings, in this study, we assessed nicotine dependence behaviors in β3 wildtype (WT) and KO male and female mice. We evaluated nicotine reward in the conditioned place preference (CPP) test and then measured nicotine withdrawal signs after chronic exposure to nicotine. The findings of this study will further elucidate the role of the nicotinic β3 subunit in nicotine dependence.
Materials and Methods
Animals
Healthy viable mice null for the β3 nicotinic subunit were provided by Dr. Jerry Stitzel at Institute for Behavioral Genetics, University of Colorado (Boulder, CO). The β3 KO and their WT littermates were bred in an animal care facility at Virginia Commonwealth University (Richmond, VA). The β3 KO mice were originally reported by Cui et al. (2003). All mice used in each experiment were backcrossed to C57BL/6J (Jackson Laboratories, Bar Harbor, ME) at least for 9 generations. Mutants and WT controls were obtained by crossing with heterozygote mice to control for any irregularities that might arise with crossing only mutant mice. Mice were group-housed (1–4 per cage) in a temperature and humidity-controlled animal care facility approved by Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Mice had free access to food and water under a 12-h light/dark cycle (lights on at 6:00 am) schedule. Male and female mice were 8–10 weeks old at the start of the experiments. All experiments were performed during the light cycle and the study was approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University. All studies were carried out in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Drugs
(−)-Nicotine hydrogen tartrate [(−)-1-methyl-2-(3-pyridyl) pyrrolidine (+)-bitartrate] was purchased from Sigma-Aldrich Inc. (St. Louis, MO, USA). All doses of nicotine refer to the free-base form. Freshly prepared solutions were given to mice at 10 ml/kg.
Nicotine conditioned place preference studies
An unbiased CPP paradigm was performed, as we previously described (Kota, Martin, Robinson, & Damaj, 2007). Briefly, the CPP apparatus consisted of three chambers in a linear arrangement (Med Associates, St Albans, VT). The CPP apparatus (Med Associates, St. Albans, VT, ENV3013) consisted of white and black chambers (20×20×20 cm each), which differed in overall color and floor texture (white mesh or black rod). These chambers were separated by a smaller gray chamber with a smooth PVC floor. Partitions could be removed to allow access from the gray chamber to the black and white chambers. On day 1, male and female mice were confined to the middle chamber for a 5-min habituation and then allowed to freely move between all three chambers for 15 min. Time spent in each chamber was recorded, and these data were used to populate groups of approximately equal bias in baseline chamber preference. Twenty-minute conditioning sessions occurred twice a day (days 2–4). During conditioning sessions, mice were confined to one of the larger chambers. The saline groups received saline in one large chamber in the morning and saline in the other large chamber in the afternoon. The nicotine group received nicotine (0.1, 0.5 or 1 mg/kg, s.c.) in one large chamber and saline in the other large chamber. Treatments were counterbalanced equally in order to ensure that some mice received the unconditioned stimulus in the morning while others received it in the afternoon. The nicotine-paired chamber was randomized among all groups. Sessions were 4hr apart and were conducted by the same investigator. On test day (day 5), mice were allowed access to all chambers for 15 min in a drug free state. The preference score was calculated by determining the difference between time spent in the drug paired side during test day versus the time in drug paired side during the baseline day. Below the specifics for the dose regimen and pretreatment time for each drug in the individual experiments are mentioned.
Spontaneous Nicotine Withdrawal Studies
Male and female mice were anesthetized by inhaling isoflurane/oxygen vapor mixture (1–3%). Alzet osmotic minipumps (model 2000; Alzet Corporation, Cupertino, CA) were then implanted subcutaneously within the animals for 14 days. The Alzet minipumps were filled with either nicotine or saline solutions and inserted by making incision parallel to the spine at shoulder level of the mouse. The wound was closed using wound clips and the mice were placed in a surgery room for recovery before using them in experiments. Post-operative care was done for 14 days by observing the animals daily. For all of the procedures, the dose of nicotine was 24 mg/kg/day, calculated according to the mouse weight. On day 15, spontaneous nicotine withdrawal was induced by removing the minipumps under isoflurane anesthesia in aseptic surgical conditions. The experiment was adapted as previously described (Damaj, Kao, & Martin, 2003; Jackson, Martin, Changeux, & Damaj, 2008). On day 15 mice were tested for anxiety-like behavior in the plus-maze test, somatic signs and hyperalgesia (day 15). On day 16 mice were tested for sucrose preference. Mice were first evaluated for 5 min in the plus maze test for anxiety-related behavior. Time spent on the open arms of the plus maze was assessed as a measure of anxiety-related response. The number of arm crosses between the open and closed arms was also counted as a measure of locomotor activity. The plus maze assessment was immediately followed by a 20-min observation of somatic signs measured as paw and body tremors, head shakes, backing, jumps, curls, and ptosis. Mice were placed in clear activity cages without bedding for the observation period. The total number of somatic signs was tallied for each mouse and the average number of somatic signs during the observation period was plotted for each test group. Hyperalgesia was evaluated using the hot plate test immediately following the somatic sign observation period. Mice were placed into a 10-cm wide glass cylinder on a hot plate (Thermojust Apparatus, Richmond, VA) maintained at 52°C. The latency to reaction time (jumping or paw licking) was recorded. On day 16, sucrose preference test was used to investigate the anhedonia-like behavior in rodents after the induction of nicotine withdrawal. In this experiment, mice were individually housed and acclimated to cages with food and water. Mice had free access to two 30 ml sipper tubes containing tap water for 3 days as a baseline. Animals then were exposed to two 30 ml sipper tubes, one with tap water and the other with 2% sucrose solution. To prevent any bias, tube placement was switched daily. The measurements of sucrose preference were taken at day 2 after removal of osmotic minipumps. Sucrose preference was determined as the percentage of 2% sucrose volume consumed over the total fluid intake volume. Sucrose preference (percentage) was calculated as follows: preference = [sucrose solution intake (ml)/total fluid intake (ml)] × 100. The experiment was adapted as previously described (Alkhlaif, Bagdas, Jackson, Park, & Damaj, 2017; Pothion, Bizot, Trovero, & Belzung, 2004; Toma et al., 2017). The specific testing sequence was chosen based on our prior studies showing that this order of testing reduced within-group variability and produced the most consistent results (Jackson et al., 2008). All studies were performed by an observer blinded to experimental treatment.
Statistical analysis
The data were analyzed with GraphPad Prism software, version 6 (GraphPad Software, Inc., La Jolla, CA), and expressed as mean ± SEM. Mice were analyzed with an ordinary two-way analysis of variance (ANOVA) test with genotype (KO versus WT) and treatment (saline versus nicotine) as between-subject factors in conjunction with a Tukey post-hoc test. Prior to the ANOVA test. All experiments animals with the same genotype were randomly allocated to experimental groups. Differences were considered significant at p <0.05.
Results
The Role of β3 nicotinic subunits in nicotine CPP
β3 WT and KO mice were conditioned with either saline or nicotine at doses of (0.1, 0.5, or 1 mg/kg; s.c.) for 3 days in the CPP paradigm. As displayed in Figure 1, nicotine treated β3 WT and KO mice showed a significant preference in comparison with saline treated counterparts [Ftreatment (3,73) = 20.29; p <0.0001]. There was no significant effect of genotype in nicotine CPP [Fgenotype (1, 73) = 0.04152; p = 0.8391]. In addition, the interaction between nicotine treatment and genotype was not significant between the same subjects [Finteraction (3, 73) = 0.2205; p = 0.8819].
Figure 1: Nicotine CPP in β3 WT and KO mice.
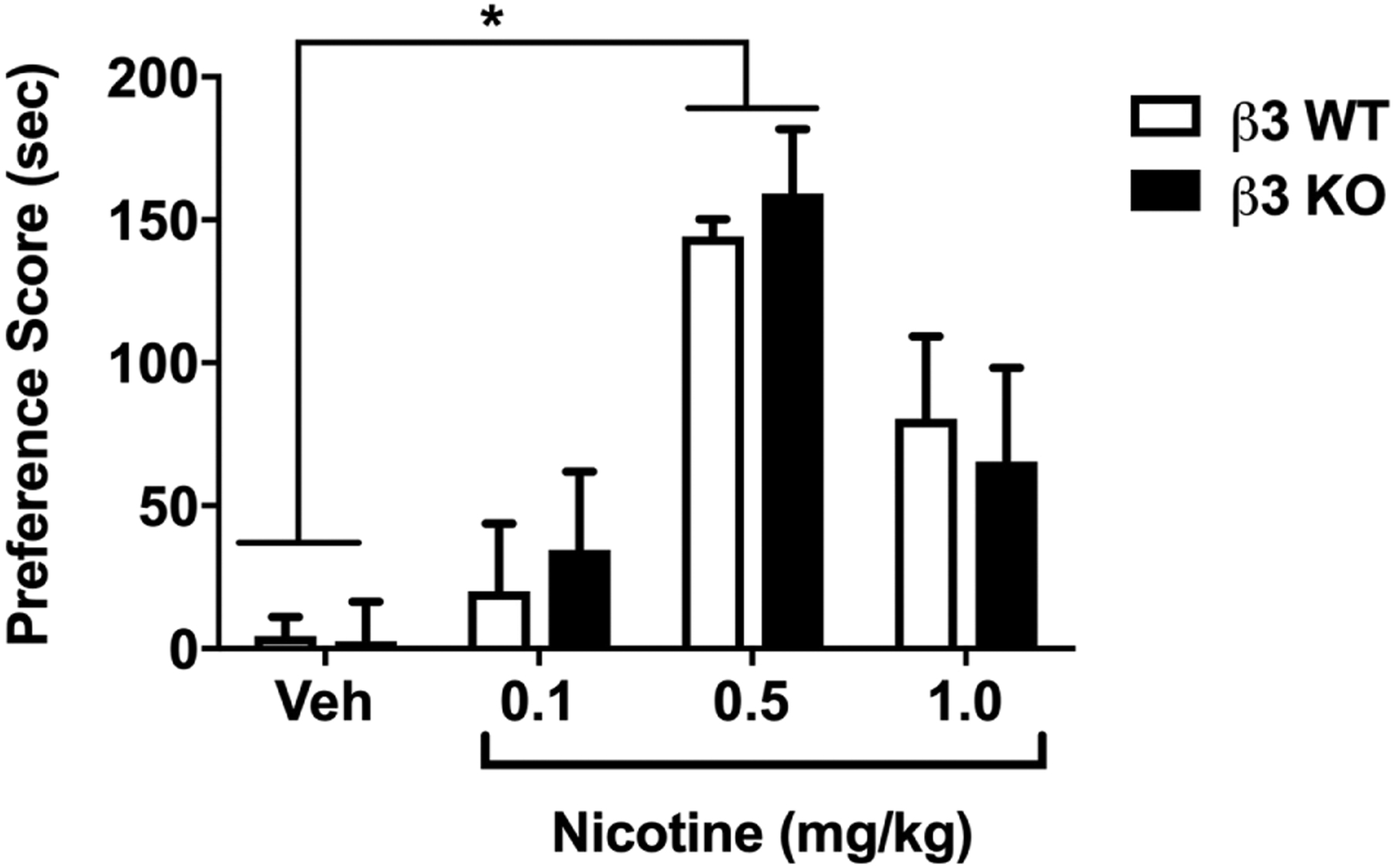
β3 WT and KO mice were conditioned with either s.c. saline or nicotine at doses of (0.1, 0.5,1 mg/kg) for 3 days. A robust CPP was observed in nicotine-conditioned β3 WT and KO mice in comparison with vehicle. *Denotes p<0.05 vs saline control. #Denotes p<0.05 nicotine dose of 0.5 mg/kg vs nicotine dose of 0.1 mg/kg in both WT and KO. Each point represents the mean±SEM of n=11 (6 male and 5 female) mice per group.
β3 nicotinic subunits are involved in affective not physical signs of nicotine withdrawal
Spontaneous nicotine withdrawal was induced in β3 WT and KO mice via removal of minipumps and nicotine withdrawals signs (anxiety-like behavior, somatic signs and hyperalgesia) were measured. In Fig. 2A, nicotine withdrawn β3 WT mice displayed anxiety-related behavior in the plus maze compared to their saline treated counterparts [Ftreatment (1, 28) = 22.08; p <0.0001]. However, β3 KO mice undergoing nicotine withdrawal did not display signs of anxiety-like behavior. Indeed, nicotine withdrawal induced anxiety-like behavior in β3 WT mice but not β3 KO mice [Fgenotype (1, 28) = 23.12; p = 0.0001]. Analyses of the average total number of arm crosses for each treatment group revealed no significant difference among groups (supplementary table 1) indicating that the observed reductions in time mice spent in the open arms of the plus maze were not related to changes in mice locomotor activity.
Figure 2: Spontaneous Nicotine Withdrawal in β3 WT and KO mice.
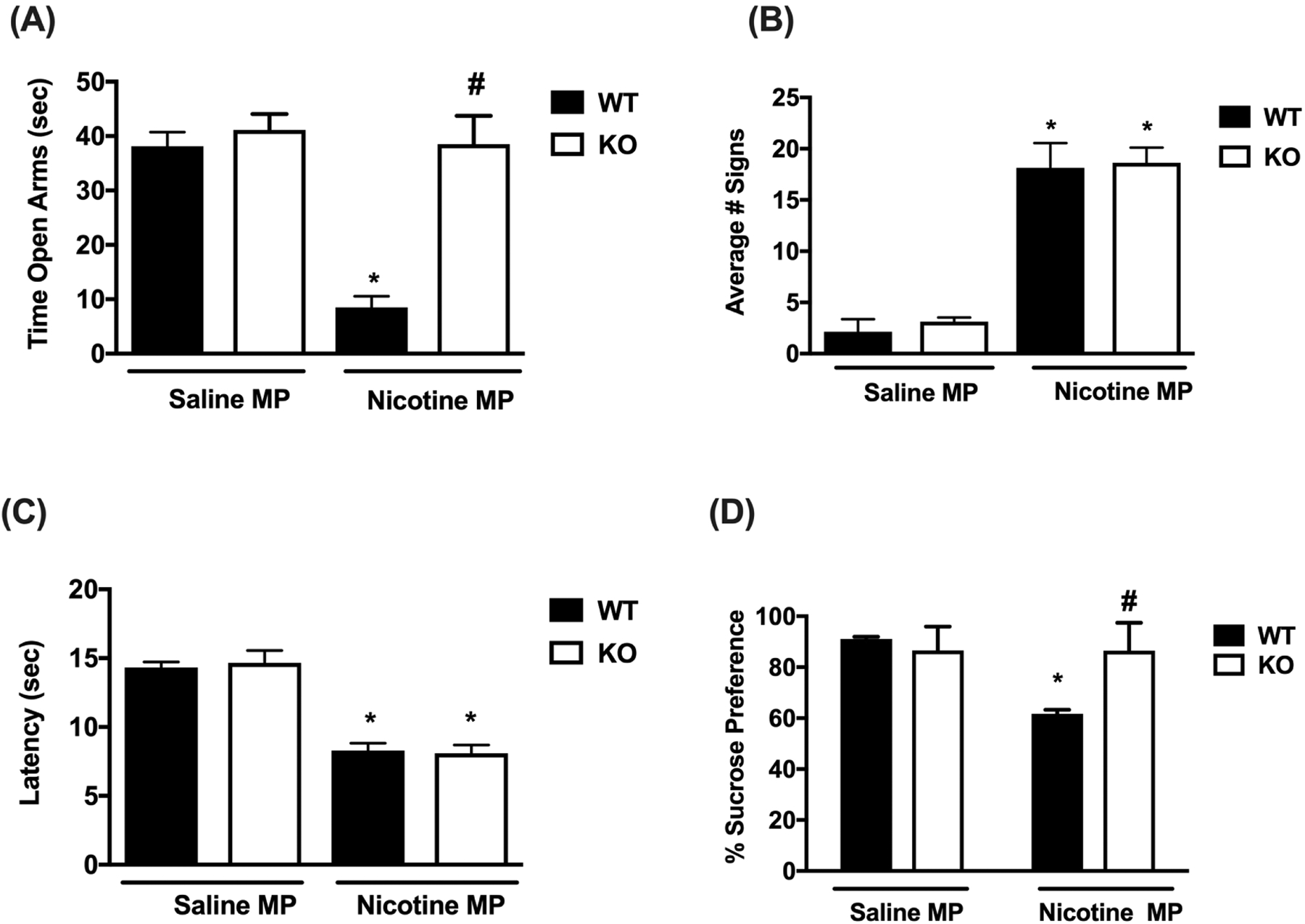
β3 WT and KO mice were chronically infused with saline or nicotine (24 mg/kg/day) for 14 days. On day 15, minipumps were removed to induce spontaneous nicotine withdrawal. On day 15 mice were tested for: A) anxiety-like behaviors (Time spent in the open arm), B) somatic signs, C) hyperalgesia (hot plate latency) and on day 16 mice were tested for: D) sucrose preference. A) Nicotine withdrawn β3 WT mice exhibit increased anxiety-like behavior, but this effect is not shown in nicotine withdrawn β3 KO mice. B) β3 WT and KO mice render nicotine dependent both displayed increased somatic signs. C) Both β3 WT and KO mice undergoing nicotine withdrawal exhibited decreased hot plate latencies. D) Nicotine withdrawn β3 WT mice exhibit decreased sucrose preference, but this effect is not shown in nicotine withdrawn β3 KO mice. Each point represents the mean ± S.E.M. of n=8 (4 male and 4 female) mice per group. * Denotes p< 0.05 vs. Saline MP group, # Denotes p< 0.05 vs. Nicotine MP group.
In addition, there was a significant interaction between genotype and treatment [Finteraction (1, 28) = 15.45; p = 0.0005]. However, as shown in Fig. 2B β3 WT and KO mice undergoing spontaneous nicotine withdrawal both expressed somatic withdrawal signs compared to control littermates [Ftreatment (1, 28) = 117.1; p = 0.0001]. There was no significant effect of genotype [Fgenotype (1, 28) = 0.2655; p = 0.6104] or interaction [Finteraction (1, 28) = 0.02950; p = 0.8649]. In addition, nicotine withdrawn β3 WT and KO mice displayed significant hyperalgesia compared to control littermates [Ftreatment (1, 28) = 94.67; p = 0.0001; Fig. 2C]. There was no significant effect of genotype [Fgenotype (1, 28) = 0.01347; p = 0.9084] or interaction [Finteraction (1, 28) = 0.1811; p = 0.6737]. Furthermore, we measured sucrose preference in β3 WT and KO mice 48 hrs after induction of spontaneous withdrawal. Nicotine infused β3 WT mice demonstrated an attenuation of sucrose preference compared to their saline controls [Ftreatment (1, 28) = 30.16; p <0.0001]. This effect was absent in nicotine withdrawn β3 KO mice, indicating a significant difference in genotype [Fgenotype (1, 28) = 14.24, p = 0.0008]. Two-way ANOVA revealed a significant effect of interaction between genotype and treatment [Finteraction (1, 28) = 29.79; p < 0.0001; Fig. 2D].
Discussion
This study sought to examine the influence of β3 nicotinic subunits on two main features of nicotine dependence, reward and withdrawal by using β3 null mutant animals. To our knowledge, this is the first report demonstrating that β3 nAChRs is not necessary for nicotine conditioned reward but seems to mediate affective but not somatic signs of withdrawal in nicotine-dependent mice. Together these data provide evidence of the importance of the β3 subunit in nicotine dependence behaviors.
We determined the role of β3 nAChRs in the CPP test of reward. In CPP experiments, the magnitude of nicotine preference achieved did not differ between the β3 WT and KO mice following drug conditioning. Indeed, the dose of 0.5 mg/kg nicotine induced significant preference in both WT and KO mice. The lack of effect of the Chrnb3 gene on nicotine reward is in contrast with a recent report (Kamens et al., 2015) that showed a reduction in voluntary oral nicotine consumption in the 2-bottle choice test in mice lacking the β3 subunit compared with wildtype animals. This difference could be related to differences in behavioral paradigms used (CPP vs oral consumption) in the two studies.
The lack of effects for β3 nAChRs in CPP behaviors is in contrast with the results reported for the α6 nicotinic subunit. This is an important comparison since the gene that codes for the β3 subunit is located adjacent to the gene coding for the α6 subunit on human chromosome 8. In addition, these subunits co-assemble to form functional receptors such as α4α6β2β3* nAChRs, in reward relevant brain regions (Cui et al., 2003; Gotti et al., 2005). Work from our labs and others using α6 KO and selective α6-containing nAChRs showed an important role for this subunits in nicotine reward and reinforcement using the CPP test (Jackson, McIntosh, Brunzell, Sanjakdar, & Damaj, 2009; Sanjakdar et al., 2015) and IVSA in mice (Pons et al., 2008) and rats (Brunzell, Boschen, Hendrick, Beardsley, & McIntosh, 2010).
An important finding of our study is the observation that β3 nAChRs are not involved in the physical signs of nicotine withdrawal, as indicated by the lack of a reduction in somatic signs and the presence of hyperalgesia in nicotine-withdrawn β3 KO mice. In addition, we assessed two affective signs of nicotine withdrawal in β3 KO mice and found that β3 nAChRs are involved in both affective measures of nicotine withdrawal. Nicotine-dependent β3 KO mice displayed a lack of anxiety-related behavior in the plus maze, as well as a lack of decrease in sucrose preference. Of interest, β3 null mutant control mice in our withdrawal studies (Fig. 2) did not display any baseline significant behavioral differences from the WT counterpart animals in the plus-maze test. This is in contrast to an earlier study, where β3 KO mice displayed decreased baseline anxiety-like behavior in the elevated plus maze, light-dark box in comparison with their wildtype counterparts (Booker, Butt, Wehner, Heinemann, & Collins, 2007). This difference can probably be explained by the differences in genetic background of β3 KO mice between the studies. The β3 KO mice were maintained on a mixed genetic background (129Svj × C57BL/6) in the Booker et al. study, while our mice were backcrossed and maintained on C57BL/6J genetic background for more than 9 generations. Indeed, The Chrnb3 gene was reported to influence nicotine oral consumption in β3 KO mice on a C57BL/6 background, but not β3 KO mice on 129S6/SvEvTac background (Kamens et al., 2015). Finally, our data on β3 KO mice in withdrawal are consistent with previous studies assessing the role of β2 and α6 nAChRs in nicotine withdrawal in mice that showed that these two subunits are important for affective but somatic signs of withdrawal (Besson et al., 2006; Jackson et al., 2008, 2009; Stoker, Marks, & Markou, 2015). This study is not without limitations. While compensatory changes in other neuronal nicotinic receptors levels or function may have occurred and could have contributed to the behavioral outcomes in this study, deletion of the β3 gene did not affect expression of mRNA for other neuronal nAChR subunits in various brain regions (Cui et al, 2003). However, given the lack of a selective β3 nAChR antagonist available, these results are important for aiding in the understanding of potential β3 subunit-dependent pathways. Of note, both male and female mice were used in this study. Since no significant sex-differences were found, results were combined. Future studies with an a priori hypothesis focusing on sex differences are warranted.
Overall our results are consistent with several human genetic association studies where SNPs in CHRNB3 were found to be associated with “dizziness” experience from the first few cigarettes (Ehringer et al., 2010), the number of quit attempts (Hoft et al., 2009), nicotine dependence but not cigarette per day (Rice et al., 2012; Thorgeirsson et al., 2010).
Supplementary Material
Funding
This research was supported by the National Institutes of Health - National Institute on Drug Abuse: R01 DA032246 (MID), and T32 DA007027 (AJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare there is no conflict of interest to declare regarding the publication of this article.
References
- Alkhlaif Y, Bagdas D, Jackson A, Park AJ, & Damaj IM (2017). Assessment of nicotine withdrawal-induced changes in sucrose preference in mice. Pharmacology Biochemistry and Behavior, 161 10.1016/j.pbb.2017.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson M, David V, Suarez S, Cormier A, Cazala P, Changeux JP, & Granon S (2006). Genetic dissociation of two behaviors associated with nicotine addiction: Beta-2 containing nicotinic receptors are involved in nicotine reinforcement but not in withdrawal syndrome. Psychopharmacology, 187(2), 189–199. 10.1007/s00213-006-0418-z [DOI] [PubMed] [Google Scholar]
- Bierut LJ (2007). Genetic variation that contributes to nicotine dependence. Pharmacogenomics, 8(8), 881–883. 10.2217/14622416.8.8.881 [DOI] [PubMed] [Google Scholar]
- Booker TK, Butt CM, Wehner JM, Heinemann SF, & Collins AC (2007). Decreased anxiety-like behavior in beta3 nicotinic receptor subunit knockout mice. Pharmacology Biochemistry and Behavior, 87, 146–157. 10.1016/j.pbb.2007.04.011 [DOI] [PubMed] [Google Scholar]
- Brunzell DH, Boschen KE, Hendrick ES, Beardsley PM, & McIntosh JM (2010). α-conotoxin MII-sensitive nicotinic acetylcholine receptors in the nucleus accumbens shell regulate progressive ratio responding maintained by nicotine. Neuropsychopharmacology, 35(3), 665–673. 10.1038/npp.2009.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LS, Baker TB, Grucza R, Wang JC, Johnson EO, Breslau N, … Bierut LJ (2012). Dissection of the phenotypic and genotypic associations with nicotinic dependence. Nicotine and Tobacco Research, 14(4), 425–433. 10.1093/ntr/ntr231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Booker TK, Allen RS, Grady SR, Whiteaker P, Marks MJ, Heinemann SF (2003). The B3 Nicotinic Receptor Subunit: A Component of α-Conotoxin MII-Binding Nicotinic Acetylcholine Receptors that Modulate Dopamine Release and Related Behaviors. Journal of Neuroscience, 23(35), 11045–11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj MI, Kao W, & Martin BR (2003). Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. The Journal of Pharmacology and Experimental Therapeutics, 307(2), 526–534. 10.1124/jpet.103.054908 [DOI] [PubMed] [Google Scholar]
- Deneris ES, Boulter J, Swansons LW, Patrick J, & Heinemann S (1989). Beta 3: A New Member of Nicotinic Acetylcholine Receptor Gene Family Is Expressed in Brain. JOURNAL OF BIOLOGICAL CHEMISTRY, 264(11), 6268–6272. [PubMed] [Google Scholar]
- Ehringer MA, McQueen MB, Hoft NR, Saccone NL, Stitzel JA, Wang JC, & Bierut LJ (2010). Association of CHRN genes with “dizziness” to tobacco. American Journal of Medical Genetics, Part B: Neuropsychiatric Genetics, 153(2), 600–609. 10.1002/ajmg.b.31027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Clementi F, Riganti L, McIntosh J, Collins A, … Whiteaker P (2005). Expression of Nigrostriatal 6-Containing Nicotinic Acetylcholine Receptors Is Selectively Reduced, but Not Eliminated, by 3 Subunit Gene Deletion. Molecular Pharmacology, 67(6), 2007–2015. 10.1124/mol.105.011940 [DOI] [PubMed] [Google Scholar]
- Grady SR, Moretti M, Zoli M, Marks MJ, Zanardi A, Pucci L, … Gotti C (2009). Rodent Habenulo-Interpeduncular Pathway Expresses a Large Variety of Uncommon nAChR Subtypes, But Only the alpha 3 beta 4*and alpha 3 beta 3 beta 4*Subtypes Mediate Acetylcholine Release. Journal of Neuroscience, 29(7), 2272–2282. 10.1523/jneurosci.5121-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC, & Marks MJ (2007). The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochemical Pharmacology, 74, 1235–1246. 10.1016/j.bcp.2007.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoft NR, Corley RP, McQueen MB, Schlaepfer IR, Huizinga D, & Ehringer MA (2009). Genetic association of the CHRNA6 and CHRNB3 genes with tobacco dependence in a nationally representative sample. Neuropsychopharmacology, 34(3), 698–706. 10.1038/npp.2008.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, & Damaj MI (2008). Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. The Journal of Pharmacology and Experimental Therapeutics, 325(1), 302–312. 10.1124/jpet.107.132977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, McIntosh JM, Brunzell DH, Sanjakdar SS, & Damaj MI (2009). The role of alpha6-containing nicotinic acetylcholine receptors in nicotine reward and withdrawal. The Journal of Pharmacology and Experimental Therapeutics, 331(2), 547–554. 10.1124/jpet.109.155457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Miyamoto J, Powers MS, Ro K, Soto M, Cox R, … Ehringer MA (2015). The β3 subunit of the nicotinic acetylcholine receptor: Modulation of gene expression and nicotine consumption. Neuropharmacology, 99, 639–649. 10.1016/j.neuropharm.2015.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kota D, Martin BR, Robinson SE, & Damaj MI (2007). Nicotine dependence and reward differ between adolescent and adult male mice. The Journal of Pharmacology and Experimental Therapeutics, 322(1), 399–407. 10.1124/jpet.107.121616 [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, … Changeux JP (1998). Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature, 391(6663), 173–177. 10.1038/34413 [DOI] [PubMed] [Google Scholar]
- Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, … Fratta W (2008). Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 28(47), 12318–12327. 10.1523/JNEUROSCI.3918-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothion S, Bizot JC, Trovero F, & Belzung C (2004). Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behavioural Brain Research, 155, 135–146. 10.1016/j.bbr.2004.04.008 [DOI] [PubMed] [Google Scholar]
- Rice JP, Hartz SM, Agrawal A, Almasy L, Bennett S, Breslau N, … Bierut LJ (2012). CHRNB3 is more strongly associated with Fagerström Test for Cigarette Dependence-based nicotine dependence than cigarettes per day: Phenotype definition changes genome-wide association studies results. Addiction, 107(11), 2019–2028. 10.1111/j.1360-0443.2012.03922.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PAF, … Bierut LJ (2007). Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Human Molecular Genetics, 16(1), 36–49. 10.1093/hmg/ddl438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas R, Pieri F, & De Biasi M (2004). Decreased signs of nicotine withdrawal in mice null for the beta4 nicotinic acetylcholine receptor subunit. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 24(45), 10035–10039. 10.1523/JNEUROSCI.1939-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjakdar SS, Maldoon PP, Marks MJ, Brunzell DH, Maskos U, McIntosh JM, … Damaj MI (2015). Differential roles of α6β2* and α4β2* neuronal nicotinic receptors in nicotine- and cocaine-conditioned reward in mice. Neuropsychopharmacology, 40(2), 350–360. 10.1038/npp.2014.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker AK, Marks MJ, & Markou A (2015). Null mutation of the β2 nicotinic acetylcholine receptor subunit attenuates nicotine withdrawal-induced anhedonia in mice. European Journal of Pharmacology, 753, 146–150. 10.1016/j.ejphar.2014.05.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, … Lester H. a. (2004). Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science (New York, N.Y.), 306(5698), 1029–1032. 10.1126/science.1099420 [DOI] [PubMed] [Google Scholar]
- Thorgeirsson TE, Gudbjartsson DF, Surakka I, Vink JM, Amin N, Geller F, … Stefansson K (2010). Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nature Genetics, 42(5), 448–453. 10.1038/ng.573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma W, Kyte SL, Bagdas D, Alkhlaif Y, Alsharari SD, Lichtman AH, … Damaj MI (2017). Effects of paclitaxel on the development of neuropathy and affective behaviors in the mouse. Neuropharmacology, 117, 305–315. 10.1016/j.neuropharm.2017.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger JS, Haberstick BC, Schlaepfer I, Collins AC, Corley RP, Crowley TJ,Ehringer MA (2008). The neuronal nicotinic receptor subunit genes (CHRNA6 and CHRNB3) are associated with subjective responses to tobacco. Human Molecular Genetics, 17(5), 724–734. 10.1093/hmg/ddm344 [DOI] [PubMed] [Google Scholar]
- Zhao-shea R, Degroot SR, Liu L, Vallaster M, Pang X, & Tapper AR (2015). Increased CRF signaling in a ventral tegmental area- interpeduncular nucleus-medial habenula circuit induces anxiety during nicotine withdrawal. Nat Commun, 6(6770), 1–20. 10.1038/ncomms7770.Increased [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao-Shea R, Liu L, Pang X, Gardner PD, & Tapper AR (2013). Activation of GABAergic neurons in the interpeduncular nucleus triggers physical nicotine withdrawal symptoms. Current Biology, 23(23), 2327–2335. 10.1016/j.cub.2013.09.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


