Abstract
Background
First line management of severe asthma exacerbations include the use of inhaled short-acting beta agonists, anticholinergics and systemic corticosteroids. Continuous intravenous ketamine given at dissociative doses may be a pharmacological option in patients who are intubated with life-threatening severe bronchospasm unresponsive to standard therapy.
Case description
We describe the case of a 44-year-old male admitted to the intensive care unit (ICU) for status asthmaticus requiring intubation and mechanical ventilation. The patient developed severe refractory hypercapnic respiratory failure necessitating additional respiratory support with veno-venous extracorporeal membrane oxygenation (VV ECMO) therapy. Ketamine was initiated at 0.5 mg/kg/hour continuous infusion on the day of admission for pain control and required up-titration to 2 mg/kg/hour by ICU day four for bronchodilation. Whole blood samples were obtained for pharmacokinetic analysis of ketamine during ECMO.
Results
The plasma concentration at steady-state was 1018.7 ng/mL with an estimated clearance of 1.96 L/hour/kg following up-titration. The estimated volume of distribution, elimination rate and half-life was 14.18 L/kg, 0.14 hr−1 and 5 hours, respectively.
Discussion
Compared to healthy adults, there was a 6.5-fold increase in the volume of distribution. However, the volume of distribution was similar compared to non-ECMO critically ill patients. Further studies should focus on the impact of ECMO on ketamine pharmacokinetics.
Keywords: Acute respiratory distress syndrome, extracorporeal membrane oxygenation, asthma exacerbation, status asthmaticus, pharmacokinetics, anesthetic
Introduction
Asthma exacerbation is one of the leading primary diagnoses in the emergency department (ED) accounting for 1.7 million visits annually in the United States.1 An estimated 25% of those patients encountered in the ED for asthma will be hospitalized with approximately 10% of those admitted to an intensive care unit (ICU).2 Although first-line treatments with oxygen, systemic corticosteroids, and bronchodilators are sufficient in reducing the symptomatology and airflow obstruction in most patients, approximately 3–5% of those admitted to the ICU will develop respiratory failure requiring intubation and mechanical ventilation.3 Extracorporeal membrane oxygenation (ECMO) therapy is a salvage therapy for severe acute asthma exacerbation when ventilation cannot be maintained with maximal medical therapies including deep sedation and neuromuscular blockade.
Ketamine is a dissociative anesthetic capable of inducing analgesia at low doses and both analgesia and anesthesia at higher doses. It exerts its effects by noncompetitively antagonizing the N-Methyl-D-aspartate receptor within the limbic and cortex system thereby producing a cataleptic dissociated state. In bronchial smooth muscle, ketamine reduces bronchospasm and airway resistance due to its sympathomimetic properties in the airways.4 In patients presenting with severe status asthmaticus, case reports have described use of intravenous ketamine in pediatric and adults as a means to avoid mechanical ventilation with bolus doses of 0.1 to 2 mg/kg followed by a continuous infusion of 0.15 to 2.5 mg/kg/hour.5, 6
The impact of ECMO on ketamine pharmacokinetics have not been described. Factors such as critical illness, varying degrees of organ dysfunction, components in the ECMO circuit, and the physicochemical properties of drugs have been implicated to contribute to the pharmacokinetic variability in this population. Importantly, interactions between the individual drug and the components of the ECMO circuitry are substantial particularly for lipophilic protein-bound drugs.7 Given the lipophilicity of ketamine (LogP = 2.9)8, adsorption of drug into the ECMO circuitry may impact its systemic disposition in patients. As a result, individual drug distribution, drug clearance and volume of distribution may be altered due to adsorption or sequestration into the ECMO circuit.
We describe the pharmacokinetics of ketamine in a critically ill patient on ECMO therapy. Pharmacokinetic parameters of interest (concentration at steady state, volume of distribution, clearance and half-life) are estimated, compared to literature, and critically discussed.
Case Report
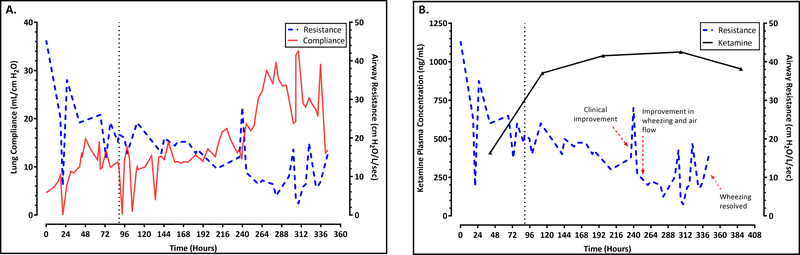
A 44-year-old African American male with a past medical history of uncontrolled moderate persistent asthma and hypertension, self-reported to be non-compliant, presented to an outside hospital with increasing shortness of breath unresponsive to a recent steroid prescription from his primary physician. His height and weight was 170 cm and 81 kg (ideal body weight = 65.9 kg), respectively. He was placed on bilevel non-invasive positive pressure ventilation for acute hypercapnic respiratory failure and quickly progressed to requiring intubation and invasive mechanical ventilation. A chest tube was placed for bilateral pneumothorax. Despite aggressive medical therapy including therapeutic neuromuscular blockade and ventilator strategies to maximize expiratory time and mitigate gas-trapping, ventilation could not be adequately maintained and the patient was placed on veno-venous (VV) ECMO via the right internal jugular vein using a 27 French Avalon cannula. The ECMO circuit consisted of a Quadrox PLS oxygenator, Rotaflow pump and tubing-set all coated in Bioline® (MAQUET Cardiopulmonary AG, Hirrlingen, Germany). In accordance with local protocol, midazolam and fentanyl was used for primary pain control. Ketamine was initiated as an adjunct for pain management as a continuous infusion on day zero at a rate of 0.5 mg/kg/hour following ECMO start at an outside hospital. Pulmonary symptoms included reduced breath sounds and wheezing requiring bronchotherapy with nebulized budesonide and ipratropium/albuterol in addition to montelukast and high-dose intravenous methylprednisolone. A respiratory pathogen PCR swab taken upon admission was positive for parainfluenza virus type 3, and sputum cultures were negative for bacterial infection. After initiation of VV ECMO, he was started on ultra-low tidal volume ventilation with a tidal volume of 200 mL (4.5 ml/kg IBW) on volume-control ventilation. The PaCO2 was kept in physiologic range with a high sweep setting on the VV ECMO circuit. On ICU day two, nebulized arformoterol was added to the regimen and ketamine was up titrated to 1 mg/kg/hour. Ketamine was subsequently titrated to 2mg/kg/hour on ICU day four, targeting a dose for treatment of severe acute asthma exacerbation. The patient’s hospital course was further complicated by Clostridium difficile colitis, ventilator associated pneumonia, Enterococcal sepsis, and gastroduodenal bleed. Over the course of ICU days eight through eleven, the wheezing and airway resistance significantly improved and his tidal volume on volume-control was increased to 330 mL by ICU day nine and reached 520 mL following ECMO decannulation. Figure 1A trends lung compliance and airway resistance over the course of ketamine infusion. Airway resistance was calculated as the difference between peak inspiratory pressure and plateau pressure divided by the flow rate. Pulmonary compliance was calculated as the ratio between the tidal volume and the difference between the peak inspiratory pressure and the positive end-expiratory pressure. All parameters were measured daily from the mechanical ventilator record and maintained by the respiratory therapist. The patient was decannulated from ECMO on ICU day thirteen and proceeded to tracheostomy on ICU day fifteen. His chest tube was removed the following day on ICU day sixteen. The ketamine infusion was gradually weaned and discontinued on hospital day twenty-seven.
Figure 1.
Airway resistance, pulmonary compliance and ketamine concentrations.
(A) A trend of airway resistance, pulmonary compliance and (B) concentration-response during ketamine infusion as a function of time. The vertical dashed line represents ketamine up-titration to 2 mg/kg/hour (ICU day four). The red arrows represents clinical observations during his course in the hospital.
Following informed consent, arterial blood sampling for ketamine analysis was obtained. A sample was taken 41 hours after the initial ketamine infusion. Additional samples were taken at 16, 100, 208, and 268 hours following ketamine up-titration on ICU day four. Ketamine plasma concentrations during the continuous infusion are shown in figure 1B together with the observed decline in airway resistance. Plasma ketamine concentrations were analyzed using a validated ultra-high performance liquid chromatography-tandem mass spectrometry following the FDA Guidance on Bioanalytical Method Validation.9
The average (range) steady-state concentration (Css) during high-dose ketamine infusion was 1018.7 (953.5–1063.6) ng/mL. This value represents the average of the three plasma samples collected at approximately 20, 42, and 54 half-lives during the continuous infusion. Patient specific pharmacokinetic parameters, including the volume of distribution (Vd), total plasma clearance (CL), elimination rate constant (ke) and half-life (t1/2) were estimated during the infusion. Total clearance was estimated by dividing the rate of infusion by the steady-state concentration [CL = Dose (mg/kg/hour)/Css]. Based on a reported plasma half-life of 4.98 hours in ICU patients, steady-state was presumed to have been reached approximately 24 hours following ketamine up-titration. As such, the first plasma sample collected (approximately 16 hours after up-titration) was used to calculate a patient specific elimination rate constant together with the mean of the plasma concentrations obtained at steady state using the following equation:
Where Cp is the plasma concentration taken at time t (first sample, 16 hours post up-titration) and Css is the steady-state ketamine plasma concentration during the continuous infusion. The estimated elimination rate constant was then used to calculate the volume of distribution using the relationship (R0/kel)/Css, where R0 is the infusion rate. Plasma half-life was estimated using t1/2 = 0.693/kel. The pharmacokinetic parameters are described in table 1 together with values reported in ICU patients and healthy adult subjects.10, 11
Table 1.
Ketamine pharmacokinetic parameters during the continuous high-dose infusion compared to published values.
| Parameter | Observed | ICU Patientsa | Healthyb |
|---|---|---|---|
| Caverage,ss (ng/mL) | 1018.69 | - | - |
| Vd (L/kg) | 14.18 | 16 | 2.18 |
| CL (L/hour/kg) | 1.96 | 2.16 | 0.89 |
| ke (hr−1) | 0.14 | 0.31 | 0.38 |
| t1/2 (hours) | 5 | 4.98 | 3.27 |
ICU = Intensive care unit, Caverage,ss = average concentration at steady-state, Vd = volume of distribution, CL = total plasma clearance, ke = elimination rate constant, t1/2 = half-life.
Mean values in 12 intensive care patients following a 2 mg/kg intravenous bolus and 2 mg/kg/hour infusion over 2 hours.10
Mean values in 10 healthy adult subjects following a 9–10 mg/kg/hour short infusion.9
After clinical improvement from a severe acute asthma exacerbation, the patient was successfully decannulated off VV ECMO and discharged from the hospital thirty-three days after ECMO removal. His follow up visit to the outpatient clinic after hospital discharge revealed no complaint of shortness of breath and was reported to be well controlled with aformoterol, nebulized budesonide and ipratropium/albuterol, and montelukast. There were no self-reported psychiatric behavior abnormalities reported during the follow up visit.
Discussion
Ketamine has several pharmacokinetic and pharmacodynamic (PKPD) advantages for initial and maintenance management of life-threatening asthma exacerbations. As an inductive agent during rapid sequence intubation, the quick onset and short duration of action makes ketamine an ideal agent for emergency airway management. In addition to its sedative and analgesic properties, ketamine has sympathomimetic activity at the bronchial smooth muscle resulting in bronchodilation. These unique PKPD properties make ketamine a useful agent for the initial management of severe asthma exacerbations requiring intubation. In cases where patients presenting with severe asthma exacerbations unresponsive to conventional therapy requiring emergency intubation, ketamine was reported to improve clinical and laboratory values following repeated administration.12–14 Infusion doses associated with efficacy ranged between 0.75 – 3 mg/kg/hour.13–15 These reports may suggest a dose-dependent bronchodilatory response often not seen with subanesthetic doses used in acute pain management. At dissociative doses, patients may be at risk for emergence phenomenon due to ketamine’s dose-related psychomimetic effects. Enhanced airway secretion and hypersalivation is also anticipated from autonomic stimulation and may be a potential downside to using ketamine.
We observed improvement in the patient’s wheezing by the fifth day after initiating high-dose ketamine with a complete resolution of symptoms ten days post up-titration. Reduction in airway resistance with an increase in pulmonary compliance were also observed during that period. These observations however, should be interpreted cautiously. Symptomatic improvement may have been contributed by other concomitant medications and interventions during the course of clinical care. This includes therapeutic agents such as high-dose corticosteroids, anticholinergics, beta2-agonists, leukotriene receptor antagonists and paralytics. Lastly, improvement may also have been possible with time during ECMO therapy.
Ketamine is commercially available as a racemic mixture. The pharmacokinetics of ketamine can be characterized by multi-compartment models. In healthy adults, the estimated volume of distribution, clearance and elimination half-life of racemic ketamine is 2.18 L/kg, 0.88 L/hour/kg and 3.27 hours following a short infusion, respectively.10 These parameters are largely altered in critically ill patients where the volume of distribution may increase by as much as 7.3 fold.11
This case is the first to report the pharmacokinetics of high-dose ketamine given to a patient unresponsive to standard asthma therapy during ECMO. The steady-state concentration during the continuous infusion was 1018.7 ng/mL. This concentration represents the average of three samples obtained approximately 20, 42 and 54 half-lives during the high-dose ketamine infusion. The concentrations achieved align with previous reports in those receiving a 2 mg/kg/hour ketamine infusion administered over two hours.11 Interestingly, the estimated pharmacokinetic parameters using the steady-state concentration did not largely differ among ICU patients. Moreover, it was hypothesized that the Vd for ketamine during ECMO would have been increased due to its lipophilicity and potential interaction with the ECMO circuit. Aside from lipophilicity, individual protein binding may also have a higher likelihood for drug sequestration during ECMO. In an ex-vivo study comparing drug lipophilicity and protein binding in ECMO circuits, drugs with higher protein binding were observed to have greater loss in the circuit despite having similar lipophilic profiles.7 Drugs having similar lipophilicity- such as ciprofloxacin and thiopentone (LogP = 2.3)- but have contrasting protein binding profiles (ciprofloxacin = 20–40%, thiopentone = 80%) were observed to have a mean loss of 4% and 88% in the circuit after 24 hours, respectively. Ketamine is lipophilic (LogP = 2.9) and is 10–30% bound to plasma proteins. The low protein binding of ketamine may in part explain the similar Vd observed in critically ill non-ECMO patients suggesting the possibility of minimal interaction between ketamine and the ECMO circuit.
Apart from its lipophilicity, ketamine is also highly water soluble.8 Although our patient appeared overweight (body mass index = 28), the patient was muscular and likely had a higher proportion of lean body mass. In patients who are overweight or obese, the impact of weight on ketamine pharmacokinetics may not be significant. Despite its large Vd, ketamine is also highly soluble in aqueous medium which may allow adequate concentrations in plasma relative to dose regardless of weight. Together, these observations should be validated in controlled ex-vivo and clinical studies.
Although therapeutic drug monitoring is not routinely performed for sedatives in the intensive care setting, the results from this report may be of use in understanding the dose-exposure relationship and the impact of the ECMO circuitry on ketamine pharmacokinetics. Such information can optimize dosing to ensure adequate safety and efficacy of ketamine when used during ECMO.
Conclusion
A continuous infusion of 2 mg/kg/hour produced a steady-state concentration of 1018.7 ng/mL. There was a 6.5-fold increase in the volume of distribution compared to healthy adults, however this increase was similarly observed in critically ill patients administered ketamine. Other pharmacokinetic parameters remained consistent as compared to non-ECMO patients in the ICU. Dedicated pharmacokinetic studies will be needed to determine optimal dosing and the potential impact of ECMO on ketamine disposition.
Acknowledgments
We thank Walter K. Kraft, MD for his comments and suggestions during the drafting of this manuscript. We thank the patient and his brother for providing informed consent for the study. Edwin Lam is supported by a National Institutes of Health (NIH) institutional training grant T32GM008562-24.
Footnotes
Conflict of interest statement
The authors have declared no conflicts of interest for this article.
References
- 1.Costanzo MR, Dipchand A, Starling R, et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29:914–956. [DOI] [PubMed] [Google Scholar]
- 2.Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. Natl Health Stat Report. 2011:1–14. [PubMed] [Google Scholar]
- 3.Nanchal R, Kumar G, Majumdar T, et al. Utilization of mechanical ventilation for asthma exacerbations: analysis of a national database. Respir Care. 2014;59:644–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White PF, Way WL, Trevor AJ. Ketamine--its pharmacology and therapeutic uses. Anesthesiology. 1982;56:119–136. [DOI] [PubMed] [Google Scholar]
- 5.Shlamovitz GZ, Hawthorne T. Intravenous ketamine in a dissociating dose as a temporizing measure to avoid mechanical ventilation in adult patient with severe asthma exacerbation. J Emerg Med. 2011;41:492–494. [DOI] [PubMed] [Google Scholar]
- 6.Goyal S, Agrawal A. Ketamine in status asthmaticus: A review. Indian J Crit Care Med. 2013;17:154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shekar K, Roberts JA, McDonald CI, et al. Protein-bound drugs are prone to sequestration in the extracorporeal membrane oxygenation circuit: results from an ex vivo study. Crit Care. 2015;19:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benet LZ, Broccatelli F, Oprea TI. BDDCS applied to over 900 drugs. AAPS J. 2011;13:519–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rochani A, Lam E, Tanjuakio J, Hirose H, Kraft WK, Kaushal G. LC-MS Method Development of Midazolam and Ketamine: Application to a Pharmacokinetic Study in Patients on Extracorporeal Membrane Oxygenation Therapy Poster Presentation at the 2018 AAPS Annual Meeting and Exposition; November 4–7, 2018; Washington, D.C: Poster W1130–03-017. [Google Scholar]
- 10.Ihmsen H, Geisslinger G, Schuttler J. Stereoselective pharmacokinetics of ketamine: R(−)-ketamine inhibits the elimination of S(+)-ketamine. Clin Pharmacol Ther. 2001;70:431–438. [DOI] [PubMed] [Google Scholar]
- 11.Hijazi Y, Bodonian C, Bolon M, Salord F, Boulieu R. Pharmacokinetics and haemodynamics of ketamine in intensive care patients with brain or spinal cord injury. Br J Anaesth. 2003;90:155–160. [DOI] [PubMed] [Google Scholar]
- 12.L’Hommedieu CS, Arens JJ. The use of ketamine for the emergency intubation of patients with status asthmaticus. Ann Emerg Med. 1987;16:568–571. [DOI] [PubMed] [Google Scholar]
- 13.Petrillo TM, Fortenberry JD, Linzer JF, Simon HK. Emergency department use of ketamine in pediatric status asthmaticus. J Asthma. 2001;38:657–664. [DOI] [PubMed] [Google Scholar]
- 14.Hemmingsen C, Nielsen PK, Odorico J. Ketamine in the treatment of bronchospasm during mechanical ventilation. Am J Emerg Med. 1994;12:417–420. [DOI] [PubMed] [Google Scholar]
- 15.Denmark TK, Crane HA, Brown L. Ketamine to avoid mechanical ventilation in severe pediatric asthma. J Emerg Med. 2006;30:163–166. [DOI] [PubMed] [Google Scholar]