Summary
We report the airway management of a patient with suspected COVID‐19 with impending airway obstruction requiring urgent surgical tracheostomy. To our knowledge, this is the first reported case of an awake tracheal intubation in a suspected COVID‐19–positive patient. Various modifications were put in place during the awake tracheal intubation and surgical tracheostomy procedures to minimise aerosol generation from the patient, such as avoiding high‐flow nasal oxygen, establishing conscious sedation with remifentanil before commencing airway topicalisation and avoiding transtracheal local anaesthetic infiltration. A multidisciplinary team discussion before performing the case highlighted aspects of both the airway management and the surgical procedure where particular care and modifications are required. There is a lack of national and international guidance for awake tracheal intubation and tracheostomy in COVID‐19 cases. This report nevertheless addresses the key procedural modifications required.
Keywords: aerosolisation, airway obstruction, awake tracheal intubation, COVID‐19
Introduction
In the presence of predictors of difficult airway management, awake tracheal intubation (ATI) should be considered 1. The incidence of failed airway management and a ‘cannot intubate cannot oxygenate’ scenario is higher in this patient group when managed asleep as compared with awake 2. Patients with confirmed or suspected coronavirus disease‐2019 (COVID‐19) who require tracheal intubation are a unique challenge, as the tracheal intubation process is a potentially infectious aerosol‐generating procedure and this may put the intubating team at risk 3. To mitigate against this risk, modification of the tracheal intubation process and donning of personal protective equipment (PPE) is imperative. However, this can make tracheal intubation time consuming and potentially more difficult, especially in the presence of anticipated difficulty.
Thorough planning of airway management and communication between all team members is essential. We report a case of ATI in a patient with suspected COVID‐19 who presented with a cough, respiratory failure and a critically obstructed airway due to a large base of tongue tumour. The key differences in performing ATI in this patient are discussed, including decision‐making; sedation; topicalisation; oxygenation; procedural performance; and planning in the event of failure. We also highlight the techniques used to minimise aerosol‐generation throughout the tracheal intubation and surgical tracheostomy process.
Report
A 54‐year‐old man with a large squamous cell carcinoma at the base of his tongue presented with a worsening cough and respiratory deterioration. He was also an intravenous (i.v.) drug user, smoked five cigarettes a day and was previously diagnosed with hepatitis C positive. He deteriorated with hypoxia, worsening stridor, an increased respiratory rate and a reduced level of consciousness. The decision was made to perform a surgical tracheostomy under general anaesthesia following ATI.
After discussions between the anaesthetic, surgical and nursing teams, we decided that full PPE, as recommended by Public Health England for aerosol‐generating procedures 3, would be worn. Using the departmental tracheal intubation action cards (Supplementary Figure S1), the anaesthetic team discussed the airway equipment required, optimal room ergonomics and the stand‐by equipment required and allocated specific roles to each individual. Then in the anaesthetic room the anaesthetic team donned their PPE, including powered air‐purifying respirators, and entered the operating theatre (hot room) to receive the patient (Fig. 1), while one member of the team remained in the anaesthetic room (clean room) as the runner, whose role was to pass any additional equipment that may be required during the ATI.
Figure 1.
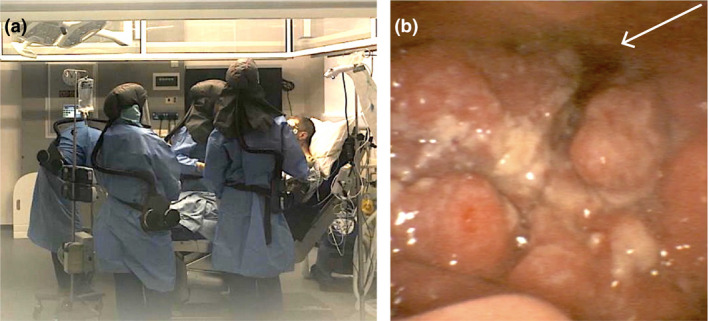
(a) Personal protective equipment worn by the team during awake tracheal intubation. (b) Flexible bronchoscopic view of the base of tongue tumour. Arrow is pointing to the glottic opening.
On arrival, standard monitoring was attached and i.v. access established. A hole was made on the side of a Hudson mask to allow for access to the patient's right nostril and carefully placed on the patient's face, and 5 l.min−1 oxygen was commenced. Conscious sedation using a target‐controlled infusion (TCI) was commenced at 0.5 μg.ml−1 propofol and 4 ng.ml−1 remifentanil.
Once conscious sedation was established, the nasopharynx was topicalized with 2.5 ml co‐phenylcaine spray and the oropharynx with 20 sprays of lignocaine 10% via a mucosal atomiser device. Gentle suctioning of the oropharynx was performed to clear any residual local anaesthetic or secretions, taking care not to cause any trauma to the base of tongue. A nasal route via the right nostril was selected and flexible bronchoscopy commenced using the Ambu® aScopeTM 4 Broncho Slim (Ambu, Copenhagen, Denmark) with a pre‐cut 6.5‐mm nasal tracheal tube. Once the flexible bronchoscope navigated past the tumour (Fig. 2b) and with the tracheal carina visualised, the tracheal tube was railroaded into the trachea (Video 1). The breathing circuit was immediately attached, the tracheal tube cuff gently inflated and the two‐point check (visualisation of the carina and capnography) 2 completed to confirm correct tracheal tube placement. The airway was secured 10 min after commencing airway topicalisation, with no coughing or positive pressure ventilation occurring throughout. Intravenous induction of anaesthesia using TCI at 3 μg.ml−1 propofol and 6 ng.ml−1 remifentanil was established and 60 mg rocuronium was given i.v.
The surgical team entered once the patient was appropriately positioned for surgical tracheostomy. After dissecting down to the trachea, before making any tracheal incisions, ventilation was ceased, the tracheal tube cuff was deflated and the tube was withdrawn by 2 cm. The surgeons then created the tracheal window and placed a size 8.0 non‐fenestrated cuffed tracheostomy tube, the cuff was inflated, the tube connected to the breathing circuit and capnography confirmed. The nasal tracheal tube was removed while the tracheostomy tube was secured.
Discussion
This case demonstrates many important issues in the management of suspected or confirmed COVID‐19 patients with an anticipated difficult airway. Careful consideration of clinical symptoms must be made if the patient is suspected to be COVID‐19–positive in the absence of laboratory test results. Meticulous planning of airway management and surgical tracheostomy in such patients must take into account the safety of the patient and that of all clinicians involved.
The complete clinical manifestations of COVID‐19 are not clear as the reported symptoms range widely. The commonest reported symptoms are fever, cough, myalgia, pneumonia and dyspnoea, whereas less common symptoms include headache, diarrhoea, runny nose and a productive cough 4. In the community, populations who are most susceptible to exposure have been identified as those aged between 35 and 55 years 5, with 59% being male 6. COVID‐19 infections occur through exposure to the virus and populations who are most at risk are those with poor immune function 6.
We considered the patient as high risk, given his symptoms of worsening cough, dyspnoea, increased work of breathing, increasing hypoxia with acute deterioration and due to his age, sex and past medical history 7. Although we could attribute many of the symptoms to impending airway obstruction, it was deemed too risky for the attending clinicians to rule out a COVID‐19 infection, therefore, the decision was made to treat his as a suspected case. The rapid deterioration of the patient meant there was no time to wait for the laboratory results. We have subsequently established that the patient was indeed COVID‐19 positive, justifying the decision to manage the case as we did.
High‐risk aerosol‐generating procedures, such as tracheal intubation and surgical tracheostomy, may put the anaesthetists, anaesthetic assistants and surgeons at risk of nosocomial infections, and this was at the forefront of the multidisciplinary discussion. We decided the patient would not tolerate a surgical tracheostomy under local anaesthesia due to his inability to closely follow instructions, lie supine and remain still for the duration of the procedure. In addition, this high‐risk aerosol‐generating procedure required controlled conditions to reduce the impact of transmitting viral load to the staff present in theatre.
Despite undergoing a debulking procedure 1 month prior in another hospital, the patient presented with a 62‐mm tumour involving his glottis and vallecula progressing to critical airway obstruction. Tracheal intubation following the induction of general anaesthesia was too risky for various reasons. He was a suspected COVID‐19–positive patient, therefore, additional precautions would have to be taken, such as donning of full PPE, avoiding facemask ventilation, avoiding multiple attempts at laryngoscopy, rapid progression to tracheal intubation through a supraglottic airway in the event of a failed tracheal intubation and then rapidly moving onto an emergency front‐of‐neck airway. In addition to the modified tracheal intubation plan, the patient had an obstructed airway and features of respiratory failure, and would, therefore, rapidly desaturate 8, 9. We opted for a modified ATI technique largely based on the principles of the Difficult Airway Society (DAS) guidelines for ATI 2, taking into consideration specific requirements for this case. To our knowledge this is the first reported case of an ATI in a suspected COVID‐19–positive patient, therefore, we were unable to follow any previously published guidance specific to this scenario.
ATI using a flexible bronchoscope via the nasal route was the chosen option due to the experience of the operator, increased likelihood of success, better tolerance by the patient and reduced risk of trauma to the friable tumour. We avoided high‐flow nasal oxygenation in this patient to reduce the risk of aerosol generation 10, instead opting for oxygenation using a Hudson mask delivered at 5 l · min−1. A higher dose of remifentanil than recommended was used, not only because the patient had a history of i.v. drug abuse but also because we wished to obtund any coughing reflex during the process. We waited for the remifentanil to take full effect before airway topicalisation was commenced to reduce coughing. Transtracheal local anaesthetic infiltration, nebulised lidocaine and spray‐as‐you‐go techniques for airway topicalisation were avoided to minimise coughing and aerosol generation. Following tracheal intubation, the cuff of the tracheal tube was inflated gently and the anaesthetic circuit immediately attached, before induction of anaesthesia to confirm correct tube placement and further reduce the risk of aerosol generation.
Planning for failure of ATI was also discussed, based on the DAS ATI guidelines 2. This must happen before the procedure and a clear plan should be made and communicated. The surgical team were scrubbed and ready in the clean room in preparation for an emergency front‐of‐neck airway if required, and the equipment for a high‐risk general anaesthesia tracheal intubation was also prepared and immediately available in the clean room. This comprised of a videolaryngoscope, bougie and surgical cricothyroidotomy kit. The incidence of failed ATI is reported to be ~1% 2; however, when performing this procedure in a COVID‐19 case the chances of failure could be higher due to all the associated additional complexities, as well as rescue procedures taking longer, therefore, planning for failure is imperative in this group.
At the time of managing this case there was no published guidance on the safest technique for surgical tracheostomy in a suspected COVID‐19 patient. We, therefore, applied the same principles that were applied to the ATI process to minimise aerosol generation during the surgical tracheostomy. Modifications to the tracheostomy process included turning off gas flows to the nasotracheal tube, deflating the cuff and withdrawing the tube proximally before the tracheal window was made. A non‐fenestrated tracheostomy tube was selected and the heat and moisture exchange filter was then immediately placed onto the tracheostomy tube once positioned in the trachea and capnography confirmed.
In summary, this report has highlighted how careful thought must be given to reduce the risk of aerosol generation in suspected COVID‐19–positive patients when performing ATI and surgical tracheostomy. We have emphasised the importance of understanding these risks and using techniques to minimise them. The planning for failure of ATI in this group of patients is also essential and must be formulated and discussed with all the team members before starting the procedure.
Supporting information
Appendix S1. The Guy’s and St Thomas’ NHS Foundation Trust COVID‐19 Tracheal Intubation action card.
Acknowledgements
IA has previously received honoraria for consulting for Ambu, honoraria and funding for travel and accommodation from Fisher & Paykel Healthcare, Ambu, BioMarin and Verathon Medical to give lectures at international meetings. No external funding or competing interests declared.
Contributor Information
I. Ahmad, Email: drimranahmad1@gmail.com, @dr_imranahmad.
S. Wade, @drstu_wade.
References
- 1. El‐Boghdadly K, Onwochei DN, Cuddihy J, et al. A prospective cohort study of awake fibreoptic intubation practice at a tertiary centre. Anaesthesia 2017; 72: 694–703. [DOI] [PubMed] [Google Scholar]
- 2. Ahmad I, El‐Boghdadly K, Bhagrath R, et al. Difficult Airway Society guidelines for awake tracheal intubation (ATI) in adults. Anaesthesia 2019; 75: 6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Department of Health and Social Care . COVID‐19 Guidance for infection prevention and control in healthcare settings. 2020. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/874316/Infection_prevention_and_control_guidance_for_pandemic_coronavirus.pdf (accessed 22/03/2020).
- 4. Adhikari SP, Meng S, Wu YJ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID‐19) during the early outbreak period: a scoping review. Infectious Diseases of Poverty 2020; 9: 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Medical expert group of Tongji hospital . Quick guide to the diagnosis and treatment of pneumonia for novel coronavirus infections (third edition). Herald Medicine. 2020. http://kns.cnki.net/kcms/detail/42.1293.r.20200130.1803.002.html (accessed 22/03/2020).
- 6. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus‐infected pneumonia. New England Journal of Medicine. 2020; [ePub ahead of print]. 10.1056/nejmoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferguson NM, Laydon D, Nedjati‐Gilani G, et al. Impact of non‐pharmaceutical interventions (NPIs) to reduce COVID‐19 mortality and healthcare demand. Imperial College COVID‐19 Response Team. 2020. https://www.imperial.ac.uk/media/imperial-college/medicine/sph/ide/gida-fellowships/Imperial-College-COVID19-NPI-modelling-16-03-2020.pdf (Accessed 22/03/20).
- 8. Cook TM, El‐Boghdadly K, McGuire B, McNarry AF, Patel A, Higgs A. Consensus guidelines for managing the airway in patients with COVID‐19: Guidelines from the Difficult Airway Society, the Association of Anaesthetists the Intensive Care Society, the Faculty of Intensive Care Medicine and the Royal College of Anaesthetists. Anaesthesia. 2020; [ePub ahead of print]. 10.1111/anae.15054 (Accessed 20/03/2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zuo MZ, Huang YG, Ma WH, et al. Expert recommendations for tracheal intubation in critically ill patients with noval coronavirus disease 2019. Chinese Medical Sciences Journal. 2020; [ePub ahead of print]. 10.24920/003724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019‐nCoV) patients. Canadian Journal of Anesthesia 2020; 67: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. The Guy’s and St Thomas’ NHS Foundation Trust COVID‐19 Tracheal Intubation action card.


