Dear Editor:
We recently published a case series of typically commensal Neisseria spp. disease among eculizumab recipients[1]. Eculizumab is a terminal complement inhibitor indicated for treatment of paroxysmal nocturnal hemoglobinuria, atypical hemolytic uremic syndrome, and certain patients with generalized myasthenia gravis or neuromyelitis optica spectrum disorder[2]. Due to complement inhibition, many different Neisseria spp. can cause invasive disease in eculizumab recipients[1, 3, 4] and eculizumab recipients are at an estimated 2000-fold increased risk of meningococcal disease (caused by Neisseria meningitidis). All eculizumab recipients should receive meningococcal vaccinations prior to therapy; however, eculizumab recipients may develop meningococcal disease or other Neisseria infections despite vaccine receipt[4–6]. For patients who cannot receive meningococcal vaccinations ≥2 weeks before starting eculizumab, U.S. eculizumab labeling recommends 2 weeks of antibiotic prophylaxis[2]. In July 2017, the Centers for Disease Control and Prevention (CDC) advised that prescribers could consider antibiotic prophylaxis in eculizumab recipients for the duration of eculizumab therapy[4]. There are no published studies evaluating the efficacy or safety of antibiotic prophylaxis in eculizumab recipients. Here, we report on potential risks and benefits of antibiotic prophylaxis for the prevention of meningococcal disease in a case series of vaccinated eculizumab recipients who developed meningococcal disease.
We searched for meningococcal disease cases in eculizumab recipients in the FDA Adverse Event Reporting System (FAERS) and the literature (PubMed, Embase) between March 2007 (U.S. approval date) and May 2017. The FAERS search retrieved 158 reports; no literature reports were identified that were not already identified in FAERS. Two FAERS reports were published in the literature after the FAERS search was conducted and were used to supplement the corresponding FAERS reports[5, 6]. Of the 158 reports, 111 were excluded for the following reasons: lack of positive test for N. meningitidis (n=59), indeterminate prophylaxis use/antibiotic not specified (n=20), insufficient clinical course/timeline details (n=16), duplicate (n=14), or trimethoprim-sulfamethoxazole use (because a high proportion of N. meningitidis isolates are resistant to trimethoprim-sulfamethoxazole[7, 8]) (n=2).
Included patients received eculizumab within the three months preceding a diagnosis of meningococcal disease, defined as a report of a symptomatic patient with a positive culture or other confirmatory test for N. meningitidis from any body site. FAERS cases were matched to CDC meningococcal disease surveillance data from the National Notifiable Diseases Surveillance system, when possible, for confirmation of infection, serogroupa, and antibiotic susceptibility testing. Time to onset (TTO) was calculated from the date of first eculizumab dose (assumed to be the date of starting antibiotic prophylaxis) to the date of the patient’s first meningococcal disease episode. For patients with multiple episodes of meningococcal disease, the calculations described below were performed using only the first meningococcal disease episode.
The series included 47 patients, of whom 15 were taking antibiotic prophylaxis at the time of meningococcal disease onset and 32 were not (Table 1). There were four fatalities due to meningococcal disease (all among patients not taking prophylaxis). All 47 patients reportedly received ≥1 dose of a meningococcal vaccine. Three of 47 patients had ≥1 episode of meningococcal disease (two patients were taking prophylaxis; one was not). TTO of first episode of meningococcal disease was reported for 11 of 15 patients taking prophylaxis and 26 of 32 not taking prophylaxis. Median TTO of first episode of meningococcal disease was 835 days in prophylaxis recipients versus 333 days in patients not taking prophylaxis. The range of TTO was large in both groups (3-1873 days in prophylaxis recipients vs. 8-2247 days in non-prophylaxis recipients). Among patients with susceptibility results available, penicillin non-susceptibility was reported more frequently in patients taking prophylaxis (5 of 6 isolates, 83%) than in patients not taking prophylaxis (2 of 9 isolates, 22%).
Table 1.
Characteristics of patients taking antibiotic prophylaxis and patients not taking antibiotic prophylaxis
 |
 |
 |
Age at first episode of meningococcal disease, rounded down to nearest year
n=2; membranoproliferative glomerulonephritis and C3 glomerulopathy
n=1 neuromyelitis optica; n=1 conflicting report in which PNH, aHUS, and renal transplant were each noted as a reason for eculizumab use; n=1 malignant hypertension; n=1 aHUS, antiphospholipid syndrome, and systemic lupus erythematous
All patients in the series reported a history of some type of meningococcal vaccination, but the serogroups covered by the vaccines (and vaccine brands) were not reported for every patient. The rows for serogroup B and serogroups A, C, W, and Y are not mutually exclusive; patients who received a serogroup B vaccine may also be counted in serogroup A, C, W, and Y vaccine row.
Including serogroup B/C vaccine (non-U.S.)
n=1 patient received meningococcal vaccine prior to developing meningococcal disease by Y serogroup, but the serogroups covered by the vaccine were not reported
Of the 6 patients with C, W, or Y disease: n=1 vaccinated patient received a serogroup B/C vaccine prior to developing serogroup C meningococcal disease; n=5 patients received ACWY vaccination prior to developing meningococcal disease by C, W, or Y serogroup. The 7th patient received serogroup C vaccine prior to serogroup W meningococcal disease
n=1 patient had two episodes of meningococcal disease: 1st episode isolate penicillin susceptible, 2nd episode isolate penicillin resistant (both are counted)
n=14 patients were taking penicillin, n=1 patient was taking amoxicillin
With the limited data available in the FAERS and published reports, it is not possible to determine whether antibiotic prophylaxis is effective in preventing meningococcal disease in eculizumab recipients. However, in this descriptive analysis we observed a prolonged TTO of first meningococcal disease episode and a higher frequency of reduced penicillin susceptibility among prophylaxis users compared to non-prophylaxis users (all previously vaccinated). Although these results suggest that antibiotic prophylaxis may delay TTO, prescribers should interpret these findings conservatively, particularly given the wide range of TTO and the substantial differences in patient age and country of residence between prophylaxis recipients and non-prophylaxis recipients.
Prescribers must weigh the potential risks of antibiotic prophylaxis, such as adverse events andantibiotic resistance, against the potential benefits. When considering the benefit-risk balance of prophylaxis use, the more frequent reports of reduced penicillin susceptibility among prophylaxis users deserve comment. If this is a true relationship, colonization by meningococcal isolates with reduced penicillin susceptibility may have precluded penicillin from preventing meningococcal disease. Reduced penicillin susceptibility could be a consequence of selective antibiotic pressure. Further complicating use of prophylaxis, eculizumab recipients are also at risk for disseminated Neisseria gonorrhoeae[3], an organism in which penicillin resistance is common.
The analysis has several limitations inherent to the data source. Cases were derived from spontaneous reports, which are subject to underreporting of outcomes, biased reporting, and variable quality. Confounding of the relationship between antibiotic prophylaxis and TTO is possible since patient characteristics, including age (Figure 1) and country of residence, differed substantially between groups. Ascertainment of prophylaxis exposure was challenging and inconsistent prophylaxis use could reduce differences in TTO between groups. Other important data deficiencies include missing information on meningococcal antimicrobial susceptibility testing results, methods, and breakpoint criteria.
Figure 1.
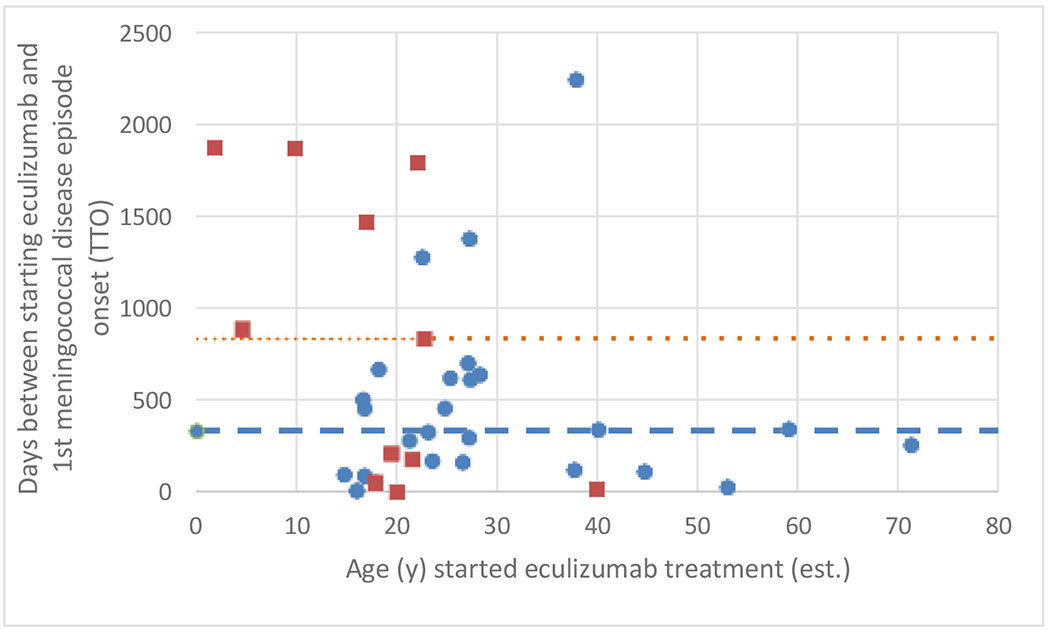
Age (years) of patients at time of starting eculizumab therapy versus TTO of first meningococcal disease episode
The relationship between age when patients started treatment and TTO of first meningococcal disease episode, for prophylaxis recipients and non-prophylaxis recipients.
Key:
Orange square = patients taking antibiotic prophylaxis with reported TTO (n=11)
Blue circle = patients not taking antibiotic prophylaxis with reported TTO (n=26)
Orange … dotted line = median TTO (835 days) for patients taking antibiotic prophylaxis with reported TTO (n=11)
Blue --- dashed line = median TTO (333 days) for patients not taking antibiotic prophylaxis with reported TTO (n=26)
Note, one patient in the “prophylaxis group” was 18 years of age with a TTO=53 days, and one patient in the “no prophylaxis group” was also 18 years of age with a TTO=53. These data points overlap on the figure.
Overall, the data are inconclusive. However, we did observe a trend towards prolonged TTO among prophylaxis users but also towards increased penicillin non-susceptibility among prophylaxis recipients. Validation of these potential associations in a larger sample, with systematic ascertainment of antibiotic exposure, could further elucidate the potential impact of prophylaxis on development of meningococcal disease among eculizumab recipients. Healthcare professionals should remain vigilant for signs of meningococcal disease among eculizumab recipients, irrespective of the preventive measures in use.
Acknowledgements
We thank Amy Blain for assistance matching FAERS reports with CDC meningococcal disease surveillance data.
Funding: None.
Footnotes
When serogroup was determined at CDC through multiple methods, slide agglutination results were used as the final serogroup
The views expressed are those of the authors and do not necessarily represent those of, nor imply endorsement from, the U.S. Food and Drug Administration, the Centers for Disease Control and Prevention, or the U.S. government.
Transparency Declaration: The authors have no potential conflicts of interest to disclose.
References
- 1.Crew PE, McNamara L, Waldron PE, McCulley L, Jones SC, Bersoff-Matcha SJ. Unusual Neisseria species as a cause of infection in patients taking eculizumab. J Infect 2019; 78 (2): 113–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soliris (eculizumab) [package insert]. Boston, MA: Alexion Pharmaceuticals, Inc; 2018. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125166s431lbl.pdf. Updated June 27, 2019 Accessed on September 6, 2019. [Google Scholar]
- 3.Crew PE, Abara WE, McCulley L, et al. Disseminated gonococcal infections in patients receiving eculizumab: a case series. Clin Infect Dis 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNamara LA, Topaz N, Wang X, Hariri S, Fox L, MacNeil JR. High Risk for Invasive Meningococcal Disease Among Patients Receiving Eculizumab (Soliris) Despite Receipt of Meningococcal Vaccine. MMWR Morb Mortal Wkly Rep 2017; 66 (27): 734–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parikh SR, Lucidarme J, Bingham C, et al. Meningococcal B Vaccine Failure With a Penicillin-Resistant Strain in a Young Adult on Long-Term Eculizumab. Pediatrics 2017; 140 (3). [DOI] [PubMed] [Google Scholar]
- 6.Nolfi-Donegan D, Konar M, Vianzon V, et al. Fatal Nongroupable Neisseria meningitides Disease in Vaccinated Patient Receiving Eculizumab. Emerg Infect Dis 2018; 24 (8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biedenbach DJ, Castanheira M, Jones RN. Determination of CEM-101 activity tested against clinical isolates of Neisseria meningitidis from a worldwide collection. Antimicrob Agents Chemother 2010; 54 (9): 4009–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jorgensen JH, Crawford SA, Fiebelkorn KR. Susceptibility of Neisseria meningitidis to 16 antimicrobial agents and characterization of resistance mechanisms affecting some agents. J Clin Microbiol 2005;43(7):3162–3171. doi: 10.1128/JCM.43.7.3162-3171.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


