Abstract
By April 7, 2020, severe acute respiratory syndrome coronavirus 2 was responsible for 1,383,436 confirmed cases of Coronavirus disease 2019 (COVID-19), involving 209 countries around the world; 378,881 cases have been confirmed in the United States. During this pandemic, the urgent surgical requirements will not stop. As an example, the most recent Centers of Disease Control and Prevention reports estimate that there are 2.8 million trauma patients hospitalized in the United States. These data illustrate an increase in the likelihood of encountering urgent surgical patients with either clinically suspected or confirmed COVID-19 in the near future. Preparation for a pandemic involves considering the different levels in the hierarchy of controls and the different phases of the pandemic. Apart from the fact that this pandemic certainly involves many important health, economic, and community ramifications, it also requires several initiatives to mandate what measures are most appropriate to prepare for mitigating the occupational risks. This article provides evidence-based recommendations and measures for the appropriate personal protective equipment for different clinical and surgical activities in various settings. To reduce the occupational risk in treating suspected or confirmed COVID-19 urgent orthopaedic patients, recommended precautions and preventive actions (triage area, ED consultation room, induction room, operating room, and recovery room) are reviewed.
In October 2019, the Department of Health and Human Services'—The Office for Civil Rights took a corrective action against an orthopaedic surgeon who unlawfully canceled an elective surgery for an HIV-positive patient. This action was justified via the requirements of Section 504 of the Rehabilitation Act of 1973 that prohibits discrimination on the basis of disability (including HIV/AIDS) in health programs or activities that receive Health and Human Service funding.1 Over the past few decades, many guidelines and recommendations have been established to reduce the occupational risk while educating surgeons to make them better prepared to operate on HIV-positive patients.2 The severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) (Coronavirus disease 2019 [COVID-19]), which seems to be highly contagious and has easily spread worldwide, is a much different virus causing a much different disease. Orthopaedic surgeons should be fully aware of the current situation regarding the COVID-19 pandemic and prepare to take proper precautions against the occupational risk of exposure, especially in asymptomatic and mildly symptomatic surgical patients. By April 7, 2020, the SARS-CoV-2 was responsible for 1,383,436 confirmed cases of COVID-19, involving 209 countries around the world; 378,881 cases have been confirmed in the United States. As an example, Centers of Disease Control and Prevention (CDC) reports an estimated 2.8 million trauma patients hospitalized in the United States. In addition, 791,000 older patients are treated in emergency departments for fall injuries each year.3 Gleaning from the trauma literature, these data suggest an increased likelihood of engaging in COVID-19 orthopaedic patients in our hospitals. Thousands of healthcare providers (HCP) have been infected with COVID-19, despite their adherence to infection control measures.4 Approximately 14% of Spain's confirmed cases are in medical professionals, per the Spanish minister of health. Despite the current definitions for diagnosing symptomatic COVID-19 patients, the transmission from an asymptomatic carrier has been documented between 25% and 50%.5
It is necessary for the orthopaedic community to be prepared for this global pandemic emergency. This is an occupational hazard not only to orthopaedic surgeons and other healthcare providers but also to the families and neighbors of exposed healthcare providers. There is still no definitive consensus of the pandemics' behavior, COVID-19 mode of transmission, diagnostic criteria, and management protocols. Preparation for a pandemic involves considering the increasing levels of protection and infection control and how they should be implemented during different phases of the pandemic. In the OR setting, these measures include the following: modification of healthcare infrastructure and processes, educating staff and patients, implementing infection control strategies, and administrative and clinical measures. The surgical management of traumatic injuries requires a complex environment with multiple stakeholders including surgeons, anesthesiologists, nurses, OR attendants, and medical staff; it can be a real challenge to align the perspectives and concerns of all parties The primary aim of this article is to help define the COVID-19 crisis and discuss effective management strategies. This article provides a brief summary of the current situation and understanding of the pandemic, diagnostic criteria, and attempts to forecast the extent and prognosis. Finally, recommending precautions and preventive actions to reduce the occupational risk in treating clinically suspected/confirmed COVID-19 surgical patients.
Recommendation and Proactive Measures for Managing the Suspected/Confirmed, Orthopaedic Patients With Coronavirus Disease 2019
General Recommendations
The CDC and World Health Organization instituted guidelines for routine infection prevention measures after the worldwide spread of the SARS-CoV-2 virus. HCP are recommended to wear a simple surgical mask and perform regular hand washing when contacting low-risk individuals to protect against contamination. HCPs in high-risk areas should adhere to infection prevention and control practices, which includes the appropriate use of engineering controls (negative pressure rooms), administrative controls, and personal protective equipment (PPE) (Figures 1–3).
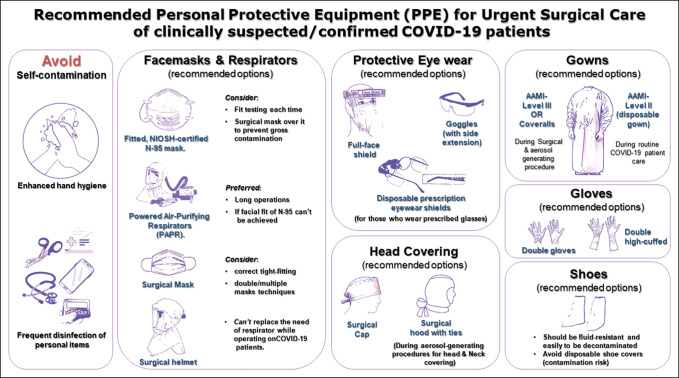
Figure 1.
Flowchart demonstrating the recommended personal protective equipment for urgent surgical care of clinically suspected/confirmed Coronavirus disease 2019 patients.
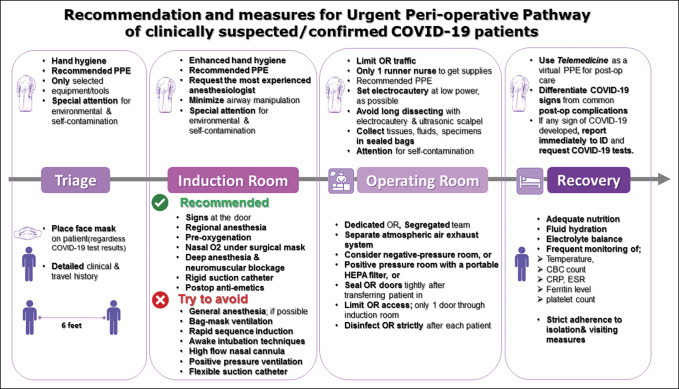
Figure 3.
Flowchart demonstrating the recommended measures for the urgent perioperative pathway of clinically suspected/confirmed COVID-19 patients. COVID = Coronavirus disease 2019, PPE = personal protective equipment
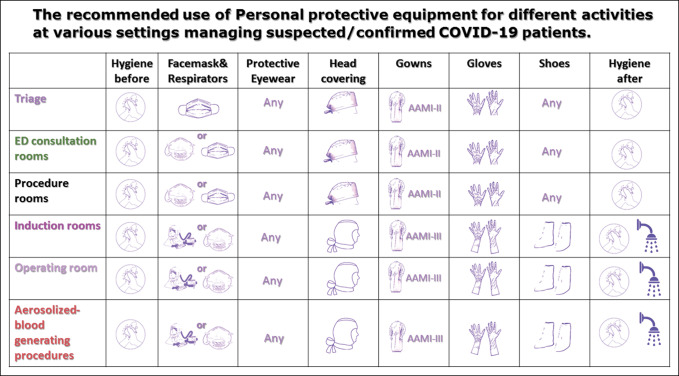
Figure 2.
Flowchart demonstrating the the recommended use of personal protective equipment for different activities at various settings managing suspected/clinically Coronavirus disease 2019 patients.
Recommended Personal Protective Equipment and Infection Control Measures
To minimize the risk of transmissibility and cross-infection, the CDC has recommended airborne, droplet, and contact precautions. This includes the mandatory use of PPE which includes gowns, gloves, face masks, and either n-95, P100, or FFP2 respirators with a face shield/googles or powered air-purifying respirator (PAPR) to minimize the risk of transmissibility and cross-infection.6 Per CDC recommendations, a clinically suspected/confirmed COVID-19 patient should wear a cloth face covering, over nose, and mouth and a surgical mask should be reserved for HCP and first responders.6 Unfortunately, these PPE recommendations for both providers and patients will fail to prevent transmission if frequent surfaces decontamination, enhanced hand hygiene, and avoiding self-contamination are not carefully considered. Providers must focus on meticulous hand hygiene and disinfecting personal items, such as stethoscopes, phones, ID tags, laptops, dictation devices, etc. A route to minimize exposure and contact between triage to induction room, OR, and then to recovery rooms should be frequently cleaned and disinfected. It is recommended for an environmental services worker to increase the frequency to disinfect the most contaminated and most touched surfaces, such as the elevator buttons, door handles, light switches, grab rails, and etc. A recent study examined the most contaminated objects and PPE in the hospitals of Wuhan, China. Of the samples collected from HCP using PPE (hand sanitizer dispensers, gloves, and protective eyewear/full-face shield), 12.9% were positive for SARS-CoV-2 RNA. The highest rates of contamination were found on hand sanitizer dispensers, gloves, goggles/face-shields at a rate of 20.3%, 15.4%, and 1.7%, respectively.7
Face masks and Respirators; Which to Use?
The CDC had recommended that HCPs closely interacting with clinically suspected/confirmed COVID-19 patients should wear n-95 respirators, along with gowns, gloves, and protective eyewear. With the current global demand and shortage of PPE, the supply chain of specialized respirators cannot meet demand but the looser fitting surgical face masks might be an acceptable alternative.6 Notably, most respirators (eg, n-95) require training to properly fit them around the maxillofacial region to ensure appropriate fitting. Our recommendations for using face masks and respirators varies depending on the setting and activity (Table 1). The available options for HCPs are the followings:
-
Fitted, NIOSH-certified N-95 masks
Each time the N-95 respirator is taken off, the wearer must double-check that it has not been soiled or damaged before donning it again.
If achieving and maintaining a very close facial fit of N-95 cannot be guaranteed during the whole time of aerosolized blood-generating procedure, PAPR is highly recommended.
Consider placing a simple surgical mask on top of the N-95 to prevent gross contamination.
-
PAPR
It is preferred for long operations (if available)
-
Surgical masks
consider double/multiple mask techniques and correct tight-fitting
It is not recommended in performing aerosolized blood-generating procedures for suspected/confirmed COVID-19 patients.
Generally, surgical masks do not provide complete protection from germs and other contaminants because of its loose facial fit. Therefore, caution should be exercised.
Table 1.
The Recommended Use of PPE for Different Activities at Various Settings Managing Suspected/Confirmed COVID-19 Patients
| Setting | Activity | Recommended PPE |
| Triage | Screening and initially examining patients | Surgical mask |
| Regular AAMI level-II gown | ||
| Goggles or full-face shields | ||
| Gloves | ||
| Hand hygiene before and after examining each patient | ||
| ED consultation rooms | Examination of patient; negative COVID-19 = no signs of respiratory illness | Surgical mask |
| Gloves | ||
| Pay attention of environmental contamination (room items, instruments, etc) | ||
| Hand hygiene before and after examining each patient | ||
| ED consultation rooms | Examination of patient; suspected/confirmed COVID-19 or patient with fever or signs of respiratory illness | Fitted, NIOSH-certified N-95 mask (if available) or surgical mask (consider double/multiple mask technique and ensure tight-fitting) |
| Regular AAMI level-II gown | ||
| Goggles or full-face shields | ||
| Gloves (double gloves should be considered in trauma level I cases) | ||
| Hand hygiene before and after examining each patient | ||
| Procedure rooms | Providing noninvasive medical care for suspected/confirmed COVID-19 patients. | Fitted, NIOSH-certified N-95 mask (if available) or surgical mask (consider double mask technique and ensure tight-fitting) |
| Regular AAMI level-II gown | ||
| Eye protection: Goggles or full-face shields (if available) | ||
| Gloves | ||
| Hand hygiene before and after examining each patient and donning/doffing PPE | ||
| Induction room | The senior anesthesiologist is performing respiratory aerosol-generating procedures for suspected/confirmed COVID-19 patient | PAPR (if available) or, |
| Fitted, NIOSH-certified N-95 mask, with | ||
| Eye protection; goggles (covered sides of eyes) or full-face shields | ||
| Disposable AAMI level-III surgical gown or coveralls | ||
| Double high-cuffed surgical gloves [alternately, vertical strips of tape can keep gloves secured to the gown] | ||
| Shoes worn should be fluid-resistant and easily to be decontaminated [disposable shoe covers might increase the risk of contamination] | ||
| Hand hygiene before and after donning/doffing PPE | ||
| A shower after respiratory aerosol-generating procedures is very prudent | ||
| Operating room | Providing emergent surgical treatment for suspected/confirmed COVID-19 patient | PAPR is preferred for long operations (if available) or, |
| Fitted, NIOSH-certified N-95 mask, with | ||
| Eye protection; goggles (covered sides of eyes) or full-face shields | ||
| Disposable AAMI level-III surgical gown or coveralls | ||
| Double high-cuffed surgical gloves [alternately, vertical strips of tape can keep gloves secured to the gown] | ||
| Shoes worn should be fluid-resistant and easily to be decontaminated [disposable shoe covers might increase risk of contamination] | ||
| Hand hygiene before and after donning/doffing PPE | ||
| Performing aerosolized blood-generating procedures for suspected/confirmed COVID-19 patient | PAPR is preferred for long operations (if available) or, | |
| Fitted, NIOSH-certified n-95 mask, with | ||
| Eye protection; goggles (covered sides of eyes) or full-face shields | ||
| Surgical hood with ties (head and neck covering) | ||
| Disposable AAMI level-III surgical gown or coveralls | ||
| Double high-cuffed surgical gloves [alternately, vertical strips of tape can keep gloves secured to the gown] | ||
| Shoes worn should be fluid-resistant and easily to be decontaminated [disposable shoe covers might increase risk of contamination] | ||
| Hand hygiene before and after donning/doffing PPE | ||
| A shower after an aerosolized- blood-generating procedure is very prudent | ||
| Recovery room | PPE doffing | Special attention is warranted to avoid self-contamination during PPE doffing |
AAMI = Association of the Advancement of Medical Instrumentation, COVID-19 = coronavirus disease 2019, PAPR = powered air-purifying respirator, PPE = personal protective equipment
Of note, the regular surgical helmet cannot replace the need of respirator while operating on suspected/confirmed COVID-19 patients.
Protective Eye Wear
Donning eye protection equipment is recommended because the inoculation of the conjunctival mucous membrane is a mode of transmission.8,9 Our recommendations for using protective eyewear are the following:
Prioritize eye protection equipment for certain selected surgical procedures, ie, during aerosol-generating procedures (splashes, sprays, etc) and where there is prolonged face-to-face or close contact with a suspected or confirmed COVID-19 patients.
Full-face shield (if available) or goggles can be issued to each provider.
Consider using safety goggles with extensions to cover the sides of eyes.
Consider using disposable prescription eyewear shields (for those who wear prescribed glasses)
During supply shortage, use eye protection equipment beyond the manufacturer-designated expiration date.
During supply shortage, follow and adhere to the manufacturer instructions for reuse and disinfection. If these instructions are not provided, consider the CDC recommendations.10
Gowns
Association of the Advancement of Medical Instrumentation (AAMI) ratings are based on the level of fluid protection in the critical zone or chest region of the surgical gown. The AAMI level should be checked on the packaging because they may come in several different designs, materials, and colors.
Nonsterile, disposable, or reusable-AAMI Level-II gowns (frequently seen as disposable isolation gowns) are appropriate for use by providers during routine COVID-19 patient care.
Surgical gowns AAMI-Level-III (typically those found in operating rooms) or coveralls should be prioritized for surgical and aerosolized blood-generating procedures.
Table 1 provides our recommendation in this regard based on different settings and activities.
Gloves
Double gloves are recommended when handling COVID-19 patients as an extra precaution to minimize contaminating ORs items, equipment, and surfaces. The outer pair should be pulled off before touching equipment or surfaces in other areas of OR.11
Surgical Head Cap
Surgical cap should be used per routine protocols.
Surgical hood with ties can be used for head and neck covering during aerosol-generating procedures.
Transfusion-transmitted Coronavirus and Aerosolized blood-Generating Procedures; What is the Risk?
COVID-19 is presumed to spread directly via infectious respiratory droplets and close contact (because SARS-CoV-2 cannot survive without a carrier).12 However, these transmission modes do not explain all cases. Recent data have shown that COVID-19 might survive and be transmitted indirectly from virus contamination of common surfaces and objects after virus aerosolization in a confined space with by infected individuals.12 The incubation period for COVID-19 is approximately 4 days, and studies suggest that it may range anywhere from 2 to 14 days.13-15 A recent study investigating SARS-CoV-2 from clinical specimens found that RNA virus detected in blood samples from confirmed COVID-19 patients (3 of 307; 1%).16 Huang et al17 reported that 15% of patients with laboratory confirmed COVID-19 had viral RNA in their plasma. The implications of these findings are still unclear, and there are no reported cases of transfusion-transmitted coronaviruses through April 5, 2020. However, continued vigilance is essential. Despite studies detecting viral RNA in the serum or plasma of confirmed/suspected COVID-19 patients, blood transmission and infectivity are still not fully understood. Because there is little evidence and vague guidelines for blood transfusion in the current setting, it is recommended to recuse anyone with symptoms or signs of respiratory illness from blood donation.18 The US FDA has suggested to retrieve and quarantine any blood products collected in the 28 days before, or after, either COVID-19 disease onset or possible exposure to individuals who are COVID-19 positive.18 Theoretically, viremia in patients with asymptomatic or confirmed COVID-19 patients could pose a risk of transmissibility to the orthopaedic team during aerosolized blood-generating procedures.19 The use of high-speed drills, bone saws, reamers, electrocautery, and ultrasonic scalpels generate significant amounts of aerosols, increasing the risk of viral contamination of the environment.20 A recent Canadian study described low-fidelity simulation training to evolve the modified PPE used for aerosol-generating procedures of suspected/confirmed COVID-19 patients and assess the sites of contamination21 The spread of the aerosolized respiratory secretions and contamination sites were visualized with a commercial powder product and ultraviolet light. They demonstrated a significant amount of contamination on the provider's neck, base of the wrist, and their lower pants and shoes. These sites, however, are probably not associated with a direct method of transmission for SARS-CoV-2. However, there are definite sources of self-contamination during PPE doffing. In addition, the disposable AAMI Level-III fluid-resistant, surgical gowns or coveralls are recommended because they detected no contamination of scrubs beneath the surgical gown compared with reusable surgical gowns (AAMI, Level-II). The AAMI, level-II gowns were permeable to aerosolized secretions. The recommended PPE for performing respiratory aerosol or aerosolized blood-generating procedures for suspected/confirmed COVID-19 patients (Table 1):
PAPR is preferred for long operations (if available)
Fitted, NIOSH-certified n-95 mask, with the following:
Eye protection; goggles (covered sides of eyes) or full-face shields (if available)
Surgical hood with ties (head and neck covering)
Disposable AAMI level-III surgical gown or coveralls
Double high-cuffed surgical gloves (alternately, vertical strips of tape can keep gloves secured to the gown and minimize exposing wrist to contamination (N.B. Circumferential taping may make doffing more challenging)22
Shoes or booties worn should be fluid-resistant and easily to be decontaminated (disposable shoe covers might increase the risk of contamination)
Hand hygiene before and after donning/doffing PPE
A shower after an aerosolized blood-generating procedure is very prudent.
Strategies for Optimizing the Supply of Personal Protective Equipment
During the initial phase of US cases of COVID-19, one Washington state hospital reported that staff changed their PPE 20 times per shift.23 Many engineering and administrative measures should be considered especially if the current pandemic becomes prolonged. These measures aim to mitigate the anticipated risk of global shortage of PPEs, such as surgical mask, N-95 mask, gowns, etc. Extended use or limited reuse are applicable alternatives to prevent the anticipated supply shortage of face masks, respirators, and protective eyewear equipment and to ensure that healthcare staff have secured access to the necessary supplies for patient care. Limited PPE reuse refers to the practice of using the same N-95 mask for multiple encounters with patients removing it between encounters and doffing to ensure proper fit and the absence of gross contamination. In the operative setting, this may be done by wearing. Consider placing a simple surgical mask over the N-95 for preventing gross contamination. N-95 respirator and change it between encounters. Reuse of protective eyewears, such as full-face shields and goggles, will be allowed if these are individually assigned to each member and regularly get disinfected. Reuse practice is permitted for a single person use (no-sharing). Reuse and reprocessing of the N-95 mask guidelines have been released by the CDC and include the use of ultraviolet light processing, hydrogen peroxide in either liquid or vaporized state, and moist heating decontamination.24 These guidelines should be reviewed at length before attempting reprocessing of equipment to prevent potentially catastrophic error. Extended use refers to the practice of using the same respirators for repeated close contact encounters with multiple patients, without doffing it between the encounters. This practice might be preferred over reuse, assuming this would reduce the risk of self-contamination through frequent donning and doffing of the same equipment. These practices vary between institutions especially for using N-95 masks. Theoretically, the HCP could extend this and tolerate wearing N-95 masks for up to 8 to 12 hours.25,26 However, most providers usually take breaks during shifts for lunch/toilets. Therefore, extended use beyond 4 hours might be impractical in most settings,27 although limited reuse practice could be adopted with negative or low-risk patients.28
The Triage Area and Preoperative Measures and Recommendations
Within crisis situations and high-risk environments, especially at crowded triage and ED, rigor in following the designed and recommended measures for all HCP and patients is crucial.29
Ask patients to wear a cloth face covering (a scarf or bandana) or face mask on the patient (regardless of the COVID-19 test results) at arrival, promoting cough etiquette, and providing tissues and for hand hygiene.
Suspected or confirmed COVID-19 patients should be in negative pressure rooms if patients are coughing or procedures that create aerosols are being done (if negative pressure rooms are available), or
Clinically suspected/confirmed COVID-19 patients should separately triaged with at least 6-feet distance from other patients or nontreating staff.
Surgeons should not approach the triage area without the minimum standard of PPE.
-
The recommended PPE in the triage area is as follows (Table 1):
Surgical mask
Regular AAMI level-II gown
Goggles or full-face shields
Gloves
Eye protection and the respirator or face mask should be removed, and hand hygiene done if they become damaged or soiled before leaving the area.
Triage personnel should have a supply of PPE, surgical face masks for the surgical team, and for the patients with symptoms of respiratory infection.
If the patient is oriented with moderate GSC score, ask for the clinical and epidemiological criteria of COVID-19 (The presence of symptoms of a respiratory infection, fever, and or contact with possible COVID-19 patients.)
It is preferred if possible to order immediate SARS-CoV-2 RT-PCR assay if patient is being admitted and especially before surgery and possible intubation.
Patient with noninjured face and/or upper respiratory tract should wear a surgical face mask and be moved along a designated route with a minimal contact with others as much as possible.
Only selected equipment and assessment tools should be brought into the triage room to minimize the number of items that need to be disinfected after the exposure.
Segregation, Restructure, and Designed Workflow
The main principles of the staff segregation, physical restructure, and the designed workflow should focus on reducing exposure and contamination, ensuring adherence to PPE, and subsequent decontamination. Ideally, two types of hospital segregation should be done. Location-based segregation of orthopaedic staff reduces the potential risk of cross-infection. Orthopaedic surgeons, for example, should not be performing screening examinations on the general public because of the risk of exposure. Geographic segregation within the OR complex limits the OR traffic, decreases the exposure, and minimizes the contamination zone.30 With the rapid increase in the number of COVID-19 patients, orthopaedic staff should be segregated into those who are treating suspected/confirmed COVID-19 patients and those who are treating noninfective patients when possible is not. This however may not be possible in smaller hospital systems and practices. Besides screening and isolation of high-risk, confirmed COVID-19 patients, strict and frequent screening of the segregated OR staff is mandatory. Members of the segregated or exposed staff should immediately report any signs of illness and must be taken off duty immediately. In addition, all contact events between patients and staff must be recorded so that contact tracing and infection control measures can be implemented quickly, in case any member of segregated staff tested positive.
Induction Room (Anesthesia Recommendations for Suspected/Confirmed Coronavirus Disease 2019
SARS-CoV-2 test should be considered in high-risk individuals, preferably before surgery and before intubation if possible.28,31-33
Signs must be placed at induction and OR doors to alert other staff not to access the dedicated unit without donning the appropriate PPE.
The most experienced anesthesiologist should intubate the patients (The rationale behind this recommendation is to make competent induction attempt in the shortest possible time, without compromising the infection control measures or the staff's safety)
Try to minimize the airway manipulation, face mask ventilation, open airway suction as much as possible.
Regional anesthesia is recommended over general anesthesia (when using regional anesthesia, patients must always wear surgical masks).
Nasal O2 should be administrated under the surgical mask.
Avoid awake intubation techniques, high flow nasal canula and positive pressure ventilation (may lead to virus aerosolization).
Avoid bag-mask ventilation and use rapid sequence induction (if it cannot be avoided, administrate small tidal volumes).
Use deep anesthesia and neuromuscular blockage.
Preoxygenation should be done via a well-fitting face mask to avoid hypoxia in critically ill COVID-19 patients with respiratory failure.
Administrate antiemetics to reduce post-op retching and vomiting that would require the mask removal.
Use a rigid suction catheter instead of a soft flexible one (reduce contamination).
The patient should recover within the OR when possible.
Operating Room Measures and Recommendations
It is a system-saving, necessary act to plan and restructure our surgical care pathways and protocol during COVID-19 pandemic to protect our community and patients and conserve our valuable resources. The restructure should mainly focus on developing a reasonable plan for operating on emergent and urgent cases. Dedicating a COVID-19 pre-, intra-, and post-operating spaces and training the administrative and surgical staff on the appropriate use of PPE and COVID-19 care pathways to the best of a hospital's ability minimizes exposure and contamination.
If there is suspected COVID-19 diagnosis, the surgical planning should be re-evaluated immediately.
Prepare and set up a separate, isolated OR with separate ventilation system (in case of confirmed COVID-19 case).
Dedicated ORs should have a separate atmospheric air inlet and outlet exhaust system. Recent studies highlighted the necessity to isolate and operate on suspected/confirmed COVID-19 patients within negative pressure OR/isolation room to disseminate the viral load.21,22,28,32 However, heating, ventilation, and air conditioning system in most of the US operating rooms is designed to provide positive pressure. Considering the current poorly controlled situation, adding a portable, self-contained high efficiency particulate air filtration system to the hospital heating, ventilation, and air conditioning systems would economically create a negative pressure that meets the OSHA and CDC TB guidelines.34
When possible, entry to the OR must be only through the anesthetic induction room.
All OR doors should be well sealed once the patient is transferred in (except one door).
The OR team involved in the surgery should be limited.
Limit the OR access and movement in and out to medically/surgically essential purposes.
Strict adherence to high standard of infection control and prevention.
Surface-tough equipment and screens within OR should be wrapped with plastic sheets to facilitate decontamination (the virus can survive within tiny grooves, under buttons, screen peripheries, etc.).
Strict adherence to the recommended high standard PPE. Our recommendations for operating on suspected/confirmed COVID-19 patients is as follows (Table 1).
PAPRs are preferred for long operations (if available) or,
Fitted, NIOSH-certified N-95 mask with;
Eye protection; goggles (covered sides of eyes) or full-face shields
Disposable AAMI level-III surgical gown or coveralls
High-cuffed surgical gloves (alternately, vertical strips of tape can keep gloves secured to the gown [double gloves may be considered but are not yet considered the standard]).35
Shoes worn should be fluid-resistant and easily to be decontaminated (disposable shoe covers might increase the risk of contamination)
Hand hygiene before and after donning/doffing PPE
Try to use disposable medical supplies/instruments as much as possible.
The settings of electrocautery should be as low as possible and avoid long dissecting times to minimize the surgical smoke.
Attention is required to avoid sharp injury or damage of PPE.
The body fluids, blood, secretions, and pathological specimens should be collected in double sealed bags for inspection or destruction.
The patient's case, surgical planning, documentation, and early recovery should be done within OR to minimize the contamination to one room.
Once the patient has been discharged or transferred, HCP, including environmental services personnel, should refrain from entering the vacated room until enough time has elapsed for enough air changers to remove potentially infectious particles.
The OR should be strictly disinfected after use.
Special attention is warranted to avoid self-contamination during PPE doffing.
All contaminated instruments and devices should be disinfected separately followed by proper labeling.
Recovery Room (Postoperative Recommendations)
Postoperative management is a crucial phase for clinically suspected/confirmed COVID-19 patients. Trauma, infection, and oncologic surgeries may be associated with slow recovery, lengthier hospital stays, and more complications. After surgery, the patient's immune system may be compromised, and there may be lower respiratory tract and/or wound infections generally associated with fever. In light of this information, surgeons must be more vigilant to differentiate common postoperative complications from COVID-19 infections. In the presence of fever and one of the symptoms of a respiratory infection (dry cough, etc), laboratory tests for COVID-19 diagnosis must be ordered. Suspected cases should be reported immediately, and under the premise of ensuring the safety of patient, transportation to an isolation ward. Surgeons, nurses, and medical staff share an equal responsibility for the postoperative management, particularly in monitoring the patients' families and visitors to ensure the strict adherence to the pandemic emergency system. When possible, it is important to limit the visitors as much as possible. In fact, most hospitals have recently discontinued visitation by anyone. Postoperatively, patients should receive adequate nutrition, fluid hydration, and electrolyte balance to promote immune recovery and rapid rehabilitation. Frequent monitoring of temperature, laboratory CBC counts, C-reactive protein, ferritin level should be done in any patient with suspected COVID-19. There is recent evidence suggesting that a subgroup of severe COVID-19 might cause a “cytokine storm syndrome,” which is an under-recognized, hyperinflammatory syndrome characterized by a fulminant and fatal hyper-cytokinemia with multiorgan failure.36,37 An increased level of ferritin occurs in approximately 50% of patients. All patients with severe COVID-19 should be screened for hyperinflammation markers such as increased ferritin, decreased platelet count, increased LDH or CRP.36
Telemedicine: A Virtual Personal Protective Equipment for Postoperative Care
One of potential consequences and systemic changes of dealing with the COVID-19 pandemic is the adoption and wide utilization of telemedicine in the surgical care of clinically suspected/confirmed COVID-19 patients. Over the past couple of months, the traditional clinics or postoperative follow-up visits have been replaced with telemedicine modalities. Telemedicine could act as a virtual postoperative PPE to provide impeccable social distancing for patients' safety and for all HCPs so critically needed in frontline against the pandemic. Orthopaedic surgeons can use telemedicine postoperatively to follow-up with COVID-19 patients in three manners38; to schedule follow-up,39-42 for routine monitoring,43-45 and management of recovery issues as needed.46-48 In addition, the use of telemedicine-based services for surgical wound care has proven to be feasible and safe in the early postoperative evaluation.49,50 Both time and cost savings contribute toward high patient satisfaction.39 A randomized controlled trial demonstrated that there was no significant difference in patient satisfaction between telemedicine and face-to-face follow-up visits in an orthopaedic trauma cohort.41 Another study after total joint arthroplasty showed that telemedicine significantly reduce the cycle “appointment” time with an average Skype follow-up call per patient that was 2.71 minutes shorter than face-to-face visits.42 Research showed that for every 23 miles away from clinic, there is 111% probability that a patient will more likely prefer using telemedicine for their postoperative follow-up.48 Surgeons form different specialties have expressed their satisfaction after using these telemedicine modalities to deliver postoperative care.44,48,50,51 Notably, telemedicine revealed high levels of provider-perceived quality of medical history (82%) and therapeutic management (85%), compared with traditional face-to-face visits (72%) and (86%), respectively.52,53 Preparation, practice, and following telemedicine start-up checklists would be useful to ensure effective implementation of telemedicine.54,55 The current focus should be directed in evolving more secured modalities to protect patient's confidentiality and keep their medical records away from any anticipated breach.
Training and Intervention of Healthcare Providers
The current infectious risk on healthcare personnel would have negative consequences, if they are not adequately prepared, trained, or equipped to mitigate the risk. Orthopaedic educators should educate their fellows, residents, students, and ancillary teams in preventing exposure to and the spreading of COVID-19. The care teams must learn how to protect themselves during a pandemic. Refresher training regarding the standard use of PPE is a necessity in this global pandemic. A recent study at the University of Illinois Chicago56 assessed the PPE doffing practices of healthcare workers. The study demonstrated that 90% of observed PPE doffing was incorrect regarding the doffing sequence, doffing technique, or use of appropriate PPE.56 Another survey showed that 14.9% of healthcare personnel did not receive previous training for appropriate PPE doffing practices.57 Training and education might flatten the curve of cross-infection and self-contamination. Training and education of the surgical staff should be continually emphasized. It is imperative that all staff be taught the proper sequential methods of donning and doffing of PPE and mask-fitting techniques to minimize the risk of self-contamination. Frequent audits of infection control must be conducted. A trained observer should be assigned for each emergent operation to identify the weaknesses and implement the necessary steps.
Simulation-based Training
Simulation-based training might be required to improve the team communication during medical crisis58 to establish competencies in PPE donning and doffing practices and workflow in induction, operating, and recovery rooms. Simulation can mimic different clinical scenarios to integrate knowledge to practice, evaluate the providers' performances, and build up self- and team-confidence for real-life cases. Lockhart et al21 demonstrated that simulation was a powerful tool to test and adapt PPE as compared to baseline recommendations alone. Using simulation-based training could be an effective method to replicate highly contiguous COVID-19 cases in a safe, yet challenging, situation without jeopardizing the team safety.
Psychological Support of Patients and Providers
COVID-19 is a major and sudden public health crisis, followed by a series of emergency response measures for community and healthcare services. The uncertainty of understanding the pandemic behavior and the definitive ways for its prevention cause psychological pressures on both the public and HCPs. The public panic and fear of getting infected can increase the burden and use of healthcare services. The surgeons should be fully aware of the psychological pressure on the patients and their families. The knowledge, counseling, education, and support may mitigate the psychological pressure in fighting the pandemic.
Summary
Apart from the fact that this pandemic certainly involves many important health, economic, and community ramifications, it also requires several initiatives to mandate what measures are most appropriate to prepare for mitigating the occupational risks. These initiatives include understanding the different aspects in disease and transmission control in the ongoing pandemic. Strict adherence to CDC and World Health Organization evidence-based guidelines for PPE and environmental hygiene enhances the safety and improves the mitigation of infection in emergent orthopaedic practice. Nevertheless, we think that these recommended measures might optimize the healthcare services provided to confirmed COVID-19 patients and should reduce the risk of occupational transmission to other patients and healthcare professionals.
Footnotes
None of the following authors or any immediate family member has received anything of value from or has stock or stock options held in a commercial company or institution related directly or indirectly to the subject of this article: Dr. Awad, Dr. Rumley, Dr. Vazquez, and Dr. Devine.
References
- References printed in bold type are those published within the past 5 years.
- 1.The U.S. Department of Health and Human Services, O.f.C.R.H.O.: HHS office for civil rights secures corrective action and ensures Florida orthopedic practice protects patients with HIV from discrimination. 2019. Available at: https://www.hhs.gov/about/news/2019/10/30/hhs-ocr-secures-corrective-action-and-ensures-fl-orthopedic-practice-protects-patients-with-hiv-from-discrimination.html. Accessed March 13, 2020.
- 2.Kuhar DT, Henderson DK, Struble KA, et al. : Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. [DOI] [PubMed]
- 3.Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: 2017.
- 4.Wang D, Hu B, Hu C, et al. : Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai Y, Yao L, Wei T, et al. : Presumed asymptomatic carrier transmission of COVID-19 . JAMA 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention: Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease 2019 (COVID-19) in healthcare settings. 2020. [Google Scholar]
- 7.Ye G, Lin H, Chen L, et al. : Environmental contamination of the SARS-CoV-2 in healthcare premises: An urgent call for protection for healthcare workers . medRxiv 2020. 10.1101/2020.03.11.20034546. [DOI] [Google Scholar]
- 8.Chang D, Xu H, Rebaza A, Sharma L, Dela Cruz CS: Protecting health-care workers from subclinical coronavirus infection . Lancet Respir Med 2020;8:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li JPO, Lam DSC, Chen Y, Ting DSW: Novel Coronavirus disease 2019 (COVID-19): The importance of recognising possible early ocular manifestation and using protective eyewear . Br J Ophthalmol 2020;104:297-298. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention: Strategies to optimize the supply of PPE and equipment. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/index.html. Accessed April 5, 2020.
- 11.Peng PWH, Wong DT, Bevan D, Gardam M: Infection control and anesthesia: Lessons learned from the Toronto SARS outbreak . Can J Anaesth 2003;50:989-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jing C, Sun W, Huang J, Gamber M, Wu J, He G: Indirect virus transmission in cluster of COVID-19 cases, Wenzhou, China, 2020 . Emerging Infect Dis J 2020;26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Q, Guan X, Wu P, et al. : Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia . N Engl J Med 2020;382:1199-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan JFW, Yuan S, Kok KH, et al. : A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster . Lancet 2020;395:514-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen N, Zhou M, Dong X, et al. : Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study . Lancet 2020;395:507-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W, Xu Y, Gao R, et al. : Detection of SARS-CoV-2 in different types of clinical specimens . JAMA 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang C, Wang Y, Li X, et al. : Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China . Lancet 2020;395:497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.U.S. Food and Drug Administration: Important information for blood establishments regarding the novel coronavirus outbreak. 2020; Available at: https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/important-information-blood-establishments-regarding-novel-coronavirus-outbreak.
- 19.Chang L, Yan Y, Wang L: Coronavirus disease 2019: Coronaviruses and blood safety . Transfus Med Rev 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeh HC, Muggenburg BA, Lundgren DL, et al. : Characterization of Aerosols Produced by Surgical Procedures. Lovelace Biomedical and Environmental Research Inst., Albuquerque, NM (United States). Inhalation Toxicology Research Inst., 1994, p. Medium: ED; Size: 584 p. [Google Scholar]
- 21.Lockhart SL, Naidu JJ, Badh CS, Duggan LV: Simulation as a tool for assessing and evolving your current personal protective equipment: Lessons learned during the coronavirus disease (COVID-19) pandemic . Can J Anaesth 2020:1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wax RS, Christian MD: Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients . Can J Anaesth 2020:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.S.J.s.P.H. System: CDC Conference call. Everett, WA. [Google Scholar]
- 24.Centers for Disease Control and Prevention: Decontamination and reuse of filtering facepiece respirators. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html. Accessed April 7, 2020.
- 25.Radonovich LJ, Cheng J, Shenal BV, Hodgson M, Bender BS: Respirator tolerance in health care workers . JAMA 2009;301:36-38. [DOI] [PubMed] [Google Scholar]
- 26.Rebmann T, Carrico R, Wang J: Physiologic and other effects and compliance with long-term respirator use among medical intensive care unit nurses . Am J Infect Control 2013;41:1218-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher EM, Shaffer RE: Considerations for recommending extended use and limited reuse of filtering facepiece respirators in health care settings . J Occup Environ Hyg 2014;11:D115-D128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong J, Goh QY, Tan Z, et al. : Preparing for a COVID-19 pandemic: A review of operating room outbreak response measures in a large tertiary hospital in Singapore . Can J Anaesth 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams JG, Walls RM: Supporting the health care workforce during the COVID-19 global epidemic . JAMA 2020. [DOI] [PubMed] [Google Scholar]
- 30.Chee VWT, Khoo MLC, Lee SF, Lai YC, Chin NM: Infection control measures for operative procedures in severe acute respiratory syndrome–related patients . Anesthesiology 2004;100:1394-1398. [DOI] [PubMed] [Google Scholar]
- 31.Kamming D, Gardam M, Chung F: Anaesthesia and SARS . Br J Anaesth 2003;90:715-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liew MF, Siow WT, MacLaren G, See KC: Preparing for COVID-19: Early experience from an intensive care unit in Singapore . Crit Care 2020;24:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tompkins BM, Kerchberger JP: Special article: Personal protective equipment for care of pandemic influenza patients: A training workshop for the powered air purifying respirator . Anesth Analg 2010;111:933-945. [DOI] [PubMed] [Google Scholar]
- 34.Elias B, Bar-Yam Y: Could Air Filtration Reduce COVID-19 Severity and Spread? New England Complex Systems Institute, 2020. [Google Scholar]
- 35.Caputo KM, Byrick R, Chapman MG, Orser BJ, Orser BA: Intubation of SARS patients: Infection and perspectives of healthcare workers . Can J Anesth 2006;53:122-129. [DOI] [PubMed] [Google Scholar]
- 36.Puja M, McAuley DF, Brown M, et al. : COVID-19: consider cytokine storm syndromes and immunosuppressio . Lancet 2020;395:1033-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seguin A, Galicier L, Boutboul D, Lemiale V, Azoulay E: Pulmonary involvement in patients with hemophagocytic lymphohistiocytosis . Chest 2016;149:1294-1301. [DOI] [PubMed] [Google Scholar]
- 38.Gunter RL, Chouinard S, Fernandes-Taylor S, et al. : Current use of telemedicine for post-discharge surgical care: A systematic review . J Am Coll Surg 2016;222:915-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams AM, Bhatti UF, Alam HB, Nikolian VC: The role of telemedicine in postoperative care . Mhealth 2018;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hwa K, Wren SM: Telehealth follow-up in lieu of postoperative clinic visit for ambulatory surgery: Results of a pilot program . JAMA Surg 2013;148:823-827. [DOI] [PubMed] [Google Scholar]
- 41.Sathiyakumar V, Apfeld JC, Obremskey WT, Thakore RV, Sethi MK: Prospective randomized controlled trial using telemedicine for follow-ups in an orthopedic trauma population: A pilot study . J Orthop Trauma 2015;29:e139-e145. [DOI] [PubMed] [Google Scholar]
- 42.Sharareh B, Schwarzkopf R: Effectiveness of telemedical applications in postoperative follow-up after total joint arthroplasty . J Arthroplasty 2014;29:918-922.e1. [DOI] [PubMed] [Google Scholar]
- 43.Yoder LH, McFall DC, Cancio LC: Use of the videophone to collect quality of life data from burn patients . Int J Burns Trauma 2012;2:135-144. [PMC free article] [PubMed] [Google Scholar]
- 44.Finkelstein SM, MacMahon K, Lindgren BR, et al. : Development of a remote monitoring satisfaction survey and its use in a clinical trial with lung transplant recipients . J Telemed Telecare 2012;18:42-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rao R, Shukla BM, Saint-Cyr M, Rao M, Teotia SS: Take two and text me in the morning: Optimizing clinical time with a short messaging system . Plast Reconstr Surg 2012;130:44-49. [DOI] [PubMed] [Google Scholar]
- 46.Pirris SM, Monaco EA, III, Tyler-Kabara EC: Telemedicine through the use of digital cell phone technology in pediatric neurosurgery: A case series . Neurosurgery 2010;66:999-1004. [DOI] [PubMed] [Google Scholar]
- 47.Sidana A, Noori S, Patil N: Utility of smartphone camera in patient management in urology . Can J Urol 2014;21:7449-7453. [PubMed] [Google Scholar]
- 48.Canon S, Shera A, Patel A, et al. : A pilot study of telemedicine for post-operative urological care in children . J Telemed Telecare 2014;20:427-430. [DOI] [PubMed] [Google Scholar]
- 49.Segura-Sampedro JJ, Rivero-Belenchón I, Pino-Díaz V, et al. : Feasibility and safety of surgical wound remote follow-up by smart phone in appendectomy: A pilot study . Ann Med Surg (Lond) 2017;21:58-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gunter R, Fernandes-Taylor S, Mahnke A, et al. : Evaluating patient usability of an image-based mobile health platform for postoperative wound monitoring . JMIR Mhealth Uhealth 2016;4:e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Albert MV, McCarthy C, Valentin J, Herrmann M, Kording K, Jayaraman A: Monitoring functional capability of individuals with lower limb amputations using mobile phones . PLoS One 2013;8:e65340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Powered air-purifying respirators.
- 53.Viers BR, Lightner DJ, Rivera ME, et al. : Efficiency, satisfaction, and costs for remote video visits following radical prostatectomy: A randomized controlled trial . Eur Urol 2015;68:729-735. [DOI] [PubMed] [Google Scholar]
- 54.American Academy of Orthopaedic Surgeons: Telemedicine startup checklist. Available at: https://www.aaos.org/quality/practice-management/telemedicine/. Accessed April 5, 2020.
- 55.Calton B, Abedini N, Fratkin M: Telemedicine in the time of coronavirus. J Pain Symptom Manage 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Phan LT, Maita D, Mortiz DC, et al. : Personal protective equipment doffing practices of healthcare workers . J Occup Environ Hyg 2019;16:575-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.John A, Tomas ME, Cadnum JL, et al. : Are health care personnel trained in correct use of personal protective equipment? Am J Infect Control 2016;44:840-842. [DOI] [PubMed] [Google Scholar]
- 58.Vortman R: Using simulation-based education to improve team communication during a massive transfusion protocol in the OR . AORN J 2020;111:393-400. [DOI] [PubMed] [Google Scholar]