Abstract
Background
This is an updated version of the original review published in Issue 4, 2004 of The Cochrane Library. Lung cancer is one of the leading causes of death globally. Despite advances in treatment, the outlook for the majority of patients remains grim and most face a pessimistic future accompanied by sometimes devastating effects on emotional and psychological health. Although chemotherapy is accepted as an effective treatment for advanced lung cancer, the high prevalence of treatment‐related side effects as well the symptoms of disease progression highlight the need for high‐quality palliative and supportive care to minimise symptom distress and to promote quality of life.
Objectives
To assess the effectiveness of non‐invasive interventions delivered by healthcare professionals in improving symptoms, psychological functioning and quality of life in patients with lung cancer.
Search methods
We ran a search in February 2011 to update the original completed review. We searched the Cochrane Central Register of Controlled Trials (The Cochrane Library 2011, Issue 2), MEDLINE (accessed through PubMed), EMBASE, PsycINFO, AMED, British Nursing Index and Archive (accessed through Ovid) and reference lists of relevant articles; we also contacted authors.
Selection criteria
Randomised or quasi‐randomised clinical trials assessing the effects of non‐invasive interventions in improving well‐being and quality of life in patients diagnosed with lung cancer.
Data collection and analysis
Two authors independently assessed relevant studies for inclusion. Data extraction and risk of bias assessment of relevant studies was performed by one author and checked by a second author.
Main results
Fifteen trials were included, six of which were added in this update. Three trials of a nursing intervention to manage breathlessness showed benefit in terms of symptom experience, performance status and emotional functioning. Four trials assessed structured nursing programmes and found positive effects on delay in clinical deterioration, dependency and symptom distress, and improvements in emotional functioning and satisfaction with care.
Three trials assessed the effect of different psychotherapeutic, psychosocial and educational interventions in patients with lung cancer. One trial assessing counselling showed benefit for some emotional components of the illness but findings were not conclusive. One trial examined the effects of coaching sensory self monitoring and reporting on pain‐related variables and found that although coaching increases the amount of pain data communicated to providers by patients with lung cancer, the magnitude of the effect is small and does not lead to improved efficacy of analgesics prescribed for each patient’s pain level. One trial compared telephone‐based sessions of either caregiver‐assisted coping skills training (CST) or education/support involving the caregiver and found that patients in both treatment conditions showed improvements in pain, depression, quality of life and self efficacy.
Two trials assessed exercise programmes; one found a beneficial effect on self empowerment and the other study showed an increase in quadriceps strength but no significant changes for any measure of quality of life. One trial of nutritional interventions found positive effects for increasing energy intake, but no improvement in quality of life. Two small trials of reflexology showed some positive but short‐lasting effects on anxiety and pain intensity.
The main limitations of the studies included were the variability of the interventions assessed and the approaches to measuring the considered outcomes, and the lack of data reported in the trials regarding allocation of patients to treatment groups and blinding.
Authors' conclusions
Nurse follow‐up programmes and interventions to manage breathlessness may produce beneficial effects. Counselling may help patients cope more effectively with emotional symptoms, but the evidence is not conclusive. Other psychotherapeutic, psychosocial and educational interventions can play some role in improving patients' quality of life. Exercise programmes and nutritional interventions have not shown relevant and lasting improvements of quality of life. Reflexology may have some beneficial effects in the short term.
Keywords: Humans, Quality of Life, Energy Intake, Exercise, Lung Neoplasms, Lung Neoplasms/nursing, Lung Neoplasms/psychology, Massage, Psychotherapy, Randomized Controlled Trials as Topic, Respiration Disorders, Respiration Disorders/nursing, Respiration Disorders/rehabilitation
Plain language summary
Non‐invasive interventions for improving well‐being and quality of life in patients with lung cancer
Despite recent advances in lung cancer treatment, the outlook for most patients is grim. Many still face a short survival time during which they may suffer physical and psychological problems associated with the cancer and with side effects of treatment. Although no cure exists, there is a need for high‐quality care to support patients and reduce symptoms as much as possible. This review found that nursing programmes and interventions to manage breathlessness may produce beneficial effects and that some psychotherapeutic, psychosocial and educational interventions can play some role in improving the quality of life of patients. Counselling may help patients to cope better with emotional symptoms and reflexology can have some short‐term beneficial effects. The main limitations of the included studies were the variability of the interventions, the way results were measured and the lack of 'blinding' (ensuring that those who are measuring the patients' outcomes are not aware of which treatment the patient actually received).
Background
This review is an update of a review previously published in The Cochrane Library 2004, Issue 4 (Solà 2004).
In 2008 lung cancer was the most commonly diagnosed cancer, as well as the leading cause of cancer death in males globally (GLOBOCAN 2008). At that time, lung cancer accounted for 13% (1.6 million) of the total cases and 18% (1.4 million) of the deaths. Lung cancer is now the leading cancer site in males, comprising 17% of total new cancer cases and 23% of total cancer deaths. Its mortality burden among females in developing countries is as high as the burden for cervical cancer (each accounting for 11% of total female cancer deaths) (Jemal 2011).
The most important single risk factor for lung cancer is smoking, accounting for about 90% of cases (Sethi 1997). The increases in tobacco use, especially amongst women and young people, that occurred during the last few decades are currently being reflected in an increased incidence of lung cancer in many countries (Jané 2002).
Over 55% of patients who present with lung cancer have distant metastases at the time of diagnosis, with 25% having regional node involvement and only about 15% having localised disease that is amenable to surgery (Fauci 1998). Although advances in chemotherapy, surgery and radiotherapy have led to a steady increase in survival, from 8% in the 1960s to 14% in the 1990s, the outlook for the great majority of people with the disease remains very poor.
Since only those patients whose disease is operable at the time of presentation have a chance of cure, between 80% and 90% of patients with lung cancer are faced with an average survival from the time of diagnosis of about eight months. During these last few months of their lives patients may undergo a range of invasive and/or toxic therapies that may include chemotherapy, radiotherapy or both, they may participate in a clinical trial of one of the novel agents being tested in phase I and II trials, or they may be unfit for any treatment other than supportive and palliative care. During this difficult time the general health of the patients with lung cancer will decline both as a consequence of the illness itself and because of side effects from the treatments they receive. In addition to physical symptoms, patients with lung cancer face a depressing and anxiety‐provoking future, an outlook that can have a devastating effect on their psychological and emotional health as well as that of their families, friends and carers (Sarna 1998).
Although chemotherapy is accepted as an effective treatment for advanced lung cancer, the high prevalence of treatment‐related side effects, as well the symptoms of disease progression, highlight the need for and importance of high‐quality palliative and supportive care to minimise symptom distress and to promote quality of care (Sarna 1998).
In 1990, the World Health Organization defined palliative care as: "the active total care of patients whose disease is not responsive to curative treatment. Control of pain, of other symptoms, and of psychological, social and spiritual problems is paramount. The goal of palliative care is the achievement of the best possible quality of life for patients and their families" (WHO 1990). In this way, palliative care is described as an approach which improves the quality of life of patients and their families facing life‐threatening illness.
Supportive care is "multi‐professional attention to the individual's overall physical, psychosocial, spiritual and cultural needs, and should be available at all stages of the illness, for patients of all ages, and regardless of the current intention of any anti‐cancer treatment" (Ahmed 2004). The main difference from palliative care is that the definition of supportive care specifies that it should be available at any stage of the illness.
Among the many interventions that may be considered as part of a holistic package of supportive and palliative care, non‐invasive interventions which improve symptoms related to the illness are of special importance. These interventions emphasise the importance of not separating the psychological and physical aspects of the symptoms (Bredin 1999) and facilitate an integrative approach to cancer care. For both palliative and supportive care, research must assess the best methods of providing patients and their families with the optimal levels of emotional, psychological and spiritual support that are coherent with their individual needs, wishes and circumstances.
In order to develop an understanding of the most effective healthcare interventions for patients with advanced lung cancer, and to work towards the development of integrated and effective care, it is necessary to draw on all forms of relevant scientific evidence. An essential step in this process is to collate and analyse the evidence from quantitative, comparative studies. This updated review assesses the available quantitative evidence on the effectiveness of non‐invasive interventions in improving the well‐being and quality of life of patients with lung cancer.
Objectives
To determine the effectiveness of non‐invasive interventions delivered by healthcare professionals in improving symptoms, psychological functioning and quality of life in patients with lung cancer.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCT) and controlled clinical trials (CCT). We considered that a trial was a CCT when it did not use a true randomisation sequence.
Types of participants
Patients of either sex and any age diagnosed with lung cancer at any stage of their illness. If studies also included some patients with other thoracic cancers (e.g. malignant pleural mesothelioma), these patients were also included. We considered studies that included patients with lung cancer as well as cancers in other sites (e.g. breast, colon, etc.) only if separate data on patients with lung cancer were reported.
Types of interventions
Non‐invasive (1) interventions (2) performed for the care of patients with lung cancer.
(1) Any physical treatment which does not require catheterisation, skin puncture, intubation, incision, drainage, endoscopy or pharmacological intervention (defined as any intervention in which a medicinal product is administered). A medicinal product, as defined in European legislation (Directive 2001/83/EC), is "any substance or combination of substances presented for treating or preventing disease in human beings. Any substance or combination of substances which may be administered with a view to making a diagnosis or to restoring, correcting or modifying physiological function is likewise considered a medicinal product". We also considered herbal remedies as medicinal products in this review (MCA 2002). In relation to food products and dietary supplements, we excluded only those ingested products that are administered via a parenteral route or by means of intubation.
(2) Any treatment or action, based on clinical judgement and knowledge, that healthcare professionals (physicians, nurses, psychologists, physiotherapists, occupational therapists, dieticians) perform to enhance patient well‐being or quality of life.
The range of possible non‐invasive interventions is huge and we recognised that some may not fall neatly within the above definitions. These definitions were therefore applied as rigorously as possible.
Types of outcome measures
1. Well‐being defined as:
a subjective or objective perception of improvement in physical health, or of symptoms related to cancer, to metastases, or to side effects of treatment of the illness; and/or
a subjective or objective perception of improvement of psychological functioning.
We only included studies in which patient well‐being defined as above had been measured by validated and specific standardised impairment, distress or psychological scales.
2. Quality of life defined as: an individual's perception of position in life in the context of the culture and value systems in which he or she lives and in relation to their goals, expectations, standards and concerns (WHO 1993), determined exclusively by means of validated scales, classifications and measurement systems.
Search methods for identification of studies
Electronic searches
We ran a search in February 2011 to update the original completed review. In this update we modified the original searches to search the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 2), MEDLINE (accessed through PubMed), EMBASE, PsycINFO, AMED, British Nursing Index and Archive (all accessed through Ovid). We include in Appendix 1 the search strategies used and the results obtained. We also report in Appendix 2 the text published for this section in the original review and the search strategies designed.
Searching other resources
We additionally checked the reference lists from relevant studies to identify further eligible studies, and contacted authors from the main studies to seek additional data on published or unpublished studies.
Data collection and analysis
Selection of studies
Two authors independently assessed all the references retrieved by the electronic searches to identify potentially relevant studies.
Data extraction and management
JR extracted data from the included studies and IS checked them. Data extracted included information on the study design, participants, a description of the intervention, outcomes measured and the characteristics of the groups of comparison, as well as the main results of the study.
Assessment of risk of bias in included studies
Two review authors (JR and IS) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). Any disagreement was resolved by discussion or involving a third author (MS or AP). We focused our assessment on the following domains:
(1) Sequence generation (checking for possible selection bias) We described for each included study the methods used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. We assessed the methods to be at:
• low risk (any truly random process, e.g. random number table; computer random number generator); • high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or • unclear risk.
(2) Allocation concealment (checking for possible selection bias) We described for each included study the method used to conceal the allocation sequence in sufficient detail and determined whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods to be at:
• low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes); • high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth); • unclear risk.
(3) Blinding (checking for possible performance bias) We described for each included study all the methods used, if any, to blind investigators, patients or outcome assessors from knowledge of which intervention a participant received. We assessed the methods to be at:
• low risk; • high risk; or • unclear risk.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations) We stated whether attrition and exclusions (and their reasons) were reported, the numbers included in the analysis (compared with the total randomised participants), and whether missing data were balanced across groups. We assessed whether each study was free of attrition bias:
• low risk; • high risk; • unclear risk.
(5) Selective outcome reporting (checking for possible reporting bias) We stated whether the reports of included studies described accurately data on all the outcomes that the trialists planned to assess originally. We assessed whether each study was free of reporting bias:
• low risk; • high risk; • unclear risk.
Data synthesis
The heterogeneity of studies, both with regard to the diversity of interventions assessed and outcomes measures used, made a quantitative pooling of the outcome data inappropriate (Petticrew 2001). This review therefore presents a narrative synthesis of the different interventions identified, as well as the main results of the included studies (see Table 1).
1. Study Results.
| Study | Results |
| Arbane 2011 |
Length of stay, mean (SD):
IG: 8.9 (3.3); CG 11.0 (8.9) days; statistically not significant differences (P value not provided) Postoperative complications (POC): Two a priori POCs defined in the active group and 3 in the control group; statistically not significant differences (P value not provided) Quality of life assessed by the EORTC QLQ‐CL13 questionnaire No significant changes for any measure of quality of life (functional, symptom and global health), either within subjects or between groups (P values not provided). Data available from 22 patients in IG and 21 in CG; measurements preoperative and 12 weeks postoperative. EORTC‐C30 (functional), mean (SD): IG: 81.0 (14.1) to 79.1 (19.1); CG: 79.4 (18.0) to 76.7 (22.7) EORTC‐C30 (symptom), mean (SD): IG: 16.1 (16.0) to 18.5 (15.2); CG: 17.8 (15.4) to 21.0 (16.4) EORTC‐C30 (global health), mean (SD): IG: 74.7 (27.3) to 68.2 (15.3); CG: 70.2 (21.7) to 68.1 (25.1) Exercise tolerance: the 6‐Minute Walking Distance (6MWD), in metres: Data available from 21 patients in IG and 16 in CG; measurements preoperative and 12 weeks postoperative. Mean (SD), IG: 466.6 (102.10) to 480.2 (110.0); CG: 455.7 (98.0) to 448.2 (95.1); (P = 0.47) Quadriceps strength, in kg: Data available from 17 patients in IG and 13 in CG; measurements preoperative and 12 weeks postoperative. Mean (SD), IG: 33.2 (15.2) to 34.2 (9.4); CG: 29.1 (10.9) to 26.4 (9.7); (P = 0.04 between groups). |
| Barton 2010 |
Patients’ self reported outcomes:
Mean (SD) change from baseline to week 4, based on 5 patients in single‐session group and 6 in 3‐session group. A negative change represents an improvement from baseline. Authors do not present estimates of statistical significance and P values, stating that their aims were to assess feasibility and to look for variability to power a larger RCT. Breathlessness: Measured by mean area under curve (AUC) from baseline to week 4 and by mean change of numerical rating scale (NRS) from baseline to week 4 Average breathlessness: Mean AUC single session 4.7; 3 sessions 3.6 (SD 2.3); NRS single session 0.8; 3 sessions ‐2.5 (SD 3.2) Worst breathlessness: Mean AUC single session 5.2; 3 sessions 3.8 (SD 2.6); NRS single session ‐1.0; 3sessions ‐3.0 (SD 2.5) Breathlessness now: Mean AUC single session 4.2; 3 sessions 3.2 (SD 2.5); NRS single session 1.8; 3 sessions ‐3.0 (SD 1.8) Distress from breathlessness: Mean AUC single session 2.4; 3 sessions 2.2 (SD 2.1); NRS single session 0.0; 3 sessions 1.2 (SD 2.2) Coping with breathlessness: Mean AUC single session 7.2; 3 sessions 6.7 (SD 1.3); NRS single session ‐2.0; 3 sessions 3.2 (SD 4.3) Satisfaction with care: Mean AUC single session 8.4; 3 sessions 9.3 (SD 0.9); NRS single session 0.6; 3 sessions 1.5 (SD 1.8) Anxiety: Single session 0.20 (SD 4.60); 3 sessions ‐1.50 (SD 5.09) Depression: Single session 0.40 (SD 2.07); 3 sessions ‐2.33 (SD 3.01) Coping strategy: Adaptive: single session) ‐0.40 (SD 4.22); 3 sessions‐0.00 (SD 7.10). Maladaptive: single session ‐1.80 (SD 4.44); 3 sessions ‐0.83 (SD 5.98) Quality of life and functional status: An EQ‐5D index was calculated, but not interpretable on so few data. EQ‐5D. EQ‐VAS scores appeared to improve in the 3‐session group but were stable in the single group, but detailed data not provided. |
| Bredin 1999 | At 8 weeks there was a favourable effect of the intervention on breathlessness at best (median change from baseline: IG = 1.3 (range: ‐7.1 to 8) versus CG = 7 (range: ‐3.3 to 8); P = 0.03), WHO performance status (median change: IG = 0 (range: ‐3 to 3) versus CG = 2 (range: ‐1 to 3); P = 0.02), physical symptom distress (median change: IG = 2.5 (range: ‐24 to 16) versus CG = 14 (range: ‐11 to 16); P = 0.04) and depression (median change from baseline: IG = 0.5 (range: ‐10 to 7) versus CG = 6 (range: ‐7 to 7); P = 0.02). Less increase in distress due to breathlessness (primary outcome) in the intervention group compared with the control group but this did not reach statistical significance (P = 0.09) No significant differences between groups in overall activity levels although a sub‐analysis of 3 specific activities on the Rotterdam checklist showed a benefit in favour of the intervention group (stair‐climbing, walking outdoors and shopping; (IG = 0 (range: ‐6 to 9) versus CG = 5.5 (range:‐3 to 9); P = 0.05)) No significant differences between groups in breathlessness at worst, psychological distress and overall quality of life Survival in patients who left the study was worse in the control group than in the intervention group (HR = 2.5; P < 0.05) |
| Chan 2011 | Symptom cluster. Doubly multivariate analysis of variance revealed a significant difference (time x group interaction effect, P = 0.003) over time between the IG and CG on the pattern of change of the symptom cluster. Significant effects on the patterns of changes in breathlessness (P = 0.002), fatigue (P = 0.011), anxiety (P = 0.001) and functional ability (P = 0.000). Estimates of the effect not provided. |
| Corner 1996 | Significant improvements from baseline in self reported breathlessness at worst, distress caused by breathlessness and functional capacity after 12 weeks in the intervention group whereas median scores in the control group were either static or worsened. Comparison of improvements from baseline between groups showed significant improvements in favour of the intervention group for breathlessness at best (intervention group (IG) = 0.5 (range: ‐0.5 to 2.8) versus control group (CG) = ‐0.5 (range: ‐5.7 to 1); P < 0.02) and at worst (IG = 3.5 (range: ‐1 to 7) versus CG = 0 (range: ‐5 to 4); P < 0.05), a decrease in the distress caused by breathlessness (IG = 5.3 (range: 0 to 9) versus CG = ‐1 (range: ‐4.5 to 3); P < 0.01), functional capacity (IG = 1.0 (range: ‐0.5 to 2) versus CG = ‐0.25 (range: ‐1 to 1), P < 0.02) and ability to undertake activities of daily living (IG = 3.0 (range: ‐3 to 8) versus CG = 0 (range: ‐3 to2); P < 0.03). No obvious survival difference between the 2 groups. |
| Evans 1987 | Results for lung cancer sample only: The interventions were effective in increasing caloric intake (SG and AG = 91% versus CG = 62%; P < 0.0001) Less weight loss in intervention group than in control group but difference not significant (SG and AG = ‐1.2% versus CG = ‐3.1%; P = 0.6) No significant differences between groups in tumour response (SG = 27.5%; AG = 20%; CG = 14.7%) Survival similar in all groups (SG = 8 months; AG = 7 months; GC = 6 months). No significant differences between control and intervention even when both intervention groups combined (P > 0.2). Although 60% of patients in both control groups required enteral nutrition by protocol only 10% in the SG and 7% in the AG received it. The main reason for which was patient refusal. Median survival times similar: (CG = 6 months; SG = 8 months; AG = 7 months) with no significant differences between control and intervention groups even when data from the 2 intervention groups were combined (P > 0.2). There were no significant differences between any of the groups in either the percent of target dose of chemotherapy achieved or in the degrees of toxicity experienced. |
| Linn 1982 | Quality of life 3 months: significant differences (P < 0.05) in mean scores in favour of intervention group for following 4/5 QOL variables (IG versus CG): Depression: 10.8 versus 14.2; F = 7.69 Alienation: 3.2 versus 4.9; F = 4.68 Life satisfaction: 36 versus 48.1; F = 9.07 Self esteem: 43.4 versus 56.3; F = 8.23 (higher mean score indicates less favourable response) 12 months: Significant differences (< 0.05) for all QOL variables in favour of intervention group F‐ratio for multivariate difference = 18.78 (P < 0.001) Functional status and degree of impairment: no significant differences Survival: no significant differences |
| McCorkle 1989 | Patients in the office care group experienced worsening of symptom distress one occasion (6 weeks) earlier than the 2 intervention groups (multivariate difference in adjusted symptom distress scale scores F = 5.01, df = 1.6; P = 0.03) A similar difference was seen for social dependency. Both intervention groups were similar but patients in the office care group deteriorated 6 weeks earlier (multivariate difference in enforced social dependency scores: F = 5.72, df = 1.6; P = 0.02) Compared with the intervention groups, the control group showed a significant improvement in self perceived health (F = 4.06, gl = 1.5; P = 0.05) OHC group had fewer hospitalisations than the other groups (mean hospitalisations: OHC = 2.08; SHC = 2.82; OC = 2.62; not significant (NS)) but mean length of stay for those patients who were admitted was longer in the OHC (mean length hospital stay OHC = 18.4 days (SD 19.7); SHC = 17.6 (SD17.7); OC = 13.6 (SD 10.3); NS). Total length of stay in the OHC group was lower than in the other 2 groups (OHC = 258 days; SHC = 317 days; OC = 272 days; NS). |
| Moore 2002 | Values are medians (interquartile range) At 3 months: ‐ dyspnoea in IG patients significantly lower than the control group: (IG = 25 (16.7 to 41.7) versus CG = 33.3 (25 to 58.3); P = 0.03) ‐ greater satisfaction with care in IG, shown by higher scores (P < 0.005) on all satisfaction subscales 78% of IG patients said that they would prefer nurse care if they had to choose, compared with 17% of CG patients who said they would prefer medical attention At 12 months: IG patients reported significantly better emotional functioning (IG = 91.7 (66.7 to 100) versus CG = 66.7 (54.2 to 87.5); P = 0.03) and less peripheral neuropathy (IG = 0 versus CG = 0 (0 to 33.3); P = 0.05). No significant survival differences between groups but there was evidence that symptomatic progression was detected sooner by the nurses than the doctors. No significant differences between groups in overall costs but the intervention changed the pattern of resource use: IG patients had fewer hospital consultations at 3 months (P = 0.004), fewer radiographs taken at 3 months (P = 0.04) and at 6 months (P = 0.03), and were more likely to have had radiotherapy treatment at 3 months (P = 0.01). More IG patients died at home (IG = 40% versus CG = 23%; P = 0.04). The cost of the nurse programme was estimated to be 150 pounds sterling per patient per month of follow‐up. |
| Porter 2011 | Analyses of patient outcome measures indicated significant main effects of time for ratings of worst pain (B = ‐ 0.15, SE = 0.13, P = 0.02); physical well‐being (B = 0.84, SE = 0.22, P = 0.0002); functional well‐being (B = 0.55, SE = 0.22, P = 0.03); lung cancer symptoms (B = 0.76, SE = 0.21, P = 0.0003); depression (B = 0.55, SE = 0.28, P = 0.05); and self efficacy (B = 2.31, SE = 1.03, P = 0.02) Patients in both CST and education/support reported improvements over time in their worst pain ratings, their physical and functional well‐being, their lung cancer symptoms, their depressive symptoms and their self efficacy for controlling symptoms. There were no significant time intervention interactions. |
| Sarna 1998 | A multivariate model showed that systematic symptom assessment was associated with less symptom distress over time: regression coefficient (in units of the symptom distress scale) = ‐1.20, standard error (SE) = 0.32, P = 0.0004). No significant relationship between the course of symptom distress and group assignment was seen on bivariate analyses of symptom distress scores with potential predictors over time (r = ‐1.20, SE = 0.32; P = 0.0004). |
| Stephenson 2000 | Mean pre‐intervention scores for anxiety were higher than the pre‐control time scores: 53.60 (SD = 30.6) versus 39.60 (SD = 29.96) but mean postintervention scores were lower than postcontrol time scores: 20 (SD = 22.36) versus 33.60 (SD = 24.94) Statistically significant reduction in anxiety scores after reflexology compared with before (post‐/pre‐score difference = ‐33.6; P = 0.002) Reduction in anxiety score at the end of the control time compared with at the beginning, but not significant (post‐/initial score difference = ‐6; P = 0.99) Difference in score reduction between control and intervention groups significant (difference = ‐27.6; P = 0.02) |
| Stephenson 2007 | The reflexology group showed a 34% reduction in pain after intervention compared to the control group's 2% reduction; 19% of the experimental group and 11% in the control group experienced pain reduction of 2 or more scale points on the 10‐point scale. In the subgroup of patients with moderate to severe pain scores prior to treatment, the reflexology group experienced a 37% reduction in pain compared to a 6% reduction in the control group; in this subgroup 50% of the of the experimental group and 20% in the control group experienced pain reduction of 2 or more scale points Anxiety scores for the total group showed a 62% improvement in the reflexology group compared to 23% in the control group; 48% of the experimental group and 32% in the control group experienced anxiety score reduction of 2 or more scale points on the 10‐point scale In the moderate to severe anxiety subgroups, scores decreased by 67% compared with the control group's 31% reduction; and 74% of the experimental group and 44% in the control group experienced anxiety score reduction of 2 or more scale points on the 10‐point scale |
| Wall 2000 | No significant differences (P = 0.08) for hope between the intervention and the control group either at baseline or at any time thereafter No significant differences between groups in power at baseline but differences were observed at both time 2 and time 3 showing a beneficial effect over time on power, of participation in the exercise programme (F(2) = 12.09; P < 0.001). Patients in the intervention group showed significant increases in power between baseline and time 2 (t(51) = ‐2068; P = 0.01), but not between time 2 and time 3 (P = 0.07). There was a steady increase in power between time 1 and time 3 in this group (t(48) = ‐3.73; P = 0.001). Power decreased in the control group between baseline and time 2 (t(50) = 2.72; P < 0.01), and this decrease was sustained between time 2 and time 3. No statistically significant differences between groups were found for the relation of hope and power |
| Wilkie 2010 |
Pain prescriptions, adequate analgesia, based on 76 patients in IG and 75 in CG:
IG 84%, CG 76% (P = 0.32) at the end of the study Pain relief: based on 76 patients in IG and 75 in CG: VAS pain relief scores (SD) at baseline and at the end of the study: IG from 66.5 (31) to 72.4 (28) (P = 0.37), CG from 66.8 (34) to 75.8 (24) VAS present pain intensity IG from 22.3 (22) to 24.3 (25), CG from 17.4 (19) to 19.4 (23) (P = 0.46) MPQ pain quality scores (SD): PRI – Sensory: IG from 11.5 (7.8) to 8.0 (7.4), CG from 9.7 (8.3) to 8.0 (8.9) (P = 0.48) PRI – Affective: IG from 1.6 (2.0) to 0.8 (1.4), CG from 1.1 (1.8) to 1.0 (2.1) (P = 0.08) PRI – Evaluative: IG from 1.8 (1.7) to 1.2 (1.4), CG from 1.4 (1.5) to 1.1 (1.4) (P = 0.79) PRI – Miscellaneous: IG from 3.04 (3.14) to 1.47 (2.27), CG from 2.39 (2.79) to 2.41 (3.67) (P = 0.01) PRI – Total: IG from 17.8 (12.5) to 11.4 (10.7), CG from 14.6 (12.6) to 12.5 (14.7) (P = 0.13) Emotional status and coping Detailed data not provided. Catastrophising coping, anxiety and depression scores were similar in the 2 study groups, both at baseline and study end. Catastrophising coping scores decreased slightly more in the coached group than in the not‐coached group, but not significantly so. Those coached are more likely than those not coached to give their providers unsolicited sensory pain information and to mention it before their providers ask for it. The mean number of pain parameters discussed during the audiotaped clinic visit is statistically larger at study end for the coached group. |
6MWD: Six‐Minute Walking Distance AG: augmented nutrition group CG: control group CST: coping skills training EORTC: European Organisation for Research and Treatment of Cancer HR: hazard ratio IG: intervention group NRS: numerical rating scale OC: Office Care Programme OHC: Specialised Oncology Home Care Programme PRI: Pain Relief Inventory QOL: quality of life RCT: randomised controlled trial SD: standard deviation SE: standard error SG: standard nutrition group SHC: Standard Home Care Programme VAS: visual analogue scale WHO: World health Organization
Results
Description of studies
See: Characteristics of included studies, Characteristics of excluded studies, Characteristics of studies awaiting classification and Characteristics of ongoing studies.
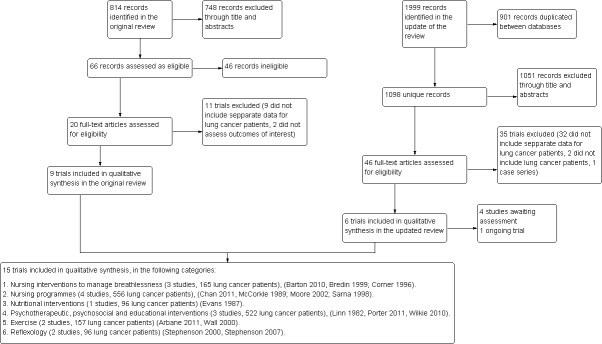
Figure 1 provides a flowchart that describes how the studies were identified in this review.
1.
Study flow diagram.
The original version of this review deemed as eligible 20 trials, of which we excluded 11 (Forester 1985; Forester 1993; Grande 1999; Grande 2000; Greer 1992; Jacobsen 2002; Li 2002; Ovesen 1993; Rawl 2002; Weinrich 1990; Wilkie 2000) leading to the inclusion of nine studies that were conducted exclusively in lung cancer patients in six trials (Bredin 1999; Corner 1996; McCorkle 1989; Moore 2002; Sarna 1998; Wall 2000), while the rest reported results in different cancer patients (Evans 1987; Linn 1982; Stephenson 2000).
The update of the bibliographic search identified 1098 unique references and we assessed in detail the full text of 46 studies. Of those, we included six new trials (Arbane 2011; Barton 2010; Chan 2011; Porter 2011; Stephenson 2007; Wilkie 2010) and excluded 35 studies (see Characteristics of excluded studies). Of the excluded studies 32 did not include disaggregated data for lung cancer patients (Armes 2007; Bakitas 2009; Borneman 2010; Brown 2006; Chan 1998; Christman 2004; Cole 2005; Cunningham 1989; de Wit 1997; Decker 1992; Dimeo 1997; Dimeo 1999; Dimeo 2004; Doorenbos 2006; Foltz 1987; Given 2002; Graham 2003; Grealish 2000; Holland 1991; McCorkle 1998; Oh 2008; Parvez 2007; Ramsay 2007; Ream 2006; Rosenbloom 2007; Schiffman 2007; Schneider 2007; Skrutkowski 2008; Soden 2004; Speca 2000; Steinhauser 2008; Wyatt 2007), two did not include lung cancer patients (Burnham 2002; Parker 1998) and one study (Cesario 2007) had a case series design. We additionally labelled four studies as 'awaiting assessment' (as these were studies that were published in Chinese: Cai 2001; Wang 2005,; Wu 2003; Zhen 2002) and included an ongoing trial (Jones 2010).
We therefore included 15 trials in the review (see Characteristics of included studies) and we categorised these into six groups according to the interventions assessed:
Nursing interventions to manage breathlessness (three studies, 165 lung cancer patients) (Barton 2010; Bredin 1999; Corner 1996).
Nursing programmes (four studies, 556 lung cancer patients) (Chan 2011; McCorkle 1989; Moore 2002; Sarna 1998).
Nutritional interventions (one study, 96 lung cancer patients) (Evans 1987).
Psychotherapeutic, psychosocial and educational interventions (three studies, 522 lung cancer patients) (Linn 1982; Porter 2011; Wilkie 2010).
Exercise (two studies, 157 lung cancer patients) (Arbane 2011; Wall 2000).
Reflexology (two studies, 96 lung cancer patients) (Stephenson 2000; Stephenson 2007).
1. Nursing interventions to manage breathlessness
Breathlessness is one of the most common symptoms reported by people with lung cancer and is recognised as being a complex condition consisting of physical, psychological, emotional and functional factors (Bredin 1999). Two studies included in the review (Bredin 1999; Corner 1996) focused on the non‐pharmacological management of breathlessness in patients with lung cancer. Corner et al (Corner 1996) undertook a pilot study in which 34 patients with lung cancer who had completed chemotherapy or radiotherapy treatment were randomised to receive a non‐pharmacological intervention to ameliorate breathlessness or to a control group which received accurate assessment of symptoms but no intervention. The nursing‐led programme was based on breathlessness rehabilitation techniques and focused on the emotional experience of symptoms as well, aiming not to separate the psychological from the physical aspects of the symptoms.
Following this pilot study a further randomised trial was then undertaken in six hospital centres around the UK to evaluate the effectiveness of the intervention in a larger and more diverse sample (Bredin 1999). This multicentre study randomised 109 patients with small cell lung cancer, non‐small cell lung cancer or mesothelioma who had completed treatment and were experiencing breathlessness, to receive the dyspnoea management intervention or standard care.
Barton 2010 assessed a breathlessness training intervention over 12 months in 22 patients with refractory breathlessness caused by malignant lung disease. The trial compared three versus a single session provided by a specialist physiotherapist or by trained nurse specialists. All patients received training in diaphragmatic breathing, pacing, anxiety management and relaxation during an hour‐long clinic session. Both groups received written and DVD/video reinforcement material and a telephone call from their therapist a week after the last training session.
2. Nursing programmes
Four studies assessed the effects of general nursing programmes and assessment on a variety of outcomes including symptomatology, psychosocial well‐being, quality of life and patient satisfaction, anxiety, depression and symptom palliation (Chan 2011; McCorkle 1989; Moore 2002; Sarna 1998).
Nurse follow‐up programmes versus standard physician follow‐up
In spite of known high levels of psychological morbidity and need for emotional support amongst cancer patients, there is little evidence that routine clinic or office appointments with a physician following completion of initial treatment are beneficial for these patients. We found two randomised studies which compared the effects of standard clinic/office (physician) follow‐up with tailored programmes of nurse follow‐up:
McCorkle et al (McCorkle 1989) compared the effect of two different home nursing care treatments and standard office physician treatment on the psychosocial well‐being of men with lung cancer (stage II or higher). Patients were randomly allocated to one of three possible programmes: i) Specialised oncology home care programme (OHC) delivered by master's level nurses trained to provide care to patients with advanced cancer; ii) Standard home care programme (SHC) delivered by an interdisciplinary team of health professionals; iii) Office care programme (OC) which served as a control, and was provided by the patient's physician without involving any specialised home nursing care.
Moore et al (Moore 2002) compared the effectiveness of nurse‐led follow‐up of patients with lung cancer who had completed their initial treatment and were expected to survive at least three months, with conventional medical follow‐up. The investigators designed a nurse‐led follow‐up service which aimed to enhance care across primary, secondary and tertiary sectors.
In the third study in this group, Sarna (Sarna 1998) hypothesised that the process of accurate symptom identification and assessment might in itself have a beneficial effect on symptom palliation. This might occur not only because accurate symptom identification is essential to enable appropriate nursing interventions to be delivered, but also because the actual process of problem identification might have a therapeutic benefit in itself by creating a sense of validation of the illness experience for the patient. This trial assessed the effects of using a structured nursing assessment protocol on symptom distress in patients with advanced lung cancer.
Nurse educational programmes versus conventional care
Chan 2011 assessed an educational package delivered by registered nurses addressed to 140 stage 3 or 4 lung cancer patients that were scheduled to receive palliative radiotherapy. The intervention, compared to usual care, consisted in a brief educational package and training in progressive muscle relaxation delivered to patients within one week prior to the beginning of the course of radiotherapy, and reinforced three weeks after commencing radiotherapy. The education package consisted of leaflets and discussion on the selected symptoms and their self care management. The intervention was delivered by experienced registered nurses.
3. Nutritional interventions
Weight loss is common in cancer patients, affects quality of life and can be a distressing sign of the disease process. Interventions to increase food intake focusing on short‐term tube feeding or parenteral nutrition have shown little effect (McGeer 1990). We found one randomised trial that assessed the effects of interventions aimed to increase oral nutritional intake, on weight, response to therapy, survival and quality of life in cancer patients:
Evans et al (Evans 1987) randomised patients with previously untreated metastatic non‐small cell lung cancer or colorectal cancer to one of three groups: i) a group who received a nutritional advice intervention described as "standard" consisting mainly of oral nutrition supplemented if necessary by enteral or parenteral support to achieve a targeted caloric intake (TCI); ii) a group who received a nutritional intervention which aimed to increase their dietary intake of protein so that 25% of the total caloric intake was from protein sources, with additional daily zinc and magnesium supplements ‐ "augmented" nutrition; and iii) a group who received no specific nutritional intervention or counselling and who followed an ad lib diet (control group).
The nutrition programmes were implemented during the first 12 weeks of antineoplastic therapy. During this time patients with lung cancer received chemotherapy treatment consisting of three consecutive cycles of vindesine and cisplatin.
4. Psychotherapeutic, psychosocial and educational interventions
We found one randomised trial which assessed the effects of counselling on patients with lung cancer. Linn et al (Linn 1982) assessed the effects of a series of counselling sessions on quality of life, functional status and survival in 120 male patients with end‐stage cancer, 64 of whom had lung cancer.
This update includes two trials about interventions assessing educational programmes. Porter 2011 assessed an intervention addressed to lung cancer caregivers to promote their coping skills with illness. The patients and their caregivers were assigned to receive telephone‐based sessions of either caregiver‐assisted coping skills training (CST) or a training programme of education and support that directly involved the caregivers. The coping skills training focused on relaxation practices, guided imagery, problem‐solving, communication and maintenance enhancement strategies. This training was complemented in one of the comparison groups with education on basic information about the illness, nutrition information, and palliative and hospice care. The intervention consisted of 14 45‐minute telephone‐based sessions conducted with the individual patient and caregiver over an eight‐month period, and were conducted by registered nurses. The study assessed the effects of this intervention on pain, psychological distress, quality of life and self efficacy for symptom management.
Wilkie 2010 assessed a sensory self monitoring and reporting coaching intervention, based on a 12‐minute videotape designed to help non‐small cell lung cancer patients to recognise the subjective nature of pain perception and to self monitor and report changes in pain perception to providers. This videotaped session was followed by three reinforcement sessions in which patients aimed to improve the pain monitoring in the following domains: patterns of pain, localisation, intensity and feelings experienced. The study included 151 patients allocated to the training intervention or to a group that did not receive the intervention, and assessed the accuracy and amount of sensory pain information communicated to care providers, and additionally pain and anxiety measures.
5. Exercise
The experience of living with a diagnosis of cancer can have devastating effects on a person's perception of physical and psychological well‐being and these perceptions may change throughout the course of the illness. The need to develop and explore strategies which might enhance feelings of well‐being in patients with lung cancer was the basis of a randomised trial by Wall (Wall 2000). The trial was designed to evaluate whether a preoperative physical exercise programme might improve perceptions of hope and perceived self power in patients with lung cancer. The theoretical basis for the trial came from Rogers' science of unitary human beings (Rogers 1986) in which the capacity of human beings to actively participate in activities to improve their sense of well‐being may be seen as a function of a patterned interaction between the individual's own values and beliefs and their environment. For the purposes of the trial "hope" was seen as an "act" by which the "temptation to despair is actively or victoriously overcome" (Marcel 1978) and "power" was defined as the capacity of an individual to knowingly participate in change.
The objectives of the trial were to assess: i) whether hope differs over time in lung cancer patients who did and did not participate in a preoperative exercise programme; ii) whether power differs over time in lung cancer patients who did and did not participate in a preoperative exercise programme; and iii) what relationship there is between hope and power in lung cancer patients who did and did not participate in a preoperative exercise programme.
Arbane 2011 compared an exercise intervention with usual care in 53 patients with non‐small cell lung cancer referred for lung resection. The intervention consisted in an early exercise intervention that included pain relief and twice‐daily additional strength and mobility training from postoperative day one through postoperative day five. The exercise training was provided by a physiotherapist and consisted of walking, marching on one spot and recumbent bike exercises (carried out at bedside). Within two weeks after discharge and once monthly for three visits in total (12 weeks postoperative), patients were followed up at home where they were encouraged to continue with their paced exercise programme (usually walking in the park or nearby streets) and an adapted home strengthening programme. The study assessed the impact of exercise in quadriceps muscle strength, quality of life, tolerance to exercise, as well as length of stay and postoperative complications.
6. Reflexology
The effects of reflexology on anxiety and pain in lung and breast cancer patients were assessed by Stephenson et al (Stephenson 2000) in a cross‐over trial in which patients served as their own controls. Patients entered the study only if they had reported anxiety.
The same researchers assessed the effects of partner‐delivered foot reflexology in 86 dyads of metastatic cancer patients and their relatives (lung cancer patients represented the 23% of the sample). The trial assessed the effects of reflexology in patients’ perceived pain and anxiety (Stephenson 2007).
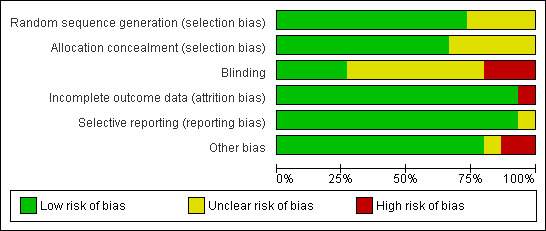
Risk of bias in included studies
The main limitation of the trials included in this review were related to the lack of data regarding the allocation of patients to treatment groups and blinding (see Figure 2 and Figure 3). Regarding blinding, some of the trials were open trials (Arbane 2011; Barton 2010; Wall 2000) and most did not provide enough information to assess if patients' allocation was known to researchers or outcome assessors (Bredin 1999; Corner 1996; Evans 1987; McCorkle 1989; Moore 2002; Sarna 1998; Stephenson 2000; Stephenson 2007). Having in mind the subjective nature of the outcomes of interest of this review, blinding could be considered as one of the most important sources of bias in the included trials.
2.
3.
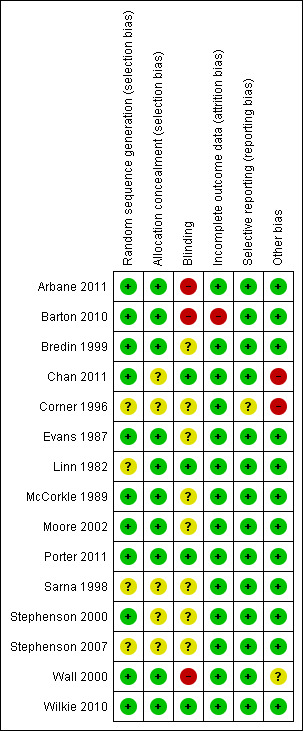
Only the trials from Chan 2011, Porter 2011 and Wilkie 2010 were free of bias in all the assessed domains. The rest of the trials did not describe details for some of the sources of bias considered (Bredin 1999; Corner 1996; Evans 1987; Linn 1982; McCorkle 1989; Moore 2002; Sarna 1998; Stephenson 2000; Stephenson 2007) or presented the commented limitation related to blinding (Arbane 2011; Barton 2010; Wall 2000). Barton 2010 was exposed to attrition bias; besides the problems experienced in recruitment that led to the inclusion of only 55% of the initially planned sample, the rapid deterioration and death of the participants led to a drop‐out rate of 40%.
Effects of interventions
Table 1 outlines the main results from the included trials. As a pooled analysis of results was not possible due to the huge heterogeneity between the studies, we present here a narrative summary of the main results.
1. Nursing interventions to manage breathlessness
Corner 1996 showed significant differences at baseline between groups for distress caused by breathlessness, difficulty in performing activities of daily living and anxiety, with the intervention group scoring higher for each of these variables.
In general terms breathlessness was experienced as an enormous limitation imposed on the patients' lives. This caused episodes of panic due to the sensation of loss of control over breathing, and severely limited such activities as walking, stair‐climbing, showering or sex. The interviews with patients in the control group at three months showed unchanged functional levels and severe limitations caused by breathing difficulties. On the other hand, patients in the intervention group reported increasing activity levels and functional capacity and felt able to control panic episodes and to face the limitations due to breathlessness. In this study after randomisation of the first 34 patients further randomisation was stopped on request of the medical and nursing staff who reported that the benefits in the intervention group were clear.
Bredin 1999 followed the pilot study of Corner 1996. A total of 119 patients were randomised but 16 patients from one centre were subsequently excluded after discovery of contamination between the control and intervention groups. A further 16 patients died during the course of the study and 28 patients withdrew. The main reason for withdrawal was because of a deterioration in condition; survival in patients who withdrew was significantly worse than in those who did not withdraw. Although survival in those who withdrew from the control group was significantly worse than in those who withdrew from the intervention group, there was no significant difference between groups in overall survival indicating that the intervention did not improve survival.
There were no differences in baseline demographics, diagnosis or outcome measures between groups and there were also no important differences in use of medication during the study period in the two groups.
Findings from this trial showed an overall favourable effect of the intervention on levels of dyspnoea and in levels of distress caused by this symptom.
Barton 2010 was a single‐centre, non‐blinded, parallel‐group, pilot study to assess the feasibility of conducting a powered RCT, so the authors did not present a statistical analysis. Twenty‐two patients were randomised over 12 months, 55% of expected recruitment from pilot data; screening logs indicated this resulted, in part, from excluding patients who were receiving or who had recently received chemotherapy or radiotherapy. There was 40% drop‐out by week four due to the rapid deterioration of included patients.
As a general pattern, the three‐session group appeared to do better than the single‐session group with regard to breathlessness severity, ability to cope with the breathlessness, satisfaction with care, quality of life (EQ‐VAS) and possibly distress caused by the breathlessness, as measured by means of numerical rating scales.
Both “worst breathlessness over the past 24 h” and “average” breathlessness improved more in the three‐session group.
Over the four weeks, distress caused by breathlessness showed little difference in the mean scores between the two groups although it was greatest in the three‐session group. Improvement in satisfaction with care appeared better in the three‐session group.
Patients in the three‐session group reported a reduction in anxiety and depression mean score from baseline to week four (17.2 at baseline to 12.3 at four weeks), whilst patients in the single‐session arm reported an increase (14.8 to 17.2). Overall, the Hospital Anxiety and Depression Scale (HADS) results are promising for the three‐session group compared with the single‐session group.
An EQ‐5D index was calculated, but not interpretable with so few data. EQ‐VAS scores appeared to improve in the three‐session group but were stable in the single‐session group.
We did not combine data from these three trials because of differences in the follow‐up periods reported and differences in the way in which data were reported in the trials.
2. Nursing programmes
Nurse follow‐up programmes versus standard physician follow‐up
In McCorkle 1989, 66% of enrolled participants failed to complete the five interviews, with death being the most frequent reason (n = 87). Statistical analysis was undertaken on the 78 (47% of the entire sample) patients who completed four interviews.
Analysis of the means for the core (baseline) measures recorded on the first occasion (prior to randomisation) showed that patients in the oncology home care group scored better on most variables. Adjusted analyses were undertaken to attempt to overcome this problem, in which data from the first occasion were treated as covariates in predicting scores on the subsequent four occasions.
Analysis of the adjusted means for symptom distress, patient dependency and health perceptions showed significant differences between groups. For symptom distress there was a significant difference in favour of the two home care groups with respect to time profiles of increasing symptom distress. A similar trend was observed for social dependency, which increased over time in all three groups but increased faster in patients in the office care group who became more dependent six weeks earlier. In contrast to the above findings, reported health perceptions increased steadily over time in the office care group, indicating a subjective improvement in perceived health experience in this group while both home care groups reported steadily worsening health perceptions.
In Moore 2002, a total of 203 patients with lung cancer were randomised, with 100 patients being allocated to nurse‐led follow‐up and 103 to conventional medical follow‐up (one patient in the intervention group was found to be ineligible after baseline assessment). At three months, data were available on 77 patients in the intervention group and 79 in the control group. Twelve months after randomisation there were 30 patients left alive in each group. There were no significant differences between groups at baseline in clinical characteristics, quality of life or patient satisfaction. The majority of patients in both groups had non‐small cell lung cancer (74%), of whom 83% were stage IIIa or higher.
Assessment, information and support
Sarna 1998 included 48 patients who had been diagnosed with lung cancer (stage III or IV) within the last two to three months. Comparative baseline data on participants in the control and experimental group is not provided in the study report and although the author has been contacted for further information this has so far not been made available.
Educational package plus coaching in progressive muscle relaxation
In Chan 2011 140 lung cancer patients receiving palliative radiotherapy were randomised patients to an educational package (leaflets and discussion on the selected symptoms and their self care management) plus coaching of progressive muscle relaxation or to control group who received usual care.
They found a statistically significant difference (time x group interaction effect, P = 0.003) over time between the psychoeducational intervention group and usual care control group on the pattern of change of the symptom cluster of anxiety, breathlessness and fatigue. Significant effects on the patterns of changes in breathlessness (P = 0.002), fatigue (P = 0.011), anxiety (P = 0.001) and functional ability (P = 0.000) were also found.
However, patients in the control group had a significantly more advanced stage of cancer (P < 0.05) when compared with the intervention group.
3. Nutritional interventions
Of the 192 patients with cancer randomised in Evans 1987, 102 had a diagnosis of non‐small cell lung cancer, of whom six were ineligible, so 96 formed the basis of the study report relating to lung cancer patients. The nutritional intervention was effective in increasing caloric intake in lung cancer patients during all three cycles of chemotherapy but in spite of this the intervention had a limited effect on weight (see Table 1). Patients in the standard and augmented groups suffered less weight loss during the first four weeks but this did not reach significance (P = 0.6). There was no evidence of the effects of counselling on the amount of total intake consumed as protein.
4. Psychotherapeutic, psychosocial and educational interventions
The counselling treatment for end‐stage lung cancer patients in Linn 1982 produced beneficial effects on quality of life for the 33 patients allocated to the counselling group compared with the 32 patients in the control group, with some of the greatest benefits being seen at three months of treatment, and with those patients who lived for a full 12 months experiencing the most significant gains in the five dimensions rated (see Table 1). There were no differences between the groups at baseline but at three months, depression, life satisfaction and self esteem were all significantly improved in the counselled group compared with the control group, although the improvements in depression were not maintained thereafter. Life satisfaction and self esteem scores remained improved in those who survived six, nine and 12 months and in those still alive at 12 months there were significant improvements in life satisfaction and self esteem and even more so for alienation and locus of control.
Objective measures of functional status were not different at any stage between the groups. At the study end there were six patients with lung cancer still alive in each group.
Wilkie 2010 examined the effects of coaching sensory self monitoring and reporting on pain‐related variables in patients with lung cancer and randomised 215 patients to coached or not‐coached groups. Of the 151 patients who completed the four‐week study, those coached were more likely than those not coached to give their providers unsolicited sensory pain information and to mention it before their providers ask for it. The mean number of pain parameters discussed during the audiotaped clinic visit was statistically larger at study end for the coached group. Scores for analgesic adequacy, all pain indices except one, anxiety, depression and catastrophising coping were not significantly different (see Table 1). Although coaching increased the amount of pain data communicated to providers by patients with lung cancer, the magnitude was small and did not lead to improved adequacy of analgesics prescribed for each patient’s pain level.
In Porter 2011 233 lung cancer patients and their caregivers were randomly assigned to receive 14 telephone‐based sessions of either caregiver‐assisted coping skills training (CST) or to education/support involving the caregiver.
Patients in both treatment conditions showed improvements in pain, physical well‐being, depression, quality of life and self efficacy, and caregivers in both conditions showed improvements in anxiety and self efficacy from baseline to four‐month follow‐up. Caregivers in both groups showed a significant improvement in anxiety and self efficacy at four months follow‐up. Results of exploratory analyses suggested that the CST intervention was more beneficial to patients/caregivers with stage II and III cancers, whereas the education/support intervention was more beneficial to patients/caregivers with stage I cancer.
5. Exercise
The exercise programme for preoperative patients (Wall 2000) included 104 patients with lung cancer and found some significant differences for power in favour of the intervention group at both time 2 and time 3 compared with baseline. There were no significant differences between groups for hope or for the relation of hope and power.
In Arbane 2011, 53 patients with non‐small cell lung cancer treated with thoracotomy were randomised to early exercise intervention (twice daily training plus usual care) or to usual care.
Quality of life assessed by the EORTC QLQ‐CL13 questionnaire showed no significant changes for any measure of quality of life (functional, symptom and global health), either within subjects or between groups (P values not provided).
Exercise tolerance assessed as the Six‐Minute Walking Distance in metres, showed significant deterioration at five days postoperatively compared with preoperatively (mean difference (SD) ‐131.6 (101.8) metres and ‐128.0 (90.7) metres in active and control groups respectively (P = 0.89 between groups)) which returned to preoperative levels by 12 weeks in both groups.
Quadriceps strength over the five‐day in‐patient period showed a decrease of ‐8.3 (11.3) kg, (mean difference (SD), in the control group compared to increase of 4.0 (21.2) kg in the intervention group (P = 0.04 between groups).
6. Reflexology
Two studies analysed the effect of reflexology on pain and anxiety in cancer patients, including 30 lung cancer patients.
There were 10 patients with lung cancer in the sample in the reflexology trial (Stephenson 2000). Pre‐intervention anxiety scores were higher than pre‐control time scores and anxiety scores were lower after the intervention than after the control time. The difference in score reduction between control and intervention groups was also significant (see Table 1). Pain scores in the lung cancer sample were not calculated since only two patients with lung cancer reported pain.
These results were similar in Stephenson 2007. After adjusting for pre‐intervention pain, significant differences were showed on postintervention pain between the patients that received reflexology and their controls (patients in the reflexology group had 34% reduction in pain from baseline to postintervention compared with a reduction of 2% in controls). This effect was also observed for anxiety; patients in the reflexology group had a 62% decrease in anxiety from baseline to postintervention measures, whereas the control group experienced a 23% decrease.
Discussion
This update of a previous review (Solà 2004) of the effectiveness of non‐invasive interventions in improving symptoms, psychological functioning and quality of life in patients with lung cancer found that interventions which have been designed and tested to date by means of randomised controlled trials reflect a broad range of interventions that we have decided to classify into six broad categories: nursing interventions to manage breathlessness, nursing programmes, nutritional interventions, psychotherapeutic interventions, exercise and reflexology.
The studies included in this review had some important limitations in their design and reporting that limits the confidence in their results. Most of the trials failed to report sufficient data to judge the accuracy of the efforts made by the researchers to generate the randomisation sequences and to conceal them. But the issue that causes more concern about the risk of bias in the included studies is related to blinding. Some of the outcomes of interest in this review entail a subjective process to record data about quality of life or symptomatology in validated scales or even asking patients to answer questions about their experiences about these outcomes. In this context the knowledge of allocated interventions should be adequately prevented for the outcome assessors at least. However, apart from four trials that were appropriately blinded (Chan 2011; Linn 1982; Porter 2011; Wilkie 2010), three of the trials included were not blinded (Arbane 2011; Barton 2010; Wall 2000) and eight did not report enough information to judge whether any effort to reach blinding had been made (Bredin 1999; Corner 1996; Evans 1987; McCorkle 1989; Moore 2002; Sarna 1998; Stephenson 2000; Stephenson 2007).
Given these circumstances and the scarcity of studies assessing each of the outcomes of interest, the quality of evidence for most of the outcomes for the compared interventions should be considered low.
We considered that a quantitative meta‐analysis would be inappropriate, given the differences in the interventions delivered, in the stage of lung cancer in participants, in the treatment undergone and in the outcome variables measured. Narrative synthesis of each of the studies does however allow us to draw some conclusions:
Nursing interventions to manage breathlessness
The review of nursing interventions for breathlessness management in lung cancer patients found two studies, although the first study was a pilot for the second. These studies found that the intervention was effective in improving the sensation of breathlessness at best and also had beneficial effects on performance status, functional ability and on depression. Although details of randomisation and other indicators of the study quality were not reported for the pilot study (Corner 1996), these were fully reported in the multi‐centre study (Bredin 1999) which was of high overall quality. Although the findings of these studies are encouraging, the multi‐centre study required the recruitment of nurses from six different centres around the UK and considerable effort was undertaken to ensure that the intervention was delivered in a uniform way. The feasibility of undertaking such training and the practicalities of delivering such an intervention under similar conditions in normal day to day clinical practice can, however, be questioned (Johnson 2003), and further research needs to address how potential barriers to successful implementation of this kind of programme in clinical practice might be overcome.
These studies were the most specific included in the review in terms of their clear focus, not only on patients with lung cancer but also on one specific symptom of this cancer, but it is of note that the investigators have themselves since questioned the validity of such a 'Cartesian' approach to understanding and managing what is essentially only one aspect of a highly complex illness experience (Krishnasamy 2001). Further research may usefully focus on more qualitative approaches to increasing these understandings. The intervention evaluated in Corner 1996 and Bredin 1999 was the only one amongst the included studies that proposed a link between the emotional and physical components of a symptom, suggesting that an improvement in the feelings associated with the symptom may have contributed to an improvement in the physical expression of that symptom. The interviews undertaken as part of the pilot study showed that patients benefited from and appreciated the opportunity to develop skills which allowed them a change their own control over their symptoms.
The modest results showed in Barton 2010 just highlight the difficulties in designing trials in patients with advanced cancer. The rapid deterioration of participants in the trial led to the completion of the study by only eight of the 22 initially randomised patients.
Nursing programmes
The interventions assessed in the group of studies which we have termed nursing programmes showed some positive results, emphasising the importance of specialised nursing care and time spent with patients whether in the home or clinic setting, giving professional support whether through thorough symptom assessment and management, emotional and psychological support or by giving information about the disease and providing education in coping strategies.
McCorkle found a delay in increase in symptom distress, patient dependency and reduced health perceptions in the two groups who received the nursing care programmes compared with the office care group. These findings suggest that providing care in the home may help to delay the onset of deterioration and increased dependency by about six weeks compared with the standard care group. Although a relatively short time, this may nevertheless have a high value for the patient at the end of their life. Values for psychosocial functioning were similar in the groups receiving care from the specialised oncology nurse team and the standard home care team, suggesting that there is little benefit to be gained from employing highly trained master's degree level nurses in this role which may be more appropriately filled by an interdisciplinary team of health professionals.
The observation of a 'notable' difference between the oncology home care (OHC) and the other two groups on the first measurement occasion was an unexpected finding which was attributed by the investigators to chance sampling error, but which nevertheless made interpretation of results more difficult. Study participants in McCorkle 1989 were younger than patients recorded on the local lung cancer registry which the study investigators explained as indicating that elderly patients may choose not to seek initial or ongoing treatment. This may also, however, have been a reflection of recruiting physicians' and/or relatives' attitudes to the participation of older people in a trial, an attitude which is prevalent in clinical research (Godlovitch 2003).
The apparently contradictory finding in the office care group of an improvement in perceived health at the same time as increasing symptom distress and general dependency is of interest. The investigators suggest that this may have been a result of one of two possible mechanisms: either a denial process on the part of the patients, or illusions created by the physician with the intention of helping them to cope better with their illness.
The study by Moore 2002 was of a high‐quality design and showed significant improvements at three months not only in patient satisfaction but also in degree of dyspnoea experienced among those in the intervention group compared with the control group. At 12 months, although numbers left in the study were smaller and findings less reliable, there were significant improvements in emotional functioning in patients who had received the nurse follow‐up programme compared with controls. There were a number of features of this nursing programme that made it different from the conventional hospital medical service, any one or combination of which could have contributed to the improvements seen in the intervention group. One of the key features was open access to the nurses thereby giving the patients some control over their care. This would fit within the theory that giving patients control or self empowerment may have beneficial effects, at least on the patient's sense of well‐being if not on objective measurements of physical health (Cartmell 2000).
Although the services provided in McCorkle and Moore were different in many aspects, not least in the provision at home versus hospital, there were nevertheless some common features in the services which may have acted on a similar mechanism to produce some relatively non‐specific but tangible improvements in the patient's illness experience. It is known that patients with cancer have considerable need for emotional and psychological support (MCA 2002), and it seems likely that the care provided in a busy hospital outpatient clinic or even a physician's private office may not be as effective in terms of providing the empathy and listening required by a patient with a diagnosis of cancer.
These two studies were conducted in different countries over 10 years apart and although their findings may give some general pointers as to the effectiveness of nurse follow‐up and management programmes in the community for patients with lung cancer, further research in different settings is needed to confirm the findings. Harmonising research such that the same outcomes are evaluated in different studies with the same measurement instruments would enable more robust conclusions to be drawn more quickly. In both studies the nurses who were giving the care underwent specific training programmes. In McCorkle the nurses had master's degrees and had in‐depth knowledge of oncology and oncological care delivery. In Moore, training of the clinical nurse specialists appeared to have been ad hoc through attendance at oncology clinics and shadowing of medical consultants. Further information is needed on what would constitute a reasonable level of training to enable a nurse to give a lung cancer patient an optimal level of psychological and emotional support while being sufficiently alert to the need for referral for medical care and follow‐up should the need arise.
Both McCorkle and Moore referred to the economic aspects of the nursing care provision as compared with conventional medical management but much more detailed work is necessary to fully understand the economic costs and benefits of these services. Costings of these complex nursing programmes need to include costs of providing sufficient initial and ongoing training and support of staff as well as the provision of resources to maintain the premises (if it is not the home) in which the intervention is delivered. Against this can be weighed the resource savings of avoiding unnecessary acute hospital admissions (Moore 2002). Differences in hospitalisations and length of stay between groups in McCorkle were non‐significant but nevertheless their findings suggest that patients who received the most intensive care in the home were also those who were least likely to be admitted to hospital but who once admitted were more seriously ill and required a longer stay than those in the other groups.
Although Sarna 1998 investigated an interesting hypothesis ‐ the effects of detailed symptom assessment on patient symptom experience ‐ lack of clarity in the study report prevented a thorough assessment of the effect of the intervention. While a significant difference in symptom distress over time on multivariate analysis between the control and intervention group is reported, it is unclear from the published data whether this had clinically significant implications. In addition to an unclear report, the study design is relatively low quality since neither the method of randomisation nor a process of allocation concealment is described. Therefore although the findings from this study give some general support to those of McCorkle that more structured nursing assessment can lead to improvements in symptom distress over time, firm conclusions are impossible on the strength of these data alone and further better designed and reported studies are essential.
The results from Chan 2011 suggested the feasibility of brief educational interventions focused on specific aspects of the illness and its management. In this study a brief educational intervention consisting of the discussion of symptomatology and self care management, complemented with training in relaxation, led to an improvement in the patterns of change in breathlessness, fatigue, anxiety and functional ability.
Nutritional interventions
Results reported in the trial that assessed nutritional interventions (Evans 1987) were not encouraging. The nutritional counselling only showed positive results in increasing energy intake but there were no beneficial effects for the remaining outcomes (weight change, tumour response and survival).
Results from this study showed that it is possible to increase the oral energy intake in patients with lung cancer but that doing so fails to result in any changes in health outcomes. This highlights the fact that current knowledge of the metabolic problems which give rise to the weight loss and cachexia of cancer are poorly understood. Effective nutritional interventions depend on improving these understandings.
Psychotherapeutic, psychosocial and educational interventions
The trial of counselling (Linn 1982) sought to influence quality of life in patients with advanced cancer by addressing the emotional components of the illness, working on the theory that these factors may play an important role in the disease process.
The results of the Linn 1982 trial suggested beneficial effects of counselling on depression levels, life satisfaction and self esteem, although these results were not maintained over a long period of time.
Porter 2011 tested the benefit of involving caregivers in a telephone‐based intervention to improve coping skills with illness. Despite the effort to entail caregivers in the process of promoting some skills addressed to better manage the symptomatology derived from the illness, the most valuable results were observed in the patients who experienced an improvement in pain, physical well‐being, lung cancer symptoms, depression and self efficacy to cope with symptomatology. Caregivers in both groups showed a significant improvement in anxiety and self efficacy at four months follow‐up. The complexity of the intervention does not allow to determine which is the factor that could have the greater effect over the patients’ outcomes, although the educational component could contribute to the observed results.
The coaching intervention of Wilkie 2010 only showed moderate effects in the communication and self monitoring of pain in patients that were instructed by means of a videotaped intervention. However, the study failed to show a benefit on pain levels. Examining the effectiveness of this kind of audiovisual aid used jointly with multifaceted and symptom‐focused interventions could be a fruitful avenue for future research.
Exercise
Wall 2000 was an interesting study that explored the potential effect of physical exercise on rather intangible notions such as hope and perceived self power and the ability of non‐invasive interventions to influence these. The findings highlight the importance of hope, illustrating that this seems to be a constant in patients with early‐stage cancer when surgery is a viable option and is seen as offering hope of cure. The capacity of patients to feel hope may be different at later stages of the disease (Fawzy 1999). While having positive findings, the study authors made the assumption that an increased ability to participate in change will de facto have a positive effect on perceived well‐being. Further research is necessary not only to confirm these positive findings but also to explore whether these positive changes in 'power' actually translate into changes in perceptions of health and well‐being and indeed whether these have any effect on objective measures of health status.
Arbane 2011 tested a physiotherapy intervention aimed to improve outcomes in patients scheduled for lung resection. Although the intervention involved a complete home‐based follow‐up of patients, the exercise training only showed moderate effects on muscle strengthening, but failed to improve patients’ capacity in terms of tolerance to exercise or quality of life.
Reflexology
The studies of reflexology (Stephenson 2000; Stephenson 2007) also showed some beneficial, if short‐lasting, effects of the interventions. These two trials suggest that there may be a positive effect of reflexology on anxiety, but higher initial scores in the intervention group in Stephenson 2000 showed that they were more stressed by having to undergo the intervention but that once they found out it was non‐invasive they were able to relax more than the control group. There was no specific information given in the study report as to how long after the intervention the positive effects lasted although the nature of the study design (pre/post cross‐over) implied that the effects were expected to be immediate but short‐lived. The design of this study was described as 'quasi‐experimental, pre/post cross‐over'. Further studies with more patients and more rigorous methods are required to confirm these findings.
Non‐invasive interventions ‐ general overview of the included studies
The link between emotions and physical health is a common theme in many of the included studies. It was explored explicitly in the breathing intervention described by Corner and Bredin and colleagues (Corner 1996, Bredin 1999) and in Sarna the hypothesis underlying the study design rested on the supposition that a "patient's emotional and physical state may influence the appraisal of symptoms and its relief" (Sarna 1998). Whatever the explanatory physiological mechanism, this theme raises interesting questions for future research: what factors impact on a person's subjective experience of health and does help in the adoption of realistic expectations contribute to a delay in physical health?
Findings from Bredin, Corner, Linn, Moore and Wall support the notion that a supportive intervention may have benefits not only on emotional and psychological states (anxiety, depression) but also on physical symptoms such as dyspnoea. Important aims of such an intervention appear to be to increase the patient's understanding of their illness and its meaning for them, to increase their ability to be active participants in their own therapy and to give them an opportunity to express and explore their feelings and concerns about their symptoms, diagnosis and future. Whatever its exact format, an intervention that enables the patient to develop a supportive and empathetic relationship with a suitably trained health professional appears to be key. Effective delivery of these kinds of intervention may also influence the balance of resource use and distribution between acute hospitals on the one hand and the home and community on the other.
The best way to deliver this kind of service (economically and practically), and who are the most appropriate professionals to provide it, is not clear from the studies included in this review. Nurses are not counsellors but have multi‐faceted skills, whereas counsellors may have the psychotherapeutic skills but have less knowledge of the disease process. This supports the need for multi‐disciplinary teams to cater for the individual needs of patients but also interdigitating with conventional medical services providing treatment and support. Such a service is likely to be resource‐intensive, not only in terms of need for professional time but also professional training and ongoing support, facilities (home or hospital ‐ if hospital where?) and practical arrangements ‐ such as the need to keep the number of visits to a minimum as these patients are very ill.
Although some of these trials have optimistic findings, there is nevertheless a need to remember that in the majority of lung cancer cases ‐ other than those with early stage disease who are eligible for surgery ‐ these patients are going to suffer a deterioration in health and die. It is therefore important to offer programmes which engender a positive approach to their illness without raising unreasonable hopes of treatment and cure.
We have concentrated for this review on lung cancer patients but many of the interventions would benefit patients with other cancers. Indeed we excluded several studies from this review because the intervention was delivered to patients with different cancers and it was not possible to separate the data on lung cancer. More qualitative research is needed to explore whether and what different illness experiences may be depending on the site of the primary cancer ‐ influenced not only by the actual physical location of that tumour (lung, breast, prostate, etc.) but also by the social, psychological and emotional meanings that arise from that cancer site. Common in lung cancer is a feeling of guilt thanks to a prevalent (occidental) societal attitude that the patient with lung cancer is responsible for having developed it because of having smoked ‐ even though this may not even have been the case.
We have categorised studies by the type of intervention delivered, but might equally have categorised studies on the basis of the stage of the illness. Interventions were different in their purpose depending on the progression of the patient's illness: at the early phase of diagnosis, interventions tested were more education‐oriented, providing patients with coping skills and guiding the management of disease‐related issues. In more advanced disease stages, the interventions assessed centred more on individual needs complemented by emotional support, while interventions tested for patients with terminal illness focused on improving patient quality of life and developing strategies to manage existential issues and topics related with mourning among relatives and carers. This alternative classification raises the question as to what kind of non‐invasive interventions are best suited at different stages of the disease for a patient with cancer.
Finally there is a need for further research into palliative care interventions ‐ and where randomised controlled trials are conducted these need to be co‐ordinated so that maximum use of scarce resources can be obtained. The difficulties in conducting randomised trials and obtaining firm evidence in palliative care are well recognised (Higginson 1999). We have concentrated only on RCTs for the purpose of this review but other valuable data from qualitative studies can also add to the insights gained from these studies and further point to the direction of future research.
Authors' conclusions
Implications for practice.
The studies of breathlessness management indicate that nurse‐led breathing programmes may produce beneficial effects and as such these should be encouraged.
The studies of nurse follow‐up suggest that this can be as effective and leads to greater patient satisfaction than physician follow‐up. An approach of interventions focused on specific aspects of illness as well as symptomatology self management could improve the patients' experience of illness and promote their functional ability.
The study of a psychotherapeutic intervention indicates that counselling may be effective in helping patients cope more effectively with the emotional symptoms associated with their disease but the most appropriate way of delivering this remains unclear. The promotion of coping skills strategies also could contribute to improve the patient’s self efficacy to cope with symptomatology and their physical well‐being.
There is no evidence that increasing oral nutritional intake in patients with lung cancer has any beneficial effect on quality of life or survival.
The findings from these studies imply a need for increased training and education of nurses and other paramedical professionals in different supportive counselling techniques and the need for sufficient resources to allow these services to complement rather than replace conventional medical interventions.
Implications for research.
Research is needed to try to understand the potential underlying mechanisms which may link the effects of psychological and emotional symptoms to physical symptoms.
There is a need to explore and test the effectiveness and feasibility of providing non‐invasive interventions for lung cancer patients within the 'real life' setting as opposed to the sometimes artificial setting of a research study.
Further research is needed to advance knowledge about nutritional interventions, in light of the insufficient evidence that exists.
Qualitative research is needed to further advance knowledge and understanding of the experience of cancer in general and lung cancer in particular and to look at ways to see how these insights can be used to improve care for patients with this disease.
Studies in this area need to be of a higher overall quality design. Many of the interventions tested in the trials included in this review had a number of different components any one or combination of which, may or may not have produced a positive or negative effect. It is essential that study designs and objectives are clear and methodology is sufficiently sound to allow the questions to be answered in a valid and robust way. Blinding in the outcome assessment should be warranted.
What's new
| Date | Event | Description |
|---|---|---|
| 19 February 2015 | Amended | Affiliation of one authors updated ‐ no change in the contain of the review |
History
Protocol first published: Issue 3, 2003 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 8 September 2011 | Amended | Inclusion criteria amended |
| 6 June 2011 | New citation required but conclusions have not changed | New first author |
Acknowledgements
The authors acknowledge the key contribution to Elinor Thompson and Consol López in the design and conduct of the first published version of the review.
Appendices
Appendix 1. Search strategies designed for identification of studies
MEDLINE (PubMed 28 February 2011)
#1 Lung Neoplasms[MH] 144509 #2 “Carcinoma, Non‐Small‐Cell Lung”[MH] 22715 #3 “Carcinoma, Small Cell”[MH] 15833 #4 Mesothelioma[MH] 9939 #5 “Small Cell Lung Carcinoma”[MH] 647 #6 lung cancer[tw] 68850 #7 small cell[tiab] AND lung[tiab] 33661 #8 non small cell[tiab] AND lung[tiab] 22969 #9 SCLC[tw] 4272 #10 NSCLC[tw] 12797 #11 mesothelioma[tw] 11897 #12 lung[tiab] AND cancer[tiab] 90003 #13 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 185889 #14 Breathing Exercises[MH] 2332 #15 Exercise[MH] 53788 #16 Exercise Therapy[MH] 22341 #17 Cancer Care Facilities[MH] 3097 #18 Complementary therapies[MH] 146059 #19 Counseling[MH] 27943 #20 Diet[MH] 160305 #21 Holistic Health[MH] 6387 #22 Home Care Services[MAJR] 25143 #23 "Life support care"[MESH] 6451 #24 Massage[MH] 4065 #25 Nursing Assessment[MH] 28688 #26 Nursing Care[MH] 108458 #27 Nutritional support[MESH] 33858 #28 Psychotherapy[MH] 130875 #29 Reflexotherapy[MH] 353 #30 Relaxation Therapy[MH] 6146 #31 Touch[MESH] 11313 #32 Spiritual Therapies[MH] 10961 #33 counselling[tiab] 14289 #34 counseling[tiab] 34260 #35 educational[tiab] 71087 #36 exercise[tw] 190701 #37 guided imagery[tiab] 394 #38 non invasive[tw] 36364 #39 non‐pharmacological[tw] 2605 #40 nursing[tw] 447461 #41 nurse led[tw] 1309 #42 nutritional[tiab] 74628 #43 psychosocial[tiab] 46401 #44 psychoeducational[tiab] 1231 #45 reflexology[tiab] 264 #46 relaxation[tiab] 75810 #47 #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 1366461 #48 “Quality of life”[MH] 87325 #49 “Power Psychology”[MH] 8177 #50 activities of daily living[tiab] 11230 #51 anxiety[tiab] 83957 #52 distress[tiab] 56168 #53 hope[tw] 23871 #54 quality of life[tw] 136543 #55 QoL[tw] 13038 #56 HRQoL[tiab] 4672 #57 Well being[tiab] 29897 #58 symptom relief[tiab] 2673 #59 health perception*[tiab] 1470 #60 Hospital Anxiety and Depression Scale[tiab] 2581 #61 WHO performance status[tiab] 248 #62 Rotterdam symptom checklist[tiab] 111 #63 Symptom Distress Scale[tiab] 132 #64 Questionnaires[MH] 227006 #65 Dyspnea[MH] 12763 #66 breathlessness[tw] 2486 #67 #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 522045 #68 #13 AND #47 AND #68 781 #69 (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) NOT (animals[mh] NOT (humans[mh] AND animals[mh])) 2355354 #70 #71 AND #81 283 #71 #68 NOT #70 498
The Cochrane Library (2011, Issue 2; 28 February 2011)
#1 MeSH descriptor Lung Neoplasms explode all trees 3931 #2 MeSH descriptor Carcinoma, Non‐Small‐Cell Lung explode all trees 1773 #3 MeSH descriptor Carcinoma, Small Cell explode all trees 752 #4 MeSH descriptor Mesothelioma explode all trees 79 #5 MeSH descriptor Small Cell Lung Carcinoma explode all trees 28 #6 lung cancer 7469 #7 small cell:ti,ab AND lung:ti,ab 4345 #8 non small cell:ti,ab AND lung:ti,ab 3444 #9 SCLC 588 #10 NSCLC 2024 #11 mesothelioma 146 #12 lung:ti,ab AND cancer:ti,ab 5721 #13 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12) 8123 #14 MeSH descriptor Breathing Exercises explode all trees 400 #15 MeSH descriptor Exercise explode all trees 7756 #16 MeSH descriptor Exercise Therapy explode all trees 4432 #17 MeSH descriptor Cancer Care Facilities explode all trees 93 #18 MeSH descriptor Complementary Therapies explode all trees 10496 #19 MeSH descriptor Counseling explode all trees 2344 #20 MeSH descriptor Diet explode all trees 9394 #21 MeSH descriptor Holistic Health explode all trees 58 #22 MeSH descriptor Home Care Services explode all trees 2115 #23 MeSH descriptor Life Support Care explode all trees 69 #24 MeSH descriptor Massage explode all trees 532 #25 MeSH descriptor Nursing Assessment explode all trees 517 #26 MeSH descriptor Nursing Care explode all trees 1490 #27 MeSH descriptor Nutritional Support explode all trees 2570 #28 MeSH descriptor Psychotherapy explode all trees 11635 #29 MeSH descriptor Reflexotherapy explode all trees 16 #30 MeSH descriptor Relaxation Therapy explode all trees 1153 #31 MeSH descriptor Touch explode all trees 463 #32 MeSH descriptor Spiritual Therapies explode all trees 653 #33 counselling 7795 #34 counseling 7795 #35 educational 7669 #36 exercise 34294 #37 guided imagery 249 #38 non invasive 4536 #39 non‐pharmacological 741 #40 nursing 19412 #41 nurse led 1417 #42 nutritional 7836 #43 psychosocial 4518 #44 psychoeducational 548 #45 reflexology 101 #46 relaxation 5300 #47 (#14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46) 100818 #48 MeSH descriptor Quality of Life explode all trees 11643 #49 MeSH descriptor Power (Psychology) explode all trees 99 #50 activities of daily living 5931 #51 anxiety 16902 #52 distress 6735 #53 hope 1723 #54 quality of life 31075 #55 QoL 2859 #56 HRQoL 791 #57 Well being 76457 #58 symptom relief 3910 #59 health perception* 3321 #60 Hospital Anxiety and Depression Scale 1428 #61 WHO performance status 3955 #62 Rotterdam symptom checklist 78 #63 Symptom Distress Scale 907 #64 MeSH descriptor Questionnaires explode all trees 12708 #65 MeSH descriptor Dyspnea explode all trees 622 #66 breathlessness 601 #67 dyspnea OR dyspnoea 2729 #68 (#48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67) 130830 #69 (#13 AND #47 AND #68) 449
EMBASE (Ovid 1980 to 2011 week 08; 28 February 2011)
1 exp lung cancer/ (138481) 2 exp lung non small cell cancer/ (36104) 3 exp small cell carcinoma/ (8399) 4 exp MESOTHELIOMA/ (8199) 5 lung cancer.mp. (105715) 6 (small cell adj5 lung).ti,ab. (38138) 7 (non small cell adj5 lung).ti,ab. (27354) 8 SCLC.mp. (5143) 9 NSCLC.mp. (16925) 10 mesothelioma.ti,ab. (10371) 11 (lung adj5 cancer).ti,ab. (88000) 12 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 (177594) 13 exp breathing exercise/ (3275) 14 exp EXERCISE/ (150766) 15 exp kinesiotherapy/ (35377) 16 exp cancer center/ (8710) 17 exp alternative medicine/ (26329) 18 exp COUNSELING/ (79621) 19 exp DIET/ (143811) 20 exp home care/ (44716) 21 exp MASSAGE/ (7495) 22 exp nursing assessment/ (26222) 23 exp nursing care/ (26871) 24 exp nutritional support/ (10336) 25 exp PSYCHOTHERAPY/ (153048) 26 exp relaxation training/ (7244) 27 counse?ing.ti,ab. (36657) 28 educational.ti,ab. (78295) 29 exercise.ti,ab. (163567) 30 guided imagery.ti,ab. (477) 31 non invasive.mp. (56320) 32 non pharmacological.mp. (3639) 33 nursing.ti,ab. (174186) 34 nurse led.ti,ab. (1537) 35 nutritional.ti,ab. (83289) 36 psychosocial.mp. (63715) 37 psychoeducational.mp. (1595) 38 reflexology.ti,ab. (304) 39 relaxation.ti,ab. (75314) 40 holistic.ti,ab. (9838) 41 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 (1177442) 42 exp "quality of life"/ (170386) 43 activities of daily living.mp. (13324) 44 anxiety.ti,ab. (101997) 45 distress.mp. (92333) 46 quality of life.mp. (198019) 47 QoL.mp. (17488) 48 HRQoL.mp. (5548) 49 Well being.mp. (36891) 50 symptom relief.mp. (3334) 51 health perception*.mp. (1804) 52 exp DYSPNEA/ (53970) 53 dyspn?ea.mp. (66012) 54 Breathlessness.mp. (2940) 55 (Hospital Anxiety and Depression Scale).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer] (4190) 56 WHO performance status.mp. (369) 57 Rotterdam symptom checklist.mp. (138) 58 Symptom Distress Scale.mp. (197) 59 exp questionnaire/ (268478) 60 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 (690010) 61 12 and 41 and 60 (1149) 62 random:.tw. or clinical trial:.mp. or exp health care quality/ (2455470) 63 61 and 62 (548)
PsycINFO (Ovid 1806 to February week 4 2011; 1 March 2011)
1 exp Neoplasms/ and exp Lung/ (251) 2 lung cancer.mp. (1137) 3 (lung adj5 cancer).ti,ab. (1239) 4 (small cell adj5 lung).ti,ab. (155) 5 (non small cell adj5 lung).ti,ab. (87) 6 SCLC.mp. (32) 7 NSCLC.mp. (38) 8 mesothelioma.mp. (25) 9 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 (1343) 10 exp Respiration/ (4059) 11 exp Exercise/ (12471) 12 exp relaxation/ (1759) 13 exp Relaxation Therapy/ (3247) 14 exp stress management/ (3549) 15 exp Progressive Relaxation Therapy/ (634) 16 exp meditation/ (2250) 17 exp Mindfulness/ (1351) 18 exp Alternative Medicine/ (4825) 19 exp Counseling/ (58829) 20 exp Diets/ (6928) 21 exp Holistic Health/ (1252) 22 exp Home Care/ (3498) 23 exp Massage/ (247) 24 exp psychotherapy/ (150532) 25 exp Physical Treatment Methods/ (131040) 26 counselling.mp. (6745) 27 counseling.mp. (69449) 28 educational.mp. (160448) 29 exercise.mp. (27902) 30 guided imagery.mp. (1100) 31 non invasive.mp. (1206) 32 non‐pharmacological.mp. (669) 33 nursing.ti,ab. (30315) 34 nurse led.ti,ab. (239) 35 nutritional.ti,ab. (5046) 36 psychosocial.mp. (71020) 37 psychoeducational.mp. (4059) 38 reflexology.ti,ab. (191) 39 relaxation.mp. (12293) 40 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 (623659) 41 exp "Quality of Life"/ (19792) 42 exp Well Being/ (16957) 43 activities of daily living.mp. (6173) 44 anxiety.mp. (116317) 45 distress.mp. (34169) 46 quality of life.mp. (29883) 47 QoL.mp. (3930) 48 HRQoL.mp. (1434) 49 well being.mp. (36397) 50 symptom relief.mp. (483) 51 health perception*.ti,ab. (697) 52 "Hospital Anxiety and Depression Scale".ti,ab. (1389) 53 WHO performance status.ti,ab. (1) 54 Rotterdam symptom checklist.ti,ab. (26) 55 Symptom Distress Scale.ti,ab. (53) 56 exp Questionnaires/ (11708) 57 exp Dyspnea/ (3225) 58 dyspn?ea.ti,ab. (732) 59 breathlessness.mp. (180) 60 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 (216897) 61 9 and 40 and 60 (143)
AMED (Allied and Complementary Medicine) (Ovid 1985 to February 2011; 1 March 2011)
1 exp Lung neoplasms/ (354) 2 lung cancer.mp. (401) 3 (lung adj5 cancer).ti,ab. (475) 4 (small cell adj5 lung).ti,ab. (150) 5 (non small cell adj5 lung).ti,ab. (119) 6 SCLC.mp. (8) 7 NSCLC.mp. (55) 8 mesothelioma.mp. (27) 9 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 (610) 10 exp Breathing exercises/ (191) 11 exp Exercise/ (7010) 12 exp Exercise therapy/ (4499) 13 exp Complementary therapies/ (42904) 14 exp Counseling/ (1553) 15 exp Diet/ (1381) 16 exp Holistic health/ (1937) 17 exp Home care services/ (1222) 18 exp Massage/ (1598) 19 exp Relaxation/ (921) 20 exp Biofeedback/ (987) 21 exp Psychosomatic therapies/ (6236) 22 exp Meditation/ (290) 23 exp Yoga/ (305) 24 exp Nursing care/ (3995) 25 exp Nutrition/ (3256) 26 exp Psychotherapy/ (7851) 27 exp Reflexology/ (176) 28 counselling.mp. (442) 29 counseling.mp. (1987) 30 educational.mp. (2986) 31 exercise.mp. (16587) 32 guided imagery.mp. (98) 33 non invasive.mp. (378) 34 non‐pharmacological.mp. (123) 35 nursing.ti,ab. (4178) 36 nurse led.ti,ab. (50) 37 nutritional.mp. (1336) 38 psychosocial.mp. (2904) 39 psychoeducational.mp. (90) 40 reflexology.mp. (221) 41 relaxation.mp. (2102) 42 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 (79639) 43 exp "Quality of life"/ (5695) 44 activities of daily living.mp. (4609) 45 anxiety.mp. (3332) 46 distress.mp. (1708) 47 quality of life.mp. (8402) 48 QoL.mp. (1056) 49 HRQoL.mp. (481) 50 well being.mp. (17728) 51 symptom relief.mp. (145) 52 health perception*.mp. (140) 53 "Hospital Anxiety and Depression Scale".mp. (257) 54 WHO performance status.mp. (3) 55 Rotterdam symptom checklist.mp. (16) 56 Symptom Distress Scale.mp. (12) 57 exp Questionnaires/ (2793) 58 exp Dyspnea/ (250) 59 dyspn?ea.mp. (756) 60 breathlessness.mp. (186) 61 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 (33853) 62 9 and 42 and 61 (52)
British Nursing Index and Archive (Ovid 1985 to February 2011; 1 March 2011)
1 lung cancer.mp. (434) 2 (lung adj5 cancer).ti,ab. (426) 3 (small cell adj5 lung).ti,ab. (36) 4 (non small cell adj5 lung).ti,ab. (28) 5 SCLC.mp. (0) 6 NSCLC.mp. (2) 7 mesothelioma.mp. (36) 8 exp Exercise/ (1501) 9 exp Diet/ (7680) 10 exp Home care services/ (1044) 11 exp Massage/ (280) 12 exp Relaxation/ (3143) 13 exp Biofeedback/ (44) 14 exp Yoga/ (1501) 15 exp Nursing care/ (10906) 16 exp Psychotherapy/ (2669) 17 counselling.mp. (2503) 18 counseling.mp. (210) 19 educational.mp. (2601) 20 exercise.mp. (2212) 21 guided imagery.mp. (63) 22 non invasive.mp. (138) 23 non‐pharmacological.mp. (216) 24 nursing.ti,ab. (39736) 25 nurse led.ti,ab. (1361) 26 nutritional.mp. (1154) 27 psychosocial.mp. (1650) 28 psychoeducational.mp. (70) 29 reflexology.mp. (104) 30 relaxation.mp. (382) 31 exp "Quality of life"/ (53365) 32 activities of daily living.mp. (165) 33 anxiety.mp. (1872) 34 distress.mp. (1307) 35 quality of life.mp. (4745) 36 QoL.mp. (79) 37 HRQoL.mp. (44) 38 well being.mp. (1080) 39 symptom relief.mp. (1242) 40 health perception*.mp. (32) 41 "Hospital Anxiety and Depression Scale".mp. (68) 42 WHO performance status.mp. (0) 43 Rotterdam symptom checklist.mp. (4) 44 Symptom Distress Scale.mp. (14) 45 exp Questionnaires/ (4560) 46 dyspn?ea.mp. (186) 47 breathlessness.mp. (159) 48 1 or 2 or 3 or 4 or 5 or 6 or 7 (470) 49 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 (69181) 50 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 (61531) 51 48 and 49 and 50 (46)
Appendix 2. Search methods for identification of studies reported in the original review
See Cochrane Lung Cancer Collaborative Review Group Strategy.
The Cochrane Central Register of Controlled Trials (The Cochrane Library Issue 4, 2003), MEDLINE (1966 to March 2003), EMBASE (1974 to March 2003), CINAHL (1982 to September 2002), CancerLit (1975 to October 2002) and PsycINFO (1873 to March 2003) were searched to identify all published randomised controlled trials and controlled clinical trials that included non‐invasive interventions for the care of lung cancer patients. The following search strategies were used for MEDLINE and adapted as appropriate for the other databases:
MEDLINE (1966 to March 2003; accessed through PubMed) 1. Oat Cell 2. SCLC OR NSCLC 3. "Carcinoma, Non‐Small‐Cell‐Lung"[MESH] 4. "Carcinoma, Small Cell"[MESH] 5. "Mesothelioma"[MESH] 6. "Pleural neoplasms"[MESH] 7. "Lung neoplasms"[MESH] 8. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 9. "Nutritional support"[MESH] 10. "Life support care" [MESH] 11. well being 12. family support 13. "Social support"[MESH] 14. "palliative care" [MESH] 15. "reflexotherapy "[MESH] 16. guided imagery 17. "Imagery (Psychotherapy)"[MESH] 18. "Complementary therapies"[MESH] 19. "Self care "[MESH] 20. "Spiritual therapies"[MESH] 21. information 22. "Aromatherapy"[MESH] 23. "Art therapy"[MESH] 24. "Dance therapy"[MESH] 25. "Music therapy"[MESH] 26. "Holistic Health"[MESH] 27. "Holistic Nursing"[MESH] 28. "diet"[MESH] 29. nutritional counselling 30. "massage"[MESH] 31. "touch"[MESH] 32. "exercise"[MESH] 33. "Motor Activity"[MESH] 34. best supportive care 35. "Relaxation techniques"[MESH] 36. psychosocial support 37. "counseling"[MESH] 38. "Psychotherapy"[MESH] 39. "nurses"[MESH] 40. nurs* 41. "nursing care"[MESH] 42. non‐pharmacological 43. "quality of life"[MESH] 44. non invasive 45. non‐invasive 46. or/ 9‐45 47. pulmonary infections 48. "muscle weakness"[MESH] 49. "loneliness"[MESH] 50. "fever"[MESH] 51. "fatigue"[MESH] 52. "cough"[MESH] 53. "cachexia"[MESH] 54. "asthenia"[MESH] 55. breathlessness 56. "Sleep Initiation and Maintenance Disorders"[MESH] 57. "anxiety"[MESH] 58. "nausea"[MESH] 59. "bodily secretions"[MESH] 60. "pain"[MESH] 61. "dyspnea"[MESH] 62. haemoptysis 63. "hemoptysis"[MESH] 64. or/ 47‐63 65. #8 AND #46 AND #64
The reference lists in relevant studies were also searched to identify further additional studies and authors of main studies were contacted to see if they were aware of any additional published or unpublished relevant study about the topic. When necessary, study authors were asked to provide complementary data about their publications.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arbane 2011.
| Methods | RCT St George's Hospital, Physiotherapy Department (London, UK) |
|
| Participants | N: 53 patients (12 weeks postoperative assessment 44, IG = 23; CG = 21) Sex: 28 male 25 female Mean age: IG = 65.4; CG = 62.6; age range: IG = 47 to 82; CG = 32 to 47) Inclusion criteria: Patients with non‐small cell lung cancer referred for lung resection via open thoracotomy or visual assisted thoracotomy (VATs) Exclusion criteria: Patients undergoing thoracotomy procedure where no lung resection is carried out (e.g. pleurectomy), patients undergoing pneumonectomy, admission greater than 48 hours to Intensive Care Unit postsurgery |
|
| Interventions | INTERVENTION: Early exercise intervention. Patients received usual care including pain relief as for the control group, plus twice‐daily additional strength and mobility training from day 1 postoperative through to day 5 postoperative ‐ provided by research physiotherapy staff and consisting of walking, as able, marching on the spot and recumbent bike exercises (carried out at bedside). Within 2 weeks of discharge and once monthly for 3 visits in total (12 weeks postoperative), patients were followed up at home where they were encouraged to continue with their paced exercise programme (usually walking in the park or nearby streets) and an adapted home‐strengthening programme. Home visits were individualised and relevant to patient hobbies (for instance one session was at the golf range for one patient). CONTROL: Control group received all usual care, pain medication as relevant was provided via patient controlled analgesia day 1 postoperative, thereafter orally as relevant. Usual care included routine physiotherapy treatments, airway clearance techniques, mobilisation as able and upper limb activities and was provided at least once daily from day 1 postsurgery. After discharge patients received monthly telephone calls up to 12 weeks from the research team, providing education only. |
|
| Outcomes | 1. Length of stay and postoperative complications (defined as X‐ray changes reported by radiologist as pneumonia, respiratory complications requiring additional ventilatory support and or necessitating a return to high dependency care) 2. Quality of life (questionnaire EORTC QLQ‐CL13 (version 2.0) 3. Exercise tolerance (6‐Minute Walking Test (6‐MWT)) 4. Quadriceps muscle strength using magnetic stimulation Measured: preoperatively, at 5 days postoperatively and at 12 weeks postoperatively |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Block randomisation. It was performed using computer generated tables after participants had consented and prior to any formal testing.” |
| Allocation concealment (selection bias) | Low risk | Quote: “The randomisation codes were kept by an independent member of the team and released after consent.” |
| Blinding | High risk | Quote: “Study was single blinded with the therapist performing assessments unaware of the randomisation although weekend treatments meant that in about 10 participants the same therapist performed the assessment and treatment.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Causes for withdrawal from the study or of missing data well reported and presented in a “CONSORT flow chart” |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but the article presents results on all outcome measures that were pre‐specified in the methods section as relevant |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Barton 2010.
| Methods | RCT (pilot study, feasibility study) Castle Hill Hospital (East Yorkshire, UK) |
|
| Participants | N: 22 patients Sex: 10 were women (6 single‐session arm) Average age: 71 years (range 58 to 85 years) Inclusion criteria: adults with refractory breathlessness caused by malignant lung disease (due to primary or secondary tumours); expected prognosis of at least 3 months and a Karnofsky Performance Scale (KPS) of > 40%. Refractory breathlessness was defined as breathlessness that persists where all identified reversible causes of the breathlessness have been treated. Exclusion criteria: any intercurrent illness or co‐morbidities making completion of the study unlikely, rapidly worsening breathlessness requiring urgent medical intervention, radical radiotherapy in the previous 6 months, palliative radiotherapy to the chest within 4 weeks or chemotherapy or a change in anti‐cancer hormone treatment in the previous 2 weeks. In addition, those with prior experience of breathlessness training were excluded. |
|
| Interventions | INTERVENTION: 3 hour‐long breathlessness management training sessions (3 weeks) CONTROL: a single breathlessness management training session only The breathing training intervention was provided by a specialist physiotherapist or 1 of 2 lung cancer trained nurse specialists. All patients received training in diaphragmatic breathing, pacing, anxiety management and relaxation during an hour‐long clinic session. Both groups received written and DVD/video reinforcement material and a telephone call from their therapist a week after the last training session. |
|
| Outcomes | 1. Breathlessness severity (numerical rating scale) 2. Breathlessness distress 3. Psychological distress (hospital anxiety and depression scale HADS) 4. Coping response (BriefCOPE and NRS coping question) 5. Quality of life and functional status (EQ‐5D and EQ‐VAS) Measured: baseline, weekly until week 4 and then at study end (week 8) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomisation was carried out by the Clinical Trials Unit (CTU), Queen’s Centre for Oncology, Hull.” |
| Allocation concealment (selection bias) | Low risk | Quote: “...telephoned the CTU where the randomisation sequence (numbered sealed opaque envelopes) was kept in a locked drawer. No member of the research team had access to the sequence and CTU staff were not involved in the recruitment of patients. The allocated arm was then telephoned to the therapist by a member of CTU staff.” |
| Blinding | High risk | Non‐blinded. Quote: “Although participant and therapist were inevitably unblinded, the RN was not given allocation information in an attempt to achieve assessor blinding. However, this was difficult to maintain as patients often discussed their treatment.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Causes for withdrawal from the study well reported. Quote: “The flow of patients through the trial is shown in the CONSORT flow diagram (Fig. 1). There were no protocol deviations. The study had difficulties in the recruitment, as only the 55% of the estimated sample was included, and experienced a high drop‐out rate (40%) |
| Selective reporting (reporting bias) | Low risk | Pilot study presents results on all outcome measures that were pre‐specified in the methods section as relevant |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Bredin 1999.
| Methods | RCT Multicentre, UK. Nursing clinics in 6 hospitals. | |
| Participants | N: 109 patients randomised (103 analysed (IG* = 51; CG = 52)
Sex: IG = 41 (80%) men CG = 35 (67%) men
Mean age: IG = 68 (range = 41 to 82); CG = 67 (range = 41 to 83) Inclusion criteria: Patients with lung cancer; treatment completed; experiencing dyspnoea. |
|
| Interventions | INTERVENTION
Nurse‐led breath management programme
Patients attended a nursing clinic once a week for between 3 to 8 weeks where they received a care package including: i) detailed assessment of breathlessness together with exacerbating/ameliorating factors; ii) advice and support on ways of managing breathlessness; iii) exploration with the patient of the meaning of their breathlessness, their disease and their view of the future; iv) training in relaxation techniques and breathing re‐training; v) goal‐setting for achieving functional and social activities and to support the development of coping strategies; vi) recognition of problems needing medical attention CONTROL: Standard care. Breathlessness and its effects were monitored. Both groups received best supportive care defined as the standard management and treatment of breathlessness, including palliative care and pharmacological treatment |
|
| Outcomes | 1. Distress due to breathlessness (visual analogue scale)
2. Emotional status (Hospital Anxiety and Depression Scale)
3. Physical status (WHO performance status scale, Rotterdam symptom checklist) Measured at: 1, 4 and 8 weeks |
|
| Notes | *See footnote for key to terms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “In each of the participating centres, once a patient from one of the participating centres had consented to take part in the trial, a telephone call was made to the Institute of Cancer Research's clinical trials office, which was responsible for independent randomisation to either intervention or control groups. The trials office informed the participating centre which group the patient had been assigned to. The patient was then asked to confirm whether he or she remained happy to participate in the study.” |
| Allocation concealment (selection bias) | Low risk | Central allocation (telephone, centrally controlled randomisation) (see above) |
| Blinding | Unclear risk | Nothing mentioned in the article. Not blinded for patients and professional. Unclear about outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data have been imputed using appropriate methods |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but the article presents results on all outcome measures that were pre‐specified in the methods section as relevant |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Chan 2011.
| Methods | RCT Tuen Mun Hospital (Hong Kong) |
|
| Participants | N: 140 patients Sex: 85% male Age data not available; 55% retired Inclusion criteria: Age 16 years or older; stage 3 or 4 lung cancer and scheduled to receive palliative radiotherapy (RT) of an average of 4.3 Gy/fraction; the ability to communicate in Chinese; signed informed consent; an Abbreviated Mental Test score of 8 or above indicating normal cognitive ability; and a Karnofsky Performance Status score of 60% or above, indicating the patient was capable of some self care and not bedridden Exclusion criteria: Patients with known psychiatric morbidity; involvement in other clinical trials |
|
| Interventions | INTERVENTION: A 40‐minute educational package plus coaching of progressive muscle relaxation (PMR) was delivered to patients within 1 week prior to the beginning of the course of RT, and reinforced 3 weeks after commencing RT. The education package consisted of leaflets and discussion on the selected symptoms and their self care management. The intervention was delivered by registered nurses with 2 years of clinical experience. CONTROL: Usual care, comprised a mandatory individual briefing of the RT procedure and about a 5 to 7‐minute discussion of side effects focusing on skin care by a therapy radiographer. Patients also were invited to attend an optional group talk given by a registered nurse and a medical social worker about general care before and/or after the commencement of RT. Patients in both intervention and control groups were offered this usual care. |
|
| Outcomes | Measured as the effect of the intervention on a symptom cluster, referred to as a composite outcome comprising the vector of means on the transformed scores of breathlessness, fatigue and anxiety across time 1. Breathlessness (visual analogue scale) 2. Fatigue (Piper Fatigue Scale) 3. Anxiety (Chinese version of the A‐state scale of the State‐Trait Anxiety Inventory) 4. Functional ability (Chinese version (HK) of the SF‐36 Health Survey) Measured: prior to the intervention, 3 weeks, 6 weeks and 12 weeks postintervention |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomised by lucky draw method to either an intervention group or control group.” |
| Allocation concealment (selection bias) | Unclear risk | Nothing mentioned in the article |
| Blinding | Low risk | Single blind. Quote: “Data were collected by a research assistant who was blinded to group allocation.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “At all time points, the sole reason for attrition was death.” |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but the article presents results on all outcome measures that were pre‐specified in the methods section as relevant |
| Other bias | High risk | Patients in the control group had a significantly more advanced stage of cancer (P < 0.05) when compared with the intervention group |
Corner 1996.
| Methods | RCT (pilot study) Nurse‐led clinic at the Institute of Cancer Research (London, UK) | |
| Participants | N: 34 patients randomised (20 analysed IG* = 11; CG = 9)
Sex: IG = 5 (45%) men; CG = 7 (77%) men
Mean age: IG = 55; CG = 69 Inclusion criteria: Patients with lung cancer; treatment completed; experiencing dyspnoea |
|
| Interventions | INTERVENTION:
Nurse‐led breath management programme
Weekly sessions over 3 to 6 weeks. Based on breathing rehabilitation strategies including counselling, breath retraining, relaxation, coping strategies, acknowledgement and management of the emotional experience of the symptom. CONTROL: Accurate symptom assessment by the patient's physician |
|
| Outcomes | 1. Breathlessness over the previous week (visual analogue scale)
2. Symptom distress (visual analogue scale)
3. Physical functioning (Functional Capacity Scale)
4. Anxiety and depression (Hospital Anxiety and Depression Scale)
5. Patients' experience of breathlessness (qualitative interviews) Measured: baseline, 4 weeks and 3 months |
|
| Notes | *See footnote for key to terms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement (judgement 'unclear'). Sequence generation process is not explained in a detailed way. |
| Allocation concealment (selection bias) | Unclear risk | Nothing mentioned in the article |
| Blinding | Unclear risk | Nothing mentioned on that topic. Not blinded for patients and professionals. Unclear about outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Causes for withdrawal from the study well reported: “Fourteen patients (eight from the intervention group and six from the control group) had to withdraw from the study due to deterioration, reflecting the limited prognosis for the majority of patients with lung cancer”. |
| Selective reporting (reporting bias) | Unclear risk | All relevant outcomes presented |
| Other bias | High risk | Premature ending of the randomisation process and nothing mentioned about existence of previous criteria for such a decision. Quote: “Results: Thirty‐four patients had consented to take part in the randomised study when randomisation was stopped in response to requests from medical nursing staff who felt they have observed a clear benefit from the intervention strategy.” |
Evans 1987.
| Methods | RCT Multicentre, USA/Canada. Emory University School of Medicine (Atlanta, Georgia), Memorial Sloan‐Kettering Cancer Center in New York (New York) and the University of Toronto (Canada) | |
| Participants | N: 192 patients randomised (180 analysed (standard nutrition (SG) = 51; augmented nutrition (AG) = 60; control group (CG) = 69); 96 of whom had lung cancer (SG = 30; AG = 30; CG = 36)
Sex (For lung cancer sample): SG = 21 (70%) men; AG = 18 (60%) men; CG = 25 (69%) men
Mean age (for lung cancer sample): SG = 57 (36 to 79); AG = 59 (37 to 68); CG = 61 (37 to 78) Inclusion criteria: Histological evidence of lung cancer Life expectancy > 16 weeks ECOG* performance status > 3 More than 21 days since major surgery On oral diet Exclusion criteria: Previous chemotherapy treatment Inadequate renal, hepatic or bone marrow function Superior vena caval obstruction Central nervous system metastases or any chronic systemic disease. Surgical complications requiring intravenous hyperalimentation Any nutritional therapy in last 6 months |
|
| Interventions | INTERVENTION:
Standard nutrition nutritional advice by dietician to achieve targeted caloric intake
Augmented nutrition: nutritional advice to ingest 25% of calories as protein in diet and by protein supplements CONTROL: Ad lib oral diet without nutritional support by dietitian |
|
| Outcomes | 1. Tumour response to therapy and survival
2. Tolerance of chemotherapy
3. Relationship between caloric intake and weight Measured: baseline and at the end of each chemotherapy cycle |
|
| Notes | *See footnote for key to terms 10% of patients in the standard group and 7% in the augmented group received enteral nutrition |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The study randomisation was performed by telephone through a central office and blocked so that the number of patients in each treatment group would be approximately equal at each centre” |
| Allocation concealment (selection bias) | Low risk | Central office and by phone |
| Blinding | Unclear risk | Nothing mentioned on that topic |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Presented data on causes of withdrawal. Missing data have been imputed using appropriate methods. Quote: “Early deaths were considered to be non responders and are included in the denominator for response rates.” “Four patients died (two cancer related; two non‐cancer related) before the first tumour evaluation. The two cancer‐related deaths were classified as disease progression”. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes presented |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Linn 1982.
| Methods | RCT General Medical Hospital in Miami (Florida, USA) | |
| Participants | N: 120 total, 64 with lung cancer (IG* = 33; CG = 32)
Sex: 100% men
Mean age: 58 (SD = 8; range = 45‐77)
Diagnosis: stage IV cancer Comments: > 50% smokers Inclusion criteria: Life expectancy > 3 months Able to communicate and give informed consent |
|
| Interventions | INTERVENTION:
Counselling. Several individual sessions per week with a single counsellor with expertise in care of the dying. Aim was to establish a trust relationship with patients, focused on reducing denial and maintaining hope. Support to families also offered. CONTROL: No intervention |
|
| Outcomes | Patient completed:
1. Quality of life measured by:
Depression (Depression Factor of the Psychiatric Outpatient Mood Scale (McNair 1971))
Self esteem (Sherwood 1965)
Life satisfaction (Cantril 1965)
Alienation (Strole 1956)
Locus of control (Rotter 1966) Nurse completed: 1. Functional status (Rapid Disability Rating Scale (Linn 1967)). Physician completed: 2. Degree of impairment (Cumulative Illness Rating Scale) Measured: baseline, 1, 3, 6, 9 and 12 months Survival |
|
| Notes | *See footnote for key to terms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement. Mentioned as “randomly assigned” but sequence generation process is not explained. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding | Low risk | Assessors blinded. Quote: “Data were collected by two psychiatric nurses blind to treatment assignment.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Presented data on causes of withdrawal “Four patients were lost to follow‐up because they moved from the area. Two other requested to be dropped from the study. Data were complete for the 114 remaining at each follow‐up as long as they lived”. |
| Selective reporting (reporting bias) | Low risk | Pre‐stated outcomes were already assessed |
| Other bias | Low risk | The study appears to be free of other sources of bias |
McCorkle 1989.
| Methods | RCT Multicentre, USA. 19 hospitals and one radiation outpatient facility in Washington state (USA). | |
| Participants | N: 166 patients with lung cancer:
Specialised oncology home care group = 45
Standard home care group = 42
Office care (control) = 46 Sex: 105 (63%) men Mean age: (66; range = 18 to 81) Clinical condition: stage II disease or higher Inclusion criteria: Registered as having lung cancer on Cancer Surveillance System Residents in King County‐Seattle (Washington, USA) Met Medicare criteria for being homebound Able to co‐operate with study requirements |
|
| Interventions | INTERVENTION:
Specialised oncology home care programme (OHC*):
Master's level nurses trained to deliver cancer care gave symptom and pain management, cancer treatment, physical and psychosocial assessment and grief management
Standard home care programme (SHC):
Interdisciplinary team consisting of registered nurses, physical therapists, home health aides, medical social worker, occupational therapist and a speech therapist. Team discussed treatment and case management CONTROL: Office care (OC): standard care provided by patient's physician |
|
| Outcomes | Psychosocial well‐being measured by:
1. Symptom distress scale (McCorkle 1978)
2. McGill‐Melzack pain questionnaire (Melzack 1987)
3. Inventory of current concerns (Weisman 1977)
4. Profile of mood states (McNair 1971)
5. Enforced social dependency scale (Benoliel 1980)
6. General health rating index (Ware 1976)
7. Medical record review instrument Measured: on 5 separate occasions over 6 months. The first data were collected between 8 and 10 weeks after diagnosis and at 6‐weekly intervals thereafter. |
|
| Notes | *see footnote for key to terms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “randomised block series in sealed envelopes” |
| Allocation concealment (selection bias) | Low risk | Quote: “randomised block series in sealed envelopes” |
| Blinding | Unclear risk | Nothing mentioned on that topic |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Presented data on causes of withdrawal |
| Selective reporting (reporting bias) | Low risk | Pre‐stated outcomes were already assessed |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Moore 2002.
| Methods | RCT Multicentre, UK. Cancer hospital and 3 cancer units. | |
| Participants | N: 202 (IG* = 99; CG = 103)
Sex: IG = 74 (75%) men; CG = 66 (64%) men
Mean age: 67(SD = 8.8; range = 45 to 89)
Clinical condition:
Patients were stratified by hospital and by type of treatment. Inclusion criteria: Patients with lung cancer Completed initial treatment Life expectancy > 3 months |
|
| Interventions | INTERVENTION:
Nurse‐led patient follow‐up. Patients assessed monthly by protocol. Protocol consisted of a programme based on open access to nurse specialists and clinics; phone assessment or clinic appointment 2 weeks after baseline and then every 4 weeks in which a clinic assessment was completed; emphasis on rapid communication with primary healthcare team when necessary; regular discussion with medical team about new symptoms. CONTROL: Medical follow‐up, with regular outpatient appointments for medical assessment and monitoring patient's progression |
|
| Outcomes | 1. Quality of life (EORTC)
2. Patient satisfaction (Newcastle Satisfaction Scale) Measured: basal and at 3, 6 and 12 months |
|
| Notes | *See footnote for key to terms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “An independent trials office was responsible for randomisation of patients to either conventional medical follow‐up or nurse‐led follow‐up. For randomisation, patients were stratified according to hospital and treatment intent.” |
| Allocation concealment (selection bias) | Low risk | Central allocation, see above |
| Blinding | Unclear risk | Nothing mentioned on this topic in the article |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reason for losses well recorded and presented in a graph. Quotes: “Most attrition was due to death or ill health.” “One patient randomised to nurse‐led follow‐up was later found to be ineligible for the study and was excluded.” |
| Selective reporting (reporting bias) | Low risk | Pre‐stated outcomes were already assessed |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Porter 2011.
| Methods | RCT Duke University Thoracic Oncology Program and several community oncology clinics in the Durham, NC area (USA) |
|
| Participants | N: 233 patients with lung cancer. IG: 117, CG: 116 Sex: 52.8% males Mean age: 65.3 years Entry criteria for patients 1) A diagnosis of early‐stage lung cancer (non‐small‐cell lung cancer stages I‐III or limited‐stage small‐cell lung cancer) 2) No other cancers in the past 5 years 3) Ability to read and speak English 4) A caregiver who also was willing to participate. "Caregiver" was broadly defined in this study as any friend or family member who provided practical and/or emotional support to the patient. To identify the patient’s primary caregiver, the patient was asked to list the people they relied on for support with things like getting to the doctor and taking medication.They were then asked to identify the main person they relied on for support. This person was identified as the primary caregiver and was invited to participate in the study. |
|
| Interventions | INTERVENTION 14 sessions of caregiver‐assisted coping skills training (CST) CONTROL 14 sessions of education/support involving the caregiver |
|
| Outcomes | 1. Pain. Assessed using 2 items from the Brief Pain Inventory (BPI) 2. Psychological Distress. Assessed using the Beck Depression Inventory (BDI) and the trait anxiety version of the State Trait Anxiety Inventory (STAI) 3. Quality of Life. QOL was measured using the Functional Assessment of Cancer Therapy‐Lung Cancer (FACT‐L) 4. Self efficacy. Self efficacy for managing pain, symptoms and function was assessed with a modified version of a standard self efficacy scale All evaluation measures were collected through a telephone interview. At each evaluation session (baseline, post‐test and 4‐month follow‐up); measures were collected from both patient and caregiver. |
|
| Notes | Comparisons of effects sizes among groups not provided. Statistical analyses were done using hierarchical linear modelling to evaluate group differences over time. Each model estimated time, intervention (CST versus education/support) and time intervention effects. The time effect assessed whether the outcome changed across the baseline, post‐test and 4‐month follow‐up assessments. Significant effects of the intervention would be indicated by a significant time intervention effect. The study also assessed measures collected from caregivers (caregiver mood brief version of the Profile of Mood States‐B (POMS‐B); Caregiver Strain Index (CSI), modified version of a standard self efficacy scale in Symptom Management) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation assignments were generated by an individual not involved in the study using a random number table." |
| Allocation concealment (selection bias) | Low risk | Quote: "Assignments were concealed in envelopes that were not opened until participants had completed their pretreatment evaluation." |
| Blinding | Low risk | Quote: "The research assistants conducting the assessments were blind to treatment condition". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Causes for withdrawal from the study or of missing data well reported and presented in a “consort flow chart” |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but the article presents results on all outcome measures that were pre‐specified in the methods section as relevant |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Sarna 1998.
| Methods | RCT Multicentre, USA. Five outpatient oncology sites in California (USA). | |
| Participants | N: 48 ‐ distribution between intervention and control groups not reported
Sex: 24 (50%) men
Mean age: 62 (SD* = 9; range = 39 to 79)
Clinical condition: stage III or IV Inclusion criteria: Patients had been diagnosed 2 or 3 months previously Aware of the diagnosis English speaker |
|
| Interventions | INTERVENTION:
Structured nursing symptom assessment protocol including an standardised symptom distress instrument
All patients completed structured instruments for the assessment of symptoms (including symptom distress)
Questionnaires from patients allocated to the experimental group (structured assessment) were summarised and shared with the nurse to help enhance the nurse's assessment of the patient's needs
The interventions which the nurses then undertook were not a focus of the study CONTROL: Forms completed by patients in the control group were placed in a sealed envelope and were not seen by the nurses caring for them. These patients received a standard nursing assessment. |
|
| Outcomes | 1. Symptom distress (Symptom Distress Scale (McCorkle 1978))
2. Emotional distress (Hospital Anxiety and Depression Scale, Zigmond 1983)
3. Physical functional status (Karnofsky 1949), Physical Functioning Scale (Stewart 1992) Measured: monthly for 6 months |
|
| Notes | *See footnote for key to terms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation process not explained |
| Allocation concealment (selection bias) | Unclear risk | No information provided on that topic |
| Blinding | Unclear risk | No information provided on that topic |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Presented data on causes of withdrawal. “Attrition during the study occurred as a result of death (n = 10) or drop‐out because of increasing physical and emotional distress (N = 17)” |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes presented |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Stephenson 2000.
| Methods | Cross‐over trial Medical‐oncology unit in a regional hospital in South Carolina (USA) | |
| Participants | N: 23 patients (10 with lung cancer (of whom half received the intervention first) Sex (lung cancer sample): 8 (80%) men Mean age: 68.7 (SD* = 2.69) Inclusion criteria: > 20 years English speaker Capable of giving informed consent Exclusion criteria: Patients not reporting anxiety Surgical intervention the previous 6 months Patient with acute cancer pain, receiving radiotherapy or with dementia |
|
| Interventions | INTERVENTION:
Reflexology
30 minutes of foot reflexology sessions divided into: 15 minutes of massage in the reflexing areas corresponding to body areas of patient's self reported pain or body part where cancer located; 10 minutes of relaxing techniques at the beginning and end of the session; and 5 minutes of reflexing all the areas of the feet to cover all the body areas CONTROL: No intervention was used during the 30‐minute control time |
|
| Outcomes | 1. Anxiety (visual analogue scale)
2. Pain (Short‐Form McGill Pain Questionnaire) Measured: before and after the intervention and before and after the control time |
|
| Notes | *See footnote for key to terms Patients were randomised to group A in which they received the intervention first, or to group B in which the intervention was received after an elapse of time (at least 48 hours elapsed between the intervention and the control time). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin toss |
| Allocation concealment (selection bias) | Unclear risk | No information provided on that topic |
| Blinding | Unclear risk | No information provided on that topic |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No mention of losses |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes presented |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Stephenson 2007.
| Methods | RCT Four hospitals in the southeastern United States (USA) |
|
| Participants | 42 experimental and 44 control subjects comprised 86 dyads of patients with metastatic cancer and their partners, representing 16 different types of cancer; 23% of patients had lung cancer, followed by breast, colorectal, head and neck cancer and lymphoma. Mean age of 58.3 years 51% female Inclusion criteria: Presence of any type of metastatic cancer Pain score of 2 or higher on a 0 to 10 pain scale Exclusion criteria: Surgery in the previous 6 weeks or had open skin wounds on the feet, foot tumours or foot metastases, radiation of the feet, radiation to the site of pain, or more than 50% loss of feeling because of peripheral neuropathy |
|
| Interventions | INTERVENTION:
15 to 30‐minute teaching session on foot reflexology to the partner by a certified reflexologist, an optional 15 to 30‐minute foot reflexology session for the partner, and a 30‐minute, partner‐delivered foot reflexology intervention for the patient CONTROL: Usual care plus 30‐minute reading session from their partners |
|
| Outcomes | 1. Pain (Brief Pain Inventory (BPI) and the Short‐Form McGill Pain Questionnaire (SF‐MPQ)) 2. Anxiety (visual analogue scale for anxiety) Measurements at baseline and postintervention |
|
| Notes | The 86 patients mentioned above were the ones with complete data. The study did not include disaggregated data for lung cancer patients. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients randomly assigned to an experimental or control group", but no more details provided in the text |
| Allocation concealment (selection bias) | Unclear risk | No information provided on that topic |
| Blinding | Unclear risk | No information provided on that topic |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Presented data on causes of withdrawal. Quote: "Three patients in the experimental group were unable to receive the intervention because they were too ill". "One control group patient...was too agitated to answer any postintervention questions". |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes presented |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Wall 2000.
| Methods | RCT Medical cancer centre in New York (New York, USA) | |
| Participants | N: 104 (IG* = 53; CG = 51)
Sex: 53.8%
Mean age: 65 (37 to 83)
Clinical condition: preoperative patients with lung cancer (stages IA‐IB‐IIA‐IIB‐IIIA) Inclusion criteria: Patients with lung cancer, able to exercise Exclusion criteria: Patients receiving chemotherapy or radiotherapy treatment |
|
| Interventions | INTERVENTION:
Preoperative exercise programme structured as a 7 to 10‐day home‐based programme that included walking (1 mile per day), stair climbing (40 steps twice per day), arm and leg exercises (combined with breathing exercises 10 times twice per day), and breathing exercises (sniff, hold breath and slow blow out as if whistling, 5 times twice per day) CONTROL: No intervention |
|
| Outcomes | 1. Hope (Herth Hope Index; Herth 1992S)
2. Power (PKPCT VII) Measured: time 1: prior to randomisation, at the time of diagnosis (about 7 to 10 days before surgery) time 2: one day before surgery, at end of exercise programme, time 3: 4 to 6 days after surgery |
|
| Notes | *See footnote for key to terms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Participants were randomly assigned to the exercise or no‐exercise group using sealed envelopes with randomisation instructions prepared by the hospital’s biostatistical department.” |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding | High risk | “Participants randomised to the exercise group were instructed on the exercises by the principal investigator” and outcome measures were subjective and evaluation was not stated as blinded, so it cannot be discarded that researcher could bias perceptions of patients |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Presented data on causes of withdrawal |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes presented |
| Other bias | Unclear risk | The study appears to be free of other sources of bias |
Wilkie 2010.
| Methods | RCT 11 clinical sites in the Puget Sound area (USA) |
|
| Participants | N: 215 (of them 151 provided study outcome data) Sex: 74% male, 26% female Age: mean 62 years old Inclusion criteria: Diagnosis of small cell or non–small cell lung cancer; spoke and read English; and had pain related to the lung cancer or to anticancer therapies during the week prior to enrolling Exclusion criteria: Pain for less than 2 months after thoracotomy; mentally or physically unable to participate. A score of 20 or less on the Mini‐Mental State Examination determined mental competence. |
|
| Interventions | INTERVENTION: Coaching of sensory self monitoring and reporting, with 5 elements (video, tool, 3 reinforcement sessions with the PAIN Report Card) CONTROL: Usual care |
|
| Outcomes | 1. Amount and accuracy of communicated sensory pain data (location, intensity, quality, temporal pattern): Audiotape Scoring Tool 2. Pain prescriptions: Pain Management Index (PMI) scores 3. Pain relief: MPQ and VAS 4. Anxiety: State‐Trait Anxiety Inventory (STAI) 5. Depression: Centre for Epidemiological Studies Depression Scale 6. Catastrophising pain coping: Coping Strategies Questionnaire Measured: baseline, 2 weeks and 4 weeks |
|
| Notes | See footnote for key to terms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “prepared in advance by the biostatistician from a computer‐generated list of random numbers”. |
| Allocation concealment (selection bias) | Low risk | Quote: “The researcher opened a sealed opaque envelope” |
| Blinding | Low risk | Quote: “A research assistant blind to group assignment abstracted all analgesics documented in the medical record during the 4‐week study.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Causes for withdrawal from the study well reported. Quote: “The main reasons some did not complete the study were as follows: (a) 10% died within the 4‐week study period, (b) 4% became too ill to finish study procedures, (c) 13% withdrew, and (d) 3% moved or were lost to follow‐up.”). Also stated that “Attrition rates and reasons for not completing were similar for both groups.” |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but the article presents results on all outcome measures that were pre‐specified in the methods section as relevant |
| Other bias | Low risk | The study appears to be free of other sources of bias |
AG: augmented nutrition group CG: control group ECOG: Eastern Co‐operative Oncology Group EORTC: European Organisation for Research and Treatment of Cancer HADS: Hospital Anxiety and Depression Scale IG: intervention group MPQ: McGill Pain Questionnaire NRS: numerical rating scale OC: Office Care Programme OHC: Specialised Oncology Home Care Programme PKPCT: Power as Knowing Participation in Change Test RCT: randomised controlled trial RN: registered nurse RT: radiotherapy SD: standard deviation SHC: Standard Home Care Programme SG: standard nutrition group VAS: visual analogue scale WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Armes 2007 | The study did not include disaggregated data for lung cancer patients |
| Bakitas 2009 | The study did not include disaggregated data for lung cancer patients |
| Borneman 2010 | The study did not include disaggregated data for lung cancer patients |
| Brown 2006 | The study did not include disaggregated data for lung cancer patients |
| Burnham 2002 | The sample did not include lung cancer patients |
| Cesario 2007 | Case series study |
| Chan 1998 | The sample included only 1 lung cancer patient |
| Christman 2004 | The study did not include disaggregated data for lung cancer patients |
| Cole 2005 | The study did not include disaggregated data for lung cancer patients |
| Cunningham 1989 | The study did not include disaggregated data for lung cancer patients |
| de Wit 1997 | The study did not include disaggregated data for lung cancer patients |
| Decker 1992 | The study did not include disaggregated data for lung cancer patients |
| Dimeo 1997 | The study did not include disaggregated data for lung cancer patients |
| Dimeo 1999 | The sample did not include lung cancer patients in one of the comparison groups |
| Dimeo 2004 | The study did not include disaggregated data for lung cancer patients |
| Doorenbos 2006 | The study did not include disaggregated data for lung cancer patients |
| Foltz 1987 | The study did not include disaggregated data for lung cancer patients |
| Forester 1985 | The study did not include disaggregated data for lung cancer patients |
| Forester 1993 | The study did not include disaggregated data for lung cancer patients |
| Given 2002 | The study did not include disaggregated data for lung cancer patients |
| Graham 2003 | The study did not include disaggregated data for lung cancer patients |
| Grande 1999 | Outcomes did not meet the inclusion criteria |
| Grande 2000 | Outcomes did not meet the inclusion criteria |
| Grealish 2000 | The study did not include disaggregated data for lung cancer patients |
| Greer 1992 | The sample did not include lung cancer patients |
| Holland 1991 | The study did not include disaggregated data for lung cancer patients |
| Jacobsen 2002 | The study did not include disaggregated data for lung cancer patients |
| Li 2002 | The study did not include disaggregated data for lung cancer patients |
| McCorkle 1998 | The study did not include disaggregated data for lung cancer patients |
| Oh 2008 | The study did not include disaggregated data for lung cancer patients |
| Ovesen 1993 | The study did not include disaggregated data for lung cancer patients |
| Parker 1998 | The sample did not include lung cancer patients |
| Parvez 2007 | The study did not include disaggregated data for lung cancer patients |
| Ramsay 2007 | The study did not include disaggregated data for lung cancer patients |
| Rawl 2002 | The study did not include disaggregated data for lung cancer patients |
| Ream 2006 | The study did not include disaggregated data for lung cancer patients |
| Rosenbloom 2007 | The study did not include disaggregated data for lung cancer patients |
| Schiffman 2007 | The study did not include disaggregated data for lung cancer patients |
| Schneider 2007 | The study did not include disaggregated data for lung cancer patients |
| Skrutkowski 2008 | The study did not include disaggregated data for lung cancer patients |
| Soden 2004 | The study did not include disaggregated data for lung cancer patients |
| Speca 2000 | The study did not include disaggregated data for lung cancer patients |
| Steinhauser 2008 | The study did not include disaggregated data for lung cancer patients |
| Weinrich 1990 | The sample did not include lung cancer patients |
| Wilkie 2000 | The study did not include disaggregated data for lung cancer patients |
| Wyatt 2007 | The study did not include disaggregated data for lung cancer patients |
Characteristics of studies awaiting assessment [ordered by study ID]
Cai 2001.
| Methods | CCT (data from abstract) |
| Participants | 208 patients (162 in the intervention group, 46 in the control group) |
| Interventions | INTERVENTION: Music therapy combined with anti‐tumour drugs (chemotherapy and Chinese drugs) CONTROL: Anti‐tumour drugs (chemotherapy and Chinese drugs) |
| Outcomes | Self rating depression scale (SDS) Self rating anxiety scale (SAS) Minnesota Multiphasic Personality Inventory (MMPI) Hamilton Rating Scale for Depression (HAMD) |
| Notes |
Wang 2005.
| Methods | RCT |
| Participants | N = 53 male patients with lung cancer IG: 28; CG: 25 |
| Interventions | INTERVENTION: Usual care plus some suitable exercises and were given corresponding instruction, they advanced step‐by‐step rehabilitation exercises according to the postoperative time and gradual reinforced strength exercises CONTROL: Usual care |
| Outcomes | Indexes of lung function including vital capacity, forced vital capacity, forced expiratory volume in first second Measurements preoperation and 1, 3 and 6 months postoperation |
| Notes | Article in Chinese. All information drawn from the Abstract. Fourth Affiliated Hospital, Hebei Medical University, Shijiazhuang, Hebei Province, China |
Wu 2003.
| Methods | RCT |
| Participants | N = 120 patients with lung cancer IG: 63 patients, 47 males and 16 females, aged 30 to 76 years old CG: 57 patients, 43 males and 14 females, aged 33 to 78 years old |
| Interventions | INTERVENTION: Psychotherapy and chemotherapy combined with radiotherapy CONTROL: Chemotherapy and radiotherapy only |
| Outcomes | Self Rating Anxiety Scale (SAS) Self Rating Depression Scale (SDS) |
| Notes | Article in Chinese. All information drawn from the Abstract. |
Zhen 2002.
| Methods | Controlled trial |
| Participants | N = 108 patients with colon, stomach, oesophagus or lung cancer IG: 61 patients (aged 32 to 69 years) CG: 47 cancer patients (aged 34 to 71 years) |
| Interventions | INTERVENTION: Patients were treated psychologically during perioperative period, and received supportive therapy, individual counselling, cognitive therapy, patients mutual therapy, relaxation training and inner idea guiding CONTROL: Usual care |
| Outcomes | State‐Trait Anxiety Inventory (STAI) Self Rating Depression Scale (SDS) Visual analogue scale (VAS) Measurements before and after surgery, 1 month after operation |
| Notes | Article in Chinese. All information drawn from the Abstract.The Second People's Hospital of Xinxiang City, Henan, China. |
CCT: controlled clinical trial CG: control group IG: intervention group RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Jones 2010.
| Trial name or title | Lung cancer exercise training study (LUNGEVITY) |
| Methods | RCT |
| Participants | 160 patients (40 patients/study arm) with histologically‐confirmed stage I‐IIIA NSCLC following curative‐intent complete surgical resection at Duke University Medical Center |
| Interventions | Four conditions: (1) aerobic training alone, (2) resistance training alone, (3) the combination of aerobic and resistance training, or (4) attention‐control (progressive stretching) |
| Outcomes | The primary study endpoint is VO2 peak. Secondary endpoints include: patient‐reported outcomes (PROs) (e.g. quality of life, fatigue, depression, etc.) and organ components of the oxygen cascade (i.e. pulmonary function, cardiac function, skeletal muscle function). |
| Starting date | |
| Contact information | Correspondence: jones442@mc.duke.edu Lee W Jones, Duke University Medical Center, Durham, NC, USA |
| Notes | Trial Registration: NCT00018255 |
NSCLC: non‐small cell lung cancer RCT: randomised controlled trial
Contributions of authors
JR screened the search results, extracted data and assessed risk of bias for all new and updated trials, and drafted the manuscript. IS screened the search results, extracted data and assessed risk of bias for all new and updated trials, and drafted the manuscript. MS and AP helped with interpretation of the results and in drafting the manuscript. All authors commented on the manuscript.
Sources of support
Internal sources
Iberoamerican Cochrane Centre, Spain.
Hospital de la Santa Creu i Sant Pau, Spain.
University of the Basque Country. Department of Preventive Medicine and Public Health. GIU 10/24, Spain.
External sources
Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III (Project no: 02/10050), Spain.
-
Cochrane Review Incentive Scheme 2010, UK.
Funded by National Institute for Health Research, awarded for the completion of the update of this review
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Arbane 2011 {published data only}
- Arbane G, Tropman D, Jackson D, Garrod R. Evaluation of an early exercise intervention after thoracotomy for non‐small cell lung cancer (NSCLC), effects on quality of life, muscle strength and exercise tolerance: randomised controlled trial. Lung Cancer 2011;71(2):229‐34. [DOI] [PubMed] [Google Scholar]
Barton 2010 {published data only}
- Barton R, English A, Nabb S, Rigby AS, Johnson MJ. A randomised trial of high vs low intensity training in breathing techniques for breathless patients with malignant lung disease: a feasibility study. Lung Cancer 2010;70(3):313‐19. [DOI] [PubMed] [Google Scholar]
Bredin 1999 {published data only}
- Bredin M, Corner J, Krishnasamy M, Plant H, Bailey C, A'Hern R. Multicentre randomised controlled trial of nursing intervention for breathlessness in patients with lung cancer. BMJ 1999;318:901‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2011 {published data only}
- Chan CW, Richardson A, Richardson J. Managing symptoms in patients with advanced lung cancer during radiotherapy: results of a psychoeducational randomized controlled trial. Journal of Pain and Symptom Management 2011;41(2):347‐57. [DOI] [PubMed] [Google Scholar]
Corner 1996 {published data only}
- Corner J, Plant H, A'Hern R, Bailey C. Non‐pharmacological intervention for breathlessness in lung cancer. Palliative Medicine 1996;10(4):299‐305. [DOI] [PubMed] [Google Scholar]
Evans 1987 {published data only}
- Evans W, Nixon D, Daly J, Ellenberg S, Gardner L, Wolfe E, et al. A randomized study of oral nutritional support versus al lib nutritional intake during chemotherapy for advanced colorectal and non‐small‐cell lung cancer. Journal of Clinical Oncology 1987;5(1):113‐24. [DOI] [PubMed] [Google Scholar]
Linn 1982 {published data only}
- Linn M, Linn B, Harris R. Effects of counseling for late stage cancer patients. Cancer 1982;16(3):1048‐55. [DOI] [PubMed] [Google Scholar]
McCorkle 1989 {published and unpublished data}
- McCorkle R, Benoliel J, Donaldson G, Georgiadou F, Moinpour C, Goodell B. A randomized clinical trial of home nursing care for lung cancer patients. Cancer 1989;64:1375‐82. [DOI] [PubMed] [Google Scholar]
- McCorkle R, Benoliel JQ, Donaldson G, Goodell B. Evaluation of cancer management. Final report of project supported by grant NU 01001. Seattle: University of Washington, 1986. [Google Scholar]
Moore 2002 {published data only}
- Moore S, Corner J, Haviland J, Wells M, Salmon E, Normand C, et al. Nurse led follow up and conventional medical follow up of patients with lung cancer: randomised trial. BMJ 2002;325:1145‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Porter 2011 {published data only}
- Porter LS, Keefe FJ, Garst J, Baucom DH, McBride CM, McKee DC, et al. Caregiver‐assisted coping skills training for lung cancer: results of a randomized clinical trial. Journal of Pain and Symptom Management 2011;41(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sarna 1998 {published data only}
- Sarna L. Effectiveness of structured nursing assessment of symptom distress in advanced lung cancer. Oncology Nursing Forum 1998;25:1041‐8. [PubMed] [Google Scholar]
Stephenson 2000 {published data only}
- Stephenson N, Weinrich S, Tavakoli A. The effects of foot reflexology on anxiety and pain in patients with breast and lung cancer. Oncology Nursing Forum 2000;27(1):67‐72. [PubMed] [Google Scholar]
Stephenson 2007 {published data only}
- Stephenson NL, Swanson M, Dalton J, Keefe FJ, Engelke M. Partner‐delivered reflexology: effects on cancer pain and anxiety. Oncology Nursing Forum 2007;34(1):127‐32. [DOI] [PubMed] [Google Scholar]
Wall 2000 {published data only}
- Wall L. Changes in hope and power in lung cancer patients who exercise. Nursing Science Quarterly 2000;13(3):234‐42. [DOI] [PubMed] [Google Scholar]
Wilkie 2010 {published data only}
- Wilkie D, Berry D, Cain K, Huang HY, Mekwa J, Lewis F, et al. Effects of coaching patients with lung cancer to report cancer pain. Western Journal of Nursing Research 2010;32(1):23‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Armes 2007 {published data only}
- Armes J, Chalder T, Addington‐Hall J, Richardson A, Hotopf M. A randomized controlled trial to evaluate the effectiveness of a brief, behaviorally oriented intervention for cancer‐related fatigue. Cancer 2007;110(6):1385–95. [DOI] [PubMed] [Google Scholar]
Bakitas 2009 {published data only}
- Bakitas M, Lyons KD, Hegel MT, Balan S, Brokaw FC, Seville J, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA 2009;302(7):741‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Borneman 2010 {published data only}
- Borneman T, Koczywas M, Sun VC, Piper BF, Uman G, Ferrell B. Reducing patient barriers to pain and fatigue management. Journal of Pain and Symptom Management 2010;39(3):486‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2006 {published data only}
- Brown P, Clark MM, Atherton P, Huschka M, Sloan JA, Gamble G, et al. Will improvement in quality of life (QOL) impact fatigue in patients receiving radiation therapy for advanced cancer?. American Journal of Clinical Oncology 2006;29(1):52–8. [DOI] [PubMed] [Google Scholar]
Burnham 2002 {published data only}
- Burnham TR, Wilcox A. Effects of exercise on physiological and psychological variables in cancer survivors. Medicine & Science in Sports & Exercise 2002;34:1863–7. [DOI] [PubMed] [Google Scholar]
Cesario 2007 {published data only}
- Cesario A, Ferri L, Galetta D, Pasqua F, Bonassi S, Clini E, et al. Post‐operative respiratory rehabilitation after lung resection for non‐small cell lung cancer. Lung Cancer 2007;57(2):175‐80. [DOI] [PubMed] [Google Scholar]
Chan 1998 {published data only}
- Chlan L. Effectiveness of a music therapy intervention on relaxation and anxiety for patients receiving ventilatory assistance. Heart and Lung 1998;27(3):169‐76. [DOI] [PubMed] [Google Scholar]
Christman 2004 {published data only}
- Christman NJ, Cain LB. The effects of concrete objective information and relaxation on maintaining usual activity during radiation therapy. Oncology Nursing Forum 2004;31(2):E39‐45. [DOI] [PubMed] [Google Scholar]
Cole 2005 {published data only}
- Cole BS. Spiritually‐focused psychotherapy for people diagnosed with cancer: a pilot outcome study. Mental Health, Religion & Culture 2005;8(3):217‐26. [Google Scholar]
Cunningham 1989 {published data only}
- Cunningham AJ, Tocco EK. A randomized trial of group psychoeducational therapy for cancer‐patients. Patient Education and Counseling 1989;14(2):101–14. [Google Scholar]
Decker 1992 {published data only}
- Decker TW, Cline‐Elsen J, Gallagher M. Relaxation therapy as an adjunct in radiation oncology. Journal of Clinical Psychology 1992;48(3):388‐93. [DOI] [PubMed] [Google Scholar]
de Wit 1997 {published data only}
- Wit R, Dam F, Zandbelt L, Buuren A, Heijden K, Leenhouts G, et al. A pain education program for chronic cancer pain patients: follow‐up results from a randomized controlled trial. Pain 1997;73(1):55–69. [DOI] [PubMed] [Google Scholar]
Dimeo 1997 {published data only}
- Dimeo F, Fetscher S, Lange W, Mertelsmann R, Keul J. Effects of aerobic exercise on the physical performance and incidence of treatment‐related complications after high‐dose chemotherapy. Blood 1997;90:3390–4. [PubMed] [Google Scholar]
Dimeo 1999 {published data only}
- Dimeo FC, Stieglitz RD, Novelli‐Fischer U, Fetscher S, Keul J. Effects of physical activity on the fatigue and psychologic status of cancer patients during chemotherapy. Cancer 1999;85:2273–7. [PubMed] [Google Scholar]
Dimeo 2004 {published data only}
- Dimeo FC, Thomas F, Raabe‐Menssen C, Pröpper F, Mathias M. Effect of aerobic exercise and relaxation training on fatigue and physical performance of cancer patients after surgery. A randomised controlled trial. Support Care Cancer 2004;12(11):774‐9. [DOI] [PubMed] [Google Scholar]
Doorenbos 2006 {published data only}
- Doorenbos A, Given B, Given C, Verbitsky N. Physical functioning: effect of behavioral intervention for symptoms among individuals with cancer. Nursing Research 2006;55(6):161‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Foltz 1987 {published data only}
- Foltz A, Besser P, Ellenberg S, Carty C, Mitchell S, Paul K, et al. Effectiveness of nutritional counseling on caloric intake, weight change, and percent protein intake in patients with advanced colorectal and lung cancer. Nutrition 1987;3(4):263‐71. [Google Scholar]
Forester 1985 {published data only}
- Forester B, Kornfeld DS, Fleiss JL. Psychotherapy during radiotherapy: effects on emotional and physical distress. American Journal of Psychiatry 1985;142(1):22‐7. [DOI] [PubMed] [Google Scholar]
Forester 1993 {published data only}
- Forester B, Kornfeld DS, Fleiss JL, Thompson S. Group psychotherapy during radiotherapy: effects on emotional and physical distress. American Journal of Psychiatry 1993;150(11):1700‐6. [DOI] [PubMed] [Google Scholar]
Given 2002 {published data only}
- Given B, Given CW, McCorkle R, Kozachik S, Cimprich B, Rahbar MH, et al. Pain and fatigue management: results of a nursing randomized clinical trial. Oncology Nursing Forum 2002;29(6):949–56. [DOI] [PubMed] [Google Scholar]
Graham 2003 {published data only}
- Graham PH, Browne L, Cox H, Graham J. Inhalation aromatherapy during radiotherapy: results of a placebo‐controlled double‐blind randomized trial. Journal of Clinical Oncology 2003;21:2372–6. [DOI] [PubMed] [Google Scholar]
Grande 1999 {published data only}
- Grande GE, Todd CJ, Barclay SI, Farquhar MC. Does hospital at home for palliative care facilitate death at home? Randomised controlled trial. BMJ 1999;319(7223):1472‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grande 2000 {published data only}
- Grande GE, Todd CJ, Barclay SI, Farquhar MC. A randomized controlled trial of a hospital at home service for the terminally ill. Palliative Medicine 2000;14(5):375‐85. [DOI] [PubMed] [Google Scholar]
Grealish 2000 {published data only}
- Grealish L, Lomasney A, Whiteman B. Foot massage: a nursing intervention to modify the distressing symptoms of pain and nausea in patients hospitalized with cancer. Cancer Nursing 2000;23:237–43. [DOI] [PubMed] [Google Scholar]
Greer 1992 {published data only}
- Greer S, Moorey S, Baruch JD, Watson M, Robertson BM, Mason A, et al. Adjuvant psychological therapy for patients with cancer: a prospective randomised trial. BMJ 1992;304(6828):675‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holland 1991 {published data only}
- Holland JC, Morrow GR, Schmale A, Derogatis L, Stefanek M, Berenson S, et al. A randomized clinical trial of alprazolam versus progressive muscle relaxation in cancer patients with anxiety and depressive symptoms. Journal of Clinical Oncology 1991;9(6):1004‐11. [DOI] [PubMed] [Google Scholar]
Jacobsen 2002 {published data only}
- Jacobsen PB, Meade CD, Stein KD, Chirikos TN, Small BJ, Ruckdeschel JC. Efficacy and costs of two forms of stress management training for cancer patients undergoing chemotherapy. Journal of Clinical Oncology 2002;20(12):2851‐62. [DOI] [PubMed] [Google Scholar]
Li 2002 {published data only}
- Li Z, Zhang H, Zhang H. Psychological intervention on mental health of perioperative patients with cancer. Chinese Mental Health Journal 2002;16(3):147‐8. [Google Scholar]
McCorkle 1998 {published data only}
- McCorkle R, Robinson L, Nuamah I, Lev E, Benoliel JQ. The effects of home nursing care for patients during terminal illness on the bereaved’s psychological distress. Nursing Research 1998;47(1):2‐10. [DOI] [PubMed] [Google Scholar]
Oh 2008 {published data only}
- Oh B, Butow P, Mullan B, Clarke S. Medical qigong for cancer patients: pilot study of impact on quality of life, side effects of treatment and inflammation. American Journal of Chinese Medicine 2008;36(3):459–72. [DOI] [PubMed] [Google Scholar]
Ovesen 1993 {published data only}
- Ovesen L, Allingstrup L, Hannibal J, Mortensen E, Hansen O. Effect of dietary counseling on food intake, body weight, response rate, survival, and quality of life in cancer patients undergoing chemotherapy: a prospective, randomized study. Journal of Clinical Oncology 1993;11(10):2043‐9. [DOI] [PubMed] [Google Scholar]
Parker 1998 {published data only}
- Parker L, Walker J. Effects of a pulmonary rehabilitation program on physiologic measures, quality of life, and resource utilization in a health maintenance organization setting. Respiratory Care 1998;43(3):177‐82. [Google Scholar]
Parvez 2007 {published data only}
- Parvez T, Alharbi TM, Mein FD. Impact of group psychotherapy in chemotherapy induced vomiting for treatment of advanced breast and lungs cancer. Journal of the College of Physicians and Surgeons ‐ Pakistan 2007;17(2):89‐93. [PubMed] [Google Scholar]
Ramsay 2007 {published data only}
- Ramsay K, Ramsay J, Main D. Both group peer counselling and individual counselling reduce anxiety and depression, and increase self‐esteem and overall life satisfaction in palliative cancer care. Counselling Psychology Quarterly 2007;20(2):157‐67. [Google Scholar]
Rawl 2002 {published data only}
- Rawl S, Given B, Given C, Champion V, Kozachik S, Barton D, el al. Intervention to improve psychological functioning for newly diagnosed patients with lung cancer. Oncology Nursing Forum 2002;29(6):967‐75. [DOI] [PubMed] [Google Scholar]
Ream 2006 {published data only}
- Ream E, RichardsonA, Alexander‐Dann C. Supportive intervention for fatigue in patients undergoing chemotherapy: a randomized controlled trial. Journal of Pain and Symptom Management 2006;31(2):148–61. [DOI] [PubMed] [Google Scholar]
Rosenbloom 2007 {published data only}
- Rosenbloom SK, Victorson DE, Hahn EA, Peterman AH, Cella D. Assessment is not enough: a randomized controlled trial of the effects of HRQL assessment on quality of life and satisfaction in oncology clinical practice. Psychooncology 2007;16(12):1069‐79. [DOI] [PubMed] [Google Scholar]
Schiffman 2007 {published data only}
- Schiffman SS, Sattely‐Miller EA, Taylor EL, Graham BG, Landerman LR, Zervakis J, et al. Combination of flavor enhancement and chemosensory education improves nutritional status in older cancer patients. Journal of Nutrition, Health & Aging 2007;11(5):439‐54. [PubMed] [Google Scholar]
Schneider 2007 {published data only}
- Schneider SM, Hood LE. Virtual reality: a distraction intervention for chemotherapy. Oncology Nursing Forum 2007;34(1):39‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skrutkowski 2008 {published data only}
- Skrutkowski M, Saucier A, Eades M, Swidzinski M, Ritchie J, Marchionni C, et al. Impact of a pivot nurse in oncology on patients with lung or breast cancer: symptom distress, fatigue, quality of life, and use of healthcare resources. Oncology Nursing Forum 2008;35(6):948‐54. [DOI] [PubMed] [Google Scholar]
Soden 2004 {published data only}
- Soden K, Vincent K, Craske S, Lucas C, Ashley S. A randomized controlled trial of aromatherapy massage in a hospice setting. Palliative Medicine 2004;18(2):87‐92. [DOI] [PubMed] [Google Scholar]
Speca 2000 {published data only}
- Speca M, Carlson LE, Goodey E, Angen M. A randomized, wait‐list controlled clinical trial: the effect of a mindfulness meditation‐based stress reduction program on mood and symptoms of stress in cancer outpatients. Psychosomatic Medicine 2000;62:613–22. [DOI] [PubMed] [Google Scholar]
Steinhauser 2008 {published data only}
- Steinhauser KE, Alexander SC, Byock IR, George LK, Olsen MK, Tulsky JA, et al. Do preparation and life completion discussions improve functioning and quality of life in seriously ill patients? Pilot randomized control trial. Journal of Palliative Medicine 2008;11(9):1234‐40. [DOI] [PubMed] [Google Scholar]
Weinrich 1990 {published data only}
- Weinrich SP, Weinrich MC. The effect of massage on pain in cancer patients. Applied Nursing Research 1990;3(4):140‐5. [DOI] [PubMed] [Google Scholar]
Wilkie 2000 {published data only}
- Wilkie D, Kampbell J, Cutshall S, Halabinsky H, Harmon H, Johnson L, et al. Effects of massage on pain intensity, analgesics and quality of life in patients with cancer pain: a pilot study of a randomized clinical trial conducted within hospice care delivery. The Hospice Journal 2000;15(3):31‐53. [PubMed] [Google Scholar]
Wyatt 2007 {published data only}
- Wyatt G, Sikorskii A, Siddiqi A, Given CW. Feasibility of a reflexology and guided imagery intervention during chemotherapy: results of a quasi‐experimental study. Oncology Nursing Forum 2007;34(3):635‐42. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Cai 2001 {published data only}
- Cai GR, Li PW, Jiao LP. Clinical observation of music therapy combined with anti‐tumor drugs in treating 116 cases of tumor patients. Zhongguo Zhong Xi Yi Jie He Za Zhi 2001;21(12):891‐4. [PubMed] [Google Scholar]
Wang 2005 {published data only}
- Wang Y, Yao JF, Yang JY. Effect of rehabilitation exercises on the recovery outcomes of lung function in postoperative patients with lung cancer. Chinese Journal of Clinical Rehabilitation 2005;9(24):66‐8. [Google Scholar]
Wu 2003 {published data only}
- Wu L, Wang SJ. Psychotherapy improving depression and anxiety of patients treated with chemotherapy combined with radiotherapy. Chinese Journal of Clinical Rehabilitation 2003;7(17):2462‐3. [Google Scholar]
Zhen 2002 {published data only}
- Zhen L, Hongmei Z, Hongya Z. Psychological intervention on mental health of perioperative patients with cancer. Chinese Mental Health Journal 2002;16(3):147‐8. [Google Scholar]
References to ongoing studies
Jones 2010 {published data only}
- Jones LW, Eves ND, Kraus WE, Potti A, Crawford J, Blumenthal JA, et al. The lung cancer exercise training study: a randomized trial of aerobic training, resistance training, or both in postsurgical lung cancer patients: rationale and design. BMC Cancer 2010;10:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Ahmed 2004
- Ahmed N, Ahmedzai S, Vora V, Hilam S, Paz S. Supportive care for patients with gastrointestinal cancer. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD003445.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Benoliel 1980
- Benoliel JQ, McCorkle R, Young K. The development of a social dependency scale. Research in Nursing & Health 1980;3:3‐10. [DOI] [PubMed] [Google Scholar]
Cantril 1965
- Cantril H. The pattern of human concerns. New Brunswick, NJ: Rutgers University Press, 1965. [Google Scholar]
Cartmell 2000
- Cartmell R, Coles A. Informed choice in cancer pain: empowering the patient. British Journal of Community Nursing 2000;5:560,562‐4. [DOI] [PubMed] [Google Scholar]
Directive 2001/83/EC
- Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use. Official Journal L 28/11/2004; Vol. 311:67‐128.
Fauci 1998
- Fauci AS, Braunwald E, Isselbacher KJ, Wilson JD, Martin JB, Kasper DL, et al (editors). Harrison's Principles of Internal Medicine. 14th Edition. NY: McGraw‐Hill, 1998. [Google Scholar]
Fawzy 1999
- Fawzy FI. Psychosocial interventions for patients with cancer: what works and what doesn't. European Journal of Cancer 1999;35(11):1559‐64. [DOI] [PubMed] [Google Scholar]
GLOBOCAN 2008
- International Agency for Research on Cancer. GLOBOCAN 2008. Cancer incidence, mortality and prevalence worldwide. http://globocan.iarc.fr/ (accessed 10 March 2011).
Godlovitch 2003
- Godlovitch G. Age discrimination in trials and treatment: old dogs and new tricks. Monash Bioethics Review 2003;22:66‐77. [DOI] [PubMed] [Google Scholar]
Herth 1992
- Herth K. Abbreviated instrument to measure hope: development and psychometric evaluation. Journal of Advanced Nursing 1992;17:1251‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org. Available from www.cochrane‐handbook.org.
Higginson 1999
- Higginson IJ. Evidence based palliative care. BMJ 1999;319:462‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jané 2002
- Jané M, Salto E, Pardell H, Tresserras R, Guayta R, Taberner JL, et al. Smoking prevalence in Catalonia (Spain), 1982‐1998: a gender perspective [Prevalencia del tabaquismo en Cataluña, 1982‐1998: una perspectiva de género]. Medicina Clínica 2002;118(3):81‐5. [DOI] [PubMed] [Google Scholar]
Jemal 2011
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: A Cancer Journal for Clinicians 2011;61(2):69‐90. [DOI] [PubMed] [Google Scholar]
Johnson 2003
- Johnson M, Moore S. Research into practice: the reality of implementing a non‐pharmacological breathlessness intervention into clinical practice. European Journal of Oncology Nursing 2003;7:33‐8. [DOI] [PubMed] [Google Scholar]
Karnofsky 1949
- Karnofsky DA, Burchenal JH. Clinical evaluation of chemotherapeutic agents in cancer. In: CM MacLeod editor(s). Evaluation of chemotherapeutic agents. New York: Columbia Press, 1949:191‐205. [Google Scholar]
Krishnasamy 2001
- Krishnasamy M, Corner J, Bredin M, Plant H, Bailey C. Cancer nursing practice development: understanding breathlessness. Journal of Clinical Nursing 2001;10(1):103‐8. [DOI] [PubMed] [Google Scholar]
Linn 1967
- Linn MW. A rapid disability rating scale. Journal of the American Geriatric Society 1967;15:211‐4. [DOI] [PubMed] [Google Scholar]
Marcel 1978
- Marcel G. Homo viator: introduction to a metaphysic of hope. Gloucester, UK: Peter Smith, 1978. [Google Scholar]
MCA 2002
- Medicines Control Agency. A guide to what is a medicinal product. MCA Guidance Note no. 8. London: Medicines Control Agency, 2002. [Google Scholar]
McCorkle 1978
- McCorkle R, Young K. Development of symptom distress scale. Cancer Nursing 1978;1:373‐8. [PubMed] [Google Scholar]
McGeer 1990
- McGeer AJ, Detsky AS, O'Rourke K. Parenteral nutrition in cancer patients undergoing chemotherapy: a meta‐analysis. Nutrition 1990;6:475‐83. [PubMed] [Google Scholar]
McNair 1971
- McNair D, Lorr M, Dropplemen L. Edits Manual: Profile of Mood States. San Diego: Educational and industrial testing services, 1971. [Google Scholar]
Melzack 1987
- Melzack R. The short form McGill Pain Questionnaire: major properties and scoring methods. Pain 1987;30:191‐7. [DOI] [PubMed] [Google Scholar]
Petticrew 2001
- Petticrew M. Systematic reviews from astronomy to zoology: myths and misconceptions. BMJ 2001;322(7278):98‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rogers 1986
- Rogers ME. Science of unitary human beings. In: Malinski, VM editor(s). Explorations on Martha Rogers’ Science of Unitary Human Beings. Norwalk, CT: Appleton‐Century‐Crofts, 1986:1‐8. [Google Scholar]
Rotter 1966
- Rotter JB. Generalised expectancies for internal versus external control of reinforcement. Psychological Monographs 1966;80:1‐28. [PubMed] [Google Scholar]
Sethi 1997
- Sethi T. Science, medicine, and the future: lung cancer. BMJ 1997;314:652‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sherwood 1965
- Sherwood JJ. Self‐identity and referents others. Sociometry 1965;28:66‐81. [PubMed] [Google Scholar]
Stewart 1992
- Stewart AL, Kamberg CJ. Physical functioning measures. In: Stewart AL, Ware JE editor(s). Measuring Functioning and Well‐Being: The Medical Outcomes Study Approach. Durham, NC: Duke University Press, 1992:86–101. [Google Scholar]
Strole 1956
- Strole L. Social integration and certain corollaries: an exploratory study. American Sociological Review 1956;21:709‐16. [Google Scholar]
Ware 1976
- Ware JE. Scales for measuring general health perceptions. Health Services Research 1976;11:396‐415. [PMC free article] [PubMed] [Google Scholar]
Weisman 1977
- Weisman AD, Worden JW. Coping and vulnerability in cancer patients. Project Omega. Boston: Harvard Medical School, 1977:37‐40. [Google Scholar]
WHO 1990
- World Health Organization. Cancer pain relief and palliative care. Technical Report Series no. 804. Genève: World Health Organization, 1990. [PubMed] [Google Scholar]
WHO 1993
- World Health Organization. Study protocol for the World Health Organization project to develop a Quality of Life assessment instrument (WHOQOL). Quality of Life Research 1993;2:153‐9. [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67:361‐70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Solà 2004
- Solà I, Thompson EM, Subirana Casacuberta M, Lopez C, Pascual A. Non‐invasive interventions for improving well‐being and quality of life in patients with lung cancer. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004282.pub2] [DOI] [PubMed] [Google Scholar]