Abstract
Background—
Adult congenital heart disease (ACHD) patients can benefit from subcutaneous implantable cardioverter-defibrillator (S-ICD).
Objective—
The goals of this study were: assess left-and right-sided S-ICD eligibility in ACHD patients, use machine learning to predict S-ICD eligibility in ACHD patients, and transform 12-lead ECG to S-ICD 3-lead ECG, and vice versa.
Methods—
ACHD outpatients (n=101; age 42±14 y; 52% female; 85% white; left ventricular ejection fraction (LVEF) 56±9%) were enrolled in a prospective study. Supine and standing 12-lead ECG were recorded simultaneously with a right- and left-sided S-ICD 3-lead ECG. Peak-to-peak QRS and T amplitudes, RR, PR, QT, QTc, QRS intervals, Tmax, and R/Tmax (31 predictor variables) were tested. Model selection, training, and testing were performed using supine ECG datasets. Validation was performed using standing ECG datasets and out-of-sample non-ACHD population (n=68; age 54±16 y; 54% female; 94% white; LVEF 61±8%).
Results—
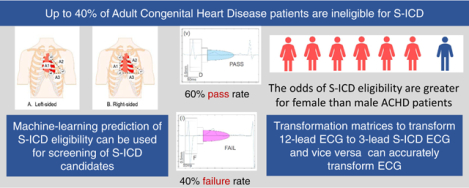
40% of participants were ineligible for S-ICD. Tetralogy of Fallot patients passed right-sided screening (57%) more often than left-sided screening (21%; McNemar’s χ2 P=0.025). Female participants had greater odds of eligibility (adjusted OR 5.9 (95%CI 1.6–21.7); P=0.008). Validation of the ridge models was satisfactory for standing left-sided [ROC AUC 0.687 (95%CI 0.582–0.791)] and right-sided [ROC AUC 0.655(95%CI 0.549–0.762)] S-ICD eligibility prediction. Validation of transformation matrices showed satisfactory agreement (<0.1 mV difference).
Conclusion—
Nearly half of the contemporary ACHD population is ineligible for S-ICD. The odds of S-ICD eligibility are greater for female than male ACHD patients. Machine-learning prediction of S-ICD eligibility can be used for screening of S-ICD candidates.
Keywords: subcutaneous ICD, electrocardiogram, eligibility
Graphical Abstract
Introduction
A Subcutaneous Implantable Cardioverter-Defibrillator (S-ICD) is a life-saving device that prevents sudden cardiac arrest in vulnerable patients.1 Approval of the S-ICD for use in the United States is significant because of the benefits it has over the traditional, transvenous ICD, which include the lack of risk for vascular occlusion, systemic infection, and adverse effects of lead extraction. S-ICD can be especially advantageous in adults with congenital heart disease (ACHD) patients who may have limited or no venous access to the heart, and in whom there is increased risk of systemic embolism when a persistent shunt is present.2, 3 These individuals are often at increased risk for sudden cardiac arrest that is higher in ACHD compared to the general population4 and frequently require thoracic surgery to place an epicardial ICD system. ACHD patients may face multiple generator changes in their lifetime, making an S-ICD a viable option due to its less-invasive placement. The 2017 AHA/ACC/HRS Guidelines5 for the prevention of sudden cardiac arrest in ACHD patients recommend S-ICD use when feasible.
S-ICD requires electrocardiographic (ECG) pre-screening before implantation to assess sensing. S-ICD pre-screening involves recording a special 3-lead ECG with ECG electrodes placed in the locations of S-ICD sensing electrodes.6 This additional step may negatively impact the utilization of S-ICD.7 Lack of confidence is the most common barrier for referral8 among physicians, and the perceived strength of the physician recommendation is the most common theme associated with ICD refusal among primary prevention candidates.9 Conversely, a 12-lead ECG is readily available and easy to obtain. Therefore, using a conventional 12-lead ECG as the tool for pre-screening eligibility would greatly improve a physician’s confidence in referral to an electrophysiologist and recommendation to suitable patients.
Our group recently developed a screening tool to predict left-sided S-ICD eligibility from a 12-lead ECG.10 Though, validation of this screening tool in an out-of-sample population has not been performed. Moreover, in ACHD patients, right-sided S-ICD implantation may improve S-ICD eligibility.11 However, very little data is available regarding right-sided S-ICD eligibility predictors in ACHD patients. Furthermore, it remains unknown whether it is feasible to transform the 12-lead ECG into left-and right-sided S-ICD 3-lead ECG, and vice versa.
We conducted this study with several goals: (1) assess left- and right-sided S-ICD eligibility in ACHD patients, (2) validate the previous10 left-sided S-ICD eligibility prediction tool, (3) use machine learning to predict right- and left-sided S-ICD eligibility in ACHD patients, and (4) develop and validate transformation matrices to transform 12-lead ECG to S-ICD 3-lead ECG, and vice versa.
Methods
MATLAB (MathWorks, Inc, Natick, MA) open-source code for ECG analyses, a user manual, and fully de-identified raw digital ECG signal data are provided at https://github.com/Tereshchenkolab/S-ICD_eligibility.
Study population
We conducted a prospective cross-sectional study at the Oregon Health & Science University (OHSU). The Institutional Review Board approved the study, and all participants signed informed consent before entering the study. Eligible adult patients that had been previously diagnosed with ACHD were invited to participate during a scheduled appointment with their cardiologist. Inclusion criteria were: (1) known congenital heart defect followed at the OHSU ACHD clinic, (2) age ≥ 18 years, (3) ability to stand on their own for the duration of ECG recording. Exclusion criteria were: (1) acute medical condition, (2) life expectancy less than one year due to a non-cardiac condition and (3) developmental delay.
Study participants were grouped based on the complexity of ACHD anatomy and physiology as described in the 2019 ACHD AP Classification12: simple (IA-B), moderate (IIB-C), or severe complexity (IIIC-D).
For out-of-sample validation of the machine learning models, we used the data of our previous S-ICD eligibility study,10 which enrolled a widely generalizable sample of the OHSU outpatient population, with a broad range of age (18–81 y), body mass index (BMI; 19–53 kg/m2), QRS duration (66–150 ms), and left ventricular ejection fraction (LVEF; 37–77%).
Assessment of S-ICD eligibility
Details of ECG recording (Figure 1), analysis, and anthropometric measurements are described in the Supplement.
Figure 1.
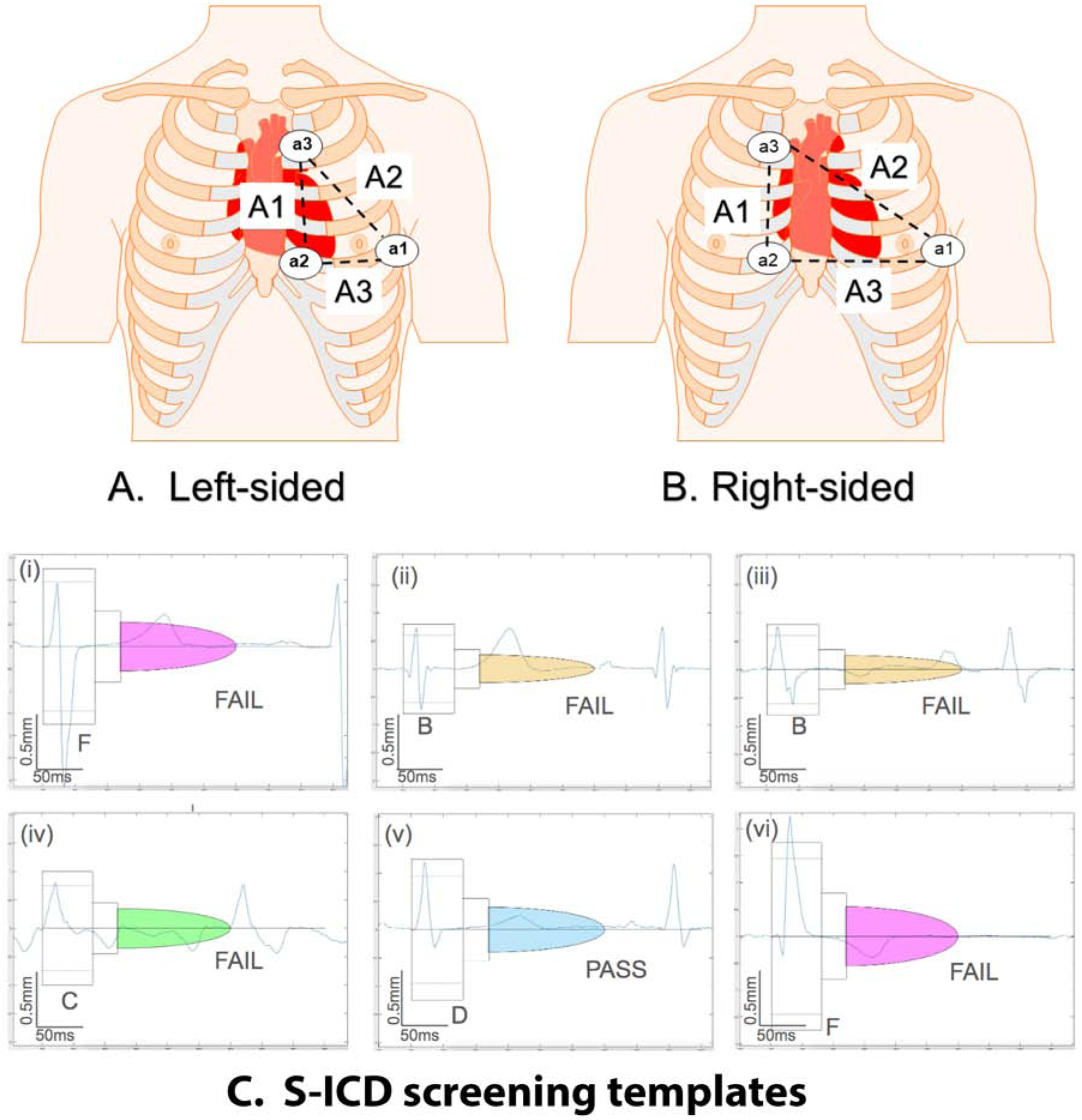
Left-sided (A) and right-sided (B) placement of a1, a2, and a3 electrodes for the 3-lead ECG to mimic the leads A1 (a2-a3), A2 (a1-a3), and A3 (a1-a2) sensing vectors of the S-ICD. (C) Representative examples of S-ICD screening template passing and failing ECG morphologies
Bipolar S-ICD leads were derived from recorded unipolar a1, a2, and a3 leads by subtraction as follows: Bipolar lead A1 = a2 – a3, Bipolar lead A2 = a1 – a3, Bipolar lead A3 = a1 – a2. Digital bipolar 3-lead left- and right-sided ECG morphologies in standing and supine position were evaluated using a digitized version of the Boston Scientific EMBLEM S-ICD Patient Screening tool6 by at least two investigators (AR, NJ, LW). A viewer for digital S-ICD eligibility assessment (Supplemental Figure 1) was developed by the investigators (NJ, KTH, AR). In the case of disagreement, the 3rd investigator (LGT) made the final determination. A sensing vector passed screening if maximum QRS amplitudes crossed the dotted line and all QRS complexes and T waves fit within a profile in all beats, in both standing and supine 10-second recording at 5–20 mm/mV gain, either on the left or right side. If applicable, the reasons for failure (high T-wave, high R-wave, deep S-wave, small QRS complex, high P, or flutter F-wave) were recorded.
Statistical analyses
After confirmation of normality, continuous variables were reported as mean ± standard deviation (SD) and compared using the t-test. The χ2 test was used to compare categorical variables. Paired t-testing was used to compare ECG measurements on the left and right side, standing, and supine. McNemar’s χ2 statistic was used for paired comparison of S-ICD ineligibility causes in different positions (standing, supine) on the left and right side.
To determine whether sex is associated with S-ICD eligibility, we constructed logistic regression, adjusted for age, race, BMI, upper and lower chest circumference, ACHD complexity, history of Fontan palliation, smoking, and use of drugs targeting hemodynamic improvement (angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, angiotensin receptor-neprilysin inhibitor, aldosterone antagonists, vasodilators, or diuretics).
Validation of the previous left-sided S-ICD eligibility tool
Accuracy of our previously developed left-sided S-ICD eligibility prediction tool10 was validated using the entire study population. We measured Area Under the Receiver Operating Characteristic Curve (ROC AUC), and calculated sensitivity and specificity of the previously defined threshold (pass if ≥ 0).
Machine learning model selection, training, testing, and validation
We applied a machine learning technique (Figure 2) to develop the prediction of left-sided and right-sided S-ICD eligibility. Supine ECG datasets were used for machine learning (training and testing), whereas standing ECG datasets and the data of our previous S-ICD eligibility study10 were used for validation. We compared logistic regression, lasso, elastic net, and ridge models in four machine learning steps, as described in the Supplement.
Figure 2.
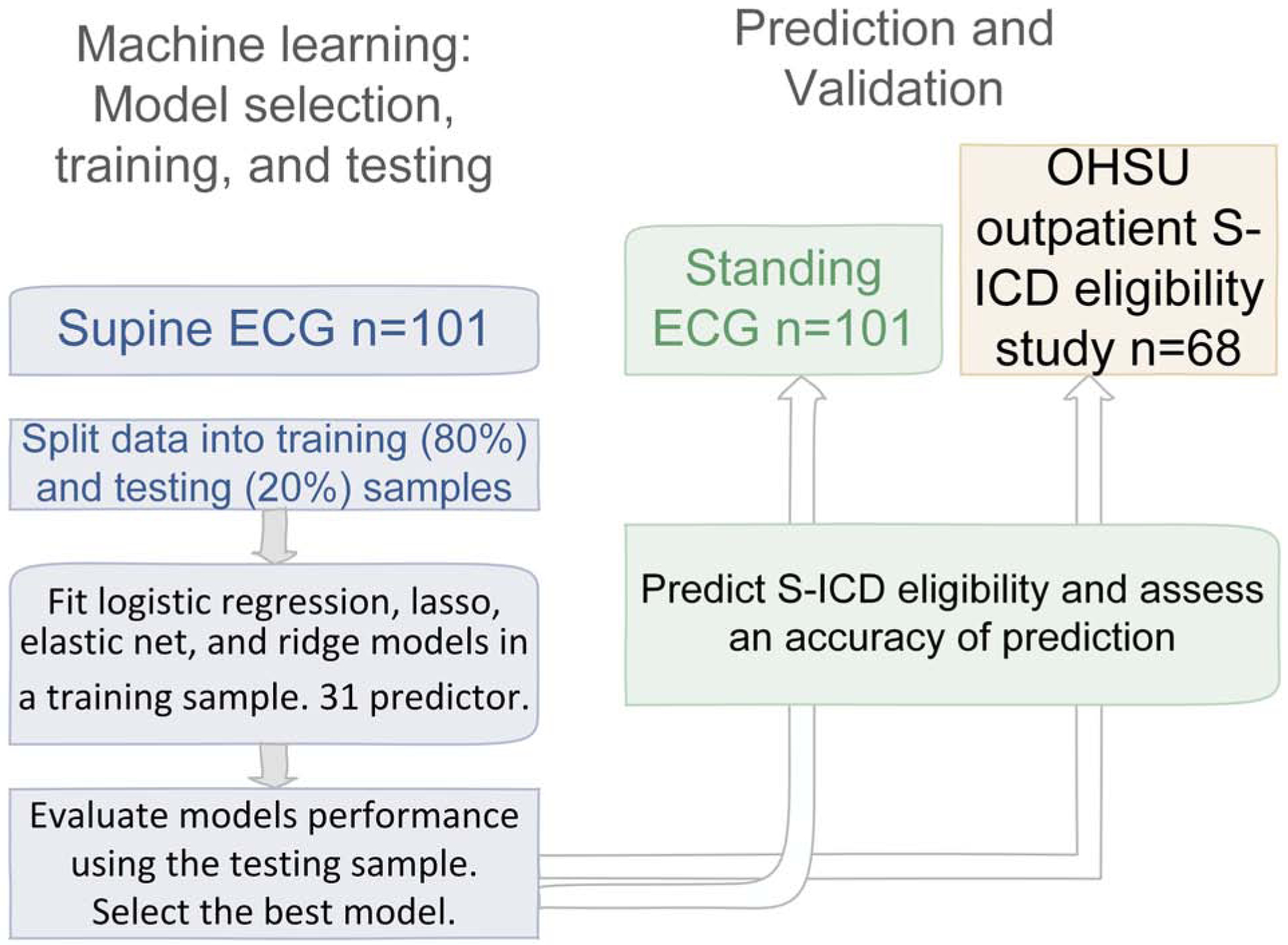
Machine learning steps: S-ICD eligibility prediction development, and validation.
Development and validation of transformation matrices is described in the Supplement.
Statistical analysis was performed using STATA MP 16.0 (StataCorp LP, College Station, TX). P-value < 0.05 was considered statistically significant.
Results
Study population
A total of 101 ACHD patients were recruited (Table 1). Most of the study participants had moderate or severe complexity ACHD with hemodynamic impairment and on average, borderline systemic ventricular function. Participants had a history of Fontan, Ross, Mustard, Senning, Rastelli, Glenn, Damus-Kaye-Sensel, and Norwood procedures. Nearly every fifth study participant already had a transvenous cardiac device implanted: more likely an ICD (65%) than a pacemaker (35%). Approximately two-thirds of participants (68%) were currently taking cardiovascular medications (Table 1), and nearly half were taking antiarrhythmic medications (beta-blockers, calcium channel blockers, sotalol, amiodarone, dofetilide, or digoxin). Almost half of the study population was on anticoagulants or antiplatelet drugs. More than half of the population took drugs targeting hemodynamic improvement.
Table 1.
Clinical characteristics of the study participants
| Characteristic | All (n=101) | Fail all (n=40) | Pass L or R (n=61) | P-value | |
|---|---|---|---|---|---|
| Age (SD), y | 41.5(14.2) | 41.0(36.1) | 41.9(13.7) | 0.763 | |
| Female, n(%) | 52(52) | 13(32.5) | 39(63.9) | 0.002 | |
| White, n(%) | 86(85) | 34(85) | 52(85) | 0.973 | |
| Height (SD), m | 1.70(0.10) | 1.70(0.10) | 1.70(0.10) | 0.260 | |
| Weight (SD), kg | 82.7(24.4) | 80.1(21.8) | 84.4(26.0) | 0.369 | |
| Body mass Index, kg/m2 | 28.9(7.9) | 27.6(6.9) | 29.7(8.4) | 0.163 | |
| Barrel shaped chest, n(%) | 19(19.6) | 8(20) | 11(19) | 0.981 | |
| Upper chest circumference (SD), cm | 99.9(14.0) | 99.3(13.4) | 100.3(14.5) | 0.749 | |
| Lower chest circumference (SD), cm | 100.9(15.3) | 98.8(13.8) | 102.4(16.1) | 0.245 | |
| Waist-to-Hip ratio(SD) | 0.89(0.10) | 0.90(0.1) | 0.88(0.11) | 0.356 | |
| Congenital heart disease complexity | simple | 15(14.9) | 6(15) | 9(15) | |
| moderate | 47(46.5) | 18(45) | 29(48) | 0.967 | |
| complex/severe | 39(38.6) | 16(40) | 23(38) | ||
| LVEF(SD), % | 56.4(9.2) | 53.4(11.3) | 58.3(7.1) | 0.067 | |
| Tetralogy of Fallot, n(%) | 16(15.8) | 8(20) | 8(13) | 0.354 | |
| History of Fontan procedure, n(%) | 10(10) | 7(18) | 3(5) | 0.038 | |
| Transposition of great arteries, n(%) | 16(15.8) | 5(13) | 11(18) | 0.456 | |
| Cardiac device implanted, n(%) | 17(18) | 8(21) | 9(16) | 0.526 | |
| Implantable cardioverter-defibrillator, n(%) | 11(11) | 6(15) | 5(8) | 0.547 | |
| Pacemaker, n(%) | 6(6) | 2(5) | 4(7) | ||
| Ventricular pacing during the study, n(%) | 10(10) | 4(10) | 6(10) | 0.978 | |
| Taking cardiovascular medications, n(%) | 68(67) | 27(68) | 41(67) | 0.976 | |
| ACEi/ARB/AA/vasodilator/diuretics, n(%) | 53(53) | 24(60) | 29(48) | 0.220 | |
| Antiarrhythmic drugs, n(%) | 48(48) | 18(45) | 30(49) | 0.681 | |
| Antiplatelet/anticoagulant, n(%) | 50(50) | 20(50) | 30(50) | 0.936 | |
| Current or past smoker, n(%) | 25(25) | 13(33) | 12(20) | 0.144 | |
| Mean heart rate(SD), bpm | 69.7(11.7) | 71.7(14.0) | 68.7(11.7) | 0.271 | |
| PR interval(SD), ms | 205.8(94.6) | 200.9(87.7) | 209.0(99.5) | 0.670 | |
| QRS duration(SD), ms | 127.0(34.5) | 126.0(30.6) | 127.7(37.1) | 0.802 | |
| QTc interval(SD), ms | 462.8(38.9) | 456.3(33.5) | 467.0(41.7) | 0.158 | |
Assessment of S-ICD eligibility
There were 61 participants (60%) that passed either left- or right-sided screening, whereas the remainder of participants (40%) were deemed non-eligible for S-ICD. Ineligible participants were more likely to be males or have had a Fontan palliation. There was a trend towards lower LVEF, the use of medications for heart failure treatment, history of past or current smoking, and lower BMI in those who failed ECG screening (Table 1). No difference in ACHD complexity or ventricular pacing was observed between those who passed versus failed screening. After adjustment for demographic and anthropometric characteristics, BMI, smoking, ACHD complexity and the use of drugs targeting hemodynamic improvement, the odds of S-ICD eligibility was greater for female as compared to male ACHD patients [odds ratio 5.9 (95%CI 1.6–21.7); P=0.008].
Overall, a similar percentage of participants was eligible for right-sided (n=49; 49%) and left-sided S-ICD (n=45; 45%; McNemar’s χ2 P=0.450). Only a third of participants (n=33; 33%) passed both left- and right-sided screening, whereas 12 (12%) passed only left-sided, and 16 (16%) passed only right-sided screening. Tetralogy of Fallot patients passed right-sided screening (8/16) more often than left-sided (3/16; McNemar’s χ2 P=0.025). Similarly, taken together Tetralogy of Fallot and Fontan procedure patients (Figure 3) passed right-sided screening more often than left-sided (McNemar’s χ2 P=0.014). No anthropometric characteristics were associated with differences in either left- or right-sided S-ICD eligibility.
Figure 3.
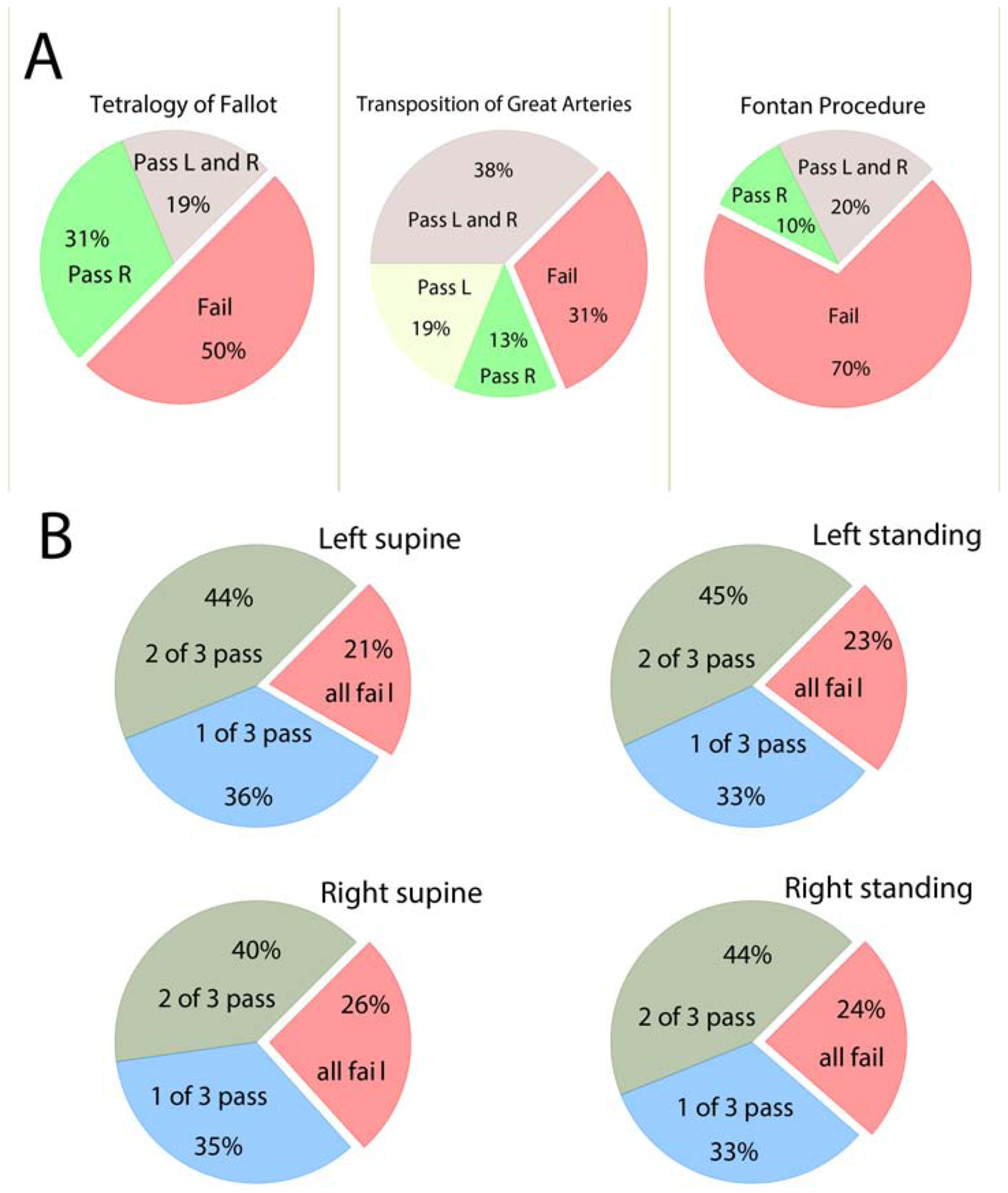
A. The proportion of patients with transposition of great arteries, Tetralogy of Fallot, and Fontan procedure with passing and failing for right (R)- and left (L)-sided sensing vectors. B. The proportion of study participants who failed all three vectors or passed 1–2 left- and right-sided vectors standing and supine.
No participants had all 3 S-ICD vectors with eligible ECG morphologies (Figure 1). In any position and any side, less than half of the participants (40–45%) had two admissible S-ICD vectors, whereas nearly a quarter of participants failed all three vectors (Figure 3).
Overall, little difference was observed in eligibility of ECG morphologies in different positions. The rates of pass/fail across complexity groups were similar for both right and left-sided vectors, either standing or supine (Figure 3). Change of the body position from supine to standing led to a slight heart rate increase, QTc lengthening, and QRS shortening (Table 2). S-ICD ineligibility due to large P (or F) waves was more likely in the left standing position than in the left supine position. S-ICD ineligibility due to a small QRS was more likely on the right side, in both supine and standing positions (Table 2).
Table 2.
Comparison of ECG measurement and causes of S-ICD ineligibility for left- and right-sided ECG, standing and supine.
| Causes | Supine Left-sided | Standing Left-sided | P Left Sup-stand | Supine Right-sided | Standing Right-sided | P Right sup-stand | PL-R supine | PL-R standing |
|---|---|---|---|---|---|---|---|---|
| Mean heart rate(SD), bpm | 70.0(12.7) | 76.1(13.6) | <0.0001 | 70.4(13.3) | 76.1(13.2) | <0.0001 | 0.070 | 0.687 |
| Mean QTc(SD), ms | 463.3(38.8) | 471.5(40.6) | <0.0001 | 461.5(40.7) | 471.3(39.2) | <0.0001 | 0.517 | 0.920 |
| Mean QRS(SD), ms | 127.1(34.7) | 120.5(34.7) | <0.0001 | 127.0(34.4) | 122.7(34.5) | 0.0001 | 0.918 | 0.080 |
| Mean PR(SD), ms | 206.3(94.9) | 211.9(107.6) | 0.399 | 211.1(95.1) | 208.7(106.2) | 0.702 | 0.344 | 0.617 |
| High P or F waves, n(%) | 5(5) | 11(11) | 0.034 | 6(6) | 10(10) | 0.248 | 0.739 | 0.739 |
| High R, n(%) | 52(52) | 54(54) | 0.637 | 45(45) | 54(54) | 0.078 | 0.108 | 1.00 |
| Deep S, n(%) | 19(19) | 20(20) | 0.763 | 22(22) | 23(23) | 0.782 | 0.467 | 0.467 |
| High T, n(%) | 61(60) | 54(54) | 0.127 | 54(54) | 48(48) | 0.083 | 0.178 | 0.289 |
| Small QRS, n(%) | 26(26) | 25(25) | 0.827 | 45(45) | 40(40) | 0.251 | 0.002 | 0.003 |
Validation of the S-ICD eligibility prediction tool
Validation ROC AUC for our previous S-ICD eligibility tool10 was unsatisfactory (0.551; 95%CI 0.493 – 0.608). The sensitivity of the pre-defined threshold (≥ 0)10 was 73%, and specificity was 35%.
Machine-learning prediction of S-ICD eligibility
Selection of the best models was performed using the supine ECG datasets. A comparison of the prediction models’ performance using testing supine ECG samples is shown in Table 3 and Figure 4. For the left-sided S-ICD eligibility prediction, the ridge model demonstrated the smallest deviance, and the largest deviance ratio, which characterizes the best cross-validation function. The elastic net model was the 2nd best, closely followed by lasso. Logistic regression showed the worst out-of-sample cross-validation function for both left-sided and right-sided prediction. Ridge and logistic regression models included all predictor variables, whereas lasso selected only four predictors (HR, QT interval, Tmax, and TV1 amplitude), and elastic net – only five predictors (HR, QT interval, Tmax, TV1, and peak-to-peak QRSV3 amplitudes). Lasso and elastic net prediction model equations are provided in the Supplement. Lasso score ≥ − 0.5 predicted left-sided S-ICD eligibility with 91% sensitivity and 30% specificity. Elastic net score ≥ − 0.5 predicted left-sided S-ICD eligibility with 96% sensitivity and 10% specificity. We provided free calculators: http://www.ecgpredictscd.org/sicd-eligibility.
Table 3.
Machine learning model selection using supine ECG datasets
| Left-sided | Right-sided | |||||
|---|---|---|---|---|---|---|
| Model | Sample | N | Deviance | Deviance ratio | Deviance | Deviance ratio |
| Logistic | Training | 81 | 0.881 | 0.364 | 0.763 | 0.445 |
| Testing | 20 | 2.835 | −1.321 | 5.395 | −3.008 | |
| Lasso | Training | 81 | 1.255 | 0.094 | 1.379 | 0 |
| Testing | 20 | 1.360 | −0.114 | 1.428 | −0.061 | |
| Elastic net | Training | 81 | 1.269 | 0.084 | 1.379 | 0 |
| Testing | 20 | 1.359 | −0.113 | 1.428 | −0.061 | |
| Ridge | Training | 81 | 1.315 | 0.050 | 1.300 | 0.057 |
| Testing | 20 | 1.350 | −0.105 | 1.436 | −0.067 | |
Figure 4.
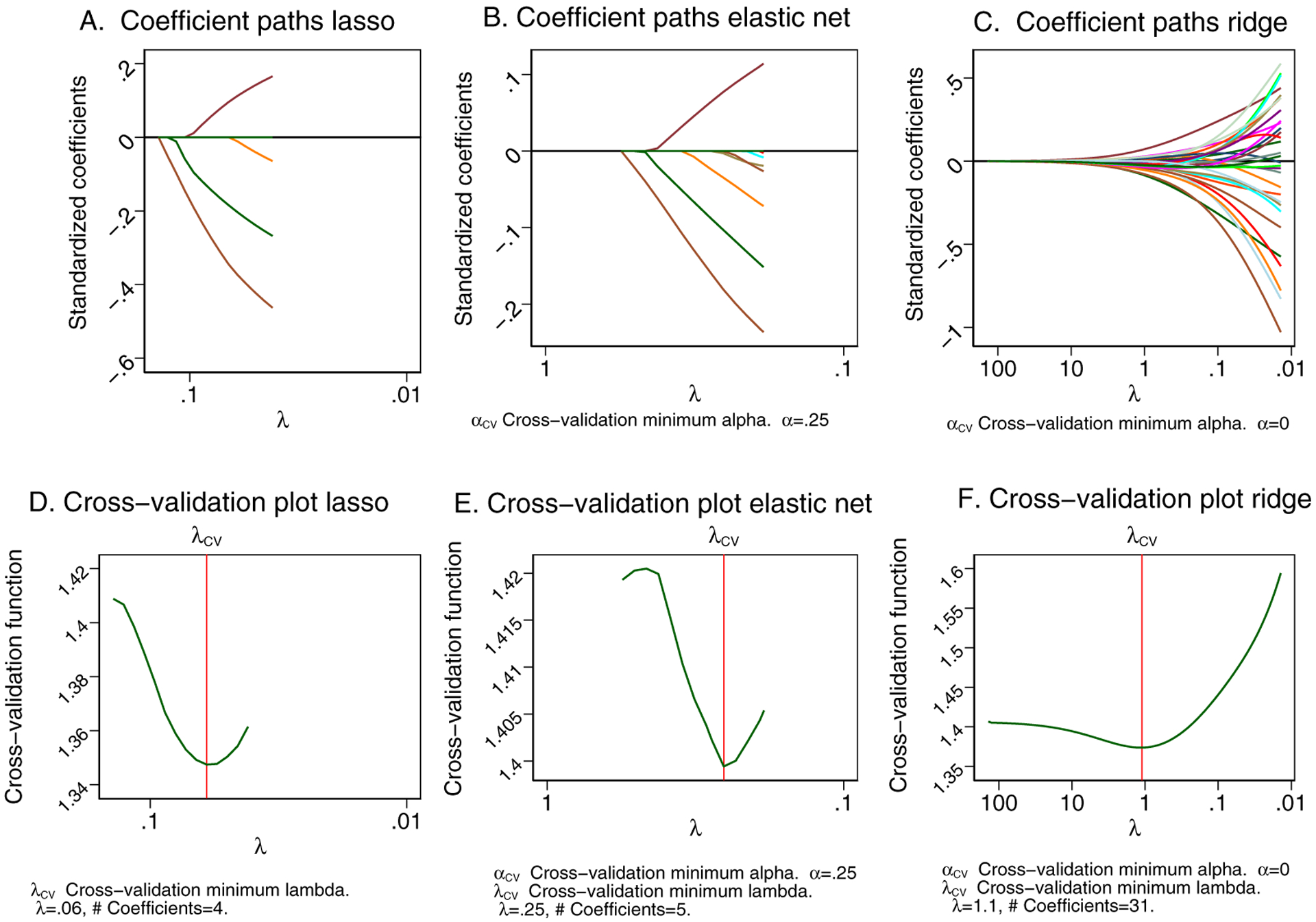
The coefficient paths after (A) lasso, (B) elastic net, (C) ridge models. A line is drawn for each coefficient that traces its value over the searched values of the lasso penalty parameter λ on a reverse logarithmic scale. Lasso is letting variables into the model based on its penalty and the current value of λ. Cross-validation (CV) function (the mean deviance in the CV samples) is plotted over the search grid for the lasso penalty parameter λ on a reverse logarithmic scale for (D) lasso, (E) elastic net, (F) ridge models. The first λ tried is on the left, and the last λ tried is on the right.
For the right-sided S-ICD eligibility prediction, both lasso and elastic net models shrunk to zero coefficients. Therefore, even if both lasso and elastic net demonstrated the minimum cross-validation function, we had to select the ridge model as the best model (Table 3). Therefore, we were not able to develop simple linear equations for right-sided S-ICD eligibility prediction.
Out-of-sample (standing ECG) validation of the ridge models was satisfactory for both left-sided [ROC AUC 0.687 (95%CI 0.582–0.791)] and right-sided [ROC AUC 0.655(95%CI 0.549–0.762)] S-ICD eligibility prediction.
Out-of-sample validation of the lasso and elastic net prediction models in the previous non-ACHD study population10 yielded high sensitivity of the pre-selected in this study threshold (≥ − 0.5): 100% for the elastic net model, and 77% for lasso model. Validation ROC AUC in a non-ACHD population was unsatisfactory for all models: specifically, for lasso (ROC AUC 0.554; 95%CI 0.355–0.754), elastic net (ROC AUC 0.548; 95%CI 0.340–0.756), and ridge model (ROC AUC 0.477; 95%CI 0.282–0.671).
Transformation of routine clinical 12-lead ECG to S-ICD 3-lead ECG, and vice versa
Validation of the transformation matrices (Supplemental Tables 1–2) showed satisfactory agreement between the originally recorded and transformed signals (Figure 5 and Supplemental Table 3). For most of the leads (52/60; 87%), the difference in the voltage was not clinically meaningful (less than 0.1 mV). We provided open-source code for transformations at https://github.com/Tereshchenkolab/S-ICD_eligibility.
Figure 5.
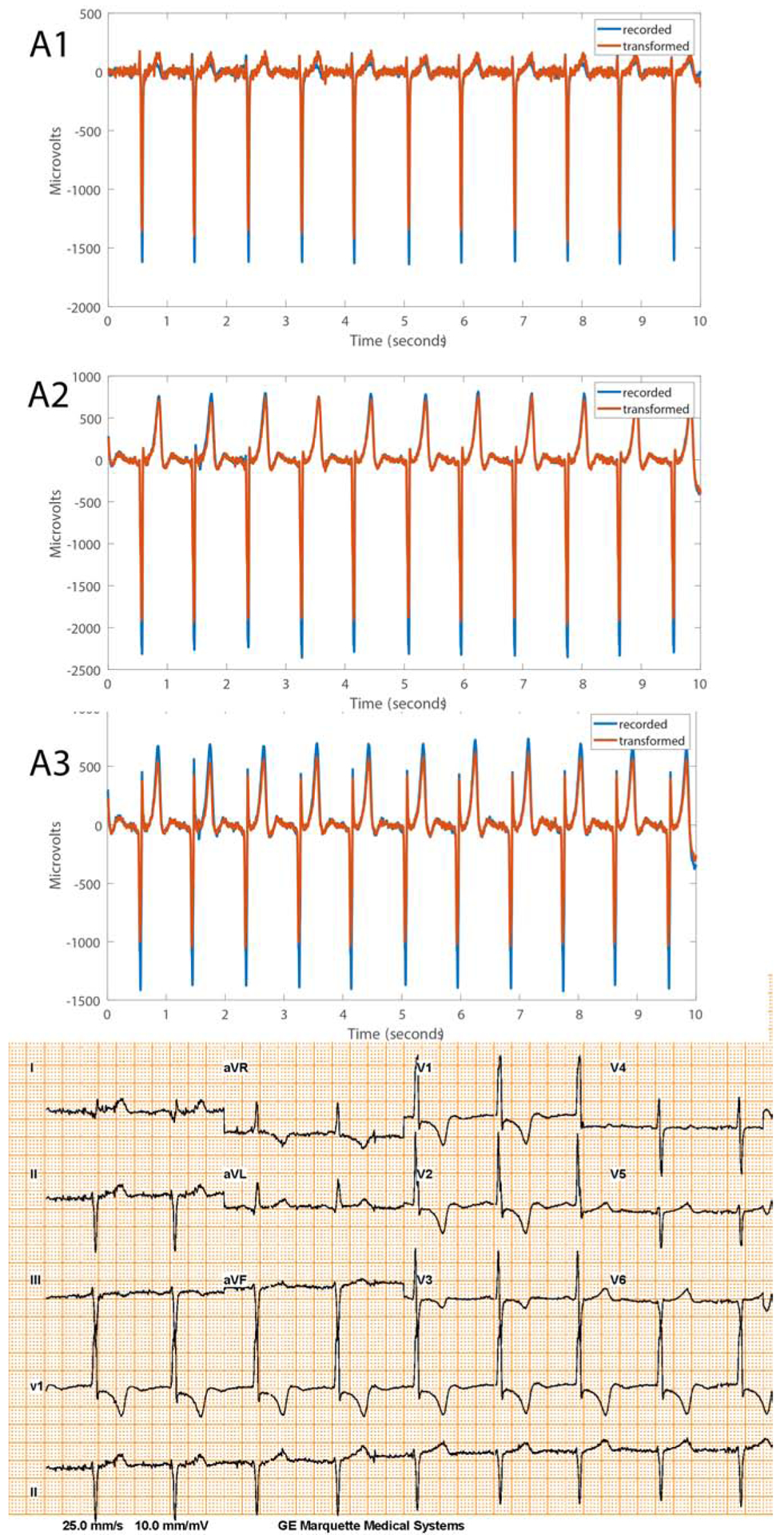
Representative examples of recorded and transformed right-sided 3-lead ECG morphologies and corresponding 12-lead ECG recorded during standing in a Fontan patient.
Discussion
This prospective study of the contemporary ACHD population revealed several important findings. First, we observed a high rate of S-ICD ineligibility: nearly half of ACHD patients were not eligible for S-ICD. Second, we noted sex differences: odds of S-ICD eligibility was nearly six fold greater for female as compared to male ACHD patients. The high rate of S-ICD ineligibility in ACHD population represents a significant barrier for the adoption of potentially advantageous and less invasive S-ICD technology for the prevention of sudden cardiac death in ACHD. Third, we used machine learning to develop and validate an S-ICD eligibility prediction tool, to simplify and make it more convenient to screen potential S-ICD candidates. We found that the most accurate prediction model suggests the use of as many as possible available 12-lead ECG features, and, therefore, is impractical for “by-hand” calculation. Instead, we were able to reliably transform 12-lead ECG into S-ICD 3-lead ECG, and vice versa. We developed and validated transformation matrices, which could facilitate future improvement of S-ICD diagnostics and the development of fully automated S-ICD eligibility assessment, using routinely recorded 12-lead ECGs. Lastly, we were able to develop and validate a simplified S-ICD prediction model for left-sided S-ICD. The simplified model includes only four or five readily available ECG features; it has high sensitivity but low specificity and can be used as a first preliminary step for S-ICD eligibility screening. It is important to emphasize that the final decision regarding S-ICD eligibility should be made by an electrophysiologist, after full clinical evaluation, as appropriate.
A large portion of the contemporary ACHD population is ineligible for S-ICD
In recent decades, the ACHD population has expanded due to the advancements in pediatric cardiology and congenital cardiac surgery; 90% of children with severe congenital heart disease now survive to 18 years of age.12 More than 1.4 million adults live with ACHD in the United States.13 Sudden cardiac death is the most frequent cause of death in ACHD.14 Patients with transposition of great arteries and tetralogy of Fallot have the highest risk of life-threatening ventricular arrhythmias.15 Since the entire S-ICD system is implanted in an extra-thoracic space, it eliminates the complications related to endo- or epicardial leads.16 The ACHD patients with no transvenous access to the heart, or those with a right-to-left shunt and increased risk of systemic emboli, can attain the utmost potential benefit17 from implantation of S-ICD. Unfortunately, our study demonstrated that 40% of the contemporary complex ACHD population is ineligible for S-ICD.
The rate of ineligibility observed in this study for both right- and left-sided S-ICD in ACHD patients (40%) is higher than the rate reported by Alonso et al.18 for tetralogy of Fallot (23%) and mixed ACHD patients11 (25%), the rate reported by Okamura et al. (12%)19, Garside et al.4 (25%; left-sided only), and Zeb et al.20 (13%; left-sided only). A higher rate of S-ICD ineligibility in our study can be due to the large size, greater complexity and heterogeneity, and more severe functional impairment of our study population.12 The results of this study underscore the need to further improve S-ICD technology to increase the number of eligible ACHD patients. Our previous study10 showed remarkable (3-fold) improvement in S-ICD eligibility after ECG filtering. In this study, high QRS and T voltage were the main reasons for S-ICD ineligibility and turning the S-ICD ECG filtering feature ON can increase the number of eligible ACHD patients.
Similar to previous studies conducted in the ACHD population11,18–20, we found more Fontan and tetralogy of Fallot participants that passed screening with the right-sided vector. Findings of improved S-ICD eligibility with the right-sided placement of S-ICD lead merit further studies comparing effectiveness in arrhythmia termination. Several case reports demonstrated successful defibrillation with 65J in ACHD patients with right-sided S-ICD lead placement.21–23 Theoretically, right-sided S-ICD lead placement can be more effective in arrhythmia termination than left-sided S-ICD lead placement, because of a more favorable S-ICD electric lead field, encompassing the whole heart (Figure 1). An in silico study reported a lower defibrillation threshold for right-sided than for left-sided S-ICD lead placement.24 An observational study in a general S-ICD population25 demonstrated similar rates of successful defibrillation with the first 65J shock (79% left-sided and 73% right-sided lead; P=NS), and similar rates of ineffective shocks (2.9% left-sided and 1.9% right-sided lead; P=NS). A randomized controlled trial is needed before right-sided S-ICD lead placement can be recommended as preferential in ACHD.
Sex-differences in S-ICD eligibility
Of note, we observed for the first time, a near sex fold greater odds of S-ICD eligibility for female than male ACHD participants. This finding requires validation in another prospective study to rule out unmeasured confounding. Of note, in our study female ACHD participants comprised 52% of the study population. Women remain underrepresented in S-ICD studies.3 This emphasizes the need for further research regarding S-ICD eligibility and effectiveness in women.26
Using 12-lead ECG for prediction of 12-lead eligibility: a machine learning approach
Results of our study demonstrating a large proportion of ACHD population being ineligible for S-ICD highlight the importance of S-ICD eligibility screening. Model selection by machine learning demonstrated that the most accurate out-of-sample prediction tool included all available ECG features. Along those lines, we developed transformation matrices to transform the entire ECG waveform from one type to another: from 12-lead ECG to 3-lead ECG and vice versa. Validation of transformation matrices demonstrated substantial agreement between originally recorded and transformed signals. Reliable signal transformation opens an avenue for further development of additional diagnostic and prognostic features that can enhance S-ICD functionality, as well as for the development of fully automated S-ICD eligibility assessment using routine 12-lead ECGs.
Machine learning indicated that no linear equation exists to describe the right-sided S-ICD eligibility prediction function accurately because of its non-linearity. However, several models were selected for the simplified prediction of left-sided S-ICD eligibility. Realizing that even using a machine learning approach, we cannot offer perfect prediction of S-ICD eligibility by a simple linear model, we tuned the developed models to high sensitivity.
Limitations
We only performed ECG screening in the supine and standing positions but did not screen during exercise or other postures, which can theoretically increase the percentage of ineligible patients. Nonetheless, as we observed very little difference in eligibility between standing and supine positions in this study, we can infer that unlike in the general population,10 body posture change in an ACHD population has little to no effect on S-ICD eligibility. Consistently with our findings, Wilson et al.27 did not detect significant differences in the R/T amplitude ratio in tetralogy of Fallot and single ventricle physiology patients in a supine, prone, left lateral, right lateral, sitting, and standing positions, whereas such differences were observed in controls. Similarly, Zeb et al.20 reported that posture change did not affect S-ICD eligibility in ACHD patients.
On the other hand, in our study, an increase in HR was associated with large P-waves as a cause of ineligibility, and overall, with less likelihood of passing the screening. As ACHD patients are prone to sinus tachycardia and supraventricular arrhythmias, future studies of S-ICD eligibility in ACHD during exercise are needed.
Although we enrolled a complex ACHD population and presented a comparable sample size4, 11, 19, our study suffered limitations typical for all ACHD studies.12 It is noteworthy that our broad inclusion also encompasses patients who would require a transvenous ICD because of indications for pacing and those who would not be considered for S-ICD. The study population was predominantly white. Future studies in ethnically diverse populations are needed.
Supplementary Material
Acknowledgments
The authors thank the study participants and staff. We thank Christopher Hamilton, BA, and Meghan Hisatomi Saito, ACNP, for help with ECG recording and enrollment. This research was supported in part by the National Institute of Health HL118277 (LGT), and the Boston-Scientific Center for the Advancement of Research (LGT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trial Registration—URL: www.clinicaltrials.gov Unique identifier: NCT03209726
Disclosure:
This physician-initiated study (PI Tereshchenko) was partially supported by the Boston-Scientific Center for the Advancement of Research. The Boston Scientific company had no role in the design, execution, analyses, and interpretation of the data and results of this study.
References:
- 1.Gold MR, Aasbo JD, El-Chami MF, et al. Subcutaneous implantable cardioverter-defibrillator Post-Approval Study: Clinical characteristics and perioperative results. Heart Rhythm 2017;14:1456–1463. [DOI] [PubMed] [Google Scholar]
- 2.Willy K, Reinke F, Bogeholz N, Kobe J, Eckardt L, Frommeyer G. The entirely subcutaneous ICDTM system in patients with congenital heart disease: experience from a large single-centre analysis. Europace 2019;21:1537–1542. [DOI] [PubMed] [Google Scholar]
- 3.D’Souza BA, Epstein AE, Garcia FC, et al. Outcomes in Patients With Congenital Heart Disease Receiving the Subcutaneous Implantable-Cardioverter Defibrillator: Results From a Pooled Analysis From the IDE Study and the EFFORTLESS S-ICD Registry. JACC Clinical electrophysiology 2016;2:615–622. [DOI] [PubMed] [Google Scholar]
- 4.Garside H, Leyva F, Hudsmith L, Marshall H, de Bono J. Eligibility for subcutaneous implantable cardioverter defibrillators in the adult congenital heart disease population. Pacing Clin Electrophysiol 2019;42:65–70. [DOI] [PubMed] [Google Scholar]
- 5.Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2018;72:e91–e220. [DOI] [PubMed] [Google Scholar]
- 6.Pulse Generator User’s Manual: Emblem™ S-ICD: Subcutaneous Implantable Cardioverter Defibrillator Model A209: Boston Scientific/Cameron Health; 2015.
- 7.Friedman DJ, Parzynski CS, Varosy PD, et al. Trends and In-Hospital Outcomes Associated With Adoption of the Subcutaneous Implantable Cardioverter Defibrillator in the United States. JAMA Cardiol 2016;1:900–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernier R, Raj SR, Tran D, et al. Assessing physician knowledge regarding indications for a primary prevention implantable defibrillator and potential barriers for referral. J Cardiovasc Electrophysiol 2017;28:1334–1341. [DOI] [PubMed] [Google Scholar]
- 9.Yuhas J, Mattocks K, Gravelin L, et al. Patients’ attitudes and perceptions of implantable cardioverter-defibrillators: potential barriers to appropriate primary prophylaxis. Pacing Clin Electrophysiol 2012;35:1179–1187. [DOI] [PubMed] [Google Scholar]
- 10.Thomas JA, Perez-Alday EA, Hamilton C, Kabir MM, Park EA, Tereshchenko LG. The utility of routine clinical 12-lead ECG in assessing eligibility for subcutaneous implantable cardioverter defibrillator. Comput Biol Med 2018;102:242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alonso P, Osca J, Rueda J, et al. Conventional and right-sided screening for subcutaneous ICD in a population with congenital heart disease at high risk of sudden cardiac death. Ann Noninvasive Electrocardiol 2017;22:e12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;139:e698–e800. [DOI] [PubMed] [Google Scholar]
- 13.Gilboa SM, Devine OJ, Kucik JE, et al. Congenital Heart Defects in the United States: Estimating the Magnitude of the Affected Population in 2010. Circulation 2016;134:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naidu P, Grigg L, Zentner D. Mortality in adults with congenital heart disease. Int J Cardiol 2017;245:125–130. [DOI] [PubMed] [Google Scholar]
- 15.Koyak Z, Harris L, de Groot JR, et al. Sudden cardiac death in adult congenital heart disease. Circulation 2012;126:1944–1954. [DOI] [PubMed] [Google Scholar]
- 16.Guerrier K, Hendrickson B, Waller BR, Wetzel GT. Diagnostic and Therapeutic Approach to Arrhythmias in Adult Congenital Heart Disease. Current treatment options in cardiovascular medicine 2019;21:44. [DOI] [PubMed] [Google Scholar]
- 17.Bordachar P, Marquie C, Pospiech T, et al. Subcutaneous implantable cardioverter defibrillators in children, young adults and patients with congenital heart disease. Int J Cardiol 2016;203:251–258. [DOI] [PubMed] [Google Scholar]
- 18.Alonso P, Osca J, Cano O, et al. The Role of Conventional and Right-Sided ECG Screening for Subcutaneous ICD in a Tetralogy of Fallot Population. Pacing Clin Electrophysiol 2017;40:145–153. [DOI] [PubMed] [Google Scholar]
- 19.Okamura H, McLeod CJ, DeSimone CV, et al. Right Parasternal Lead Placement Increases Eligibility for Subcutaneous Implantable Cardioverter Defibrillator Therapy in Adults With Congenital Heart Disease. Circ J 2016;80:1328–1335. [DOI] [PubMed] [Google Scholar]
- 20.Zeb M, Curzen N, Veldtman G, et al. Potential eligibility of congenital heart disease patients for subcutaneous implantable cardioverter-defibrillator based on surface electrocardiogram mapping. Europace 2015;17:1059–1067. [DOI] [PubMed] [Google Scholar]
- 21.Chan NY, Yuen HC, Mok NS. Right Parasternal Electrode Configuration Converts a Failed Electrocardiographic Screening to a Pass for Subcutaneous Implantable Cardioverter-Defibrillator Implantation. Heart Lung Circ 2015;24:e203–205. [DOI] [PubMed] [Google Scholar]
- 22.Luker J, Sultan A, Sreeram N, Brockmeier K, Steven D. Implantation of a subcutaneous implantable cardioverter defibrillator with right parasternal electrode position in a patient with D-transposition of the great arteries and concomitant AAI pacemaker: a case report. Eur Heart J Case Rep 2018;2:yty099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wakabayashi Y, Mitsuhashi T, Fujita H, Momomura S-i. Usefulness of lead repositioning from left to right sternal border for a patient with subcutaneous implantable cardioverter defibrillator showing high defibrillation threshold. Journal of arrhythmia 2019;35:133–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noro M, Zhu X, Enomoto Y, et al. Efficacy and Myocardial Injury With Subcutaneous Implantable Cardioverter Defibrillators – Computer Simulation of Defibrillation Shock Conduction -. Circulation Journal 2016;80:85–92. [DOI] [PubMed] [Google Scholar]
- 25.Bettin M, Dechering D, Frommeyer G, et al. Right versus left parasternal electrode position in the entirely subcutaneous ICD. Clinical Research in Cardiology 2018;107:389–394. [DOI] [PubMed] [Google Scholar]
- 26.Dhruva SS, Redberg RF. Sex-Specific Data for the Subcutaneous Implantable Cardioverter-Defibrillator. Journal of the American College of Cardiology 2016;68:133. [DOI] [PubMed] [Google Scholar]
- 27.Wilson DG, Zeb M, Veldtman G, Dimitrov BD, Morgan JM. Left and Right Parasternal Sensing for the S-ICD in Adult Congenital Heart Disease Patients and Normal Controls. Pacing Clin Electrophysiol 2016;39:282–290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


