SUMMARY
Sensory neurons initiate defensive reflexes that ensure airway integrity. Dysfunction of laryngeal neurons is life-threatening, causing pulmonary aspiration, dysphagia, and choking, yet relevant sensory pathways remain poorly understood. Here, we discover rare throat-innervating neurons (~100 neurons/mouse) that guard the airways against assault. We used genetic tools that broadly cover a vagal/glossopharyngeal sensory neuron atlas to map, ablate, and control specific afferent populations. Optogenetic activation of vagal P2RY1 neurons evokes a coordinated airway defense program- apnea, vocal fold adduction, swallowing, and expiratory reflexes. Ablation of vagal P2RY1 neurons eliminates protective responses to laryngeal water and acid challenge. Anatomical mapping revealed numerous laryngeal terminal types, with P2RY1 neurons forming corpuscular endings that appose laryngeal taste buds. Epithelial cells are primary airway sentinels that communicate with second-order P2RY1 neurons through ATP. These findings provide mechanistic insights into airway defense, and a general molecular/genetic roadmap for internal organ sensation by the vagus nerve.
Graphical Abstract

In brief
Prescott, Umans et al. profile throat-innervating neurons and discover 37 subtypes of vagal sensory cells, including rare P2RY1-expressing neurons that associate with taste bud structures in the upper airway to detect water and acid threats and initiate important defensive reflexes.
INTRODUCTION
The vagus nerve and other cranial nerves ensure the integrity of several major physiological systems. Peripheral sensory neurons monitor numerous vital parameters like blood pressure, airway volume, stomach distension, and circulating oxygen levels, and if potentially harmful deviations from equilibrium are detected, neural circuits are engaged to initiate corrective physiological and behavioral responses. Neuronal surveillance of internal organs is extensive but not comprehensively charted, with many neuronal safeguards likely remaining so far hidden. Moreover, dysfunction of organ-to-brain communication can be lethal. One of the most fundamental functions of the vagus nerve is to provide the first line of defense that protects the airways from injury. Defects in vagally mediated reflexes in the upper airways cause some of the most pervasive and severe clinical problems associated with aging including dysphagia (difficulty swallowing), weight loss, choking, speech impairment, and respiratory tract infections such as aspiration pneumonia (Jadcherla et al., 2010; Ludlow, 2015; Santoso et al., 2019). Furthermore, hyperactivity or sensitization of upper airway neurons may contribute to asthma, chronic cough, and Sudden Infant Death Syndrome (SIDS). Here, we used information and genetic tools resulting from a comprehensive survey of vagal sensory neuron diversity to gain insights into physiological mechanisms underlying airway defense.
The larynx, as gateway to the respiratory system, is a hotspot for neuronal innervation (Ludlow, 2015; Steele and Miller, 2010). The larynx adjoins the digestive tract, and ingested food and water, as well as expelled stomach contents, must transit over the larynx without penetrating the airways. A complex network of laryngeal neurons guards the airways against infiltration by evoking characteristic occlusion and expulsion reflexes (Coleridge et al., 1989; Ludlow, 2015; Mazzone and Undem, 2016; Steele and Miller, 2010). For example, each swallow evokes reflexive responses that include (1) stopping inspiration to prevent pulmonary aspiration, (2) vocal fold adduction, and (3) laryngeal vestibule closure through hyoid bone elevation and epiglottis descent. If foreign material does enter the airways, secondary clearance reflexes are evoked, including cough (in some species), gag, expiratory reflexes, and pharyngeal swallow.
The upper airways are densely innervated by the vagus nerve through the superior laryngeal (SLN) and recurrent laryngeal (RLN) nerve branches (Figure 1A) (Coleridge et al., 1989; Ludlow, 2015; Mazzone and Undem, 2016; Steele and Miller, 2010). The internal branch of the SLN provides the major sensory innervation of the larynx, from the vocal folds to the epiglottis, as well as the caudal pharynx. RLN afferents instead predominate below the vocal folds, with dense innervation of the trachea and lung. Loss of SLN function by nerve injury or transient application of local anesthetic causes dysphagia, incomplete laryngeal closure, and risk of airway aspiration (Jadcherla et al., 2010; Jafari et al., 2003; Santoso et al., 2019). Electrically stimulating the SLN evokes hallmarks of airway protection including cough, apnea (breathing pause), vocal fold adduction, and fictive swallowing (Bolser, 1991; Doty, 1951). These studies highlighted the general importance of neuronal feedback for airway protection, but did not distinguish the contributions of various vagal neuron subtypes, or the molecular mechanisms they employ.
Figure 1. Evoking swallows and expiratory reflexes with laryngeal stimulation and light.

(A) Cartoon of upper airway innervation. (B) Schematic for perfusion of laryngeal stimuli. (C) Representative respiratory rhythms during (blue shading) delivery of isotonic saline (control), water, high salt (1500 mOsm) and citric acid (25 mM), with swallows (red triangles) and expiratory reflexes (blue triangles) noted. (D) Swallows (n=6–13, mean ± sem) were counted during laryngeal application of stimuli (see methods). (E) Swallows were counted before (control, gray) or after (SLN-cut, red) acute bilateral SLN transection (n=2–31, mean ± sem). (F) Cartoon (left) depicting optogenetics paradigm under urethane, and (right) changes in tracheal pressure (i: inspiration; e: expiration), digastric muscle electromyography (EMG), and pharyngeal pressure during a representative swallow and expiratory reflex. (G) Optogenetic stimulation (vagal trunk) in Vglut2-ires-Cre; loxP-Chr2 mice resulted in swallows (red triangles) and expiratory reflexes (blue triangles). Representative traces of tracheal pressure, digastric muscle EMG, and pharyngeal pressure over time are shown, yellow shading: light stimulation (20 Hz).
Airway threats are detected throughout the larynx, with complex neuronal arbors found on the epiglottis and vocal folds, and in the postcricoid and arytenoid regions (Feindel, 1956; Mu and Sanders, 2000; Travers and Nicklas, 1990; Yamamoto et al., 1997). A multitude of laryngeal sensory terminals have been described, including submucosal and epithelial free endings, ivy-like laminar (hederiform) endings, corpuscular endings, cough receptors, and endings near taste buds. The functions of particular terminal morphologies have been debated, with models based largely on the proximity of terminals to mechanosensory or chemosensory hotspots. However, different terminal types typically display overlapping regions of innervation in the larynx, and consistent anatomical nomenclature is lacking. As a result, previous descriptors may redundantly refer to the same terminal type, or incorrectly group together sensory neurons with different response profiles. We reasoned that genetic tools might provide more consistency across studies, and also allow for specific functional manipulation to resolve the sensory roles of different laryngeal terminals.
Nerve recordings revealed that laryngeal sensory neurons detect various mechanical and chemical stimuli (Harding et al., 1978; Sampson and Eyzaguirre, 1964; Sant’Ambrogio et al., 1991; Smith and Hanamori, 1991; Storey, 1968), including (1) water, salt, and pharyngeal/laryngeal force from ingested food and drink, (2) acid, bile, and esophageal force from regurgitated stomach contents, and (3) invasive threats, such as pathogens and irritants like smoke, oxidants and dust particles. Various mechanisms have been proposed for irritant and pathogen detection (Baral et al., 2018; Bessac and Jordt, 2008; Canning et al., 2004; Han et al., 2018; Krasteva et al., 2011; Tizzano et al., 2010), but less is understood about the diversity and organization of sensory pathways that protect the airways from penetration by food, drink, and gastric reflux. Water, salt, acid, and force stimulate the SLN (Smith and Hanamori, 1991), and also evoke defensive reflexes including apnea, vocal fold adduction, pharyngeal swallow, and cough (Canning et al., 2004; Kovar et al., 1979; Shingai et al., 1989). Water responses are particularly robust in the larynx, yet primary sensory cells are unknown. It is unclear whether water-sensing and acid-sensing laryngeal afferents are first-order or second-order neurons, and furthermore, whether various other cell types, such as laryngeal taste cells, solitary chemosensory cells, neuroendocrine cells, or other epithelial or immune cell types may play an upstream role.
Here, we used molecular and genetic approaches to provide insights into sensory pathways that protect the upper airways. Single-cell RNA sequencing revealed a remarkable diversity of vagal afferents, and Cre mice were obtained for genetic control over most neuron subtypes individually or in small clusters, enabling links between cell identity, terminal morphology, sensory response, and physiological function. Cell type-specific optogenetics revealed that rare vagal P2RY1 neurons (~100 per mouse) elicit a suite of airway protective responses including expiratory reflexes, vocal fold adduction, apnea, and fictive swallow. Moreover, ablation of vagal P2RY1 neurons impairs defensive responses to laryngeal challenges from acid and water, but not salt or force. Genetically guided anatomical tracing revealed that vagal P2RY1 neurons densely innervate laryngeal epithelium, closely apposing laryngeal taste buds. Laryngeal chemosensory reflexes are mimicked by optogenetic activation of epithelial cells, and are lost in knockout mice lacking ionotropic purinergic receptors. Thus, Cre-guided mapping, optogenetics, and cell ablation provide evidence that one of ~37 identified vagal cell subtypes functions as a second-order sensor for specific chemical threats in the larynx.
RESULTS
Reflexes evoked by laryngeal stimulation
Swallowing is a complex motor action with distinct oral, pharyngeal, and esophageal phases (Steele and Miller, 2010). The pharyngeal phase of swallowing plays an active role in airway defense by clearing infiltrate away from the airways while propelling food, water, and other ingested contents safely past the larynx into the esophagus. (Pharyngeal swallow is also termed ‘secondary swallow’ when initiated in the absence of an oral phase). Chemical and mechanical stimulation of the larynx evokes guarding reflexes like apnea, vocal fold adduction, and laryngeal vestibule closure, and also clearance reflexes like pharyngeal swallow and cough. The same sensory stimulus can variably evoke a pharyngeal swallow, persistent apnea, and cough, with responses differing across species, age, depth of anesthesia, and stimulus magnitude (Nishino et al., 1990; Troche et al., 2014). The contributions of different laryngeal chemoreceptors and mechanoreceptors in these complex sensorimotor transitions are not well understood.
To monitor airway defense reflexes, we developed an in vivo preparation in anesthetized mice to record swallowing responses to laryngeal stimuli (Figure 1B). Solutions were introduced at a very slow rate (100 μl/min) through a cannula inserted past the tongue and directly over the larynx, with an exit port placed in the trachea below the vocal folds. A tracheal pressure transducer was inserted below the exit port to monitor breathing rhythms. Evoked swallows were quantified by visually observing hyoid elevation, and were not observed under baseline conditions involving continuous slow perfusion of saline (PBS). Furthermore, based on control trials with PBS, swallowing was not evoked by flow alterations that may be associated with switching perfusion solutions. We found that robust and repetitive swallowing was evoked by transient perfusion of several test stimuli through the larynx, including water, acid, and high salt (Figure 1C, 1D). Swallowing was also evoked by mechanical stimulation of the larynx by direct physical probing. In contrast, laryngeal perfusion of TRPA1 or TRPV1 agonists, as well as bitter, sweet, or umami tastants (dissolved in PBS) did not evoke pharyngeal swallows.
Water, high salt, and acid also evoked transient apneas with each swallow, as well as occasional expiratory reflexes (Figure 1C). An airway expiratory reflex is a sudden forced airway evacuation reminiscent of a cough, but unlike a cough is not associated with a prior deep inspiration (Ludlow, 2015). Transection of the SLN eliminated swallowing to laryngeal water, acid, and high salt, and decreased the frequency of force-evoked swallow, consistent with a key role for larynx-innervating neurons (Figure 1E). Together, these findings reveal at least four modalities of upper airway stimuli that evoke reflexive secondary swallow: water, acid, high salt, and force. Thus, chemosensory responses in the larynx can be robustly elicited in mice, and are at least partially distinct from classical taste responses of gustatory nerves.
Evoking fictive swallows by sensory neuron optogenetics
We used optogenetics to identify sensory neurons involved in airway defense. The throat is densely innervated by the vagus and glossopharyngeal nerves, and in mice afferent soma from these nerves form a pair of fused nodose/jugular/petrosal (NJP) superganglia, each containing ~2,300 sensory neurons clustered near the jugular foramen at the base of the skull (Fox et al., 2001). We crossed mice harboring a Cre-dependent Channelrhodopsin allele (loxP-ChR2) to Vglut2-ires-Cre and Chat-ires-Cre mice, which respectively label the vast majority of sensory and motor fibers in vagal and glossopharyngeal nerves. Optogenetic stimulation was achieved in offspring by focal illumination of the vagus nerve trunk or particular nerve branches (Figure 1F).
Activating all vagal sensory neurons in Vglut2-ires-Cre; loxP-ChR2 mice evoked repetitive swallowing (Figure 1G, Movie S1), with an average of 4.3 swallows per 10 second photostimulation trial. Pharyngeal swallows were observed and distinguished from primary swallows based on a lack of tongue movement and from esophageal swallows based on a lack of esophageal peristalsis. These findings indicate that pharyngeal swallow can be separately initiated and controlled, even under anesthesia. Pharyngeal swallows were further distinguished from other potential throat movements (such as cough or gaping), as they were associated with transient increases in pharyngeal pressure, contraction of the digastric muscle (which elevates the hyoid bone) as recorded by electromyography (EMG), and laryngeal elevation (Figure 1F). Analysis of tracheal pressure also revealed a transient apnea that occurred with each optogenetically evoked swallow (Figure 1F, 1G).
Bulk optogenetic stimulation of NJP sensory neurons, in addition to evoking swallow, also evoked occasional airway expiratory reflexes (1.5 events per 10 second trial, Figure 1F, 1G). Expiratory reflexes sometimes occurred immediately after a swallow, and other times were observed as isolated events. Light-evoked swallows and expiratory reflexes were not observed in control loxP-ChR2 mice or during vagal motor neuron stimulation in Chat-ires-Cre; loxP-ChR2, as motor control of these reflexes is conveyed by other efferent nerves.
Sweeping genetic control over vagal sensory neuron subtypes
We next defined NJP sensory neurons involved in airway protection. We used single-cell RNA sequencing for unbiased investigation of sensory neuron diversity in the vagus and glossopharyngeal nerves (Figure 2A). Sensory neurons were acutely harvested from NJP ganglia of wild type (8-week old, male, C57BL6/J) mice, enriched by centrifugation in a Percoll gradient, and encapsulated in nanoliter droplets containing primer-decorated beads for barcoded cDNA synthesis. Single-cell cDNA was then amplified and sequenced to generate single cell transcriptomes.
Figure 2. An atlas of sensory neuron subtypes from vagal and glossopharyngeal nerves.

(A) Experimental pipeline for single-cell transcriptome analysis. (B) Uniform Manifold Approximation and Projection (UMAP) plots indicating cell subtype diversity across 25,117 NJP sensory neurons. Neuron clusters from jugular (J1-J10) and nodose/petrosal (NP1-NP27) ganglia are color coded. (C) Normalized expression levels (blue-gray scale) of cluster-defining signature genes (see Table S1 for genes 1–185) across all NJP sensory neurons analyzed. (D) UMAP plots from single-cell transcriptomes (left) and RNA in situ hybridization in cryosections of NJP ganglia (right) showing gene expression in sensory neuron subsets, scale bars: 100 μm. See also Figure S1 and Table S1.
Transcriptome data (average: 6,937 unique reads spanning 2,293 genes) were obtained on 46,712 cells, including 25,117 sensory neurons as well as macrophages, stromal cells and various types of glial cells (Figure S1A–S1C). Sensory neurons were defined based on expression of several neuronal markers, including Vglut2 (Slc17a6), a glutamate transporter expressed in >99% of vagal and glossopharyngeal sensory neurons but not in other non-neuronal cell types of sensory ganglia (Chang et al., 2015). Unsupervised clustering analysis revealed ~37 classes of sensory neurons (Figure 2B), each ranging in size from 12 to 151 neurons per NJP ganglion (Figure S1C). Additional rare vagal sensory neuron subtypes were uncovered here, compared with other recent single-cell sequencing studies (Bai et al., 2019; Kupari et al., 2019; Mazzone et al., 2019), likely because of the larger number of cells we analyzed. 27 cell clusters, representing 79% of NJP sensory neurons (19,756/25,117), were derived from nodose and inferior petrosal ganglia based on expression of Phox2b, a marker for epibranchial placode-derived sensory neurons (Figure S1D). The remaining 10 clusters, representing 21% of neurons, did not express Phox2b but instead expressed Prdm12, a neural crest transcription factor and marker for sensory neurons of the jugular and superior glossopharyngeal ganglia. Markers that broadly distinguish petrosal neurons from nodose/jugular neurons are not available, although at least one large placode-derived neuron cluster (NP9) lacks expression of Hoxb4 (Figure S1D), and is likely part of the glossopharyngeal nerve.
We defined cell clusters by singular or combinatorial expression of signature genes (Figure 2C); see Table S1 for signature genes used to define each cell cluster. In situ hybridization verified expression of selected markers in dispersed subsets of sensory neurons (Figure 2D). GO term analysis on differentially expressed genes revealed that sensory neuron subtypes differed primarily by expression of various cell surface receptors such as G Protein-Coupled Receptors (GPCRs), ion channels such as TRPs and PIEZOs, and neuropeptides (Figure S1D, S1E). Single-cell transcriptome data of vagal/glossopharyngeal sensory neurons are publicly available (GEO accession number GSE145216).
The 37 classes of vagal sensory neurons may differ with regards to organ-targeting pattern, response property, and/or physiological function. We previously characterized various classes of sensory neurons that innervate the lungs, stomach, intestine, and aorta, and differentially control breathing, heart rate, blood pressure, and gut motility (Chang et al., 2015; Min et al., 2019; Williams et al., 2016). For example, in the digestive system, stomach and intestine mechanoreceptors express several gut hormone receptors, and are marked in Glp1r-ires-Cre mice (Williams et al., 2016); cell clusters that express Glp1r include NP4, NP8, NP26, and NP27. Other sensory neurons marked in Gpr65-ires-Cre mice function as chemosensory neurons that innervate intestinal villi and stomach mucosa (Williams et al., 2016), with Gpr65 highly expressed in cluster NP5. Some vagal afferents in the lung and lower airways contain PIEZO2, sense airway stretch and evoke a classic apnea reflex termed the Hering-Breuer inspiratory reflex (Nonomura et al., 2017). There are multiple classes of PIEZO2 neurons (Figure 3A), and it is unclear which transcriptome-defined cluster detects airway stretch, and which others may mediate different internal senses such as arterial baroreception (Min et al., 2019; Zeng et al., 2018). Some vagal P2RY1 neurons also innervate the lung, with arbors that appose clusters of lung secretory cells termed neuroepithelial bodies (Chang et al., 2015).
Figure 3. A genetic toolkit for accessing small groups of NJP sensory neurons.

(A) UMAP plots indicating expression of genes in NJP sensory neurons. (B) Native reporter fluorescence in cryosections of NJP ganglia from mice with Cre alleles and Cre-dependent reporter genes (L10-GFP, except tdTomato for Calb1-ires-Cre and Vglut-ires-Cre). tdTomato images were pseudocolored to match GFP images; far-red fluorescent Nissl counterstain (gray), scale bars: 100 μm. The image from Npy2r-ires-Cre mice was previously published (Chang et al., 2015). See also Figure S2.
Single-cell sequencing revealed that visceral sensations of the vagus and glossopharyngeal nerves depend on a striking diversity of cell types. This estimate of cell diversity is conservative, and some co-clustered cells may perform similar sensory functions such as mechanosensation or cytokine detection in different organs. Thus, recognized cell type diversity may be even greater once physiological roles are understood for each neuron group. Moreover, most vagal and glossopharyngeal neuron subtypes have not been accessed previously through selective genetic approaches.
To obtain genetic control over newly identified NJP sensory neuron subtypes, we constructed Gabra1-ires-Cre and Crhr2-ires-Cre mice; two-color analysis validated that Cre recombinase drove reporter expression to the correct neurons in these mice (Figure S2). We also obtained other published mouse lines, Calb1-ires-Cre and Npy1r-Cre mice, and observed that they target subsets of NJP sensory neurons. Together with knock-in lines we previously reported to target NJP ganglia (P2ry1-ires-Cre, Gpr65-ires-Cre, Glp1r-ires-Cre, Piezo2-ires-Cre, and Npy2r-ires-Cre mice), this genetic toolkit provides access to 33 out of the 37 NJP neuronal populations, with each Cre line marking different small groups of neurons (Figure 3). Moreover, cell types colabeled by one Cre line are often differentially labeled by a second Cre line; for example, NP17 is labeled by Piezo2-ires-Cre and P2ry1-ires-Cre, NP18 is labeled by Piezo2-ires-Cre but not P2ry1-ires-Cre, and NP19 is labeled by P2ry1-ires-Cre but not Piezo2-ires-Cre. By using this large panel of Cre lines, and treating each Cre line as an independent linear variable, we reasoned that it could be possible to deduce the functions of particular transcriptome-defined sensory neurons.
Vagal P2RY1 neurons evoke a coordinated airway defense program
We used optogenetics to identify NJP sensory neuron subtypes that evoke swallowing. We crossed loxP-ChR2 mice to Calb1-ires-Cre, Piezo2-ires-Cre, Gabra1-ires-Cre, Gpr65-ires-Cre, Npy1r-Cre, Npy2r-ires-Cre, Crhr2-ires-Cre, Glp1r-ires-Cre, and P2ry1-ires-Cre that label different groups of sensory neurons, and wild type mice for control experiments. Optogenetic stimulation was achieved in offspring by focal illumination of the nerve trunk or particular nerve branches.
Light-induced stimulation of vagal P2RY1 neurons evoked frequent pharyngeal swallowing and expiratory reflexes (5.7 and 3.0 per 10 second stimulation, Figure 4A, 4B). Swallows occurred (maximal likelihood) with a latency of 0.67 seconds after optogenetic stimulation and an interswallow interval of 1.65 seconds. Both reflexes were evoked by branch-selective optogenetics involving focal SLN illumination in P2ry1-ires-Cre; loxP-ChR2 mice, but not pharyngeal or glossopharyngeal nerve illumination, indicating involvement of laryngeal neurons (Figure S3A). Furthermore, optogenetic stimulation of vagal P2RY1 neurons in a vagus nerve stump above a trunk transection, but not below, induced swallowing, demonstrating a role for sensory neurons; furthermore, responses persisted after bilateral transection of SLN motor neurons (trunk transection below the illumination point with contralateral SLN transection), consistent with a role for non-vagal motor neurons (Figure S3B). Swallows and expiratory reflexes were more rarely observed following optogenetic activation of vagal NPY1R neurons, and never observed following stimulation of vagal PIEZO2, CALB1, GABRA1, CRHR2, GPR65, NPY2R, or GLP1R neurons (Figure 4A, 4B).
Figure 4. P2RY1 neurons elicit a multi-faceted airway defense program.

(A) Swallows and (B) expiratory reflexes were counted during vagus nerve optogenetics experiments in mice expressing ChR2 from Cre drivers indicated, mean ± sem. (C) UMAP plot indicating NP19 neurons, which are the only neurons that express P2ry1, but not Piezo2, Crhr2, Gpr65, or Gabra1. (D) Cartoon (top) depicting vocal fold dynamics, with images (bottom) of glottic area (green) during abduction and adduction, scale bar: 500 μm. (E) Representative graphs of glottic area over time during (yellow shading) vagus nerve optogenetics; triangles: swallows. (F) In vivo calcium imaging in NJP ganglia from 5 Piezo2-GCaMP* mice (left) and 3 P2ry1-GCaMP* mice (right). Rows indicate responses (ΔF/F, color scale) over time of individual neurons to lung stretch (15 seconds). Magenta and black bars represent tdTomato-positive and negative neurons. Not all unresponsive tdTomato-negative neurons are depicted; numbers at Y-axis base indicate total number of viable imaged neurons. See also Figure S3, S4.
Based on prior RNA in situ hybridization analysis, P2ry1 is expressed in 11.6% of NJP sensory neurons, or ~250 neurons per ganglion (Chang et al., 2015). Single-cell RNA sequencing data indicated abundant levels of P2ry1 transcript in five neuron subtypes (J3, NP16, NP17, NP19, and NP26) and lower levels in four other neuron subtypes (NP5, NP9, NP13, and NP23). Four of these nine P2RY1 neuron subtypes also express Piezo2 (J3, NP16, NP17, and NP26), and all of them except for NP19 express Piezo2, Crhr2, Gpr65 and/or Gabra1. Eight of nine P2RY1 neuron populations are targeted by Cre lines that do not evoke swallowing, pinpointing a particular P2RY1-expressing cell subtype, NP19 neurons (~50 neurons per NJP ganglion), as a candidate mediator of laryngeal reflexes (Figure 4C). Single-cell sequencing of NP19 neurons revealed expression of Phox2b, indicating placodal origin, signature genes that include P2ry1, Slc18a3, Gm2990, Runx3, and Pappa2 (Figure S3C), but not Trpa1 or Trpv1, which are implicated in irritant responses.
Airway challenges evoke coordinated motor responses that also include vocal fold adduction and breathing inhibition, so we asked whether activating vagal P2RY1 neurons evoked these additional hallmarks of airway defense. Vocal fold adduction provides a physical barrier to prevent passage of threatening substances from the larynx to the trachea (Jadcherla et al., 2010). The vocal folds were visualized with a miniaturized fiber endoscope (Movie S2) or by light microscopy (Figure 4D), and normal glottic movements were observed across the respiratory cycle, including full abduction during inspiration and partial adduction during expiration. Optogenetic activation of all vagal sensory neurons in Vglut2-ires-Cre; loxP-ChR2 mice induced sustained and complete glottic closure, while activating P2RY1 or PIEZO2 neurons differentially controlled vocal fold dynamics (Figure 4E). Light-induced stimulation of vagal P2RY1 neurons caused brief, sporadic episodes of complete glottic closure that were synchronized with each swallow. For comparison, optogenetic activation of PIEZO2 neurons decreased the extent of glottic abduction during inspiratory events, without changing the extent of glottic adduction during expiratory events. These findings indicate that vagal P2RY1 neurons evoke episodes of complete vocal fold adduction, a stereotyped signature of airway protection.
Laryngeal challenges also suppress respiration to prevent unintended aspiration of foreign materials into the lungs and upper airways. We noted above that optogenetic activation of P2RY1 neurons caused a transient apnea that occurred with each swallow. Under deeper anesthesia, stimulating P2RY1 or VGLUT2 neurons no longer evoked frequent swallowing, but instead caused a prolonged apnea and reduction in minute volume (Figure S4), as reported previously (Chang et al., 2015). The observation that physiological responses (swallow, cough, or apnea) vary with depth of anesthesia is consistent with previously reported anesthesia-dependent responses mediated by the SLN (Nishino et al., 1990). We note that multiple classes of apnea-promoting neurons were identified by optogenetics under deep anesthesia (Chang et al., 2015; Nonomura et al., 2017), including neurons that express PIEZO2 and function as airway stretch sensors. ~50% of vagal P2RY1 neurons co-express PIEZO2, and optogenetic activation of vagal P2RY1 neurons also inhibits respiration (Chang et al., 2015). It is possible that airway stretch sensors are encompassed by both Piezo2-ires-Cre mice and P2ry1-ires-Cre mice; however, activating PIEZO2 neurons did not evoke swallowing and associated transient apnea under lighter anesthesia, nor expiratory reflexes, suggesting that vagal P2RY1 and PIEZO2 neurons mediate distinct apnea-related reflexes.
To exclude the possibility that P2RY1-evoked apnea is related to airway stretch reception, we imaged calcium transients in individual genetically defined neurons of vagal ganglia (Figure 4F). We generated P2ry1-ires-Cre; loxP-tdTomato; ROSA26-GCaMP3 (P2ry1-GCaMP* mice) and Piezo2-ires-Cre; loxP-tdTomato; ROSA26-GCaMP3 (Piezo2-GCaMP* mice), in which all vagal sensory neurons express GCaMP3 from a constitutive promoter and Cre-expressing cells also express tdTomato. We then imaged single-cell calcium transients in NJP ganglia during trials of airway stretch stimulation, as described previously (Williams et al., 2016). Sensory neurons responsive to airway stretch were rare, dispersed, and marked by tdTomato expression in Piezo2-GCaMP* mice (17/19 neurons), but not in P2ry1-GCaMP* mice (2/23 neurons), Thus, airway stretch sensation is mediated by PIEZO2-positive, P2RY1-negative sensory neurons. Together, these findings reveal separate subtypes of apnea-promoting vagal afferents that can be distinguished using genetic tools. Vagal PIEZO2 neurons mediate airway stretch-induced apnea, while vagal P2RY1 neurons instead evoke a coordinated motor program with multiple signatures of airway defense, including not only apnea, but also vocal fold adduction, pharyngeal swallowing, and expiratory reflexes.
Ablation of vagal P2RY1 neurons eliminates laryngeal responses to airway challenge
Optogenetic activation of P2RY1 neurons evokes a suite of protective reflexes similar to those elicited by various airway threats. We next used targeted neuron ablation to ask whether P2RY1 neurons are required for physiological responses to any particular laryngeal challenge. Targeted ablation of NJP sensory neurons was achieved using Cre-based expression of diphtheria toxin receptor (DTR), as reported previously (Min et al., 2019; Trankner et al., 2014). Mouse cells are normally resistant to diphtheria toxin (DT), but can be rendered sensitive by expression of DTR (Saito et al., 2001). We generated P2ry1-ires-Cre; loxP-DTR mice and injected DT bilaterally into NJP ganglia (P2ry1-ABLATE mice), resulting in efficient ablation of vagal P2RY1 neurons, while sparing other intermingled NJP sensory neurons (Figure 5A, S5). To control for non-specific ablation, we also injected DT into NJP ganglia of loxP-DTR mice lacking a Cre driver (DT-injected control mice), as well as Npy1r-Cre; loxP-DTR (Npy1r-ABLATE) and Gabra1-ires-Cre; loxP-DTR (Gabra1-ABLATE) mice.
Figure 5. P2RY1 neurons mediate responses to laryngeal water and acid challenge.
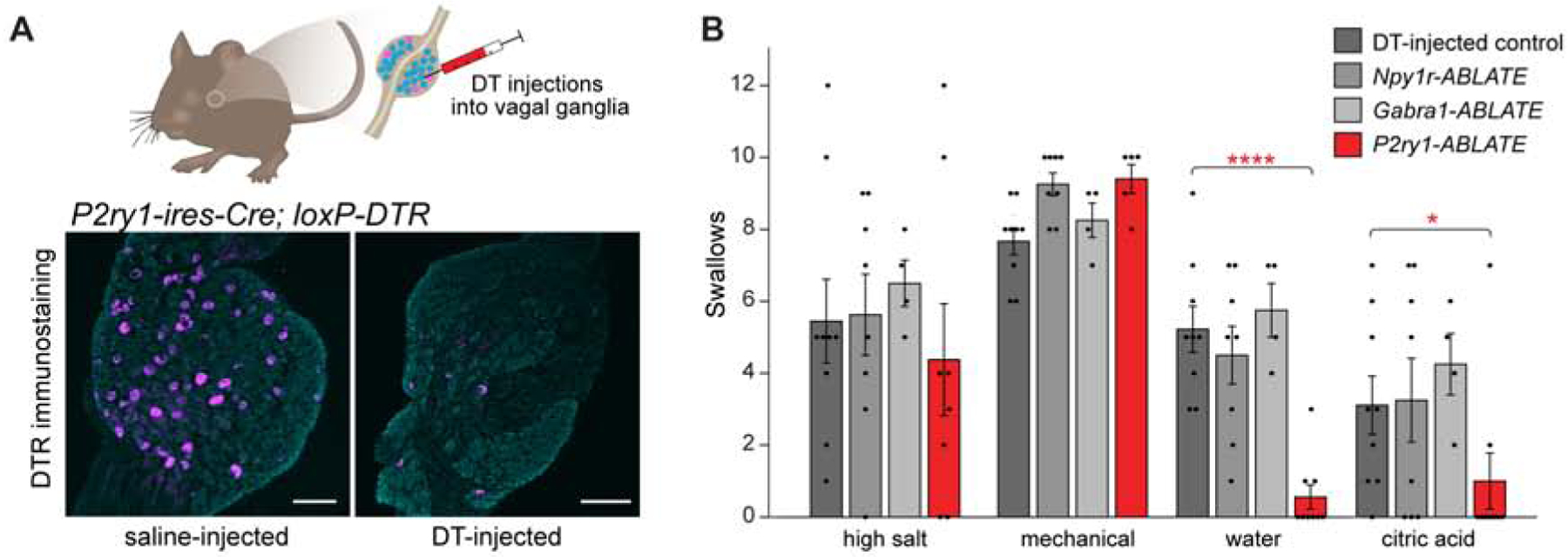
(A) Cartoon (top) of DT-mediated ablation of Cre-expressing cells. DTR immunostaining of NJP ganglia from P2ry1-ires-Cre; loxP-DTR mice 3 weeks after intraganglionic saline (left) or DT (right) injection, far-red fluorescent Nissl counterstain (gray), scale bars: 100 μm. (B) Swallows over 20 second perfusion or 10 mechanical trials in mice indicated, n=4–9 mice, mean ± sem, *p<0.05, **p<0.005, ****p<0.00005. See also Figure S5.
Next, we asked whether swallowing responses to various airway assaults were intact in P2ry1-ABLATE mice using our in vivo preparation for laryngeal perfusion where we observed responses to water, acid, high salt, and force. We found that P2ry1-ABLATE mice, but not other mice analyzed, had impaired swallowing responses to both laryngeal acid and water (Figure 5B). However, swallowing to laryngeal force and high salt was normal in P2ry1-ABLATE mice, with levels similar to control mice, Npy1r-ABLATE mice, and Gabra1-ABLATE mice. These findings indicate that laryngeal P2RY1 neurons are required for sensing specific chemosensory challenges to upper airway integrity.
Vagal P2RY1 neurons densely innervate laryngeal taste buds
Many types of laryngeal terminals have been described, but those that mediate specific aspects of airway defense are unknown due to a lack of genetic tools. Next, we asked whether vagal P2RY1 neurons might innervate the larynx, and if so, whether they form particular terminal structures.
We previously developed a genetic approach to trace the anatomical projections of Cre-expressing vagal sensory neurons (Chang et al., 2015; Min et al., 2019; Williams et al., 2016). Cre-dependent Adeno-associated viruses (AAVs) encoding fluorescent (AAV-flex-tdTomato) or alkaline phosphatase (AAV-flex-AP) reporters were directly injected into NJP ganglia of Cre knock-in mice, and labeled fibers subsequently visualized in peripheral tissues (Figure 6A). Bulk labeling of NJP sensory neurons using Cre-independent AAVs or Cre-dependent AAVs in Vglut2-ires-Cre mice revealed a complex network of neuronal terminals throughout the larynx, with particularly dense innervation of the epithelium of the epiglottis, arytenoids, vocal folds, and subglottis underneath the thyroid cartilage. Diverse terminal morphologies were congested and intermingled across the laryngeal surface, explaining the prior challenges of linking terminal morphology to physiological function.
Figure 6. P2RY1 neurons innervate laryngeal taste buds.

(A) Cartoon depicting Cre-based anatomical mapping of vagal sensory neurons. (B) P2RY1 neuron terminals visualized in an open-book preparation of the larynx after injecting AAV-flex-AP into NJP ganglia of P2ry1-ires-Cre mice, scale bar: 500 μm. (C) Diagram depicting terminals of various neuron types in the larynx after AAV mapping. Red boxes i to vi show regions of larynx highlighted below. (D, E) Representative images of terminals formed by neuron subtypes captured by wholemount analysis of an open-book preparation (top) or in larynx cryosections (bottom) following tdTomato immunostaining, scale bars: 50 μm. (F) Taste buds visualized in larynx cryosections by KRT8 immunohistochemistry (green), scale bar: 100 μm. (G) Two-color immunohistochemistry for KRT8 (green) and tdTomato (magenta) in cryosections of larynx from P2ry1-ires-Cre mice injected with AAV-flex-tdTomato in NJP ganglia, scale bar: 50 μm. See also Figure S6.
We next surveyed the types of upper airway terminals that could be selectively marked using specific Cre lines. We injected NJP ganglia of Npy1r-Cre, Npy2r-ires-Cre, Calb1-ires-Cre, Piezo2-ires-Cre, Gabra1-ires-Cre and P2ry1-ires-Cre mice bilaterally with reporter AAVs, and visualized neuronal endings in the larynx. In each of these six mouse lines, labeled neurites densely innervated the larynx (Figure 6C, S6A), while neurites labeled by similar approaches in Gpr65-ires-Cre mice did not. Moreover, CALB1, P2RY1, NPY1R and NPY2R neurons formed different terminal types in laryngeal epithelium (Figure 6D). CALB1 neurons displayed striking chandelier-shaped terminals concentrated on the ciliated epithelium of the epiglottis and subglottis below the thyroid cartilage. NPY1R and NPY2R neurons, which are transcriptionally distinct subsets of TRPV1 neurons predominantly derived from jugular and nodose ganglia respectively, formed free endings in different airway regions: NPY1R terminals were observed in epiglottis, arytenoids, and trachea, while NPY2R terminals were only found in the trachea up to the cricoid cartilage and not in the vocal folds or above. PIEZO2 and GABRA1 neurons did not access the epithelium, but instead formed distinct subepithelial endings. PIEZO2 fibers encircled laryngeal mucosal glands, and also ramified within intrinsic laryngeal muscles, while GABRA1 neurons displayed large, isolated and branched terminals in the trachealis muscle.
Next, we focused on anatomical characterization of vagal P2RY1 neurons, given their role as laryngeal chemoreceptors. Vagal P2RY1 neurons deposited dense and complex neuronal arbors in ciliated epithelium, including the laryngeal surface of the epiglottis and subglottis, and more dispersed and distinctive endings in squamous epithelium near the vocal folds, arytenoid cartilages and aryepiglottal folds (Figure 6B, S6B). The terminals of P2RY1 neurons in squamous epithelium formed rare, characteristic corpuscles of ~40 μm diameter (Figure 6E). Interestingly, P2RY1 neuron corpuscles directly apposed laryngeal taste buds, as visualized by immunohistochemistry for the taste cell marker KRT8 (Figure 6F, 6G). Every P2RY1 corpuscle in the larynx appeared to innervate a taste bud, and quantitative analysis revealed that the majority of taste buds (33/46, 6 mice) were innervated by a P2RY1 corpuscle; this is likely an underestimation of taste bud innervation based on the partial efficiency of AAV infection (~60%) in NJP ganglion neurons. We also note that some P2RY1 terminals, presumably from the pharyngeal branch of the glossopharyngeal nerve, contacted taste buds in the oropharynx, as well as lingual taste buds in foliate and circumvallate papillae (Figure S6C). Together, these observations suggest that vagal P2RY1 neurons function as second-order chemosensory neurons in the larynx and in other locations.
Epithelial cells as primary sentinels for airway defense
Lingual afferents that mediate bitter, sweet, sour, umami, and salt taste are second-order neurons that receive input from dedicated taste cells on the tongue (Yarmolinsky et al., 2009). In the larynx, it has been debated whether water and acid sensation occurs in upstream sentinel cells or directly by neurons, and adding complexity, multiple laryngeal sensory pathways have been proposed for both stimuli (Harding et al., 1978; Kollarik and Undem, 2002; Sant’Ambrogio et al., 1991). We observed that vagal P2RY1 neurons innervate laryngeal epithelium including taste buds, and are also required for physiological responses to laryngeal acid and water challenge. These findings raise the possibility that upstream sentinel cells first detect water and/or acid in the larynx, and relay information to P2RY1 neurons through specific neurotransmitters. We next sought to test this model by both stimulating and blocking epithelial cell-to-neuron communication.
First, we asked whether optogenetic stimulation of epithelial cells in the larynx triggered airway defense responses. We generated Krt8-CreER; loxP-ChR2 mice, which drives Channelrhodopsin expression in epithelial cells, and within squamous epithelium, selectively to laryngeal taste cells (Figure 7A); reporter expression was not observed in vagal sensory neurons (Figure S7A, S7B). Optogenetic stimulation of epithelial cells in different throat regions was achieved by selective optic fiber positioning (Figure 7B). Light-evoked swallowing was observed by illumination of the larynx (light directed towards the inferior edge of the arytenoids and vocal folds), but not the upper trachea (between thyroid and cricoid cartilages) or posterior oral cavity (Figure 7C). Swallowing was also not evoked by illuminating NJP ganglia in Krt8-CreER; loxP-ChR2 mice. Together, these findings suggest that stimulation of upstream epithelial cells in the larynx is sufficient to activate the neural arc resulting in secondary swallow.
Figure 7. Laryngeal reflexes involve epithelial cell-neuron communication.

(A) Two-color immunohistochemistry for KRT8 (green) and eYFP (yellow) in cryosections of arytenoid epithelium from tamoxifen-treated Krt8-CreER; loxP-ChR2 mice (ChR2 encodes a ChR2-YFP fusion protein), scale bar: 100 μm. (B) Cartoon of epithelium optogenetics in Krt8-CreER; loxP-ChR2 mice, with illumination of oral cavity (1), larynx (2), trachea (3) or NJP ganglion. (C) Swallows per optogenetics trial (10 Hz) in tamoxifen-treated Krt8-CreER; loxP-ChR2 mice (n=3–9, mean ± sem), with light fiber positions indicated in (B). (D) Swallows evoked in wild type (control, n=13–31) and P2×2/P2×3−/− mice (n=6) by test stimuli, mean ± sem, **p<0.005, ***p<5×10−10. (E) Swallows evoked in wild type (control) and P2×2/P2×3−/− mice by laryngeal perfusion (20 seconds) of NaCl solutions of various concentration in water, n=8–31 control mice, 3–6 P2×2/P2×3−/− mice, mean ± sem, **p<0.005, ***p<5×10−10. See also Figure S7.
Next, we asked whether eliminating epithelial cell-neuron communication blocks physiological responses to particular laryngeal stimuli. Lingual taste cells communicate with second-order gustatory fibers using ATP as a neurotransmitter, and responses to sweet, salty, sour, bitter, and umami tastants are disrupted in knockout mice lacking the ionotropic ATP receptors P2×2 and P2×3 (Finger et al., 2005). P2×3-immunoreactive nerve terminals are abundant in laryngeal epithelium (Takahashi et al., 2016); moreover, P2×2 and P2×3 are highly expressed in NP19 neurons, the P2RY1 neuron subset implicated by optogenetics experiments to be involved in laryngeal defense (Figure S7C), raising the possibility that certain laryngeal sensations require ATP signaling as well. We measured swallowing to laryngeal challenge in P2×2/P2×3 knockout mice, and observed that although responses to laryngeal force and high salt were normal, responses to acid were diminished, and responses to laryngeal water were lost (Figure 7D, 7E). Thus, activation of laryngeal epithelial cells is sufficient to evoke swallowing, while knockout of ionotropic ATP receptors impairs swallowing responses to particular laryngeal stimuli. We also noted that water responses were intact in mice lacking phospholipase C β2 (PLCβ2, Figure S7D); PLCβ2 is utilized by solitary chemosensory cells as well as taste cells that detect sweet, bitter, and umami chemicals, but not salty or sour chemicals (Krasteva et al., 2011; Zhang et al., 2003). Together, these findings indicate that certain chemical challenges in the larynx are first detected by epithelial sentinel cells and then communicated to P2RY1 neurons (NP19 neurons) through ATP, ultimately evoking a suite of defensive responses including apnea, vocal fold adduction, and pharyngeal swallow.
DISCUSSION
Ingested food and drink pass directly over the airways en route to the esophagus. The larynx is a critical sensory hotspot for neuronal detection of airway threats and coordination of defensive reflexes to secure airway integrity. Vagal sensory neurons display a multitude of sensory terminals in the larynx, but their functions have been difficult to disentangle without specific genetic reagents. Here, we used single-cell RNA sequencing and a large genetic toolkit to interrogate the cellular diversity of vagal and glossopharyngeal nerves. Cre-guided sensory neuron mapping, ablation, and optogenetics revealed that a small cluster of P2RY1 neurons mediates a suite of stereotyped airway protection responses that include pharyngeal swallow, apnea, expiratory reflexes, and vocal fold adduction. Moreover, P2RY1 neurons form corpuscular terminals that appose laryngeal taste buds, and physiological responses to laryngeal water challenge involve epithelial sentinel cells and ATP signaling.
Each mouse NJP ganglion is small, with ~2,300 neurons, yet rich in diversity, with ~37 molecularly distinct sensory neuron subtypes. This cellular diversity is presumably needed as the vagus nerve innervates vast terrain, projecting to many internal organs within the body, and detects an assortment of sensory cues. Our findings also suggest that a small number of vagal sensory neurons may be sufficient to mediate a particular physiological function; for example, there are only ~50 NP19 neurons in each ganglion devoted to an aspect of airway defense. For comparison, the somatosensory system is much larger in mice, involving hundreds of thousands of sensory neurons located across numerous dorsal root ganglia (DRG) (Gjerstad et al., 2002). Single-cell sequencing of DRG neurons revealed various neuron subtypes involved in touch, pain, proprioception, temperature sensation and itch (Li et al., 2016; Usoskin et al., 2015). The dramatic increase in the total number of DRG neurons presumably enables highly specific spatial discrimination of sensory stimuli, which may not be needed for at least some vagally mediated internal organ sensations. Other sensory systems rely on a smaller number of input channels; in the visual system, there are a few principal sensory cells: rods, cones, and melanopsin-utilizing retinal ganglion cells, while the gustatory system involves five classical taste modalities- sweet, salty, sour, bitter and umami (Yarmolinsky et al., 2009). At the other extreme, the olfactory system contains >1,000 sensory receptors in mice and has the power to detect and discriminate a tremendous diversity of odors (Buck and Axel, 1991). The number of sensations mediated by the vagus nerve has not previously been quantified, but is presumably large based on the underlying cellular diversity. Furthermore, it seems likely that some sensory neurons characterized here mediate internal organ sensory functions that have yet to be determined. Approaches used here provide a technical blueprint for studying the roles of sensory neurons in other aspects of interoception.
Among the many unresolved questions in interoception, laryngeal cell types and terminal morphologies that sense water have remained elusive. We observed numerous terminal structures in the larynx that can be distinguished using various Cre lines, including epithelial chandelier terminals, corpuscular terminals, and free endings, as well as terminals in muscle and around submucosal glands. The sensory functions of most laryngeal terminal types remain unclear. Several prior studies proposed that water was directly sensed in the larynx by first-order free nerve endings rather than specialized terminal structures, although direct functional perturbations were lacking (Anderson et al., 1990; Storey and Johnson, 1975). Here, we present several pieces of evidence demonstrating that a physiological response to laryngeal water instead involves second-order neurons that communicate with upstream epithelial sentinel cells. Genetically guided anatomical mapping revealed that vagal P2RY1 neurons form large corpuscular terminals that appose laryngeal taste buds, which are marked in Krt8-CreER; loxP-ChR2 mice. Ablation of vagal P2RY1 neurons, or knockout of ionotropic ATP receptors, eliminates defensive responses to laryngeal water challenge. Furthermore, optogenetic stimulation of vagal P2RY1 neurons, or laryngeal epithelial cells, evokes hallmarks of airway defense. Based on these findings, we posit that airway epithelial cells first detect water, and then communicate with second-order P2RY1 afferents through ATP action to relay the signal centrally. We do note that SLN nerve responses to water and acid reportedly persist in P2×2/P2×3 knockout mice (Ohkuri et al., 2012), raising the possibility that additional detection pathways exist for these cues, perhaps with relevance for distinct physiological effects.
ATP is utilized as a neurotransmitter by lingual taste buds for communication with afferent nerves (Finger et al., 2005), and may serve a similar epithelial cell-neuron communication role in other organs, such as the skin, bladder and carotid body (Cockayne et al., 2000; Dussor et al., 2009; Piskuric and Nurse, 2013). Here, we show that in the larynx, a water-evoked reflex also requires ionotropic ATP signaling. The functions of laryngeal taste buds have been mysterious, and findings here raise them to be prime candidates involved in airway protection. A recent study proposed that water sensation can occur through sour taste cells in the oral cavity, as marked by Pkd2l1 expression (Zocchi et al., 2017); however, we note that laryngeal water responses did not require bicarbonate washout, that secondary swallowing was not evoked by optogenetic stimulation of glossopharyngeal neurons that innervate lingual taste buds, and that Pkd2l1 could potentially mark discrete taste cells in the larynx. Other classes of epithelial sentinel cells could additionally (1) be innervated by P2RY1 neurons, (2) be targeted in Krt8-CreER; loxP-ChR2 mice, and (3) utilize ATP as a neurotransmitter. Other rare airway epithelial cells include solitary chemosensory cells and neuroendocrine cells. Solitary chemosensory cells express bitter taste receptors that detect irritants, including bacterial molecules involved in quorum sensing, and in response, mediate respiratory depression (Finger et al., 2005; Krasteva et al., 2011). However, bitter receptor agonists administered in the larynx do not evoke swallow, water-evoked swallow persists in Plcβ2 knockout mice, and solitary chemosensory cells reportedly utilize acetylcholine rather than ATP to communicate with afferent nerves.
Multiple chemosensory pathways emanate from the larynx and are relevant for airway defense. Pathogen sensation is proposed to involve not only upstream epithelial cells, but also direct engagement of sensory neurons, for example through formyl peptide receptors or Mas-related G Protein-Coupled Receptors (MRGs) (Chiu et al., 2013; Han et al., 2018). Airway irritants include cigarette smoke, inhaled dust, TRPA1-activating oxidants such hypochlorite and hydrogen peroxide, and the TRPV1 agonist capsaicin (Bessac and Jordt, 2008; Bessac et al., 2008; Canning et al., 2004). Single-cell RNA sequencing indicated that vagal NP19 neurons are nodose-derived, and distinct from sensory neurons that express TRPA1, TRPV1, or MRGs; channelrhodopsin-assisted conduction velocity measurements also indicated that most P2RY1 neurons are A fibers (Chang et al., 2015), and distinct from classical nociceptive C fibers. We note that rarer swallowing was observed following activation of NPY1R neurons, a jugular population that does express both TRPA1 and TRPV1. Furthermore, a population of P2RY1-negative neurons is sufficient to mediate salt sensation; laryngeal salt responses occur at a much higher stimulus threshold (750 mM, Figure 7E) than appetitive salt taste responses (3–30 mM) (Ishiwatari and Bachmanov, 2012), and also do not require P2×2/P2×3, suggesting distinct sensory pathways for oral and laryngeal salt sensation. Together, there appear to be various chemosensory neurons in the upper airways responsible for detecting pathogens, respiratory irritants, and airway challenges from ingested or refluxed food and drink.
Sensorimotor transformations underlying airway protection have been poorly defined, as linking specific reflexes to particular laryngeal neurons is technically challenging in the absence of genetic tools. One model is that various sensory stimuli engage separate information processing streams to mediate different motor responses. Alternatively, various airway challenges might engage common brain command centers that provide a general alarm signal and orchestrate multiple components of a stereotyped motor program (Jean, 2001). The observation that distinct laryngeal challenges (water, acid, high salt, and force) evoke similar physiological reactions (cough, apnea, pharyngeal swallow, and vocal fold adduction) suggests that at least some sensory inputs might converge in responding neural circuits. Here, optogenetic stimulation of a small cluster of transcriptionally defined vagal sensory neurons (NP19 neurons) is sufficient to evoke multiple aspects of airway defense. We observe that motor control centers apparently evoke the command to swallow in a probabilistic manner, with a refractory period between swallows. AAV-based mapping of vagal P2RY1 axons previously revealed preferential innervation of lateral subdivisions within the nucleus of the solitary tract (NTS) in the brainstem (Chang et al., 2015), and NTS neurons display polymodal responses to certain mechanical and chemical stimuli in the larynx (Takagi et al., 1995). However, it is debated whether different sensory neuron subtypes converge in the NTS, or whether polymodality arises earlier in the periphery. Single unit studies in laryngeal afferent nerves reported a continuous response spectrum including both multimodal and unimodal responses, with the frequency of polymodal responses varying across studies (Smith and Hanamori, 1991; Storey, 1968; Takagi et al., 1995). Here, we observed that ablation of P2RY1 neurons impairs responses to challenge from laryngeal water and acid, but not to challenges from high salt or force, indicating that a functional division of labor does exist between at least some sensory neurons in the periphery.
Optogenetic studies revealed distinct classes of apnea-promoting vagal afferents. Vagal PIEZO2 neurons mediate lower airway stretch-induced apnea (Nonomura et al., 2017), a classical reflex termed the Hering Breuer inspiratory reflex, while vagal P2RY1 neurons instead induce apnea as part of an upper airway protective motor program. Other NJP afferents also likely cause apnea, including esophageal distension receptors that protect the airways during acid reflux and vomiting, as well as pharyngeal mechanoreceptors (Lang et al., 2001; Shaker and Hogan, 2000). Yet other NJP sensory neurons instead enhance respiration, such as carotid body-innervating chemoreceptors (Chang, 2017). Thus, respiratory control centers can be differentially toggled by various afferent pathways from vagal and glossopharyngeal nerves.
Stimulating vagal P2RY1 neurons also evokes expiratory reflexes, forced airway evacuations that help expel infiltrate and are nicknamed ‘laryngeal coughs’ (Ludlow, 2015). While mice do not display audible coughing behavior, laryngeal water and acid sensation potently produce cough in other species, including humans (Canning et al., 2004). Since mouse sensory neurons can detect laryngeal stimuli that evoke cough in other species, one model is that sensory pathways for airway threats such as those described here are evolutionarily conserved between mouse and human, even if evoked motor programs have diverged. Consistent with this model, human and mouse contain a similar diversity of laryngeal taste cells (Jette et al., 2020). It is thus intriguing to note that we observed a loss of physiological responses to laryngeal water perfusion in P2×2/P2×3 knockout mice. The P2×3 antagonist AF-219 is an effective suppressant of chronic cough in human patients, although mechanisms have been elusive (Abdulqawi et al., 2015). One prior explanation for AF-219 antitussive action involved blockade of ATP-mediated inflammatory signals that may chronically sensitize cough receptors (Abdulqawi et al., 2015). Data here suggest an alternative model that P2X antagonism directly disrupts laryngeal sensation by some vagal afferents through inhibition of epithelial cell-neuron communication. Multiple types of cough neurons have been proposed, including both A and C fibers with varying sensitivity to capsaicin and anesthetics (Canning et al., 2004; Mazzone and Undem, 2016). Consistent with the notion of multiple afferent pathways for cough, proposed cough receptor terminals in the trachea are distinct from the corpuscular terminals of P2RY1 neurons described here. We note that laryngeal responses to water were lost following P2RY1 neuron ablation and P2×2/P2×3 knockout, but responses to force and high salt were not, further indicating multiple types of airway protection neurons. Since knockout of ionotropic ATP receptors does not block activity of all laryngeal defense pathways, AF-219 may very well have more efficacy as a cough suppressant in some patients than others, depending on which sensory pathways are sensitized or chronically engaged. Understanding the diversity of airway sensory neurons, and which pathways go awry across patient populations, should help optimize clinical treatments.
In addition to chronic cough, persistent or impaired activity in laryngeal sensory neurons can cause other severe clinical problems. Persistent engagement of laryngeal water receptors causes reflexive cough and/or swallowing in adults (Canning et al., 2004; Shingai et al., 1989), but in newborn animals (puppies, kittens and lambs) causes an unadapting apnea which can be fatal if the offending stimulus is not removed (Boggs and Bartlett, 1982; Kovar et al., 1979). In human babies, physical obstruction or fluid accumulation in the larynx may similarly cause unrelenting engagement of airway defense pathways, and seemingly has the potential to cause a sustained apnea that results in Sudden Infant Death Syndrome (SIDS). At the other extreme, breakdown of airway protection leads to respiratory infections such as aspiration pneumonia, which is among the leading causes of death in the elderly. Loss of laryngeal sensory neuron function also causes dysphagia and associated weight loss, speech impairments, and risk of choking, and severely impacts quality of life. Understanding the diversity of airway sensory neurons, and mechanisms by which they function, may provide new insights into human physiology in health and disease.
STAR METHODS
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for resources and reagents should be directed and will be fulfilled by the Lead Contact, Stephen Liberles (Stephen_Liberles@hms.harvard.edu). Mouse lines generated in this study will be deposited at Jackson Laboratory.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
All animal husbandry and procedures were performed in compliance with institutional animal care and use committee guidelines. Crhr2-ires-Cre and Gabra1-ires-Cre mice were constructed as described below; Gpr65-ires-Cre, Glp1r-ires-Cre, Npy2r-ires-Cre, P2ry1-ires-Cre and P2×2/P2×3−/− mice were described before (Chang et al., 2015; Finger et al., 2005; Williams et al., 2016); Vglut2-ires-Cre and loxP-L10-GFP mice were gifts from Bradford Lowell (Beth Israel Deaconess Medical Center); and wild type C57BL/6J (000664), Calb1-ires-Cre (028532), Chat-ires-Cre (006410), Chat-GFP (007902), Krt8-CreER (017947), Npy1r-Cre (030544), Piezo2-EGfp-ires-Cre (027719), Plcβ2−/− (018064), loxP-tdTomato (007908), loxP-ChR2 (012569), and loxP-DTR (007900) mice were purchased (Jackson Laboratory). The constitutive GCaMP3 allele (Rosa26-GCaMP3) was generated by breeding loxP-GCaMP3 (014538) with E2a-Cre mice (Jackson, 003314), and then crossing out the E2a-Cre allele. Single-cell sequencing was performed on male C57BL/6J mice; both male and female mice between 8–24 weeks old were used for all other studies, and no differences based on sex were observed.
METHOD DETAILS
Single-cell RNA sequencing
NJP ganglia were acutely harvested from 40 adult male C57BL/6J mice (across 4 technical replicates), and dissociated (75 min, 37°C, nutation, 1 ml dissociation solution per 10 mice). Dissociation solution is Dulbecco’s Modified Eagle’s Medium containing liberase (55 mg/ml, Roche 05401135001) and DNAse (0.004%, Worthington, LS002007). Cells were pelleted (3 min, 300g, 4°C), suspended (0.2% ovomucoid, 0.004% DNAse, PBS, 200 μl), triturated with a P200 pipette tip, filtered through a 100 μm mesh cell strainer, diluted (300 μl PBS passed also through the strainer), and pelleted again (3 min, 300g, 4°C). Cells were re-suspended (1 ml, PBS-Ca2+/Mg2+, which is PBS supplemented with calcium and magnesium) and loaded onto a Percoll gradient (Sigma-Aldrich, P1644) in a 14 ml round-bottom tube with three layers: 2 ml 55% Percoll in PBS-Ca2+/Mg2+ (bottom), 2 ml 25% Percoll in PBS-Ca2+/Mg2+ (middle) and 1 ml dissociated cells (top). After centrifugation (20 min, 800g, 4°C), the cell-enriched fraction (~1 ml) was collected at the 25%–55% Percoll interface, diluted (4 ml PBS) and centrifuged (8 min, 800g, 4°C). The cell pellet was re-suspended (20 μl PBS-Ca2+/Mg2+), and aliquots removed for cell counting and viability analysis.
Cells (10,000 per sample) were individually encapsulated in small droplets using a 10X Genomics platform (v3 chemistry). Single-cell cDNA was prepared using manufacturer’s protocols, and sequenced on an Illumina NextSeq High Output platform. For analysis, fastqs from each replicate were aligned separately using Cell Ranger to a mm10 reference transcriptome that was custom modified to extend the Glp1r 3′ UTR. The feature-barcode matrices were loaded into Seurat (v3.1.0) for quality control, preprocessing, normalization and clustering (resolution=1.5, PCAs=1:25) using a standard workflow that excluded cells with >25% mitochondrial reads or <200 unique features. Cells from the four replicates were integrated using Seurat’s IntegrateData function. Sensory neurons were identified for subsequent subcluster analysis based on expression of multiple neuronal markers including Vglut2, Prph, Tubb3, Nefl, Uchl1, Phox2b, and Prdm12. Gene ontology enrichments were calculated using Enrichr (https://amp.pharm.mssm.edu/Enrichr/) on the ten most enriched genes in each cluster, ranked by significance (-p value) with exclusion of broadly expressed genes (pct.2 <0.25).
Generating Cre knock-in mice
Crhr2-ires-Cre mice were generated by electroporating a vector containing an ires-Cre cassette and a neomycin selection cassette into 129S4/SvJae-derived J1 ES cells and selected for neomycin resistance. Appropriately targeted cells were identified by Southern blot and PCR analysis, and injected into C57BL/6 blastocysts to generate chimeric animals. The neomycin resistance gene was subsequently removed by crossing with Act-Flpe (Jackson, 003800). Gabra1-ires-Cre mice were generated by pronuclear injection of Cas9 protein, CRISPR sgRNAs targeting the Gabra1 locus 3’ UTR, and a dsDNA vector containing an ires-Cre cassette with long (~4 kb) arms of homology into C57BL/6 embryos. Transgenic pups were screened by PCR analysis, and correct expression of the transgene was verified by two-color RNA in situ hybridization. All Cre driver lines used are viable and fertile, and abnormal phenotypes were not detected. Genotyping primers for Crhr2-ires-Cre mice were TTCCACAGCATCAAGCAGAC (common 5′ primer), ATGGAAAGCAAGGAGCAGAG (wt allele 3′ primer), and AGGAACTGCTTCCTTCACGA (ires-Cre allele 3′ primer), with differentially sized PCR products for the wild type allele (464 bp) and knock-in allele (263 bp). Genotyping primers for Gabra1-ires-Cre mice were: TAACAGCGTCAGCAAAATCG (common 5′ primer), ATTCTCGGTGCAGAGGACTG (wt allele 3′ primer), and GAGGAACTGCTTCCTTCACG (Gabra1-ires-Cre allele 3′ primer), with differentially sized PCR products for the wild type allele (178 bp) and knock-in allele (375 bp).
Optogenetics
For vagus nerve optogenetics, animals were anesthetized with urethane (2 mg/g) unless otherwise specified and the left NJP superganglion was surgically exposed. An optic fiber (200 μm core, Thorlabs) coupled to a DPSS laser light source (473 nm, 150 mW, Ultralaser) was positioned for focal illumination of the vagal trunk (over the ganglion), superior laryngeal branch, petrosal branch, or the glossopharyngeal nerve respectively. Stimulation (10 sec, 10–50 Hz, 5 msec pulses, 120–130 mW/mm2) was controlled by a shutter system (Uniblitz). For epithelial cell optogenetics, Krt8-CreER; loxP-ChR2 mice were treated twice over three days with tamoxifen (100 μg/g in corn oil, Sigma T5648, oral gavage) ten days before testing. Animals were then anesthetized with urethane (2 mg/g), the trachea was surgically exposed, and a transverse incision was made below the ventral thyroid cartilage to expose the inferior aspect of the vocal folds. The optic fiber was positioned over the vocal folds and arytenoids, dorsal tracheal surface, or posterior oral cavity for illumination (10 sec, 10 Hz, 5 msec pulses, 120–130 mW/mm2) as described above.
Physiological measurements
Digastric muscle contraction was characterized by electromyographic recording (Biopac, EMG100C); tracheal pressure was measured by a differential pressure transducer (Harvard Apparatus, Part.No 73–0064), and was used for display of respiratory rhythms; and pharyngeal pressure was monitored by transoral insertion of a pressure transducer (Biopac TSD104A) connected to a size 3 balloon (Harvard Apparatus) coupled to an amplifier and data acquisition system (Biopac MP150). Swallows were identified by digastric muscle contraction, increases in pharyngeal pressure, transient apnea, and hyoid elevation. Subsequent quantification was performed by observing hyoid bone elevation, which was visually observable and occurred concordantly with swallows measured through physiological measurements (Movie S1). Expiratory reflexes were identified and quantified based on elevations of tracheal pressure without concurrent changes in digastric muscle EMG and pharyngeal pressure. Tongue movement and esophageal peristalsis were assessed by visual observation. Vocal fold dynamics were visualized from the inferior aspect through an incision below the cricoid cartilage using a fiber endoscope (Milliscope II, AIT) or stereomicroscope camera (Amscope).
Larynx perfusions
In animals anesthetized with urethane (2 mg/g), the trachea was surgically exposed and opened with a transverse incision 4–5 cartilaginous rings below the thyroid cartilage. A stimulus delivery port was inserted in the back of the mouth over the larynx, the distal trachea was cannulated to provide a breathing tube, and proximal trachea was cannulated to provide a stimulus exit port. Delivery and exit ports were connected to a peristaltic pump to deliver constant perfusion (0.1 ml/min) of saline or various stimuli. This slow flow rate avoids mechanical responses in nerve recordings, and the inserted oral cannula was secured with Kwik-Sil silicone adhesive to prevent leakage or cannula movement-induced responses. The upper airways were washed for 10 minutes, and stimuli were introduced (20 seconds, RT) with PBS washes between stimuli. Stimuli included water, saline (PBS: 137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, and 2 mM KH2PO4, pH 7.4, ~320 mOsmolar), high salt (5× PBS: 685 mM NaCl, 13.5 mM KCl, 40 mM Na2HPO4, and 10 mM KH2PO4, pH 7.4, ~1500 mOsmolar; or, for some experiments, 750 mM NaCl in water, ~1500 mOsmolar), citric acid (25 mM citric acid, pH 2.6, PBS), saccharin (30 mM, PBS), sucrose (100 mM in 2/3X PBS, ~320 mOsmolar), monosodium glutamate (100 mM in 1/3X PBS, ~320 mOsmolar), alanine (100 mM in 2/3X PBS, ~320 mOsmolar), denatonium (1 mM, PBS), quinine (1 mM, PBS), allyl isothiocyanate (3 mM, PBS) or capsaicin (16.4 μM, PBS). All perfused solutions contained Fast Green FCF (0.0025%) to track stimulus delivery. Each perfused stimulus was insulated by air to prevent mixing in the perfusion tubing; swallowing to PBS or air bubbles was never observed. Each mouse was given three stimulus trials, and the largest trial response was recorded. Trials were excluded from analysis if continuous perfusion was disrupted, for example due to mucus-induced clogging, and mice were rarely excluded if depth of urethane-induced anesthesia caused low breathing rate. For mechanical stimulation, PE-50 tubing (exit port) was advanced anteriorly toward the pharynx ten times, and the stimulation trials that evoked swallowing counted.
In situ hybridization
Single-color RNA in situ hybridization (ISH) was performed on cryosections (10 μm) of freshly frozen NJP ganglia as described previously (Chang et al., 2015; Montmayeur et al., 2001), and involved digoxigenin-conjugated cRNA riboprobes, alkaline phosphatase-conjugated anti-digoxigenin antibody (Roche AB_514497) and staining with NBT/BCIP (Thermo Scientific 34042). Two color ISH was performed as described previously with minor modifications. Digoxigenin-conjugated cRNA riboprobes were visualized with alkaline phosphatase-conjugated anti-digoxigenin antibody (Roche AB_514497) and Fast Red (Sigma-Aldrich F4648–50SET), while fluorescein-conjugated cRNA riboprobes were visualized with peroxidase-conjugated anti-fluorescein antibodies and TSA-Cy5.5 (Perkin-Elmer NEL766001KT). High-stringency washes used 0.2X SSC (2×20 minutes, 70°C, VWR 45001–046) and subsequent washes and antibody incubations used maleic acid buffer with 0.05% Tween-20. Phosphatase and peroxidase reporter reactions were performed according to manufacturer’s protocols. Fluorescent images were analyzed with a Leica SP5 II confocal microscope, and colorimetric images were analyzed with a Nikon Ti2 Inverted microscope. cRNA probes were synthesized with fluorescein- (Roche 11685619910) or digoxigenin-conjugated (Roche 11277073910) dNTPs and transcribed in vitro following manufacturer’s protocols (MegaScript T7 or SP6 Kits, Invitrogen AM1334 or AM1330). Probes (listed 5′ to 3′) were amplified using the following primer pairs: Cckar (994 bp) AGGAGGAAGATGGAAGGACC and GCTACTTATTAAGTGAGTCCC, Gabra1 (1128 bp) TGGACAGCCCTCCCAAGATG and TCAGGCTTGACTTCTTTCGGTTC, Crhr2 (835 bp) AAGGACCAGGGCCCAGTGCA and GGGTGGTGCAGATGCCACCC, Mrgprd (966 bp) AGAAGGGAGAGGCTACCAGG and GTTGGGATGCCAGAATTGGACG, Rarres1 (772 bp) GCTGCGCTGCACTTCTTCAAC and CAGGGAGCCCAATCAGGAAAG, Gfra3 (824 bp) ACCTCCACTGCTGATGATCCTG and ACTGCTCAGTTGCACAAGTCC, Cpne6 (822 bp) TGGAGCAGCGAGGAAGTCAG and TTCCTGGAATGTGCTGGTGAAC, Lamp5 (682 bp) GGACCGTTCCCAGTACAAGC and CCCTCACTTGGAACCGACAC, Cysltr2 (930 bp) ATGGAAGTAACTGGGACCC and CTATAGATGAACTTTGCTGAAT, Slc17a7 (906 bp) TCCCTCGTCGCTACATCATCG and TCTGGCCTCCAATGGGTACG, Uts2b (553 bp) AACCTCTCTCTGGTGTGGACTC and CATTCCAGAGTGCAGCCAGTG, and P2ry1 (1122 bp) ATGACCGAGGTGCCTTGG and TCACAAACTCGTGTCTCC.
Histology and immunochemistry
Native GFP fluorescence was analyzed in cryosections of NJP ganglia (Figure 3B, S2A, S2B), except images in Figure 3 from Vglut2-ires-Cre and Calb1-ires-Cre mice, which involved native tdTomato fluorescence. For Figures 5A, 6D (cross-section, bottom), 6E (cross-section, bottom) 6F, 6G, 7A, S6B (cross-section), S6C, and S7A, immunohistochemistry was performed on fixed cryosections of NJP ganglia or larynx. Larynxes were obtained from mice after intracardial perfusion with cold fixative (4% paraformaldehyde, PBS) and cryopreservation (30% sucrose, PBS, 4°C, overnight) while NJP ganglia were harvested fresh and fixed ex vivo (4% paraformaldehyde, PBS, RT, 20 min). Tissue was sectioned (larynx: 35 μm, NJP ganglia: 12 μm), fixed (4% paraformaldehyde, PBS, RT, 10 min), washed (3xPBS), blocked (PBS, 5% donkey serum, 0.1% Triton X-100), and incubated with primary antibody diluted in blocking buffer. Antibody solutions were Chicken-anti-GFP (Abcam, AB_300798, 1:1000), Rabbit-anti-RFP (for tdTomato, Rockland, AB_2209751, 1:500), Goat-anti-HB-EGF (for DTR, R&D Systems, AF-259-NA, 1:250–1:500) or Rat-anti-KRT8/TROMA-I (The Developmental Studies Hybridoma Bank, AB_531826, 1:500). Specimens were washed (blocking buffer) and then incubated with fluorophore-conjugated secondary antibodies (PBS, 5% donkey serum). For open-book wholemount images (Figure 6D top, 6E top, S6B top) larynxes were collected fresh without fixation, cut along the ventral axis for open-book visualization, pinned flat and fixed (4% paraformaldehyde/PBS either 1 hr, RT or overnight, 4°C). Tissue was then stained for tdTomato as above, except antibody incubations (36 hours, 4°C), and subsequent washes (3×12 hours, 4°C) were longer. For visualizing alkaline phosphatase (Figures 6B, S6A), animals were perfused with PBS, and larynxes were collected, fixed (4% paraformaldehyde, 1 hr, RT), and washed in cold PBS. Tissues were then incubated (70°C, 2 hours) in alkaline phosphatase (AP) buffer (0.1 M Tris HCl pH 9.5, 0.1 M NaCl, 50 mM MgCl2, 0.1% Tween20, 5 mM levamisole) and washed twice in AP buffer. AP activity was visualized with NCT/BCIP solution (ThermoFisher Scientific 34042) according to manufacturer’s protocols. Stained samples were post-fixed (4% paraformaldehyde, overnight, 4°C), dehydrated through a series of ethanol washes, and cleared using a 1:2 mixture of benzyl alcohol (Sigma-Aldrich 402834–500ML): benzyl benzoate (Sigma-Aldrich B6630–1L). The tissue was then cut along the ventral axis for open-book visualization. Fluorescence was captured on Leica SP5 II and Nikon Ti2 Inverted Fluorescence microscopes; whole-mount colorimetric stainings were analyzed by light microscopy (Zeiss AxioZoom).
Calcium imaging in vagal ganglia
In vivo NJP ganglion imaging was performed as described previously (Williams et al., 2016). Briefly, Piezo2-GCaMP* and P2ry1-GCaMP* mice were anesthetized by isoflurane inhalation and the left ganglion was surgically exposed after severing central connections and immobilized on a stable platform for confocal imaging (Leica SP5 II) of calcium transients. Stretch of the lower airways was achieved by gas infusion of (1 liter/min, 15 sec) through a tracheal cannula (PE10 tubing, Braintree Scientific) inserted below the thyroid cartilage and advanced to the level of the carina. The baseline activity for each neuron was defined as the average GCaMP3 fluorescence intensity over a three-minute period preceding stimulus delivery, and cells were excluded if they failed to display maximal responses to electrical stimulation greater than seven standard deviations above their baseline means at the conclusion of the experiment. Cells were identified as responsive if (1) maximum GCaMP3 fluorescence exceeded seven standard deviations above the baseline mean, or (2) mean GCaMP3 fluorescence during the stimulation period exceeded three standard deviations above the baseline mean. Response analysis was performed blind to neuron identity.
Cell ablations and nerve transections
Targeted cell ablations were achieved by direct DT injection into NJP ganglia, as reported previously (Min et al., 2019) with minor modifications. Surgically exposed NJP superganglia were serially injected (10 × 10 nl) with DT injection solution (DT Sigma D0564, 0.05% Fast Green FCF Dye, PBS) using a Nanoject III Injector (Drummond). Different lots of DT had different minimal effective doses (0.08 to 2.0 ng), as determined in control titration experiments involving DTR immunostaining as a readout for efficiency. Animals recovered from surgery for 3–4 weeks prior to use in physiological assays. After physiological measurements, NJP ganglia were collected and the extent of ablation quantified by in situ hybridization and/or DTR immunofluorescent labeling. For Figure 1E, the SLN was transected bilaterally below the carotid artery before the branchpoint of the internal and external SLN branches; for Figure S3B, nerve transections were performed unilaterally or bilaterally as indicated. SLN-transected mice displayed normal vocal dynamics across the respiratory cycle, consistent with preservation of the RLN.
AAV-guided anatomical mapping
AAV injections into NJP ganglia were done as reported previously (Chang et al., 2015), with minor modifications. Surgically exposed NJP superganglia were serially injected (10 × 10 nl) with AAV solutions containing 0.05% Fast Green FCF Dye (Sigma) using a Nanoject III Injector (Drummond). AAV-flex-tdTomato was AAV9.CAG.Flex.tdTomato.WPRE.BGH (Addgene viral prep #51503-AAV9, ~1013 genome copies/ml). AAV-flex-AP was AAV9.CAG.flex.PLAP.WPRE.BGh (custom virus, Boston Children’s Hospital Viral Core, ~1013 genome copies/ml). AAV-flex-tdTomato virus solution was diluted 1:1 with a Cre-independent AAV-GFP virus solution (pENN.AAV.CB7.CI.eGFP.WPRE.rBG, Addgene viral prep #105542-AAV9, ~1013 genome copies/ml) prior to injection. Animals recovered from surgery and were sacrificed 4 weeks later for histology.
QUANTIFICATION AND STATISTICAL ANALYSIS
Swallows were identified by digastric muscle contraction, increases in pharyngeal pressure, transient apnea, and hyoid elevation. Subsequent quantification was performed by observing hyoid bone elevation. Expiratory reflexes were identified and quantified based on elevations of tracheal pressure without concurrent changes in digastric muscle EMG and pharyngeal pressure. During quantification of swallowing and expiratory reflexes, multiple trials (2–3) were performed per animal and since transient mucus secretion sometimes caused false negatives in individual trials, the trial with the highest number of events was used for each mouse. Respiratory minute volume was quantified by summing tidal volumes for each breath over the measurement period; custom Matlab scripts were used to identify individual breaths by automated peak detection (at least 20% resting tidal volume) and to calculate tidal volume by integrating the area under the curve from the tracheal pressure trace. Minute volume was measured during light stimulation (ten seconds), and compared to the ten-second period preceding stimulation. Glottic area was defined based on pixel intensity, and measured in each video frame using customized Matlab scripts. For in vivo ganglion imaging experiments, quantification was performed as described (Williams et al., 2016) using customized Matlab scripts.
Data in graphs are represented as means ± sem. All tests of statistical significance were performed using a one-sided Student’s t-test with R software, with the exception of analysis of Figure S4D which used a two-sided Student’s t-test. Data points in Figures 1, 4, 5, 7C, 7D, S3A, S4B and S4D are each derived from a different mouse; data points in Figure S5A are each derived from a different ganglion; data points in Figure S5B are each derived from different sections, with each bar representing a different mouse. Sample sizes from left to right: Figure 1D (6, 6, 6, 13, 6, 6, 6, 6, 6, 6, 6, 6, 6), Figure 1E (8, 2, 31, 7, 13, 5, 20, 6, 17, 6), Figure 4A (2, 10, 4, 3, 8, 2, 2, 3, 2, 2, 14, 5), Figure 4B (4, 4, 4, 3, 3, 2, 2, 3, 2, 2, 8, 5), Figure 5B (9, 8, 4, 9, 9, 8, 4, 9, 9, 8, 4, 9, 9, 8, 4, 9), Figure 7C (5, 9, 5, 3), Figure 7D (17, 3, 13, 6, 20, 6, 31, 6), Figure 7E (Control: 31, 8, 7, 8, 8, 13, 8; P2×2/×3 KO: 6, 3, 3, 3, 3, 6, 3), Figure S3A (4, 3, 3, 5–6, 2, 1, 3, 2, 2, 4–5, 4–5), Figure S3B (14, 5, 5, 4), Figure S4B (4, 3, 4, 10), Figure S4D (6, 4, 6, 5, 6), Figure S5A (6, 12), Figure S5B (9, 9, 9, 7, 6, 9, 6, 11, 10, 6, 4) and Figure S7D (31, 6, 6). Calculations for latency to first swallow and interswallow interval were based on 248 swallows from 40 optogenetic stimulation trials in 4 P2ry1-ires-Cre; loxP-ChR2 mice.
DATA AND CODE AVAILABILITY
Single-cell transcriptome data from vagal and glossopharyngeal sensory neurons are publicly available through GEO (GSE145216); other primary images and data are available at Mendeley (https://dx.doi.org/10.17632/4gfm8jp6dr.1).
Supplementary Material
Figure S1. Single-cell transcriptomes of NJP sensory neurons, Related to Figure 2 (A) UMAP plots derived from single-cell transcriptomes indicating all cell type diversity within pooled samples (46,712 cells, 4 replicates, 10 mice per replicate). (B) UMAP plots pseudocolored by biological replicate identity, unique gene counts (nFeature_RNA), mitochondrial sequence content (%), and read counts (nCount_RNA) across sensory neuron populations. (C) Transcriptome statistics and cell type distributions across all cells (left) and sensory neurons (right). (D) UMAP plots from single-cell transcriptomes show expression of indicated genes across sensory neurons. (E) Gene ontology enrichment was calculated using Enrichr for the top 10 most differentially expressed genes from each sensory neuron cluster.
Figure S2. Validation of Gabra1-ires-Cre and Crhr2-ires-Cre mice, Related to Figure 3 Two-color analysis in cryosections of NJP ganglia from Gabra1-ires-Cre; loxP-L10-GFP (A) and Crhr2-ires-Cre; loxP-L10-GFP mice (B). Fluorescence signal is from RNA in situ hybridization (red) using cRNA probes that recognize Gabra1 (A) and Crhr2 (B) and native GFP fluorescence (green). GFP fluorescence was imaged prior to initiation of RNA in situ hybridization experiments, and was superimposed post hoc, scale bar, 100 μm. The numbers of cells expressing the indicated mRNA alone (red), GFP alone (green) or both (yellow) were counted.
Figure S3. Nerve branch-specific optogenetics, Related to Figure 4 (A) Swallows evoked by focal illumination (20 Hz) of the superior laryngeal nerve (left), pharyngeal branch of the vagus nerve (middle), and glossopharyngeal nerve (right) in loxP-ChR2 (CONTROL), Vglut2-ires-Cre; loxP-ChR2 (VGLUT2), Calb1-ires-Cre; loxP-ChR2 (CALB1), Piezo2-ires-Cre; loxP-ChR2 (PIEZO2), Gabra1-ires-Cre; loxP-ChR2 (GABRA1), Gpr65-ires-Cre; loxP-ChR2 (GPR65), Npy2r-ires-Cre; loxP-ChR2 (NPY2R), Chat-ires-Cre; loxP-ChR2 (CHAT), Crhr2-ires-Cre; loxP-ChR2 (CRHR2), Glp1r-ires-Cre; Vglut2-ires-FlpO; RCFL-CatCh (GLP1R), P2ry1-ires-Cre; loxP-ChR2 (P2RY1), and Npy1r-Cre; loxP-ChR2 (NPY1R) mice, n=1–6 mice, mean ± sem. (B) Swallows evoked in optogenetics experiments in P2ry1-ires-Cre; loxP-ChR2 mice involving illumination regions and nerve transections indicated, including vagus nerve (1) trunk illumination in intact mice, (2) trunk illumination above vagus nerve transection, (3) trunk illumination below vagus nerve transection, and (4) trunk illumination above vagus nerve transection with contralateral SLN transection, n=4–14, mean ± sem. (C) UMAP plots from single-cell transcriptomes show expression of indicated genes.
Figure S4. Anesthesia depth alters optogenetically-evoked reflexes, Related to Figure 4 (A) Representative respiratory rhythms in Vglut2-ires-Cre; loxP-ChR2 mice were measured before, during (yellow shading) and after vagus nerve optogenetic activation (10 seconds, 5 msec pulses) under different anesthetics: 1%, 1.5% or 2% isoflurane (inhaled) or urethane (2 mg/g intraperitoneal injection) and frequencies (left: 20 Hz, right: 50 Hz). (B) Swallows evoked during optogenetic stimulation (20 Hz) of vagal sensory neurons in Vglut2-ires-Cre; loxP-ChR2 mice using anesthetics indicated. (C) Representative respiratory rhythms (changes in tracheal pressure over time) before, during (yellow shading), and after focal illumination (20 Hz, 1.5% isoflurane) of the vagus nerve trunk in loxP-ChR2 (CONTROL), Vglut2-ires-Cre; loxP-ChR2 (VGLUT2), Gabra1-ires-Cre; loxP-ChR2 (GABRA1), P2ry1-ires-Cre; loxP-ChR2 (P2RY1), and Piezo2-ires-Cre; loxP-ChR2 (PIEZO2) mice. (D) Quantification of respiratory minute volume changes induced by optogenetic activation (10 sec) of neurons indicated, compared with pre-stimulus baseline (10 sec), n: 4–6, mean ± sem, *p<0.01, ***p<0.0005.
Figure S5. Validating cell-specific ablation, Related to Figure 5 (A) Number of DTR-positive neurons counted following DTR immunohistochemistry in cryosections of NJP ganglia from P2ry1-ires-Cre; loxP-DTR mice injected with saline (−DT, n=6 ganglia) or DT (+DT, n=12 ganglia), mean ± sem, ****p<8×10−5. (B) Two-color fluorescence in situ hybridization using cRNA probes to P2ry1 and Cckar in cryosections of NJP ganglia from P2ry1-ires-Cre; loxP-DTR mice injected intraganglionically with saline (control, gray, n=5 ganglia), or DT (red, P2ry1-ABLATE, n=6 ganglia). Each bar indicates labeled cells from a single mouse, with each dot indicating cell counts from one section, mean ± sem. (C) Representative images from cryosections of NJP ganglia from control and P2ry1-ABLATE mice after two-color fluorescence in situ hybridization using cRNA probes to P2ry1 (cyan) and Cckar (magenta), scale bars: 100 μm.
Figure S6. Diversity of upper airway afferent terminal morphology, Related to Figure 6 (A) Neuron terminals were visualized in an open-book preparation of the larynx using an alkaline phosphatase substrate after injection of AAV-flex-AP into NJP ganglia of Npy1r-Cre (NPY1R), Npy2r-ires-Cre (NPY2R), Piezo2-ires-Cre (PIEZO2), Calb1-ires-Cre (CALB1) and Gabra1-ires-Cre (GABRA1) mice, scale bar: 500 μm. (B) Terminals in ciliated epithelium of the larynx observed following immunohistochemistry for tdTomato after injecting AAV-flex-tdTomato virus into NJP ganglia of P2ry1-ires-Cre mice, scale bars: 50 μm. (C) Two-color immunohistochemistry for KRT8 (green) and tdTomato (magenta) in cryosections of oropharynx from P2ry1-ires-Cre mice injected with AAV-flex-tdTomato in NJP ganglia, scale bar: 50 μm.
Figure S7. Expression of ChR2 in Krt8-CreER; loxP-ChR2 mice; NP19 neurons express ionotropic ATP receptors, Related to Figure 7 (A) Two-color immunohistochemistry for KRT8 (green) and eYFP (yellow) in cryosections of vagal ganglia (left), laryngeal squamous epithelium (middle), and laryngeal ciliated epithelium (right) from tamoxifen-injected Krt8-CreER; loxP-ChR2 mice which express a ChR2-YFP fusion protein, scale bars: 100 μm (vagal ganglia) and 50 μm (epithelia). (B, C) UMAP plots showing expression of Krt8, P2×2, or P2×3 in vagal sensory neurons; NP19 neurons are circled. (D) Swallows to laryngeal water perfusion (20 seconds) in mice indicated, n=6–31, mean ± sem, n.s. not significant, p>0.05.
Table S1. Signature genes for NJP sensory neuron subtypes, Related to Figure 2 A list of the five most enriched genes for each NJP sensory neuron cluster, with gene numbers corresponding to rows in Figure 2C.
Movie S2. Evoking vocal fold adduction by optogenetics, Related to Figure 4 Representative movie of vocal fold adduction during optogenetic activation (blue laser light) of the vagus nerve in a Vglut2-ires-Cre; loxP-ChR2 mice. Vocal fold dynamics were visualized using a miniaturized fiber endoscope.
Movie S1. Evoking swallows by optogenetics, Related to Figure 1 Representative movie of evoked swallowing during optogenetic activation (blue laser light) of the vagus nerve in a Vglut2-ires-Cre; loxP-ChR2 mice. The hyoid bone elevates with each swallow.
HIGHLIGHTS.
Broad genetic access enables functional dissection of a vagal sensory neuron atlas
Rare P2RY1 sensory neurons guard the airways by engaging an airway defense program
P2RY1 neurons innervate the laryngeal epithelium and appose laryngeal taste buds
Epithelial cells detect laryngeal water/acid and communicate to P2RY1 neurons via ATP
ACKNOWLEDGMENTS
We thank David Ginty, Jay Rajagopal, Isaac Chiu, John Flanagan, Rui Chang, Phillip Song and Marie Bao for manuscript comments, Brad Lowell for mice, and David Strochlic for images. Silvia Huerta and Yandan Wang assisted with anatomical analysis, Jian Shu and Eric Lander with pilot single-cell RNA sequencing, Eli Weinstein with analyzing vocal fold dynamics, the HMS Nikon Imaging Center with microscopy, the Boston Children’s Hospital Mouse Gene Manipulation Core with making mice, and the HMS Biopolymers Facility with single-cell sequencing. The work was supported by NIH grants to SDL (DP1 AT009497 and R01 HL132255), a Faculty Scholars Award from the Howard Hughes Medical Institute to SDL, an American Diabetes Association postdoctoral fellowship to RDB, NIH grant F31 HL132645 to BDU, and NIH grant F30 CA177170 to EKW. SLP is an Open Philanthropy Fellow of the Life Sciences Research Foundation. SDL is an investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
SDL is a consultant for Kallyope, Inc.
REFERENCES
- Abdulqawi R, Dockry R, Holt K, Layton G, McCarthy BG, Ford AP, and Smith JA (2015). P2×3 receptor antagonist (AF-219) in refractory chronic cough: a randomised, double-blind, placebo-controlled phase 2 study. Lancet 385, 1198–1205. [DOI] [PubMed] [Google Scholar]
- Anderson JW, Sant’Ambrogio FB, Mathew OP, and Sant’Ambrogio G (1990). Water-responsive laryngeal receptors in the dog are not specialized endings. Respir Physiol 79, 33–43. [DOI] [PubMed] [Google Scholar]
- Bai L, Mesgarzadeh S, Ramesh KS, Huey EL, Liu Y, Gray LA, Aitken TJ, Chen Y, Beutler LR, Ahn JS, et al. (2019). Genetic Identification of Vagal Sensory Neurons That Control Feeding. Cell 179, 1129–1143 e1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baral P, Umans BD, Li L, Kirschbaum T, Wei Y, Zhou Y, Yipp BG, Liberles SD, and Chiu IM (2018). Nociceptor sensory neurons suppress neutrophil and gamma delta T cell responses in bacterial lung infections and lethal pneuomonia. Nature Medicine in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessac BF, and Jordt SE (2008). Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda) 23, 360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessac BF, Sivula M, von Hehn CA, Escalera J, Cohn L, and Jordt SE (2008). TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest 118, 1899–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs DF, and Bartlett D Jr. (1982). Chemical specificity of a laryngeal apneic reflex in puppies. J Appl Physiol Respir Environ Exerc Physiol 53, 455–462. [DOI] [PubMed] [Google Scholar]
- Bolser DC (1991). Fictive cough in the cat. J Appl Physiol (1985) 71, 2325–2331. [DOI] [PubMed] [Google Scholar]
- Buck L, and Axel R (1991). A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell 65, 175–187. [DOI] [PubMed] [Google Scholar]
- Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, and Undem BJ (2004). Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol 557, 543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AJ (2017). Acute oxygen sensing by the carotid body: from mitochondria to plasma membrane. J Appl Physiol (1985) 123, 1335–1343. [DOI] [PubMed] [Google Scholar]
- Chang RB, Strochlic DE, Williams EK, Umans BD, and Liberles SD (2015). Vagal Sensory Neuron Subtypes that Differentially Control Breathing. Cell 161, 622–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, Wainger B, Strominger A, Muralidharan S, Horswill AR, et al. (2013). Bacteria activate sensory neurons that modulate pain and inflammation. Nature 501, 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, et al. (2000). Urinary bladder hyporeflexia and reduced pain-related behaviour in P2×3-deficient mice. Nature 407, 1011–1015. [DOI] [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JC, and Schultz HD (1989). Afferent pathways involved in reflex regulation of airway smooth muscle. Pharmacology & therapeutics 42, 1–63. [DOI] [PubMed] [Google Scholar]
- Doty RW (1951). Influence of stimulus pattern on reflex deglutition. Am J Physiol 166, 142–158. [DOI] [PubMed] [Google Scholar]
- Dussor G, Koerber HR, Oaklander AL, Rice FL, and Molliver DC (2009). Nucleotide signaling and cutaneous mechanisms of pain transduction. Brain research reviews 60, 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feindel W (1956). The neural pattern of the epiglottis. J Comp Neurol 105, 269–285. [DOI] [PubMed] [Google Scholar]
- Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, and Kinnamon SC (2005). ATP signaling is crucial for communication from taste buds to gustatory nerves. Science (New York, NY 310, 1495–1499. [DOI] [PubMed] [Google Scholar]
- Fox EA, Phillips RJ, Baronowsky EA, Byerly MS, Jones S, and Powley TL (2001). Neurotrophin-4 deficient mice have a loss of vagal intraganglionic mechanoreceptors from the small intestine and a disruption of short-term satiety. J Neurosci 21, 8602–8615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerstad MD, Tandrup T, Koltzenburg M, and Jakobsen J (2002). Predominant neuronal B-cell loss in L5 DRG of p75 receptor-deficient mice. J Anat 200, 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Limjunyawong N, Ru F, Li Z, Hall OJ, Steele H, Zhu Y, Wilson J, Mitzner W, Kollarik M, et al. (2018). Mrgprs on vagal sensory neurons contribute to bronchoconstriction and airway hyper-responsiveness. Nature neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding R, Johnson P, and McClelland ME (1978). Liquid-sensitive laryngeal receptors in the developing sheep, cat and monkey. J Physiol 277, 409–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwatari Y, and Bachmanov AA (2012). NaCl taste thresholds in 13 inbred mouse strains. Chemical senses 37, 497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadcherla SR, Hogan WJ, and Shaker R (2010). Physiology and pathophysiology of glottic reflexes and pulmonary aspiration: from neonates to adults. Semin Respir Crit Care Med 31, 554–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari S, Prince RA, Kim DY, and Paydarfar D (2003). Sensory regulation of swallowing and airway protection: a role for the internal superior laryngeal nerve in humans. J Physiol 550, 287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean A (2001). Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiological reviews 81, 929–969. [DOI] [PubMed] [Google Scholar]
- Jette ME, Clary MS, Prager JD, and Finger TE (2020). Chemical receptors of the arytenoid: A comparison of human and mouse. Laryngoscope 130, 423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollarik M, and Undem BJ (2002). Mechanisms of acid-induced activation of airway afferent nerve fibres in guinea-pig. J Physiol 543, 591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar I, Selstam U, Catterton WZ, Stahlman MT, and Sundell HW (1979). Laryngeal chemoreflex in newborn lambs: respiratory and swallowing response to salts, acids, and sugars. Pediatr Res 13, 1144–1149. [DOI] [PubMed] [Google Scholar]
- Krasteva G, Canning BJ, Hartmann P, Veres TZ, Papadakis T, Muhlfeld C, Schliecker K, Tallini YN, Braun A, Hackstein H, et al. (2011). Cholinergic chemosensory cells in the trachea regulate breathing. Proceedings of the National Academy of Sciences of the United States of America 108, 9478–9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupari J, Haring M, Agirre E, Castelo-Branco G, and Ernfors P (2019). An Atlas of Vagal Sensory Neurons and Their Molecular Specialization. Cell Rep 27, 2508–2523 e2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang IM, Medda BK, and Shaker R (2001). Mechanisms of reflexes induced by esophageal distension. American journal of physiology Gastrointestinal and liver physiology 281, G1246–1263. [DOI] [PubMed] [Google Scholar]
- Li CL, Li KC, Wu D, Chen Y, Luo H, Zhao JR, Wang SS, Sun MM, Lu YJ, Zhong YQ, et al. (2016). Somatosensory neuron types identified by high-coverage single-cell RNA-sequencing and functional heterogeneity. Cell Res 26, 967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow CL (2015). Laryngeal Reflexes: Physiology, Technique, and Clinical Use. J Clin Neurophysiol 32, 284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone SB, Tian L, Moe AAK, Trewella MW, Ritchie ME, and McGovern AE (2019). Transcriptional Profiling of Individual Airway Projecting Vagal Sensory Neurons. Mol Neurobiol. [DOI] [PubMed] [Google Scholar]
- Mazzone SB, and Undem BJ (2016). Vagal Afferent Innervation of the Airways in Health and Disease. Physiological reviews 96, 975–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min S, Chang RB, Prescott SL, Beeler B, Joshi NR, Strochlic DE, and Liberles SD (2019). Arterial baroreceptors sense blood pressure through decorated aortic claws. Cell Reports 29, 2192–2201 e2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montmayeur JP, Liberles SD, Matsunami H, and Buck LB (2001). A candidate taste receptor gene near a sweet taste locus. Nature neuroscience 4, 492–498. [DOI] [PubMed] [Google Scholar]
- Mu L, and Sanders I (2000). Sensory nerve supply of the human oro- and laryngopharynx: a preliminary study. Anat Rec 258, 406–420. [DOI] [PubMed] [Google Scholar]
- Nishino T, Hiraga K, and Yokokawa N (1990). Laryngeal and respiratory responses to tracheal irritation at different depths of enflurane anesthesia in humans. Anesthesiology 73, 46–51. [DOI] [PubMed] [Google Scholar]
- Nonomura K, Woo SH, Chang RB, Gillich A, Qiu Z, Francisco AG, Ranade SS, Liberles SD, and Patapoutian A (2017). Piezo2 senses airway stretch and mediates lung inflation-induced apnoea. Nature 541, 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuri T, Horio N, Stratford JM, Finger TE, and Ninomiya Y (2012). Residual chemoresponsiveness to acids in the superior laryngeal nerve in “taste-blind” (P2×2/P2×3 double-KO) mice. Chemical senses 37, 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskuric NA, and Nurse CA (2013). Expanding role of ATP as a versatile messenger at carotid and aortic body chemoreceptors. J Physiol 591, 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Iwawaki T, Taya C, Yonekawa H, Noda M, Inui Y, Mekada E, Kimata Y, Tsuru A, and Kohno K (2001). Diphtheria toxin receptor-mediated conditional and targeted cell ablation in transgenic mice. Nature biotechnology 19, 746–750. [DOI] [PubMed] [Google Scholar]
- Sampson S, and Eyzaguirre C (1964). Some Functional Characteristics of Mechanoreceptors in the Larynx of the Cat. J Neurophysiol 27, 464–480. [DOI] [PubMed] [Google Scholar]
- Sant’Ambrogio G, Anderson JW, Sant’Ambrogio FB, and Mathew OP (1991). Response of laryngeal receptors to water solutions of different osmolality and ionic composition. Respir Med 85 Suppl A, 57–60. [DOI] [PubMed] [Google Scholar]
- Santoso LF, Kim DY, and Paydarfar D (2019). Sensory dysphagia: A case series and proposed classification of an under recognized swallowing disorder. Head Neck 41, E71–E78. [DOI] [PubMed] [Google Scholar]
- Shaker R, and Hogan WJ (2000). Reflex-mediated enhancement of airway protective mechanisms. Am J Med 108 Suppl 4a, 8S–14S. [DOI] [PubMed] [Google Scholar]
- Shingai T, Miyaoka Y, Ikarashi R, and Shimada K (1989). Swallowing reflex elicited by water and taste solutions in humans. Am J Physiol 256, R822–826. [DOI] [PubMed] [Google Scholar]
- Smith DV, and Hanamori T (1991). Organization of gustatory sensitivities in hamster superior laryngeal nerve fibers. J Neurophysiol 65, 1098–1114. [DOI] [PubMed] [Google Scholar]
- Steele CM, and Miller AJ (2010). Sensory input pathways and mechanisms in swallowing: a review. Dysphagia 25, 323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey AT (1968). A functional analysis of sensory units innervating epiglottis and larynx. Exp Neurol 20, 366–383. [DOI] [PubMed] [Google Scholar]
- Storey AT, and Johnson P (1975). Laryngeal water receptors initiating apnea in the lamb. Exp Neurol 47, 42–55. [DOI] [PubMed] [Google Scholar]
- Takagi S, Umezaki T, and Shin T (1995). Convergence of laryngeal afferents with different natures upon cat NTS neurons. Brain Res Bull 38, 261–268. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Nakamuta N, and Yamamoto Y (2016). Morphology of P2×3-immunoreactive nerve endings in the rat laryngeal mucosa. Histochem Cell Biol 145, 131–146. [DOI] [PubMed] [Google Scholar]
- Tizzano M, Gulbransen BD, Vandenbeuch A, Clapp TR, Herman JP, Sibhatu HM, Churchill ME, Silver WL, Kinnamon SC, and Finger TE (2010). Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proceedings of the National Academy of Sciences of the United States of America 107, 3210–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trankner D, Hahne N, Sugino K, Hoon MA, and Zuker C (2014). Population of sensory neurons essential for asthmatic hyperreactivity of inflamed airways. Proceedings of the National Academy of Sciences of the United States of America 111, 11515–11520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers SP, and Nicklas K (1990). Taste bud distribution in the rat pharynx and larynx. Anat Rec 227, 373–379. [DOI] [PubMed] [Google Scholar]
- Troche MS, Brandimore AE, Godoy J, and Hegland KW (2014). A framework for understanding shared substrates of airway protection. J Appl Oral Sci 22, 251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling-Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV, et al. (2015). Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nature neuroscience 18, 145–153. [DOI] [PubMed] [Google Scholar]
- Williams EK, Chang RB, Strochlic DE, Umans BD, Lowell BB, and Liberles SD (2016). Sensory Neurons that Detect Stretch and Nutrients in the Digestive System. Cell 166, 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Hosono I, Atoji Y, and Suzuki Y (1997). Morphological study of the vagal afferent nerve endings in the laryngeal mucosa of the dog. Ann Anat 179, 65–73. [DOI] [PubMed] [Google Scholar]
- Yarmolinsky DA, Zuker CS, and Ryba NJ (2009). Common sense about taste: from mammals to insects. Cell 139, 234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng WZ, Marshall KL, Min S, Daou I, Chapleau MW, Abboud FM, Liberles SD, and Patapoutian A (2018). PIEZOs mediate neuronal sensing of blood pressure and the baroreceptor reflex. Science 362, 464–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, and Ryba NJ (2003). Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112, 293–301. [DOI] [PubMed] [Google Scholar]
- Zocchi D, Wennemuth G, and Oka Y (2017). The cellular mechanism for water detection in the mammalian taste system. Nature neuroscience 20, 927–933. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Single-cell transcriptomes of NJP sensory neurons, Related to Figure 2 (A) UMAP plots derived from single-cell transcriptomes indicating all cell type diversity within pooled samples (46,712 cells, 4 replicates, 10 mice per replicate). (B) UMAP plots pseudocolored by biological replicate identity, unique gene counts (nFeature_RNA), mitochondrial sequence content (%), and read counts (nCount_RNA) across sensory neuron populations. (C) Transcriptome statistics and cell type distributions across all cells (left) and sensory neurons (right). (D) UMAP plots from single-cell transcriptomes show expression of indicated genes across sensory neurons. (E) Gene ontology enrichment was calculated using Enrichr for the top 10 most differentially expressed genes from each sensory neuron cluster.
Figure S2. Validation of Gabra1-ires-Cre and Crhr2-ires-Cre mice, Related to Figure 3 Two-color analysis in cryosections of NJP ganglia from Gabra1-ires-Cre; loxP-L10-GFP (A) and Crhr2-ires-Cre; loxP-L10-GFP mice (B). Fluorescence signal is from RNA in situ hybridization (red) using cRNA probes that recognize Gabra1 (A) and Crhr2 (B) and native GFP fluorescence (green). GFP fluorescence was imaged prior to initiation of RNA in situ hybridization experiments, and was superimposed post hoc, scale bar, 100 μm. The numbers of cells expressing the indicated mRNA alone (red), GFP alone (green) or both (yellow) were counted.
Figure S3. Nerve branch-specific optogenetics, Related to Figure 4 (A) Swallows evoked by focal illumination (20 Hz) of the superior laryngeal nerve (left), pharyngeal branch of the vagus nerve (middle), and glossopharyngeal nerve (right) in loxP-ChR2 (CONTROL), Vglut2-ires-Cre; loxP-ChR2 (VGLUT2), Calb1-ires-Cre; loxP-ChR2 (CALB1), Piezo2-ires-Cre; loxP-ChR2 (PIEZO2), Gabra1-ires-Cre; loxP-ChR2 (GABRA1), Gpr65-ires-Cre; loxP-ChR2 (GPR65), Npy2r-ires-Cre; loxP-ChR2 (NPY2R), Chat-ires-Cre; loxP-ChR2 (CHAT), Crhr2-ires-Cre; loxP-ChR2 (CRHR2), Glp1r-ires-Cre; Vglut2-ires-FlpO; RCFL-CatCh (GLP1R), P2ry1-ires-Cre; loxP-ChR2 (P2RY1), and Npy1r-Cre; loxP-ChR2 (NPY1R) mice, n=1–6 mice, mean ± sem. (B) Swallows evoked in optogenetics experiments in P2ry1-ires-Cre; loxP-ChR2 mice involving illumination regions and nerve transections indicated, including vagus nerve (1) trunk illumination in intact mice, (2) trunk illumination above vagus nerve transection, (3) trunk illumination below vagus nerve transection, and (4) trunk illumination above vagus nerve transection with contralateral SLN transection, n=4–14, mean ± sem. (C) UMAP plots from single-cell transcriptomes show expression of indicated genes.
Figure S4. Anesthesia depth alters optogenetically-evoked reflexes, Related to Figure 4 (A) Representative respiratory rhythms in Vglut2-ires-Cre; loxP-ChR2 mice were measured before, during (yellow shading) and after vagus nerve optogenetic activation (10 seconds, 5 msec pulses) under different anesthetics: 1%, 1.5% or 2% isoflurane (inhaled) or urethane (2 mg/g intraperitoneal injection) and frequencies (left: 20 Hz, right: 50 Hz). (B) Swallows evoked during optogenetic stimulation (20 Hz) of vagal sensory neurons in Vglut2-ires-Cre; loxP-ChR2 mice using anesthetics indicated. (C) Representative respiratory rhythms (changes in tracheal pressure over time) before, during (yellow shading), and after focal illumination (20 Hz, 1.5% isoflurane) of the vagus nerve trunk in loxP-ChR2 (CONTROL), Vglut2-ires-Cre; loxP-ChR2 (VGLUT2), Gabra1-ires-Cre; loxP-ChR2 (GABRA1), P2ry1-ires-Cre; loxP-ChR2 (P2RY1), and Piezo2-ires-Cre; loxP-ChR2 (PIEZO2) mice. (D) Quantification of respiratory minute volume changes induced by optogenetic activation (10 sec) of neurons indicated, compared with pre-stimulus baseline (10 sec), n: 4–6, mean ± sem, *p<0.01, ***p<0.0005.
Figure S5. Validating cell-specific ablation, Related to Figure 5 (A) Number of DTR-positive neurons counted following DTR immunohistochemistry in cryosections of NJP ganglia from P2ry1-ires-Cre; loxP-DTR mice injected with saline (−DT, n=6 ganglia) or DT (+DT, n=12 ganglia), mean ± sem, ****p<8×10−5. (B) Two-color fluorescence in situ hybridization using cRNA probes to P2ry1 and Cckar in cryosections of NJP ganglia from P2ry1-ires-Cre; loxP-DTR mice injected intraganglionically with saline (control, gray, n=5 ganglia), or DT (red, P2ry1-ABLATE, n=6 ganglia). Each bar indicates labeled cells from a single mouse, with each dot indicating cell counts from one section, mean ± sem. (C) Representative images from cryosections of NJP ganglia from control and P2ry1-ABLATE mice after two-color fluorescence in situ hybridization using cRNA probes to P2ry1 (cyan) and Cckar (magenta), scale bars: 100 μm.
Figure S6. Diversity of upper airway afferent terminal morphology, Related to Figure 6 (A) Neuron terminals were visualized in an open-book preparation of the larynx using an alkaline phosphatase substrate after injection of AAV-flex-AP into NJP ganglia of Npy1r-Cre (NPY1R), Npy2r-ires-Cre (NPY2R), Piezo2-ires-Cre (PIEZO2), Calb1-ires-Cre (CALB1) and Gabra1-ires-Cre (GABRA1) mice, scale bar: 500 μm. (B) Terminals in ciliated epithelium of the larynx observed following immunohistochemistry for tdTomato after injecting AAV-flex-tdTomato virus into NJP ganglia of P2ry1-ires-Cre mice, scale bars: 50 μm. (C) Two-color immunohistochemistry for KRT8 (green) and tdTomato (magenta) in cryosections of oropharynx from P2ry1-ires-Cre mice injected with AAV-flex-tdTomato in NJP ganglia, scale bar: 50 μm.
Figure S7. Expression of ChR2 in Krt8-CreER; loxP-ChR2 mice; NP19 neurons express ionotropic ATP receptors, Related to Figure 7 (A) Two-color immunohistochemistry for KRT8 (green) and eYFP (yellow) in cryosections of vagal ganglia (left), laryngeal squamous epithelium (middle), and laryngeal ciliated epithelium (right) from tamoxifen-injected Krt8-CreER; loxP-ChR2 mice which express a ChR2-YFP fusion protein, scale bars: 100 μm (vagal ganglia) and 50 μm (epithelia). (B, C) UMAP plots showing expression of Krt8, P2×2, or P2×3 in vagal sensory neurons; NP19 neurons are circled. (D) Swallows to laryngeal water perfusion (20 seconds) in mice indicated, n=6–31, mean ± sem, n.s. not significant, p>0.05.
Table S1. Signature genes for NJP sensory neuron subtypes, Related to Figure 2 A list of the five most enriched genes for each NJP sensory neuron cluster, with gene numbers corresponding to rows in Figure 2C.
Movie S2. Evoking vocal fold adduction by optogenetics, Related to Figure 4 Representative movie of vocal fold adduction during optogenetic activation (blue laser light) of the vagus nerve in a Vglut2-ires-Cre; loxP-ChR2 mice. Vocal fold dynamics were visualized using a miniaturized fiber endoscope.
Movie S1. Evoking swallows by optogenetics, Related to Figure 1 Representative movie of evoked swallowing during optogenetic activation (blue laser light) of the vagus nerve in a Vglut2-ires-Cre; loxP-ChR2 mice. The hyoid bone elevates with each swallow.
Data Availability Statement
Single-cell transcriptome data from vagal and glossopharyngeal sensory neurons are publicly available through GEO (GSE145216); other primary images and data are available at Mendeley (https://dx.doi.org/10.17632/4gfm8jp6dr.1).


