Abstract
Cerebrovascular reactivity (CR) is a mechanism that maintains table blood flow supply to the brain. Pressure reactivity index (PRx), the correlation coefficient between slow waves of invasive arterial blood pressure (ABP) and intracranial pressure (ICP) has been validated for CR assessment. However, in clinical ward, not every subarachnoid hemorrhage (SAH) patient has invasive ABP monitoring. Pulse transit time (PTT), the propagation time of a pulse wave travelling from the heart to peripheral arteries, has been suggested as a surrogate measure of ABP. In this study, we proposed to use PTT instead of invasive ABP to monitor CR. Forty-five SAH patients with simultaneous recordings of invasive ABP, ICP, oxygen saturation level (SpO2) and electrocardiograph (ECG) were included. PTT was calculated as the time from the ECG R-wave peak to the onset of SpO2.PTT based pressure reactivity index (tPRx) was calculated as the correlation coefficient between slow waves of PTT and ICP. Wavelet tPRx (wtRx) was calculated as the cosine of wavelet phase shift between PTT and ICP. Meanwhile, PRx and wPRx were also calculated using ABP and ICP as input. The result showed a negative relationship between PTT and ABP (r = −0.58, p<0.001). tPRx negatively correlated with PRx (r = −0.51, p = 0.003).Wavelet method correlated well with correlation method demonstrated through positive relationship between wPRx and PRx (r = 0.82, p<0.001) as well as wtPRx and tPRx (r = 0.84, p<0.001). PTT demonstrates great potential as a useful tool for CR assessment when invasive ABP is unavailable.
Keywords: Pulse Transit Time, Intracranial Pressure, Non-invasive Arterial Blood Pressure, Cerebrovascular Reactivity
Introduction
Cerebrovascular reactivity (CR) is an important mechanism to Maintains table oxygen and nutrition supply to the brain through regulation of cerebral blood flow (CBF) over a wide range of arterial blood pressure (Addison 2015, Caldas et al 2018).Currently, there is no gold standard to assess dynamic CR, neither for the experimental protocol nor for how to process the recorded clinical signals including arterial blood pressure (ABP), intracranial pressure (ICP) or CBF (Claassen et al 2016).Methods for CR assessment are still controversial: on the one hand, some studies suggest that spontaneous fluctuations should be used to assess the relationship between ABP and CBF whenever possible (Tzeng and Panerai 2018); on the other hand, Other investigators Suggest that more robust results can be obtained using manoeuvers that induce larger changes in ABP than normally observed at rest (Simpson and Claassen 2018). However, on a practical level, the spontaneous fluctuations are favored due to its convenience, ease of operation, high availability and continuity in intensive care units (ICU).
Over the past two decades, considerable progress has been made in assessing CR through spont aneous waves, including correlation methods (Czosnyka et al2017, Highton et al 2015, Da Costa et al 2015, Lee et al 2011), frequency domain methods (e.g. transfer function method) (Blaber et al 1997, Panerai et al 2002, Meel-van den Abeelen et al 2014) and time-frequency domain methods(e.g. wavelet methodology) (Liu et al 2017, Tian et al 2016, Addison 2015, Highton et al 2014). Among all these methods, pressure reactivity index (PRx), calculated as The correlation coefficient between slow waves of invasive ABP and ICP, has been widely used in different cohorts of patients (Steiner et al 2003, Lewis et al 2015, Tseng et al 2006, Brady et al 2009), and proved to be related to patient outcome after head injury (Balestreri et al 2006).PRx has also been used to determine optimal cerebral perfusion pressure value for traumatic brain injury Patients (Aries et al 2012, Steiner et al 2002).
However, not every patient in clinical ward has invasive ABP monitoring. Standard monitoring in ICU includes electrocardiograph (ECG, for heart rate and rhythm monitoring) and pulse oximetry (for oxygen saturation level (Sp O2) measurement) (Perkins et al 2003, Van Oostrom and Melker 2004). Invasive ABP is normally measured in ventilated patients or moderate-to high-risk patients by inserting an arterial line, most often in the radial or femoral artery (Svensen et al 2018). An alternative method to invasive ABP measurement is pulse transit time (PTT) (Singham et al 2003, Sugo et al 2010), defined as the propagation time of a pulse wave from the heart to the peripheral arteries (e.g. a finger or a toe) and is generally calculated as the time between the R-peak of the ECG and the onset of the corresponding pulse wave at the given peripheral site (Fig.1) (van Velzen et al 2017, Vlahandonis et al 2014, Foo et al 2005).Because a portion of PTT is determined by peripheral arterial stiffness that is also under influence of systemic blood pressure, a large body of research has been carried out to develop noninvasive beat-to-beat estimation of blood pressure from PTT(Pitson and Stradling 1998, Galland et al 2007, Xu et al 2011). While challenges still exist in obtaining accurate estimation of absolute ABP from PTT due to various reasons, e.g. motion artifacts, the assumption that the component of pre-ejection period in PTT is not related to ABP (Zhang et al 2011, Payne 2006, Buxi et al 2015), it is possible that relative changes in PTT can substitute changes in ABP as input to assess CR. CR assessment through PTT has advantages over invasive ABP, as it avoids bleeding and infection risk, and can be used outside of the ICU (Lehman et al 2013). However, to our knowledge, no study has used PTT to assess CR previously. Moreover, while the relationship between ABP and PTT has been studied in normal subjects and in patients with sleep disorders, hypertension (Drinnan et al 2001, Kim et al 2013, Vlahandonis et al 2014), it has not been studied for patients with aneurysmal subarachnoid hemorrhage (SAH). In addition to hydrocephalus, SAH patients are at risk to develop cerebral vasospasm and infarction in the days and weeks following aneurysm rupture (Jun et al 2010). Having a noninvasive method to identify SAH patients with cerebral vasospasm—such as through the measurement of cerebrovascular reactivity—would improve triage of ICU patients to be taken to the angiography suite where endovascular therapies can reverse cerebral vasospasm and prevent infarction.
Fig 1.
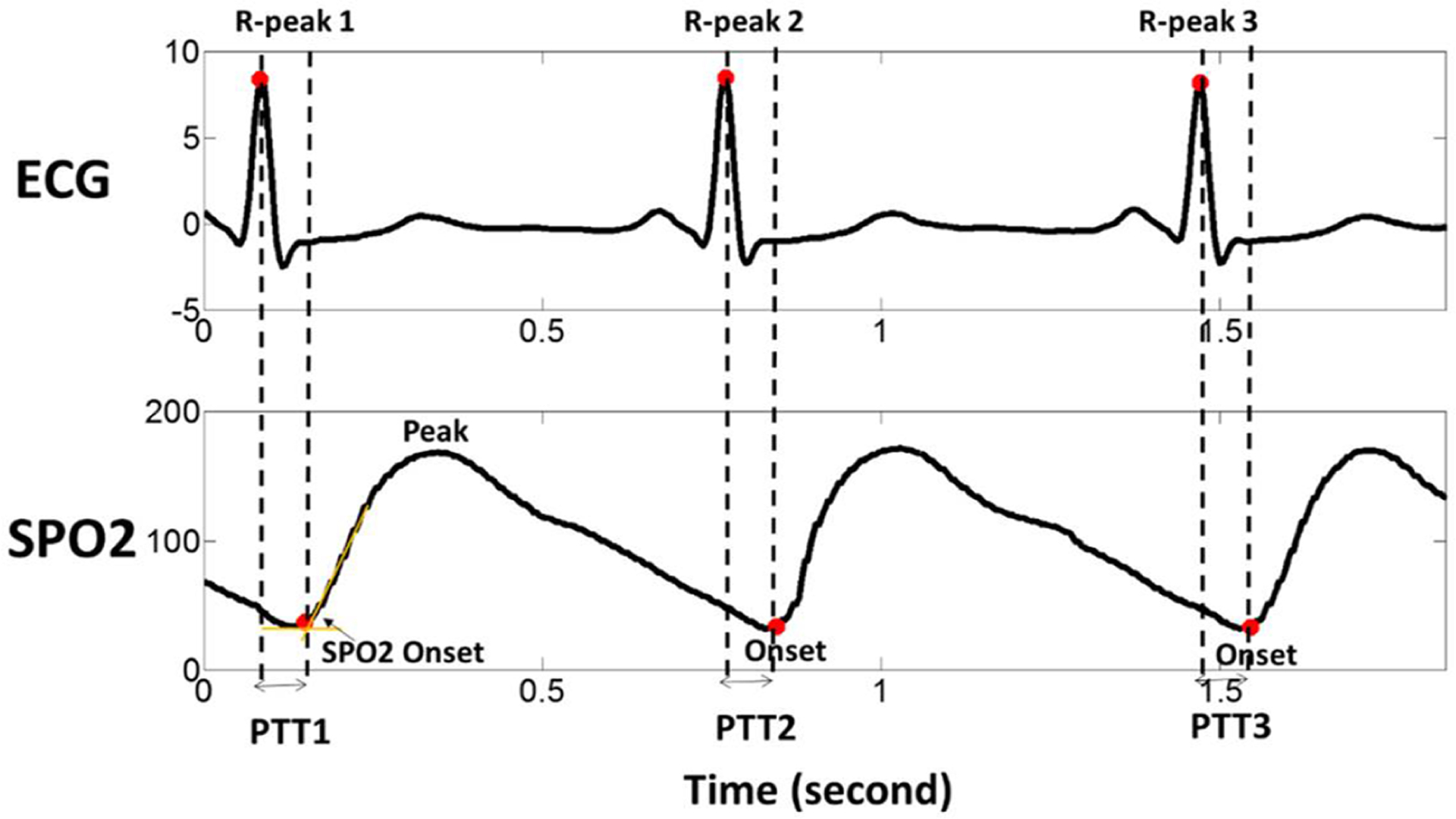
Graphical explanation of pulse transit time (PTT) calculation when using the pulse wave onset as the landmark that indicates arrival of the pulse wave. ECG: electrocardiogram, SpO2: blood oxygen saturation.
Hence, we conducted the present study with the following objectives: (1) to test if changes in invasive ABP are correlated with changes in noninvasive PTT in SAH patients; (2) to evaluate the potential of using PTT to estimate CR.
Materials and Methods
A total of 181 nontraumatic aneurysmal SAH patients admitted to University of California, San Francisco Medical Center(San Francisco, CA, USA) between March 2013 and September 2016 were studied retrospectively. To be included in the study, subjects must have continuous monitoring of ICP, SpO2, ECG and ABP. 45of 181 SAH patients met the inclusion and exclusion criteria and were selected for this study. The data were collected during external ventricular drain (EVD)clamping trials.
Continuous ICP was monitored through EVD (LimiTorr 20 or 30 mL, Integra, New Jersey, USA) while it is closed to cerebral spinal fluid drainage; SpO2 was monitored through a pulse oximeter (PILSOX-1, Konica Minolta Sensing, Osaka, Japan); ECG was recorded using 5-lead configuration for all patients (Drew et al 2014); ABP was monitored invasively through the radial or femoral artery using a standard pressure monitoring kit (Baxter Healthcare, CardioVascular Group, Irvine, CA). All data were recorded through BedMasterEx software (Excel Medical Electronics, Inc, Jupiter, FL) at a sampling rate of 240 Hz (Drew et al 2014). Artifacts due to patient movement or bedside interventions were removed manually through ICM+ software (University of Cambridge, Cambridge Enterprise, Cambridge, UK, http://www.neurosurg.cam.ac.uk/icmplus). The data were retrieved from a continuous archival of patient monitoring data under an approved institutional review board (IRB) protocol. Each patient provided written informed consent. The result of the clamping test was solely clinically based without any influence from this study.
1). Calculation of PTT, beat-to-beat mean ICP and ABP
As shown in Fig. 1, PTT was calculated as the time from the ECG R-wave peak to the onset point of the pulse oximeter wave (SpO2). The onset point of SpO2 was determined using an algorithm as described previously for finding onset of intracranial pressure pulses (Hu et al 2008).This algorithm is robust to the presence of missed or spurious ECG beat detections, which is a critical feature for handling signals recorded in clinical settings. Parameters in the original algorithm were tweaked to match the range of SpO2PTT because these parameters were designed for ICP pulse. In our data set, beat-to-beat mean value of ICP and ABP was calculated by one beat ahead of PTT calculation to synchronize the data, which means the average value of ICP, ABP between R-peak 1 and R-peak 2, was aligned with PTT2 rather than PTT1 in Fig 1.
2). Cerebrovascular Reactivity
PRx and Transit PRx
PRx was calculated as a moving Pearson correlation coefficient between 10-second averages of ABP and ICP, using a 300-second data window (Czosnyka et al 1997). Transit PRx (tPRx) was calculated as a moving Pearson correlation coefficient between 10-second averages of PTT and ICP, using a 300-second window.
Wavelet PRx and wavelet tPRx
We used the wavelet transform method to assess CR (Addison 2002), as it has recently been proved to show more stable result than PRx in a cohort of TBI patients (Liu et al 2017) and also in animal experiments (Liu et al 2018). In this study, the wavelet transform phase shift (WTP) between ABP and ICP was calculated in the frequency of 0.0067 Hz to 0.05 Hz by using Morlet mother wave (Liu et al 2017). An 800-second window was used to calculate WTP, in order to allow enough data points to be calculated after removing the edge effect. More details about the criteria of selecting frequency band and about the edge effect can be found in our previous publication (Liu et al 2017). The calculation of WTP was updated every 10 seconds. The cosine of the WTP angle was calculated afterwards, termed wPRx. The same calculation was also done by using PTT and ICP as input, rendering wavelet tPRx (wtPRx). More details about the algorithm were described in our previous publications (Liu et al 2017, 2018).
3). Statistical analysis
Statistical analyses were performed using Matlab software (ver. R2012A, MathWorks, Inc.) and SPSS (version 25.0, IBM, NY, USA). To analyze the inter-individual correlation between different parameters, the averaged values of PTT, ABP, PRx and tPRx were calculated across the whole monitoring period for each patient (one value per patient). Spearman’s correlation coefficient (R) between the Mean values was calculated. A simple linear regression was used to describe the relationship between the mean values of the tested parameters. p < 0.05 was considered to be significant relationship.
In order to analyze the intra-individual correlation between these parameters, 5-min averaged data were calculated, resulting in multiple measurements for each patient. Then the linear mixed-effects Models with random intercepts and slopes were conducted to assess the intra-individual correlations of repeated measurements between PTT and ABP, and between PRx and tPRx (Magezi 2015, Zhang et al 2017, Twisk 2006). In a linear mixed-effects model, Yij = β0+ β1Xij +μi0+μi1Xij + εij, where Yij (PTT or tPRx) is the response of j-th measurement of i-th subject; β0 is the fixed intercept for the β1 regression model; is the fixed slope for the regression model Xij; (ABP or PRx)is the predictor for j-th measurement of i-th subject; is the random intercept for the i-th subject, iid means independent and identically distributed and σ is standard deviation ; is the random slope for the subject, is a Gaussian error term. The correlation coefficient was calculated through the formula below:
Here, the covariance refers to the covariance of the intercepts and the fixed effects.
Standard deviation (SD) of each parameter was calculated across the whole monitoring period (one value per patient) to assess the variability of the parameters. Student t test was applied to compare whether there is significant difference in the variability of the tested parameters.
Results
The group of patients included 27 females and 18 males. Their mean age was 59.3± 16.3 (mean ± SD) years old. The mean recording time per patient after artifact removal was 73minutes (range from 17 minutes to 233 minutes).The average ABP and ICP of this cohort was79.8± 20.5mmHg and 10.9± 9.9mmHg, respectively. The average PTT was 193.7± 124.9 millisecond (ms). An example of time trends of ABP, ICP, PTT, PRx, and tPRx Is shown in Fig 2.
Fig 2.
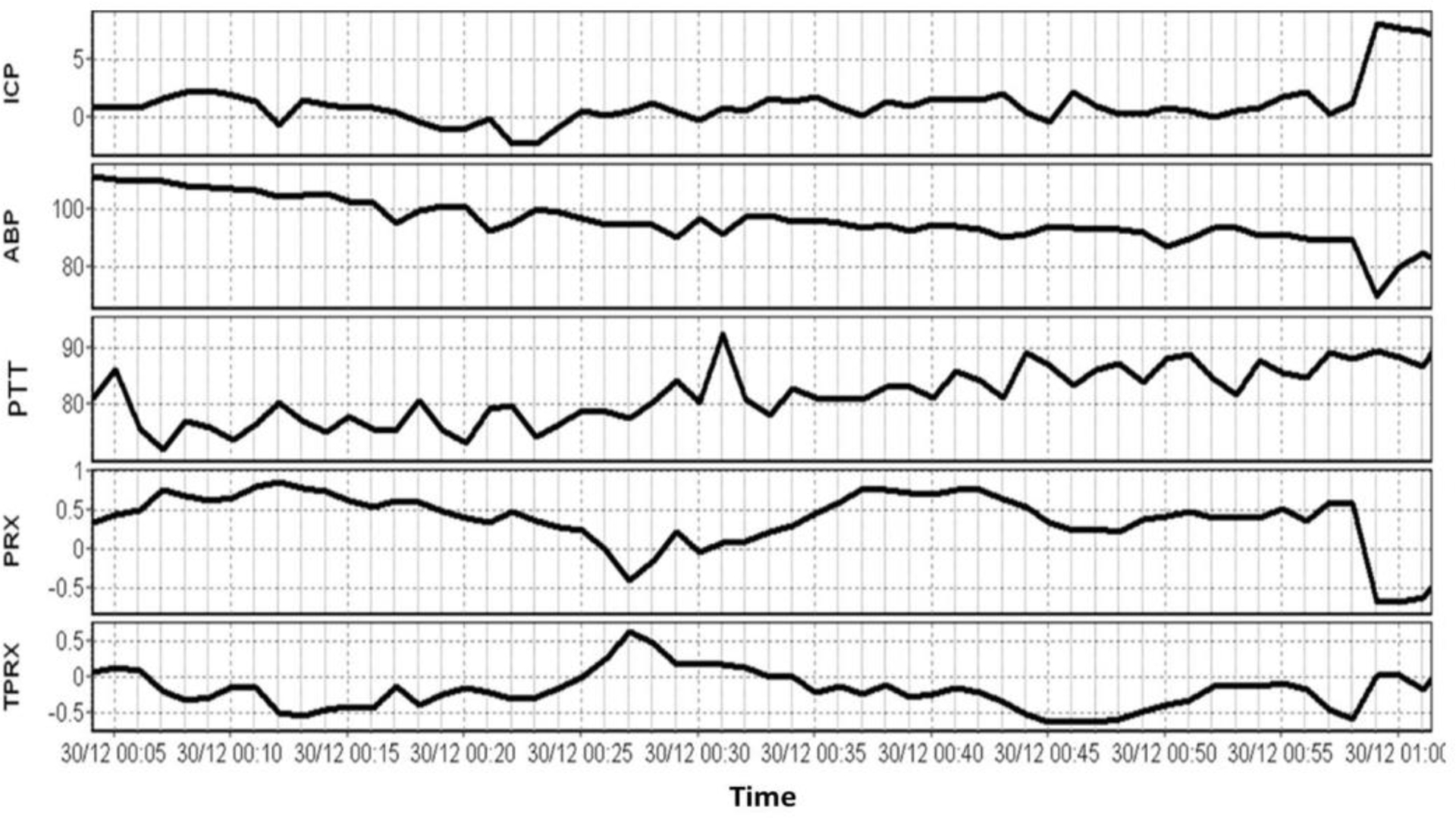
An example of time trends of ABP, ICP, PTT, PRx, and tPRx. ABP: arterial blood pressure; ICP: intracranial pressure; PTT: pulse transit time; PRx: pressure reactivity index; tPRx: transit PRx.
The inter-individual correlation between the tested parameters
The average value of each tested parameter was calculated across the whole recording of each patient (one value one patient), and the correlation was studied using Spearman’s correlation coefficient (R) as shown in Fig 3 (n=45). There was a statistically significant, negative, correlation between PTT and ABP, which can be described through a linear regression PTT=477.0–3.55× ABP (Fig 3A, R =−0.58, p<0.001, n=45); PRx negatively correlated with tPRx (Fig 3B, tPRx = 0.03–0.36×PRx, R = − 0.51 p=0.003, n=45). Wavelet method correlated well with correlation method demonstrated through linear, positive relationship between wPRx and PRx (Fig 3C, R =0.82, p<0.001, n=45) as well as wtPRx and tPRx (Fig 3D, R =0.84, p<0.001, n=45).
Fig.3.
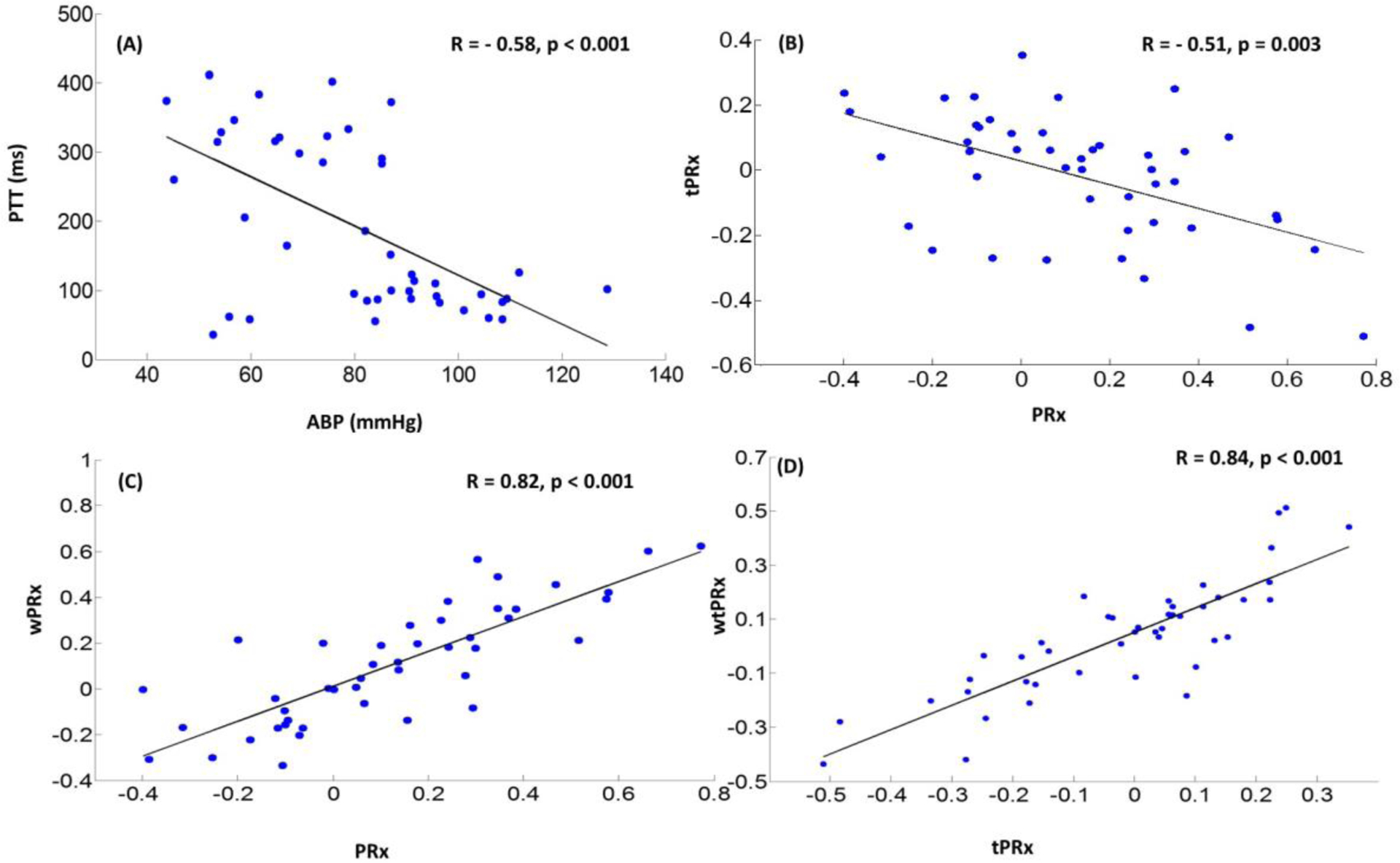
Inter -individual relationship between the tested parameters. (A) A statistically significant, negative correlation exists between PTT and ABP;(B)A statistically significant, negative correlation exists between PRx and tPRx; (C) There is a linear relationship between wPRx and PRx; (D)A linear relationship exists between wtPRx and tPRx(n=45). ABP: arterial blood pressure; PTT: pulse transit time; ICP: intracranial pressure; PRx: pressure reactivity index, correlation coefficient between slow waves of ABP and ICP; tPRx: transit PRx, correlation coefficient between slow waves of PTT and ICP; wPRx: wavelet phase shift between ABP and ICP; wtPRx: wavelet phase shift between PTT and ICP.
The intra-individual association between PRx and tPRx
The linear mixed-effects models analysis showed a significant, negative relationship between ABP and PTT(Fig 4A, p<0.001). A unit increase in ABP was associated with 2.36 unit decrease in PTT (95% CI: −3.56 ~ −1.13). The correlation coefficient between ABP and PTT calculated through the linear mixed-effects models was −0.94 (covariance of intercept and ABP was −781.3 [−1269.4 ~ −293.3], the variance of intercept and ABP was 76430.4[42663~136924]and 9 [4.82 ~16.83] respectively).
Fig 4.
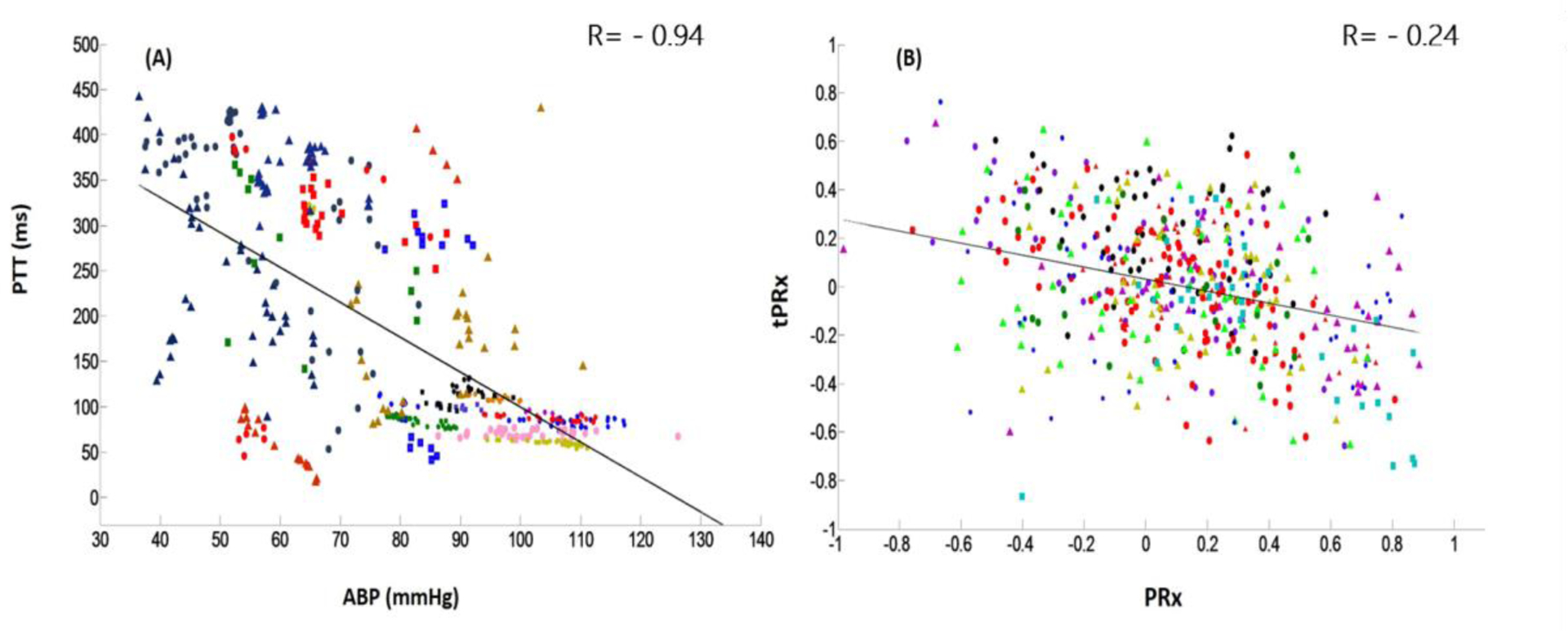
Intra-individual association between PTT and ABP, as well as between PRx and tPRx. (A) A negative correlation exists between PTT and ABP. (B) A negative correlation exists between tPRx and PRx. ABP: arterial blood pressure; PTT: pulse transit time; PRx: pressure reactivity index, correlation coefficient between slow waves of ABP and ICP; tPRx: transit PRx, correlation coefficient between slow waves of PTT and ICP. One color marker represents one patient.
There was a significant, negative relationship between PRx and tPRx (Fig 4B, p=0.001). A unit increase in PRx was associated with 0.21 unit decrease in tPRx (95% CI: −0.32 ~ −0.09). The correlation coefficient between PRx and tPRx calculated through the linear mixed-effects models was −0.24 (covariance of intercept and ABP was −0.007[−0.025~ 0.01], the variance of intercept and ABP was 0.014 [0.007~ 0.028] and 0.06 [0.02 ~0.17] respectively).
Variability of wavelet method and correlation method
In order to compare the variability of wavelet method and correlation method, we compared the standard deviation of the tested parameters of each patient across the whole monitoring time using student t test. The result showed wPRx was more stable than PRx (mean SD of wPRx was 0.22vs.mean SD of PRx was 0.33, p<0.001). Similar conclusion can also be drawn for wtPRx and tPRx (mean SD of wtPRx was 0.23vs mean SD of tPRx was 0.31, p<0.001).
Discussion
Noninvasive and cuffless measurement ABP is desirable for patient monitoring. Various methods have been developed to measure ABP noninvasively and continuously, which include arterial tonometry (e.g. Colin® tonometer), volume clamp method (e.g. Finapres) and PPT-based method (Teng and Zhang 2006). However, the arterial tonometer suffers from relatively high cost, and its accuracy decreased by wrist movement (Teng and Zhang 2006); and the volume clamp method needs a small inflatable cuff to measure beat-to-beat noninvasive ABP (Aitken et al 1991).Derived from routinely bedside signals (ECG and photoplethysmography, PPG), the PPT-Based noninvasive ABP avoids the danger of cessation of perfusion to finger that might be caused by volume clamp method (Teng and Zhang 2006). However, the use of PTT as a surrogate measurement of ABP is still controversial. Some studies have shown a direct relationship between ABP and PTT in healthy patients and hypertensive patients (Foo et al 2005, Pitson et al 1994, Kim et al 2013); while others concluded in verse results (Zhang et al 2011, Payne 2006). Our study Demonstrated a significant Negative correlation between beat-to-beat PTT and invasive ABP in SAH patients. An increase in ABP causes a change in the geometric and Mechanical properties of the arterial wall (e.g. vascular constriction), leading to increased stiffness (Vlahandonis et al 2014, Foo and Wilson 2009). This, in turn, causes pulse waves to travel faster, and consequently leads to a reduction in PTT (Foo and Wilson 2009, Galland et al 2007). On the contrary, a decrease in ABP leads to increase in PTT.
However, as measured at the peripheral arteries, PTT is also influenced by many other factors such as individual differences in vascular compliance, pre-ejection period, arm length, etc. (Vlahandonis et al 2014), the PTT-based noninvasive ABP might be different from the invasive ABP measured from intravascular catheter in terms of waveforms and also systolic and diastolic values (Chin and Panerai 2013). In the calculation of CR, we care more about the relative changes of PTT rather than the absolute value, Where peripheral measurements of ABP are not very problematic (Sammons et al 2007). Our Results showed That PTT can Replace direct ABP measurement in continuous monitoring of CR through the significant negative relationship between PRx and tPRx (p=−0.51). For PRx, a negative Value indicates a pressure-active vascular bed with preserved cerebrovascular reactivity, whereas a positive value indicates a pressure-passive vascular bed with impaired reactivity (Kvandal et al 2013). While for tPRx, high tPRx means high ICP is related with high PTT, which is normally due to low ABP; thus, high tPRx actually reflects close relationship between high ICP and low ABP and it refers to good CR; and vice versa. This study Suggests a potential method of using PTT for CR measurement in SAH patients. Because of its ease of use, portability and applicability in a wide range of clinical settings of pulse oximeters (Lipnick et al 2016), PTT might bring benefits for future CR assessment.
Moreover, this study compared PRx with tPRx using data collected from 45 SAH patients. Although a significant negative correlation was found, both PRx and tPRx make use of the same ICP data and are not therefore completely independent. This may lead to a spurious correlation. Therefore, in order to clear this suspect, we randomly shuffled each patient’s ICP, and used the original ABP and shuffled ICP to calculate ‘artificial’ PRx and used the original PTT and shuffled ICP to calculate ‘artificial’ tPRx. There is no significant relationship between the ‘artificial’ PRx and tPRx. The mean value of the PRx and tPRx using shuffled ICP was obtained for each patient, and the inter-individual correlation between the mean values of PRx and tPRx was 0.04 (n = 45, p=0.81, Supplementary Fig 1). Therefore, the relationship between PRx and tPRx using real ABP or PTT and real ICP is not spurious. However, we still need larger numbers of patients to support the conclusion that the tPRx is a good surrogate for PRx. The study also Utilized a recently validated method, wavelet method for CR calculation. The wavelet method has shown more stable results than correlation-based method in TBI patients and it was more strongly associated with patient outcome. This study validated the wavelet method in SAH patients. Big wPRx values reflect small phase shifts between fluctuations in ABP and ICP, indicating direct changes in ICP following ABP because of impaired cerebrovascular function. The good correlation between PRx and wPRx matched our previous findings in a TBI cohort (Liu et al 2017). Moreover, we compared the standard deviation (SD) of the tested parameters in this SAH cohort, the result showed smaller SD of wPRx than the SD of PRx (p< 0.001), and smaller SD of wtPRx than the SD of tPRx (p< 0.001).The improved precision of wavelet method versus correlation method may be explained by the robustness of the wavelet method for determining phase shift from nonstationary signals (Keissar et al 2009). While correlation measurement takes all the information into calculation without removing any uncertain noise, wavelet method by using wavelet coherence as a filter, guarantees a reliable relationship between input and output. By removing points that represent noise, wavelet method produces a more stable estimation of pressure reactivity than traditional correlation method.
Limitation
In this study, we only collected data from 45 SAH patients, which is a rather small cohort. Further analysis needs to be carried out on a larger Cohort of patients than in this study and on patients with other conditions. Calculation of PTT in this study does not take cardiac pre-ejection period (PEP) into account, which represents the total duration of the electrical and mechanical events prior to the ejection of blood from the left ventricle (i.e. the electrical mechanical delay) defined by the opening of the aortic valve (Newlin and Levenson 1979). Payne et al demonstrated that PEP accounts for 12 to 35% of PTT variation (Payne 2006), but there were also Other studies showing that the PEP variations can be considered negligible (Masè et al 2011) PEP during a resting state, such as SAH patients in our study, should have a rather low contribution (Vlahandonis et al 2014) to PTT demonstrated by the significant, negative relationship between PTT and ABP in our results. The method is a step toward non-invasive continuous monitoring of CR. If ICP measurement can be substituted with total hemoglobin NIRS measurement, this objective may be achieved.
Conclusion
In summary, beat-to-beat PTT showed good correlation with invasive ABP in SAH patients. Beat-to-beat PTT may be useful for cerebrovascular reactivity assessment when invasive ABP is unavailable in SAH patients.
Supplementary Material
Key points.
Pulse transit time (PTT), defined as the propagation time of a pulse wave travelling from the heart to the peripheral arteries, has been proposed as a surrogate measure of arterial blood pressure (ABP).The relationship between PTT and ABP in subarachnoid hemorrhage (SAH) patients remains unknown.
Cerebrovascular reactivity (CR) assessment through PTT has advantages over invasive ABP, as it avoids bleeding and infection risk, and can be used outside of the ICU.
We introduced a new method to assess CR using PTT and intracranial pressure (ICP) through correlation based method and wavelet based method.
We found that beat-to-beat PTT was Negatively related with invasive ABP in SAH patients. A significant linear relationship exists between PTT-based CR parameter and a well validated method, pressure reactivity index (PRx). PTT demonstrates great potential as a useful tool for CR assessment when invasive ABP is unavailable in SAH patients.
Acknowledgement
A sincere thank you to Dr. Bruce Cooper for his great help with our statistical analysis.
Funding
This work was partially supported by Middle Career Scientist Award, UCSF Institute for Computational Health Sciences, and National Institutes of Health Awards (R01NS076738 and NS106905A1).
Footnotes
Competing Interests
ICM+ Software is licensed by Cambridge Enterprise, Cambridge, UK (http://icmplus.neurosurg.cam.ac.uk). M.C. and P.S. have a financial interest in a fraction of the licensing fee.
References
- Addison PS 2015. A Review of Wavelet Transform Time-Frequency Methods for NIRS-based Analysis of Cerebral Autoregulation. IEEE Rev. Biomed. Eng PP1. [DOI] [PubMed] [Google Scholar]
- Addison PS 2002. The Illustrated Wavelet Transform Handbook: Introductory Theory and Applications in Science, Engineering, Medicine and Finance 1st Edition( Boca Raton: CRC Press; ) [Google Scholar]
- Aitken HA, Todd JG and Kenny GNC 1991. Comparison of the finapres and direct arterial pressure monitoring during profound hypotensive anaesthesia Br. J. Anaesth [DOI] [PubMed] [Google Scholar]
- Aries MJH, Czosnyka M, Budohoski KP, Steiner L a., Lavinio A, Kolias AG, Hutchinson PJ, Brady KM, Menon DK, Pickard JDand Smielewski P 2012. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury Crit. Care Med 40 2456–63 [DOI] [PubMed] [Google Scholar]
- Balestreri M, Czosnyka M, Hutchinson P, Steiner LA, Hiler M, Smielewski P and Pickard JD 2006. Impact of intracranial pressure and cerebral perfusion pressure on severe disability and mortality after head injury Neurocrit. Care 4 8–13 [DOI] [PubMed] [Google Scholar]
- Blaber a P, Bondar RL, Stein F, Dunphy PT, Moradshahi P, Kassam MS and Freeman R 1997. Transfer function analysis of cerebral autoregulation dynamics in autonomic failure patients. Stroke. 28 1686–92 [DOI] [PubMed] [Google Scholar]
- Brady KM, Shaffner DH, Lee JK, Easley RB, Smielewski P, Czosnyka M, Jallo GI and Guerguerian A-M 2009. Continuous monitoring of cerebrovascular pressure reactivity after traumatic brain injury in children. Pediatrics 124 e1205–12 [DOI] [PubMed] [Google Scholar]
- Buxi D, Redouté J-M and Yuce MR 2015. A survey on signals and systems in ambulatory blood pressure monitoring using pulse transit time Physiol. Meas [DOI] [PubMed] [Google Scholar]
- Caldas JR, Haunton VJ, Panerai RB, Hajjar LA and Robinson TG 2018. Cerebral autoregulation in cardiopulmonary bypass surgery: A systematic review Interact. Cardiovasc. Thorac. Surg 26 [DOI] [PubMed] [Google Scholar]
- Chin KY and Panerai RB 2013. A new noninvasive device for continuous arterial blood pressure monitoring in the superficial temporal artery Physiol. Meas [DOI] [PubMed] [Google Scholar]
- Claassen JA, Meel-van den Abeelen AS, Simpson DM, Panerai RB and international Cerebral Autoregulation Research Network (CARNet) 2016. Transfer function analysis of dynamic cerebral autoregulation: A white paper from the International Cerebral Autoregulation Research Network. J. Cereb. Blood Flow Metab 36 665–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Costa CS, Czosnyka M, Smielewski P, Mitra S, Stevenson GN and Austin T 2015. Monitoring of cerebrovascular reactivity for determination of optimal blood pressure in preterm infants J. Pediatr 167 86–91 [DOI] [PubMed] [Google Scholar]
- Curran PJ and Bauer DJ 2010. The Disaggregation of Within-Person and Between-Person Effects in Longitudinal Models of Change [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czosnyka M, Czosnyka Z and Smielewski P 2017. Pressure reactivity index: journey through the past 20 years Acta Neurochir. (Wien) 159 2063–5 [DOI] [PubMed] [Google Scholar]
- Czosnyka M, Smielewski P, Kirkpatrick P, Laing RJ, Menon D and Pickard JD 1997. Continuous assessment of the cerebral vasomotor reactivity in head injury Neurosurgery 41 11–9 [DOI] [PubMed] [Google Scholar]
- Drew BJ, Harris P, Zègre-emsey JK, Mammone T, Schindler D, Salas-Boni R, Bai Y, Tinoco A, Ding Q and Hu X 2014. Insights into the problem of alarm fatigue with physiologic monitor devices: A comprehensive observational study of consecutive intensive care unit patients PLoS One 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinnan MJ, Allen J and Murray A 2001. Relation between heart rate and pulse transit time during paced respiration Physiol. Meas 22 425–32 [DOI] [PubMed] [Google Scholar]
- Foo J Y a and Wilson SJ 2009. Clinical applications of pulse transit time in paediatric critical care. J. Med. Eng. Technol 33 79–86 [DOI] [PubMed] [Google Scholar]
- Foo JYA, Wilson SJ, Dakin C, Williams G, Harris MA and Cooper D 2005. Variability in time delay between two models of pulse oximeters for deriving the photoplethysmographic signals Physiol. Meas 26 531–44 [DOI] [PubMed] [Google Scholar]
- Galland BC, Tan E and Taylor BJ 2007. Pulse transit time and blood pressure changes following auditory-evoked subcortical arousal and waking of infants. Sleep 30 891–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highton D, Ghosh A, Tachtsidis I, Elwell C and Smith M 2014. Analysis of slow wave oscillations in cerebral haemodynamics and metabolism following subarachnoid haemorrhage Adv. Exp. M ed. Biol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highton D, Ghosh A, Tachtsidis I, Panovska-Griffiths J, Elwell CE and Smith M 2015. Monitoring cerebral autoregulation after brain injury: Multimodal assessment of cerebral slow-wave oscillations using near-infrared spectroscopy Anesth. Analg 121 198–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Xu P, Lee DJ, Vespa P, Baldwin K and Bergsneider M 2008. An algorithm for extracting intracranial pressure latency relative to electrocardiogram R wave Physiol. Meas 29 459–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun P, Ko NU, English JD, Dowd CF, Halbach VV.,Higashida RT, Lawton MT and Hetts SW 2010. Endovascular treatment of medically refractory cerebral vasospasm following aneurysmal subarachnoid hemorrhage. AJNR. Am. J. Neuroradiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keissar K, Davrath LR and Akselrod S 2009. Coherence analysis between respiration and heart rate variability using continuous wavelet transform. Philos. Trans. A. Math. Phys. Eng. Sci 367 1393–406 [DOI] [PubMed] [Google Scholar]
- Kim SH, Song JG, Park JH, Kim JW, Park YS and Hwang GS 2013. Beat-to-beat tracking of systolic blood pressure using noninvasive pulse transit time during anesthesia induction in hypertensive patients Anesth. Analg 116 94–100 [DOI] [PubMed] [Google Scholar]
- Kvandal P, Sheppard L, Landsverk S a, Stefanovska A and Kirkeboen K a 2013. Impaired cerebrovascular reactivity after acute traumatic brain injury can be detected by wavelet phase coherence analysis of the intracranial and arterial blood pressure signals. J. Clin. Monit. Comput 27 375–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Brady KM, Mytar JO, Kibler KK, Carter EL, Hirsch KG, Hogue CW, Easley RB, Jordan LC, Smielewski P, Czosnyka M, Shaffner DH and Koehler RC 2011. Cerebral blood flow and cerebrovascular autoregulation in a swine model of pediatric cardiac arrest and hypothermia. Crit Care Med 39 2337–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Leeden R 1998. Multilevel analysis of repeated measures data Qual. Quant. [Google Scholar]
- Lehman LWH, Saeed M, Talmor D, Mark R and Malhotra A 2013. Methods of blood pressure measurement in the ICU Crit. Care Med 41 34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PM, Czosnyka M, Carter BG, Rosenfeld JV., Paul E, Singhal Nand Butt W 2015. Cerebrovascular Pressure Reactivity in Children With Traumatic Brain Injury* Pediatr. Crit. Care Med 16 739–49 [DOI] [PubMed] [Google Scholar]
- Lipnick MS, Feiner JR, Au P, Bernstein M and Bickler PE 2016. The Accuracy of 6 Inexpensive Pulse Oximeters Not Cleared by the Food and Drug Administration: The Possible Global Public Health Implications Anesth. Analg 123 338–45 [DOI] [PubMed] [Google Scholar]
- Liu X, Czosnyka M, Donnelly J, Cardim D, Cabeleira M, Hutchinson PJ, Hu X, Smielewski P and Brady K 2018. Wavelet pressure reactivity index: A validation study J. Physiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Donnelly J, Czosnyka M, Aries MJH, Brady K, Cardim D, Robba C, Cabeleira M, Kim D-J, Haubrich C, Hutchinson PJ and Smielewski P 2017. Cerebrovascular pressure reactivity monitoring using wavelet analysis in traumatic brain injury patients: A retrospective study PLOS Med. 14 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magezi DA. Linear mixed-effects models for within-participant psychology experiments: An introductory tutorial and free, graphical user interface (LMMgui) Front. Psychol. 2015 doi: 10.3389/fpsyg.2015.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masè M, Mattei W, Cucino R, Faes L and Nollo G 2011. Feasibility of cuff-free measurement of systolic and diastolic arterial blood pressure J. Electrocardiol 44 201–7 [DOI] [PubMed] [Google Scholar]
- Meel-van den Abeelen ASS, Simpson DM, Wang LJY, Slump CH, Zhang R, Tarumi T, Rickards, Payne S, Mitsis GD, Kostoglou K, Marmarelis V, Shin, Tzeng YC, Ainslie PN, Gommer E, Müller M, D orado AC, Smielewski P, Yelicich B, Puppo C, Liu X, Czosnyka M, Wang CY, Novak V, Panerai RBand Claassen JAHR 2014. Between-centre variability in transfer function analysis, a widely used method for linear quantification of the dynamic pressure-flow relation: The CARNet study Med. Eng. Phys 36 620–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minalu G, Aerts M, Coenen S, Versporten A, Muller A, Adriaenssens N, Beutels P, Molenberghs G, Goossens H and Hens N 2011. Application of mixed-effects models to study the country-specific outpatient antibiotic use in Europe: A tutorial on longitudinal data analysis J. Antimicrob. Chemother [DOI] [PubMed] [Google Scholar]
- Newlin DB and Levenson RW 1979. Pre-ejection Period: Measuring Beta-adrenergic Influences Upon the Heart Psychophysiology 16 546–52 [DOI] [PubMed] [Google Scholar]
- Van Oostrom JH and Melker RJ 2004. Comparative Testing of Pulse Oximeter Probes Anesth. Analg. 98 1354–8 [DOI] [PubMed] [Google Scholar]
- Panerai RB, Hudson V, Fan L, Mahony P, Yeoman PM, Hope T and Evans DH 2002. Assessment of dynamic cerebral autoregulation based on spontaneous fluctuations in arterial blood pressure and intracranial pressure Physiol Meas 23 59–72 [DOI] [PubMed] [Google Scholar]
- Payne RA 2006. Pulse transit time measured from the ECG: an unreliable marker of beat-to-beat blood pressure J. Appl. Physiol 100 136–41 [DOI] [PubMed] [Google Scholar]
- Perkins GD, McAuley DF, Giles S, Routledge H and Gao F 2003. Do changes in pulse oximeter oxygen saturation predict equivalent changes in arterial oxygen saturation? Crit. Care 7 R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitson D, Chhina N, Knijn S and Stradling J 1994. Changes in pulse transit time and pulse rate as markers of arousal from sleep in normal subjects Clin. Sci 87 269 273. [DOI] [PubMed] [Google Scholar]
- Pitson DJ and Stradling JR 1998. Value of beat-to-beat blood pressure changes, detected by pulse transit time, in the management of the obstructive sleep apnoea/hypopnoea syndrome. Eur. Respir. J 12 685–92 [DOI] [PubMed] [Google Scholar]
- Sammons EL, Samani NJ, Smith SM, Rathbone WE, Bentley S, Potter JF and Panerai RB 2007. Influence of noninvasive peripheral arterial blood pressure measurements on assessment of dynamic cerebral autoregulation. J. Appl. Physiol [DOI] [PubMed] [Google Scholar]
- Simpson D and Claassen J 2018. CrossTalk opposing view: dynamic cerebral autoregulation should be quantified using induced (rather than spontaneous) blood pressure fluctuations J. Physiol 596 7–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singham S, Voss L, Barnard J and Sleigh J 2003. Nociceptive and anaesthetic-induced changes in pulse transit time during general anaesthesia Br. J. Anaesth 91 662–6 [DOI] [PubMed] [Google Scholar]
- Steiner LA, Coles JP, Johnston AJ, Chatfield DA, Smielewski P, Fryer TD, Aigbirhio FI, Clark JC, Pickard JD, Menon DK and Czosnyka M 2003. Assessment of cerebrovascular autoregulation in head-injured patients: A validation study Stroke 34 2404–9 [DOI] [PubMed] [Google Scholar]
- Steiner LA, Czosnyka M, Piechnik SK, Smielewski P, Chatfield D, Menon DK and Pickard JD 2002. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury Crit. Care Med 30 733–8 [DOI] [PubMed] [Google Scholar]
- Sugo Y, Ukawa T, Takeda S, Ishihara H, Kazama T and Takeda J 2010. A novel continuous cardiac output monitor based on pulse wave transit time IEEE Eng. Med. Biol. Soc 2010 2853–6 [DOI] [PubMed] [Google Scholar]
- Svensen CH, Prough DS, Feldman LS and Gan TJ 2018. Fluid Therapy for the Surgical Patient (NW: CRC Press, Taylor & Francis Group; ) [Google Scholar]
- Teng XF and Zhang YT 2006. An evaluation of a PTT-based method for noninvasive and cuffless estimation of arterial blood pressure Annual International Conference of the IEEE Engineering in Medicine and Biology -Proceedings [DOI] [PubMed] [Google Scholar]
- Tian F, Tarumi T, Liu H, Zhang R and Chalak L 2016. Wavelet coherence analysis of dynamic cerebral autoregulation in neonatal hypoxic-ischemic encephalopathy NeuroImage Clin. 11 124–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng MY, Czosnyka M, Richards H, Pickard JD and Kirkpatrick PJ 2006. Effects of acute treatment with statins on cerebral autoregulation in patients after aneurysmal subarachnoid hemorrhage Neurosurg Focus 21 E10. [DOI] [PubMed] [Google Scholar]
- Twisk JWR. Applied Multilevel Analysis: A Practical Guide for Medical Researchers 2006 [Google Scholar]
- Tzeng YC and Panerai RB 2018. CrossTalk proposal: dynamic cerebral autoregulation should be quantified using spontaneous blood pressure fluctuations J. Physiol 596 3–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Velzen MHN, Loeve AJ, Niehof SPand Mik EG 2017. Increasing accuracy of pulse transit time measurements by automated elimination of distorted photoplethysmography waves Med. Biol. Eng. Comput 55 1989–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahandonis A, Biggs SN, Nixon GM, Davey MJ, Walter LM and Horne RSC 2014. Pulse transit time as a surrogate measure of changes in systolic arterial pressure in children during sleep J. Sleep Res 23 406–13 [DOI] [PubMed] [Google Scholar]
- Xu D, Ryan KL, Rickards CA, Zhang G, Convertino VA and Mukkamala R 2011. Improved pulse transit time estimation by system identification analysis of proximal and distal arterial waveforms AJP Hear. Circ. Physiol [DOI] [PubMed] [Google Scholar]
- Zhang G, Gao M, Xu D, Olivier NB and Mukkamala R 2011. Pulse arrival time is not an adequate surrogate for pulse transit time as a marker of blood pressure J. Appl. Physiol 111 1681–6 [DOI] [PubMed] [Google Scholar]
- Zhang J, Cavallari JM, Fang SC, Weisskopf MG, Lin X, Mittleman MA and Christiani DC 2017. Application of linear mixed-effects model with LASSO to identify metal components associated with cardiac autonomic responses among welders: a repeated measures study Occup. Environ. Med [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


