Abstract
The adoption of the goal of universal health coverage and the growing burden of cancer in low- and middle-income countries makes it important to consider how to provide cancer care. Specific interventions can strengthen health systems while providing cancer care within a resource-stratified perspective (similar to the WHO tiered approach). Four specific topics are discussed: essential medicines/essential diagnostics lists; national cancer plans; provision of affordable essential public services (either at no cost to users, or through national health insurance); and finally, how a nascent breast cancer program can build on existing programs. A case study of Zambia (a country with a core level of resources for cancer care, using the Breast Health Global Initiative typology) discusses how a breast cancer program was built on a cervical cancer program, which in turn had evolved from the HIV/AIDS program. A case study of Brazil (which has enhanced resources for cancer care) describes how access to breast cancer care evolved as universal health coverage expanded. A case study of Uruguay shows how breast cancer outcomes have improved as the country shifted from a largely private system, to a single-payer national health insurance system, in the transition to becoming a country with maximal resources for cancer care. The final case study describes an exciting initiative, the city cancer challenge, and how that may lead to improved cancer services.
Keywords: Breast cancer, national health insurance, essential medicines list, national cancer control plans, diagnostic*
Precis:
We discuss how providing care for breast cancer can benefit, and benefit from, health system strengthening efforts. We discuss the role of Essential Medicines/Essential Diagnostics Lists, national cancer plans, financing of a specific set of public services, and how breast cancer programs can build on various other programs, along with some examples from country experience.
Introduction
The emphasis on health systems and Universal Health Coverage (UHC) in the Sustainable Development Goals provides impetus to improve cancer care and survival. The longstanding earlier focus of global health on communicable disease, “quick wins,” and vertical programs left cancer—which requires many components of health systems to work together—lower on the priority list. The previous WHO “Best Buys” for tackling non-communicable diseases (NCDs) paid scant attention to cancer treatment, focusing primarily on prevention (hepatitis B vaccination, and attention to diet, exercise, and use of alcohol and tobacco).1 The only cancer-related treatment included was for pre-cancerous cervical lesions; by contrast, treatment options for cardiovascular disease were included.
The increasing burden of cancer especially in low- and middle-income countries (LMICs) and the adoption of UHC as a goal in 2015 has created an opening for progress on cancer. The updated WHO “Best Buys” for NCDs now include treatment of stages I and II colorectal, cervical, and breast cancers as “Good Buys”.2 The 70th World Health Assembly included a resolution on cancer prevention and control3 and in response, WHO and IARC are developing a cancer costing and prioritization tool that will cover seven adult cancers, including breast cancer, and selected pediatric cancers.4 The goal is to develop a tiered framework similar in approach to the BHGI Resource-Stratified Guidelines,5 where different approaches are adopted according to the affordability in each country. UHC promises access to effective and cost-effective, lifesaving cancer treatment without catastrophic financial consequences, at least for some cancers initially, and with increasing population and disease coverage over time. All these developments are important for making progress on breast cancer, which accounts for more disability-adjusted life-years (DALYs) lost by women globally than any other cancer.6
Building a health system that integrates breast cancer care in a developing health system is a significant undertaking. Previous studies from the BHGI have discussed some of the health system components needed to sustain breast cancer programs within a resource-stratified framework.7,8 This paper begins with some concepts and definitions, and then discusses four additional components to be integrated into the health system, namely: national Essential Medicines and Diagnostics Lists, national cancer plans, national health insurance, and possible platforms that can be used as a basis for launching a breast cancer control program. We locate these components within a resource-stratified framework (Table 1) and provide case studies of three countries whose average resource availability fit the Core, Enhanced, and Maximal models, respectively (Boxes 1, 2, and 3). Figure 1 illustrates the importance of planning and financing for the many components in the continuum linking the identification of patients with cancer to the continuum of care.
Table 1.
Summary of selected support systems for low- and middle-income countries
| Resource level | Cancer plan | Coverage of financing for cancer care | Platform for breast cancer program | Essential Medicines List |
|---|---|---|---|---|
| Basic | Cancer included in NCD plan, e.g. Mozambique prior to 2019 | Payment largely out-of-pocket | Cancer care focuses mainly on cervical cancer | Includes basic cancer medicines |
| Core | Separate cancer plan; e.g. Ghana plan 2012–2016 with one paragraph on breast cancer; Zambia 2016–2021 with two paragraphs on breast cancer | Selected cancers covered by growing national insurance, e.g. Ghana covers basic breast & cervical cancers treatment | Breast cancer care added onto cervical cancer platform (e.g. Zambia); or onto reproductive health platform (e.g. India pilot) in low income states | Includes basic cancer medicines and some may be publicly subsidized; includes basic hormonal therapies (e.g. Ghana) |
| Enhanced | Cancer plan articulated to include cancer-specific goals, e.g. Malaysia’s 2016–2020 plan with several pages specifically on breast cancer resources | Broader coverage of cancer types overall in NHI; broader coverage of breast cancer stages and therapies (e.g. Malaysia, Brazil) | Breast cancer integrated into broader health system; National Cancer Centre develops network with regional centres (India; Mexico, Cuba) | Includes more advanced therapies, on-patent and costly therapies supply is limited (Malaysia; Brazil) |
| Maximal | Comprehensive cancer plans with targets and metrics | NHI covers full range of cancers and therapies, subject to national clinical guidelines (e.g. Uruguay) | Includes organized mammographic screening, diagnosis and multidisciplinary treatment (e.g. Uruguay) | Full range of therapies including those on-patent, as determined by national health technology assessments |
Box 1. Building on Success in Zambia (Draws on37,38).
Zambia, a lower middle-income country of 16 million people in southern Africa, has recently begun to phase in a program for early breast cancer detection and surgical treatment through the public healthcare system. Even in its first year 2016, 1955 women received services of some kind. The program was able to get up and running quickly because it was built on a decade-old cervical cancer screening and treatment program with services available nationwide. The decision to leverage this existing delivery platform was the result of a comprehensive assessment of cervical and breast cancer control infrastructure. With the new services and equitable access, Zambia is providing core resource-level breast cancer control.
The Cervical Cancer Prevention Program in Zambia (CCPPZ) had itself evolved from an HIV/AIDS-focused program, developed with support initially from the US President’s Emergency Plan for AIDS Relief beginning in 2005, to providing services to women in 60 government-operated clinics with nationwide coverage. Each year, 50,000 women are screened for cervical cancer using visual inspection with acetic acid and those with precancerous lesions are treated in a single visit. Breast cancer screening by clinical breast examination (CBE)—the most appropriate wide-scale intervention at the existing level of resources—could be delivered, after training, by the same nurses who screened for cervical cancer at primary health-level clinics, without new infrastructure or additional personnel costs. New facilities and use and upgrading of existing higher-level facilities, as well as newly-trained professionals would be required to handle referrals of women with possible abnormalities for full diagnoses.
Development of the plan involved first bringing the five active breast and cervical advocacy groups in Zambia into a consortium called Cancer Prevention Alliance Zambia—CAPRAZ— an independent nonprofit organization, which has been taking the process forward, with assistance from US-based academic consultants. The first step CAPRAZ took was an assessment of breast cancer early detection, diagnosis, and surgical treatment capacity, which formed the basis of a needs assessment. The next step was curriculum development to fill the identified gaps, and then curriculum implementation in a one-week practicum. Benchmarks for evaluation were then put in place.
At the 6-month mark:
four of the existing CCPPZ clinics had integrated CBE and breast cancer education into their routine work.
two new breast cancer diagnostic centers were operating, providing CBE, breast ultrasound, ultrasound-guided core needle biopsies, and needle aspiration
1,955 women were screened by CBE in the four clinics, and 256 with palpable breast masses were referred to a diagnostic center; 176 attended. Palpable breast masses were confirmed in 59; 55 of 59 had ultrasound-guided biopsies, and pathology results were available for 45; breast cancer was confirmed in 17 women, benign lesions in 24, and normal or inclusive results in 4. Breast conserving surgery was performed on 20 women, of whom eight had sentinel lymph node biopsies.
The Zambia strategy stands in striking contrast to the concept of starting with a center of excellence and moving outward, by starting with a successful distributed primary-care level service that is already established and developing the higher-level capacities. Not every country has such a program on which to build, but early signs of success suggest the viability and scalability of this approach.
Box 2. Brazil – expanded UHC improves equity of access.
The Brazilian government undertook a series of steps towards UHC following the establishment of health as a civil right in the 1988 Constitution.39 Other steps included decentralization of healthcare within a referral system to improve access, tax incentives to non-profit medical providers to favor a good standard of care, and specific financial health supports targeting small and mid-size cities to improve basic care.
All citizens are covered by a unified global free-of-charge health system. Cancer screening is recommended by the Brazilian National Cancer Institute (INCA) and offered at basic and intermediate levels. All data from mammographic screening and Pap smear screening are collected in a centralized unit at INCA (the SISMAMA and SISCOLO programs, respectively). Mammographic screening is offered biannually to all women 50 to 69 years old. Women age 40 to 49 are also included if additional risk factors (e.g., family history) are present40,41. Limitations regarding network and accessibility to a referral network have been addressed in multiple ways, depending on the local resources and political interests. There is no governmental organized screening, but publications in different regions suggest the network is adequate and includes quality assurance measurements.
There is no requirement for compulsory notification of a diagnosis of cancer, but all cancer drug regimens, radiation therapy, and surgeries do need an International Classification of Diseases (ICD) coding that can be tracked in the system. Registered referral institutions receive higher reimbursement per treatment than non-referral institutions. Cancer centers are classified into four levels of complexity, with most third and fourth level units are concentrated in the southern part of the country. INCA has provided cancer estimates since 1995, based on regional and institutional cancer registry databases.42 Breast cancer has the highest incidence among non-skin cancers in women in 4 out of 5 Brazilian regions. As a result of enormous regional social disparities, breast cancer incidence is the most common female cancer in the more developed South and Southeast states, but in underserved regions in some North and Northeast states, it is second to cervical cancer, which still has high mortality rates.
Brazil has emphasized health technology assessment in order to prioritize health spending. Cancer technology acquisition for the public health system undergoes a thorough evaluation by a committee at the Ministry of Health, and if approved, it should be available nationwide at cancer centers providing the level of care required by the technology. However, there is a shortfall of radiotherapy units in the country: the Brazilian Radiation Oncology Society estimates there is only 60% coverage by the system. Accessibility to chemotherapy and surgery are described as adequate, although more than 80% of the providers are concentrated in the South. Despite a national law stating that all patients should receive first treatment within 60 days of diagnosis, estimated waiting time for radiation oncology is 113 days.43
In addition to the public health system, there is a parallel private sector, covering 47 million Brazilians (although coverage is falling). A separate institution (ANS: Supplementary National Health Agency) determines technology acquisition. There is a lack of information regarding drug regimens, types of breast surgery performed, and oncological outcomes in the private sector.
There are centers of excellence across the country, with access to personalized therapy, research protocols, and multidisciplinary teams of subspecialties. Major referral centers report quality assurance metrics and aim to achieve the highest standard of care. But these centers are mainly located in South and Southeast, and some offer therapy mostly for the private sector. The challenge for the next years will be to reduce the disparities among regions and centers.
Box 3. Uruguay: Single-Payer Public Financing Leads to Universal Cancer Care.
Cancer services have been among the defined benefits of Uruguay’s health care system since it began a major reform in 2005, and following laws establishing the National Integrated Health System (NIHS) and National Health Insurance in 2007.44 Prior to the reform, access to good healthcare was based largely on wealth, with the well-off able to buy good coverage and the poor served by an under-resourced public sector. In the decade that has followed, the economy continued to strengthen and the country transitioned from middle- to high-income, according to the World Bank’s classification. Uruguay was the first country in Latin America to offer an explicit, comprehensive, and equal health care plan for its entire population, which corresponds to the “maximal” level for breast cancer control in BGHI’s resource-appropriate ladder. Arriving at the maximal level was achieved through providing equal access to existing resources rather than creating new services (although the quantity of services has also been expanded).
The reform brought changes to the broad healthcare model, management, and financing. The guiding principles of the reform are universal coverage, access, equity and continuity of health benefits, and sustainability of health services. Users pay an income-based amount into a national fund (averaging $80 per inhabitant in April 2019), which also receives contributions from companies (a payroll tax) and from the State. The National Fund pays all providers—public and private, 43 comprehensive health care providers in total—according to a capitation system, plus an amount based on “pay for performance” (PfP). Users choose their own comprehensive health care plan (public or private), which is the entity responsible for providing and coordinating all health care. People can also opt to pay other providers, including for “VIP services.” There are no co-pays with public providers—which serve 40% of the population—but private providers can collect a co-pay up to a government-set maximum.44
Each Comprehensive Health Care Provider signs a management contract with the National Board of Health, which lasts for two years. This contract includes the PfP goals which are continually ratcheted up. PfP goals for cancer currently are:
training health team members
improvement of breast, colorectal, and cervical cancer screening results entered in to registry
improvement of cancer screening coverage
improvement in the percentage of patients with positive screening studies who have timely diagnosis
improvement in percentage of diagnosed patients with timely and appropriate treatment
By law, women have the right to one day of paid leave to get a screening mammography and Pap smear with a recommended periodicity of every two years, and both tests are free of charge. In fact, women are required to complete these screening tests for employment, unless they decline with a formal “informed refusal.”
Uruguay’s breast cancer profile is similar to that of longstanding high-income countries: breast cancer has the highest incidence and mortality of any cancer among females, incidence has been stable over the last decade and death rates have fallen by an average of 1.1% per year from 1990–2015, and almost half of all cases are diagnosed in stage I and a quarter in stage II.
Starting with a well-developed, largely private health care system, Uruguay has deployed public policy to extend equitable access to the entire population with all care (both public and private) financed through the National Health Insurance. Actions against cancer are coordinated by the National Cancer Control Program, created in 2005, which prioritizes cancer prevention and appropriate and timely access to diagnosis and treatment, as well as access to palliative care. This includes access to high-cost medications such as trastuzumab, pertuzumab, and TDM1 for breast cancer. The country is pursuing continual improvement in cancer control, not only in access, but in results, monitored through a well-functioning cancer registry.
Figure 1.
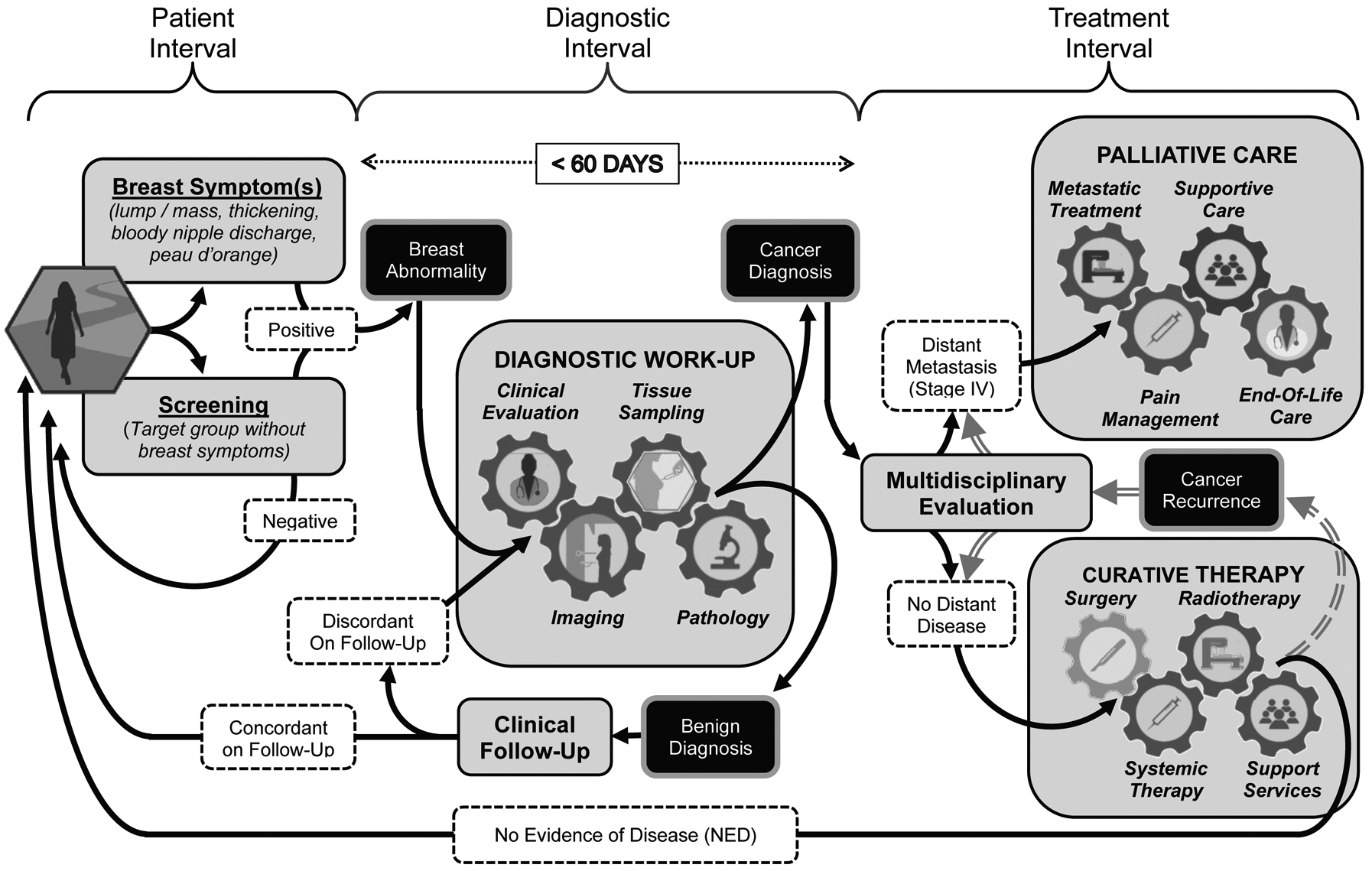
Breast Patient Pathway Overview
Universal patient pathway for breast cancer management in three sequential intervals of care including the Patient Interval the Diagnostic Interval, and the Treatment Interval. The Patient Interval spans the time from initial presentation based on onset of clinical symptoms or asymptomatic screening to the point where a breast abnormality warranting further evaluation is detected. During the Diagnostic Interval, the identified breast abnormality undergoes the ‘triple test’ work-up based on clinical evaluation, imaging and tissue sampling to achieve a definitive benign or malignant diagnosis. The health system should endeavor to complete the diagnostic work-up within a 60 day (2 month) period, because worsened survival outcomes can result from diagnostic delays extending significantly beyond 3 months. During the Treatment Interval, each patient undergoes individualized evaluation and treatment planning for curative therapy or palliative management, the selection of which depends on the extent of disease and potential for meaningful clinical improvement based on the application of realistically available resources.
Concepts and definitions
The World Health Organization laid out a framework for action on strengthening health systems, with six building blocks: service delivery; health workforce; information; medical products, vaccines, and technologies; financing; and leadership and governance.9 Health system strengthening was defined as:
improving these six health system building blocks and managing their interactions in ways that achieve more equitable and sustained improvements across health services and health outcomes. It requires both technical and political knowledge and action
(p4).
At the same time, it was noted that scaling up did not simply involve replicating existing models.
It is important to take note of what did and did not work in the past. Careful analysis is needed about which local initiatives are genuinely amenable for replication and expansion. Multiple barriers cannot all be addressed or overcome at once.
(p8).
The present paper draws on examples from individual countries and international initiatives to explore the ways in which phased implementation of breast cancer interventions can succeed. The focus is on the financing and governance components of the WHO building blocks.
According to the WHO:10
UHC means that all people and communities can use the promotive, preventive, curative, rehabilitative, and palliative health services they need, of sufficient quality to be effective, while also ensuring that the use of these services does not expose the user to financial hardship.
The path to UHC consists of expanding availability of services for different conditions, expanding the population coverage of these services, and decreasing reliance on out-of-pocket payments. All of this requires additional resources and ensuring that resources are allocated appropriately to achieve maximum benefit. WHO has provided some guidance on fairness in progressive realization of UHC.11 Different strategies to achieve UHC have different implications for equity, an issue that is taken up in the concluding section of this review.
Governance
It is tempting to think of expanding cancer services straight away by training more specialized health workers, providing more resources to acquire radiation machines or more chemotherapy agents, or buying mammography machines, but it is important first to think about the system in which these resources are embedded. If the environment is not conducive, pilot and demonstration programs may succeed within a narrow framework, but sustainable scale-up is unlikely. In this section, we look at the role of governance tools such as the Essential Medicines List (EML) and the new Essential Diagnostics List (EDL) as well as national cancer plans. The EML and EDL were developed by WHO as global guides, to be adapted by each country according to needs and resources.
The Essential Medicines List and the Essential Diagnostics List
The WHO EML is taking an increasingly important role in listing cancer medicines, as it has historically for medicines for infectious diseases. In 2015, 16 new cancer medicines were added to the EML “complementary list,” nearly doubling the number of listed drugs, which stands at 37. Some have argued that the inclusion of trastuzumab for breast cancer among these additions was particularly notable, on par with the addition of antiretrovirals in 2002, in that it demonstrated that high cost should not preclude the inclusion of biologically effective medicines12. Others argue that it has been more of a distraction from improving access to surgery and radiotherapy, without which chemotherapy is much less effective. For infectious diseases, the EML has played a role in increasing quality standards and drug availability, through mechanisms such as prequalification and pooled procurement, but this has had yet happened for cancer medicines.
The introduction of a WHO EDL in 2018 has the potential for far-reaching consequences. Appropriate treatment is dependent on appropriate and timely diagnosis.13 For cancer, the addition of histopathology, cytology, and immunohistochemistry tests in the 2019 WHO EDL is a significant step forward.
These international recommendations become truly effective only if they are nationally adapted and adopted and complemented by national treatment guidelines. Countries with lower national incomes tend to include fewer of the higher-priced breast cancer medicines from the WHO EML list in their lists, implicitly following a resource-stratified approach. A recent study of 75 LMICs found that 71–78% of national EMLs included tamoxifen and first-generation chemotherapies, thereby making possible treatment for “Luminal A” early breast cancer.14 Only 60% of low-income countries included these drugs, however. HER-2 targeted therapies, taxanes and aromatase inhibitors were considerably less likely to be included in LMIC lists. One study of drug availability for seven cancers (including both early-stage and metastatic breast cancer) found that even if cancer medicines were included on national EMLs, they were not necessarily publicly funded or subsidized, particularly in lower-income countries.15
Even if cancer medicines are included as benefits of national health insurance (NHI), cancer diagnostics are frequently not publicly funded in practice. Thus, only those people able to afford diagnostic tests can access publicly funded medicines. As national EDLs are developed, and diagnostics are included in NHI, this barrier may be reduced.
National cancer control plans
National cancer control plans (NCCPs) are another key aspect of an implementation framework, and the International Cancer Control Partnership provides support for their development and maintains an online NCCP repository (https://www.iccp-portal.org/). The first global analysis of existing country-level NCCPs noted that the number of countries with either an NCD plan including cancer, or a stand-alone NCCP, increased steadily between 2000 and 2015, although low-income countries were markedly less likely than high-income ones to have an NCCP.16 Over the period from 2000 to 2015, NCCPs became more likely to be scored more highly on comprehensiveness, consistency, and coherency; to have cancer-specific goals; to address survivorship; to mention cancer registries; and to be correlated with declines in male smoking rates. A major gap identified was that only 10% of plans had associated costing or budgeting components. Table 1 provides some examples of how content of the plans evolves as country resources increase, with reference specifically to breast cancer.
The role of national health insurance
In the absence of insurance or publicly subsidized service provision, breast cancer diagnosis and treatment creates financial hardship for all but the wealthiest patients. Broad access to the full range of services available depends heavily on UHC. In some countries this takes the form of certain services being provided free in public facilities (financed by taxation), while in many countries this takes the form of NHI. NHI generally means that legislation exists ensuring that the national population is covered for a set of defined health services. The way that services are provided (publicly or privately) and how payment for the services is made, vary widely. Countries that lack either no-user fee provision of services or NHI almost always have poor cancer outcomes related to late cancer presentation, delayed cancer diagnosis, and inadequate or incomplete cancer treatment, which, even as it fails to provide high-quality care to the patient, can leave families impoverished.
In the lower-middle-income countries in sub-Saharan Africa, NHI is gradually being established and coverage expanded. Ghana has one of the longer-established NHI schemes in the region, dating from 2003 legislation. Breast and cervical cancers are the only cancers that are covered, and although by legislation many aspects of diagnostics and treatment should be covered, in actual practice there are limitations. Mammography is covered for diagnostic purposes although not widely available, along with breast tissue biopsy and histopathology, but other diagnostics such as investigation of receptors and immunohistochemistry are not specifically included.17 Among therapies, tamoxifen and anastrozole (an aromatase inhibitor) are covered, along with half a dozen older chemotherapies,18 out of the longer list of cancer therapies on Ghana’s Essential Medicines List.19 Although surgery is usually covered, anesthesia and radiotherapy are not always. Some issues have arisen in the implementation of insurance. In some cases, because of slow payment by the insurance fund, service providers require patients to pay for services rather than accepting insurance cards. In other cases, service providers argue that the NHI rates for certain services are below the cost of providing services and require additional payments from patients. Building up new insurance schemes takes time, and it takes time and expertise to get the details correct. Evidence from the United States suggests that among women with health insurance that covers breast cancer services, higher out-of-pocket costs (because of high deductible health plans) cause delays in accessing all aspects of care, from screening through beginning treatment.20
In other sub-Saharan countries with less mature insurance schemes, any breast cancer coverage tends to be more recent, more variable (for example insurance varies by county in Kenya), and is more accessible to groups such as public servants than the general population. It is too soon to assess the impact of insurance on cancer survival in this group of countries.
The upper-middle income countries in Latin America and Asia have broader population enrolment in their NHI schemes, and correspondingly better coverage of breast cancer diagnosis and treatment. Thailand is an example of fairly broad NHI and Malaysia is an example where provision of basic cancer services is provided at no cost to the user in public facilities. The Thai NHI scheme emerged following elections in 2001 and merged the two previous schemes covering the poor, who constitute 74% of those covered. 17% of the population is covered by a contributory scheme for the formal sector, and 9% by the civil servants’ scheme. The three schemes are financed by a mixture of contributions as well as general taxation.21 The benefits cover diagnostics and treatment for breast cancer as well as clinical breast exams, but not mammography screening.
In Malaysia, public and private systems of care co-exist. The public system provides free services but has longer waiting times. The services available publicly for diagnosis and treatment have expanded over time. Trastuzumab is covered, but budget limitations mean that not all those who are eligible receive the drug.22 Once patients present to health facilities, access to diagnosis, surgery, and treatment is timely, and those who choose to receive treatment (75–80% of those presenting), do so.22 However, late presentation (40% of those presenting, a number which has not changed over a decade) is a problem, with significant variation among the three main ethnic groups.23
Latin America has generally tended more towards two-tier systems of UHC. Colombia is an example where 80% of the population is covered by some form of insurance. A subsidized regime for the poor offers low-complexity care and catastrophic coverage but limited coverage of hospital care, whereas a contributory regime offers more comprehensive benefits and access to higher quality care in the private sector. There are big differences in the proportion of those presenting with early-stage breast cancer by sector: 53.3% in the private as compared to only 19.7% in the public sector.24 By comparison, the UHC systems in middle-income Brazil (Box 2) and Peru, and high-income Uruguay (Box 3) are unusual for Latin America, with broader UHC coverage, falling into the top quintile worldwide for coverage, along with the OECD countries.25
Expanding access alone is a necessary but not sufficient condition for improving breast cancer outcomes. Emphasizing quality is also vital.26
Platforms for implementation and synergies within an expanding health infrastructure
Phasing in the systems required to detect, diagnose, and treat breast cancer – and to finance all it – is a daunting task. It requires expanding shared services such as diagnostics, surgery and radiotherapy, and as well as training specialized health workers. Countries have used a variety of paths, depending on the services available at their starting point; there is no one-size-fits-all model. New innovations are being tried, such as the City Cancer Challenge, for which it is too early to see the outcomes (Box 4). A major constraint on financial planning for expansion is the almost complete lack of data from LMICs of the costs of new services, making budgeting very difficult. Some progress is being made in this area, including better costing studies27 and the development of “investment cases” for cancer.
Box 4. The City Cancer Challenge.
Most of the world’s population lives in cities, and most of the world’s cancer resources are concentrated in them. But cancer services, particularly in LMICs may be uncoordinated, of variable quality, and financially inaccessible to many or most inhabitants. City Cancer Challenge (C/Can) was established by the Union for International Cancer Control in January 2017 (and is now an independent non-profit) to provide a framework and resources to guide the development of a data-driven plan for high quality, accessible, city-wide, coordinated cancer services.45 All analytical work and planning is carried out by the local partners, including INGOs, professional associations, UN Agencies, bilateral and multilateral agencies, private companies, government, and city leaders, based on a memorandum of understanding including all local parties and C/Can.
Seven cities have started the process: Cali, Colombia; Asunción, Paraguay; Yangon, Myanmar; Kumasi, Ghana; Porto Alegre, Brazil; Kigali, Rwanda; and Tbilisi, Georgia and implementation is just beginning. C/Can’s goal is to initiate the process in 20 cities (each with over 1 million population) by 2020. Each city is guided by its own City Executive Committee, and an assessment of existing capacities and needs, gaps and priorities for cancer diagnosis, treatment, and care is carried out by a technical committee. C/Can’s role to provide the tools and resources for local experts to complete the preliminary tasks, as advisors, and to help find funding partners.
Cali, Asuncion, and Yangon are at various stages of starting to implement their projects. By the end of year one, the goal is that these cities should have: operational multidisciplinary teams for cervical, breast, and colorectal cancer in the hospitals treating these kinds of cancer patients; locally-adapted cancer management guidelines and treatment protocols for those cancers, with the intention of expanding to other cancers; an updated essential oncology medicine list for adults, paediatric, and palliative care patients; a cancer care human resources training plan; a plan for radiotherapy development; quality assurance programs in pathology and radiotherapy and a manual of standard operating procedures for pathology; and a palliative care strategy. Measurable results in cancer care access and outcomes should start to be available in a few years.
Centre of excellence model
Providing a basic structure for the proper diagnosis and treatment of cancer is essential for the success of cancer programs in LMICs.28 One successful strategy is to create public or not-for-profit, centralized diagnostic and treatment centers—cancer centers—strategically located in certain geographic regions, where imaging facilities, diagnostic services, surgical procedures, basic radiotherapy equipment, and essential anticancer agents for the treatment of highly curable forms of cancer and agents for pain control are available. The centers can range from departments in existing facilities to dedicated new construction.
Starting in the 1940s, several countries in Latin America began to create National Cancer Institutes, offering specialized treatment to cancer patients, but also promoting the education and training of qualified professionals and developing research capacity. Almost all of these institutes had the intention of becoming health policymaking focal points for cancer. To expand cancer care nationally, some institutes began to promote the creation of cancer centres outside the capital. Guided by the Mexican Institute of Cancer (INCan), this process began in Mexico during the 1980s. Expansion stopped, however, after the inauguration of just two centers, in the states of Guerrero and Colima. In the 1970s, the Cuban National Institute of Oncology and Radiobiology (INOR) was able to integrate two cancer centres located in the centre and east of the country (Camagüey and Santiago de Cuba) with the newly created Cancer Units in four other provinces in a cancer care network. In Brazil, Colombia, Panama, Paraguay, Peru, and Uruguay, national institutes or programs were also created under Ministries of Health, but no cancer care network was created. National institutes were also created in Guatemala and El Salvador, but by non-governmental organizations, whose potential influence on national policy was weaker. Although several of the institutes played important roles as national reference and training centres for cancer care, most of them failed to consolidate a true network of care centres. Most of the institutes were intended to be the managers for the implementation of cancer policies nationally. While some institutes have achieved this goal, especially those linked to the implementation of National Programs for Cancer Control (Colombia, Cuba, and Peru), unfortunately for most, this has not been achieved.
Building on existing disease platforms
Several lower-middle income countries are using what might be termed a diagonal approach,29 2010), such as building a breast cancer program on a cervical cancer platform (for example, Zambia and Tanzania); or on a reproductive health platform (for example demonstration projects in two states in India). Several cervical cancer programs have, in turn, been built on HIV services. In this approach, the expansion into breast cancer care puts pressure on the health system to expand other services such as radiotherapy and diagnostics, which benefit both other cancers and some other diseases.
Building on a cervical cancer platform has advantages because of common resource needs in cancer treatment, as demonstrated in the Zambia case study (Box 1). The newly developed program benefitted from radiotherapy services at The Cancer Hospital in Lusaka, which had been established with IAEA support in 2007, and the integration of breast cancer clinic staff in the rural outreach camps for cervical cancer prevention.30 An important factor is that the Zambian government covers the screening and treatment costs at public facilities. There is potential to expand this model in other sub-Saharan African countries, where international donors have provided funding to expand cervical cancer services, for example, new initiatives for cervical cancer in Mozambique and Malawi.31
Building on a maternal and child health platform has advantages, since these platforms are widely available and were strengthened during the Millennium Development Goals. Jhpiego has an ongoing demonstration project (Ginsburg et al, paper 4) to build a breast healthcare pathway in two of the lower-income states in India. This project developed the capacity of the ashas (community health workers) who provide maternal and child health services, to increase awareness of signs of breast cancer, and to refer women for evaluation. India has extensive national plans and frameworks for cancer care. Jhpiego also supported the integration of breast health care with cervical cancer services in Tanzania (Ginsburg et al, paper 4).
A recent pilot program in Cali, Colombia, has approached improving breast and cervical cancer control by first assessing barriers to effective screening, diagnosis, and treatment, and customizing solutions in three healthcare delivery sectors of the city. The goal is integration with the existing public health system. The work was organized by a partnership of two non-profit organizations, one from the United States and one Colombia. The major components instituted in response to the barriers identified are 1) a patient navigation system, 2) adding infrastructure (e.g., diagnostic technology) where needed, 3) a community education program, and 4) professional education. The results have been impressive, in reduced waiting times for all aspects of care and steady increases in women served, in a program now under public control. The program has begun expanding to other cities in Colombia and appears to be a successful and replicable model.32
Synergies in adding resources for cancer and other unmet or rising healthcare needs
Cancer control cannot be successful unless fully integrated into the healthcare infrastructure, where sharing of human and material resources can take place, and where improvements in the management of one condition can benefit a range of others, either directly or by bootstrapping. At the low-resource end, Zambia provides a good example of a well-funded primary care-level HIV/AIDS program that became the platform for a limited and then population-wide cervical cancer screening and treatment, and then a platform for breast cancer screening, which laid the groundwork for more specialized breast cancer management at higher levels in the healthcare system (see Box 1). The latter investments will have positive spillover not only for other cancers, but for other NCDs, in particular.
One of the scarcest human resources are surgeons, who provide the frontline intervention for all early solid tumors, including breast cancer. According to the recent Lancet Commission on Global Surgery, surgery and anesthesia are prerequisite to attaining national and global health goals “in areas as diverse as cancer, injury, cardiovascular disease, infection, and reproductive, maternal, neonatal, and child health”.33 Clearly, many surgeons are required, but they need not mainly be specialists. Instead, general surgeons can be trained to perform basic cancer surgery procedures such as the modified radical mastectomy. Other investments that will benefit cancer care but also will result in generally raising the level of healthcare delivery and coverage include system-wide information systems, various types of imaging, pharmacy, and laboratory services.
Cancer control also requires some dedicated technologies and resources. Radiotherapy is the most prominent and costly element; others include diagnostic breast imaging (such as mammography) and surgical pathology, none of which is broadly useful for other conditions.
The practical implication of recognizing synergies among disease investments is that planning—including national cancer control planning—will fail to achieve the greatest efficiencies if conducted in silos. The same holds for advocacy for investments, both with governments, international organizations, and NGOs. Even advocacy for cancer-specific elements might benefit from a more focused funding campaign.
Conclusions
This review has discussed aspects of governance and financing of health systems, as well as some examples of how a breast cancer program can be built within an existing health system – and can in turn help to strengthen the health system. The country examples show that there are some common patterns of phasing in care, dictated by resource availability. At the same time, context matters. We conclude here by discussing how the choices that countries make can have big impacts on equity in access to care and affordability.
Inequities in geographic access to breast cancer care abound, since some of the aspects of care have to be located in specialized facilities, usually in urban areas (radiotherapy, breast clinics, specialized diagnostics, mammography machines). Even in high income countries, patients in rural and remote areas are disadvantaged in access to cancer care. Socioeconomic inequities also abound.
If key components of care (such as specialized diagnostics and radiotherapy in Ghana) are not covered by health insurance, this disadvantages poorer households. Many countries have some combination of public and private provision of services, and public and private insurance. The Uruguay case study suggests that outcomes are more equitable when there is a strong component of payment from a single (public) payer, which facilitates holding both public and private service providers to uniform standards of coverage and care. The Malaysian example likewise confirms that commitment to funding and quality in the public system is important for equity. By contrast, the more common Latin American model of a lower quality, underfunded, public system of care coexisting with a separate private system, results in poorer cancer outcomes across the population.
Affordability of cancer care is important not only for individual patients and their families, but also for the public budget. Trastuzumab, mentioned several times, is an example of a therapy that, when provided as part of a complete treatment regimen, is very effective for women with cancers that overexpress the HER2 oncogene. It was added to the WHO EML as adjuvant therapy in patients with early-stage HER2-positive breast cancer. At the same time, the medication is expensive. In the US, a course of trastuzumab was estimated to cost $70,000 in 2012.34 Even as trastuzumab began to come off patent, a course of the Indian biosimilar Biocon still cost almost $10,000 in 2014.35 In both countries, the one-year treatment cost more than per capita national income. Recent guidelines36 on advanced breast cancer commented that biosimilars should first pass the development and validation processes of a strict authority such as the European Medicines Agency or the Food and Drug Administration and urged that “the price of biosimilars should be substantially lower than the original compounds” (p 1639) to increase availability of treatment. When costly antiretrovirals for HIV/AIDS were added to national EMLs, public pressure led to tiered pricing from manufacturers to render them more affordable in LMICs – something that did not happen even after trastuzumab was added to the WHO EML.
As nearly all countries move toward UHC, broad access to the full range of breast (and other) cancer services can take a variety of paths. In all cases, the expansion has serious financial implications for governments, but it is clear from experience in different countries that the way the expansion is structured can make it more or less successful and more or less costly. In the past, at least in some countries, cancer services have expanded in an uncoordinated fashion, with largely unsatisfactory results. Basing the architecture of the expansion on an assessment of the strengths and weaknesses of the existing health infrastructure and workforce, and other local factors, can produce a feasible and, ultimately sustainable system. Stewards of NCCPs can expect continued challenges to achieve and maintain broad access to breast cancer services, to rein in costs as ever more expensive, but effective, drugs come on line, and to maintain adequate support in the face of the competing demands of other health conditions.
Acknowledgements:
The authors thank Andre Ilbawi of WHO and Lai Meng Looi, Inaugural Distinguished Professor, Malaysia for helpful references.
Funding:
The BHGI Global Summit was funded by grants from the Fred Hutchinson Cancer Research Center; Susan G. Komen (GSP18BHGI001); National Comprehensive Cancer Network; US National Institutes of Health (1R13CA224776-01A1); National Cancer Institute Center for Global Health; American Society of Clinical Oncology; American Society of Clinical Pathology; Journal of Global Oncology; National Breast Cancer Foundation, Inc.; pH Trust; Seattle Cancer Care Alliance; Union for International Cancer Control; University of Washington Department of Global Health. Support from unrestricted educational grants came from Cepheid; GE Healthcare; Novartis; Pfizer Inc.; and UE LifeSciences. Additional funding to cover publication costs was provided by GE Healthcare and Novartis.
The Susan G. Komen Leadership Grant (SAC170082) supported BOA, CD and AD. No additional funding was provided for writing this particular paper.
Footnotes
Conflict of interest statement:
The authors declare no conflicts of interest.
References
- 1.World Economic Forum and WHO. From Burden to “Best Buys”: Reducing the Economic Impact of Non-Communicable Diseases in Low- and Middle-Income Countries. World Economic Forum, 2011. https://www.who.int/nmh/publications/best_buys_summary.pdf Accessed April 2, 2019. [Google Scholar]
- 2.WHO. Tackling NCDs: ‘best buys’ and other recommended interventions for the prevention and control of noncommunicable diseases. 2017b. Geneva: World Health Organization; https://apps.who.int/iris/bitstream/handle/10665/259232/WHO-NMH-NVI-17.9-eng.pdf?sequence=1 Accessed April 2, 2019. [Google Scholar]
- 3.World Health Assembly. Resolution WHA70.12, Cancer prevention and control in the context of an integrated approach. Seventieth World Health Assembly, 31 May 2017. http://apps.who.int/gb/ebwha/pdf_files/WHA70/A70_R12-en.pdf Accessed October 24, 2019. [Google Scholar]
- 4.Meheus F. Planning and costing cancer control interventions: a health economics perspective Presentation to UICC World Cancer Leaders Summit, 15–17 October 2019, Nur-Sultan, Kazakhstan: https://www.uicc.org/sites/main/files/atoms/files/Filip%20Meheus_keynote%20presentation.pdf Accessed December 19, 2019. [Google Scholar]
- 5.Anderson BO, Yip CH, Smith RA, et al. Guideline implementation for breast healthcare in low-income and middle-income countries: overview of the Breast Health Global Initiative Global Summit 2007. Cancer 2008;113(8 Suppl):2221–2243 [DOI] [PubMed] [Google Scholar]
- 6.IHME (International Health Metrics and Evaluation) GBD Compare: Viz Hub (data for 2017): https://vizhub.healthdata.org/gbd-compare/ Accessed April 2, 2019.
- 7.Harford J, Azavedo E, Fischietto M. Guideline Implementation for Breast Healthcare in Low- and Middle-Income Countries: Breast Healthcare Program Resource Allocation. Cancer 113;2008(Supp):2282–2296. [DOI] [PubMed] [Google Scholar]
- 8.Harford JB, Otero IV, Anderson BO, Cazap E, Gradishar WJ, Gralow JR et al. Problem solving for breast health care delivery in low and middle resource countries (LMCs): consensus statement from the Breast Health Global Initiative. The Breast 2011;20:S20–S29. [DOI] [PubMed] [Google Scholar]
- 9.WHO. Everybody’s business — Strengthening health systems to improve health outcomes. WHO’s framework for action 2007. Geneva: World Health Organization; http://www.who.int/healthsystems/strategy/everybodys_business.pdf Accessed October 24, 2019. [Google Scholar]
- 10.WHO (2019). What is health financing for universal coverage. https://www.who.int/health_financing/universal_coverage_definition/en/ Accessed September 3, 2019.
- 11.WHO Consultative Group. Making fair choices on the path to universal health coverage Final report of the WHO Consultative Group on Equity and Universal Health Coverage, 2014. https://www.who.int/choice/documents/making_fair_choices/en/ Accessed May 5, 2019.
- 12.Jarvis J, Kishore S. WHO takes a big step to promote cancer treatment worldwide. The Lancet United States of Health blog May 18, 2015. https://marlin-prod.literatumonline.com/pb-assets/Lancet/lancet/TL_USA_Posts.pdf Accessed April 2, 2019. [Google Scholar]
- 13.Pai M, Walia K, Boehme K. Essential medicines and essential diagnostics: a package deal. Lancet, 2019,4(1)):e492. [DOI] [PubMed] [Google Scholar]
- 14.Bazargani YT, de Boer A, Schellens JHM, Leufkens HGM, Mantel_Teeuwisse AK. Essential medicines for breast cancer in low and middle income countries BMC Cancer 2015;15:591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cherny NI, Sullivan R, Torode J, Saar M, Eniu A. ESMO International Consortium Study on the availability, out-of-pocket costs and accessibility of antineoplastic medicines in countries outside of Europe. Ann Oncol 2017;28:2633–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romero Y, Trapani D, Johnson S, Tittenbrun Z, Given L, Hohman K, Stevens L, Torode JS, Boniol Ilbawi AM. National cancer control plans: a global analysis. Lancet Oncol 2018;19:e546–555. [DOI] [PubMed] [Google Scholar]
- 17.NHIS (National Health Insurance Scheme) (Ghana). G-DRG Revised Tariffs 2019: Tariffs for diagnostic centre. Accra: National Health Insurance Scheme; 2019. [Google Scholar]
- 18.NHIS (National Health Insurance Scheme) (Ghana) Medicines List. 2018. http://www.nhis.gov.gh/files/2018%20NHIS%20ML.pdf Accessed December 2, 2018.
- 19.Ministry of Health, Republic of Ghana: Ghana National Drugs Programme. Ghana Essential Medicines List (7th edition), 2017. Accra, Ghana: Ministry of Health; 2017. [Google Scholar]
- 20.Wharam JF, Zhang F, Lu CY, Wagner AK, Nekhlyudov L, Earle CC, et al. Breast Cancer Diagnosis and Treatment After High- Deductible Insurance Enrollment. J Clin Oncol. 2019;36:1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Center for Global Development. Millions Saved: Thailand’s Universal Coverage scheme. 2015. http://millionssaved.cgdev.org/case-studies/thailands-universal-coverage-scheme Accessed April 14, 2019.
- 22.Lim GCC, Aina EN, Cheah SK, Ismail F, Ho GF, Tho LM et al. (2014). Closing the global cancer divide- performance of breast cancer care services in a middle income developing country. BMC Cancer. 2014;14:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yip CH, Bhoo Pathy N, Teo SH. A Review of Breast Cancer Research in Malaysia. Med J Malaysia 2014;69:(Supp A):8–14. [PubMed] [Google Scholar]
- 24.Murillo R, Diaz S, Sánchez O, Perry F, Piñeros M, Povedad C et al. Pilot Implementation of Breast Cancer Early Detection Programs in Colombia. Breast Care. 2008;3:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO. Universal Health Coverage. Monitoring report, 2017 2017a. https://www.who.int/healthinfo/universal_health_coverage/report/2017/en/ Accessed April 14, 2019.
- 26.Kruk ME, Gage AD, Arsenault C, Jordan K, Leslie HH, Roder-DeWan S et al. Quality Health Systems in the Sustainable Development Goals era: time for a revolution. Lancet Glob Health. 2018;6:e1196–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horton S, Gupta S. Costing Methods Applied to Cancer. J Glob Oncol. 2019;5:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson BO. Global Summit Consensus Conference on International Breast Health Care: guidelines for countries with limited resources. Breast J. 2003;9(Suppl 2):S40–41. [DOI] [PubMed] [Google Scholar]
- 29.Frenk J The global health system: strengthening national health systems as the next step for global progress. PLoS Med 2010;7:e1000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kapambwe S, Mwanahamuntu M, Pinder LF, Chisele S, Chirwa SC, Parham GP. Partnering with traditional Chiefs to expand access to cervical cancer prevention services in rural Zambia Int J Gynaecol Obstet. 2019;144:297–301. [DOI] [PubMed] [Google Scholar]
- 31.USAID Office of Press Relations. USAID announces $12 million for the prevention of cervical cancer in Sub-Saharan Africa. News Release, January 31, 2019. https://www.usaid.gov/news-information/press-releases/jan-31-2019-usaid-announces-12-million-prevention-cervical-cancer-sub-saharan-africa Accessed April 15, 2019.
- 32.Sardi A, Orozco-Urdaneta M, Velez-Mejia C, Perez-Bustos AH. Overcoming Barriers in the Implementation of Programs for Breast and Cervical Cancers in Cali, Colombia: A Pilot Model special article abstract. J Glob Oncol. 2019;5:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan R, Alatise OI, Anderson BO, et al. Global cancer surgery: delivering safe, affordable, and timely cancer surgery. Lancet Oncol. 2015;16:1193–1224. [DOI] [PubMed] [Google Scholar]
- 34.Nordqvist C One Year on Herceptin for breast cancer ideal. [Internet] Medical News Today Newsletter October 1 2012. https://www.medicalnewstoday.com/articles/250912.php Accessed April 26, 2019. [Google Scholar]
- 35.Menon-Sen K Campaign for Affordable Trastuzumab: Bio-similar of breast cancer drug launched but still out of reach for most, Jan 20, 2014. [Internet]. https://donttradeourlivesaway.wordpress.com/2014/01/22/campaign-for-affordable-trastuzumab-bio-similar-of-breast-cancer-drug-launched-but-still-out-of-reach-for-most/ Accessed April 25, 2019.
- 36.Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, Andre F et al. 4th ESO–ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4). Annals Oncol 2018;29:1634–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chibwesha C, Pinder LF, Musonda A, Sikombe K, Matambo J, Bateman AC, et al. A comprehensive assessment of breast and cervical cancer control infrastructure in Zambia. J Cancer Policy. 2017;13:24–9. [Google Scholar]
- 38.Pinder LF, Henry-Tillman R, Linyama D, Nzayisenga J-B, Shibemba A, Sahasrabuddhe V, et al. Leverage of an Existing Cervical Cancer Prevention Service Platform to Initiate Breast Cancer Control Services in Zambia: Experiences and Early Outcomes. J Glob Oncol. 2017;4:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Constituição da República Federativa do Brasil de 1988. Vide Emenda Constitucional n° 91, de 2016 [Google Scholar]
- 40.INCA (Instituto Nacional de Câncer José Alencar Gomes da Silva). Diretrizes para a detecção precoce do câncer de mama no Brasil. Rio de Janeiro: Ministério do Saúde; 2015. [Google Scholar]
- 41.Migowski A et al. Diretrizes para detecção precoce do câncer de mama no Brasil. II - Novas recomendações nacionais, principais evidências e controvérsias. Cad Saúde Pública [online]. 2018;34:e00074817. [DOI] [PubMed] [Google Scholar]
- 42.INCA. Estimativa 2018: incidência de câncer no Brasil. Coordenação de Prevenção e Vigilância Rio de Janeiro: Ministério do Saúde; 2018. [Google Scholar]
- 43.Amendola B, Quarneti A, Rosa AA, Sarria G, Amendola M. Perspectives on Patient Access to Radiation Oncology Services in South America. Semin Radiat Oncol. 2017;27:169–175. [DOI] [PubMed] [Google Scholar]
- 44.Arbulo V, Castelao G, Oreggioni I, Pagano JP. Uruguay. Building up the national integrated health system Geneva: WHO Health Systems and Governance: Improving Health Systems Efficiency; https://apps.who.int/iris/bitstream/handle/10665/187934/WHO_HIS_HGF_CaseStudy_15.10_eng.pdf;jsessionid=BF34CF8358C1D697CD205BB8B94768E6?sequence=1 Accessed Oct 24, 2019. [Google Scholar]
- 45.City Cancer Challenge. [Website] 2019. http://www.citycancerchallenge.org Accessed May 5, 2019.


